Abstract
INTRODUCTION:
Black women with breast cancer have lower survival rates and higher recurrence rates compared with white women. Here we compare treatment and survival outcomes in black and white women at a highly specialized tertiary-care cancer center.
METHODS:
We performed an IRB-approved retrospective institutional database review to identify all black women treated for invasive breast cancer between 2005-2010. We excluded women with prior history of breast cancer, stage IV, or bilateral breast cancer. White women had similar exclusion criteria applied and were then matched to black women 1:1 by age and diagnosis year. Clinicopathologic and treatment variables were compared by race. Kaplan-Meier methodology estimated overall (OS) and disease-free survival (DFS) survival; multivariable analysis was conducted with Cox regression models.
RESULTS:
Our study group consisted of 1,332 women (666 black). Median tumor size was larger in black women (1.6cm versus 1.3cm, p<0.001). Black women had more nodal disease (41.1% vs 32%, p<0.001), and had tumors that were more frequently of estrogen receptor-negative (32.9% vs 15%, p<0.001), progesterone receptor-negative (47.1% vs 30.2%, p<0.001), and triple negative (TN) subtype (24% vs 8.9%, p<0.001) compared with white. Black women also had inferior DFS and OS; race was not an independent prognostic indicator in multivariable analysis.
CONCLUSIONS:
Black women had more advanced disease and adverse prognostic indicators at diagnosis, but race was not an independent predictor of outcome. Black women were significantly more likely to have TN breast cancer. Further research is necessary to help understand the differences in tumor biology associated with race.
Keywords: breast cancer, race, survival, black women, African-American, disparities, triple negative breast cancer
Condensed abstract:
Our study is a retrospective, matched comparison of black women and white women treated for invasive breast cancer at a tertiary care center. We found black women had more advanced disease and were more likely to have triple negative breast cancer compared to white women with worse overall and disease-free survival; race was not found to be an independent predictor for overall or disease-free survival.
INTRODUCTION
Despite recent advances in treatment and prognosis, the racial gap in breast cancer death rates has widened,1, 2 with black women having consistently lower survival rates3-7 and lower disease-specific survival rates compared with white women8, 9; black women are currently 39% more likely to die from breast cancer than their white counterparts.10 Multiple studies report higher mortality rates for black women at all disease stages, even after adjusting for age, tumor size, nodal status, hormone receptor status, and histology.11, 12
Hypotheses for inferior outcomes in black women include adverse tumor biology,6, 11 differences in access to care,5, 13 lack of prescription of appropriate adjuvant therapy,14, 15 and/or adherence to treatment.16, 17
Here we examine treatment and survival outcomes for breast cancer in black women compared with white women at a highly specialized tertiary-care cancer center. Our setting, where women actively seek specialized care and have insurance, minimizes access-to-care issues, suboptimal treatment recommendations, and adherence to treatment that would affect black women in a disproportionate way in other health care systems. By minimizing socioeconomic variables in our setting, we are uniquely able to examine race-related differences in disease biology and outcomes.
METHODS
We performed an institutional review board-approved retrospective review of our institutional database to identify all black women treated for invasive breast cancer between 01/2005-12/2010. We excluded women with prior history of breast cancer, stage IV cancer, or bilateral breast cancer. White women were matched 1:1 to black women by age and year of diagnosis, after applying the same exclusion criteria. Clinical, pathologic, and treatment variables were collected. Local recurrence (LR) and distant recurrence (DR) events were recorded.
Data were summarized using median (range) for continuous variables, and number (percentage) for categorical variables. Univariable between-group comparisons were made with McNemar’s test for binary variables, Friedman’s test for categorical variables, and the Wilcoxon signed rank test for continuous variables, to account for the matched nature of the data. Disease-free survival times were computed from date of surgery, and overall survival times from date of diagnosis. For overall survival (OS), women still alive were censored at their last status date. Recurrences were examined as disease-free survival (DFS) where the first event of LR, DR, or death from disease counted as an event, and those alive without recurrence or dead from other causes were censored at their last status date. If a DR occurred within 30 days after an LR, the DR was considered the event of interest. Between-group comparisons of survival outcomes were made using a Cox regression model stratified by match set, to account for the paired nature of the data. Multivariable models for survival outcomes included potential confounders determined a priori, and were stratified by match set.
In subset analysis among women who had an Oncotype DX (Genomic Health, Redwood City, CA) test, differences in Oncotype DX score according to race were tested using Fisher’s exact test, as the matched design of the study no longer holds in subset analyses.
A p-value <0.05 was considered statistically significant. Statistical analyses were conducted using R software version 3.2.5 (R Core Development Team, Vienna, Austria), including the “survival” package.
RESULTS
Clinical and Pathologic Characteristics
Our study consisted of 1,332 women (Table 1). Median age at diagnosis was 55 years (range 24-94). Cancer diagnosis was by physical findings or mammography, and this did not differ by race (p=0.446). The median time from diagnosis date to surgery date was 55 days (range 0-644) for black women compared to 43 days (range 0-493 days) for white women (p<.001), excluding women who received neoadjuvant chemotherapy. All patients had health insurance. Black women had less private or commercial insurance (47.7% vs 56.3%, p<0.001), were more likely to reside in the New York City boroughs of Brooklyn, Queens, and the Bronx, and less likely to reside in a state other than New York (p<0.001).
