HY5 interacts with HDA15 to form a key transcription regulatory node involved in repressing hypocotyl cell elongation-related genes and promoting photomorphogenesis in Arabidopsis seedlings.
Abstract
Photomorphogenesis is a critical plant developmental process that involves light-mediated transcriptome and histone modification changes. The transcription factor ELONGATED HYPOCOTYL5 (HY5) acts downstream of multiple families of photoreceptors to promote photomorphogenesis by regulating the expression of light-responsive genes. However, the molecular mechanism for HY5-mediated transcriptional regulation remains largely unclear. Here, we demonstrated that HY5 directly interacts with a Reduced Potassium Dependence3/Histone Deacetylase1 (HDA1)-type histone deacetylase, HDA15, both in vitro and in vivo. Phenotypic analysis revealed that HDA15 is a negative regulator of hypocotyl cell elongation under both red and far-red light conditions in Arabidopsis (Arabidopsis thaliana) seedlings. The enzymatic activity of HDA15 is required for inhibition of hypocotyl elongation. Furthermore, HDA15 and HY5 act interdependently in the repression of hypocotyl cell elongation in photomorphogenesis. Genome-wide transcriptome analysis revealed that HDA15 and HY5 corepress the transcription of a subset of cell wall organization and auxin signaling-related genes. In addition, HDA15 is required for the function of HY5 in the repression of genes related to hypocotyl cell elongation in Arabidopsis seedlings. Moreover, HY5 recruits HDA15 to the promoters of target genes and represses gene expression by decreasing the levels of histone H4 acetylation in a light-dependent manner. Our study revealed a key transcription regulatory node in which HY5 interacts with HDA15 involved in repressing hypocotyl cell elongation to promote photomorphogenesis.
As sessile organisms, plants evolved highly plastic developmental programs in response to changing environmental conditions. Light is one of the most important external signals that governs plant growth and development throughout the entire life cycle from germination to flowering (Smith, 2000). In the dark, seedlings undergo skotomorphogenesis, a process that is characterized by elongated hypocotyls, closed cotyledons and apical hooks, and undifferentiated chloroplasts. Upon light irradiation, seedlings undergo photomorphogenic development, including cotyledon opening, repression of hypocotyl elongation, and biosynthesis of mature chloroplasts (McNellis and Deng, 1995). To monitor the surrounding light conditions, plants have evolved at least four classes of photoreceptors: red/far-red light-sensing phytochromes (PHY), blue/UV-A light-sensing cryptochromes and phototropins, and UV-B light-sensing UVR8 (Chen et al., 2004; Rizzini et al., 2011).
In Arabidopsis (Arabidopsis thaliana), PHYA to PHYE regulate light responses by initiating the transcriptional cascades that alter the expression at least 10% of the entire transcriptome (Ma et al., 2001; Tepperman et al., 2001). Emerging evidence suggests that PHYs promote photomorphogenesis by suppressing two main branches of light signaling. A subset of basic helix-loop-helix type transcription factors, named PHYTOCHROME-INTERACTING FACTORs (PIFs) including PIF1, PIF3, PIF4, and PIF5, negatively regulate light responses by sequence-specific binding to the G-box or PIF-binding E-box motifs of the light-responsive genes in etiolated seedlings (Zhang et al., 2013; Leivar and Monte, 2014). Upon light irradiation, photoactivated PHYs interact with PIFs and induce their phosphorylation and degradation, allowing the initiation of photomorphogenesis (Al-Sady et al., 2006; Shen et al., 2008). Furthermore, a group of CONSTITUTIVELY PHOTOMORPHOGENIC (COP)/DEETIOLATED/FUSCA proteins act as the central repressors of photomorphogenesis downstream of the PHYs (Wei and Deng, 1996). COP1, a ring finger protein, possesses E3 ubiquitin ligase activity toward a number of photomorphogenesis-promoting factors, including ELONGATED HYPOCOTYL5 (HY5) and its homolog HY5 HOMOLOG (HYH), facilitating their targeted degradation through the 26S proteasome pathway (Lau and Deng, 2012). Photoreceptors promote photomorphogenic development by decreasing the nuclear abundance of COP1 or inhibiting its E3 ubiquitin-ligase activity, leading to HY5 accumulation during the de-etiolation process (Osterlund and Deng, 1998; Wang et al., 2001; Yang et al., 2001; Hofmann, 2015; Sheerin et al., 2015).
HY5 is a basic leucine-zipper type transcription factor that plays a major role in promoting photomorphogenesis (Oyama et al., 1997). Mutations in HY5 cause aberrant phenotypes, including an elongated hypocotyl, reduced chlorophyll, and anthocyanin accumulation in greening seedlings (Oyama et al., 1997; Holm et al., 2002). HY5 specifically binds to the G-box motif of the light-responsive promoters (Chattopadhyay et al., 1998). Genome-wide transcriptome and binding analyses revealed that HY5 binds to >9,000 genes and affects the expression of >1,100 genes, either positive or negatively (Zhang et al., 2011). However, the HY5-mediated transcriptional regulatory mechanism remains unclear.
The basic repeating unit of chromatin is the nucleosome, typically composed of an octamer of four core histones (H2A, H2B, H3, and H4) and 146 bp of DNA wrapped around the histones (Luger et al., 1997). Each histone is composed of a structured domain and an unstructured amino-terminal “tail” of 25–40 residues. The histone tails provide sites for a variety of posttranslational modifications, including acetylation, methylation, phosphorylation, and ubiquitination (Wu and Grunstein, 2000; Liu et al., 2014). Reversible acetylation and deacetylation of Lys residues in the N terminus of histone tails provide a flexible mechanism for regulation of gene expression. Hyperacetylation of histones relaxes chromatin structure and is associated with transcriptional activation, whereas hypoacetylation of histones induces chromatin compaction and is related to gene repression (Berger, 2007). Histone acetylation and deacetylation are catalyzed by histone acetyltransferases and histone deacetylases (HDACs), respectively (Pandey et al., 2002). Three families of HDACs were identified in Arabidopsis, including Reduced Potassium Dependence3/Histone Deacetylase1 (RPD3/HDA1), Silent Information Regulator2, and plant-specific Histone Deacetylase2 (HDA2) type HDACs (Pandey et al., 2002).
Emerging evidence revealed involvement of histone acetylation in light-responsive gene expression. It was reported that the levels of histone H3 acetylation at lysine 9 of the light-responsive genes are regulated by changing light conditions in Arabidopsis seedlings (Guo et al., 2008). Genome-wide histone modification analysis revealed that activation of photosynthetic genes correlates with dynamic acetylation of H3K9 and H3K27 in response to light (Charron et al., 2009). Furthermore, phenotypic analysis of HDAC mutants suggested a possible role of HDACs in photomorphogenesis. Loss of function of HDA19 seedlings results in a shorter hypocotyl phenotype under various light conditions, whereas mutations of HDA15 lead to increased chlorophyll biosynthesis gene expression in etiolated seedlings (Benhamed et al., 2006; Liu et al., 2013). Moreover, HDA19 was shown to repress the expression of PHYA by decreasing the histone H3K9 and H3K14 acetylation levels during dark-to-light transition (Jang et al., 2011). However, the mechanism for HDAC-regulated photomorphogenic growth remains unelucidated.
In this work, we determined that HY5 directly interacts with HDA15 both in vitro and in vivo. HY5 and HDA15 act interdependently in the repression of hypocotyl cell elongation under both red and far-red light conditions. Furthermore, HY5 recruits HDA15 to repress cell wall organization and auxin signaling-related genes by decreasing the levels of histone H4 acetylation in Arabidopsis seedlings. These findings revealed a key transcription regulatory node in which HY5 interacts with HDA15 involved in repressing hypocotyl cell elongation in photomorphogenesis.
RESULTS
HDA15 Physically Interacts with HY5
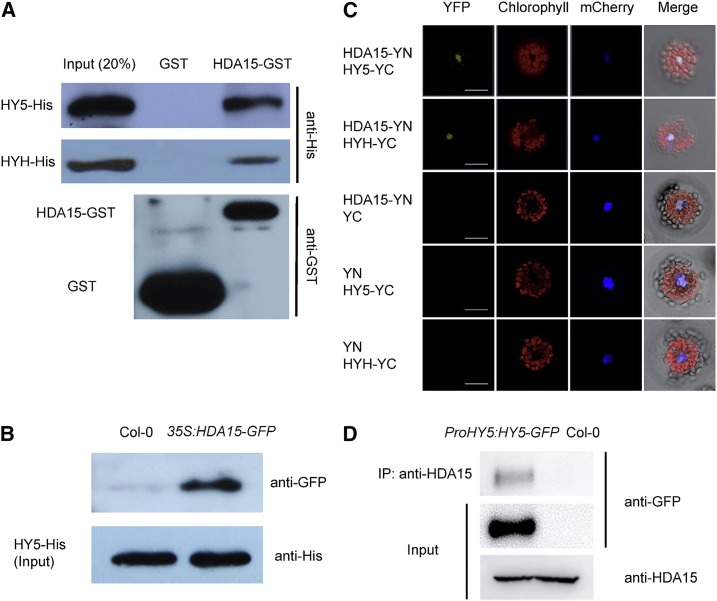
Previously, it was reported that Arabidopsis HDA15 interacts with PIF3 and PIF1 to repress chlorophyll biosynthesis in etiolated seedlings and light-regulated seed germination, respectively (Liu et al., 2013; Gu et al., 2017). We further analyzed whether HDA15 can interact with other transcription factors involved in photomorphogenesis such as HY5 and its homolog HYH in de-etiolated seedlings. We found that HDA15 could directly interact with HY5 and HYH by in vitro and semi-in vivo pull-down assays (Fig. 1, A and B). For in vitro pull-down assays, the purified recombinant HDA15-GST (glutathione S-transferase) protein was incubated with HY5-His and HYH-His, respectively. As shown in Figure 1A, HY5-His and HYH-His were pulled down by HDA15-GST, but not by GST. Semi-in vivo pull-down analysis displayed that HDA15-GFP from the total protein extracts of 35S:HDA15-GFP (Liu et al., 2013) seedlings was pulled down by recombinant HY5-His (Fig. 1B). Together, these data suggested a direct interaction between HDA15 and HY5/HYH.
Figure 1.
HDA15 interacts with HY5 in vitro and in vivo. A, In vitro pull-down analysis of HDA15-HY5/HYH interaction. Resin-bound HY5/HYH-His recombinant protein was incubated with GST or HDA15-GST. The precipitated protein was detected by an anti-His antibody. The lower displays the bait proteins of GST and HDA15-GST detected by an anti-GST antibody. B, Semi-in vivo pull-down analysis of HDA15-HY5 interaction. Resin-bound HY5-His protein was incubated with total protein extracted from 2-d–old Col-0 and 35S:HDA15-GFP seedlings grown under red light (13.12 μmol m−2 s−1), respectively. The precipitated protein was detected by an anti-GFP antibody. HY5-His was used as a loading control. C, BiFC analysis of HDA15-HY5/HYH interaction in vivo. HDA15 and HY5/HYH fused with YN and YC of YFP were cotransformed into protoplasts. mCherry was used as nuclear marker. Bars = 25 μm. D, Co-IP analysis of HDA15-HY5 interaction. Total proteins were extracted from Col-0 and ProHY5:HY5-GFP seedlings grown under red light (13.12 μmol m−2 s−1) for 2 d. A polyclonal anti-HDA15 antibody was used for immunoprecipitation. The precipitated protein and the input samples were detected with anti-GFP and anti-HDA15 antibodies. IP, immunoprecipitation.
