Abstract
Autism spectrum disorder (ASD) is more prevalent in males than females. Previous research indicates females camouflage ASD symptoms more than males, potentially contributing to the difference in prevalence. This study investigated sex/gender differences in behavioral phenotypes in 17 males and 11 females with ASD, as well camouflaging in ASD, in an attempt to partially replicate findings from Lai et al. (Autism 21(6):690–702, 2017). Overall ASD symptoms were measured by the autism spectrum quotient (AQ). Mean AQ in females with ASD was higher than males with ASD, with the difference approaching statistical significance. Camouflaging was found to be more common in females with ASD, and not associated to social phobia. Furthermore, camouflaging correlated negatively with emotional expressivity in females, but not males, with ASD. These findings strengthen previous findings regarding camouflaging being more common in females and add to the literature on how camouflaging may be different in females versus males.
Keywords: Autism spectrum disorder (ASD), Sex/gender differences, Camouflaging
The prevalence of autism spectrum disorder (ASD) is considerably higher in males than females, especially in individuals with higher IQ (Werling and Geschwind 2013). According to the most recent report from the United States Center for Disease Control and Prevention, one in 38 boys and one in 152 girls aged eight years were diagnosed with ASD (Baio et al. 2018). The same report revealed that, although the average prevalence of males to females with a diagnosis is 4:1, a significantly higher proportion of males with ASD have average or above average IQ as compared to females with ASD. Given the noted gender imbalance, there is a distinct possibility that the typical presentation of ASD is biased toward males (Kirkovski et al. 2013). Females may need to exhibit a greater number of or more intense symptoms in order to receive a diagnosis given that many diagnostic tools were originally tested with male participants (Kreiser and White 2014). Additionally, females with ASD have been shown to engage in “camouflaging” (i.e. masking their autistic symptoms) (Hull et al. 2017b) more than males. This strongly suggests that ASD symptoms might be more difficult to detect in females and, consequently, a significant portion of females may be misdiagnosed, diagnosed after a significant delay, or not diagnosed altogether, resulting in lack of treatment and support. It is therefore vital to elucidate potentially distinctive aspects of ASD presentation in females.
The discrepancy in prevalence rates may indicate a distinct phenotypic difference between males and females with ASD. Results of the studies that explored sex/gender differences in core ASD symptoms as well as co-occurring symptoms have been inconsistent thus far, with majority of studies focusing on children and adolescents. (Note that we use the term “sex/gender” to be consistent with Lai et al. (2015), given that the concepts of “sex” and “gender” may be ambiguous and have different meanings for different people. For example, “sex” traditionally refers to one’s biological sex at birth, whereas “gender” refers to one’s identity as male, female, or non-binary. However, these traditional distinctions may not apply to everyone, so we choose not to make the distinction in order to be more inclusive.) For instance, in a study in which 28 European datasets of children and adults (N = 2684) with ASD were analyzed, it was found that females had lower restricted and repetitive behaviors (RRBs) in childhood than males, but no differences in socio-communicative functioning, either earlier in childhood or at present (Tillmann et al. 2018). On the other hand, in a study of 499 toddlers with ASD, girls showed more impairment in communication on the Autism Diagnostic Observation Schedule-Generic (ADOS-G; Lord et al. 2000) than boys, whereas boys showed more impairment in RRB (Hartley and Sikora 2009). A range of other studies reported on one hand higher RRBs (e.g., Mandy et al. 2012; Van Wijngaarden-Cremers et al. 2014) and poorer socio-communicative functioning (Head et al. 2014) in males, and on the other hand, higher severity of ASD traits in females (e.g. Carter et al. 2007; Holtmann et al. 2007; Rynkiewicz et al. 2016). Still several other studies found no sex/gender effects on the severity of core ASD symptoms (Andersson et al. 2013; Holtmann et al. 2007). A recent meta-analysis by Hull et al. (2017a) that included 13 studies of both children and adults found no evidence for gender differences in either socio-communicative skills or RRBs. The inconsistencies across reviewed studies, in addition to often-limited sample sizes and inherent ASD heterogeneity, may be partially attributable to differences in measurement, as some studies used clinician-observations (e.g. ADOS and Autism Diagnostic Interview-Revised [ADI-R; Lord et al. 1994]), whereas others focused more on parent-report measures.