TABLE 1.
Clinical and Pathologic Characteristics
| Parameter | Black, n (%) (n=666) |
White, n (%) (n=666) |
p-value |
|---|---|---|---|
| Mode of diagnosis | 0.446 | ||
| Mammogram | 255 (38.3%) | 298 (44.7%) | |
| Ultrasound | 5 (0.8%) | 17 (2.6%) | |
| MRI | 5 (0.8%) | 13 (2%) | |
| Physical findings | 287 (43.1%) | 304 (45.6%) | |
| Incidental | 0 (0) | 7 (1.1%) | |
| Missing | 114 (17.1%) | 27 (4.1%) | |
| Insurance | |||
| Government | 348 (52.3%) | 291 (43.7%) | <0.001 |
| Commercial/Private | 318 (47.7%) | 375 (56.3%) | |
| Address | |||
| Manhattan | 69 (10.4%) | 66 (10%) | |
| Brooklyn | 201 (30.3%) | 46 (6.9%) | <0.001 |
| Queens | 90 (13.6%) | 44 (6.6%) | |
| Bronx | 73 (11%) | 8 (1.2%) | |
| Other New York | 118 (17.8%) | 233 (35.2%) | |
| New Jersey | 53 (8%) | 159 (24%) | |
| Other state | 60 (9%) | 106 (16%) | |
| Missing | 2 (0.3%) | 4 (0.6%) | |
| Tumor size (cm) | 1.6 (0.1, 15.5) | 1.3 (0, 11) | <0.001 |
| AJCC Stage | <0.001 | ||
| Stage I | 311 (46.7%) | 395 (59.3%) | |
| Stage II | 226 (33.9%) | 179 (26.9%) | |
| Stage III | 105 (15.8%) | 69 (10.4%) | |
| NA | 24 (3.6%) | 23 (3.5%) | |
| ER | <0.001 | ||
| Negative | 219 (32.9%) | 100 (15%) | |
| Positive | 442 (66.4%) | 560 (84.1%) | |
| NA | 5 (0.8%) | 6 (0.9%) | |
| PR | <0.001 | ||
| Negative | 314 (47.1%) | 201 (30.2%) | |
| Positive | 345 (51.8%) | 458 (68.8%) | |
| NA | 7 (1.1%) | 7 (1.1%) | |
| HER2 | 0.878 | ||
| Equivocal | 9 (1.4%) | 2 (0.3%) | |
| Negative | 526 (79%) | 542 (81.4%) | |
| Positive | 106 (15.9%) | 95 (14.3%) | |
| NA | 25 (3.8%) | 27 (4.1%) | |
| Grade (Histological) | 0.949 | ||
| 1 | 25 (3.8%) | 79 (11.9%) | |
| 2 | 109 (16.4%) | 172 (25.8%) | |
| 3 | 446 (67%) | 361 (54.2%) | |
| NA | 86 (12.9%) | 54 (8.1%) | |
| Grade (Nuclear) | <0.001 | ||
| Low | 14 (2.1%) | 62 (9.3%) | |
| Intermediate | 186 (27.9%) | 252 (37.8%) | |
| High | 290 (43.5%) | 207 (31.1%) | |
| NA | 176 (26.4%) | 145 (21.8%) | |
| Nodal Status | <0.001 | ||
| Negative | 390 (58.6%) | 444 (66.7%) | |
| Positive | 274 (41.1%) | 213 (32%) | |
| NA | 2 (0.3%) | 9 (1.4%) | |
| ECE | 0.115 | ||
| No | 523 (78.5%) | 572 (85.9%) | |
| Yes | 102 (15.3%) | 87 (13.1%) | |
| NA | 41 (6.2%) | 7 (1.1%) | |
| LVI | 0.598 | ||
| No | 439 (65.9%) | 463 (69.5%) | |
| Yes | 202 (30.3%) | 200 (30%) | |
| NA | 25 (3.8%) | 3 (0.5%) | |
| Molecular Subtype | <0.001 | ||
| HR−/HER2− | 160 (24%) | 59 (8.9%) | |
| HR−/HER2+ | 45 (6.8%) | 30 (4.5%) | |
| HR+/HER2− | 366 (55%) | 483 (72.5%) | |
| HR+/HER2+ | 61 (9.2) | 64 (9.6%) | |
| NA | 34 (5.1%) | 30 (4.5%) |
Abbreviations: ER, estrogen receptor; PR, progesterone receptor; NA, not available; ECE, extracapsular extension; LVI, lymphovascular invasion; HR, hormone receptor
Although the majority of women in our study had stage I disease (53%) a significant difference in stage at presentation was noted, with more stage II/III (p<0.001) disease in black women. Black women had larger tumors (p<0.001) and more frequently had estrogen receptor (ER) negative tumors (p<0.001), progesterone receptor (PR) negative tumors (p<0.001), triple negative (TN) subtype tumors (24% versus 8.9%), nodal disease (p<0.001) and high nuclear-grade disease (p<0.001) compared with white women. Histologic grade, HER2 overexpression, and presence of lymphovascular invasion (LVI) was similar. Despite TN breast cancer being more common in black women, incidence of genetic testing was less frequent, being done in 69/666 (10%) black women as compared to 494/666 (74%) white women. Mutations in BRCA1/2 occurred in 10/69 (14%) of black women tested compared with 2% of white women tested. Statistical comparison was not performed because of the small number of women with identified mutations.
