Abstract
Age-related bone impairment often leads to fragility fractures in the elderly. Although excellent surgical care is widely provided, diagnosis and treatment of the underlying bone disorder are often not kept in mind. The interplay of the three major bone cells – osteoblasts, osteoclasts, and osteocytes – is normally well regulated via the secretion of messengers to control bone remodeling. Possible imbalances that might occur in the elderly are partly due to age, genetic risk factors, and adverse lifestyle factors but importantly also due to imbalances in calcium homeostasis (mostly due to vitamin D deficiency or hypochlorhydria), which have to be eliminated. Therefore, the cooperation between the trauma surgeon and the osteologist is of major importance to diagnose and treat the respective patients at risk. We propose that any patient suffering from fragility fractures is rigorously screened for osteoporosis and metabolic bone diseases. This includes bone density measurement by dual-energy X-ray absorptiometry, laboratory tests for calcium, phosphate, vitamin D, and bone turnover markers, as well as additional diagnostic modalities if needed. Thereby, most risk factors, including vitamin D deficiency, can be identified and treated while patients who meet the criteria for a specific therapy (i.e. antiresorptive and osteoanabolic) receive such. If local health systems succeed to manage this process of secondary fracture prevention, morbidity and mortality of fragility fractures will decline to a minimum level.
Keywords: bone remodeling, DXA, fragility fractures, HR-pQCT, osteoporosis, secondary fracture prevention, vitamin D
Introduction
Osteoporosis is the most common skeletal disorder characterized by low bone mineral density (BMD), impaired bone quality, and fragility fractures [1]. It is furthermore regarded as a polygenic condition in which aging, lifestyle factors, and several unfavorable single nucleotide polymorphisms (SNPs) as well as other genetic variants lead to increased skeletal fragility [2]. Fragility fractures are very common and affect one in two older women and one in three older men [3]. Whereas vertebral fractures are the most frequent osteoporotic fractures, proximal femur fractures have the most severe outcome, causing 24.5%–36% mortality in the first year after fracture [4, 5]. In the aging population, fracture rates have reached epidemic levels in the Western Hemisphere. The subsequent loss of mobility and autonomy often constitute a drastic drop in the quality of life.
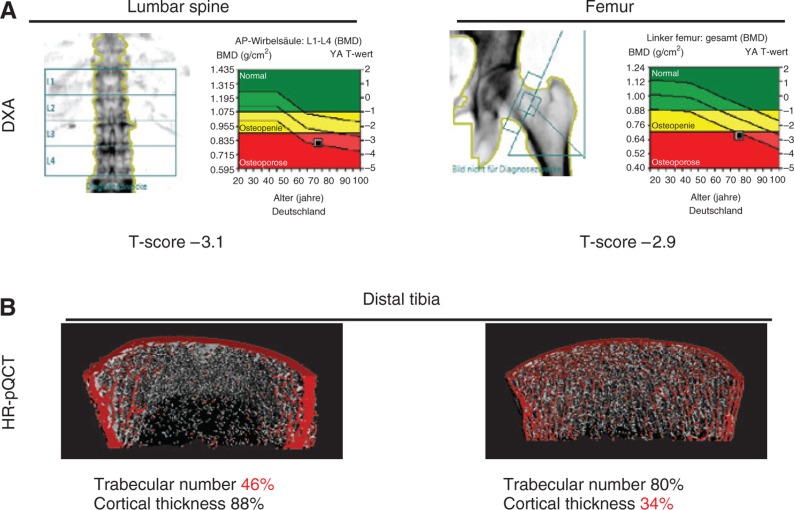
The diagnostic gold standard of fracture risk assessment is a BMD scan by dual-energy X-ray absorptiometry (DXA), measuring the proximal femur and the lumbar spine. The World Health Organization defines osteoporosis as BMD of lower than 2.5 standard deviations below average as assessed by DXA measurements (T-score; Figure 1A). However, fracture risk depends on many more factors such as age, gender, or hormonal status. Therefore, there is a relevant proportion of patients with a T-score higher than –2.5 suffering from fragility fractures. Although age is a risk factor for osteoporosis, it is often mistakenly assumed that osteoporosis might be a natural phenomenon if one lives long enough [6]. In Germany, 6.3 million patients suffer from osteoporosis, of which only one quarter receives specific treatment [7]. The surgical treatment of fractures is performed on a very high level nowadays, although the underlying causes for fractures remain underdiagnosed.
Figure 1:
Diagnostic modalities of osteoporosis.
(A) DXA bone scans of the lumbar spine and proximal femur reveal a low BMD. A standard deviation of 2.5 below the average of a young adult (T-score <–2.5) is defined as osteoporosis. However, a statement on the volumetric parameters cannot be made. (B) HR-pQCT offers a possibility to assess the patient’s bone structure in terms of cortical and/or trabecular bone loss, whereas both lead to increased risk of fracture (% compared to reference values).
