Abstract
Data suggest psychedelics such as psilocybin and lysergic acid diethylamide (LSD) may hold therapeutic potential in the treatment of addictions, including tobacco dependence. This retrospective cross-sectional anonymous online survey characterized 358 individuals (52 females) who reported having quit or reduced smoking after ingesting a psychedelic in a non-laboratory setting ≥1 year ago. On average, participants smoked 14 cigarettes/day for 8 years, and had 5 previous quit attempts before their psychedelic experience. Of the 358 participants, 38% reported continuous smoking cessation after psychedelic use (quitters). Among quitters, 74% reported >2 years abstinence. Of the 358 participants, 28% reported a persisting reduction in smoking (reducers), from a mode of 300 cigarettes/month before, to a mode of 1 cigarette/month after the experience. Among reducers, 62% reported >2 years of reduced smoking. Finally, 34% of the 358 participants (relapsers) reported temporary smoking reduction before returning to baseline smoking levels, with a mode time range to relapse of 3–6 months. Relapsers rated their psychedelic experience significantly lower in personal meaning and spiritual significance than both other groups. Participants across all groups reported less severe affective withdrawal symptoms (e.g. depression, craving) after psychedelic use compared with previous quit attempts, suggesting a potential mechanism of action for psychedelic-associated smoking cessation/reduction. Changes in life priorities/values were endorsed as the most important psychological factor associated with smoking cessation/reduction. Results suggest psychedelics may hold promise in treating tobacco addiction as potentially mediated by spiritual experience, changed priorities/values, and improved emotional regulation.
Keywords: hallucinogen, tobacco, smoking cessation, nicotine, addiction, psilocybin, psychedelic, mystical experience
Introduction
Converging evidence suggests that structured administration of serotonin 2A receptor (5-HT2AR) agonist hallucinogens (i.e. psychedelics) may be an effective aid in the treatment of addiction (Bogenschutz et al., 2015; Garcia-Romeu et al., 2016; Johnson et al., 2014; Ross, 2012; Sessa and Johnson, 2015). Observational studies have reported addiction recovery associated with the ceremonial use of 5-HT2AR agonists such as peyote and ayahuasca among indigenous cultures and syncretic religions (Albaugh and Anderson, 1974; Bergman, 1971; Blum et al., 1977; Calabrese, 1997; de Rios et al., 2002; Garrity, 2000; Halpern, 1996; Pascarosa and Futterman, 1976; Prue, 2013; Roy, 1973; Thomas et al., 2013). A meta-analysis of randomized clinical trials from the 1960s and 1970s also found that structured administration of lysergic acid diethylamide (LSD) in the treatment of alcoholism resulted in significantly less alcohol misuse than randomized control conditions at initial follow-up, with a robust effect size (odds ratio: 1.96; Krebs and Johansen, 2012).
Research with psilocybin in healthy volunteers also shows effects that may be consistent with therapeutic potential for addiction treatment, including positive behavior change as assessed by blinded community observers (Griffiths et al., 2006, 2011), increased personality openness (MacLean et al., 2011), and high ratings of psychedelic session personal meaning and spiritual significance present 14 months (Griffiths et al., 2008), and 25 years (Doblin, 1991; Pahnke, 1963) after psilocybin administration. Moreover, neuroscientific studies have discovered biological effects of psychedelics, including alterations in default mode network activation and changes in amygdala and anterior cingulate cortex reactivity to negative cues (Carhart-Harris et al., 2012, 2016; Kraehenmann et al., 2014; Palhano-Fontes et al., 2015; Petri et al., 2014; Preller et al., 2016), and it is plausible that these may mediate acute and persisting beneficial effects of psychedelics.
The authors recently conducted an open-label pilot-study suggesting that the 5-HT2AR agonist psilocybin may be a safe and promising adjunct to cognitive-behavioral smoking cessation treatment, with 80% of participants (N=15) demonstrating biologically verified smoking abstinence at 6-month follow-up (Johnson et al., 2014). The lack of control condition and open-label study design prohibit definitive conclusions about efficacy. However, these success rates substantially exceed those for the most efficacious accepted smoking cessation treatments (typically <35% abstinence rates at 6 months; Cahill et al., 2014; Mottillo et al., 2009).
No reports in the scientific literature have described tobacco smoking cessation resulting from non-clinical use of 5-HT2AR agonists, although anecdotal evidence suggests that such accounts are not uncommon. Clinical psychologist Leo Zeff described how spontaneous reflections about tobacco smoking during an LSD experience in the 1960s prompted his own subsequent life-long smoking abstinence (Stolaroff, 2004). Similar reports attributing smoking cessation to psilocybin or other psychedelic use have appeared on websites related to psychoactive substances such as Erowid (http://www.erowid.org).
These anecdotal reports of smoking cessation or reduction attributed to use of psychedelics in non-treatment contexts complement clinical studies that suggest psychedelics have anti-addiction effects. Therefore, to inform the clinical application of psychedelics in treating addiction, the present survey study characterized reports of smoking cessation or reduction attributed to psychedelic use among psychedelic users. The study examined factors associated with longer durations of abstinence, sustained abstinence vs. relapse, and cessation vs. reduction. We hypothesized that more personally meaningful and spiritually significant experiences would be associated with greater smoking cessation success. Online surveys have been used successfully in the past to identify trends in the natural ecology of drug use that would have otherwise been unknown from clinical laboratory research (McCambridge et al., 2005; Winstock et al., 2011, 2014). Although such a survey by itself cannot directly address the potential causal role of psychedelics in smoking cessation, it can complement laboratory research, for example, by identifying conditions for which psychedelic use is associated with persisting tobacco smoking cessation.
Method
This anonymous (i.e. no name or IP address recorded) survey was conducted online via SurveyMonkey (http://www.surveymonkey.com) from September 2013 to May 2014. Recruitment advertisements which included a link to the survey were distributed via social media (http://www.facebook.com, http://www.reddit.com), and on websites visited by individuals interested in psychedelics, including Erowid, Shroomery (http://www.shroomery.org), and the Multidisciplinary Association for Psychedelic Studies website (http://www.maps.org). Recruitment materials solicited individuals who had, “quit or reduced smoking after a psychedelic experience.” The stated goal of the survey was “to learn more information about whether psychedelic drugs are associated with reduction or cessation of cigarette smoking… [and] to characterize people’s experiences in non-laboratory settings in which taking a psychedelic may have led to reducing or quitting smoking.”
The survey took approximately 40 minutes to complete. Only individuals who reported quitting or reducing cigarette smoking, even temporarily, after the use of the following 5-HT2AR agonist psychedelics were included: psilocybin (“magic”) mushrooms, LSD, morning glory seeds, mescaline, peyote cactus, San Pedro cactus, dimethyltryptamine (DMT), and ayahuasca. Other inclusion criteria were that participants be at least 18 years of age, and were able to speak, read, and write English fluently. Participants received no compensation for their response. This study was approved by the Institutional Review Board of the Johns Hopkins University School of Medicine, and all participants provided informed consent by choosing to complete the survey after the presentation of introductory information.
Measures.
The full survey is available online (Supporting Information). Demographic information, current non-psychedelic drug use, and lifetime psychedelic use were collected. The survey assessed participants’ previous smoking behavior, including number of cigarettes smoked per day (CPD) before the psychedelic experience to which they attributed their subsequent smoking cessation or reduction (hereafter referred to as “reference psychedelic experience”). Questions also assessed previous serious quit attempts (≥ 1 day) and associated withdrawal symptoms. Participants indicated categorically whether they were totally abstinent from smoking since their reference psychedelic experience, had a persisting reduction in their smoking since the reference psychedelic experience, or had a temporary reduction in their smoking after the reference psychedelic experience that later ended in relapse. Those who endorsed a persisting reduction in smoking since the reference psychedelic experience were asked to characterize their current smoking rate as falling into one of these four categories: ≤ 1 cig./month, ≤ 1 cig./week, ≤ 1 cig./day, or ≥ 1 cig./day.
