Abstract
Post-traumatic stress disorder (PTSD) is a debilitating mental disorder precipitated by trauma exposure. However, only some persons exposed to trauma develop PTSD. There are sex differences in risk; twice as many women as men develop a lifetime diagnosis of PTSD. Methylomic profiles derived from peripheral blood are well-suited for investigating PTSD because DNA methylation (DNAm) encodes individual response to trauma and may play a key role in the immune dysregulation characteristic of PTSD pathophysiology. In the current study, we leveraged recent methodological advances to investigate sex-specific differences in DNAm-based leukocyte composition that are associated with lifetime PTSD. We estimated leukocyte composition on a combined methylation array dataset (483 participants, ~450k CpG sites) consisting of two civilian cohorts, the Detroit Neighborhood Health Study and Grady Trauma Project. Sex-stratified Mann-Whitney U test and two-way ANCOVA revealed that lifetime PTSD was associated with a significantly higher monocyte proportions in males, but not in females (Holm-adjusted p-val < 0.05). No difference in monocyte proportions was observed between current and remitted PTSD cases in males, suggesting that this sex-specific difference may reflect a long-standing trait of lifetime history of PTSD, rather than current state of PTSD. Associations with lifetime PTSD or PTSD status were not observed in any other leukocyte subtype and our finding in monocytes was confirmed using cell estimates based on a different deconvolution algorithm, suggesting that our sex-specific findings are robust across cell estimation approaches. Overall, our main finding of elevated monocyte proportions in males, but not in females with lifetime history of PTSD provides evidence for a sex-specific difference in peripheral blood leukocyte composition that is detectable in methylomic profiles and that may reflect long-standing changes associated with PTSD diagnosis.
Keywords: PTSD, monocytes, leukocyte composition, DNA methylation, sex differences
1. INTRODUCTION
Post-traumatic stress disorder (PTSD) is a debilitating mental disorder that is precipitated by a traumatic experience involving direct or indirect exposure to actual or threatened death, serious injury, or sexual violence1. PTSD presents with intrusive and persistent re-experiencing of the traumatic event, avoidance of distressing, trauma-associated stimuli, negative alterations in cognition and mood, and alterations in arousal/reactivity that persist for longer than a month1. While most individuals are exposed to a potentially traumatic event at some point in their lives, only some develop PTSD2–8, suggesting that the disorder reflects a distinct inability to reinstate homeostasis after psychological trauma in vulnerable individuals9.
Epidemiological studies have identified sex to be a significant vulnerability factor for developing PTSD, with women twice as likely to have lifetime PTSD than men, even when risk of exposure and types of trauma are taken into consideration3,4,8,10–12. This sex bias in disease prevalence is also observed in other stress-related mood and anxiety disorders13, including depression14. Preclinical and clinical investigations have identified sexual dimorphism in stress response systems that may be involved in the increased prevalence of stress-related psychopathologies in women15,16. Furthermore, in addition to sexual dimorphism in the neurobiological underpinnings of stress/trauma response, recent animal studies suggest that behavioral response to traumatic stress is fundamentally different between males and females and should be considered in interpretation of results17. In humans, response to stress/trauma exposure involves both biological and social contributors corresponding to sex and gender-related variables. While the effects of sex and gender are difficult to disentangle, investigations stratified by biological sex, understood to interact with gender-related variables, are warranted to improve our currently limited understanding of the sex-specific biological processes dysregulated in PTSD pathophysiology.
Mounting evidence suggests a key role for stress-induced inflammation and immune alteration in the development and maintenance of PTSD and other stress-related psychiatric disorders. Although findings from human literature have been mixed, PTSD has generally been associated with increased pro-inflammatory tone, basally and in response to immune challenge, via both cytokine signaling and changes in immune cell distribution/function18–34. Investigations in animal models, primarily in male rodent studies, have provided mechanistic insights into how peripheral immune cell response/signaling and distribution is linked with microglial activation and neuroinflammatory dynamics to trigger stress-induced anxiety behavior and memory impairment35–41.
Epigenetic mechanisms have emerged as important regulators of PTSD-associated immune dysregulation and inflammation42–47, and are particularly significant for the study of PTSD because they capture the interactions among pre-disposing genetic/environmental risk factors and the precipitating trauma exposure. Individual response to trauma exposure can be encoded as short-lived or persistent epigenetic changes that reflect and may contribute to posttraumatic physiological changes, some of which remain after remission of PTSD symptoms. DNA methylation (DNAm) has been the most widely studied epigenetic mechanism and evidence from both animal and human models point to its key role in stress regulation9,48–50, fear memory51–55, and immune function44–47,56–59, in both brain and blood. Exploring PTSD-associated DNAm profiles in blood may inform our understanding of mechanisms underlying immune dysregulation, particularly those that coordinate peripheral immune-neuroimmune crosstalk60,61; moreover, given the well-known sex difference in PTSD prevalence and in immune response62,63, sex-stratified investigation relating to these dual factors is warranted to identify potential differences in variability by sex that may be missed in analyses combining both sexes.
Peripheral blood-based methylomic profiles are comprised of two dynamic components: 1) profiles reflecting proportions of immune cell subtypes (i.e., leukocyte composition) and 2) alterations in DNAm levels at CpG sites genome-wide (i.e., differential methylation). Epigenome-wide association studies (EWAS) often seek to identify dynamic differential methylation marks and treat cellular heterogeneity as a major confound that must be addressed to improve signal detection. However, differences in leukocyte subtypes provide key insights into immunological changes and warrant examination themselves. Recent developments in deconvolution algorithms and cell-type discriminating reference databases have improved estimates64–67 and enabled utility of DNAm-based leukocyte subtype estimates as proxies for white blood cell differential-based metrics68,69. Leveraging these recently developed methods, here we use leukocyte-derived methylomic profiles combined from two civilian cohorts to investigate our hypothesis that PTSD is associated with sex-specific differences in leukocyte composition, detectable by DNAm-based estimates. To our knowledge, this study is the first to investigate leukocyte composition profiles in PTSD using these new DNAm-based approaches for immune profiling.
2. MATERIALS AND METHODS
2.1. Study Participants
Samples from trauma-exposed, adult participants with available Illumina HumanMethylation450 (450K) BeadChip array data were selected from two predominantly African-American, community-based cohorts examining biological and environmental correlates of PTSD, namely the Detroit Neighborhood Health Study (DNHS; n=192) and Grady Trauma Project (GTP; n=422). The DNHS, based in Detroit, MI, was approved by the institutional review boards of the University of Michigan and University of North Carolina at Chapel Hill. The GTP, based in Atlanta, GA, was approved by the institutional review boards of Emory University School of Medicine and Grady Memorial Hospital. All participants provided written informed consent prior to data collection. Details regarding the DNHS44,70,71 and GTP72–74 were published previously. While neither study excluded participants based on illness, women known to be pregnant (in the GTP) were excluded from estimation and analyses, due to well-known/known significant differences in leukocyte composition during pregnancy75. Collected demographic data included self-reported gender, race, age, and current smoking, which was defined as any cigarette smoking in the past 30 days.
2.2. Assessment of PTSD
Study participants were assessed for PTSD, as defined by the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV)1. In the DNHS, study participants were assessed for PTSD using the well-validated self-report PTSD Checklist, Civilian Version (PCL-C)76–79 and additional questions about duration, timing, and impairment due to symptoms, via structured telephone interviews10,44,80. Participants who met all six DSM-IV criteria in reference to their worst traumatic event or to a randomly selected traumatic event (if the participant experienced more than one trauma), were considered affected by lifetime PTSD. Those that met criteria based on symptoms reported within the past month were considered affected by current PTSD. Analysis of data from the clinical interviews showed that the PTSD instrument used during structured telephone interviews had excellent internal consistency and high concordance44,81. The PCL-C yielded a Cronbach coefficient alpha (α) of 0.93. Using cluster scoring based on DSM-IV criteria (i.e. to be a case, the participant’s symptoms had to meet all six criteria), the instrument had a specificity (SP) of 0.97, sensitivity (SE) of 0.24, positive predictive value (PPV) of 0.80, negative predictive value (NPV) 0.72, and an area under the ROC curve (AUC) of 0.76, as previously reported44,81. In the GTP, 82study participants were assessed for lifetime and current PTSD using the Clinician-Administered Post-traumatic Stress Disorder Scale (CAPS, DSM-IV), a structured interview administered by a clinician that has previously been shown to have excellent reliability (i.e., consistent scores across items, raters, testing occasions) and excellent convergent and discriminant validity in large scale psychometric studies83,84. For lifetime analyses, participants meeting criteria for PTSD at any point (including current and past PTSD) were considered cases and compared to trauma-exposed controls who lacked a history of PTSD at any point in their lifetime. For comparisons of current PTSD with remitted PTSD and trauma exposed controls, participants with lifetime PTSD were further separated into two groups: those with current PTSD and those with lifetime (but not current) PTSD.
2.3. Sample Processing
In the DNHS, samples were obtained via an in-home blood draw performed by a clinician, processed in the lab within two hours, and stored at −20°C until testing. Detailed methods regarding biospecimen processing in the DNHS are available in a separate publication85. In the GTP, DNA was extracted from whole blood, aliquoted and frozen at −80°C within two hours of collection. Genomic DNA was extracted from peripheral blood using the DNA Mini Kit (Qiagen, Germantown, MD) in the DNHS and the Gentra Puregene Kit (Qiagen, Germantown, MD) in the GTP. Both studies bisulfite-converted the DNA samples using the Zymo EZ-96 DNA methylation kit (Zymo Research, Irvine, CA) and used 500 ng of DNA per sample for whole-genome amplification, fragmentation, and hybridization to the Illumina Human Methylation 450K BeadChip array (Illumina, San Diego, CA), according to the manufacturers’ recommended protocols. Sample processing procedures have been published previously for both the DNHS and GTP59,74,86–88.
2.4. Quality control and pre-processing of 450K Data
The raw .idat files were imported into R (version 3.5.1)89, using the minfi90 Bioconductor (version 3.7)91,92 package, for all subsequent data processing and analyses. After quality control (QC)90,93,94, data pre-processing95–100 was conducted on all QC’ed samples (DNHS: n = 187; GTP: n = 416). This included duplicates (n=12) in the DNHS and participants with known pregnancy (n = 26) or missing PTSD phenotype data (n = 82) in the GTP. Analyses were conducted on unique participants that passed QC, as described below, and had PTSD data available (Table 1).