The interaction of HDA15 with HY5/HYH was further examined in vivo by bimolecular fluorescence complementation (BiFC) and coimmunoprecipitation (Co-IP) assays (Fig. 1, C and D). For the BiFC assay, HDA15 and HY5/HYH were fused to the pEarley gate-YN (YN) vector and the pEarley gate-YC(YC) vector (Lu et al., 2010), respectively. The constructs were codelivered into Arabidopsis protoplasts by polyethylene glycol-mediated transfection and the protoplasts were incubated under white light conditions. Strong YFP signals were observed in the nucleus (Fig. 1C), suggesting that HDA15 interacts with HY5/HYH in Arabidopsis. The constructs were also codelivered into tobacco (Nicotiana tabacum) leaves by Agrobacterium tumefaciens (GV3101)-mediated transformation. The interaction between HDA15 and HY5/HYH was also detected in the nucleus of tobacco epidermal cells (Supplemental Fig. S1).
For the Co-IP assay, we generated ProHY5:HY5-GFP transgenic plants expressing HY5 in the hy5-215 mutant background (Oyama et al., 1997) under the control of its native promoter. The transgenic line with the HY5 expression level similar to the endogenous level in Col-0 was selected for further analysis (Supplemental Fig. S2). Two-d–old Col-0 and ProHY5:HY5-GFP seedlings grown under red light conditions were harvested for the Co-IP analysis. An anti-HDA15 antibody (Liu et al., 2013) was used for immunoprecipitation and an anti-GFP antibody was then used for immunoblot analysis. As shown in Figure 1D, the HY5-GFP protein was precipitated by the anti-HDA15 antibody in ProHY5:HY5-GFP seedlings. Taken together, these results indicated that HDA15 interacts with HY5 both in vitro and in vivo.
HDA15 Is a Negative Regulator of Hypocotyl Elongation in Photomorphogenesis
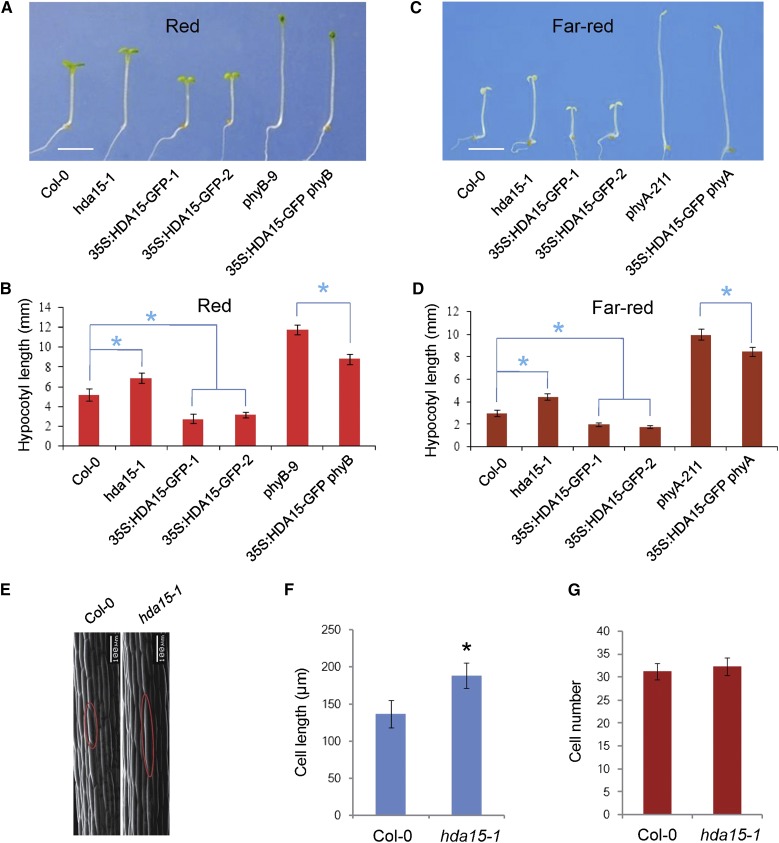
We previously observed that the hda15 mutant, hda15-1, exhibits a longer hypocotyl phenotype under various light conditions (Liu et al., 2013). In contrast, 35S:HDA15-GFP transgenic lines display a significantly shorter hypocotyl phenotype compared with wild-type (Col-0) under red and far-red light conditions (Fig. 2, A–D; Supplemental Figs. S3 and S4), suggesting a negative role of HDA15 in hypocotyl elongation in photomorphogenesis. To test the genetic relationship between HDA15 and the photoreceptors, PHYA and PHYB, 35S:HDA15-GFP was introduced into phyA-211 and phyB-9 mutants by crossing (Nagatani et al., 1993; Reed et al., 1993). 35S:HDA15-GFP phyA and 35S:HDA15-GFP phyB seedlings displayed shorter hypocotyls compared to phyA and phyB mutants under far-red and red light conditions (Fig. 2, A–D; Supplemental Figs. S3 and S4). Moreover, 35S:HDA15-GFP phyA and 35S:HDA15-GFP phyB seedlings exhibited a similar hypocotyl length as wild-type seedling grown under dark conditions (Supplemental Fig. S5). Together, these findings suggested that HDA15 might act independently or downstream of PHYA and PHYB in photomorphogenesis.
Figure 2.
HDA15 is a negative regulator of hypocotyl cell elongation in photomorphogenesis. A, Phenotypes of 4-d–old Col-0, hda15-1, 35S:HDA15-GFP, phyB-9, and 35S:HDA15-GFP phyB seedlings grown under red light (13.12 μmol m−2 s−1). 35S:HDA15-GFP-1 and 35S:HDA15-GFP-2 are two independent HDA15 transgenic lines. Bar = 2 mm. B, Quantification of the hypocotyl length as indicated in (A). Asterisks indicate the significant difference (P < 0.05, Student’s t test; n > 20). C, Phenotypes of 4-d–old Col-0, hda15-1, 35S:HDA15-GFP, phyA-211, and 35S:HDA15-GFP phyA seedlings grown under far-red light (3.82 μmol m−2 s−1). Bar = 2 mm. D, Quantification of the hypocotyl length as indicated in (C). Asterisks indicate the significant difference (P < 0.05, Student’s t test; n > 20). E, Scanning electron microscopy analysis of hypocotyl cells of 4-d–old Col-0 and hda15-1 seedlings grown under red light (13.12 μmol m−2 s−1). Bar = 100 μm. F, Quantification of the cell length as indicated in (E). Asterisk indicates the significant difference (P < 0.05, Student’s t test; n > 30). G, Quantification of the cell number as indicated in (E; n > 30).
Because plant growth involves both cell elongation and proliferation, we further determined the cell length and cell number of 4-d–old Col-0 and had15-1 seedlings grown under red light conditions. The average cell length of hda15-1 hypocotyls was significantly longer than that of wild type. In contrast, there was no significant difference in the cell number between hda15-1 and wild type (Fig. 2, E–G). These data suggested that HDA15 predominantly regulates hypocotyl cell elongation in Arabidopsis seedlings.
Enzymatic Activity of HDA15 Is Required for Inhibition of Hypocotyl Elongation
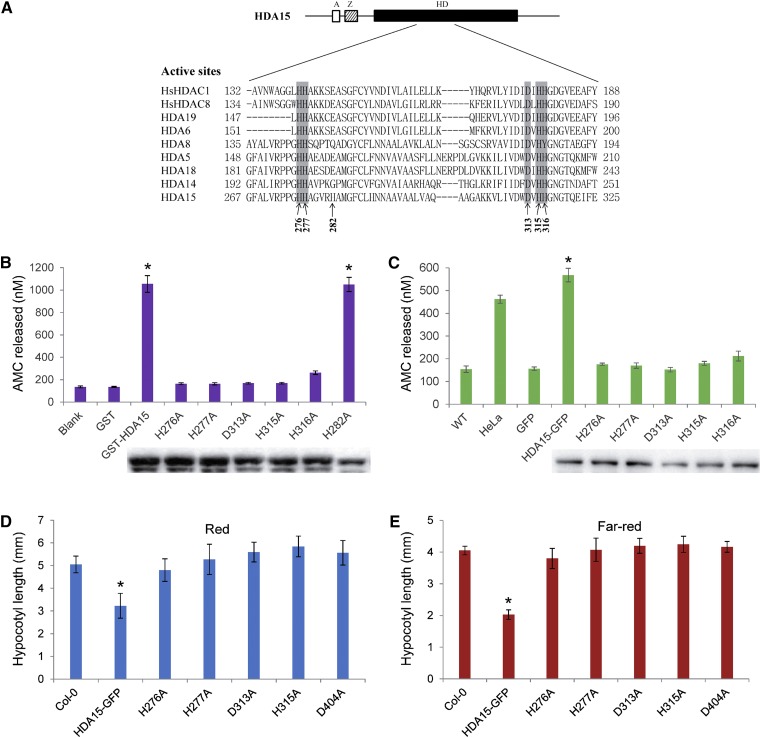
Previous studies showed that the RPD3/HDA1-type HDACs, HDA5, HDA6, HDA15, and HDA19, have histone deacetylase activity (Earley et al., 2006; Fong et al., 2006; Liu et al., 2013; Luo et al., 2015). We further determined the amino acid residues required for the enzymatic activity of HDA15. Sequence alignment of the histone deacetylase domains (HDs) of human HDAC1 (hsHDAC1) and HDAC8 (hsHDAC8) as well as Arabidopsis RPD3/HDA1-type HDACs revealed that the amino acid residues, H276, H277, D313, H315, and H316 in HDA15, are conserved among these HDACs (Fig. 3A). To analyze whether these conserved amino acids are important for the catalytic activity of HDA15, the plasmids bearing the mutations of the conserved amino acids (H276A, H277A, D313A, H315A, and H316A) were constructed and expressed in Escherichia coli. As a control, a mutation on the nonconserved amino acid (H282A) was also analyzed in parallel. The wild-type GST-HDA15 recombinant protein showed strong HDAC activity, whereas the proteins mutated in the conserved sites displayed no measurable HDAC activity (Fig. 3B). In contrast, the protein mutated in the nonconserved amino acid H282 sustained strong catalytic activity (Fig. 3B).
Figure 3.
