Abstract
Bacteroides fragilis (BF) is an integral component of the human colonic commensal microbiota. BF is also the most commonly isolated organism from clinical cases of intra-abdominal abscesses, suggesting its potential to induce proinflammatory responses upon accessing the systemic compartment. Hence, we examined the impact of mucosal and systemic exposures to BF on type 1 diabetes (T1D) incidence in NOD mice. The impact of intestinal exposure to BF under a chemically induced enhanced gut permeability condition, which permits microbial translocation, in T1D was also examined. While oral administration of heat-killed (HK) BF to prediabetic mice caused enhanced immune regulation and suppression of autoimmunity, resulting in delayed hyperglycemia, mice that received HK BF by intravenous injection showed rapid disease progression. Importantly, polysaccharide A–deficient BF failed to produce these opposing effects upon oral and systemic deliveries. Furthermore, BF-induced modulation of disease progression was observed in wild-type, but not TLR2-deficient, NOD mice. Interestingly, oral administration of BF under enhanced gut permeability conditions resulted in accelerated disease progression and rapid onset of hyperglycemia in NOD mice. Overall, these observations suggest that BF-like gut commensals can cause proinflammatory responses upon gaining access to the systemic compartment and contribute to T1D in at-risk subjects.
Introduction
While gut commensal microbes contribute profoundly to human health and immune system maturation (1,2), many reports also have supported the notion that gut commensals can contribute to the pathogenesis of autoimmune diseases like type 1 diabetes (T1D) (3–5). It has been proposed that three mutually linked features, namely, 1) aberrant gut microbiota, 2) compromised intestinal epithelial barrier, and 3) altered immune responsiveness, are linked to the contribution of commensal organisms to these diseases (6). Gut pathobionts and proinflammatory immune responses can compromise gut integrity, leading to the translocation of microbial components to the systemic compartment and, consequently, to systemic inflammation and autoimmune progression (7–9).
Bacteroides fragilis (BF), a Gram-negative anaerobe and an integral component of the colonic commensal microbiota of many mammals (10), has been widely studied as a model organism to determine the influence of commensal bacteria in immune regulation (11–13). Although BF represents a very small portion of the gut microbes (14), it is the most commonly isolated bacterium from clinical cases of intra-abdominal abscesses (15,16), perhaps because of its ability to colonize the colonic crypt (11). While capsular polysaccharides of BF are found to be essential for abscess formation (16,17) as well as for growth (18), these components, polysaccharide A (PSA) in particular, have been demonstrated to have the ability to promote immune regulation by directly acting on dendritic cells (DCs) and regulatory T cells (Tregs) in a toll-like receptor (TLR) 2–dependent manner (11,19–21). These paradoxical effects of BF suggest that intestinal mucosa and systemic compartments may produce different responses upon exposure to this commensal. Although the association between intra-abdominal abscesses and T1D incidence in humans is unknown or uninvestigated, many recent clinical and preclinical studies, including those involving large longitudinal cohorts of children of The Environmental Determinants of Diabetes in the Young (TEDDY) study, have associated higher prevalence of Bacteroides (phylum Bacteroidetes) members, including BF, with T1D disease incidence/progression in humans and preclinical models (4,22–32). Furthermore, it has been shown that increased abundance of Bacteroides species (spp.) precedes T1D onset in children (32). However, whether gut commensals, such as BF or other Bacteroides members, directly affect T1D autoimmune susceptibility has not been tested directly.
Using BF as a model, we show that intestinal and systemic exposures to specific commensal organisms have opposing effects on autoimmune progression and T1D incidence in the NOD mouse model. While oral administration of heat-killed (HK) BF to prediabetic mice caused enhanced immune regulation and significant suppression of autoimmunity, resulting in delayed hyperglycemia, mice that received small amounts of HK BF by intravenous (i.v.) injection showed rapid disease progression. Interestingly, only the wild-type (WT), but not the PSA-deficient (ΔPSA), BF produced opposing disease outcomes upon oral and systemic administrations. Furthermore, these effects were observed only in WT, but not in TLR2-deficient, NOD mice. Most importantly, oral administration of BF in NOD mice under enhanced gut permeability recapitulated the T1D accelerating effect of systemic administration with HK BF. These results suggest that systemic access by a gut symbiont, such as BF, in the event of compromised gut barrier function can act as a trigger and catalyst for autoimmunity in T1D in at-risk subjects.
Research Design and Methods
Mice and Bacteria
WT NOD/ShiLtJ (NOD), NOD-BDC2.5 TCR transgenic (NOD-BDC2.5), and NOD-Rag1–deficient (NOD-Rag1) mice were purchased from The Jackson laboratory (Bar Harbor, ME). Foxp3-GFP-knockin (Foxp3-GFP) mice in the B6 background were provided by Dr. Vijay Kuchroo (Harvard Medical School). NOD-WT mice from our breeding colony were used in this study. NOD-Foxp3-GFP mice were generated by backcrossing B6-Foxp3-GFP mice to the NOD background for 12 generations. NOD-BDC2.5-Foxp3-GFP mice were generated by crossing NOD-BDC2.5 mice with NOD-Foxp3-GFP mice. NOD-TLR2 knockout (NOD-TLR2-KO) mice were provided by Dr. Alexander Chervonsky (The University of Chicago). All animal studies were approved by the animal care and use committee of the Medical University of South Carolina. To detect hyperglycemia, glucose levels in blood collected from the tail vein were determined at timely intervals using the Ascensia Contour Microfill Blood Glucose Monitoring System (Bayer, Pittsburgh, PA). Mice with a glucose level of >250 mg/dL for two consecutive tests were considered diabetic.
BF (ATCC 25285; National Collection of Type Cultures 9343) and isogenic ΔPSA-BF (provided by Dr. Laurie Comstock, Harvard Medical School) were cultured from single colonies in brain-heart infusion (BHI) medium under anaerobic conditions for up to 72 h, diluted 50-fold using complete medium, and cultured for an additional 16 h. Bacterial cells were pelleted and washed with PBS twice by centrifugation and incubated at 65°C for 30 min for heat inactivation. HK bacterial preparations were tested for viable bacteria, if any, by plating and culturing aliquots of these preparations on BHI agar plates for 72 h. Listeria monocytogenes (LM) (ATCC 19111) cultured in BHI medium under aerobic conditions was also processed similarly. These HK bacterial preparations were subjected to an additional quick wash in sterile distilled water to minimize salt content, and the pellet was air-dried and dry weight determined, before suspending in 20 mg/mL (w/v) PBS, as stock suspension. These stock solutions containing whole bacteria were diluted in sterile PBS as needed for in vivo studies.
Peptide Antigens, Cell Lines, and Antibodies
Immunodominant β-cell antigen peptides—viz., 1) insulin B (9–23), 2) GAD65 (206–220), 3) GAD65 (524–543), 4) IA-2β (755–777), 5) IGRP (123–145), and 6) BDC2.5 TCR-reactive peptide (YVRPLWVRME, referred to as BDC peptide)—were custom synthesized (GenScript). Peptides 1–5 were pooled at an equal molar ratio and used as β-cell antigen peptide cocktail as described in our earlier study (33). Phorbol myristic acid (PMA), ionomycin, brefeldin A, monensin, magnetic bead–based T-cell and DC enrichment kits, ELISA and magnetic bead–based multiplex cytokine kits and unlabeled and fluorochrome-labeled antibodies, FITC-dextran, and other key reagents were purchased from Sigma-Aldrich, BD Biosciences, eBioscience, Invitrogen, Millipore, Miltenyi Biotec, STEMCELL Technologies, R&D Systems, and BioLegend. ELISAs were read using a Bio-Rad iMark 96-well plate reader. Luminex technology–based multiplex assays were read using FLEXMAP 3D or Bio-Plex 100 instruments. Real-time PCR assays were performed using transcript-specific or bacterial 16S rDNA–specific primer sets and SYBR Green PCR master mix. Flow cytometry data were acquired using FACSCalibur, FACSVerse, or CyAn ADP instruments and analyzed using Summit or Cytobank applications.
Treatment of Mice With HK BF and Peptides
Female NOD-WT mice were administered HK BF (WT or ΔPSA) orally (500 μg/mouse/day for 15 days) or injected intravenously (i.v.) (10 μg/mouse/day every 3rd day for 15 days) and examined for blood glucose levels every week over 30 weeks posttreatment initiation to detect hyperglycemia. Control mice were left untreated or received PBS as carrier buffer. In some experiments, mice that were treated as described above for 15 days or for 3 consecutive days were euthanized either 24 h or 15 days posttreatment. In some experiments, NOD-WT, NOD-Foxp3-GFP, or NOD-BDC2.5-Foxp3-GFP mice were administered orally or by i.v. injection bacteria alone or along with β-cell antigen peptide cocktail or BDC2.5 peptide. Spleen, pancreatic lymph node (PnLN), mesenteric lymph node (MLN), Peyer patch (PP), small intestinal lamina propria (SiLP), and/or large intestinal lamina propria (LiLP) cells from treated and control mice were examined for cytokine responses and/or T-cell phenotypes. In some experiments, serum samples were subjected to Luminex multiplex assays to detect cytokines.
