Abstract
Purpose:
Patients with human epidermal growth factor receptor 2-positive (HER2+) breast cancer have a high incidence of brain metastases, and trastuzumab emtansine (T-DM1) is often employed. Stereotactic radiosurgery (SRS) is frequently utilized, and case series report increased toxicity with combination SRS and T-DM1. We provide an update of our experience of T-DM1 and SRS evaluating risk of clinically significant radionecrosis (CSRN) and propose a mechanism for this toxicity.
Methods:
Patients with breast cancer who were ≤45 years regardless of HER2 status or had HER2+ disease regardless of age and underwent SRS for brain metastases were included. Rates of CSRN, SRS data, and details of T-DM1 administration were recorded. Proliferation and astrocytic swelling studies were performed to elucidate mechanisms of toxicity.
Results:
A total of 45 patients were identified; 66.7% were HER2+, and 60.0% were ≤ 45 years old. Of the entire cohort, 10 patients (22.2%) developed CSRN, 9 of whom received T-DM1. CSRN was observed in 39.1% of patients who received T-DM1 vs. 4.5% of patients who did not. Receipt of T-DM1 was associated with a 13.5-fold (p = 0.02) increase in CSRN. Mechanistically, T-DM1 targeted reactive astrocytes and increased radiation-induced cytotoxicity and astrocytic swelling via upregulation of Aquaporin-4 (Aqp4).
Conclusion:
The strong correlation between development of CSRN after SRS and T-DM1 warrants prospective studies controlling for variations in timing of T-DM1 and radiation dosing to further stratify risk of CSRN and mitigate toxicity. Until such studies are completed, we advise caution in the combination of SRS and T-DM1.
Keywords: radiosurgery, radionecrosis, breast cancer, HER2, trastuzumab emtansine
Introduction
The estimated number of new cases of breast cancer in the United States in 2018 was 266,1201 with approximately 20% being HER2-positive(HER2+)2. Overall, 5–15% of breast cancer patients will develop brain metastases in their lifetime3, although these rates may be 2–4 times higher in patients with HER2+ disease4. HER2-positivity and young age have both been associated with increased risk of developing intracranial metastases in patients with breast cancer5. Stereotactic radiosurgery (SRS) plays a critical role in the contemporary management of brain metastases, with excellent local control rates and an improved toxicity profile compared to whole brain radiation (WBRT). Systemic therapies including trastuzumab emtansine T-DM1 can be effective in treating patients with HER2+ breast cancer with CNS disease, with T-DM1 showing improved median survival without increased risk for CNS progression compared to capecitabine-lapatinib in the EMILIA trial6,7. Jacot, et al, have also shown T-DM1 to be safe and effective in patients with HER2+ breast cancer with brain metastases8.
Although both SRS and T-DM1 are important therapies for HER2+ populations with brain metastases, a number of series, including a previous report from our institution, have suggested increased rates of clinically significant radiation necrosis with their combination9,10. Given clinical findings consistent primarily with edema, and the known role of Aquaporin-4 (Aqp4) in neuroinflammation, we further focused on Aqp4 changes after therapy. In this analysis, we update our institutional series and provide pre-clinical evidence suggesting that unintended T-DM1 targeting of reactive astrocytes surrounding brain metastases is a mechanism underlying T-DM1/SRS-induced toxicity.
Materials and Methods
Patient Selection
Under Institutional Review Board (IRB) approval, utilizing the MOSAIQ® Radiation Oncology software, institutional records were queried to select for female patients with a diagnosis of breast cancer (utilizing ICD-9 and ICD-10 codes) treated with radiosurgery to the brain during the years 2004–2017. Fraction number was limited to five fractions or less to account for fractionated SRS (fSRS). Chart review was then completed to select for patients less than or equal to 45 years of age with any HER2 status as well as patients who had HER2+ tumors regardless of age given the association of increased risk of brain metastases in both of these subgroups5. These two groups represent a population in our institution who received multiple systemic treatment courses and SRS over their extended lifetime.
Patient Demographics and Treatment Variables
Pertinent patient demographics and treatment characteristics were included in this series. The American Joint Committee on Cancer (AJCC) staging system was utilized to determine stage at diagnosis11. Age, hormone receptor status (ER/PR status), HER2 status, and systemic therapy were noted in the retrospective review. Dates of both primary diagnosis and diagnosis of brain metastases were recorded. Utilization of T-DM1 in patients’ treatment course, including dates of treatment, were documented. Patients were defined as having received concurrent T-DM1 if they received the drug within ≤ 4 weeks of any course of SRS. Charts were queried for diagnosis of CSRN and treatment was captured for each case. CSRN was defined as neurologic symptoms warranting hospital admission and treatment. If craniotomy was a modality of treatment, pathology was recorded. In cases with a diagnosis of CSRN, imaging and prior SRS treatments were reviewed to correlate the lesion associated with CSRN. Dosimetric data from the SRS course resulting in CSRN were recorded including prescription dose, fraction number, prescription isodose line, maximum point dose, volume treated, and planning target volume (PTV) margin for each case of CSRN. For all patients, total number of SRS courses, total lifetime number of lesions treated, and receipt of WBRT were documented.
