Abstract
Background
Randomized controlled trials (RCTs) have yielded conflicting results regarding the ability of beta‐blockers to influence perioperative cardiovascular morbidity and mortality. Thus routine prescription of these drugs in unselected patients remains a controversial issue. A previous version of this review assessing the effectiveness of perioperative beta‐blockers in cardiac and non‐cardiac surgery was last published in 2018. The previous review has now been split into two reviews according to type of surgery. This is an update and assesses the evidence in cardiac surgery only.
Objectives
To assess the effectiveness of perioperatively administered beta‐blockers for the prevention of surgery‐related mortality and morbidity in adults undergoing cardiac surgery.
Search methods
We searched CENTRAL, MEDLINE, Embase, CINAHL, Biosis Previews and Conference Proceedings Citation Index‐Science on 28 June 2019. We searched clinical trials registers and grey literature, and conducted backward‐ and forward‐citation searching of relevant articles.
Selection criteria
We included RCTs and quasi‐randomized studies comparing beta‐blockers with a control (placebo or standard care) administered during the perioperative period to adults undergoing cardiac surgery. We excluded studies in which all participants in the standard care control group were given a pharmacological agent that was not given to participants in the intervention group, studies in which all participants in the control group were given a beta‐blocker, and studies in which beta‐blockers were given with an additional agent (e.g. magnesium). We excluded studies that did not measure or report review outcomes.
Data collection and analysis
Two review authors independently assessed studies for inclusion, extracted data, and assessed risks of bias. We assessed the certainty of evidence with GRADE.
Main results
We included 63 studies with 7768 participants; six studies were quasi‐randomized and the remaining were RCTs. All participants were undergoing cardiac surgery, and in most studies, at least some of the participants were previously taking beta‐blockers. Types of beta‐blockers were: propranolol, metoprolol, sotalol, esmolol, landiolol, acebutolol, timolol, carvedilol, nadolol, and atenolol. In twelve studies, beta‐blockers were titrated according to heart rate or blood pressure. Duration of administration varied between studies, as did the time at which drugs were administered; in nine studies this was before surgery, in 20 studies during surgery, and in the remaining studies beta‐blockers were started postoperatively. Overall, we found that most studies did not report sufficient details for us to adequately assess risk of bias. In particular, few studies reported methods used to randomize participants to groups. In some studies, participants in the control group were given beta‐blockers as rescue therapy during the study period, and all studies in which the control was standard care were at high risk of performance bias because of the open‐label study design. No studies were prospectively registered with clinical trials registers, which limited the assessment of reporting bias. We judged 68% studies to be at high risk of bias in at least one domain.
Study authors reported few deaths (7 per 1000 in both the intervention and control groups), and we found low‐certainty evidence that beta‐blockers may make little or no difference to all‐cause mortality at 30 days (risk ratio (RR) 0.95, 95% confidence interval (CI) 0.47 to 1.90; 29 studies, 4099 participants). For myocardial infarctions, we found no evidence of a difference in events (RR 1.05, 95% CI 0.72 to 1.52; 25 studies, 3946 participants; low‐certainty evidence). Few study authors reported cerebrovascular events, and the evidence was uncertain (RR 1.37, 95% CI 0.51 to 3.67; 5 studies, 1471 participants; very low‐certainty evidence). Based on a control risk of 54 per 1000, we found low‐certainty evidence that beta‐blockers may reduce episodes of ventricular arrhythmias by 32 episodes per 1000 (RR 0.40, 95% CI 0.25 to 0.63; 12 studies, 2296 participants). For atrial fibrillation or flutter, there may be 163 fewer incidences with beta‐blockers, based on a control risk of 327 incidences per 1000 (RR 0.50, 95% CI 0.42 to 0.59; 40 studies, 5650 participants; low‐certainty evidence). However, the evidence for bradycardia and hypotension was less certain. We found that beta‐blockers may make little or no difference to bradycardia (RR 1.63, 95% CI 0.92 to 2.91; 12 studies, 1640 participants; low‐certainty evidence), or hypotension (RR 1.84, 95% CI 0.89 to 3.80; 10 studies, 1538 participants; low‐certainty evidence).
We used GRADE to downgrade the certainty of evidence. Owing to studies at high risk of bias in at least one domain, we downgraded each outcome for study limitations. Based on effect size calculations in the previous review, we found an insufficient number of participants in all outcomes (except atrial fibrillation) and, for some outcomes, we noted a wide confidence interval; therefore, we also downgraded outcomes owing to imprecision. The evidence for atrial fibrillation and length of hospital stay had a moderate level of statistical heterogeneity which we could not explain, and we, therefore, downgraded these outcomes for inconsistency.
Authors' conclusions
We found no evidence of a difference in early all‐cause mortality, myocardial infarction, cerebrovascular events, hypotension and bradycardia. However, there may be a reduction in atrial fibrillation and ventricular arrhythmias when beta‐blockers are used. A larger sample size is likely to increase the certainty of this evidence. Four studies awaiting classification may alter the conclusions of this review.
Plain language summary
Beta‐blockers to prevent death or serious events after heart surgery
This review assessed evidence of whether beta‐blockers given around the time of surgery can reduce death or other serious events for people undergoing heart surgery.
Background
People undergoing heart surgery are at greater risk of complications and death. Heart surgery increases the amount of stress in the body, causing the release of the hormones adrenaline and noradrenaline. This stress can lead to serious events including death, heart attacks, stroke, or an irregular heartbeat. Beta‐blockers are drugs that block the action of adrenaline and noradrenaline on the heart. Beta‐blockers can slow down the heart and reduce blood pressure, and this effect may reduce the risk of serious events. However, they can also lead to a very low heart rate or very low blood pressure, and this effect may increase the risk of death or a stroke. Prevention of complications around the time of surgery is an important safety consideration for people undergoing heart surgery.
Study characteristics
The evidence is current to 28 June 2019. We included 63 studies with 7768 adults who were undergoing heart surgery, including coronary artery bypass graft and valve replacement surgery. Studies were mostly randomized controlled studies, and six were quasi‐randomized (participants were allocated to groups by methods such as using hospital record numbers or dates of birth). The types of beta‐blockers were: propranolol, metoprolol, sotalol, esmolol, landiolol, acebutolol, timolol, carvedilol, nadolol, and atenolol. These beta‐blockers were compared with either a placebo (disguised to look like a beta‐blocker but containing no medicine) or with standard care. Beta‐blockers were started before surgery, during surgery or at the latest by the end of the first day after surgery. The length of time beta‐blockers were given varied between studies. In most studies, at least some of the people were already taking beta‐blockers, which would be expected for people who had conditions that needed heart surgery.
Key results
Beta‐blockers probably make little or no difference to the number of people who die (29 studies, 4099 participants) or have a heart attack (25 studies, 3946 participants) within 30 days of surgery. This was supported by low‐certainty evidence. Few studies reported on people who had a stroke, and we were uncertain whether or not beta‐blockers reduced strokes because the certainty of the evidence was very low (5 studies, 1471 participants). Beta‐blockers may reduce atrial fibrillation, which is an irregular heartbeat starting in the atrial chambers of the heart that increases the risk of stroke if untreated (40 studies, 5650 participants; low‐certainty evidence). Beta‐blockers may also reduce ventricular arrhythmias, which are potentially life‐threatening irregular heartbeat rhythms originating in the main chambers of the heart, and which may need immediate medical treatment (12 studies, 2296 participants). We found that beta‐blockers may make little or no difference to whether people experience a very low heart rate or very low blood pressure.
We were uncertain whether beta‐blockers made a difference to the number of deaths up to a year after surgery (3 studies, 511 participants), to death because of the heart (4 studies, 320 participants), or to people who had heart failure (3 studies, 311 participants). The certainty of this evidence was very low. People who took beta‐blockers had a shorter hospital stay by about half a day (14 studies, 2450 participants; low‐certainty evidence).
No studies assessed whether people on beta‐blockers had a better quality of life after heart surgery.
Certainty of the evidence
The certainty of the evidence in this review was mostly low. We found that many studies reported methods that we believed could influence the results. For example, many studies did not use a placebo‐control and the doctors might, therefore, have treated people differently in each group. We were unable to explain some of the differences that we found in the data for atrial fibrillation. We also needed to have evidence from a larger number of participants to be very confident in our findings.
Conclusion
Beta‐blockers may be beneficial for people who are undergoing cardiac surgery because they may reduce the number of people who experience atrial fibrillation and ventricular arrhythmias. Beta‐blockers may make little or no difference to the other outcomes in this review, including death, heart attacks or stroke.
Summary of findings
Summary of findings for the main comparison. Perioperative beta‐blockers for preventing surgery‐related mortality and morbidity in adults undergoing cardiac surgery.
| Beta‐blockers compared to placebo or standard care for preventing perioperative mortality and morbidity in adults undergoing cardiac surgery | |||||
| Population: adults undergoing cardiac surgery under general anaesthesia Setting: hospitals in: Australia, Austria, Belgium, Brazil, Canada, China, Denmark, France, Germany, Israel, Italy, Japan, Netherlands, New Zealand, Norway, Portugal, Spain, Sweden, Switzerland, Turkey, UK, USA Intervention: beta‐blockers (metoprolol, propranolol, sotalol, esmolol, timolol, landiolol, nadolol, acebutolol, atenolol) Comparison: placebo or standard care | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with control | Risk with beta‐blockers | ||||
|
All‐cause mortality (within 30 days) |
Study population | RR 0.95 (0.47 to 1.90) |
4099 (29 studies) | ⊕⊕⊝⊝ Lowa |
|
| 7 per 1000 | 7 per 1000 (3 to 14) | ||||
|
Acute myocardial infarction (within 30 days) |
Study population | RR 1.05 (0.72 to 1.52) | 3946 (25 studies) | ⊕⊕⊝⊝ Lowa |
|
| 29 per 1000 | 30 per 1000 (21 to 43) | ||||
|
Cerebrovascular events (within 30 days) |
Study population | (RR 1.37, 95% CI 0.51 to 3.67) | 1471 (5 studies) | ⊕⊝⊝⊝ Very lowb | |
| 10 per 1000 | 14 per 1000 (5 to 36) | ||||
|
Ventricular arrhythmias (within 30 days) |
Study population | RR 0.40
(0.25 to 0.63) NNTB: 31 (25 to 50) |
2296 (12 studies) | ⊕⊕⊝⊝ Lowc |
|
| 54 per 1000 | 22 per 1000 (13 to 34) | ||||
|
Atrial fibrillation or flutter, or both (within 30 days) |
Study population | RR 0.50
(0.42 to 0.59) NNTB: 6 (5 to 7) |
5650 (40 studies) | ⊕⊕⊝⊝ Lowd |
|
| 327 per 1000 | 164 per 1000 (137 to 193) | ||||
|
Bradycardia (within 30 days; as defined by study authors, minimum heart rate < 60 beats per minute or requiring medication) |
Study population | RR 1.63 (0.92 to 2.91) | 1640 (12 studies) | ⊕⊕⊝⊝ Lowe | |
| 30 per 1000 | 48 per 1000 (27 to 87) | ||||
|
Hypotension (within 30 days; as defined by study authors, minimum systolic blood pressure < 90 mmHg or requiring medication) |
Study population | RR 1.84 (0.89 to 3.80) | 1538 (10 studies) | ⊕⊕⊝⊝ Lowe | |
| 15 per 1000 | 28 per 1000 (14 to 58) | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; NNTB: number needed to treat for an additional beneficial outcome | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | |||||
aWe downgraded by two levels: one level for study limitations owing to the inclusion of several studies at high risk of bias in at least one domain; and one level for imprecision owing to a wide CI in the effect estimate and because the evidence was from too few participants; our estimate for the required number of participants was taken from a calculation in a previous version of the review (Blessberger 2018). bWe downgraded by three levels: one level for study limitations owing to the inclusion of several studies at high risk of bias; and two levels for imprecision owing to the very wide CI in the effect estimate and because the evidence was from too few participants. Our estimate for the required number of participants was taken from a calculation in a previous version of the review (Blessberger 2018). cWe downgraded by two levels: one level for study limitations owing to the inclusion of several studies at high risk of bias; and one level for imprecision because the evidence was from too few participants; our estimate for the required number of participants was taken from a calculation in a previous version of the review (Blessberger 2018). dWe downgraded by two levels: one level for study limitations owing to the inclusion of several studies at high risk of bias; and one level for inconsistency owing to moderate level of statistical heterogeneity which we were unable to explain through subgroup analysis. eWe downgraded by two levels: one level for study limitations owing to the inclusion of several studies at high risk of bias in at least one domain; and one level for imprecision owing to the very wide confidence interval and because the evidence was from too few participants. Our estimate for the required number of participants was taken from a calculation in a previous version of the review (Blessberger 2018).
Background
Description of the condition
Cardiovascular mortality and morbidity are prevalent and costly in people undergoing cardiac surgery, and prevention of early postoperative complications remains a major issue (Oprea 2019). Surgery for acquired cardiac disease has a mortality rate of up to 1% to 4% (Sanagou 2012; SCTS 2015), a perioperative myocardial infarction rate up to 9% (Chen 2007) and overall complication rates of 15%, depending on the type of operation and a person's co‐morbidities (Crawford 2017).
Description of the intervention
There are various pharmacological agents that may be used to protect people undergoing surgery against adverse cardiovascular events (Lewis 2018), and alpha‐2 adrenergic agents have also been evaluated for their use perioperatively (Duncan 2018). In addition, the choice of anaesthetic drugs and techniques can also affect cardiovascular outcomes (Hristovska 2017).
This review, however, evaluates the effectiveness of beta‐adrenoceptor blocking agents, or beta‐blockers, for this purpose. These pharmacological agents block the actions of the stress hormones epinephrine (adrenaline) and norepinephrine (noradrenaline). They are typically used to manage abnormal heart rhythms, heart failure, coronary heart disease, and their effectiveness has been assessed for hypertension (Wiysonge 2017) and for secondary prevention of stroke (De Lima 2014).
How the intervention might work
Cardiac adverse events appear to be related to the persistently exaggerated sympathetic response that is associated with substantial increases in heart rate and myocardial oxygen consumption. Drugs that block beta‐adrenergic receptors, and thus the sympathetic response, are capable of preventing cardiac complications in people with acute myocardial infarction, silent ischaemia and heart failure (Oprea 2019; Thaper 2018). Perioperative blockade of beta‐adrenergic receptors, therefore, has been proposed to reduce the risk of perioperative complications in people after cardiac surgery but its routine use is still a matter of debate (Sousa‐Uva 2017).
Why it is important to do this review
Current guidelines on the use of beta‐blockers in aortocoronary bypass surgery suggest that people with an ejection fraction greater than 30% should receive beta‐blockers preoperatively to reduce in‐hospital mortality (Hillis 2011). This class IIa recommendation was based on smaller RCTs and observational studies indicating reduced mortality with the use of beta‐blockers in bypass surgery. Besides this, all people undergoing aortocoronary bypass surgery are diagnosed with coronary heart disease and therefore should receive beta‐blockers if not contraindicated for other reasons (class Ia recommendation; Montalescot 2013). However, evidence from a previous version of this review evaluating all‐cause mortality within 30 days demonstrated little or no difference in mortality for people undergoing cardiac surgery (Blessberger 2018). We aim to incorporate new evidence into this review, to increase the certainty of the effect of beta‐blockers in this population.
Our previous review assessed the effectiveness of beta‐blockers in both cardiac and non‐cardiac surgery (Blessberger 2018). The previous review has now been split into two reviews according to type of surgery. This is an update, in which we assess the evidence in cardiac surgery only. We report the evidence for non‐cardiac surgery elsewhere (Blessberger 2019).
Objectives
To assess the effectiveness of perioperatively administered beta‐blockers for the prevention of surgery‐related mortality and morbidity in adults undergoing cardiac surgery.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) and quasi‐randomized studies in which investigators used methods to allocate participants to groups such as hospital record number or date of birth.
Types of participants
We included studies that assessed the effects of beta‐blockers on adults who were 18 years of age or older and who were undergoing cardiac surgery under general anaesthesia.
Types of interventions
We included studies in which beta‐adrenoceptor‐blockers (beta‐blockers) were administered during the perioperative period; we defined the perioperative period as 30 days before surgery to 30 days after surgery. Beta‐blockers could be started before surgery, during surgery or at the latest by the end of the first day after surgery. Beta‐blockers were given intravenously, orally or via a feeding tube, and were compared with a control (placebo or standard care). We excluded studies in which all participants in the standard care control group were given a pharmacological agent that was not given to participants in the intervention group; similarly, we excluded studies in which all participants in the control group were given a beta‐blocker.
We excluded studies (or intervention groups within a multi‐arm study) in which the beta‐blocker was given with a supplementary agent (e.g. magnesium) unless the agent was given in both groups as part of standard care management.
Types of outcome measures
We excluded studies that did not measure review outcomes (see Differences between protocol and review). Except for long‐term all‐cause mortality, length of hospital stay, and quality of life, we aimed to collect outcome data that were measured within 30 days postoperatively or before hospital discharge (whichever occurred later). We removed some outcomes in this update (see Differences between protocol and review).
Primary outcomes
Early all‐cause mortality
Secondary outcomes
Long‐term all‐cause mortality, occurring later than 30 days postoperatively
Death due to cardiac causes
Acute myocardial infarction, as defined by study authors. We included only non‐fatal myocardial infarctions if a distinction was possible.
Cerebrovascular events: transient ischaemic attack (TIA), prolonged reversible ischaemic neurological deficit (PRIND), or stroke, as defined by study authors. We included only non‐fatal cerebrovascular events if a distinction was possible.
Ventricular arrhythmias: ventricular tachycardias and ventricular fibrillation
Atrial fibrillation or atrial flutter (or both)
Bradycardia, as defined by study authors (minimum criteria: below 60 beats per minute or requiring medical intervention)
Hypotension, as defined by study authors (minimum criteria: below 90 mmHg systolic blood pressure or requiring medical intervention)
Congestive heart failure, as defined by study authors
Length of hospital stay
Quality of life, as defined by study authors
Search methods for identification of studies
Electronic searches
We identified RCTs through literature searching with systematic and sensitive search strategies, as outlined in Chapter 6.4 of theCochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011). We applied no restrictions to language or publication status. We searched the following databases for relevant trials:
Cochrane Central Register of Controlled Trials (CENTRAL; 2019; Issue 6) in the Cochrane Library (searched 28 June 2019);
MEDLINE (Ovid SP; 1946 to 28 June 2019);
Embase (Ovid SP; 1974 to 28 June 2019);
CINAHL (EBSCOhost: 1981 to 28 June 2019);
Biosis Previews (1969 to 28 June 2019);
Web of Science (SCI‐EXPANDED; 1900 to 28 June 2019);
Conference Proceedings Citation Index‐Science (CPCI‐S; 1990 to 28 June 2019).
We developed a subject‐specific search strategy in MEDLINE and other listed databases, in consultation with the Information Specialist for Cochrane Anaesthesia. We subtracted the previous review's search results (up to and including 2012) from the new search. Search strategies can be found in: Appendix 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6; Appendix 7.
We scanned the following clinical trials registers for ongoing and unpublished trials:
World Health Organization International Clinical Trials Registry Platform (www.who.int/ictrp/en/) on 22 March 2019;
ClinicalTrials.gov (www.clinicaltrials.gov) on 22 March 2019.
Searching other resources
We carried out citation searching of identified included studies published since 2012 in Web of Science on 22 March 2019 (apps.webofknowledge.com). We conducted a search of grey literature using Opengrey on 5 April 2019 (www.opengrey.eu/). In addition, we scanned reference lists of relevant systematic reviews which were published since 2015.
Data collection and analysis
Two review authors (SL, and LF or MP) independently selected studies and extracted data from new included studies. We compared decisions at each stage. In cases of disagreement, we reassessed the respective studies to reach consensus, and if necessary included a third review author (HB) for resolution.
Selection of studies
We used reference management software to collate the results of searches and to remove duplicates (Endnote). We used Covidence 2019 software to screen results of the search of titles and abstracts and to identify potentially relevant studies. We sourced the full texts of all potentially relevant studies and considered whether they met the inclusion criteria (see Criteria for considering studies for this review). We reviewed abstracts at this stage and included them in the review only if they provided sufficient information and relevant results that included denominator figures for the intervention and control groups. We recorded the number of papers retrieved at each stage and report this information using a PRISMA flow chart (see Figure 1). We report in the review brief details of closely related but excluded papers.
Data extraction and management
We used a data extraction form to collect information and outcome data from studies (Appendix 8). We collected the following information.
Methods: type of study design, setting, dates of study, funding sources and study author declarations of interest
Participants: number randomized to each group; number of losses; number analysed in each group and whether intention‐to‐treat analysis was used; baseline characteristics (age, gender, American Society of Anesthesiologists (ASA) grade of other measure of health status, type of surgery, history of coronary heart disease, myocardial infarction, hypertension, reduced ejection fraction, chronic obstructive pulmonary disease (COPD), preoperative use of beta‐blockers)
Intervention: details of beta‐blocker (type; dose; time; duration; route of administration; goal‐directed or fixed‐dose), and details of control (placebo or standard care)
Outcomes: data for all reported review outcomes including study author definitions, measurement tools, and time points.
We considered the applicability of information from individual studies and generalizability of the data to our intended study population (i.e. the potential for indirectness in our review).
In multi‐arm studies, we did not collect data on any groups that were not eligible for inclusion in the review.
Assessment of risk of bias in included studies
We assessed study quality, study limitations, and the extent of potential bias using the Cochrane 'Risk of bias' tool (Higgins 2017). We assessed the following domains.
Sequence generation (selection bias)
Allocation concealment (selection bias)
Blinding of participants, personnel, and outcomes assessors (performance and detection bias)
Incomplete outcome data (attrition bias)
Selective outcome reporting (reporting bias)
Other risks of bias
For each domain, two review authors (SL, and MP or LF) judged whether study authors made sufficient attempts to minimize bias in their study design. We made judgements using three measures ‐ high, low, or unclear risk of bias. We recorded this in 'Risk of bias' tables and present summary 'Risk of bias' figures (see Figure 2 and Figure 3).
For other risks of bias, we considered the effect of beta‐blockers given as 'rescue therapy' to treat specified conditions. We judged studies to have a high risk of other bias if administration of such 'rescue therapy' had the potential to influence outcome data.
Measures of treatment effect
We collected dichotomous data for mortality outcomes, acute myocardial infarction, cerebrovascular events, ventricular arrhythmias, atrial fibrillation and atrial flutter, bradycardia, hypotension, and congestive heart failure. We collected continuous data for length of hospital stay.
For dichotomous data, we reported risk ratios (RRs) to compare groups, and continuous data as mean difference (MDs). We reported 95% confidence intervals (CIs).
Unit of analysis issues
For multi‐arm studies, which included different types of beta‐blockers or different doses of beta‐blockers, we combined dichotomous data to create a single beta‐blocker group, and we used these composite data in the primary analysis. During subgroup analysis by type of beta‐blocker, we included data separately for each type of beta‐blocker, and used the 'halving method' with data in the control group to avoid a unit of analysis error (Deeks 2017).
If multi‐arm studies had included continuous data for length of stay, we planned to calculate combined mean and standard deviation values according to the formula provided in Chapter 7.7.3.8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
If information on both study group allocation and respective outcomes was available, we re‐included withdrawn participants in keeping with the intention‐to‐treat principle. If information was not available, we performed an available case analysis. We did not perform imputation techniques.
We excluded continuous data assessing length of stay if a range of dispersion (standard deviation or standard error) was not provided along with mean values. When both measures of spread (standard deviation and standard error) were presented, we used the standard deviation as the measure of choice. We did not apply imputation techniques.
We attempted contact with some study authors for additional information; we reported this information in the Notes section of the Characteristics of included studies.
Assessment of heterogeneity
We assessed whether evidence of inconsistency was apparent in our results by considering heterogeneity. We assessed clinical and methodological heterogeneity by comparing similarities in our included studies between study designs, participants, interventions, and outcomes, and used the data collected from the full‐text reports (as stated in Data collection and analysis). We explored clinical and methodological heterogeneity through subgroup analysis. We assessed statistical heterogeneity by calculating the Chi2 test or I2 statistic (Higgins 2003), and judged any heterogeneity above an I2 value of 40% and a Chi2 P value less than or equal to 0.05 to indicate moderate to substantial statistical heterogeneity (Deeks 2017). We did not conduct meta‐regression to explore heterogeneity in this updated review (see Differences between protocol and review).
As well as to looking at statistical results, we considered point estimates and overlap of CIs. If CIs overlap, then results are more consistent. However, combined studies may show a large consistent effect but with significant heterogeneity. We, therefore, planned to interpret heterogeneity with caution (Guyatt 2011a).
Assessment of reporting biases
We attempted to source published protocols for each of our included studies by using clinical trials registers. We planned to compare published protocols with published study results, to assess the risk of selective reporting bias. In addition, we appraised reporting bias through visual assessment of funnel plots (Egger 1997). We planned to include figures of funnel plots in which we identified possible reporting bias from visual assessment; in this review, no reporting bias was found.
Data synthesis
We presented a statistical summary of treatment effects in the absence of significant clinical or methodological heterogeneity. We used the statistical calculator in Review Manager 5 (RevMan 5) to perform meta‐analysis (Review Manager 2014).
For dichotomous outcomes, we used the Mantel‐Haenszel random‐effects model to account for potential variability in participant conditions between studies (Borenstein 2010). For continuous outcomes, we used inverse variance with a random‐effects model.
We calculated CIs at 95% and used a P value less than or equal to 0.05 to judge whether a result was statistically significant; for statistically significant results, we also reported the number needed to treat for an additional beneficial outcome (NNTB). We considered imprecision in the results of analyses by assessing the CI around an effect measure; a wide CI would suggest a higher level of imprecision in our results. A small number of studies may also reduce precision (Guyatt 2011b).
Subgroup analysis and investigation of heterogeneity
In subgroup analysis, we evaluated the effect of the start of beta‐blocker therapy (i.e. before surgery, during surgery, or after surgery) and the type of beta‐blocking agent. In multi‐arm studies of more than one type of beta‐blocking agent, we compared each type of beta‐blocker using the halving method for the control group data (Deeks 2017); thus, we avoided a unit of analysis error.
We planned to complete subgroup analysis in which we found more than 10 studies (Deeks 2017), for the following outcomes.
Early all‐cause mortality
Acute myocardial infarction
Cerebrovascular events
Ventricular arrhythmias
Atrial fibrillation
Bradycardia
Hypotension
Sensitivity analysis
We explored the potential effect of decisions made as part of the review process. In each sensitivity analysis, we compared the effect estimate with the main analysis. We reported these effect estimates only if they indicated a difference in interpretation of the effect. We performed the following sensitivity analysis.
We excluded studies in which the control group was standard care rather than placebo.
We excluded studies that we judged at high or unclear risk of selection bias.
We excluded studies that we judged to have high risk of attrition bias because of missing data with a loss of more than 10% participants, data that were unbalanced between groups, or that were unexplained.
As well as sensitivity analyses performed in an earlier version of the review (Blessberger 2014), we also used sensitivity analysis to explore the potential effect of studies in which the control group were given beta‐blockers as rescue therapy. The review included several studies published before 2000, in which clinical management may differ from current standards. Accordingly, we made a post‐hoc decision to explore the potential effect of these early studies on the outcomes; in sensitivity analysis, we excluded studies published before 2000.
We calculated RRs using a random‐effects model for all analyses in the review. Although the random‐effects model accounted for potential variation in the population, this statistical tool did not account for outcomes with rare events. In sensitivity analysis, we evaluated the effect of outcomes with events fewer than 1% using Peto odds ratio (Deeks 2017).
We assessed the effect of these potential biases on the following outcomes.
Early all‐cause mortality
Acute myocardial infarction
Cerebrovascular events
Ventricular arrhythmias
Atrial fibrillation and flutter
Bradycardia
Hypotension
'Summary of findings' table and GRADE
One review author (SL) used the GRADE system to assess the certainty of the body of evidence and construct a 'Summary of findings' table associated with the following outcomes (Guyatt 2008).
Early all‐cause mortality
Acute myocardial infarction
Cerebrovascular events
Ventricular arrhythmias
Atrial fibrillation and atrial flutter
Bradycardia
Hypotension
The GRADE approach appraises the certainty of a body of evidence, based on the extent to which we can be confident that an estimate of effect or association reflects the item being assessed. Evaluation of the certainty of a body of evidence considers within‐study risk of bias, directness of the evidence, heterogeneity of the data, precision of the effect estimates, and risk of publication bias. We constructed a 'Summary of findings' table using GRADEpro GDT.
We used the GRADE approach to appraise the certainty of the body of evidence for the remaining outcomes, but we did not construct a 'Summary of findings' table for these outcomes.
Results
Description of studies
Results of the search
After the removal of duplicates from the search results, we screened 5972 titles and abstracts, which included forward‐ and backward‐citation searches, clinical trials registers and grey literature. We sourced 37 full‐text reports to assess eligibility. Figure 1
1.
Study flow diagram for updated search on 28 June 2019
Included studies
See Characteristics of included studies.
We included 63 studies with 7768 participants (Abel 1983; Ali 1997; Arar 2007; Auer 2004; Babin‐Ebell 1996; Bert 2001; Bignami 2017; Booth 2004; But 2006; Connolly 2003; Cork 1995; Daudon 1986; De Azevedo Lúcio 2003; Dy 1998; Evrard 2000; Forlani 2002; Gandhi 2007; Girard 1986; Gomes 1999; Graham 1996; Hammon 1984; Harrison 1987; Ivey 1983; Jacquet 1994; Janssen 1986; Khuri 1987; Kurian 2001; Lamb 1988; Liu 2016; Martinussen 1988; Matangi 1985; Matangi 1989; Materne 1985; Matsuura 2001; Mohr 1981; Myhre 1984; Neto 2013; Neustein 1994; Nicolson 1990; Nyström 1993; Ogawa 2013; Oka 1980; Ormerod 1984; Osada 2012; Paull 1997; Pfisterer 1997; Reves 1990; Rubin 1987; Sakaguchi 2012; Salazar 1979; Serruys 2000; Sezai 2011; Sezai 2012; Silverman 1982; Skiba 2013; Stephenson 1980; Sun 2011; Suttorp 1991; Vecht 1986; Wenke 1999; White 1984; Williams 1982; Yazicioglu 2002). Six studies were quasi‐randomized (Abel 1983; Matsuura 2001; Mohr 1981; Silverman 1982; Stephenson 1980; Williams 1982); the remaining studies were RCTs. We included two studies for which we could only source the abstract and this limited the details of study characteristics that we were able to extract (Dy 1998; Graham 1996). We sourced the full text of all remaining studies.
This review includes ten new studies (Arar 2007; Bignami 2017; Gandhi 2007; Girard 1986; Liu 2016; Neto 2013; Nicolson 1990; Serruys 2000; Skiba 2013; Yazicioglu 2002). The remaining studies were previously included in Blessberger 2018.
Study population
All participants were scheduled for cardiac surgery, which included coronary artery bypass graft (CABG) and valve replacement.
We collected data from study reports on additional risk factors for included participants; we used information reported in the baseline characteristics tables and in the study inclusion and exclusion criteria. We summarized these factors in a table (Appendix 9).
Nine studies included only participants who were already taking beta‐blocking agents preoperatively (Abel 1983; Ali 1997; Arar 2007; Ivey 1983; Matangi 1985; Mohr 1981; Myhre 1984; Oka 1980; Salazar 1979), whilst three studies excluded participants who were taking beta‐blockers preoperatively (Harrison 1987; Neto 2013; Neustein 1994). Other studies reported in baseline characteristics tables that at least some participants were taking beta‐blockers pre‐operatively; only 10 studies did not report preoperative beta‐blocker use (Dy 1998; Gandhi 2007; Hammon 1984; Janssen 1986; Kurian 2001; Ormerod 1984; Osada 2012; Paull 1997; Sun 2011; Yazicioglu 2002).
Study setting
All studies were conducted in a hospital setting and four were multi‐centre studies (Gandhi 2007; Gomes 1999; Khuri 1987; Serruys 2000).
Interventions and comparisons
Four studies were multi‐arm studies and included more than one type of beta‐blocker (Auer 2004; Janssen 1986; Sezai 2012) or different doses of the same beta‐blocker (Graham 1996). One study had two control groups according to time of withdrawal of existing propranolol treatment (Oka 1980). Types of beta‐blockers assessed were:
propranolol versus a placebo (Hammon 1984; Ivey 1983; Martinussen 1988), or standard care (Abel 1983; Babin‐Ebell 1996; Bert 2001; Matangi 1985; Mohr 1981; Myhre 1984; Oka 1980; Ormerod 1984; Rubin 1987; Salazar 1979; Silverman 1982; Stephenson 1980; Williams 1982);
metoprolol versus a placebo (Auer 2004; Booth 2004; Connolly 2003; Dy 1998; Graham 1996; Paull 1997), or standard care (De Azevedo Lúcio 2003; Janssen 1986; Neto 2013; Skiba 2013; Wenke 1999);
sotalol versus a placebo (Auer 2004; Gomes 1999; Pfisterer 1997; Suttorp 1991), or standard care (Evrard 2000; Forlani 2002; Jacquet 1994; Janssen 1986; Matsuura 2001; Nyström 1993);
esmolol versus a placebo (Arar 2007; Bignami 2017; But 2006; Cork 1995; Girard 1986; Harrison 1987; Liu 2016; Neustein 1994; Nicolson 1990; Reves 1990; Sun 2011), or standard care (Kurian 2001);
landiolol versus a placebo (Sezai 2011; Sezai 2012), or standard care (Ogawa 2013; Osada 2012; Sakaguchi 2012);
acebutolol versus standard care (Daudon 1986; Materne 1985);
timolol versus a placebo (Vecht 1986; White 1984);
carvedilol with a placebo (Serruys 2000), or standard care (Gandhi 2007);
nadolol with a placebo (Khuri 1987);
atenolol with a placebo (Matangi 1989), or with standard care (Lamb 1988).
In one study, the type of beta‐blocking agent was at the discretion of the treating clinician and included metoprolol, atenolol, sotalol, or inderal; these were compared with standard care (Ali 1997).
In twelve studies, beta‐blockers were titrated according to heart rate or blood pressure (Auer 2004; Daudon 1986; De Azevedo Lúcio 2003; Gandhi 2007; Gomes 1999; Jacquet 1994; Kurian 2001; Liu 2016; Ogawa 2013; Paull 1997; Sakaguchi 2012; Skiba 2013). In the remaining studies, beta‐blockers were given at a fixed dose.
