Abstract
Background:
Cystic Fibrosis (CF), one of the most frequent genetic diseases, is characterized by the production of viscous mucus in several organs. In the lungs, mucus clogs the airways and traps bacteria, leading to recurrent/resistant infections and lung damage. For cystic fibrosis patients, respiratory failure is still lethal in early adulthood since available treatments display incomplete efficacy.
Objective:
The objective of this review is to extend the current knowledge in the field of available treat-ments for cystic fibrosis. A special focus has been given to inhaled peptide-based drugs.
Methods:
The current review is based on recent and/or relevant literature and patents already available in various scientific databases, which include PubMed, PubMed Central, Patentscope and Science Direct. The information obtained through these diverse databases is compiled, critically interpreted and presented in the current study. An in-depth but not systematic approach to the specific research question has been adopted.
Results:
Recently, peptides have been proposed as possible pharmacologic agents for the treatment of respiratory diseases. Of note, peptides are suitable to be administered by inhalation to maximize efficacy and reduce systemic side effects. Moreover, innovative delivery carriers have been developed for drug administration through inhalation, allowing not only protection against proteolysis, but also a prolonged and controlled release.
Conclusion:
Here, we summarize newly patented peptides that have been developed in the last few years and advanced technologies for inhaled drug delivery to treat cystic fibrosis.
Keywords: Alpha-1-antitrypsin, Cystic fibrosis, CFTR, ENaC, Nebulizer, RhDNase
1. INTRODUCTION
Cystic Fibrosis (CF) is one of the most common among rare diseases, affecting over 75000 patients worldwide. CF is caused by congenital mutations disrupting the function of the CF Transmembrane Conductance Regulator (CFTR) channel [1, 2]. More than 2000 mutations in the CFTR gene have been identified to date. Among these, the loss of the amino acid phenylalanine (F) at the 508th position (F508-del mutation) accounts for approximately 70% of disease-related mutations in CF patients. The mutated F508-del CFTR channel is not properly folded, displaying minimal membrane levels and gating in CF epithelial cells. This defect, in turn, results in the production of abnormally viscous mucus, sweat and digestive fluids, which obstruct airways, intestine, pancreas and liver.
Improvements in the available therapies contributed to such an extension in life expectancy that the current view is to no more consider CF a pediatric disease, but rather a pathology of the young adult (some patients reaching 50 years of age). However, respiratory failure is still lethal in early adulthood, due to limited availability and incomplete efficacy of current treatments [3]. Indeed, although most CF patients have disease manifestations in multiple organs, respiratory failure remains the leading cause of morbidity and mortality, due to irreversible airways obstruction, recurrent and chronic infections, and progressive loss in pulmonary function.
In this review, we summarize the most up to date and relevant peptide-based approaches for CF therapy via the inhalation route. Unbiased search for “peptide” and “Cystic Fibrosis” keywords in Patentscope database retrieved patented inventions spanning from those improving mucus clearance, to those targeting the primary defect in CFTR function (Fig. 1). Some remarkable insights on carriers and available medical devices for inhaled delivery, as retrieved by PubMed investigation, are also provided. An in-depth albeit not systematic approach has been used to retrieve salient information that is considered useful for the reader. A critical perspective has been used to comment on available data and provide suggestions for future research.
Fig. (1).
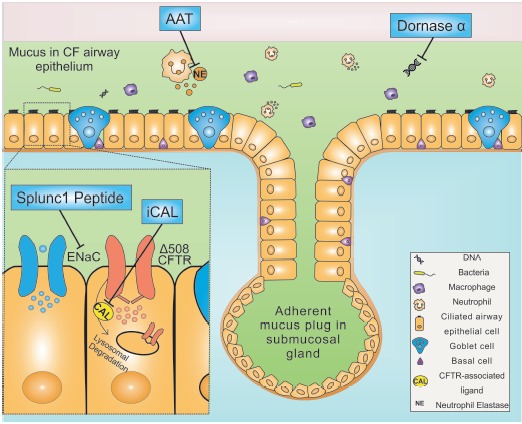
In Cystic fibrosis, depletion of the airway surface liquid is due to the absence of Cystic Fibrosis Transmembrane Conductance Regulator (CFTR)-mediated fluid secretion, accompanied by increased absorption via ENaC epithelial sodium channel. The dehydration of the liquid layer associated with a mutant CFTR contributes to mucus stasis, and eventually to plugging in the submucosal gland or in proximity of the originating goblet cells. These events lead to the onset of a proinflammatory airway environment that promotes proliferation of bacteria and attracts inflammatory cells, including neutrophils and macrophages. Neutrophils, in turn, release proteases, which further enhance mucus viscosity and ultimately lead to lung damage.
2. CURRENT TREATMENTS OF CYSTIC FIBROSIS
Current treatments for CF combine several agents which delay either pulmonary (mucolytics, bronchodilators, antibiotics, corticosteroids, chest physiotherapy, airway clearance, and exercise) or gastroenteric dysfunctions (pancreatic enzyme replacement, fat-soluble vitamin replacement and high-caloric density diets). As a consequence, adherence to such complex and time-consuming therapies is often incomplete [4, 5].
Moreover, most of the available therapies achieve only a symptomatic relief and lack a mechanistic rationale, possibly due to the gaps in the comprehension of CFTR complex biology. Accordingly, only three medicinal products targeting the molecular defects were developed, so far, by Vertex Pharma, by means of high throughput screening and massive money investments. These molecules, which aim at restoring normal salt/water transport across epithelia, have been approved in the EU, and are marketed as Kalydeco® (which is effective in a minor subset of patients), Orkambi®, and the recently developed Tezacaftor [6, 7].
Orkambi® has been recently approved for the treatment of CF patients carrying the genetic F508del mutation in homozygosity, occurring in around 40% of the cases. Notably, Orkambi® is the combination of a CFTR corrector and a potentiator. However, its clinical efficacy is unsatisfactory: treatment shows only limited improvement in lung function, not all the patients respond to therapy, and drug costs are elevated. Despite the recent approval of these new compounds restoring CFTR function, the quality of life and life expectancy of CF patients remain poor, and the current mean age of death is around 30 years [3].
However, clinical results with Orkambi® indicate that the abnormal CFTR is per se a druggable target. This prompted the development of new drugs for oral CF treatment, including the Tezacaftor/Ivacaftor combined therapy, which is now in Phase III (NCT03150719), for patients carrying the homozygous F508-del CFTR gene who discontinued treatment with Orkambi®. Unfortunately, the cost of such regimens is elevated, possibly leading to failure of reimbursement agreements. Thus, effective and affordable drugs for CF therapy are still an unmet need.