Research on sex/gender differences in adults with ASD has resulted in similarly inconsistent findings. For example, in an early study, Baron-Cohen et al. (2001) found no significant sex/gender differences in Autism Spectrum Quotient (AQ) scores (though typically developing [TD] males and females did differ significantly). The same group found that males with ASD exhibited significantly more severe autism symptoms and less empathy but higher capacity to systematize (i.e. the drive to analyze) than females with ASD. This pattern was similar in the TD sample, though differences were attenuated in the ASD sample (Baron-Cohen et al. 2014). Pisula et al. (2013), however, found that males only scored higher on only the communication subscale of the AQ, whereas another study found that females with ASD actually scored higher on the total score of AQ than males (Lai et al. 2011). Using the ADI-R, Wilson et al. (2016) found that high-functioning adults did not differ by sex/gender in socio-communicative symptoms, but males did have more RRBs than females. Post-hoc analyses found that, among those with more severe autism symptoms, males scored higher on average on the social and communication domains of the ADI-R, though among those with atypical autism (or pervasive development disorder—not otherwise specified (PDD-NOS)), females had more socio-communicative deficits. There were no sex/gender differences on the ADOS. On the other hand, Lai et al. (2011) found that females with ASD showed fewer socio-communicative deficits than males with ASD in a clinician observation, though self-reported more autistic traits on the AQ. These results indicate that sex/gender differences in individuals with ASD may manifest differently depending on the type of measurement used (e.g. clinician observation, parent-, or self-report), though inconsistencies still exist within each measurement type.
As noted, part of the difficulty in assessing sex/gender differences may be due to the masking of certain symptoms. It has been suggested that females are better able to “camouflage” their ASD symptoms than males, thus making it more difficult for clinicians to diagnose ASD in females (Bargiela et al. 2016; Hull et al. 2017b; Rynkiewicz et al. 2016). Such camouflaging may come in the form of modifying one’s outward social expression, such as forcing oneself to display appropriate facial expressions and eye contact or even playing a character or role to appear more typical (Hull et al. 2017a) or in suppressing inappropriate and idiosyncratic behaviors (Wiskerke et al. 2018). Even young girls with ASD may camouflage their symptoms (Dean et al. 2017), which may diminish the chance of getting a diagnosis (Dworzynski et al. 2012).
As a way of quantifying camouflaging, Lai et al. (2017) subtracted the standardized clinician-rated ADOS scores from the standardized self-report measures of ASD symptoms and their Reading the Mind in the Eyes score, and found that females with ASD engaged in camouflaging more than males with ASD. Additionally, camouflaging was positively correlated with severity of mood symptoms in males with ASD and with executive functioning in females with ASD. This suggests that the same ASD-related phenomenon, camouflaging in this case, may manifest differently in males versus females, resulting in a potential differential risk for psychopathology. Furthermore, the phenotype presented by women with ASD may not necessarily reflect how they experience the world. This, along with the delay in diagnosis in females (Begeer et al. 2013), can have detrimental effects on well-being and quality of life.
The current study aimed to further characterize sex/gender differences in adults with ASD without co-occurring intellectual disability. In particular, we aimed to replicate the gender differences in camouflaging reported by Lai et al. (2017). Drawing from previous findings, we hypothesized that camouflaging would be more common in females with ASD than males with ASD, and that it would not be related to anxiety. Given Lai et al.’s finding that camouflaging was related to executive functioning in females, we also hypothesized that camouflaging would be related to the Working Memory (WM) score of the Stanford-Binet in females, but not males. We also sought to extend previous work on correlates of camouflaging by exploring whether camouflaging was related emotional expressivity. As conceptualized by Gross and John (1997), an individual’s emotional expressivity includes both the strength of an emotional impulse and its valence (positive vs negative). We have focused on this particular construct as previous research indicates that individuals with ASD have difficulties with emotional expression (Gordon et al. 2014). In TD individuals, Burgin et al. (2012) found that increased emotional expressivity was associated with better social functioning and happiness, suggesting that emotional expressivity, which may be suppressed by those attempting to camouflage, could be related to quality of life in ASD. However, no studies to date have explored the relation between expressivity and camouflaging in ASD.
Methods
Participants
The study included a total of 62 adults, 28 with ASD (11 females) and 34 without (15 females) (see Table 1 for demographics). The current analyses focus on the ASD group, though questionnaire scores from the TD group can be found in the supplementary materials.
Table 1.
