Abstract
Gene expression and DNA methylation levels affect the outcomes of patients with cancer. The present study aimed to establish a multigene risk model for predicting the outcomes of patients with cervical cancer (CerC) treated with or without radiotherapy. RNA sequencing training data with matched DNA methylation profiles were downloaded from The Cancer Genome Atlas database. Patients were divided into radiotherapy and non-radiotherapy groups according to the treatment strategy. Differently expressed and methylated genes between the two groups were identified, and 8 prognostic genes were identified using Cox regression analysis. The optimized risk model based on the 8-gene signature was defined using the Cox's proportional hazards model. Kaplan-Meier survival analysis indicated that patients with higher risk scores exhibited poorer survival compared with patients with lower risk scores (log-rank test, P=3.22×10−7). Validation using the GSE44001 gene set demonstrated that patients in the high-risk group exhibited a shorter survival time comprared with the low-risk group (log-rank test, P=3.01×10−3). The area under the receiver operating characteristic curve values for the training and validation sets were 0.951 and 0.929, respectively. Cox regression analyses indicated that recurrence and risk status were risk factors for poor outcomes in patients with CerC treated with or without radiotherapy. The present study defined that the 8-gene signature was an independent risk factor for the prognosis of patients with CerC. The 8-gene prognostic model had predictive power for CerC prognosis.
Keywords: cervical cancer, radiotherapy, DNA methylation, risk model, overall survival
Introduction
Cervical cancer (CerC) is a leading cause of cancer-associated mortality in women worldwide (1,2). Surgery in combination with chemotherapy and radiotherapy is the most common strategy for CerC treatment. Radiotherapy significantly improves CerC patient prognosis (3). However, the overall survival of patients with CerC diagnosed at advanced stages remains poor, with the 5-year survival rate ≤50%, despite advanced surgical protocols and diagnostic methods (4).
The general prognosis criteria of the International Federation of Gynecology and Obstetrics (FIGO) classification do not include all prognostic factors, including histologic subtypes and lymph node metastasis, which are effective for the prediction of CerC prognosis (5). Molecular markers and clinical parameters are crucial for the prediction of clinical outcomes and deciding treatment strategies (2,5). In addition, the identification of biomarkers associated with radiotherapy response is of great importance for understanding the molecular mechanisms of CerC and developing novel strategies.
Radiotherapy significantly benefits patients with CerC (3). The methylation status in the promoters of a number of genes is associated with patient outcomes after radiotherapy (6–8). For example, Dunn et al (6) demonstrated that the O6-methyxlguanine-DNA-methyltransferase (MGMT) promoter methylation level was positively associated with the progression-free survival and overall survival of patients with glioblastomas treated with temozolomide and radiotherapy (6). Huang et al (7) indicated that the combined Ras association domain family member (RASSF) 1A/RASSF2A methylation level was negatively correlated with the disease-free survival (DFS) of radiotherapy-treated squamous cell carcinoma. Widschwendter et al (9) revealed that the methylated myoblast determination protein 1 (MYOD1) in CerC was associated with poor DFS (9).
An increasing number of studies have indicated the prognostic power of gene signatures for diease prognosis, metastasis and recurrence. Okayama et al (10) identified a 4 gene signature with prediction power for stage I lung cancer prognosis (10); Cheng et al (11) described an 8-gene classifier with predictive power for locoregional recurrence of breast cancer in patients post-mastectomy (11). Therefore, the predictive power of multigene sigatures for disease development may be of great clinical interest. A 12-gene classifier has been used for the clinical diagnosis of low and high metastasis of in uveal melanoma (12,13). In addition, the DNA methylation level is a significant factor in disease development (6–8). However, to the best of our knowledge, there have been few studies investigating gene methylation signatures for prognosis in radiotherapy-treated patients with CerC.
The present study was designed to explore a novel risk model for predicting outcome of patients with CerC by analyzing RNA sequencing (RNA-seq) data in combination with matched DNA methylation profiles from The Cancer Genome Atlas (TCGA) database. A multigene risk model that predicted the outcomes of patients with CerC treated with or without radiotherapy was identified.
Materials and methods
TCGA and Gene Expression Omnibus (GEO) dataset
Training data were downloaded from TCGA database (https://gdc-portal.nci.nih.gov/) in June 2018. A total of 307 mRNA-seq profiles (Illumina Hiseq2000) and 312 DNA methylation profiles (Illumina Infinium Human Methylation 450 BeadChip) were downloaded. Paired mRNA-seq and methylation data were included in the present study. Clinical features including age, pathologic stage and grade, and survival rate of patients with CerC were extracted and used for subsequent analysis.
Validation dataset GSE44001 (GPL14951 Illumina HumanHT-12 WG-DASL V4.0 R2 expression beadchip) (14) was downloaded from the National Center of Biotechnology Information GEO database (http://www.ncbi.nlm.nih.gov/geo/). The GSE44001 dataset consists of 300 patients with primary early CerC (FIGO stage I–II). Prognostic data were available for the training and validation sets. The study design presented in Fig. 1.
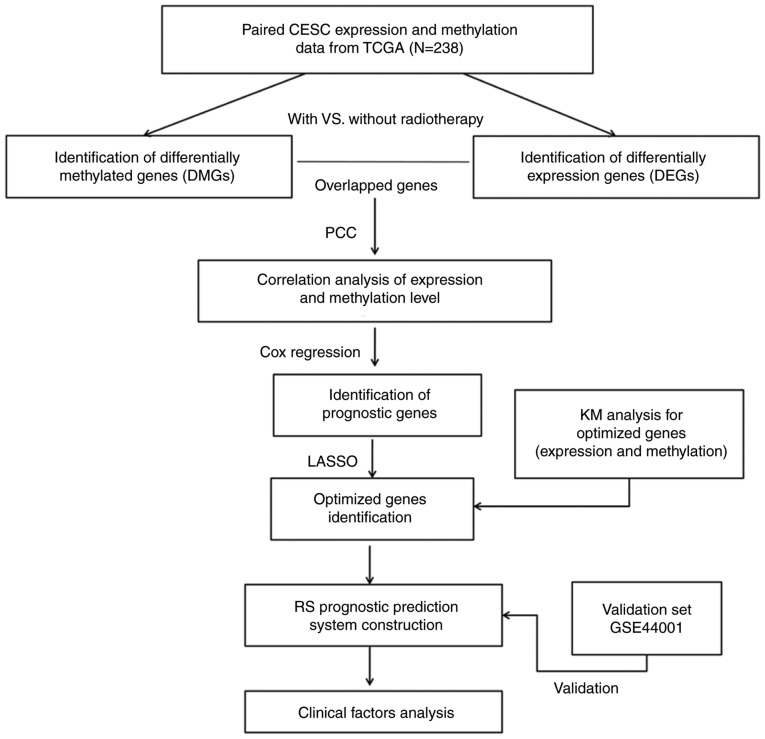
Figure 1.
Flow diagram of the study design. KM, Kaplan-Meier; Lasso, least absolute shrinkage and selection operator. PCC, Pearson correlation coefficient analysis.
Identification of differentially expressed and methylated genes
Samples from the TCGA training set were assigned into two groups according to radiotherapy treatment (with and without radiotherapy). Differentially expressed genes (DEGs) and differentially methylated genes (DMGs) between the two groups were identified using Linear Models for Microarray Data (Limma) package (version 3.34.7) in R (https://bioconductor.org/packages/release/bioc/html/limma.html). A false discovery rate (FDR) <0.05 and |log2 fold change (FC) | >0.263 (>1.2 FC) were set as the cutoffs. DEGs with differential methylation were selected.
Correlation analysis between gene expression and methylation level
Pearson's correlation between gene expression and the methylation level of DEGs was calculated using Cor. Test function (https://stat.ethz.ch/R-manual/R-devel/library/stats/html/cor.test.html) in R. DEGs with significantly correlated expression and methylation level (P<0.05) were included as candidate genes for subsequent analysis.
Selection of prognostic DEGs
Univariate Cox regression analysis in survival package of R (version 2.41.3; http://CRAN.R-project.org/package=survival) (15) was used to screen the DEGs and DMGs associated with the prognosis of patients with CerC. P<0.05, determined by a Kaplan-Meier log-rank test, was defined as the significant cutoff value.