In addition to DXA, extended laboratory tests of calcium homeostasis and bone turnover are useful to exclude secondary causes for fragility fractures. Here, prominent examples are osteomalacia caused by vitamin D deficiency [low 25-OH-D3, elevated alkaline phosphatase, elevated parathyroid hormone (PTH)], calcium malabsorption due to hypochlorhydria (low calcium, elevated gastrin), multiple myeloma (high calcium, high creatinine, γ-peak in protein electrophoresis), primary hyperparathyroidism (elevated calcium, elevated PTH), or renal osteodystrophy (decreased 1,25-OH2-D3, increased PTH). Table 1 lists the relevant serum markers in the differential diagnosis of osteoporosis.
Table 1:
Laboratory parameters to identify the causes for imbalances in calcium homeostasis and osteoporosis (only selected conditions).
| Parameter | Low | High |
|---|---|---|
| Calcium | Vitamin D deficiency, hypochlorhydria, hypoparathyroidism, renal insufficiency (renal loss), secondary hyperparathyroidism | Primary hyperparathyroidism, tumor (i.e. multiple myeloma), sarcoidosis |
| Phosphate | Vitamin D deficiency, hyperparathyroidism, renal insufficiency (renal loss) | Elevated intake, rhabdomyolysis, renal insufficiency |
| Alkaline phosphatase | Hypophosphatasia | Liver disease, Paget’s disease, osteomalacia |
| 25-OH-D3 | Insufficient intake or production in the skin | Vitamin D intoxication (very rare) |
| PTH | Hypoparathyroidism | Primary, secondary, or tertiary hyperparathyroidism |
| Osteocalcin/P1NP | Low turnover osteoporosis | Teriparatide |
| Deoxypyridinoline/β-CTX (urine) | High turnover osteoporosis, vitamin D insufficiency, hyperparathyroidism, hyperthyroidism |
P1NP, Procollagen type 1 amino-terminal propeptide; β-CTX, β-crosslaps.
Apart from DXA, another helpful tool for osteoporosis diagnostics is high-resolution peripheral quantitative computed tomography (HR-pQCT; Scanco Medical, Switzerland), which assesses the three-dimensional bone microstructure in the cortical and trabecular bony compartment as well as bone mineralization (Figure 1B). This way, trabecular and cortical bone loss syndromes can be uncovered, while both may lead to increased risk of fracture [8]. In patients with degenerative diseases such as osteoarthritis in the lumbar spine, where DXA BMD values are falsely high, HR-pQCT offers a valid diagnostic alternative.
In general, it is of high importance to perform osteologic diagnostics after any fragility fracture has occurred to provide the best long-term medical care. Also, the diagnostic procedures have to be carried out even before the first fracture, especially if any risk factors such as immobilization, diabetes, or corticosteroid therapy are present. Because fragility fractures affect most commonly elderly patients, the German guidelines recommend the basic osteoporosis diagnostics independently from risk factors for women ages 70 years above and men ages 80 years and above. In the following sections, we outline the basics of bone biology, which are fundamental to understand the differential diagnostics and therapeutic options of osteoporotic fractures. The optimization of fracture healing and long-term therapy of osteoporosis often go hand in hand.
Bone remodeling
Bone remodeling in the adult skeleton serves for a continuous bone replacement [9]. Under optimal conditions, this cellular process keeps bone mass constant from puberty throughout life. In the current understanding, three major bone cells are involved in this process. All three cell types communicate with each other through the secretion of messengers, while the balance of bone formation and bone resorption is aimed to be well regulated. The bone cells involved in bone remodeling are also the protagonists for fracture healing.
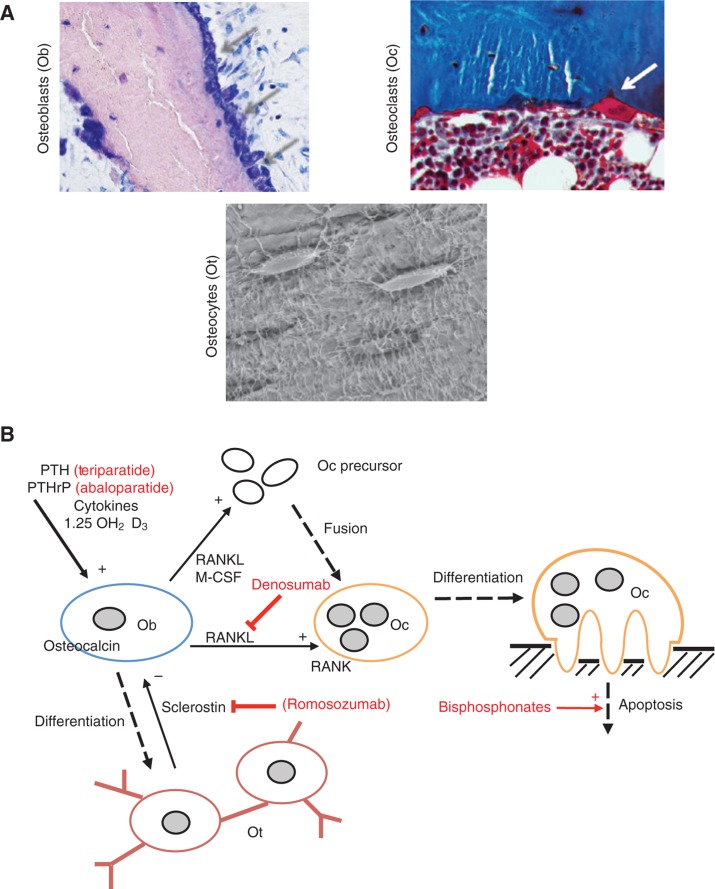
Osteoblasts are bone-forming cells derived from mesenchymal stem cells (Figure 2A). Bone formation is enhanced by vitamin D as well as PTH (Figure 2B).