Participants provided detailed information regarding their reference psychedelic experience. This included data on the drug used, the setting in which the experience took place, the intention for taking the drug, and the incidence of any adverse effects, or other behavioral changes attributed to this psychedelic experience. Participants were asked whether or not they found their reference psychedelic experience to be personally meaningful and spiritual or mystical in nature, and to retrospectively rate the personal meaning and spiritual significance of their reference psychedelic experience. Participants were also asked to endorse potential mechanisms of change attributed to their psychedelic-associated smoking cessation or reduction. Additional measures described below were used to probe relationships between constructs hypothesized to affect smoking cessation outcomes or the nature of the reference psychedelic experience. A series of hypothetical decision-making assessments were also conducted, which are not reported here.
Fagerström Test for Cigarette Dependence (FTCD).
The FTCD is a 6-item questionnaire widely used to characterize the level of dependence of cigarette smokers (Heatherton et al., 1991; Fagerström, 2012). The FTCD was completed retrospectively, with items referring to smoking behavior in the 6-month period prior to the reference psychedelic experience (e.g. “In the 6 months prior to your psychedelic-occasioned smoking cessation or reduction, how soon after waking up did you want to smoke your first cigarette?”).
Questionnaire on Smoking Urges (QSU).
The QSU is a 32-item multidimensional assessment of smoking craving with demonstrated sensitivity to smoking cessation (Tiffany and Drobes, 1991). Participants were asked to complete the QSU twice, once retrospectively using a modified version of the assessment (e.g. “In the 6 months prior to my psychedelic-occasioned smoking cessation or reduction, my desire to smoke would have seemed overpowering at a time such as now”), and once using the standard questionnaire with items in the present tense (e.g. “My desire to smoke seems overpowering”). Although the QSU has not been psychometrically validated for retrospective use in this manner, our intention was to examine QSU data for evidence of within-subjects changes in smoking craving following an individual’s reference psychedelic experience.
Toronto Alexithymia Scale (TAS-20).
The TAS-20 is a 20-item assessment of alexithymia, a personality trait associated with difficulty identifying and describing one’s internal emotional state (Bagby et al., 1994). Individuals scoring higher on the TAS-20 have shown greater tobacco craving during a smoking cessation attempt (Sutherland et al., 2013). Therefore, we hypothesized that individuals high in alexithymia may exhibit less success in maintaining long-term abstinence.
Mystical Experience Questionnaire (MEQ30).
The MEQ30 is a 30-item measure designed to assess the occurrence and intensity of mystical-type experiences occasioned by 5-HT2AR agonists (Barrett et al., 2015; MacLean et al., 2012). Previous research has found associations between mystical-type effects of psilocybin and increased personality openness in healthy volunteers (MacLean et al., 2011), as well as sustained smoking abstinence in individuals undergoing psilocybin-facilitated smoking cessation treatment (Garcia-Romeu et al., 2015). Thus we hypothesized that individuals who scored higher on the MEQ30 would be more successful in maintaining long-term abstinence. The MEQ30 consists of four dimensions: unity, noetic quality, and sacredness (Factor 1), positive mood (Factor 2), transcendence of space/time (Factor 3), and ineffability (Factor 4). The MEQ30 was completed with respect to the reference psychedelic experience.
Tellegen Absorption Scale (TAS).
The TAS is a 34-item measure of absorption, a personality trait linked to suggestibility and general predisposition to altered states of consciousness (Glisky et al., 1991; Tellegen and Atkinson, 1974). Given that higher scores on the TAS have been shown to predict pleasant and mystical-type experiences during psilocybin administration in the laboratory (Studerus et al., 2012), we predicted that participants scoring high on trait absorption would also score higher on the MEQ30 and subsequently report greater rates of long-term smoking abstinence.
Data Analysis.
Participants were categorized into three groups according to smoking cessation outcome: Quit (smoking abstinence since the reference psychedelic experience), Reduce (persisting reduction in smoking since the reference psychedelic experience), and Relapse (temporary reduction in smoking after the reference psychedelic experience, but ultimately resumed smoking at baseline rate). Data from continuous and interval outcome measures (e.g. age, FTCD score) were assessed for normality of distribution using D’Agostino-Pearson omnibus tests (D’Agostino et al., 1990). For normally distributed data, one-way analysis of variance (ANOVA) was performed to assess between-group differences. For non-normally distributed data, Kruskal-Wallis tests by ranks were performed to assess between-group differences. Chi-square analyses were used to test for differences between groups in categorical variables (e.g. sex, intention to quit). Categories with few participants were combined with related categories for chi-square analyses to ensure test validity (e.g., morning glory, mescaline, peyote, San Pedro were classified as “other”). Post hoc pairwise comparisons using the Tukey method for normally distributed data, or Dunn multiple comparison method for non-normally distributed data were used to examine ANOVAs and Kruskal-Wallis tests reaching statistical significance. Finally, a Spearman rank correlation analysis was conducted to assess the relationship between mystical experience as measured by the MEQ30, and trait absorption as assessed by the TAS. This relationship was examined because previous research indicated that individuals higher in trait absorption were more likely to have mystical-type experiences when administered psilocybin in a laboratory setting (Studerus et al., 2012). Due to the exploratory nature of these analyses, no corrections were made for multiple comparisons. Statistical analyses were conducted using GraphPad Prism 6.05 for Mac (GraphPad Software, Inc., La Jolla, CA).
Results
Participant information.
A total of 1273 individuals completed the survey. Among the 1273 individuals, individuals were removed from data analysis for a variety of reasons. A group of 163 individuals were removed because they reported smoking cessation or reduction following use of a non-serotonergic drug (e.g. ketamine). Another 329 individuals were removed because they provided inconsistent data (e.g. endorsed total smoking abstinence since the experience, but also indicated smoking in the 7 days prior to taking the survey). Another 423 individuals whose reference psychedelic experience occurred less than 12 months before taking the survey were removed in order for analyses to focus on long-term outcomes of psychedelic-associated smoking cessation and reduction. Thus, the final study sample consisted of 358 participants. Table 1 contains participant demographic and smoking-related information.
Table 1.
Participant demographic and smoking-related information.