Definition and validation of prognostic risk model
Cox's proportional hazards (Cox-PH) model based on the L1-penalized least absolute shrinkage and selection operator regression algorithm in the penalized package (version 0.9.50) was used for optimizing a prediction model with a linear gene signature (16). The optimized parameter ‘lamba’ was obtained by 1,000 rounds of cross-validated likelihood (cvl) circular calculation. The risk score of each sample was defined as the linear combination of prognostic gene expression level and Cox-PH regression coefficient: Risk score=∑coefgene × Expression (Methylation) gene. Patients were assigned into high-risk and low-risk groups according to the median value of risk score. The overall survival difference between the two groups was evaluated using Kaplan-Meier and log-rank methods in survival package of R (version 2.41.3; http://CRAN.R-project.org/package=survival). The GSE44001 dataset was used to validate the performance and predictive power of the prognostic risk model. The area under the time-independent receiver operating characteristic curve (AUC) was used for evaluation (2).
Selection and stratification analyses of potential clinical prognostic factors
The independent prognostic risk factors among clinical variables in TCGA patients were selected using univariate and multivariate Cox regression analysis in survival package of R (https://CRAN.R-project.org/package=survival). P<0.05, determined by a Kaplan-Meier log-rank test, was set as the significant cutoff value. Stratification analysis was performed for patients with and without radiotherapy, with a significant threshold of P<0.05, as determined by a log-rank test.
Bioinformatic analysis of prognostic DEGs
Patients within the training set were assigned into high-risk (samples with higher risk scores than the median) and low-risk (samples with lower risk scores than the median) groups according to the computed risk scores. DEGs between the two groups (FDR <0.05 and |logFC| >0.263) were identified using Limma package in R. Hierarchical clustering analysis of DEGs was analyzed using the Pheatmap package (version 1.0.8; http://CRAN.R-project.org/package=pheatmap) in R (17,18). Gene Set Enrichment Analysis (GSEA) (19,20) was performed to identify the Kyoto Encyclopedia of Genes and Genomes (KEGG) (21) pathways significantly (P<0.05) associated with DEGs between the two groups.
Statistical analysis
Continuous clinical variables, including age and overall survival, are presented as the mean ± standard deviation (SD), and differences between groups were analyzed using Student's t-test. Differences in categorical variables, including mortality and pathological characteristics, between two groups were analyzed using Fisher's exact test. Univariate Cox regression analysis was employed for the identification of independent prognostic genes, and a two-step Cox regression analysis was used to identify independent prognostic factors among clinical variables. In the stratified analysis, prognostic differences between the high-risk and low-risk patients stratification analysis were analyzed using Kaplan-Meier survival analysis. All analyses were performed in R (version 3.4.1; http://www.r-project.org/), and P<0.05 was considered to indicate a statistically significantly difference.
Results
Baseline characteristics of patients with CerC
A total of 238 patients with CerC with paired mRNA-seq and DNA methylation profiles from TCGA were used in the present study. Table I describes the baseline characteristics of the included 238 patients. A total of 64 and 174 patients were assigned into non-radiotherapy and radiotherapy groups, respectively. Significant differences in age (P=3.12×10−2), pathologic N stage (P=2.15×10−3), pathologic T stage (P=1.90×10−5), pathologic stage (P=9.84×10−5), new tumor incidence (recurrence; P=2.20×10−16) and therapy strategy (P=3.16×10−10) were observed between patients with and without radiotherapy. There was no difference in overall survival and survival rate between the two groups (Table I).
Table I.
Baseline characteristics of The Cancer Genome Atlas patients with cervical cancer treated with or without radiotherapy.
| Clinical characteristics | Without radiotherapy (N=64) | With radiotherapy (N=174) | P-value |
|---|---|---|---|
| Age, years, mean ± SD | 45.09±11.49 | 49.01±14.19 | 3.12×10−2a |
| Pathologic M (M0/M1/NA) | 29/1/34 | 57/9/108 | 1.65×10−1b |
| Pathologic N (N0/N1/NA) | 45/7/12 | 58/35/81 | 2.15×10−3b |
| Pathologic T (T1/T2/T3/T4/NA) | 47/9/0/4/4 | 62/46/17/5/44 | 1.902×10−5b |
| Pathologic stage (I/II/III/IV/NA) | 49/8/2/4/1 | 78/47/29/16/4 | 9.842×10−5b |
| Pathologic grade (1/2/3/4/NA) | 6/27/26/0/5 | 10/79/66/1/18 | 6.92×10−1b |
| Smoking (reformed/current/never/NA) | 7/12/38/7 | 33/43/86/12 | 2.06×10−2b |
| New tumor (yes/no/-) | 51/12/1 | 31/142/1 | 2.20×10−16b |
| Targeted molecular therapy (yes/no/NA) | 5/25/34 | 127/37/10 | 3.164×10−10b |
| Death (dead/alive) | 15/49 | 51/123 | 4.17×10−1b |
| Overall survival months, mean ± SD | 35.11±43.03 | 38.75±38.92 | 5.54×10−1a |
Student's t test.
Fisher's exact test. NA, not available; SD, standard deviation.
Identification of DEGs and DMGs in patients with CerC
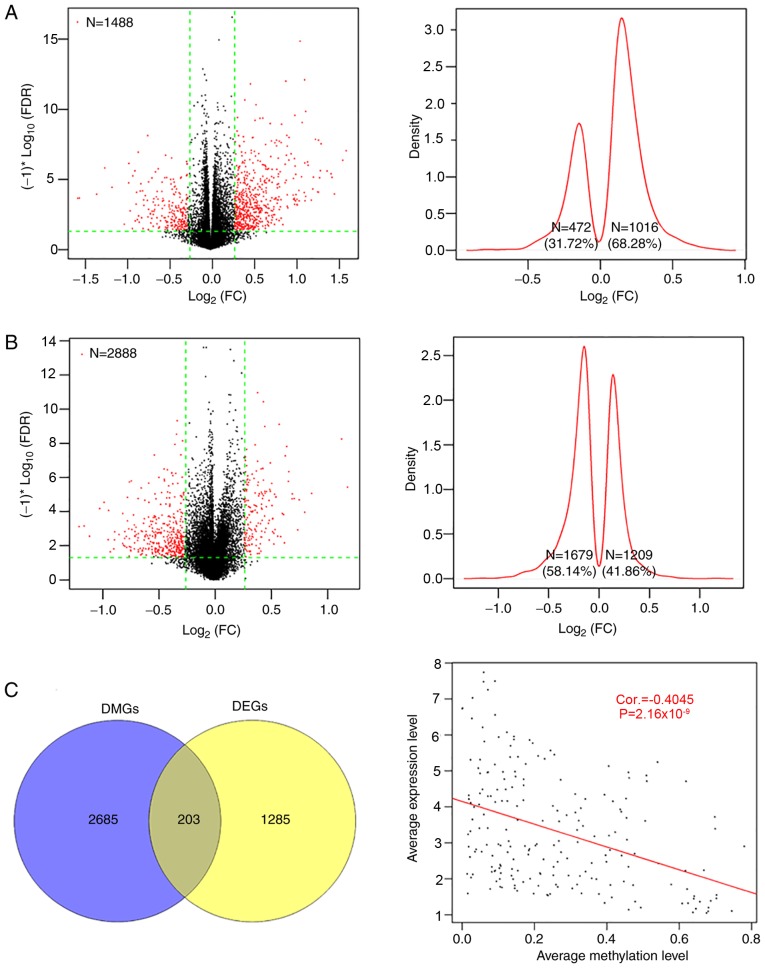
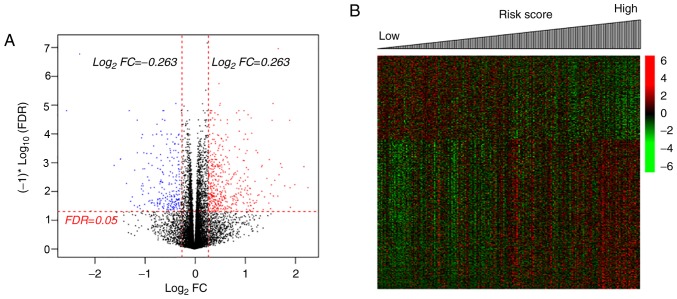
There were 1,488 DEGs and 2,888 DMGs identified between the two groups (Fig. 2); the majority of the DEGs (62.28%, 1,016/1,488) were upregulated and the majority of the DMGs (58.14%, 1,679/2,888) were hypomethylated by radiotherapy, compared with the non-radiotherapy group (Fig. 2A and B). There were 203 overlapping genes, with an overall negative correlation between average expression and methylation levels (Cor=−0.4045; P=2.16×10−9; Fig. 2C). Pearson's correlation analysis identified 107 genes (including 83 up- and 24 downregulated genes, Table SI) with negatively correlated expression and methylation levels.
