Abstract
Introduction:
The purpose of this study is to use real world evidence on treatment use to evaluate drug superiority within the same treatment group.
Methods:
Retrospective cohort analysis using the Spanish Database for Pharmacoepidemiological Research in Primary Care (BIFAP). Data includes longitudinal routine clinical data extracted from practice records of 7,890,485 patients. All subjects with an incident diagnosis of COPD in the database BIFAP between January 1 2010 and December 31 2012 were included in the cohort study. Cox regression analysis was performed to compare the hazard of COPD exacerbation outcome of the four principal cohorts (no therapy, monotherapy, double therapies with and without corticoids, and triple therapy) and within each principal cohort between the different treatment combinations.
Results:
27,739 patients with COPD were included in the analysis. The median age was 64 years, male proportion was 69% and 70% were smokers. 58,042.9 person--years of follow-up were obtained for the cohort with a mean follow-up of 2.09 years per subject. The strongest factor associated with an increased risk of exacerbation was suffering an exacerbation the previous year (HR = 1.82[1.76--1.87 95%CI]). No differences were found between the most frequent monotherapies, double therapies without corticoid, or triple therapy. When comparing the different combinations of double therapies with corticoid, salmeterol/fluticasone combination (HR = 1.16[1.08--1.24]) revealed a higher adjusted hazard of exacerbation when compared with formoterol/budesonide.
Conclusions:
Treatment with a combination of budesonide/formoterol was associated with lower exacerbations than the treatment with fluticasone/salmeterol. The analysis did not reveal any differences in terms of exacerbation in monotherapy, double therapy without corticoids, and triple therapy combinations.
Keywords: Chronic obstructive pulmonary disease, exacerbations, inhaled corticosteroids, inhaled therapy, long-acting β2-agonist, long-acting muscarinic antagonist, observational study, primary care
Introduction
Chronic obstructive pulmonary disease (COPD) is a progressive, chronic lung disease that is characterized by airflow limitation. COPD currently has no cure, so health efforts are focused on reducing chronic symptoms of cough, excessive sputum production, and dysnea.[1,2] COPD treatment aims to improve life quality and reduce exacerbations, since recurrent exacerbations are associated with rapid decline in lung function, life quality decrease, increase in hospitalization, and increase of death.[3]
Maintenance COPD therapy is based on inhaled long-acting bronchodilator therapy, including long-acting muscarinic antagonist (LAMA), long-acting beta2 agonist (LABA), and inhaled corticoids (ICS), as single therapy or in combination. Practice guidelines treatment algorithms describe different scenarios and propose different treatment options, with no drug or drug combination preference. The reviews of the studies that compare the different treatment options within the same group have shown differences among them. Costa-Scharplatz et al. concluded that glycopyrronium was more cost-effective than tiotropium.[4] A recent Italian study revealed that the fixed combination budesonide/formoterol offered better health results than fluticasone/salmeteraol.[5] The studies that compared LAMA/LABA fixed combination show that indacaterol/glycopyrronium is superior to umeclidinium/vilanterol in exacerbation prevention and life quality improvement,[6] but Kerwin et al.[7] did not reveal clinical differences between them; Feldman et al.[8] revealed that umeclidinium/vilanterol was superior to tiotropium/olodaterol improving respiration function tests. The use of real-world evidence on treatment for patients with COPD is valuable to discover the real benefits of the different treatment options. The Spanish Database for Pharmacoepidemiological Research in Primary Care (BIFAP http://bifap.aemps.es/)[9] database contains anonymised research--quality data from patients being treated by approximately 4,910 Family Physicians and 842 Primary Care Pediatricians in Spain. The data includes longitudinal routine clinical data extracted from practice records of 7,890,485 patients. The purpose of this study is to use real-world evidence on COPD treatment use to evaluate drug superiority within the same treatment group, measured as a decrease in the hazard of COPD exacerbations.
Methods
Information from BIFAP database was used to run this cohort study. In BIFAP database, all the prescriptions are associated with the ICPC code (that can be a condition or a symptom). All subjects with an incident diagnosis of COPD between January 1 2010 and December 31 2012 were included in the cohort study. Subjects with a diagnosis of COPD prior to the study period or patients with a history of cancer were excluded. Incident diagnosis of cancer was censored.