3. RECENT DEVELOPMENTS ON PEPTIDE-BASED INHALED DRUGS
Given their attractive properties, such as high specificity, excellent safety and tolerability, several peptides have been proposed as therapeutic agents for respiratory diseases. Some of these are currently under development for CF systemic therapy, including the Vasoactive Intestinal Peptide (VIP) and an amino acid fragment of the CB subunit of crotoxin from Crotalus durrissus terrificus venom. VIP is indeed able to rescue F508-del CFTR trafficking to the apical cell membrane and restore protein function [8] while CB directly interacts with F508-del CFTR NBD1 domain and behaves as both potentiator and corrector [9]. Moreover, antimicrobial peptides have been considered for the treatment of CF-related infections, including the tetra-branched M33 peptide (and its derivatives) [10-12] and Esculentin-1a-Derived Peptides, currently under evaluation for their activity against Pseudomonas aeruginosa lung infections [13].
Interestingly, the antibiotic Tobramycin, which was initially delivered via the parenteral route, was lately administered as an inhaled medication that was recently optimized by the development of Tobramycin Inhalation Powder (TOBI Podhaler®) [14]. Therefore, we cannot exclude that peptides that have been initially tested for systemic and/or topical delivery might be implemented for inhaled delivery.
3.1. Peptides Reducing Mucus Viscosity
3.1.1. ENaC Inhibition
In CF, accumulation of de-hydrated mucus in the airways is a major pathogenic process. The balance of Cl- secretion and Na+ absorption across the airway is critical for the regulation of Airway Surface Liquid (ASL) volume [15, 16]. Indeed, airway epithelia can both secrete chloride (Cl-) through the CFTR and absorb sodium (Na+) through the Epithelial Sodium Channel (ENaC), the apical channel responsible for Na+ absorption across a wide range of epithelia, including renal, gastrointestinal, and airway [17]. Therefore, modulation of ENaC by peptide-based drugs emerged as a promising therapeutic approach to maintain ASL in respiratory diseases [18].
In CF, the absence of CFTR results in ENaC hyperactivity, leading to uncontrolled absorption of Na+ and depletion of ASL volume [19], further resulting in lack of chloride and bicarbonate transport. The consequent defect in mucus clearance promotes the formation of biofilms colonized by pathogens, like Pseudomonas aeruginosa, Staphylococcus aureus and Haemophilus influenza. Notably, when submerged in biofilms, bacteria are less targetable by drugs, thus promoting the development of antibiotic resistance.
Short palate lung and nasal epithelium clone 1 (Splunc1) is a ~25kDa protein which acts as a volume sensor of ASL, and contains an ENaC inhibitory domain, the so-called S18 region [20-24]. Unlike conventional ion channel antagonists blocking the pore, Splunc1 inhibits ENaC by inducing allosteric modulation and endocytosis [25-28]. Since Splunc1 failed to regulate ENaC in CF lungs due to protease degradation, Spyryx Biosciences is currently developing SPX-10®, a Splunc1-derived peptide, which functions in CF airways as an ENaC inhibitor [29-33]. The restored ASL hydration is predicted to improve mucociliary clearance and decrease infection/inflammation in CF lungs [34, 35]. Inhaled SPX-101® already concluded a Phase I study in CF patients (NCT03056989), and the HOPE-1 randomized, double-blind, placebo-controlled Phase II study for efficacy and safety is ongoing (NCT03229252).
Interestingly, S18-derived peptides are intrinsically disordered thanks to their peculiar amino acidic sequence and display a high degree of flexibility in their polypeptide chain, which lacks a stable 3D structure. As a consequence, they are heat-stable and can achieve a high contact area and binding efficiency with their target proteins [36]. In addition, S18-derived peptides are protease resistant, do not freely cross the respiratory epithelium barrier, and do not reach kidneys, thus avoiding the undesired induction of hyperkalemia caused by small molecule ENaC antagonists like amiloride [37, 38]. For many peptide-based drugs, the inability to cross the respiratory epithelium and reach the bloodstream after inhaled delivery may be an advantage, as it would result in low, if not absent, systemic bioavailability, thus reducing both on-target and off-target effects in other organs. For example, in the case of CF, inhaled antibiotics achieve higher concentrations with fewer side effects than orally delivered antibiotics [39]. Chronic inhalation of peptides could eventually induce a local immunogenic response, but, given that Splunc1-derived peptides are naturally occurring, at least in normal lungs, immunogenicity is unlikely for SPX-101® [22].
3.1.2. Neutrophil Elastase (NE) Inhibition
Airway recruitment of neutrophils is another major hallmark of CF lung disease and is responsible for tissue remodeling and, ultimately, destruction as a consequence of the excessive release of Neutrophil Elastases (NEs). The activity and level of NEs are closely related to the ongoing inflammation, and NE levels are elevated in the Bronchoalveolar Lavage (BAL) fluid of CF patients [40]. Besides inducing lung damage, NEs cleave and activate ENaC, further reducing mucus hydration and clearance [41-44]. A number of NE inhibitors have been described [45]; among these, hypersulfated disaccharides were disclosed for the treatment of CF.
Alpha-1-Antitrypsin (AAT), also known as Alpha-1-Proteinase Inhibitor (API), is an endogenous NE inhibitor, which is predicted to improve pulmonary function [46, 47]. In CF, AAT levels are normal, but the burden of NEs is so large that it overwhelms AAT protective effect. To date, clinical grade AAT is obtained by extraction and purification from human plasma, a procedure that has been patented about three decades ago [48]. Five patents recently addressed the production of AAT (years 2013 to 2015) [49-53], as summarized in [54].
Intravenous infusion of AAT purified from human plasma is indicated for the chronic therapy of adults affected by severe hereditary API deficiency [55]. However, the need for prolonged and repeated intravenous access, as recommended for AAT deficiency-associated lung diseases, as well as for chronic diseases like CF, implicates an increased risk of thrombosis and infections [41]. To overcome these major issues, an inhaled biological therapy based on human AAT has been developed, and completed a phase I trial in CF patients (NCT01347190). Three phase II trials were also completed (NCT01684410 and NCT00486837 sponsored by Grifols Therapeutics; NCT00499837 sponsored by Kamada) and one was terminated (NCT02010411). Overall, inhaled AAT has been shown to be safe and well tolerated, albeit its clinical efficacy is still debated [41, 56-59].