DCs and T Cells, and In Vitro and In Vivo Assays
CD11c+ DCs were enriched from spleen cells using magnetic separation kits and reagents from Miltenyi Biotec and/or STEMCELL Technologies. SiLP and LiLP cells were prepared by collagenase digestion and density gradient centrifugation for enriching immune cells (34), and followed by CD11c+ DCs by magnetic separation. T cells were enriched from spleens of treated and untreated control mice using negative selection kits. In antigen presentation assays, DCs that were exposed to HK BF in vivo were pulsed with BDC2.5 peptide, washed, and incubated with CD4+ T cells from NOD-BDC2.5 or NOD-BDC2.5-Foxp3-GFP mice in 96-well plates. After 4 days of culture, cells were stained for CD4 and examined for Foxp3 or GFP expression and/or intracellular cytokines (after 4 h stimulation using PMA and ionomycin) by FACS.
Adoptive T-Cell Transfer Experiment
Total T cells isolated from spleens of control and HK BF–treated mice were transferred i.v. into 8-week-old NOD-Rag1–deficient mice (1 × 106 cells/mouse) and tested for blood glucose levels every week to determine the diabetogenic properties of T cells.
Induction of Increased Gut Permeability
Mice were given 0.5% (w/v) dextran sulfate sodium (DSS) in drinking water for 5 days and switched to regular water for the rest of the monitoring period. Control and DSS-treated mice were given HK BF (500 μg/mouse/day) by oral gavage every day for up to 15 days starting day 0. Cohorts of these mice were euthanized on day 6 or day 30 or monitored for up to 20 weeks posttreatment. To determine gut permeability, in some experiments, DSS-treated and control mice were given FITC-dextran (4 kDa, 10 mg/mouse) on day 6 by oral gavage and euthanized after 4 h, and plasma samples were examined for FITC-dextran concentration by fluorimetry against control samples spiked with a known concentration of FITC-dextran. In some assays, whole blood collected from different groups of euthanized (by cardiac puncture using a sterile syringe) mice into sodium citrate vials were subjected to quantitative PCR (qPCR) assay using universal as well as BF-specific 16S rDNA–specific primer sets to determine the levels of bacterial DNA in circulation.
Histochemical and Immunofluorescence Analysis of Pancreatic Tissues
Pancreata were fixed in 10% formaldehyde, and 5-μm paraffin sections were made and stained with hematoxylin-eosin (H-E). Stained sections were analyzed using a 0–4 grading system as described in our earlier studies (35). At least 100 islets were examined for every group. In some experiments, pancreatic sections were stained using anti-insulin antibody followed by Alexa Fluor 488– or 568–linked secondary antibodies and DAPI and scored for insulitis on the basis of DAPI-positive cells in islet areas and insulin expression. Insulitis was scored as described for H-E–stained sections, and insulin-positive and -negative islets were counted.
Statistical Analysis
Statistical significance (P value) was calculated using GraphPad Prism and/or online statistical applications. Log-rank analysis (http://bioinf.wehi.edu.au/software/russell/logrank) was performed to compare T1D incidence (hyperglycemia). Fisher exact test was used for comparing the total number of severely infiltrated islets (grades ≥3) relative to the total number of islets with low or no infiltration (grades ≤2) in test versus control groups. Mann-Whitney (nonparametric, two-tailed) or unpaired t test (two-tailed) was used, unless otherwise specified, for values from ex vivo and in vitro assays. Paired t test (parametric, two-tailed) was used for cumulative values of multiple experiments that were not performed in parallel. Values of assays done in duplicate or triplicate were averaged for each mouse or pool of mice before calculating statistical significance. P ≤ 0.05 was considered statistically significant.
Data and Resource Availability
The data sets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request. No new resources were generated or analyzed during the current study.
Results
Exposure of Gut Mucosa to BF Results in Suppression of Autoimmune Progression
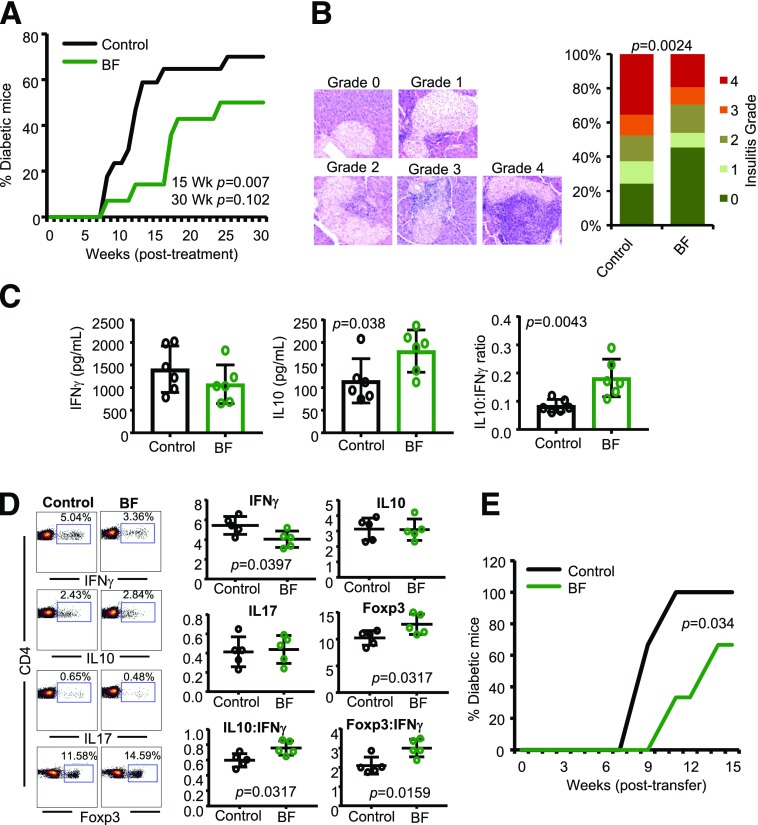
Hyperglycemia is detected in female NOD mice as early as 12 weeks of age, and 80–100% of the mice turn overtly hyperglycemic before the age of 30 weeks. Here, we used 10-week-old prediabetic NOD mice to determine the impact of oral administration of HK BF on autoimmune progression and T1D incidence. As shown in Fig. 1A, oral administration of NOD mice with HK BF (500 μg/day) for 15 days resulted in delayed hyperglycemia in majority of the mice, as indicated by significantly reduced T1D incidence in treated mice compared with controls at 15 and 30 weeks posttreatment. While >80% of the mice remained diabetes free at 15 weeks posttreatment, >60% of the control mice developed diabetes within this period. To determine the impact of orally administered BF on islet autoimmunity, one set of euglycemic mice was euthanized 30 days posttreatment, and the pancreatic tissues were examined for degree of insulitis. Low incidence of the disease in BF-treated mice was found to correlate with suppressed immune cell infiltration and a profoundly higher number of islets with no or modest immune cell infiltration (grades ≤2 insulitis) (Fig. 1B).
Figure 1.
Oral administration of BF results in suppression of autoimmunity and protection of NOD mice from T1D. Ten-week-old prediabetic female NOD mice were given HK BF for 15 consecutive days (500 μg/mouse/day; dry-weight equivalent). A: Cohorts of control (n = 17) and BF-treated (n = 14) mice were examined for blood glucose levels every week, and the mice with glucose levels of >250 mg/dL for 2 consecutive weeks were considered diabetic. The percentage of mice that developed diabetes at weeks (Wk) posttreatment initiation are shown. Statistical significance was assessed by log-rank test for up to 15 and 30 weeks of the monitoring periods. B: Cohorts of mice (n = 5/group) were euthanized 30 days posttreatment, and pancreatic tissue sections were subjected to H-E staining and examined for insulitis. Examples of islets with different insulitis grades (left) and the percentage islets with different insulitis grades (right) are shown. Grades are as follow: 0 = no evidence of infiltration, 1 = peri-islet infiltration (<5%), 2 = 5–25% islet infiltration, 3 = 25–50% islet infiltration, and 4 = >50% islet infiltration. At least 100 islets from intermittent sections were examined for each group. Statistical significance was assessed by Fisher exact test comparing relative numbers of islets with insulitis grades ≤2 and ≥3 between groups. C: PnLN cells of mice euthanized 15 days posttreatment (n = 6 mice/group) were cultured in triplicate in the presence of β-cell antigen peptide cocktail for 48 h, and the spent media was examined for self-antigen–induced cytokine release by Luminex multiplex assay. Cytokine concentrations and relative ratios were compared, and the statistical significance values were calculated by Mann-Whitney test. D: Fresh spleen cells from mice euthanized at 15 days posttreatment (n = 5 mice/group) were activated ex vivo in duplicate wells with PMA and ionomycin for 4 h and stained for intracellular cytokine levels or examined for Foxp3 levels without ex vivo activation. Percentage and relative ratios of cells positive for specific markers are shown, and the statistical significance was assessed by Mann-Whitney test. Experiments shown in panels C and D were repeated using four to six mice/group at least once with similar statistical trends in results. E: Enriched splenic T cells from control and BF-treated mice were adoptively transferred into 6-week-old NOD-Rag1 KO mice (1 × 106 cells/mouse), and the mice were monitored (n = 6 mice/group) for hyperglycemia as described above. Log-rank test was used for determining statistical significance.