Figure 2:
Bone remodeling.
(A) Microscopic views of bone-forming osteoblasts (toluidine blue staining) as well as bone-resorbing osteoclasts (trichrome Goldner staining). Osteocytes (bottom row) are connected via canalicular extensions and are able to sense mechanical strain to signal osteoblasts and osteoclasts. (B) Simplified bone remodeling scheme demonstrates the interplay between the bone cells. Ob, Osteoblasts; Oc, osteoclasts; Ot, osteocytes; (+), stimulation; (–), inhibition. Osteoporosis drugs and their targets are in red.
Osteoclasts are mostly multinucleated and represent the bone-resorbing cells (Figure 2A). Osteoclast precursor cells become activated multinucleated osteoclasts through their activation by the receptor activator of nuclear factor-κB ligand (RANKL) as well as macrophage colony-stimulating factor (M-CSF; Figure 2B).
Osteocytes are cells entrapped in the bone matrix and able to sense mechanical strain (90%–95% of all bone cells) [10]. They originate from osteoblasts, which represent the primary bone-forming cells [11]. Osteocytes are well connected via canaliculi, thereby communicating with each other and the bone surface [12] (Figure 2A). They express several factors that regulate phosphate metabolism [i.e. fibroblast growth factor-23 (FGF-23)], which indicates that they are involved in matrix mineralization. Furthermore, osteocytes produce sclerostin to inhibit the Wnt signaling pathway with subsequent negative influence on bone formation [13]. Interestingly, circulating sclerostin levels have been found to increase with age [14].
In the two bony compartments, trabecular and cortical bone, different physiological and pathophysiological mechanisms have to be differentiated, which also have clinical relevance for osteoporosis treatment [15]. Trabecular bone with its thin plates has a low matrix volume and a large surface area, which is why signaling molecules are thought to have more effects on remodeling. Cortical bone has comparably smaller surfaces but contributes to 80% of the weight of the human skeleton. Especially in long bones, including the femur, cortical bone is found. In aging cortical bone, bone loss from the inner (endocortical) surface exceeds deposition of bone on the outer (periosteal) surface, which leads to bone fragility [16]. Furthermore, the accumulation of microdamage increases and the number of osteocyte lacunae decreases with age in cortical bone, which might jeopardize the skeletal integrity and bone repair in aged cortical bone [17].
Calcium homeostasis
Next to possible imbalances in the cellular bone remodeling process, an intact calcium homeostasis and bone mineralization is essential for fracture prevention and postfracture care. Inadequate calcium supply leads to mineralization defects of the skeleton, whereas calcium homeostasis in highly dependent on vitamin D status [18]. Active vitamin D (1,25-OH2-D3) increases intestinal calcium absorption and reduces excessive bone remodeling via direct and indirect pathways, therefore leading to decreased risk of fracture [19]. In Germany, there is an unequivocal endemic vitamin D deficiency affecting 60%–89% of the population according to the Robert Koch Institute (RKI) [20]. Any vitamin D supplementation should aim 25-OH-D3 serum levels of more than 30 μg/L (75 nmol/L), as this has been shown to be the threshold to exclude any mineralization defect and osteomalacia [21]. In the histologic observation of bone biopsies, an accumulation of unmineralized bone matrix, called osteoid, covering more than 20% of the bone surface is called osteomalacia (Figure 3A). We have shown previously that osteomalacia affects up to 25% of the assumed-to-be healthy population [21] and that osteomalacia is more common in patients with hip fracture than in patients without hip fracture [22]. Another consequence of vitamin D deficiency is also a secondary hyperparathyroidism, which leads to the subsequent activation of bone resorption with inadequate fracture healing (Figure 3B). Of note, 25-OH-D3 is the best predictor of vitamin D status and active 1,25-OH2-D3 should only be tested in patients with renal failure due to the inadequate activation of 25-OH-D3 in the kidneys.