HDA15 enzymatic activity is required for the inhibition of hypocotyl elongation. A, Multiple alignment of HDA15 and related HDACs in vicinity of the active site. “A,” “Z,” and “HD” indicate aldehyde dehydrogenase, zinc finger, and histone deacetylase domains, respectively. The gray boxes indicate the conserved amino acids in HD domains. Arrows indicate amino acids mutated to alanines. B, In vitro HDAC activity of GST-HDA15 proteins and mutated versions. Recombinant GST-HDA15 and mutated versions (H276A, H277A, D313A, H315A, H316A, and H282A) of proteins were detected by immunoblot analysis using an anti-GST antibody. GST protein was used as a control. Asterisks indicate the HDAC activity of GST-HDA15 and GST-HDA15 (H282A) is significantly different from that of GST (P < 0.05, Student’s t test; n = 3). C, Ala substitutions of conserved active site amino acids abolish in vivo HDA15 enzymatic activity. Transgenic plants of GFP-tagged HDA15 (HDA15-GFP) and single Ala substitution mutants (H276A, H277A, D313A, H315A, and H316A) were constructed in hda15-1 mutant background. Total proteins were extracted from transgenic plants, and then immunoprecipitated with anti-GFP antibody for enzymatic analysis. Hela nuclear extracts that contain a blend of HDACs served as the positive control, and the proteins extracted from 35S:GFP transgenic plants were used as a negative control. GFP-tagged proteins in the transgenic plants were detected by immunoblot analysis using an anti-GFP antibody. Asterisk indicates the HDAC activity of HDA15-GFP proteins is significantly different from that of 35S:GFP (P < 0.05, Student’s t test; n = 3). D, The hypocotyl length of 4-d–old wild type (WT) and the transgenic plants expressing HDA15-GFP and variant substitution mutants grown under red light (13.12 μmol m−2 s−1). Asterisks indicate the significant difference (P < 0.05, Student’s t test; n > 20). E, The hypocotyl length of 4-d–old wild type and the transgenic plants expressing HDA15-GFP and variant substitution mutants grown under far-red light (3.82 μmol m−2 s−1). Asterisk indicates the significant difference (P < 0.05, Student’s t test; n > 20).
We further generated transgenic plants expressing the GFP-tagged HDA15 bearing mutations of the amino acids (H276A, H277A, D313A, H315A, and H316A) in the hda15 mutant. The HDAC activities of the wild-type and mutated HDA15-GFP proteins purified from the transgenic plants were also measured. The HDA15-GFP protein isolated from HDA15-GFP transgenic plants showed significantly strong HDAC activity. In contrast, the mutated versions of HDA15-GFP proteins showed no measurable HDAC activity (Fig. 3C). Collectively, these data suggested that the amino acids H276, H277, D313, H315, and H316 of HDA15 are required for its enzymatic activity.
We further determined the phenotypes of the transgenic plants overexpressing the mutated HDA15-GFP proteins. The seedlings were grown under continuous red and far-red light conditions for 4 d. 35S:HDA15-GFP seedlings showed a significantly shorter hypocotyl length compared with wild-type, whereas the transgenic plants bearing mutated HDA15-GFP displayed a hypocotyl length similar to wild-type under both red and far-red light conditions (Fig. 3, D and E). Taken together, our data suggested that the enzymatic activity of HDA15 is required for inhibition of hypocotyl elongation in Arabidopsis seedlings.
HDA15 and HY5 Act Interdependently in Repressing Hypocotyl Elongation in Photomorphogenesis
The physical interaction of HDA15 with HY5 prompted us to determine their genetic interaction. 35S:HDA15-GFP was introduced into the hy5-215 allele by crossing (Oyama et al., 1997). The hypocotyls of 4-d–old 35S:HDA15-GFP hy5 were significantly longer than those of 35S:HDA15-GFP under both red and far-red light conditions (Fig. 4, A and B), revealing that the role of HDA15 in repressing of hypocotyl elongation is partially dependent on HY5.
Figure 4.
HDA15 and HY5 act interdependently in repressing hypocotyl elongation. A, Hypocotyl length of 4-d–old Col-0, hy5-215, 35S:HDA15-GFP, and 35S:HDA15-GFP hy5 seedlings grown under red light (13.12 μmol m−2 s−1). Two independent lines of 35S:HDA15-GFP and 35S:HDA15-GFP hy5 were analyzed. Asterisks indicate the significant difference (P < 0.05, Student’s t test; n > 20). B, Hypocotyl length of 4-d–old Col-0, hy5-215, 35S:HDA15-GFP, and 35S:HDA15-GFP hy5 seedlings grown under far-red light (3.82 μmol m−2 s−1). Two independent lines of 35S:HDA15-GFP and 35S:HDA15-GFP hy5 were analyzed. Asterisks indicate the significant difference (P < 0.05, Student’s t test; n > 20). C, Hypocotyl length of 4-d–old Col-0, hda15-1, 35S:HY5-GFP-1, and 35S:HY5-GFP hda15-1 seedlings grown under red light (13.12 μmol m−2 s−1). Asterisks indicate the significant difference (P < 0.05, Student’s t test; n > 20). D, Hypocotyl length of 4-d–old Col-0, hda15-1, 35S:HY5-GFP-1, and 35S:HY5-GFP hda15-1 seedlings grown under far-red light (3.82 μmol m−2 s−1). Asterisks indicate the significant difference (P < 0.05, Student’s t test; n > 20). E, Hypocotyl length of 4-d–old Col-0, hda15-1, hy5-215, and hda15 hy5 double mutants grown under red light (13.12 μmol m−2 s−1). Asterisks indicate the hypocotyl length of these mutants is significantly different from that of Col-0 (P < 0.05, Student’s t test; n > 20). F, Hypocotyl length of 4-d–old Col-0, hda15-1, hy5-215, and hda15 hy5 double mutants grown under far-red light (3.82 μmol m−2 s−1). Asterisks indicate the hypocotyl length of these mutants is significantly different from that of Col-0 (P < 0.05, Student’s t test; n > 20).
Next, 35S:HY5-GFP was also introduced into the hda15 background by crossing. As reported in Ang et al. (1998), 35S:HY5-GFP seedlings displayed a shorter hypocotyl phenotype. The hypocotyls of 35S:HY5-GFP hda15 were significantly longer than those of 35S: HY5-GFP hda15 under both red and far-red light conditions (Fig. 4, C and D), suggesting that HDA15 is also required for the role of HY5 in repressing hypocotyl elongation. In addition, all of these seedlings displayed a similar hypocotyl phenotype as wild type when grown in darkness (Supplemental Fig. S6).
We also constructed the hda15 hy5 double mutant. hda15 hy5 seedlings displayed a longer hypocotyl phenotype compared with hda15 and hy5 single mutants under red and far-red light conditions (Fig. 4, E and F), suggesting an additive role of HDA15 and HY5 in repressing hypocotyl elongation. In addition, hda15 hy5 seedlings exhibited a similar hypocotyl length as hda15 and hy5 in the dark (Supplemental Fig. S7). Taken together, these data indicated that HDA15 and HY5 may act interdependently in the regulation of hypocotyl elongation in photomorphogenesis.
Genome-wide Transcriptome Analysis of HDA15 and HY5 Coregulated Genes under Red Light Conditions
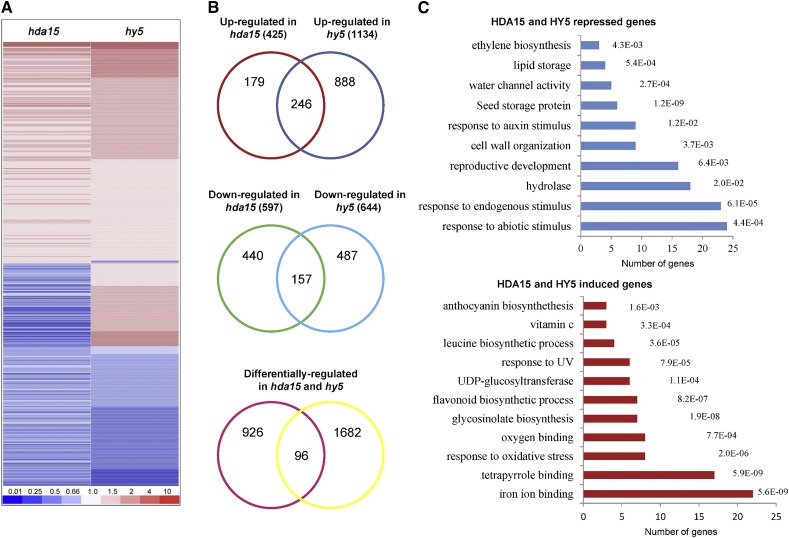
To further examine the role of HDA15 and HY5 in hypocotyl elongation, we analyzed the HDA15- and HY5-regulated transcriptome changes by mRNA deep sequencing (RNA-seq). Two-d–old Col-0, hda15-1, and hy5-215 seedlings grown under continuous red light conditions were harvested for analysis. To obtain reliable sequencing results, three independent biological replicate samples were prepared for high-throughput sequencing. We used a cutoff of a 1.5-fold change for statistical significance (False Discovery Rate < 0.05). Compared with wild-type, 425 and 1,134 genes were upregulated whereas 579 and 644 genes were downregulated in the hda15 and hy5 mutants, respectively (Supplemental Tables S1–S4). In addition, 499 genes were coregulated by HDA15 and HY5 (Fig. 5, A and B; Supplemental Tables S5–S7). Among these coregulated genes, 246 genes were upregulated and 157 genes were downregulated in both hda15 and hy5 mutants (Figs. 5, A and B; Supplemental Tables S5–S7). In eukaryotes, HDACs are identified to be components of multiprotein corepressor complexes, such as Sin3, Nucleosome-Remodeling HDAC, and corepressor for REST (Yang and Seto, 2008). The HDAC-containing corepressor complexes lead to chromatin condensation and transcription repression by removing acetyl groups from the histone tail of target genes (Yang and Seto, 2008). HDA15 may directly repress the expression of some of the upregulated genes by histone deacetylation, or indirectly regulate the expression of the downregulated genes in Arabidopsis seedlings. To validate the RNA-seq data, a number of randomly selected up- or downregulated genes were further analyzed by reverse transcription quantitative PCR (RT-qPCR) analyses (Supplemental Fig. S8).
Figure 5.
Genome-wide transcriptome analysis of HDA15 and HY5 coregulated genes under red light conditions. A, Cluster analysis of HDA15 and HY5 coregulated genes under red light conditions (13.12 μmol m−2 s−1). The bar represents the fold change. B, Venn diagrams show the overlaps of the upregulated, downregulated, and differentially regulated genes in hda15 and hy5 mutants. C, DAVID functional clustering of HDA15 and HY5 repressed and induced genes. The P values corresponding to the categories are indicated.
Previous work showed that HY5 can bind to 3,894 loci in the Arabidopsis genome (Lee et al., 2007). We found that 420 (23.6%) HY5-regulated genes are the direct targets of HY5 (Supplemental Fig. S9; Supplemental Table S8). Among these target genes, 120 of them were repressed whereas 300 were induced in hy5 seedlings (Supplemental Fig. S9; Supplemental Table S8), suggesting that HY5 may act both as a transcription repressor and activator under red light conditions. In addition, a comparison of the RNA-seq data with the HY5 binding sites data demonstrated that 26 genes that are downregulated in both hda15 and hy5 are also direct targets of HY5 (Supplemental Table S8), suggesting that HY5 may also act with HDA15 to activate gene expression. A recent study indicated that HDACs can also activate gene expression by regulating transcriptional elongation in mammals (Greer et al., 2015). Further research is required to investigate the molecular mechanism of the involvement of HDA15 in gene activation.
Gene ontology analysis of the HDA15 and HY5 coregulated genes by using the Database for Annotation, Visualization, and Integrated Discovery (DAVID) resource (Huang et al., 2009) revealed that HDA15 and HY5 corepressed genes are preferentially associated with responses to abiotic stimulus, responses to endogenous stimulus, hydrolases, reproductive development, cell wall organization, and responses to the auxin stimulus (Fig. 5C). In contrast, HDA15 and HY5 induced genes are enriched with iron ion binding, tetrapyrrole binding, responses to oxidative stress, and oxygen binding (Fig. 5C). These findings suggested that HDA15 may act with HY5 to regulate multiple developmental processes and pathways in de-etiolated seedlings.
