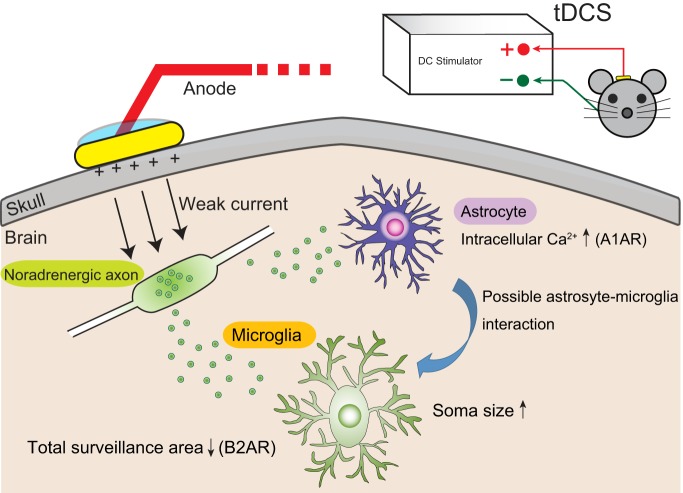
Visual Abstract
Keywords: iba1, in vivo, microglia, norepinephrine, tDCS, two-photon imaging
Abstract
Transcranial direct current stimulation (tDCS) has been reported for its beneficial effects on memory formation and various brain disorders. While the electrophysiological readout of tDCS effects is subtle, astrocytes have been demonstrated to elicit Ca2+ elevations during tDCS in a rodent model. This study aimed to elucidate the effects of tDCS on another major glial cell type, microglia, by histology and in vivo imaging. tDCS performed in awake conditions induced a significant change in the pixel intensity distribution of Iba-1 immunohistochemistry, and microglial somata were enlarged when examined 3 h after tDCS. These effects were blocked by adrenergic receptor antagonists or in IP3R2 (inositol trisphosphate receptor type 2)-deficient mice, which lack large cytosolic Ca2+ elevations in astrocytes. No obvious changes were observed in isoflurane-anesthetized mice. Furthermore, in vivo two-photon imaging of microglia showed a reduction of motility that was blocked by a β2-adrenergic receptor antagonist. Our observations add support for the influence of noradrenaline in tDCS and suggest possible interactions between microglia and astrocytes to express functional changes associated with tDCS.
Significance Statement
Transcranial direct current stimulation (tDCS) is a neuromodulation procedure in which a weak electric direct current is delivered through the brain for tens of minutes. Despite reported positive effects, the mechanisms of tDCS stimulation are not yet well understood. Here, we examined microglial morphology in the mouse cortex after tDCS. We find that the morphology and morphologic dynamics of microglia are altered by tDCS in a manner dependent on adrenergic receptors, supporting the notion that (nor)adrenergic signaling is involved in tDCS.
Introduction
Noninvasive neuromodulation is a subject of intense research because of its potential for treating patients with neuropsychiatric and neurologic conditions. Transcranial direct current stimulation (tDCS) is the application of a constant and weak electric current to the brain through the skull. Typical parameters applied in humans are 1 mA over ∼30 cm2 for 10–30 min (Bikson et al., 2016). A fair sized body of published literature suggests that tDCS has positive effects on cognitive abilities and could be an alternative treatment for various brain disorders (Fregni and Pascual-Leone, 2007; Nitsche et al., 2008, 2009; Brunoni et al., 2012; Dedoncker et al., 2016). On the other hand, there is a notable degree of skepticism due to mixed outcomes of tDCS experiments (Horvath et al., 2015a,b; Jalali et al., 2017; Medina and Cason, 2017; Kunzelmann et al., 2018; Turkakin et al., 2018). The skepticism has been, in part, strengthened by a recent study that suggested negligible tDCS-induced membrane potential changes in cerebral cortical neurons (Vöröslakos et al., 2018), implying limited involvement of neuronal discharge as the prevalent mechanism of tDCS.
The circuit and cellular mechanisms for tDCS remain to be understood. Glial cells represent electrically nonexcitable cells in the nervous system. They have been regarded as “support cells” for the normal function of neurons. Among glial cell types, astrocytes and microglia maintain the extracellular milieu by ion homeostasis and phagocytosis, respectively. Additionally, astrocytes and microglia have been reported to interact with neuronal synapses (Wake et al., 2013; Araque et al., 2014). We recently reported that astrocytic Ca2+ surges occur during tDCS in mice. Moreover, tDCS-induced astrocytic Ca2+ surges were shown to promote cortical plasticity and have beneficial effects in a mouse model of depression (Monai et al., 2016; Monai and Hirase, 2016, 2018). The recruitment of Ca2+ activities in astrocytes has prompted us to investigate another major glial cell type, microglia.
Microglia are sensitive to brain tissue damage and transform to reactive microglia on inflammation. Iba1 (ionized calcium binding adaptor molecule 1) immunohistochemistry (IHC) visualizes the morphology of microglia, which is profoundly altered in reactive microglia. Following the published observation that reported the lack of pronounced microglial reactivity after tDCS (Monai et al., 2016), here we investigated Iba1 IHC in detail by digital image analysis. We report subtle, but significant effects of tDCS in an awake condition, but not under anesthesia, that depended on adrenergic receptors. Subsequently, we examined microglial motility by in vivo two-photon imaging and found that tDCS reduces microglial motility.
Materials and Methods
All animal procedures were performed in accordance with the RIKEN animal experimental committee regulations.
Animals
Adult C57BL/6J and IP3R2 (inositol trisphosphate receptor type 2) knock-out (KO) mice (Futatsugi et al., 2005) were used for immunohistochemical experiments (male, 2–4 months old). BAC-GLT1-G-CaMP7 line 817 mice (male, 2–5 months old; catalog #G7NG817, RIKEN BioResource Research Center; resource ID: RBRC09650) were used for transcranial macroscopic imaging of neuronal and astrocytic Ca2+ activity (Monai et al., 2016). Iba1-GFP mice (male, 3–10 months old; Hirasawa et al., 2005) were used for in vivo two-photon imaging of microglial morphology.
Surgical procedures
Mice were deeply anesthetized with isoflurane (1.5–2.0%), and their scalps were exposed by shaving. Each mouse was fixed on a stereotaxic apparatus (Narishige) under isoflurane anesthesia. Throughout the surgery and experiments with anesthetized mice, the body temperature was kept at 37°C with a heating blanket (BWT-100A, Bio Research Center). After topical application of xylocaine ointment (2% lidocaine) on the scalp, the skull above the sensory cortex was exposed by incision of the scalp and temporal muscle. A custom-made chamber ring was glued to the skull with cyanoacrylate superglue. After the glue settled, we applied dental cement (Fuji LUTE BC, GC Corporation; Super-Bond C&B, Sun Medical) for reinforcement. For two-photon imaging, the inner cavity of the chamber ring was reinforced with additional dental cement to secure the interface for an objective lens. Once the chamber ring was rigidly attached, the mouse was fixed on a custom-made stage via the chamber ring. Thereafter, a small craniotomy (φ = 3 mm, with intact dura) was carefully made using a dental drill.
Habituation to head restraint
The postsurgical recovery period was at least 3 d for IHC experiments and 2 weeks for in vivo two-photon imaging experiments. Following the recovery period, mice were placed on a water restriction schedule and subjected to an acclimatization procedure for head restraint (Fig. 1B). Food was given ad libitum. The acclimatization procedure was performed for 7 d.
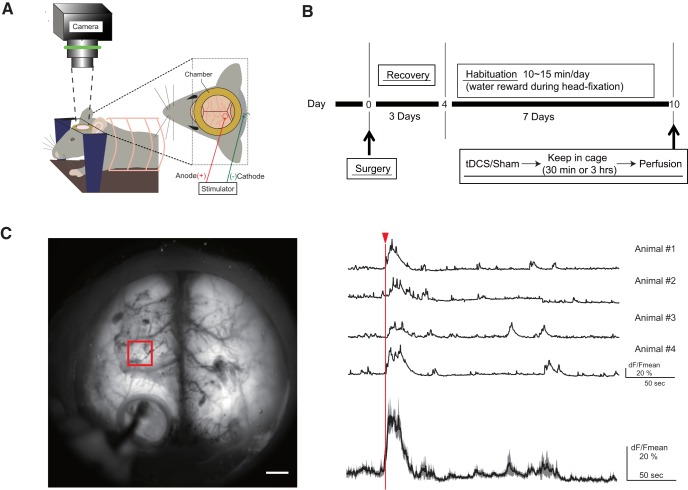
Figure 1.