However, a potential limitation of AAT is that several other proteases, including cathepsins and metalloproteases, are upregulated in CF and may contribute to lung damage, without being blocked by AAT [60].
3.1.3. DNA Clearance
CF airways are characterized by high levels of DNA and actin in the lung lumen, which are released by necrotic neutrophils [61-63]. Extracellular DNA accumulation, in turn, adversely alters mucus rheology, leading to decreased mucociliary clearance.
The first patented invention concerning the ability of DNase1 to cleave extracellular DNA, and thus decrease mucus viscosity, in CF lungs has been described in the nineties [64, 65]. Dornase alfa, or rhDNAse, is a recombinant version of human DNase1 protein used as a therapeutic for CF [66]. Pulmozyme® is a FDA/EMA approved recombinant version of human DNase1 marketed by Genentech for the treatment of CF (Phase IV trial completed in 2013, NCT01712334), where it has been shown to reduce the incidence of infections [67]. Pulmozyme® is administered via nebulization by means of different nebulizers, including Hudson T Up-draft II® and Marquest Acorn II® with Pulmo-Aide®, PARI LC® Plus or PARI BABY™ with PARI PRONEB®, Durable Sidestream® with Mobilaire™ or Porta-Neb®, and the eRapid™ Nebulizer System.
rhDNAse is nowadays considered a gold-standard drug. Notably, it was the first peptide-based drug showing the safety and efficacy requirements needed to be successfully developed and marketed as an inhaled therapeutic for CF.
3.2. Peptides Targeting the Primary CFTR Defect
3.2.1. NEG2
The pioneer of peptide-based drugs for CF is a short peptide derived from the R domain of CFTR (amino acids 817-838), named NEG2 peptide, which was shown to play a critical role in channel activity regulation. Exogenous NEG2 peptide derivatives were shown to interact with CFTR, exerting both stimulatory and inhibitory effects on the function of the channel. Unfortunately, despite leading to a remarkable progress in the knowledge of CFTR biology, NEG2-derived peptides were not developed further for CF therapy [68, 69].
3.2.2. Thymosin Alpha 1
Thymosin Alpha 1 (Tα1) is a naturally occurring polypeptide of 28 amino acids. Tα1 is well known for its immunoregulatory properties [70], and has been suggested as an anti-inflammatory agent and a CFTR modulator in CF [71].
Tα1 composition may be adapted for oral, parenteral, topical and inhaled administration [72]. Of note, the ability of Tα1 to correct/potentiate the F508-del CFTR mutant has been reported [71]. However, this finding has been questioned by a recent publication showing that Tα1 does not correct F508-del CFTR in CF airway epithelia, raising concerns on the future development of Tα1 as a CFTR modulator [73], but rather as an anti-inflammatory agent.
3.2.3. iCAL
In CF patients, F508-del CFTR is improperly folded, resulting in increased Endoplasmic Reticulum (ER) degradation and decreased Golgi trafficking. Moreover, the residual CFTR is removed from the apical membrane and degraded in lysosomes due to pathological interactions with CFTR-Associated Ligand (CAL), a CFTR-interacting PDZ domain protein that associates predominantly with Golgi [74]. Recently, CAL Competitive Inhibitor (iCAL) peptides have been shown to selectively bind to CAL PDZ domain, preventing the interaction with CFTR [75]. iCALs selectively inhibit CAL, but not NHERF, increasing F508-del CFTR at the membrane, resulting in increased Cl- conductance in the bronchial epithelium of CF patients [75, 76].
Unfortunately, these isolated sequences are not suitable as drugs, due to their inability to penetrate cell membranes and reach their intracellular CAL target. CT007 is an optimized iCAL drug compound that represents a breakthrough for the selective stabilization of F508-del CFTR in CF and that acts by maintaining functional CFTR at the cell surface. CT007 shows additivity or even synergy in combination therapy with VX-809. The compound displays cell permeability, solubility, targeted intracellular release, enhanced in vivo stability, low immunogenicity and physical-chemical properties suitable for the formulation into an inhaled dry powder or solution form that allows topical dosing and immediate uptake in airway epithelial cells [77, 78].
3.3. Nanocarriers
Albeit promising in terms of efficacy, peptides might be difficult to handle, because of their instability in body fluids due to the action of proteases, and during storage or delivery due to interactions with surfaces, air/water interface, presence of proteolytic enzymes, and sensitivity to ultrasounds and shear stress. Accordingly, some protein-based therapies failed during clinical translation, being labile and prone to proteolysis in the blood, compared to conventional small molecules [79]. Combining peptides with nanocarriers was therefore explored as a strategy to provide both protection against proteolysis and prolonged/controlled release [80, 81].
Several types of nanocarriers have been developed, including liposomes, which are able to carry active substances both inside and in/on their surface, as well as chitosan-modified liposomes [82, 83]. Thanks to this successful strategy, a number of nebulizable liposomal formulations have reached clinical trials, including Arikace® (liposomal amikacin) and Pulmaquin® (liposomal ciprofloxacin) antibacterial formulations, which are currently in advanced stages of development.
Alternative to liposomes, PLGA, alginate-chitosan hybrid, and hyaluronan particles have been designed to prolong residence in the lung milieu [79, 80]. PLGA nanoparticles can be used, for example, as carriers of triplex molecules that bind or hybridize to a target sequence in the human CFTR gene [84]. However, peptides that either target pathogens or epithelial cells need to penetrate the biofilm, a matrix composed of polysaccharides and DNA that contribute to increased mucus viscosity. Interestingly, when tested, the efficiency of compounds conjugated to liposomes or PLGA particles was enhanced in most cases, compared with the corresponding free drugs [81, 85].
3.4. Inhaled Drug Delivery
The preferred route of administration of drugs targeting respiratory dysfunctions in CF is ideally inhalatory. Notably, inhalation is not only preferable for the diminished side effects, compared to oral and/or systemic delivery, but is also convenient considering the large surface area and high vascularization of the lungs [82, 83].
On the other side, to be suitable for inhalation therapy, drug particles should meet specific requirements, including adequate ADME features (pharmacokinetics and pharmacology for absorption, distribution, metabolism, and excretion) and an appropriate respirable size, namely 1-5μm, which depends also on the performance of the device used for delivery [4].