| Whole sample (N = 358) |
Quit group (n = 137) |
Reduce group (n = 100) |
Relapse group (n = 121) |
|||
|---|---|---|---|---|---|---|
| Sex: | n (%) | n (%) | n (%) | n (%) | Test statistic | P |
| Male | 306 (85.5) | 115 (83.9) | 89 (89.0) | 102 (84.3) | χ2=1.40 | 0.50 |
| Female | 52 (14.5) | 22 (16.1) | 11 (11.0) | 19 (15.7) | ||
| Race: | n (%) | n (%) | n (%) | n (%) | ||
| White | 307 (85.8) | 116 (84.7) | 82 (82.0) | 109 (90.1) | χ2=4.68 | 0.32 |
| American Indian / Alaska Native | 6 (1.7) | 4 (2.9) | 1 (1.0) | 1 (0.8) | ||
| Asian | 6 (1.7) | 3 (2.2) | 2 (2.0) | 1 (0.8) | ||
| Mixed | 26 (7.3) | 9 (6.6) | 11 (11.0) | 6 (5.0) | ||
| Other | 13 (3.7) | 5 (3.6) | 4 (4.0) | 4 (3.3) | ||
| Education: | n (%) | n (%) | n (%) | n (%) | ||
| Some high school or less | 14 (3.9) | 4 (2.9) | 4 (4.0) | 6 (5.0) | χ2=6.41 | 0.78 |
| High school or equivalent | 40 (11.2) | 14 (10.2) | 10 (10.0) | 16 (13.2) | ||
| Some college | 153 (42.7) | 60 (43.8) | 41 (41.0) | 52 (43.0) | ||
| College degree | 73 (20.4) | 27 (19.7) | 22 (22.0) | 24 (19.8) | ||
| Some grad./professional school | 34 (9.5) | 12 (8.8) | 14 (14.0) | 8 (6.6) | ||
| Graduate/professional degree | 44 (12.3) | 20 (14.6) | 9 (9.0) | 15 (12.4) | ||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Test statistic | P | |
| Age (years) | 31.1 (11.2) | 31.2 (10.9) | 30.2 (10.5) | 31.7 (12.0) | H=0.55 | 0.76 |
| Age started smoking | 15.6 (3.2) | 15.5 (3.4) | 15.4 (2.9) | 15.8 (3.3) | H=0.38 | 0.83 |
| Years smoking | 7.7 (7.1) | 8.2 (7.9) | 7.2 (5.8) | 7.7 (7.0) | H=0.15 | 0.93 |
| Cigs./day before psychedelic exp. | 14.0 (9.6) | 13.9 (10.1) a,b | 12.8 (9.1) a | 15.2 (9.3) b | H=7.21 | 0.03 |
| Previous quit attempts | 4.9 (5.3) | 4.3 (4.6) | 5.2 (5.5) | 5.3 (5.7) | H=1.62 | 0.45 |
| FTCD (smoking dependence) | 3.7 (2.4) | 3.7 (2.4) | 3.3 (2.5) | 4.0 (2.4) | H=4.72 | 0.09 |
| QSU (craving) pre-quit/reduction | 111.6 (26.1) | 109.3 (27.6) | 114.2 (24.6) | 111.9 (25.4) | F=1.05 | 0.35 |
| QSU (craving) post-quit/reduction | 87.5 (37.3) | 61.7 (17.4)a | 87.5 (31.5)b | 116.8 (36.8)c | H=149 | <0.001 |
Note: Between-groups chi-square analyses are shown for sex, race, and education. For all other categories, between-groups one-way ANOVA analyses or Kruskal-Wallis tests are shown. For all analyses, significant p values (p < 0.05) are shown in bold. For variables with significant main effects, group values not sharing a common letter are significantly different based on Tukey-corrected pairwise comparison or Dunn’s multiple comparisons. FTCD = Fagerström Test for Cigarette Dependence. QSU = Questionnaire on Smoking Urges.
The majority of participants (65.1%, n = 233) learned about the survey through advertisements on www.erowid.org, with the remainder being referred via www.shroomery.org (8.4%, n = 30), www.facebook.com (4.2%, n = 15), other unspecified sources (19.0%, n = 68), and related websites (e.g., www.maps.org, n < 10). Participants resided in 27 different countries, with most coming from the United States (69.6%, n = 249), Canada (7.5%, n = 27), and the United Kingdom (7.0%, n = 25). Table 2 presents data on lifetime psychedelic drug use. Psilocybin mushrooms (95.3%) and LSD (88.8%) were the most commonly used psychedelics, with a mode of 2–5 lifetime uses (i.e. separate occasions) among individuals who had used these substances.
Table 2.
Participants’ self-reported total number of lifetime uses of serotonergic psychedelics (N = 358).
| Lifetime use | Psilocybin mushrooms, n (%) | LSD, n (%) | morning glory seeds, n (%) | mescaline (pc), n (%) | peyote cactus, n (%) | San Pedro cactus, n (%) | DMT (pc), n (%) | Ayahuasca, n (%) |
|---|---|---|---|---|---|---|---|---|
| Never | 17 (4.7) | 40 (11.2) | 217 (60.6) | 288 (80.4) | 307 (85.8) | 285 (79.6) | 210 (58.7) | 295 (82.4) |
| 1 occasion | 25 (7.0) | 23 (6.4) | 61 (17.0) | 34 (9.5) | 23 (6.4) | 26 (7.3) | 38 (10.6) | 29 (8.1) |
| 2 5 separate occasions | 87 (24.3) | 83 (23.2) | 57 (15.9) | 19 (5.3) | 20 (5.6) | 29 (8.1) | 47 (13.1) | 17 (4.7) |
| 6 – 10 separate occasions | 66 (18.4) | 51 (14.2) | 11 (3.1) | 4 (1.1) | 1 (0.3) | 8 (2.2) | 25 (7.0) | 6 (1.7) |
| 11 – 20 separate occasions | 69 (19.3) | 50 (14.0) | 9 (2.5) | 2 (0.6) | 5 (1.4) | 6 (1.7) | 15(4.2) | 4 (1.1) |
| 21 – 50 separate occasions | 53 (14.8) | 39 (10.9) | 2 (0.6) | 2 (0.6) | 0 (0) | 2 (0.6) | 13 (3.6) | 5 (1.4) |
| 51 – 100 separate occasions | 22 (6.1) | 36 (10.1) | 1 (0.3) | 5 (1.4) | 1 (0.3) | 2 (0.6) | 5 (1.4) | 1 (0.3) |
| More than 100 separate occasions | 19 (5.3) | 36 (10.1) | 0 (0) | 4 (1.1) | 1 (0.3) | 0 (0) | 5 (1.4) | 1 (0.3) |
Note: Modal responses in bold. pc = Pure compound. LSD = Lysergic acid diethylamide. DMT = Dimethyltryptamine.
Smoking outcomes and group comparisons.
Figure 1 shows duration of long-term abstinence or reduction in the Quit and Reduce groups. One hundred thirty-seven participants (38.3%; Quit group) reported complete smoking cessation after their reference psychedelic experience, with 102 of the 137 (74.5%) reporting >2 years abstinence. One hundred participants (27.9%; Reduce group) reported a persisting reduction in smoking, from a mode of 300 cigarettes/month before (n = 20), to a mode of ≤ 1 cigarette/month after the experience (n = 44), with 62 of the 100 (62%) reporting >2 years reduction in smoking. Among the Reduce group, 44 participants reported current smoking ≤ 1 cig./month, 19 reported smoking ≤ 1 cig./week, 14 reported smoking ≤ 1 cig./day, and 23 reported smoking ≥ 1 cig./day. Finally, 121 individuals (33.8%; Relapse group) reported temporary reduction in their smoking before returning to baseline smoking levels, with a mode of 3–6 months to relapse (n = 21). Only 30 of the 358 respondents (8.4%) reported going into their reference psychedelic experience with a premeditated intention to quit or reduce smoking (Table 3).
Figure 1.
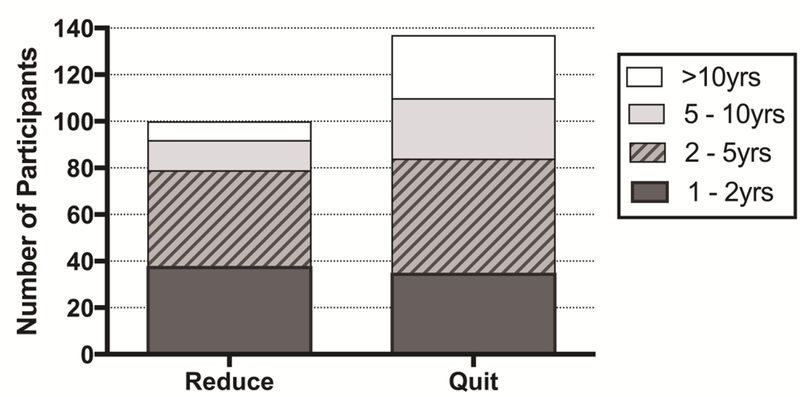
Distribution of participants reporting persisting smoking reduction (Reduce group) by duration of reduction is shown on the left. Distribution of participants reporting ongoing smoking abstinence (Quit group) by duration of abstinence is shown on the right.
Table 3.