To determine whether the autoreactivity of immune cells is modulated in mice orally administered with HK BF, PnLN cells were activated ex vivo using β-cell antigen peptide cocktail. PnLN cells from BF-treated mice produced significantly higher amounts of interleukin (IL) 10, and showed a profoundly higher IL-10:interferon (IFN)-γ production ratio compared with these cells from control mice (Fig. 1C). Oral BF treatment-induced modulation of systemic immune function was also assessed by examining the splenic T cells for their expression of cytokines and Foxp3. As shown in Fig. 1D, IFN-γ+ T-cell frequencies were lower and Foxp3+ cell frequencies were higher in BF-treated mice. Furthermore, overall IL-10+:IFN-γ+ and Foxp3+:IFN-γ+ cell ratios were significantly higher in these mice compared with controls. To further assess whether oral administration of BF affects the diabetogenic function of systemic immune cells, splenic T cells from BF-treated mice were adoptively transferred to NOD-Rag1 mice. Figure 1E shows that NOD-Rag1 mice that received T cells from BF-fed mice developed hyperglycemia at a significantly slower rate than mice that received T cells from control mice. Overall, these observations suggest that exposure of gut mucosa to BF results in enhanced immune regulation in the systemic compartment, including the pancreatic microenvironment, resulting in suppression of T1D incidence in NOD mice.
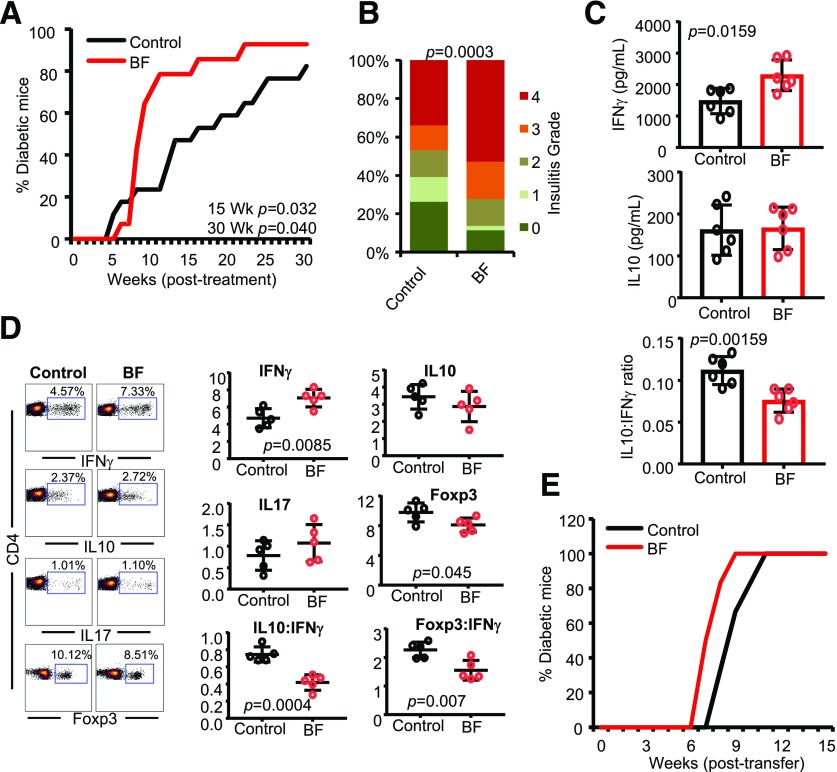
Exposure of Systemic Compartment to BF Results in Enhanced Proinflammatory Response and Accelerated T1D Onset
Because BF is known to cause peritonitis, intra-abdominal infections and abscesses upon gaining access to these compartments (15–17,36), and correlations of T1D autoimmunity with higher Bacteroides spp. abundances as well as overall microbial translocation (4,9,22–32,37), we determined the effect of systemic exposure to small amounts of HK BF on autoimmune progression and T1D incidence. Ten-week-old prediabetic NOD mice were treated i.v. with small amounts of BF (10 μg/day) every 3 days for 15 days and monitored for hyperglycemia. As observed in Fig. 2A, compared with controls, mice that received an i.v. injection of HK BF showed rapid onset of hyperglycemia and significantly higher overall diabetes incidence. Consistent with this observation, mice that were treated i.v. showed profoundly higher degrees of insulitis at 30 days posttreatment (Fig. 2B). Importantly, PnLN cells from mice that received systemic treatment showed significantly higher IFN-γ production and an overall low IL-10:IFN-γ production ratio upon ex vivo activation using β-cell antigen peptide cocktail compared with cells from control mice (Fig. 2C). Furthermore, compared with control mice, significantly higher IFN-γ+ and lower Foxp3+ T-cell frequencies were detected in the spleens of mice that were exposed to BF systemically (Fig. 2D). In addition, NOD-Rag1 mice that received splenic T cells from BF-injected mice showed a relatively faster onset of hyperglycemia, albeit not significant statistically, compared with those that received cells from control mice (Fig. 2E).
Figure 2.
Systemic (i.v.) administration of BF results in aggravated autoimmune response and rapid T1D onset in NOD mice. Ten-week-old prediabetic female NOD mice were given HK BF every 3rd day during a 15-day period (10 μg/mouse/injection; dry-weight equivalent) by i.v. injection and studied as detailed in Fig. 1. A: Cohorts of control (n = 17) and BF-treated (n = 14) mice were monitored for hyperglycemia as described for Fig. 1. Statistical significance was calculated by log-rank test for up to 15 and 30 weeks (Wk) of the monitoring periods. B: Cohorts of mice (n = 5/group) were euthanized 30 days posttreatment, pancreatic tissue sections were examined for insulitis, and the statistical significance was calculated by Fisher exact test. C: PnLN cells of mice (n = 6/group) were examined for self-antigen–induced cytokine levels by Luminex multiplex assay, and the statistical significance values were calculated by Mann-Whitney test. D: Fresh spleen cells from BF-treated mice (n = 5/group) and controls, 15 days posttreatment, were examined for intracellular cytokines and Foxp3 levels, and the statistical significance was calculated by Mann-Whitney test. Experiments shown in panels C and D were repeated using four to six mice/group at least once with similar statistical trends in results. E: Enriched splenic T cells from control and BF-treated mice were adoptively transferred into 6-week-old NOD-Rag1 KO mice (n = 6/group) and monitored for hyperglycemia.
The systemic effects of i.v. injection with BF in NOD mice (Fig. 2) are opposite to that produced by oral treatment with this agent (Fig. 1). To determine whether such contrasting effects are produced by other gut bacteria, prediabetic NOD mice were treated with HK LM by oral gavage or i.v. injection and examined for T1D incidence and insulitis. As observed in Supplementary Fig. 1, orally and systemically administered HK LM produced disease incidence and insulitis trends in NOD mice similar to that of HK BF–treated mice; however, these observations were, unlike those from BF-treated mice, not significant statistically by log-rank test and Fisher exact test.
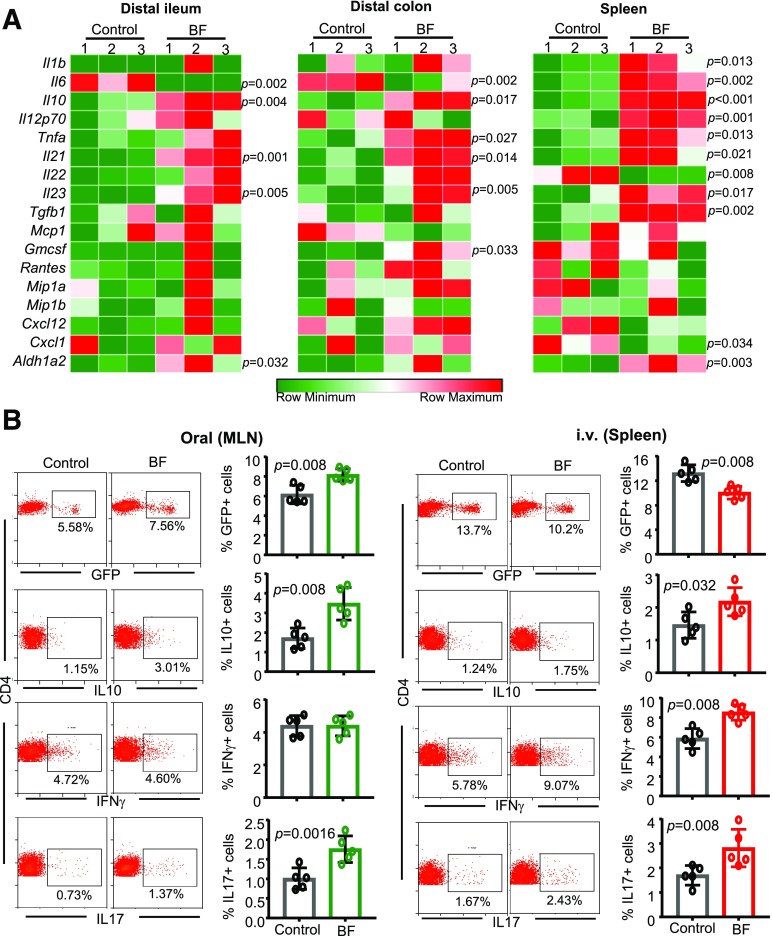
Orally and Systemically Administered BF Induces Distinct Immune Responses in the Gut Mucosa and Spleen
Because NOD mice that were treated with HK BF orally and systemically showed opposing effects on insulitis and hyperglycemia onset, intestinal and splenic immune responses of mice that were exposed to BF for 3 days were determined. qPCR assay of distal ileum and distal colon from mice that received BF orally and spleen cells from mice that received BF systemically show that BF exposure triggers the expression of mRNA for a large number of cytokines, chemokines, and immune-regulatory enzymes, such as Aldh1A2, in both systemic and intestinal compartments. Notably, compared with controls, higher Il10 mRNA expression was induced in BF-exposed intestine as well as in spleen. However, most intriguingly, Il6 expression was profoundly higher in the spleen of mice that received i.v. injection of BF, but significantly lower in the intestine of oral treatment recipients, compared with their control counterparts. Furthermore, BF exposure–associated Il12 and Il1b mRNA expressions were significantly higher in the spleen of i.v. injected mice but not in the intestine of orally administered mice (Fig. 3A).