To this aim, new formulations were engineered to improve lung targeting and dose consistency, so that could be adapted to the high number of delivery systems that are now available, including portable inhalers and nebulizers [86].
In 1956, Riker Laboratories developed the first pressurized Metered Dose Inhaler (MDI) [87]. The introduction of the pMDI denoted the beginning of modern aerosol industry, which now provides also Dry-Powder (DPI) and Soft-Mist Inhalers (SMI), ultrasonic and jet nebulizers, vibrating-mesh as well as smart devices [88].
pMDIs, SMIs and DPIs can deliver relatively small quantities of medication to the lungs, when compared to medical nebulizers. As an example, pMDI and DPI usually deliver small drug amounts to the airways, so they require repetitive delivery to provide effective doses [89]. An exception might be the preparation in the form of pulmospheres [86], which allows loading of drug particles up to 95% in weight [90] and guarantees low interpatient variability, enabling treatment of patients of any age, even in the presence of severe airflow obstruction [91]. Moreover, use of pMDI and DPI inhalers can be challenging, potentially leading to improper handling that can significantly reduce drug delivery to the lungs and therapeutic effectiveness [92].
Ultrasonic nebulizers are not suitable for nebulization of proteins such as AAT, because the molecule is destroyed by frictional forces. On the contrary, jet nebulizers, such as the Pari Boy® S with the LC Plus® nebulizer (Pari), are suitable, although able to deliver only up to 40% of the aerosol to the lungs [93]. Vibrating-mesh devices, such as the MicroAir NE-U22V® nebulizer (Omrom Healthcare), the AeroNeb® OnQ aerosol generator (Inspiration Medical), and the eFlow® (Pari), conveniently generate aerosols by perforated meshes actuated by piezo-electric vibrational crystal or ceramic elements.
Even at high outputs, the delivery rates to the lungs can be further improved by timing drug delivery to the inspiratory phase of the respiration cycle [94, 95]. To this aim, a smart card has been designed to optimize aerosol pulse to the patient’s pulmonary function. Currently, two smart devices equipped with such technology are available: The I-neb, a small stand-alone nebulizer, and the Akita®, hooked to a Pari jet or eFlow nebulizer [94, 96]. However, devices equipped with such technology are still very expensive.
Unfortunately, the shearing provided during nebulization of aerosol droplets may induce physical stress on liposome bilayers and peptides themselves, causing a loss in therapeutic efficacy. Hence, formulation composition, nebulizer design and an adequate training program of patients should be optimized, with the aim of i) reducing the detrimental effect of shearing on the drug, and ii) maximizing deposition in the lower airways [97, 98].
CONCLUSION
Peptides are versatile therapeutic candidates for the systemic and inhaled treatment of several diseases. Accordingly, during the past few years, peptide-based therapeutics have been developed for the treatment of CF.
Evidence discussed above highlights the emerging peptide-based drugs as promising, albeit challenging, therapeutic agents for the inhalatory treatment of CF patients.
CURRENT & FUTURE DEVELOPMENTS
An emblematic case recapitulating the challenges in the development of peptide-drugs for inhaled delivery is that of insulin [99]. Massive efforts in developing inhaled insulin formulations were pushed on one side by the global burden of diabetes worldwide, and, on the other side, by the need to overcome the distress of multiple daily injections. This new formulation was expected to be successful, especially when Exubera® was approved by the FDA/EMA and released to the market in 2006/2007.
A number of other inhaled insulin formulations were in late stage of clinical development and expected to reach the market. However, when Pfizer withdrew Exubera® from the market due to limited commercial success, inhaled insulin was removed from several pipelines, despite positive clinical results and high acceptance from patients. MannKind was the only one to progress with Afrezza® inhaled insulin. Sanofi brought this product to the market in 2014, but returned it to MannKind shortly afterward, because of poor sales. As learned from the insulin story, a successful development of peptide-based drugs for inhaled delivery might be challenging even in the case of a non-rare disease like diabetes.
Of note, CF-related diabetes is a major co-morbidity of CF, affecting over half of middle-aged CF patients [100]. However, inhaled delivery for CF therapy, per se, has an additional, intrinsic challenge: given the thick mucus layer and the chronic inflammation in the lungs, it is likely that peptide-based drugs, like insulin and others, do not efficiently reach the target cells. Nevertheless, peptides can survive sufficiently long in the mucus layer to diffuse and reach their targets. In addition, reduction of the mucus burden with different physiotherapeutic techniques prior to the administration of the compound can further help to overcome the potential problem of delivery through the mucus.
Future trials in CF should be therefore grounded on such concepts and take in serious consideration the need of optimization in the delivery of peptidic drugs in a potentially adverse environment [85].