Participant demographics
| Group (N) | Males (17) | Females (11) |
|---|---|---|
| Age | 23 (4.09) | 33 (9.72) |
| FSIQ | 102 (16.77) | 101 (16.01) |
| VIQ | 106 (16.90) | 101 (20.34) |
| NVIQ | 99 (17.04) | 101 (12.51) |
| Ethnicity | ||
| White | 11 | 10 |
| Asian | 1 | 1 |
| Hispanic | 1 | 0 |
| Black | 0 | 0 |
| Unknown | 4 | 0 |
Age and IQ scores are presented as mean (standard deviation) for each group. Ethnicity breakdown is presented as number of participants endorsing each ethnicity
FSIQ full scale IQ, VIQ verbal IQ, NVIQ nonverbal IQ
All were recruited for either a molecular neuroimaging study focusing on the GABAergic system or a systemic biomarker study investigating neurosteroids in individuals with ASD. The current analysis is a post hoc study of available data from these two studies. Participants were eligible to participate if they were 18–55 years old, had a full-scale IQ (FSIQ) of 70 or above, and were in good physical health with no serious mental illness (e.g. a diagnosis of bipolar disorder, schizophrenia, or currently experiencing a major depressive episode). Participants in the ASD group either had an established diagnosis before joining the study or were diagnosed by the corresponding author before participating. ASD diagnosis was confirmed with diagnostic testing (see below).
Procedures
All procedures of this study were approved by the Institutional Review Board at Stanford University. Informed consent was obtained from all participants before beginning study procedures. Participants were recruited via referral from the Autism and Developmental Disabilities Clinic at Stanford Children’s Health and flyers posted at colleges within the Bay Area. To determine eligibility based on FSIQ, the Stanford-Binet Intelligence Scales, Fifth Edition (Roid 2003) was administered. Participants in the ASD group were also administered the ADOS-2 module four (Lord, et al. 2012). If possible, parents of individuals in the ASD group completed the ADI-R. Participants completed questionnaires online through a secure, HIPAA compliant online system (Harris et al. 2009).
Measures
All participants completed a questionnaire assessing ASD symptoms, the AQ (Baron-Cohen et al. 2001). The AQ is a 50-question measure assessing social skill, attention switching, attention to detail, communication, and imagination. Higher scores indicate more severe symptoms. Though not used in the current analyses, participants also completed the Ritvo Autism-Asperger Diagnostic Scale Revised (RAADS-R; Ritvo et al. 2011) and the Social Responsiveness Scale, Second Edition (SRS-2; Constantino and Gruber 2012); mean scores for male and female participants in both the ASD and TD groups can be found in the supplementary materials. Demographics for the current analyses were gathered through the demographic section of the RAADS-R, which included a question phrased “Your Gender:” with the answer choices male or female.
Participants also completed the Berkeley Expressivity Questionnaire (BEQ; Gross and John 1995) and the Social Phobia and Anxiety Inventory (SPAI; Turner et al. 1989). The BEQ is comprised of sixteen 7-point Likert scale items designed to measure emotional expressivity. The scale produces an overall emotional expressivity (EE) score, as well as positive (PE) and negative expressivity (NE) scores and an impulse strength (IS) score (i.e. the strength of overt emotional displays). The SPAI is a 32-item measure designed to assess social phobia and anxiety. Only the overall social phobia score was used in the current study. All questionnaires were self-report instruments.
Data Analysis
Independent samples t-tests were used to explore sex/gender differences on the AQ. To quantify camouflaging, AQ and ADOS scores were first mean centered and then scaled (following Lai et al. 2017) to create standardized AQ (SAQ) and ADOS (SADOS) scores. Camouflage (CAM) scores were obtained by subtracting SADOS from SAQ. Sex/gender differences in CAM were assessed with independent samples t-tests. Pearson correlations between CAM and SPAI Social Phobia and Stanford-Binet Working Memory were assessed for males and females. For the aforementioned six tests, Bonferroni corrections were applied to correct for multiple comparisons, with an alpha level set at 0.008. Bonferroni corrections were not applied when correlations between CAM and BEQ scores (overall emotional expressivity, positive expressivity, negative expressivity, and impulse control) were assessed, given that these analyses were exploratory in nature (i.e. no previous research has investigated the relationship between camouflaging and these constructs). Effect sizes were calculated using Cohen’s d (Cohen 1988). In order to provide more robust estimates, 95% confidence intervals are reported after using bootstrapping with 5000 repeats (Efron and Tibshirani 1993; Tabachnick and Fidell 2007).