Duration of administration varied across studies. In nine studies, administration started before surgery (we defined this as any time up to 15 minutes before the surgery; Ali 1997; Auer 2004; Gomes 1999; Lamb 1988; Myhre 1984; Neto 2013; Nyström 1993; Serruys 2000; Yazicioglu 2002). In twenty studies, administration started during surgery (Abel 1983; Arar 2007; Bignami 2017; Booth 2004; But 2006; Cork 1995; Dy 1998; Girard 1986; Harrison 1987; Kurian 2001; Liu 2016; Neustein 1994; Nicolson 1990; Ogawa 2013; Pfisterer 1997; Reves 1990; Sezai 2011; Sezai 2012; Skiba 2013; Sun 2011). In the remaining studies, administration started after surgery.
Outcomes
All studies included at least one review outcome, as this formed part of the inclusion criteria for this review. We reported numbers of studies for each outcome in Effects of interventions.
Funding
We found that most studies did not report sources of support or conflicts of interest. Eight studies reported support from pharmaceutical companies (Cork 1995; Hammon 1984; Ivey 1983; Khuri 1987; Matangi 1989; Reves 1990; Serruys 2000; Vecht 1986), and nine studies reported departmental or other sources of funding, which we assumed to be independent (Booth 2004; Connolly 2003; Girard 1986; Gomes 1999; Harrison 1987; Rubin 1987; Sezai 2011; Sezai 2012; White 1984). One study declared support from both pharmaceutical and independent sources (Auer 2004).
Excluded studies
We excluded 21 studies following assessment of full texts (Figure 1).
We reported the details of 12 of these excluded studies (De Bruijn 1987; Deng 2002; Efe 2014; Fujii 2012; Hamaguchi 2014; Imren 2007; Newsome 1986; O'Dwyer 1993; Rees 2015; Sezai 2015; Tempe 1999; Yazicioglu 2012). Seven of these 12 studies did not measure or report outcomes relevant to the review (De Bruijn 1987; Deng 2002; Efe 2014; Newsome 1986; O'Dwyer 1993; Tempe 1999; Yazicioglu 2012). We excluded one study in which all participants in the intervention group were also given magnesium (Rees 2015), and three studies in which all participants, including those in the control groups, were given beta‐blockers (Fujii 2012; Imren 2007; Sezai 2015). In one study all participants in both groups were given catecholamines but those in the control group were given a lower dose and thus we considered the catecholamine to be control group variable that could influence the result (Hamaguchi 2014). See Characteristics of excluded studies.
This review does not include studies that were previously excluded; details of previous exclusions can be found elsewhere (Blessberger 2014; Blessberger 2018).
Studies awaiting classification
We found four studies awaiting classification (Bozotlan 2013; Ishigaki 2012; NCT00959569; PACTR201801003026226). Two were published only as abstracts with insufficient information to assess eligibility or extract outcome data (Bozotlan 2013; Ishigaki 2012), and two were described as completed on a clinical trials register but results are not yet published (NCT00959569; PACTR201801003026226). See Characteristics of studies awaiting classification.
Ongoing studies
We found two ongoing studies; one compared landiolol versus standard care with an expected recruitment of 60 participants (UMIN000004216), and one compared esmolol to a placebo, with an expected recruitment of 144 participants (Chictr‐ior‐16009203). See Characteristics of ongoing studies.
Risk of bias in included studies
See Characteristics of included studies, Figure 2 and Figure 3.
2.
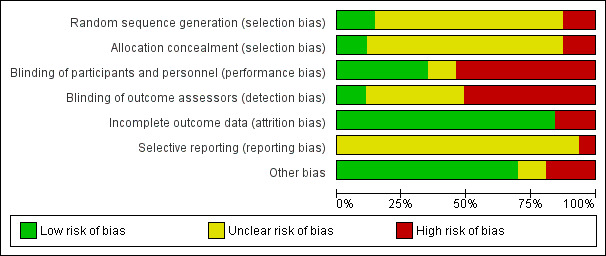
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies
3.
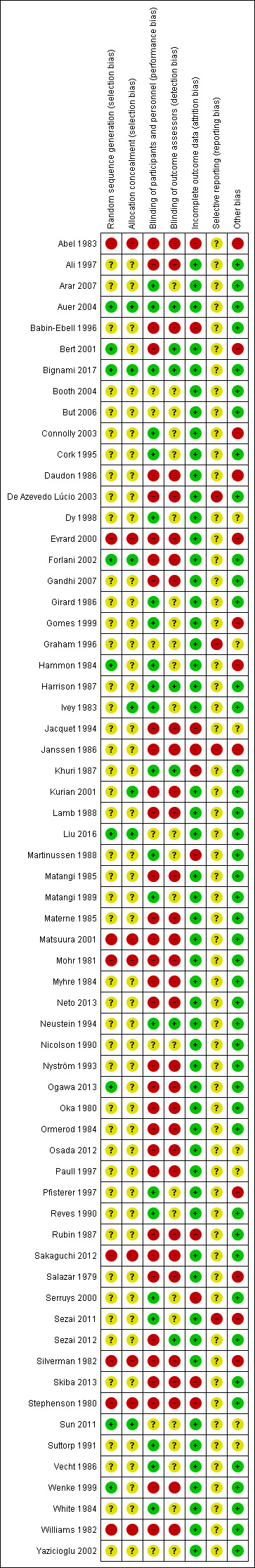
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study
Overall, we judged 68% studies to be at high risk of bias in at least one domain.
Allocation
For random sequence generation, we found that few studies reported sufficient methods used to randomize participants, and we judged only nine studies to be at low risk of selection bias (Auer 2004; Bert 2001; Bignami 2017; Forlani 2002; Hammon 1984; Liu 2016; Ogawa 2013; Sun 2011; Wenke 1999).
We judged six quasi‐randomized studies to be at high risk of selection bias (Abel 1983; Matsuura 2001; Mohr 1981; Silverman 1982; Stephenson 1980; Williams 1982). One study described randomization as being open, and another study used a method of coin‐tossing but we were not confident that this method had been used as described because the number of participants in each group was equal; we judged both studies to be at high risk of selection bias (Evrard 2000; Sakaguchi 2012). We judged the remaining studies to have an unclear risk of selection bias for random sequence generation.
For allocation concealment, we judged only seven to be at low risk of bias (Auer 2004; Bignami 2017; Forlani 2002; Ivey 1983; Kurian 2001; Liu 2016; Sun 2011). We judged the quasi‐randomized studies to be at high risk of bias for allocation concealment, and we judged the remaining studies to have an unclear risk of bias because of inadequate reporting of methods to conceal allocation.
Blinding
Many of the studies in this review compared a beta‐blocker with standard care, and because it was not feasible to blind personnel to the intervention, we judged all studies with a standard care control group to be at high risk of performance bias because of this open‐label study design. In addition, we judged Sezai 2012 to be at high risk of performance bias because personnel were aware of the use of bisoprolol in one of the study arms.
We found insufficient information to confirm blinding of personnel in only seven studies (Booth 2004; But 2006; Graham 1996; Liu 2016; Nicolson 1990; Sun 2011; Yazicioglu 2002); we judged these to have an unclear risk of performance bias. We assumed that studies described as double‐blinded had used appropriate methods to blind personnel to study treatments, and similarly if the comparison was described as a placebo agent, we also assumed that this was blinded, and we judged these remaining studies to be at low risk of performance bias.
Only seven studies reported that outcome assessors were blinded and we judged these studies to be at low risk of detection bias (Auer 2004; Bert 2001; Bignami 2017; Harrison 1987; Khuri 1987; Neustein 1994; Sezai 2012). Twenty‐two studies did not report whether outcome assessors were blinded and we judged detection bias to be unclear (Booth 2004; But 2006; Connolly 2003; Cork 1995; Dy 1998; Girard 1986; Gomes 1999; Graham 1996; Hammon 1984; Ivey 1983; Liu 2016; Martinussen 1988; Matangi 1989; Nicolson 1990; Pfisterer 1997; Reves 1990; Sezai 2011; Sun 2011; Suttorp 1991; Vecht 1986; White 1984; Yazicioglu 2002). Because the remaining open‐label studies did not describe the blinding of outcome assessors, we assumed that blinding had not occurred and, therefore, judged these to be at high risk of detection bias.
Incomplete outcome data
Most studies did not report losses, and we assumed that these studies had no losses; in these studies we used the numbers of randomized participants to inform the assumed number of analysed participants. In addition, most studies did not report whether investigators used intention‐to‐treat analysis. When studies reported losses but did not state whether investigators had used intention‐to‐treat analysis, we assumed the use of per‐protocol analysis. We reported this information in the Characteristics of included studies.
We judged 10 studies to be at high risk of attrition bias (Abel 1983; Babin‐Ebell 1996; Jacquet 1994; Janssen 1986; Khuri 1987; Martinussen 1988; Rubin 1987; Serruys 2000; Skiba 2013; Stephenson 1980); these studies lost more than 10% of participants, loss of participants was imbalanced between groups, or exclusions were unclearly reported.
We judged the remaining studies to be at low risk of attrition bias because study authors reported no losses or losses were fewer than 10% and we did not expect the loss to influence outcome data. In the event that study authors did not report whether all participants were included in the outcomes, we assumed that there were no losses.
Selective reporting
No studies were prospectively registered with clinical trials registers and this limited our ability to effectively assess the risk of reporting bias. Two studies provided clinical trial registration numbers but the time point of registration was unclear (Sezai 2011; Sezai 2012). We assessed one of these studies to be at high risk of reporting bias because we noted that additional outcomes were reported that were not included in the clinical trials register documents (Sezai 2011). We assessed Sezai 2012 to be at unclear risk of reporting bias, because we could not be certain whether registration was retrospective.
We judged risk of selective reporting to be high in three studies; two studies reported statistically significant results only (Graham 1996; Janssen 1986), and in another study, we noted that study group allocation of participants with certain adverse events (acute myocardial infarction, stroke) remained unclear and we considered this to be evidence of selective under‐reporting (De Azevedo Lúcio 2003).
We judged the remaining studies to be at unclear risk of reporting bias.
Other potential sources of bias
Eleven studies reported the use of beta‐blockers as rescue therapy in the control group (Abel 1983; Bert 2001; Connolly 2003; Daudon 1986; Evrard 2000; Hammon 1984; Janssen 1986; Pfisterer 1997; Salazar 1979; Sezai 2011; Silverman 1982). We believed that this introduced considerable bias to the data and we judged all these studies to be at high risk of bias. In addition, we judged Gomes 1999 to be at high risk of bias because more participants in the control group were taking beta‐blockers before entry into the study.
Three studies were reported only as abstracts and these short reports were insufficient to judge risks of other bias, and as such, we judged these to be unclear (Dy 1998; Graham 1996; Osada 2012). Similarly, three studies had limited information in the report and it was also not feasible to assess risks of other bias in these studies (Paull 1997; Sun 2011; Suttorp 1991). In Jacquet 1994, we noted a difference in the number of participants taking beta‐blockers preoperatively and we were uncertain of this effect on the data. We did not identify any other sources of bias in the remaining study reports.
Effects of interventions
See: Table 1
Early all‐cause mortality
Twenty‐nine studies reported mortality data (Abel 1983; Ali 1997; Auer 2004; Bert 2001; Connolly 2003; Cork 1995; De Azevedo Lúcio 2003; Evrard 2000; Forlani 2002; Gomes 1999; Hammon 1984; Ivey 1983; Janssen 1986; Liu 2016; Martinussen 1988; Matangi 1989; Matsuura 2001; Mohr 1981; Myhre 1984; Neto 2013; Nyström 1993; Oka 1980; Paull 1997; Sezai 2011; Sezai 2012; Skiba 2013; Suttorp 1991; White 1984; Yazicioglu 2002). We noted that 12 of these studies had no deaths in either group (Bert 2001; Connolly 2003; Forlani 2002; Hammon 1984; Ivey 1983; Janssen 1986; Liu 2016; Martinussen 1988; Matsuura 2001; Nyström 1993; Paull 1997; Suttorp 1991).
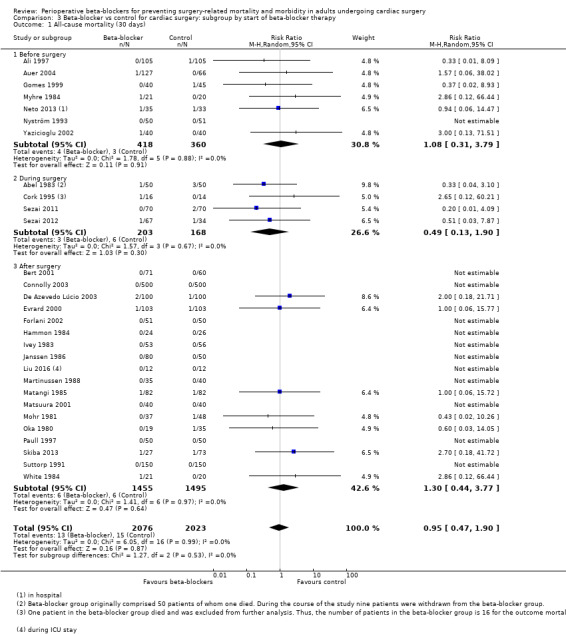
We found that studies reported few events, and beta‐blockers probably make little or no difference to all‐cause mortality at 30 days (RR 0.95, 95% CI 0.47 to 1.90; I2 = 0%; 4099 participants; low‐certainty evidence; Analysis 1.1). We used GRADE to downgrade the certainty of the evidence by two levels: one level for study limitations owing to the inclusion of several studies at high risk of bias in at least one domain; and one level for imprecision owing to the wide CI and because the evidence was from too few participants. Our estimate for the required number of participants was taken from a calculation in a previous version of the review (Blessberger 2018). See Table 1 and Figure 4.
1.1. Analysis.
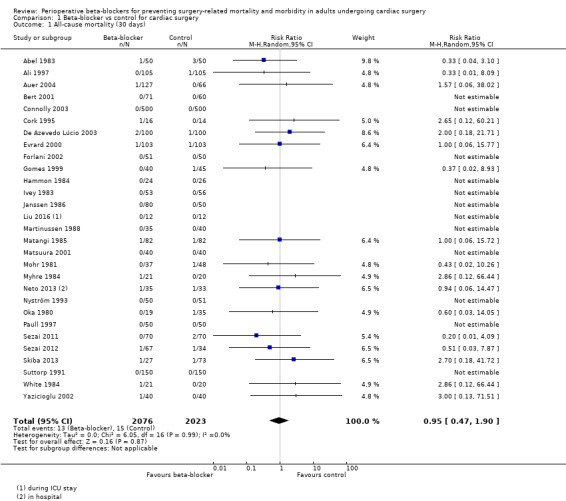
Comparison 1 Beta‐blocker vs control for cardiac surgery, Outcome 1 All‐cause mortality (30 days).
4.
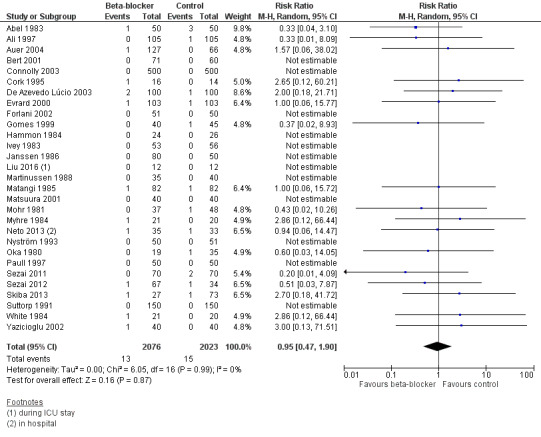
Forest plot of comparison 1. Beta‐blocker vs control for cardiac surgery, outcome: 1.1 All‐cause mortality (30 days)
Long‐term mortality
Three studies reported mortality at one year (Bignami 2017), at six months (Gandhi 2007), and at seven months (Serruys 2000). We found little or no difference in effect between groups (RR 0.90, 95% CI 0.29 to 2.79; I2 = 0%; 511 participants; very low‐certainty evidence; Analysis 1.2). We used GRADE to downgrade the certainty of the evidence by three levels: one level for study limitations because one of the three studies was at high risk of bias in two domains; and two levels for imprecision owing to the wide CI and because the evidence was from only three studies with few participants.
1.2. Analysis.
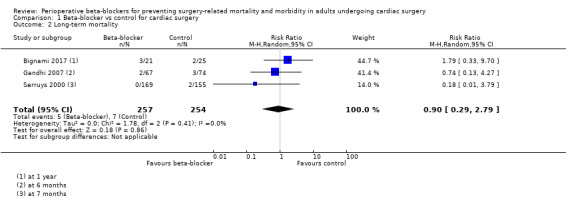
Comparison 1 Beta‐blocker vs control for cardiac surgery, Outcome 2 Long‐term mortality.
Death due to cardiac causes
Four studies reported death due to cardiac causes (Gomes 1999; Oka 1980; Sezai 2011; White 1984), with little or no difference in effect between groups (RR 0.84, 95% CI 0.14 to 5.19; I2 = 0%; 320 participants; very low‐certainty evidence; Analysis 1.3). Gomes 1999 reported no events in either group. We used GRADE to downgrade the certainty of the evidence by three levels: one level for study limitations because most of the studies were at high risk of bias in at least one domain, and two levels of imprecision owing to the wide CI and because the evidence was from few studies with few participants.
1.3. Analysis.
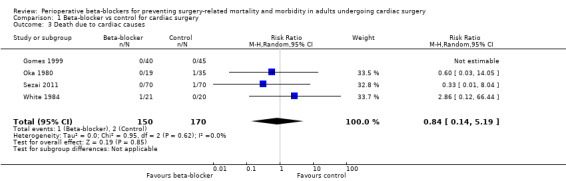
Comparison 1 Beta‐blocker vs control for cardiac surgery, Outcome 3 Death due to cardiac causes.
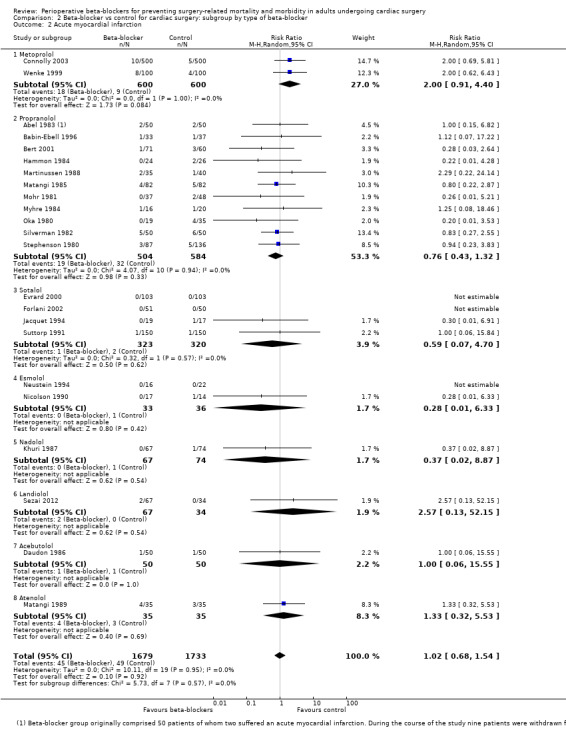
Acute myocardial infarction
Twenty‐five studies reported participants who had a myocardial infarction during the perioperative period or within 30 days of surgery (Abel 1983; Ali 1997; Babin‐Ebell 1996; Bert 2001; Connolly 2003; Daudon 1986; Evrard 2000; Forlani 2002; Hammon 1984; Jacquet 1994; Khuri 1987; Martinussen 1988; Matangi 1985; Matangi 1989; Mohr 1981; Myhre 1984; Neustein 1994; Nicolson 1990; Oka 1980; Serruys 2000; Sezai 2012; Silverman 1982; Stephenson 1980; Suttorp 1991; Wenke 1999). We noted no events in three of these studies (Evrard 2000; Forlani 2002; Neustein 1994).
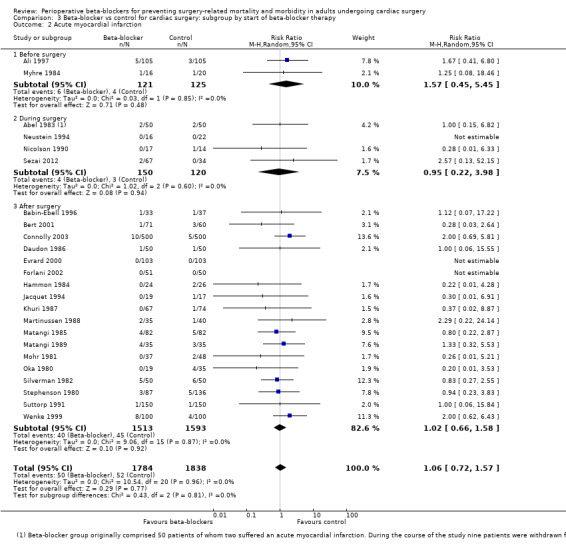
We found little or no difference in the number of people having a myocardial infarction when beta‐blockers were used (RR 1.05, 95% CI 0.72 to 1.52; I2 = 0%: 3946 participants; low‐certainty evidence; Analysis 1.4). We used GRADE to downgrade the certainty of the evidence by two levels: one level for study limitations owing to the inclusion of several studies at high risk of bias in at least one domain; and one level for imprecision owing to the wide confidence interval in the effect estimate and because the evidence was from too few participants. Our estimate for the required number of participants was taken from a calculation in a previous version of the review (Blessberger 2018). See Table 1.
1.4. Analysis.
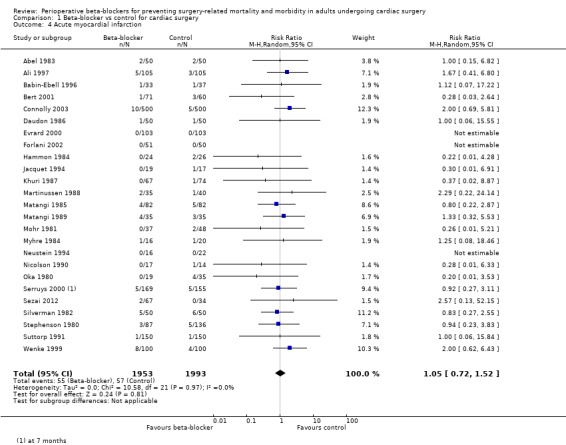
Comparison 1 Beta‐blocker vs control for cardiac surgery, Outcome 4 Acute myocardial infarction.
Cerebrovascular events
Seven studies reported the number of participants who had a cerebrovascular event (Ali 1997; Auer 2004; Connolly 2003; Matangi 1989; Neto 2013; Rubin 1987; Sezai 2011). We did not include data for Rubin 1987 in the analysis because study authors did not report to which group two participants with cerebrovascular events belonged. We did not include data for Ali 1997, in which one participant in the control group had a stroke, because this event was reported as fatal by study authors.
Beta‐blockers may make little or no difference to cerebrovascular events, however, few studies reported events; seven out of 704 participants experienced an event in the control group compared to 10 out of 767 participants who experienced an event when beta‐blockers were used (RR 1.37, 95% CI 0.51 to 3.67; I2 = 0%; 5 studies, 1471 participants; very low‐certainty evidence; Analysis 1.5). We used GRADE to downgrade the certainty of the evidence by three levels: one level for study limitations owing to the inclusion of several studies at high risk of bias; and two levels for imprecision owing to the very wide CI and because the evidence was from too few participants. Our estimate for the required number of participants was taken from a calculation in a previous version of the review (Blessberger 2018). See Table 1.
1.5. Analysis.
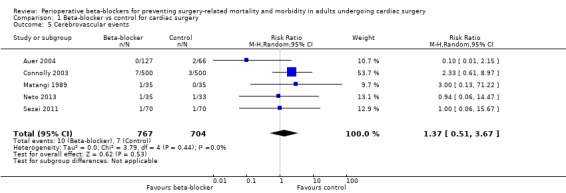
Comparison 1 Beta‐blocker vs control for cardiac surgery, Outcome 5 Cerebrovascular events.
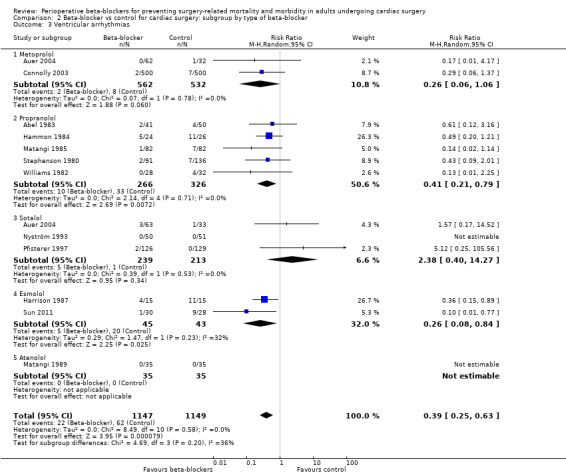
Ventricular arrhythmias
Thirteen studies reported ventricular arrhythmias (Abel 1983; Auer 2004; Connolly 2003; Gomes 1999; Hammon 1984; Harrison 1987; Matangi 1985; Matangi 1989; Nyström 1993; Pfisterer 1997; Stephenson 1980; Sun 2011; Williams 1982). We did not include one study in meta‐analysis because we could not be certain whether events were recorded in the control group (Gomes 1999).
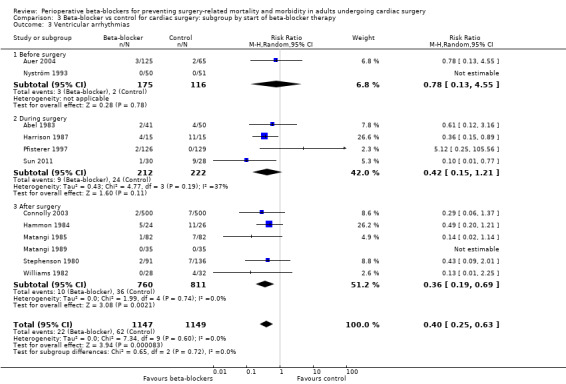
In meta‐analysis of the remaining studies, we found that beta‐blockers probably reduce episodes of ventricular arrhythmias (RR 0.40, 95% CI 0.25 to 0.63; I2 = 0%; 12 studies, 2296 participants; low‐certainty evidence; Analysis 1.6). In absolute terms, we found 32 fewer episodes per 1000 when beta‐blockers were used, based on a control risk of 54 per 1000; the number needed to treat for a beneficial outcomes (NNTB) was 31 (95% CI 25 to 50). We used GRADE to downgrade the certainty of the evidence by two levels: one level for study limitations owing to the inclusion of several studies at high risk of bias, and one level for imprecision because the evidence was from too few participants. Our estimate for the required number of participants was taken from a calculation in a previous version of the review (Blessberger 2018). See Table 1.
1.6. Analysis.
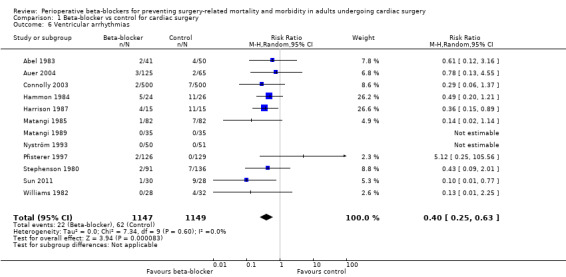
Comparison 1 Beta‐blocker vs control for cardiac surgery, Outcome 6 Ventricular arrhythmias.
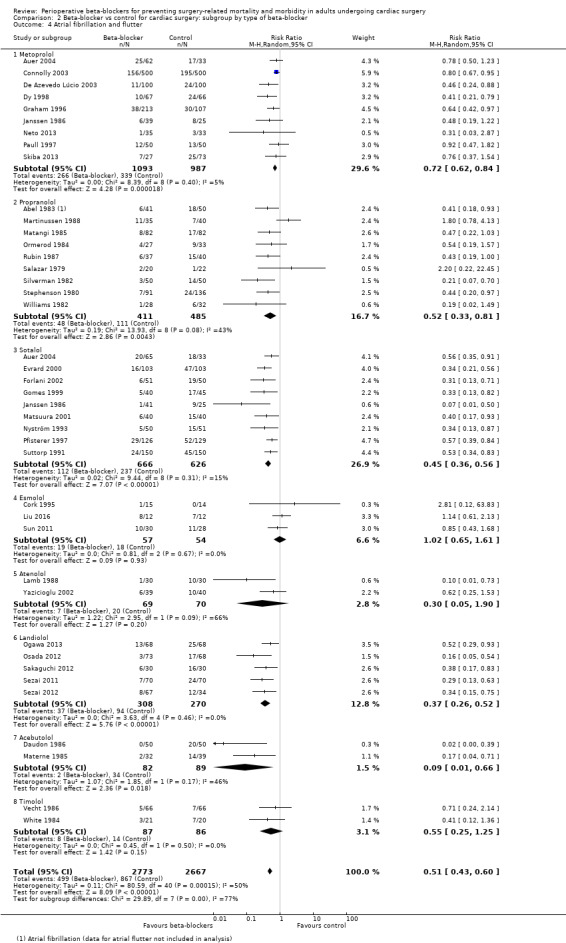
Atrial fibrillation or flutter, or both
Forty studies reported atrial fibrillation or atrial flutter (Abel 1983; Ali 1997; Auer 2004; Connolly 2003; Cork 1995; Daudon 1986; De Azevedo Lúcio 2003; Dy 1998; Evrard 2000; Forlani 2002; Gomes 1999; Graham 1996; Janssen 1986; Lamb 1988; Liu 2016; Martinussen 1988; Matangi 1985; Materne 1985; Matsuura 2001; Neto 2013; Nyström 1993; Ogawa 2013; Ormerod 1984; Osada 2012; Paull 1997; Pfisterer 1997; Rubin 1987; Sakaguchi 2012; Salazar 1979; Sezai 2011; Sezai 2012; Silverman 1982; Skiba 2013; Stephenson 1980; Sun 2011; Suttorp 1991; Vecht 1986; White 1984; Williams 1982; Yazicioglu 2002).
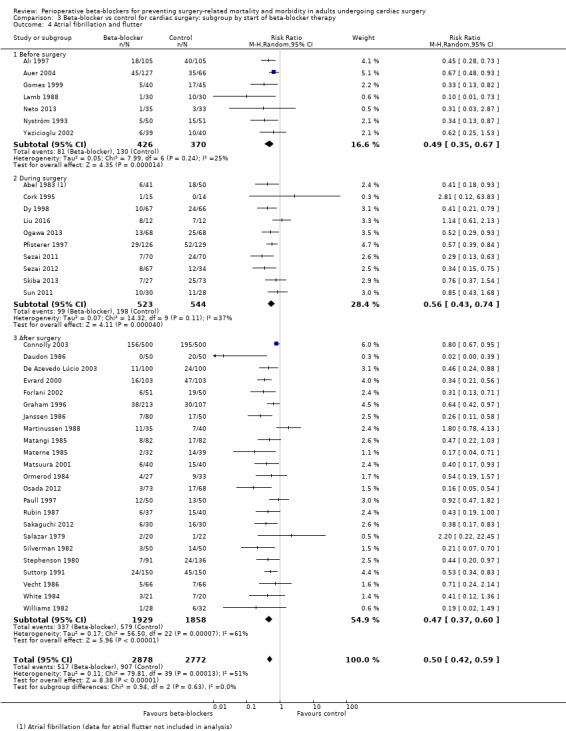
We found that beta‐blockers reduce the number of participants who experience atrial fibrillation or atrial flutter (RR 0.50, 95% CI 0.42 to 0.59; I2 = 51%; 5650 participants; low‐certainty evidence; Analysis 1.7). In absolute terms, we found 163 fewer incidences per 1000 when beta‐blockers were used, based on a control risk of 327 per 1000; the NNTB was 6 (95% CI 5 to 7). We used GRADE to downgrade the certainty of the evidence by two levels: one level for study limitations owing to the inclusion of several studies at high risk of bias, and one level for inconsistency owing to a moderate level of statistical heterogeneity which we were unable to explain through subgroup analysis. See Table 1 and Figure 5.
1.7. Analysis.
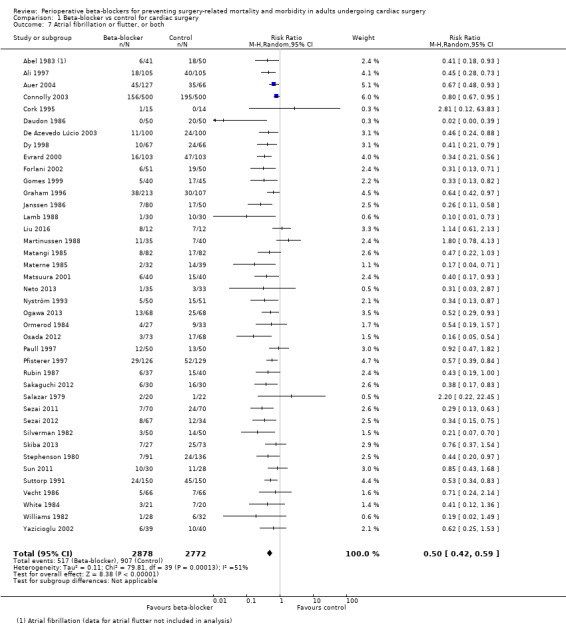
Comparison 1 Beta‐blocker vs control for cardiac surgery, Outcome 7 Atrial fibrillation or flutter, or both.
5.