Figure 2.
DEGs and DMGs between patients with and without radiotherapy. (A) Volcano plot of the DEGs (left) and the kernel density curve plot (right). (B) Volcano plot of the DMGs (left) and the kernel density curve plot (right). Green lines indicate the thresholds of FDR<0.05 (horizontal) and |log2FC| >0.263 (vertical), respectively. (C) Identification of DEGs and DMGs levels between the two groups (right), and the Pearson's correlation analysis for average expression and methylation levels of the 203 genes. DEGs, differentially expressed genes; DMGs, differentially methylated genes; FDR, false discovery rate; FC, fold change.
Identification of prognostic genes
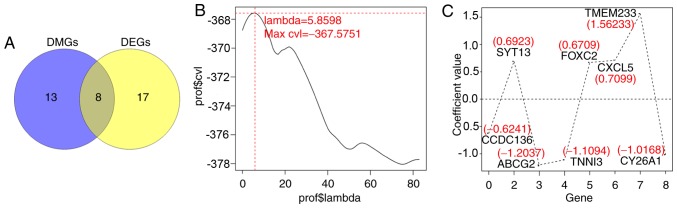
Using univariate Cox regression analysis in the survival package of R, a total of 25 prognostic DEGs and 21 prognostic DMGs were identified from the aforementioned 107 genes (Table SII), including 8 overlapped genes, which were identified to be candidate genes associated with the prognosis of patients with CerC (Fig. 3A). The optimal 8-gene matrix was obtained using Cox-PH model (max ‘lambda’=5.8598, max cvl=−367.5751; Fig. 3B). The Cox-PH regression coefficients are indicated in Fig. 3C and Table II. Accordingly, 238 patients in the training set were stratified into high-expression (n=119) and low-expression (n=119) groups, according to the median expression value of each gene.
Figure 3.
Selection of optimal prognostic genes using the Cox-PH model. (A) Venn diagram of overlapped DEGs and DMGs, FDR <0.05 and |log2FC| >0.263. (B) The optimal ‘lambda’ parameters by cvl circular calculation. (C) The Cox-PH regression coefficients distribution of the 8 genes in the optimal matrix. Cox-PH, Cox's proportional hazards; DEGs, differentially expressed genes; DMGs, differentially methylated genes; cvl, Cross-validation likelihood; CCDC136, coiled-coil domain containing 136 gene; ABCG2, ATP binding cassette subfamily G member 2 gene; SYT13, synaptotagmin XIII gene; TNNI3, cardiac troponin I gene; FOXC2, Forkhead 1 gene; CXCL5, epithelial neutrophil-activating peptide-78 gene; TMEM233, transmembrane protein 233 gene; CY26A1, cytochrome P450 26A1 gene.
Table II.
Cox's proportional hazards regression coefficients of the 8 signature genes.
| Gene | Correlation coefficient | HR (95% CI) | P-value |
|---|---|---|---|
| CCDC136 | −0.6241 | 0.917 (0.758–0.991) | 2.71×10−2 |
| ABCG2 | −1.2037 | 0.847 (0.699–0.925) | 4.76×10−2 |
| CYP26A1 | −1.0168 | 0.889 (0.786–0.998) | 6.00×10−3 |
| TNNI3 | −1.1094 | 0.881 (0.799–0.971) | 4.87×10−2 |
| SYT13 | 0.6923 | 1.076 (1.008–1.168) | 4.52×10−2 |
| FOXC2 | 0.6709 | 1.066 (1.056–1.189) | 2.12×10−3 |
| CXCL5 | 0.7099 | 1.075 (1.006–1.160) | 2.48×10−3 |
| TMEM233 | 1.5623 | 1.234 (1.007–1.526) | 2.61×10−4 |
HR, hazard ratio; CI, confidence interval; CCDC136, coiled-coil domain containing 136 gene; ABCG2, ATP binding cassette subfamily G member 2 gene; CY26A1, cytochrome P450 26A1 gene; TNNI3, cardiac troponin I gene; SYT13, synaptotagmin XIII gene; FOXC2, Forkhead 1 gene; CXCL5, epithelial neutrophil-activating peptide-78 gene; TMEM233, transmembrane protein 233 gene.
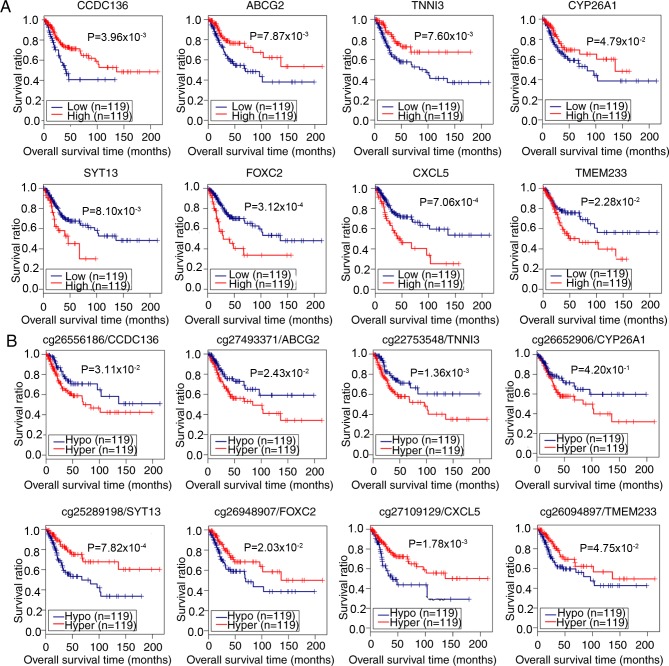
Subsequent Kaplan-Meier survival analyses demonstrated that the expression of coiled-coil domain containing 136 gene (CCDC136), ATP binding cassette subfamily G member 2 gene (ABCG2), cardiac troponin I gene (TNNI3) and Cytochrome P450 26A1 gene (CYP26A1) were positively associated with survival of patients with CerC, whereas the expression of Synaptotagmin XIII gene (SYT13), Forkhead 1 gene (FOXC2), epithelial neutrophil-activating peptide-78 gene (CXCL5) and transmembrane protein 233 gene (TMEM233) were negatively correlated (log-rank test; P<0.05; Fig. 4A; Table II). For methylation levels, analysis indicated the hypermethylation of CCDC136, ABCG2, TNNI3, and CYP26A1 genes, and the hypomethylation of SYT13, FOXC2, CXCL5, and TMEM233 genes was associated with the poor survival of patients with CerC (P<0.05, log-rank test; Fig. 4B). These data demonstrated that the expression and methylation levels of these 8 genes were potential independent risk factors for prognosis in patients with CerC.
Figure 4.
Correlation analysis of the expression and methylation levels of 8 potential prognostic genes with survival of patients with CerC. (A) Correlation between the expression of 8 genes and the prognosis of patients with CerC. Red and blue lines indicate high and low expression levels, respectively. (B) Correlation between the DNA methylation level and prognosis of patients with CerC. The number prior to the gene symbols indicates the methylation loci. Red and blue lines denote hyper- and hypomethylation, respectively. Correlation analysis was performed using Kaplan-Meier survival analysis. CCDC136, coiled-coil domain containing 136 gene; ABCG2, ATP binding cassette subfamily G member 2 gene; SYT13, synaptotagmin XIII gene; TNNI3, cardiac troponin I gene; FOXC2, Forkhead 1 gene; CXCL5, epithelial neutrophil-activating peptide-78 gene; TMEM233, transmembrane protein 233 gene; CY26A1, cytochrome P450 26A1 gene; CerC, cervical cancer.