Participant questionnaire data and psychedelic-related information.
| Whole sample (N = 358) |
Quit group (n = 137) |
Reduce group (n = 100) |
Relapse group (n = 121) |
|||
|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Test statistic | P | |
| Tellegen Absorption Scale | 23.2 (6.4) | 22.9 (6.8) | 24.1 (5.8) | 23.0 (6.3) | H=1.52 | 0.47 |
| Toronto Alexithymia Scale | 48.4 (12.2) | 46.1 (11.9) a | 49.0 (11.7) a,b | 50.4 (12.4) b | H=10.32 | 0.006 |
| Mystical Experience Questionnaire (MEQ30) | 72.8 (19.0) | 74.4 (19.9) | 74.5 (16.7) | 69.5 (19.6) | H=5.58 | 0.06 |
| Personal Meaning of psychedelic experience | 5.7 (1.6) | 5.9 (1.6) a | 5.7 (1.6) a,b | 5.4 (1.6) b | H=6.29 | 0.04 |
| Spiritual Significance of psychedelic experience | 3.1 (1.3) | 3.2 (1.4) a | 3.3 (1.1) a | 2.8 (1.4) b | H=10.89 | 0.004 |
| Age of psychedelic experience | 23.3 (7.2) | 24.1 (7.9) a | 23.7 (6.3) a | 22.0 (6.8) b | H=9.95 | 0.007 |
| Confidence to abstain from smoking | 76.3 (31.5) | 97.5 (5.4) a | 70.9 (28.9) b | 56.1 (36.4) b | H=147.1 | <0.001 |
| Premeditated intention to quit: | n (%) | n (%) | n (%) | n (%) | χ2=4.13 | 0.13 |
| Yes | 30 (8.4) | 12 (8.8) | 4 (4.0) | 14 (11.6) | ||
| No | 328 (91.6) | 125 (91.2) | 96 (96.0) | 107 (88.4) | ||
| Drug associated with smoking cessation/reduction: | n (%) | n (%) | n (%) | n (%) | χ2=8.09 | 0.09 |
| Psilocybin | 157 (43.9) | 64 (46.7) | 47 (47.0) | 46 (38.0) | ||
| Lysergic acid diethylamide (LSD) | 157 (43.9) | 57 (41.6) | 36 (36.0) | 64 (52.9) | ||
| Dimethyltryptamine (DMT) | 14 (3.9) | 7 (5.1) | 6 (6.0) | 1 (0.8) | ||
| Ayahuasca | 17 (4.7) | 8 (5.8) | 4 (4.0) | 5 (4.1) | ||
| Other (morning glory, mescaline, peyote, San Pedro) | 13 (3.6) | 1 (0.7) | 7 (7.0) | 5 (4.1) | ||
| Where did this experience take place? | n (%) | n (%) | n (%) | n (%) | ||
| At home | 218 (60.9) | 88 (64.2) | 51 (51.0) | 79 (65.3) | χ2=5.73 | 0.06 |
| At a party | 33 (9.2) | 13 (9.5) | 6 (6.0) | 14 (11.6) | χ2=2.05 | 0.36 |
| In a public place (e.g. shopping mall, movie theater) | 38 (10.6) | 15 (10.9) | 10 (10.0) | 13 (10.7) | χ2=0.06 | 0.97 |
| At a concert or festival | 26 (7.3) | 9 (6.6) | 11 (11.0) | 6 (5.0) | χ2=3.13 | 0.21 |
| Outdoors in nature | 145 (40.5) | 48 (35.0) | 41 (41.0) | 56 (46.3) | χ2=3.39 | 0.18 |
| In a religious / spiritual setting (e.g. ritual ceremony) | 18 (5.0) | 7 (5.1) | 4 (4.0) | 7 (5.8) | χ2=0.37 | 0.83 |
| Intention for taking the psychedelic? | n (%) | n (%) | n (%) | n (%) | ||
| Because other people were, but no serious intention | 17 (4.7) | 8 (5.8) | 3 (3.0) | 6 (5.0) | χ2=1.05 | 0.59 |
| Curiosity without any other serious intention | 62 (17.3) | 22 (16.1) | 15 (15.0) | 25 (20.7) | χ2=1.47 | 0.48 |
| Recreational | 205 (57.3) | 77 (56.2) | 60 (60.0) | 68 (56.2) | χ2=0.42 | 0.81 |
| A serious intention for psychological self-exploration | 187 (52.2) | 66 (48.2) | 58 (58.0) | 63 (52.1) | χ2=2.24 | 0.33 |
| A serious intention to explore spirituality / the sacred | 137 (38.3) | 49 (35.8) | 40 (40.0) | 48 (39.7) | χ2=0.59 | 0.74 |
| Other behavioral changes after this psychedelic session? | n (%) | n (%) | n (%) | n (%) | ||
| Reduced or quit drinking alcohol | 152 (42.5) | 57 (41.6) | 45 (45.0) | 50 (41.3) | χ2=0.37 | 0.83 |
| Reduced or quit other drugs | 100 (27.9) | 37 (27.0) | 33 (33.0) | 30 (24.8) | χ2=1.93 | 0.38 |
| Negative effects from this psychedelic session? | n (%) | n (%) | n (%) | n (%) | χ2=3.33 | 0.50 |
| Yes (typically acute anxiety or physical discomfort) | 43 (12) | 17 (12.4) | 16 (16.0) | 10 (8.3) | ||
| No | 281 (78.5) | 107 (78.1) | 74 (74.0) | 100 (82.6) | ||
| Not sure | 34 (9.5) | 13 (9.5) | 10 (10.0) | 11 (9.1) |
Note: Between-groups Kruskal-Wallis tests are shown for absorption, alexithymia, mystical experience, personal meaning, spiritual significance, age of psychedelic experience, and confidence to abstain. For all other categories, between-groups chi-square analyses are shown. For items with mutually exclusive responses, modal responses are shown in bold. For all analyses, significant p values (p < 0.05) are shown in bold.
Values not sharing a common letter are significantly different based on Dunn’s multiple comparisons.
DMT: dimethyltryptamine; LSD: lysergic acid diethylamide; MEQ30: Mystical Experience Questionnaire; TAS: Tellegen Absorption Scale; TAS-20: Toronto Alexithymia Scale.
Demographic data are presented in Table 1. There were no significant between-group differences in demographic variables including age, sex distribution, racial composition, educational achievement, prevalence of self-reported psychiatric diagnoses, number of previous quit attempts, years smoking, or retrospective cigarette dependence scores. Kruskal-Wallis tests found significant between-groups differences in alexithymia scores (p = 0.006), cigarettes per day prior to the reference psychedelic experience (p = 0.03), confidence to abstain from smoking (p < 0.001), and current craving for tobacco (p < 0.001; Table 3). Dunn’s multiple comparisons found that individuals in the Relapse group scored significantly higher on alexithymia than those in the Quit group. Participants in the Relapse group smoked significantly more cigarettes per day prior to the reference psychedelic experience than those in the Reduce group (Table 1). Confidence to abstain from smoking was significantly higher in the Quit than Reduce and Relapse groups. Additionally, current tobacco craving scores on the QSU showed significant between-groups differences, with the Relapse group reporting greater craving at the time of the survey than the Quit and Reduce groups (Figure 2), and the Reduce group scoring significantly higher than the Quit group.
Figure 2.
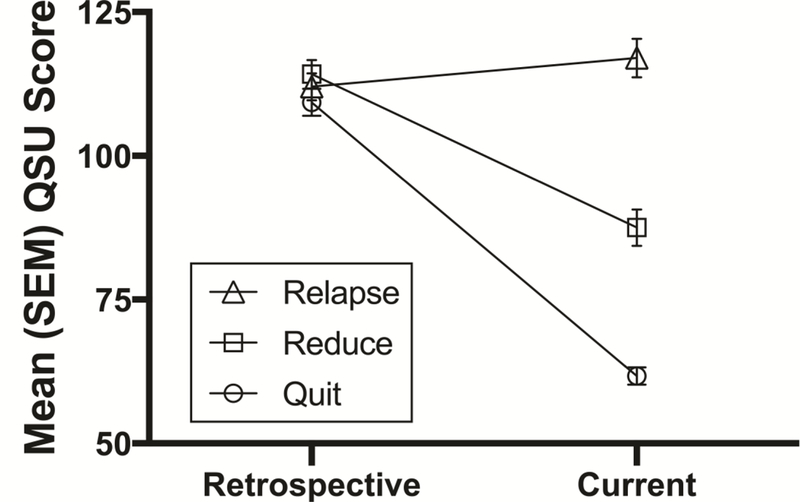
Group differences in smoking craving as assessed by the Questionnaire on Smoking Urges (QSU) from pre- to post-reference psychedelic experience. Retrospective ratings showed no significant differences among the groups. Current ratings showed significant difference among all three of the groups (p < 0.001).