Table 1:
Key demographic characteristics of the DNHS and GTP
| Female (n = 330) | Male (n = 153) | Total (n = 483) | |
|---|---|---|---|
| Study | |||
| DNHS | 111 (33.6%) | 64 (41.8%) | 175 (36.2%) |
| GTP | 219 (66.4%) | 89 (58.2%) | 308 (63.8%) |
| Race | |||
| AA | 291 (88.2%) | 140 (91.5%) | 431 (89.2%) |
| CA | 33 (10.0%) | 11 (7.2%) | 44 (9.1%) |
| Other | 6 (1.8%) | 2 (1.3%) | 8 (1.7%) |
| Median Age | |||
| 49.0 (38.0 – 55.0) | 48.0 (36.0 – 56.0) | 48.0 (37.5 – 55.0) | |
| Current Smoking | |||
| no | 210 (63.6%) | 72 (47.1%) | 282 (58.4%) |
| yes | 109 (33.0%) | 78 (51.0%) | 187 (38.7%) |
| Missing | 11 (3.3%) | 3 (2.0%) | 14 (2.9%) |
| Lifetime PTSD | |||
| no | 135 (40.9%) | 71 (46.4%) | 206 (42.7%) |
| yes | 195 (59.1%) | 82 (53.6%) | 277 (57.3%) |
This table describes the subset of participants included in primary analyses investigating sex-specific associations between DNAm-based cell estimates and lifetime PTSD, by sex. For the 2-way ANCOVA, 14 participants were excluded due to missing current smoking data.
DNHS: Detroit Neighborhood Health Study; GTP: Grady Trauma Project
For data quality assessment, samples were checked for 1) low total signal (mean signal intensity less than half of the overall median, after setting probes with detection p-value > 0.001 or < 2,000 arbitrary units to missing); 2) > 1% of failed probes (detection p-value > 0.001); 3) outlying beta value distribution (i.e., smaller than three times interquartile range (IQR) from the lower quartile or larger than 3 times IQR from the upper quartile); 4) greater than three standard deviations of the mean bisulfite conversion control probe signal intensity67. Additionally, samples were checked for gender discordance based on median total intensity of X and Y-chromosome mapped probes (as implemented in minfi90) and removed if predicted sex differed from self-reported gender. Five samples were removed among DNHS samples for gender discordance, and six samples were removed among GTP samples (two for data quality and four for gender discordance). After within-array background correction and dye-bias equalization using out-of-band control probes (ssNoob96,101; minfi), probes with detection p-value > 0.001 in more than 10% of samples93 and cross-reactive probes97 (i.e., cross-hybridized between autosomes and sex chromosomes) were removed. Beta-mixture quantile (BMIQ) normalization (ChAMP99,100) was used to correct for type II probe bias98.
To control for technical artifacts (e.g., sample processing and imaging batch effects), principal components (PCs) based on non-negative control probe signal intensity94 were removed from BMIQ-normalized M-values (i.e., logit-transformed beta-values) separately for each study. PC correlation heatmaps were used to check for successful removal/reduction of batch effects, especially chip and row effects, while maintaining signal from biological variables. The DNHS and GTP datasets were then combined and an empirical Bayes method (i.e., ComBat102 in the sva package103) was used on the combined M-values to account for study effects while controlling for sex and age. Data quality assessment, QC probe filtering, and first step of batch removal were study-specific, while pre-processing steps (ssNoob+BMIQ) implemented within-array approaches unaffected by study. Only probes that passed QC in both studies (n = 455,072 probes) were included in the combined dataset.
2.5. Leukocyte Composition Estimation
Leukocyte composition was estimated on ComBat-adjusted beta-values using the EpiDISH65 reference database, which is informed by cell-type specific DNase hypersensitive sites (DHS; based on the NIH Epigenomics Roadmap database104) and is optimized for discriminating granulocytes, CD14+ monocytes, CD8+ T cells, CD4+ T cells, CD19+ B cells, and CD56+ natural killer cells. Robust partial correlation (RPC; robust multivariate linear regression, non-constrained projection) was used as the primary deconvolution algorithm and EpiDISH’s implementation of linear, constrained projection (CP), originally introduced by Houseman et al. (2012)105, was used to calculate a second set of estimates for comparison.
2.6. Ancestry Estimation
DNAm-based ancestry PCs were derived on cleaned beta-values after regressing out sex and age from batch-adjusted M-values. Ancestry PCs were calculated on a subset of 2,317 ancestry informative CpG probes included in two published ancestry informative CpG lists that accounted for confounders106 and that included probes within 10 base pairs (bp) of single nucleotide polymorphisms (SNPs)107. The first 2 PCs based on this subset of probes were used as ancestry PCs (ancPCs) after checking for strong association with self-reported race and effective separation of self-reported races.
2.7. Statistical Analysis
The Shapiro-Wilk test was used to assess normality and Levene’s test was used to compare equality of variance among groups. Since cell estimates had dissimilar, non-normal distributions, non-parametric tests were used for all initial group comparisons of leukocyte subtypes. The two-sample Kolmogorov-Smirnov (KS) test was used to compare distribution of cell estimates when variances were unequal between groups and Mann-Whitney U test was used to compare mean ranks of cell estimates otherwise. Spearman’s rank correlation was used to assess agreement between estimates based on RPC and CP deconvolution approaches. A threshold of 0.05 was used for p-values and p-values were adjusted for multiple comparisons using Holm’s method108, unless otherwise specified.
To test our main hypothesis—that PTSD is associated with sex-specific differences in leukocyte composition—initial sex-stratified analyses were conducted on all leukocyte subtypes using the non-parametric Mann-Whitney U test. For leukocyte subtypes determined to be significantly associated with lifetime PTSD in either sex based on initial Mann-Whitney U tests, a two-way analysis of covariance (ANCOVA; Type III) controlling for age, ancestry (based on DNAm ancestry PCs), and current smoking, was performed with post-hoc comparison of estimated marginal means to examine the effects of sex and lifetime PTSD on transformed cell estimates. Transformation for cell estimate was conducted to meet modeling assumptions for ANCOVA and was informed by Tukey’s Ladder of Powers. Power parameter (λ) was computed to maximize normality based on the Shapiro-Wilks W statistic. Sex-stratified Kruskal-Wallis and post-hoc Dunn tests were conducted as additional follow-up to investigate possible differences in cell proportions by PTSD status (i.e., trauma-exposed controls, remitted PTSD, and current PTSD).
3. RESULTS
3.1. Demographic characteristics of sampled study participants from the DNHS and GTP
The demographic characteristics of study participants included in primary analyses investigating sex-specific associations between DNAm-based cell estimates and lifetime PTSD are presented in Table 1. Of the 483 participants from the combined DNHS and GTP sample, 57.3% had a lifetime diagnosis of PTSD, 68.3% were female, and 38.7% were current smokers. The study population was predominantly African-American (89.2%), based on self-reported race, and the median age was 48 years (IQR: 17.5; 37.5–55 years).
3.2. Comparison of leukocyte subtype estimates by deconvolution approach
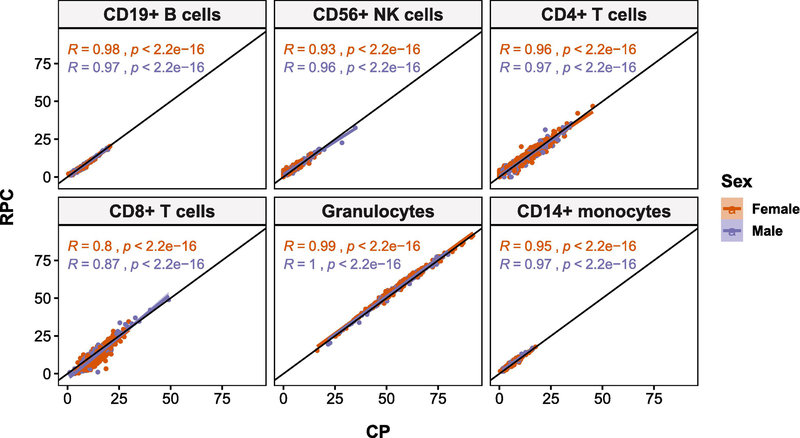
Good overall agreement was observed between RPC and CP estimates, as measured by Spearman’s correlation (i.e., RPC-CP correlation), but CD8T cells showed much poorer agreement, ρs(481) = 0.83, relative to the other leukocyte subtypes, ρs(481) > 0.94 (Figure 1). Since the main objective of this study was to investigate sex-specific differences in leukocyte composition, comparison of RPC and CP estimates was stratified by sex. Sex-stratified RPC-CP correlation revealed that the largest difference in RPC-CP correlation between sexes was also found in CD8T cells, |∆ρs| = 0.07, such that females showed poorer correlation, ρs(328) = 0.80, than males, ρs(151) = 0.87. For the other leukocyte subtypes, the difference in RPC-CP correlation between sexes (|∆ρs|), ranged from 0.01 to 0.03, with CD56+ natural killer (NK) cells having the second largest difference in correlation between sexes (female: ρs(328) = 0.93; male: ρs(151) = 0.96). In all leukocyte subtypes, except CD19+ B cells, females had lower correlation coefficients than males. Detailed results for RPC-based cell estimates are reported below and corresponding results based on CP-based estimates are reported in supplementary materials, due to strong agreement between findings from both sets of estimates.
Figure 1:
Correlation (Spearman’s rho) between cell estimates derived using robust partial correlation (RPC) and constrained projection (CP) deconvolution algorithms is high in all leukocyte subtypes, with CD8+ T cells (CD8T) showing the worst agreement at R = 0.8 in females and R = 0.87 in males. CD8T cells also showed the largest discrepancy in sex-specific correlation of estimates. RPC-CP correlation was > 0.9 and difference in RPC-CP correlation between sexes was between 0.01 and 0.03 for all other leukocyte subtypes.