Head-restraint tDCS experiment. A, Experimental setup for tDCS. B, Experimental schedule of immunohistochemical experiment. C, Top view of a BAC-GLT1-G7 Line 817 (G7NG817) mouse. Fluorescent Ca2+ signal is transcranially observable. Signals ∼3 mm anterior to the anodal site (1 × 1 mm2 red square) are plotted from four mice (right, top traces). The bold trace on the bottom is the mean of the four traces, and the shaded areas represent SE. The red arrowhead and line indicate the onset of tDCS. Scale bar, 1 mm. tDCS-induced Ca2+ elevations were not observed in isoflurane-anesthetized mice (Extended Data Fig. 1-1).
On day 1, each mouse was held in the experimenter’s hands and water was given via a syringe (∼0.2 ml). During handling, we let the mouse explore until it entered into a body tube similar to the one used with the tDCS apparatus. If the mouse entered the body tube, we repeated the procedure four to five times. The total handling time was 10 min for each mouse. From day 2, the mouse continued to be acclimatized to the experimenter and apparatus with a water reward (0.1–0.2 ml) for each entry to the body tube. At this point, the head of the mouse was quickly (<10–20 s) fixed to the apparatus via the chamber ring with its body in the tube. Additional water and sunflower seeds were provided during head fixation (10–15 min). The total amount of water given during head fixation was 1.0 ml/d. In some mice, for in vivo two-photon imaging acclimatization was performed for >7 d.
Transcranial DC stimulation
tDCS was applied on mice under anesthesia (2% isoflurane) or in awake conditions. In either condition, the anode (stainless wire, φ = 350 μm) was placed on a sodium chloride-based conductive gel interface (Z101BA, NIHON-KODEN) spread over a circular area (φ = ∼2 mm) above the primary visual cortex (anteroposterior, −2.9 mm; mediolateral 2.0 mm). The cathode was connected to the neck skin after topical application of xylocaine ointment. DC (0.1 mA, 10 min) was applied with a custom-made isolated constant-current supply.
Histology
After tDCS application, mice were kept for 30 min or 3 h before they were killed. After deep anesthesia by urethane, they were first perfused with 0.9% NaCl and later with fixative solution (4.0% paraformaldehyde in 0.1 m phosphate buffer, pH 7.4). Following brain removal and overnight postfixation in the same fixative, coronal slices (60 μm) were prepared using a microslicer (PRO 7, Dosaka). For Iba1 staining, sections were incubated in a buffer containing the primary antibody (Tris-buffered saline with 0.1% Triton X-1000; 1:2000; catalog #019-19741, Wako) overnight. The sections were subsequently washed in PBS and incubated with the Cy3-conjugated secondary antibody (Invitrogen) for 2 h for fluorescent labeling. To evaluate DSP4 [N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine hydrochloride] efficacy, noradrenergic fibers were labeled by anti-tyrosine hydroxylase (TH) antibody (1:1000; catalog #AB152, Millipore) using sagittal slices (60 μm). For positive control of microglial reactivity, Escherichia coli lipopolysaccharide (LPS; 0.5 mg/kg) was administered by intraperitoneal injection 2 d before the mice were killed.
Confocal imaging
Immunolabeled cortical microglia (V2 area) were examined using a confocal microscope (FV1000, Olympus). Images were acquired with a 60× water-immersion objective (UPlanSApo; numerical aperture, 1.20) at an excitation wavelength of 559 nm. Imaged areas covered 211.761 × 211.761 μm2 (1024 × 1024 pixels) with an optical sectioning of 0.5 μm. Images were scanned with the one-way mode (8 μs/pixel exposure).
Drug administration
In some experiments, the following drugs were administered before tDCS by intraperitoneal injection: ICI81551 (5 mg/kg body weight, 30 min before; Tocris); and Prazosin (10 mg/kg, 30 min before; Sigma-Aldrich). For the ablation of noradrenergic neurons, DSP4 (Sigma-Aldrich) was injected 11 and 7 d before tDCS application (50 mg/kg, i.p., each time). Drugs were dissolved in 0.9% NaCl.
In vivo imaging of microglial morphology
Adult Iba1-EGFP transgenic mice (Hirasawa et al., 2005), in which EGFP is expressed exclusively in microglia, were used to monitor microglial morphologic dynamics. All mice were habituated to the experimental apparatus for >7 d. On the day of imaging, the mouse was set on a custom-made stage under a two-photon microscope (B-Scope, Thorlabs). Microglia located >50 μm below the pial surface were imaged under awake conditions at a wavelength of 920 nm. The laser power was adjusted to ∼12 mW at the preparation (Hines et al., 2009; Wake et al., 2009; Pfeiffer et al., 2016). Depth stacks (24–26 slices, 2 μm z interval, 512 × 512 pixels corresponding to 101 × 101 μm2 or 201 × 201 μm2) were acquired every 60 s.
Analysis
Iba1 IHC image analysis
Confocal images were used for pixel intensity analysis. Image stacks extending to 15 μm thickness were collapsed into 2D images by maximum intensity projection. Pixel intensities were converted to z scores, and the cumulative distribution was computed for each collapsed 2D image.
For soma size analysis, confocal image stacks (45–50 μm thickness) were first filtered with a 3 × 3 × 3 median filter. The resultant image stacks were collapsed into 2D images by maximum intensity projection. To correct for uneven background, the rolling ball method with a radius of 30 pixels was used for background subtraction. Thereafter, the images were subjected to a 3 × 3 2D median filter followed by binarization with Yen’s thresholding method (ImageJ, National Institutes of Health) for soma extraction. In some cases, manual adjustments of threshold were needed. Extracted somata were approximated to ellipses. Following these automated procedures in ImageJ, extracted somata were validated by manual inspection. The median of microglial soma size distribution from each mouse was taken as a data point for statistical comparisons.
TH image analysis
The efficacy of DSP4 was evaluated by calculating the mean intensity of posterior cortical layer 1 TH-positive (TH+) innervation using ImageJ. Briefly, sagittal brain section images (60 μm thickness) were acquired by a Keyence microscope (BZ-X710; 0.37 μm pixel size). Ten to 12 contiguous regions of interest (ROIs; 100 × 100 μm each) were allocated to occupy layer 1. A background intensity value was calculated from a neighboring parenchymal area that does not contain TH+ axons. The mean TH+ signal intensity of each ROI was computed as the mean pixel intensity minus the background intensity.
Microglial motility assessment
Quantification of microglial surveillance was performed using custom-written ImageJ and MATLAB programs (MathWorks). The maximum intensity projection image was computed for each time point of xyzt image stack. The resultant xyt image stack was registered for xy motion correction. Next, each slice of the xyt stack was processed by the ImageJ “Subtract Background” plugin to subtract smooth continuous background with a ball size of 30 pixels. Thereafter, images were treated with a 2D 3 × 3 median filter. After this preprocessing, rectangular areas containing the morphologic extent of single microglia were extracted as separate image stacks. These cell-wise image stacks were then binarized with a single threshold determined by Li’s Minimum Cross Entropy method (ImageJ). Noise reduction was then performed by a cycle of erosion and dilation. The normalized surveillance area at time t was calculated as the number of pixels that were occupied by the microglia at least once since the beginning of imaging until a given time t, divided by the number of pixels occupied by the microglia at the beginning. Normalized surveillance area is therefore a monotonically increasing function of time (see Fig. 6C,D, example). The surveillance index is defined as the ratio of normalized surveillance areas of a microglia in two different sessions [e.g., control (“Before”) vs post-tDCS (“After”)].
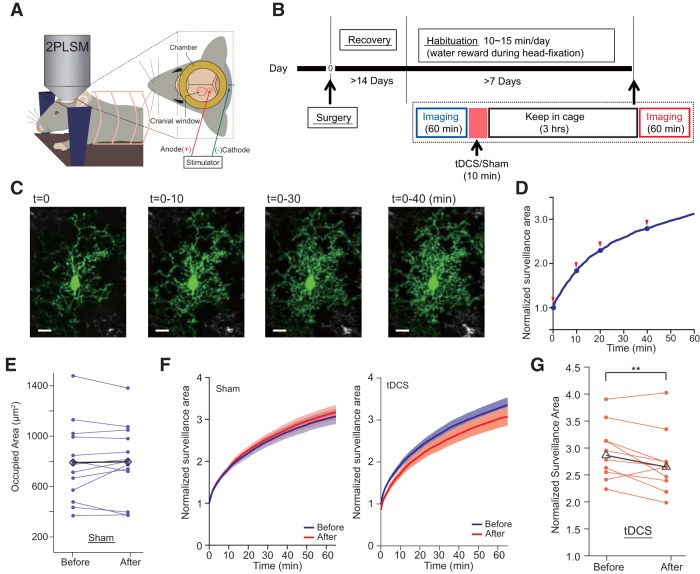
Figure 6.