Mechanism of Toxicity
De-identified samples from Her2+ brain metastases were obtained from archival paraffin embedded tissue under an approved IRB protocol at the University of Colorado. Informed written consent was obtained from donors and studies were conducted in accordance with recognized ethical guidelines. Human adult astrocytes immortalized by hTERT and SV40 (referred as THV cells) were a kind gift of Dr. Paul B. Fisher (Virginia Commonwealth University). THV cells were maintained in high glucose Dulbecco’s Modified Eagle Medium (Life Technologies) supplemented with 10% FBS (Life Technologies) at 37°C in 5% CO2.
Proliferation assays
THV cells were plated at 1000/cell per well in 96 well plates and treated with indicated doses of radiation (RT) in a Rad Source RS2000 irradiator. Following irradiation, cells were treated with vehicle (PBS), 1 μg/ml trastuzumab or 1 μg/ml T-DM1 (Genetech), 10μM cisplatin, or 0.1 μM paclitaxel as indicated, and cells were imaged over time using Live Cell Incucyte Imaging (Essen Bioscience). Cell confluence per well was calculated in 3 fields per well in at least 4 replicates per treatment and three independent experiments.
Immunohistochemistry and digital imaging
Immunostaining was performed using rat anti-GFAP (13–0300, Invitrogen) and rabbit anti-aquaporin 4 (AB3594, Millipore). For immunofluorescence analysis, images were collected using a Nikon Eclipse Ti-S inverted microscope. ROIs were made using Confocal Uniovi 1–51 and an ImageJ bundle, and then digital images were exported as tiff files to Adobe Photoshop. Minor linear adjustments to brightness and contrast were performed identically and in parallel. For quantification of astrocytes cell size, a minimum of 50 cells per treatment in two independent experiments were quantified. Cell area was manually delineated using the drawing tool of NIS-Elements software (version 4.30.01), and cell area was calculated using NIS-Elements software. Western blots were imaged and quantified using Odyssey CLx Imaging System and Image Studio Software v.5.2.5 LI-COR Biosciences.
Statistical Analysis
Statistical analyses were performed using SPSS version 24.0 (SPSS Inc., Chicago, IL). Logistic regression models were used to estimate odds ratios (OR) for the risk of CSRN associated with receipt of T-DM1 at any point throughout the patient’s treatment course, receipt of T-DM1 concurrent with SRS, age, total number of SRS courses, total number of lesions treated, and receipt of WBRT.
For in vitro studies, statistics were done using Graphpad Prism 7.3 software (GraphPad Software Inc, La Jolla, CA). One-way ANOVA or repeated measures ANOVA followed by multiple comparison post hoc tests were performed, as appropriate. P<0.05 was considered significant, and test assumptions were checked for all analyses. Adjusted p values are shown in all graphs.
Results
Overall Patient and Treatment Characteristics
A total of 45 patients meeting the aforementioned inclusion criteria were treated with SRS from 2004–2017 and were included in this series. Table 1 displays patient, disease, and treatment characteristics. The median age of the cohort was 45 years (range 28–66). The most common clinical stage at diagnosis was Stage III (24.4%), and 30 patients (66.7%) were HER2+. Seven patients (15.6%) had triple negative breast cancer (ER/PR/HER2-negative). Only one patient (2.2%) had brain metastases at diagnosis. Just over half (23, 51.1%) of patients received T-DM1 as a component of therapy with 16 patients (35.6%) receiving T-DM1 concurrently with SRS. The median number of total SRS courses was 1 (range 1–5). The median number of total intracranial lesions treated from total SRS courses per patient was 5 (range 1–17). One-third of patients also received WBRT as a component of their intracranial radiotherapy during the course of their disease.
Table 1.