Establishment and evaluation of the risk model
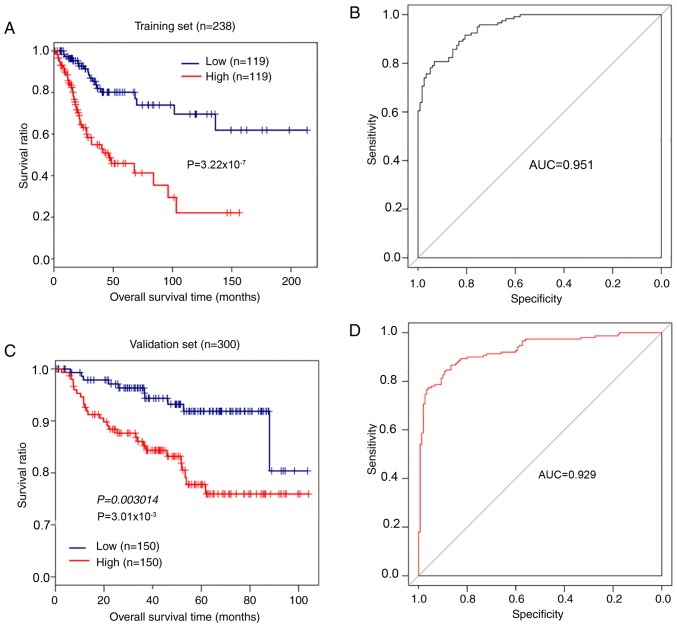
The mRNA prognostic model based on the combination of Cox-PH regression coefficients and gene expression levels was established as: Risk score=(−0.6241) × ExpCCDC136+ (−1.2037) × ExpABCG2 + (−1.0168) × ExpCYP26A1+ (−1.1094) × ExpTNNI3+ (0.6923) × ExpSYT13 + (0.6709) × ExpFOXC2+ (0.7099) × ExpCXCL5 + (1.5623) × ExpTMEM233. According to the median risk score, 238 patients with CerC in the training set were assigned into high-risk and low-risk groups. Kaplan-Meier survival analysis indicated that patients with low-risk scores exhibited longer overall survival compared with patients with high-risk scores (P=3.22×10−7, log-rank test; Fig. 5A). The AUC was 0.951 (Fig. 5B). Analysis of the validation set GSE44001 demonstrated that patients with CerC with high-risk scores exhibited significantly shorter overall survival times compared with patients with low-risk scores (P=3.01×10−3, log-rank test; Fig. 5C), and the AUC was 0.929 (Fig. 5D). These results demonstrated that the 8-gene signature had performance and predictive power for outcomes of patients with CerC.
Figure 5.
Kaplan-Meier survival analysis and ROC curves for the 8-gene signature in patients with cervical cancer. (A) Kaplan-Meier survival analysis and (B) ROC curve analysis for patients in TCGA training set (n=238). (C) Kaplan-Meier survival analysis and (D) ROC curve analysis for patients in TCGA GSE44001 validation set (n=300) based on the 8-gene signature risk model. ROC, receiver operating characteristic; TCGA, The Cancer Genome Atlas; AUC, area under the curve.
Prognostic value of clinical variables
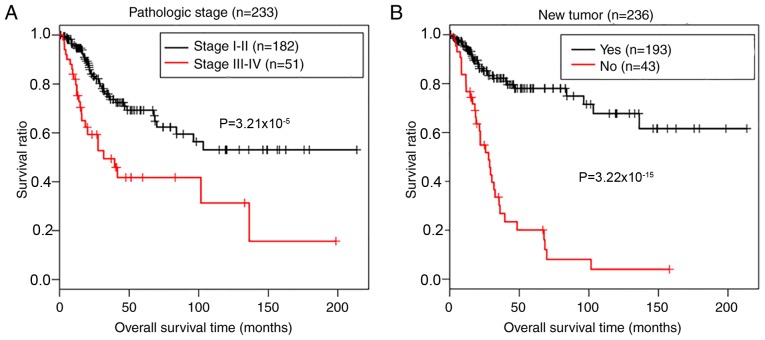
A two-step Cox regression analyses (univariate and multivariate) were used to define the potential prognostic values of clinical variables, including age, pathologic stage and grade, smoking, radiotherapy, recurrence and risk status, in patients from the TCGA data. Table III demonstrates that 3 independent risk factors, including pathologic stage [hazard ratio (HR)=2.386; 95% confidence interval (CI), 1.097–5.192; P=0.0284), new tumor (recurrence; HR=7.333; 95% CI, 1.833–12.235; P=3.21×10−9) and risk status (HR=1.359; 95% CI, 1.702–8.905; P=1.28×10−3) were of prognostic value for the outcomes of patients with CerC. Kaplan-Meier survival analysis determined the prognostic potential of pathologic stage and tumor recurrence. As presented in Fig. 6, there was a significantly shorter overall survival time in patients with advanced (III–IV) pathological stages (P=3.21×10−5, log-rank test; Fig. 6A) and recurrence (P=9.22×10−15, log-rank test; Fig. 6B), compared with patients in early stage disease without recurrence.
Table III.
Cox regression analyses for the prognostic value of clinical variables.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Age (≤45/>45) | 1.014 (0.996–1.032) | 0.138 | – | – |
| Pathologic M (M0/M1) | 4.101 (1.356–12.41) | 0.0677 | – | – |
| Pathologic N (N0/N1) | 2.923 (1.440–5.932) | 1.86×10−3 | 1.535 (0.711–3.315) | 2.76×10−1 |
| Pathologic T (T1/T2/T3/T4) | 1.986 (1.473–2.678) | 2.69×10−6 | 1.900 (0.986–3.662) | 5.51×10−2 |
| Pathologic stage (I/II/III/IV) | 1.594 (1.277–1.989) | 2.20×10−5 | 2.386 (1.097–5.192) | 2.84×10−2 |
| Pathologic grade (1/2/3/4) | 0.943 (0.612–1.452) | 7.89×10−1 | – | – |
| Smoking (reformed/current/never) | 0.984 (0.719–1.346) | 9.18×10−1 | – | – |
| New tumor (yes/no) | 5.637 (3.446–9.22) | 9.22×10−15 | 7.33 (1.833–12.235) | 3.22×10−9 |
| Targeted molecular therapy (yes/no) | 0.953 (0.547–1.659) | 8.64×10−1 | – | – |
| Risk status (high/low) | 3.736 (2.177–6.411) | 3.22×10−7 | 1.359 (1.702–8.905) | 1.28×10−3 |
HR, hazard ratio; CI, confidence interval.
Figure 6.
Kaplan-Meier survival analysis of (A) pathological stage and (B) tumor recurrence for predicting outcomes of patients in The Cancer Genome Atlas training set. Significant differences were analyzed using Kaplan-Meier log-rank test (P<0.05).
Stratification analysis for risk factors associated with radiotherapy
To additionally confirm the risk factors associated with radiotherapy, stratified analysis for patients with radiotherapy and without radiotherapy was performed. A two-step Cox regression analyses indicated that pathologic N stage (HR=4.247; 95% CI, 1.3651–6.216; P=1.25×10−2), pathologic stage (HR=2.275; 95% CI, 1.052–3.868; P=4.53×10−2), recurrence (HR=3.841; 95% CI, 1.332–5.122; P=2.27×10−5) and risk status (HR=5.110; 95% CI, 1.578–6.547; P=6.51×10−3) were risk factors for radiotherapy-treated patients, whereas recurrence (HR=4.665; 95% CI, 2.367–9.463; P=1.58×10−3) and risk status (HR=7.546; 95% CI, 1.177–8.364; P=3.30×10−2) were risk factors for patients treated without radiotherapy (Table IV). These results demonstrated that recurrence and 8-gene signature risk status were independent risk factors for predicting the prognosis of patients with CerC.
Table IV.