In vivo monitoring of microglial morphologic dynamics. A, B, Experimental setup (A) and time schedule of in vivo two-photon imaging (B). C, Representative images of a microglia overlaid from t = 0 to respective time points (10, 30, and 40 min). D, Normalized surveillance area curve during 60 min imaging period. Red arrowheads show the time points for the images in C. Scale bar, 10 μm. E, Initial microglial area at t = 0 of Before and After sessions are similar in Sham mice (13 cells from 8 mice; p = 0.82i). Blue lines represent data from individual microglia, and the black line represents averaged data. F, Normalized surveillance area curves during the 60 min imaging period before (blue) and after (red) stimulation in the sham (left) and tDCS (right) mice. Data are represented as the mean ± SEM. G, Normalized surveillance area at t = 40 min in Before and After sessions in tDCS-treated mice (normalized by surveillance area at t = 0/Before). Red lines represent data from individual microglia, and the black line represents averaged data. p = 0.014j, paired t test. **p < 0.03.
Statistical analyses
Statistical analyses were performed using Igor Pro (WaveMetrics). Student’s paired t tests and Wilcoxon-Mann–Whitney rank sum tests were used for the comparison of two sample populations with matched data and unmatched data, respectively, unless otherwise noted. Data are expressed as the mean ± SEM, and p values <0.05 were considered statistically significant. Statistical values are reported in Table 1.
Table 1:
Statistical table
| Sample number: cells (animals) | Test type | p Value | Power | |
|---|---|---|---|---|
| a | Sham: 334(7), Ctl: 315(7) | Mann–Whitney Wilcoxon rank sum test | 0.1 | |
| b | Sham: 315(7), tDCS: 314(7) | Mann–Whitney Wilcoxon rank sum test | 0.16 | |
| c | Sham: 309(7), tDCS: 301(7) | Mann–Whitney Wilcoxon rank sum test | **0.017 | |
| d | Sham: 278(6), tDCS: 296(7) | Mann–Whitney Wilcoxon rank sum test | 0.95 | |
| e | Sham: 285(7), tDCS: 319(7) | Mann–Whitney Wilcoxon rank sum test | 0.073 | |
| f | Sham: 238(6), tDCS: 356(7) | Mann–Whitney Wilcoxon rank sum test | 0.73 | |
| g | Sham: 274(7), tDCS: 310(7) | Mann–Whitney Wilcoxon rank sum test | 0.8 | |
| h | Sham: 266(6), tDCS: 282(6) | Mann–Whitney Wilcoxon rank sum test | 0.48 | |
| i | Sham: 13(8) | Paired t test | 0.82 | 0.055 |
| j | tDCS: 11(8) | Paired t test | **0.014 | 0.77 |
| k | Sham: 13(8), tDCS: 11(8) | Mann–Whitney Wilcoxon rank sum test | ***0.006 | |
| l | Sham: 11(3), tDCS: 9(2) | Mann–Whitney Wilcoxon rank sum test | **0.015 | |
| m | Sham: 9(3), tDCS: 12(3) | Mann–Whitney Wilcoxon rank sum test | **0.023 |
*p < 0.05, **p < 0.03, ***p < 0.01
Results
First, we confirmed tDCS-induced cortex-wide Ca2+ elevations (Monai et al., 2016) in the present setting using G7NG817 transgenic mice that express the G-CaMP7 Ca2+ sensor in astrocytes and a subpopulation of neurons. Mice had been acclimatized to be rigidly fixed to a head-restraint platform, where tDCS (0.1 mA, 10 min) and transcranial fluorescence imaging were performed (Fig. 1A,B; see Materials and Methods). Cortical Ca2+ signals elevated immediately after the passage of the DC current. The peak amplitude of the G-CaMP7 response measured ∼3 mm anterior to the anodal position was 39.7 ± 4.1% (Fig. 1C; N = 4 mice), showing that tDCS-induced Ca2+ elevation is observable with the head chamber-ring configuration. Notably, tDCS-induced Ca2+ elevations were not observed in isoflurane-anesthetized mice (Extended Data Fig. 1-1). Having demonstrated the effectiveness of tDCS, we used C57BL/6 mice to investigate microglial morphology after tDCS by Iba1 IHC. Mice were killed either 30 min or 3 h after tDCS for perfusion fixation.
Cortical Ca2+ activity during tDCS in mice under deep isoflurane anesthesia. G-CaMP7 signal was transcranially measured from isoflurane-anesthetized (1.5–2.0%) BAC-GLT1-G7 Line 817 (G7NG817) mice. The top trace is for Sham stimulation (−3.14 ± 0.02%), and the lower trace is for tDCS (0.1 mA, 10 min; −4.30 ± 0.02%). Bold traces represent the mean of 11 traces from 9 mice. Shaded areas represent the SE. The red arrowhead and vertical line indicate the onset of tDCS or sham stimulation. Download Extended Data 1, EPS file (1.6MB, eps) .
Iba1 IHC patterns are affected by tDCS in awake mice
Iba1 IHC visualized highly ramified microglial morphology throughout brain slices of sham-operated, LPS-treated, and tDCS mice (Fig. 2A,B,I,J). To investigate the impact of tDCS on the wide-field appearance of Iba1 IHC, we computed the pixel intensity distribution, which is a proxy of global morphologic changes. We analyzed layer 2 and 3 of the visual cortex located below the anode, since a previous study demonstrated that tDCS-mediated plasticity occurs in these layers (Monai et al., 2016). Pixel intensities were converted to z scores with which the cumulative distributions were plotted. We compared head ring-implanted, unrestrained control mice (Ctl group) with head ring-implanted, acclimatized, 25 min head-restrained mice (Sham group) to evaluate the possible effects of head restraint. In Figure 2C, we demonstrate that cumulative pixel intensity distribution is similar between the Ctl and Sham groups, whereas the pixel intensity distribution shifted significantly in mice with reactive microglia caused by LPS. These results suggest that the head-restraining procedure in acclimatized mice does not cause reactivity in cortical microglia.
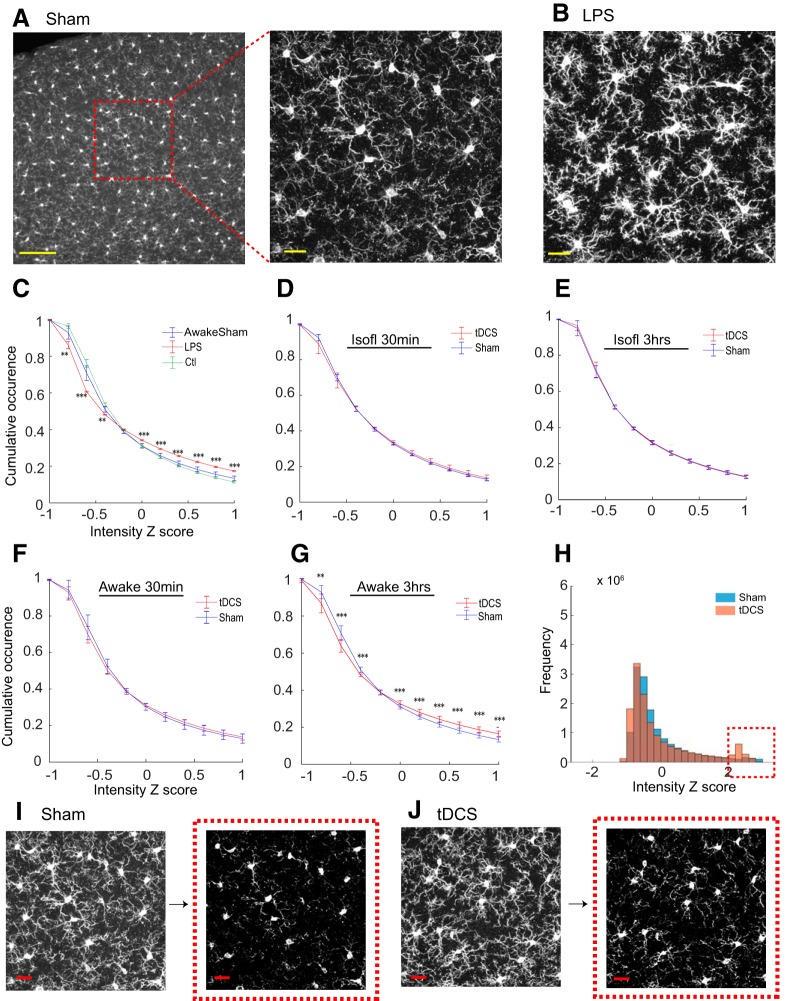
Figure 2.