Patient, Disease, and Treatment Characteristics
| All Patients | CSRN | No CSRN | ||||
|---|---|---|---|---|---|---|
| Variables | No. | % | No. | % | No. | % |
| Age at Diagnosis (years) | ||||||
| ≤ 45 | 27 | 60.0 | 3 | 30.0 | 24 | 68.6 |
| > 45 | 18 | 40.0 | 7 | 70.0 | 11 | 31.4 |
| AJCC 7th Edition Clinical Stage | ||||||
| 0 | 2 | 4.4 | 1 | 10.0 | 1 | 2.9 |
| 1 | 3 | 6.7 | 1 | 10.0 | 2 | 5.7 |
| 2 | 10 | 22.2 | 1 | 10.0 | 9 | 25.7 |
| 3 | 11 | 24.4 | 2 | 20.0 | 9 | 25.7 |
| 4 | 10 | 22.2 | 2 | 20.0 | 8 | 22.9 |
| Unknown | 9 | 20.0 | 3 | 30.0 | 6 | 17.1 |
| Brain Metastases at Diagnosis | ||||||
| No | 42 | 93.3 | 10 | 100.0 | 32 | 91.4 |
| Yes | 1 | 2.2 | 0 | 0.0 | 1 | 2.9 |
| Unknown | 2 | 4.4 | 0 | 0.0 | 2 | 5.7 |
| HER2 Status | ||||||
| Negative | 15 | 33.3 | 0 | 0.0 | 15 | 42.9 |
| Positive | 30 | 66.7 | 10 | 100.0 | 20 | 57.1 |
| Receipt of T-DM1 | ||||||
| No | 22 | 48.9 | 1 | 10.0 | 21 | 60.0 |
| Yes | 23 | 51.1 | 9 | 90.0 | 14 | 40.0 |
| Timing of T-DM1 with SRS | ||||||
| Sequential | 7 | 15.6 | 3 | 30.0 | 4 | 11.4 |
| Concurrent | 16 | 35.6 | 6 | 60.0 | 10 | 28.6 |
| N/A (no T-DM1) | 22 | 48.9 | 1 | 10.0 | 21 | 60.0 |
| Total Number SRS Courses | ||||||
| 1 | 27 | 60.0 | 3 | 30.0 | 24 | 68.6 |
| 2 | 9 | 20.0 | 1 | 10.0 | 8 | 22.9 |
| 3 | 4 | 8.9 | 3 | 30.0 | 1 | 2.9 |
| 4 | 4 | 8.9 | 2 | 20.0 | 2 | 5.7 |
| 5 | 1 | 2.2 | 1 | 10.0 | 0 | 0.0 |
| Total Number Lesions Treated | ||||||
| 1–5 | 26 | 57.8 | 5 | 50.0 | 21 | 60.0 |
| > 5 | 19 | 42.2 | 5 | 50.0 | 14 | 40.0 |
| Receipt of WBRT | ||||||
| No | 30 | 66.7 | 7 | 70.0 | 23 | 65.7 |
| Yes | 15 | 33.3 | 3 | 30.0 | 12 | 34.3 |
Clinically Significant Radionecrosis
A total of ten patients (22.2%) developed clinically significant radionecrosis. CSRN was observed in 9 of 23 (39.1%) patients who received T-DM1 compared with only 1 of 22 (4.5%) patients who did not receive T-DM1. Six patients receiving T-DM1 concurrent with any course of SRS, and four receiving concurrent T-DM1 with the course of SRS directed to the lesion, later developed CSRN. Symptoms included seizures, headaches, blurred vision, ataxia, dizziness, altered mental status, and dysarthria. Three patients required only corticosteroid treatment during their admission. One case required hospital admission with supportive care only. In the entire cohort, six patients required therapeutic craniotomy with pathologic confirmation of radionecrosis in all cases.
Table 2 displays the dosimetric characteristics of patients with CSRN. The median treated volume was 0.70 cc (corresponding sphere diameter of 1.10 cm) with a range of 0.09–41.55 cc. The median prescription dose and fraction number were 2000 cGy (range 1800–2500 cGy) and 1 (range 1–5), respectively.
Table 2.
Dosimetric Characteristics of Patients and Respective Lesions with Radionecrosis
| Patient | Target Volume (cc) |
Prescription Dose (cGy) |
Number of Fractions |
Prescription Isodose Line (%) |
Maximum Point Dose (cGy) |
PTV Margin (mm) |
Dose of Prior Whole Brain RT (cGy) |
Total SRS Courses |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.09 | 2,400 | 1 | 80 | 3192 | 0 | 0 | 4 |
| 2 | 0.55 | 200 | 1 | 80 | 2660 | 0 | 0 | 4 |
| 6.93 | 2,500 | 5 | 88 | 2921 | 1 | 0 | 4 | |
| 3 | 6.65 | 1,800 | 1 | 64 | 2880 | 0 | 0 | 2 |
| 4 | 41.55 | 2,000 | 5 | 81 | 2634 | 5 | 2000 | 3 |
| 5 | 0.77 | 2,000 | 1 | 77 | 2707 | 0 | 3500 | 1 |
| 0.51 | 2,000 | 1 | 77 | 2707 | 0 | 3500 | 1 | |
| 0.70 | 2,000 | 1 | 77 | 2630 | 0 | 3500 | 1 | |
| 0.42 | 2,000 | 1 | 77 | 2630 | 0 | 3500 | 1 | |
| 6 | 0.89 | 2,000 | 1 | 60 | 3380 | 0 | 0 | 5 |
| 7 | 4.77 | 2,000 | 1 | 60 | 3072 | 0 | 0 | 3 |
| 0.49 | 2,000 | 1 | 80 | 2580 | 0 | 0 | 3 | |
| 1.51 | 2,000 | 1 | 60 | 3400 | 0 | 0 | 3 | |
| 8 | 0.20 | 2,000 | 1 | 80 | 2600 | 0 | 3000 | 1 |
| 9 | 20.93 | 2,400 | 3 | 73 | 3370 | 2 | 0 | 3 |
| 10 | 0.09 | 1,800 | 1 | 80 | 2380 | 0 | 0 | 1 |
| 0.16 | 2,000 | 1 | 80 | 2740 | 0 | 0 | 1 | |
| 0.22 | 2,000 | 1 | 80 | 2780 | 0 | 0 | 1 | |
| 0.24 | 2,000 | 1 | 80 | 2380 | 0 | 0 | 1 |
Table 3 shows systemic therapies received by patients who had CSRN as well as timing of SRS in relation to the delivery of T-DM1. The median time from SRS of the affected lesion to CSRN was 16 months (range 1–79 months). In those patients who received sequential T-DM1 there was a large range in timing. For those who received T-DM1 prior to SRS, the time range was 77–131 days. The cohort who received T-DM1 after SRS ranged 420–1426 days. Also displayed in Table 3 is the time from T-DM1 to development of CSRN with a range of 8–532 days.