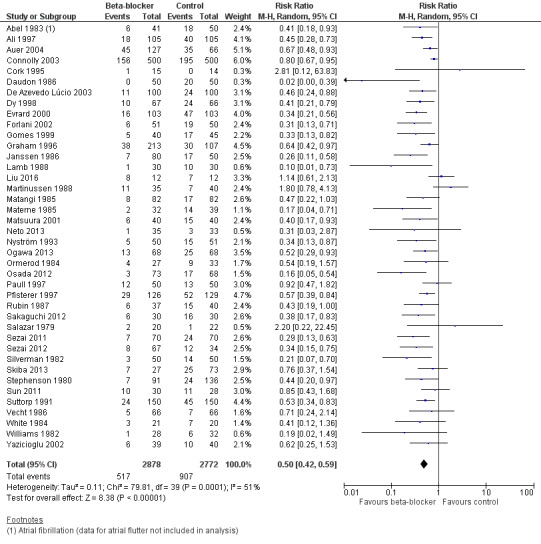
Forest plot of comparison 1. Beta‐blocker vs control for cardiac surgery, outcome: 1.7 Atrial fibrillation or flutter, or both
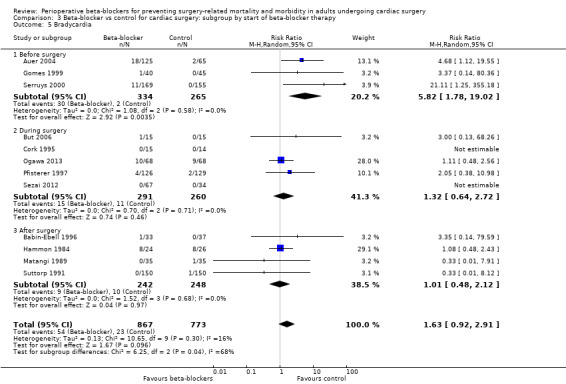
Bradycardia
Twenty‐three studies reported bradycardia (Abel 1983; Arar 2007; Auer 2004; Babin‐Ebell 1996; But 2006; Cork 1995; Girard 1986; Gomes 1999; Hammon 1984; Jacquet 1994; Matangi 1989; Matsuura 2001; Neto 2013; Neustein 1994; Nyström 1993; Ogawa 2013; Pfisterer 1997; Reves 1990; Serruys 2000; Sezai 2012; Silverman 1982; Stephenson 1980; Suttorp 1991). However, data for bradycardia were not clearly reported in 11 studies and we were uncertain whether some of these study authors had collected data for bradycardia in the control group (Abel 1983; Arar 2007; Girard 1986; Jacquet 1994; Matsuura 2001; Neto 2013; Neustein 1994; Nyström 1993; Reves 1990; Silverman 1982; Stephenson 1980). Therefore, we did not include these studies in meta‐analysis; for these studies, we have reported data for bradycardia, as presented by study authors, in Characteristics of included studies.
In analysis of the remaining studies, we found no evidence of a difference in bradycardia when beta‐blockers were used (RR 1.63, 95% CI 0.92 to 2.91; I2 = 16%; 12 studies; 1640 participants; low‐certainty evidence; Analysis 1.8). We used the GRADE approach to downgrade the certainty of the evidence by two levels: one for study limitations owing to the inclusion of several studies at high risk of bias in at least one domain, and one level for imprecision owing to the very wide confidence interval and because the evidence was from too few participants. Our estimate for the required number of participants was taken from a calculation in a previous version of the review (Blessberger 2018). See Table 1.
1.8. Analysis.
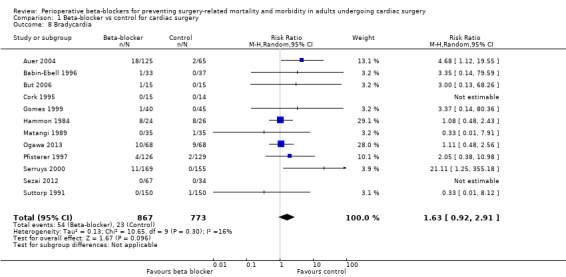
Comparison 1 Beta‐blocker vs control for cardiac surgery, Outcome 8 Bradycardia.
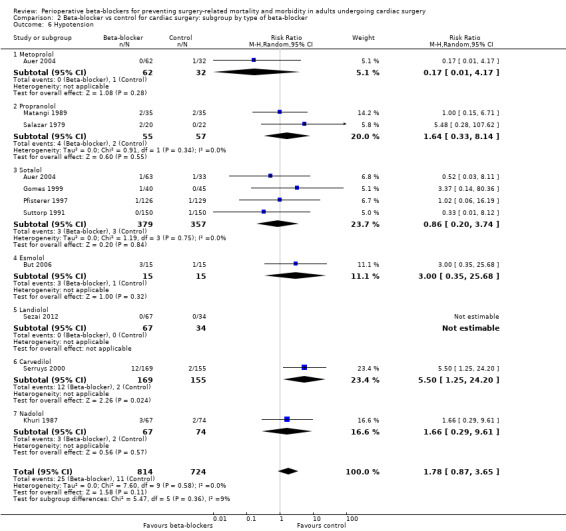
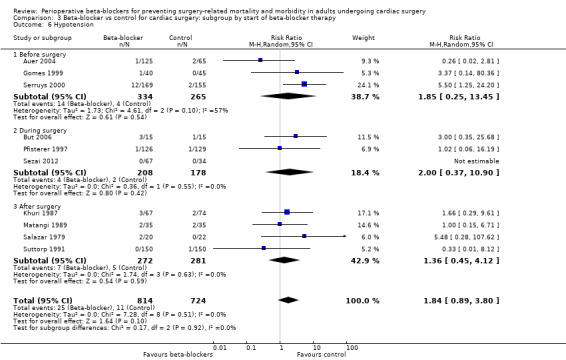
Hypotension
Nineteen studies reported hypotension (Abel 1983; Arar 2007; Auer 2004; But 2006; Gomes 1999; Jacquet 1994; Khuri 1987; Kurian 2001; Matangi 1989; Matsuura 2001; Neustein 1994; Nyström 1993; Pfisterer 1997; Reves 1990; Rubin 1987; Salazar 1979; Serruys 2000; Sezai 2012; Suttorp 1991). However, nine studies did not clearly report data for hypotension and we were uncertain whether study authors had collected data for hypotension in the control group (Abel 1983; Arar 2007; Jacquet 1994; Kurian 2001; Matsuura 2001; Neustein 1994; Nyström 1993; Reves 1990; Rubin 1987). Therefore, we did not include these studies in meta‐analysis; for these studies, we have reported data for hypotension, as presented by study authors, in Characteristics of included studies.
In analysis of the remaining studies, we found no evidence of a difference in hypotension when beta‐blockers were used (RR 1.84, 95% CI 0.89 to 3.80; I2 = 0%; 10 studies, 1538 participants; low‐certainty evidence; Analysis 1.9). We used GRADE to downgrade the certainty of the evidence by two levels: one level for study limitations owing to the inclusion of several studies at high risk of bias in at least one domain, and one level owing to the very wide confidence interval and because the evidence was from too few participants. Our estimate for the required number of participants was taken from a calculation in a previous version of the review (Blessberger 2018). See Table 1.
1.9. Analysis.
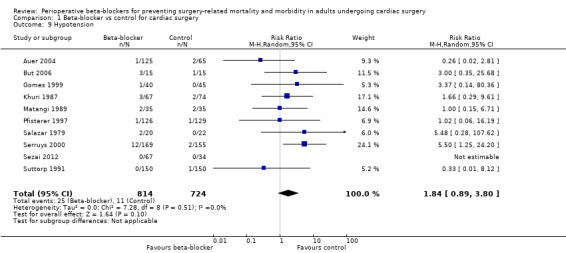
Comparison 1 Beta‐blocker vs control for cardiac surgery, Outcome 9 Hypotension.
Congestive heart failure
Three studies reported congestive heart failure (Matangi 1989; Sezai 2011; Sezai 2012). We found little or no difference between groups in the number of participants who had heart failure (RR 0.22, 95% CI 0.04 to 1.36; I2 = 0%; 331 participants; very low‐certainty evidence; Analysis 1.10). We used GRADE to downgrade the certainty of the evidence by three levels; one level for study limitations because two of the three studies were at high risk of bias in at least one domain, and two levels for imprecision because the evidence was from few studies with few participants.
1.10. Analysis.
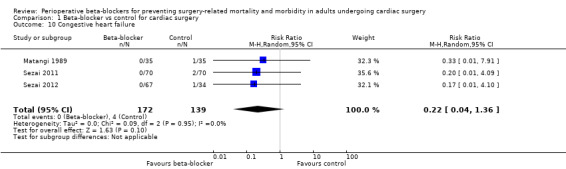
Comparison 1 Beta‐blocker vs control for cardiac surgery, Outcome 10 Congestive heart failure.
Length of hospital stay
Eighteen studies reported length of hospital stay (Auer 2004; Bert 2001; Bignami 2017; Booth 2004; But 2006; Connolly 2003; Cork 1995; Evrard 2000; Forlani 2002; Gomes 1999; Jacquet 1994; Matsuura 2001; Pfisterer 1997; Rubin 1987; Sezai 2011; Sezai 2012; Skiba 2013; Wenke 1999). We did not include data from four studies in meta‐analysis because the data were not reported in equivalent values (mean and standard deviation; Bignami 2017; Evrard 2000; Rubin 1987; Skiba 2013).
In meta‐analysis of remaining studies, we found that length of stay was shorter when participants were treated with beta‐blockers (MD −0.54 days, 95% CI −0.90 to −0.19; I2 = 58%; 14 studies, 2450 participants; low‐certainty evidence; Analysis 1.11). We used GRADE to downgrade the certainty of the evidence by two levels: one level for study limitations owing to the inclusion of several studies at high risk of bias in at least one domain, and one level for inconsistency owing to a moderate level of statistical heterogeneity. We did not explore possible causes of this heterogeneity through formal subgroup analysis.
1.11. Analysis.
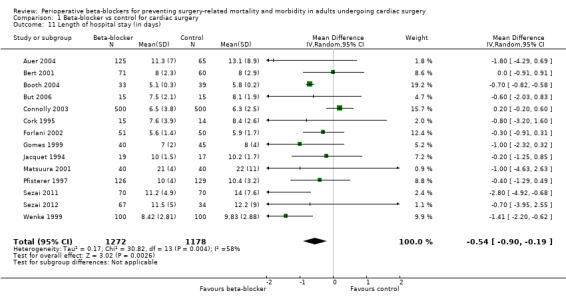
Comparison 1 Beta‐blocker vs control for cardiac surgery, Outcome 11 Length of hospital stay (in days).
Quality of life
No studies reported outcome data for quality of life.
Subgroup analysis
We did not complete subgroup analysis for cerebrovascular events, because we found fewer than 10 studies for this outcome.
Type of beta‐blocker
We did not include Ali 1997 in subgroup analysis by type of beta‐blocker because participants in the intervention group were given a variety of types of beta‐blockers and study authors did not report data according to type of beta‐blocker. For subgroup analysis of Auer 2004 and Janssen 1986, in which both studies had two beta‐blocker groups, we halved the control group data.
Early all‐cause mortality: we found no evidence of difference between subgroups (P = 0.74, I2 = 0%; Analysis 2.1). Each type of agent showed little or no difference in all‐cause mortality, which was consistent with our primary analysis: metoprolol (RR 1.72, 95% CI 0.44 to 6.69; I2 = 0%; 7 studies, 1627 participants); propranolol (RR 0.67, 95% CI 0.19 to 2.37; I2 = 0%; 9 studies, 809 participants); sotalol (RR 0.65, 95% CI 0.08 to 5.25; I2 = 0%; 8 studies, 1037 participants); esmolol (RR 2.65, 95% CI 0.12 to 60.21; 2 studies, 54 participants); timolol (RR 2.86, 95% CI 0.12 to 66.44; 1 study, 41 participants); landiolol (RR 0.33, 95% CI 0.04 to 2.53; I2 = 0%; 2 studies, 241 participants); and atenolol (RR 3.00, 95% CI 0.13 to 71.51; 1 study, 80 participants).
Acute myocardial infarction: we found no evidence of a difference between subgroups (P = 0.57, I2 = 0%; Analysis 2.2). Each type of agent showed little or no difference in myocardial infarction, which was consistent with our primary analysis: metoprolol (RR 2.00, 95% CI 0.91 to 4.40; I2 = 0%; 2 studies, 1200 participants); propranolol (RR 0.76, 95% CI 0.43 to 1.32; I2 = 0%; 11 studies, 1088 participants); sotalol (RR 0.59, 95% CI 0.07 to 4.70; I2 = 0%; 4 studies, 643 participants); esmolol (RR 0.28, 95% CI 0.01 to 6.33; 2 studies, 69 participants); nadolol (RR 0.37, 95% CI 0.02 to 8.87; 1 study, 141 participants); landiolol (RR 2.57, 95% CI 0.13 to 52.15; 1 study, 101 participants); acebutolol (RR 1.00, 95% CI 0.06 to 15.55; 1 study, 100 participants); and atenolol (RR 1.33, 95% CI 0.32 to 5.53; 1 study, 70 participants).
Ventricular arrhythmias: we found no evidence of a difference between subgroups (P = 0.20, I2 = 36.0%; Analysis 2.3). Studies of metoprolol showed little or no difference between groups in ventricular arrhythmias (RR 0.26, 95% CI 0.06 to 1.06; I2 = 0%; 2 studies, 1094 participants), and, similarly, we found little or no difference between groups for sotalol (RR 2.38, 95% CI 0.40 to 14.27; I2 = 0%; 3 studies, 452 participants). Analysis of the remaining studies according to type of beta‐blocker showed findings consistent with the primary analysis: propranolol (RR 0.41, 95% CI 0.21 to 0.79; I2 = 0%; 5 studies, 592 participants); and esmolol (RR 0.26, 95% CI 0.08 to 0.84; I2 = 32%; 2 studies, 88 participants). Only one study reported data for atenolol, in which study authors reported no events (Matangi 1989).
Atrial fibrillation or flutter, or both: we found differences in incidences of atrial fibrillation according to the type of beta‐blocker that participants were given (P < 0.0001, I2 = 76.6%; Analysis 2.4). However, types of agents that demonstrated an effect that differed from the primary analysis had few studies: esmolol (RR 1.02, 95% CI 0.65 to 1.61; I2 = 0%; 3 studies, 111 participants); timolol (RR 0.55, 95% CI 0.25 to 1.25; I2 = 0%; 2 studies, 173 participants); and atenolol (RR 0.30, 95% CI 0.05 to 1.90; I2 = 66%; 2 studies, 139 participants). Analysis of the remaining studies was consistent with the primary analysis: metoprolol (RR 0.72, 95% CI 0.62 to 0.84; I2 = 5%; 9 studies, 2080 participants); propranolol (RR 0.52, 95% CI 0.33 to 0.81; I2 = 43%; 9 studies, 896 participants); sotalol (RR 0.45, 95% CI 0.36 to 0.56; I2 = 15%; 9 studies, 1292 participants); landiolol (RR 0.37, 95% CI 0.26 to 0.52; I2 = 0%; 5 studies, 578 participants); and acebutolol (RR 0.09, 95% CI 0.01 to 0.66; I2 = 46%; 2 studies, 171 participants).
Bradycardia: we found no evidence of a difference between subgroups (P = 0.30, I2 = 16.6%; Analysis 2.5). Each type of agent showed little or no difference in incidences of bradycardia, which was consistent with our primary analysis: metoprolol (RR 5.16, 95% CI 0.69 to 38.55; 1 study, 94 participants); propranolol (RR 1.16, 95% CI 0.53 to 2.54; I2 = 0%; 2 studies, 120 participants); sotalol (RR 2.16, 95% CI 0.70 to 6.65; I2 = 0%; 4 studies, 736 participants); esmolol (RR 3.00, 95% CI 0.13 to 68.26; 2 studies, 59 participants); landiolol (RR 1.11, 95% CI 0.48 to 2.56; 2 studies, 237 participants); atenolol (RR 0.33, 95% CI 0.01 to 7.91; 1 study, 70 participants); carvedilol (RR 21.11, 95% CI 1.25 to 355.18; 1 study, 324 participants).
Hypotension: we found no evidence of a difference between subgroups (P = 0.36, I2 = 8.7%; Analysis 2.6). Only carvedilol showed an increase in hypotension when beta‐blockers were given, but this evidence was from a single study (RR 5.50, 95% CI 1.25 to 24.20; 324 participants). The remaining agents showed little or no difference in incidences of hypotension, which was consistent with our primary analysis: metoprolol (RR 0.17, 95% CI 0.01 to 4.17; 1 study, 94 participants); propranolol (RR 1.64, 95% CI 0.33 to 8.14; I2 = 0%; 2 studies, 112 participants); sotalol (RR 0.86, 95% CI 0.20 to 3.74; I2 = 0%; 4 studies, 736 participants); esmolol (RR 3.00, 95% CI 0.35 to 25.68; 1 study, 30 participants); nadolol (RR 1.66, 95% CI 0.29 to 9.61; 1 study, 141 participants). Only one study reported data for landiolol, in which study authors reported no events (Sezai 2012).
2.1. Analysis.

Comparison 2 Beta‐blocker vs control for cardiac surgery: subgroup by type of beta‐blocker, Outcome 1 All‐cause mortality (30 days).
2.2. Analysis.
Comparison 2 Beta‐blocker vs control for cardiac surgery: subgroup by type of beta‐blocker, Outcome 2 Acute myocardial infarction.
2.3. Analysis.
Comparison 2 Beta‐blocker vs control for cardiac surgery: subgroup by type of beta‐blocker, Outcome 3 Ventricular arrhythmias.
2.4. Analysis.
Comparison 2 Beta‐blocker vs control for cardiac surgery: subgroup by type of beta‐blocker, Outcome 4 Atrial fibrillation and flutter.
2.5. Analysis.

Comparison 2 Beta‐blocker vs control for cardiac surgery: subgroup by type of beta‐blocker, Outcome 5 Bradycardia.
2.6. Analysis.
Comparison 2 Beta‐blocker vs control for cardiac surgery: subgroup by type of beta‐blocker, Outcome 6 Hypotension.
Start of beta‐blocker therapy
Early all‐cause mortality (30 days): we found no evidence of a difference between subgroups according to the time at which beta‐blockers were first given (P = 0.53, I2 = 0%; Analysis 3.1). Evidence at each time point was consistent with the primary analysis: before surgery (RR 1.08, 95% CI 0.31 to 3.79; I2 = 0%; 7 studies, 778 participants); during surgery (RR 0.49, 95% CI 0.13 to 1.90; I2 = 0%; 4 studies, 371 participants); and after surgery (RR 1.30, 95% CI 0.44 to 3.77; I2 = 0%; 18 studies, 2950 participants).
Acute myocardial infarction: we found no evidence of a difference between subgroups according to the time at which beta‐blockers were first given (P = 0.81, I2 = 0%; Analysis 3.2). Evidence at each time point was consistent with the primary analysis: before surgery (RR 1.57, 95% CI 0.45 to 5.45; I2 = 0%; 2 studies, 246 participants); during surgery (RR 0.95, 95% CI 0.22 to 3.98; I2 = 0%; 4 studies, 270 participants); after surgery (RR 1.02, 95% CI 0.66 to 1.58; I2 = 0%; 18 studies, 3106 participants).
Ventricular arrhythmias: we found no evidence of a difference between subgroups according to the time at which beta‐blockers were first given (P = 0.72, I2 = 0%; Analysis 3.3). We found no evidence of a difference in ventricular arrhythmias when beta‐blockers were given before surgery (RR 0.78, 95% CI 0.13 to 4.55; 291 participants); however, this subgroup included only two studies of which one reported no events. We found only slightly fewer incidences of ventricular arrhythmias when beta‐blockers were given during surgery in four studies (RR 0.42, 95% CI 0.15 to 1.21; I2 = 37%; 434 participants). Analysis of studies in which beta‐blockers were given after surgery included a larger number of studies and was consistent with the primary analysis (RR 0.36, 95% CI 0.19 to 0.69; I2 = 0%; 6 studies, 1571 participants).
Atrial fibrillation or flutter, or both: we found no evidence of a difference between subgroups according to the time at which beta‐blockers were first given (P = 0.63, I2 = 0%; Analysis 3.4). Evidence at each time point was consistent with the primary analysis: before surgery (RR 0.49, 95% CI 0.35 to 0.67; I2 = 25%; 7 studies, 796 participants); during surgery (RR 0.56, 95% CI 0.43 to 0.74; I2 = 37%; 10 studies, 1067 participants); and after surgery (RR 0.47, 95% CI 0.37 to 0.60; I2 = 61%; 23 studies, 3787 participants).
Bradycardia: the test for subgroup differences indicated a difference between groups (P = 0.04, I2 = 68.0%; Analysis 3.5). We found more incidences of bradycardia when beta‐blockers were started before surgery, but this evidence was from few studies with a very wide CI (RR 5.82, 95% CI 1.78 to 19.02; I2 = 0%; 3 studies, 599 participants). The remaining studies were consistent with the primary analysis and showed little or no difference in bradycardia when beta‐blockers were given during surgery (RR 1.32, 95% CI 0.64 to 2.72; I2 = 0%; 5 studies, 551 participants), or after surgery (RR 1.01, 95% CI 0.48 to 2.12; I2 = 0%; 4 studies, 490 participants).
Hypotension: we found no evidence of a difference between subgroups according to the time at which beta‐blockers were first given (P = 0.92, I2 = 0%; Analysis 3.6). Evidence at each time point was consistent with the primary analysis: before surgery (RR 1.85, 95% CI 0.25 to 13.45; I2 = 57%; 3 studies, 599 participants); during surgery (RR 2.00, 95% CI 0.37 to 10.90; I2 = 0%; 3 studies, 386 participants); after surgery (RR 1.36, 95% CI 0.45 to 4.12; I2 = 0%; 4 studies, 553 participants).
3.1. Analysis.
Comparison 3 Beta‐blocker vs control for cardiac surgery: subgroup by start of beta‐blocker therapy, Outcome 1 All‐cause mortality (30 days).
3.2. Analysis.
Comparison 3 Beta‐blocker vs control for cardiac surgery: subgroup by start of beta‐blocker therapy, Outcome 2 Acute myocardial infarction.
3.3. Analysis.
Comparison 3 Beta‐blocker vs control for cardiac surgery: subgroup by start of beta‐blocker therapy, Outcome 3 Ventricular arrhythmias.
3.4. Analysis.
Comparison 3 Beta‐blocker vs control for cardiac surgery: subgroup by start of beta‐blocker therapy, Outcome 4 Atrial fibrillation and flutter.
3.5. Analysis.
Comparison 3 Beta‐blocker vs control for cardiac surgery: subgroup by start of beta‐blocker therapy, Outcome 5 Bradycardia.
3.6. Analysis.
Comparison 3 Beta‐blocker vs control for cardiac surgery: subgroup by start of beta‐blocker therapy, Outcome 6 Hypotension.
Sensitivity analysis
Standard care control
Early all‐cause mortality (30 days): we excluded 15 studies from analysis in which the control group comparison was standard care (Abel 1983; Ali 1997; Bert 2001; De Azevedo Lúcio 2003; Evrard 2000; Forlani 2002; Janssen 1986; Matangi 1985; Matsuura 2001; Mohr 1981; Myhre 1984; Neto 2013; Nyström 1993; Oka 1980; Skiba 2013). This did not alter the interpretation of the effect for this outcome.
Acute myocardial infarction: we excluded 14 studies from analysis in which the control group comparison was standard care (Abel 1983; Ali 1997; Babin‐Ebell 1996; Bert 2001; Daudon 1986; Evrard 2000; Forlani 2002; Jacquet 1994; Matangi 1985; Mohr 1981; Myhre 1984; Oka 1980; Silverman 1982; Wenke 1999). This did not alter the interpretation of the effect for this outcome.
Cerebrovascular events: we excluded one study from analysis in which the control group comparison was standard care (Neto 2013). This did not alter the interpretation of the effect for this outcome.
Ventricular arrhythmias: we excluded five studies from analysis in which the control group comparison was standard care (Abel 1983; Matangi 1985; Nyström 1993; Stephenson 1980; Williams 1982). This did not alter the interpretation of the effect for this outcome.
Atrial fibrillation or flutter, or both: we excluded 23 studies from analysis in which the control group comparison was standard care (Abel 1983; Ali 1997; Daudon 1986; De Azevedo Lúcio 2003; Evrard 2000; Forlani 2002; Janssen 1986; Lamb 1988; Matangi 1985; Materne 1985; Matsuura 2001; Neto 2013; Nyström 1993; Ogawa 2013; Ormerod 1984; Osada 2012; Rubin 1987; Sakaguchi 2012; Salazar 1979; Silverman 1982; Skiba 2013; Stephenson 1980; Williams 1982). This did not alter the interpretation of the effect for this outcome.
Bradycardia: we excluded two studies from analysis in which the control group comparison was standard care (Babin‐Ebell 1996; Ogawa 2013). This did not alter the interpretation of the effect for this outcome.
Hypotension: we excluded one study from analysis in which the control group comparison was standard care (Salazar 1979). This did not alter the interpretation of the effect for this outcome.
Risk of selection bias
Overall, we judged only nine studies to have a low risk of selection bias for sequence generation (Auer 2004; Bert 2001; Bignami 2017; Forlani 2002; Hammon 1984; Liu 2016; Ogawa 2013; Sun 2011; Wenke 1999). This limited sensitivity analysis because there were few remaining studies in analyses once we excluded studies with a high or unclear risk of selection bias.
Early all‐cause mortality (30 days): once we excluded studies with a high or unclear risk of selection bias, only five studies remained in analysis (Auer 2004; Bert 2001; Forlani 2002; Hammon 1984; Liu 2016), of which only one study had event data (Auer 2004). Meaningful sensitivity analysis could not be conducted for this outcome.
Acute myocardial infarction: once we excluded studies with a high or unclear risk of selection bias, only four studies remained in analysis (Bert 2001; Forlani 2002; Hammon 1984; Wenke 1999). Analysis with only these studies did not alter the interpretation of the effect for this outcome.
Cerebrovascular events: once we excluded studies with a high or unclear risk of selection bias, only one study remained, and this prevented meaningful sensitivity analysis.
Ventricular arrhythmias: once we excluded studies with a high or unclear risk of selection bias, only three studies remained in analysis (Auer 2004; Hammon 1984; Sun 2011). Whilst analysis without the studies at high or unclear risk of bias showed that fewer participants had incidences of ventricular arrhythmias, the effect was less certain (RR 0.41, 95% CI 0.16 to 1.05).
Atrial fibrillation or flutter, or both: once we excluded studies with a high or unclear risk of selection bias, only five studies remained in analysis (Auer 2004; Forlani 2002; Liu 2016; Ogawa 2013; Sun 2011). Analysis with only these studies did not alter the interpretation of the effect for this outcome.
Bradycardia: once we excluded studies with a high or unclear risk of selection bias, only three studies remained in analysis (Auer 2004; Hammon 1984; Ogawa 2013). Analysis with only these studies did not alter the interpretation of the effect for this outcome.
Hypotension: once we excluded studies with a high or unclear risk of selection bias, only one study remained (Auer 2004), and this prevented meaningful sensitivity analysis.
Risk of attrition bias
Early all‐cause mortality (30 days): we excluded four studies with a high risk of attrition bias (Abel 1983; Janssen 1986; Martinussen 1988; Skiba 2013). This did not alter the interpretation of the effect for this outcome.
Acute myocardial infarction: we excluded five studies with a high risk of attrition bias (Abel 1983; Babin‐Ebell 1996; Jacquet 1994; Khuri 1987; Martinussen 1988). This did not alter the interpretation of the effect for this outcome.
Cerebrovascular events: we found no studies with a high risk of attrition bias for this outcome.
Ventricular arrhythmias: we excluded one study with a high risk of attrition bias (Abel 1983). This did not alter the interpretation of the effect for this outcome.
Atrial fibrillation or flutter, or both: we excluded five studies with a high risk of attrition bias (Abel 1983; Janssen 1986; Martinussen 1988; Rubin 1987; Skiba 2013). This did not alter the interpretation of the effect for this outcome.
Bradycardia: we excluded one study with a high risk of attrition bias (Babin‐Ebell 1996). This did not alter the interpretation of the effect for this outcome.
Hypotension: we excluded one study with a high risk of attrition bias (Khuri 1987). This did not alter the interpretation of the effect for this outcome.
Beta‐blockers given as rescue therapy
Early all‐cause mortality (30 days): we excluded seven studies in which participants in the control group were given beta‐blockers as rescue therapy (Abel 1983; Bert 2001; Connolly 2003; Evrard 2000; Hammon 1984; Janssen 1986; Sezai 2011). This did not alter the interpretation of the effect for this outcome.
Acute myocardial infarction: we excluded seven studies in which participants in the control group were given beta‐blockers as rescue therapy (Abel 1983; Bert 2001; Connolly 2003; Daudon 1986; Evrard 2000; Hammon 1984; Silverman 1982). This did not alter the interpretation of the effect for this outcome.
Cerebrovascular events: we excluded two studies in which participants in the control group were given beta‐blockers as rescue therapy (Connolly 2003; Sezai 2011). This did not alter the interpretation of the effect for this outcome.
Ventricular arrhythmias: we excluded four studies in which participants in the control group were given beta‐blockers as rescue therapy (Abel 1983; Connolly 2003; Hammon 1984; Pfisterer 1997). This did not alter the interpretation of the effect for this outcome.
Atrial fibrillation or flutter, or both: we excluded nine studies in which participants in the control group were given beta‐blockers as rescue therapy (Abel 1983; Connolly 2003; Daudon 1986; Evrard 2000; Janssen 1986; Pfisterer 1997; Salazar 1979; Sezai 2011; Silverman 1982). This did not alter the interpretation of the effect for this outcome.
Bradycardia: we excluded two studies in which participants in the control group were given beta‐blockers as rescue therapy (Hammon 1984; Pfisterer 1997). This did not alter the interpretation of the effect for this outcome.
Hypotension: we excluded one study in which participants in the control group were given beta‐blockers as rescue therapy (Pfisterer 1997). This did not alter the interpretation of the effect for this outcome.
Studies published before 2000
Early all‐cause mortality (30 days): we included in analysis only studies published since 2000 (Auer 2004; Bert 2001; Connolly 2003; De Azevedo Lúcio 2003; Evrard 2000; Forlani 2002; Matsuura 2001; Neto 2013; Sezai 2011; Sezai 2012; Skiba 2013; Yazicioglu 2002). This did not alter the interpretation of the effect for this outcome.
Acute myocardial infarction: we included in analysis only studies published since 2000 (Bert 2001; Connolly 2003; Evrard 2000; Forlani 2002; Serruys 2000; Sezai 2012). This did not alter the interpretation of the effect for this outcome.
Cerebrovascular events: only one study was published before 2000, and we excluded this study (Matangi 1989). This did not alter the interpretation of the effect for this outcome.
Ventricular arrhythmias: we included in analysis only studies published since 2000 (Auer 2004; Connolly 2003; Sun 2011). This did not alter the interpretation of the effect for this outcome.
Atrial fibrillation or flutter, or both: we included in analysis only studies published since 2000 (Auer 2004; Connolly 2003; De Azevedo Lúcio 2003; Evrard 2000; Forlani 2002; Liu 2016; Matsuura 2001; Neto 2013; Ogawa 2013; Osada 2012; Sakaguchi 2012; Sezai 2011; Sezai 2012; Skiba 2013; Sun 2011; Yazicioglu 2002). This did not alter the interpretation of the effect for this outcome.
Bradycardia: we included in analysis only studies published since 2000 (Auer 2004; But 2006; Ogawa 2013; Serruys 2000; Sezai 2012). Whilst the effect continued to show little or no difference between groups in the incidence of bradycardia, we noted that the CI was much wider when analysis included fewer and only more recently published studies (RR 3.11, 95% CI 0.81 to 11.95).
Hypotension: we included in analysis only studies published since 2000 (Auer 2004; But 2006; Serruys 2000; Sezai 2012). Whilst the effect continued to show little or no difference between groups in the incidence of hypotension, we noted that the CI was much wider when analysis included fewer and only more recently published studies (RR 1.95, 95% CI 0.35 to 10.99).
Outcomes with rare events
In studies reporting all‐cause mortality, fewer than 1% participants had an event within 30 days. In sensitivity analysis, we used Peto odds ratio. This did not alter the interpretation of the effect.
Discussion
Summary of main results
We found 63 studies in which beta‐blockers were given during the perioperative period to adults undergoing cardiac surgery. In addition, we identified four studies awaiting classification (two are reported only as an abstract and two are completed trials; we await publication of the full reports for these studies) and two ongoing studies.
We found low‐certainty evidence that beta‐blockers make little or no difference to all‐cause mortality within 30 days, however, we found few deaths in the control and the beta‐blocker groups. We found no evidence of a difference in the number of people who have an acute myocardial infarction (low‐certainty evidence). We were uncertain whether these agents make little or no difference to cerebrovascular events because we assessed the certainty of the evidence to be very low. We found low‐certainty evidence that taking beta‐blockers perioperatively may reduce ventricular arrhythmias and atrial fibrillation or flutter. Beta‐blockers may make little or no difference to hypotension or bradycardia; although we assessed the evidence for these outcomes to be low certainty.
We found little or no difference in long‐term mortality from two studies, as well as little or no difference in death due to cardiac causes in four studies. Similarly, we found little or no difference in the number of people who had congestive failure in three studies. However, we assessed the certainty of the evidence for these outcomes to be very low. We found low‐certainty evidence that length of stay was shorter when participants were treated with beta‐blockers but analysis identified moderate statistical heterogeneity; we did not explore reasons for this heterogeneity through further subgroup analysis. No studies assessed quality of life.
Overall completeness and applicability of evidence
We identified 63 studies with 7768 participants, of which ten were identified in the most recent search. All studies were in adult participants undergoing cardiac surgery.
Because of the type of surgery, we assumed that all participants were at high risk of cardiac complications. We noted differences between studies with regard to whether participants were taking beta‐blockers preoperatively, and previous history of conditions such as hypertension or myocardial infarctions. However, these differences would be consistent with a general population of people scheduled to undergo cardiac surgery.
We included in the review several studies published prior to 2000, and these studies may have used clinical management strategies that differ from current practice. We explored this in sensitivity analysis but found no changes to the interpretation of the effect for the main review outcomes when we included only studies published since 2000.
For most outcomes, we noted little evidence of heterogeneity in the effects. Exploration of subgroups according to the type of beta‐blocker or whether the intervention was given before, during, or after surgery did not strongly demonstrate that these differences were likely to influence the results.
Quality of the evidence
We used GRADE to assess the certainty of the evidence. In a previous version of the review (Blessberger 2018), we calculated optimal information sizes for each outcome and we used these calculations in the current update to assess that we had insufficient numbers of participants for our main outcomes (except for atrial fibrillation). In addition, we noted a wide confidence interval for the effects in some outcomes. Subsequently, we downgraded the certainty of the evidence owing to imprecision.
