Abstract
Prior research suggests that warfarin, when given concomitantly with some sulfonylureas, may increase the risk of serious hypoglycemia. However, the clinical significance remains unclear. We examined rate ratios (RRs) for the association between serious hypoglycemia and concomitant use of warfarin with either sulfonylureas or metformin using a self-controlled case series design and US Medicaid claims (supplemented with Medicare claims) from 1999-2011. Across all risk windows combined, warfarin was associated with an elevated rate of serious hypoglycemia when given concomitantly with glimepiride (RR:1.47; 95% confidence interval:1.07, 2.02) and metformin (1.73; 1.38, 2.16). Particularly in the late risk window (>120 days since beginning concomitancy), most of the RRs for warfarin were elevated: glipizide (1.72; 1.29, 2.29), glyburide (1.57; 1.15, 2.15), metformin (2.26; 1.67, 3.05), and glimepiride (1.56; 0.97, 2.50). These results are consistent with a previously hypothesized hypoglycemic effect of warfarin in type-2 diabetes patients through inhibition of the carboxylation of osteocalcin.
Keywords: sulfonylureas, glimepiride, glipizide, glyburide, metformin, warfarin, hypoglycemia, self-controlled case series study, drug interactions, Medicaid, pharmacoepidemiology
Introduction
The US National Action Plan for Adverse Drug Event Prevention1 focuses on three common, clinically significant, and preventable adverse drug events: hypoglycemia from diabetes agents; bleeding from anticoagulants; and overdoses, over-sedation, and respiratory depression from opioids. Among other measures, the Plan calls for research on drug-drug interactions that may lead to these adverse events. Sulfonylureas and warfarin are among the drugs that are most frequently associated with adverse drug events.1-6 Han and colleagues conducted a series of high-throughput screening studies to identify potential drug-drug interactions involving sulfonylureas that may lead to serious hypoglycemia.7 That paper suggested that warfarin may be associated with an increased risk of serious hypoglycemia among persons taking glimepiride or glipizide. Further, because cytochrome P450 (CYP) 2C9 is involved in the metabolism of both sulfonylureas8-13 and warfarin,13-15 there is a potential pharmacokinetic mechanism for such an interaction. However, clinical significance of the potential interactions between these drugs remains unclear.
We studied whether warfarin is associated with an elevated rate of serious hypoglycemia when used concomitantly with sulfonylureas using real-world, large population data. In a drug-drug interaction, the object drug is the drug whose pharmacokinetics or pharmacodynamics is affected; the precipitant drug is the drug that affects the pharmacokinetics or pharmacodynamics of the object drug.16 We measured the association between warfarin (the precipitant drug) and serious hypoglycemia in users of the most commonly used sulfonylureas (glimepiride, glipizide, and glyburide) as object drugs. Because we knew of no well-described mechanism by which warfarin could interact with metformin to increase the risk of hypoglycemia, we also included metformin as a negative control object drug, which is drug that is used for similar indications as the object drugs under study, but is not believed to interact pharmacologically with the precipitant drugs.16
Results
We identified 495, 1123, 1049, and 800 individuals who experienced serious hypoglycemia at least once while receiving glimepiride, glipizide, glyburide, or metformin, respectively. Table 1 shows the characteristics of these individuals by object drug.
Table 1.
Characteristics of persons experiencing serious hypoglycemia by antidiabetes object drug
| Object drug | |||||
|---|---|---|---|---|---|
| glimepiride | glipizide | glyburide | metformin (control object) |
||
| Number of persons, total | 495 | 1,123 | 1,049 | 800 | |
| warfarin-triggered groupa | 161 | 345 | 315 | 232 | |
| antidiabetes-triggered groupb | 143 | 287 | 288 | 219 | |
| combination-triggered groupc | 39 | 121 | 120 | 79 | |
| Person-days of observation time, total | 263,305 | 625,872 | 606,369 | 554,690 | |
| Time exposed to warfarin | 95,321 | 209,497 | 197,031 | 176,094 | |
| Time unexposed to warfarin | 167,984 | 416,375 | 409,338 | 378,596 | |
| Person-days of observation time by concomitancy-triggering drug | |||||
| warfarin-triggered group | 47,150 | 106,638 | 94,581 | 82,118 | |
| antidiabetes-triggered group | 37,847 | 75,876 | 82,848 | 78,704 | |