Table 3.
Systemic Treatments Details in Patients with Radionecrosis
| Patient | Prior Systemic Treatment | T-DM1 delivered concurrent with SRS |
Interval any SRS course from T-DM1 (days) |
Interval to CSRN from T-DM1 (days) |
|---|---|---|---|---|
| 1 | D, P, T, Ca, L, S, Px, ONT | No | 77* | 221 |
| 2 | Px, T, A, C, D, P, L, ONT, Ca, Pz | Yes | 17 | 116 |
| No | 131* | 374 | ||
| 3 | A, C, Ev, T, E, Az, Px, Pz, L, Ca | Yes | 14 | 15 |
| 4 | Px, T, Az, L, ONT, F, Pz, G, S, E | Yes | 10 | 529 |
| 5 | Px, T, Pz, ONT, S | Yes | 16 | 532 |
| 6 | A, Px, T, V, X, G, Ca, L | Yes | 3 | 16 |
| 7 | A, C, Px, T, Tam, Lz, S, Fx, L, Ex, Ca, S with Px, V, ONT | Yes | 3 | 25 |
| 8 | Az, T, Px, L, Ca, Ex | No | 420 | 92 |
| 9 | D, P, T, E, A, C, S with Px, Ca | NA (did not receive T-DM1) | NA | NA |
| 10 | P, G, T, Ca, L | No | 1426 | 8 |
; in those who did not receive T-DM1 concurrent with SRS, the * denotes that SRS was delivered after receipt of T-DM1.
D, docetaxel; P, carboplatin; T, trastuzumab; Ca, capecitabine; L, lapatinib; S, study drug; Px, paclitaxel; ONT, ONT-380; A, doxorubicin; C, cyclophosphamide; Pz, pertuzumab; Ev, everolimus; E, eribulin; Az, anstrozole; F, fulvestrant; G, gemcitabine; V, vinorelbine; X, abraxane; Tam, tamoxifen; Lz, letrozole; Fx, fasldoex; Ex, exemestane.
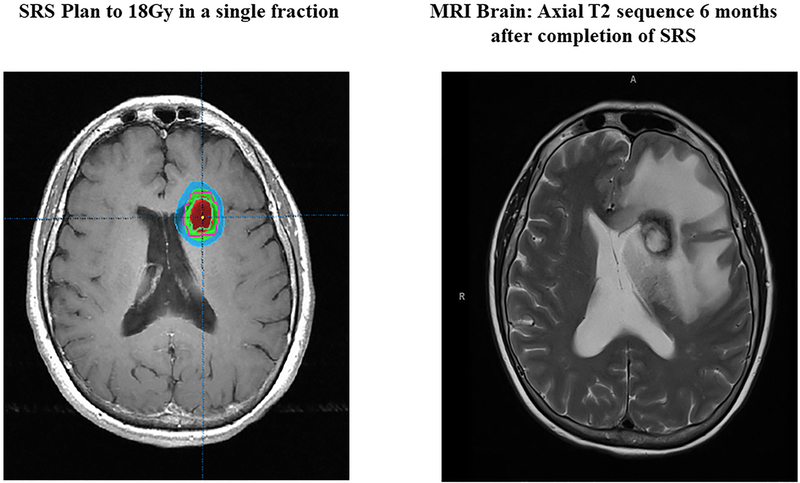
Figure 1 shows a representative example of radiographic changes associated with CSRN. The SRS plan is displayed in the left panel with magnetic resonance imaging (MRI) at the time of CSRN diagnosis in the right panel. In this case, SRS was completed 14 days prior to starting T-DM1. CSRN developed while on T-DM1, 6 months after initiation of the drug.
Figure 1:
(Left) SRS plan; (Right) MRI imaging, Axial T2 Sequence representing CSRN
In analysis of the entire cohort on logistic regression (Table 3), receipt of T-DM1 was associated with a 13.5-fold increased risk of CSRN (p=0.02, 95% CI 1.5–118.7). In those who did receive T-DM1, 9 patients developed CSRN and 6 of those had received T-DM1 concurrently. Treatment with increasing number of SRS courses (OR 2.7, 95% CI 1.3–5.3, p < 0.01) and age >45 years (OR 5.1, 95% CI 1.1–23.5, p = 0.04) were both associated with increased risk of developing CSRN. Risk of CSRN was not altered by an increase in the number of treated lesions (p = 0.57) or receipt of WBRT (p = 0.80). The per-lesion rate of CSRN in the overall cohort was 7.1% (19/268 lesions).