In general, we found that very few included studies had used adequate reporting methods to describe methods of sequence generation and allocation concealment, and none of the included studies were prospectively registered with clinical trial registers. Many studies compared perioperative beta‐blockers with standard care rather than with a placebo and this open‐label study design introduced performance bias which was not present in most of the placebo‐controlled trials. We noted that some studies allowed clinicians to use beta‐blockers to treat arrhythmias regardless of the study group to which participants were allocated; therefore, in some studies participants in the control group received beta‐blockers as rescue therapy during the study period. We downgraded the certainty of the evidence owing to these study limitations.
Potential biases in the review process
Our previous review assessed beta‐blockers in both cardiac and non‐cardiac surgery (Blessberger 2018); we split this review into two reviews according to type of surgery. This version incorporates data from studies of cardiac surgery and includes an updated search. We conducted a thorough search in the update and used two review authors to assess study eligibility, extract data, and assess risk of bias in included studies; therefore, we reduced potential bias in the review process.
During the updating process, we made changes to the review to meet current Cochrane standards. This included minor clarifications to the inclusion criteria of the review, and changes to the wording of the sections of the methods within Data collection and analysis. In particular, this version of the review includes fewer outcomes. Data for myocardial ischaemia, supraventricular arrhythmias (except atrial fibrillation or flutter), bronchospasm and cost of care are reported only in the previous version (Blessberger 2018). In reducing the number of outcomes, our intention was to improve the usability of the review; therefore, we selected outcomes that we considered to be most important to users of the review. We used the work of Myles and colleagues to support this decision‐making process (Myles 2016), and sought advice from a Cochrane Editor before agreeing to a reduction in outcomes.
In addition, we did not include meta‐regression as a method to explore heterogeneity in this review. We explored heterogeneity only through subgroup analysis that we decided in the review protocol. We conducted analyses using a random‐effects model, rather than choosing the effects model based on statistical heterogeneity (Borenstein 2010); we evaluated in sensitivity analysis the effect of using Peto odds ratio for rare events, which uses a fixed‐effect model. Again, we made these decisions following advice from the Cochrane Editorial team.
We included only trials that investigated at least one outcome specified in the Methods section. Because of the vast scope of the literature search and the large number of search results and included trials, this approach was justified in order to improve the management of the review. Whilst this may have introduced a source of bias, we judged the impact of potentially missing studies as small because the available set of data was derived from a large number of trials.
Whilst we attempted to collect definitions of outcomes from study reports (e.g. for hypotension), we could not account for the variation in individual clinical management of participants in which agents such as vasopressors or inotropes may affect outcome definition. We also found that some studies did not specify a definition of all outcomes.
Furthermore, we could not contact all study authors to gather further information about trial design or data analysis (e.g. performance of an intention‐to‐treat analysis) because of the large number of studies. However, it is likely that study authors, if contacted, may over report trial design quality criteria.
Agreements and disagreements with other studies or reviews
The addition of 10 new studies in this update did not alter the interpretation of our findings; these results are consistent with those reported for cardiac surgery in the previous version of this review (Blessberger 2018). They are also largely consistent with those of other systematic reviews which include both RCTs and observational studies (Crystal 2002; Tamura 2017; Thein 2018; Wang 2018).
We identified moderate statistical heterogeneity in the effect estimate for atrial fibrillation or flutter, and this heterogeneity was not present in most of our other outcomes. We were unable to explain this inconsistency in the data, and noted that another large systematic review similarly reported unexplained heterogeneity for this outcome (Crystal 2002).
A reduction in heart rate and blood pressure are inherent properties of beta‐blockers, and it could be argued that it is expected that use of beta‐blockers during the perioperative period would induce bradycardia and hypotension. However, people undergoing cardiac surgery, in particular, are likely to be under stricter controls to keep haemodynamic variables stable within a small range; we could not always ascertain whether and how participants were managed in all studies and could not be certain whether other clinical management may have influenced the results for bradycardia and hypotension.
Although we used subgroup analysis to assess the effects of the type of beta‐blocker or the time at which the treatment was initiated, we were not confident in the findings from this analysis. Any differences between the primary analyses and subgroup analyses were in subgroups for which there were very few studies and participants and subsequently, the effect was much less precise.
Authors' conclusions
Implications for practice.
We found no evidence of a difference in early all‐cause mortality, myocardial infarction, cerebrovascular events, hypotension and bradycardia. However, our evidence showed a reduction in atrial fibrillation when beta‐blockers were used, and they probably also reduce ventricular arrhythmias. A larger sample size would increase the certainty of this evidence. Four studies awaiting classification may alter the conclusion of this review.
Implications for research.
In order to increase the certainty in the effects for most outcomes in this review, a larger sample is required. More research in this field would also enable a more precise exploration of subgroups. In particular, the timing of beta‐blocker application may have an important influence on their effect (plaque stabilization, haemodynamic adaptation).
We noted that no studies measured quality of life. From a patient perspective, this is likely an important outcome, and from a study investigator perspective, evaluation can be conducted easily using established questionnaires. We suggest that future trials should also focus their attention on this endpoint.
The review assessed only direct comparisons. Given the different types of beta‐blockers, and continuing research that we expect to contribute to this field, a network meta‐analysis should be considered in future review updates. A network meta‐analysis would explore indirect comparisons, as well as direct comparisons, and may be useful to clinicians by providing a more comprehensive analysis of the effects of each type of beta‐blocker, and further improve the precision of the estimates.
What's new
| Date | Event | Description |
|---|---|---|
| 7 October 2019 | Amended | Complete reference to companion review (Blessberger 2019) added. It was in progress at the time of initial publication. |
History
Review first published: Issue 9, 2019
| Date | Event | Description |
|---|---|---|
| 1 September 2019 | New search has been performed |
|
| 1 September 2019 | New citation required but conclusions have not changed |
|
| 22 February 2018 | New citation required but conclusions have not changed | Removal of retracted study in non‐cardiac surgery (Suttner 2009); conclusions unchanged. |
| 22 February 2018 | Amended | Following publication of the first version of this systematic review, a previously included study was retracted (Suttner 2009). This trial has now been excluded and all relevant analyses and results have been amended accordingly. All NNTB/Hs were recalculated according to the formula in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Guideline recommendations were updated in the Discussion section. |
Notes
None.
Acknowledgements
We would like to thank the authors involved in previous versions of the review (Danyel Azar, Martin Schillinger, Franz Wiesbauer), people who offered editorial support or advice in preparation of the protocol (M. Müllner, L. Richard, K. Schroeder and M.J. Zeitler), or editorial support in previous versions of the review (Jane Cracknell, Managing Editor; Harald Herkner, Content Editor; Cathal Walsh, Statistical Editor; Janet Wale, Consumer Editor). We thank peer review support in previous versions of the review (G. Guyatt, F. Botto, G. Lurati, M. Mrkobrada, P. Foex and J. Wetterslev), and assistance with translation of articles in Chinese (Y. Wang; formally of Linz General Hospital, Austria).
We would like to thank Anna Lee (Content Editor), Susanne Schmitz (Statistical Editor), Ben Gibbison (Peer Reviewer), Janet Wale (Consumer Editor), Janne Vendt (Information Specialist), Jane Cracknell and Teo Quay (Managing Editors), and Andrew Smith (Co‐ordinating Editor) for their help and editorial advice during the preparation of this systematic review.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Adrenergic beta‐Antagonists] explode all trees
#2 MeSH descriptor: [Bendroflumethiazide] explode all trees
#3 (acebutolol* or adimolol* or afurolol* or alpha hydroxymetoprolol* or alprenolol* or amosulalol* or arotinolol* or atenolol* or befunolol* or bendroflumethiazide* or betaxolol* or bevantolol* or bfe 55 or bisoprolol* or bopindolol* or bornaprolol* or brefonalol* or bromoacetylalprenololmenthane* or bucindolol* or bucumolol* or bufetolol* or bufuralol* or bunitrolol* or bunolol* or bupranolol* or butofilolol* or butoxamine* or carazolol* or carteolol* or carvedilol* or celiprolol* or cetamolol* or cloranolol* or cyanoiodopindolol* or cyanopindolol* or deacetylmetipranolol* or diacetolol* or dihydroalprenolol* or dilevalol* or epanolol* or esmolol* or exaprolol* or falintolol* or flestolol* or flusoxolol* or hydroxybenzylpindolol* or indenolol* or iodocyanopindolol* or iodopindolol* or iprocrolol* or isamoltane* or isoxaprolol* or labetalol* or landiolol* or levobunolol* or levomoprolol* or medroxalol* or mepindolol* or mercuderamide* or metipranolol* or metoprolol* or moprolol* or nadolol* or nebivolol* or nifenalol* or nipradilol* or oxprenolol* or pafenolol* or pamatolol* or penbutolol* or pindolol* or practolol* or primidolol* or prizidilol* or pronetalol* or propranolol* or proxodolol* or ridazolol* or salcardolol* or sandoz 204545 or soquinolol* or sotalol* or spirendolol* or talinolol* or tertatolol* or tienoxolol* or tilisolol* or timolol* or tolamolol* or toliprolol* or trasitensin* or trepress* or tribendilol* or viskaldix* or xibenolol* or zolertine or adaprolol* or bamosiran* or bendacalol* or dramedilol* or ersentilide* or oberadilol* or procinolol* or zoleprodolol*):ti,ab,kw
#4 (beta* near (adrenergic* or blocker* or blockade* or blocking)):ti,ab,kw
#5 (#1 OR #2 OR #3 OR #4)
#6 MeSH descriptor: [Premedication] explode all trees
#7 MeSH descriptor: [Preanesthetic Medication] explode all trees
#8 MeSH descriptor: [Intraoperative Period] explode all trees
#9 MeSH descriptor: [Intraoperative Complications] explode all trees
#10 MeSH descriptor: [Perioperative Period] explode all trees
#11 MeSH descriptor: [Postoperative Complications] explode all trees
#12 MeSH descriptor: [Postoperative Period] explode all trees
#13 MeSH descriptor: [Preoperative Period] explode all trees
#14 (intraoperat* or perioperat* or peroperat* or postoperat* or preoperat* or premedicat* or intra operat* or peri operat* or per operat* or post operat* or pre operat* or pre medicat*):ti,ab,kw or ((during or before or prior or undergo* or following or after) NEAR/3 (surg* or operat* or infusion* or procedur* or bypass or intubat* or anaesth* or anesth*)):ti,ab,kw
#15 (#6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14)
#16 MeSH descriptor: [Specialties, Surgical] explode all trees
#17 MeSH descriptor: [Anesthesia, General] explode all trees
#18 MeSH descriptor: [Coronary Artery Bypass] explode all trees
#19 (surg* or operat* or anesth* or anaesth* or bypass):ti,ab,kw
#20 (#16 OR #17 OR #18 OR #19)
#21 (#5 AND #15 AND #20)
#22 #21 in Trials
Appendix 2. MEDLINE search strategy
exp Adrenergic beta‐Antagonists/ or exp Bendroflumethiazide/ or (acebutolol* or adimolol* or afurolol* or alpha hydroxymetoprolol* or alprenolol* or amosulalol* or arotinolol* or atenolol* or befunolol* or bendroflumethiazide* or betaxolol* or bevantolol* or bfe 55 or bisoprolol* or bopindolol* or bornaprolol* or brefonalol* or bromoacetylalprenololmenthane* or bucindolol* or bucumolol* or bufetolol* or bufuralol* or bunitrolol* or bunolol* or bupranolol* or butofilolol* or butoxamine* or carazolol* or carteolol* or carvedilol* or celiprolol* or cetamolol* or cloranolol* or cyanoiodopindolol* or cyanopindolol* or deacetylmetipranolol* or diacetolol* or dihydroalprenolol* or dilevalol* or epanolol* or esmolol* or exaprolol* or falintolol* or flestolol* or flusoxolol* or hydroxybenzylpindolol* or indenolol* or iodocyanopindolol* or iodopindolol* or iprocrolol* or isamoltane* or isoxaprolol* or labetalol* or landiolol* or levobunolol* or levomoprolol* or medroxalol* or mepindolol* or mercuderamide* or metipranolol* or metoprolol* or moprolol* or nadolol* or nebivolol* or nifenalol* or nipradilol* or oxprenolol* or pafenolol* or pamatolol* or penbutolol* or pindolol* or practolol* or primidolol* or prizidilol* or pronetalol* or propranolol* or proxodolol* or ridazolol* or salcardolol* or sandoz 204545 or soquinolol* or sotalol* or spirendolol* or talinolol* or tertatolol* or tienoxolol* or tilisolol* or timolol* or tolamolol* or toliprolol* or trasitensin* or trepress* or tribendilol* or viskaldix* or xibenolol* or zolertine or adaprolol* or bamosiran* or bendacalol* or dramedilol* or ersentilide* or oberadilol* or procinolol* or zoleprodolol* or (beta* adj3 (adrenergic* or blocker* or blockade* or blocking))).ti,ab,kf.
exp Premedication/ or Preanesthetic Medication/ or Intraoperative Period/ or Intraoperative Complications/ or Perioperative Period/ or Postoperative Complications/ or Postoperative Period/ or Preoperative Period/ or (intraoperat* or perioperat* or peroperat* or postoperat* or preoperat* or premedicat* or intra operat* or peri operat* or per operat* or post operat* or pre operat* or pre medicat*).ti,ab,hw,kf. or ((during or before or prior or undergo* or following or after) adj3 (surg* or operat* or infusion* or intubat* or an?esth* or procedur* or bypass)).ti,ab,kf.
exp Specialties, Surgical/ or General Surgery/ or coronary artery bypass/ or su.xs. or (surg* or operat* or bypass).ti,ab,hw,kf. or exp Anesthesia, General/ or an?esth*.ti,ab,hw,kf.
((randomized controlled trial or controlled clinical trial).pt. or random*.ab. or placebo.ab. or clinical trials as topic.sh. or random allocation.sh. or trial.ti.) not (exp animals/ not humans.sh.)
1 and 2 and 3 and 4
Appendix 3. Embase search strategy
exp beta adrenergic receptor blocking agent/ or bendroflumethiazide/ or (acebutolol* or adimolol* or afurolol* or alpha hydroxymetoprolol* or alprenolol* or amosulalol* or arotinolol* or atenolol* or befunolol* or bendroflumethiazide* or betaxolol* or bevantolol* or bfe 55 or bisoprolol* or bopindolol* or bornaprolol* or brefonalol* or bromoacetylalprenololmenthane* or bucindolol* or bucumolol* or bufetolol* or bufuralol* or bunitrolol* or bunolol* or bupranolol* or butofilolol* or butoxamine* or carazolol* or carteolol* or carvedilol* or celiprolol* or cetamolol* or cloranolol* or cyanoiodopindolol* or cyanopindolol* or deacetylmetipranolol* or diacetolol* or dihydroalprenolol* or dilevalol* or epanolol* or esmolol* or exaprolol* or falintolol* or flestolol* or flusoxolol* or hydroxybenzylpindolol* or indenolol* or iodocyanopindolol* or iodopindolol* or iprocrolol* or isamoltane* or isoxaprolol* or labetalol* or landiolol* or levobunolol* or levomoprolol* or medroxalol* or mepindolol* or mercuderamide* or metipranolol* or metoprolol* or moprolol* or nadolol* or nebivolol* or nifenalol* or nipradilol* or oxprenolol* or pafenolol* or pamatolol* or penbutolol* or pindolol* or practolol* or primidolol* or prizidilol* or pronetalol* or propranolol* or proxodolol* or ridazolol* or salcardolol* or sandoz 204545 or soquinolol* or sotalol* or spirendolol* or talinolol* or tertatolol* or tienoxolol* or tilisolol* or timolol* or tolamolol* or toliprolol* or trasitensin* or trepress* or tribendilol* or viskaldix* or xibenolol* or zolertine or adaprolol* or bamosiran* or bendacalol* or dramedilol* or ersentilide* or oberadilol* or procinolol* or zoleprodolol* or (beta* adj3 (adrenergic* or blocker* or blockade* or blocking))).ti,ab,kw,hw.
premedication/ or intraoperative period/ or peroperative complication/ or perioperative period/ or postoperative complication/ or postoperative period/ or preoperative period/ or (intraoperat* or perioperat* or peroperat* or postoperat* or preoperat* or premedicat* or intra operat* or peri operat* or per operat* or post operat* or pre operat* or pre medicat*).ti,ab,hw,kw. or ((during or before or prior or undergo* or following or after) adj3 (surg* or operat* or infusion* or intubat* or an?esth* or procedur* or bypass)).ti,ab,kw.
exp surgery/ or exp general anesthesia/ or coronary artery bypass graft/ or (surg* or operat* or bypass or an?esth*).ti,ab,hw,kw.
(placebo.sh. or controlled study.ab. or random*.ti,ab. or trial*.ti,ab. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab.) not (animals not (humans and animals)).sh.
1 and 2 and 3 and 4
Appendix 4. CINAHL search strategy
S1 (MH "Adrenergic Beta‐Antagonists+") OR TX ( acebutolol* or adimolol* or afurolol* or alpha hydroxymetoprolol* or alprenolol* or amosulalol* or arotinolol* or atenolol* or befunolol* or bendroflumethiazide* or betaxolol* or bevantolol* or bfe 55 or bisoprolol* or bopindolol* or bornaprolol* or brefonalol* or bromoacetylalprenololmenthane* or bucindolol* or bucumolol* or bufetolol* or bufuralol* or bunitrolol* or bunolol* or bupranolol* or butofilolol* or butoxamine* or carazolol* or carteolol* or carvedilol* or celiprolol* or cetamolol* or cloranolol* or cyanoiodopindolol* or cyanopindolol* or deacetylmetipranolol* or diacetolol* or dihydroalprenolol* or dilevalol* or epanolol* or esmolol* or exaprolol* or falintolol* or flestolol* or flusoxolol* or hydroxybenzylpindolol* or indenolol* or iodocyanopindolol* or iodopindolol* or iprocrolol* or isamoltane* or isoxaprolol* or labetalol* or landiolol* or levobunolol* or levomoprolol* or medroxalol* or mepindolol* or mercuderamide* or metipranolol* or metoprolol* or moprolol* or nadolol* or nebivolol* or nifenalol* or nipradilol* or oxprenolol* or pafenolol* or pamatolol* or penbutolol* or pindolol* or practolol* or primidolol* or prizidilol* or pronetalol* or propranolol* or proxodolol* or ridazolol* or salcardolol* or sandoz 204545 or soquinolol* or sotalol* or spirendolol* or talinolol* or tertatolol* or tienoxolol* or tilisolol* or timolol* or tolamolol* or toliprolol* or trasitensin* or trepress* or tribendilol* or viskaldix* or xibenolol* or zolertine or adaprolol* or bamosiran* or bendacalol* or dramedilol* or ersentilide* or oberadilol* or procinolol* or zoleprodolol*) OR TX ( (beta* and (adrenergic* or blocker* or blocking or blockade*)))
S2 ((MH "Premedication") OR (MH "Intraoperative Period") OR (MH "Postoperative Period") OR (MH "Intraoperative Complications") OR (MH "Preoperative Period") OR (MH "Postoperative Complications")) OR TX ( intraoperat* or perioperat* or peroperat* or postoperat* or preoperat* or premedicat* or intra operat* or peri operat* or per operat* or post operat* or pre operat* or pre medicat*) or TX ((during or before or prior or undergo* or following or after) N3 (surg* or operat* or infusion* or intubat* or anaesth* or anesth* or procedur* or bypass))
S3 (MH "Specialties, Surgical+") OR (MH "Anesthesia, General+") OR (MH "Coronary Artery Bypass+") OR TX (surg* or operat* or bypass or an*esth*)
S4 S1 AND S2 AND S3
S5 ((MH "Randomized Controlled Trials") OR (MH "Clinical Trials+") OR (MH "Random Assignment") OR (MH "Prospective Studies+") OR (MH "Clinical Trial Registry") OR (MH "Double‐Blind Studies") OR (MH "Single‐Blind Studies") OR (MH "Triple‐Blind Studies") OR (MH "Multicenter Studies") OR (MH "Placebos")) OR TX (random* or placebo* or trial*)
S6 S4 AND S5
Appendix 5. BIOSIS Previews search strategy
#1 TS=(acebutolol* or adimolol* or afurolol* or alpha hydroxymetoprolol* or alprenolol* or amosulalol* or arotinolol* or atenolol* or befunolol* or bendroflumethiazide* or betaxolol* or bevantolol* or bfe 55 or bisoprolol* or bopindolol* or bornaprolol* or brefonalol* or bromoacetylalprenololmenthane* or bucindolol* or bucumolol* or bufetolol* or bufuralol* or bunitrolol* or bunolol* or bupranolol* or butofilolol* or butoxamine* or carazolol* or carteolol* or carvedilol* or celiprolol* or cetamolol* or cloranolol* or cyanoiodopindolol* or cyanopindolol* or deacetylmetipranolol* or diacetolol* or dihydroalprenolol* or dilevalol* or epanolol* or esmolol* or exaprolol* or falintolol* or flestolol* or flusoxolol* or hydroxybenzylpindolol* or indenolol* or iodocyanopindolol* or iodopindolol* or iprocrolol* or isamoltane* or isoxaprolol* or labetalol* or landiolol* or levobunolol* or levomoprolol* or medroxalol* or mepindolol* or mercuderamide* or metipranolol* or metoprolol* or moprolol* or nadolol* or nebivolol* or nifenalol* or nipradilol* or oxprenolol* or pafenolol* or pamatolol* or penbutolol* or pindolol* or practolol* or primidolol* or prizidilol* or pronetalol* or propranolol* or proxodolol* or ridazolol* or salcardolol* or sandoz 204545 or soquinolol* or sotalol* or spirendolol* or talinolol* or tertatolol* or tienoxolol* or tilisolol* or timolol* or tolamolol* or toliprolol* or trasitensin* or trepress* or tribendilol* or viskaldix* or xibenolol* or zolertine or adaprolol* or bamosiran* or bendacalol* or dramedilol* or ersentilide* or oberadilol* or procinolol* or zoleprodolol* or (beta* NEAR (adrenergic* or blocker* or blocking)))
#2 TS=(intraoperat* or perioperat* or peroperat* or postoperat* or preoperat* or premedicat* or “intra operat*” or “peri operat*” or “per operat*” or “post operat*” or “pre operat*” or “pre medicat*”) or TS= ((during or before or prior or undergo* or following or after) NEAR/3 (surg* or operat* or infusion* or procedur* or bypass or intubat* or anaesth* or anesth*))
#3 TS=(surg* or operat* or anesth* or anaesth* or bypass)
#4 TS=(placebo* or random* or control* or prospectiv* or volunteer* or (clin* NEAR/3 trial*) or (compar* NEAR/3 stud*) or ((singl* or doubl* or trebl* or tripl*) NEAR/3 (blind* or mask*)))
#5 #4 AND #3 AND #2 AND #1
Appendix 6. Web of Science search strategy
#1 TS=(acebutolol* or adimolol* or afurolol* or alpha hydroxymetoprolol* or alprenolol* or amosulalol* or arotinolol* or atenolol* or befunolol* or bendroflumethiazide* or betaxolol* or bevantolol* or bfe 55 or bisoprolol* or bopindolol* or bornaprolol* or brefonalol* or bromoacetylalprenololmenthane* or bucindolol* or bucumolol* or bufetolol* or bufuralol* or bunitrolol* or bunolol* or bupranolol* or butofilolol* or butoxamine* or carazolol* or carteolol* or carvedilol* or celiprolol* or cetamolol* or cloranolol* or cyanoiodopindolol* or cyanopindolol* or deacetylmetipranolol* or diacetolol* or dihydroalprenolol* or dilevalol* or epanolol* or esmolol* or exaprolol* or falintolol* or flestolol* or flusoxolol* or hydroxybenzylpindolol* or indenolol* or iodocyanopindolol* or iodopindolol* or iprocrolol* or isamoltane* or isoxaprolol* or labetalol* or landiolol* or levobunolol* or levomoprolol* or medroxalol* or mepindolol* or mercuderamide* or metipranolol* or metoprolol* or moprolol* or nadolol* or nebivolol* or nifenalol* or nipradilol* or oxprenolol* or pafenolol* or pamatolol* or penbutolol* or pindolol* or practolol* or primidolol* or prizidilol* or pronetalol* or propranolol* or proxodolol* or ridazolol* or salcardolol* or sandoz 204545 or soquinolol* or sotalol* or spirendolol* or talinolol* or tertatolol* or tienoxolol* or tilisolol* or timolol* or tolamolol* or toliprolol* or trasitensin* or trepress* or tribendilol* or viskaldix* or xibenolol* or zolertine or adaprolol* or bamosiran* or bendacalol* or dramedilol* or ersentilide* or oberadilol* or procinolol* or zoleprodolol* or (beta* NEAR (adrenergic* or blocker* or blocking)))
#2 TS=(intraoperat* or perioperat* or peroperat* or postoperat* or preoperat* or premedicat* or “intra operat*” or “peri operat*” or “per operat*” or “post operat*” or “pre operat*” or “pre medicat*”) or TS= ((during or before or prior or undergo* or following or after) NEAR/3 (surg* or operat* or infusion* or procedur* or bypass or intubat* or anaesth* or anesth*))
#3 TS=(surg* or operat* or anesth* or anaesth* or bypass)
#4 TS=(placebo* or random* or control* or prospectiv* or volunteer* or (clin* NEAR/3 trial*) or (compar* NEAR/3 stud*) or ((singl* or doubl* or trebl* or tripl*) NEAR/3 (blind* or mask*)))
#5 #4 AND #3 AND #2 AND #1
Appendix 7. Conference Proceedings Citation Index‐Science search strategy
#1 TS=(acebutolol* or adimolol* or afurolol* or alpha hydroxymetoprolol* or alprenolol* or amosulalol* or arotinolol* or atenolol* or befunolol* or bendroflumethiazide* or betaxolol* or bevantolol* or bfe 55 or bisoprolol* or bopindolol* or bornaprolol* or brefonalol* or bromoacetylalprenololmenthane* or bucindolol* or bucumolol* or bufetolol* or bufuralol* or bunitrolol* or bunolol* or bupranolol* or butofilolol* or butoxamine* or carazolol* or carteolol* or carvedilol* or celiprolol* or cetamolol* or cloranolol* or cyanoiodopindolol* or cyanopindolol* or deacetylmetipranolol* or diacetolol* or dihydroalprenolol* or dilevalol* or epanolol* or esmolol* or exaprolol* or falintolol* or flestolol* or flusoxolol* or hydroxybenzylpindolol* or indenolol* or iodocyanopindolol* or iodopindolol* or iprocrolol* or isamoltane* or isoxaprolol* or labetalol* or landiolol* or levobunolol* or levomoprolol* or medroxalol* or mepindolol* or mercuderamide* or metipranolol* or metoprolol* or moprolol* or nadolol* or nebivolol* or nifenalol* or nipradilol* or oxprenolol* or pafenolol* or pamatolol* or penbutolol* or pindolol* or practolol* or primidolol* or prizidilol* or pronetalol* or propranolol* or proxodolol* or ridazolol* or salcardolol* or sandoz 204545 or soquinolol* or sotalol* or spirendolol* or talinolol* or tertatolol* or tienoxolol* or tilisolol* or timolol* or tolamolol* or toliprolol* or trasitensin* or trepress* or tribendilol* or viskaldix* or xibenolol* or zolertine or adaprolol* or bamosiran* or bendacalol* or dramedilol* or ersentilide* or oberadilol* or procinolol* or zoleprodolol* or (beta* NEAR (adrenergic* or blocker* or blocking)))
#2 TS=(intraoperat* or perioperat* or peroperat* or postoperat* or preoperat* or premedicat* or “intra operat*” or “peri operat*” or “per operat*” or “post operat*” or “pre operat*” or “pre medicat*”) or TS= ((during or before or prior or undergo* or following or after) NEAR/3 (surg* or operat* or infusion* or procedur* or bypass or intubat* or anaesth* or anesth*))
#3 TS=(surg* or operat* or anesth* or anaesth* or bypass)
#4 TS=(placebo* or random* or control* or prospectiv* or volunteer* or (clin* NEAR/3 trial*) or (compar* NEAR/3 stud*) or ((singl* or doubl* or trebl* or tripl*) NEAR/3 (blind* or mask*)))
#5 #4 AND #3 AND #2 AND #1
Appendix 8. Data extraction form
| Completed by: | |
| Date completed: | |
| Study ID | |
| Methods | |
| Participants |
Total number of randomized participants: Inclusion criteria: Exclusion criteria: Type of surgery: Baseline characteristics Intervention group
Control group
Country: Setting: |
| Interventions | Intervention group
Control group
|
| Outcomes |
Outcomes measured/reported by study authors: Outcomes relevant to the review: |
| Notes |
Funding/declarations of interest: Study dates: Notes: |
Outcome data
(Complete tables for all available relevant outcome data)
| Name of outcome: | |
| Time point of measurement: | |
| Intervention group | |
| Number of events | Total number of participants in the group |
| Control group | |
| Number of events | Total number of participants in the group |
| Name of outcome: | Length of stay | |
| Intervention group | ||
| Mean | SD | Total number of participants in the group |
| Control group | ||
| Mean | SD | Total number of participants in the group |
Risk of bias table
| Domain | High/Low/ Unclear |
Judgement |
| Random sequence generation (selection bias) | ||
| Allocation concealment (selection bias) |
||
| Blinding of participants and personnel (performance bias) | ||
| Blinding of outcome assessors (detection bias) | ||
| Incomplete outcome data (attrition bias) | ||
| Selective reporting (reporting bias) |
||
| Other bias |
Appendix 9. Summary of risk factors in included studies
| Study ID | Age in years, mean (SD)a,b |
Gender, M/F, nb |
Previous MI, %*b |
History of hypertension, %b |
Ejection fraction, mean (SD)* as %b |
Preoperative beta‐blockers, %b |
| Abel 1983 | I: 56.8 (SEM ± 1.3) C: 56.4 (SEM ± 1.2) |
I: 44/6 C: 39/11 |
NR | NR | NR | NR |
| Ali 1997 | I: 65.1 (± 8.4) C: 63.3 (± 7.2) |
I: 74/31 C: 69/36 |
NR | NR | I: 59 (± 14) C: 56 (± 8) | 100 |
| Arar 2007 | I: 57.46 (± 8.03) C: 59.18 (± 9.91) |
I: 17/23 C:16/24 |
NR | NR | Overall: > 40 | 100 |
| Auer 2004 | I: 68 (± 9) I: 66 (± 10) C: 63 (± 12) |
I: 37/25 I: 40/23 C: 38/27 |
I: 21 I: 15.9 C: 15.4 |
I: 66.1 I: 66.7 C: 55.4 |
I: 69 (± 9) I: 69 (± 9) C: 68 (± 8) |
I: 38.7 I: 39.7 C: 33.8 |
| Babin‐Ebell 1996 | I: 61.4 (± 8.7) C: 64.3 (± 9.1) |
I: 25/8 C: 31/6 |
I: 57 C: 35 |
I: 66 C: 49 |
Overall: > 40 | I: 61 C: 65 |
| Bert 2001 | I: 63.8 (± 10.7) C: 63.6 (± 9.6) |
I: 54/17 C: 50/10 |
NR | NR | I: 49 (± 10) C: 49 (± 11) |
I: 76.1 C: 71.7 |
| Bignami 2017 | I: 62 (± 10.8) C: 63 (± 14.5) |
I: 18/3 C: 25/5 |
NR | I: 57.1 C: 46.7 |
I: 37 (± 7.1) C: 38 (± 8.9) |
I: 61.9 C: 50 |
| Booth 2004 | I: 64 (± 1.6) C: 59 (± 1.7) |
I: 21/12 C: 25/14 |
NR | NR | I: 52 (± 2) C: 56 (± 2) |
I: 59 C: 63 |
| But 2006 | I: 58 (± 6) C: 57 (± 5) |
I: 8/7 C: 6/9 |
I: 46.7 C: 33.3 |
I: 33.3 C: 40 |
I: 49 (± 5) C: 51 (± 5) |
I: 26.7 C: 20 |
| Connolly 2003 | I: 63 (± 10) C: 62 (± 10) |
I: 390/10 C: 400/10 |
NR | NR | NR | I: 82 C: 79 |
| Cork 1995 | I: 60 (± 2.7) C: 63.2 (± 2.1) |
I: 11/5 C: 8/6 |
NR | NR | I: 51.3 (± 4.9) C: 57.6 (± 4.0) |
I: 37.5 C: 7.1 |
| Daudon 1986 | I: 51.8 (± 8) C: 56 (± 9) |
I: 49/1 C: 46/4 |
NR | NR | I: 59 (± 12) C: 61 (± 11) |
I: 74 C: 70 |
| De Azevedo Lúcio 2003 | I: 59 (± 10) C: 62 (± 11) |
I: 72/28 C: 74/26 |
I: 42 C: 42 |
I: 59 C: 63 |
I: > 0.50 (85% participants); 0.35 to 0.50 (15% participants) C: > 0.50 (82% participants); 0.35 to 0.50 (17% participants) |
I: 65 C: 63 |
| Dy 1998 | NR | NR | NR | NR | Overall: < 30 | NR |
| Evrard 2000 | I: 61 (± 9) C: 61 (± 9) |
I: 92/11 C: 92/11 |
I: 38 C: 40 |
NR | I: 61 (± 13) C: 60 (± 12) |
I: 67 C: 68 |
| Forlani 2002 | I: 64 (± 10) C: 64 (± 9) |
I: 42/9 C: 44/6 |
I: 51 C: 65 |
I: 72 C: 62 |
I: 54.7 (± 9.5) C: 54.6 (± 9.5) |
I: 45 C: 38 |
| Gandhi 2007 | I: 59.14 (± 1.12) C: 56.96 (± 1.09) |
I: 66/1 C: 68/6 |
I: 61.2 C: 56.8 |
NR | Overall: ≤ 30 | NR |
| Girard 1986 | I: 62 (± 9) C: 59 (± 5) |
I: 8/1 C: 4/4 |
I: 44.4 C: 62.5 |
I: 22.2 C: 62.5 |
I: 65 (± 14) C: 61 (± 23) |
I: 44.4 C: 62.5 |
| Gomes 1999 | I: 61 (± 10) C: 69 (± 10) |
I: 27/13 C: 28/17 |
NR | NR | I: 50 (± 9) C: 48 (± 9) |
I: 20 C: 46.6 |
| Graham 1996 | NR | NR | NR | NR | NR | NR |
| Hammon 1984 | NR | NR | NR | NR | NR | NR |
| Harrison 1987 | I: 56.7 (± 2.06) C: 56.0 (± 2.16) |
I: 13/2 C: 14/1 |
NR | NR | NR | NR |
| Ivey 1983 | I: 54.4 (± 9.7) C: 59.1 (± 10) |
NR | NR | NR | Overall: > 40 | Overall: 100 |
| Jacquet 1994 | I: 59.3 (± 9.2) C: 61.7 (± 6) |
I: 21/4 C: 16/1 |
I: 36 C: 52.9 |
NR | I: 56 (± 11.5) C: 60.3 (± 13.7) |
I: 64 C: 52.9 |
| Janssen 1986 | I: 58 (range 31 to 74) C: 57.5 (range 37 to 68) |
I: 34/7 C: 31/8 |
I: 26.8 C: 41 |
NR | Overall: > 30 | NR |
| Khuri 1987 | I: 60.4 (± 0.8) C: 59.5 (± 1.0) |
NR | I: 32.8 C: 37.8 |
NR | NR | I: 86.6 C: 93.2 |
| Kurian 2001 | I: 60.2 (± 6.69) C: 61.1 (± 7.47) |
I: 25/6 C: 33/4 |
NR | NR | NR | NR |
| Lamb 1988 | I: 52.7 (± 7.8) C: 57.1 (± 7.3) |
I: 27/3 C: 25/5 |
I: 60 C: 63.3 |
NR | Overall: > 40 | I: 53.3 C: 46.7 |
| Liu 2016 | I: 58.9 (± 9.8) C: 62.1 (± 7.1) |
I: 8/4 C: 6/6 |
Overall, within 4 weeks: 0 | I: 66.7 C: 66.7 |
I: 52.7 (± 6) C: 55.8 (± 3.2) |
I: 8.3 C: 0 |
| Martinussen 1988 | I: 57 (±.1.1) C: 54 (± 0.9) |
I: 42/10 C: 47/9 |
Mean (SD) per participant I: 0.8 (± 0.1) C: 0.9 (0.1) |
NR | I: 68.4 (± 1.9) C: 63.0 (± 2.0) |
I: 50 C: 57 |
| Matangi 1985 | I: 54.6 (± 9.3) C: 55.7 (± 9.9) |
I: 67/15 C: 63/19 |
I: 50 C: 65.9 |
I: 41/82 C: 44/82 |
Overall: > 35 | Overall: 100 |
| Matangi 1989 | I: 58.9 (± 8.1) C: 59.4 (± 8.6) |
I: 28/7 C: 27/8 |
I: 54.9 C: 48.6 |
I: 45.7 C: 42.9 |
I: 64.6 (± 9.2) C: 64.5 (± 8.8) |
I: 77.1 C: 65.7 |
| Materne 1985 | I: 55.1 (± 7.6) C: 57.9 (± 6.9) |
I: 28/4 C: 32/7 |
NR | NR | Overall > 40 | I: 65.6 C: 58.9 |
| Matsuura 2001 | I: 62 (± 10) C: 60 (± 9) |
I: 32/8 C: 33/7 |
NR | I: 55 C: 50 |
I: 56 (± 15) C: 55 (± 14) |
I: 50 C: 40 |
| Mohr 1981 | I: 56 (range 38 to 71) C: 57 (range 37 to 72) |
I: 33/4 C: 39/9 |
NR | I: 48.6 C: 37.5 |
I: 50% ‐ 17; 35% to 50% ‐ 16; 30% to 35% ‐ 4 C: 50% ‐ 19; 35% to 50% ‐ 23; 30% to 35% ‐ 6 |
Overall: 100 |
| Myhre 1984 | I: 53 (± 8.9) C: 59 (± 8.5) | I: 15/5 C: 17/3 |
NR | NR | I: 67.4 (± 11.6) C: 65.4 (± 16.1) |
Overall: 100 |
| Neto 2013 | I: 57.9 (± 1.4) C: 59 (± 1.7) |
NR | Overall, within 30 days: 0 | NR | I: 66.3 (SEM ± 1.1) C: 64 (SEM ± 1) |
Overall: 0 |
| Neustein 1994 | I: 68 (± 8) C: 61 (± 12) |
I: 12/5 C: 15/8 |
NR | NR | NR | Overall: 0 |
| Nicolson 1990 | I: 58.2 (± 2) C: 61.3 (± 3) |
I: 14/3 C: 15/2 |
NR | NR | Overall: > 45 | I: 23.5 C: 17.6 |
| Nyström 1993 | I: 59 (range 33 to 75) C: 60 (range 43 to 71) |
I: 45/5 C: 43/8 |
Mean (SD) per participant: I: 1 (± 1.3) C: 1 (± 0.9) |
NR | I: 60 (± 10) C: 60 (± 20) |
I: 84 C: 78.4 |
| Ogawa 2013 | I: 69.3 (± 6.3) C: 71.6 (± 7.8) |
I: 49/19 C: 56/12 |
I: 42.6 C: 54.4 |
I: 67.6 C: 76.5 |
I: 59.6 (± 11.5) C: 53.9 (± 11.9) |
I: 50 C: 22 |
| Oka 1980 | I: 56 (± 2) C: 56 (± 2) C: 55 (± 2) |
I: 11/8 C: 11/6 C: 12/6 |
I: 26 C: 23 C: 28 |
I: 32 C: 41 C: 28 |
NR | Overall: 100 |
| Ormerod 1984 | I: 54.9 C: 51.8 |
I: 23/4 C: 30/3 |
I: 44.4 C: 36.4 |
NR | Overall: > 40 | NR |
| Osada 2012 | NR | NR | NR | NR | NR | NR |
| Paull 1997 | NR | NR | NR | NR | Overall: 30 | NR |
| Pfisterer 1997 | I: 61 (± 9) C: 60 (± 9) |
I: 101/9 C: 94/16 |
I: 58 C: 60 |
I: 50 C: 58 |
I: 61 (± 10) C: 60 (± 13) |
I: 82 C: 72 |
| Reves 1990 | I: 57 (± 9) C: 55 (± 10) |
I: 14/2 C: 13/1 |
NR | NR | I: 55 (± 12) C: 48 (± 15) |
Overall: 100 |
| Rubin 1987 | I: 55.0 (± 8.6) C: 55.8 (± 2) |
NR | NR | I: 37.8 C: 72.5 |
Overall: > 50 | I: 75.7 C: 72.5 |
| Sakaguchi 2012 | I: 69.3 (± 8.6) C: 68.7 (± 10) |
I: 15/15 C: 17/13 |
NR | I: 70 C: 63.3 |
I: 57.5 (± 9.3) C: 60.5 (± 7.6) |
I: 3.3 C: 10 |
| Salazar 1979 | NR | NR | NR | NR | NR | Overall: 100 |
| Serruys 2000 | I: 57.9 (± 10.0) C: 58.6 (± 9.7) |
I: 147/22 C: 137/18 |
I: 49.7 C: 36.1 |
I: 29.6 C: 27.7 |
NR | I: 62.1 C: 62.6 |
| Sezai 2011 | I: 68.5 (± 4.7) C: 66.7 (± 8.9) |
I: 62/8 C: 66/4 |
I: 47.1 C: 45.7 |
I: 82.9 C: 71.4 |
I: 54.5 (± 14.2) C: 55.6 (± 13.5) |
I: 24.3 C: 35.7 |
| Sezai 2012 | I: 68.5 (± 9.6) I: 68.1 (± 8.2) C: 68.2 (± 7.5) |
I: 26/8 I: 26/7 C: 30/4 |
I: 35.3 I: 48.5 C: 38.2 |
I: 76.5 I: 82.4 C: 82.4 |
I: 60.4 (± 10.1) I: 53.9 (± 14.5) C: 60.0 (± 13.6) |
I: 26.5 I: 21.1 C: 26.5 |
| Silverman 1982 | I: 55.2 (± 1.7) C: 58.2 (± 1.5) |
I: 48/2 C: 45/5 |
I: 56 C: 64 |
I: 44 C: 54 |
NR | Overall: 100 |
| Skiba 2013 | I: 67.5 (± 1.8) C: 63.3 (± 1.2) |
I: 56/19 C: 60/13 |
I: 28 C: 26 |
I: 57 C: 60 |
Overall: > 30 | I: 17.3 C: 5.5 |
| Stephenson 1980 | I: 54 C: 56 |
I: 80/7 C: 122/14 |
NR | NR | NR | I: 72.4 C: 63.2 |
| Sun 2011 | NR | NR | NR | NR | NR | NR |
| Suttorp 1991 | I: 62 (± 8.4) C: 62 (± 9.5) |
I: 121/29 C: 113/37 |
I: 50 C: 51.3 |
NR | Overall: > 40% | I: 78.7 C: 72 |
| Vecht 1986 | I: 54.2 (range 38‐70) C: 54.0 (range 34‐71) |
I: 60/6 C: 61/5 |
NR | NR | NR | I: 86.4 C: 83.3 |
| Wenke 1999 | I: 63.17 (± 9.2) C: 6.9 (± 9.5) |
I: 79/21 C: 75/25 |
NR | NR | I: 63.4 (± 13.1) C: 62.2 (± 14.0) |
I: 61 C: 56 |
| White 1984 | I: 55 (± 9) C: 56 (± 10) |
I: 17/4 C: 17/3 |
NR | NR | I: 63 (± 11) C: 62 (± 12) |
I: 100 C: 90 |
| Williams 1982 | I: 55.3 C: 55.3 |
I: 25/3 C: 24/8 |
NR | NR | NR | I: 89.3 C: 84.3 |
| Yazicioglu 2002 | I: 57.1 (± 7.3) C: 55.3 (± 8.1) |
I: 32/8 C: 30/10 |
I: 10 C: 12.5 |
I: 30 C: 22.5 |
I: 52 (± 6.1) C: 50 (± 5.7) |
NR |
| C: control group; I: intervention group; ID: identification; M/F: male/female; MI: myocardial infarction; NR: not reported; SD: standard deviation; SEM: standard error of the mean | ||||||
aUnless otherwise stated. bData were collected from baseline characteristics tables, or overall data were taken from study inclusion or exclusion criteria.