No significant differences were observed with regard to lifetime hallucinogen use (between-groups data not shown). Furthermore, there were no significant between-group differences in the substance associated with the reference psychedelic experience, intention for the experience, or setting in which the experience took place (Table 3). However, there was a significant between-groups difference in the age at which the reference psychedelic experience occurred (p = 0.007). Dunn’s multiple comparisons found participants in the Relapse group were significantly younger when their reference psychedelic experience occurred than those in the Quit and Reduce groups, with a mean age of 22 at the time of the reference psychedelic experience for those in the Relapse group as compared to a mean age of 24.1 in the Quit group, and 23.7 in the Reduce group at the time of the reference psychedelic experience. Additionally, chi-square analyses found significant between-groups differences in duration of longest prior quit (χ2=34.9, p = 0.004), with 53% of longest previous quit attempts lasting 2 weeks or less in the Quit group, compared to 37% in the Reduce, and 28% in the Relapse group (data not shown).
Withdrawal symptoms.
Table 4 shows participant ratings of withdrawal symptoms after their psychedelic-associated smoking cessation or reduction in comparison to previous quit attempts. The majority of withdrawal symptoms (e.g. weight gain, headaches, insomnia) were rated as similar in severity to previous quit attempts according to mode responses. Conversely, affective features of withdrawal (i.e. restlessness, depression, irritability, craving) were largely rated “much less severe” after the reference psychedelic experience in comparison to other quit attempts. Individuals reporting greater severity of withdrawal symptoms after their reference psychedelic experience compared to previous quit attempts were in the minority (n ≤ 16). Chi-square analyses found no significant differences between groups in reported severity of withdrawal symptoms (data not shown).
Table 4.
Withdrawal severity after psychedelic-associated smoking cessation or reduction in comparison to previous quit attempts. Modal responses shown in bold.
| Withdrawal Symptoms’ Severity (n)a | Much less severe, n (%) | Less severe, n (%) | Same, n (%) | More severe, n (%) | Much more severe, n (%) |
|---|---|---|---|---|---|
| Weight gain (258) | 77 (29.8) | 49 (19.0) | 121 (46.9) | 8 (3.1) | 3 (1.2) |
| Increased eating (269) | 71 (26.4) | 61 (22.7) | 121 (45.0) | 14 (5.2) | 2 (0.7) |
| Digestive problems (242) | 69 (28.5) | 40 (16.5) | 127 (52.5) | 6 (2.5) | 0 (0) |
| Nausea (240) | 74 (30.8) | 41 (17.1) | 118 (49.2) | 7 (2.9) | 0 (0) |
| Headaches (255) | 84 (32.9) | 54 (21.2) | 106 (41.6) | 9 (3.5) | 2 (0.8) |
| Drowsiness (251) | 80 (31.9) | 42 (16.7) | 123 (49.0) | 6 (2.4) | 0 (0) |
| Fatigue (257) | 74 (28.8) | 55 (21.4) | 119 (46.3) | 8 (3.1) | 1 (0.4) |
| Insomnia (257) | 76 (29.6) | 51 (19.8) | 122 (47.5) | 5 (1.9) | 3 (1.2) |
| Heart pounding / sweating (237) | 78 (32.9) | 29 (12.2) | 125 (52.7) | 4 (1.7) | 1 (0.4) |
| Difficulty concentrating (268) | 88 (32.8) | 67 (25.0) | 104 (38.8) | 7 (2.6) | 2 (0.7) |
| Anxiety (268) | 93 (34.7) | 72 (26.9) | 93 (34.7) | 6 (2.2) | 4 (1.5) |
| Restlessness (272) | 92 (33.8) | 76 (27.9) | 90 (33.1) | 7 (2.6) | 7 (2.6) |
| Depression / low mood (271) | 114 (42.1) | 69 (25.5) | 80 (29.5) | 7 (2.6) | 1 (0.4) |
| Irritability (277) | 118 (42.6) | 73 (26.4) | 73 (26.4) | 10 (3.6) | 3 (1.1) |
| Craving tobacco (284) | 133 (46.8) | 89 (31.3) | 52 (18.3) | 4 (1.4) | 6 (2.1) |
Sample size varies by symptom (range = 237–284), as some participants reported no previous quit attempts as a basis for comparison, and others had never experienced particular withdrawal symptoms. Percentages were calculated based on the number of individuals who reported a particular withdrawal symptom.
Mechanisms of change, personal meaning, and spiritual significance.
As shown in Table 5, participants endorsed “changing life priorities or values, such that smoking was no longer more important than quitting” (88.5%, n = 317), “changing your orientation toward the future, so that long-term benefits outweighed immediate desires” (85.2%, n = 305), and “strengthening your belief in your own ability to quit” (79.1%, n = 283) as the most important potential mechanisms leading to quitting or reducing smoking after their reference psychedelic experience.
Table 5.
Smoking cessation or reduction mechanisms attributed to reference psychedelic experience.
| Mechanism a | Endorsed item, n (%) | Ranked most important, n (%) b |
|---|---|---|
| Changing life priorities or values, such that smoking was no longer more important than quitting | 317 (88.5) | 95 (26.5) |
| Strengthening your belief in your own ability to quit. | 283 (79.1) | 65 (18.2) |
| Changing your orientation toward the future, so that long-term benefits outweighed immediate desires. | 305 (85.2) | 60 (16.8) |
| Reducing stress involved with quitting. | 287 (80.2) | 44 (12.3) |
| Reframing quitting as a spiritual task | 252 (70.4) | 44 (12.3) |
| Increasing space between the desire to smoke and taking action. | 281 (78.5) | 21 (5.9) |
Participants were asked to endorse any relevant mechanisms they believed played a role in their psychedelic-associated smoking cessation or reduction. These mechanisms are based on our laboratory research (Johnson et al., 2014).
For each mechanism endorsed, participants were asked to rank order the items in terms of their importance to helping them quit or reduce smoking.
In total, 336 (93.9%) participants characterized their reference psychedelic experience as personally meaningful with 218 (60.1% of entire study sample) considering it among the 10 most personally meaningful experiences of their lives. Additionally, 281 (78.5%) characterized their psychedelic experience as spiritual or mystical in nature (i.e. “a moment of sudden spiritual awakening or insight”), with 161 (45.0% of the entire sample) rating it among the 5 most spiritually significant experiences of their lives (data not shown).
Significant between-groups differences were found in ratings of personal meaning (p = 0.03) and spiritual significance (p = 0.004) of the reference psychedelic experience (Table 3). Participants in the Relapse group rated the reference psychedelic experience as significantly less personally meaningful than those in the Quit group. Individuals in the Relapse group additionally rated their reference psychedelic experience as less spiritually significant than those in both the Quit and Reduce groups. Among the Relapse group 33.9% (n = 41) rated their reference psychedelic experience among the 5 most spiritually significant of their lives as compared to 50% (n = 50) in the Reduce, and 51.1% (n = 70) in the Quit group. No significant differences were observed between groups on Mystical Experience Questionnaire (MEQ30) scores, although a near-significant effect was found for the MEQ30 (p = 0.06), with the Relapse group scoring the lowest. No significant differences were observed between groups on self-reported intentions for the reference psychedelic experience (Table 3). A significant positive correlation was found between MEQ30 and Tellegen Absorption Scale scores (r = 0.44, p < 0.001; Figure 3).
Figure 3.