Figure 3.
Orally and systemically administered BF induces distinct immune responses. Eight-week-old female NOD mice were given HK BF for 3 consecutive days by oral gavage (500 μg/mouse/day) or by i.v. injection (10 μg/mouse/injection). A: On day 4, one set of mice was euthanized, and cDNA prepared from distal ileum and distal colon of control and BF-fed (oral) mice and spleen cells from control and BF-injected (systemic) mice were subjected to qPCR assay and the expression levels of cytokines and noncytokine factors compared. Expression levels relative to β-actin expression were plotted as heat maps using the Morpheus application. The assay was performed in triplicate (n = 3 mice/group), and for each mouse, the average values were used for each lane. This experiment was repeated using three mice/group at least once with similar statistical trends in outcomes. B: A set of NOD-Foxp3-GFP mice from a similar experiment (treated with BF for 3 days) was euthanized on day 7. MLN cells from BF-fed mice and spleen cells from BF-injected and control mice were examined for GFP+CD4+ cells or ex vivo stimulated with PMA and ionomycin for 4 h and examined for intracellular cytokines by FACS. Representative FACS plots (left) and mean ± SD of percentage of cells that are positive for specific markers (right). The assay was performed in duplicate for each mouse (n = 5 mice/group). This experiment was repeated at least twice (using three or four mice/group) with similar statistical trends in outcomes. The statistical significance was assessed by Mann-Whitney test for all panels. Supplementary Fig. 2 shows Foxp3+, IFN-γ+, IL-10+, and IL-17+ CD4 T-cell frequencies from spleen of orally treated mice and MLN of i.v.-treated mice used for panel B. Supplementary Fig. 3 shows Foxp3+, IFN-γ+, and IL-10+ CD4+ T-cell frequencies of PP, SiLP, LiLP, and spleen of mice that received BF orally and spleen and PP of mice that received i.v. injection of BF for an extended period.
To determine the impact of BF-induced intestinal and systemic immune responses on T-cell phenotype, MLN cells from NOD-Foxp3-GFP mice that received oral administration of BF and spleens from mice that received systemic administration of BF for 3 days were compared for Foxp3 (GFP) and intracellular cytokine-positive T-cell frequencies on day 7. As observed in Fig. 3B, while MLN cells of mice that received BF orally showed significantly higher frequencies of GFP+, IL-10+, and IL-17+ T cells compared with their control counterparts, spleens of mice that received systemic BF showed profoundly lower frequencies of GFP+ cells and higher frequencies of IFN-γ+ cells compared with controls. Furthermore, mice that received BF systemically for 3 days also showed higher splenic IL-17+ and IL-10+ T-cell frequencies. Spleen cells and MLN cells from mice that were treated for 3 days with HK BF orally and systemically, respectively, showed only a modest effect on T-cell phenotype (Supplementary Fig. 2). In a separate experiment, prediabetic mice were also subjected to prolonged (15 day) oral and systemic treatments as described for Figs. 1 and 2, euthanized within 24 h, and examined for T-cell phenotype. Supplementary Fig. 3 shows significantly higher Foxp3+ and IL-10+, and lower IFN-γ+ CD4+ cells, in the gut-associated lymphoid tissues (GALT) as well as in the spleens of mice that received prolonged treatment. On the other hand, prolonged systemic treatment caused significantly reduced Foxp3+ and IL-10+, and increased IFN-γ+, CD4+ T-cell frequencies primarily in the spleen but not in the PP. Overall, these observations, along with the cytokine expression profile data of Fig. 3A, suggest an overall regulatory immune response by gut mucosa and proinflammatory immune response by systemic immune cells upon exposure of those compartments to BF.
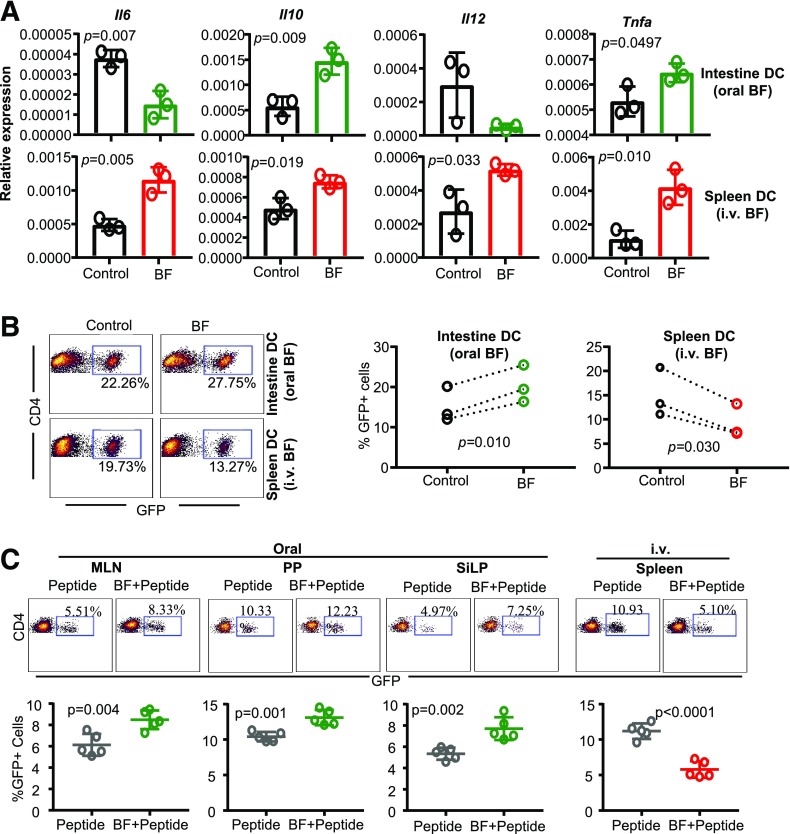
BF-Exposed Intestinal and Splenic Antigen-Producing Cells Produce Opposing Effects on Tregs Upon Antigen Presentation Ex Vivo and In Vivo
Because exposure to HK BF resulted in contrasting immune profiles in the intestinal mucosa and systemic compartment, impacts of exposure to BF on intestinal and splenic antigen presenting cells (APCs) and antigen-specific T-cell activation were determined. NOD mice were treated with HK BF for 3 consecutive days and euthanized on day 4 as described in Fig. 3A. CD11c+ DCs were enriched from the small intestine of BF-fed mice and the spleen of BF-injected mice along with respective control mice and used in qPCR and antigen presentation assays. Although showing low overall levels, relative to abundantly expressed actin, perhaps due to weak activation of a small fraction of total DC preparation in vivo, cells isolated from the intestine of mice that received BF orally expressed significantly less Il6 and Il12 and more Il10 and Tnfa mRNA compared with cells from control mouse intestines (Fig. 4A). On the other hand, splenic DCs of mice that received systemic treatment with BF expressed higher Il6, Il12, Il10, and Tnfa mRNA compared with their counterparts from control mice. However, splenic DCs from orally treated mice and intestinal DCs from i.v.-treated mice showed comparable cytokine expression profiles to those of their control counterparts (Supplementary Fig. 4).
Figure 4.
BF-exposed intestinal and splenic APCs produce opposing effects on Tregs upon antigen presentation ex vivo and in vivo. Eight-week-old female NOD mice were given HK BF for 3 consecutive days by oral gavage or by i.v. injection as described in Fig. 3. A: One set of mice was euthanized within 24 h of treatment, and CD11c+ DCs were enriched from the ileum of control and BF-fed (oral) mice and from spleen cells of control and BF-injected (systemic) mice and subjected to qPCR assay to detect the expression levels of cytokines. Parallel independent assays (n = 3) used DCs enriched and pooled from two mice/group, and each assay was performed in triplicate. The statistical significance was assessed by t test (unpaired, parametric, two-tailed). This experiment was repeated at least once with similar statistical trends in outcomes. Supplementary Fig. 4 shows expression levels of various factors in DCs isolated from the intestine of i.v.-treated mice and spleen of orally treated mice used for panel A. B: DCs were enriched from cohorts of mice similar to that of panel A, pulsed with BDC2.5 peptide, and cultured with T cells enriched from BDC2.5-Foxp3-GFP mice for 4 days and examined for GFP+CD4+ cells by FACS. Independent assays (n = 3) used DCs enriched and pooled from at least two mice/group in each. Each assay was performed in triplicate, and the cumulative values of three independent assays are shown. The statistical significance value was determined by t test (paired, parametric, two-tailed), which compared control and BF groups within each assay as indicated by the dotted lines. DCs isolated from large intestine showed similar properties in terms of cytokine expression and T-cell activation (data not shown). Supplementary Fig. 5A shows GFP+CD4+ T-cell frequencies in cultures where T cells were activated using intestinal DCs from BF-injected and spleen DCs from BF-fed mice. Supplementary Fig. 5B shows cytokine levels in the supernatants from the cultures described for panel B of this figure, tested by ELISA. C: BDC2.5-Foxp3-GFP mice that were treated for 3 days as described above received BDC2.5 peptide by oral gavage (20 μg/mouse) or by i.v. injection (2 μg/mouse) on days 2 and 3. These mice were euthanized on day 7, and GFP+CD4+ T-cell frequencies in MLN, PP, and SiLP of orally treated and spleen cells of i.v.-treated mice were determined by FACS. The assay was performed in duplicate for each mouse (n = 5/group). This experiment was repeated at least once (using four mice/group) with similar statistical trends in outcomes. The statistical significance was assessed by t test (unpaired, parametric, two-tailed).