Stratification analysis for risk factors associated with radiotherapy in patients from The Cancer Genome Atlas training set.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
| With radiotherapy (n=174) | ||||||
| Age (≤45/>45) | 1.006 | 0.986–1.026 | 5.48×10−1 | – | – | – |
| Pathologic M (M0/M1) | 5.457 | 1.436–10.73 | 5.13×10−2 | – | – | – |
| Pathologic N (N0/N1) | 4.651 | 1.769–12.23 | 6.31×10−4 | 4.247 | 1.3651–6.216 | 1.25×10−2 |
| Pathologic T (T1/T2/T3/T4) | 1.652 | 1.135–2.407 | 7.65×10−3 | 1.564 | 0.7868–3.107 | 2.02×10−1 |
| Pathologic stage (I/II/III/IV) | 2.320 | 1.327–4.056 | 2.36×10−3 | 2.275 | 1.052–3.868 | 4.53×10−2 |
| Pathologic grade (1/2/3/4) | 0.878 | 0.540–1.428 | 6.01×10−1 | – | – | – |
| Smoking (reformed/current/never) | 0.998 | 0.705–1.415 | 9.95×10−1 | – | – | – |
| New tumor (yes/no) | 5.191 | 2.986–9.026 | 7.89×10−11 | 3.841 | 1.332–5.122 | 2.27×10−5 |
| Targeted molecular therapy (yes/no) | 1.06 | 0.539–2.081 | .66×10−1 | – | – | – |
| Risk status (high/low) | 3.198 | 1.762–5.804 | 5.51×10−5 | 5.11 | 1.578–6.547 | 6.51×10−3 |
| Without radiotherapy (n=64) | ||||||
| Age (≤45/>45) | 1.051 | 1.004–1.1 | 3.13×10−2 | 1.031 | 0.977–1.089 | 2.67×10−1 |
| Pathologic M (M0/M1) | – | – | – | – | – | – |
| Pathologic N (N0/N1) | 1.348 | 0.287–2.337 | 7.05×10−1 | – | – | – |
| Pathologic T (T1/T2/T3/T4) | 3.638 | 1.999–6.623 | 2.29×10−9 | 1.187 | 0.122–1.598 | 8.83×10−1 |
| Pathologic stage (I/II/III/IV) | 2.462 | 1.281–3.148 | 4.53×10−5 | 2.655 | 1.243–4.007 | 4.05×10−1 |
| Pathologic grade (1/2/3/4) | 1.192 | 0.438–3.245 | 7.31×10−1 | – | – | – |
| Smoking (reformed/current/never) | 0.863 | 0.395–1.882 | 7.10×10−1 | – | – | – |
| New tumor (yes/no) | 7.802 | 2.53–14.06 | 2.52×10−5 | 4.665 | 2.367–9.463 | 1.58×10−3 |
| Targeted molecular therapy (yes/no) | 1.408 | 0.998–2.181 | 2.77×10−1 | – | – | – |
| Risk status (high/low) | 6.762 | 1.804–10.35 | 1.40×10−3 | 7.546 | 1.177–8.364 | 3.30×10−2 |
HR, hazard ratio; CI, confidence interval. Risk status was predicated based on the 8-gene risk model.
Identification of DEGs and KEGG pathways associated with risk status of patients with CerC
To define the gene profiles between patients with high and low risk status, 490 DEGs were identified (Table SIII) in the high-risk group, compared with the low-risk group (Fig. 7A), including 313 upregulated DEGs (63.88%, including CXCL5, SYT13, FOXC2, ITGB3 and TMEM233) and 177 downregulated DEGs (36.18%, including CYP26A1 and TNNI3) in the high-risk group. Fig. 7B demonstrates the markedly altered expression profiles of these DEGs in patients with low and high risk scores. GSEA KEGG pathway analysis indicated that these genes (including CYP26A1 and CXCL5) were associated with pathways including ‘ECM Receptor Interaction’, ‘Retinol Metabolism’, ‘Focal Adhesion’, ‘Hedgehog Signaling Pathway’, ‘NOD-like Receptor Signaling Pathway’ and ‘Chemokine Signaling Pathway’ (Table V).
Figure 7.
DEGs between patients with high- and low-risk scores. (A) Volcano plot differentially expressed genes (FDR <0.05 and |log2FC| >0.263). (B) The heat map of the DEGs in The Cancer Genome Atlas patients. DEGs, differentially expressed genes; FDR, false discovery rate; FC, fold change.
Table V.
Gene Set Enrichment Analysis of the KEGG pathways associated with differentially expressed genes between patients with cervical cancer with high- and low-risk scores.
| KEGG term | ES | NES | NOM P-value | Gene |
|---|---|---|---|---|
| ECM receptor interaction | 0.7695 | 1.2517 | 1.88×10−2 | LAMA1, COL11A1, ITGB3, IBSP |
| Retinol Metabolism | −0.8045 | −1.2455 | 2.05×10−2 | ADH7, CYP26A1, CYP26C1, UGT2A1 |
| Focal adhesion | 0.6963 | 1.2640 | 2.14×10−2 | LAMA1, COL11A1, ITGB3 |
| Hedgehog signaling pathway | −0.7371 | −1.2183 | 2.34×10−2 | WNT3A, BMP7 |
| NOD-like receptor signaling pathway | 0.7413 | 1.1071 | 3.98×10−2 | CXCL2, IL6, IL1B |
| Chemokine signaling pathway | 0.5071 | 0.9989 | 4.72×10−2 | CXCL2, CXCL6, ADCY1, CXCL3, CXCL5 |
KEGG, Kyoto Encyclopedia of Genes and Genomes; ECM, extracellular matrix; ES, enrichment score; NES, normalized enrichment score; NOM, nominal.
Discussion
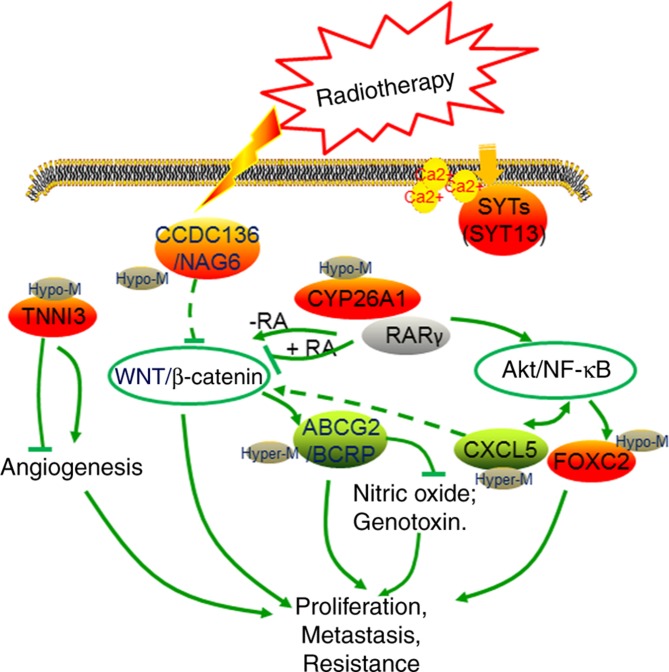
Identification of molecular biomarkers associated with radiotherapy may aid in devising strategies for improving radiotherapy response (22). In the present study, a large-scale analysis of RNA-seq from TCGA CerC samples, in combination with matched DNA methylation profiles, was performed, and an 8-gene risk model was identified (CCDC136, ABCG2, CYP26A1, TNNI3, CXCL5, SYT13 FOXC2, ITGB3, and TMEM233) to predict the risk status of patients with CerC. This 8-gene signature was defined to be an independent prognostic factor, with predictive power for prognosis of patients with CerC. Among these 8 genes, 4 hypermethylated genes (CCDC136, ABCG2, CYP26A1 and TNNI3) were positively associated the overall survival of patients with CerC, and 4 hypomethylated genes (SYT13, FOXC2, CXCL5 and TMEM233) were negatively associated the overall survival of patients with CerC.
The 4 hypermethylated genes (CCDC136, ABCG2, CYP26A1 and TNNI3) had previously been identified to be dysregulated in various human cancer tissues (23–26). Among these, TNNI3 is an angiogenesis inhibitor responsible for the inhibition of endothelial cell tube formation (27,28). Kern et al (28) suggested that metastasis was decreased in a mouse model of pancreatic cancer in response to troponin I treatment, compared with control mice. Downregulated troponin I inhibits cancer cell proliferation, as it is required for tumor growth (29). CCDC136, also known as nasopharyngeal carcinoma-associated gene 6, is located at chromosome 7q31-32. It is commonly deleted in a number of types of malignant human cancer, and has been recognized to function as a putative tumor suppressor in gastric tumor and nasopharyngeal carcinoma (23,30). Wei et al (30) suggested that CCDC136 negatively regulated the Wnt/β-catenin signaling pathway in zebrafish embryos. Wnt/β-catenin signaling is oncogenic and confers cancer cell proliferation, drug resistance and metastasis in various types of human cancer, including ovarian cancer and CerC (31–34) (Fig. 8). In the present study, it was identified that CCDC136 and TNNI3 were upregulated in the radiotherapy group, compared with the non-radiotherapy group. This may be associated with the decreased angiogenesis and downregulated Wnt/β-catenin signaling in patients treated with radiotherapy, which in turn is associated with the lower recurrence and improved prognosis observed in the radiotherapy group.
Figure 8.