Results
Table 2 includes mean scores for all participants on questionnaires.
Table 2.
Assessment scores by sex/gender
| Group (n) | ASD M (17) | ASD F (11) |
|---|---|---|
| AQ total | 29.35 (5.26) | 35.45 (6.70)* |
| CAM | 0.03 (.20) | 0.34 (.24)** |
| SPAI social phobia total | 106.71 (35.90) | 115.91 (41.35) |
| ADOS total score | 13.53 (5.50) | 8.09 (4.57) |
| SB-5 WM | 96.41 (18.76) | 96.00 (16.21) |
| BEQ EE | 67.29 (18.08) | 81.18 (18.61) |
| BEQ PE | 17.94 (5.83) | 21.91 (4.93) |
| BEQ NE | 23.71 (6.02) | 23.64 (11.58) |
| BEQ IS | 25.65 (9.21) | 35.64 (5.37) |
Only 15 males had an ADOS module 4 (and therefore camouflaging score). We were unable to obtain one ADOS; the other participant completed a module 3 years ago in a previous research study
AQ Autism Spectrum Quotient, SPAI Social Phobia and Anxiety Inventory, BEQ Berkeley Expressivity Questionnaire, CAM Camouflaging score. Scores are presented as mean (standard deviation)
p < 0.05,
p < 0.008 (Bonferroni corrected alpha level)
Females with ASD scored higher than males on the AQ, though the difference was not significant after Bonferroni correction, t(17.79) = − 2.56, p = 0.02, d = 1.01, BCa 95% CI [− 10.43, − 1.14].
Females had significantly higher CAM scores (M = .34, SD = .24) than males (M = 0.03, SD = 0.05), t(18.87) = − 3.45, p = 0.003, d = 1.79, BCa 95% CI [− 0.476, −0.139]. In both males and females, CAM did not correlate with social phobia (females: r = 0.266, p = 0.429, BCa 95% CI [− 0.371, 0.759]; males: r=0.353, p = 0.237, BCa 95% CI [− 0.177, 0.767]). CAM was also not correlated with WM in either females (r = .470, p = 0.170, BCa 95% CI [− 0.434, 0.837]) or males (r = .368, p = 0.178, BCa 95% CI [− 0.242−.775]).
As shown in Fig. 1a, in females, CAM correlated negatively with EE (r = − 0.607, p = 0.048, BCa 95% CI [− 0.952, 0.014]). In males, CAM did not correlate with EE (r=0.039, p = 0.891, BCa 95% CI [− 0.427, 0.518]). In females, CAM correlated negatively with PE (r = − 0.676, p = 0.022, −0.910, − 0.253]; see Fig. 1b), but did not correlate with NE (r = − 0.530, p = 0.093, BCa 95% CI [− 0.931, 0.117]) or IS (r = − 0.340, p = 0.306, BCa 95% CI [− 0.765, 0.313]). CAM in males did not correlate with PE (r = − 0.224, p = 0.422, BCa 95% CI [− 0.635, 0.443]), NE (r = 0.035, p = 0.903, BCa 95% CI [− 0.480, 0.613]), or IS (r = 0.200, p = 0.475, BCa 95% CI [− 0.280, 0.611]). As stated in the methods, because camouflaging’s relationship with emotional expressivity was a novel topic, Bonferroni corrections were not applied to these analyses.
Fig. 1.