Subjects were then followed until death, change to another healthcare system not included in BIFAP or the end of the study period fixed on December 31, 2016.
Criteria to define COPD exacerbation in this study was: (1) presence of a prescription of any of the oral antibiotics commonly used in a COPD exacerbation in Spain associated with respiratory disease or symptom or (2) the presence of a new prescription or change in the dosage of oral corticoids, again associated at the time with respiratory disease diagnosis or symptom.
A washout period of 30 days was used to consider a new exacerbation episode, so any new antibiotic or change in corticoid therapy initiated on the 30 days following days after a exacerbation were considered to be from the same episode.
In detail, COPD exacerbation was defined as a new prescription of amoxicilin (J01CA04), amoxicilin/clavulanate (J01CR02), moxifloxacin (J01MA14), levofloxacin (J01MA12), ciprofloxacin (J01MA02), cefditoren (J01DD16), cefuroxim (J01DC02), telitromicin (J01FA15), azitromicin (J01FA10), claritromicin (J01FA09), eritromicin (J01FA01), or a new prescription predinsone (H02AB07), dexametasone (H02AB02), or an increase of at least 50% in the daily dosage of these corticoids. Only prescriptions associated in the clinical record with a diagnosis or symptom of respiratory disease were considered.
The different treatments received by subjects for COPD were used to build the different cohorts, taking into account that a subject could change his treatment throughout the time (changing exposure cohorts). The treatment included as exposures were: salbutamol R03AC02, R03CC02, terbutaline R03AC03, R03CC03, salmeterol R03AC12, formoterol R03AC13, indacaterol R03AC18, olodaterol R03AC19, bambuterol R03CC12, salmeterol/fluticasone R03AK06, formoterol/budesonide R03AK07, formoterol/beclomethasone R03AK08, vilanterol/fluticasone R03AK10, formoterol/fluticasone R03AK11, salbutamol/beclomethasone R03AK13, salbutamol/ipratropium R03AL02, vilanterol/umeclidinium R03AL03, indacaterol/glicopirronium R03AL04, formoterol/aclidinium R03AL05, olodaterol/tiotropium R03AL06, beclomethasone R03BA01, budesonide R03BA02, fluticasone R03BA05, mometasone R03BA07, ipratropium R03BB01, tiotropium R03BB04, aclidinium R03BB05, glicopirronium R03BB06, and umeclidinium R03BB07.
Adherence treatment was calculated by analyzing the duration of each prescription registered in an electronic prescription system. To estimate the duration of the treatment, the unit dosage, amount of units of the drug presentation, and the dosage described in the prescription were used. Stock piling was considered to fill the potential gaps between prescriptions. A gap of more than 7 days was considered as discontinuation of the treatment.
The different treatment combinations including those periods where the subjects did not receive any therapy were grouped in five general categories to allow comparison within different levels of treatment: no therapy, monotherapy (b2 agonist or anticholinergic), double therapy without corticoid (b2 agonist + anticholinergic), double therapy with corticoid (corticoid + b2 agonist or anticholinergic), and triple therapy (b2 agonist + anticholinergic + corticoid).
Within each of these principal cohorts, except for the no therapy group, a subcohort was created for each treatment combination that was represented by at least 10% of the person--years of that level. The rest were grouped as “other combination” in each of the four principal cohorts.
Age, sex, history at the moment of inclusion in the cohort of diabetes mellitus, hepatic cirrhosis, chronic kidney disease, ischemic heart disease, hypertension, dyslipidemia, stroke, heart failure, smoke and obstructive sleep apnea syndrome were included as covariables for adjustment. The commorbidities covariables were updated at the beginning of each change in the COPD therapy. The number of COPD exacerbations in the previous year and the number of COPD therapy changes in the previous years were also calculated at the beginning of each COPD therapy period.
Statistical analysis
Cox regression analysis was performed to compare the hazard of COPD exacerbation outcome of the four principal cohorts (no therapy, monotherapy, double therapies with and without corticoids, and triple therapy) and within each principal cohort between the different treatment combinations. Hazard ratio was adjusted by age, sex, history at the moment of inclusion in the cohort of diabetes mellitus, hepatic cirrhosis, chronic kidney disease, ischemic heart disease, hypertension, dyslipidemia, stroke, heart failure, smoke, obstructive sleep apnea syndrome, number of previous COPD exacerbations during the previous year, and number of COPD therapy changes during the previous year.