To examine the impact of ex vivo antigen presentation by APCs that were exposed to BF in vivo, BDC2.5 peptide–pulsed DCs were cultured with T cells from NOD-BDC2.5-Foxp3-GFP mice, and the Foxp3 (GFP)+ T-cell frequencies were determined in these cultures. As shown in Fig. 4B, although intestinal DCs from mice that received BF orally caused a significant increase in GFP+ CD4 T cells compared with these cells from control mice, GFP+ CD4 T-cell frequencies were significantly lower in cultures where splenic DCs from BF-injected mice were used. In addition, as shown in Supplementary Fig. 3, activation of antigen-specific T cells by intestinal DCs from BF-fed mice resulted in relatively lower IFN-γ and significantly high IL-10 release, and an overall higher IL-10:IFN-γ ratio, in the cultures. On the other hand, activation of T cells by splenic DCs from BF-injected mice resulted in higher IFN-γ and lower IL-10 release, and a profoundly low IL-10:IFN-γ ratio, in the cultures. Of note, effects of splenic DCs from BF-fed mice and intestinal DCs from BF-injected mice on T cells were comparable to those of respective DC preparations from control mice (Supplementary Fig. 5A). Overall, these observations, along with the data of Figs. 1 and 2 and Supplementary Figs. 2, 3, and 5, suggest that while short-term systemic and oral administrations with HK BF have significant impacts primarily on systemic lymphoid tissues and GALT, respectively, immune modulation associated with prolonged exposure of gut mucosa to BF is also detectable in systemic organs, including PnLNs and spleen.
To determine the impact of antigen presentation by BF-exposed APCs in vivo, NOD-BDC2.5-Foxp3-GFP mice were treated with BDC2.5 peptide alone or along with BF administered orally or systemically and examined for GFP+ CD4 T-cell frequencies in GALT and spleen, respectively. Compared with the mice that received BDC2.5 peptide alone, mice that received oral gavage of BDC2.5 peptide along with BF showed significantly higher frequencies of GFP+ CD4 cells in the GALT, including MLN, PP, and SiLP (Fig. 4C). However, mice that received systemic injection of BDC2.5 peptide along with BF had profoundly diminished frequencies of GFP+ CD4 T cells in the spleen than those that received BDC2.5 peptide alone. These observations, in association with the results on T1D incidences in BF-treated mice (shown in Figs. 1 and 2), indicate that while BF-induced immune response in the gut mucosa promotes immune regulation, systemic immune response against BF promotes acceleration of the autoimmune process in T1D.
Oral or Systemic Administration of ΔPSA-BF Failed to Affect Autoimmune Progression and T1D Incidence in NOD Mice
Because PSA has been considered as the major symbiotic factor and host immune modulator of BF (11), we determined whether the isogenic ΔPSA mutant strain (38) of BF produces immune responses in the intestinal and systemic compartments similar to that by BF. Cohorts of mice were treated orally or systemically with HK BF and ΔPSA-BF for 3 days, and cytokine expression profiles in the intestine and spleen, respectively, were determined by qPCR. Supplementary Fig. 6 shows that mice that received ΔPSA-BF orally for 3 days, compared with BF recipients, showed significantly higher expression of Il6 and Il12 mRNA and lower expression of Il10 mRNA in the intestine. On the other hand, spleen cells of mice that received ΔPSA-BF systemically showed diminished expression of mRNA for most cytokines, albeit not statistically significant except for Il10, and profoundly increased expression of Il23 compared with spleen cells from BF recipient counterparts.
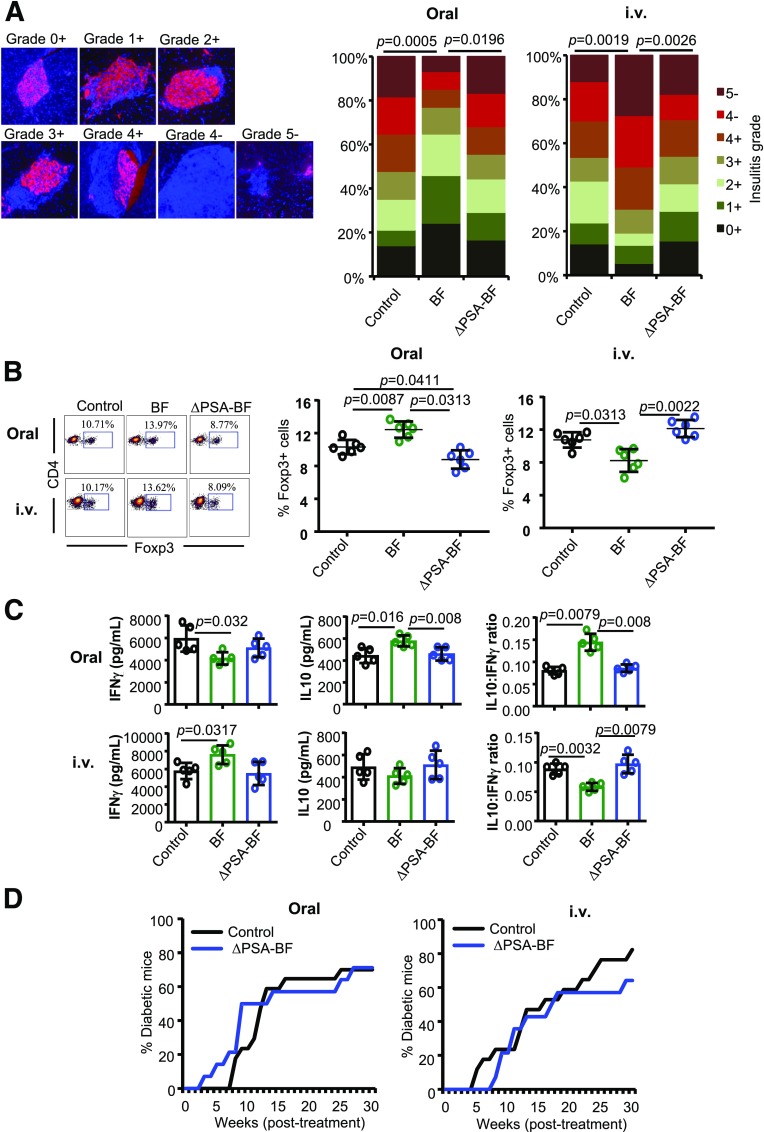
To determine the intestinal and systemic impacts of PSA deficiency in BF on T1D incidence, prediabetic female NOD mice were given HK BF or its ΔPSA mutant by oral gavage or by i.v. injection for 15 days as described for Figs. 1 and 2. Pancreatic tissues from cohorts of these mice euthanized 30 days posttreatment were examined for the degree of immune cell infiltration and insulin-positive islets. Figure 5A shows, as observed in Fig. 1B, significant suppression of insulitis as well as higher frequencies of insulin-positive islets in mice that were orally administered with BF. Mice that received BF systemically showed, as observed in Fig. 2B, more severe insulitis as well as lower frequencies of insulin-positive islets. Interestingly, mice that received ΔPSA orally and systemically showed islet function and immune cell infiltration comparable to that of control mice. Examination of PnLN cells showed that Foxp3+ T-cell frequencies were significantly increased upon oral treatment and diminished upon systemic administration compared with controls in BF, but not ΔPSA-BF, recipient mice (Fig. 5B). Furthermore, only PnLN cells from BF, but not ΔPSA-BF, recipient mice showed significant differences in the β-cell antigen activation–induced release of IL-10 and IFN-γ compared with these cells from control mice (Fig. 5C). Moreover, systemic and intestinal exposures to ΔPSA-BF induced different cytokine expression profiles compared with that induced by WT BF (Supplementary Fig. 6). In concurrence with these observations in immune cell phenotypes, insulitis, and insulin-positive islets, the timing of hyperglycemia and the disease incidence rate in NOD mice that received ΔPSA-BF orally and systemically were comparable to that of their control counterparts (Fig. 5D). Overall, these studies using HK ΔPSA-BF suggest that PSA is the primary factor responsible for the direct effect of BF-induced opposing responses of gut mucosa and systemic immune cells and the overall disease outcomes.
Figure 5.