Figure 3:
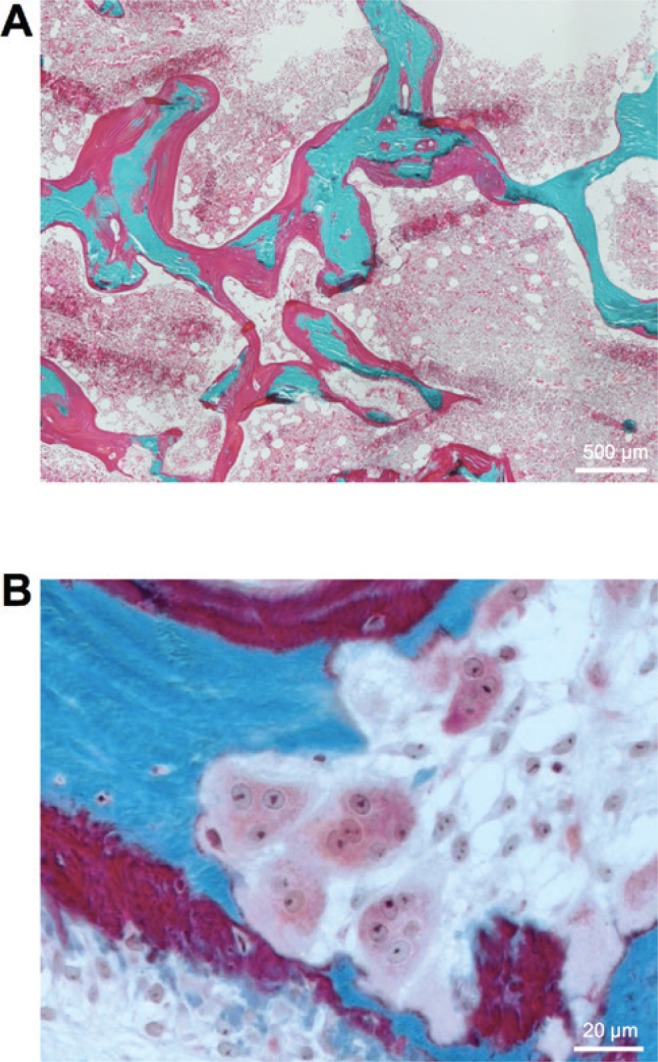
Osteomalacia is an underdiagnosed differential diagnosis of osteoporosis.
(A) A diagnostic iliac crest bone biopsy shows an accumulation of unmineralized bone called osteoidosis. Surface (>20%) and volume (>2%) osteoidosis are the diagnostic hallmarks of osteomalacia. (B) Activated bone resorption in a patient with secondary hyperparathyroidism caused by vitamin D deficiency. Multinucleated osteoclasts can be seen. Trichrome Goldner staining of 5-μm-thick undercalcified sections (green-blue, mineralized bone; red, osteoid).
Of major importance in the treatment of elderly patients is also hypochlorhydria, which constitutes a deficiency of gastric acid. Impaired gastric acidification negatively affects calcium homeostasis and bone mass [23, 24]. Therefore, the use of proton pump inhibitors (PPIs) is associated with the increased risk of fracture. In fact, a meta-analysis including 1,084,560 patients with 62,210 PPI users demonstrated an increased risk of hip or any-site fractures among PPI users [25]. In the case of fracture, hypochlorhydria leads to increased mobilization of calcium from the skeleton and posttraumatic bone loss, which may be responsible for the increased risk of further fractures after the initial fracture event [26]. Importantly, only calcium citrate and calcium gluconate supplements, which can be ingested independently from gastric acid, are shown to balance calcium homeostasis and increase BMD in patients with hypochlorhydria [27]. If no defective intestinal calcium absorption is present, a nutritional daily calcium intake of 1000–1500 mg calcium should be guaranteed, whereas no additional calcium supplementation is needed. That a balanced interplay of calcium, vitamin D, and PTH is not only crucial to provide a healthy skeleton from the beginning but also for fracture healing is the fact that adequate calcium supply is essential for callus mineralization and the bridging of the fracture gap [26].
Make the first fracture the last – implications for treatment
Closing the gap in between surgical fracture treatment and follow-up examination and therapy is of major importance to prevent further fractures. Therefore, any clinician dealing with fractures in elderly patients should initiate the basic osteologic diagnostics. Here, the implementation of coordinator-based fracture liaison services (FLS) in health-care systems has been shown to raise the postfracture assessment rate from 20% to 80%–90% [3].
With regard to the outlined bone remodeling process and possible imbalances in the elderly, a postfracture diagnostic and treatment algorithm should be implemented. After any low traumatic fracture has occurred in patients older than 60 years, basic diagnostics, including detailed medical history, assessment of risk factors, DXA, and laboratory tests, should be arranged. In the case of low traumatic vertebral or hip fracture and exclusion of secondary causes for osteoporosis, a specific osteological treatment has to be started afterwards. The most commonly used and approved osteoporosis medications in North America and Europe include bisphosphonates (antiresorptive), denosumab (antiresorptive), and teriparatide (osteoanabolic). Of note, any specific therapy requires the normalization of calcium homeostasis.
Nitrogen-containing bisphosphonates (including alendronate, ibandronate, risedronate, and zoledronate) are the oldest and most experienced osteoporosis drugs and have been shown to be highly effective in primary and secondary fracture prevention. They interact with the mevalonate biosynthesis and inhibit the farnesyl pyrophosphate synthase, which eventually leads to the disruption of the cytoskeleton in osteoclasts and subsequent apoptosis. In postmenopausal women with a T-score lower than –2.5, long-term treatment with alendronate leads to a 50% reduction of nonvertebral fractures [28].