HDA15 and HY5 Corepress the Expression of Cell Wall Organization and Auxin Signaling-Related Genes in Seedlings under Red Light
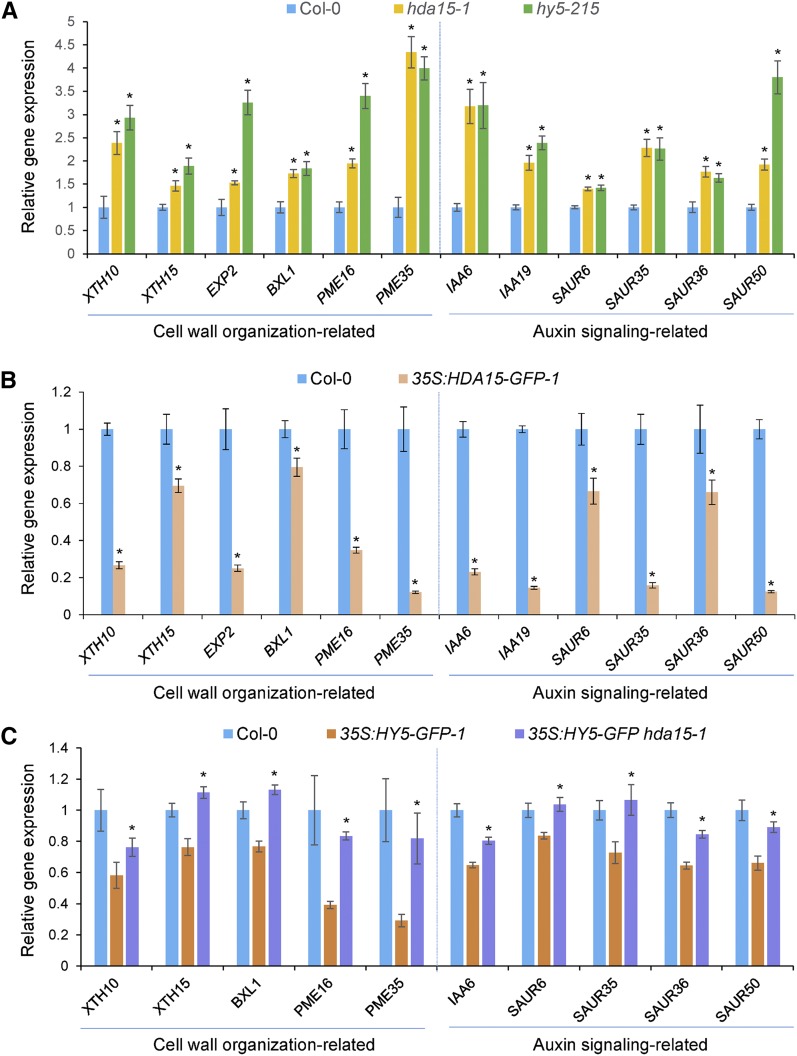
RNA-seq data revealed that HDA15 and HY5 corepress a number of genes involved in cell wall organization, such as XYLOGLUCAN ENDOTRANSGLYCOSYLASE/HYDROLASEs (XTH10, XTH15, and XTH17), BETA-XYLOSIDASE1 (BXL1), EXPANSIN2 (EXP2), ALPHA/BETA-HYDROLASEs (AT1G02660, AT2G39400, and AT4G17470), and PECTIN METHYL ESTERASEs (PME16 and PME35; Supplemental Table S9). RT-qPCR analysis confirmed that the expression of XTH10, XTH15, XTH17, EXP2, BXL1, PME16, and PME35 was upregulated in hda15 and hy5 mutants (Fig. 6A), supporting the negative role of HDA15 and HY5 in hypocotyl elongation.
Figure 6.
HDA15 and HY5 corepress the expression of cell wall organization and auxin signaling-related genes in Arabidopsis seedlings. Two-d–old seedlings grown under red light conditions (13.12 μmol m−2 s−1) were harvested for analysis. UBQ10 was used as an internal control. A, RT-qPCR analysis of the expression levels of cell wall organization and auxin signaling-related genes in 2-d–old Col-0, hda15-1, and hy5-215 seedlings. Asterisks indicate the significant difference from that of Col-0 (P < 0.05, Student’s t test; n = 3). B, RT-qPCR analysis of the expression levels of the genes involved in cell wall organization and auxin signaling in Col-0 and 35S:HDA15-GFP-1 seedlings. Asterisks indicate the significant difference (P < 0.05, Student’s t test; n = 3). C, RT-qPCR analysis of the expression levels of cell wall organization and auxin signaling-related genes in Col-0, 35S:HY5-GFP-1, and 35S:HY5-GFP hda15-1 seedlings. Asterisks indicate statistically significant differences between 35S:HY5-GFP-1 and 35S:HY5-GFP hda15-1 (P < 0.05, Student’s t test; n = 3).
Furthermore, HDA15 and HY5 also corepress a cluster of genes related to auxin signaling, such as INDOLE-3-ACETIC ACID INDUCIBLE6 (IAA6), IAA19, and SMALL AUXIN UP RNAs (SAUR6, SAUR35, SAUR36, and SAUR50; Fig. 6A; Supplemental Table S9). IAAs and SAURs are early auxin-responsive genes (Hagen and Guilfoyle, 2002; Chae et al., 2012). Previous studies showed that IAA19 plays a role in promoting hypocotyl elongation and shy1-1, the gain-of-function mutant of IAA6, displays a reduced hypocotyl elongation phenotype (Kim et al., 1996; Tatematsu et al., 2004). Increased expression of the auxin-responsive genes in hda15 and hy5 mutants indicated that HDA15 and HY5 may repress hypocotyl elongation through the auxin signaling pathway.
Because 35S:HDA15-GFP seedlings exhibited a short-hypocotyl phenotype in red light, we examined the expression levels of the cell wall organization and auxin signaling-related genes in 2-d–old 35S:HDA15-GFP seedlings. In contrast to the hda15 mutant, the transcripts of these genes were downregulated in 35S:HDA15-GFP compared with wild type (Fig. 6B), confirming the negative role of HDA15 in repression of the genes involved in hypocotyl cell elongation.
Because our genetics analysis demonstrates that HDA15 is required for the role of HY5 in repressing hypocotyl elongation, we further detected the expression levels of the cell wall organization and auxin signaling-related genes in 35S:HY5-GFP and 35S:HY5-GFP hda15 seedlings grown in red light conditions. As shown in Figure 6C, the expression of the cell wall organization and auxin signaling-related genes was downregulated in 35S:HY5-GFP seedlings, whereas the transcription of these genes was partly or fully recovered in 35S:HY5-GFP hda15 seedlings. These data revealed that HDA15 is required for the repression of cell wall organization and auxin signaling-related genes regulated by HY5.
HY5 Recruits HDA15 to the Promoters of Cell Wall Organization and Auxin Signaling-Related Genes in a Light-Dependent Manner
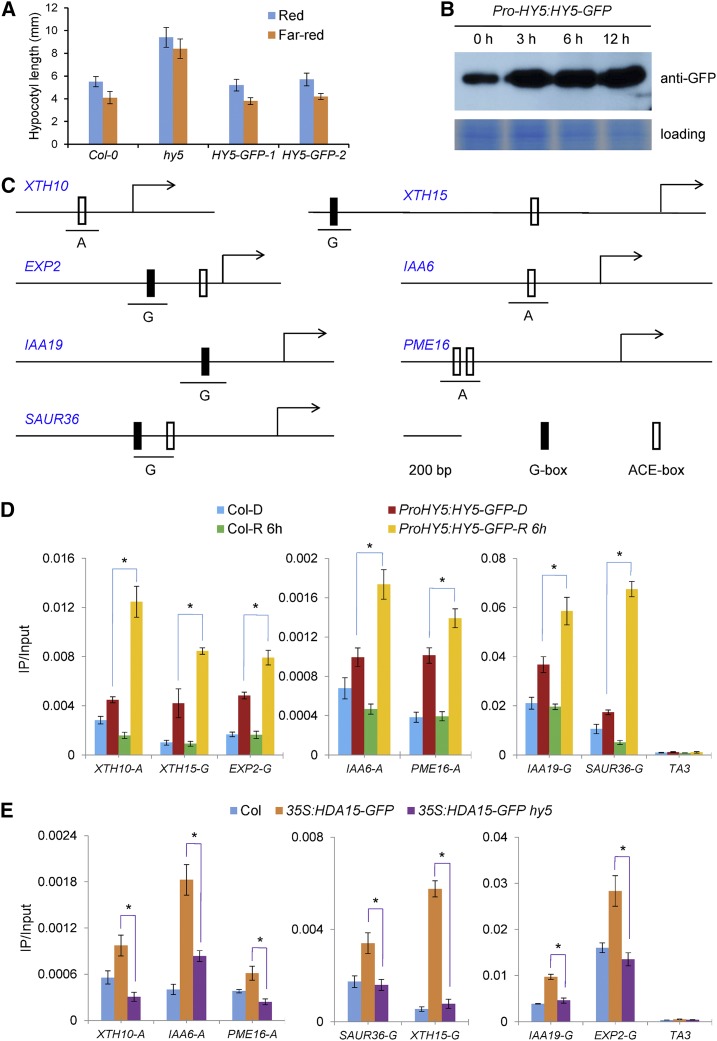
To determine whether these cell wall organization and auxin signaling-related genes are the direct targets of HY5 in vivo, the chromatin immunoprecipitation (ChIP) assay was performed by using the ProHY5:HY5-GFP transgenic plants expressing HY5-GFP driven by the native HY5 promoter. Expression of HY5-GFP in hy5 seedlings could fully recover the hypocotyl phenotype of the hy5 mutant under both red and far-red light conditions (Fig. 7A), suggesting that the HY5-GFP fusion protein is functional in vivo. Similar to previous reports (Osterlund et al., 2000; Lau and Deng, 2012), the HY5-GFP protein level was increased when 2-d–old dark-grown seedlings were transferred to red light for 3, 6, and 12 h (Fig. 7B). Furthermore, the ChIP analysis demonstrated that the promoter regions in XTH10, XTH15, EXP2, IAA6, IAA19, PME16, and SAUR36 containing the G-box (CACGTG) or ACGT-containing–element motif were enriched in ProHY5:HY5-GFP seedlings (Fig. 7, C, and D). Moreover, consistent with the HY5 protein level changes, the binding of HY5 to its targets was significantly increased when dark-grown ProHY5:HY5-GFP seedlings were transferred to red light for 6 h (Fig. 7D). Taken together, these data revealed that HY5 directly binds to the genes related to the hypocotyl cell elongation in a light-dependent manner.
Figure 7.