Treatment
We found a significant difference in treatment variables, with black women having more mastectomies (48.6% versus 43.2%, p=0.028), axillary lymph node dissections (ALNDs)(44.6% versus 32.9%, p<0.001), and chemotherapy (64.9% versus 57.7%, p=0.004), and less endocrine therapy (61.3% versus 78.5%, p<0.001) compared with white women. The receipt of radiation therapy was similar (p=1.00)(Table 2). Oncotype DX testing was sent on 251 women with ER positive, HER2 negative, node-negative breast cancer (Table 3). There was no difference among black or white women in recurrence score categories (p=0.897).
TABLE 2.
Treatment
| Black, n (%) (n=666) |
White, n (%) (n=666) |
p-value | |
|---|---|---|---|
| Breast surgery | 0.028 | ||
| Lumpectomy | 340 (51.1%) | 378 (56.8%) | |
| Mastectomy | 324 (48.6%) | 288 (43.2%) | |
| No breast surgery | 2 (0.3%) | 0 (0) | |
| Axillary surgery | <0.001 | ||
| SLNB | 352 (52.9%) | 438 (65.8%) | |
| ALND | 297 (44.6%) | 219 (32.9%) | |
| Axillary sampling | 3 (0.5%) | 0 (0) | |
| NA | 14 (2.1%) | 9 (1.4%) | |
| Chemotherapy | 0.004 | ||
| No | 234 (35.1%) | 282 (42.3%) | |
| Yes | 432 (64.9%) | 384 (57.7%) | |
| Endocrine therapy | <0.001 | ||
| No | 258 (38.7%) | 143 (21.5%) | |
| Yes | 408 (61.3%) | 523 (78.5%) | |
| Radiation therapy | 1 | ||
| No | 230 (34.5%) | 232 (34.8%) | |
| Yes | 422 (63.4%) | 431 (64.7%) | |
| NA | 14 (2.1%) | 3 (0.5%) |
Abbreviations: SLNB, sentinel lymph node biopsy; ALND, axillary lymph node dissection; NA, not available
TABLE 3.
Oncotype DX Recurrence Scores among the subset of patients who had testing done
| Oncotype DX RS | Overall (n=251) |
Black (n=97) |
White (n=154) |
p-value |
|---|---|---|---|---|
| 0.897 | ||||
| High (RS ≥ 31) | 25 (10%) | 6 (6.2%) | 19 (12.3%) | |
| Intermediate (RS 18-30) | 87 (34.7%) | 38 (39.2%) | 49 (31.8%) | |
| Low (RS < 18) | 139 (55.4%) | 53 (54.6%) | 86 (55.8%) |
Abbreviations: RS, recurrence score
Outcomes
With a median follow-up of 6.3 years (range 0-11.9) among survivors, 180 patients died from any cause. There were 52 LRs and 117 DRs.
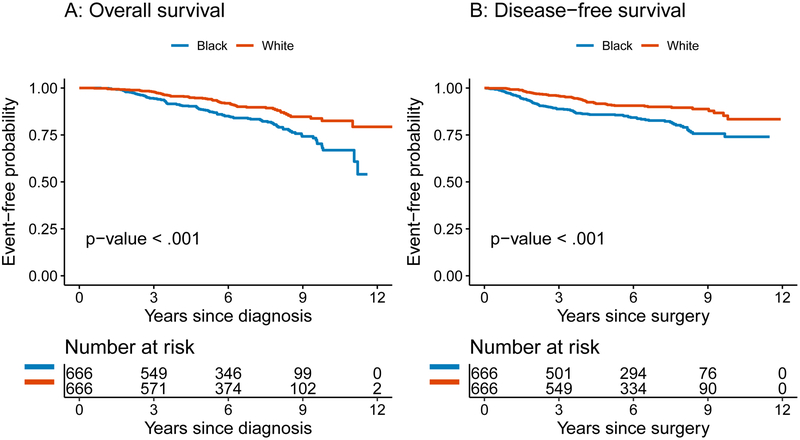
The 5-year DFS probability was 86% (95% confidence interval [CI] 83-89%) for black women compared with 91% (95% CI 89-93%) for white women (p<0.001)(Figure 1b). On univariable analysis, increasing tumor size, higher American Joint Committee on Cancer (AJCC) stage, ER and PR negativity, higher histologic grade, nodal positivity, presence of extracapsular extension (ECE), presence of LVI, hormone receptor negative/HER2+ subtype, mastectomy versus breast-conserving surgery (BCS), and chemotherapy were associated with increased hazard of recurrence or death from disease (Table 4, all p<0.05). Women diagnosed by physical findings compared to mammography had increased hazard of recurrence or death from disease (p=0.001). Insurance type was not significantly associated with DFS (p=0.87). On multivariable analysis, only AJCC stage at diagnosis (p=0.032) and tumor subtype (p=0.002) were independently associated with DFS (Table 4).
Figure 1.
(a) Overall and (b) disease-free survival by race.
TABLE 4.