RT + T-DM1 Mechanism of Brain Edema
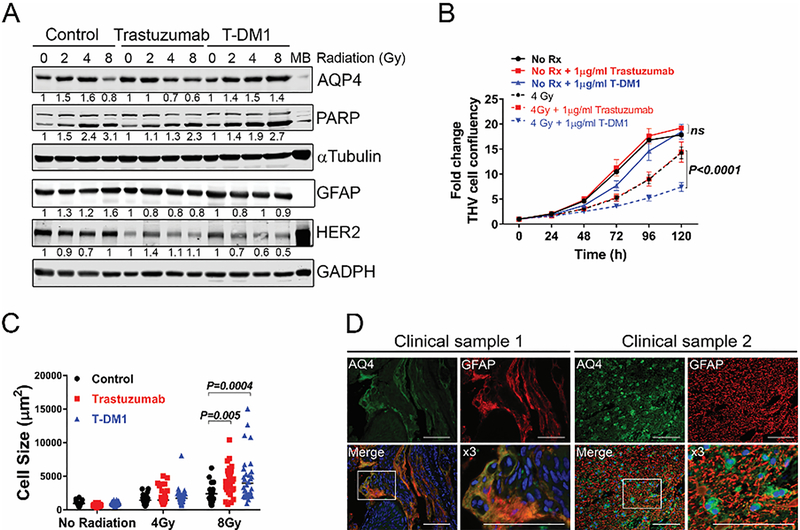
Given clinical findings consistent primarily with edema, we sought to investigate whether T-DM1 in combination with RT promoted water flow dysfunction in the brain niche. Reactive astrocytes are key modulators of the neuroinflammatory response during brain metastasis and regulate water flow across the blood-brain barrier through modulation of the water transporter Aqp412–16. Reactive astrocytes can pose as targets for T-DM1 since human astrocytes (THV) expressed normal levels of HER2 (Figure 2A). Astrocytic HER2 was downregulated by both T-DM1 and trastuzumab suggesting both HER2-targeting agents are taken up by astrocytes. To assess whether HER2-targeted therapies impacted Aqp4 expression and induced astrocytic swelling (a measurement of water flow impairment), THV cells were treated with increasing doses of RT (0, 2, 4, 8 Gy) alone or in combination with 1 μg/mL trastuzumab or T-DM1. Western blots showed that while trastuzumab reduced the RT-induced upregulation in Aqp4, T-DM1 further exacerbated RT-induced Aqp4 upregulation, and increased PARP activation (Figure 2A).Consistent with these results, astrocyte survival measured using Incucyte Live Cell Imaging over 5 days showed that RT alone decreased astrocyte-survival by 35%, and trastuzumab did not affect survival of astrocytes or show synergistic effects with 4 Gy (Figure 2B). By contrast, in the absence of RT, T-DM1 decreased confluence of astrocytes by 8.8% (91.3±0.8 vs 99.8%±0.2, respectively, p>0.01, at 5 days) as compared to control astrocytes and by 32.1% as compared to control astrocytes pre-treated with 4Gy (32.8 ± 3% vs 64.9 ± 13.3% respectively, p<0.0001, at 5 days) (Figure 2B). These results suggest that TDM-1 enhances the cytotoxic effects of RT on reactive astrocytes, concomitant with dysregulation of Aqp4 expression.
Figure 2:
(A) Representative Western blot showing regulation of aquaporin 4 (Aqp4) and Poly (ADP-ribose) polymerase (PARP) in the setting of trastuzumab, T-DM1 and radiation. THV cells were plated in DMEM 10% FBS and treated with 0, 2, 4, 8 Gy as indicated. Cell lysates were analyzed 48hr later. GAPDH was used as loading control. Numbers represent protein levels normalized to GAPDH and relative to untreated control cells. (B) Astrocyte survival with varying combinations of trastuzumab, T-DM1 and radiation (RT). Cells were treated with 0 (No RT) or 4 Gy RT, and cell confluence was measured over time using live imaging Incucyte system. Graph shows fold change in confluence relative to time 0. Graph shows mean ± SEM for each time point. Data was analyzed using two-way ANOVA followed by Dunn’s post-hoc analysis. Adjusted p values are shown. (C) Astrocyte cell size in the setting of trastuzumab or T-DM1 with or without RT. Cells were plated in coverslips and treated with indicated doses of RT. Cells were stained for Aqp4 and cell size measure in at least 50 individual cells per treatment, in two independent experiments. (D) Double immunofluorescence staining of reactive astrocytes (GFAP, red) and Aqp4 (Green) in brain metastases from two patients with HER2+ disease. Dapi marks nuclei.
Since upregulation of Aqp4 is reported to result in astrocytic swelling15,16, the cell size of astrocytes treated with 4 and 8 Gy alone or in combination with trastuzumab or T-DM1 was assessed. Immunofluorescence staining showed that at high dose RT (8Gy) T-DM1/RT increased astrocytic cell size in a subpopulation of astrocytes (p=0.004) to a larger extent than equivalent RT dose in combination with trastuzumab (p=0.005) (Figure 2C). Moreover, immunostaining of brain metastases from patients with HER2+ disease who were treated with T-DM1/SRS showed enlarged reactive astrocytes expressing high levels of Aqp4 (Figure 2D). Other chemotherapeutic agents (paclitaxel and cisplatin) did not increase Aqp4 protein levels, had no synergistic effects with 4Gy RT in reducing astrocytic survival, nor did they result in increased astrocytic swelling (Supplementary Figure 1a, b, c ) in vitro. Taken together, these results suggest that uptake of T-DM1 by reactive astrocytes enhances RT-induced cytotoxic edema in astrocytes during SRS.