Denosumab, which is a human monoclonal antibody against RANKL, the main regulator of osteoclastogenesis, showed a reduction of new vertebral fractures by 68% and hip fractures by 40% [29]. A main advantage of denosumab, particularly in elderly patients with declining renal function, is that it is not eliminated by the kidneys and therefore also suitable in patients with renal insufficiency [30]. The osteoanabolic drug teriparatide (PTH-1-34) is less commonly used and generally reserved for patients with severe osteoporosis. Its use is furthermore limited to 18–24 months, as the osteoanabolic effect of recombinant PTH is limited to this time called the “anabolic window” [31]. Therefore, the treatment of patients with severe osteoporosis requires the sequential use of several drugs, for example, both teriparatide and denosumab [32]. Switching from teriparatide to denosumab is hereby associated with a continuous increase in BMD. For the action mechanism of these three drugs, see also Figure 2B. A promising drug target is also sclerostin and its inhibition, which is currently subject of clinical trials [33].
The effect of osteoporosis drugs on fracture healing itself is another point that has often been discussed [34] and will only shortly be addressed here. Importantly, there is no evidence for a significant negative effect of bisphosphonates or denosumab on fracture healing in humans. Teriparatide has been shown to accelerate fracture repair in small clinical trials [35], but a further investigation is needed. Therefore, the main focus should lie on the initiation of osteoporosis diagnostics and treatment after fracture, whereas risk factors for nonunion such as diabetes, nicotine, glucocorticoids, or chemotherapy have to be considered [36].
We conclude that the basic diagnostic procedures (assessment of medical history, DXA, laboratory tests, and HR-pQCT if required) are all relatively easy to carry out. This way, patients suffering from fragility fractures can be diagnosed and the appropriate treatment can be started leading to a significantly decreased risk of further fractures. Our knowledge of bone biology has advanced in the last years, which is why different pathways can be antagonized specifically to stop further bone loss and treat patients with osteoporosis or other skeletal diseases. One has to keep in mind that a balanced vitamin D and calcium homeostasis is the fundament for any further specific therapy.
Supporting Information
Supplemental Material
The article (DOI: iss-2016-0025) offers reviewer assessments as supplementary material.
Author Statement
Funding: Authors state no funding involved. Conflict of interest: Authors state no conflict of interest. Informed consent: Informed consent is not applicable. Ethical approval: The conducted research is not related to either human or animals use.
Author Contributions
Writing of the manuscript: Tim Rolvien; Michael Amling.
Publication Funding
The German Society of Surgery funded the article processing charges of this article.
References
- [1].van den Bergh JP, van Geel TA, Geusens PP. Osteoporosis, frailty and fracture: implications for case finding and therapy. Nat Rev Rheumatol 2012;8:163–172. [DOI] [PubMed]; van den Bergh JP, van Geel TA, Geusens PP. Osteoporosis, frailty and fracture: implications for case finding and therapy. Nat Rev Rheumatol. 2012;8:163–172. doi: 10.1038/nrrheum.2011.217. [DOI] [PubMed] [Google Scholar]
- [2].Estrada K, Styrkarsdottir U, Evangelou E, et al. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat Genet 2012;44:491–501. [DOI] [PMC free article] [PubMed]; Estrada K, Styrkarsdottir U, Evangelou E. et al. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat Genet. 2012;44:491–501. doi: 10.1038/ng.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Eisman JA, Bogoch ER, Dell R, et al. Making the first fracture the last fracture: ASBMR task force report on secondary fracture prevention. J Bone Miner Res 2012;27:2039–2046. [DOI] [PubMed]; Eisman JA, Bogoch ER, Dell R. et al. Making the first fracture the last fracture: ASBMR task force report on secondary fracture prevention. J Bone Miner Res. 2012;27:2039–2046. doi: 10.1002/jbmr.1698. [DOI] [PubMed] [Google Scholar]
- [4].Hu F, Jiang C, Shen J, Tang P, Wang Y. Preoperative predictors for mortality following hip fracture surgery: a systematic review and meta-analysis. Injury 2012;43:676–685. [DOI] [PubMed]; Hu F, Jiang C, Shen J, Tang P, Wang Y. Preoperative predictors for mortality following hip fracture surgery: a systematic review and meta-analysis. Injury. 2012;43:676–685. doi: 10.1016/j.injury.2011.05.017. [DOI] [PubMed] [Google Scholar]