ACKNOWLEDGEMENTS
We acknowledge Paolo Ettore Porporato for critical reading. Valentina Sala, Alessandra Murabito and Alessandra Ghigo analyzed data and wrote the manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No Animals/Humans were used for studies that are base of this research.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
This work has been supported by the Italian Cystic Fibrosis Foundation FFC#6/2016 and FFC#11/2017 and Cariplo Foundation #2015-0880 to AG.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Angelis A., Tordrup D., Kanavos P. Socio-economic burden of rare diseases: A systematic review of cost of illness evidence. Health Policy. 2015;119(7):964–979. doi: 10.1016/j.healthpol.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 2.Syed B.A., Hamad B. The cystic fibrosis drug market. Nat. Rev. Drug Discov. 2014;13(10):721–722. doi: 10.1038/nrd4434. [DOI] [PubMed] [Google Scholar]
- 3.Elborn J.S., Bell S.C., Madge S.L., Burgel P-R., Castellani C., Conway S., et al. Report of the European Respiratory Society/European Cystic Fibrosis Society Task Force on the care of adults with cystic fibrosis. Eur. Respir. J. 2016;47(2):420–428. doi: 10.1183/13993003.00592-2015. [DOI] [PubMed] [Google Scholar]
- 4.Labiris N.R., Dolovich M.B. Pulmonary drug delivery. Part I: Physiological factors affecting therapeutic effectiveness of aerosolized medications. Br. J. Clin. Pharmacol. 2003;56(6):588–599. doi: 10.1046/j.1365-2125.2003.01892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fellner R.C., Terryah S.T., Tarran R. Inhaled protein/peptide-based therapies for respiratory disease. Mol. Cell Pediatr. 2016;3(1):16. doi: 10.1186/s40348-016-0044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Livraghi A., Randell S.H. Cystic fibrosis and other respiratory diseases of impaired mucus clearance. Toxicol. Pathol. 2007;35(1):116–129. doi: 10.1080/01926230601060025. [DOI] [PubMed] [Google Scholar]
- 7.da Silva A.L., Cruz F.F., Rocco P.R.M., Morales M.M. New perspectives in nanotherapeutics for chronic respiratory diseases. Biophys. Rev. 2017;9(5):793–803. doi: 10.1007/s12551-017-0319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnold S., Balance D. Methods and compositions for treating Cystic Fibrosis. WO2015172046. 2015 [Google Scholar]
- 9.Odolczyk N., Edelman A., Zielenkiewicz P., Faure-Kuzminska G. Methods and compositions for modifying cystic fibrosis transmembrane conductance regulator activity. WO2017187274. 2017 [Google Scholar]
- 10.Pini A., Falciani C., Bracci L. Antimicrobial peptide, branched forms thereof and their use in the treatment of bacterial infections. WO2012010266. 2012 [Google Scholar]
- 11.Pini A, Falciani C, Bracci L. Antimicrobial peptide, branched forms thereof and their use in the treatment of bacteria infections. US20130130969. 2013 [Google Scholar]
- 12.Bracci L., Giuliani A., Pini A., Neri P. Antibacterial peptides and analogues thereof. US20090053151. 2001 [Google Scholar]
- 13.McDermott A., Mangoni M. Esculentin 1a derivatives and uses thereof. US20150104492. 2015 [Google Scholar]
- 14.Vazquez-Espinosa E., Marcos C., Alonso T., Giron R.M., Gomez-Punter R.M., Garcia-Castillo E., et al. Tobramycin inhalation powder (TOBI Podhaler) for the treatment of lung infection in patients with cystic fibrosis. Expert Rev. Anti Infect. Ther. 2016;14(1):9–17. doi: 10.1586/14787210.2016.1118344. [DOI] [PubMed] [Google Scholar]
- 15.Knowles M.R., Boucher R.C. Mucus clearance as a primary innate defense mechanism for mammalian airways. J. Clin. Invest. 2002;109(5):571–577. doi: 10.1172/JCI15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ratjen F. Restoring airway surface liquid in cystic fibrosis. N. Engl. J. Med. 2006;354(3):291–293. doi: 10.1056/NEJMe058293. [DOI] [PubMed] [Google Scholar]
- 17.Garty H., Palmer L.G. Epithelial sodium channels: Function, structure, and regulation. Physiol. Rev. 1999;77(2):359–396. doi: 10.1152/physrev.1997.77.2.359. [DOI] [PubMed] [Google Scholar]
- 18.Schwameis R., Eder S., Pietschmann H., Fischer B., Mascher H., Tzotzos S., et al. A FIM study to assess safety and exposure of inhaled single doses of AP301-A specific ENaC channel activator for the treatment of acute lung injury. J. Clin. Pharmacol. 2014;54(3):341–350. doi: 10.1002/jcph.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stutts M.J., Canessa C.M., Olsen J.C., Hamrick M., Cohn J.A., Rossier B.C., et al. CFTR as a cAMP-dependent regulator of sodium channels. Science. 1995;269(5225):847–850. doi: 10.1126/science.7543698. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Caballero A., Rasmussen J.E., Gaillard E., Watson M.J., Olsen J.C., Donaldson S.H., et al. SPLUNC1 regulates airway surface liquid volume by protecting ENaC from proteolytic cleavage. Proc. Natl. Acad. Sci. USA. 2009;106(27):11412–11417. doi: 10.1073/pnas.0903609106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hobbs C.A., Blanchard M.G., Kellenberger S., Bencharit S., Cao R., Kesimer M., et al. Identification of SPLUNC1's ENaC-inhibitory domain yields novel strategies to treat sodium hyperabsorption in cystic fibrosis airways. FASEB J. 2012;26(10):4348–4359. doi: 10.1096/fj.12-207431. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Hobbs C.A., Blanchard M.G., Alijevic O., Tan C.D., Kellenberger S., Bencharit S., et al. Identification of the SPLUNC1 ENaC-inhibitory domain yields novel strategies to treat sodium hyperabsorption in cystic fibrosis airway epithelial cultures. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013;305(12):990–1001. doi: 10.1152/ajplung.00103.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tarran R., Stutts M., Donaldson S. Regulation of sodium channels by PLUNC proteins. US20140228276. 2014 [Google Scholar]
- 24.Tarran R., Stutts M., Donaldson S. Regulation of sodium channels by PLUNC proteins. US20160159879. 2016 [Google Scholar]