| combination-triggered group | 10,324 | 26,983 | 19,602 | 15,272 | |
| Person-days of observation time, median per individual (Q1; Q3) | 298 (83; 748) | 324 (93; 813) | 309 (93; 816) | 421 (91; 1,023) | |
| warfarin-triggered group | 476 (181; 994) | 573 (225; 1,117) | 531 (206; 1,137) | 749 (350; 1,462) | |
| antidiabetes-triggered group | 223 (70; 581) | 286 (124; 713) | 326 (129; 765) | 533 (194; 1,131) | |
| combination-triggered group | 139 (48; 727) | 222 (78; 616) | 204 (85; 628) | 342 (117; 664) | |
| Number of outcome occurrence during observation time | 654 | 1,554 | 1,405 | 1,197 | |
| Exposed time | 255 | 531 | 482 | 435 | |
| Unexposed time | 399 | 1,023 | 923 | 762 | |
| Number of outcome occurrence during observation time by concomitancy-triggering drug | |||||
| warfarin-triggered group | 121 | 235 | 177 | 177 | |
| antidiabetes-triggered group | 104 | 203 | 209 | 190 | |
| combination-triggered group | 30 | 93 | 96 | 68 | |
| Demographic characteristics | Category | % of persons (unless otherwise noted) | |||
| Age in years at start of observation time | Median (Q1; Q3) | 74.7 (66.3; 82.9) | 71.1 (60.5; 79.2) | 73.4 (64.4; 80.4) | 65.1 (52.1; 74.2) |
| Sex | Female | 65.5 | 65.3 | 63.5 | 64.9 |
| Race/ethnicity | White | 57.8 | 40.2 | 43.6 | 46.1 |
| Black | 14.7 | 25.7 | 21.7 | 20.6 | |
| Hispanic/Latino | 12.9 | 17.4 | 17.3 | 18.4 | |
| Other/unknown | 14.5 | 16.7 | 17.3 | 14.9 | |
| State of residence | CA | 39.0 | 45.6 | 52.6 | 47.0 |
| FL | 8.9 | 9.6 | 6.5 | 7.3 | |
| NY | 18.6 | 22.4 | 20.3 | 23.9 | |
| OH | 21.4 | 10.6 | 12.7 | 12.6 | |
| PA | 12.1 | 11.8 | 7.9 | 9.3 | |
| Calendar year at start of observation time | 1999 | d | 4.6 | 6.8 | 2.5 |
| 2000 | 6.9 | 9.3 | 13.6 | 5.9 | |
| 2001 | 4.6 | 9.4 | 10.7 | 7.3 | |
| 2002 | 6.9 | 10.6 | 10.3 | 6.4 | |
| 2003 | 10.5 | 8.1 | 10.9 | 8.6 | |
| 2004 | 11.1 | 7.1 | 9.2 | 10.0 | |
| 2005 | 10.5 | 10.0 | 8.9 | 10.9 | |
| 2006 | 12.7 | 13.0 | 10.8 | 11.4 | |
| 2007 | 9.3 | 7.8 | 6.3 | 9.8 | |
| 2008 | 8.5 | 6.9 | 4.8 | 10.1 | |
| 2009 | 8.5 | 6.6 | 3.2 | 7.8 | |
| 2010 | 5.7 | 4.0 | 3.3 | 7.0 | |
| 2011 | d | 2.5 | 1.4 | 2.5 | |
| Dually-enrolled for Medicare anytime during baseline period | Yes | 87.1 | 81.2 | 83.3 | 76.4 |
| Exposure to Precipitant Drug | Category | % of person-days | |||
| Warfarin | Yes | 36.2 | 33.5 | 32.5 | 31.7 |
| Pre-specified time-varying covariates |
Category | % of person-days (unless otherwise noted) | |||
| Acute infection in prior 15 days | Yes | 10.8 | 11.1 | 10.8 | 9.8 |
| ACE inhibitors in prior 31 days | Yes | 28.4 | 30.7 | 30.7 | 33.8 |
| Angiotensin II receptor antagonists in prior 31 days | Yes | 18.5 | 14.3 | 13.4 | 14.7 |
| Atypical antipsychotics in prior 31 days | Yes | 5.6 | 7.8 | 5.2 | 12.5 |
| Average daily dose, in milligrams | Median (Q1; Q3) | 2.0 (1.0; 4.0) | 5.0 (5.0; 10.0) | 5.0 (2.5; 5.0) | 1,000 (1,000; 1,000) |
| Beta-blockers in prior 31 days | Yes | 37.7 | 38.8 | 33.2 | 34.6 |
| Calcineurin inhibitors in prior 31 days | Yes | 0.2 | 0.4 | 0.2 | 0.1 |
| Corticosteroids in prior 31 days | Yes | 6.2 | 6.3 | 4.9 | 5.5 |
| CYP2C9 inhibitors in prior 31 days | Yes | 6.0 | 2.8 | 2.5 | 3.5 |
| CYP3A4 inhibitors in prior 31 days | Yes | 19.4 | 15.8 | 13.7 | 21.6 |
| MAO inhibitors in prior 31 days | Yes | 0.0 | 0.4 | 0.4 | 0.0 |
| Other drugs that can cause hypoglycemia in prior 31 days | Yes | 2.8 | 5.3 | 3.8 | 3.2 |
| Protease inhibitors in prior 31 days | Yes | 0.6 | 0.2 | 0.2 | 0.1 |
| Quinolones in prior 15 days | Yes | 3.6 | 2.9 | 2.8 | 2.8 |
| Retinoids in prior 31 days | Yes | 0.0 | 0.0 | 0.0 | 0.2 |
| Salicylates in prior 31 days | Yes | 7.9 | 10.5 | 9.5 | 9.2 |
| Thiazide diuretics in prior 31 days | Yes | 14.7 | 12.7 | 13.4 | 16.6 |
ACE: angiotensin converting enzyme. MAO: monoamine oxidase. CYP: cytochrome P450 enzyme.
Warfarin-triggered group: observation time (person-days) in which the concomitant use of object and precipitant drugs was initiated by warfarin during the ongoing antidiabetic treatment.
Antidiabetes-triggered group: observation time (person-days) in which the concomitant use of object and precipitant drugs was initiated by an antidiabetic drug during the ongoing warfarin treatment.
Combination-triggered group: observation time (person-days) in which the concomitant use of object and precipitant drugs was initiated by warfarin and an antidiabetic drug on the same day, i.e., prescriptions of warfarin and an antidiabetic drug were dispensed on the same day.