HY5 and HDA15 cotarget cell wall organization and auxin signaling-related genes. A, Phenotypic analysis of ProHY5:HY5-GFP transgenic plants. ProHY5:HY5-GFP lines were constructed in the hy5-215 background. The hypocotyl length of seedlings was measured after growth under red (13.12 μmol m−2 s−1) or far-red light (3.82 μmol m−2 s−1) for 4 d, respectively. Error bars = sd (n > 20). B, Detection of the HY5 protein levels in 2-d–old ProHY5:HY5-GFP seedlings grown in darkness and the seedlings transferred to red light (13.12 μmol m−2 s−1) for 3, 6, and 12 h. The proteins were detected by immunoblot analysis using an anti-GFP antibody. C, Schematic diagram of the HDA15-HY5–coregulated genes for ChIP analysis. Black and gray blocks indicate G-box and ACGT-containing element (ACE), respectively. D, ChIP analysis of enrichment of HY5 in the promoters of the cell elongation-related genes. Two-d–old Col-0 and ProHY5:HY5-GFP seedlings grown in darkness and the seedlings transferred to red light (13.12 μmol m−2 s−1) for 6 h were harvested for analysis. An anti-GFP antibody was used for immunoprecipitation. TA3 was used as a negative control. E, ChIP analysis of enrichment of HDA15 in the promoters of the cell elongation-related genes in 2-d–old 35S:HDA15-GFP and 35S:HDA15-GFP hy5 seedlings grown in red light (13.12 μmol m−2 s−1). An anti-GFP antibody was used for immunoprecipitation. TA3 was used as a negative control. Asterisks indicate the significant difference (P < 0.05, Student’s t test, n = 3).
We next examined whether HDA15 also targets these genes in vivo. ChIP assays were performed by using 2-d–old Col-0, 35S:HDA15-GFP, and 35S:HDA15-GFP hy5 seedlings grown under red light conditions. Much higher enrichment of the promoter fragments of XTH10, XTH15, EXP2, IAA6, IAA19, PME16, and SAUR36 was detected in 35S:HDA15-GFP seedlings compared with Col-0. However, the enrichment of these promoter fragments was significantly decreased in 35S:HDA15-GFP hy5 compared with 35S:HDA15-GFP seedlings (Fig. 7E), indicating that HY5 is required for the binding of HDA15 to its target genes. In addition, no significant enrichment of these fragments was detected in either 35S:HDA15-GFP or 35S:HDA15-GFP hy5 seedlings grown in dark conditions (Supplemental Fig. S10). Taken together, these data indicated that HY5 may recruit HDA15 to the promoters of hypocotyl elongation-related genes in a light-dependent manner.
HY5 and HDA15 Decrease the Histone H4 Acetylation Levels of the Target Genes
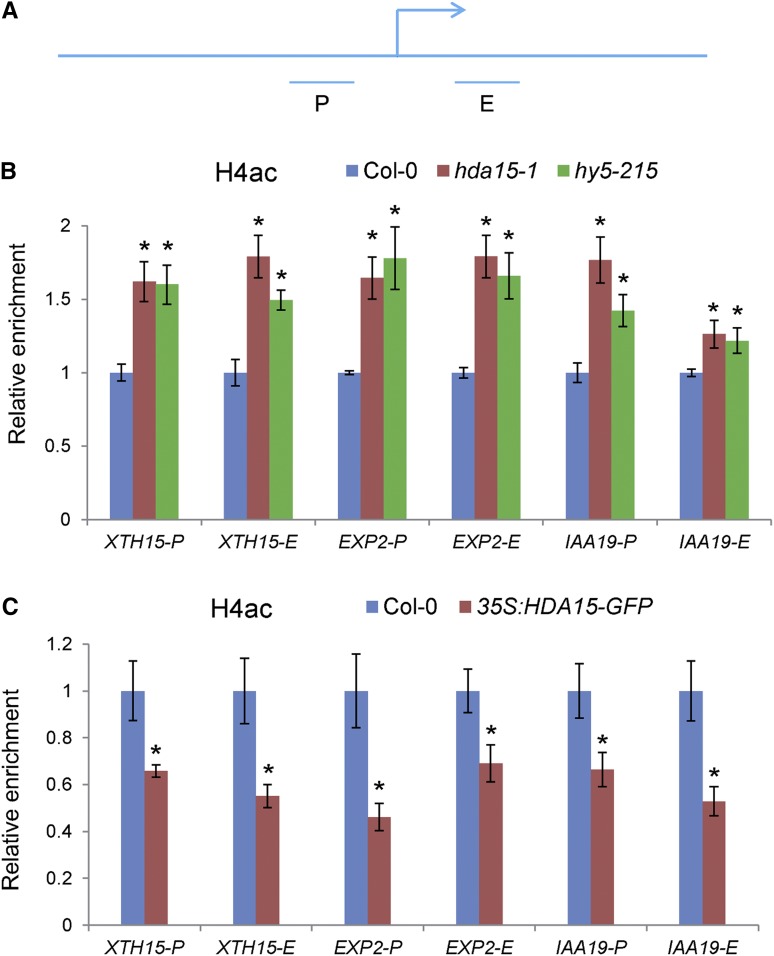
Since HDA15 has histone deacetylase activity and is required for the repression of hypocotyl elongation, we further examined the histone H3 and H4 acetylation levels of the HDA15 and HY5 coregulated genes, XTH15, EXP2, and IAA19, in Col-0, hda15, and hy5 seedlings by ChIP assays. An increase in histone H4 acetylation (H4ac) levels in the proximal promoter and the first exon regions of these genes was detected in hda15 and hy5 seedlings compared with wild type (Fig. 8, A and B). However, histone H3 acetylation levels of these genes were not significantly altered in hda15 and hy5 mutants compared with wild-type (Supplemental Fig. S11). In addition, the H4ac levels of these target genes were decreased in 35S:HDA15-GFP seedlings compared with wild-type (Fig. 8C). These data indicated that HDA15 and HY5 may repress the expression of these target genes by decreasing the levels of histone H4 acetylation.
Figure 8.
HDA15 and HY5 decrease the histone H4ac levels of the target genes under red light conditions. A, Schematic diagram of the regions for ChIP analysis. “P” and “E” indicate proximal promoter and first exon regions, respectively. B, ChIP analysis of the histone H4ac levels of XTH15, EXP2, and IAA19 in Col-0, hda15-1, and hy5-215 mutants grown under red light (13.12 μmol m−2 s−1) for 2 d. ACT2 was used as an internal control. C, ChIP analysis of the histone H4ac levels of XTH15, EXP2, and IAA19 in Col-0 and 35S:HDA15-GFP-1 seedlings grown under red light (13.12 μmol m−2 s−1) for 2 d. ACT2 was used as an internal control. Asterisks indicate the significant difference from that of Col-0 (P < 0.05, Student’s t test, n = 3).
DISCUSSION
The Histone Deacetylase Activity of HDA15 Is Important for Its Function
In yeast and mammalian cells, most members of the RPD3/HDA1-type HDACs are subunits of the multiprotein corepressor complexes, such as SIN3, Nucleosome-Remodeling HDAC, and corepressor for REST (Yang and Seto, 2008). However, the functions of HDAC corepressor complexes in plants are poorly understood. In this work, we showed that HDA15, a RPD3/HDA1-type HDAC member, plays an important role in repressing the gene expression involved in hypocotyl cell elongation. Furthermore, the association of HDA15 to its target genes requires the transcription factor HY5. These findings revealed that HY5 may recruit HDA15-associated corepressor complexes to repress the transcription of the target loci. Similarly, LEUNIG_HOMOLOG, a Groucho family transcriptional corepressor, interacts with PIF1 and coregulates gene expression in light-dependent seed germination processes (Lee et al., 2015). Furthermore, the repressor activity of LEUNIG is dependent on HDAC activity and LEUNIG directly interacts with the histone deacetylase HDA19 in Arabidopsis (Sridhar et al., 2004; Gonzalez et al., 2007). It remains to be determined whether LEUNING/LEUNIG_HOMOLOG and HDA15 can also act in the same corepressor complex in repression of light-responsive gene expression. Further identification and characterization of the subunits of the HDAC corepressor complexes could be useful to reveal the transcriptional regulatory mechanisms in photomorphogenesis.
The enzymatic deacetylase activity is crucial for HDAC-mediated transcriptional repression. Similar to HDA5, HDA6, and HDA19 (Earley et al., 2006; Fong et al., 2006; Luo et al., 2015), we showed that HDA15 possesses histone deacetylase activity, suggesting a potential role of these HDACs in transcriptional repression. Mutations of the conserved amino acids H276, H277, D313, H315, and H316 of HDA15 abolish its enzymatic activity. 35S:HDA15-GFP seedlings showed a significantly shorter hypocotyl length compared with wild-type, whereas the transgenic plants bearing mutated HDA15-GFP displayed a hypocotyl length similar to wild-type under both red and far-red light conditions. These data confirmed that these conserved amino acids are important for the enzymatic activity and function of HDA15 in plant development. In mammals, crystal structure analysis of human HDAC8 showed that the conserved amino acids D176, D178, H180, S199, and L200 are arranged in a distorted octahedral geometry, which is required for potassium ion (K+) binding (Vannini et al., 2004). Furthermore, the presence of K+ in the active sites of HDAC8 was proposed to influence the structural stability and deacetylation activity (Vannini et al., 2004). The amino acid residues D313 and H315 of HDA15 correspond to D178 and H180 of human HDAC8, which are conserved in all RPD3/HDA1-type HDACs. Mutations of these two sites may abolish K+ binding of HDA15 and result in reduced histone deacetylation activity. Further crystal structural analysis of HDA15 will help to elucidate the roles of these conserved amino acids in maintenance of its function and activity.
HDA15 Interacts with HY5 and Is a Negative Regulator in Hypocotyl Elongation
Gain of function of HDA15 in Arabidopsis caused reduced hypocotyl elongation, whereas loss of function of hda15 mutants displayed elongated hypocotyls, confirming a negative role of HDA15 in hypocotyl growth. In comparison, HDA19 was reported to act as a positive regulator of hypocotyl elongation under different light conditions (Benhamed et al., 2006). These findings indicated that different HDACs may have distinct functions in hypocotyl growth. It was shown that HDA19 decreases the histone acetylation and expression level of PHYA in Arabidopsis seedlings during dark-to-light transition (Jang et al., 2011), indicating that HDA19 may promote hypocotyl elongation by repressing PHYA gene expression during the early seedling development stage. The distinct roles played by HDA15 and HDA19 in hypocotyl growth may be due to the fact that they target different genes in photomorphogenesis.
Distinct histone modifications, such as histone acetylation and methylation, often act sequentially or in combination to form the “histone code” (Strahl and Allis, 2000). Acetylation of histones is usually associated with transcription activation (Sterner and Berger, 2000), whereas histone H3 Lys-27 trimethylation (H3K27me3) is often related to transcription repression (Jenuwein and Allis, 2001; Li et al., 2007). A recent study showed that HY5 represses the hypocotyl cell elongation-related genes by decreasing the levels of H3K27me3 (Jing et al., 2013). The Polycomb-repressive Complex2 (PRC2) is a global repressor of transcription catalyzing the trimethylation of H3K27 (Schubert et al., 2006). In mammalian cells, EZH2, the key subunit of PRC2, is reported to interact with the histone deacetylases HDAC1 and HDAC2 (van der Vlag and Otte, 1999; Tonini et al., 2004; Caretti et al., 2005), suggesting a direct link between HDACs and the PRC2 complex. In this work, we showed that HDA15 and HY5 corepress hypocotyl cell elongation-related genes by decreasing histone acetylation levels. Furthermore, decreased H3K27me3 levels in the proximal promoter and exon regions of XTH15, EXP2, and IAA19 were detected in hda15 and hy5 mutants (Supplemental Fig. S12). These findings indicated that HY5 may recruit multiple chromatin modifiers including HDA15 and the PRC2 complex in repression of hypocotyl cell elongation-related genes in photomorphogenesis.