Intensity analysis of microglial confocal images. A, B, Representative images of Cy3-labeled Iba1 IHC by maximum intensity projection obtained in Sham- and LPS-treated mice. Yellow scale bars: A, left, 100 μm; A, right, B, 20 μm. C, Cumulative pixel intensity distributions from unrestrained Ctl and head-restrained Sham groups were similar and distinct from the LPS-treated group. D–G, Intensity was compared between tDCS- and Sham-treated groups under the isoflurane-anesthetized (D, E) or awake (F, G) conditions, perfused at 30 min or 3 h after sham/tDCS. H, In awake mice, the pixel intensity histogram indicates that there is a cluster at z score >2 (i.e., mean + 2 SDs) region in the tDCS group (dotted red square). I, J, Representative images from a sham-treated mouse and a tDCS-treated mouse. Images in the red squares correspond to the thresholded images on the left at the mean + 2 SDs. Red scale bars, 20 μm. **p < 0.01, ***p < 0.001.
Next, we compared tDCS and sham-treated mice. The combination of two conditions [isoflurane-anesthetized (isofl) or awake] and two time points (30 min and 3 h after tDCS) were investigated (Fig. 2D–G). Pixel intensity distribution was similar between sham and tDCS for isofl 30 min, isofl 3 h, and awake 30 min experiments; however, the awake 3 h tDCS data exhibited a visible deviation from the sham group (Fig. 2G). This deviation was caused by a higher proportion of pixels at the high-intensity end. For instance, tDCS had a relatively large presence of pixels that had a z score >0.6 (21.2 ± 1.6% vs 18.2 ± 1.4%; p = 1.7e-5, t test). Moreover, a high-intensity cluster that has a z score >2 was apparent in the pixel intensity histogram (Fig. 2H). Consistent with this observation, thresholding with z > 2 preserved more microglial structures in awake 3 h tDCS images than the sham counterpart (Fig. 2I,J). While the cumulative pixel intensity histogram of awake 3 h tDCS deviated in the same direction as LPS, microglial morphology appeared normal with fine ramified processes throughout the extent of the cortex in all tDCS experiments. Thus, tDCS does not appear to cause inflammatory responses.
tDCS enlarges microglial somata in awake mice
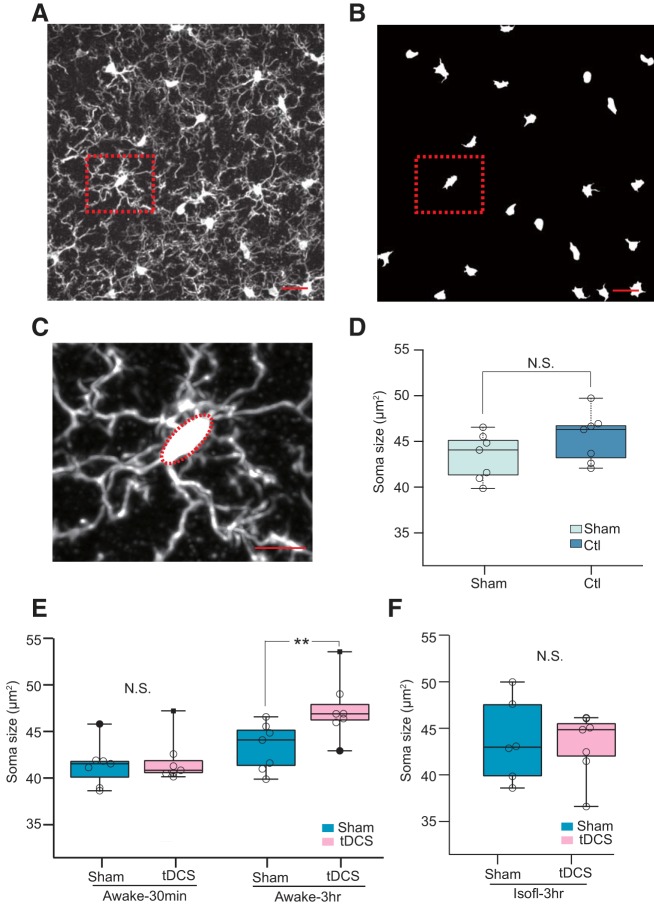
While the z score-based pixel intensity distribution analysis detected changes in the global appearance of images, it falls short of providing information on specific aspects of morphologic alterations. Microglial soma size has been reported to be sensitive to brain environmental changes (Kongsui et al., 2015). Therefore, we measured microglial soma size from Iba1 IHC images (Fig. 3A–C; see Materials and Methods). First, we compared the median microglial soma size of individual animals (43 cells per mouse on average) for unrestrained control and head-restrained sham groups as we did in Figure 2C. Figure 3D indicates that microglia soma sizes are similar between the control and sham groups (Ctl group: 45.4 ± 1.0 μm2, 7 mice; Sham group: 43.4 ± 1.0 μm2, 7 mice; p = 0.16b, Mann–Whitney Wilcoxon test), suggesting that the microglial soma size of the Sham group serves as a valid control for tDCS experiments.
Figure 3.
Quantification and comparison of microglial soma size. A, Example image of an Iba1 IHC confocal image stack collapsed by maximum intensity projection. Scale bar, 20 μm. B, Digitally processed image of A for soma extraction. C, Example of the elliptic approximation of soma (A, B, red dotted square). D, Comparison of median values of microglial soma areas between Sham-stimulated and unrestrained control mice (p = 0.1a, Mann–Whitney Wilcoxon rank sum test). Scale bars: A, B, 10 μm; C, 20 μm. E, Comparison of microglial soma size in awake mice with/without tDCS treatment at different time points (30 min or 3 h) after tDCS. Microglial soma size was larger in the tDCS group in the awake 3 h experiment (p = 0.017c, Mann–Whitney Wilcoxon rank sum test). Each group contains seven mice. F, Microglial soma size comparison in isoflurane-anesthetized mice (Isofl-3hr). **p < 0.03, N.S. not significant.
Soma size did not differ significantly between the awake 30 min tDCS and Sham groups (Sham group: 41.5 ± 0.92 μm2, 7 mice; tDCS group: 41.9 ± 0.9 μm2, 7 mice; Fig. 3E; p = 1.0a). In awake 3 h experiments, soma size was significantly larger in the tDCS group (p = 0.017e; Sham group: 43.4 ± 1.0 μm2, 7 mice; tDCS group: 47.5 ± 1.2 μm2, 7 mice; Fig. 3E). On the other hand, soma size did not differ significantly when tDCS was performed on isoflurane-anesthetized mice (3 h group: p = 0.95f, Sham group: 43.7 ± 1.8 μm2, 6 mice; vs tDCS group: 43.2 ± 1.3 μm2, 7 mice; Fig. 3F). These results were consistent with the pixel intensity distribution analysis (Fig. 2) and suggest that isoflurane anesthesia hampers tDCS-induced microglial soma enlargement.
tDCS-induced microglial soma enlargement is dependent on adrenergic receptors
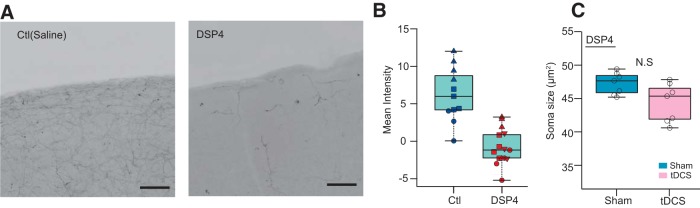
Recent human and animal studies have implicated the involvement of noradrenaline in tDCS (Monai et al., 2016; Kuo et al., 2017; Monai and Hirase, 2018; Souza et al., 2018). To examine the possible contribution of noradrenaline to tDCS-induced microglia soma size, we ablated noradrenergic cells in the locus ceruleus using the neurotoxin DSP4 (Bekar et al., 2008), which was confirmed by TH staining in the sensory cortex (Fig. 4A,B). Following noradrenergic neuron ablation, we performed tDCS using the awake 3 h protocol. As a result, DSP4-treated mice did not show a microglial soma enlargement after tDCS (Fig. 4C; Sham group: 47.3 ± 0.6 μm2, 7 mice; tDCS group: 44.5 ± 1.1 μm2, 7 mice; p = 0.073e).
Figure 4.
tDCS-induced microglial somatic enlargement depends on noradrenaline. A, Example of cortical image (inverted grayscale) from saline- (left) or DSP4- (right) pretreated mice stained with TH antibody. B, Mean intensity analysis of TH+ fiber. Each group contains data from three mice. Data from the same animals are plotted with the same symbol and color. Scale bars, 100 μm. C, Comparison between median glial soma size from sham- and tDCS-treated mice (Sham group: 7 mice; tDCS: 7 mice; p = 0.073e, Mann–Whitney Wilcoxon rank sum test). N.S. not significant.