3.3. Comparison of leukocyte subtype estimates by sex and lifetime PTSD
Cell estimates were compared by sex, lifetime PTSD, and study in each leukocyte subtype to establish overall differences. Significant overall sex differences were observed in the distributions of CD56+ natural killer (NK) cell (KS: D = 0.19, adj. p = 0.007) and CD8+ T cell (CD8T) proportions (KS: D = 0.16; adj. p = 0.04) in RPC estimates. Males showed greater variability than females for both NK and CD8T cells (male vs. female - IQRNK: 6.2% vs. 4.15%; IQRCD8T: 9.5% vs. 6.2%), as well as higher median NK (5.5% vs. 4.4%) and lower median CD8T (9.0% vs. 9.7%) cell proportions (Figure 2). No significant overall differences (i.e., in both sexes combined) were observed between lifetime PTSD cases and trauma-exposed controls in any leukocyte subtype (Mann-Whitney). Additional analyses comparing leukocyte subtype proportions between participating cohorts and assessing age effects in each cell type are reported in supplementary materials.
Figure 2:
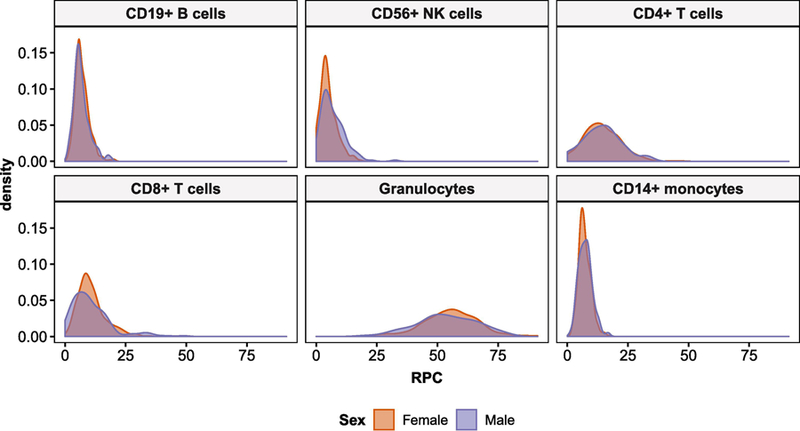
Distribution of leukocyte subtypes based on robust partial correlation (RPC) estimates, by sex. Sex differences in CD8+ T and CD56+ NK cell distributions were found to be prominent.
3.4. Elevated monocyte proportions were associated with lifetime PTSD in males, but not females
Sex-stratified Mann-Whitney U tests revealed a significant difference in monocyte proportions between PTSD cases and controls in males (Figures 3 and 4). Males with lifetime PTSD had higher median monocyte proportions than trauma-exposed controls, U = 2100, Z = − 2.9, p = 0.004, adj. p = 0.026, r = 0.23. In contrast, no difference in monocyte estimates was found between groups in females, U = 13000, Z = −0.58, p = 0.6, adj. p = 1, r = 0.03. Lifetime PTSD-associated differences were not observed in any other leukocyte subtypes in either sex.
Figure 3:
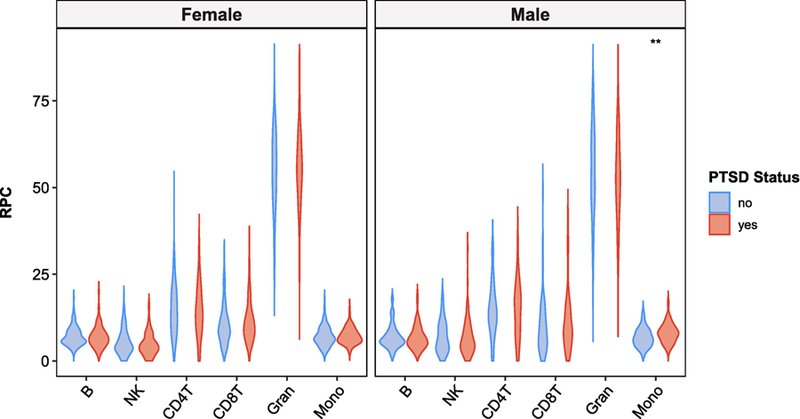
Violin plots of RPC estimates for lifetime PTSD cases and controls, stratified by sex. Only monocyte proportions in males showed statistically significant difference between lifetime PTSD cases and controls, based on Mann-Whitney U test (p-value = 0.004; Holm-adjusted p-value = 0.026). For figure labels on x-axis: B = CD19+ B cells; NK = CD56+ NK cells; CD4T = CD4+ T cells; CD8T = CD8+ T cells; Gran = Granulocytes; Mono = CD14+ monocytes.
Figure 4:
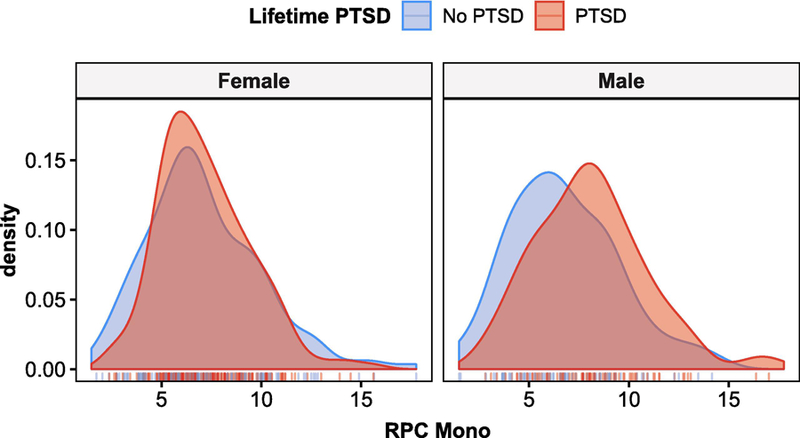
Density plots for RPC monocyte estimates in lifetime PTSD cases and controls, stratified by sex, show distinctly higher monocyte levels in males with lifetime PTSD compared to trauma-exposed controls. This difference in monocyte levels between lifetime PTSD case and controls is not observed in females.
A two-way ANCOVA was conducted to investigate whether sex moderated the effects of lifetime PTSD on transformed monocyte estimates, while accounting for age, ancestry, and current smoking (Table 2). Monocyte estimates were square root transformed for the ANCOVA to meet model assumptions (i.e., normality) and were informed by Tukey’s Ladder of Powers (RPC: λ = 0.43; CP λ = 0.5). A significant interaction was found between sex and lifetime PTSD, F(1, 461) = 4.89, p = 0.027, ηp2 = 0.011. Post-hoc comparison of estimated marginal means (EMMs) for lifetime PTSD by sex (Figure 5; Table 3) showed a significant mean difference between lifetime PTSD cases and controls in males, ∆EMM = 0.26, SE = 0.08, t(461) = 3.32, p = 0.001, where mean monocyte estimates were higher in lifetime PTSD cases than controls. No significant mean difference was observed between PTSD cases and controls in females, ∆EMM = 0.05, SE = 0.05, t(461) = 0.89, p = 0.37, confirming findings from initial sex-stratified analyses. Together, our results suggest that male PTSD cases have significantly elevated monocyte proportions compared to trauma-exposed controls and that this lifetime PTSD-associated difference is not observed in females.
Table 2:
Two-way ANCOVA Table for RPC monocyte estimates (n = 469)
| Terms | Type III Sum of Squares | df | Mean Square | F | P | partial η2 |
|---|---|---|---|---|---|---|
| Sex | 0.336 | 1 | 0.336 | 1.469 | 0.226 | 0.003 |
| PTSDlife | 2.379 | 1 | 2.379 | 10.407 | 0.001*** | 0.022 |
| Age | 2.309 | 1 | 2.309 | 10.101 | 0.002*** | 0.021 |
| ancPC1 | 0.000 | 1 | 0.000 | 0.001 | 0.977 | 0.000 |
| ancPC2 | 0.219 | 1 | 0.219 | 0.957 | 0.329 | 0.002 |
| Smoking | 0.060 | 1 | 0.060 | 0.261 | 0.610 | 0.001 |
| Sex:PTSDlife | 1.146 | 1 | 1.146 | 5.011 | 0.026* | 0.011 |
| Residuals | 105.399 | 461 | 0.229 |
p < 0.05
p < 0.01
p < 0.005
Figure 5:
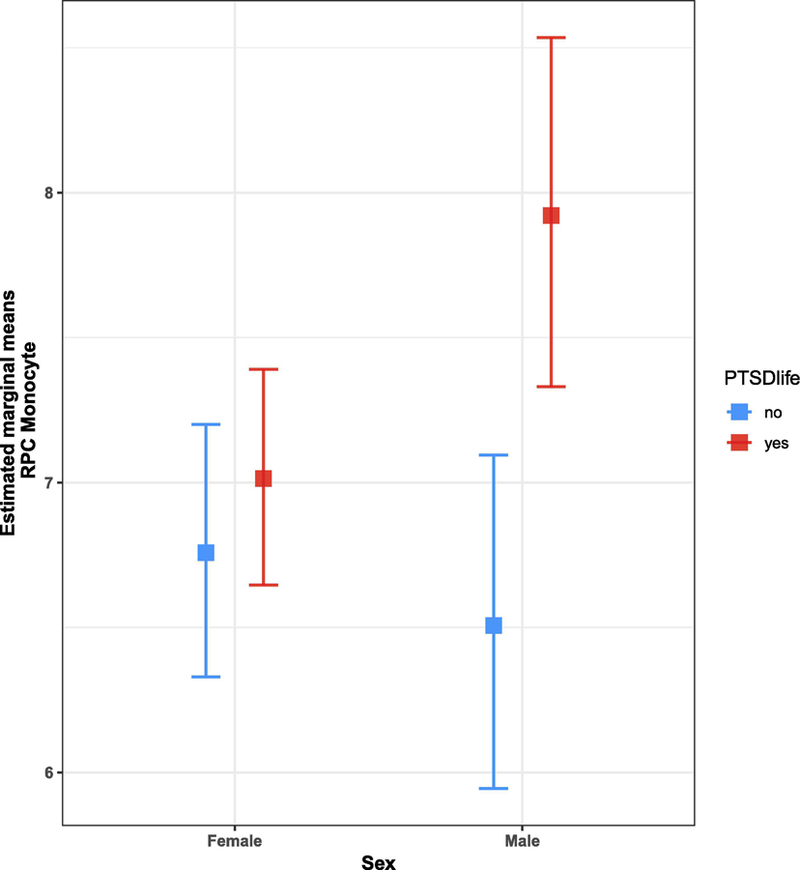
Lifetime PTSD by sex interaction plot for estimated marginal means (EMMs) of RPC monocyte estimates. Interaction plot shows a significant EMM difference between lifetime PTSD cases (red) and controls (blue) in males, where mean monocyte estimates are higher in cases than controls. No significant EMM difference was observed between PTSD cases and controls in females.