- [5].Abrahamsen B, van Staa T, Ariely R, Olson M, Cooper C. Excess mortality following hip fracture: a systematic epidemiological review. Osteoporos Int 2009;20:1633–1650. [DOI] [PubMed]; Abrahamsen B, van Staa T, Ariely R, Olson M, Cooper C. Excess mortality following hip fracture: a systematic epidemiological review. Osteoporos Int. 2009;20:1633–1650. doi: 10.1007/s00198-009-0920-3. [DOI] [PubMed] [Google Scholar]
- [6].Gruber R, Koch H, Doll BA, Tegtmeier F, Einhorn TA, Hollinger JO. Fracture healing in the elderly patient. Exp Gerontol 2006;41:1080–1093. [DOI] [PubMed]; Gruber R, Koch H, Doll BA, Tegtmeier F, Einhorn TA, Hollinger JO. Fracture healing in the elderly patient. Exp Gerontol. 2006;41:1080–1093. doi: 10.1016/j.exger.2006.09.008. [DOI] [PubMed] [Google Scholar]
- [7].Hadji P, Klein S, Gothe H, et al. The epidemiology of osteoporosis – Bone Evaluation Study (BEST): an analysis of routine health insurance data. Dtsch Arztebl Int 2013;110:52–57. [DOI] [PMC free article] [PubMed]; Hadji P, Klein S, Gothe H. et al. The epidemiology of osteoporosis – Bone Evaluation Study (BEST): an analysis of routine health insurance data. Dtsch Arztebl Int. 2013;110:52–57. doi: 10.3238/arztebl.2013.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wang J, Stein EM, Zhou B, et al. Deterioration of trabecular plate-rod and cortical microarchitecture and reduced bone stiffness at distal radius and tibia in postmenopausal women with vertebral fractures. Bone 2016;88:39–46. [DOI] [PMC free article] [PubMed]; Wang J, Stein EM, Zhou B. et al. Deterioration of trabecular plate-rod and cortical microarchitecture and reduced bone stiffness at distal radius and tibia in postmenopausal women with vertebral fractures. Bone. 2016;88:39–46. doi: 10.1016/j.bone.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Parfitt AM. Osteonal and hemi-osteonal remodeling: the spatial and temporal framework for signal traffic in adult human bone. J Cell Biochem 1994;55:273–286. [DOI] [PubMed]; Parfitt AM. Osteonal and hemi-osteonal remodeling: the spatial and temporal framework for signal traffic in adult human bone. J Cell Biochem. 1994;55:273–286. doi: 10.1002/jcb.240550303. [DOI] [PubMed] [Google Scholar]
- [10].Bonewald LF. Mechanosensation and transduction in osteocytes. Bonekey Osteovis 2006;3:7–15. [DOI] [PMC free article] [PubMed]; Bonewald LF. Mechanosensation and transduction in osteocytes. Bonekey Osteovis. 2006;3:7–15. doi: 10.1138/20060233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bonewald LF. Osteocytes as dynamic multifunctional cells. Ann NY Acad Sci 2007;1116:281–290. [DOI] [PubMed]; Bonewald LF. Osteocytes as dynamic multifunctional cells. Ann NY Acad Sci. 2007;1116:281–290. doi: 10.1196/annals.1402.018. [DOI] [PubMed] [Google Scholar]
- [12].Milovanovic P, Zimmermann EA, Hahn M, et al. Osteocytic canalicular networks: morphological implications for altered mechanosensitivity. ACS Nano 2013;7:7542–7551. [DOI] [PubMed]; Milovanovic P, Zimmermann EA, Hahn M. et al. Osteocytic canalicular networks: morphological implications for altered mechanosensitivity. ACS Nano. 2013;7:7542–7551. doi: 10.1021/nn401360u. [DOI] [PubMed] [Google Scholar]
- [13].Ellies DL, Viviano B, McCarthy J, et al. Bone density ligand, sclerostin, directly interacts with LRP5 but not LRP5G171V to modulate Wnt activity. J Bone Miner Res 2006;21:1738–1749. [DOI] [PubMed]; Ellies DL, Viviano B, McCarthy J. et al. Bone density ligand, sclerostin, directly interacts with LRP5 but not LRP5G171V to modulate Wnt activity. J Bone Miner Res. 2006;21:1738–1749. doi: 10.1359/jbmr.060810. [DOI] [PubMed] [Google Scholar]
- [14].Hay E, Bouaziz W, Funck-Brentano T, Cohen-Solal M. Sclerostin and bone aging: a mini-review. Gerontology 2016;62:618–623. [DOI] [PubMed]; Hay E, Bouaziz W, Funck-Brentano T, Cohen-Solal M. Sclerostin and bone aging: a mini-review. Gerontology. 2016;62:618–623. doi: 10.1159/000446278. [DOI] [PubMed] [Google Scholar]