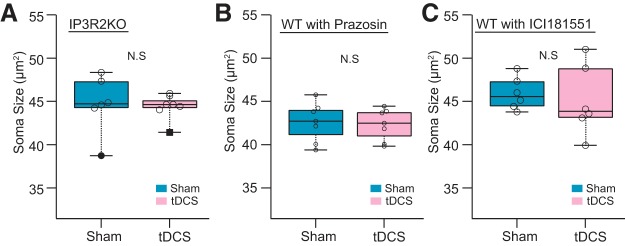
Since astrocytes exhibit profound α1-adrenergic receptor (A1AR)-mediated Ca2+ elevations by tDCS (Monai et al., 2016), astrocytic Ca2+ signaling possibly plays a role in the microglial soma enlargement via an intercellular communication. To examine this possibility, we used IP3R2 knock-out mice in which Gq GPCR (e.g., A1AR)-activated intracellular Ca2+ elevation is diminished in astrocytes. Awake 3 h tDCS did not result in significant microglial soma size changes in IP3R2 KO mice (p = 0.73f; Sham group: 44.6 ± 1.4 μm2, 6 mice; tDCS group: 44.4 ± 0.56 μm2, 7 mice; Fig. 5A). We next examined the involvement of A1AR using the specific antagonist prazosin in wild-type C57BL/6J mice. Similar to IP3R2 KO mice, prazosin-treated mice did not display tDCS-induced microglial soma enlargement compared with the Sham control group that also received the antagonist pretreatment (p = 0.8 × g; Sham group: 42.6 ± 0.9 μm2, 7 mice; tDCS group: 42.3 ± 0.7 μm2, 7 mice; Fig. 5B). These results suggest that tDCS-triggered noradrenaline release affects microglial soma enlargement via A1AR activation and the downstream astrocytic IP3R2-dependent Ca2+ signaling pathway.
Figure 5.
tDCS-induced microglial somatic enlargement depends on B2AR and A1AR pathways. A, Comparison between median microglial soma size between Sham- and tDCS-treated IP3R2 KO mice (Sham group: 6 mice; tDCS group: 7 mice; p = 0.73f). B, C, Comparison of microglial soma size between Sham- and tDCS-treated wild-type strain C57BL/6J with prazosin (B; Sham group: 7 mice; tDCS group: 7 mice; p = 0.8 × g), or ICI181551 pretreatment (C; Sham group: 6 mice; tDCS group: 6 mice; p = 0.48h). N.S. not significant.
Furthermore, we asked whether activation of β-adrenergic receptors is also involved. In particular, microglia are known for high levels of β2-adrenergic receptor (B2AR) expression (Tanaka et al., 2002, Gyoneva and Traynelis, 2013). Accordingly, mice were pretreated with ICI181551, a selective B2AR blocker, and soma sizes were compared. In the ICI181551 group, tDCS-induced soma size enlargement was not observed (p = 0.48h; Sham group: 45.9 ± 0.8 μm2, 6 mice; tDCS group: 45.2 ± 1.7 μm2, 6 mice; Fig. 5C). These results are indicative of noradrenergic involvement in tDCS-induced microglial changes and suggest that both A1ARs and B2BRs are involved in tDCS-induced microglial soma enlargement.
tDCS decreases microglial surveillance area in vivo
One of the striking features of microglia is the motility of their ramified processes. Here, we directly examined the morphologic dynamics of individual microglia in the cortex of awake mice using a two-photon microscope (Fig. 6A). We used the Iba1-EGFP mouse, in which EGFP is exclusively expressed in microglia (Hirasawa et al., 2005). We confirmed that microglia showed surveillance activities by continual extension and retraction of their processes in all directions (Davalos et al., 2005; Nimmerjahn et al., 2005). For example, the overlay of 60 min imaging resulted in an extensive coverage of the area within ∼60 μm from the soma, while the soma position remained unmoved (Fig. 6C). We defined normalized surveillance area as the proportion of cumulative microglia-occupied area at a given time relative to a start time (Fig. 6D). To check whether laser scanning has an impact on microglial morphology, we compared the occupied area of each monitored microglia at the beginnings of Before and After imaging sessions of the Sham-treated group (Fig. 6E). We found no significant difference, suggesting that the effect of laser irradiation on microglial morphologic dynamics is negligible.
While the evolution of a normalized surveillance area varied considerably among individual microglia, the average trace converged to a gradually decelerating curve (Fig. 6F). The mean surveillance area after 60 min did not differ significantly between before and after sham stimulation. Remarkably, the mean surveillance area index curve of tDCS mice (i.e., After session) is plotted lower than the control condition (i.e., Before session). We assessed the surveillance area change of individual microglia by taking the ratio of the surveillance area indices during Before and After sessions, demonstrating a significant decrease of surveillance area by tDCS (t = 40 min, p = 0.014j, paired t test; Fig. 6G).
Furthermore, we addressed whether noradrenergic signaling is involved in this tDCS-induced microglial surveillance reduction by prazosin or ICI181551 pretreatment in awake mice (Fig. 7A). As a reference, we computed the surveillance index comparing Before and After sessions at 40 min after the start of respective sessions. As expected from the previous analysis (Fig. 6G), the surveillance index of tDCS experiments was significantly reduced (Fig. 7A). Prazosin-treated mice showed a similar significant reduction of surveillance index after tDCS (Fig. 7B). By contrast, ICI181551 treatment abolished tDCS-induced reduction of microglia surveillance, and a trend for increased surveillance was apparent (Fig. 7C). These results point to a significant role of the B2AR in the inhibition of microglial surveillance activity after tDCS.
Figure 7.
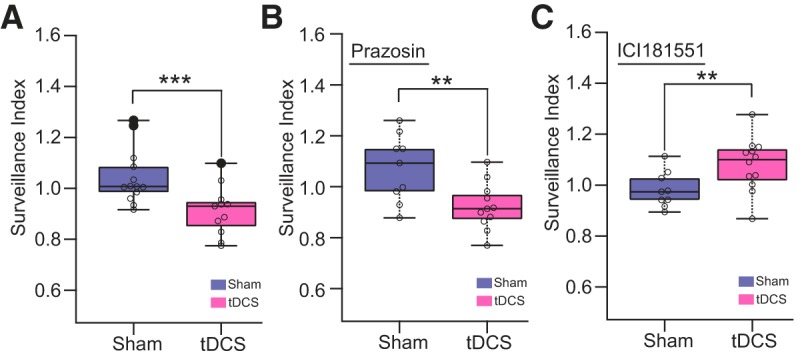
Microglial surveillance is compromised by tDCS. A, Surveillance index at t = 40 min after sham/tDCS treatment in no drug-treated animals (Sham group: 13 cells from 8 mice; tDCS group: 11 cells from 8 mice; p = 0.006k). B, Surveillance index comparison in prazosin pretreated mice (Sham group: 9 cells from 2 mice; tDCS group: 11 cells from 3 mice; p = 0.015l). C, Surveillance index comparison in ICI181551-pretreated mice (Sham group: 9 cells from 3 mice; tDCS group: 12 cells from 3 mice; p = 0.023m) Mann–Whitney Wilcoxon rank sum test. **p < 0.03; ***p < 0.01.
Discussion
The present experiments report that tDCS induces subtle, but significant, alterations of Iba1 distribution and microglial motility in the cerebral cortex in awake mice. Furthermore, these alterations were dependent on (nor)adrenergic receptors, which is in line with the results of an earlier study that described tDCS-induced A1AR-dependent astrocytic Ca2+ surges (Monai et al., 2016). Notably, while astrocytic Ca2+ responses occur during tDCS, morphologic alterations of microglia occurred after a few hours.
We demonstrated that the microglial soma is enlarged after tDCS. Remarkably, the soma enlargement occurs only in awake mice. It is well established that microglial morphology is radically altered by LPS-induced inflammation (Kondo et al., 2011; Kozlowski and Weimer, 2012; Kongsui et al., 2015). LPS-induced microglial alterations are obvious even with a low dosage of 100 μg/kg, whereby ∼20% soma enlargement has been reported in the prefrontal cortex (Kongsui et al., 2015). The tDCS-induced microglial soma enlargement of a mere several percentage points in the current study is relatively modest. Moreover, no obvious change was detected in ramified processes. As general anesthesia compromises astrocytic Ca2+ activation, in particular noradrenergically driven large-scale and synchronized Ca2+ surges (Thrane et al., 2012; Ding et al., 2013), microglial changes by tDCS conceivably depend on the elevated noradrenergic tone during awake states. On the other hand, some studies have reported significant changes in anesthetized mice that underwent tDCS. For instance, one study reported that enhancements of GFAP and brain-derived neurotrophic factor (BDNF) in anesthesia changed gene expression (de Souza Nicolau et al., 2018). Another study showed long-lasting antidepressive behavioral effects (Peanlikhit et al., 2017). However, these studies used stronger stimulation in terms of stimulus current, duration, and/or frequency. Moreover, the anesthesia condition used in the current study is deeper than that in the study by Peanlikhit et al. (2017). Considering the lack of astrocytic Ca2+ surges in this condition (Extended Data Fig. 1-1), our results support the involvement of volume-transmitted neuromodulators in tDCS.