Oral or systemic administration of ΔPSA-BF has no major impact on autoimmune progression and T1D incidence in NOD mice. Ten-week-old female NOD mice were given HK BF or ΔPSA-BF by oral gavage or by i.v. injection and studied as described for Figs. 1 and 2. A: Cohorts of four mice/group were euthanized 30 days posttreatment, and pancreatic tissue sections were stained using anti-insulin antibody (red) and the nuclear stain DAPI (blue) and examined for insulitis and insulin-positive islets. Insulitis was graded 0–5 on the basis of islet structure and immune cell infiltration (on the basis of DAPI) and insulin staining (+ or −), as follows: 0+ (no infiltration/insulin positive), 1+ (<5% infiltration/insulin positive), 2+ (5–25% infiltration/insulin positive), 3+ (25–50% infiltration/insulin positive), 4+ (50–100% infiltration/insulin positive), 4− (50–100% infiltration/insulin negative), and 5− (only islet remnants left/insulin negative). At least 100 islets from multiple intermittent sections were examined for each group. Statistical significance was calculated by Fisher exact test comparing relative numbers of islets with insulitis grades ≤2+ and ≥3+ between control vs. BF and BF vs. ΔPSA-BF groups. B: PnLN cells of mice (n = 5/group) euthanized 15 days posttreatment were examined for Foxp3+CD4+ T-cell frequencies by FACS, and the statistical significance was calculated by Mann-Whitney test. C: PnLN cells (n = 5 mice/group) were also cultured overnight in the presence of soluble anti-CD3 antibody for 24 h, and the supernatants were tested for cytokine levels by Luminex multiplex assay. Cytokine levels and relative ratios are shown, and the statistical significance was calculated by Mann-Whitney test. Experiments of panels C and D were repeated at least twice (using three or four mice/group) with similar statistical trends in results. D: Cohorts of control (n = 17) and BF-treated (n = 14) mice were monitored for hyperglycemia as described for Figs. 1 and 2. Statistical significance was calculated by log-rank test. This set of experiments was carried out in parallel with the experiments of Figs. 1A and 2A, and the BF group was not included in D to avoid repeated presentation of the same data. Supplementary Fig. 6 shows the cytokine mRNA expression profile of intestinal tissues from mice that were fed or injected with BF and ΔPSA-BF for 3 days.
TLR2-Deficient NOD Mice, Unlike WT Mice, Failed to Show Opposing T1D Modulatory Effects Upon Systemic and Oral Administration of BF
Interaction of BF-PSA with TLR2 plays a key role in the symbiotic function of BF (11). Therefore, the contribution of TLR2 in protecting NOD mice from T1D upon oral administration and accelerating the disease progression upon systemic administration of HK BF was determined using NOD-TLR2-KO mice. Ten-week-old TLR2-KO and WT NOD mice were given HK BF orally or i.v. as described for Figs. 1 and 2 and monitored for hyperglycemia. Supplementary Fig. 7 shows that, as reported by others (3,5,39), NOD-TLR2-KO mice developed hyperglycemia at a relatively lower pace than the WT mice. However, unlike WT NOD mice, neither systemic nor oral treatment with BF produced a modulatory effect on the onset of hyperglycemia or T1D incidence in TLR2-KO mice. Similarly, examination of pancreatic tissues of similarly treated mice 30 days posttreatment showed that control and BF-treated NOD-TLR2-KO mice had comparable insulitis. These results, in conjunction with the data shown in Fig. 5, suggest a direct mechanistic role for a PSA-TLR2 interaction in the opposing T1D modulatory effects observed upon exposure of the gut mucosa and systemic compartment to BF.
Oral Administration of HK BF Under Enhanced Gut Permeability Results in Accelerated T1D
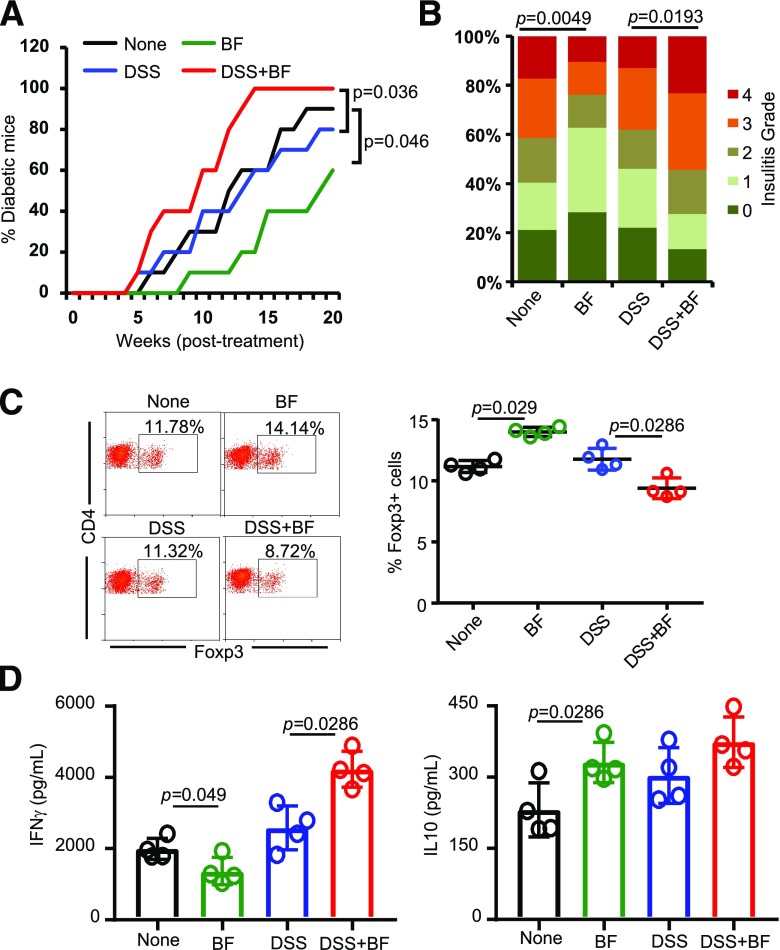
Compromised gut epithelial barrier function and translocation of microbial components to the systemic compartment can occur under many circumstances, including stress and medications. Recent reports have suggested that leaky gut and microbial translocation may contribute to initiation and/or progression of various autoimmune conditions (8,9,37,40–43). Because systemic administrations of HK BF caused rapid disease onset in NOD mice, we determined the impact of oral administration of this agent under enhanced gut permeability conditions. To enhance gut permeability, we used a chemical approach of epithelial barrier injury. Prediabetic NOD mice were given low-dose DSS in drinking water before oral administration of HK BF. DSS-treated mice showed higher translocation of orally administered FITC-dextran as well as microbial DNA into blood (Supplementary Fig. 8), suggesting that they have higher gut permeability. While HK BF recipients, as observed in Fig. 1, showed protection from T1D, DSS treatment alone did not affect the disease progression in NOD mice of our specific-pathogen-free (SPF) facility (Fig. 6A). However, compared with mice that received DSS alone, mice that received both DSS and HK BF showed rapid progression of the disease and early onset of hyperglycemia. Furthermore, pancreas of mice that received both DSS and BF showed a significantly higher number of islets with severe insulitis compared with DSS recipient controls (Fig. 6B). Examination of PnLN cells revealed significantly low Foxp3+ T-cell frequency (Fig. 6C) and profoundly higher β-cell antigen–induced IFN-γ production (Fig. 6D) in DSS- and BF-treated mice compared with DSS-treated controls. Overall, these results suggest that while transient translocation of gut microbial components normally may not have a profound impact on autoimmune progression, systemic access to specific gut microbes such as BF, especially under gut permeability–compromised conditions, can accelerate the disease process.
Figure 6.
Oral administration of BF under enhanced gut permeability results in accelerated autoimmune progression and early onset of hyperglycemia in NOD mice. Eight-week-old female NOD mice were maintained on regular drinking water or drinking water containing DSS (0.5% w/v) for 5 days and switched to regular water. A: Cohorts of these mice (n = 10/group) were left untreated or given daily oral gavage of HK BF (500 μg/day/mouse) for 15 days (during and after DSS treatment) and monitored for hyperglycemia as described for Figs. 1 and 2. Statistical significance was calculated by log-rank test. B: Cohorts of mice (n = 4/group) were euthanized 30 days posttreatment, H-E–stained pancreatic tissue sections were examined for insulitis as described for Fig. 1, and the statistical significance was calculated by Fisher exact test. C: PnLN cells of mice (n = 4/group) euthanized 30 days posttreatment initiation were examined for Foxp3+CD4+ T-cell frequencies by FACS, and the statistical significance was assessed by Mann-Whitney test. D: PnLN cells (n = 4 mice/group) were also cultured overnight in the presence of β-cell antigen peptide cocktail for 48 h, and the supernatants were tested for cytokine levels by Luminex multiplex assay. The statistical significance was calculated by Mann-Whitney test. Experiments of panels C and D were repeated at least once (using three mice/group) with similar statistical trends in results. Supplementary Fig. 8 shows the degree of gut permeability in low-dose DSS-treated and control mice. High-dose (2.5% w/v), but not low-dose (0.5%), DSS-treated NOD mice showed transient loss of body weight, and the weight loss was very modest compared with B6 mice (data not shown).