Cox Regression for the Association Between Race and DFS, Adjusted for Potential Confounders Determined A Priori
| Univariable | Multivariable | |||
|---|---|---|---|---|
| Parameter | HR (95% CI) | p-value | HR (95% CI) | p-value |
| White race | 0.51 (0.35-0.73) | <0.001 | 0.85 (0.44-1.64) | 0.631 |
| Mode of diagnosis | 0.001 | -- | -- | |
| Mammogram | 1.00 | |||
| Other | 0.49 (0.09-2.57) | |||
| Physical findings | 3.67 (1.82-7.39) | |||
| Insurance | 0.869 | -- | -- | |
| Government | 1.00 | |||
| Commercial/Private | 0.95 (0.5-1.81) | |||
| Tumor size (cm) | 1.57 (1.25-1.98) | <0.001 | 1.25 (0.9-1.74) | 0.177 |
| AJCC Stage | <0.001 | 0.032 | ||
| Stage I | 1.00 | 1.00 | ||
| Stage II | 2.57 (1.4-4.73) | 1.66 (0.37-7.34) | ||
| Stage III | 10.27 (4.02-26.22) | 8.61 (1.03-72.2) | ||
| ER+ | 0.26 (0.13-0.5) | <0.001 | -- | -- |
| PR+ | 0.27 (0.14-0.52) | <0.001 | -- | -- |
| HER2+ | 0.7 (0.35-1.39) | 0.306 | -- | -- |
| Grade (Histological) | 0.001 | -- | -- | |
| 1 | 1.00 | |||
| 2 | 0.45 (0.05-3.66) | |||
| 3 | 5.88 (1.06-32.67) | |||
| Nodal positivity | 3.29 (1.91-5.67) | <0.001 | 0.65 (0.15-2.84) | 0.565 |
| ECE | 3.33 (1.58-7.02) | 0.002 | 1.75 (0.38-7.96) | 0.47 |
| LVI | 2.5 (1.4-4.46) | 0.002 | 1.13 (0.37-3.43) | 0.826 |
| Molecular subtype | 0.001 | 0.002 | ||
| HR+/HER2− | 1.00 | 1.00 | ||
| HR−/HER2+ | 4.99 (2.29-10.89) | 2.56 (0.39-16.63) | ||
| HR−/HER2− | 1.67 (0.49-5.7) | 7.48 (2.21-25.28) | ||
| HR+/HER2+ | 0.76 (0.31-1.88) | 0.71 (0.15-3.28) | ||
| Mastectomy vs BCS | 1.9 (1.11-3.27) | 0.02 | 0.99 (0.4-2.44) | 0.975 |
| Chemotherapy | 7.0 (2.74-17.87) | <0.001 | 1.87 (0.47-7.43) | 0.375 |
Abbreviations: DFS, disease-free survival; HR, hazard ratio; CI, confidence interval; AJCC, American Joint Committee on Cancer; ER, estrogen receptor, ECE, extracapsular extension; LVI, lymphovascular invasion; BCS, breast-conserving surgery
The 5-year OS probability was 88% (95% CI 86-91%) for black women and 94% (95% CI 93-96%) for white women (p<0.001)(Figure 1a). On univariable analysis, increasing tumor size, higher AJCC stage, ER and PR negativity, nodal positivity, presence of ECE, presence of LVI, TN subtype, mastectomy versus BCS, and chemotherapy were all significantly associated with increased hazard of death from any cause (Table 5, all p<0.05). Patients diagnosed by physical findings had worse OS compared with those diagnosed by mammography (p<.001). Insurance type was not significantly associated with OS (p=0.31). On multivariable analysis, there were no significant associations with OS (Table 5).
TABLE 5.
Cox Regression for the Association Between Race, and OS, Adjusted for Potential Confounders Determined A Priori
| Univariable | Multivariable | |||
|---|---|---|---|---|
| Parameter | HR (95% CI) | p-value | HR (95% CI) | p-value |
| White race | 0.51 (0.35-0.74) | <0.001 | 0.79 (0.43, 1.45) | 0.443 |
| Mode of diagnosis | <0.001 | -- | -- | |
| Mammogram | 1.00 | |||
| Other | 0.18 (0.02-1.5) | |||
| Physical findings | 4.18 (1.93-9.03) | |||
| Insurance | 0.31 | -- | -- | |
| Government | 1.00 | |||
| Commercial/Private | 1.43 (0.72-2.83) | |||
| Tumor size (cm) | 1.47 (1.17-1.85) | 0.001 | 1.46 (0.93, 2.31) | 0.102 |
| AJCC Stage | <0.001 | 0.247 | ||
| Stage I | 1.00 | 1.00 | ||
| Stage II | 1.97 (1.07-3.64) | 0.69 (0.18, 2.68) | ||
| Stage III | 7.69 (3-19.68) | 2.12 (0.28, 16.26) | ||
| ER+ | 0.31 (0.16-0.59) | <0.001 | -- | -- |
| PR+ | 0.54 (0.32-0.9) | 0.018 | -- | -- |
| HER2+ | 0.67 (0.3-1.48) | 0.321 | -- | -- |
| Grade (Histological) | 0.118 | -- | -- | |
| 1 | 1.00 | |||
| 2 | 1.63 (0.37-7.16) | |||
| 3 | 2.82 (0.74-10.68) | |||
| Nodal positivity | 2.71 (1.55-4.72) | <0.001 | 1.35 (0.37, 4.87) | 0.651 |
| ECE | 2.73 (1.37-5.44) | 0.004 | 1.41 (0.35, 5.75) | 0.633 |
| LVI | 2.64 (1.43-4.89) | 0.002 | 1.02 (0.31, 3.3) | 0.974 |
| Molecular subtype | 0.009 | 0.196 | ||
| HR+/HER2− | 1.00 | 1.00 | ||
| HR−/HER2+ | 1.05 (0.27-4.02) | 0.5 (0.06, 3.89) | ||
| HR−/HER2− | 3.42 (1.66-7.06) | 3.88 (1.32, 11.46) | ||
| HR+/HER2+ | 1.03 (0.37-2.89) | 0.53 (0.11, 2.45) | ||
| Mastectomy vs BCS | 2.05 (1.19-3.55) | 0.001 | 1.80 (0.73, 4.48) | 0.205 |
| Chemotherapy | 2.38 (1.25-4.56) | 0.009 | 1.98 (0.61, 6.41) | 0.253 |
Abbreviations: OS, overall survival; HR, hazard ratio; CI, confidence interval; AJCC, American Joint Committee on Cancer; ER, estrogen receptor, ECE, extracapsular extension; LVI, lymphovascular invasion; BCS, breast-conserving surgery