- 25.Butler R., Hunt T., Smith N.J. ENaC inhibitors for the treatment of cystic fibrosis. Pharm. Pat. Anal. 2015;4(1):17–27. doi: 10.4155/ppa.14.51. [DOI] [PubMed] [Google Scholar]
- 26.Smith N.J., Solovay C.F. Epithelial Na+ channel inhibitors for the treatment of cystic fibrosis. Pharm. Pat. Anal. 2017;6(4):179–188. doi: 10.4155/ppa-2017-0009. [DOI] [PubMed] [Google Scholar]
- 27.Kim CS, Ahmad S, Wu T, Walton WG, Redinbo MR, Tarran R. SPLUNC1 is an allosteric modulator of the epithelial sodium channel. FASEB J. 2018 doi: 10.1096/fj.201701126R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rollins B.M., Garcia-Caballero A., Stutts M.J., Tarran R. SPLUNC1 expression reduces surface levels of the epithelial sodium channel (ENaC) in Xenopus laevis oocytes. Channels (Austin) 2010;4(4):255–259. doi: 10.4161/chan.4.4.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garland A.L., Walton W.G., Coakley R.D., Tan C.D., Gilmore R.C., Hobbs C.A., et al. Molecular basis for pH-dependent mucosal dehydration in cystic fibrosis airways. Proc. Natl. Acad. Sci. USA. 2013;110(40):15973–15978. doi: 10.1073/pnas.1311999110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scott D.W., Walker M.P., Sesma J., Wu B., Stuhlmiller T.J., Sabater J.R., et al. SPX-101 is a novel epithelial sodium channel-targeted therapeutic for cystic fibrosis that restores mucus transport. Am. J. Respir. Crit. Care Med. 2017;196(6):734–744. doi: 10.1164/rccm.201612-2445OC. [DOI] [PubMed] [Google Scholar]
- 31.Terryah S.T., Fellner R.C., Ahmad S., Moore P.J., Reidel B., Sesma J.I., et al. Evaluation of a SPLUNC1-derived peptide for the treatment of cystic fibrosis lung disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018;314(1):192–205. doi: 10.1152/ajplung.00546.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tarran R., Christensen D. Improved peptide inhibitors of sodium channels. WO2016057795. 2016 [Google Scholar]
- 33.Tarran R., Wu T. Peptide inhibitors of calcium channels. WO2017147128. 2017 [Google Scholar]
- 34.Kurbatova P., Bessonov N., Volpert V., Tiddens H.A., Cornu C., Nony P., et al. Model of mucociliary clearance in cystic fibrosis lungs. J. Theor. Biol. 2015;372:81–88. doi: 10.1016/j.jtbi.2015.02.023. [DOI] [PubMed] [Google Scholar]
- 35.Boucher R.C. New concepts of the pathogenesis of cystic fibrosis lung disease. Eur. Respir. J. 2004;23(1):146–158. doi: 10.1183/09031936.03.00057003. [DOI] [PubMed] [Google Scholar]
- 36.Berlow R.B., Dyson H.J., Wright P.E. Functional advantages of dynamic protein disorder. FEBS Lett. 2015;589(19):2433–2440. doi: 10.1016/j.febslet.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirsh A.J. Altering airway surface liquid volume: Inhalation therapy with amiloride and hyperosmotic agents. Adv. Drug Deliv. Rev. 2002;54(11):1445–1462. doi: 10.1016/s0169-409x(02)00161-8. [DOI] [PubMed] [Google Scholar]
- 38.Hirsh A.J., Molino B.F., Zhang J., Astakhova N., Geiss W.B., Sargent B.J., et al. Design, synthesis, and structure-activity relationships of novel 2-substituted pyrazinoylguanidine epithelial sodium channel blockers: Drugs for cystic fibrosis and chronic bronchitis. J. Med. Chem. 2006;49(14):4098–4115. doi: 10.1021/jm051134w. [DOI] [PubMed] [Google Scholar]
- 39.Rubin B.K., Williams R.W. Aerosolized antibiotics for non-cystic fibrosis bronchiectasis. Respiration. 2014;88(3):177–184. doi: 10.1159/000366000. [DOI] [PubMed] [Google Scholar]
- 40.Majewski P., Majchrzak-Gorecka M., Grygier B., Skrzeczynska-Moncznik J., Osiecka O., Cichy J. Inhibitors of serine proteases in regulating the production and function of neutrophil extracellular traps. Front. Immunol. 2016;7:261–266. doi: 10.3389/fimmu.2016.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Griese M., Kappler M., Gaggar A., Hartl D. Inhibition of airway proteases in cystic fibrosis lung disease. Eur. Respir. J. 2008;32(3):783–795. doi: 10.1183/09031936.00146807. [DOI] [PubMed] [Google Scholar]
- 42.Greene C.M., McElvaney N.G. Proteases and antiproteases in chronic neutrophilic lung disease: Relevance to drug discovery. Br. J. Pharmacol. 2009;158(4):1048–1058. doi: 10.1111/j.1476-5381.2009.00448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Low T.B., Greene C.M., O’Neill S.J., McElvaney N.G. Quantification and evaluation of the role of antielastin autoantibodies in the emphysematous lung. Pulm. Med. 2011;8:261–266. doi: 10.1155/2011/826160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Randell S.H., Boucher R.C. Effective mucus clearance is essential for respiratory health. Am. J. Respir. Cell Mol. Biol. 2006;35(1):20–28. doi: 10.1165/rcmb.2006-0082SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsai Y.F., Hwang T.L. Neutrophil elastase inhibitors: a patent review and potential applications for inflammatory lung diseases (2010 - 2014). Expert Opin. Ther. Pat. 2015;25(10):1145–1158. doi: 10.1517/13543776.2015.1061998. [DOI] [PubMed] [Google Scholar]
- 46.Griese M., Latzin P., Kappler M., Weckerle K., Heinzlmaier T., Bernhardt T., et al. Alpha1-antitrypsin inhalation reduces airway inflammation in cystic fibrosis patients. Eur. Respir. J. 2007;29(2):240–250. doi: 10.1183/09031936.00047306. [DOI] [PubMed] [Google Scholar]
- 47.Travis J. Structure, function, and control of neutrophil proteinases. Am. J. Med. 1988;84(6A):37–42. doi: 10.1016/0002-9343(88)90156-8. [DOI] [PubMed] [Google Scholar]
- 48.Gadek J.E., Klein H.G., Holland P.V., Crystal R.G. Replacement therapy of alpha 1-antitrypsin deficiency. Reversal of protease-antiprotease imbalance within the alveolar structures of PiZ subjects. J. Clin. Invest. 1981;68(5):1158–1165. doi: 10.1172/JCI110360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brinkman E., Hack C., Van D.N.I. Recombinant human alpha1- antitrypsin. US20120214747. 2012 [Google Scholar]
- 50.Brinkman N., Bigler D., Bolli R., Foertsch V. Methods for purification of alpha-1-antitrypsin andapolipoprotein A-1. US8436152 (2013) & US8653245 (2014) & US8962802. 2015 [Google Scholar]