Small numbers of observation are suppressed by the cell suppression policy of the Centers for Medicare and Medicaid Services (data source).
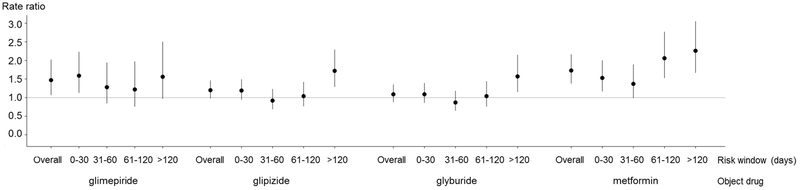
Table 2 and Figure 1 present rate ratios (RRs, with 95% confidence intervals [CIs]), defined as the rate of serious hypoglycemia during the concomitant use of the antidiabetes object drug with warfarin divided by the rate during the use of the antidiabetes object drug without warfarin. Across all risk windows combined, warfarin was associated with a statistically elevated rate of serious hypoglycemia when used concomitantly with glimepiride (RR: 1.47; 95% CI: 1.07, 2.02) and metformin (RR: 1.73; 95% CI: 1.38, 2.16), and a nearly statistically significantly elevated rate in users of glipizide (RR 1.20; 95% CI: 0.98, 1.46). In the late risk window (>120 days since the beginning of concomitancy), the RR for warfarin was elevated with concomitant glipizide (RR: 1.72; 95% CI: 1.29, 2.29), glyburide (RR: 1.57; 95% CI: 1.15, 2.15), and metformin (RR: 2.26; 95% CI: 1.67, 3.05), and nearly statistically elevated with concomitant glimepiride (RR: 1.56; 95% CI: 0.97, 2.50). The results of sensitivity analyses – i.e., adjusting for average daily dose of object drugs (Table S1); stratifying on concomitancy-triggering drug (Table S2); excluding individuals who had death outcome during the observation time (Table S3); and excluding individuals with potentially incomplete data (Table S4) – were substantively similar.
Table 2.
Primary analysis: Confounder-adjusted outcome occurrence rate ratios for the association between warfarin and serious hypoglycemia when used concomitantly with a sulfonylurea or metformin
| Object drug (N=number of outcomesa) |
Risk Window (days)b | Rate ratioc (95% CI) |
|---|---|---|
| glimepiride (N=654) |
Overall | 1.47 (1.07, 2.02) |
| 0-30 | 1.59 (1.13, 2.23) | |
| 31-60 | 1.28 (0.85, 1.94) | |
| 61-120 | 1.22 (0.76, 1.97) | |
| >120 | 1.56 (0.97, 2.50) | |
| glipizide (N=1,554) |
Overall | 1.20 (0.98, 1.46) |
| 0-30 | 1.19 (0.95, 1.49) | |
| 31-60 | 0.92 (0.69, 1.23) | |
| 61-120 | 1.04 (0.77, 1.42) | |
| >120 | 1.72 (1.29, 2.29) | |
| glyburide (N=1,405) |
Overall | 1.09 (0.88, 1.35) |
| 0-30 | 1.09 (0.86, 1.39) | |
| 31-60 | 0.87 (0.65, 1.18) | |
| 61-120 | 1.04 (0.76, 1.44) | |
| >120 | 1.57 (1.15, 2.15) | |
| metformin (N=1,197) |
Overall | 1.73 (1.38, 2.16) |
| 0-30 | 1.53 (1.17, 2.00) | |
| 31-60 | 1.37 (0.99, 1.89) | |
| 61-120 | 2.06 (1.53, 2.77) | |
| >120 | 2.26 (1.67, 3.05) |
CI: confidence interval.
Number of outcomes: number of serious hypoglycemia occurrence during the observation time for each object-precipitant drug pair.
Risk window (days): days within the exposed time (i.e., concomitant-use period) in the observation time since the initiation of the concomitant-use time.
Rate ratio: [(outcome occurrence rate of exposed time) / (outcome occurrence rate of unexposed time)], for each object-precipitant drug pair.
Figure 1.
Primary analysis: Confounder-adjusted outcome occurrence rate ratios and 95% confidence intervals for the association between warfarin and serious hypoglycemia when used with a sulfonylurea or metformin
Discussion
We found that the RR for the association between warfarin and serious hypoglycemia across all temporal risk windows combined was statistically elevated in users of glimepiride and in users of metformin, and nearly so in users of glipizide. In a prior study, warfarin use was associated with an elevated risk of serious hypoglycemia in users of either glipizide or glimepiride (odds ratio for both sulfonylureas considered collectively: 1.22; 95% CI: 1.05, 1.42), and a similar, nearly statistically significantly elevated odds ratio in users of metformin.17 In the present study, the association between warfarin and serious hypoglycemia appeared to be strongest after 120 days of concomitant use of warfarin with most of the antidiabetes drugs examined, including metformin. Given the lack of known pharmacokinetic or pharmacodynamic mechanisms by which warfarin could interact with metformin (which itself is associated with a low risk of hypoglycemia18) to increase the risk of hypoglycemia, what could explain these findings?