Discussion
With promising rates of improved survival with T-DM1 in patients with brain metastases, there remains a paucity of data addressing the safety of combination T-DM1 and SRS. We previously described an unanticipated increase in toxicity with combination of SRS and T-DM19. This manuscript updates our institutional findings and reports a suggested mechanism that the T-DM1 targeting of reactive astrocytes surrounding brain metastases underlies T-DM1/SRS-induced toxicity. To our knowledge, this series represents the largest published experience of the use of SRS in patients with intracranial metastases from breast cancer receiving T-DM1. In this single-institution experience of 45 total patients with brain metastases from breast cancer, receipt of T-DM1 was associated with a 13.5-fold increase in risk of developing CSRN when combined with SRS. CSRN was observed in 39.1% of patients that received T-DM1 compared with 4.5% of those who did not receive T-DM1. Increasing total number of SRS courses delivered and older age also portended a higher risk of developing CSRN.
Historical published controls suggest much lower rates of significant radionecrosis varying from 5–17% in reported studies17–20. Kondziolka, et al, published a study of 350 women with breast cancer undergoing SRS for brain metastases (total 1535 lesions) with symptomatic adverse radiation effects occurring in just 6% of patients with only 3 patients requiring therapeutic resection17. In 2011, Minniti described patients (18% of total cohort with breast cancer) who underwent SRS for brain metastases18. Symptomatic radionecrosis was observed in 10% of patients. Severe neurologic complications (RTOG grade 3 or 4) were noted in 5.8% of patients and required surgery or medical treatment. Another study analyzed outcomes of women with breast cancer with 1–3 brain metastases treated with SRS. Pathologic radionecrosis was identified in 8.6% of cases19. In contrast, in our study, among patients that received T-DM1, 39.1% developed clinically significant radionecrosis with five patients requiring surgical resection. In patients who did not receive T-DM1, the rate of CSRN (4.5%) was more consistent with published rates of CSRN in historical SRS series17–20. However, we acknowledge that the incidence of CSRN in patients in our cohort with HER2- negative breast cancer (who would not have received T-DM1) may not be accurately captured given historically short survival in this subgroup21 and short median survival after brain metastases in our series (9.7 months; range 2.9–65.7). Nevertheless, CSRN remains an important clinical outcome in the HER2+ cohort with overall longer survival.
Patients and treatment factors in this series were quite heterogeneous with varying intervals to CSRN, duration of T-DM1, dose and fractionation schemes, volume of treatment, and receipt of WBRT. The interval from SRS to the lesion later presenting with CSRN varied greatly with the shortest interval of 30 days and the longest delay of just over 6.5 years. Most patients did receive only single fraction SRS; however, some patients did undergo fSRS. Three of the patients who developed CSRN received fSRS. As evidenced in Table 2, the median volume of treated tumors was 0.70cc. Notably, one patient had a large volume of 41.55cc treated in a fractionated course to a total dose of 2000 cGy. This is outside the usual consideration of radiosurgery in terms of dosing and fractionation; however, the patient had CSRN evidenced by new onset seizures and hemorrhage requiring craniotomy and resection with pathology consistent with radionecrosis without any viable tumor. The patient had prior courses of intracranial directed RT including WBRT and single fraction radiosurgery. This case was pertinent to include as this suggests a risk of CSRN at a lower dose threshold and fractionation scheme than may be classically considered. Three patients had also received WBRT as a course of CNS-directed RT. The concurrent vs sequential delivery of T-DM1 as well as total T-DM1 duration was variable. Total duration of T-DM1 ranged from 22–981 days in patients with CSRN. Most patients underwent T-DM1 concurrent with any SRS treatment, though this was not always concurrent with the course of SRS which later resulted in CSRN. A recent publication by Geraud and colleagues retrospectively reviewed twelve patients treated for brain metastases with T-DM1, four of whom received this concurrently with SRS and eight sequentially10. In the concurrent group 50% of patients (2 patients) had radionecrosis as compared to only 28.6% in the sequential group. Carlson and colleagues at our institution published a series of 13 patients of whom 7 patients had HER2+ disease and received T-DM19. The rate of CSRN in the T-DM1 treatment group was 57%.