Subset analysis of 219 patients with TN breast cancer found no difference between white and black women with respect to DFS (p=0.6) or OS (p=0.9). Subset analysis of 849 patients with ER positive, HER2 negative disease, did identify a significant difference between black and white women with respect to DFS and OS (p<.001),
DISCUSSION
Breast cancer in black women is associated with a worse prognosis than in white women Possible explanations include lack of access to care, more advanced disease at presentation, and differences in tumor biology in black women. In this study, we examine the impact of race on outcomes within a highly specialized tertiary-care cancer center. We found black women had more-advanced disease and were more often diagnosed with TN breast cancer than their white counterparts, and had worse disease-free and overall survival. We found that when stage and breast cancer subtype were accounted for, race ceased to be an independent prognostic factor.
Recent studies using Surveillance, Epidemiology, and End Results data report black women have larger mean tumor size, lower rates of localized disease, and higher rates of ER negativity compared with white women,11, 18 consistent with our findings.
TN breast cancer is associated with poor overall and recurrence-free survival. Numerous studies have demonstrated disproportionately higher rates of TN breast cancer in black women,6, 7, 19-22 consistent with our findings.
There is evidence to suggest that TN breast cancer is not the same disease in black women compared to white women. Black women with TN breast cancer have been shown to have a higher risk of nodal recurrence than white women with TN breast cancer, despite receiving similar treatment.23 Within TN breast cancers, intra-tumor heterogeneity and expression of basal genes have been shown to be higher in black woman compared to white women. A study of Nigerian black women with breast cancer also noted a higher expression of biomarkers associated with aggressive disease, such as basal cytokeratins and EGFR (epidermal growth factor receptor) compared with similarly matched white women from the United Kingdom.19 However, in subset analysis of 219 patients with TN breast cancer, we could not identify a significant difference between white and black women with respect to DFS (p=0.6) or OS (p=0.9). This highlights the poor prognosis associated with TN breast cancer, independent of race.
The proportion of black women in our study who had genetic testing was lower than white women despite the higher prevalence of TN disease amongst black women. In our study, we found that although fewer black women were referred for genetic testing, a greater proportion (14%) had identification of a mutation compared to white women. The time period of our study was prior to the 2011 National Comprehensive Cancer Network guidelines recommending testing for women with TN breast cancer <60 years of age. However, it is likely that this does not completely address the disparity in referral and testing we noted. In this retrospective analysis, we are not able to elucidate whether the disparity in genetic testing rates was due to differences in the proportion offered testing or differences in uptake of testing by black women.
Our findings are consistent with other studies showing a lower rate of genetic testing among black women with breast cancer versus white women.24, 25 Whether the low rate of testing is attributable to a lower perceived benefit, as has been suggested in some studies, is not discernable in our study.24, 25
Pal et al reported identification of BRCA mutations was higher in young black women with breast cancer compared with white women. Black women are disproportionately affected with TN breast cancer and should be referred for genetic testing as a result. However, beyond the presence of TN breast cancer, it has been reported that >40% of young black women with cancer with BRCA mutations do not have close relatives with breast or ovarian cancer, suggesting that family history alone may not be enough to identify black women at high risk of having BRCA mutations.26 BRCA mutations specific to African-American families have also been identified.27 It is possible that the criteria used for genetic testing referral may not be predictive for mutations in black women. The findings in our study support strategies to maximize referral and uptake of genetic testing for young black women with invasive breast cancer, in addition to women with TN breast cancer.