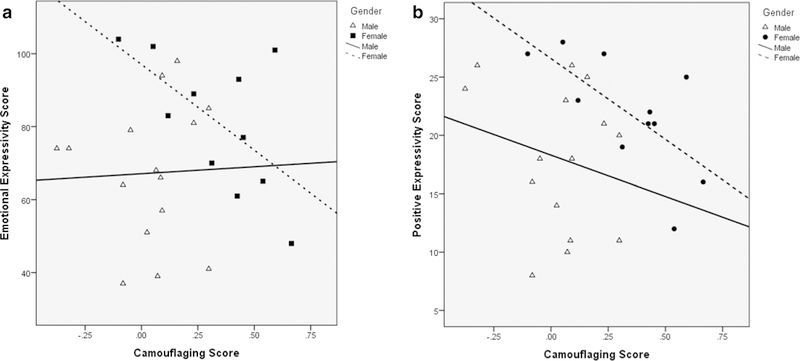
a Correlation between camouflaging and BEQ emotional expressivity in males and females with ASD. The correlation was significant in females (r = − 0.607, p = 0.048), but not males (r = 0.039, p = 0.891). b Correlation between camouflaging and BEQ positive emotionality in males and females with ASD. The correlation was significant in females (r = − 0.676, p = 0.022), but not males (r = − 0.224, p = 0.422)
Discussion
This study aimed to explore sex/gender differences in camouflaging in adults with ASD without comorbid intellectual disability, with a focus on replicating the findings from Lai et al. (2017). Though females with ASD in our sample selfreported more ASD symptoms on the AQ than males, the relationship was not significant after Bonferroni correction. However, the females in our sample did camouflage their autism symptoms significantly more than males, supporting the theory that females with ASD are masking their symptoms more so than males. This is in line with the finding by Lai et al. (2017). The gender difference in camouflaging may be due at least in part to the societal pressures females face to conform to gender roles (Kreiser and White 2014). Females with ASD may face more stigma and rebuke for exhibiting characteristics that are stereotypically more male, such as being disruptive or less empathic (Goldman 2013). On the other hand, females may have learned better masking strategies than males as a result of their socialization as the more social sex.
Further replicating the findings by Lai et al. (2017), we also found that camouflaging was not related to anxiety. However, our findings diverged from those of Lai et al., as camouflaging in the current study was not related to executive functioning, as measured by the working memory score from the SB-5. Importantly, we found that camouflaging correlated negatively with emotional expressivity, and positive expressivity in particular, in females but not in males with ASD. Though causation cannot be inferred from this correlation, it is possible that women with ASD who camouflage tend to be more aware of their emotional displays and are more likely to inhibit them in order to appear more “typical.” For example, if females are particularly excited about a circumscribed interest, they may be more aware than males that it is unusual to show their fascination, and therefore reduce their emotional displays. On the other hand, the toll of needing to camouflage may be dampening the females’ positive affect, in that they are so concerned with masking their ASD symptoms that they exhibit fewer positive emotions. Males, alternatively, may be engaging in other camouflaging behaviors not picked up by the emotional expressivity construct. More research, especially longitudinal studies, will need to determine how and why females and males camouflage and what effects different types of camouflaging have on well-being.
Though the current study did successfully replicate many of the findings from Lai et al. (2017), there were a few differences, particularly in the measures used in each study. Firstly, Lai et al. quantified camouflaging in two ways: (1) by subtracting standardized ADOS scores from standardized AQ scores and (2) by subtracting standardized ADOS scores from standardized RMET scores. A final camouflaging score was determined by principal component analysis. Though our quantification of camouflaging matches the first approach (i.e., SAQ-SADOS), we did not replicate the second, as our participants were not assessed with RMET. Additionally, though both studies looked at camouflaging’s relationship to anxiety, Lai et al. used the Beck Anxiety Inventory, whereas we used the SPAI to look at social phobia in particular. Though these two scales are not interchangeable with each other, we view it as a strength that the lack of relationship between camouflaging and anxiety was replicated using slightly different methods than Lai et al. In terms of executive functioning, we used the working memory standard score from the SB-5, whereas Lai et al. used the Go/ No-Go task. The divergence between our findings and theirs may indicate that the relationship between camouflaging and executive functioning is more tenuous and/or specific than its relationship with anxiety. Beyond the replication, we were also able add a novel finding to the existing camouflaging literature by exploring its association with emotional expressivity. These findings need further investigation, given that females who are camouflaging more have decreased positive expressivity, which could be related to other constructs, such as social skills and happiness (Burgin et al. 2012).
It is important to consider the findings reported here in light of several limitations. Firstly, the sample was one of convenience and was of modest size, rendering our analyses generally underpowered to detect small differences. However, it is also important to highlight that the inclusion of bootstrapped confidence intervals provided more robust statistics. It will also be beneficial to have multiple informants, especially parental reports, in order to compare parents’ observations with self-report, especially given the importance of camouflaging in this population. Additionally, camouflaging is still a crude concept that has not been extensively researched, and more conceptual clarity is needed. An initial version of a camouflaging self-report measure was published during the publication of this manuscript (the Camouflaging Autistic Traits Questionnaire; Hull et al. 2019), which will be a useful tool in future camouflaging investigations. It is also important for future studies to include additional measures that assess traits like self-awareness, alexithymia, emotion regulation, and coping strategies that may contribute to camouflaging and other sex/gender differences in ASD. Additionally, the results of this investigation indicate that other more sensitive tools will be useful in further characterizing different aspects of camouflaging that individuals with ASD engage in. One such measure could be digital phenotyping (Onnela and Rauch 2016), which relies on objective data gathered from one’s phone. Asking participants to report on more nuanced aspects of gender, such as status as non-binary or transgender, as opposed to having a forced-choice male/female option, will also help elucidate subtle differences in gender identities in ASD.