Graphical presentation of the radiotherapy-relevant 8-gene signature in cervical cancer. Green and red points indicate downregulated and upregulated genes in the radiotherapy group, respectively. SYT13, synaptotagmin XIII gene; CCDC136/ NAG6, coiled-coil domain containing 136 gene; TNNI3, cardiac troponin I gene; CY26A1, cytochrome P450 26A1 gene; RA, retinoic acid; RARγ, RA receptor γ; ABCG2/BCRP, ATP binding cassette subfamily G member 2 gene; CXCL5, epithelial neutrophil-activating peptide-78 gene; FOXC2, Forkhead 1 gene.
ABCG2 encodes an multidrug transporter protein, breast cancer resistance protein (BCRP), which contributes to drug resistance in cancer cell lines and tumors (35,36). It has been reported that ABCG2 is downstream of Wnt/β-catenin signaling and is responsible for chemoresistance (37). Downregulated BCRP/ABCG2 is common in tumor tissues, including CerC, which may function in tumorigenesis by promoting the accumulation of genotoxins and nitric oxide (24,38,39). In addition, ABCG2 promoter methylation has been described in multiple myeloma tissues (40). The demethylation of ABCG2 increases its expression and enhances multidrug resistance in cancer cells (40,41). CYP26A1 is an oncogenic protein in breast cancer, cervical squamous neoplasia, ovarian cancer, and head and neck cancer (25,26,42). CYP26A1 is a metabolizing enzyme for retinoic acids (RAs) (25). RAs induce the differentiation of various types of stem cells (43), and the RA receptor γ (RARγ) is associated with the Akt/NF-κB and Wnt/β-catenin signaling pathways in tumorigenesis (44). Yasuhara et al (45) suggested that RARγ enhances and inhibits Wnt/β-catenin signaling in RA-free and RA-treated conditions, respectively. Demethylation and hypermethylation of CYP26A1 had been demonstrated in the CYP26A1-positive T47D cell line, which exhibits low rates of metastasis, and the CYP26A1-negative T47D cell line, which exhibits high rates of metastasis, respectively (46). It has been suggested that increased methylation levels in the CYP26A1 promoter is associated with poor survival in patients with prostate cancer (47). In the present study, the expression levels of ABCG2 and CYP26A1 were downregulated and upregulated, respectively, in patients in the radiotherapy group compared with the non-radiotherapy group. These two genes were identified to be positively associated with the prognosis of patients with CerC, and their hypermethylation was correlated with poor survival. In addition, it was also observed that CYP26A1 was associated with the ‘Retinol Metabolism’ GSEA KEGG pathway, which was associated with RA metabolism in cancer cells (48). These results suggested the complex roles of these genes in response to radiotherapy, and their potential prognostic value.
Among the 4 hypomethylated genes (SYT13, FOXC2, CXCL5 and TMEM233), SYT13, FOXC2 and CXCL5 have been demonstrated to be associated with tumorigenesis. FOXC2 is a downstream target of the Akt/NF-κB signaling pathway and is critical for tumor metastasis (49). The inhibition of FOXC2 results in the suppression of tumor metastasis and chemoresistance in lung cancer cells, nasopharyngeal carcinomas and CerC cells (49–51). Synaptotagmins are a family of Ca2+ sensors that function in promoting membrane fusion (52,53). Overexpression of synaptotagmin has previously been described in human cancer (54–57). Kanda et al (58) demonstrated that SYT13 was upregulated in gastric cancer and was associated with metastatic status. CXCL5 is a CXC-type chemokine, and is involved in angiogenesis and associated with poor prognosis in cancer patients (59–62). In addition, CXCL5 expression activated the Akt/NF-κB and Wnt/β-catenin signaling pathways (63–65). FOXC2 and CXCL5 were upregulated in the patients treated with radiotherapy compared with patients without radiotherapy, and their expression was associated with poor survival in patients with CerC. These demonstrated the potential prognostic value of SYT13, FOXC2 and CXCL5 for predicting patients with high risk status or poor outcomes.
In conclusion, a significant difference in survival was observed between the patients with CerC with high- and low-risk scores according to the 8-gene signature. The AUC and survival analysis in the training and validation set revealed the performance and predictive power of the 8-gene signature risk model for predicting survival of patients with CerC. Cox regression analysis indicated that the 8-gene signature was an independent risk factor for the prognosis of patients with CerC. Validation with more and larger clinical cohorts may additionally verify the potential prognostic value of the 8-gene signature in patients with CerC.
Supplementary Material
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the Double Tenth Engineering of Major Research Project of Jilin Provincial Science and Technology Department (grant no. 20140201012YY) and the Major Development Programs for New Drugs of the Chinese Academy of Sciences during the 12th Five-Year Plan Period (grant no. 2011ZX09102-001-36).
Availability of data and materials
The results published here are in part based upon data generated by the TCGA Research Network (https://www.cancer.gov/tcga). The Gene Expression Omnibus dataset GSE44001 is available at http://www.ncbi.nlm.nih.gov/geo/. All data generated during this study are included in this published article.
Authors' contributions
FX, DD and GT were responsible for the conception and design of the research. ND, LG, WN, HY, NZ, JJ and GL acquired and analyzed the data. FX and DD drafted the manuscript. LG, WN, HY, NZ, JJ and GL revised important intellectual content. All authors agreed with the final revision.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Cancer Genome Atlas Research Network; Albert Einstein College of Medicine; Analytical Biological Services; Barretos Cancer Hospital; Baylor College of Medicine; Beckman Research Institute of City of Hope; Buck Institute for Research on Aging; Canada's Michael Smith Genome Sciences Centre; Harvard Medical School, corp-author. Helen F, et al. Integrated genomic and molecular characterization of cervical cancer. Nature. 2017;543:378–384. doi: 10.1038/nature21386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang K, Sun H, Li X, Hu T, Yang R, Wang S, Jia Y, Chen Z, Tang F, Shen J, et al. Prognostic risk model development and prospective validation among patients with cervical cancer stage IB2 to IIB submitted to neoadjuvant chemotherapy. Sci Rep. 2016;6:27568. doi: 10.1038/srep27568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jadon R, Pembroke CA, Hanna CL, Palaniappan N, Evans M, Cleves AE, Staffurth J. A systematic review of organ motion and image-guided strategies in external beam radiotherapy for cervical cancer. Clin Oncol (R Coll Radiol) 2014;26:185–196. doi: 10.1016/j.clon.2013.11.031. [DOI] [PubMed] [Google Scholar]
- 4.White A, Joseph D, Rim SH, Johnson CJ, Coleman MP, Allemani C. Colon cancer survival in the United States by race and stage (2001–2009): Findings from the CONCORD-2 study. Cancer. 2017;123(Suppl 24):S5014–S5036. doi: 10.1002/cncr.31076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Obrzut B, Kusy M, Semczuk A, Obrzut M, Kluska J. Prediction of 5-year overall survival in cervical cancer patients treated with radical hysterectomy using computational intelligence methods. BMC Cancer. 2017;17:840. doi: 10.1186/s12885-017-3806-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunn J, Baborie A, Alam F, Joyce K, Moxham M, Sibson R, Crooks D, Husband D, Shenoy A, Brodbelt A, et al. Extent of MGMT promoter methylation correlates with outcome in glioblastomas given temozolomide and radiotherapy. Br J Cancer. 2009;101:124–131. doi: 10.1038/sj.bjc.6605127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang KH, Huang SF, Chen IH, Liao CT, Wang HM, Hsieh LL. Methylation of RASSF1A, RASSF2A, and HIN-1 is associated with poor outcome after radiotherapy, but not surgery, in oral squamous cell carcinoma. Clin Cancer Res. 2009;15:4174–4180. doi: 10.1158/1078-0432.CCR-08-2929. [DOI] [PubMed] [Google Scholar]