Differences in the treatment received by black and white women in our study were noted and likely due to the higher stage and disease burden noted in black women compared to white. On multivariable analysis, after adjustment for disease characteristics, no treatment variables were independently associated with OS or DFS. Black women had larger tumor size and were thus more likely to have mastectomy than their white counterparts. Recent data from the National Cancer Database showed that although black women were more likely than white women to have mastectomy, when stratified by tumor size, black women had higher rates of BCS.28
Some studies suggest that black women are less likely to be offered sentinel node biopsy (SLNB) even when presenting with stage I disease.29, 30 Black women in our cohort were also more likely than white women to undergo ALND because of higher incidence of nodal disease (41% vs 32%), but all women with clinically negative nodes were offered SLNB. Additionally, the high rate of ALND in our study is a reflection of its time period occurring before the publication of the American College of Surgeons Oncology Group Z0011 study.31 As expected, because of higher rates of TN breast cancer and nodal disease in black women in our study, they received more chemotherapy and less endocrine therapy compared with white women. Our high rate of chemotherapy for black women contrasts with a recent study reporting that black women are less likely to receive appropriate adjuvant therapy compared to white.32 This findings difference is likely due to our study setting being that of a tertiary care center.
Several other studies15, 33, 34 have shown that black women are significantly more likely to experience early termination of chemotherapy and delays in treatment, less likely to complete courses of trastuzumab,14 and less likely to start endocrine therapy.17 Economic factors are also thought to contribute to low rates of adherence to endocrine therapy in black women.16 A study from Atlanta indicated that black women were less likely to receive Oncotype DX testing, but more likely to be categorized as high risk in comparison with white women based on tumor factors.35 In our study, 251 women had Oncotype DX testing. The distribution of Oncotype DX scores did not differ significantly between black and white women in our study (p =0.9). This is consistent with the data presented by Albain et al at the 41st Annual San Antonio Breast Cancer Symposium, December 4-8, 2018, San Antonio, TX consisting of race-stratified results from the TAILORx trial and showed that although recurrence scores were similar in black and white women, despite comparable adjuvant systemic therapy, black women had worse outcomes.36 We performed a subset analysis of 849 patients with ER positive, HER2 negative disease, and did identify a significant difference between black and white women with respect to DFS and OS (p<.001), which supports data presented by Albain et al suggesting that recurrence scores may have less prognostic accuracy in black women. Further research is necessary to address this discrepancy in outcomes for black women with ER positive disease.
Adverse survival outcomes for black women with breast cancer have been observed in numerous previous studies.2, 5, 6, 8, 10, 13, 18, 37 Here we also note a worse OS and DFS, with higher rates of local and distant relapse in the black women in our study. In a previous study at our institution comparing black and white women undergoing breast-conservation therapy for TN breast cancer, race was a prognostic indicator for regional nodal failure, but not for local control.23 Race was not an independent prognostic indicator in our current study. Previous studies have suggested that socioeconomic factors contribute to the racial gap in breast cancer outcomes. We examined zip code and insurance data, and although black women were less likely to have private or commercial insurance, type of insurance was not a predictor of OS or DFS. Although, the black women in our study were more likely to be from New York City boroughs outside of Manhattan, less likely to be from out of New York State, and less likely to have private insurance, they were all insured and thus differences in socioeconomic status were likely to be small. However, we did note a significant difference between date of diagnosis and surgery, with a slightly longer interval for black women. This may be a surrogate for differences in ease of access to a tertiary care center for the black women. The retrospective nature of this study does not allow for further interpretation of this difference in the time interval from diagnosis to surgery.
When Tao et al13 controlled for socioeconomic and insurance status, higher mortality was still seen in black women. Lu et al38 found that the mortality risk of black women with breast cancer was almost double that of white women, but that this effect decreased when education and study site location were added to the Cox model. Results from the Florida Cancer Data System5 found that a black survival disadvantage was sustained in its multivariate analysis, which included socioeconomic status. It is likely that as all the women in this study sought out care in a tertiary referral center, suboptimal treatment recommendations, lack of access to care, and adherence to recommended treatments were less likely to affect the black women in our study in a disproportionate way. Therefore, by minimizing the socioeconomic factors that contributed to disparities in outcomes in other studies, this study offers a unique focus on disease biology.
CONCLUSIONS
Although we did not identify race to be an independent prognostic indicator, it is difficult to separate the fact that TN breast cancer was more prevalent in black women. Additionally, we see a significant difference in stage at presentation, suggesting that there is likely a difference in breast cancer screening patterns for our groups. We did not have information regarding screening patterns prior to women entering our health care system. Referral and treatment at a highly specialized tertiary-care cancer center may also have its own inherent biases. We do see that black women appropriately received more adjuvant treatment as dictated by their advanced stage of disease, higher tumor burden, and molecular subtype in our setting. These traditional adverse risk factors continue to be significant prognostic factors for survival outcomes.
It is important to highlight the importance of early diagnosis based on our findings. We noted that with advanced disease presentation, and even with treatment at a highly specialized tertiary-care cancer center with aggressive surgery and adjuvant therapies, survival outcome was adversely affected. The importance of education and outreach to facilitate breast cancer screening in black women is supported by the findings in our study. We found more TN breast cancer in black women, highlighting the need for additional research to understand this relationship in hopes of elucidating more effective therapies.
Acknowledgments
FUNDING SUPPORT
The preparation of this study was supported in part by NIH/NCI Cancer Center Support Grant P30CA008748 to Memorial Sloan Kettering Cancer Center.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
Dr. Monica Morrow has received speaking honoraria from Roche and Genomic Health.