Results
A total of 27,739 subjects diagnosed with COPD met the eligibility criteria and were included in the analysis. The summary subject characteristics are provided in Table 1. The median age was 64 years, the proportion of males was 69% and most subjects were smokers (70%). A total of 58,042.9 person--years of follow-up were obtained for the cohort with a mean follow-up of 2.09 years per subject. The follow-up was divided in periods, and each time a patient had an exacerbation or a change in its treatment a new period was created. A total of 138,131 free of exacerbation periods were created with a mean duration of 153 days (SD = 244) and a median of 64 days. Figure 1 shows the survival curve for COPD exacerbation (as defined in this study) for the whole cohort. Figure 2 shows the survival curve for COPD exacerbation for each of the five principal cohorts: no therapy, monotherapy, double therapy without corticoid, double therapy with corticoid, and triple therapy.
Table 1.
Basal characteristics of 27,739 patients with a diagnosis of COPD included in the analysis
| Variable | Proportion |
|---|---|
| Men | 69.4% |
| Age (years)1 | 63.98 (SD=9.94) |
| Smoker2 | 70.53% |
| Dyslipidemia | 46.96% |
| Hypertension | 41.56% |
| Diabetes | 16.99% |
| Ischemic heart disease | 8.06% |
| Hepatopathy | 5.26% |
| Stroke | 3.03% |
| Heart failure | 3.14% |
| Obstructive sleep apnea syndrome | 3.06% |
| Chronic kidney disease | 2.02% |
1Mean and standard deviation. 2Proportion over those patients with information about tobacco use. In 31.6% of the patients this information was missing
Figure 1.
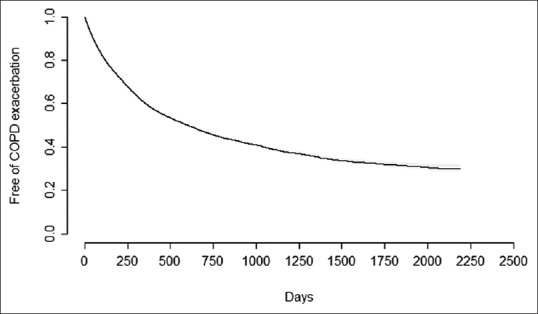
Survival curve for COPD exacerbation for a total of 138,131 periods analyzed over 27,739 patients
Figure 2.
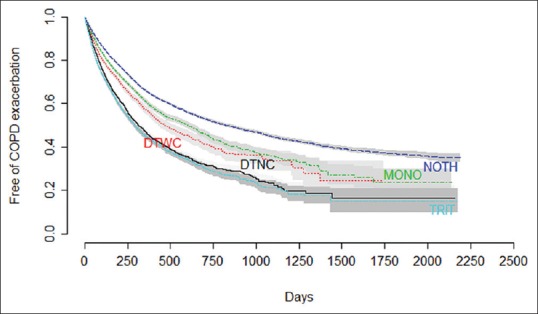
Unadjusted survival curves for COPD exacerbation for a total of 138,131 periods grouped by type of treatment analyzed over 27,739 patients. NOTH: No therapy, MONO: Monotherapy, DTNC: Double therapy without corticoid, DTWC: Double therapy with corticoid, TRIT: Triple therapy
Cox regression grouped by principal cohorts is detailed in Table 2. The strongest factor associated with an increased risk of exacerbation is having other exacerbation in the previous year (HR = 1.82 [1.76--1.87 95% CI]) for a previous exacerbation and HR = 4.86 [4.65--5.09] for five or more exacerbations in the previous year. Other basal characteristics associated with a higher risk of exacerbation were age (HR = 1.07 [1.02--1.13] for subjects over 70 years old compared with 50 years old), ischemic heart disease (HR = 1.08 [1.04--1.13]), diabetes (HR = 1.05 [1.03--1.13]), and dyslipidemia (HR = 1.03 [1.01--1.08]). Hepatic cirrhosis, chronic kidney disease, hypertension, stroke, heart failure, and obstructive sleep apnea syndrome did not show association with risk of exacerbation in this study. Being a smoker was associated with a lower risk of exacerbation (HR = 0.85 [0.83--0.88]; 3 or more changes in the treatment was also associated with a lower risk of exacerbation (HR = 0.93 [0.89--0.97]). The hazards of exacerbation between the principal levels of treatments revealed that the “no therapy group” showed the lowest adjusted risk of exacerbation and the higher the intensity of treatment was, the higher the hazard of exacerbation was observed including monotherapy (HR = 1.24 [1.20--1.28]), double therapy without corticoid (HR = 1.40 [1.34--1.47]), double therapy with corticoid (HR = 1.64 [1.58--1.69]), and triple therapy (HR = 1.75 [1.69--1.81]).