Data and analyses
Comparison 1. Beta‐blocker vs control for cardiac surgery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality (30 days) | 29 | 4099 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.47, 1.90] |
| 2 Long‐term mortality | 3 | 511 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.29, 2.79] |
| 3 Death due to cardiac causes | 4 | 320 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.14, 5.19] |
| 4 Acute myocardial infarction | 25 | 3946 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.72, 1.52] |
| 5 Cerebrovascular events | 5 | 1471 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.51, 3.67] |
| 6 Ventricular arrhythmias | 12 | 2296 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.25, 0.63] |
| 7 Atrial fibrillation or flutter, or both | 40 | 5650 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.42, 0.59] |
| 8 Bradycardia | 12 | 1640 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [0.92, 2.91] |
| 9 Hypotension | 10 | 1538 | Risk Ratio (M‐H, Random, 95% CI) | 1.84 [0.89, 3.80] |
| 10 Congestive heart failure | 3 | 311 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.04, 1.36] |
| 11 Length of hospital stay (in days) | 14 | 2450 | Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐0.90, ‐0.19] |
Comparison 2. Beta‐blocker vs control for cardiac surgery: subgroup by type of beta‐blocker.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality (30 days) | 28 | 3889 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.49, 2.04] |
| 1.1 Metoprolol | 7 | 1627 | Risk Ratio (M‐H, Random, 95% CI) | 1.72 [0.44, 6.69] |
| 1.2 Propranolol | 9 | 809 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.19, 2.37] |
| 1.3 Sotalol | 8 | 1037 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.08, 5.25] |
| 1.4 Esmolol | 2 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 2.65 [0.12, 60.21] |
| 1.5 Timolol | 1 | 41 | Risk Ratio (M‐H, Random, 95% CI) | 2.86 [0.12, 66.44] |
| 1.6 Landiolol | 2 | 241 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.04, 2.53] |
| 1.7 Atenolol | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.13, 71.51] |
| 2 Acute myocardial infarction | 23 | 3412 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.68, 1.54] |
| 2.1 Metoprolol | 2 | 1200 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.91, 4.40] |
| 2.2 Propranolol | 11 | 1088 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.43, 1.32] |
| 2.3 Sotalol | 4 | 643 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.07, 4.70] |
| 2.4 Esmolol | 2 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.01, 6.33] |
| 2.5 Nadolol | 1 | 141 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.02, 8.87] |
| 2.6 Landiolol | 1 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 2.57 [0.13, 52.15] |
| 2.7 Acebutolol | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.06, 15.55] |
| 2.8 Atenolol | 1 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.32, 5.53] |
| 3 Ventricular arrhythmias | 12 | 2296 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.25, 0.63] |
| 3.1 Metoprolol | 2 | 1094 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.06, 1.06] |
| 3.2 Propranolol | 5 | 592 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.21, 0.79] |
| 3.3 Sotalol | 3 | 452 | Risk Ratio (M‐H, Random, 95% CI) | 2.38 [0.40, 14.27] |
| 3.4 Esmolol | 2 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.08, 0.84] |
| 3.5 Atenolol | 1 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Atrial fibrillation and flutter | 39 | 5440 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.43, 0.60] |
| 4.1 Metoprolol | 9 | 2080 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.62, 0.84] |
| 4.2 Propranolol | 9 | 896 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.33, 0.81] |
| 4.3 Sotalol | 9 | 1292 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.36, 0.56] |
| 4.4 Esmolol | 3 | 111 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.65, 1.61] |
| 4.5 Atenolol | 2 | 139 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.05, 1.90] |
| 4.6 Landiolol | 5 | 578 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.26, 0.52] |
| 4.7 Acebutolol | 2 | 171 | Risk Ratio (M‐H, Random, 95% CI) | 0.09 [0.01, 0.66] |
| 4.8 Timolol | 2 | 173 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.25, 1.25] |
| 5 Bradycardia | 12 | 1640 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [0.93, 2.64] |
| 5.1 Metoprolol | 1 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 5.16 [0.69, 38.55] |
| 5.2 Propranolol | 2 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.53, 2.54] |
| 5.3 Sotalol | 4 | 736 | Risk Ratio (M‐H, Random, 95% CI) | 2.16 [0.70, 6.65] |
| 5.4 Esmolol | 2 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.13, 68.26] |
| 5.5 Landiolol | 2 | 237 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.48, 2.56] |
| 5.6 Atenolol | 1 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.91] |
| 5.7 Carvedilol | 1 | 324 | Risk Ratio (M‐H, Random, 95% CI) | 21.11 [1.25, 355.18] |
| 6 Hypotension | 10 | 1538 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [0.87, 3.65] |
| 6.1 Metoprolol | 1 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.01, 4.17] |
| 6.2 Propranolol | 2 | 112 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [0.33, 8.14] |
| 6.3 Sotalol | 4 | 736 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.20, 3.74] |
| 6.4 Esmolol | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.35, 25.68] |
| 6.5 Landiolol | 1 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.6 Carvedilol | 1 | 324 | Risk Ratio (M‐H, Random, 95% CI) | 5.50 [1.25, 24.20] |
| 6.7 Nadolol | 1 | 141 | Risk Ratio (M‐H, Random, 95% CI) | 1.66 [0.29, 9.61] |
Comparison 3. Beta‐blocker vs control for cardiac surgery: subgroup by start of beta‐blocker therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality (30 days) | 29 | 4099 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.47, 1.90] |
| 1.1 Before surgery | 7 | 778 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.31, 3.79] |
| 1.2 During surgery | 4 | 371 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.13, 1.90] |
| 1.3 After surgery | 18 | 2950 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.44, 3.77] |
| 2 Acute myocardial infarction | 24 | 3622 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.72, 1.57] |
| 2.1 Before surgery | 2 | 246 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [0.45, 5.45] |
| 2.2 During surgery | 4 | 270 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.22, 3.98] |
| 2.3 After surgery | 18 | 3106 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.66, 1.58] |
| 3 Ventricular arrhythmias | 12 | 2296 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.25, 0.63] |
| 3.1 Before surgery | 2 | 291 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.13, 4.55] |
| 3.2 During surgery | 4 | 434 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.15, 1.21] |
| 3.3 After surgery | 6 | 1571 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.19, 0.69] |
| 4 Atrial fibrillation and flutter | 40 | 5650 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.42, 0.59] |
| 4.1 Before surgery | 7 | 796 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.35, 0.67] |
| 4.2 During surgery | 10 | 1067 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.43, 0.74] |
| 4.3 After surgery | 23 | 3787 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.37, 0.60] |
| 5 Bradycardia | 12 | 1640 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [0.92, 2.91] |
| 5.1 Before surgery | 3 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 5.82 [1.78, 19.02] |
| 5.2 During surgery | 5 | 551 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.64, 2.72] |
| 5.3 After surgery | 4 | 490 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.48, 2.12] |
| 6 Hypotension | 10 | 1538 | Risk Ratio (M‐H, Random, 95% CI) | 1.84 [0.89, 3.80] |
| 6.1 Before surgery | 3 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 1.85 [0.25, 13.45] |
| 6.2 During surgery | 3 | 386 | Risk Ratio (M‐H, Random, 95% CI) | 2.00 [0.37, 10.90] |
| 6.3 After surgery | 4 | 553 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.45, 4.12] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abel 1983.
| Methods | Quasi‐randomized trial, parallel design | |
| Participants |
Total number of randomized participants: 100 Inclusion criteria: participants of either gender receiving beta‐adrenergic blocking agents and undergoing isolated autocoronary saphenous vein bypass procedures without concomitant valve replacement or left ventricular resection and with preoperative left ventricular ejection fractions Exclusion criteria: intolerance to beta‐adrenergic blocking agents or severe bronchospastic symptoms Type of surgery: elective CABG Baseline characteristics Intervention group (propranolol)
Control group (standard care)
Country: USA Setting: hospital; single centre |
|
| Interventions |
Intervention group (propranolol)
Control group (standard care)
|
|
| Outcomes |
Outcomes measured/reported by study authors: mortality (in‐hospital); MI; ventricular arrhythmias; supraventricular arrhythmias (to include overall, and data for atrial fibrillation and atrial flutter); reasons for treatment withdrawal (intervention group only) ‐ severe hypotension, preoperative MI, bradycardia, cardiac arrest and biventricular failure Outcomes relevant to the review: mortality (in‐hospital); MI; ventricular arrhythmias; AF; hypotension and bradycardia (see notes below) |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomization (last digit of hospital record number) |
| Allocation concealment (selection bias) | High risk | See above |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessors (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 9 withdrawals in beta‐blocker group due to bradycardia (2), severe hypotension (3), preoperative myocardial infarction (2), biventricular failure (1), and cardiac arrest (1). No losses reported in the control group |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | High risk | Quote: "In the postoperative period, 26 of the 50 control patients (52%) received propranolol therapy for at least one dose because of a variety of therapeutic indications". Comment: this may influence outcome data in the control group |
Ali 1997.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 210 Inclusion criteria: participants undergoing elective CABG, taking beta‐blockers (metoprolol, atenolol, sotalol, or inderal) preoperatively Exclusion criteria: chronic obstructive lung airway disease, preoperative ejection fraction < 35%, history of AF, SVT, or bradycardia (< 50 bpm) Type of surgery: elective CABG Baseline characteristics Intervention group (various)
Control group (standard care)
Country: Canada Setting: hospital; single centre |
|
| Interventions |
Intervention group (various)
Control group (standard care)
|
|
| Outcomes |
Outcomes measured/reported by study authors: AF; MI; mortality; cerebrovascular events (stroke, leading to death) Outcomes relevant to the review: AF; MI; mortality |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessors (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Arar 2007.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 80 Inclusion criteria: scheduled for CABG surgery, with planned extubation in the ICU Exclusion criteria: preoperative ejection fraction < 40%; history of asthma; receiving vasodilator and inotropic support by infusion; allergic to study drugs Type of surgery: elective CABG Baseline characteristics Intervention group (esmolol)
Control group (placebo)
Country: Turkey Setting: hospital; single centre |
|
| Interventions |
Intervention group (esmolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic variables; hypotension; bradycardia Outcomes relevant to the review: hypotension (see notes); bradycardia (see notes) |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The drugs were prepared by one anaesthesiologist and administered by another who did not know its identity" |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Auer 2004.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 193 Inclusion criteria: haemodynamically stable, available at least 1 day before surgery, > 19 years of age, in normal sinus rhythm with no evidence of preoperative AF, baseline corrected QT interval of ≤ 400 ms Exclusion criteria: chronic or paroxysmal AF, MI < 2 weeks before surgery, unstable angina, ejection fraction < 35%, HR < 55 bpm, advanced heart block of severe conduction disturbance, history of amiodarone toxicity, implantable defibrillator, or treatment with certain possibly interacting drugs, untreated thyroid disease, serum aspartate aminotransferase or alanine aminotransferase concentrations > 3 times normal, impaired renal function Type of surgery: cardiac surgery (CABG and valvular surgery) Baseline characteristics Intervention group (metoprolol)
Intervention group (sotalol)
Control group (placebo)
Country: Austria Setting: single centre; hospital (tertiary care centre) |
|
| Interventions |
Intervention group (metoprolol)
Intervention group (sotalol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: AF, cerebrovascular accident (stroke, TIA), ventricular tachycardia, bradycardia (HR < 40 bpm), hypotension (SBP < 90 mmHg), postoperative infection, mortality, length of hospital stay. Outcome monitoring during hospital stay Outcomes relevant to the review: mortality, cerebrovascular accident (stroke, TIA), ventricular tachycardia, AF, bradycardia, hypotension, length of hospital stay |
|
| Notes |
Funding/declarations of interest: study drugs provided by pharmaceutical company, all other funding from intramural divisional funds Study dates: January 2001‐May 2002 Notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization table was used for sequence generation |
| Allocation concealment (selection bias) | Low risk | Randomization schedule was sealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Only the nurse and unmasked study controller (who reviewed safety issues) had knowledge of drug group allocation, and neither had patient contact nor extracted data" |
| Blinding of outcome assessors (detection bias) All outcomes | Low risk | Quote: "Only the nurse and unmasked study controller (who reviewed safety issues) had knowledge of drug group allocation, and neither had patient contact nor extracted data" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants were excluded from analysis after randomization (1 participant refused surgery, 1 was refused to be operated on by the surgeon and 1 suffered from a stroke before the time of surgery ‐ study authors provided exact group allocation). Small number of losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Babin‐Ebell 1996.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 70 Inclusion criteria: scheduled for CABG Exclusion criteria: unstable angina, bradycardia (HR < 50 bpm), COPD, ejection fraction < 0.4, history of SVT and additional surgical procedures Type of surgery: elective CABG Baseline characteristics Intervention group (propranolol)
Control group (standard care)
Country: Germany Setting: hospital; single centre |
|
| Interventions |
Intervention group (propranolol)
Control group (standard care)
|
|
| Outcomes |
Outcomes measured/reported by study authors: supraventricular tachycardia, haemodynamic parameters, cardiac enzyme levels, use of catacholamines (to manage low cardiac output, or hypotension) and leading to withdrawal from treatment, MI, bradycardia. Outcomes were assessed up to 7 days following surgery Outcomes relevant to the review: acute MI, bradycardia |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessors (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6 participants were excluded from analysis of supraventricular arrhythmias ‐ we re‐included these lost participants for data for bradycardia, hypotension, MI). Losses are imbalanced between groups |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Bert 2001.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 131 Inclusion criteria: scheduled for primary CABG surgery Exclusion criteria: history of atrial or ventricular arrhythmias or any other than sinus rhythm on ECG obtained evening before surgery; severe left ventricular dysfunction, bronchospastic airway disease, renal failure Type of surgery: elective CABG Baseline characteristics Intervention group (propranolol)
Control group (standard care)
Country: USA Setting: single centre; hospital |
|
| Interventions |
Intervention group (propranolol)
Control group (standard care)
|
|
| Outcomes |
Outcomes measured/reported by study authors: postoperative atrial tachyarrhythmias, time to extubation, MI, duration of hospital stay, mortality, ventricular ectopic activity Outcomes relevant to the review: MI, length of hospital stay, mortality |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported (study duration 36 months) Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessors (detection bias) All outcomes | Low risk | Quote: "All arrhythmia tracings were reviewed by a cardiologist unaware of the patient's treatment group to confirm arrhythmia classification and to quantify the initial ventricular response rate" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | We noted that 3 participants were excluded after enrolment because they did not undergo surgery; study authors did not report to which group these participants belonged. All other participants were included in analysis |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | High risk | The study end‐point was reached if a participant had postoperative atrial tachycardia. At this point, clinicians were free to treat participants with other agents to include beta‐blockers. Because participants in the control group may have received beta‐blockers, this may influence data for other outcomes |
Bignami 2017.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 46 Inclusion criteria: undergoing cardiac surgery; ≥ 18 years of age; preoperative left ventricular end diastolic diameter > 60 mm and left ventricular ejection fraction < 50% Exclusion criteria: previous unusual response to esmolol; esmolol administration in previous 30 days; emergency surgery; inclusion in other RCTs Type of surgery: elective cardiac surgery (coronary, mitral valve, aortic valve) Baseline characteristics Intervention group (esmolol)
Control group (placebo)
Country: Italy Setting: single centre; teaching hospital |
|
| Interventions |
Intervention group (esmolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: myocardial damage; troponin levels, ventricular fibrillation, need for inotropic support, ICU and hospital length of stay, mortality (at 1 year) Outcomes relevant to the review: hospital length of stay (see notes below), mortality (at 1 year) |
|
| Notes |
Funding/declarations of interest: study authors received no funding, and declare no conflicts Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Use of sealed, numbered, opaque envelopes for group assignment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All personnel were blinded to group assignment. Study drugs prepared so that they were identical in appearance |
| Blinding of outcome assessors (detection bias) All outcomes | Low risk | Outcome assessors blinded to group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Booth 2004.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 72 Inclusion criteria: scheduled for primary CABG Exclusion criteria: history of severe hepatic dysfunction, history of bronchospasm requiring daily bronchodilator therapy, pregnancy, preoperative inotropic drug use, off‐pump coronary surgery Type of surgery: elective CABG Baseline characteristics Intervention group (metoprolol)
Control group (placebo)
Country: USA Setting: single centre, hospital |
|
| Interventions |
Intervention group (metoprolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: beta‐adrenergic receptor function, inotropic support, supraventricular arrhythmias, haemodynamic variables, length of hospital stay, duration of ventilation Outcomes relevant to the review: length of hospital stay |
|
| Notes |
Funding/declarations of interest: in part, by NIH grants and the Duke Clinical Research Centers Program Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
But 2006.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 30 Inclusion criteria: people with type II diabetes mellitus undergoing CABG surgery Exclusion criteria: type I diabetes mellitis; ejection fraction < 40%, liver and kidney insufficiency; bleeding diathesis; valvular heart disease Type of surgery: CABG Baseline characteristics Intervention group (esmolol)
Control group (placebo)
Country: Turkey Setting: single centre; hospital |
|
| Interventions |
Intervention group (esmolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: amount of glucose‐insulin‐potassium infusion consumption; blood glucose levels; bradycardia (not defined), hypotension (not defined), hypertension; recovery times; length of hospital stay; dysrhythmias; inotropic support Outcomes relevant to the review: bradycardia; hypotension; length of hospital stay |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Although the control group is given normal saline, it is not clear whether this is given as a placebo, and whether anaesthetists are aware of group allocation |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Connolly 2003.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 1000 Inclusion criteria: scheduled for heart surgery with CABG, residing at home before hospital admission Exclusion criteria: emergency surgery, previous adverse reaction to beta‐blockers, COPD, junctional rhythm or 2nd‐ or 3rd‐degree atrioventricular block, long‐term preoperative amiodarone therapy. Additional postoperative exclusion criteria: sinus bradycardia; cardiac index < 2.3 L/min/m²; need for IV inotropic agent; evidence of bronchospasm Type of surgery: elective heart surgery (CABG) Baseline characteristics Intervention group (metoprolol)
Control group (placebo)
Country: Canada Setting: single centre; hospital Mean age: 62.5 years Percentage of female participants: 21 |
|
| Interventions |
Intervention group (metoprolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: length of stay, arrhythmias (supraventricular and ventricular arrhythmias), MI, cost of care, stroke, death, haemodynamic parameters Outcomes relevant to the review: length of hospital stay, arrhythmias (AF and ventricular arrhythmias), MI, stroke, death |
|
| Notes |
Funding/declarations of interest: supported by a grant from Canadian Institutes for Health Research Study dates: January 1997‐September 1999 Notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Randomized, placebo‐controlled, double‐blind trial |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | High risk | Non‐study beta‐blockers were given to 146 participants in the intervention group, and 199 participants in the placebo group (main indication for this was to treat postoperative AF). This may influence outcome data |
Cork 1995.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 30 Inclusion criteria: scheduled for heart surgery involving cardiopulmonary bypass and aortic cross‐clamping Exclusion criteria: ASA status V, MI within 1 week, evidence of renal or hepatic failure, history of severe asthma, history of allergy or idiosyncratic reaction to beta‐blockers Type of surgery: elective heart surgery involving cardiopulmonary bypass and aortic cross‐clamping Baseline characteristics Intervention group (esmolol)
Control group (placebo)
Country: USA Setting: single centre; hospital |
|
| Interventions |
Intervention group (esmolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic and laboratory parameters, dysrhythmias (supraventricular and ventricular), length of stay and cost of care, mortality Outcomes relevant to the review: length of hospital stay; mortality |
|
| Notes |
Funding/declarations of interest: supported in part by a grant from DuPont Pharmaceuticals Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Randomized, placebo‐controlled, double‐blind trial |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant died, and was not included in data for arrhythmia outcomes. No other losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Daudon 1986.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 100 Inclusion criteria: scheduled for elective CABG without additional cardiac surgical procedures Exclusion criteria: contraindication to beta‐blockers, left ventricular aneurysm, major renal failure, history of cardiac arrhythmias, cardiac arrhythmias during immediate 36 h postoperative period, low cardiac output still requiring catecholamine support at 36 h after surgery Type of surgery: elective CABG Baseline characteristics Intervention group (acebutolol)
Control group (standard care)
Country: France Setting: single centre; hospital |
|
| Interventions |
Intervention group (acebutolol)
Control group (standard care)
|
|
| Outcomes |
Outcomes measured/reported by study authors: AF and atrial flutter; MI Outcomes relevant to the review: AF and atrial flutter; MI |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessors (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | High risk | Study authors noted a statistically significant difference between ages (participants in acebutolol group were younger). This age difference did not appear to be clinically significant and we did not expect it to influence the data. However, we noted that some participants (3) in the control group were given beta‐blocking therapy to treat supraventricular tachyarrhythmias. Although only a small number, this could have influenced data for other outcomes |
De Azevedo Lúcio 2003.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 200 Inclusion criteria: participants underwent isolated CABG surgery Exclusion criteria: history of bronchospasm, left ventricular ejection fraction < 35% in preoperative period, implantable cardiac pacemaker, chronic AF, history of supraventricular arrhythmias, using amiodarone, congestive heart failure, low cardiac output, dependence on inotropic drugs, bradyarrhythmias Type of surgery: elective CABG Baseline characteristics Intervention group (metoprolol)
Control group (standard care)
Country: Brazil Setting: single centre; hospital |
|
| Interventions |
Intervention group (metoprolol)
Control group (standard care)
|
|
| Outcomes |
Outcomes measured/reported by study authors: AF/flutter, death, MI (group affiliation not specified), stroke (group affiliation not specified) Outcomes relevant to the review: AF/flutter, death, MI (group affiliation not specified ‐ see notes below), stroke (group affiliation not specified ‐ see notes below) |
|
| Notes |
Funding/declarations of interest: not reported Study dates: February 1997‐October 1998 Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessors (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | High risk | We noted that study group allocation of participants with certain adverse events (AMI, stroke) remained unclear; we considered this to demonstrate evidence of selective reporting |
| Other bias | Low risk | Not detected |
Dy 1998.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 135 Inclusion criteria: scheduled for isolated CABG Exclusion criteria: ejection fraction < 30%, prior atrial or ventricular arrhythmias, severe COPD, serum creatinine > 2.0, severe bradycardia Type of surgery: elective CABG Baseline characteristics not reported in abstract Country: USA Setting: not reported in abstract |
|
| Interventions |
Intervention group (metoprolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: AF (period of observation is unclear) Outcomes relevant to the review: AF |
|
| Notes |
Funding/declarations of interest: not reported Study dates: January 1995‐May 1997 Notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Randomized, placebo‐controlled, double‐blind trial |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Unclear risk | Limited detail in abstract. Not feasible to assess other risks of bias from this report |
Evrard 2000.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 206 Inclusion criteria: undergone CABG without cardiac concomitant procedures Exclusion criteria: left ventricular ejection fraction < 35%, history of obstructive lung disease, known intolerance to beta‐blockers, history of recurrent or persistent SVT, ventricular tachycardia or fibrillation, postoperative aortic balloon pumping, renal failure, digoxin or other anti‐arrhythmic agents between surgery and randomization Type of surgery: elective CABG Baseline characteristics Intervention group (sotalol)
Control group (standard care)
Country: Belgium Setting: single centre; hospital |
|
| Interventions |
Intervention group (sotalol)
Control group (standard care)
|
|
| Outcomes |
Outcomes measured/reported by study authors: supraventricular and ventricular arrhythmias (to include 'runs of ventricular tachycardia'), length of hospital stay, mortality; MI; adverse events leading to discontinuation of treatment (to include asthma, mild heart failure, bradycardia, reduction in cardiac index, sinus tachycardia, moderate hypertension, ventricular extrasystoles) Outcomes relevant to the review: AF; length of hospital stay (see notes below), mortality; MI |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Blocked randomization in a prospective open manner" |
| Allocation concealment (selection bias) | High risk | Blocked randomization in an unblinded trial |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessors (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | High risk | Quote: "In patients with sustained SVT, trial medication was stopped and patients were treated according to the physician in charge by amiodarone or digitalization, or both, beta‐blockers, sotalol, or an increased dose of sotalol" Comment: in the control group, 15 participants were treated with beta‐blockers as rescue therapy for different reasons. This could influence outcome data |
Forlani 2002.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 102 Inclusion criteria: first‐time isolated CABG with cardiopulmonary bypass Exclusion criteria: preoperative ejection fraction < 0.40, sick sinus syndrome and atrioventricular node disease, a corrected QT interval > 440 ms, preoperative use of antiarrhythmic drugs (except beta‐blockers), history of supraventricular arrhythmias, severe COPD, serum creatinine levels > 2.0 mg/dL Type of surgery: elective CABG Baseline characteristics Intervention group (sotalol)
Control group (standard care)
Country: USA Setting: single centre; hospital |
|
| Interventions |
Intervention group (sotalol)
Control group (standard care)
|
|
| Outcomes |
Outcomes measured/reported by study authors: AF, length of hospital stay, MI, all‐cause mortality; haemodynamic variables; QTc values; calcium and potassium serum levels Outcomes relevant to the review: AF, length of hospital stay, MI, all‐cause mortality |
|
| Notes |
Funding/declarations of interest: not reported Study dates: January 2001‐July 2001 Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessors (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | More than 2 intervention groups |
Gandhi 2007.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 141 Inclusion criteria: scheduled for CABG surgery; ischaemic left ventricular systolic dysfunction (ejection fraction ≤ 30% as depicted by 2D echocardiography) Exclusion criteria: > 70 years of age; previous or recent history of 2nd‐ or 3rd‐degree atrioventricular block; renal failure; hepatic dysfunction; cerebrovascular events; previous history of revascularization or valve replacement surgery Type of surgery: elective CABG surgery Intervention group (carvedilol)
Control group (standard care)
Country: India Setting: multi‐centre; 2 hospitals |
|
| Interventions |
Intervention group (carvedilol)
Control group (standard care)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic parameters; changes in left ventricular ejection fraction; changes to NYHA class; mortality (at 6 months) Outcomes relevant to the review: mortality (at 6 months) |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessors (detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Girard 1986.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 17 Inclusion criteria: scheduled for myocardial revascularization Exclusion criteria: severe congestive heart failure; valvular heart disease; MI within 1 month of surgery; not in sinus rhythm Type of surgery: elective myocardial revascularization Baseline characteristics Intervention group (esmolol)
Control group (placebo)
Country: USA Setting: single‐centre; hospital |
|
| Interventions |
Intervention group (esmolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic parameters, discontinuation of study drug (due to bradycardia with HR < 50 bpm) Outcomes relevant to the review: bradycardia (see notes below) |
|
| Notes |
Funding/declarations of interest: supported by a grant from American Critical Care Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinded solutions were provided by hospital pharmacy |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Gomes 1999.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 85 Inclusion criteria: scheduled for elective CABG with or without valve replacement Exclusion criteria: emergent open heart surgery, prior history of AF of atrial flutter, left ventricular ejection fraction < 28% or clinically active congestive heart failure, 1st‐degree or higher degrees of atrioventricular block, QTc > 450 ms, impaired renal function, COPD, current use of anti‐arrhythmic drugs Type of surgery: elective CABG ± valve replacement Baseline characteristics Intervention group (sotalol)
Control group (placebo)
Country: USA Setting: multi‐centre; 2 hospitals |
|
| Interventions |
Intervention group (sotalol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: AF, mortality, adverse events (to include bradycardia, hypotension, ventricular tachycardia), length of hospital stay. Follow‐up until hospital discharge Outcomes relevant to the review: AF, mortality, length of hospital stay, bradycardia (not defined), hypotension (not defined), ventricular tachycardia (see notes below) |
|
| Notes |
Funding/declarations of interest: supported by Electrophysiology Section Research Funds Study dates: February 1997‐August 1997 Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Randomized, placebo‐controlled, double‐blind trial. Pharmacy at each institution dispensed medication and placebo pills for all participants |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | High risk | Significantly more participants from the control group than from the treatment group were receiving long‐term beta‐blocker treatment before they entered the study |
Graham 1996.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 320 Inclusion criteria: participants undergoing isolated CABG Exclusion criteria: no details Type of surgery: elective CABG Baseline characteristics not reported in abstract Country: USA Setting: unknown if single‐ or multi‐centre study; hospital |
|
| Interventions |
Intervention group (metoprolol 25 mg)
Intervention group (metoprolol 50 mg)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: AF Outcomes relevant to the review: AF |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | High risk | We noted that study authors appeared to only report statistically significant results in the abstract |
| Other bias | Unclear risk | Limited data in abstract and it is not feasible to assess risk of other bias |