Osteocalcin is a 49-amino acid protein that is produced by osteoblasts during bone formation. Its uncarboxylated form stimulates the secretion of insulin both through a direct effect on pancreatic islet cells, and indirectly by stimulating the secretion of glucagon-like peptide-1 from the small intestine.19 In addition to stimulating insulin secretion through these mechanisms, uncarboxylated osteocalcin also increases insulin sensitivity,20 the combined effect of which would be expected to reduce blood glucose. The conversion of osteocalcin to its carboxylated form, which lacks effects on glucose metabolism, is vitamin K-dependent and inhibited by warfarin.21 Thus, there is a previously postulated mechanism for warfarin to lower blood glucose. Although warfarin’s effects on blood glucose have not been studied in human trials,19 in diabetic rats it increases insulin secretion and reduces fasting glucose.22 Moreover, a positive feedback loop has been postulated in which uncarboxylated ostocalcin stimulates insulin secretion, which further stimulates osteoblasts to produce uncarboxylated osteocalcin, the metabolism of which is inhibited by warfarin.19 Such a positive feedback loop would be expected to result in a more marked hypoglycemic effect of warfarin after prolonged exposure, as is seen in Figure 1. The increased rate of serious hypoglycemia with warfarin observed in users of metformin (which we had originally included as a negative control object drug) supports a previously hypothesized hypoglycemic effect of warfarin. It also suggests that the increased risk may be attributable primarily to a hypoglycemic effect of warfarin itself, rather than to a drug interaction between warfarin and either sulfonylureas or metformin, considering that the magnitude of the elevated risk was similar between sulfonylureas and metformin both in the current study and the earlier study.17 Further research is needed to better understand underlying mechanisms.
This study has important strengths. It employed a large healthcare database, which allowed examination of antidiabetes drugs individually rather grouping them, and to examine associations over different risk windows of concomitancy. The study used an established, well-performing algorithm to identify serious hypoglycemia.23-24 The self-controlled case series research design inherently avoids confounding by time-invariant patient factors, and permits control for potential time-varying confounders, including apparent dose of the antidiabetes drug. Further, we conducted several sensitivity analyses that suggest that our results are robust.
This study also has limitations. First, it used administrative claims data, which lack information on actual ingestion of prescribed drugs, non-medical or non-prescription drug therapy, dietary habits, and other health behaviors. However, such factors could have influenced the observed associations only if and to the degree that they varied within person and were temporally associated with warfarin use within individual. Further, the magnitude of association seen in Medicaid enrollees, who tend to be vulnerable, may not reflect the magnitude in other populations. Therefore, further research in other populations is warranted. However, there is no reason to believe that the relationships should be dramatically different in commercially-insured populations.25
In conclusion, we found that warfarin was associated with an elevated rate of serious hypoglycemia when used concomitantly with either a sulfonylurea or metformin, and that this elevation was especially pronounced after prolonged concomitant therapy. These results are consistent with a previously hypothesized hypoglycemic effect of warfarin in persons with type-2 diabetes, which might be explained by warfarin’s inhibition of the carboxylation of osteocalcin and a positive feedback loop. Our findings suggest that physicians and patients need to be vigilant about the potential elevated risk of serious hypoglycemia associated with the use of warfarin during sulfonylurea or metformin therapy, especially with prolonged concomitant therapy.
Methods
Overview of study design and data
We examined the RRs for serious hypoglycemia associated with warfarin as the precipitant drug among users of widely-used sulfonylureas (glimepiride, glipizide, and glyburide (which account for more than 99% of all sulfonylureas in our data)) as object drugs and in users of metformin as a negative control object drug. The use of a negative control object drug helps to distinguish a drug-drug interaction from the inherent effect of the precipitant drug itself and to avoid confounding by indication.16 We used the self-controlled case series research design,26-28 which includes only persons who experienced the outcome of interest within the observation time (in this case, time exposed to a sulfonylurea or metformin), using each person as their own control. This design inherently eliminates confounding by factors that do not change within individual over the observation period.29 We also conducted sensitivity analyses to examine the robustness of our primary results by 1) controlling for average daily dose of antidiabetes drugs; 2) performing the analysis by concomitancy-triggering drug (i.e., warfarin-triggered, antidiabetes-triggered, and combination-triggered group)16; 3) excluding individuals who had death outcome during the observation time; and 4) excluding individuals with potentially incomplete data (operational definition: individuals with a managed care plan, a private health insurance, restricted benefits, or among Medicaid-Medicare dual-enrollees, individuals enrolled in a group health organization or a Medicare Advantage plan for which the Centers for Medicare and Medicaid Services does not process provider claims).
We used data from five US Medicaid programs (California, Florida, New York, Ohio, and Pennsylvania) from 1999 to 2011,25 supplemented with Medicare claims for the Medicaid-Medicare dual enrollees for the same period (for better capture of health care claims30), including Medicare Part D data for 2006-2011 (the Medicare Part D program began in 2006). These five states include nearly 40% of the nationwide Medicaid population.31 We linked these data to the Social Security Administration Death Master File to ascertain deaths. Adults (18 ≤ age < 100 years) who had continuous enrollment in Medicaid for at least six months before the first observation time were included.