HDA15 and HY5 Compose an Important Transcriptional Module in Integration of Light, Plant Hormone, and Environmental Stress Responses
Genome-wide transcriptome and binding site analyses revealed that HY5 is a global regulator involved in multiple plant development processes (Lee et al., 2007; Zhang et al., 2011). HY5 mediates crosstalk among light, hormone signaling, and stress responses (Lau and Deng, 2012). In this work, we showed that HDA15 and HY5 corepress the expression of IAA6 and IAA19, two primary auxin-responsive genes, suggesting that the HY5-HDA15 interaction plays an important role in integration of light and auxin signals in photomorphogenesis. Furthermore, transcriptome analysis revealed that HDA15 and HY5 also coregulate the expression of genes related to response to abiotic stimuli (Fig. 5C; Supplemental Table S5). The involvement of HY5 in drought and ABA responses during seed germination and early seedling development has been reported (Chen et al., 2008). Therefore, HDA15 and HY5 may interact to integrate light and ABA signaling during the early growth stage of Arabidopsis. HY5 also plays a role in ethylene-mediated hypocotyl growth in the light and acts as a repressor of ethylene biosynthesis (Li et al., 2011). Our RNA-seq analysis showed that the expression of ethylene biosynthetic genes such as ACS2, ACS7, and ACS11 was upregulated in both hda15 and hy5 mutants (Supplemental Table S5), revealing a key role of the HY5-HDA15 module in the crosstalk between light and ethylene signaling pathways in Arabidopsis seedlings. Collectively, these findings revealed that HDA15 and HY5 may act as an important transcriptional module in integration of light, plant hormone, and environmental stress responses.
HY5 is regulated by multiple photoreceptors and the COP/DEETIOLATED/FUSCA protein degradation machinery (Lau and Deng, 2012). In the dark, COP1 targets HY5 for ubiquitination and degradation, leading to suppression of photomorphogenesis. Consistent with the HY5 protein level changes, we showed that the enrichment of HY5 to the hypocotyl elongation-related genes was increased when etiolated seedlings were transferred to red light conditions, suggesting that the association of HY5 to the targets depends on its protein level. In this work, we showed that there was no significant difference in hypocotyl elongation in the hda15, hy5, and hda15 hy5 mutants compared with wild-type grown in the dark conditions (Supplemental Fig. S7). The levels of expression as well as histone H4 acetylation and H3K27 trimethylation of the cell wall organization and auxin signaling-related genes were also not significantly altered in the hda15 and hy5 seedlings compared with wild type grown in dark conditions (Supplement Supplemental Fig. S13). Collectively, these data support that HY5 and HDA15 interact to repress the expression of hypocotyl elongation-related genes in a light-dependent manner.
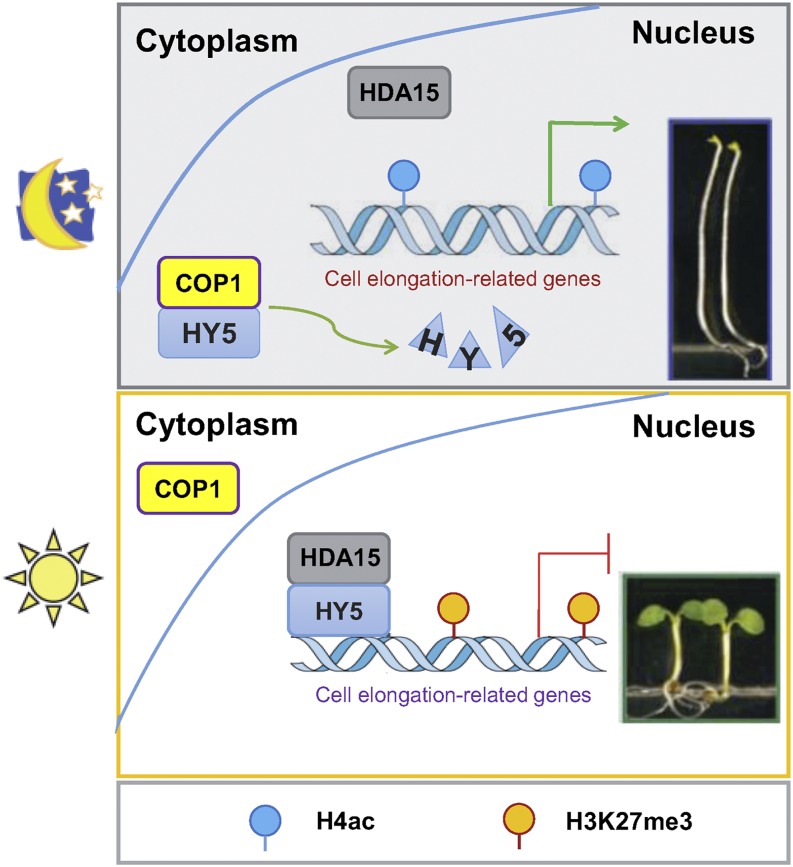
In summary, we propose a working model of how the HY5-HDA15 module functions in regulating hypocotyl elongation (Fig. 9). Under soil (in the dark), COP1 accumulates in the nucleus and interacts with HY5, resulting in HY5 degradation. When seedlings break through the soil (in the light), COP1 shuttles to the cytoplasm, resulting in HY5 accumulation in the nucleus. Furthermore, HY5 recruits HDA15 and other chromatin modifiers such as the PRC2 complex to repress the expression of hypocotyl cell elongation-related genes by decreasing the levels of histone acetylation and increasing the levels of H3K27me3, leading to the inhibition of hypocotyl elongation.
Figure 9.
A proposed model of HY5-HDA15 module functions in regulating hypocotyl elongation in Arabidopsis. In the dark, COP1 targets HY5 for ubiquitination and degradation, resulting in hypocotyl elongation. After seedlings emerge from the soil, COP1 shuttles to the cytoplasm, leading to HY5 accumulation. HY5 recruits HDA15 and other chromatin modifiers to repress the expression of cell wall organization and auxin signaling-related genes by decreasing the levels of H4ac whereas increasing levels of H3K27me3 leads to the inhibition of hypocotyl elongation.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
hy5-215 (Oyama et al., 1997) and phyB-9 (Reed et al., 1993) Arabidopsis (Arabidopsis thaliana) mutants were obtained from Nottingham Arabidopsis Stock Centre (http://arabidopsis.info/). hda15-1 (Liu et al., 2013) and phyA-211 (Nagatani et al., 1993) mutants were obtained from Arabidopsis Biological Resource Center (http://www.arabidopsis.org/). The transfer-DNA mutants were confirmed by PCR genotyping. All alleles used in this work are in the Col-0 ecotype. Double mutants were generated by genetic crossing. To generate mutated versions of 35S:HDA15-GFP transgenic lines, the mutated versions of HDA15 complementary DNA (cDNA) were subcloned into the PK7WGF2 binary vector. To generate 35S:HY5-GFP transgenic lines, the full length cDNA of HY5 was subcloned into the pCAMBIA1302 binary vector. To generate ProHY5:HY5-GFP lines, the native promoter and cDNA sequence of HY5 were subcloned into the pCAMBIA1302 binary vector. The transgenic plants were generated using the floral dip method (Clough and Bent, 1998).
For measurement of morphogenetic phenotypes, sterilized seeds were sowed on half-strength Murashige–Skoog medium (Sigma-Aldrich) agar plates containing 0.3% (w/v) Suc and imbibed for 3 d at 4°C in the dark. After germination was induced by white light for 6 h (100 μmol m−2 s−1), the plates were placed under various light conditions at 22°C for the indicated time. Hypocotyl length of 4-d–old seedlings grown under red and far-red light conditions were measured using the software ImageJ (Schneider et al., 2012). All experiments were performed in triplicate, with >20 seedlings measured for each sample.
Gene Expression Analysis
Total RNA was extracted with Trizol reagent (Invitrogen) according to the manufacture’s protocol. The first-strand cDNA was synthesized using reverse transcriptase (Takara). RT-qPCR analysis was performed using the SYBR Green PCR Supermix (Bio-Rad Laboratories) on an ABI7500 Real-Time PCR System (Applied Biosystems). Each sample was quantified at least in triplicate and normalized using UBIQUITIN10 (UBQ10) as an internal control. The gene-specific primer pairs for RT-qPCR are listed in Supplemental Table S10. Three biological replicates were performed for RT-qPCR analysis and representative results from one biological replicate were shown.
Measurement of HDAC Activity
HDAC activity was detected using the HDAC activity fluorometric assay kit (BioVision) following the manufacturer’s instructions. Briefly, 4 μg of purified proteins were diluted to 103 μL, and 12-μL 10×HDAC assay buffer and 5-μL substrate (0.02-mm Boc-Lys[Ac]-AMC) were added to each well and incubated at 37°C for 1 h. The reaction was stopped by adding 80-μL Lys developer and incubating at 37°C for 30 min. The HDAC activity was then measured by fluorescence plate reader (Excitation/Emission = 360/460 nm) using the 96-well black plate. A standard curve was prepared using the known amount of the AMC Standard (range from 0 to 2 μM). AMC released was used to stand for HDAC activity.
RNA-Seq Assays
For whole genomic transcriptome analysis, total RNA was extracted by Trizol reagent, and an mRNA-seq library was prepared by using an mRNA Seq Kit (Illumina). RNA-seq was performed by Genepioneer Biotechnologies with triplicate biological samples. All raw tags from each sample were mapped to the Arabidopsis genome TAIR10 and its corresponding annotated gene profile. Up to two mismatches were allowed during the mapping. Tags mapped to a unique location were used for the downstream analysis, whereas those mapped to multiple loci were discarded to avoid ambiguity. Cuffdiff (http://cufflinks.cbcb.umd.edu/manual.html) was applied to detect differentially expressed genes in mutant compared with wild-type. Genes with >1.5-fold changes with statistical significance (False Discovery Rate < 0.05) were selected. Functional classification was performed using the DAVID functional annotation clustering tool (http://david.abcc.ncifcrf.gov/home.jsp; Huang et al., 2009). The functional clusters enrichment analysis was calculated by comparing the whole Arabidopsis genome, and the highest classification stringency was chosen for clustering.
In Vitro Pull-Down Assays
In vitro pull-down assays were performed as described in Liu et al. (2013). His-HY5 or His-HYH recombinant protein was incubated with 50-μL GST-Bind Resin (Cat. no. 70541-3; Novagen) in a P buffer (10-mm Na2HPO4, 10-mm NaH2PO4, 500-mm NaCl, and 10-mm imidazole) for 2 h at 4°C, the binding reaction was washed three times with the P buffer, and then HDA15-GST or GST was added and incubated for 2 h at 4°C. After washing three times with the P buffer, the pulled-down proteins were eluted by boiling, separated by 10% SDS-PAGE, and detected by immunoblot analysis using an anti-His antibody (Cat. no. AbM59012; BGI).
Semi-In Vivo Pull-Down Assays
HY5-His recombinant protein (0.5 μg) was added to 1 mL of pull-down buffer with 50 μL of His Ni-NTA Agarose (Cat. no. R901-15; Invitrogen) and the mixtures were preincubated at 4°C for 1 h. Total proteins (1 mg) extracted from Col-0 or 35S: HY5-GFP seedlings grown under red light (13.12 μmol m−2 s−1) for 2 d were added into the mixtures and incubated for 3 h. After washing three times, the pulled-down proteins were detected by immunoblotting using an anti-GFP antibody (Cat. No. ab290; Abcam).