Hammon 1984.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 50 Inclusion criteria: scheduled for CABG, with stable angina pectoris Exclusion criteria: congestive heart failure, history of bronchospasm, previous sensitivity to propranolol Type of surgery: elective CABG Baseline characteristics not reported Country: USA Setting: single centre; hospital |
|
| Interventions |
Intervention group (propranolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic parameters, atrial and ventricular arrhythmias, death, MI, bradycardia (HR < 60 bpm) Outcomes relevant to the review: ventricular arrhythmias, death, MI, bradycardia |
|
| Notes |
Funding/declarations of interest: study drug provided by pharmaceutical company (Ayerst Laboratories) Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Randomized, placebo‐controlled, double‐blind trial |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | High risk | Study authors do not report baseline characteristics and we could not be certain whether these characteristics were balanced between groups. In addition, we noted that antiarrhythmic therapy, which included propranolol, was given to 11 participants in the control group and 5 participants in the intervention group. This may have influenced outcome data |
Harrison 1987.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 30 Inclusion criteria: elective myocardial revascularization Exclusion criteria: pregnancy; AF or atrial flutter; atrioventricular conduction block > 1st degree; conditions that preclude beta‐adrenergic blocker treatment; MI within previous 3 months; severe hepatic or renal disease; SBP < 100 mmHg or cardiogenic shock; severe electrolyte imbalance; adrenergic augmenting drugs; long‐term beta‐adrenergic blocking drugs; calcium channel blocking agents Type of surgery: elective CABG Baseline characteristics Intervention group (esmolol)
Control group (placebo)
Country: USA Setting: single centre; hospital |
|
| Interventions |
Intervention group (esmolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic parameters, intraoperative myocardial ischaemia, intraoperative ventricular arrhythmias Outcomes relevant to the review: intraoperative ventricular arrhythmias |
|
| Notes |
Funding/declarations of interest: supported by a grant from American Critical Care Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Randomized, placebo‐controlled, double‐blind trial |
| Blinding of outcome assessors (detection bias) All outcomes | Low risk | Quote: "The ST trends and ECG strips were evaluated for myocardial ischaemia independently by a cardiologist who had no knowledge of the patient's treatment group" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Ivey 1983.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 116 Inclusion criteria: scheduled for CABG surgery, receiving ≥ 80 mg of propranolol daily for treatment of angina Exclusion criteria: previously received digoxin, quinidine, or procainamide; ejection fraction of at least 0.4; no preoperative history of SVT Type of surgery: CABG Baseline characteristics Intervention group (propranolol)
Control group (placebo)
Country: USA Setting: single centre; hospital |
|
| Interventions |
Intervention group (propranolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: SVT, mortality Outcomes relevant to the review: mortality |
|
| Notes |
Funding/declarations of interest: plasma assays performed by pharmaceutical company Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed by the hospital pharmacy in a double‐blind manner." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Randomized, placebo‐controlled, double‐blind trial. Control group participants "received an exact propranolol placebo on an identical schedule" |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss of 7 participants after consent; study authors do not report to which group these participants belonged and we could not re‐include data in analysis. Reasons for losses include potential outcome data (postoperative bradycardia and hypotension) |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Jacquet 1994.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 42 Inclusion criteria: scheduled for CABG surgery without a concomitant procedure, in sinus rhythm, preoperative left ventricular ejection fraction of > 35%, no contraindication to use of beta‐blockers, HR > 50 bpm, SBP > 100 mmHg, cardiac index > 2.8 L/min/m², pulmonary capillary wedge pressure < 15 mmHg without inotropic support Exclusion criteria: history of recurrent SVA, atrioventricular conduction disturbances, prolonged QT interval, chronic obstructive airway diseases treated with aerosolized beta‐sympathomimetic drugs, significant renal or hepatic dysfunction Type of surgery: elective CABG Baseline characteristics Intervention group (sotalol)
Control group (standard care)
Country: Belgium Setting: single centre; hospital |
|
| Interventions |
Intervention group (sotalol)
Control group (standard care)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic parameters, supraventricular arrhythmias, length of hospital stay, perioperative MI, discontinuation of intervention due to hypotension (SBP < 90 mmHg) and bradycardia (HR < 50 bpm) Outcomes relevant to the review: length of hospital stay, perioperative MI, hypotension (see notes below), bradycardia (see notes below) |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessors (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Withdrawal of treatment in 6 participants in the treatment group (because of bradycardia and hypotension). Analysis of arrhythmias did not included these participants. Loss is < 10%, but is imbalanced between groups |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Unclear risk | We noted more participants in the intervention group were taking beta‐blockers preoperatively |
Janssen 1986.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 151 Inclusion criteria: scheduled for CABG surgery, with left ventricular ejection fraction at least 30% Exclusion criteria: preoperative MI, inotropic support with dopamine, postoperative death, severe sinus bradycardia, inappropriate data (no additional details) Type of surgery: elective CABG Baseline characteristics Intervention group (sotalol)
Intervention group (metoprolol)
Control group (standard care)
Country: Netherlands Setting: single centre; hospital |
|
| Interventions |
Intervention group (sotalol)
Intervention group (metoprolol)
Control group (standard care)
|
|
| Outcomes |
Outcomes measured/reported by study authors: supraventricular tachycardias, atrial fibrillation Outcomes relevant to the review: AF, mortality |
|
| Notes |
Funding/declarations of interest: not reported Study dates: October 1983‐January 1984
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Use of sealed envelopes; no additional details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessors (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 21 participants excluded from analysis (2 MI, 12 inotropic support after CABG, 1 death, 1 bradycardia, 5 inappropriate data). It is not clear to which group these participants belonged |
| Selective reporting (reporting bias) | High risk | We noted that study authors appeared to only report statistically significant results |
| Other bias | High risk | All participants were given beta‐blockers, if required, to treat supraventricular tachycardia. In the control group, 10 participants were given sotalol, and 4 participants were given metoprolol. This may influence outcome data for mortality |
Khuri 1987.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 148 Inclusion criteria: scheduled for CABG surgery Exclusion criteria: COPD, bronchial asthma or severe lower extremity claudication; significant GI disease, insulin‐dependent diabetes mellitus, renal failure, hyperthyroidism, conditions leading to non‐compliance, sinus bradycardia, heart block > 1st degree, sick sinus syndrome, congestive heart failure, inotropic support, therapeutic IAB counterpulsation Type of surgery: elective CABG Baseline characteristics Intervention group (nadolol)
Control group (placebo)
Country: USA Setting: multi‐centre; 2 hospitals |
|
| Interventions |
Intervention group (nadolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic variables, postoperative arrhythmias, hypotension (leading to discontinuation of study medication), MI Outcomes relevant to the review: hypotension, MI |
|
| Notes |
Funding/declarations of interest: grant from ER Squibb and Sons Inc., and the Richard Warren Surgical Research and Educational Fund Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Randomized, placebo‐controlled, double‐blind trial |
| Blinding of outcome assessors (detection bias) All outcomes | Low risk | Outcome assessor blinded ("analyzed by an independent observer") |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 7 participants were excluded from analysis because of "insufficient postoperative data". It is not clear to which groups these participants belonged and whether losses were balanced between groups |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Kurian 2001.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 72 Inclusion criteria: scheduled for coronary artery surgery Exclusion criteria: conditions that would make ST segment monitoring unreliable (digoxin therapy, left ventricular hypertrophy, left bundle branch block, presence of a pacemaker), contraindications to beta‐adrenoceptor blocker, asthma, intolerance to beta‐adrenergic blockade, 1st‐degree heart block, beta‐adrenergic agonist infusion at start of study, preoperative serum creatinine > 120 µmol/L Type of surgery: elective CABG Baseline characteristics Intervention group (esmolol)
Control group (standard care)
Country: UK Setting: single centre; hospital |
|
| Interventions |
Intervention group (esmolol)
Control group (standard care)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic variables, discontinuation of treatment due to hypotension (not defined), perioperative myocardial ischaemia (120 min before until 180 min after tracheal extubation) Outcomes relevant to the review: hypotension (see notes below) |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Low risk | Numbered, sealed envelopes, provided by hospital pharmacy |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessors (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 participants were withdrawn (2 because of hypotension in the esmolol group, 1 in the esmolol group and 1 in the control group because of insufficient monitoring). Clearly reported, < 10% loss |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Lamb 1988.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 60 Inclusion criteria: scheduled for CABG surgery Exclusion criteria: history of arrhythmia, asthma, peripheral vascular disease, congestive cardiac failure, left ventricular ejection fraction < 0.4 Type of surgery: elective CABG Baseline characteristics Intervention group (atenolol)
Control group (standard care)
Country: UK Setting: single centre; hospital |
|
| Interventions |
Intervention group (atenolol)
Control group (standard care)
|
|
| Outcomes |
Outcomes measured/reported by study authors: supraventricular arrhythmias; AF Outcomes relevant to the review: AF |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessors (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Liu 2016.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 24 Inclusion criteria: 40‐80 years of age; undergoing elective primary cardiac surgery; NYHA class II or III; no evidence of myocardial ischaemia or elevated serum levels of myocardial markers within 24 h prior to surgery Exclusion criteria: diagnosis of acute MI within the last 4 weeks; activated phase of rheumatic diseases; left ventricular ejection fraction < 40%; intracardiac shunt; haematocrit < 30%; severe systemic diseases (including pulmonary diseases, hepatic, renal, musculoskeletal diseases or immune system illnesses; receiving oral hypoglycaemic agents or theophyllines Type of surgery: cardiac surgery (CABG or valve replacement) Baseline characteristics Intervention group (esmolol)
Control group (saline)
Country: China Setting: single centre; hospital |
|
| Interventions |
Intervention group (esmolol)
Control group (saline)
|
|
| Outcomes |
Outcomes measured/reported by study authors: changes in serum markers for myocardial injury; haemodynamic parameters; use of vasoactive treatment; adverse events (neurological complications; pulmonary infection; incision infection; pericardial tamponade; open‐chest haemostasis; death); AF; length of stay in the ICU Outcomes relevant to the review: mortality (during ICU stay); AF |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of computer‐generated randomization |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Control group is not described as using a placebo. It is unclear whether anaesthetists were blinded to study treatments |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Martinussen 1988.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 108 Inclusion criteria: undergoing CABG surgery, normotensive, in sinus rhythm, no history of SVT or atrioventricular nodal block Exclusion criteria: congestive heart failure despite medical treatment, obstructive lung disease, undergoing concomitant valve surgery, anti‐arrhythmic surgery, aneurysmectomy Type of surgery: elective CABG Baseline characteristics Intervention group (propranolol)
Control group (placebo)
Country: Denmark Setting: single centre; hospital |
|
| Interventions |
Intervention group (propranolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: supraventricular tachyarrhythmias (AF), MI, mortality Outcomes relevant to the review: MI, mortality |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Randomized, placebo‐controlled, double‐blind trial |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 33 participants already randomly assigned were excluded from analysis (in 14 participants, a nasogastric tube for drug administration could not be placed, 19 participants developed disorders as specified in the discontinuation criteria); originally 108 participants were randomly assigned |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Matangi 1985.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 168 Inclusion criteria: scheduled for CABG surgery, preoperative use of beta‐blockers Exclusion criteria: ejection fraction 35% or less, no preoperative use of beta‐blockers, SVT, postoperative inotropic support, congestive heart failure, postoperative heart block, involvement in another study where propranolol was contraindicated, sick sinus syndrome, known reaction to propranolol Type of surgery: elective CABG Baseline characteristics Intervention group (propranolol)
Control group (standard care)
Country: New Zealand Setting: single centre; hospital |
|
| Interventions |
Intervention group (propranolol)
Control group (standard care)
|
|
| Outcomes |
Outcomes measured/reported by study authors: supraventricular arrhythmias (AF and atrial tachycardia), ventricular arrhythmias, ventricular premature beats, acute MI, mortality Outcomes relevant to the review: AF, ventricular arrhythmias, MI, mortality |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Use of sealed envelopes. Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessors (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In 4 participants, beta‐blocker therapy was discontinued because of side effects (low cardiac output, bronchospasm, nightmares) but were analysed according to ITT. 4 participants were excluded owing to clinical events specified in exclusion criteria; these participants were not included in analyses. However, loss is < 10% |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Matangi 1989.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 70 Inclusion criteria: scheduled for CABG surgery Exclusion criteria: > 70 years of age, 2nd or subsequent CABG operation, clinically significant congestive heart failure, preoperative ejection fraction < 30%, preoperative use of digitalis or other anti‐arrhythmic agent, preoperative hypotension, concomitant left ventricular aneurysm resection or valve replacement, persistent bradycardia, 2nd‐ or 3rd‐degree atrioventricular block, postoperative haemodynamic complication requiring continued vasopressor support or intra‐aortic balloon pump counter pulsation, asthma or > moderate COPD, brittle type I diabetes mellitus, inability to discontinue calcium channel blocking agents for 24 h preoperatively Type of surgery: CABG Baseline characteristics Intervention group (atenolol)
Control group (placebo)
Country: Canada Setting: single centre; hospital |
|
| Interventions |
Intervention group (atenolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: ventricular and supraventricular arrhythmias, acute MI, adverse events, bradycardia (≤ 40 bpm), hypotension (not defined), congestive heart failure Outcomes relevant to the review: ventricular arrhythmias, acute MI, bradycardia, hypotension, congestive heart failure |
|
| Notes |
Funding/declarations of interest: grant from Stuart Pharmaceuticals Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Randomized, placebo‐controlled, double‐blind trial |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses after randomization |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Materne 1985.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 71 Inclusion criteria: elective CABG Exclusion criteria: left ventricular ejection fraction < 40%, previous treatment with amiodarone, major perioperative complication (MI, cardiac tamponade), use of another anti‐arrhythmic drug Type of surgery: elective CABG Baseline characteristics (acebutolol) Intervention group
Control group (standard care)
Country: Belgium Setting: single centre; hospital |
|
| Interventions |
Intervention group (acebutolol)
Control group (standard care)
|
|
| Outcomes |
Outcomes measured/reported by study authors: supraventricular arrhythmias (to include AF), ventricular extrasystoles, haemodynamic parameters Outcomes relevant to the review: AF |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessors (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Matsuura 2001.
| Methods | Quasi‐randomized trial, parallel design | |
| Participants |
Total number of randomized participants: 80 Inclusion criteria: scheduled for CABG surgery Exclusion criteria: history of AF or atrial flutter, contraindications to beta‐blockers (such as asthma; or concomitant valvular, anti‐arrhythmic, or aortic surgery), severe bradycardia, hypotension Type of surgery: elective CABG Baseline characteristics Intervention group (sotalol)
Control group (standard care)
Country: Japan Setting: single centre; hospital |
|
| Interventions |
Intervention group (sotalol)
Control group (standard care)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic variables, plasma creatinine concentrations, AF, length of stay, mortality, adverse events relating to discontinuation of intervention drug (to include bradycardia and hypotension) Outcomes relevant to the review: AF; length of stay; mortality; bradycardia and hypotension (see notes below) |
|
| Notes |
Funding/declarations of interest: not reported Study dates: February 1999‐December 2000 Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Patients were randomised alternately" Comment: quasi‐randomization |
| Allocation concealment (selection bias) | High risk | See above |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessors (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Mohr 1981.
| Methods | Quasi‐randomized trial, parallel design | |
| Participants |
Total number of randomized participants: 85 Inclusion criteria: scheduled for CABG surgery, all taking beta‐blockers before surgery. Study included participants who were poor risk (left ventricular aneurysm, low ejection fraction, or congestive heart failure) Exclusion criteria: undergoing concomitant valve replacement Type of surgery: elective CABG Baseline characteristics Intervention group (propranolol)
Control group (standard care)
Country: Israel Setting: single centre; hospital |
|
| Interventions |
Intervention group (propranolol)
Control group (standard care)
|
|
| Outcomes |
Outcomes measured/reported by study authors: supraventricular arrhythmias, MI, perioperative death, bronchospasm Outcomes relevant to the review: MI, perioperative death |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Odd or even last numbers on medical records" Comment: quasi‐randomization |
| Allocation concealment (selection bias) | High risk | See above |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessors (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 participants died and were, therefore, not included in analysis of other outcomes |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Myhre 1984.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 41 Inclusion criteria: undergoing CABG, with stable angina pectoris treated with beta‐blocking agents, normotensive and in sinus rhythm Exclusion criteria: not reported Type of surgery: elective CABG Baseline characteristics Intervention group (propranolol)
Control group (standard care)
Country: Norway Setting: single centre; hospital |
|
| Interventions |
Intervention group (propranolol)
Control group (standard care)
|
|
| Outcomes |
Outcomes measured/reported by study authors: supraventricular tachyarrhythmias, acute MI, mortality, hypotension Outcomes relevant to the review: acute MI, mortality |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessors (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant died and was excluded from the analysis (we included this participant in analysis of mortality). An additional participant was added to the study after this loss. We used the total number of randomized participants as 41, rather than 40, to account for this. Loss was < 10% |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Neto 2013.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 68 Inclusion criteria: scheduled for CABG surgery Exclusion criteria: previous use of beta‐blockers; contraindication for beta‐blockers; clinical signs of systolic heart failure; ejection fraction < 50%; CABG associated with other procedures; presence of new Q waves on ECG during ICU stay; MI in previous 30 days Type of surgery: elective CABG surgery Baseline characteristics Intervention group (metoprolol)
Control group (standard care)
Country: Brazil Setting: single centre; hospital |
|
| Interventions |
Intervention group (metoprolol)
Control group (standard care)
|
|
| Outcomes |
Outcomes measured/reported by study authors: myocardial injury (assessed by troponin I concentrations); inotropes > 24 h; intubation > 24 h; stroke; AF; death (in the ICU and in hospital); bradycardia (HR < 50 bpm) Outcomes relevant to the review: stroke; AF; death (in hospital); bradycardia (see notes below) |
|
| Notes |
Funding/declarations of interest: no sources of funding Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessors (detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss of 2 participants after randomization because of meeting exclusion criteria (new Q waves on ECG). We did not include these participants in the number randomized |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Neustein 1994.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 40 Inclusion criteria: scheduled for CABG surgery Exclusion criteria: chronic preoperative beta‐adrenergic blocker therapy, asthma, COPD, severe myocardial dysfunction, intraventricular conduction delay, bundle‐branch block, pre‐existing ST segment depression Type of surgery: elective CABG Baseline characteristics Intervention group (esmolol)
Control group (placebo)
Country: USA Setting: single centre; hospital |
|
| Interventions |
Intervention group (esmolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: intraoperative myocardial ischaemia, bradycardia and hypotension (not defined), MI, haemodynamic variables Outcomes relevant to the review: bradycardia and hypotension (see notes below), MI |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Randomized, triple‐blind, placebo‐controlled trial |
| Blinding of outcome assessors (detection bias) All outcomes | Low risk | Randomized, triple‐blind, placebo‐controlled trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 participants were excluded from analysis (1 in the beta‐blocker group because of bradycardia and hypotension and 1 in the control group as the result of a wide QRS complex that did not allow interpretation of intraoperative myocardial ischaemia). We included data for bradycardia and hypotension in analysis. Loss < 10% |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Nicolson 1990.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 34 Inclusion criteria: good left ventricular function (ejection fraction > 45% or left ventricular end‐diastolic pressure < 12 mmHg); resting HR > 70 bpm; scheduled for elective myocardial revascularization Exclusion criteria: bronchospastic lung disease; having undergone a previous median sternotomy Type of surgery: elective myocardial revascularization Baseline characteristics Intervention group (esmolol)
Control group (placebo)
Country: USA Setting: single centre; hospital |
|
| Interventions |
Intervention group (esmolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic variables, supplemental sufentanil, postoperative events (MI) Outcomes relevant to the review: MI |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization using "a series of sealed envelopes". Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Placebo‐controlled trial. However, it is unclear whether anaesthetists were aware of drug allocation |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss of 3 participants in the control group because of tachycardia. Although all losses were in the control group, overall the number of losses were few |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Nyström 1993.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 101 Inclusion criteria: scheduled for CABG surgery because of severe angina pectoris Exclusion criteria: repeat CABG surgery, if preoperative rhythm was not sinus, or if HR < 45 bpm, known intolerance to beta‐blocking agents, significant pulmonary disease or uncompensated heart failure Type of surgery: elective CABG Baseline characteristics Intervention group (sotalol)
Control group (standard care)
Country: Sweden Setting: single centre; hospital |
|
| Interventions |
Intervention group (sotalol)
Control group
|
|
| Outcomes |
Outcomes measured/reported by study authors: AF, HR, bradycardia (not defined), hypotension (not defined), mortality, ventricular arrhythmias Outcomes relevant to the review: AF, mortality, ventricular arrhythmias, bradycardia and hypotension (see notes below) |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessors (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Ogawa 2013.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 136 Inclusion criteria: undergone isolated off‐pump CABG Exclusion criteria: NYHA class IV; serious cardiac hypofunction with left ventricular ejection fraction < 30%; history of surgery for acute MI, or low tolerance to beta‐blockers Type of surgery: off‐pump CABG (16.9% emergency surgery) Baseline characteristics Intervention group (landiolol)
Control group (standard care)
Country: Japan Setting: not reported |
|
| Interventions |
Intervention group (landiolol)
Control group (standard care)
|
|
| Outcomes |
Outcomes measured/reported by study authors: AF, laboratory markers of ischaemia and inflammation, HR, bradycardia (HR < 50 bpm) Outcomes relevant to the review: AF |
|
| Notes |
Funding/declarations of interest: not reported Study dates: January 2008‐May 2010 Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment group allocation was performed using "a random number program" |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessors (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Oka 1980.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 54 Inclusion criteria: people with stable angina pectoris, scheduled for long‐term propranolol therapy Exclusion criteria: resting HR < 55 bpm; no additional criteria reported Type of surgery: elective CABG Baseline characteristics Intervention group (propranolol continuation)
Control group (withdrawal of existing propranolol 48 h preoperatively)
Control group (withdrawal of existing propranolol 10 h preoperatively)
Country: USA Setting: single centre; hospital |
|
| Interventions |
Intervention group (propranolol)
Control group (withdrawal of existing propranolol 48 h preoperatively)
Control group (withdrawal of existing propranolol 10 h preoperatively)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic variables; supraventricular arrhythmias, mortality, acute MI Outcomes relevant to the review: mortality, acute MI |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessors (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Ormerod 1984.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 60 Inclusion criteria: undergoing CABG surgery for angina pectoris Exclusion criteria: ejection fraction < 40%, known contraindications to propranolol or digoxin. Participants were withdrawn if they had postoperative low cardiac output or continued assisted mechanical ventilation (preventing oral treatment) Type of surgery: elective CABG Baseline characteristics Intervention group (propranolol)
Control group (standard care)
Country: UK Setting: single centre; hospital |
|
| Interventions |
Intervention group (propranolol)
Control group (standard care)
|
|
| Outcomes |
Outcomes measured/reported by study authors: AF, ventricular extrasystoles, bundle branch block Outcomes relevant to the review: AF |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessors (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 participants were excluded after the surgery because oral drug administration was impossible (low cardiac output or assisted ventilation after operation), it is not clear to which group these participants belonged. However, overall loss is < 10% |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | 3 intervention arms; results were presented identically for all groups |
Osada 2012.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 141 Inclusion criteria: scheduled for open‐heart surgery Exclusion criteria: previous AF, undergoing emergency surgery Type of surgery: open‐heart surgery (CABG, valve surgery, thoracic aorta surgery) Baseline characteristics not reported. Study authors state "no significant differences between the two groups in baseline patient characteristics". Country: Japan Setting: single centre; hospital |
|
| Interventions |
Intervention group (landiolol)
Control group (standard care)
|
|
| Outcomes |
Outcomes measured/reported by study authors: AF Outcomes relevant to the Review: AF |
|
| Notes |
Funding/declarations of interest: not reported Study dates: May 2010‐January 2012 Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessors (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses |
| Selective reporting (reporting bias) | Unclear risk | Not detected (in abstract) |
| Other bias | Unclear risk | Limited detail in abstract and therefore, it is not feasible to assess risks of other bias |
Paull 1997.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 100 Inclusion criteria: scheduled for CABG surgery Exclusion criteria: presence of 2nd‐ or 3rd‐degree heart block, bradycardia, asthma requiring bronchodilator therapy, SVT, severe hypoglycaemic episodes, ejection fraction < 30%, need for inotropic support preoperatively Type of surgery: elective CABG Baseline characteristics not reported by group: study authors report "The two study groups were comparable with respect to age, sex, ventricular function, number of coronary grafts, and preoperative beta blocker use" Country: USA Setting: single centre; hospital |
|
| Interventions |
Intervention group (metoprolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: AF, mortality Outcomes relevant to the review: AF, mortality |
|
| Notes |
Funding/declarations of interest: not reported Study dates: August 1990‐April 1995 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Single‐blind trial. We assumed, therefore, that personnel were not blinded to treatment allocation |
| Blinding of outcome assessors (detection bias) All outcomes | High risk | Single‐blind trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Unclear risk | Short report with limited information. Not feasible to assess risks of other bias. In addition, study authors report "Patients who developed AF during the study were treated according to the preferences of their cardiologists". We could not be certain whether participants in either group were treated with additional beta‐blockers. |
Pfisterer 1997.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 255 Inclusion criteria: scheduled for CABG or aortic valve surgery Exclusion criteria: additional procedures (mitral valve replacement or aneurysmectomy), sinus or atrioventricular nodal disease, AF before surgery, severe COPD, or if anti‐arrhythmic therapy was mandatory to control severe ventricular arrhythmias Type of surgery: elective CABG or aortic valve surgery Baseline characteristics Intervention group (sotalol)
Control group (placebo)
Country: Switzerland Setting: single centre; hospital |
|
| Interventions |
Intervention group (sotalol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: supraventricular tachyarrhythmias (AF), ventricular arrhythmias, side effects (to include bradycardia and hypotension ‐ not defined), length of hospital stay Outcomes relevant to the review: AF, ventricular arrhythmias, bradycardia, hypotension, length of hospital stay |
|
| Notes |
Funding/declarations of interest: not reported Study dates: June 1994‐March 1995 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Randomized, placebo‐controlled, double‐blind trial |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | High risk | We noted that participants could be treated with esmolol during surgery. More participants in the placebo group received intraoperative esmolol, which could influence outcome data |
Reves 1990.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 30 Inclusion criteria: scheduled for myocardial revascularization, male and non‐pregnant female patients; SBP > 140 and < 180 mmHg and HR > 70 and < 105 bpm; SBP < 180 mmHg and HR > 80 and < 150 bpm; maintained on beta‐adrenergic blocker until night before surgery Exclusion criteria: < 21 years of age, American Surgical Association class V, presence of AF or flutter, presence of atrioventricular conduction block > 1st degree, history of myocardial infarction within 1 week, clinical or laboratory evidence of severe renal or hepatic failure, history of bronchial asthma, history of drug allergy to beta‐adrenergic blocking drugs, receiving monoamine oxidase inhibitors within 1 month of study, people taking cocaine, heroin, LSD, or other mood‐altering drugs Type of surgery: elective CABG Baseline characteristics Intervention group (esmolol)
Control group (placebo)
Country: USA Setting: single centre; hospital |
|
| Interventions |
Intervention group (esmolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic variables, resolution of tachycardia and hypertension, myocardial ischaemia, hypotension, bradycardia Outcomes relevant to the review: hypotension and bradycardia (see notes below) |
|
| Notes |
Funding/declarations of interest: supported in part by DuPont Critical Care Inc. Study dates: not reported Notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Randomized, double‐blind, placebo‐controlled trial |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Rubin 1987.