Discussion
Many years of studies have demonstrated unique immune-modulatory/regulatory properties and the symbiotic nature of a key human colonic bacterium belonging to Bacteroidetes phylum, BF (11,12). However, BF is also the primary microbe detected in most clinical intra-abdominal infections/abscesses (15,16,36,44). This suggested to us that although the association between intra-abdominal infections and T1D has never been investigated/reported, BF-like human gut commensals may promote immune regulation when restricted to the gut, while their escape to the systemic compartment could produce strong proinflammatory response and may affect autoimmune progression. Here, we tested this notion in a NOD mouse model of T1D using HK BF and oral and i.v. administrations to mimic gut mucosa and systemic exposures to this organism. We also used a chemical approach to establish enhanced gut permeability and test the translocation of orally administered BF components to the systemic compartment for assessing the potential impacts of systemic exposure to BF on autoimmunity. We show that in contrast to exposure of gut mucosa to BF, which produced protection from T1D, exposure of the systemic compartment to BF resulted in a proinflammatory response, rapid insulitis progression, and early onset of hyperglycemia. Using ΔPSA-BF and NOD-TLR2-KO mice, we also show that these opposing effects of BF on T1D, upon oral and systemic administrations, are primarily bacterial PSA and host TLR2 dependent.
It has now been established that the gut microbiota is an important environmental factor that modulates T1D disease outcomes (1,3,6). Studies in human subjects and mouse models have shown the occurrence of dysbiosis very close to disease onset (24,32). In general, higher abundance of Bacteroidetes phylum members and a reduction in firmicutes are associated with T1D (23,24,28). However, protection from T1D has also been positively correlated with a higher abundance of multiple species belonging to Bacteroidetes (24,26). The fact that BF, which is known to colonize the colonic crypt and promote gut immune regulation (11,18), is the most commonly isolated gut bacterium in clinical intra-abdominal infections suggests its potential impacts on autoimmune outcomes, especially upon reaching the systemic compartment. In fact, higher gut permeability and microbial translocation have been detected in T1D-prone individuals long before the disease onset (41,45). Furthermore, it has been shown that mucosal-associated invariant T cells play a critical role in maintaining gut integrity and alterations in the function of these cells, including enhanced cytotoxic properties. This leads to higher gut permeability occurrence in patients with T1D and in NOD mice before the onset of clinical disease (46), supporting the notion that compromised gut integrity and microbial translocation contribute to T1D. It is possible that BF is one of the major gut commensals that gains access to the systemic compartment under enhanced gut permeability in T1D-susceptible subjects. However, it is not known whether and how the systemic immune response to this gut symbiont contributes to autoimmunity. In this regard, our observations from studies using HK BF in NOD mice, for the first time in our knowledge, show that exposure of the systemic compartment to this agent leads to a profound proinflammatory response, diminished Treg function, and accelerated autoimmune progression. Our observations from studies using HK BF also substantiate some of the previously reported immune-regulatory features (11) of gut colonization by live BF.
The ability of gut and systemic immune cells to produce different types of immune responses has been studied in the past, and the gut mucosa is generally considered tolerant to commensals and dietary antigens (47,48). Hence, although the impacts on T1D disease outcomes are intriguing, our observation that distinct immune responses are induced against HK BF by mucosal and systemic immune compartments is not surprising. Nevertheless, it is important to note that not all gut microbial factors promote T1D upon exposing the systemic compartment to them. This notion has been supported by our observation that enhanced gut permeability and translocation of components of normal gut microbiota of NOD mice in our SPF facility alone does not affect T1D incidence. Furthermore, it has been shown that injecting pathogenic bacteria and their components, such as lipopolysaccharide (LPS) (TLR4 ligand), induces protection from T1D in NOD mice (49). A modest impact of systemically administered HK LM and no effect of mutant BF on T1D in NOD mice suggest that our observation of an accelerated autoimmune process upon systemic administration is, perhaps, unique to BF-like microbes and the degree and specificity of host-microbe molecular interactions. In this regard, although HK LM, at a dose comparable to that of BF, produced only a modest modulation of T1D in NOD mice, more profound impacts upon treatments with higher doses of this microbe cannot be ruled out.
Importantly, the opposing effects of systemic and oral administrations of HK BF in the mouse model of T1D appear to be primarily driven by PSA and its likely interaction with TLR2. BF PSA interacts with TLR2 on APCs as well as on T cells and is known to promote immune regulation (11,19–21). The ability of PSA to activate conventional and plasmacytoid DCs that further activate/induce Tregs has been described (20). PSA is also known to be critical for gut colonization by BF and to suppress the colonization by pathogenic bacteria (11,30). Interestingly, it has been shown that not only does TLR2 deficiency protect NOD mice from T1D but also the TLR2–gut microbiota interaction promotes autoimmunity progression in NOD mice (3,5,39). Hence, our observation that opposing effects on T1D disease outcomes upon exposure of the gut mucosa and the systemic compartment to BF is detectable only in WT, but not in TLR2-KO, NOD mice validates the previous reports on the involvement of TLR2 in T1D. Our observations also suggest a paradoxical role for TLR2 signaling in the gut mucosa and in the systemic compartment.
Unlike human gut Gram-negative bacteria such as Escherichia coli that produce highly immunogenic LPS, which activates TLR4 signaling, LPS of BF is considered a weak immune activator and does not require TLR4 (50). In fact, we observed that low-dose HK BF produced only a modest proinflammatory immune response in the systemic compartment compared with E. coli LPS (Supplementary Fig. 9). Recently, it has been shown that variation in microbiome and LPS immunogenicity can affect T1D in humans (4). Immune response to E. coli LPS, but not the LPS of Bacteroides spp. (Bacteroides dorei particularly), is associated with early immune education and protection from T1D in young children (4). In agreement with these reports, not only do TLR4-KO NOD mice show rapid onset of hyperglycemia (3) but also TLR4-binding LPS preparations from Gram-negative bacteria protect NOD mice from T1D (49). Our observations that oral or systemic administrations of ΔPSA-BF failed to affect insulitis or hyperglycemia in NOD mice indicate, in addition to the previously reported low immunogenicity and the passive role of its BF LPS, that PSA plays a role in modulating T1D disease outcomes.
Overall, our observations from this study suggest that certain gut microbes such as BF, while promoting immune regulation when restricted to its natural habitat in the distal colon, can contribute to the initiation and/or progression of autoimmunity in at-risk subjects upon accessing the systemic compartment. This is evident from the fact that while mice that received HK BF orally under normal conditions were protected from T1D, administration of this agent under enhanced gut permeability accelerated disease progression. While NOD mice housed in an SPF facility may not encounter many gut permeability–inducing events, leaky gut–inducing conditions, such as stress, medications, and/or infection, alone may be sufficient to cause transient microbial translocation and produce inflammatory effects and instigate autoimmunity in at-risk subjects. Importantly, our observations that enhanced gut permeability and microbial translocation alone may not affect disease outcomes in NOD mice also indicate that specific molecular interactions and immune responses may be critical to influence disease initiation and progression. Of note, a higher abundance of Bacteroidetes phylum members, including Bacteroides spp., has been detected not only in the gut of rodent models of T1D but also in patients with T1D and at-risk children who have progressed toward developing disease symptoms (4,22–32), prompting the suggestion that specific Bacteroides members could exert proautoimmune effects under T1D susceptibility. However, while additional systematic studies in SPF and germ-free NOD mice at different ages and disease stages using live BF and other Bacteroides members are needed for a full understanding, our results obtained using an HK BF model and previous studies suggest that colonic symbiotic bacteria such as BF play a critical role in gut and systemic immune regulation under normal circumstances but could promote autoimmunity under circumstances such as abnormal epithelial barrier function and higher gut permeability.
Supplementary Material
Article Information
Acknowledgments. The authors are thankful to the Cell and Molecular Imaging, Pathology, Proteomics, Immune Monitoring and Discovery, and Flow Cytometry Cores of the Medical University of South Carolina for histology service, microscopy, FACS, and multiplex assay instrumentation support.
Funding. This work was supported by unrestricted research funds from the Medical University of South Carolina and by National Institutes of Health grant R21AI133798.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. M.H.S. researched and analyzed data. B.M.J. researched data. R.R.G. researched and analyzed data and edited the manuscript. A.J. and M.-C.G. assisted in experiments. C.V. designed experiments, researched and analyzed data, and wrote/edited the manuscript. C.V. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db19-0211/-/DC1.