BiFC Assays
Full-length cDNA fragments of HDA15 and HY5/HYH were subcloned into the pCR8/GW/TOPO vectors (Invitrogen) and then recombined into the YN and YC vectors (Lu et al., 2010). The constructed vectors were transformed into Arabidopsis protoplasts by polyethylene-glycol–mediated transfection for transient expression (Yoo et al., 2007). To detect the interaction in tobacco, leaves of 2- to 4-week–old tobacco plants (Nicotiana benthamiana) were infiltrated with Agrobacterium tumefaciens strains (GV3101) containing HDA15 and HY5/HYH BiFC construct pairs. Transfected cells were imaged using a TCS SP5 Confocal Spectral Microscope Imaging System (Leica).
Co-IP Assays
Co-IP assays were performed as described in Liu et al. (2013). Two-d–old Col-0 and ProHY5:HY5-GFP-1 seedlings grown under red light conditions (13.12 μmol m−2 s−1) were harvested for analysis. Total proteins were extracted in an extraction buffer (50-mm Tris-HCl at pH 7.4, 150-mm NaCl, 2-mm MgCl2, 1-mm dithiothreitol, 20% glycerol, and 1% CA-630) containing protease inhibitor cocktail (Roche). Protein extracts were incubated with the polyclonal anti-HDA15 antibody (Liu et al., 2013) overnight at 4°C. Protein A Mag Sepharose beads (Cat. No. SC-2001; Santa Cruz) were then added. After incubation for 2 h at 4°C, the beads were centrifuged and washed three times with a wash buffer (50-mm Tris-HCl at pH 7.4, 150-mm NaCl, 2-mm MgCl2, 1-mm dithiothreitol, 10% [v/v] glycerol, and 1% [v/v] CA-630). The input and immunoprecipitated proteins were detected by SDS-PAGE gel with an anti-GFP antibody (Cat. No. ab290; Abcam).
ChIP Assays
ChIP assays were performed as described in Gendrel et al. (2005). After fixation with formaldehyde, the chromatin was extracted and then sheared to an average length of 500 bp by sonication. The chromatin was immunoprecipitated with specific antibodies including anti-H4ac (Cat. No. 06-866; Millipore), anti-H3K27me3 (Cat. No. 07-449; Millipore), and anti-GFP (Cat. No. ab290; Abcam). After cross-linking reversed, the amount of each precipitated DNA fragment was detected by real-time qPCR using specific primers listed in Supplemental Table S10. Three biological replicates were performed, and three technical repeats were carried out for each biological replicate. Representative results from one biological replicate were shown.
Statistical Analyses
Statistical differences were assessed by a Student’s t test. Values of P < 0.05 were considered statistically significant. Results are presented as mean ± sd.
Accession Numbers
Sequence data from this work can be found in the Arabidopsis Genome initiative or GenBank/EMBL databases under the following accession numbers: HDA15 (AT3G18520), HY5 (AT5G11260), HYH (AT3G17609), PHYA (AT1G09570), PHYB (AT2G18790), COP1 (AT2G32950), PIF1 (AT2G20180), PIF3 (AT1G09530), PIF4 (AT2G43010), PIF5 (AT3G59060), XTH10 (AT2G14620), XTH15 (AT4G14130), XTH17 (AT1G65310), BXL1 (AT5G49360), EXP2 (AT5G05290), PME16 (AT2G43050), PME35 (AT3G59010), IAA6 (AT1G52830), IAA19 (AT3G15540), SAUR6 (AT2G21210), SAUR35 (AT4G12410), SAUR36 (AT2G45210), SAUR50 (AT4G34760), ACT2 (AT3G18780), UBQ10 (AT4G05320), and TA3 (AT1G37110).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. HDA15 interacts with HY5/HYH in tobacco epidermal cells.
Supplemental Figure S2. RT-qPCR analysis of the expression level of HY5 in Col-0 and hy5-215 and ProHY5:HY5-GFP-1 seedlings.
Supplemental Figure S3. Hypocotyl lengths of 4-d–old Col-0, hda15-1, 35S:HDA15-GFP-1, phyB-9, 35S:HDA15-GFP phyB, and hy5-215 seedlings grown under various fluences of red light conditions.
Supplemental Figure S4. Hypocotyl lengths of 4-d–old Col-0, hda15-1, 35S:HDA15-GFP-1, phyA-211, 35S:HDA15-GFP phyA, and hy5-215 seedlings grown under various fluences of far-red light conditions.
Supplemental Figure S5. Hypocotyl lengths of 4-d–old Col-0, hda15-1, 35S:HDA15-GFP-1, phyA-211, phyB-9, 35S:HDA15-GFP phyA, and 35S:HDA15-GFP phyB seedlings grown in the dark conditions.
Supplemental Figure S6. Hypocotyl lengths of 4-d–old Col-0, 35S:HDA15-GFP, 35S:HY5-GFP, 35S:HDA15-GFP, 35S:HY5-GFP hda15, and 35S:HDA15-GFP hy5 seedlings grown under dark conditions.
Supplemental Figure S7. The hda15, hy5, and hda15 hy5 double mutants display similar hypocotyl length as wild type in the dark conditions.
Supplemental Figure S8. Validation of the RNA-seq data.
Supplemental Figure S9. A Venn diagram of HY5-regulated genes and HY5-associated genes.
Supplemental Figure S10. ChIP analysis of enrichment of HDA15 in the promoters of the cell wall organization and auxin signaling-related genes in 2-d–old 35S:HDA15-GFP and 35S:HDA15-GFP hy5 seedlings grown in the dark.
Supplemental Figure S11. ChIP analysis of histone H3 acetylation levels of representative cell elongation-related genes in Col-0, hda15-1, and hy5-215 mutants.
Supplemental Figure S12. ChIP analysis of the histone H3K27 trimethylation (H3K27me3) levels of XTH15, EXP2, and IAA19 in Col-0, hda15-1, and hy5-215 seedlings grown under red light (13.12 μmol m−2 s−1) for 2 d.
Supplemental Figure S13. Gene expression and histone modifications of hypocotyl cell elongation-related genes in hda15 and hy5 seedlings grown in the dark.
Supplemental Table S1. Upregulated genes in hda15.
Supplemental Table S2. Downregulated genes in hda15.
Supplemental Table S3. Upregulated genes in hy5.
Supplemental Table S4. Downregulated genes in hy5.
Supplemental Table S5. Upregulated genes in both hda15 and hy5 mutants.
Supplemental Table S6. Downregulated genes in both hda15 and hy5 mutants.
Supplemental Table S7. Differentially regulated genes in hda15 and hy5 mutants.
Supplemental Table S8. HY5-associated and HY5-regulated genes.
Supplemental Table S9. HDA15 and HY5 corepressed cell elongation-related genes.
Supplemental Table S10. Primers used in this study.
Acknowledgments
We thank Mrs. Xiaoping Pan and Rufang Deng at South China Botanical Garden, Chinese Academy of Sciences for their assistance in RT-qPCR and electron microscope assays. The National Botanical Gardens, Chinese Academy of Sciences for their support. We also thank Technology Commons, College of Life Science, National Taiwan University for the convenient use of the Bio-Rad Real-Time PCR System and the Confocal Spectral Microscope Imaging System.
Footnotes
This work was supported by the National Natural Science Foundation of China (grants no. 31771366 and no. 31371308), the Youth Innovation Promotion Association, Chinese Academy of Sciences (grant no. 2014319), the Science and Technology Foundation of Guangzhou City (grant no. 201707010335), the Ministry of Science and Technology of Taiwan (grants no. 105-2311-B-002-012-MY3 and no. 107-2313-B-002-001), and National Taiwan University (grant no. NTU-AS-108L104310).
Articles can be viewed without a subscription.
References
- Al-Sady B, Ni W, Kircher S, Schäfer E, Quail PH (2006) Photoactivated phytochrome induces rapid PIF3 phosphorylation prior to proteasome-mediated degradation. Mol Cell 23: 439–446 [DOI] [PubMed] [Google Scholar]
- Ang L-H, Chattopadhyay S, Wei N, Oyama T, Okada K, Batschauer A, Deng X-W (1998) Molecular interaction between COP1 and HY5 defines a regulatory switch for light control of Arabidopsis development. Mol Cell 1: 213–222 [DOI] [PubMed] [Google Scholar]
- Benhamed M, Bertrand C, Servet C, Zhou DX (2006) Arabidopsis GCN5, HD1, and TAF1/HAF2 interact to regulate histone acetylation required for light-responsive gene expression. Plant Cell 18: 2893–2903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger SL. (2007) The complex language of chromatin regulation during transcription. Nature 447: 407–412 [DOI] [PubMed] [Google Scholar]
- Caretti G, Di Padova M, Micales B, Lyons GE, Sartorelli V (2005) The Polycomb Ezh2 methyltransferase regulates muscle gene expression and skeletal muscle differentiation. Genes Dev 18: 2627–2638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae K, Isaacs CG, Reeves PH, Maloney GS, Muday GK, Nagpal P, Reed JW (2012) Arabidopsis SMALL AUXIN UP RNA63 promotes hypocotyl and stamen filament elongation. Plant J 71: 684–697 [DOI] [PubMed] [Google Scholar]
- Charron JB, He H, Elling AA, Deng XW (2009) Dynamic landscapes of four histone modifications during deetiolation in Arabidopsis. Plant Cell 21: 3732–3748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S, Ang LH, Puente P, Deng XW, Wei N (1998) Arabidopsis bZIP protein HY5 directly interacts with light-responsive promoters in mediating light control of gene expression. Plant Cell 10: 673–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Zhang J, Neff MM, Hong SW, Zhang H, Deng XW, Xiong L (2008) Integration of light and abscisic acid signaling during seed germination and early seedling development. Proc Natl Acad Sci USA 105: 4495–4500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Chory J, Fankhauser C (2004) Light signal transduction in higher plants. Annu Rev Genet 38: 87–117 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Earley K, Lawrence RJ, Pontes O, Reuther R, Enciso AJ, Silva M, Neves N, Gross M, Viegas W, Pikaard CS (2006) Erasure of histone acetylation by Arabidopsis HDA6 mediates large-scale gene silencing in nucleolar dominance. Genes Dev 20: 1283–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong PM, Tian L, Chen ZJ (2006) Arabidopsis thaliana histone deacetylase 1 (AtHD1) is localized in euchromatic regions and demonstrates histone deacetylase activity in vitro. Cell Res 16: 479–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendrel AV, Lippman Z, Martienssen R, Colot V (2005) Profiling histone modification patterns in plants using genomic tiling microarrays. Nat Methods 2: 213–218 [DOI] [PubMed] [Google Scholar]
- Gonzalez D, Bowen AJ, Carroll TS, Conlan RS (2007) The transcription corepressor LEUNIG interacts with the histone deacetylase HDA19 and mediator components MED14 (SWP) and CDK8 (HEN3) to repress transcription. Mol Cell Biol 27: 5306–5315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer CB, Tanaka Y, Kim YJ, Xie P, Zhang MQ, Park I-H, Kim TH (2015) Histone deacetylases positively regulate transcription through the elongation machinery. Cell Reports 13: 1444–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu D, Chen CY, Zhao M, Zhao L, Duan X, Duan J, Wu K, Liu X (2017) Identification of HDA15-PIF1 as a key repression module directing the transcriptional network of seed germination in the dark. Nucleic Acids Res 45: 7137–7150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Zhou J, Elling AA, Charron JBF, Deng XW (2008) Histone modifications and expression of light-regulated genes in Arabidopsis are cooperatively influenced by changing light conditions. Plant Physiol 147: 2070–2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen G, Guilfoyle T (2002) Auxin-responsive gene expression: Genes, promoters and regulatory factors. Plant Mol Biol 49: 373–385 [PubMed] [Google Scholar]