- [15].Li J, Bao Q, Chen S, et al. Different bone remodeling levels of trabecular and cortical bone in response to changes in Wnt/β-catenin signaling in mice. J Orthop Res 2016. DOI: 10.1002/jor.23339 [Epub ahead of print]. [DOI] [PubMed]; Li J, Bao Q, Chen S. et al. Different bone remodeling levels of trabecular and cortical bone in response to changes in Wnt/β-catenin signaling in mice. J Orthop Res. 2016 doi: 10.1002/jor.23339. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- [16].Szulc P, Seeman E, Duboeuf F, Sornay-Rendu E, Delmas PD. Bone fragility: failure of periosteal apposition to compensate for increased endocortical resorption in postmenopausal women. J Bone Miner Res 2006;21:1856–1863. [DOI] [PubMed]; Szulc P, Seeman E, Duboeuf F, Sornay-Rendu E, Delmas PD. Bone fragility: failure of periosteal apposition to compensate for increased endocortical resorption in postmenopausal women. J Bone Miner Res. 2006;21:1856–1863. doi: 10.1359/jbmr.060904. [DOI] [PubMed] [Google Scholar]
- [17].Busse B, Djonic D, Milovanovic P, et al. Decrease in the osteocyte lacunar density accompanied by hypermineralized lacunar occlusion reveals failure and delay of remodeling in aged human bone. Aging Cell 2010;9:1065–1075. [DOI] [PubMed]; Busse B, Djonic D, Milovanovic P. et al. Decrease in the osteocyte lacunar density accompanied by hypermineralized lacunar occlusion reveals failure and delay of remodeling in aged human bone. Aging Cell. 2010;9:1065–1075. doi: 10.1111/j.1474-9726.2010.00633.x. [DOI] [PubMed] [Google Scholar]
- [18].Amling M, Priemel M, Holzmann T, et al. Rescue of the skeletal phenotype of vitamin D receptor-ablated mice in the setting of normal mineral ion homeostasis: formal histomorphometric and biomechanical analyses. Endocrinology 1999;140:4982–4987. [DOI] [PubMed]; Amling M, Priemel M, Holzmann T. et al. Rescue of the skeletal phenotype of vitamin D receptor-ablated mice in the setting of normal mineral ion homeostasis: formal histomorphometric and biomechanical analyses. Endocrinology. 1999;140:4982–4987. doi: 10.1210/endo.140.11.7110. [DOI] [PubMed] [Google Scholar]
- [19].Chapuy MC, Arlot ME, Duboeuf F, et al. Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med 1992;327:1637–1642. [DOI] [PubMed]; Chapuy MC, Arlot ME, Duboeuf F. et al. Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med. 1992;327:1637–1642. doi: 10.1056/NEJM199212033272305. [DOI] [PubMed] [Google Scholar]
- [20].Hintzpeter B, Mensink GB, Thierfelder W, Muller MJ, Scheidt-Nave C. Vitamin D status and health correlates among German adults. Eur J Clin Nutr 2008;62:1079–1089. [DOI] [PubMed]; Hintzpeter B, Mensink GB, Thierfelder W, Muller MJ, Scheidt-Nave C. Vitamin D status and health correlates among German adults. Eur J Clin Nutr. 2008;62:1079–1089. doi: 10.1038/sj.ejcn.1602825. [DOI] [PubMed] [Google Scholar]
- [21].Priemel M, von Domarus C, Klatte TO, et al. Bone mineralization defects and vitamin D deficiency: histomorphometric analysis of iliac crest bone biopsies and circulating 25-hydroxyvitamin D in 675 patients. J Bone Miner Res 2010;25:305–312. [DOI] [PubMed]; Priemel M, von Domarus C, Klatte TO. et al. Bone mineralization defects and vitamin D deficiency: histomorphometric analysis of iliac crest bone biopsies and circulating 25-hydroxyvitamin D in 675 patients. J Bone Miner Res. 2010;25:305–312. doi: 10.1359/jbmr.090728. [DOI] [PubMed] [Google Scholar]
- [22].Seitz S, Koehne T, Ries C, et al. Impaired bone mineralization accompanied by low vitamin D and secondary hyperparathyroidism in patients with femoral neck fracture. Osteoporos Int 2013;24:641–649. [DOI] [PubMed]; Seitz S, Koehne T, Ries C. et al. Impaired bone mineralization accompanied by low vitamin D and secondary hyperparathyroidism in patients with femoral neck fracture. Osteoporos Int. 2013;24:641–649. doi: 10.1007/s00198-012-2011-0. [DOI] [PubMed] [Google Scholar]
- [23].Schinke T, Schilling AF, Baranowsky A, et al. Impaired gastric acidification negatively affects calcium homeostasis and bone mass. Nat Med 2009;15:674–681. [DOI] [PubMed]; Schinke T, Schilling AF, Baranowsky A. et al. Impaired gastric acidification negatively affects calcium homeostasis and bone mass. Nat Med. 2009;15:674–681. doi: 10.1038/nm.1963. [DOI] [PubMed] [Google Scholar]
- [24].Keller J, Schinke T. The role of the gastrointestinal tract in calcium homeostasis and bone remodeling. Osteoporos Int 2013;24:2737–2748. [DOI] [PubMed]; Keller J, Schinke T. The role of the gastrointestinal tract in calcium homeostasis and bone remodeling. Osteoporos Int. 2013;24:2737–2748. doi: 10.1007/s00198-013-2335-4. [DOI] [PubMed] [Google Scholar]