Despite the noted limitations, the current study provides an important contribution to the literature on camouflaging in ASD by replicating the finding of Lai et al. (2017) that females camouflage their ASD symptoms more than males. As the second study to quantify camouflaging and use this quantification to reveal gender differences, this study represents a step forward in studying this burgeoning topic. The findings herein also highlight areas in which males and females with ASD may differ in their autism presentations and suggests that men and women with ASD may cope with and manifest their symptoms differently. Our findings regarding emotional expressivity will need to be replicated and extended with a larger sample using more objective assessments in order to demonstrate causal relationship between camouflaging and expressivity, and explore how camouflaging is affecting individuals’ quality of life. Though more work on camouflaging is needed, it is vital for clinicians to be aware of such gender differences in ASD, as their judgments, even when supplemented by parental report, may not accurately reflect the true experiences of the women on the spectrum.
Supplementary Material
Acknowledgments
This study was funded by the National Institute of Mental Health (K08MH111750; awarded to LKF). The authors would like to thank Mirko Uljarevic for providing input regarding statistical analyses.
Footnotes
Compliance with Ethical Standards
Conflict of Interest All authors declare they have no conflicts of interest.
Ethics Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent Informed consent was obtained from all individual participants included in the study.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s10803-019-03998-y) contains supplementary material, which is available to authorized users.
References
- Andersson GW, Gillberg C, & Miniscalco C (2013). Pre-school children with suspected autism spectrum disorders: Do girls and boys have the same profiles? Research in Developmental Disabilities, 34(1), 413–422. [DOI] [PubMed] [Google Scholar]
- Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, … Dowling NF. (2018). Prevalence of autism spectrum disorder among children aged 8 years: Autism and developmental disabilities monitoring network, 11 Sites, United States, 2014. MMWR Surveillance Summaries, 67(6), 1–23. 10.15585/mmwr.ss6706a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargiela S, Steward R, & Mandy W (2016). The experiences of late-diagnosed women with autism spectrum conditions: An investigation of the female autism phenotype. Journal of Autism and Developmental Disorders, 46(10), 3281–3294. 10.1007/s10803-016-2872-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Cassidy S, Auyeung B, Allison C, Achoukhi M, Robertson S, … Lai M-C. (2014). Attenuation of typical sex differences in 800 adults with autism vs. 3,900 controls. PLoS ONE, 9(7), e102251. 10.1371/journal.pone.0102251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Skinner R, Martin J, & Clubley E (2001). The autism-spectrum quotient (AQ): Evidence from asperger syndrome/high-functioning autism, malesand females, scientists and mathematicians. Journal of Autism and Developmental Disorders, 31(1), 5–17. [DOI] [PubMed] [Google Scholar]
- Begeer S, Mandell D, Wijnker-Holmes B, Venderbosch S, Rem D, Stekelenburg F, et al. (2013). Sex differences in the timing of identification among children and adults with autism spectrum disorders. Journal of Autism and Developmental Disorders, 43(5), 1151–1156. 10.1007/s10803-012-1656-z. [DOI] [PubMed] [Google Scholar]
- Burgin CJ, Brown LH, Royal A, Silvia PJ, Barrantes-Vidal N, & Kwapil TR (2012). Being with others and feeling happy: Emotional expressivity in everyday life. Personality and Individual Differences, 53(3), 185–190. 10.1016/j.paid.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter AS, Black DO, Tewani S, Connolly CE, Kadlec MB, & Tager-Flusberg H (2007). Sex differences in toddlers with autism spectrum disorders. Journal of Autism and Developmental Disorders, 37(1), 86–97. 10.1007/s10803-006-0331-7. [DOI] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical power analysis for the behavioral sciences (2nd ed., Vol. 4). London: Routledge. [Google Scholar]
- Constantino JN, & Gruber CP (2012). Social responsiveness scale (SRS). Torrance: Western Psychological Services. [Google Scholar]