A few studies have examined cortical microglia after tDCS. For instance, Rueger et al. (2012) reported that multisession tDCS of 5–10 d induced a mild sign of microglial activation as observed by an upregulation of Iba1 immunohistochemical signals. The current density used in the study by Rueger et al. (2012) was ∼150 A/m2, whereas that used in the current study is <30 A/m2. Considering the study by Gellner et al. (2016), which reported a microglial activation threshold of 30–50 A/m2 with light isoflurane anesthesia, it is conceivable that our experiments were performed in near-threshold conditions. The tDCS-induced microglial soma enlargement and Iba1 signal intensity distribution shift are different from the microglial morphologic alterations reported in a rodent model of electroconvulsive therapy (ECT), in which obvious reductions in process ramification and Iba1 expression occur (Jinno and Kosaka, 2008). The pronounced alterations of microglia by ECT are most likely caused by the high-intensity electric stimulation that induces seizures. By contrast, cortical neuronal discharge activity remains undisturbed by tDCS (Monai et al., 2016; Vöröslakos et al., 2018).
We find that tDCS-induced soma enlargement is dependent on noradrenergic signaling. Moreover, the prazosin and IP3R2-KO mouse (which lacks astrocytic Ca2+ surges) experiments suggest a key mechanism linked to A1AR activation. The previous reports of relative abundance of A1ARs in astrocytes over microglia (Hertz et al., 2010; Zhang et al., 2014) and A1AR-dependent tDCS-induced astrocytic Ca2+ surges (Monai et al., 2016) support the idea that astrocytic activation exerts effects on microglia. While this is intriguing, neither the prazosin nor the IP3R2 KO mouse experiment is cell type specific; therefore, it is possible that direct noradrenergic activation of microglia causes soma enlargement. Indeed, B2AR inhibition by ICI181551 also disrupted microglial somatic enlargement. Functional and transcriptomic evidence underwrites the enriched expression of B2ARs in microglia (Tanaka et al., 2002; Gyoneva and Traynelis, 2013; Zhang et al., 2014).
By imaging microglial morphology in awake mice, we found that tDCS attenuates microglial motility. This effect was also dependent on B2ARs, but not on A1ARs. The inhibitory effect of microglial B2ARs on motility is consistent with the in vitro observation by Gyoneva and Traynelis (2013) and recent in vivo observations in awake mice (Liu et al., 2019; Stowell et al., 2019). It is tempting to speculate that the brake on microglial surveillance creates an opportunity for relevant synapses to establish an initial stage of synaptic plasticity. Microglia have been demonstrated to be a source for BDNF (Parkhurst et al., 2013), a pivotal neurotrophin for synaptic plasticity and neurogenesis. Interestingly, tDCS upregulates Bdnf (de Souza Nicolau et al., 2018), promotes BDNF-dependent synaptic plasticity (Fritsch et al., 2010), and causes epigenetic modification to Bdnf genomic regions (Podda et al., 2016). It remains to be shown whether BDNF synthesis is promoted by (nor)adrenergic activation as is reported in astrocytes (Jurič et al., 2008). In addition to astrocyte–neuron interactions (Monai and Hirase, 2016; Cocco et al., 2018), our results advocate for the inclusion of microglia as a functional component of the tDCS mechanism via adrenergic receptor activation.
One of the limitations of the current study is the lack of microglia-specific molecular manipulations. While it remains undetermined whether the microglial changes observed in this study have causal roles for positive outcomes of tDCS, several groups have consistently reported inflammation-associated microglial soma enlargement (Chen et al., 2012; Kozlowski et al., 2012; Kongsui et al., 2015). Brain inflammation activates microglia and leads to the production of proinflammatory molecules such as TNF-α, IL-1β, and IL-6 (Hanisch, 2002). It is possible that these cytokines are involved in the synaptic plasticity induced by tDCS. For instance, it has been demonstrated that the glial TNF-α has a pivotal role in the regulation of homeostatic synaptic plasticity (Stellwagen and Malenka, 2006). Future studies should address the causal relationship, for instance by microglial B2AR knock-out mice combined with tDCS and behavioral performance.
Acknowledgments
Acknowledgments: We thank the members of the laboratory for comments on this manuscript. We also thank the RIKEN CBS-Olympus Collaboration Center for confocal imaging equipment and software, Keyence for fluorescence microscopy support, and Dr. Kazuhiro Sohya (National Center of Neurology and Psychiatry, Japan) for providing transgenic mice.
Synthesis
Reviewing Editor: Maiken Nedergaard, University of Rochester Medical Center
Decisions are customarily a result of the Reviewing Editor and the peer reviewers coming together and discussing their recommendations until a consensus is reached. When revisions are invited, a fact-based synthesis statement explaining their decision and outlining what is needed to prepare a revision will be listed below. The following reviewer(s) agreed to reveal their identity: Marco Aurélio Romano-Silva, Takahiro Kato.
The two reviewers and I are in agreement that this is a potential novel and important observation and that only minor revisions are required.
Reviewer #1
There is a great body of literature associated to tDCS effectiveness, mostly applied through clinical trials showing cognitive and functional rescue in diseased patients or enhancement in healthy volunteers as well as for several pre-clinical studies. The key point of this article is focused on elucidating components for tDCS mechanism which may further explain why tDCS is effective in cognitive modulation. There has been a great demand for such kind of studies seen that there is much we don't know about tDCS. The current study successfully demonstrates microglial morphological and surveillance alterations induced by tDCS. Furthermore, it highlights possible receptors that were significantly shown to be involved in these results. Although the data does show us that microglia is somehow altered in tDCS, it fails to associate these alterations to an actual mechanism. None of the presented results for microglia nor the references to it actually demonstrate if such alterations are in fact associated to tDCS' mechanism, being it may, these alterations could even be discussed as a collateral effect of the electrical current being applied to the animal's brain. To further corroborate what the authors conclude in the last paragraph of the discussion, the microglia inhibition should be shown together with the elimination of tDCS' effects such as cognitive alterations (behavioral assessment) or plasticity alterations (electrophysiological assessment). I do think the study brings important results to the field and that the experiments were well conducted, but I also feel that the discussion should be reviewed.
Statistics I see no problems concerning data analysis.
Visual Abstract(Required) N/A
Extended Data N/A
Software Comments N/A
Concerns about human or animal subjects I have no concerns regarding experimental conduct nor ethical issues.
Multimedia Comments N/A
Comments to the Authors (Required) I have no major concerns regarding this study.
There are a couple of minor considerations that should be assessed.
Lines 68 - 80: By providing this information this study will further assist other researches in replicating or continuing this investigation.
1. Please refer to the stereotaxic coordinates of the electrodes as well as its dimensions and material.
2. What conducting medium was used between the electrode and the scalp?
Line 196:
1. Sentence: “Iba1 IHC visualized......brain slices of tDCS and sham-treated mice (Fig. 2A,B)” seems not to refer to the correct figures since fig. 2 A-B depict control and LPS microglial activation. I think the correct images are 2 I-J
Lines 204-206 Fig. 2C:
1. Significance is presented in text but not shown in figure 2C. I understand the difference is in the LPS group, which is working as a positive control group, visually I see that there is a difference, but I still think it should be indicated in the figure.