- 8.Miousse IR, Kutanzi KR, Koturbash I. Effects of ionizing radiation on DNA methylation: From experimental biology to clinical applications. Int J Radiat Biol. 2017;93:457–469. doi: 10.1080/09553002.2017.1287454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Widschwendter A, Müller HH, Fiegl H, Ivarsson L, Wiedemair A, Müller-Holzner E, Goebel G, Marth C, Widschwendter M. DNA methylation in serum and tumors of cervical cancer patients. Clin Cancer Res. 2004;10:565–571. doi: 10.1158/1078-0432.CCR-0825-03. [DOI] [PubMed] [Google Scholar]
- 10.Okayama H, Schetter AJ, Ishigame T, Robles AI, Kohno T, Yokota J, Takenoshita S, Harris CC. The expression of four genes as a prognostic classifier for stage I lung adenocarcinoma in 12 independent cohorts. Cancer Epidemiol Biomarkers Prev. 2014;23:2884–2894. doi: 10.1158/1055-9965.EPI-14-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng SH, Horng CF, Huang TT, Huang ES, Tsou MH, Shi LS, Yu BL, Chen CM, Huang AT. An eighteen-gene classifier predicts locoregional recurrence in post-mastectomy breast cancer patients. EBioMedicine. 2016;5:74–81. doi: 10.1016/j.ebiom.2016.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harbour JW. A prognostic test to predict the risk of metastasis in uveal melanoma based on a 15-gene expression profile. Methods Mol Biol. 2014;1102:427–440. doi: 10.1007/978-1-62703-727-3_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Field MG, Decatur CL, Kurtenbach S, Gezgin G, van der Velden PA, Jager MJ, Kozak KN, Harbour JW. PRAME as an independent biomarker for metastasis in Uveal melanoma. Clin Cancer Res. 2016;22:1234–1242. doi: 10.1158/1078-0432.CCR-15-2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee YY, Kim TJ, Kim JY, Choi CH, Do IG, Song SY, Sohn I, Jung SH, Bae DS, Lee JW, Kim BG. Genetic profiling to predict recurrence of early cervical cancer. Gynecol Oncol. 2013;131:650–654. doi: 10.1016/j.ygyno.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Wang P, Wang Y, Hang B, Zou X, Mao JH. A novel gene expression-based prognostic scoring system to predict survival in gastric cancer. Oncotarget. 2016;7:55343–55351. doi: 10.18632/oncotarget.10533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goeman JJ. L1 penalized estimation in the Cox proportional hazards model. Biom J. 2010;52:70–84. doi: 10.1002/bimj.200900028. [DOI] [PubMed] [Google Scholar]
- 17.Wang L, Cao C, Ma Q, Zeng Q, Wang H, Cheng Z, Zhu G, Qi J, Ma H, Nian H, Wang Y. RNA-seq analyses of multiple meristems of soybean: Novel and alternative transcripts, evolutionary and functional implications. BMC Plant Biol. 2014;14:169. doi: 10.1186/1471-2229-14-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstråle M, Laurila E, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 21.Kanehisa M, Furumichi M, Tanabe M, Sato Y, Morishima K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017;45:D353–D361. doi: 10.1093/nar/gkw1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu FF, Shi W, Done SJ, Miller N, Pintilie M, Voduc D, Nielsen TO, Nofech-Mozes S, Chang MC, Whelan TJ, et al. Identification of a Low-Risk luminal a breast cancer cohort that may not benefit from breast radiotherapy. J Clin Oncol. 2015;33:2035–2040. doi: 10.1200/JCO.2014.57.7999. [DOI] [PubMed] [Google Scholar]
- 23.Zhang XM, Sheng SR, Wang XY, Bin LH, Wang JR, Li GY. Expression of tumor related gene NAG6 in gastric cancer and restriction fragment length polymorphism analysis. World J Gastroenterol. 2004;10:1361–1364. doi: 10.3748/wjg.v10.i9.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta N, Martin PM, Miyauchi S, Ananth S, Herdman AV, Martindale RG, Podolsky R, Ganapathy V. Down-regulation of BCRP/ABCG2 in colorectal and cervical cancer. Biochem Biophys Res Commun. 2006;343:571–577. doi: 10.1016/j.bbrc.2006.02.172. [DOI] [PubMed] [Google Scholar]
- 25.Osanai M, Lee GH. Increased expression of the retinoic acid-metabolizing enzyme CYP26A1 during the progression of cervical squamous neoplasia and head and neck cancer. BMC Res Notes. 2014;7:697. doi: 10.1186/1756-0500-7-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Downie D, Mcfadyen MC, Rooney PH, Cruickshank ME, Parkin DE, Miller ID, Telfer C, Melvin WT, Murray GI. Profiling cytochrome P450 expression in ovarian cancer: Identification of prognostic markers. Clin Cancer Res. 2005;11:7369–7375. doi: 10.1158/1078-0432.CCR-05-0466. [DOI] [PubMed] [Google Scholar]
- 27.Wang D, Li T, Cui H, Zhang Y. Analysis of the indicating value of cardiac troponin I, tumor necrosis factor-α, interleukin-18, Mir-1 and Mir-146b for viral myocarditis among Children. Cell Physiol Biochem. 2016;40:1325–1333. doi: 10.1159/000453185. [DOI] [PubMed] [Google Scholar]
- 28.Kern BE, Balcom JH, Antoniu BA, Warshaw AL, Fernández-del Castillo C. Troponin I peptide (Glu94-Leu123), a cartilage-derived angiogenesis inhibitor: In vitro and in vivo effects on human endothelial cells and on pancreatic cancer. J Gastrointest Surg. 2003;7:961–969. doi: 10.1016/j.gassur.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Casas-Tintó S, Maraver A, Serrano M, Ferrús A. Troponin-I enhances and is required for oncogenic overgrowth. Oncotarget. 2016;7:52631–52642. doi: 10.18632/oncotarget.10616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei S, Shang H, Cao Y, Wang Q. The coiled-coil domain containing protein Ccdc136b antagonizes maternal Wnt/β-catenin activity during zebrafish dorsoventral axial patterning. J Genet Genomics. 2016;43:431–438. doi: 10.1016/j.jgg.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Kopczynski J, Kowalik A, Chłopek M, Wang ZF, Góźdź S, Lasota J, Miettinen M. Oncogenic activation of the Wnt/β-catenin signaling pathway in signet ring stromal cell tumor of the ovary. Appl Immunohistochem Mol Morphol. 2016;24:e28–e33. doi: 10.1097/PAI.0000000000000271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagaraj AB, Joseph P, Kovalenko O, Singh S, Armstrong A, Redline R, Resnick K, Zanotti K, Waggoner S, DiFeo A. Critical role of Wnt/β-catenin signaling in driving epithelial ovarian cancer platinum resistance. Oncotarget. 2015;6:23720–23734. doi: 10.18632/oncotarget.4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Emons G, Spitzner M, Reineke S, Auslander N, Kramer F, Rave-Fraenk M, Gaedcke J, Ghadimi M, Ried T, Grade M. Abstract 4760: Wnt/β-catenin signaling mediates resistance of colorectal cancer cell lines to chemoradiotherapy. Cancer Res. 2017;77:4760–4760. [Google Scholar]
- 34.Lan K, Zhao Y, Fan Y, Ma B, Yang S, Liu Q, Linghu H, Wang H. Sulfiredoxin may promote cervical cancer metastasis via Wnt/β-catenin signaling pathway. Int J Mol Sci. 2017;18(pii):E917. doi: 10.3390/ijms18050917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarkadi B, Ozvegy-Laczka C, Német K, Váradi A. ABCG2-a transporter for all seasons. FEBS Lett. 2004;567:116–120. doi: 10.1016/j.febslet.2004.03.123. [DOI] [PubMed] [Google Scholar]
- 36.Elkind NB, Szentpétery Z, Apáti A, Ozvegy-Laczka C, Várady G, Ujhelly O, Szabó K, Homolya L, Váradi A, Buday L, et al. Multidrug transporter ABCG2 prevents tumor cell death induced by the epidermal growth factor receptor inhibitor Iressa (ZD1839, Gefitinib) Cancer Res. 2005;65:1770–1777. doi: 10.1158/0008-5472.CAN-04-3303. [DOI] [PubMed] [Google Scholar]
- 37.Chau WK, Ip CK, Mak AS, Lai HC, Wong AS. c-Kit mediates chemoresistance and tumor-initiating capacity of ovarian cancer cells through activation of Wnt/β-catenin-ATP-binding cassette G2 signaling. Oncogene. 2013;32:2767–2781. doi: 10.1038/onc.2012.290. [DOI] [PubMed] [Google Scholar]