Table 2.
Cox regression model for hazard of exacerbation over 138,131 periods of follow-up over 27,739 patients with a total of 29,417 COPD exacerbations identified. Adjusted for stroke, heart failure, obstructive sleep apnea syndrome, chronic kidney disease, chronic hepatopathy, and hypertension
| HR | CI 95% | P | |
|---|---|---|---|
| Sex | |||
| Woman | 1.00 | ||
| Men | 0.81 | 0.80--0.84 | <.001 |
| Age | |||
| 40--49 years | 1.00 | ||
| 50--59 years | 1.02 | 0.97--1.07 | 0.48 |
| 60--69 years | 1.05 | 0.99--1.10 | 0.066 |
| 70--79 years | 1.07 | 1.02--1.13 | 0.01 |
| Medical History | |||
| Diabetes | 1.05 | 1.02--1.08 | 0.002 |
| Dyslipidemia | 1.03 | 1.01--1.08 | 0.01 |
| Ischemic heart disease | 1.08 | 1.04--1.13 | < 0.001 |
| Smoker | 0.85 | 0.83--0.88 | < 0.001 |
| Smoking status missing | 0.90 | 0.87--0.93 | < 0.001 |
| Exacerbation in the previous year | |||
| None | 1.00 | ||
| One | 1.82 | 1.76--1.87 | < 0.001 |
| Two | 2.39 | 2.30--2.49 | < 0.001 |
| Three | 3.10 | 2.96--3.24 | < 0.001 |
| Four | 3.44 | 3.25--3.64 | < 0.001 |
| Five or more | 4.86 | 4.65--5.09 | < 0.001 |
| Changes in the COPD treatment in the previous year | |||
| None | 1.00 | ||
| One | 1.00 | 0.97--1.04 | 0.83 |
| Two | 0.97 | 0.93--1.01 | 0.059 |
| Three | 0.93 | 0.89--0.97 | 0.001 |
| Four | 0.87 | 0.83--0.92 | < 0.001 |
| Five or more | 0.78 | 0.75--0.81 | < 0.001 |
| Type of treatment | |||
| No treatment | 1.00 | ||
| Monotherapy | 1.24 | 1.20--1.28 | < 0.001 |
| Double therapies without corticoid | 1.40 | 1.34--1.47 | < 0.001 |
| Double therapies with corticoid | 1.64 | 1.58--1.69 | < 0.001 |
| Triple therapy | 1.75 | 1.69--1.81 | < 0.001 |
The comparison between the different monotherapies is detailed in Table 3. No differences were found between the most frequent monotherapies: indacaterol, tiotropio, and aclidinium. The group of other therapies that included treatments with less than a 10% of presence in the cohort revealed a higher hazard of exacerbation when compared with indacaterol (HR = 1.24 [1.12--1.36]).
Table 3.
Cox model for hazard of exacerbation over 30,082 periods of follow-up under monotherapy with 4,622 COPD exacerbations identified. Adjusted for age, sex, number of exacerbations during the previous year, changes in treatment during the previous year, diabetes, dyslipidemia, ischemic heart disease, obstructive sleep apnea syndrome, chronic kidney disease, chronic hepatopathy and hypertension
| HR | CI 95% | P | |
|---|---|---|---|
| Monotherapy | |||
| Indacaterol | 1.00 | ||
| Tiotropium | 0.92 | 0.84--1.01 | 0.093 |
| Aclidinium | 1.02 | 0.91--1.15 | 0.729 |
| Other monotherapies | 1.24 | 1.12--1.36 | <.001 |
Double therapy without corticoid is detailed in Table 4 and with corticoid is detailed in Table 5. No differences between indacaterol + glicopirronio and indacaterol + tiotropio were revealed (HR = 1.12 [0.98--1.27]. When comparing the different combinations of double therapies with corticoid, salmeterol + fluticasone combination (HR = 1.16 [1.08--1.24]) revealed a higher adjusted hazard of exacerbation when compared with formoterol + budesonide.