Discussion:
1. With the exception of Ca++ influx alterations in astrocytes which has been previously demonstrated to have a direct influence on tDCS' mechanism and effectiveness in the 2016 paper by Monai and collaborators, there is no actual evidence that the microglia itself have a direct involvement. This conclusion may be achieved by the following factors: The use Transcranial Direct Current Stimulation is mainly driven by the necessity of treating neuropsychiatric and neurologic diseases and the vast number of cognitive deficiencies associated to them, and with some additional attempts of cognitive enhancement. Although this paper does show effects evoked by tDCS, it fails to provide if these alterations are simply collateral effects of the electrical current being applied to the animal's brain or that these effects actually have any involvement in how tDCS is able to modulate cognition. By inhibiting possible associated receptors such as B2AR and A1AR they were able to modulate microglial response to the electrical current, but by not showing that doing so also inhibited classical behavioral alterations or plasticity, fails to convince on microglia's involvement. This study shows that microglia response happens at some point between 30 minutes and above or up to 3h, while no effect is seen for the tDCS isoflurane exposed group. There are a couple of articles in the body of literature demonstrating successful behavioral alterations in tDCS stimulated mice in spite of isoflurane activity (Peanlikhit, 2017). The same is seen for tDCS induced calcium events in astrocytes (Monai, 2016) and gene expression (Nicolau, 2018) alterations, in which both situations, animals were exposed to isoflurane. I do believe the study was well conducted and that data itself is important for future investigations, but I recommend that the authors better discuss the data seeing that these results may have no connection to tDCS' actual mechanism, considering that it may as well be a collateral effect. To do so further experiments would be necessary.
General Text:
1. There are some grammar mistakes and missing words in the text, I believe it is nothing that would blur text comprehension but I do suggest the authors review the text.
Reviewer #2
tDCS is mainly understood from the viewpoint of neuron and astrocytes. In this study, the authors has focused on microglia in the process of tDCS, which is very novel approach.
Statistics NA
Visual Abstract(Required) NA
Extended Data NA
Software Comments NA
Concerns about human or animal subjects NA
Multimedia Comments NA
Comments to the Authors (Required) Majority of studies regarding tDCS has been focused only on neurons and astrocytes, and this study has shown us a novel study approach to clarify the microglial roles in tDCS.
The authors have revealed that microglial somata were enlarged when examined 3 hr after tDCS, and these effects were blocked by adrenergic receptor antagonists or in IP3R2 (inositol trisphosphate receptor type 2)-deficient mice which lack large cytosolic Ca2+ elevations in astrocytes. In addition, they showed that tDCS decreases microglial surveillance area in vivo. Overall, this study has nicely been conducted with appropriate study designs. The following comments/suggestions will improve the impact of this study.
1) The authors have indicated the importance of astrocyte-microglia interaction in the tDCS. However, this study did not show us the impact of microglial changes on the therapeutic effects of tDCS. In the initial part of the introduction, they highlighted that tDCS has positive effects on cognitive abilities. However, in this study, this therapeutic aspect has been ignored. Do the microglial somata enlargement and/or decreased microglial surveillance induce positive effects of tDCS on cognitive functions? Cognitive tests will help to answer the above fundamental questions, which will enhance the impact of this study.
2) The authors have estimated the activities of microglia by iba-1 staining and two-photon imaging. What do the enlargement of microglial somata mean? Other activation profiles such as cytokine release or mRNA expression of microglial cells may help to understand what kinds of activations are induced by tDCS.
References
- Araque A, Carmignoto G, Haydon PG, Oliet SHR, Robitaille R, Volterra A (2014) Gliotransmitters travel in time and space. Neuron 81:728–739. 10.1016/j.neuron.2014.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekar LK, He W, Nedergaard M (2008) Locus coeruleus α-adrenergic-mediated activation of cortical astrocytes in vivo. Cereb Cortex 18:2789–2795. 10.1093/cercor/bhn040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikson M, Grossman P, Thomas C, Zannou AL, Jiang J, Adnan T, Mourdoukoutas AP, Kronberg G, Truong D, Boggio P, Brunoni AR, Charvet L, Fregni F, Fritsch B, Gillick B, Hamilton RH, Hampstead BM, Jankord R, Kirton A, Knotkova H, et al. (2016) Safety of transcranial direct current stimulation: evidence based update 2016. Brain Stimul 9:641–661. 10.1016/j.brs.2016.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunoni AR, Nitsche MA, Bolognini N, Bikson M, Wagner T, Merabet L, Edwards DJ, Valero-Cabre A, Rotenberg A, Pascual-Leone A, Ferrucci R, Priori A, Boggio PS, Fregni F (2012) Clinical research with transcranial direct current stimulation (tDCS): challenges and future directions. Brain Stimul 5:175–195. 10.1016/j.brs.2011.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Jalabi W, Shpargel KB, Farabaugh KT, Dutta R, Yin X, Kidd GJ, Bergmann CC, Stohlman SA, Trapp BD (2012) Lipopolysaccharide-induced microglial activation and neuroprotection against experimental brain injury is independent of hematogenous TLR4. J Neurosci 32:11706–15. 10.1523/JNEUROSCI.0730-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocco S, Podda MV, Grassi C (2018) Role of BDNF signaling in memory enhancement induced by transcranial direct current stimulation. Front Neurosci 12:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan W-B (2005) ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 8:752–758. 10.1038/nn1472 [DOI] [PubMed] [Google Scholar]
- de Souza Nicolau E, de Alvarenga KAF, Tenza-Ferrer H, Nogueira MCA, Rezende FD, Nicolau NF, Collodetti M, de Miranda DM, Magno LAV, Romano-Silva MA (2018) Transcranial direct current stimulation (tDCS) in mice. J Vis Exp (139):e58517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedoncker J, Brunoni AR, Baeken C, Vanderhasselt M-A (2016) A systematic review and meta-analysis of the effects of transcranial direct current stimulation (tDCS) over the dorsolateral prefrontal cortex in healthy and neuropsychiatric samples: influence of stimulation parameters. Brain Stimul 9:501–517. 10.1016/j.brs.2016.04.006 [DOI] [PubMed] [Google Scholar]
- Ding F, O’Donnell J, Thrane AS, Zeppenfeld D, Kang H, Xie L, Wang F, Nedergaard M (2013) α1-Adrenergic receptors mediate coordinated Ca2+signaling of cortical astrocytes in awake, behaving mice. Cell Calcium 54:387–394. 10.1016/j.ceca.2013.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregni F, Pascual-Leone A (2007) Technology insight: noninvasive brain stimulation in neurology—perspectives on the therapeutic potential of rTMS and tDCS. Nat Clin Pract Neurol 3:383–393. 10.1038/ncpneuro0530 [DOI] [PubMed] [Google Scholar]
- Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, Cohen LG, Lu B (2010) Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron 66:198–204. 10.1016/j.neuron.2010.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futatsugi A, Nakamura T, Yamada MK, Ebisui E, Nakamura K, Uchida K, Kitaguchi T, Takahashi-Iwanaga H, Noda T, Aruga J, Mikoshiba K (2005) IP3 receptor types 2 and 3 mediate exocrine secretion underlying energy metabolism. Science 309:2232–2234. 10.1126/science.1114110 [DOI] [PubMed] [Google Scholar]
- Gellner A-K, Reis J, Fritsch B (2016) Glia: a neglected player in non-invasive direct current brain stimulation. Front Cell Neurosci 10:188. 10.3389/fncel.2016.00188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyoneva S, Traynelis SF (2013) Norepinephrine modulates the motility of resting and activated microglia via different adrenergic receptors. J Biol Chem 288:15291–15302. 10.1074/jbc.M113.458901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch UK (2002) Microglia as a source and target of cytokines. Glia 40:140–155. 10.1002/glia.10161 [DOI] [PubMed] [Google Scholar]
- Hertz L, Lovatt D, Goldman SA, Nedergaard M (2010) Adrenoceptors in brain: cellular gene expression and effects on astrocytic metabolism and [Ca(2+)]i. Neurochem Int 57:411–420. 10.1016/j.neuint.2010.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines DJ, Hines RM, Mulligan SJ, Macvicar BA (2009) Microglia processes block the spread of damage in the brain and require functional chloride channels. Glia 57:1610–1618. 10.1002/glia.20874 [DOI] [PubMed] [Google Scholar]
- Hirasawa T, Ohsawa K, Imai Y, Ondo Y, Akazawa C, Uchino S, Kohsaka S (2005) Visualization of microglia in living tissues using Iba1-EGFP transgenic mice. J Neurosci Res 81:357–362. 10.1002/jnr.20480 [DOI] [PubMed] [Google Scholar]