- 38.Liu HG, Pan YF, You J, Wang OC, Huang KT, Zhang XH. Expression of ABCG2 and its significance in colorectal cancer. Asian Pac J Cancer Prev. 2010;11:845–848. [PubMed] [Google Scholar]
- 39.Sari FM, Yanar HT, Ozhan G. Investigation of the functional single-nucleotide polymorphisms in the BCRP transporter and susceptibility to colorectal cancer. Biomed Rep. 2015;3:105–109. doi: 10.3892/br.2014.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turner JG, Gump JL, Zhang C, Cook JM, Marchion D, Hazlehurst L, Munster P, Schell MJ, Dalton WS, Sullivan DM. ABCG2 expression, function, and promoter methylation in human multiple myeloma. Blood. 2006;108:3881–3889. doi: 10.1182/blood-2005-10-009084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bram EE, Stark M, Raz S, Assaraf YG. Chemotherapeutic drug-induced ABCG2 promoter demethylation as a novel mechanism of acquired multidrug resistance 1 2. Neoplasia. 2009;11:1359–1370. doi: 10.1593/neo.91314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang CL, Hong E, Lao-Sirieix P, Fitzgerald RC. A novel role for the retinoic acid-catabolizing enzyme CYP26A1 in Barrett's associated adenocarcinoma. Oncogene. 2008;27:2951–2960. doi: 10.1038/sj.onc.1210969. [DOI] [PubMed] [Google Scholar]
- 43.Tang XH, Gudas LJ. Retinoids, retinoic acid receptors, and cancer. Annu Rev Pathol. 2011;6:345–364. doi: 10.1146/annurev-pathol-011110-130303. [DOI] [PubMed] [Google Scholar]
- 44.Huang GL, Luo Q, Rui G, Zhang W, Zhang QY, Chen QX, Shen DY. Oncogenic activity of retinoic acid receptor γ is exhibited through activation of the Akt/NF-κB and Wnt/β-catenin pathways in cholangiocarcinoma. Mol Cell Biol. 2013;33:3416–3425. doi: 10.1128/MCB.00384-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yasuhara R, Yuasa T, Williams JA, Byers SW, Shah S, Pacifici M, Iwamoto M, Enomoto-Iwamoto M. Wnt/beta-catenin and retinoic acid receptor signaling pathways interact to regulate chondrocyte function and matrix turnover. J Biol Chem. 2010;285:317–327. doi: 10.1074/jbc.M109.053926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Francesca C, Stefano R, Gaia B, Ren M, Nicoletta S. Derangement of a factor upstream of RARalpha triggers the repression of a pleiotropic epigenetic network. PLoS One. 2009;4:e4305. doi: 10.1371/journal.pone.0004305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu LY. University of Toronto (Canada); 2012. Association of tissue promoter methylation levels of APC, RASSF1A, CYP26A1 and TBX15 with prostate cancer progression (unpublished PhD thesis) [Google Scholar]
- 48.García-Mariscal A, Peyrollier K, Basse A, Pedersen E, Rühl R, van Hengel J, Brakebusch C. RhoA controls retinoid signaling by ROCK dependent regulation of retinol metabolism. Small GTPases. 2018;9:433–444. doi: 10.1080/21541248.2016.1248272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu YH, Chen HA, Chen PS, Cheng YJ, Hsu WH, Chang YW, Chen YH, Jan Y, Hsiao M, Chang TY, et al. MiR-520h-mediated FOXC2 regulation is critical for inhibition of lung cancer progression by resveratrol. Oncogene. 2013;32:431–443. doi: 10.1038/onc.2012.74. [DOI] [PubMed] [Google Scholar]
- 50.Zheng CH, Quan Y, Li YY, Deng WG, Shao WJ, Fu Y. Expression of transcription factor FOXC2 in cervical cancer and effects of silencing on cervical cancer cell proliferation. Asian Pac J Cancer Prev. 2014;15:1589–1595. doi: 10.7314/APJCP.2014.15.4.1589. [DOI] [PubMed] [Google Scholar]
- 51.Zhou Z, Zhang L, Xie B, Wang X, Yang X, Ding N, Zhang J, Liu Q, Tan G, Feng D, Sun LQ. FOXC2 promotes chemoresistance in nasopharyngeal carcinomas via induction of epithelial mesenchymal transition. Cancer Lett. 2015;363:137–145. doi: 10.1016/j.canlet.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 52.Martens S, Kozlov MM, Mcmahon HT. How synaptotagmin promotes membrane fusion. Science. 2007;316:1205–1208. doi: 10.1126/science.1142614. [DOI] [PubMed] [Google Scholar]
- 53.Geppert M, Goda Y, Hammer RE, Li C, Rosahl TW, Stevens CF, Südhof TC. Synaptotagmin I: A major Ca2+ sensor for transmitter release at a central synapse. Cell. 1994;79:717–727. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- 54.Jahn JE, Coleman WB. Phenotypic normalization of GN6TF rat liver tumor cells results from WT1 expression following transfection of human SYT13-containing BACs. FASEB J. 2006;20:A1091. [Google Scholar]
- 55.Kanda M, Shimizu D, Tanaka H, Tanaka C, Kobayashi D, Hayashi M, Iwata N, Niwa Y, Yamada S, Fujii T, et al. Significance of SYT8 For the detection, prediction, and treatment of peritoneal metastasis from gastric cancer. Ann Surg. 2016;267:495–503. doi: 10.1097/SLA.0000000000002096. [DOI] [PubMed] [Google Scholar]
- 56.Sung HY, Han J, Ju W, Ahn JH. Synaptotagmin-like protein 2 gene promotes the metastatic potential in ovarian cancer. Oncol Rep. 2016;36:535–541. doi: 10.3892/or.2016.4835. [DOI] [PubMed] [Google Scholar]
- 57.Jin H, Xu G, Zhang Q, Pang Q, Fang M. Synaptotagmin-7 is overexpressed in hepatocellular carcinoma and regulates hepatocellular carcinoma cell proliferation via Chk1-p53 signaling. Onco Targets Ther. 2017;10:4283–4293. doi: 10.2147/OTT.S143619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kanda M, Shimizu D, Tanaka H, Tanaka C, Kobayashi D, Hayashi M, Takami H, Niwa Y, Yamada S, Fujii T, et al. Synaptotagmin XIII expression and peritoneal metastasis in gastric cancer. Br J Surg. 2018;105:1349–1358. doi: 10.1002/bjs.10876. [DOI] [PubMed] [Google Scholar]
- 59.Li A, King J, Moro A, Sugi MD, Dawson DW, Kaplan J, Li G, Lu X, Strieter RM, Burdick M, et al. Overexpression of CXCL5 is associated with poor survival in patients with pancreatic cancer. Am J Pathol. 2011;178:1340–1349. doi: 10.1016/j.ajpath.2010.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park JY, Park KH, Bang S, Kim MH, Lee JE, Gang J, Koh SS, Song SY. CXCL5 overexpression is associated with late stage gastric cancer. J Cancer Res Clin Oncol. 2007;133:835–840. doi: 10.1007/s00432-007-0225-x. [DOI] [PubMed] [Google Scholar]
- 61.Kawamura M, Toiyama Y, Tanaka K, Saigusa S, Okugawa Y, Hiro J, Uchida K, Mohri Y, Inoue Y, Kusunoki M. CXCL5, a promoter of cell proliferation, migration and invasion, is a novel serum prognostic marker in patients with colorectal cancer. Eur J Cancer. 2012;48:2244–2251. doi: 10.1016/j.ejca.2011.11.032. [DOI] [PubMed] [Google Scholar]
- 62.Begley L, Kasina S, Mehra R, Adsule S, Admon AJ, Lonigro RJ, Chinnaiyan AM, Macoska JA. CXCL5 promotes prostate cancer progression. Neoplasia. 2008;10:244–254. doi: 10.1593/neo.07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang C, Li A, Yang S, Qiao R, Zhu X, Zhang J. CXCL5 promotes mitomycin C resistance in non-muscle invasive bladder cancer by activating EMT and NF-κB pathway. Biochem Biophys Res Commun. 2018;498:862–868. doi: 10.1016/j.bbrc.2018.03.071. [DOI] [PubMed] [Google Scholar]
- 64.Guan Z, Li C, Fan J, He D, Li L. Androgen receptor (AR) signaling promotes RCC progression via increased endothelial cell proliferation and recruitment by modulating AKT→NF-κB→CXCL5 signaling. Sci Rep. 2016;6:37085. doi: 10.1038/srep37085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao J, Ou B, Han D, Wang P, Zong Y, Zhu C, Liu D, Zheng M, Sun J, Feng H, Lu A. Tumor-derived CXCL5 promotes human colorectal cancer metastasis through activation of the ERK/Elk-1/Snail and AKT/GSK3β/β-catenin pathways. Mol Cancer. 2017;16:70. doi: 10.1186/s12943-017-0629-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The results published here are in part based upon data generated by the TCGA Research Network (https://www.cancer.gov/tcga). The Gene Expression Omnibus dataset GSE44001 is available at http://www.ncbi.nlm.nih.gov/geo/. All data generated during this study are included in this published article.