Table 3:
Summary for RPC monocyte estimates by group
| Sex | PTSD | n | mean | SE | EMM | SEEMM | lower.CL | upper.CL |
|---|---|---|---|---|---|---|---|---|
| Female | no | 135 | 7.113 | 0.2443 | 6.758 | 0.2214 | 6.330 | 7.200 |
| Female | yes | 184 | 7.182 | 0.1691 | 7.014 | 0.1893 | 6.647 | 7.391 |
| Male | no | 70 | 6.803 | 0.3228 | 6.507 | 0.2926 | 5.945 | 7.095 |
| Male | yes | 80 | 8.103 | 0.3147 | 7.921 | 0.3063 | 7.331 | 8.535 |
This table describes untransformed RPC monocyte estimates by group (i.e., sex and lifetime PTSD); n = count per group; EMM = estimated marginal means (i.e., least squares means); SE = standard errors for regular means; SEEMM = standard errors for EMM.
Lower and upper confidence limits are for 95% level. EMM and intervals were back-transformed from the square root scale to the original scale of cell subtype proportions (%). Significance level of alpha = 0.05 was used for EMM comparisons. Results for pairwise comparison were averaged over levels for current smoking. Degree of freedom was 461 and male lifetime PTSD cases were significantly different from other groups.
3.5. Association between monocyte proportions and lifetime PTSD in males is independent of current PTSD status
To investigate whether participants with current PTSD exhibited a different monocyte profile from those with remitted PTSD, a sex-stratified Kruskal-Wallis test was conducted for PTSD status (i.e., trauma-exposed controls, remitted PTSD, and current PTSD; Figure 6). A significant difference in monocyte estimates was observed in males, H(2) = 8.2, p = 0.017, but not in females, H(2) = 1.1, p = 0.59, confirming findings from analyses for lifetime PTSD. The post-hoc Dunn test revealed significant differences between PTSD case groups and trauma-exposed controls (current PTSD vs. controls: Z = 2.31, p = 0.021, adj. p = 0.042, r = 0.23; remitted PTSD vs controls: Z = 2.40, p = 0.016, adj. p = 0.049, r = 0.22), but no significant difference between current and remitted PTSD groups, Z = 0.18, p = 0.86, adj. p = 0.86, r = 0.02. These findings suggest that the association between monocyte proportions and lifetime PTSD in males is independent of current PTSD state and may reflect long-standing changes associated with lifetime history of PTSD diagnosis.
Figure 6:
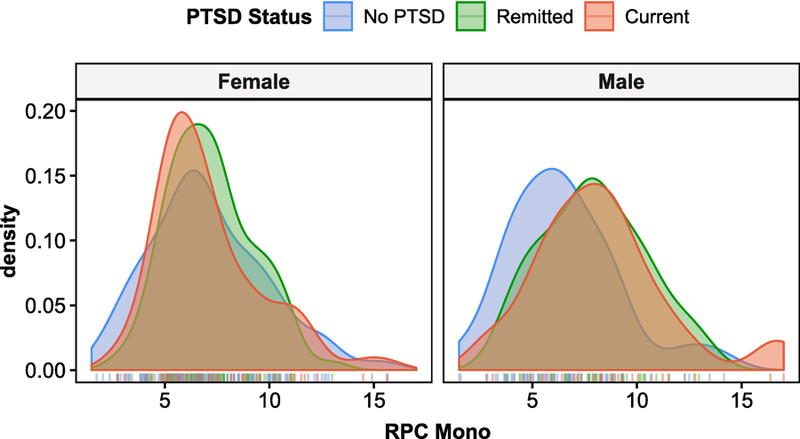
Density plots for RPC monocyte estimates comparing those with current PTSD, remitted PTSD, and trauma-exposed controls, stratified by sex. Distinguishing between current and remitted PTSD cases suggests that the significant peak difference in male PTSD cases may be associated with long-standing PTSD trait, rather than current PTSD state. Corresponding post-hoc Dunn test revealed no significant difference between current and remitted PTSD cases and significant differences between PTSD case groups and trauma-exposed controls. Again, no significant differences were observed in females.
Comparative analyses based on CP monocyte estimates showed similar results to RPC-based results (see Supplementary Materials); however, CP-based results consistently presented smaller effect sizes than RPC-based results across all analyses and follow-up comparisons between PTSD case groups and trauma-exposed controls did not reach significance after p-value adjustment in post-hoc Dunn test using CP monocyte estimates in males.
4. DISCUSSION
Methylomic profiles derived from peripheral blood offer a wealth of information and can be harnessed to detect two dynamic measures of immune state: 1) differences in leukocyte composition (i.e., proportions of peripheral immune cell subtypes); and 2) true alterations in methylation involved in epigenetic regulation of immune processes. They are particularly well-suited for investigating PTSD because DNAm encodes individual response to trauma and may play a key role in PTSD-associated immune dysregulation. Given the prominent sex differences in both PTSD prevalence3,4,8,10–12 and immune response62,63, the primary goal of the present study was to investigate whether PTSD is associated with sex-specific differences in leukocyte composition, detectable by DNAm-based estimates. We found that males with lifetime PTSD showed significantly higher monocyte proportions than trauma-exposed males without PTSD; this difference was not observed in females. No difference in monocyte proportions was observed between current and remitted PTSD cases in males, suggesting that this sex-specific difference may reflect a long-standing trait of lifetime history of PTSD diagnosis, rather than current state of PTSD. These findings were observed in both the primary RPC and comparison CP-based sets of cell estimates, which were derived using non-constrained vs. constrained projection deconvolution algorithms, respectively. Overall, our main finding of elevated monocyte proportions in males, but not females with lifetime history of PTSD provides evidence for a sex-specific difference in peripheral blood leukocyte composition that may reflect long-standing changes associated with PTSD diagnosis and is detectable in methylomic profiles, consistently across different deconvolution algorithms.
In our study, we leveraged recent advances in reference-based deconvolution methods65–67 – specifically the EpiDISH algorithm65, which (i) employs DNase hypersensitive site (DHS) data of leukocyte subtypes to inform probe selection for their reference database and (ii) introduces RPC, a non-constrained projection approach for reference-based deconvolution. A comparative validation study on in-silico mixtures of purified cell DNAm profiles previously showed this newer RPC approach to consistently perform better than the widely used CP approach105, based on root mean square error (RMSE) and R2, at low noise levels65 typically encountered in real data65,67. Relevant to our results, the study showed the difference in RMSE and R2 to be the most prominent in CD8T cells65. This is consistent with our comparison between RPC and CP estimates, which showed CD8T cells to exhibit poorer correlation between RPC and CP estimates relative to other leukocyte subtypes and the largest difference in RPC-CP correlation between sexes. Similarly, the validation study reported better performance of RPC, compared to CP, in monocytes, with higher RMSE and lower R2 in CP compared to RPC, suggesting RPC-based estimates were more robustly associated with true weights. In light of the validation study, this suggests that our RPC-based estimates were better able to resolve male-specific association of monocyte proportions with lifetime PTSD. In all, our results were consistent with the previously published validation study65 and favored use of RPC estimates for modeling leukocyte composition. However, as these methods have been developed recently, further validation and comparative studies are warranted.
Comparison of leukocyte subtype estimates by sex revealed significant baseline sex differences in the distributions of NK cell and CD8T cell proportions, with males showing greater median NK and lower median CD8T cell proportions, using both RPC and CP based estimates. This finding is consistent with a previous study that reported sex differences in both leukocyte subtypes using estimates based on minfi’s implementation of the Houseman approach109 and with immunology literature that reported higher NK cell counts and proportions in males compared to females110. A recent study that modeled cell-specific methylation profiles also reported robust sex differences in CD56+ NK methylation patterns111, suggesting that this leukocyte subtype may be regulated by DNAm in a sex-specific manner. Additionally, DNAm dynamics have been found to drive effector functions in CD8T cells after stimulation112,113. Development of reference databases that resolve the six main leukocyte subtypes to consider proportions of subsets with shared lineage but different functionality/phenotype (e.g., naïve vs memory vs regulatory subtypes) may allow us to explore this hypothesis and would greatly enrich our understanding of immune activity.
Our main finding of higher monocyte proportions in male lifetime PTSD cases is consistent with a previous study of Gulf War Illness (GWI) in a predominantly male veteran cohort, which reported an association between GWI and increase in monocyte count114.
However, comparable studies reporting monocyte counts from differential leukocyte count are generally lacking; the majority of immune studies of PTSD have focused on inflammatory markers (e.g., cytokine levels) and cellular activity, including spontaneous and stimulated cytokine production, and studies of cell counts/proportions have focused on lymphocytes, particularly T-cell subsets18,20,115–118. Two PTSD studies that reported monocyte counts based on white blood cell differential count found no significant difference in monocyte proportions119,120, which is consistent with our results in both sexes, but did not conduct sex-stratified analyses. Additionally, a small study in adult females that matched PTSD participants and controls for phase of menstrual cycle agreed with our female-specific results and reported no difference between PTSD subjects and controls in percentage of any lymphocyte subsets or total numbers of leukocytes, neutrophils, lymphocytes, or monocytes121. PTSD studies investigating peripheral lymphocyte numbers have reported mixed findings21,43,116,117,119,122–126, but more recent studies that further resolved T-cell subpopulations support PTSD-associated differences in T-cell composition indicative of pro-inflammatory skew30,43,126 and immunological aging27. Many of these studies were conducted predominantly in one sex (often in male veteran cohorts43,122–126 and those based on both sexes did not conduct sex-stratified analyses27,30,119. To our knowledge, no authoritative study of sex differences in complete blood counts in PTSD has yet been published, and studies of sex differences in PTSD have generally been lacking, with a number of large-scale studies conducted in predominantly male military cohorts (or on female-only cohorts, e.g. Nurses’ Health Study II127,128).