Furthermore, we explored the mechanism for the observed increase in toxicity. Studies have asserted that the integrity of the blood-brain barrier (BBB) is compromised by metastatic CNS disease, and T-DM1 has been found to cross the BBB22. Since the radionecrosis associated with T-DM1/SRS treatment is accompanied by significant cerebral edema, we sought to investigate the mechanism underlying this toxicity. While SRS and brain tumor burden can cause toxicity via upregulation of VEGF and disruption of the BBB23,24, our preclinical data suggest that additional unintended targeting of HER2-positive reactive astrocytes25 by T-DM1, enhances RT-induced cytotoxic edema, a pre-morbid cellular process that results in osmotic expansion of cells and leads to necrotic cell death12,26. Our data show that enhanced Aqp4 expression and astrocytic swelling is specific to T-DM1 but not trastuzumab or other chemotherapeutic agents. Given that emtansine-related compounds have been reported to enhance irradiation-induced cell death27, it is possible that T-DMI induced cytotoxicity results from the uptake of emtansine in HER2-positive astrocytes. Taken together, our studies suggest that a critical targeting of astrocytes and induction of cytotoxic edema is a mechanism underlying the significant toxicity associated with T-DM1/SRS. However, it remains to be investigated whether Aqp4 and astrocytic swelling are required and sufficient events that explain T-DM1/RT-induced toxicity. Further studies are needed to fully decipher molecular and cellular mechanisms resulting in radionecrosis and edema.
The finding that the most significant changes in Aqp4 upregulation and astrocytic swelling occur at higher doses of RT but not at lower RT doses suggests that lower dose fSRS, as opposed to higher-dose single fraction SRS schedules, might be needed to diminish the T-DM1/SRS toxic effect on astrocytes and reduce risk of clinically significant cerebral edema and radionecrosis. However, further testing and observation in these patients is needed given the case of CSRN in a fSRS course reported here. Targeting changes in Aqp4 expression may offer additional options to prevent the observed toxicity. FDA-approved anti-epileptic drugs that target Aqp4 might represent rapidly translatable candidates to this end28.
The patients captured in this series represent a highly complex group with multiple prior lines of treatment and aggressive management due to prolonged survival. With T-DM1 increasing not only overall survival but, specifically, survival in patients with CNS disease, the safety and efficacy of SRS with T-DM1 should be a focus of future trials. Due to the heterogeneity displayed by this cohort, it is imperative that future prospective trials assessing the risks of combination SRS and T-DM1 consider dose, fractionation, number of SRS courses, treatment volume, and timing of T-DM1. With the delay in development of CSRN, the need for protracted follow-up in future studies remains of utmost importance.
In our series, the combination of T-DM1 and SRS results in alarming rates of clinically significant radionecrosis in patients with brain metastases from breast cancer. Given the considerable level of heterogeneity and overall small patient numbers, it is difficult to ascertain which variables may significantly elevate the risk of developing CSRN, though the association between receipt of T-DM1 and SRS is clear. Prospective trials are necessary to determine the safety of the combination of T-DM1 and SRS. However, caution is warranted in this specific cohort until such trials are completed.
Supplementary Material
Table 4.
Logistic Regression Predicting for CSRN
| Univariate | |||
|---|---|---|---|
| Variables | OR | 95% CI | P |
| Age at Diagnosis (years) | |||
| ≤ 45 | 1 | ||
| > 45 | 5.091 | 1.103–23.493 | 0.037 |
| HER2 Status | |||
| Negative | 1 | ||
| Positive | - | - | 1 |
| Receipt of T-DM1 | |||
| No | 1 | ||
| Yes | 13.500 | 1.535–118.692 | 0.019 |
| Total Number SRS Courses | |||
| continuous | 2.658 | 1.329–5.314 | 0.006 |
| Total Number Lesions Treated | |||
| 1–5 | |||
| >5 | 1.500 | 0.365–6.157 | 0.574 |
| Receipt of WBRT | |||
| No | 1 | ||
| Yes | 0.821 | 0.179–3.763 | 0.800 |
Translational Relevance.
Patients with human epidermal growth factor receptor 2-positive (HER2+) breast cancer represent a highly complex group with multiple prior lines of treatment and aggressive management due to prolonged survival. With trastuzumab emtansine (T-DM1) increasing not only overall survival but, specifically, survival in patients with CNS disease, the safety and efficacy of stereotactic radiosurgery (SRS) with T-DM1 needs to be reported and the mechanism elucidated. Here we review the strong correlation of clinically significant radionecrosis (CSRN) with the combination of SRS and T-DM1 and propose a mechanism for this toxicity. This experience provides critical information for providers utilizing this combination and our mechanism, supported by pre-clinical and clinical data, suggests potential pathways for intervention and mitigation of CSRN.
Acknowledgements:
We thank the Brain tumor Biorepository, University of Colorado Cancer Center Shared Resources supported by NCI P30CA046934 and CTSA UL1TR001082 Center grants. This work was supported by Cancer League of Colorado and DoD BCRP W81XWH-19–1-0033(MJC). R37 CA227984 and DoD BCRP W81XWH-15–1-0352 supported DMC. R01CA205044supported PK. University of Colorado Cancer Center Shared Resources were supported by NCI P30CA046934 and CTSA UL1TR001082 Center grants.
Financial Support: This work was supported by Cancer League of Colorado and DoD BCRP W81XWH-19–1-0033(MJC). R37 CA227984 and DoD BCRP W81XWH-15–1-0352 supported DMC. R01CA205044 supported PK. University of Colorado Cancer Center Shared Resources were supported by NCI P30CA046934 and CTSA UL1TR001082 Center grants.