- [25].Yu EW, Bauer SR, Bain PA, Bauer DC. Proton pump inhibitors and risk of fractures: a meta-analysis of 11 international studies. Am J Med 2011;124:519–526. [DOI] [PMC free article] [PubMed]; Yu EW, Bauer SR, Bain PA, Bauer DC. Proton pump inhibitors and risk of fractures: a meta-analysis of 11 international studies. Am J Med. 2011;124:519–526. doi: 10.1016/j.amjmed.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Haffner-Luntzer M, Heilmann A, Heidler V, et al. Hypochlorhydria-induced calcium malabsorption does not affect fracture healing but increases post-traumatic bone loss in the intact skeleton. J Orthop Res 2016. DOI: 10.1002/jor.23221 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]; Haffner-Luntzer M, Heilmann A, Heidler V. et al. Hypochlorhydria-induced calcium malabsorption does not affect fracture healing but increases post-traumatic bone loss in the intact skeleton. J Orthop Res. 2016 doi: 10.1002/jor.23221. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Krause M, Keller J, Beil B, et al. Calcium gluconate supplementation is effective to balance calcium homeostasis in patients with gastrectomy. Osteoporos Int 2015;26:987–995. [DOI] [PubMed]; Krause M, Keller J, Beil B. et al. Calcium gluconate supplementation is effective to balance calcium homeostasis in patients with gastrectomy. Osteoporos Int. 2015;26:987–995. doi: 10.1007/s00198-014-2965-1. [DOI] [PubMed] [Google Scholar]
- [28].Eriksen EF, Diez-Perez A, Boonen S. Update on long-term treatment with bisphosphonates for postmenopausal osteoporosis: a systematic review. Bone 2014;58:126–135. [DOI] [PubMed]; Eriksen EF, Diez-Perez A, Boonen S. Update on long-term treatment with bisphosphonates for postmenopausal osteoporosis: a systematic review. Bone. 2014;58:126–135. doi: 10.1016/j.bone.2013.09.023. [DOI] [PubMed] [Google Scholar]
- [29].Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 2009;361:756–765. [DOI] [PubMed]; Cummings SR, San Martin J, McClung MR. et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–765. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- [30].Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet 2011;377:1276–1287. [DOI] [PMC free article] [PubMed]; Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011;377:1276–1287. doi: 10.1016/S0140-6736(10)62349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Pleiner-Duxneuner J, Zwettler E, Paschalis E, Roschger P, Nell-Duxneuner V, Klaushofer K. Treatment of osteoporosis with parathyroid hormone and teriparatide. Calcif Tissue Int 2009;84:159–170. [DOI] [PubMed]; Pleiner-Duxneuner J, Zwettler E, Paschalis E, Roschger P, Nell-Duxneuner V, Klaushofer K. Treatment of osteoporosis with parathyroid hormone and teriparatide. Calcif Tissue Int. 2009;84:159–170. doi: 10.1007/s00223-009-9218-x. [DOI] [PubMed] [Google Scholar]
- [32].Leder BZ, Tsai JN, Uihlein AV, et al. Denosumab and teriparatide transitions in postmenopausal osteoporosis (the DATA-Switch study): extension of a randomised controlled trial. Lancet 2015;386:1147–1155. [DOI] [PMC free article] [PubMed]; Leder BZ, Tsai JN, Uihlein AV. et al. Denosumab and teriparatide transitions in postmenopausal osteoporosis (the DATA-Switch study): extension of a randomised controlled trial. Lancet. 2015;386:1147–1155. doi: 10.1016/S0140-6736(15)61120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].McClung MR, Grauer A, Boonen S, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med 2014;370:412–420. [DOI] [PubMed]; McClung MR, Grauer A, Boonen S. et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med. 2014;370:412–420. doi: 10.1056/NEJMoa1305224. [DOI] [PubMed] [Google Scholar]
- [34].Barvencik F. Medication and bone metabolism: clinical importance for fracture treatment. Unfallchirurg 2015;118:1017–1024. [DOI] [PubMed]; Barvencik F. Medication and bone metabolism: clinical importance for fracture treatment. Unfallchirurg. 2015;118:1017–1024. doi: 10.1007/s00113-015-0109-5. [DOI] [PubMed] [Google Scholar]
- [35].Aspenberg P, Genant HK, Johansson T, et al. Teriparatide for acceleration of fracture repair in humans: a prospective, randomized, double-blind study of 102 postmenopausal women with distal radial fractures. J Bone Miner Res 2010;25:404–414. [DOI] [PubMed]; Aspenberg P, Genant HK, Johansson T. et al. Teriparatide for acceleration of fracture repair in humans: a prospective, randomized, double-blind study of 102 postmenopausal women with distal radial fractures. J Bone Miner Res. 2010;25:404–414. doi: 10.1359/jbmr.090731. [DOI] [PubMed] [Google Scholar]
- [36].Bishop JA, Palanca AA, Bellino MJ, Lowenberg DW. Assessment of compromised fracture healing. J Am Acad Orthop Surg 2012;20:273–282. [DOI] [PubMed]; Bishop JA, Palanca AA, Bellino MJ, Lowenberg DW. Assessment of compromised fracture healing. J Am Acad Orthop Surg. 2012;20:273–282. doi: 10.5435/JAAOS-20-05-273. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.