Exposure of interest and covariates
The exposure of interest was the concomitant use of warfarin with either a sulfonylurea or metformin during observation time (described below). Since the self-controlled case series design inherently controls for static patient factors, we adjusted only for the time-varying covariates listed in Table 3, including: 1) drug that may cause hypoglycemia (e.g., angiotensin-converting enzyme inhibitors; angiotensin II receptor antagonists; beta blockers; quinolones; monoamine oxidase inhibitors, etc.); 2) drugs that may cause hyperglycemia (e.g., thiazide and thiazide-like diuretics; corticosteroids; protease inhibitors; retinoid; atypical antipsychotics; calcineurin inhibitors); 3) drugs that interact with sulfonylureas (e.g., CYP2C9 inhibitors and CYP3A4 inhibitors); 4) major acute condition that may affect hypoglycemia (e.g., acute infection); and 5) additionally in the sensitivity analysis, average daily dose of object antidiabetes drugs as a continuous variable, which was defined as the multiplication of the quantity and strength of the prescribed drug divided by the days’ supply of the prescribed drug. In this sensitivity analysis, we excluded as potentially implausible average daily doses greater than two times maximum daily dose of each object drug; glimepiride 16 mg/day; glipizide 80 mg/day; glyburide 40 mg/day; and metformin 5100 mg/day.
Table 3.
Pre-specified time-varying covariates included in the conditional Poisson regression model
| Category | Component | Description | Identification method |
|---|---|---|---|
| Drugs that interact with sulfonylureas | ACE inhibitorsa | benazepril, enalapril, lisinopril, perindopril, ramipril, captopril, fosinopril, moexipril, quinapril, trandolapril | NDC, dispensing date, days' supply |
| ARBsa | losartan, candesartan, valsartan, irbesartan, eprosartan, olmesartan, telmisartan, azilsartan | NDC, dispensing date, days' supply | |
| Beta blockersa | acebutolol, atenolol, betaxolol, bisoprolol, carteolol, carvedilol, labetalol, metoprolol, nadolol, nebivolol, penbutolol, pindolol, propranolol, sotalol, timolol | NDC, dispensing date, days' supply | |
| MAO inhibitorsa | isocarboxazid, phenelzine, selegiline, tranylcypromine | NDC, dispensing date, days' supply | |
| Quinolonesb | cinoxacin, ciprofloxacin, enoxacin, gatifloxacin, gemifloxacin, grepafloxacin, levofloxacin, lomefloxacin, moxifloxacin, nalidixic acid, norfloxacin, ofloxacin, sparfloxacin, trovafloxacin | NDC, dispensing date, days' supply | |
| Salicylatesa | aspirin, choline salicylate, magnesium salicylate, magnesium salicylate tetrahydrate, salsalate, sodium salicylate | NDC, dispensing date, days' supply | |
| Othersa | sulfamethoxazole, trimethoprim, haloperidol, quinidine, quinine, pentamidine, chloramphenicol, chloroquine, clofibrate, disopyramide | NDC, dispensing date, days' supply | |
| Drugs that can cause hyperglycemia | Atypical antipsychoticsa | aripiprazole, clozapine, iloperidone, lurasidone, olanzapine, paliperidone, quetiapine, risperidone, ziprasidone | NDC, dispensing date, days' supply |
| Calcineurin inhibitorsa | cyclosporine, sirolimus, tacrolimus | NDC, dispensing date, days' supply | |
| Corticosteroidsa | betamethasone, budesonide, cortisone, dexamethasone, fluodrocortisone, hydrocortisone, methylprednisolone, prednisolone, prednisone, triamcinolone | NDC, dispensing date, days' supply | |
| Protease inhibitorsa | amprenavir, atazanavir, darunavir, fosamprenavir, indinavir, lopinavir, nelfinavir, ritonavir, saquinavir, tipranavir | NDC, dispensing date, days' supply | |
| Retinoida | tretinoin, isotretinoin, acitretin | NDC, dispensing date, days' supply | |
| Thiazide and thiazide-like diureticsa | bendroflumethiazide, benthiazide, chlorothiazide, chlorthalidone, hydrochlorothiazide, hydroflumethiazide, indapamide, methyclothiazide, metolazone, polythiazide, trichlormethiazide | NDC, dispensing date, days' supply | |
| Drugs that interact with sulfonylureas | CYP2C9 inhibitors | fluconazoleb, cotrimoxazoleb, fenofibratea | NDC, dispensing date, days' supply |
| CYP3A4 inhibitors | azithromycinb, clarithromycinb, erythromycinb, simvastatina, gemfibrozila | NDC, dispensing date, days' supply | |
| Major non-chronic condition that may affect hypoglycemia | Acute infectionb | acute infection identified at any position of discharge diagnosis on inpatient or outpatient claims | ICD-9-CM diagnosis codes, admission or service date |
| Average daily dose of object drugs* | Average daily dose of sulfonylureas and metforminc | defined by [(prescription quantity × strength) / (prescription days’ supply)] | NDC, dispensing date, days' supply, quantity, strength |
NDC: National Drug Code. CYP: cytochrome P450 enzyme. ICD-9-CM: International Classification of Diseases 9th Revision Clinical Modification. CPT: Current Procedural Terminology. HCPCS: Healthcare Common Procedure Coding System. ACE: angiotensin-converting enzyme. ARB: angiotensin II receptor antagonists. MAO: monoamine oxidase.
Measured as a day-level binary variable indicating being dispensed on the current day (refers to each day during the observation time as current) or any time during the 31 days prior to the current day.
Measured as a day-level binary variable indicating being dispensed on the current day or any time during the 15 days prior to the current day.
Measured as a day-level continuous variable on the current day, based on the prescription active on the current day.
covariates additionally adjusted in the sensitivity analysis.