Table 4.
Cox model for hazard of exacerbation over 11,664 periods of follow-up under double therapy without corticoid with 2,065 COPD exacerbations identified. Adjusted for age, sex, number of exacerbations during the previous year, changes in treatment during the previous year, diabetes, dyslipidemia, ischemic heart disease, obstructive sleep apnea syndrome, chronic kidney disease, chronic hepatopathy and hypertension
| HR | CI 95% | P | |
|---|---|---|---|
| Double therapy without corticoid | |||
| Indacaterol/glicopirronium | 1.00 | ||
| Indacaterol/tiotropium | 1.12 | 0.98--1.27 | 0.10 |
| Other double therapies without corticoid | 1.33 | 1.19--1.49 | 0.73 |
Table 5.
Cox model for hazard of exacerbation over 23,473 periods of follow-up under a double therapy with corticoid treatment with 4,633 COPD exacerbations identified. Adjusted for age, sex, number of exacerbations during the previous year, changes in treatment during the previous year, diabetes, dyslipidemia, ischemic heart disease, obstructive sleep apnea syndrome, chronic kidney disease, chronic hepatopathy and hypertension
| HR | CI 95% | P | |
|---|---|---|---|
| Double therapy with corticoid | |||
| Formoterol/Budesonide | 1.00 | ||
| Salmeterol/Fluticasone | 1.16 | 1.08--1.24 | <.001 |
| Other double therapy with corticoid | 1.31 | 1.22--1.41 | <.001 |
Results for triple therapy is described in Table 6, formoterol + budesonide + tiotropium and salmeterol + fluticasone + tiotropium did not show differences between them in terms of exacerbation adjusted hazard (HR = 1.16 [0.97--1.13]). When analyzed as a group, the rest of triple therapies showed a higher adjusted hazard of exacerbation (HR = 1.27 [1.18-1.36]) when compared with formoterol + budesonide + tiotropium combination.
Table 6.
Cox model for hazard of exacerbation over 21,459 periods of follow-up under a triple therapy with 5,123 COPD exacerbations identified. Adjusted for age, sex, number of exacerbations during the previous year, changes in treatment during the previous year, diabetes, dyslipidemia, ischemic heart disease, obstructive sleep apnea syndrome, chronic kidney disease, chronic hepatopathy and hypertension
| HR | CI 95% | P | |
|---|---|---|---|
| Triple Therapy | |||
| Formoterol/Budesonida + Tiotropium | 1.00 | ||
| Salmeterol/Fluticasone + Tiotropium | 1.05 | 0.97--1.14 | 0.22 |
| Other triple therapies | 1.27 | 1.18--1.36 | <.001 |
Discussion
This is a “real-world” retrospective cohort study of COPD treatments, where subjects and physicians are not controlled, revealing daily practice. In this study, the median age and sex was similar to other studies of similar databases. The high proportion of smokers (70%) is remarkable considering that in most COPD studies it is around 50%. When analyzing the factors associated with an increased risk of exacerbation, surprisingly being smoker was associated with a lower risk of exacerbation (HR = 0.85 [0.83--0.88]); this finding could be due to a severity bias being that more severe cases of COPD have a greater perception of disease and as a consequence have a higher probability of quitting smoking. The distinction between never-smoker and ex-smokers was not feasible due to quality of registry limitations of the database; in fact a 31% of subjects had a missing value regarding smoking status. During our analysis, we treated those missing smoking status as a separate category given the unexpected nature of the results.
Another interesting finding is the decrease in the risk of exacerbation associated with an increase in the number of changes in the pharmacologic treatment of the COPD during the previous year. This protective effect is significant from 3 or more recent changes in the treatment (HR = 0.93 [0.89--0.97]) and is stronger as more treatment changes occur. This finding could suggest that in those subjects where the treatment is adjusted more frequently subjects are better controlled than those with the same characteristics but without this treatment adjustment.
The comparisons between the hazards of exacerbation between the principal levels of treatments to our understanding suggest a potential indication bias; the no therapy group showed the lowest adjusted risk of exacerbation and the increase in therapy intensity was associated with an increase in the hazard of exacerbation.