- Dean M, Harwood R, & Kasari C (2017). The art of camouflage: Gender differences in the social behaviors of girls and boys with autism spectrum disorder. Autism, 21(6), 678–689. 10.1177/1362361316671845. [DOI] [PubMed] [Google Scholar]
- Dworzynski K, Ronald A, Bolton P, & Happé F (2012). How different are girls and boys above and below the diagnostic threshold for autism spectrum disorders? Journal of the American Academy of Child and Adolescent Psychiatry, 51(8), 788–797. 10.1016/j.jaac.2012.05.018. [DOI] [PubMed] [Google Scholar]
- Efron B, & Tibshirani RJ (1993). An introduction to the bootstrap. Boca Raton: Chapman & Hall; Retrieved from https://cds.cern.ch/record/526679/files/0412042312_TOC.pdf [Google Scholar]
- Goldman S (2013). Opinion: Sex, gender and the diagnosis of autism—A biosocial view of the male preponderance. Research in Autism Spectrum Disorders, 7(6), 675–679. 10.1016/j.rasd.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon I, Pierce MD, Bartlett MS, & Tanaka JW (2014). Training facial expression production in children on the autism spectrum. Journal of Autism and Developmental Disorders, 44(10), 2486–2498. 10.1007/s10803-014-2118-6. [DOI] [PubMed] [Google Scholar]
- Gross JJ, & John OP (1995). Facets of emotional expressivity: Three self-report factors and their correlates. Personality and Individual Differences, 19(4), 555–568. [Google Scholar]
- Gross JJ, & John OP (1997). Revealing feelings: Facets of emotional expressivity in self-reports, peer ratings, and behavior. Journal of Personality and Social Psychology, 72(2), 435 10.1037/0022-3514.72.2.435. [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, & Conde JG (2009). Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42(2), 377–381. 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley SL, & Sikora DM (2009). Sex differences in autism spectrum disorder: an examination of developmental functioning, autistic symptoms, and coexisting behavior problems in toddlers. Journal of Autism and Developmental Disorders, 59(12), 1715–1722. 10.1007/s10803-009-0810-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head AM, McGillivray JA, & Stokes MA (2014). Gender differences in emotionality and sociability in children with autism spectrum disorders. Molecular Autism, 5(1), 19 10.1186/2040-2392-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmann M, Bolte S, & Poustka F (2007). Autism spectrum disorders: sex differences in autistic behaviour domains and coexisting psychopathology. Developmental Medicine and Child Neurology, 49(5), 361–366. 10.1111/j.1469-8749.2007.00361.x. [DOI] [PubMed] [Google Scholar]
- Hull L, Mandy W, Lai M-C, Baron-Cohen S, Allison C, Smith P, et al. (2019). Development and validation of the camouflaging autistic traits questionnaire (CAT-Q). Journal of Autism and Developmental Disorders, 49(3), 819–833. 10.1007/s10803-018-3792-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull L, Mandy W, & Petrides K (2017a). Behavioural and cognitive sex/gender differences in autism spectrum condition and typically developing males and females. Autism, 21(6), 706–727. 10.1177/1362361316669087. [DOI] [PubMed] [Google Scholar]
- Hull L, Petrides KV, Allison C, Smith P, Baron-Cohen S, Lai M-C, et al. (2017b). “Putting on My Best Normal”: social camouflaging in adults with autism spectrum conditions. Journal of Autism and Developmental Disorders, 47(8), 2519–2534. 10.1007/s10803-017-3166-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkovski M, Enticott PG, & Fitzgerald PB (2013). A review of the role of female gender in autism spectrum disorders. Journal of Autism and Developmental Disorders, 45(11), 2584–2603. 10.1007/s10803-013-1811-1. [DOI] [PubMed] [Google Scholar]
- Kreiser NL, & White SW (2014). ASD in females: Are we overstating the gender difference in diagnosis? Clinical Child and Family Psychology Review, 17(1), 67–84. 10.1007/s10567-013-0148-9. [DOI] [PubMed] [Google Scholar]