- Horvath JC, Forte JD, Carter O (2015a) Evidence that transcranial direct current stimulation (tDCS) generates little-to-no reliable neurophysiologic effect beyond MEP amplitude modulation in healthy human subjects: a systematic review. Neuropsychologia 66:213–236. 10.1016/j.neuropsychologia.2014.11.021 [DOI] [PubMed] [Google Scholar]
- Horvath JC, Forte JD, Carter O (2015b) Quantitative review finds no evidence of cognitive effects in healthy populations from single-session transcranial direct current stimulation (tDCS). Brain Stimul 8:535–550. 10.1016/j.brs.2015.01.400 [DOI] [PubMed] [Google Scholar]
- Jalali R, Miall RC, Galea JM (2017) No consistent effect of cerebellar transcranial direct current stimulation on visuomotor adaptation. J Neurophysiol 118:655–665. 10.1152/jn.00896.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinno S, Kosaka T (2008) Reduction of Iba1-expressing microglial process density in the hippocampus following electroconvulsive shock. Exp Neurol 212:440–447. 10.1016/j.expneurol.2008.04.028 [DOI] [PubMed] [Google Scholar]
- Jurič DM, Lončar D, Čarman-Kržan M (2008) Noradrenergic stimulation of BDNF synthesis in astrocytes: mediation via α1- and β1/β2-adrenergic receptors. Neurochem Int 52:297–306. 10.1016/j.neuint.2007.06.035 [DOI] [PubMed] [Google Scholar]
- Kondo S, Kohsaka S, Okabe S (2011) Long-term changes of spine dynamics and microglia after transient peripheral immune response triggered by LPS in vivo. Mol Brain 4:27. 10.1186/1756-6606-4-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongsui R, Johnson SJJ, Graham BAA, Nilsson M, Walker FRR (2015) A combined cumulative threshold spectra and digital reconstruction analysis reveal structural alterations of microglia within the prefrontal cortex following low-dose LPS administration. Neuroscience 310:629–640. 10.1016/j.neuroscience.2015.09.061 [DOI] [PubMed] [Google Scholar]
- Kozlowski C, Weimer RM (2012) An automated method to quantify microglia morphology and application to monitor activation state longitudinally in vivo. PLoS One 7:e31814. 10.1371/journal.pone.0031814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunzelmann K, Meier L, Grieder M, Morishima Y, Dierks T (2018) No effect of transcranial direct current stimulation of the auditory cortex on auditory-evoked potentials. Front Neurosci 12:880. 10.3389/fnins.2018.00880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo H-I, Paulus W, Batsikadze G, Jamil A, Kuo M-F, Nitsche MA (2017) Acute and chronic effects of noradrenergic enhancement on transcranial direct current stimulation-induced neuroplasticity in humans. J Physiol 595:1305–1314. 10.1113/JP273137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Li Y, Eyo UB, Chen T, Umpierre A, Zhu J, Bosco DB, Dong H, Wu L-J (2019) Neuronal network activity controls microglial process surveillance in awake mice via norepinephrine signaling. bioRxiv. Advance online publication. Retrieved August 24, 2019. doi:10.1101/557686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina J, Cason S (2017) No evidential value in samples of transcranial direct current stimulation (tDCS) studies of cognition and working memory in healthy populations. Cortex 94:131–141. 10.1016/j.cortex.2017.06.021 [DOI] [PubMed] [Google Scholar]
- Monai H, Hirase H (2016) Astrocytic calcium activation in a mouse model of tDCS—extended discussion. Neurogenesis 3:e1240055 10.1080/23262133.2016.1240055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monai H, Hirase H (2018) Astrocytes as a target of transcranial direct current stimulation (tDCS) to treat depression. Neurosci Res 126:15–21. 10.1016/j.neures.2017.08.012 [DOI] [PubMed] [Google Scholar]
- Monai H, Ohkura M, Tanaka M, Oe Y, Konno A, Hirai H, Mikoshiba K, Itohara S, Nakai J, Iwai Y, Hirase H (2016) Calcium imaging reveals glial involvement in transcranial direct current stimulation-induced plasticity in mouse brain. Nat Commun 7:11100. 10.1038/ncomms11100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F (2005) Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308:1314–1318. 10.1126/science.1110647 [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, Paulus W, Hummel F, Boggio PS, Fregni F, Pascual-Leone A (2008) Transcranial direct current stimulation: state of the art 2008. Brain Stimul 1:206–223. 10.1016/j.brs.2008.06.004 [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Boggio PS, Fregni F, Pascual-Leone A (2009) Treatment of depression with transcranial direct current stimulation (tDCS): a review. Exp Neurol 219:14–19. 10.1016/j.expneurol.2009.03.038 [DOI] [PubMed] [Google Scholar]
- Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR, Lafaille JJ, Hempstead BL, Littman DR, Gan W-B (2013) Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 155:1596–1609. 10.1016/j.cell.2013.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peanlikhit T, Van Waes V, Pedron S, Risold P-Y, Haffen E, Etiévant A, Monnin J (2017) The antidepressant-like effect of tDCS in mice: a behavioral and neurobiological characterization. Brain Stimul 10:748–756. [DOI] [PubMed] [Google Scholar]
- Pfeiffer T, Avignone E, Nägerl UV (2016) Induction of hippocampal long-term potentiation increases the morphological dynamics of microglial processes and prolongs their contacts with dendritic spines. Sci Rep 6:32422 10.1038/srep32422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podda MV, Cocco S, Mastrodonato A, Fusco S, Leone L, Barbati SA, Colussi C, Ripoli C, Grassi C (2016) Anodal transcranial direct current stimulation boosts synaptic plasticity and memory in mice via epigenetic regulation of Bdnf expression. Sci Rep 6:22180. 10.1038/srep22180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueger MA, Keuters MH, Walberer M, Braun R, Klein R, Sparing R, Fink GR, Graf R, Schroeter M (2012) Multi-session transcranial direct current stimulation (tDCS) elicits inflammatory and regenerative processes in the rat brain. PLoS One 7:e43776. 10.1371/journal.pone.0043776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza A, Martins DF, Medeiros LF, Nucci-Martins C, Martins TC, Siteneski A, Caumo W, dos Santos ARS, Torres ILS (2018) Neurobiological mechanisms of antiallodynic effect of transcranial direct current stimulation (tDCS) in a mice model of neuropathic pain. Brain Res 1682:14–23. 10.1016/j.brainres.2017.12.005 [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Malenka RC (2006) Synaptic scaling mediated by glial TNF-α. Nature 440:1054–1059. 10.1038/nature04671 [DOI] [PubMed] [Google Scholar]
- Stowell RD, Grayson OS, Ryan PD, Hanna NB, Lordy AK, Jean MB, Edward B, Mriganka S, Ania KM (2019) Noradrenergic signaling in wakeful states inhibits microglial surveillance and synaptic plasticity in the mouse visual cortex. bioRxiv. Advance online publication. Retrieved August 24, 2019. doi:10.1101/556480. [Google Scholar]
- Tanaka KF, Kashima H, Suzuki H, Ono K, Sawada M (2002) Existence of functional beta1- and beta2-adrenergic receptors on microglia. J Neurosci Res 70:232–237. 10.1002/jnr.10399 [DOI] [PubMed] [Google Scholar]
- Thrane AS, Thrane VR, Zeppenfeld D, Lou N, Xu Q, Nagelhus EA, Nedergaard M, Rangroo Thrane V (2012) General anesthesia selectively disrupts astrocyte calcium signaling in the awake mouse cortex. Proc Natl Acad Sci U S A 109:18974–18979. 10.1073/pnas.1209448109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkakin E, Akbıyık S, Akyol B, Gürdere C, Çakmak YÖ, Balcı F (2018) Differential bilateral primary motor cortex tDCS fails to modulate choice bias and readiness in perceptual decision making. Front Neurosci 12:410. 10.3389/fnins.2018.00410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vöröslakos M, Takeuchi Y, Brinyiczki K, Zombori T, Oliva A, Fernández-Ruiz A, Kozák G, Kincses ZT, Iványi B, Buzsáki G, Berényi A (2018) Direct effects of transcranial electric stimulation on brain circuits in rats and humans. Nat Commun 9:483. 10.1038/s41467-018-02928-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J (2009) Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci 29:3974–80. 10.1523/JNEUROSCI.4363-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake H, Moorhouse AJ, Miyamoto A, Nabekura J (2013) Microglia: actively surveying and shaping neuronal circuit structure and function. Trends Neurosci 36:209–217. 10.1016/j.tins.2012.11.007 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, Deng S, Liddelow SA, Zhang C, Daneman R, Maniatis T, Barres BA, Wu JQ (2014) An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci 34:11929–11947. 10.1523/JNEUROSCI.1860-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cortical Ca2+ activity during tDCS in mice under deep isoflurane anesthesia. G-CaMP7 signal was transcranially measured from isoflurane-anesthetized (1.5–2.0%) BAC-GLT1-G7 Line 817 (G7NG817) mice. The top trace is for Sham stimulation (−3.14 ± 0.02%), and the lower trace is for tDCS (0.1 mA, 10 min; −4.30 ± 0.02%). Bold traces represent the mean of 11 traces from 9 mice. Shaded areas represent the SE. The red arrowhead and vertical line indicate the onset of tDCS or sham stimulation. Download Extended Data 1, EPS file (1.6MB, eps) .