When analyzing single inhaled therapy, no difference in COPD control was found between the most frequent monotherapies: indacaterol, tiotropium, and aclidinium. Our finding is contrary to what Decreamer et al.[10] and Price et al.[11] conclude, where indacaterol proved to give a better COPD control over tiotropium and contrary to what Volgelmeir et al.[12] concluded, where tiotropium and salmeterol also revealed differences in COPD control.[12] Our findings are consistent with current guidelines of first step treatment LAMA or LABA with no preference and choosing among the patient and physician preference.[1,2]
Double therapy without corticoid (LAMA + LABA) revealed no differences between indacaterol/glicopirronium and indacaterol/tiotropium; recent Cochrane review revealed that indacaterol/glicopirronium was superior to umeclidinium/vilanterol,[6] but these findings were not supported by Kerwin et al.,[7] although in this study the dosage of indacaterol/glicopirronum was not the standard 24-h lasting COPD treatment. Umeclidinium/vilanterol did prove to have better improvement of respiratory function tests when compared with tiotropium/olodaterol in the study done by Feldman et al.[8]
Budesonide/formoterol was associated with fewer COPD exacerbations than fluticasone/salmeterol, which is consistent with the findings from Perrone et al.[5] in national Italian database and in contrast with the previous double therapy with corticoid research studies, where no differences could be proven among the different combinations.[13,14,15,16,17]
In terms of triple therapy, combination of formoterol/budesonide/tiotropium was not superior to salmeterol/fluticasone/tiotropium. No triple therapy combination comparison research was found to compare with our findings. TRILOGY study[18] concluded that beclometasona/formoterol/glicopirronium was better than double therapy with corticoid beclometasone/formoterol, TRINITY study[19] concluded that it was also better than LAMA monotherapy with glicopirronium, TRIBUTE study[20] concluded that it was better than double therapy without corticoid indacateraol/glycopyrronium, further research also concluded that furoate/umeclidinium/vilanterol was better than double therapy with corticoid budesonide/formoterol[21] and fluticasone furoate/vilanterol[22] or double therapy without corticoid umeclidinium--vilanterol.[22] These studies compared single and double therapy against triple therapy, proving triple therapy to offer better control, in the line of associating agents proposed by the guidelines in noncontrolled patients[1,2] and by recent studies towards long-acting triple therapy.[23,24,25]
There are some limitations in our study derived from the nature of the data sources used in the analysis. The use of the BIFAP database is only based on the medical data registered by family physicians, so specialized intervention could be incompletely recorded in some cases. Analysis was based on administrative data, so a COPD diagnosis could not be verified beyond the codification performed by clinicians during their activity. Also, no spirometry registry was included in the analysis, which could impact on the diagnosis, classification, and severity of COPD patients. Certain underregistration of COPD diagnosis is expected and as a result the results are not a good indicator of COPD incidence in the population. We do feel that the strong point of our research and the database is the use of “real-world” uncontrolled data based on daily practice.
Conclusion
Our findings show “real-world” data of the COPD population. The most relevant factor related with the risk of exacerbation is the number of exacerbations in the previous year. In our cohort, subjects treated with a combination of budesonide/formoterol showed a lower hazard of COPD exacerbations requiring antibiotics therapy or increases in the corticoid dosage than patients under fluticasone/salmeterol treatment. No differences in terms of COPD exacerbation were found with monotherapy, double therapy without corticoids, and triple therapy. There are not too many studies that compare drugs within the same group and the available ones do not reveal a clear superiority. Further studies are needed to compare the different molecules in order to discover if any drug or combination is better than any other in the same group. Currently, selecting COPD treatment is based on patient and physician preference.
Author contributions
All authors contributed towards data analysis, drafting, and critically revising the paper and agree to be accountable for all aspects of the work.
Data used in this work is part of the database BIFAP managed by Agencia Española de Medicamentos y Productos Sanitarios (AEMPS). The results, discussion, and conclusions of this work are only of the authors and do not represent an official position of the AEMPS.
Ethics Review
The scientific committee of BIFAP granted a positive opinion to the study protocol. The investigators had access to only fully anonymized data, and under this condition, no specific ethics review was required according to Spanish law.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
Authors would like to acknowledge the excellent collaboration of general practitioners and pediatricians and the support of regional governments taking part in BIFAP.