REFERENCES
- 1.DeSantis CE, Siegel RL, Sauer AG, Miller KD, Fedewa SA, Alcaraz KI, et al. Cancer statistics for African Americans, 2016: Progress and opportunities in reducing racial disparities. CA Cancer J Clin. 2016;66(4):290–308. [DOI] [PubMed] [Google Scholar]
- 2.Richardson LC, Henley SJ, Miller JW, Massetti G, Thomas CC. Patterns and Trends in Age-Specific Black-White Differences in Breast Cancer Incidence and Mortality - United States, 1999-2014. MMWR Morb Mortal Wkly Rep. 2016;65(40):1093–8. [DOI] [PubMed] [Google Scholar]
- 3.Howard-McNatt M, Lawrence J, Melin SA, Levine EA, Shen P, Stewart JHt. Race and recurrence in women who undergo neoadjuvant chemotherapy for breast cancer. Am J Surg. 2013;205(4):397–401. [DOI] [PubMed] [Google Scholar]
- 4.Rugo HS, Brufsky AM, Ulcickas Yood M, Tripathy D, Kaufman PA, Mayer M, et al. Racial disparities in treatment patterns and clinical outcomes in patients with HER2-positive metastatic breast cancer. Breast Cancer Res Treat. 2013;141(3):461–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tannenbaum SL, Koru-Sengul T, Miao F, Byrne MM. Disparities in survival after female breast cancer diagnosis: a population-based study. Cancer Causes Control. 2013;24(9):1705–15. [DOI] [PubMed] [Google Scholar]
- 6.Warner ET, Tamimi RM, Hughes ME, Ottesen RA, Wong YN, Edge SB, et al. Racial and Ethnic Differences in Breast Cancer Survival: Mediating Effect of Tumor Characteristics and Sociodemographic and Treatment Factors. J Clin Oncol. 2015;33(20):2254–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wright JL, Reis IM, Zhao W, Panoff JE, Takita C, Sujoy V, et al. Racial disparity in estrogen receptor positive breast cancer patients receiving trimodality therapy. Breast. 2012;21(3):276–83. [DOI] [PubMed] [Google Scholar]
- 8.Ademuyiwa FO, Gao F, Hao L, Morgensztern D, Aft RL, Ma CX, et al. US breast cancer mortality trends in young women according to race. Cancer. 2015;121(9):1469–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akinyemiju T, Moore JX. Racial disparities in individual breast cancer outcomes by hormone-receptor subtype, area-level socio-economic status and healthcare resources. 2016;157(3):575–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeSantis CE, Ma J, Goding Sauer A, Newman LA, Jemal A. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J Clin. 2017;67(6):439–48. [DOI] [PubMed] [Google Scholar]
- 11.Newman LA, Bunner S, Carolin K, Bouwman D, Kosir MA, White M, et al. Ethnicity related differences in the survival of young breast carcinoma patients. Cancer. 2002;95(1):21–7. [DOI] [PubMed] [Google Scholar]
- 12.Christiansen N, Chen L, Gilmore J, Pechar D, Szabo S. Association between African American race and outcomes in patients with nonmetastatic triple-negative breast cancer: a retrospective analysis by using results from the Georgia Cancer Specialist Database. Clin Breast Cancer. 2012;12(4):270–5. [DOI] [PubMed] [Google Scholar]
- 13.Tao L, Gomez SL, Keegan TH, Kurian AW, Clarke CA. Breast Cancer Mortality in African-American and Non-Hispanic White Women by Molecular Subtype and Stage at Diagnosis: A Population-Based Study. Cancer Epidemiol Biomarkers Prev. 2015;24(7):1039–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freedman RA, Hughes ME, Ottesen RA, Weeks JC, He Y, Wong YN, et al. Use of adjuvant trastuzumab in women with human epidermal growth factor receptor 2 (HER2)-positive breast cancer by race/ethnicity and education within the National Comprehensive Cancer Network. Cancer. 2013;119(4):839–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hershman D, McBride R, Jacobson JS, Lamerato L, Roberts K, Grann VR, et al. Racial disparities in treatment and survival among women with early-stage breast cancer. J Clin Oncol. 2005;23(27):6639–46. [DOI] [PubMed] [Google Scholar]
- 16.Hershman DL, Tsui J, Wright JD, Coromilas EJ, Tsai WY, Neugut AI. Household net worth, racial disparities, and hormonal therapy adherence among women with early-stage breast cancer. J Clin Oncol. 2015;33(9):1053–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reeder-Hayes KE, Meyer AM, Dusetzina SB, Liu H, Wheeler SB. Racial disparities in initiation of adjuvant endocrine therapy of early breast cancer. Breast Cancer Res Treat. 2014;145(3):743–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iqbal J, Ginsburg O, Rochon PA, Sun P, Narod SA. Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the United States. Jama. 2015;313(2):165–73. [DOI] [PubMed] [Google Scholar]
- 19.Agboola AJ, Musa AA, Wanangwa N, Abdel-Fatah T, Nolan CC, Ayoade BA, et al. Molecular characteristics and prognostic features of breast cancer in Nigerian compared with UK women. Breast Cancer Res Treat. 2012;135(2):555–69. [DOI] [PubMed] [Google Scholar]