Outcome of interest
The outcome of interest was serious hypoglycemia, and ascertained by the following International Classification of Diseases 9th Revision Clinical Modification (ICD-9-CM) discharge diagnosis codes appearing in the principal position of an inpatient claim or in any position of an emergency department claim: 251.0 (hypoglycemic coma); 251.1 (other specific hypoglycemia); 251.2 (hypoglycemia, unspecified); or 280.8X (diabetes with other specified manifestations), unless accompanied by one of the exclusionary diagnosis codes suggesting manifestations other than hypoglycemia (presented in Table 4). This algorithm has a positive predictive value of 89%23 in emergency department claims and 78%24 in inpatient claims.
Table 4.
Operational definition of the outcome of interest and performance measure of the ascertainment algorithm
| Outcome | ICD-9-CM code | Diagnosis description | Diagnosis position and claim type |
Performance of algorithm |
|
|---|---|---|---|---|---|
| Serious hypoglycemia | 251.0 | hypoglycemic coma | Any position of discharge diagnosis on ED claim or principal position on inpatient claim | PPV ~89%23 (ED claim); 78%24 (inpatient claim) | |
| 251.1 | other specific hypoglycemia | ||||
| 251.2 | hypoglycemia, unspecified | ||||
| 250.8X | diabetes with other specified manifestations | ||||
| Exclusionary diagnosis codes in the occurrences of 250.8X23-24 | |||||
| 259.8 | Other specified endocrine disorders | Secondary diabetic glycogenosis | Any position of discharge diagnosis on ED claim or principal position on inpatient claim | ||
| 272.7 | Mixed hyperlipidemia | Diabetic lipidosis | |||
| 681.XX | Cellulitis and abscess of finger and toe | Cellulitis | |||
| 682.XX | Other cellulitis and abscess | ||||
| 686.9 | Unspecified local infection of skin and subcutaneous tissue | ||||
| 707.1X | Ulcer of lower limbs, except decubitus ulcer | Ulcers of the lower extremity | |||
| 707.2X | Pressure ulcer stages | ||||
| 707.8 | Chronic ulcer of other specified sites | ||||
| 707.9 | Chronic ulcer of unspecified site | ||||
| 709.3 | Degenerative skin disorders | Necrobiosis lipoidica diabeticorum | |||
| 730.0X | Acute osteomyelitis | Osteomyelitis | |||
| 730.1X | Chronic osteomyelitis | ||||
| 730.2X | Unspecified osteomyelitis | ||||
| 731.8 | Other bone involvement in diseases classified elsewhere | ||||
ED: emergency department. ICD-9-CM: International Classification of Diseases, 9th Revision, Clinical Modification. PPV: positive predictive value.
Study cohorts
We constructed our study cohorts for each object drug (glimepiride, glipizide, glyburide, or metformin) separately. We constructed an object drug’s episodes by using dispensing date and days’ supply fields from prescription claims. An episode was defined as a unit of continuous prescriptions that only allowed a grace period to allow for potential incomplete adherence, a 14-day gap in our study, between contiguous prescriptions and at the end of the last prescription. Warfarin episodes were constructed by the same method. The object drug episodes served as the basis of observation time, and individuals were allowed to contribute more than one episode and more than one outcome occurrence within an episode included in the analysis if inclusion/exclusion and baseline period criteria were met. We included only observation episodes during which the outcome of interest occurred at least once (i.e., once or more; not restricted to incident events), regardless of whether it occurred during a period of warfarin exposure or non-exposure.
Observation time and baseline periods
Observation time comprised of object antidiabetes drug episodes in which at least one outcome event occurred. An object drug’s episode began at the first dispensing date of that episode and ended by the first occurrence of the following: a) end of days’ supply of the episode, including a 14-day grace period); b) Medicaid enrollment discontinuation; c) death; and d) end of dataset (i.e., December 31, 2011). The occurrence of the outcome of interest (i.e., serious hypoglycemia) did not end the object drug’s episode based on the independent exposure assumption of the self-controlled case series design to avoid bias from reverse-causality. As death is a reason that permanently ends the observation time, we also performed a sensitivity analysis (described below) that only included individuals alive throughout the observation time. Person-days with concomitant use of object and precipitant drugs were categorized into four pre-specified risk windows by the number of days since the initiation of concomitant use: a) 0-30 days, b) 31-60 days, c) 61-120 days, and d) >120 days.
To reduce potential bias in estimation from the uncertain, incomplete capture of data (e.g., unseen prescriptions during the Medicaid disenrollment period that do not enable us to identify the correct start date of an object drug episode and/or the concomitant use of a precipitant drug from the Medicaid claims data), we applied a 6-month baseline period criterion immediately before the first object drug’s episode, which was devoid of: a) Medicaid enrollment gap; b) death; and c) a prescription claim for the object drug. In case an individual dis-enrolled from Medicaid after contributing to an observation time and re-enrolled in Medicaid later, we applied again the baseline period criterion immediately before the first object drug episode identified since the re-enrollment and the requirement of at least one outcome occurrence during the observation time.
Statistical analysis
We performed a conditional Poisson regression analysis,9 estimating occurrence RRs (i.e., outcome occurrence rate during exposed time vs. unexposed time; exposed time refers to time of concomitant use of the antidiabetes drug with warfarin, and unexposed time refers to time of use of the antidiabetes drug without warfarin) and 95% confidence intervals. The dependent variable was an indicator variable for the outcome occurrence. Independent variables were: indicator of concomitant use (i.e., exposure to warfarin) status; and the pre-specified time-varying covariates presented in Table 1 and Table 3. The unit of analysis was a person-day of observation time. For each antidiabetes drug, we examined the RR for warfarin across all risk windows combined and within the pre-specified risk windows listed above.
All analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC). This study was approved by the institutional review board of the University of Pennsylvania, which waived the obtainment of informed consent.
Supplementary Material
Table S1. Sensitivity analysis: Confounder-adjusted outcome occurrence rate ratios for the association between warfarin and serious hypoglycemia when used concomitantly with a sulfonylurea or metformin with additional adjustment for the average daily dose of object drug
Table S2. Sensitivity analysis: Outcome occurrence rate ratios for the association between warfarin and serious hypoglycemia when used concomitantly with a sulfonylurea or metformin, by concomitancy-triggering drug
Table S3. Sensitivity analysis: Confounder-adjusted outcome occurrence rate ratios for the association between warfarin and serious hypoglycemia when used concomitantly with a sulfonylurea or metformin, excluding individuals who had death outcome during the observation time
Table S4. Sensitivity analysis: Confounder-adjusted outcome occurrence rate ratios for the association between warfarin and serious hypoglycemia when used concomitantly with a sulfonylurea or metformin, excluding individuals with potentially incomplete data
Study Highlights.
-
What is the current knowledge on the topic?
Warfarin, when given concomitantly with some sulfonylureas, may increase the risk of serious hypoglycemia. However, the clinical importance of this potential effect is unclear.
-
What question did this study address?
This study investigated whether warfarin use in combination with either sulfonylureas or metformin increases the risk of serious hypoglycemia.
-
What does this study add to our knowledge?
This study found that warfarin was associated with an increased risk of serious hypoglycemia when used concomitantly with either a commonly used sulfonylurea (glimepiride, glipizide, or glyburide) or metformin, and that the increase in the risk was especially pronounced after prolonged concomitant therapy.
-
How might this change clinical pharmacology or translational science?
The results are consistent with a previously hypothesized hypoglycemic effect of warfarin in type-2 diabetes patients, which might be explained by warfarin’s inhibition of the carboxylation of osteocalcin and a positive feedback loop. Physicians and patients will need to be vigilant about the potential increase in the risk of serious hypoglycemia associated with the use of warfarin during sulfonylurea or metformin therapy, especially with prolonged concomitant therapy.
Acknowledgments
The authors thank Ms. Qing Liu and Ms. Min Du of the Center for Clinical Epidemiology and Biostatistics, University of Pennsylvania, for their assistance with biostatistics computer programming.
Funding: This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK102694; Recipient: SH) and the National Institute on Aging (R01AG025152; Recipient: SH) of the National Institutes of Health. These organizations had no role in the design and conduct of the study, data collection and analysis, interpretation of the results, writing and review of the manuscript, or the decision to submit the manuscript for publication.
Footnotes
Conflict of Interest/Disclosure
S.H. consulted for Merck Research Laboratories on topics unrelated to antidiabetes drugs, and leads a training program that receives support from Pfizer Inc. and Sanofi unrelated to this submitted work. All the other authors declare no conflict of interest.
References
- 1.U.S. Department of Health and Human Services, Office of Disease Prevention and Health Promotion. National Action Plan for Adverse Drug Event Prevention < https://health.gov/hcq/pdfs/ade-action-plan-508c.pdf> (2014). Accessed 6 December 2017.
- 2.Holstein A, Holstein JD, Patzer OM, Stumvoll M, Machalke K & Kovacs P. Substantial increase in incidence of severe hypoglycemia between 1997-2000 and 2007-2010. Diabetes Care. 35(5), 972–975 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Budnitz DS, Lovegrove MC, Shehab N & Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N. Engl. J. Med. 365, 2002–2012 (2011). [DOI] [PubMed] [Google Scholar]
- 4.Gurwitz JH. et al. Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA. 289, 1107–1116 (2003). [DOI] [PubMed] [Google Scholar]
- 5.Budnitz DS, Pollock DA, Weidenbach KN, Mendelsohn AB, Schroeder TJ & Annest JL. National surveillance of emergency department visits for outpatient adverse drug events. JAMA. 296, 1858–1866 (2006). [DOI] [PubMed] [Google Scholar]
- 6.Shehab N, Lovegrove MC, Geller AI, Rose KO, Weidle NJ & Budnitz DS. US emergency department visits for outpatient adverse drug events, 2013-2014. JAMA. 316(20), 2115–2125 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han X. et al. Biomedical informatics approaches to identifying drug-drug interactions: Application to insulin secretagogues. Epidemiol. 28(3), 459–468 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirchheiner J, Roots I, Goldammer M, Rosenkranz B & Brockmoller J. Effect of genetic polymorphisms in cytochrome P450 (CYP) 2C9 and CYP2C8 on the pharmacokinetics of oral antidiabetic drugs: clinical relevance. Clin. Pharmacokinet. 44(12), 1209–1225 (2005). [DOI] [PubMed] [Google Scholar]
- 9.Niemi M. et al. Glyburide and glimepiride pharmacokinetics in subjects with different CYP2C9 genotypes. Clin. Pharmacol. Ther. 72(3), 326–332 (2002). [DOI] [PubMed] [Google Scholar]