References
- 1.Global initiative for chronic Obstructive Lung Disease (GOLD) [homepage on the Internet] Global Strategy for the Diagnosis, Management and Prevention of COPD [2019 REPORT] [Last accessed on 2019 May 26]. Available from: http://www.goldcopd.org/
- 2.Miravitlles M, Soler-Cataluña JJ, Calle M, Molina J, Almagro P, Quintano JA, et al. Spanish Guidelines for Management of Chronic Obstructive Pulmonary Disease (GesEPOC) 2017. Pharmacological treatment of stable phase. Arch Bronconeumol. 2017;53:324–35. doi: 10.1016/j.arbres.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 3.Duffy SP, Criner GJ. Chronic obstructive pulmonary disease: Evaluation and management. Med Clin North Am. 2019;103:453–61. doi: 10.1016/j.mcna.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Costa-Scharplatz M, Ställberg B, Goyal P, Asukai Y, Gruenberger JB, Price D. Cost-effectiveness of glycopyrronium bromide compared with tiotropium in patients with chronic obstructive pulmonary disease in Sweden. Appl Health Econ Health Policy. 2015;13:637–45. doi: 10.1007/s40258-015-0193-2. [DOI] [PubMed] [Google Scholar]
- 5.Perrone V, Sangiorgi D, Buda S, Degli Esposti L. Comparative analysis of budesonide/formoterol and fluticasone/salmeterol combinations in COPD patients: Findings from a real-world analysis in an Italian setting. Int J Chron Obstruct Pulmon Dis. 2016;11:2749–55. doi: 10.2147/COPD.S114554. eCollection 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horita N, Goto A, Shibata Y, Ota E, Nakashima K, Nagai K, et al. Long-acting muscarinic antagonist (LAMA) plus long-acting beta-agonist (LABA) versus LABA plus inhaled corticosteroid (ICS) for stable chronic obstructive pulmonary disease (COPD) Cochrane Database of Systematic Reviews. 2017;2:CD012066. doi: 10.1002/14651858.CD012066.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kerwin E, Ferguson GT, Sanjar S, Goodin T, Yadao A, Fogel R, et al. Dual bronchodilation with indacaterol maleate/glycopyrronium bromide compared with umeclidinium bromide/vilanterol in patients with moderate-to-severe COPD: Results from two randomized, controlled, cross-over studies. Lung. 2017;195:739–47. doi: 10.1007/s00408-017-0055-9. [DOI] [PubMed] [Google Scholar]
- 8.Feldman GJ, Sousa AR, Lipson DA, Tombs L, Barnes N, Riley JH, et al. Comparative efficacy of once-daily umeclidinium/vilanterol and tiotropium/olodaterol therapy in symptomatic chronic obstructive pulmonary disease: A randomized study. Adv Ther. 2017;34:2518–33. doi: 10.1007/s12325-017-0626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salvador Rosa A, Moreno Pérez JC, Sonego D, García Rodríguez LA, de Abajo Iglesias FJ. The BIFAP project: Database for pharmaco-epidemiological research in primary care. Aten Primaria. 2002;30:655–61. doi: 10.1016/S0212-6567(02)79129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Decramer ML, Chapman KR, Dahl R, Frith P, Devouassoux G, Fritscher C, et al. Once-daily indacaterol versus tiotropium for patients with severe chronic obstructive pulmonary disease (INVIGORATE): A randomised, blinded, parallel-group study. Lancet Respir Med. 2013;1:524–33. doi: 10.1016/S2213-2600(13)70158-9. [DOI] [PubMed] [Google Scholar]
- 11.Price D, Asukai Y, Ananthapavan J, Malcolm B, Radwan A, Keyzor I. A UK-based cost-utility analysis of indacaterol, a once-daily maintenance bronchodilator for patients with COPD, using real world evidence on resource use. Appl Health Econ Health Policy. 2013;11:259–74. doi: 10.1007/s40258-013-0021-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vogelmeier CF, Asijee GM, Kupas K, Beeh KM. Tiotropium and salmeterol in COPD patients at risk of exacerbations: A post hoc analysis from POET-COPD(®) Adv Ther. 2015;32:537–47. doi: 10.1007/s12325-015-0216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yip E, Karimi S, Pien LT. Evaluation of a therapeutic interchange from fluticasone/salmeterol to mometasone/formoterol in patients with chronic obstructive pulmonary disease. J Manag Care Spec Pharm. 