While not for PTSD, a study of depression that examined white blood cell differential count noted a significant increase in monocyte count and proportions among males with major depressive disorder (MDD), but not females, and a significant sex by diagnosis interaction129. Likewise, a separate longitudinal study following MDD inpatients also reported elevated monocyte counts in patients compared to controls, and this was driven by men130. Additionally, a decrease in depression severity was associated with a decrease in monocyte counts130, suggesting that monocytes may be related to clinical improvement. Similarly, the presence and severity of atherosclerosis, another condition linked to PTSD via systemic low-grade inflammatory state131, are also associated with an increase in monocyte count in males, but not females132.
Further prospective investigation of PTSD is needed to determine whether the higher monocyte proportion observed in males reflects an increased susceptibility for developing PTSD or if it reflects an immunological shift in response to the precipitating trauma associated with PTSD psychopathology. However, studies in a male rodent model provide strong evidence for the latter and have been important for establishing the relationship between peripheral immune cells and the brain in the context of psychosocial stress and associated behavior. Repeated social defeat (RSD) was found to induce myelopoiesis and release inflammatory (Ly6Chi) monocytes into circulation via sympathetic signaling, and this increased level of circulating peripheral monocytes was associated with recruitment of pro-inflammatory monocytes/macrophages to the brain and neuroinflammation35,133,134. Increased proportion of these peripheral monocytes and macrophage recruitment to the brain were also demonstrated to correspond with development, maintenance, and re-establishment of RSD-induced anxiety-like behavior; blockade of this recruitment (via splenectomy or β-adrenergic receptor blockage) before RSD was found to prevent development of anxiety-like behavior37,38. Additionally, a recent paper discerned that stress-induced anxiety-like behavior and social avoidance are dependent on an increase in IL-6 after stress exposure, which induces a primed transcriptional profile in monocytes recruited to the brain and propagates IL-1β mediated inflammation associated with anxiety-like behavior135. These studies implicate peripheral monocytes in directly affecting relevant PTSD-like behavior after stress exposure in males136,137. Very recently, the first study using a modified version of the RSD paradigm was conducted in female mice and reported a similar onset of anxiety-like and social avoidance behavior, increase in myelopoiesis, increase in peripheral monocyte proportions, and recruitment of peripheral myeloid cells to the brain, 14 hours after the last RSD cycle41. Continued work based on this paradigm at multiple time points may be fruitful for investigating if there are sex differences in the kinetics of leukocyte trafficking and tissue distribution, especially since recent investigations in other PTSD-relevant rodent models suggest fundamental sex differences in neurobiological response to trauma exposure17 and in regulation of stress/trauma-induced neuroinflammatory priming/neuroimmune alterations138,139. Furthermore, a social stress paradigm in pregnant rats reported lower numbers of monocytes in stressed females than control females rats140, illustrating the importance of considering different paradigms and breeds/species.
Although no studies of PTSD have investigated sex differences in monocyte counts or methylomic profiles, chronic PTSD-associated sex differences were noted in transcriptional regulation141 and gene expression142 of CD14+ monocytes isolated from peripheral blood. Given the inherent sex differences in innate immune response62,143, as understood in the context of infection, injury, and treatment of inflammatory disorders, sexually dimorphic dynamics and effects may also exist in the context of neuroimmune response to stress/trauma exposure139. One relevant sex difference in monocytes involves the expression of IL-6, which was suggested to be important for stress-induced anxiety-like behavior and social avoidance in the RSD model135. Independent of reproductive hormones (i.e., estradiol, dehydroepiandrosterone [DHEA], progesterone), women were shown to have greater monocyte expression of IL-6 across a circadian period than men, and sympathovagal balance was negatively associated with monocyte IL-6 expression only in women144. On the other hand, a study examining sex differences in regulation of inflammatory cell recruitment and cytokine synthesis found that ovarian hormones regulate phenotype, function, and numbers of macrophages, but not T lymphocytes, in females145. This fundamental sex difference may underlie more efficient recognition and elimination of infectious stimuli without recruitment of circulating neutrophils or excessive cytokine production in females, compared to males145, and may also have implications in the context of psychosocial stress exposure. Relatedly, statins, which have anti-inflammatory activity, modulate monocyte migration in a sex-specific manner, such that both spontaneous and LPS-induced migration of isolated monocytes were found to be inhibited by statins in women, but not men146.
Our observation of male-specific increase in monocyte proportions associated with lifetime PTSD may reflect fundamental sex differences in leukocyte trafficking, tissue distribution, and thus composition in blood, with implications for stress/trauma-induced neuroimmune alterations and behavior. Of note, while the effect size detected in males translates to an absolute difference of only 1.3% in monocyte proportions between participants with vs. without PTSD (~8.1% vs. 6.8%; Table 3), it also corresponds to an increase of approximately 19% in overall monocytes among men with lifetime PTSD. Furthermore, the lack of difference between remitted and current PTSD observed in males may have a number of implications for PTSD pathophysiology, including adverse health consequences associated with PTSD across the life course in men, which may be distinct from PTSD-associated health trajectories in women147–149.
Although the current dataset combined two cohorts and known pregnancies were excluded from our study, sample size and unavailable phenotype data on pregnancy, timing of the menstrual cycle, hormonal birth control use, well-harmonized measures of depression, and health status, as well as gender-related variables, such as coping mechanisms, are all limitations of this study. Future studies that account for hormone levels and other fundamental physiological sex differences may help identify female-specific associations between PTSD and leukocyte composition and clarify if hormone-dependent processes influence leukocyte composition dynamics. Additionally, both cohorts included in this study are civilian, urban, and predominantly African-American, so generalizability of our findings may be limited to this population.
Overall, our study implements current state-of-the-art methods to illustrate feasibility of using DNAm-based leukocyte composition estimates to probe immune profiles. Our literature-supported finding of higher DNAm-based monocyte proportions in males may be an informative metric to include as part of a diagnostic biomarker panel for PTSD in males, and future study in females, with consideration for hormonal status, may elucidate a female-specific panel as well. Differential methylation markers discovered in sex-stratified EWAS, which account for these cell estimates as covariates, are other prime candidates to be included in such sex-specific biomarker panels. Furthermore, in addition to being able to infer leukocyte composition when complete blood count data is not available, these DNAm-based estimates of leukocyte composition can be used to determine cell-specific differential methylation profiles. In fact, methods and validation for cell-specific differential methylation analyses have been published very recently150,151 and may enable the next significant advance in extracting insights from methylomic profiles by contextualizing how differential methylation in specific leukocyte subtypes alter regulatory dynamics in the immune system. Ultimately, this work may help to shape future studies designed to determine whether sex-specific methylomic metrics of peripheral immune status can inform us about sex differences in neuroinflammation and corresponding behavior in response to trauma exposure.
5. CONCLUSION
By combining DNA methylation datasets from two civilian cohorts, the current study found significantly higher monocyte proportions in males with lifetime PTSD compared to trauma-exposed controls, a difference that was not observed in females. This sex-specific difference in peripheral blood leukocyte composition may reflect a long-standing trait of PTSD diagnosis, rather than current state of PTSD. While this finding was confirmed using two different cell estimation approaches (i.e., deconvolution algorithms), the recently developed non-constrained projection approach (RPC) appears better suited for modeling leukocyte composition. Methylome-based characterization of immune profiles holds special promise for the study of PTSD and continued development of reference databases and validation of methods will build on these recent improvements to enrich our understanding of sex-specific immune dysregulation associated with PTSD.
Supplementary Material
Highlights:
Higher monocyte proportion is associated with PTSD in males, but not females.
The difference may reflect a long-standing trait of PTSD, rather than current state.
A second cell estimation approach corroborated this sex-specific finding.
Recently developed estimation approaches may improve on previous methods.
This is the first combined study of DNAm-based leukocyte composition in PTSD.
ACKNOWLEDGMENTS
We appreciate the time and effort of study participants, staff and volunteers of the Detroit Neighborhood Health Study, supported by the National Institutes of Health [R01DA022720, R01DA022720-S1, RC1MH088283, R01 MD011728, 3R01MD011728–02S1], and the Grady Trauma Project, supported by the National Institute of Mental Health [MH096764 and MH071537]. This work was also supported by the CompGen Fellowship (University of Illinois; GSK).
Funding: NIH grants: R01MD011728; 3R01MD011728–02S1; R01 DA022720; DA022720-S1; RC1MH088283; MH096764; MH071537; University of Illinois: CompGen Fellowship
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
REFERENCES
- 1.Association, A. P. Diagnostic and statistical manual of mental disorders 5 edn, (2013).