- 51.Dinarello C., Crapo J., Kim S. Compositions, methods and uses for alpha-1 antitrypsin fusion molecules.. US20140341899. 2014 [Google Scholar]
- 52.Kee S., Cook P., Smith J., Fowler S., Weber D. Methods of treatment using alpha-1-antitrypsin compositions. US20150320846. 2015 [Google Scholar]
- 53.Kumpalume P., Podmore A., Dalton J. Method for the purification of alpha-1-antitrypsin. US8580931. 2013 [Google Scholar]
- 54.Lior Y., Geyra A., Lewis E.C. Therapeutic compositions and uses of alpha1-antitrypsin: A patent review. Expert Opin. Ther. Pat. 2016;26(5):581–589. doi: 10.1517/13543776.2016.1165210. [DOI] [PubMed] [Google Scholar]
- 55.Chapman K.R., Burdon J.G., Piitulainen E., Sandhaus R.A., Seersholm N., Stocks J.M., et al. Intravenous augmentation treatment and lung density in severe alpha1 antitrypsin deficiency (RAPID): A randomised, double-blind, placebo-controlled trial. Lancet. 2015;386(9991):360–368. doi: 10.1016/S0140-6736(15)60860-1. [DOI] [PubMed] [Google Scholar]
- 56.Brand P., Schulte M., Wencker M., Herpich C.H., Klein G., Hanna K., et al. Lung deposition of inhaled alpha1-proteinase inhibitor in cystic fibrosis and alpha1-antitrypsin deficiency. Eur. Respir. J. 2009;34(2):354–360. doi: 10.1183/09031936.00118408. [DOI] [PubMed] [Google Scholar]
- 57.Gaggar A., Chen J., Chmiel J.F., Dorkin H.L., Flume P.A., Griffin R., et al. Inhaled alpha1-proteinase inhibitor therapy in patients with cystic fibrosis. J. Cyst. Fibros. 2016;15(2):227–233. doi: 10.1016/j.jcf.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaner Z., Ochayon D.E., Shahaf G., Baranovski B.M., Bahar N., Mizrahi M., et al. Acute phase protein alpha1-antitrypsin reduces the bacterial burden in mice by selective modulation of innate cell responses. J. Infect. Dis. 2015;211(9):1489–1498. doi: 10.1093/infdis/jiu620. [DOI] [PubMed] [Google Scholar]
- 59.McElvaney N.G. Alpha-1 Antitrypsin therapy in cystic fibrosis and the lung disease associated with alpha-1 antitrypsin deficiency. Ann. Am. Thorac. Soc. 2016;13:S191–S196. doi: 10.1513/AnnalsATS.201504-245KV. [DOI] [PubMed] [Google Scholar]
- 60.Kryczka J., Boncela J. Proteases revisited: Roles and therapeutic implications in fibrosis. Mediators Inflamm. 2017;25:7015–7024. doi: 10.1155/2017/2570154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vasconcellos C.A., Allen P.G., Wohl M.E., Drazen J.M., Janmey P.A., Stossel T.P. Reduction in viscosity of cystic fibrosis sputum in vitro by gelsolin. Science. 1994;263(5149):969–971. doi: 10.1126/science.8310295. [DOI] [PubMed] [Google Scholar]
- 62.Lethem M.I., James S.L., Marriott C., Burke J.F. The origin of DNA associated with mucus glycoproteins in cystic fibrosis sputum. Eur. Respir. J. 1990;3(1):19–23. [PubMed] [Google Scholar]
- 63.Matthews L.W., Spector S., Lemm J., Potter J.L. Studies on pulmonary secretions. The over-all chemical composition of pulmonary secretions from patients with cystic fibrosis, bronchiectasis, and laryngectomy. Am. Rev. Respir. Dis. 1963;88:199–204. doi: 10.1164/arrd.1963.88.2.199. [DOI] [PubMed] [Google Scholar]
- 64.Fuchs H.J., Borowitz D.S., Christiansen D.H., Morris E.M., Nash M.L., Ramsey B.W., et al. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. The Pulmozyme Study Group. N. Engl. J. Med. 1994;331(10):637–642. doi: 10.1056/NEJM199409083311003. [DOI] [PubMed] [Google Scholar]
- 65.Felgner P., Abai A., Manthorpe M. Composition and method for treating cystic fibrosis. WO1993003709. 1993 [Google Scholar]
- 66.Wagener J.S., Kupfer O. Dornase alfa (Pulmozyme). Curr. Opin. Pulm. Med. 2012;18(6):609–614. doi: 10.1097/MCP.0b013e328358d51f. [DOI] [PubMed] [Google Scholar]
- 67.Sawicki G.S., Chou W., Raimundo K., Trzaskoma B., Konstan M.W. Randomized trial of efficacy and safety of dornase alfa delivered by eRapid nebulizer in cystic fibrosis patients. J. Cyst. Fibros. 2015;14(6):777–783. doi: 10.1016/j.jcf.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 68.Xie J., Adams L.M., Zhao J., Gerken T.A., Davis P.B., Ma J. A short segment of the R domain of cystic fibrosis transmembrane Conductance regulator contains channel stimulatory and inhibitory activities that are separable by sequence modification. J. Biol. Chem. 2002;277(25):23019–23027. doi: 10.1074/jbc.M201661200. [DOI] [PubMed] [Google Scholar]
- 69.Adams L., Davis P., Ma J. Enhancers of CFTR chloride channel function. WO2000050591 (2000) & AU2000032419 (2000) & US6770739 (2000) & US20040121957. 2004 [Google Scholar]
- 70.Goldstein A.L. From lab to bedside: Emerging clinical applications of thymosin alpha 1. Expert Opin. Biol. Ther. 2009;9(5):593–608. doi: 10.1517/14712590902911412. [DOI] [PubMed] [Google Scholar]
- 71.Romani L., Oikonomou V., Moretti S., Iannitti R.G., D’Adamo M.C., Villella V.R., et al. Thymosin alpha1 represents a potential potent single-molecule-based therapy for cystic fibrosis. Nat. Med. 2017;23(5):590–600. doi: 10.1038/nm.4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Romani L., Garaci E. Thymosin alpha 1 for use in treatment of cystic fibrosis. US20180036381 (2018) & WO2016129005 (2016) & AU2016217473 (2016) & CA2976062. 2018 [Google Scholar]
- 73.Tomati V., Caci E., Ferrera L., Pesce E., Sondo E., Cholon D.M., et al. Thymosin alpha-1 does not correct F508del-CFTR in cystic fibrosis airway epithelia. JCI Insight. 2018;3(3):11–19. doi: 10.1172/jci.insight.98699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cheng J., Wang H., Guggino W.B. Modulation of mature cystic fibrosis transmembrane regulator protein by the PDZ domain protein CAL. J. Biol. Chem. 2004;279(3):1892–1898. doi: 10.1074/jbc.M308640200. [DOI] [PubMed] [Google Scholar]