2016;22:316–23. doi: 10.18553/jmcp.2016.22.4.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kern DM, Davis J, Williams SA, Tunceli O, Wu B, Hollis S, et al. Comparative effectiveness of budesonide/formoterol combination and fluticasone/salmeterol combination among chronic obstructive pulmonary disease patients new to controller treatment: A US administrative claims database study. Respir Res. 2015;16:52. doi: 10.1186/s12931-015-0210-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Postma DS, Roche N, Colice G, Israel E, Martin RJ, van Aalderen WM, et al. Comparing the effectiveness of small-particle versus large-particle inhaled corticosteroid in COPD. Int J Chron Obstruct Pulmon Dis. 2014;17(9):1163–86. doi: 10.2147/COPD.S68289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohar JA, Crater GD, Emmett A, Ferro TJ, Morris AN, Raphiou I, et al. Fluticasone propionate/salmeterol 250/50 μg versus salmeterol 50 μg after chronic obstructive pulmonary disease exacerbation. Respir Res. 2014;15:105. doi: 10.1186/s12931-014-0105-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larsson K, Janson C, Lisspers K, Jørgensen L, Stratelis G, Telg G, et al. Combination of budesonide/formoterol more effective than fluticasone/salmeterol in preventing exacerbations in chronic obstructive pulmonary disease: The PATHOS study. J Intern Med. 2013;273:584–94. doi: 10.1111/joim.12067. [DOI] [PubMed] [Google Scholar]
- 18.Singh D, Papi A, Corradi M, Pavlišová I, Montagna I, Francisco C, et al. Single inhaler triple therapy versus inhaled corticosteroid plus long-acting β2-agonist therapy for chronic obstructive pulmonary disease (TRILOGY): A double-blind, parallel group, randomised controlled trial. Lancet. 2016;388:963–73. doi: 10.1016/S0140-6736(16)31354-X. [DOI] [PubMed] [Google Scholar]
- 19.Vestbo J, Papi A, Corradi M, Blazhko V, Montagna I, Francisco C, et al. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): A double-blind, parallel group, randomised controlled trial. Lancet. 2017;389:1919–29. doi: 10.1016/S0140-6736(17)30188-5. [DOI] [PubMed] [Google Scholar]
- 20.Papi A, Vestbo J, Fabbri L, Corradi M, Prunier H, Cohuet G, et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): A double-blind, parallel group, randomised controlled trial. Lancet. 2018;391:1076–84. doi: 10.1016/S0140-6736(18)30206-X. [DOI] [PubMed] [Google Scholar]
- 21.Bremner PR, Birk R, Brealey N, Ismaila AS, Zhu CQ, Lipson DA. Single-inhaler fluticasone furoate/umeclidinium/vilanterol versus fluticasone furoate/vilanterol plus umeclidinium using two inhalers for chronic obstructive pulmonary disease: A randomized non-inferiority study. Respir Res. 2018;19:19. doi: 10.1186/s12931-018-0724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lipson DA, Barnhart F, Brealey N, Brooks J, Criner GJ, Day NC, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378:1671–80. doi: 10.1056/NEJMoa1713901. [DOI] [PubMed] [Google Scholar]
- 23.Alcázar-Navarrete B, Castellano Miñán F, Romero Palacios PJ. The future of triple therapy in chronic obstructive pulmonary disease. Arch Bronconeumol. 2018;54:63–4. doi: 10.1016/j.arbres.2017.06.019. [DOI] [PubMed] [Google Scholar]
- 24.Vetrano DL, Zucchelli A, Bianchini E, Cricelli C, Piraino A, Zibellini M, et al. Triple inhaled therapy in COPD patients: Determinants of prescription in primary care. Respir Med. 2019;154:12–7. doi: 10.1016/j.rmed.2019.05.022. [DOI] [PubMed] [Google Scholar]
- 25.Papi A, Petruzzelli S, Vezzoli S, Georges G, Fabbri LM. Triple therapy for all patients with severe symptomatic COPD at risk of exacerbations. Eur Respir J. 2019;53 doi: 10.1183/13993003.00147-2019. pii: 1900147. [DOI] [PubMed] [Google Scholar]