| Methods | RCT, parallel design | |
| Participants |
Total number of analysed participants: 77 (number randomized is unclearly reported) Inclusion criteria: scheduled for CABG surgery Exclusion criteria: prior history of AF, lung disease with bronchospasm, brittle diabetes, previous severe bradycardia, high‐degree atrioventricular block or known sensitivity to digoxin or propranolol; ejection fraction < 0.50. Participants who had an intraoperative MI or cerebral vascular accident, who died intraoperatively, or who developed a problem necessitating discontinuation of the study drug were excluded from analysis Type of surgery: elective CABG Baseline characteristics Intervention group (propranolol)
Control group (standard care)
Country: USA Setting: single centre; hospital |
|
| Interventions |
Intervention group (propranolol)
Control group (standard care)
|
|
| Outcomes |
Outcomes measured/reported by study authors: AF, length of hospital stay (not reported by groups); mortality; MI; cerebrovascular events; bronchospasm; hypotension Outcomes relevant to the review: mortality, cerebrovascular events, bronchospasm, hypotension, late mortality (due to MI), and length of hospital stay (see notes below); AF |
|
| Notes |
Funding/declarations of interest: supported by a grant from the Dr I Fund Foundation, New York, USA Study dates: not reported Notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised by lot" Comment: no additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessors (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 27 participants were excluded from analysis after randomization. We were unable to re‐include data for these participants because study authors did not report to which group these participants belonged. |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | No other sources of bias identified |
Sakaguchi 2012.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 60 Inclusion criteria: undergone valve replacement or valvuloplasty; had sinus rhythm on admission to the ICU Exclusion criteria: people with left ventricular ejection fraction < 40% in preoperative ECG or a history of 2nd‐ or 3rd‐degree atrioventricular block Type of surgery: heart valve surgery Baseline characteristics Intervention group (landiolol)
Control group (standard care)
Country: Japan Setting: single centre; hospital |
|
| Interventions |
Intervention group (landiolol)
Control group (standard care)
|
|
| Outcomes |
Outcomes measured/reported by study authors: AF, haemodynamic parameters Outcomes relevant to the review: AF |
|
| Notes |
Funding/declarations of interest: not reported Study dates: April 2008‐July 2010 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "60 subjects were randomised into 2 groups by the coin toss method" Comment: coin tossing is a valid method used to generate a randomization sequence. In our opinion, however, it is highly unlikely that coin tossing would result in exactly 30 participants in each study group. With such a low number of participants, we would expect an unequal number of participants in each trial arm if the randomization sequence was generated with coin flipping |
| Allocation concealment (selection bias) | High risk | See above |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessors (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Salazar 1979.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 42 Inclusion criteria: scheduled for CABG surgery; all participants taking beta‐blocker therapy preoperatively Exclusion criteria: additional procedures such as valve replacement or aneurysmectomy, or people with complicated postoperative courses such as re‐exploration for bleeding or perioperative infarction Type of surgery: elective CABG Baseline characteristics not reported by group: study authors state "There were no statistically significant differences between the two group with respect to age, sex, extent of disease, number of grafts, dosage of propranolol preoperatively, or other pertinent variables" Country: USA Setting: single centre; hospital |
|
| Interventions |
Intervention group (propranolol)
Control group (standard care)
|
|
| Outcomes |
Outcomes measured/reported by study authors: sinus tachycardia; paroxysmal atrial tachycardia, flutter‐fibrillation; multiple atrial/nodal premature contractions; hypotension (SBP < 100 mmHg) Outcomes relevant to the review: AF; hypotension |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessors (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | High risk | Study authors reported no difference in baseline characteristics, however, no table was presented in the study report to allow for comparison. In addition, we noted that participants in either group were given supplemental propranolol to control arrhythmias. This may have influenced outcome data. |
Serruys 2000.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 406 Inclusion criteria: stable or unstable angina pectoris who were scheduled to undergo elective directional coronary atherectomy of a single native primary coronary stenosis Exclusion criteria: contraindications to carvedilol (bradycardia < 50 bpm, 2nd‐ or 3rd‐degree atrioventricular block, obstructive airway disease, insulin‐dependent diabetes): contraindications to a discontinuation of existing beta‐blocker therapy; ineligibility for directional coronary atherectomy. Concomittant treatment with beta‐blockers, alpha‐blockers, anti‐arrhythmics, antioxidants, antiproliferative agents, drugs that influence the pharmacodynamics or kinetics of carvedilol, or anticoagulants was not allowed during the trial Type of surgery: elective directional coronary atherectomy Baseline characteristics (reported for 324 participants) Intervention group (carvedilol)
Control group (placebo)
Countries: Austria; Belgium; France; Germany; Netherlands; Portugal; Spain; Sweden Setting: hospitals; multicentre |
|
| Interventions |
Intervention group (carvedilol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: success of surgery; major adverse events (mortality, MI, need for CABG, repeat procedure. Measured at 7 months postoperatively); adverse events (bradycardia; hypotension ‐ not defined). Study follow‐up at 1, 5, 6, and 7 months Outcomes relevant to the review: mortality; MI; hypotension; bradycardia |
|
| Notes |
Funding/declarations of interest: educational grant from Boehringer Mannheim, Germany Study dates: December 1994‐February 1997 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as double‐blinded study; we assumed that clinicians were not aware of group allocation |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss of 20% participants. Reasons for losses were not reported by group, and it was not feasible to assess whether the reasons were balanced between groups |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Sezai 2011.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 142 Inclusion criteria: scheduled to undergo CABG on cardiopulmonary bypass Exclusion criteria: people with cardiogenic shock; sinus bradycardia, 2nd‐ or 3rd‐degree atrioventricular block, clinical hypothyroidism or hyperthyroidism, history of arrhythmias; undergoing off‐pump surgery Type of surgery: elective CABG Baseline characteristics Intervention group (landiolol)
Control group (placebo)
Country: Japan Setting: single centre; hospital |
|
| Interventions |
Intervention group (landiolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: AF (within 1 week of surgery); mortality; total cost of hospital treatment; multiple laboratory parameters indicating myocardial ischaemia or inflammation; haemodynamic parameters; fluid balance; length of stay; complications (to include congestive heart failure, and stroke); hypotension and bradycardia leading to discontinuation of treatment (cut‐off points not defined) Outcomes relevant to the review: AF; length of hospital stay; mortality; congestive heart failure; stroke |
|
| Notes |
Funding/declarations of interest: supported by grants from the Japanese Ministry of Education, Culture, Sports, Science, and Technology; and from Nihon University School of Medicine. Study authors declare no conflicts Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants "were randomised into two groups by the lottery method". Insufficient detail |
| Allocation concealment (selection bias) | Unclear risk | See above |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Agents were given "in a blinded manner" |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 participants were withdrawn after randomization because of "lack of sufficient data". Losses < 10%, and balanced between groups |
| Selective reporting (reporting bias) | High risk | Study reports clinical trial registration (UMIN000001792); however it is not clear whether study was registered prospectively. We noted that additional outcomes are reported by study authors that are not included in clinical trial registration documents |
| Other bias | High risk | Participants from either group could be treated with beta‐blockers for conditions such as left ventricular dysfunction. 20 participants in the placebo group and 14 participants in the intervention group were treated with beta‐blockers during the perioperative period |
Sezai 2012.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 101 Inclusion criteria: scheduled for CABG surgery under cardiopulmonary bypass Exclusion criteria: people with cardiogenic shock; sinus bradycardia, 2nd‐ or 3rd‐degree atrioventricular block, clinical hypothyroidism or hyperthyroidism, history of arrhythmias; undergoing off‐pump surgery Type of surgery: elective CABG Baseline characteristics Intervention group (landiolol)
Intervention group (landiolol + bisoprolol)
Control group (placebo)
Country: Japan Setting: single centre; hospital |
|
| Interventions | Intervention group (landiolol)
Intervention group (landiolol + bisoprolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: AF (in 1st postoperative week); haemodynamic parameters, multiple lab parameters indicating myocardial ischaemia or inflammation, mortality, acute MI, congestive heart failure, length of hospital stay; hypotension and bradycardia leading to discontinuation of treatment (cut‐off points not defined) Outcomes relevant to the review: AF, mortality, MI, congestive heart failure; length of hospital stay; hypotension and bradycardia leading to discontinuation of treatment (cut‐off points not defined) |
|
| Notes |
Funding/declarations of interest: supported by grants from the Japanese Ministry of Education, Culture, Sports, Science, and Technology; from Takeda Science Foundation; and from Nihon University School of Medicine. Study authors declare no conflicts Study dates: not reported Notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised into three groups by the lottery method'" Comment: insufficient details |
| Allocation concealment (selection bias) | Unclear risk | See above |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Medical staff were blinded for landiolol and placebo treatments but not for group that was given bisoprolol |
| Blinding of outcome assessors (detection bias) All outcomes | Low risk | Personnel involved in outcome measurements were blinded to study groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 participants were excluded after randomization (off‐pump CABG, other surgery done concomitantly), however this was part of the exclusion criteria and we have not included these participants as randomized |
| Selective reporting (reporting bias) | Unclear risk | Study reports clinical trial registration (UMIN000002489). However, it is unclear whether the study is prospectively registered and it is not feasible to assess risk of reporting bias from these documents |
| Other bias | Low risk | Not detected |
Silverman 1982.
| Methods | Quasi‐randomized trial, parallel design | |
| Participants |
Total number of randomized participants: 100 Inclusion criteria: scheduled for CABG surgery without additional cardiac surgical procedures Exclusion criteria: not reported Type of surgery: elective CABG Baseline characteristics Intervention group (propranolol)
Control group (standard care)
Country: USA Setting: single centre; hospital |
|
| Interventions |
Intervention group (propranolol)
Control group (standard care)
|
|
| Outcomes |
Outcomes measured/reported by study authors: supraventricular arrhythmias (to include AF and atrial flutter); perioperative MI, bradycardia or bronchospasm (necessitating discontinuation of study medication) Outcomes relevant to the review: AF and atrial flutter; perioperative MI; bradycardia and bronchospasm (see notes below) |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Randomization was by birthdate" Comment: quasi‐randomization |
| Allocation concealment (selection bias) | High risk | See above |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessors (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | High risk | Either group were given digoxin and propranolol to control supraventricular tachycardias ‐ this affected 6 participants in the control group and 5 participants in the intervention group. This may influence outcome data |
Skiba 2013.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 148 Inclusion criteria: > 18 years of age; in normal sinus rhythm preoperatively; scheduled for cardiac surgery: ejection fraction > 30%; not in NYHA class IV heart failure Exclusion criteria: thyroid disease; elevated serum aspartate aminotransferase or alanine aminotransferase, or gastrointestinal disorders, which may interfere with drug absorption Type of surgery: cardiac surgery (CABG, valve, or both) Baseline characteristics Intervention group (metoprolol)
Control group (standard therapy)
Country: Australia Setting: single centre; hospital |
|
| Interventions |
Intervention group (metoprolol)
Control group (standard therapy)
|
|
| Outcomes |
Outcomes measured/reported by study authors: AF, length of hospital stay; mortality (time point not specified) Outcomes relevant to the review: AF, length of hospital stay; mortality |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Use of sealed envelopes, opened in theatre. No additional details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessors (detection bias) All outcomes | High risk | Open‐label |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study authors reported a large number of losses due to treatment not being given at each time point. However, because doses were given according to haemodynamic parameters, the loss of participants may relate to haemodynamic stability. |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Stephenson 1980.
| Methods | Quasi‐randomized trial, parallel design | |
| Participants |
Total number of analysed participants: 223 (number of randomized participants not clearly reported) Inclusion criteria: scheduled for CABG surgery without additional cardiac surgical procedures Exclusion criteria: cardiac arrhythmias in immediate 18 h postoperative period; bradycardia requiring a pacemaker; low cardiac output requiring catecholamine support Type of surgery: elective CABG Baseline characteristics Intervention group (propranolol)
Control group (standard care)
Country: USA Setting: single centre; hospital |
|
| Interventions |
Intervention group (propranolol)
Control group (standard care)
|
|
| Outcomes |
Outcomes measured/reported by study authors: postoperative cardiac arrhythmias (supraventricular and ventricular) Outcomes relevant to the review: postoperative ventricular arrhythmias; MI; bradycardia (see notes below) |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomized ‐ randomization by date of birth |
| Allocation concealment (selection bias) | High risk | See above |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessors (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Number of randomized participants was unclearly reported with an unspecified number of exclusions in the propranolol group. We re‐included data for 4 excluded participants in the propranolol group for arrhythmia data |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Sun 2011.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 60 Inclusion criteria: people with rheumatic heart disease undergoing elective single mitral valve replacements Exclusion criteria: not reported Type of surgery: elective single mitral valve replacement in participants with rheumatic heart disease Baseline characteristics not reported Country: China Setting: single centre; hospital |
|
| Interventions |
Intervention group (esmolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: cardiac recovery after cardiopulmonary bypass as assessed by occurrence of arrhythmias (to include AF and ventricular arrhythmias), haemodynamic parameters and vasoactive drug use Outcomes relevant to the review: AF, ventricular arrhythmias |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned to two groups by a computer program" |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 control group participants were excluded from the study as the result of pericarditis after randomization. It is not clear to which group these participants belonged, or the number of randomized participants in each group. However, losses are < 10% |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Unclear risk | Short report with limited information; it is not feasible to assess risks of other bias |
Suttorp 1991.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 303 Inclusion criteria: people who had undergone CABG surgery without concomitant procedures (valve replacement, ventricular aneurysmectomy or others), in sinus rhythm Exclusion criteria: people with left ventricular ejection fraction < 40% or postoperative resting ventricular rate < 50 bpm; history of obstructive lung disease, recurrent or persistent ST during 1st 4 h after surgery, conduction disturbances, ventricular tachycardia or fibrillation immediately after surgery, postoperative intra‐aortic balloon pumping, inotropic support for ≥ 6 h, renal failure, potassium ion concentration not between 3.5 and 5.3 mmol/L, or contraindications to beta‐blockers Type of surgery: elective CABG Baseline characteristics Intervention group (sotalol)
Control group (placebo)
Country: Netherlands Setting: single centre; hospital |
|
| Interventions |
Intervention group (sotalol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: supraventricular tachyarrhythmias (to include AF, atrial tachycardia, SVT), mortality, MI (perioperative); adverse events requiring discontinuation of study medication (to include hypotension with bradycardia) Outcomes relevant to the review: AF, mortality, MI (perioperative); adverse events (to include hypotension with bradycardia) |
|
| Notes |
Funding/declarations of interest: not reported Study dates: September 1989‐February 1990 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Randomized, double‐blind, placebo‐controlled trial |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants were excluded from analysis because of protocol violations (per‐protocol analysis); 303 participants enrolled. Few losses (< 10%) |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Unclear risk | Quote: "In patients with persistent sinus tachycardia, trial medication was stopped and treatment with a beta‐blocking agent was begun". Comment: it is unclear from the study results whether any participants had persistent sinus tachycardia |
Vecht 1986.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 132 Inclusion criteria: people undergoing exclusive CABG surgery Exclusion criteria: cardiogenic shock, hypotension, bradycardia, atrioventricular dissociation and supraventricular arrhythmias occurring before randomization Type of surgery: elective CABG Baseline characteristics Intervention group (timolol)
Control group (placebo)
Country: UK Setting: single centre; hospital |
|
| Interventions |
Intervention group (timolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: supraventricular tachyarrhythmias, haemodynamic parameters Outcomes relevant to the review: AF |
|
| Notes |
Funding/declarations of interest: supported by a grant from Merck, Sharp and Dohme (who supplied study drug), and by the Leventis Foundation. We noted that one study author was an employee of Merck, Sharp and Dohme Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Randomized, double‐blind, placebo‐controlled trial |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Wenke 1999.
| Methods | RCT, parallel group | |
| Participants |
Total number of randomized participants: 200 Inclusion criteria: undergoing CABG surgery Exclusion criteria: left ventricular ejection fraction < 30%, bronchial asthma, bradycardia, atrioventricular block, with unstable circulation Type of surgery: elective CABG Baseline characteristics Intervention group (metoprolol)
Control group (standard care)
Country: Germany Setting: single centre; hospital |
|
| Interventions |
Intervention group (metoprolol)
Control group (standard care)
|
|
| Outcomes |
Outcomes measured/reported by study authors: supraventricular arrhythmias, acute MI, length of hospital stay Outcomes relevant to the review: acute MI, length of hospital stay |
|
| Notes |
Funding/declarations of interest: not reported Study dates: January 1998‐June 1998 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessors (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
White 1984.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 41 Inclusion criteria: undergoing CABG surgery Exclusion criteria: contraindications to beta‐blockers; 2nd‐ or 3rd‐degree atrioventricular block; resting sinus bradycardia < 56 bpm; diabetes mellitis; spontaneous hypoglycaemia; allergic rhinitis; bronchospasm of any cause; COPD; treatment with digitalis Type of surgery: elective CABG Baseline characteristics Intervention group (timolol)
Control group (placebo)
Country: USA Setting: single centre; hospital |
|
| Interventions |
Intervention group (timolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: supraventricular tachyarrhythmias (to include AF), mortality, death due to cardiac causes (MI) Outcomes relevant to the review: AF, mortality |
|
| Notes |
Funding/declarations of interest: primary author supported by Odlin Research Fellowship of the Royal Australasian College of Physicians Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Randomized, placebo‐controlled, double‐blind trial |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Williams 1982.
| Methods | Quasi‐randomized trial, parallel design | |
| Participants |
Total number of randomized participants: 50 Inclusion criteria: scheduled for CABG surgery without additional cardiac procedures Exclusion criteria: heart failure requiring use of intra‐aortic balloon pump Type of surgery: elective CABG Baseline characteristics Intervention group (propranolol)
Control group (standard care)
Country: USA Setting: single centre; hospital |
|
| Interventions |
Intervention group (propranolol)
Control group (standard care)
|
|
| Outcomes |
Outcomes measured/reported by study authors: postoperative arrhythmias (supraventricular and ventricular, to include AF) Outcomes relevant to the review: ventricular arrhythmias, AF |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Patients were randomised by odd or even birthdate" Comment: quasi‐randomization |
| Allocation concealment (selection bias) | High risk | See above |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessors (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 participants were excluded from analysis because of postoperative heart failure requiring IABP perioperatively, which we assumed to be an a priori exclusion criteria; we have not included these in the overall number of randomized participants |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Yazicioglu 2002.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 80 Inclusion criteria: people who had undergone elective CABG surgery Exclusion criteria: undergone re‐operation, concomitant valve surgery, ventricular aneurysm resection or other major cardiac procedures; 2nd‐ or 3rd‐degree atrioventricular block; bradycardia; asthma necessitating bronchodilator therapy; COPD; history of preoperative AF and AF episodes; diabetes mellitus; renal failure; left ventricular aneurysm; left ventricular ejection fraction < 30%; needing inotropic support preoperatively Type of surgery: elective CABG surgery Baseline characteristics Intervention group (atenolol)
Control group (placebo)
Country: Turkey Setting: single centre; hospital |
|
| Interventions |
Intervention group (atenolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: mortality (due to stroke); AF, return to sinus rhythm, HR, side effects (to include bradycardia and hypotension) Outcomes relevant to the review: mortality; AF |
|
| Notes |
Funding/declarations of interest: not reported Study dates: March 1999‐December 1999 Notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Although study includes a placebo, it is not reported whether anaesthetists were blinded to study drugs |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant in the intervention group was excluded from further analysis due to death. We re‐included this participant in analysis of mortality. |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
2D: two‐dimensional; ACE inhibitor: angiotensin‐converting‐enzyme inhibitor; AF: atrial fibrillation; AMI: acute myocardial infarction; ASA: American Society of Anesthesiologists; bpm: beats per minute; BP: blood pressure; CABG: coronary artery bypass graft; COPD: chronic obstructive pulmonary disease; CPB: cardiopulmonary bypass; CVA: cerebrovascular accident; ECG: electrocardiogram; GA: general anaesthesia; GI: gastrointestinal; HR: heart rate; IAB: intra‐aortic balloon; IABP: intra‐aortic balloon pump; ICU: intensive care unit; IQR: interquartile range; ITT: intention‐to‐treat; IV: intravenous(ly); LSD: lysergic acid diethylamide; MACE: major adverse cardiovascular event; M/F: male/female; MI: myocardial infarction; NIH: National Institutes of Health; NYHA: New York Heart Association; PCWP: pulmonary capillary wedge; pressure; Q waves: name given to a wave on an electrocardiogram; QRS: a measure of three waves on an electrocardiogram; QTc: corrected QT interval; QT: interval measurement on an electrocardiogram; RCT: randomized controlled trial; SBP: systolic blood pressure; SD: standard deviation; SE: standard error; SEM: standard error of the mean; ST segment: a period between waves on an electrocardiogram; SVA: supraventricular arrhythmia; SVT: supraventricular tachycardia; TIA: transient ischaemic attack
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| De Bruijn 1987 | RCT, adults undergoing CABG surgery. Esmolol vs 5% dextrose in water. Study does not measure or report outcomes relevant to the review |
| Deng 2002 | RCT, beating heart surgery with cardiopulmonary bypass. Esmolol vs standard care. Study does not measure or report outcomes relevant to the review |
| Efe 2014 | RCT, adults undergoing CABG surgery. Esmolol 0.5 mg/kg/min vs 1.5 mg/kg/min vs control. Study does not measure or report outcomes relevant to the review |
| Fujii 2012 | RCT, adults undergoing CABG surgery. Landiolol vs control. We excluded this study because participants in both groups were given oral carvedilol 2.5‐5 mg/day postoperatively. |
| Hamaguchi 2014 | RCT, adults undergoing CABG surgery. Landiolol vs control. We excluded this study because both groups also received catecholamines with all participants in the control group receiving a lower dose with the intention of reducing heart rate; therefore, different doses of catecholamines may influence the results. |
| Imren 2007 | RCT, adults undergoing CABG surgery. Metoprolol vs placebo. We excluded this study because all participants in both groups were also given esmolol to reduce heart rate. |
| Newsome 1986 | RCT, adults undergoing CABG surgery. Esmolol vs placebo. Study does not measure or report outcomes relevant to the review |
| O'Dwyer 1993 | RCT, adults undergoing CABG surgery. Esmolol vs placebo. Study does not measure or report outcomes relevant to the review |
| Rees 2015 | RCT, adults undergoing CABG surgery. Sotalol vs placebo. We excluded this study because the intervention group were also given magnesium. |
| Sezai 2015 | RCT, landiolol vs control. We excluded this study because we noted that participants in both groups were given an oral beta‐blocker as soon as oral treatment was tolerated, which continued during period of outcome assessment, and therefore standard treatment included beta‐blocker treatment. |
| Tempe 1999 | RCT, adults undergoing CABG surgery. Esmolol vs placebo. Study does not measure or report outcomes relevant to the review |
| Yazicioglu 2012 | RCT, adults undergoing CABG surgery. Esmolol vs placebo. Study does not measure or report outcomes relevant to the review |
CABG: coronary artery bypass graft; RCT: randomized controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Bozotlan 2013.
| Methods | RCT, parallel design |
| Participants |
Total number of randomized participants: 32 Inclusion criteria: adults undergoing CABG |
| Interventions | Esmolol infusion for 24 h after cross‐clamp removal vs control (not described) |
| Outcomes |
Outcomes measured/reported by study authors: troponin‐1; AF Outcomes relevant to the review: AF |
| Notes | Study published as an abstract with insufficient information to fully assess eligibility and extract outcome data. We await publication of the full report to assess eligibility |
Ishigaki 2012.
| Methods | RCT, parallel design |
| Participants |
Total number of randomized participants: 49 Inclusion criteria: people with drug‐resistant AF, undergoing catheter ablation Country: Japan |
| Interventions | Landiolol vs placebo, given after the procedure and continued for 3 days |
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic variables; AF Outcomes relevant to the review: AF |
| Notes | Study published as an abstract with insufficient information to fully assess eligibility. We await publication of the full report to assess eligibility |
NCT00959569.
| Methods | RCT, parallel design |
| Participants |
Anticipated number of randomized participants: 200 Inclusion criteria: undergoing cardiac surgery end diastolic diameter > 60 mm and/or an ejection fraction < 50%; written informed consent; >18 years of age Exclusion criteria: previous unusual response to esmolol; inclusion in other randomized studies; esmolol administration in the previous 30 days; emergency operation Country: Italy Setting: hospital; single centre |
| Interventions | Esmolol, 1‐3 mg/kg during cardiac surgery vs placebo |
| Outcomes |
Outcomes measured by study authors: composite endpoint of mortality or number of participants requiring prolonged ICU stay, or both; ventricular fibrillation; low cardiac output syndrome; need for inotropic support; peak postoperative cardiac troponin levels Outcomes relevant to the review: mortality |
| Notes | Study is completed. Awaiting publication of study results in order to assess eligibility for the review |
PACTR201801003026226.
| Methods | RCT, parallel design |
| Participants |
Anticipated number of randomized participants: 70 Inclusion criteria: 18‐70 years of age; people with rheumatic heart disease undergoing valve replacement therapy Exclusion criteria: with pericarditis Country: Egypt |
| Interventions | Esmolol vs normal saline |
| Outcomes | Outcomes measured by study authors: recovery time; ventricular fibrillation |
| Notes | Study is completed. Awaiting publication of study results in order to assess eligibility for the review |
AF: atrial fibrillation; ASA: American Society of Anesthesiologists; BIS: bispectral index; RCT: randomized controlled trial
Characteristics of ongoing studies [ordered by study ID]
Chictr‐ior‐16009203.
| Trial name or title | Perioperative esmolol injection and acute kidney injury associated with CPB after cardiac surgery |
| Methods | RCT, parallel design |
| Participants |
Anticipated number of randomized participants: 144 Inclusion criteria: ≥ 18 years of age; undergoing heart surgery, CABG with CPB, valve surgery with CPB, CABG with other heart surgery, valve surgery with other heart surgery; agreement to participate Exclusion criteria: emergency heart surgery; heart surgery without CPB; heart surgery with deep hypothermia; ascending aortic surgery; correction of congenital heart disease; allergy to esmolol; liver and kidney dysfunction; severe dehydration and severe malnutrition or Hb < 10 g/dL; mental or other reasons; participating in other clinical drug research in the past 3 months; cardiac shock or placement of IABP Country: China Setting: hospital |
| Interventions |
|
| Outcomes | Acute kidney injury; mortality at 30 days |
| Starting date | Not specified |
| Contact information | Aijun XU; ajxu@tjh.tjmu.edu.cn |
| Notes |
UMIN000004216.
| Trial name or title | Efficacy of beta‐blocker on the autonomic nervous activities and the onset of atrial fibrillation or flutter in patients after cardiac surgery |
| Methods | RCT, parallel design |
| Participants |
Anticipated number of randomized participants: 60 Inclusion criteria: undergoing cardiac surgery Exclusion criteria: chronic or paroxysmal atrial fibrillation; receiving antiarrhythmic agents: Ia, IIc and IV groups; sick sinus syndrome; > 2nd‐degree atrioventricular block: ejection fraction < 30%; bronchial asthma; receiving beta‐blockers Country: Japan Setting: hospital; single centre |
| Interventions |
|
| Outcomes | AF/flutter; changes to HR |
| Starting date | 1 November 2009 |
| Contact information | Kazuma Kanetsuki; kkazuma@med.shimane‐u.ac.jp |
| Notes |
AF: atrial fibrillation; CABG: coronary artery bypass; CPB: cardiopulmonary bypass; HR: heart rate; IABP: intra‐aortic balloon pump; IV: intravenous(ly); RCT: randomized controlled trial
Differences between protocol and review
Differences between the previous review and the updated review
The previous version of the review assessed the effectiveness of perioperative beta‐blockers in cardiac and non‐cardiac surgery (Blessberger 2018). The previous version has now been split into two reviews according to type of surgery. This review assesses the evidence in cardiac surgery only; we made appropriate changes throughout the review to reflect evidence for only this type of surgery. Data for non‐cardiac surgery can be found in Blessberger 2019. Whilst updating the review, we made changes to ensure that it met current Cochrane standards (Methodological Expectations of Cochrane Intervention Reviews (MECIR)), adding additional subheadings where necessary. In addition, we made the following changes.
Authors: we added three new review authors (Sharon Lewis, Michael Pritchard, Lizzy Fawcett), and we removed three review authors who had been involved in previous updates (Danyel Azar, Martin Schillinger, Franz Wiesbauer).
Objectives: we re‐worded these to account for the inclusion of only cardiac surgery.
Types of studies: we clarified the inclusion of quasi‐randomized studies.
Types of interventions: we clarified the routes by which beta‐blockers could be given; previously, we had stated 'any route', but we did not intend to include beta‐blockers that were administered topically. We clarified the exclusion of studies (or study arms within a multi‐arm study) in which a supplementary agent was given with a beta‐blocker; we used this criteria in the previous version of the review. We added an exclusion criteria for the standard care control group, and excluded studies in which participants were given an agent that was not given to those in the intervention or in which all participants in the control group were given a beta‐blockers; this was because we expected such comparison groups could introduce too much contamination in the results.
Types of outcome measures: in this section, we clarified the exclusion of studies that did not measure the review outcomes (this was a change from protocol made in the previous version of the review). We removed some outcomes from this update (myocardial ischaemia, supraventricular arrhythmias (except for atrial fibrillation), ventricular extrasystoles, bronchospasm, and cost of care). This decision was made in order to increase the usability, manageability, and focus of the review. We used the work of Myles and colleagues as a guide to select outcomes that we considered to be most important to the user of the review (Myles 2016), and we sought advice from the Cochrane editorial team to approve this change. Data from these outcomes are available in the previous publication of this review (Blessberger 2018). We included atrial fibrillation and atrial flutter as a distinct outcome that was previously reported with data for supraventricular arrhythmias.
Search methods: we made changes to the search strategies to include terms for beta‐blockers. We did not search additional databases as part of a search of other resources, nor did we search the National Research Register or the Meta register of controlled trials.
Data extraction and management: we used an amended template for collecting data that was more familiar to the new review authors who were responsible for data extraction in this review. In order to improve transparency, we added additional detail to the tables in Characteristics of included studies, and we created a summary table of the participant risk factors (Appendix 9).
Unit of analysis issues: we clarified that we used the 'halving method' for the control group in multi‐arm studies during subgroup analysis by the type of beta‐blocker, if necessary.
Assessment of reporting biases: we did not conduct a 'trim and fill' method after generation of funnel plots. We conducted assessment of reporting bias only through visual inspection of funnel plots.
Assessment of heterogeneity: we did not use meta‐regression to explore heterogeneity. Previously, we identified effect modifiers and assessed them in meta‐regression. We could not be certain of our confidence in the findings from so many effect modifiers, and felt that this additional analysis detracted from the primary analysis. In the review, we explained that we considered clinical and methodological differences between studies as well as consideration of statistical heterogeneity. We collected clinical differences between study participants and presented this in a table. In addition, we expanded the information reported in the Characteristics of included studies tables to present information to the readers of possible effect modifiers.
Data synthesis: we used a random‐effects model, which allowed for the potential variation between participants (Borenstein 2010). We did not calculate optimal information sizes using trial sequential analysis (TSA); for optimal information sizes we used data previously calculated (Blessberger 2018).
Sensitivity analysis: we evaluated the decision to include studies published prior to 2000; these studies may use clinical management practices that are not consistent with current standards. We evaluated the decision to use RR with a random‐effects model for effects in which events were rare; in sensitivity analysis, for all‐cause mortality, we used the Peto odds ratio.
'Summary of findings' table: we changed the outcomes, replacing supraventricular arrhythmias with atrial fibrillation.
Differences between protocol and previous review versions
Changes relevant to Blessberger 2014 and Blessberger 2018.
Change to inclusion criteria regarding general anaesthesia. The review included studies if more than 100 randomly assigned participants were operated on under general anaesthesia, or more than 70% of participants received general anaesthesia. This change is not relevant to the current review because cardiac surgery would always be conducted under general anaesthesia.
Bronchospasm and cost of care were added as secondary outcomes. These outcomes have been removed in the current version (see above)
Meta‐regression was conducted to evaluate potential effect modifiers. This was not completed in the current version (see above).
Trial sequential analysis was conducted. This was not completed in the current version (see above).
Risk ratio was used, rather than the odds ratio.
Data were reported separately for cardiac and non‐cardiac surgeries.
Contributions of authors
Contributions made in previous versions of the review can be found in Blessberger 2014 and Blessberger 2018.
Hermann Blessberger (HB), Sharon Lewis (SL), Michael Pritchard (MP), Lizzy Fawcett (LF), Juergen Kammler (JK), Hans Domanovits (HD), Oliver Schlager (OS), Brigitte Wildner (BW), Clemens Steinwender (CS)
Conceiving of the review: previous review author F Wiesbauer
Co‐ordinating the review: SL
Undertaking manual searches: SL, BW, Janne Vendt (Information Specialist, Cochrane Anaesthesia)
Screening search results: SL, MP
Organizing retrieval of papers: SL, MP
Screening retrieved papers against inclusion criteria: SL, MP, HB
Appraising quality of papers: SL, MP, HB
Abstracting data from papers: SL, MP, LF
Managing data for the review: SL
Entering data into Review Manager 5 (RevMan 5; Review Manager 2014): SL, MP, LF
Analysing RevMan 5 statistical data: SL, HB
Interpreting data: SL, HB, JK, CS, OS, HD
Making statistical inferences: SL, HB, JK, CS
Writing the review: SL, HB
Securing funding for the review: Cochrane Anaesthesia
Performing previous work that was the foundation of the present study: E. Villanueva, R. Johnston and A. Rauli (preparation of first protocol draft)
Serving as guarantor for the review (one author): HB
Taking responsibility for reading and checking the review before submission: HB
Sources of support
Internal sources
None, Other.
External sources
NIHR Cochrane Incentive Awards Scheme, 2018, UK.
Declarations of interest
Hermann Blessberger: none known
Sharon Lewis: none known
Michael Pritchard: none known
Lizzy Fawcett: none known
Hans Domanovits: none known
Oliver Schlager: none known
Brigitte Wildner: none known
Juergen Kammler: none known
Clemens Steinwender: I have received speaker's honoraria from MSD, Sanofi‐Aventis, Boehringer‐Ingelheim, Bayer, Medtronic, Biotronic, Abbott, St. Jude Medical and Boston Scientific. Sanofi‐Aventis, Boehringer‐Ingelheim Europe, Medtronic, Biotronik, Bayer Austria, Abbott Vascular, St. Jude Medical and Boston Scientific do not produce, market or distribute any of the studied drug entities. MSD have one beta‐blocker (timolol) in their portfolio.