References
- 1.Chervonsky AV. Microbiota and autoimmunity. Cold Spring Harb Perspect Biol 2013;5:a007294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiba T, Seno H. Indigenous clostridium species regulate systemic immune responses by induction of colonic regulatory T cells. Gastroenterology 2011;141:1114–1116 [DOI] [PubMed] [Google Scholar]
- 3.Wen L, Ley RE, Volchkov PY, et al. . Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature 2008;455:1109–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vatanen T, Kostic AD, d’Hennezel E, et al.; DIABIMMUNE Study Group . Variation in microbiome LPS immunogenicity contributes to autoimmunity in humans. Cell 2016;165:842–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burrows MP, Volchkov P, Kobayashi KS, Chervonsky AV. Microbiota regulates type 1 diabetes through Toll-like receptors. Proc Natl Acad Sci U S A 2015;112:9973–9977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaarala O, Atkinson MA, Neu J. The “perfect storm” for type 1 diabetes: the complex interplay between intestinal microbiota, gut permeability, and mucosal immunity. Diabetes 2008;57:2555–2562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaarala O. Gut microbiota and type 1 diabetes. Rev Diabet Stud 2012;9:251–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manfredo Vieira S, Hiltensperger M, Kumar V, et al. . Translocation of a gut pathobiont drives autoimmunity in mice and humans. Science 2018;359:1156–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costa FR, Françozo MC, de Oliveira GG, et al. . Gut microbiota translocation to the pancreatic lymph nodes triggers NOD2 activation and contributes to T1D onset. J Exp Med 2016;213:1223–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ley RE, Hamady M, Lozupone C, et al. . Evolution of mammals and their gut microbes. Science 2008;320:1647–1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Round JL, Lee SM, Li J, et al. . The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 2011;332:974–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiu CC, Ching YH, Wang YC, et al. . Monocolonization of germ-free mice with Bacteroides fragilis protects against dextran sulfate sodium-induced acute colitis. BioMed Res Int 2014;2014:675786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 2005;122:107–118 [DOI] [PubMed] [Google Scholar]
- 14.Moore WE, Holdeman LV. Human fecal flora: the normal flora of 20 Japanese-Hawaiians. Appl Microbiol 1974;27:961–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nichols RL, Schumer W, Nythus LM, Bartlett JG, Gorbach SL. Anaerobic infections. Am Fam Physician 1976;14:100–110 [PubMed] [Google Scholar]
- 16.Bartlett JG, Onderdonk AB, Louie T, Kasper DL, Gorbach SL. A review. Lessons from an animal model of intra-abdominal sepsis. Arch Surg 1978;113:853–857 [DOI] [PubMed] [Google Scholar]
- 17.Onderdonk AB, Kasper DL, Cisneros RL, Bartlett JG. The capsular polysaccharide of Bacteroides fragilis as a virulence factor: comparison of the pathogenic potential of encapsulated and unencapsulated strains. J Infect Dis 1977;136:82–89 [DOI] [PubMed] [Google Scholar]
- 18.Liu CH, Lee SM, Vanlare JM, Kasper DL, Mazmanian SK. Regulation of surface architecture by symbiotic bacteria mediates host colonization. Proc Natl Acad Sci U S A 2008;105:3951–3956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kayama H, Takeda K. Polysaccharide A of Bacteroides fragilis: actions on dendritic cells and T cells. Mol Cell 2014;54:206–207 [DOI] [PubMed] [Google Scholar]
- 20.Dasgupta S, Erturk-Hasdemir D, Ochoa-Reparaz J, Reinecker HC, Kasper DL. Plasmacytoid dendritic cells mediate anti-inflammatory responses to a gut commensal molecule via both innate and adaptive mechanisms. Cell Host Microbe 2014;15:413–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Q, McLoughlin RM, Cobb BA, et al. . A bacterial carbohydrate links innate and adaptive responses through Toll-like receptor 2. J Exp Med 2006;203:2853–2863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vatanen T, Franzosa EA, Schwager R, et al. . The human gut microbiome in early-onset type 1 diabetes from the TEDDY study. Nature 2018;562:589–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cinek O, Kramna L, Lin J, et al. . Imbalance of bacteriome profiles within the Finnish Diabetes Prediction and Prevention study: parallel use of 16S profiling and virome sequencing in stool samples from children with islet autoimmunity and matched controls. Pediatr Diabetes 2017;18:588–598 [DOI] [PubMed] [Google Scholar]
- 24.Alkanani AK, Hara N, Gottlieb PA, et al. . Alterations in intestinal microbiota correlate with susceptibility to type 1 diabetes. Diabetes 2015;64:3510–3520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis-Richardson AG, Ardissone AN, Dias R, et al. . Bacteroides dorei dominates gut microbiome prior to autoimmunity in Finnish children at high risk for type 1 diabetes. Front Microbiol 2014;5:678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mejía-León ME, Petrosino JF, Ajami NJ, Domínguez-Bello MG, de la Barca AM. Fecal microbiota imbalance in Mexican children with type 1 diabetes. Sci Rep 2014;4:3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Goffau MC, Luopajärvi K, Knip M, et al. . Fecal microbiota composition differs between children with β-cell autoimmunity and those without. Diabetes 2013;62:1238–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sofi MH, Gudi R, Karumuthil-Melethil S, Perez N, Johnson BM, Vasu C. pH of drinking water influences the composition of gut microbiome and type 1 diabetes incidence. Diabetes 2014;63:632–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Goffau MC, Fuentes S, van den Bogert B, et al. . Aberrant gut microbiota composition at the onset of type 1 diabetes in young children. Diabetologia 2014;57:1569–1577 [DOI] [PubMed] [Google Scholar]
- 30.Stewart CJ, Ajami NJ, O’Brien JL, et al. . Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature 2018;562:583–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Endesfelder D, Engel M, Davis-Richardson AG, et al. . Towards a functional hypothesis relating anti-islet cell autoimmunity to the dietary impact on microbial communities and butyrate production. Microbiome 2016;4:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kostic AD, Gevers D, Siljander H, et al.; DIABIMMUNE Study Group . The dynamics of the human infant gut microbiome in development and in progression toward type 1 diabetes. Cell Host Microbe 2015;17:260–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perez N, Karumuthil-Melethil S, Li R, Prabhakar BS, Holterman MJ, Vasu C. Preferential costimulation by CD80 results in IL-10-dependent TGF-beta1(+) -adaptive regulatory T cell generation. J Immunol 2008;180:6566–6576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goodyear AW, Kumar A, Dow S, Ryan EP. Optimization of murine small intestine leukocyte isolation for global immune phenotype analysis. J Immunol Methods 2014;405:97–108 [DOI] [PubMed] [Google Scholar]
- 35.Karumuthil-Melethil S, Perez N, Li R, Vasu C. Induction of innate immune response through TLR2 and dectin 1 prevents type 1 diabetes. J Immunol 2008;181:8323–8334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horn J, Bender BS, Bartlett JG. Role of anaerobic bacteria in perimandibular space infections. Ann Otol Rhinol Laryngol Suppl 1991;154:34–39 [DOI] [PubMed] [Google Scholar]
- 37.Sapone A, de Magistris L, Pietzak M, et al. . Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes 2006;55:1443–1449 [DOI] [PubMed] [Google Scholar]
- 38.Coyne MJ, Tzianabos AO, Mallory BC, Carey VJ, Kasper DL, Comstock LE. Polysaccharide biosynthesis locus required for virulence of Bacteroides fragilis. Infect Immun 2001;69:4342–4350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim HS, Han MS, Chung KW, et al. . Toll-like receptor 2 senses beta-cell death and contributes to the initiation of autoimmune diabetes. Immunity 2007;27:321–333 [DOI] [PubMed] [Google Scholar]
- 40.Fasano A. Leaky gut and autoimmune diseases. Clin Rev Allergy Immunol 2012;42:71–78 [DOI] [PubMed] [Google Scholar]
- 41.Bosi E, Molteni L, Radaelli MG, et al. . Increased intestinal permeability precedes clinical onset of type 1 diabetes. Diabetologia 2006;49:2824–2827 [DOI] [PubMed] [Google Scholar]
- 42.Meddings JB, Jarand J, Urbanski SJ, Hardin J, Gall DG. Increased gastrointestinal permeability is an early lesion in the spontaneously diabetic BB rat. Am J Physiol 1999;276:G951–G957 [DOI] [PubMed] [Google Scholar]
- 43.Ho J, Reimer RA, Doulla M, Huang C. Effect of prebiotic intake on gut microbiota, intestinal permeability and glycemic control in children with type 1 diabetes: study protocol for a randomized controlled trial. Trials 2016;17:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Polk BF, Kasper DL. Bacteroides fragilis subspecies in clinical isolates. Ann Intern Med 1977;86:569–571 [DOI] [PubMed] [Google Scholar]
- 45.Kuitunen M, Saukkonen T, Ilonen J, Akerblom HK, Savilahti E. Intestinal permeability to mannitol and lactulose in children with type 1 diabetes with the HLA-DQB1*02 allele. Autoimmunity 2002;35:365–368 [DOI] [PubMed] [Google Scholar]
- 46.Rouxel O, Da Silva J, Beaudoin L, et al. . Cytotoxic and regulatory roles of mucosal-associated invariant T cells in type 1 diabetes. Nat Immunol 2017;18:1321–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pabst O, Mowat AM. Oral tolerance to food protein. Mucosal Immunol 2012;5:232–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Castro-Sánchez P, Martín-Villa JM. Gut immune system and oral tolerance. Br J Nutr 2013;109(Suppl. 2):S3–S11 [DOI] [PubMed] [Google Scholar]
- 49.Caramalho I, Rodrigues-Duarte L, Perez A, Zelenay S, Penha-Gonçalves C, Demengeot J. Regulatory T cells contribute to diabetes protection in lipopolysaccharide-treated non-obese diabetic mice. Scand J Immunol 2011;74:585–595 [DOI] [PubMed] [Google Scholar]
- 50.Tezuka H, Ohteki T. Regulation of intestinal homeostasis by dendritic cells. Immunol Rev 2010;234:247–258 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.