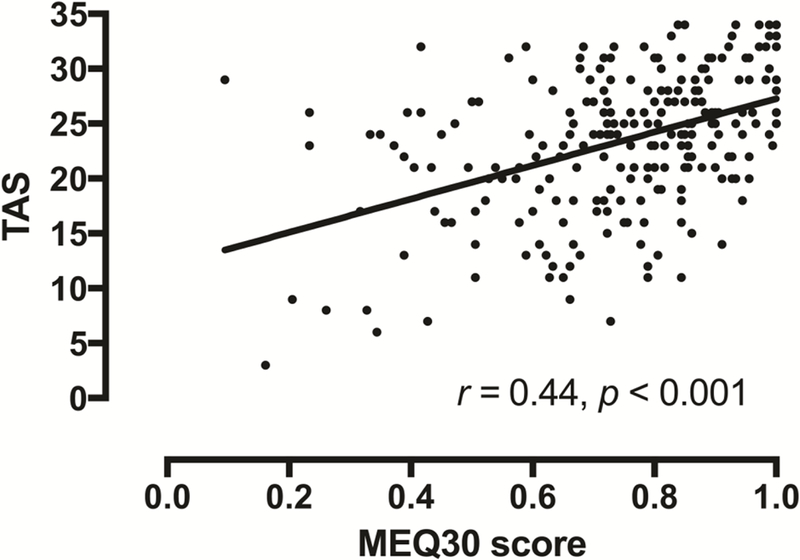
Relationship between Mystical Experience Questionnaire (MEQ30) and Tellegen Absorption Scale scores. Data points show data from each of the 358 individual participants with best-fit linear regression.
Other outcomes attributed to reference psychedelic experience.
In addition to tobacco smoking cessation or reduction, some participants also reported reductions in use of alcohol (42.5%, n = 152), and other drugs (27.9%, n = 100) attributed to the reference psychedelic experience, with no significant between-group differences (Table 3). The majority of participants (78.5%, n = 281) reported no negative effects from their reference psychedelic experience, some responded that they were unsure whether the experience had any negative effects (9.5%, n = 34), and others did report negative effects of their experience (12.0%, n = 43). Negative effects were typically described as acute anxiety or dysphoria, as well as feelings of physical discomfort such as gastrointestinal distress during drug effects. Prevalence of negative effects did not differ significantly between groups.
Discussion
This online survey study provides detailed information on a sample of 358 individuals who reported smoking cessation or reduction after using a serotonergic psychedelic in a non-laboratory setting ≥1 year ago. Individual smoking outcomes varied, including total smoking abstinence after the reference psychedelic experience (Quit group), persisting reductions in previous smoking rate (Reduce group), and reductions in regular daily smoking ultimately culminating in relapse to baseline smoking levels (Relapse group). Participants were predominantly young White males who reported using a psychedelic recreationally or for the purposes of psychological exploration, with no premeditated intention to decrease their smoking. However, participants claimed that they experienced a subsequent cessation or reduction in their smoking that they attributed in part to their reference psychedelic experience. These findings are consistent with laboratory data finding that controlled administration of 5-HT2AR agonists may hold therapeutic potential in treating tobacco and other substance use disorders (Bogenschutz et al., 2015; Johnson et al., 2014; Krebs and Johansen, 2012), as well as anthropological reports suggesting that structured (i.e. religious) psychedelic use may confer benefits against addiction (Albaugh and Anderson, 1974; Bergman, 1971; Blum et al., 1977; Calabrese, 1997; de Rios et al., 2002; Garrity, 2000; Halpern, 1996; Pascarosa and Futterman, 1976; Prue, 2013; Roy, 1973). It should be noted though that volunteers who underwent psilocybin-facilitated smoking cessation treatment in a prior laboratory study were purposefully attempting to quit smoking (Johnson et al., 2014), while participants in this survey were largely not intending to reduce their smoking. If psychedelics are capable of prompting smoking cessation or reduction in those not seeking to reduce smoking, potential efficacy may be even more likely in those who are treatment seeking and under therapeutic supervision.
Several group differences highlight potentially relevant clinical factors regarding smoking cessation and relapse. Ratings of personal meaning and spiritual significance of the reference psychedelic experience differed statistically between groups, with individuals who maintained long-term smoking abstinence rating their reference psychedelic experience as more personally meaningful and spiritually significant than those who relapsed to smoking, and those who reported persisting smoking reductions rating their reference psychedelic experience more spiritually significant than those who relapsed. Thus, these results support prior laboratory research demonstrating psychedelics’ potential to occasion highly meaningful and spiritually significant experiences (Griffiths et al., 2006, 2008, 2011; MacLean et al, 2011), and suggest a mediating role for spiritual experience in promoting long-term smoking abstinence (Garcia-Romeu et al., 2015).
Moreover, participants in the Relapse group reported smoking more cigarettes per day on average prior to the reference psychedelic experience than those in the Quit group. Therefore, it is possible that the greater rate of smoking and the younger age at which the reference psychedelic experience occurred in the Relapse group may account for their failure to maintain abstinence/reduction, rather than variance in alexithymia or personal meaning and spiritual significance of the psychedelic experience.
Groups did not differ significantly with respect to demographics (aside from differences in cigarettes smoked per day discussed above), the psychedelic used, setting, or intention for use during the reference psychedelic experience. Although negative effects were reported at similar rates across groups, these were typically confined to the period of acute drug effects, and were predominantly described as either psychological (i.e. dysphoria, anxiety), or physical discomfort (e.g. stomachache, headache). Adverse effects of psychedelics are relatively well documented, and represent risks to the user, especially in uncontrolled settings (Johnson et al, 2012; Ungerleider et al., 1968). Furthermore, illegal drugs acquired on the black-market are rarely verified with regard to substance, purity, and dose, representing an additional serious risk factor (Burrai et al., 2014). Thus, we do not encourage psychedelic use outside of medical or research settings that can provide the appropriate screening, structure, and supervision to ensure safety (Johnson et al., 2008). The majority of participants reported 10 or fewer lifetime uses of the psychedelics examined (Table 2), consistent with the low addictive potential of this drug class (Johnson et al., 2008).
Regarding potential mechanisms of action, decreased affective withdrawal symptoms after psychedelic-associated compared to other, non-psychedelic-associated quit attempts suggest an important role for psychedelics in attenuating low mood and craving, which are known to precipitate relapse (Piper, 2015). Such effects may be related to psychedelics’ serotonergic mechanism of action at the 5-HT2A and possibly 5-HT1A receptors (Bogenshutz and Pommy, 2012; Kraehenmann et al., 2014; Preller et al., 2016; Ross, 2012; Vollenweider and Kometer, 2010).
Participants also reported other behavioral changes as a result of their psychedelic session, with individuals across all three groups reporting reduction or cessation of alcohol (≥41%) or other drug use (≥25%) after their reference psychedelic experience (Table 3). These data support the hypothesis that psychedelic experiences may result in behaviorally plastic states in which habitual or addictive behaviors can be re-evaluated. These findings suggest that psychedelics may provide benefits against a range of substance use disorders, rather than being specific to tobacco per se.
The insights and processes that appear to be prompted by psychedelics, and which may lead to smoking cessation or reduction, may not be unique to psychedelics. For example, abstinence from tobacco and other drugs is sometimes prompted by naturalistic spiritual or insightful experiences (Miller, 2004; Miller & C’de Baca, 2001). Also, survey data indicate that smokers generally consider spirituality to be relevant to smoking cessation (Gonzales et al., 2007). It is our hypothesis that administration of psychedelics under structured conditions may strongly increase the likelihood of motivational insights leading to persisting behavior change such as smoking cessation. Further, we propose that while these motivational insights from psychedelics occur and sometimes prompt people to quit smoking in recreational or non-clinical contexts, such effects are likely to lead to substantially higher probability of persisting behavior change (abstinence) when smoking cessation is the a priori goal of the psychedelic experience, and when combined with effective behavioral therapy (Garcia-Romeu et al., 2015; Johnson et al., 2014).