- 10.Kidd RS. et al. Pharmacokinetics of chlorpheniramine, phenytoin, glipizide and nifedipine in an individual homozygous for the CYP2C9*3 allele. Pharmacogenetics. 9(1), 71–80 (1999). [DOI] [PubMed] [Google Scholar]
- 11.Zharikova OL. et al. Identification of the major human hepatic and placental enzymes responsible for the biotransformation of glyburide. Biochem. Pharmacol. 78(12), 1483–1490 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou L. et al. Contributions of human cytochrome P450 enzymes to glyburide metabolism. Biopharm. Drug. Dispos. 31(4), 228–242 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rettie AE & Jones JP. Clinical and toxicological relevance of CYP2C9: Drug-Drug Interactions and Pharmacogenetics. Annu. Rev. Pharmacol. Toxicol. 45, 477–494 (2005). [DOI] [PubMed] [Google Scholar]
- 14.Miners JO & Birkett DJ. Cytochrome P4502C9: An enzyme of major importance in human drug metabolism. Br. J. Clin. Pharmacol. 45, 525–538 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams PA, Cosme J, Ward A, Angove HC, Vinkovic DM & Jhoti H. Crystal structure of human cytochrome P450 2C9 with bound warfarin. Nature. 424(6947), 464–468 (2003). [DOI] [PubMed] [Google Scholar]
- 16.Hennessy S. et al. Pharmacoepidemiologic methods for studying the health effects of drug-drug interactions. Clin. Pharmacol. Ther. 99(1), 92–100 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romley JA. et al. Association between use of warfarin with common sulfonylureas and serious hypoglycemic events: retrospective cohort analysis. BMJ. 351, h6223 (2015). doi: 10.136/bmj.h6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leonard CE. et al. Comparative risk of serious hypoglycemia with oral antidiabetes monotherapy: A retrospective cohort study. Pharmacoepidemiol. Drug Saf. 27(1), 9–18 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mizokami A, Kawakubo-Yasukochi T & Hirata M. Osteocalcin and its endocrine functions. Biochem. Pharmacol. 132, 1–8 (2017). doi: 10.1016/j.bcp.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Fulzele K. et al. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell. 142, 309–319 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferron M, Wei J, Yoshizawa T, Ducy P & Karsenty G. An ELISA-based method to quantify osteocalcin carboxylation in mice. Biochem. Biophys. Res. Com. 397(4), 691–696 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gamal SM, Sadek NB, Rashed LA, Shawky HM & Gamal El-Din. Effect of gamma-carboxylase inhibition on serum osteocalcin may be partially protective against developing diabetic cardiomyopathy in type 2 diabetic rats. Diab. Vasc. Dis. Res. 13(6), 405–417 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Ginde AA, Blanc PG, Lieberman RM & Camargo CA Jr.. Validation of ICD-9-CM coding algorithm for improved identification of hypoglycemia visits. BMC. Endocr. Disord. 8:4 (2008). doi: 10.1186/1472-6823-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schelleman H, Bilker WB, Brensinger CM, Wan F & Hennessy S. Anti-infectives and the risk of severe hypoglycemia in users of glipizide or glyburide. Clin. Pharmacol. Ther. 88(2), 214–222 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hennessy S, Freeman CP & Cunningham F 2012. US Government Claims Databases In Pharmacoepidemiology 5th ed (ed. Strom BL). 209-223 (John Wiely & Sons, Sussex, United Kingdom, 2012). ISBN: 978-0-470-65475-0. [Google Scholar]
- 26.Whitaker HJ, Farrington CP, Spiessens B & Musonda P. Tutorial in biostatistics: The self-controlled case series method. Stat. Med. 25, 1768–1797 (2006). [DOI] [PubMed] [Google Scholar]
- 27.Whitaker HJ, Hocine MN & Farrington CP. The methodology of self-controlled case series studies. Stat. Methods. Med. Res. 18, 7–26 (2009). [DOI] [PubMed] [Google Scholar]
- 28.Weinberg CR. Invited commentary: Self-control is a virtue. Am. J. Epidemiol. 185(11), 1184–1186 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Petersen I, Douglas I & Whitaker H. Self controlled case series methods: an alternative to standard epidemiological study designs. BMJ. 354, i4515 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Hennessy S, Leonard CE, Weber A & Strom BL. Descriptive analyses of the integrity of a US Medicaid claims database. Pharmacoepidemiol. Drug Saf. 12(2), 103–111 (2003). [DOI] [PubMed] [Google Scholar]
- 31.Kaiser Family Foundation. Medicaid State Fact Sheets. < https://www.kff.org/interactive/medicaid-state-fact-sheets/> (2017). Accessed 19 January 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Sensitivity analysis: Confounder-adjusted outcome occurrence rate ratios for the association between warfarin and serious hypoglycemia when used concomitantly with a sulfonylurea or metformin with additional adjustment for the average daily dose of object drug
Table S2. Sensitivity analysis: Outcome occurrence rate ratios for the association between warfarin and serious hypoglycemia when used concomitantly with a sulfonylurea or metformin, by concomitancy-triggering drug
Table S3. Sensitivity analysis: Confounder-adjusted outcome occurrence rate ratios for the association between warfarin and serious hypoglycemia when used concomitantly with a sulfonylurea or metformin, excluding individuals who had death outcome during the observation time
Table S4. Sensitivity analysis: Confounder-adjusted outcome occurrence rate ratios for the association between warfarin and serious hypoglycemia when used concomitantly with a sulfonylurea or metformin, excluding individuals with potentially incomplete data