Edited (no change to conclusions)
References
References to studies included in this review
Abel 1983 {published data only}
- Abel RM, Gelder HM, Pores IH, Liguori J, Gielchinsky I, Parsonnet V. Continued propranolol administration following coronary bypass surgery. Antiarrhythmic effects. Archives of Surgery 1983;118(6):727‐31. [PUBMED: 6601941] [DOI] [PubMed] [Google Scholar]
Ali 1997 {published data only}
- Ali IM, Sanalla AA, Clark V. Beta‐blocker effects on postoperative atrial fibrillation. European Journal of Cardio‐Thoracic Surgery 1997;11(6):1154‐7. [PUBMED: 9237602] [DOI] [PubMed] [Google Scholar]
Arar 2007 {published data only}
- Arar C, Colak A, Alagol A, Uzer SS, Ege T, Turan N, et al. The use of esmolol and magnesium to prevent haemodynamic responses to extubation after coronary artery grafting. European Journal of Anaesthesiology 2007;24(10):826‐31. [PUBMED: 17583595] [DOI] [PubMed] [Google Scholar]
Auer 2004 {published data only}
- Auer J, Weber T, Berent R, Puschmann R, Hartl P, Ng C, et al. A comparison between oral antiarrhythmic drugs in the prevention of atrial fibrillation after cardiac surgery: the pilot study of prevention of postoperative atrial fibrillation (SSPAF), a randomized, placebo‐controlled trial. American Heart Journal 2004;147(4):636‐43. [PUBMED: 15077078] [DOI] [PubMed] [Google Scholar]
Babin‐Ebell 1996 {published data only}
- Babin‐Ebell J, Keith PR, Elert O. Efficacy and safety of low‐dose propranolol versus diltiazem in the prophylaxis of supraventricular tachyarrhythmia after coronary artery bypass grafting. European Journal of Cardio‐Thoracic Surgery 1996;10(6):412‐6. [PUBMED: 8817135] [DOI] [PubMed] [Google Scholar]
Bert 2001 {published data only}
- Bert A, Reinert SE, Singh AK. A beta‐blocker, not magnesium, is effective prophylaxis for atrial tachyarrhythmias after coronary artery bypass graft surgery. Journal of Cardiothoracic and Vascular Anesthesia 2001;15(2):204‐9. [PUBMED: 11312480] [DOI] [PubMed] [Google Scholar]
Bignami 2017 {published data only}
- Bignami E, Guarnieri M, Franco A, Gerli C, Luca M, Monaco F, et al. Esmolol before cardioplegia and as cardioplegia adjuvant reduces cardiac troponin release after cardiac surgery. A randomized trial. Perfusion 2017;32(4):313‐20. [PUBMED: 27932571] [DOI] [PubMed] [Google Scholar]
Booth 2004 {published data only}
- Booth JV, Ward EE, Colgan KC, Funk BL, El‐Moalem H, Smith MP, et al. Metoprolol and coronary artery bypass grafting surgery: does intraoperative metoprolol attenuate acute β‐adrenergic receptor desensitization during cardiac surgery?. Anaesthesia and Analgesia 2004;98(5):1224‐31. [PUBMED: 15105192] [DOI] [PubMed] [Google Scholar]
But 2006 {published data only}
- But AK, Yapici E, Erdil F, Öztürk E, Gedik E, Durmus M, et al. The effects of esmolol and magnesium on blood glucose regulation in the patients with type II diabetes mellitus undergoing CABG surgery. Gogus‐Kalp‐Damar Anestezi ve Yogun Bakim Dernegi Dergesi 2006;124(4):143‐8. [Google Scholar]
Connolly 2003 {published data only}
- Connolly SJ, Cybulsky I, Lamy A, Roberts RS, Tech M, O'Brien B, et al. Double‐blind, placebo‐controlled, randomized trial of prophylactic metoprolol for reduction of hospital length of stay after heart surgery: the beta‐blocker length of stay (BLOS) study. American Heart Journal 2003;145(2):226‐32. [PUBMED: 12595838] [DOI] [PubMed] [Google Scholar]
Cork 1995 {published data only}
- Cork RC, Kramer TH, Dreischmeier B, Behr S, DiNardo JA. The effect of esmolol given during cardiopulmonary bypass. Anesthesia and Analgesia 1995;80(1):28‐40. [PUBMED: 7802296] [DOI] [PubMed] [Google Scholar]
Daudon 1986 {published data only}
- Daudon P, Corcos T, Gandjabakhch I, Levasseur JP, Cabrol A, Cabrol C. Prevention of atrial fibrillation or flutter by acebutolol after coronary bypass grafting. American Journal of Cardiology 1986;58(10):933‐6. [PUBMED: 3535474] [DOI] [PubMed] [Google Scholar]
De Azevedo Lúcio 2003 {published data only}
- Azevedo Lúcio E, Flores A, Blacher C, Leaes PE, Lucchese FA, Pinto Ribeiro J. Effectiveness of metoprolol in preventing atrial fibrillation and flutter in the postoperative period of coronary artery bypass graft surgery. Arquivos Brasileiros de Cardiologia 2003;82(1):37‐41. [PUBMED: 14978593] [PubMed] [Google Scholar]
Dy 1998 {published data only}
- Dy J, Jayasundera T, Kapadala D, Cuperman C, Whitman G, DiSesa V, et al. Post‐operative atrial fibrillation—a randomized trial of metoprolol, flecainide, and placebo. Journal of the American College of Cardiology 1998;31(Suppl A):324A. [Google Scholar]
Evrard 2000 {published data only}
- Evrard P, Gonzalez M, Jamart J, Malhomme B, Blommaert D, Eucher P, et al. Prophylaxis of supraventricular and ventricular arrhythmias after coronary artery bypass grafting with low‐dose sotalol. Annals of Thoracic Surgery 2000;70(1):151‐6. [PUBMED: 10921700] [DOI] [PubMed] [Google Scholar]
Forlani 2002 {published data only}
- Forlani S, Paulis R, Notaris S, Nardi P, Tomai F, Proietti I, et al. Combination of sotalol and magnesium prevents atrial fibrillation after coronary artery bypass grafting. Annals of Thoracic Surgery 2002;74(3):720‐6. [PUBMED: 12238830] [DOI] [PubMed] [Google Scholar]
Gandhi 2007 {published data only}
- Gandhi PS, Goyal RK, Jain AR, Mallya BS, Gupta VM, Shah DS, et al. Beneficial effects of carvedilol as a concomitant therapy to angiotensin‐converting enzyme inhibitor in patients with ischemic left ventricular systolic dysfunction. Canadian Journal of Physiology and Pharmacology 2007;85(2):193‐9. [PUBMED: 17487260] [DOI] [PubMed] [Google Scholar]
Girard 1986 {published data only}
- Girard D, Shulman BJ, Thys DM, Mindich BP, Mikula SK, Kaplan JA. The safety and efficacy of esmolol during myocardial revascularization. Anesthesiology 1986;65(2):157‐64. [PUBMED: 3526984] [DOI] [PubMed] [Google Scholar]
Gomes 1999 {published data only}
- Gomes JA, Ip J, Santoni‐Rugiu F, Mehta D, Ergin A, Lansman S, et al. Oral d,l sotalol reduces the incidence of postoperative atrial fibrillation in coronary artery bypass surgery patient: a randomized, double‐blind, placebo‐controlled study. Journal of the American College of Cardiology 1999;34(2):334‐9. [PUBMED: 10440141] [DOI] [PubMed] [Google Scholar]
Graham 1996 {published data only}
- Graham SP, Hasnain S, Celano J. Low dose but not medium dose beta blockers reduce post‐operative atrial fibrillation. Circulation 1996;94(8 Suppl 1):190‐1. [Google Scholar]
Hammon 1984 {published data only}
- Hammon JW, Wood AJ, Prager RL, Wood M, Muirhead J, Bender HW Jr. Perioperative beta blockade with propranolol: reduction in myocardial oxygen demands and incidence of atrial and ventricular arrhythmias. Annals of Thoracic Surgery 1984;38(4):363‐7. [PUBMED: 6385890] [DOI] [PubMed] [Google Scholar]
Harrison 1987 {published data only}
- Harrison L, Ralley FE, Wynands E, Robbins GR, Sami M, Ripley R, et al. The role of an ultra short‐acting adrenergic blocker (esmolol) in patients undergoing coronary artery bypass surgery. Anesthesiology 1987;66(4):413‐8. [PUBMED: 2881505] [DOI] [PubMed] [Google Scholar]
Ivey 1983 {published data only}
- Ivey MF, Ivey TD, Bailey WW, Williams DB, Hessel EA, Miller DW. Influence of propranolol on supraventricular tachycardia early after coronary artery revascularization. Journal of Cardiovascular Surgery 1983;85(2):214‐8. [PUBMED: 6337306] [PubMed] [Google Scholar]
Jacquet 1994 {published data only}
- Jacquet L, Evenepoel M, Marenne F, Evrard P, Verhelst R, Dion R, et al. Hemodynamic effects and safety of sotalol in the prevention of supraventricular arrhythmias after coronary artery bypass surgery. Journal of Cardiothoracic and Vascular Anesthesia 1994;8(4):431‐6. [PUBMED: 7948800] [DOI] [PubMed] [Google Scholar]
Janssen 1986 {published data only}
- Janssen J, Loomans L, Harink J, Taams M, Brunninkhuis L, Starre P, et al. Prevention and treatment of supraventricular tachycardia shortly after coronary artery bypass grafting: a randomized open trial. Angiology 1986;37(8):601‐9. [PUBMED: 2874755] [DOI] [PubMed] [Google Scholar]
Khuri 1987 {published data only}
- Khuri SF, Okike N, Josa M, Salm TJ, Assoussa S, Leone L, et al. Efficacy of nadolol in preventing supraventricular tachycardia after coronary artery bypass grafting. American Journal of Cardiology 1987;60(6):51‐8D. [PUBMED: 3498356] [DOI] [PubMed] [Google Scholar]
Kurian 2001 {published data only}
- Kurian SM, Evans R, Fernandes NO, Sherry KM. The effect of an infusion of esmolol on the incidence of myocardial ischaemia during tracheal extubation following coronary artery surgery. Anaesthesia 2001;56(12):1163‐8. [PUBMED: 11736772] [DOI] [PubMed] [Google Scholar]
Lamb 1988 {published data only}
- Lamb RK, Prabhakar G, Thorpe JA, Smith S, Norton R, Dyde JA. The use of atenolol in the prevention of supraventricular arrhythmias following coronary artery surgery. European Heart Journal 1988;9(1):32‐6. [PUBMED: 3257915] [PubMed] [Google Scholar]
Liu 2016 {published data only}
- Liu X, Shao F, Yang L, Jia Y. A pilot study of perioperative esmolol for myocardial protection during on‐pump cardiac surgery. Experimental and Therapeutic Medicine 2016;12(5):2990‐6. [PUBMED: 27882105] [DOI] [PMC free article] [PubMed] [Google Scholar]
Martinussen 1988 {published data only}
- Martinussen HJ, Lolk A, Szczepanski C, Alstrup P. Supraventricular tachyarrhythmias after coronary bypass surgery ‐ a double blind randomized trial of prophylactic low dose propranolol. Thoracic Cardiovascular Surgeon 1988;36(4):206‐7. [PUBMED: 2903581] [DOI] [PubMed] [Google Scholar]
Matangi 1985 {published data only}
- Matangi MF, Neutze JM, Graham KJ, Hill DG, Kerr AR, Barratt‐Boyes BG. Arrhythmia prophylaxis after aorta‐coronary bypass. The effect of minidose propranolol. Journal of Thoracic and Cardiovascular Surgery 1985;89(3):439‐43. [PUBMED: 3871883] [PubMed] [Google Scholar]
Matangi 1989 {published data only}
- Matangi MF, Strickland J, Garbe GJ, Habib N, Basu A, Burgess JJ, et al. Atenolol for the prevention of arrhythmias following coronary artery bypass grafting. Canadian Journal of Cardiology 1989;5(4):229‐34. [PUBMED: 2659151] [PubMed] [Google Scholar]
Materne 1985 {published data only}
- Materne P, Larbuisson R, Collignon P, Limet R, Kulbertus H. Prevention by acebutolol of rhythm disorders following coronary bypass surgery. International Journal of Cardiology 1985;8(3):275‐83. [PUBMED: 3894250] [DOI] [PubMed] [Google Scholar]
Matsuura 2001 {published data only}
- Matsuura K, Takahara Y, Sudo Y, Ishida K. Effect of sotalol in the prevention of atrial fibrillation following coronary artery bypass grafting. Japanese Journal of Thoracic and Cardiovascular Surgery 2001;49(10):614‐7. [PUBMED: 11692587] [DOI] [PubMed] [Google Scholar]
Mohr 1981 {published data only}
- Mohr R, Smolinsky A, Goor DA. Prevention of supraventricular tachyarrhythmia with low‐dose propranolol after coronary bypass. Journal of Thoracic and Cardiovascular Surgery 1981;81(6):840‐5. [PUBMED: 7015021] [PubMed] [Google Scholar]
Myhre 1984 {published data only}
- Myhre ES, Sorlie D, AArbakke J, Hals PA, Straume B. Effects of low dose propranolol after coronary bypass surgery. Journal of Cardiovascular Surgery 1984;25(4):348‐51. [PUBMED: 6148345] [PubMed] [Google Scholar]
Neto 2013 {published data only}
- Neto RJ, Gun C, Ramos RF, Almeida AF, Issa M, Amato VL, et al. Myocardial protection with prophylactic oral metoprolol during coronary artery bypass grafting surgery: evaluation by troponin I. Brazilian Journal of Cardiovascular Surgery 2013;28(4):449‐54. [PUBMED: 24598948] [DOI] [PMC free article] [PubMed] [Google Scholar]
Neustein 1994 {published data only}
- Neustein SM, Bronheim DS, Lasker S, Reich DL, Thys DM. Esmolol and intraoperative myocardial ischemia: a double‐blind study. Journal of Cardiothoracic and Vascular Anesthesia 1994;8(3):273‐7. [PUBMED: 7914754] [DOI] [PubMed] [Google Scholar]
Nicolson 1990 {published data only}
- Nicolson SC, Jobes DR, Quinlan JJ. Cardiovascular effects of esmolol in patients anesthetized with sufentanil‐pancuronium for myocardial revascularization. Journal of Cardiothoracic Anesthesia 1990;5(Suppl 2):55‐8. [Google Scholar]
Nyström 1993 {published data only}
- Nyström U, Edvardsson N, Berggren H, Pizzarelli GP, Radergran K. Oral sotalol reduces the incidence of atrial fibrillation after coronary artery bypass surgery. Thoracic and Cardiovascular Surgeon 1993;41(1):34‐7. [PUBMED: 8103611] [DOI] [PubMed] [Google Scholar]
Ogawa 2013 {published data only}
- Ogawa S, Okawa Y, Goto Y, Aoki M, Baba H. Perioperative use of beta blocker in coronary artery bypass grafting. Asian Cardiovascular and Thoracic Annals 2013;21(3):265‐9. [PUBMED: 24570490] [DOI] [PubMed] [Google Scholar]
Oka 1980 {published data only}
- Oka Y, Frishman W, Becker RM, Kadish A, Strom J, Matsumoto M, et al. Clinical pharmacology of the new beta‐adrenergic blocking drugs. Part 10. Beta‐adrenoceptor blockade and coronary artery surgery. American Heart Journal 1980;99(2):255‐69. [PUBMED: 6101516] [DOI] [PubMed] [Google Scholar]
Ormerod 1984 {published data only}
- Ormerod OJ, McGregor CG, Stone DL, Wisbey C, Petch MC. Arrhythmias after coronary bypass surgery. British Heart Journal 1984;51(6):618‐21. [PUBMED: 6610435] [DOI] [PMC free article] [PubMed] [Google Scholar]
Osada 2012 {published data only}
- Osada H, Nakajima H, Masuyama S, Morishima M, Su T. Landiolol hydrochloride: prevention of atrial fibrillation after open‐heart surgery. European Heart Journal 2012;33(Suppl 1):65. [Google Scholar]
Paull 1997 {published data only}
- Paull DL, Tidwell SL, Guyton SW, Harvey E, Woolf RA, Holmes JR, et al. Beta‐blockade to prevent atrial dysrhythmias following coronary bypass surgery. American Journal of Surgery 1997;173(5):419‐21. [PUBMED: 9168080] [DOI] [PubMed] [Google Scholar]
Pfisterer 1997 {published data only}
- Pfisterer ME, Klöter‐Weber UC, Huber M, Osswald S, Buser PT, Skarvan K, et al. Prevention of supraventricular tachyarrhythmias after open heart operation by low‐dose sotalol: a prospective, double‐blind, randomized, placebo‐controlled study. Annals of Thoracic Surgery 1997;64(4):1113‐9. [PUBMED: 9354537] [DOI] [PubMed] [Google Scholar]
Reves 1990 {published data only}
- Reves JG, Croughwell ND, Hawkins E, Smith LR, Jacobs JR, Rankin S, et al. Esmolol for treatment of intraoperative tachycardia and/or hypertension in patients having cardiac operations. Bolus loading technique. Journal of Thoracic and Cardiovascular Surgery 1990;100(2):221‐7. [PUBMED: 1974664] [PubMed] [Google Scholar]
Rubin 1987 {published data only}
- Rubin DA, Nieminski KE, Reed GE, Herman MV. Predictors, prevention, and long‐term prognosis of atrial fibrillation after coronary artery bypass graft operation. Journal of Thoracic and Cardiovascular Surgery 1987;94(3):331‐5. [PUBMED: 3306163] [PubMed] [Google Scholar]
Sakaguchi 2012 {published data only}
- Sakaguchi M, Sasaki Y, Hirai H, Hosono M, Nakahira A, Seo H, et al. Efficacy of landiolol hydrochloride for prevention of atrial fibrillation after heart valve surgery. International Heart Journal 2012;53(6):359‐63. [PUBMED: 23258136] [DOI] [PubMed] [Google Scholar]
Salazar 1979 {published data only}
- Salazar C, Frishman W, Friedman S, Patel J, Lin YT, Oka Y, et al. Beta‐blockade therapy for supraventricular tachyarrhythmias after coronary surgery: a propranolol withdrawal syndrome?. Angiology 1979;30(12):816‐9. [PUBMED: 316976] [DOI] [PubMed] [Google Scholar]
Serruys 2000 {published data only}
- Serruys PW, Foley DP, Hofling B, Puel J, Glogar HD, Seabra‐Gomes R, et al. Carvedilol for prevention of restenosis after directional coronary atherectomy: final results of the European carvedilol atherectomy restenosis (EUROCARE) trial. Circulation 2000;101(13):1512‐8. [PUBMED: 10747343] [DOI] [PubMed] [Google Scholar]
Sezai 2011 {published data only}
- Sezai A, Minami K, Nakai T, Hata M, Yoshitake I, Wakui S, et al. Landiolol hydrochloride for prevention of atrial fibrillation after coronary artery bypass grafting: new evidence from the PASCAL trial. Journal of Thoracic and Cardiovascular Surgery 2011;141(6):1478‐87. [PUBMED: 21269646] [DOI] [PubMed] [Google Scholar]
Sezai 2012 {published data only}
- Sezai A, Nakai T, Hata M, Yoshitake I, Shiono M, Kunimoto S, et al. Feasibility of landiolol and bisoprolol for prevention of atrial fibrillation after coronary artery bypass grafting: a pilot study. Journal of Thoracic and Cardiovascular Surgery 2012;144(5):1241‐8. [PUBMED: 22858430] [DOI] [PubMed] [Google Scholar]
Silverman 1982 {published data only}
- Silverman NA, Wright R, Levitsky S. Efficacy of low‐dose propranolol in preventing postoperative supraventricular tachyarrhythmias: a prospective, randomized study. Annals of Surgery 1982;196(2):194‐7. [PUBMED: 6979982] [DOI] [PMC free article] [PubMed] [Google Scholar]
Skiba 2013 {published data only}
- Skiba MA, Pick AW, Chaudhuri K, Bailey M, Krum H, Kwa LJ, et al. Prophylaxis against atrial fibrillation after cardiac surgery: beneficial effect of perioperative metoprolol. Heart Lung and Circulation 2013;22(8):627‐33. [PUBMED: 23465653] [DOI] [PubMed] [Google Scholar]
Stephenson 1980 {published data only}
- Stephenson LW, MacVaugh H, Tomasello DN, Josephson ME. Propranolol for prevention of postoperative cardiac arrhythmias: a randomized study. Annals of Thoracic Surgery 1980;29(2):113‐6. [PUBMED: 6965579] [DOI] [PubMed] [Google Scholar]
Sun 2011 {published data only}
- Sun J, Ding Z, Qian Y. Effect of short‐acting beta blocker on the cardiac recovery after cardiopulmonary bypass. Journal of Cardiothoracic Surgery 2011;6(99):no pagination. [PUBMED: 21854625] [DOI] [PMC free article] [PubMed] [Google Scholar]
Suttorp 1991 {published data only}
- Suttorp MJ, Kingma JH, Peels HO, Koomen EM, Tijssen JG, Hemel NM, et al. Effectiveness of sotalol in preventing supraventricular tachyarrhythmias shortly after coronary artery bypass grafting. American Journal of Cardiology 1991;68(11):1163‐9. [PUBMED: 1951075] [DOI] [PubMed] [Google Scholar]
Vecht 1986 {published data only}
- Vecht RJ, Nicolaides EP, Ikeuke JK, Liassides C, Cleary J, Cooper WB. Incidence and prevention of supraventricular tachyarrhythmias after coronary bypass surgery. International Journal of Cardiology 1986;13(2):125‐34. [PUBMED: 3539826] [DOI] [PubMed] [Google Scholar]
Wenke 1999 {published data only}
- Wenke K, Parsa MH, Imhof M, Kemkes BM. Efficacy of metoprolol in prevention of supraventricular arrhythmias after coronary artery bypass grafting. Zeitschrift für Kardiologie 1999;88(9):647‐52. [PUBMED: 10525926] [DOI] [PubMed] [Google Scholar]
White 1984 {published data only}
- White HD, Antman EM, Glynn MA, Collins JJ, Cohn LH, Shemin RJ, et al. Efficacy and safety of timolol for prevention of supraventricular tachyarrhythmias after coronary artery bypass surgery. Circulation 1984;70(3):479‐84. [PUBMED: 6378423] [DOI] [PubMed] [Google Scholar]
Williams 1982 {published data only}
- Williams JB, Stephenson LW, Holford FD, Langer T, Dunkman WB, Josephson ME. Arrhythmia prophylaxis using propranolol after coronary artery surgery. Annals of Throacic Surgery 1982;34(4):435‐8. [PUBMED: 6982689 ] [DOI] [PubMed] [Google Scholar]
Yazicioglu 2002 {published data only}
- Yazicioglu L, Eryilmaz S, Sirlak M, Inan MB, Aral A, Tasoz R, et al. The effect of preoperative digitalis and atenolol combination on postoperative atrial fibrillation incidence. European Journal of Cardio‐Thoracic Surgery 2002;22(3):397‐401. [PUBMED: 12204730] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
De Bruijn 1987 {published data only}
- Bruijn NP, Croughwell N, Reves JG. Hemodynamic effects of esmolol in chronically beta‐blocked patients undergoing aortocoronary bypass surgery. Anesthesia and Analgesia 1987;66(2):137‐41. [PUBMED: 2880530] [PubMed] [Google Scholar]
Deng 2002 {published data only}
- Deng YK, Wei F, Li ZL, Zhang DG, Yang SY. Esmolol protects the myocardium and facilitates direct version intracardiac operation with a beating heart. Circulation Journal 2002;66(8):715‐7. [PUBMED: 12197593] [DOI] [PubMed] [Google Scholar]
Efe 2014 {published data only}
- Efe EM, Bilgin BA, Alanoglu Z, Akbaba M, Denker C. Comparison of bolus and continuous infusion of esmolol on hemodynamic response to laryngoscopy, endotracheal intubation and sternotomy in coronary artery bypass graft. Brazilian Journal of Anesthesiology 2014;64(4):247‐52. [PUBMED: 24998108] [DOI] [PubMed] [Google Scholar]
Fujii 2012 {published data only}
- Fujii M, Bessho R, Ochi M, Shimizu K, Terajima K, Takeda S. Effect of postoperative landiolol administration for atrial fibrillation after off pump coronary artery bypass surgery. Journal of Cardiovascular Surgery 2012;53(3):369‐74. [CN‐00881488; PUBMED: 22249647] [PubMed] [Google Scholar]
Hamaguchi 2014 {published data only}
- Hamaguchi S, Nagao M, Takahashi Y, Ikeda T, Yamaguchi S. Low dose landiolol combined with catecholamine can decrease heart rate without suppression of cardiac contraction after cardiopulmonary bypass. Dokkyo Journal of Medical Sciences 2014;41(1):27‐33. [373250136] [Google Scholar]
Imren 2007 {published data only}
- Imren Y, Benson AA, Zor H, Tasoglu I, Ereren E, Sinci V. Preoperative beta‐blocker use reduces atrial fibrillation in off‐pump coronary bypass surgery. Australian and New Zealand Journal of Surgery 2007;77(6):429‐32. [CN‐00866547; PUBMED: 17501880] [DOI] [PubMed] [Google Scholar]
Newsome 1986 {published data only}
- Newsome LR, Roth JV, Hug CC, Nagle D. Esmolol attenuates hemodynamic responses during fentanyl‐pancuronium anesthesia for aortocoronary bypass surgery. Anesthesia and Analgesia 1986;65(5):451‐6. [PUBMED: 3485937] [PubMed] [Google Scholar]
O'Dwyer 1993 {published data only}
- O'Dwyer JP, Yorukoglu D, Harris MN. The use of esmolol to attenuate the haemodynamic response when extubating patients following cardiac surgery ‐ a double‐blind controlled study. European Heart Journal 1993;14(5):701‐4. [PUBMED: 8099549] [DOI] [PubMed] [Google Scholar]
Rees 2015 {published data only}
- Rees C, Sultani Z, Theologou T, Poon S, Field LM, Kuduvalli M, et al. Prophylactic treatment of atrial fibrillation post coronary artery bypass grafting. A randomised controlled trial of sotalol and magnesium versus placebo. European Surgical Research 2015;1:89. [71958303] [Google Scholar]
Sezai 2015 {published data only}
- Sezai A, Osaka S, Yaoita H, Ishii Y, Arimoto M, Hata H, et al. Safety and efficacy of landiolol hydrochloride for prevention of atrial fibrillation after cardiac surgery in patients with left ventricular dysfunction: prevention of atrial fibrillation after cardiac surgery with landiolol hydrochloride for left ventricular dysfunction (PLATON) trial. Journal of Thoracic and Cardiovascular Surgery 2015;150(4):957‐64. [PUBMED: 26254752] [DOI] [PubMed] [Google Scholar]
Tempe 1999 {published data only}
- Tempe DK, Mulchandani P, Tandon MS, Mehta N, Tomar AS, Banerjee A, et al. Control of tachycardia and hypertension following coronary artery bypass graft surgery: efficacy and haemodynamic effects of esmolol. Indian Heart Journal 1999;51(3):294‐300. [PUBMED: 10624069] [PubMed] [Google Scholar]
Yazicioglu 2012 {published data only}
- Yazicioglu H, Ozgok A, Balaban F, Doganay B, Unver S. Single dose esmolol attenuates bispectral index scale response to intubation during fentanyl‐midazolam anesthesia for cardiac surgery. Turkish Journal of Thoracic and Cardiovascular Surgery 2012;20(1):72‐8. [DOI: 10.5606/tgkdc.dergisi.2012.012; WOS:000299866900012] [DOI] [Google Scholar]
References to studies awaiting assessment
Bozotlan 2013 {published data only}
- Bozotlan O, Gultekin Y, Mese B, Erdem K, Erotlu E, Yasim A. The effect of esmolol on prevention of postoperative atrial fibrillation after coronary artery bypass graft surgery. International Journal of Cardiology 2013;1:S142. [71039415] [Google Scholar]
Ishigaki 2012 {published data only}
- Ishigaki D, Arimoto T, Iwayama T, Yashiro Y, Kutsuzawa D, Tamura H, et al. Landiolol, an ultra‐short‐acting beta‐blocker, for prevention of atrial fibrillation after catheter ablation. European Heart Journal 2012;33(Suppl 1):352. [Google Scholar]
NCT00959569 {published data only}
- NCT00959569. Esmolol in cardiac surgery. clinicaltrials.gov/ct2/show/NCT00959569 (first received 14 August 2009).
PACTR201801003026226 {published data only}
- PACTR201801003026226. Cardiac recovery after single, low esmolol dose in patients undergoing valve replacement surgery. A randomized controlled double‐blind study. apps.who.int/trialsearch/Trial2.aspx?TrialID=PACTR201801003026226 (first received 28 January 2018).
References to ongoing studies
Chictr‐ior‐16009203 {published data only}
- Chictr‐ior‐16009203. Perioperative esmolol injection and acute kidney injury associated with CPB after cardiac surgery. www.chictr.org.cn/showproj.aspx?proj=15579 (first received 13 August 2016).
UMIN000004216 {published data only}
- UMIN000004216. Efficacy of beta‐blocker on the autonomic nervous activities and the onset of atrial fibrillation or flutter in patients after cardiac surgery. upload.umin.ac.jp/cgi‐open‐bin/ctr_e/ctr_view.cgi?recptno=R000005063 (first received 1 October 2010).
Additional references
Blessberger 2019
- Blessberger H, Lewis SR, Pritchard MW, Fawcett LJ, Domanovits H, Schlager O, et al. Perioperative beta‐blockers for preventing surgery‐related mortality and morbidity in adults undergoing non‐cardiac surgery. Cochrane Database of Systematic Reviews 2019, Issue 9. [DOI: 10.1002/14651858.CD013438] [DOI] [PMC free article] [PubMed] [Google Scholar]
Borenstein 2010
- Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed‐effect and random‐effects models for meta‐analysis. Research Synthesis Methods 2010;1(2):97‐111. [PUBMED: 26061376] [DOI] [PubMed] [Google Scholar]
Chen 2007
- Chen JC, Kaul P, Haverich A, Menasche P, Smith PK, Carrier M, et al. Myocardial infarction following coronary artery bypass graft surgery increases healthcare resource utilization. Critical Care Medicine 2007;35(5):1296‐301. [PUBMED: 17414091] [DOI] [PubMed] [Google Scholar]
Covidence 2019 [Computer program]
- Veritas Health Innovation. Covidence. Version accessed 2 January 2019. Melbourne, Australia: Veritas Health Innovation.
Crawford 2017
- Crawford TC, Magruder JT, Grimm JC, Suarez‐Pierre A, Sciortino CM, Mandal K, et al. Complications after cardiac operations: all are not created equal. Annals of Thoracic Surgery 2017;103:32‐40. [PUBMED: 27884410] [DOI] [PubMed] [Google Scholar]
Crystal 2002
- Crystal E, Connolly SJ, Sleik K, Ginger TJ, Yusuf S. Interventions on prevention of postoperative atrial fibrillation in patients undergoing heart surgery: a meta‐analysis. Circulation 2002;106(1):75‐80. [PUBMED: 12093773] [DOI] [PubMed] [Google Scholar]
De Lima 2014
- Lima LG, Saconato H, Atallah ÁN, Silva EM. Beta‐blockers for preventing stroke recurrence. Cochrane Database of Systematic Reviews 2014, Issue 10. [DOI: 10.1002/14651858.CD007890.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JP, Altman DG, editor(s) on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS editor(s), Cochrane Handbook for Systematic Reviews of Interventions. Version 5.2.0 (updated June 2017), The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Duncan 2018
- Duncan D, Sankar A, Beattie W, Wijeysundera DN. Alpha‐2 adrenergic agonists for the prevention of cardiac complications among adults undergoing surgery. Cochrane Database of Systematic Reviews 2018, Issue 3. [DOI: 10.1002/14651858.CD004126.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [PUBMED: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
Endnote [Computer program]
- Thomson Reuters. Endnote X5. Philadelphia (PA): Thomson Reuters, 2011.
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 28 June 2019. Hamilton (ON): McMaster University (developed by Evidence Prime).
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [PUBMED: 18436948] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011a
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence ‐ inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. [PUBMED: 21803546] [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence ‐ imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Higgins 2017
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available fromwww.training.cochrane.org/handbook.
Hillis 2011
- Hillis LD, Smith PK, Anderson JL, Bittl JA, Bridges CR, Byrne JG, et al. 2011 ACCF/AHA guidelines for coronary artery bypass graft surgery: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2011;124(23):e652‐e735. [PUBMED: 22064599] [DOI] [PubMed] [Google Scholar]
Hristovska 2017
- Hristovska AM, Duch P, Allingstrup M, Afshari A. Efficacy and safety of sugammadex versus neostigmine in reversing neuromuscular blockade in adults. Cochrane Database of Systematic Reviews 2017, Issue 8. [DOI: 10.1002/14651858.CD012763] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Lewis 2018
- Lewis SR, Pritchard MW, Schofield‐Robinson OJ, Alderson P, Smith AF. Continuation versus discontinuation of antiplatelet therapy for bleeding and ischaemic events in adults undergoing non‐cardiac surgery. Cochrane Database of Systematic Reviews 2018, Issue 7. [DOI: 10.1002/14651858.CD012584.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Montalescot 2013
- Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, et al. 2013 ESC guidelines in the management of stable coronary artery disease. European Heart Journal 2013;34(38):2949‐3003. [PUBMED: 23996286] [DOI] [PubMed] [Google Scholar]
Myles 2016
- Myles PS, Grocott MP, Boney O, Moonesinghe SR, COMPAC‐StEP Group. Standardizing end points in perioperative trials: towards a core and extended outcome set. British Journal of Anaesthesia 2016;116(5):586‐9. [PUBMED: 27106961] [DOI] [PubMed] [Google Scholar]
Oprea 2019
- Oprea AD, Lombard FW, Kertai MD. Perioperative beta‐adrenergic blockade in noncardiac and cardiac surgery: a clinical update. Journal of Cardiothoracic and Vascular Anesthesia 2019;33(3):817‐32. [PUBMED: 29934209] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sanagou 2012
- Sanagou M, Wolfe R, Forbes A, Reid CM. Hospital‐level associations with 30‐day patient mortality after cardiac surgery: a tutorial on the application and interpretation of marginal and multilevel logistic regression. BMC Medical Research Methodology 2012;12(28):1‐10. [PUBMED: 22409732] [DOI] [PMC free article] [PubMed] [Google Scholar]
SCTS 2015
- Society for Cardiothoracic Surgery in Great Britain & Ireland. Blue Book Online. www.bluebook.scts.org (accessed 22 August 2019).
Sousa‐Uva 2017
- Sousa‐Uva M, Head SJ, Milojevic M, Collet JP, Landoni G, Castella M, et al. 2017 EACTS guidelines on perioperative medication in adult cardiac surgery. European Journal of Cardio‐Thoracic Surgery 2018;53(1):5‐33. [PUBMED: 29029110] [DOI] [PubMed] [Google Scholar]
Tamura 2017
- Tamura T, Yatabe T, Yokoyama M. Prevention of atrial fibrillation after cardiac surgery using low‐dose landiolol: a systematic review and meta‐analysis. Journal of Clinical Anesthesia 2017;42:1‐6. [617384012; PUBMED: 28962938] [DOI] [PubMed] [Google Scholar]
Thaper 2018
- Thaper A, Kulik A. Rationale for administering beta‐blocker therapy to patients undergoing coronary artery bypass surgery: a systematic review. Expert Opinion on Drug Safety 2018;17(8):805‐13. [PUBMED: 30037300] [DOI] [PubMed] [Google Scholar]
Thein 2018
- Thein PM, White K, Banker K, Lunny C, Mirzaee S, Nasis A. Preoperative use of oral beta‐adrenergic blocking agents and the incidence of new‐onset atrial fibrillation after cardiac surgery. A systematic review and meta‐analysis. Heart Lung and Circulation 2018;27(3):310‐21. [619169404; PUBMED: 29129562] [DOI] [PubMed] [Google Scholar]
Wang 2018
- Wang LS, Wang H, Hou XT. Short‐term effects of preoperative beta‐blocker use for isolated coronary artery bypass grafting: a systematic review and meta‐analysis. Journal of Thoracic and Cardiovascular Surgery 2018;155(2):620‐9. [PUBMED: 28918203] [DOI] [PubMed] [Google Scholar]
Wiysonge 2017
- Wiysonge CS, Bradley HA, Volmink J, Mayosi BM, Opie LH. Beta‐blockers for hypertension. Cochrane Database of Systematic Reviews 2017, Issue 1. [DOI: 10.1002/14651858.CD002003.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Blessberger 2014
- Blessberger H, Kammler J, Domanovits H, Schlager O, Wildner B, Azar D, et al. Perioperative beta‐blockers for preventing surgery‐related mortality and morbidity. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD004476.pub2] [DOI] [PubMed] [Google Scholar]
Blessberger 2018
- Blessberger H, Kammler J, Domanovits H, Schlager O, Wildner B, Azar D, et al. Perioperative beta‐blockers for preventing surgery‐related mortality and morbidity. Cochrane Database of Systematic Reviews 2018, Issue 3. [DOI: 10.1002/14651858.CD004476.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