The results presented here are limited due to participant self-selection, and the retrospective nature of the data, which are subject to recall bias. No definitive conclusions can be drawn about the role of psychedelics in smoking cessation or reduction as data were collected via online survey, and cannot be verified. Additionally, information on the identity and dosage of the psychedelics used by participants cannot be confirmed due to limitations of the online study format.
Although efforts were made to allow only one response per computer per web browser, and to exclude inconsistent and nonsensical responses, it cannot be confirmed whether some individuals responded more than once, though we judge it unlikely due to the length of the survey. The current study design cannot address absolute prevalence or efficacy of psychedelic-associated smoking cessation or reduction, as negative cases were not taken into account (i.e. when psychedelic use did not affect, or exacerbated smoking). An additional design limitation was the omission of an item probing average cigarettes per day intake after the reference psychedelic experience, which made it impossible to quantify the magnitude of daily smoking reduction among the Reduce group. The possibility of Type I error should be noted, as alpha was not adjusted for multiple comparisons given the exploratory nature of the study aims. Moreover, most participants were White, college-educated males, consistent with epidemiological data on global psychedelic use (Winstock et al., 2014), raising the possibility that results may not generalize to other groups. Despite these limitations, our findings, in combination with pilot laboratory results (Garcia-Romeu et al., 2015; Johnson et al., 2014), suggest that psilocybin and other serotonergic psychedelics may hold considerable potential in the treatment of tobacco, and possibly other substance use disorders, and should therefore continue to be examined as a pharmacological aid in the treatment of addiction.
Supplementary Material
Acknowledgements
Grant Glatfelter, BS, Toni White, BA, and Jefferson Mattingly assisted in data collection. Dr. Frederick S. Barrett provided valuable comments on the manuscript. Earth Erowid and Fire Erowid at www.Erowid.org provided key recruitment support for this project.
Funding/Support: The Beckley Foundation provided initial funding for this research, with continued funding provided by Heffter Research Institute. Support for Dr. Garcia-Romeu and Dr. Patrick Johnson was provided by National Institute on Drug Abuse Grant T32DA07209. Support for Dr. Griffiths was provided in part by NIDA Grant R01DA003889.
Financial Disclosure: Dr. Griffiths is on the board of directors of the Heffter Research Institute, Santa Fe, NM, USA.
References:
- Albaugh BJ and Anderson PO (1974). Peyote in the treatment of alcoholism among American Indians. American Journal of Psychiatry, 131(11), 1247–1250. [DOI] [PubMed] [Google Scholar]
- Bagby RM, Parker JD, and Taylor GJ (1994). The 20-item Toronto Alexithymia Scale: 1. Item selection and cross-validation of the factor structure. Journal of Psychosomatic Research, 38(1), 23–32. [DOI] [PubMed] [Google Scholar]
- Barrett FS, Johnson MW, and Griffiths RR (2015). Validation of the revised Mystical Experience Questionnaire in experimental sessions with psilocybin. Journal of Psychopharmacology, 29(11), 1182–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman RL (1971). Navajo peyote use: Its apparent safety. American Journal of Psychiatry, 128(6), 695–699. [DOI] [PubMed] [Google Scholar]
- Blum K, Futterman SFL, and Pascarosa P (1977). Peyote, a potential ethnopharmacologic agent for alcoholism and other drug dependencies: Possible biochemical rationale. Clinical Toxicology, 11(4), 459–472. [DOI] [PubMed] [Google Scholar]
- Bogenschutz MP, Forcehimes AA, Pommy JA, Wilcox CE, Barbosa PCR, and Strassman RJ (2015). Psilocybin-assisted treatment for alcohol dependence: A proof-of-concept study. Journal of Psychopharmacology, 29(3), 289–299. [DOI] [PubMed] [Google Scholar]
- Bogenschutz MP, and Pommy JM (2012). Therapeutic mechanisms of classic hallucinogens in the treatment of addictions: From indirect evidence to testable hypotheses. Drug testing and analysis, 4(7–8), 543–555. [DOI] [PubMed] [Google Scholar]
- Burdick BV and Adinoff B (2013). A proposal to evaluate mechanistic efficacy of hallucinogens in addiction treatment. The American Journal of Drug and Alcohol Abuse, 39(5), 291–297. [DOI] [PubMed] [Google Scholar]
- Burrai L, Nieddu M, Palomba M, Pirisi MA (2014). Identification and quantitation of 4-bromo-2, 5-dimethoxyamphetamine in seized blotters. Legal Medicine, 17(1), 56–59. [DOI] [PubMed] [Google Scholar]
- Cahill K, Stevens S, and Lancaster T (2014). Pharmacological Treatments for Smoking Cessation. JAMA, 311(2), 193–194. [DOI] [PubMed] [Google Scholar]
- Calabrese J (1997). Spiritual healing and human development in the Native American Church: Toward a cultural psychiatry of peyote. Psychoanalytic Review, 84(2), 237–255. [PubMed] [Google Scholar]
- Carhart-Harris RL, Erritzoe D, Williams T, Stone JM, Reed LJ, Colasanti A, ... and Hobden P (2012). Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proceedings of the National Academy of Sciences, 109(6), 2138–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Muthukumaraswamy S, Roseman L, Kaelen M, Droog W, Murphy K, ... and Leech R (2016). Neural correlates of the LSD experience revealed by multimodal neuroimaging. Proceedings of the National Academy of Sciences, 201518377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Agostino RB, Belanger A, and D’Agostino RB Jr (1990). A suggestion for using powerful and informative tests of normality. The American Statistician, 44(4), 316–321. [Google Scholar]
- de Rios MD, Grob CS, and Baker JR (2002). Hallucinogens and redemption. Journal of Psychoactive Drugs, 34(3), 239–248. [DOI] [PubMed] [Google Scholar]
- Doblin R (1991). Pahnke’s Good Friday experiment: a long-term follow-up and methodological critique. The Journal of Transpersonal Psychology, 23(1), 1–28. [Google Scholar]
- Fagerström K (2012). Determinants of tobacco use and renaming the FTND to the Fagerström Test for Cigarette Dependence. Nicotine & Tobacco Research, 14(1), 75–78. [DOI] [PubMed] [Google Scholar]
- Garcia-Romeu A, Griffiths RR, and Johnson MW (2015). Psilocybin-occasioned mystical experiences in the treatment of tobacco addiction. Current Drug Abuse Reviews, 7(3), 157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Romeu A, Kersgaard B and Addy PH (2016). Clinical applications of hallucinogens: A review. Experimental and Clinical Psychopharmacology, 24(4), 229–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrity JF (2000). Jesus, peyote, and the holy people: Alcohol abuse and the ethos of power in Navajo healing. Medical Anthropology Quarterly, 14, 521–542. [DOI] [PubMed] [Google Scholar]
- Glisky ML, Tataryn DJ, Tobias BA, Kihlstrom JF, and McConkey KM (1991). Absorption, openness to experience, and hypnotizability. Journal of Personality and Social Psychology, 60(2), 263–272. [DOI] [PubMed] [Google Scholar]
- Gonzales D, Redtomahawk D, Pizacani B, Bjornson WG, Spradley J, Allen E, & Lees P (2007). Support for spirituality in smoking cessation: results of pilot survey. Nicotine & Tobacco Research, 9(2), 299–303. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Johnson MW, Richards WA, Richards BD, McCann U, and Jesse R (2011). Psilocybin occasioned mystical-type experiences: immediate and persisting dose-related effects. Psychopharmacology (Berl), 218(4), 649–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Richards WA, Johnson MW, McCann U, and Jesse R (2008). Mystical-type experiences occasioned by psilocybin mediate the attribution of personal meaning and spiritual significance 14 months later. Journal of Psychopharmacology, 22(6), 621–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Richards WA, McCann U, and Jesse R (2006). Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology (Berl),187(3): 268–283. [DOI] [PubMed] [Google Scholar]