- 20.Chu QD, Henderson AE, Ampil F, Li BD. Outcome for patients with triple-negative breast cancer is not dependent on race/ethnicity. Int J Breast Cancer. 2012;2012:764570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Brien KM, Cole SR, Tse CK, Perou CM, Carey LA, Foulkes WD, et al. Intrinsic breast tumor subtypes, race, and long-term survival in the Carolina Breast Cancer Study. Clin Cancer Res. 2010;16(24):6100–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sturtz LA, Melley J, Mamula K, Shriver CD, Ellsworth RE. Outcome disparities in African American women with triple negative breast cancer: a comparison of epidemiological and molecular factors between African American and Caucasian women with triple negative breast cancer. BMC Cancer. 2014;14:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perez CA, Zumsteg ZS, Gupta G, Morrow M, Arnold B, Patil SM, et al. Black race as a prognostic factor in triple-negative breast cancer patients treated with breast-conserving therapy: a large, single-institution retrospective analysis. Breast Cancer Res Treat. 2013;139(2):497–506. [DOI] [PubMed] [Google Scholar]
- 24.Cragun D, Weidner A, Lewis C, Bonner D, Kim J, Vadaparampil ST, et al. Racial disparities in BRCA testing and cancer risk management across a population-based sample of young breast cancer survivors. Cancer. 2017;123(13):2497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones T, McCarthy AM, Kim Y, Armstrong K. Predictors of BRCA1/2 genetic testing among Black women with breast cancer: a population-based study. Cancer Med. 2017;6(7):1787–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pal T, Bonner D, Cragun D, Monteiro AN, Phelan C, Servais L, et al. A high frequency of BRCA mutations in young black women with breast cancer residing in Florida. Cancer. 2015;121(23):4173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao Q, Neuhausen S, Cummings S, Luce M, Olopade OI. Recurrent germ-line BRCA1 mutations in extended African American families with early-onset breast cancer. Am J Hum Genet. 1997;60(5):1233–6. [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas P, Killelea BK, Horowitz N, Chagpar AB, Lannin DR. Racial Differences in Utilization of Breast Conservation Surgery: Results from the National Cancer Data Base (NCDB). Ann Surg Oncol. 2016;23(10):3272–83. [DOI] [PubMed] [Google Scholar]
- 29.Reeder-Hayes KE, Bainbridge J, Meyer AM, Amos KD, Weiner BJ, Godley PA, et al. Race and age disparities in receipt of sentinel lymph node biopsy for early-stage breast cancer. Breast Cancer Res Treat. 2011;128(3):863–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Black DM, Jiang J, Kuerer HM, Buchholz TA, Smith BD. Racial disparities in adoption of axillary sentinel lymph node biopsy and lymphedema risk in women with breast cancer. JAMA Surg. 2014;149(8):788–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giuliano AE, Ballman KV, McCall L, Beitsch PD, Brennan MB, Kelemen PR, et al. Effect of Axillary Dissection vs No Axillary Dissection on 10-Year Overall Survival Among Women With Invasive Breast Cancer and Sentinel Node Metastasis: The ACOSOG Z0011 (Alliance) Randomized Clinical Trial. Jama. 2017;318(10):918–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bickell NA, Wang JJ, Oluwole S, Schrag D, Godfrey H, Hiotis K, et al. Missed opportunities: racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006;24(9):1357–62. [DOI] [PubMed] [Google Scholar]
- 33.Hershman DL, Unger JM, Barlow WE, Hutchins LF, Martino S, Osborne CK, et al. Treatment quality and outcomes of African American versus white breast cancer patients: retrospective analysis of Southwest Oncology studies S8814/S8897. J Clin Oncol 2009;27(13):2157–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chavez-MacGregor M, Clarke CA, Lichtensztajn DY, Giordano SH. Delayed Initiation of Adjuvant Chemotherapy Among Patients With Breast Cancer. JAMA Oncol. 2016;2(3):322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lund MJ, Mosunjac M, Davis KM, Gabram-Mendola S, Rizzo M, Bumpers HL, et al. 21-Gene recurrence scores: racial differences in testing, scores, treatment, and outcome. Cancer. 2012;118(3):788–96. [DOI] [PubMed] [Google Scholar]
- 36.Albain K GR, Sparano JA, Makower DF, Pritchard KI, Hayes DF, Geyer CE Jr., Dees EC, Goetz MP, Olson JA Jr., Lively T, Badve SS, Saphner TJ, Wagner LI, Whelan TJ, Ellis MJ, Paik S, Wood WC, Ravdin PM, Keane MM, Gomez HL, Reddy PS, Goggins TF, Mayer IA, Brufsky AM, Toppmeyer DL, Kaklamani VG, Berenberg JL, Abrams J, Sledge GW Jr.. Race, ethnicity and clinical outcomes in hormone receptor-positive, HER2-negative, node-negative breast cancer: results from the TAILORx trial. Abstract No. GS4-07. 41st Annual San Antonio Breast Cancer Symposium, December 4-8, 2018, San Antonio, TX 2018. [Google Scholar]
- 37.Akinyemiju T, Sakhuja S, Waterbor J, Pisu M, Altekruse SF. Racial/ethnic disparities in de novo metastases sites and survival outcomes for patients with primary breast, colorectal, and prostate cancer. Cancer Med. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu Y, Ma H, Malone KE, Norman SA, Sullivan-Halley J, Strom BL, et al. Obesity and survival among black women and white women 35 to 64 years of age at diagnosis with invasive breast cancer. J Clin Oncol. 2011;29(25):3358–65. [DOI] [PMC free article] [PubMed] [Google Scholar]