- Hofmann NR. (2015) A mechanism for inhibition of COP1 in photomorphogenesis: direct interactions of phytochromes with SPA proteins. Plant Cell 27: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm M, Ma LG, Qu LJ, Deng XW (2002) Two interacting bZIP proteins are direct targets of COP1-mediated control of light-dependent gene expression in Arabidopsis. Genes Dev 16: 1247–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Sherman BT, Lempicki RA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4: 44–57 [DOI] [PubMed] [Google Scholar]
- Jang IC, Chung PJ, Hemmes H, Jung C, Chua NH (2011) Rapid and reversible light-mediated chromatin modifications of Arabidopsis phytochrome A locus. Plant Cell 23: 459–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenuwein T, Allis CD (2001) Translating the histone code. Science 293: 1074–1080 [DOI] [PubMed] [Google Scholar]
- Jing Y, Zhang D, Wang X, Tang W, Wang W, Huai J, Xu G, Chen D, Li Y, Lin R (2013) Arabidopsis chromatin remodeling factor PICKLE interacts with transcription factor HY5 to regulate hypocotyl cell elongation. Plant Cell 25: 242–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BC, Soh MC, Kang BJ, Furuya M, Nam HG (1996) Two dominant photomorphogenic mutations of Arabidopsis thaliana identified as suppressor mutations of hy2. Plant J 9: 441–456 [DOI] [PubMed] [Google Scholar]
- Lau OS, Deng XW (2012) The photomorphogenic repressors COP1 and DET1: 20 years later. Trends Plant Sci 17: 584–593 [DOI] [PubMed] [Google Scholar]
- Lee J, He K, Stolc V, Lee H, Figueroa P, Gao Y, Tongprasit W, Zhao H, Lee I, Deng XW (2007) Analysis of transcription factor HY5 genomic binding sites revealed its hierarchical role in light regulation of development. Plant Cell 19: 731–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N, Park J, Kim K, Choi G (2015) The transcriptional coregulator LEUNIG_HOMOLOG inhibits light-dependent seed germination in Arabidopsis. Plant Cell 27: 2301–2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivar P, Monte E (2014) PIFs: Systems integrators in plant development. Plant Cell 26: 56–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Carey M, Workman JL (2007) The role of chromatin during transcription. Cell 128: 707–719 [DOI] [PubMed] [Google Scholar]
- Li Z, Zhang L, Yu Y, Quan R, Zhang Z, Zhang H, Huang R (2011) The ethylene response factor AtERF11 that is transcriptionally modulated by the bZIP transcription factor HY5 is a crucial repressor for ethylene biosynthesis in Arabidopsis. Plant J 68: 88–99 [DOI] [PubMed] [Google Scholar]
- Liu X, Chen CY, Wang KC, Luo M, Tai R, Yuan L, Zhao M, Yang S, Tian G, Cui Y, et al. (2013) PHYTOCHROME INTERACTING FACTOR3 associates with the histone deacetylase HDA15 in repression of chlorophyll biosynthesis and photosynthesis in etiolated Arabidopsis seedlings. Plant Cell 25: 1258–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Yang S, Zhao M, Luo M, Yu CW, Chen CY, Tai R, Wu K (2014) Transcriptional repression by histone deacetylases in plants. Mol Plant 7: 764–772 [DOI] [PubMed] [Google Scholar]
- Lu Q, Tang X, Tian G, Wang F, Liu K, Nguyen V, Kohalmi SE, Keller WA, Tsang EW, Harada JJ, et al. (2010) Arabidopsis homolog of the yeast TREX-2 mRNA export complex: Components and anchoring nucleoporin. Plant J 61: 259–270 [DOI] [PubMed] [Google Scholar]
- Luger K, Mäder AW, Richmond RK, Sargent DF, Richmond TJ (1997) Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature 389: 251–260 [DOI] [PubMed] [Google Scholar]
- Luo M, Tai R, Yu CW, Yang S, Chen CY, Lin WD, Schmidt W, Wu K (2015) Regulation of flowering time by the histone deacetylase HDA5 in Arabidopsis. Plant J 82: 925–936 [DOI] [PubMed] [Google Scholar]
- Ma L, Li J, Qu L, Hager J, Chen Z, Zhao H, Deng XW (2001) Light control of Arabidopsis development entails coordinated regulation of genome expression and cellular pathways. Plant Cell 13: 2589–2607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNellis TW, Deng XW (1995) Light control of seedling morphogenetic pattern. Plant Cell 7: 1749–1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatani A, Reed JW, Chory J (1993) Isolation and initial characterization of Arabidopsis mutants that are deficient in phytochrome a. Plant Physiol 102: 269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterlund MT, Deng XW (1998) Multiple photoreceptors mediate the light-induced reduction of GUS-COP1 from Arabidopsis hypocotyl nuclei. Plant J 16: 201–208 [DOI] [PubMed] [Google Scholar]
- Osterlund MT, Hardtke CS, Wei N, Deng XW (2000) Targeted destabilization of HY5 during light-regulated development of Arabidopsis. Nature 405: 462–466 [DOI] [PubMed] [Google Scholar]
- Oyama T, Shimura Y, Okada K (1997) The Arabidopsis HY5 gene encodes a bZIP protein that regulates stimulus-induced development of root and hypocotyl. Genes Dev 11: 2983–2995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey R, Müller A, Napoli CA, Selinger DA, Pikaard CS, Richards EJ, Bender J, Mount DW, Jorgensen RA (2002) Analysis of histone acetyltransferase and histone deacetylase families of Arabidopsis thaliana suggests functional diversification of chromatin modification among multicellular eukaryotes. Nucleic Acids Res 30: 5036–5055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JW, Nagpal P, Poole DS, Furuya M, Chory J (1993) Mutations in the gene for the red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell 5: 147–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzini L, Favory JJ, Cloix C, Faggionato D, O’Hara A, Kaiserli E, Baumeister R, Schäfer E, Nagy F, Jenkins GI, et al. (2011) Perception of UV-B by the Arabidopsis UVR8 protein. Science 332: 103–106 [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D, Primavesi L, Bishopp A, Roberts G, Doonan J, Jenuwein T, Goodrich J (2006) Silencing by plant Polycomb-group genes requires dispersed trimethylation of histone H3 at lysine 27. EMBO J 25: 4638–4649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheerin DJ, Menon C, zur Oven-Krockhaus S, Enderle B, Zhu L, Johnen P, Schleifenbaum F, Stierhof YD, Huq E, Hiltbrunner A (2015) Light-activated phytochrome A and B interact with members of the SPA family to promote photomorphogenesis in Arabidopsis by reorganizing the COP1/SPA complex. Plant Cell 27: 189–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Zhu L, Castillon A, Majee M, Downie B, Huq E (2008) Light-induced phosphorylation and degradation of the negative regulator PHYTOCHROME-INTERACTING FACTOR1 from Arabidopsis depend upon its direct physical interactions with photoactivated phytochromes. Plant Cell 20: 1586–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. (2000) Phytochromes and light signal perception by plants—an emerging synthesis. Nature 407: 585–591 [DOI] [PubMed] [Google Scholar]
- Sridhar VV, Surendrarao A, Gonzalez D, Conlan RS, Liu Z (2004) Transcriptional repression of target genes by LEUNIG and SEUSS, two interacting regulatory proteins for Arabidopsis flower development. Proc Natl Acad Sci USA 101: 11494–11499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterner DE, Berger SL (2000) Acetylation of histones and transcription-related factors. Microbiol Mol Biol Rev 64: 435–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahl BD, Allis CD (2000) The language of covalent histone modifications. Nature 403: 41–45 [DOI] [PubMed] [Google Scholar]
- Tatematsu K, Kumagai S, Muto H, Sato A, Watahiki MK, Harper RM, Liscum E, Yamamoto KT (2004) MASSUGU2 encodes Aux/IAA19, an auxin-regulated protein that functions together with the transcriptional activator NPH4/ARF7 to regulate differential growth responses of hypocotyl and formation of lateral roots in Arabidopsis thaliana. Plant Cell 16: 379–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepperman JM, Zhu T, Chang HS, Wang X, Quail PH (2001) Multiple transcription-factor genes are early targets of phytochrome a signaling. Proc Natl Acad Sci USA 98: 9437–9442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonini T, Bagella L, D’Andrilli G, Claudio PP, Giordano A (2004) Ezh2 reduces the ability of HDAC1-dependent pRb2/p130 transcriptional repression of cyclin A. Oncogene 23: 4930–4937 [DOI] [PubMed] [Google Scholar]
- van der Vlag J, Otte AP (1999) Transcriptional repression mediated by the human Polycomb-group protein EED involves histone deacetylation. Nat Genet 23: 474–478 [DOI] [PubMed] [Google Scholar]
- Vannini A, Volpari C, Filocamo G, Casavola EC, Brunetti M, Renzoni D, Chakravarty P, Paolini C, De Francesco R, Gallinari P, et al. (2004) Crystal structure of a eukaryotic zinc-dependent histone deacetylase, human HDAC8, complexed with a hydroxamic acid inhibitor. Proc Natl Acad Sci USA 101: 15064–15069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Ma LG, Li JM, Zhao HY, Deng XW (2001) Direct interaction of Arabidopsis cryptochromes with COP1 in light control development. Science 294: 154–158 [DOI] [PubMed] [Google Scholar]
- Wei N, Deng XW (1996) The role of the COP/DET/FUS genes in light control of Arabidopsis seedling development. Plant Physiol 112: 871–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Grunstein M (2000) 25 years after the nucleosome model: Chromatin modifications. Trends Biochem Sci 25: 619–623 [DOI] [PubMed] [Google Scholar]
- Yang XJ, Seto E (2008) The Rpd3/Hda1 family of lysine deacetylases: From bacteria and yeast to mice and men. Nat Rev Mol Cell Biol 9: 206–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HQ, Tang RH, Cashmore AR (2001) The signaling mechanism of Arabidopsis CRY1 involves direct interaction with COP1. Plant Cell 13: 2573–2587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SD, Cho YH, Sheen J (2007) Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat Protoc 2: 1565–1572 [DOI] [PubMed] [Google Scholar]
- Zhang H, He H, Wang X, Wang X, Yang X, Li L, Deng XW (2011) Genome-wide mapping of the HY5-mediated gene networks in Arabidopsis that involve both transcriptional and post-transcriptional regulation. Plant J 65: 346–358 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Mayba O, Pfeiffer A, Shi H, Tepperman JM, Speed TP, Quail PH (2013) A quartet of PIF bHLH factors provides a transcriptionally centered signaling hub that regulates seedling morphogenesis through differential expression-patterning of shared target genes in Arabidopsis. PLoS Genet 9: e1003244. [DOI] [PMC free article] [PubMed] [Google Scholar]