- Halpern JH (1996). The use of hallucinogens in the treatment of addiction. Addiction Research, 4(2), 177–189. [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, and Fagerström K (1991). The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction, 86(9), 1119–1127. [DOI] [PubMed] [Google Scholar]
- Hollister LE, Shelton J, and Krieger G (1969). A controlled comparison of lysergic acid diethylamide (LSD) and dextroamphetamine in alcoholics. American Journal of Psychiatry, 125(10), 1352–7. [DOI] [PubMed] [Google Scholar]
- Johnson MW, Garcia-Romeu A, Cosimano MP, and Griffiths RR (2014). Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. Journal of Psychopharmacology, 28(1), 983–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Richards WA, and Griffiths RR (2008). Human hallucinogen research: Guidelines for safety. Journal of Psychopharmacology, 22(6), 603–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Sewell RA, and Griffiths RR (2012). Psilocybin dose-dependently causes delayed, transient headaches in healthy volunteers. Drug and Alcohol Dependence, 123(1), 132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraehenmann R, Preller KH, Scheidegger M, Pokorny T, Bosch OG, Seifritz E, and Vollenweider FX (2014). Psilocybin-induced decrease in amygdala reactivity correlates with enhanced positive mood in healthy volunteers. Biological Psychiatry, 78(8), 572–581. [DOI] [PubMed] [Google Scholar]
- Krebs TS and Johansen PØ (2012). Lysergic acid diethylamide (LSD) for alcoholism: meta-analysis of randomized controlled trials. Journal of Psychopharmacology, 26(7), 994–1002. [DOI] [PubMed] [Google Scholar]
- Ludwig A, Levine J, Stark L, and Lazar R (1969). A clinical study of LSD treatment in alcoholism. American Journal of Psychiatry, 126(1): 59–69. [DOI] [PubMed] [Google Scholar]
- MacLean KA, Johnson MW, and Griffiths RR (2011). Mystical experiences occasioned by the hallucinogen psilocybin lead to increases in the personality domain of openness. Journal of Psychopharmacology (Berl), 25(11), 1453–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean KA, Leoutsakos JMS, Johnson MW, and Griffiths RR (2012). Factor analysis of the mystical experience questionnaire: A study of experiences occasioned by the hallucinogen psilocybin. Journal for the scientific study of religion, 51(4), 721–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangini M (1998). Treatment of alcoholism using psychedelic drugs: a review of the program of research. Journal of Psychoactive Drugs, 30(4), 381–418. [DOI] [PubMed] [Google Scholar]
- McCambridge J, Mitcheson L, Winstock AR, and Hunt N (2005). Five-year trends in patterns of drug use among people who use stimulants in dance contexts in the United Kingdom. Addiction, 100, 1140–49. [DOI] [PubMed] [Google Scholar]
- Miller WR (2004). The phenomenon of quantum change. Journal of clinical psychology, 60(5), 453–460. [DOI] [PubMed] [Google Scholar]
- Miller WR C’de Baca J (2001). Quantum Change When Epiphanies and Sudden Insights Transform Ordinary Lives. The Guilford Press, New York, NY. [Google Scholar]
- Mottillo S, Filion KB, Bélisle P, Joseph L, Gervais A, O’Loughlin J, ... and Tremblay M (2009). Behavioural interventions for smoking cessation: a meta-analysis of randomized controlled trials. European heart journal, 30(6), 718–730. [DOI] [PubMed] [Google Scholar]
- Pahnke W (1963). Drugs and mysticism: An analysis of the relationship between psychedelic drugs and the mystical consciousness [Unpublished doctoral dissertation]. Harvard University: Boston, MA. [Google Scholar]
- Palhano-Fontes F, Andrade KC, Tofoli LF, Santos AC, Crippa JAS, Hallak JE, ... and de Araujo DB (2015). The psychedelic state induced by ayahuasca modulates the activity and connectivity of the default mode network. Plos one, 10(2), e0118143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascarosa P and Futterman S (1976). Ethnopsychedelic therapy for alcoholics: Observations in the peyote ritual of the Native American Church. Journal of Psychoactive Drugs, 8(3), 215–221. [Google Scholar]
- Petri G, Expert P, Turkheimer F, Carhart-Harris R, Nutt D, Hellyer PJ, and Vaccarino F (2014). Homological scaffolds of brain functional networks. Journal of The Royal Society Interface, 11(101), 20140873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME (2015). Withdrawal: expanding a key addiction construct. Nicotine & Tobacco Research, 17(12), 1405–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preller KH, Pokorny T, Hock A, Kraehenmann R, Stämpfli P, Seifritz E, ... and Vollenweider FX (2016). Effects of serotonin 2A/1A receptor stimulation on social exclusion processing. Proc Natl Acad Sci USA 113: 5119–5124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prue B (2013). Indigenous supports for recovery from alcoholism and drug abuse: The Native American Church. Journal of Ethnic And Cultural Diversity in Social Work, 22(3–4), 271–287. [Google Scholar]
- Ross S (2012). Serotonergic hallucinogens and emerging targets for addiction pharmacotherapies. Psychiatric Clinics of North America, 35(2), 357–374. [DOI] [PubMed] [Google Scholar]
- Roy C (1973). Indian Peyotists and Alcohol. American Journal of Psychiatry, 130(10), 329–330. [DOI] [PubMed] [Google Scholar]
- Sessa B, and Johnson MW (2015). Can psychedelic compounds play a part in drug dependence therapy?. The British Journal of Psychiatry, 206(1), 1–3. [DOI] [PubMed] [Google Scholar]
- Smart RG, Storm T, Baker EFW, Solursh L (1966). A controlled study of lysergide in the treatment of alcoholism: The effects on drinking behavior. Quarterly Journal of Studies on Alcohol, 27(3): 469–482. [PubMed] [Google Scholar]
- Stolaroff M (2004). The Secret Chief Revealed: Conversations with Leo Zeff, Pioneer in the Underground Psychedelic Therapy Movement. Sarasota, FL: Multidisciplinary Association for Psychedelic Studies. [Google Scholar]
- Studerus E, Gamma A, Kometer M, and Vollenweider FX (2012). Prediction of psilocybin response in healthy volunteers. PLoS One, 7(2): e30800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MT, Carroll AJ, Salmeron BJ, Ross TJ, and Stein EA (2013). Insula’s functional connectivity with ventromedial prefrontal cortex mediates the impact of trait alexithymia on state tobacco craving. Psychopharmacology (Berl), 228(1), 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellegen A and Atkinson G (1974). Openness to absorbing and self-altering experiences (“absorption”), a trait related to hypnotic susceptibility. Journal of Abnormal Psychology, 83(3), 268–277. [DOI] [PubMed] [Google Scholar]
- Thomas G, Lucas P, Capler NR, Tupper KW and Martin G (2013). Ayahuasca-assisted therapy for addiction: Results from a preliminary observational study in Canada. Current Drug Abuse Reviews, 6(1), 30–42. [DOI] [PubMed] [Google Scholar]
- Tiffany ST and Drobes DJ (1991). The development and initial validation of a questionnaire on smoking urges. British Journal of Addiction, 86(11), 1467–1476. [DOI] [PubMed] [Google Scholar]
- Ungerleider JT, Fisher DD, Fuller M, and Caldwell A (1968). The ‘bad trip’: The etiology of the adverse LSD reaction. American Journal of Psychiatry, 124(11), 1483–1490 [DOI] [PubMed] [Google Scholar]
- Vollenweider FX and Kometer M (2010). The neurobiology of psychedelic drugs: Implications for the treatment of mood disorders. Nature Reviews Neuroscience, 11(9), 642–651. [DOI] [PubMed] [Google Scholar]
- Winstock AR, Kaar S, and Borschmann R (2014). Dimethyltryptamine (DMT): Prevalence, user characteristics and abuse liability in a large global sample. Journal of Psychopharmacology, 28(1), 49–54. [DOI] [PubMed] [Google Scholar]
- Winstock AR, Mitcheson LR, Deluca P, Davey Z, Corazza O, and Schifano F (2011). Mephedrone, new kid for the chop? Addiction, 106(1), 154–161. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