- 75.Cushing P.R., Vouilleme L., Pellegrini M., Boisguerin P., Madden D.R. A stabilizing influence: CAL PDZ inhibition extends the half-life of DeltaF508-CFTR. Angew. Chem. Int. Ed. Engl. 2010;49(51):9907–9911. doi: 10.1002/anie.201005585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Roberts K.E., Cushing P.R., Boisguerin P., Madden D.R., Donald B.R. Computational design of a PDZ domain peptide inhibitor that rescues CFTR activity. PLOS Comput. Biol. 2012;8(4):e1002477. doi: 10.1371/journal.pcbi.1002477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Calista T.I. Composition and methods of use for cell targeted inhibitors of the cystic fibrosis transmembrane regulator associated ligand. AU2015234367. 2015 [Google Scholar]
- 78.Mallon A.P., Alvin C.B.I. Compositions and methods of use for cell targeted inhibitors of the cystic fibrosis transmembrane regulator associated ligand. US20140296164. 2014 [Google Scholar]
- 79.Hwang S.M., Kim D.D., Chung S.J., Shim C.K. Delivery of ofloxacin to the lung and alveolar macrophages via hyaluronan microspheres for the treatment of tuberculosis. J. Control. Release. 2008;129(2):100–106. doi: 10.1016/j.jconrel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 80.Ahmad Z., Sharma S., Khuller G.K. Inhalable alginate nanoparticles as antitubercular drug carriers against experimental tuberculosis. Int. J. Antimicrob. Agents. 2005;26(4):298–303. doi: 10.1016/j.ijantimicag.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 81.Cheow W.S., Hadinoto K. Factors affecting drug encapsulation and stability of lipid-polymer hybrid nanoparticles. Colloids Surf. B Biointerfaces. 2011;85(2):214–220. doi: 10.1016/j.colsurfb.2011.02.033. [DOI] [PubMed] [Google Scholar]
- 82.Changsan N., Chan H.K., Separovic F., Srichana T. Physicochemical characterization and stability of rifampicin liposome dry powder formulations for inhalation. J. Pharm. Sci. 2009;98(2):628–639. doi: 10.1002/jps.21441. [DOI] [PubMed] [Google Scholar]
- 83.Zaru M., Manca M.L., Fadda A.M., Antimisiaris S.G. Chitosan-coated liposomes for delivery to lungs by nebulisation. Colloids Surf. B Biointerfaces. 2009;71(1):88–95. doi: 10.1016/j.colsurfb.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 84.Glazer P., Saltzman W.M., Egan M., McNeer N.A. Compositions and methods for treatment of cystic fibrosis. WO2017143061. 2017 [Google Scholar]
- 85.d’Angelo I., Conte C., La Rotonda M.I., Miro A., Quaglia F., Ungaro F. Improving the efficacy of inhaled drugs in cystic fibrosis: Challenges and emerging drug delivery strategies. Adv. Drug Deliv. Rev. 2014;75:92–111. doi: 10.1016/j.addr.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 86.Weers J., Tarara T. The PulmoSphere platform for pulmonary drug delivery. Ther. Deliv. 2014;5(3):277–295. doi: 10.4155/tde.14.3. [DOI] [PubMed] [Google Scholar]
- 87.Stein S.W., Thiel C.G. The history of therapeutic aerosols: A chronological review. J. Aerosol Med. Pulm. Drug Deliv. 2017;30(1):20–41. doi: 10.1089/jamp.2016.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ibrahim M., Verma R., Garcia-Contreras L. Inhalation drug delivery devices: Technology update. Med. Devices (Auckl.) 2015;8:131–139. doi: 10.2147/MDER.S48888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Griese M., Scheuch G. Delivery of alpha-1 antitrypsin to airways. Ann. Am. Thorac. Soc. 2016;13(Suppl. 4):S346–S351. doi: 10.1513/AnnalsATS.201507-469KV. [DOI] [PubMed] [Google Scholar]
- 90.Geller D.E., Weers J., Heuerding S. Development of an inhaled dry-powder formulation of tobramycin using PulmoSphere technology. J. Aerosol Med. Pulm. Drug Deliv. 2011;24(4):175–182. doi: 10.1089/jamp.2010.0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Weers J., Ung K., Le J., Rao N., Ament B., Axford G., et al. Dose emission characteristics of placebo PulmoSphere(R) particles are unaffected by a subject’s inhalation maneuver. J. Aerosol Med. Pulm. Drug Deliv. 2013;26(1):56–68. doi: 10.1089/jamp.2012.0973. [DOI] [PubMed] [Google Scholar]
- 92.Usmani OS, Biddiscombe MF, Yang S, Meah S, Oballa E, Simpson JK, et al. The topical study of inhaled drug (salbutamol) delivery in idiopathic pulmonary fibrosis. Respir Res. 2018;6; 19(1):25. doi: 10.1186/s12931-018-0732-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hertel S.P., Winter G., Friess W. Protein stability in pulmonary drug delivery via nebulization. Adv. Drug Deliv. Rev. 2011;93:79–94. doi: 10.1016/j.addr.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 94.Geller D.E., Kesser K.C. The I-neb adaptive aerosol delivery system enhances delivery of alpha1-antitrypsin with controlled inhalation. J. Aerosol Med. Pulm. Drug Deliv. 2010;23(Suppl. 1):S55–S59. doi: 10.1089/jamp.2009.0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fischer A., Stegemann J., Scheuch G., Siekmeier R. Novel devices for individualized controlled inhalation can optimize aerosol therapy in efficacy, patient care and power of clinical trials. Eur. J. Med. Res. 2009;14(Suppl. 4):71–77. doi: 10.1186/2047-783X-14-S4-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hertel S., Pohl T., Friess W., Winter G. Prediction of protein degradation during vibrating mesh nebulization via a high throughput screening method. Eur. J. Pharm. Biopharm. 2014;87(2):386–394. doi: 10.1016/j.ejpb.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 97.Elhissi A. Liposomes for pulmonary drug delivery: The role of formulation and inhalation device design. Curr. Pharm. Des. 2017;23(3):362–372. doi: 10.2174/1381612823666161116114732. [DOI] [PubMed] [Google Scholar]
- 98.Nikander K., von Hollen D., Larhrib H. The size and behavior of the human upper airway during inhalation of aerosols. Expert Opin. Drug Deliv. 2017;14(5):621–630. doi: 10.1080/17425247.2016.1227780. [DOI] [PubMed] [Google Scholar]
- 99.Heinemann L., Baughman R., Boss A., Hompesch M. Pharmacokinetic and pharmacodynamic properties of a novel inhaled insulin. J. Diabetes Sci. Technol. 2017;11(1):148–156. doi: 10.1177/1932296816658055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Norris A.W. Is Cystic Fibrosis related diabetes reversible? New data on CFTR potentiation and insulin secretion. Am. J. Respir. Crit. Care Med. 2018;••• doi: 10.1164/rccm.201808-1501ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