- 2.Breslau N The epidemiology of trauma, PTSD, and other posttrauma disorders. Trauma Violence Abuse 10, 198–210, 10.1177/1524838009334448 (2009). [DOI] [PubMed] [Google Scholar]
- 3.Kessler RC, Sonnega A, Bromet E, Hughes M & Nelson CB Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry 52, 1048–1060 (1995). [DOI] [PubMed] [Google Scholar]
- 4.Kilpatrick DG et al. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J Trauma Stress 26, 537–547, 10.1002/jts.21848 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu H et al. Association of DSM-IV Posttraumatic Stress Disorder With Traumatic Experience Type and History in the World Health Organization World Mental Health Surveys. JAMA Psychiatry 74, 270–281, 10.1001/jamapsychiatry.2016.3783 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benjet C et al. The epidemiology of traumatic event exposure worldwide: results from the World Mental Health Survey Consortium. Psychol Med 46, 327–343, 10.1017/S0033291715001981 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kessler RC et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62, 593–602, 10.1001/archpsyc.62.6.593 (2005). [DOI] [PubMed] [Google Scholar]
- 8.Kessler RC & Wang PS The descriptive epidemiology of commonly occurring mental disorders in the United States. Annu Rev Public Health 29, 115–129, 10.1146/annurev.publhealth.29.020907.090847 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Yehuda R, Koenen KC, Galea S & Flory JD The role of genes in defining a molecular biology of PTSD. Dis Markers 30, 67–76, 10.3233/DMA-2011-0794 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breslau N et al. Trauma and posttraumatic stress disorder in the community: the 1996 Detroit Area Survey of Trauma. Arch Gen Psychiatry 55, 626–632 (1998). [DOI] [PubMed] [Google Scholar]
- 11.Breslau N, Davis GC, Andreski P, Peterson EL & Schultz LR Sex differences in posttraumatic stress disorder. Arch Gen Psychiatry 54, 1044–1048 (1997). [DOI] [PubMed] [Google Scholar]
- 12.Tolin DF & Foa EB Sex differences in trauma and posttraumatic stress disorder: a quantitative review of 25 years of research. Psychol Bull 132, 959–992, 10.1037/0033-2909.132.6.959 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Kessler RC et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry 51, 8–19 (1994). [DOI] [PubMed] [Google Scholar]
- 14.Kessler RC, McGonagle KA, Swartz M, Blazer DG & Nelson CB Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. J Affect Disord 29, 85–96 (1993). [DOI] [PubMed] [Google Scholar]
- 15.Hodes GE Sex, stress, and epigenetics: regulation of behavior in animal models of mood disorders. Biol Sex Differ 4, 1, 10.1186/2042-6410-4-1 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bangasser DA & Valentino RJ Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front Neuroendocrinol 35, 303–319, 10.1016/j.yfrne.2014.03.008 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pooley AE et al. Sex differences in the traumatic stress response: PTSD symptoms in women recapitulated in female rats. Biol Sex Differ 9, 31, 10.1186/s13293-018-0191-9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Passos IC et al. Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression. Lancet Psychiatry 2, 1002–1012, 10.1016/S2215-0366(15)00309-0 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Michopoulos V, Powers A, Gillespie CF, Ressler KJ & Jovanovic T Inflammation in Fear- and Anxiety-Based Disorders: PTSD, GAD, and Beyond. Neuropsychopharmacology 42, 254–270, 10.1038/npp.2016.146 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Z & Young MR PTSD, a Disorder with an Immunological Component. Front Immunol 7, 219, 10.3389/fimmu.2016.00219 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawamura N, Kim Y & Asukai N Suppression of cellular immunity in men with a past history of posttraumatic stress disorder. Am J Psychiatry 158, 484–486, 10.1176/appi.ajp.158.3.484 (2001). [DOI] [PubMed] [Google Scholar]
- 22.Altemus M, Cloitre M & Dhabhar FS Enhanced cellular immune response in women with PTSD related to childhood abuse. Am J Psychiatry 160, 1705–1707, 10.1176/appi.ajp.160.9.1705 (2003). [DOI] [PubMed] [Google Scholar]
- 23.Glover DA, Steele AC, Stuber ML & Fahey JL Preliminary evidence for lymphocyte distribution differences at rest and after acute psychological stress in PTSD-symptomatic women. Brain Behav Immun 19, 243–251, 10.1016/j.bbi.2004.08.002 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gill JM, Saligan L, Woods S & Page G PTSD is Associated With an Excess of Inflammatory Immune Activities. Perspectives in Psychiatric Care 45, 262–277, 10.1111/j.1744-6163.2009.00229.x (2009). [DOI] [PubMed] [Google Scholar]
- 25.Gotovac K et al. Natural killer cell cytotoxicity and lymphocyte perforin expression in veterans with posttraumatic stress disorder. Prog Neuropsychopharmacol Biol Psychiatry 34, 597–604, 10.1016/j.pnpbp.2010.02.018 (2010). [DOI] [PubMed] [Google Scholar]
- 26.Lindqvist D et al. Proinflammatory milieu in combat-related PTSD is independent of depression and early life stress. Brain Behav Immun 42, 81–88, 10.1016/j.bbi.2014.06.003 (2014). [DOI] [PubMed] [Google Scholar]
- 27.Aiello AE et al. PTSD is associated with an increase in aged T cell phenotypes in adults living in Detroit. Psychoneuroendocrinology 67, 133–141, 10.1016/j.psyneuen.2016.01.024 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindqvist D et al. Increased pro-inflammatory milieu in combat related PTSD - A new cohort replication study. Brain Behav Immun 59, 260–264, 10.1016/j.bbi.2016.09.012 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Sondergaard HP, Hansson LO & Theorell T The inflammatory markers C-reactive protein and serum amyloid A in refugees with and without posttraumatic stress disorder. Clin Chim Acta 342, 93–98, 10.1016/j.cccn.2003.12.019 (2004). [DOI] [PubMed] [Google Scholar]
- 30.Sommershof A et al. Substantial reduction of naive and regulatory T cells following traumatic stress. Brain Behav Immun 23, 1117–1124, 10.1016/j.bbi.2009.07.003 (2009). [DOI] [PubMed] [Google Scholar]
- 31.Hoge EA et al. Broad spectrum of cytokine abnormalities in panic disorder and posttraumatic stress disorder. Depress Anxiety 26, 447–455, 10.1002/da.20564 (2009). [DOI] [PubMed] [Google Scholar]
- 32.Michopoulos V, Norrholm SD & Jovanovic T Diagnostic Biomarkers for Posttraumatic Stress Disorder: Promising Horizons from Translational Neuroscience Research. Biol Psychiatry 78, 344–353, 10.1016/j.biopsych.2015.01.005 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bersani FS et al. A population of atypical CD56(−)CD16(+) natural killer cells is expanded in PTSD and is associated with symptom severity. Brain Behav Immun 56, 264–270, 10.1016/j.bbi.2016.03.021 (2016). [DOI] [PubMed] [Google Scholar]
- 34.Wang Z, Mandel H, Levingston CA & Young MRI An exploratory approach demonstrating immune skewing and a loss of coordination among cytokines in plasma and saliva of Veterans with combat-related PTSD. Hum Immunol 77, 652–657, 10.1016/j.humimm.2016.05.018 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wohleb ES et al. beta-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci 31, 6277–6288, 10.1523/JNEUROSCI.0450-11.2011 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wohleb ES, Powell ND, Godbout JP & Sheridan JF Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J Neurosci 33, 13820–13833, 10.1523/JNEUROSCI.1671-13.2013 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wohleb ES et al. Re-establishment of anxiety in stress-sensitized mice is caused by monocyte trafficking from the spleen to the brain. Biol Psychiatry 75, 970–981, 10.1016/j.biopsych.2013.11.029 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McKim DB et al. Sympathetic Release of Splenic Monocytes Promotes Recurring Anxiety Following Repeated Social Defeat. Biol Psychiatry 79, 803–813, 10.1016/j.biopsych.2015.07.010 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McKim DB et al. Neuroinflammatory Dynamics Underlie Memory Impairments after Repeated Social Defeat. J Neurosci 36, 2590–2604, 10.1523/JNEUROSCI.2394-15.2016 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wohleb ES & Delpech JC Dynamic cross-talk between microglia and peripheral monocytes underlies stress-induced neuroinflammation and behavioral consequences. Prog Neuropsychopharmacol Biol Psychiatry 79, 40–48, 10.1016/j.pnpbp.2016.04.013 (2017). [DOI] [PubMed] [Google Scholar]
- 41.Yin W et al. Repeated social defeat in female mice induces anxiety-like behavior associated with enhanced myelopoiesis and increased monocyte accumulation in the brain. Brain Behav Immun 78, 131–142, 10.1016/j.bbi.2019.01.015 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bam M et al. Evidence for Epigenetic Regulation of Pro-Inflammatory Cytokines, Interleukin-12 and Interferon Gamma, in Peripheral Blood Mononuclear Cells from PTSD Patients. J Neuroimmune Pharmacol 11, 168–181, 10.1007/s11481-015-9643-8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou J et al. Dysregulation in microRNA expression is associated with alterations in immune functions in combat veterans with post-traumatic stress disorder. PLoS One 9, e94075, 10.1371/journal.pone.0094075 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uddin M et al. Epigenetic and immune function profiles associated with posttraumatic stress disorder. Proc Natl Acad Sci U S A 107, 9470–9475, 10.1073/pnas.0910794107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rusiecki JA et al. PTSD and DNA Methylation in Select Immune Function Gene Promoter Regions: A Repeated Measures Case-Control Study of U.S. Military Service Members. Front Psychiatry 4, 56, 10.3389/fpsyt.2013.00056 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bam M et al. Dysregulated immune system networks in war veterans with PTSD is an outcome of altered miRNA expression and DNA methylation. Sci Rep 6, 31209, 10.1038/srep31209 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith AK et al. Differential immune system DNA methylation and cytokine regulation in post-traumatic stress disorder. Am J Med Genet B Neuropsychiatr Genet 156B, 700–708, 10.1002/ajmg.b.31212 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weaver IC et al. Epigenetic programming by maternal behavior. Nat Neurosci 7, 847–854, 10.1038/nn1276 (2004). [DOI] [PubMed] [Google Scholar]
- 49.McGowan PO et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci 12, 342–348, 10.1038/nn.2270 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stankiewicz AM, Swiergiel AH & Lisowski P Epigenetics of stress adaptations in the brain. Brain Res Bull 98, 76–92, 10.1016/j.brainresbull.2013.07.003 (2013). [DOI] [PubMed] [Google Scholar]