- Lai M-C, Lombardo MV, Auyeung B, Chakrabarti B, & Baron-Cohen S (2015). Sex/gender differences and autism: setting the scene for future research. Journal of the American Academy of Child and Adolescent Psychiatry, 54(1), 11–24. 10.1016/j.jaac.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M-C, Lombardo MV, Pasco G, Ruigrok ANV, Wheel-wright SJ, Sadek SA, … Baron-Cohen S. (2011). A behavioral comparison of male and female adults with high functioning autism spectrum conditions. PLoS ONE, 6(6), e20835. 10.1371/journal.pone.0020835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M-C, Lombardo MV, Ruigrok AN, Chakrabarti B, Auyeung B, Szatmari P, … MRC AIMS Consortium. (2017). Quantifying and exploring camouflaging in men and women with autism. Autism, 21(6), 690–702. 10.1177/1362361316671012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, … Rutter M. (2000). The autism diagnostic observation schedule—generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders, 50(3), 205–223. [PubMed] [Google Scholar]
- Lord Catherine, Rutter M, DiLavore PC, Risi S, Gotham K, & Bishop S. (2012). Autism diagnostic observation schedule (2nd ed.). Torrance: Western Psychological Services. [Google Scholar]
- Lord C, Rutter M, & Le Couteur A (1994). Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24(5), 659–685. [DOI] [PubMed] [Google Scholar]
- Mandy W, Chilvers R, Chowdhury U, Salter G, Seigal A, & Skuse D (2012). Sex differences in autism spectrum disorder: Evidence from a large sample of children and adolescents. Journal of Autism and Developmental Disorders, 42(7), 1304–1313. 10.1007/s10803-011-1356-0. [DOI] [PubMed] [Google Scholar]
- Onnela J-P, & Rauch SL (2016). Harnessing smartphone-based digital phenotyping to enhance behavioral and mental health. Neuropsychopharmacology, 41(7), 1691–1696. 10.1038/npp.2016.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisula E, Kawa R, Szostakiewicz Ł, Łucka I, Kawa M, & Rynkiewicz A (2013). Autistic traits in male and female students and individuals with high functioning autism spectrum disorders measured by the polish version of the autism-spectrum quotient. PLoS ONE, 8(9), e75236. 10.1371/journal.pone.0075236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritvo RA, Ritvo ER, Guthrie D, Ritvo MJ, Hufnagel DH, McMahon W, … Attwood T. (2011). The Ritvo Autism Asperger Diagnostic Scale-Revised (RAADS-R): a scale to assist the diagnosis of autism spectrum disorder in adults: an international validation study. Journal of Autism and Developmental Disorders, 41(8), 1076–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roid GH (2003). Stanford-Binet intelligence scales. Itasca: Riverside. [Google Scholar]
- Rynkiewicz A, Schuller B, Marchi E, Piana S, Camurri A, Lassalle A, & Baron-Cohen S (2016). An investigation of the ‘female camouflage effect’ in autism using a computerized ADOS-2 and a test of sex/gender differences. Molecular Autism, 7(1). 10.1186/s13229-016-0073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabachnick BG, & Fidell LS (2007). Using multivariate statistics (5th ed.). Needham Heights: Allyn & Bacon. [Google Scholar]
- Tillmann J, Ashwood K, Absoud M, Bolte S, Bonnet-Brilhault F, Buitelaar JK, … Charman T. (2018). Evaluating sex and age differences in ADI-R and ADOS scores in a large European multisite sample of individuals with autism spectrum disorder. Journal of Autism and Developmental Disorders, 48(7), 2490–2505. 10.1007/s10803-018-3510-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner SM, Beidel DC, Dancu CV, & Stanley MA (1989). An empirically derived inventory to measure social fears and anxiety: The Social Phobia and Anxiety Inventory. Psychological Assessment: A Journal of Consulting and Clinical Psychology, 1(1), 35. [Google Scholar]
- Van Wijngaarden-Cremers PJM, van Eeten E, Groen WB, Van Deurzen PA, Oosterling IJ, & Van der Gaag RJ (2014). Gender and age differences in the core triad of impairments in autism spectrum disorders: A systematic review and meta-analysis. Journal of Autism and Developmental Disorders, 44(3), 627–635. 10.1007/s10803-013-1913-9. [DOI] [PubMed] [Google Scholar]
- Werling DM, & Geschwind DH (2013). Sex differences in autism spectrum disorders. Current Opinion in Neurology, 26(2), 146–153. 10.1097/WCO.0b013e32835ee548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CE, Murphy CM, McAlonan G, Robertson DM, Spain D, Hayward H, … Murphy DG. (2016). Does sex influence the diagnostic evaluation of autism spectrum disorder in adults? Autism, 20(7), 808–819. 10.1177/1362361315611381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiskerke J, Stern H, & Igelstrom K (2018). Camouflaging of repetitive movements in autistic female and transgender adults. https://doi.org/10.110¼12619. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