Footnotes
Conflicts of Interest: Ryan Ormond- Research Funding- Synaptive; Peter Kabos-Research Funding: Angiochem, AstraZeneca, Eli Lilly, Pfizer
References
- 1.Surveillance E, and End Results Program: Cancer Stat Facts: Female Breast Cancer, 2017 [Google Scholar]
- 2.Owens MA, Horten BC, Da Silva MM: HER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6556 breast cancer tissues. Clin Breast Cancer 5:63–9, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Leyland-Jones B: Human epidermal growth factor receptor 2-positive breast cancer and central nervous system metastases. J Clin Oncol 27:5278–86, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Brufsky AM, Mayer M, Rugo HS, et al. : Central nervous system metastases in patients with HER2-positive metastatic breast cancer: incidence, treatment, and survival in patients from registHER. Clin Cancer Res 17:4834–43, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Weil RJ, Palmieri DC, Bronder JL, et al. : Breast cancer metastasis to the central nervous system. Am J Pathol 167:913–20, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verma S, Miles D, Gianni L, et al. : Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 367:1783–91, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krop IE, Lin NU, Blackwell K, et al. : Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer and central nervous system metastases: a retrospective, exploratory analysis in EMILIA. Ann Oncol 26:113–9, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacot W, Pons E, Frenel JS , et al: Efficacy and safety of trastuzumab emtansine (T-DM1) in patients with HER2-positive breast cancer with brain metastases. Breast Cancer Res Treat 157:307–318, 2016 [DOI] [PubMed] [Google Scholar]
- 9.Carlson JA, Nooruddin Z, Rusthoven C, et al. : Trastuzumab emtansine and stereotactic radiosurgery: an unexpected increase in clinically significant brain edema. Neuro Oncol 16:1006–9, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geraud A, Xu HP, Beuzeboc P, et al. : Preliminary experience of the concurrent use of radiosurgery and T-DM1 for brain metastases in HER2-positive metastatic breast cancer. J Neurooncol 131:69–72, 2017 [DOI] [PubMed] [Google Scholar]
- 11.Edge SB, Compton CC: The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 17:1471–4, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Klatzo I: Evolution of brain edema concepts. Acta Neurochir Suppl (Wien) 60:3–6, 1994 [DOI] [PubMed] [Google Scholar]
- 13.Papadopoulos MC, Verkman AS: Aquaporin-4 and brain edema. Pediatr Nephrol 22:778–84, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nielsen S, Nagelhus EA, Amiry-Moghaddam M, et al. : Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci 17:171–80, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stokum JA, Kurland DB, Gerzanich V, et al. : Mechanisms of astrocyte-mediated cerebral edema. Neurochem Res 40:317–28, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thrane AS, Rappold PM, Fujita T, et al. : Critical role of aquaporin-4 (AQP4) in astrocytic Ca2+ signaling events elicited by cerebral edema. Proc Natl Acad Sci U S A 108:846–51, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kondziolka D, Kano H, Harrison GL, et al. : Stereotactic radiosurgery as primary and salvage treatment for brain metastases from breast cancer. Clinical article. J Neurosurg 114:792–800, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Minniti G, Clarke E, Lanzetta G, et al. : Stereotactic radiosurgery for brain metastases: analysis of outcome and risk of brain radionecrosis. Radiat Oncol 6:48, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang TJ, Oh JH, Folkert MR, et al. : Outcomes and prognostic factors in women with 1 to 3 breast cancer brain metastases treated with definitive stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 90:518–25, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Rhun E, Dhermain F, Vogin G, et al. : Radionecrosis after stereotactic radiotherapy for brain metastases. Expert Rev Neurother 16:903–14, 2016 [DOI] [PubMed] [Google Scholar]
- 21.Kim YJ, Kim JS, Kim IA: Molecular subtype predicts incidence and prognosis of brain metastasis from breast cancer in SEER database. J Cancer Res Clin Oncol 144:1803–1816, 2018 [DOI] [PubMed] [Google Scholar]
- 22.Dijkers EC, Oude Munnink TH, Kosterink JG, et al. : Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin Pharmacol Ther 87:586–92, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Jiang S, Xia R, Jiang Y, et al. : Vascular endothelial growth factors enhance the permeability of the mouse blood-brain barrier. PLoS One 9:e86407, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winkler F, Kozin SV, Tong RT, et al. : Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell 6:553–63, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Vartanian T, Goodearl A, Viehover A, et al. : Axonal neuregulin signals cells of the oligodendrocyte lineage through activation of HER4 and Schwann cells through HER2 and HER3. J Cell Biol 137:211–20, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang D, Bhatta S, Gerzanich V, et al. : Cytotoxic edema: mechanisms of pathological cell swelling. Neurosurgical focus 22:E2–E2, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edwards A, Gladstone M, Yoon P, et al. : Combinatorial effect of maytansinol and radiation in Drosophila and human cancer cells. Disease Models & Mechanisms 4:496–503, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huber VJ, Tsujita M, Kwee IL, et al. : Inhibition of aquaporin 4 by antiepileptic drugs. Bioorg Med Chem 17:418–24, 2009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.