- 51.Miller CA, Campbell SL & Sweatt JD DNA methylation and histone acetylation work in concert to regulate memory formation and synaptic plasticity. Neurobiol Learn Mem 89, 599–603, 10.1016/j.nlm.2007.07.016 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maddox SA, Schafe GE & Ressler KJ Exploring epigenetic regulation of fear memory and biomarkers associated with post-traumatic stress disorder. Front Psychiatry 4, 62, 10.3389/fpsyt.2013.00062 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zovkic IB & Sweatt JD Epigenetic mechanisms in learned fear: implications for PTSD. Neuropsychopharmacology 38, 77–93, 10.1038/npp.2012.79 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Malan-Muller S, Seedat S & Hemmings SM Understanding posttraumatic stress disorder: insights from the methylome. Genes Brain Behav 13, 52–68, 10.1111/gbb.12102 (2014). [DOI] [PubMed] [Google Scholar]
- 55.Kwapis JL & Wood MA Epigenetic mechanisms in fear conditioning: implications for treating post-traumatic stress disorder. Trends Neurosci 37, 706–720, 10.1016/j.tins.2014.08.005 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alvarez-Errico D, Vento-Tormo R, Sieweke M & Ballestar E Epigenetic control of myeloid cell differentiation, identity and function. Nat Rev Immunol 15, 7–17, 10.1038/nri3777 (2015). [DOI] [PubMed] [Google Scholar]
- 57.Sun B et al. DNA methylation perspectives in the pathogenesis of autoimmune diseases. Clin Immunol 164, 21–27, 10.1016/j.clim.2016.01.011 (2016). [DOI] [PubMed] [Google Scholar]
- 58.Chen L et al. Genetic Drivers of Epigenetic and Transcriptional Variation in Human Immune Cells. Cell 167, 1398–1414 10.1016/j.cell.2016.10.026 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wolf EJ et al. Traumatic stress and accelerated DNA methylation age: A meta-analysis. Psychoneuroendocrinology 92, 123–134, 10.1016/j.psyneuen.2017.12.007 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Irwin MR & Cole SW Reciprocal regulation of the neural and innate immune systems. Nat Rev Immunol 11, 625–632, 10.1038/nri3042 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pfau ML & Russo SJ Peripheral and Central Mechanisms of Stress Resilience. Neurobiol Stress 1, 66–79, 10.1016/j.ynstr.2014.09.004 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klein SL & Flanagan KL Sex differences in immune responses. Nat Rev Immunol 16, 626–638, 10.1038/nri.2016.90 (2016). [DOI] [PubMed] [Google Scholar]
- 63.Osborne BF, Turano A & Schwarz JM Sex Differences in the Neuroimmune System. Curr Opin Behav Sci 23, 118–123, 10.1016/j.cobeha.2018.05.007 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Titus AJ, Gallimore RM, Salas LA & Christensen BC Cell-type deconvolution from DNA methylation: a review of recent applications. Hum Mol Genet 26, R216–R224, 10.1093/hmg/ddx275 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Teschendorff AE, Breeze CE, Zheng SC & Beck S A comparison of reference-based algorithms for correcting cell-type heterogeneity in Epigenome-Wide Association Studies. BMC Bioinformatics 18, 105, 10.1186/s12859-017-1511-5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koestler DC et al. Improving cell mixture deconvolution by identifying optimal DNA methylation libraries (IDOL). BMC Bioinformatics 17, 120, 10.1186/s12859-016-0943-7 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Salas LA et al. An optimized library for reference-based deconvolution of whole-blood biospecimens assayed using the Illumina HumanMethylationEPIC BeadArray. Genome Biol 19, 64, 10.1186/s13059-018-1448-7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wiencke JK et al. Immunomethylomic approach to explore the blood neutrophil lymphocyte ratio (NLR) in glioma survival. Clin Epigenetics 9, 10, 10.1186/s13148-017-0316-8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koestler DC et al. DNA Methylation-Derived Neutrophil-to-Lymphocyte Ratio: An Epigenetic Tool to Explore Cancer Inflammation and Outcomes. Cancer Epidemiol Biomarkers Prev 26, 328–338, 10.1158/1055-9965.EPI-16-0461 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goldmann E et al. Pervasive exposure to violence and posttraumatic stress disorder in a predominantly African American Urban Community: the Detroit Neighborhood Health Study. J Trauma Stress 24, 747–751, 10.1002/jts.20705 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meyers JL et al. Frequency of alcohol consumption in humans; the role of metabotropic glutamate receptors and downstream signaling pathways. Transl Psychiatry 5, e586, 10.1038/tp.2015.70 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gillespie CF et al. Trauma exposure and stress-related disorders in inner city primary care patients. Gen Hosp Psychiatry 31, 505–514, 10.1016/j.genhosppsych.2009.05.003 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Binder EB et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA 299, 1291–1305, 10.1001/jama.299.11.1291 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zannas AS et al. Lifetime stress accelerates epigenetic aging in an urban, African American cohort: relevance of glucocorticoid signaling. Genome Biol 16, 266, 10.1186/s13059-015-0828-5 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Luppi P How immune mechanisms are affected by pregnancy. Vaccine 21, 3352–3357, 10.1016/S0264-410x(03)00331-1 (2003). [DOI] [PubMed] [Google Scholar]
- 76.Blanchard EB, Jones-Alexander J, Buckley TC & Forneris CA Psychometric properties of the PTSD Checklist (PCL). Behav Res Ther 34, 669–673 (1996). [DOI] [PubMed] [Google Scholar]
- 77.Grubaugh AL, Elhai JD, Cusack KJ, Wells C & Frueh BC Screening for PTSD in public-sector mental health settings: the diagnostic utility of the PTSD checklist. Depress Anxiety 24, 124–129, 10.1002/da.20226 (2007). [DOI] [PubMed] [Google Scholar]
- 78.Wilkins KC, Lang AJ & Norman SB Synthesis of the psychometric properties of the PTSD checklist (PCL) military, civilian, and specific versions. Depress Anxiety 28, 596–606, 10.1002/da.20837 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ruggiero KJ, Del Ben K, Scotti JR & Rabalais AE Psychometric properties of the PTSD Checklist-Civilian Version. J Trauma Stress 16, 495–502, 10.1023/A:1025714729117 (2003). [DOI] [PubMed] [Google Scholar]
- 80.Koenen KC et al. SLC6A4 methylation modifies the effect of the number of traumatic events on risk for posttraumatic stress disorder. Depress Anxiety 28, 639–647, 10.1002/da.20825 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Uddin M et al. Gene expression and methylation signatures of MAN2C1 are associated with PTSD. Dis Markers 30, 111–121, 10.3233/DMA-2011-0750 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jovanovic T et al. Impaired fear inhibition is a biomarker of PTSD but not depression. Depress Anxiety 27, 244–251, 10.1002/da.20663 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Blake DD et al. The development of a Clinician-Administered PTSD Scale. J Trauma Stress 8, 75–90 (1995). [DOI] [PubMed] [Google Scholar]
- 84.Weathers FW, Keane TM & Davidson JR Clinician-administered PTSD scale: a review of the first ten years of research. Depress Anxiety 13, 132–156 (2001). [DOI] [PubMed] [Google Scholar]
- 85.Weckle A et al. Rapid Fractionation and Isolation of Whole Blood Components in Samples Obtained from a Community-based Setting. J Vis Exp, 10.3791/52227 (2015). [DOI] [PMC free article] [PubMed]
- 86.Ratanatharathorn A et al. Epigenome-wide association of PTSD from heterogeneous cohorts with a common multi-site analysis pipeline. Am J Med Genet B Neuropsychiatr Genet 174, 619–630, 10.1002/ajmg.b.32568 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Uddin M et al. Epigenetic meta-analysis across three civilian cohorts identifies NRG1 and HGS as blood-based biomarkers for post-traumatic stress disorder. Epigenomics 10, 1585–1601, 10.2217/epi-2018-0049 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mehta D et al. Childhood maltreatment is associated with distinct genomic and epigenetic profiles in posttraumatic stress disorder. Proc Natl Acad Sci U S A 110, 8302–8307, 10.1073/pnas.1217750110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, Austria, 2018). [Google Scholar]
- 90.Aryee MJ et al. Minfi: a flexible and comprehensive Bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics 30, 1363–1369, 10.1093/bioinformatics/btu049 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huber W et al. Orchestrating high-throughput genomic analysis with Bioconductor. Nat Methods 12, 115–121, 10.1038/nmeth.3252 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gentleman RC et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 5, R80, 10.1186/gb-2004-5-10-r80 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Barfield RT, Kilaru V, Smith AK & Conneely KN CpGassoc: an R function for analysis of DNA methylation microarray data. Bioinformatics 28, 1280–1281, 10.1093/bioinformatics/bts124 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xu Z, Niu L, Li L & Taylor JA ENmix: a novel background correction method for Illumina HumanMethylation450 BeadChip. Nucleic Acids Res 44, e20, 10.1093/nar/gkv907 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu J & Siegmund KD An evaluation of processing methods for HumanMethylation450 BeadChip data. BMC Genomics 17, 469, 10.1186/s12864-016-2819-7 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fortin JP, Triche TJ Jr. & Hansen KD Preprocessing, normalization and integration of the Illumina HumanMethylationEPIC array with minfi. Bioinformatics 33, 558–560, 10.1093/bioinformatics/btw691 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen YA et al. Discovery of cross-reactive probes and polymorphic CpGs in the Illumina Infinium HumanMethylation450 microarray. Epigenetics 8, 203–209, 10.4161/epi.23470 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Teschendorff AE et al. A beta-mixture quantile normalization method for correcting probe design bias in Illumina Infinium 450 k DNA methylation data. Bioinformatics 29, 189–196, 10.1093/bioinformatics/bts680 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Morris TJ et al. ChAMP: 450k Chip Analysis Methylation Pipeline. Bioinformatics 30, 428–430, 10.1093/bioinformatics/btt684 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tian Y et al. ChAMP: updated methylation analysis pipeline for Illumina BeadChips. Bioinformatics 33, 3982–3984, 10.1093/bioinformatics/btx513 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Triche TJ Jr., Weisenberger DJ, Van Den Berg D, Laird PW & Siegmund KD Low-level processing of Illumina Infinium DNA Methylation BeadArrays. Nucleic Acids Res 41, e90, 10.1093/nar/gkt090 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Johnson WE, Li C & Rabinovic A Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 8, 118–127, 10.1093/biostatistics/kxj037 (2007). [DOI] [PubMed] [Google Scholar]
- 103.Leek JT, Johnson WE, Parker HS, Jaffe AE & Storey JD The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics 28, 882–883, 10.1093/bioinformatics/bts034 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Roadmap Epigenomics, C. et al. Integrative analysis of 111 reference human epigenomes. Nature 518, 317–330, 10.1038/nature14248 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Houseman EA et al. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinformatics 13, 1–16, 10.1186/1471-2105-13-86 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rahmani E et al. Genome-wide methylation data mirror ancestry information. Epigenetics Chromatin 10, 1, 10.1186/s13072-016-0108-y (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Barfield RT et al. Accounting for population stratification in DNA methylation studies. Genet Epidemiol 38, 231–241, 10.1002/gepi.21789 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Holm S A Simple Sequentially Rejective Multiple Test Procedure. Scandinavian Journal of Statistics 6, 65–70 (1979). [Google Scholar]
- 109.Inoshita M et al. Sex differences of leukocytes DNA methylation adjusted for estimated cellular proportions. Biol Sex Differ 6, 11, 10.1186/s13293-015-0029-7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Abdullah M et al. Gender effect on in vitro lymphocyte subset levels of healthy individuals. Cell Immunol 272, 214–219, 10.1016/j.cellimm.2011.10.009 (2012). [DOI] [PubMed] [Google Scholar]
- 111.White N et al. Accounting for cell lineage and sex effects in the identification of cell-specific DNA methylation using a Bayesian model selection algorithm. PLoS One 12, e0182455, 10.1371/journal.pone.0182455 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Scharer CD, Barwick BG, Youngblood BA, Ahmed R & Boss JM Global DNA methylation remodeling accompanies CD8 T cell effector function. J Immunol 191, 3419–3429, 10.4049/jimmunol.1301395 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Suarez-Alvarez B, Rodriguez RM, Fraga MF & Lopez-Larrea C DNA methylation: a promising landscape for immune system-related diseases. Trends Genet 28, 506–514, 10.1016/j.tig.2012.06.005 (2012). [DOI] [PubMed] [Google Scholar]
- 114.Johnson GJ, Slater BC, Leis LA, Rector TS & Bach RR Blood Biomarkers of Chronic Inflammation in Gulf War Illness. PLoS One 11, e0157855, 10.1371/journal.pone.0157855 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Speer K, Upton D, Semple S & McKune A Systemic low-grade inflammation in post-traumatic stress disorder: a systematic review. J Inflamm Res 11, 111–121, 10.2147/JIR.S155903 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pace TW & Heim CM A short review on the psychoneuroimmunology of posttraumatic stress disorder: from risk factors to medical comorbidities. Brain Behav Immun 25, 6–13, 10.1016/j.bbi.2010.10.003 (2011). [DOI] [PubMed] [Google Scholar]
- 117.Ironson G, Cruess D & Kumar M in Psychoneuroimmunology (Fourth Edition) (ed Robert Ader) 531–547 (Academic Press, 2007). [Google Scholar]
- 118.Wong CM Post-traumatic stress disorder: advances in psychoneuroimmunology. Psychiatr Clin North Am 25, 369–383, vii (2002). [DOI] [PubMed] [Google Scholar]
- 119.Rohleder N, Joksimovic L, Wolf JM & Kirschbaum C Hypocortisolism and increased glucocorticoid sensitivity of pro-Inflammatory cytokine production in Bosnian war refugees with posttraumatic stress disorder. Biol Psychiatry 55, 745–751, 10.1016/j.biopsych.2003.11.018 (2004). [DOI] [PubMed] [Google Scholar]
- 120.Gola H et al. Posttraumatic stress disorder is associated with an enhanced spontaneous production of pro-inflammatory cytokines by peripheral blood mononuclear cells. BMC Psychiatry 13, 10.1186/1471-244x-13-40 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Altemus M, Dhabhar FS & Yang R Immune function in PTSD. Ann N Y Acad Sci 1071, 167–183, 10.1196/annals.1364.013 (2006). [DOI] [PubMed] [Google Scholar]
- 122.Boscarino JA & Chang J Higher abnormal leukocyte and lymphocyte counts 20 years after exposure to severe stress: research and clinical implications. Psychosom Med 61, 378–386 (1999). [DOI] [PubMed] [Google Scholar]
- 123.de Kloet CS et al. Leukocyte glucocorticoid receptor expression and immunoregulation in veterans with and without post-traumatic stress disorder. Mol Psychiatry 12, 443–453, 10.1038/sj.mp.4001934 (2007). [DOI] [PubMed] [Google Scholar]
- 124.Vidovic A et al. Repeated assessments of endocrine- and immune-related changes in posttraumatic stress disorder. Neuroimmunomodulation 18, 199–211, 10.1159/000322869 (2011). [DOI] [PubMed] [Google Scholar]
- 125.Vidovic A et al. Circulating lymphocyte subsets, natural killer cell cytotoxicity, and components of hypothalamic-pituitary-adrenal axis in Croatian war veterans with posttraumatic stress disorder: cross-sectional study. Croat Med J 48, 198–206 (2007). [PMC free article] [PubMed] [Google Scholar]
- 126.Jergovic M et al. Patients with posttraumatic stress disorder exhibit an altered phenotype of regulatory T cells. Allergy Asthma Clin Immunol 10, 43, 10.1186/1710-1492-10-43 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Koenen KC et al. Protocol for investigating genetic determinants of posttraumatic stress disorder in women from the Nurses’ Health Study II. BMC Psychiatry 9, 29, 10.1186/1471-244X-9-29 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sumner JA et al. Cross-Sectional and Longitudinal Associations of Chronic Posttraumatic Stress Disorder With Inflammatory and Endothelial Function Markers in Women. Biol Psychiatry 82, 875–884, 10.1016/j.biopsych.2017.06.020 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Maes M et al. Leukocytosis, monocytosis and neutrophilia: hallmarks of severe depression. J Psychiatr Res 26, 125–134, 10.1016/0022-3956(92)90004-8 (1992). [DOI] [PubMed] [Google Scholar]
- 130.Seidel A et al. Major depressive disorder is associated with elevated monocyte counts. Acta Psychiat Scand 94, 198–204, 10.1111/j.1600-0447.1996.tb09849.x (1996). [DOI] [PubMed] [Google Scholar]
- 131.Brouwers CJ, Wolf JM & von Känel R in Comprehensive Guide to Post-Traumatic Stress Disorder Ch. Chapter 54–1, 1–13 (2015).
- 132.Huang ZS et al. Correlations between peripheral differential leukocyte counts and carotid atherosclerosis in non-smokers. Atherosclerosis 158, 431–436, 10.1016/S0021-9150(01)00445-2 (2001). [DOI] [PubMed] [Google Scholar]
- 133.Engler H, Bailey MT, Engler A & Sheridan JF Effects of repeated social stress on leukocyte distribution in bone marrow, peripheral blood and spleen. J Neuroimmunol 148, 106–115, 10.1016/j.jneuroim.2003.11.011 (2004). [DOI] [PubMed] [Google Scholar]
- 134.Weber MD, Godbout JP & Sheridan JF Repeated Social Defeat, Neuroinflammation, and Behavior: Monocytes Carry the Signal. Neuropsychopharmacology 42, 46–61, 10.1038/npp.2016.102 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Niraula A, Witcher KG, Sheridan JF & Godbout JP Interleukin-6 Induced by Social Stress Promotes a Unique Transcriptional Signature in the Monocytes That Facilitate Anxiety. Biol Psychiatry 85, 679–689, 10.1016/j.biopsych.2018.09.030 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bierhaus A et al. A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci U S A 100, 1920–1925, 10.1073/pnas.0438019100 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Grisanti LA et al. Pro-inflammatory responses in human monocytes are beta1-adrenergic receptor subtype dependent. Mol Immunol 47, 1244–1254, 10.1016/j.molimm.2009.12.013 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fonken LK et al. Neuroinflammatory priming to stress is differentially regulated in male and female rats. Brain Behav Immun 70, 257–267, 10.1016/j.bbi.2018.03.005 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bekhbat M & Neigh GN Sex differences in the neuro-immune consequences of stress: Focus on depression and anxiety. Brain Behav Immun 67, 1–12, 10.1016/j.bbi.2017.02.006 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Stefanski V, Raabe C & Schulte M Pregnancy and social stress in female rats: influences on blood leukocytes and corticosterone concentrations. J Neuroimmunol 162, 81–88, 10.1016/j.jneuroim.2005.01.011 (2005). [DOI] [PubMed] [Google Scholar]
- 141.O’Donovan A et al. Transcriptional control of monocyte gene expression in post-traumatic stress disorder. Dis Markers 30, 123–132, 10.3233/DMA-2011-0768 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Neylan TC et al. Suppressed monocyte gene expression profile in men versus women with PTSD. Brain Behav Immun 25, 524–531, 10.1016/j.bbi.2010.12.001 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nunn CL, Lindenfors P, Pursall ER & Rolff J On sexual dimorphism in immune function. Philos Trans R Soc Lond B Biol Sci 364, 61–69, 10.1098/rstb.2008.0148 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.O’Connor MF, Motivala SJ, Valladares EM, Olmstead R & Irwin MR Sex differences in monocyte expression of IL-6: role of autonomic mechanisms. Am J Physiol Regul Integr Comp Physiol 293, R145–151, 10.1152/ajpregu.00752.2006 (2007). [DOI] [PubMed] [Google Scholar]
- 145.Scotland RS, Stables MJ, Madalli S, Watson P & Gilroy DW Sex differences in resident immune cell phenotype underlie more efficient acute inflammatory responses in female mice. Blood 118, 5918–5927, 10.1182/blood-2011-03-340281 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ruggieri A et al. Statin-induced impairment of monocyte migration is gender-related. J Cell Physiol 229, 1990–1998, 10.1002/jcp.24657 (2014). [DOI] [PubMed] [Google Scholar]
- 147.Dedert EA, Calhoun PS, Watkins LL, Sherwood A & Beckham JC Posttraumatic stress disorder, cardiovascular, and metabolic disease: a review of the evidence. Ann Behav Med 39, 61–78, 10.1007/s12160-010-9165-9 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mitchell KS et al. PTSD and obesity in the Detroit neighborhood health study. Gen Hosp Psychiatry 35, 671–673, 10.1016/j.genhosppsych.2013.07.015 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.McLean CP, Asnaani A, Litz BT & Hofmann SG Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. J Psychiatr Res 45, 1027–1035, 10.1016/j.jpsychires.2011.03.006 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zheng SC, Breeze CE, Beck S & Teschendorff AE Identification of differentially methylated cell types in epigenome-wide association studies. Nat Methods 15, 1059–1066, 10.1038/s41592-018-0213-x (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Li Z, Wu Z, Jin P & Wu H Dissecting differential signals in high-throughput data from complex tissues. Bioinformatics, 10.1093/bioinformatics/btz196 (2019). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.