Abstract
Selective autophagy sequesters cytoplasmic cargo for lysosomal degradation via the binding of autophagy receptors to Atg8 (autophagy-related 8) family proteins on the autophagic membrane. The sole yeast Atg8 gene has six mAtg8 (mammalian Atg8) homologs, including the MAP1LC3 (microtubule-associated protein-1 light chain 3) family and the GABA receptor–associated proteins. Selective autophagy receptors interact with two conserved hydrophobic pockets (termed the W-site and L-site) of mATG8 proteins through a linear motif called the LC3-interacting region (LIR) with the general composition (W/F/Y)XX(I/L/V). To address a lack in our knowledge regarding LIR peptide specificity toward each mATG8 homolog, here we used competitive time-resolved FRET to sensitively and quantitatively characterize the interactions between LIRs and mAtg8. We report that 14 representative LIR-containing peptides display differential binding affinities toward the mAtg8 proteins and identified the LIR domain peptide of TP53INP1 as exhibiting high affinity for all six mATG8 proteins. Using peptide truncation studies, we found that both N- and C-terminal acidic residues, as well as the C-terminal Cys residue of the TP53INP1 LIR peptide, are required for its high-affinity binding to LC3A and LC3B, whereas binding to the GABARAP subfamily proteins was facilitated by residues either N-terminal or C-terminal to the core motif. Finally, we used NMR chemical shift perturbation analysis to gain molecular insights into these findings. Collectively, our results may aid in the development of molecules that selectively disrupt specific mATG8–LIR interactions to dissect the biological roles of the six mATG8 homologs for potential therapeutic applications.
Keywords: autophagy, peptide interaction, nuclear magnetic resonance (NMR), fluorescence resonance energy transfer (FRET), mitophagy, protein-protein interaction, Atg8, LC3, LIR
Introduction
Autophagy is a highly conserved intracellular lysosomal degradation process that can be thought of as the cell's quality control and recycling system. It has become clear that autophagy plays an important role in a variety of physiological and pathological processes including development, differentiation, immunity, obesity, neurodegeneration, and tumorigenesis. The autophagic process can be broken down into five steps: phagophore formation, elongation, closure to form a double-membrane vesicle called autophagosome, autophagosome-lysosome fusion to form an autolysosome, and cargo degradation (1). Autophagy that is induced in response to starvation is generally considered to be an adaptive, nonselective degradative process that recycles cellular components to generate molecules for energy production and macromolecular synthesis, thereby promoting cell survival. However, autophagy can also be selective in delivering a variety of substrates including, among many others, protein aggregates, ferritin, mitochondria, and pathogens to the lysosome for degradation (2). Selective autophagy sequesters cytoplasmic cargo for lysosomal degradation via the binding of cargo-interacting proteins, known as selective autophagy receptors, to Atg8 family proteins, which are covalently conjugated to phosphatidylethanolamine in the autophagic membrane during autophagosome biogenesis. The first autophagy receptor to be identified was p62 (sequestosome-1), a well-known scaffolding protein that was found to accumulate in ubiquitin-containing protein inclusions in a number of disease states (3–5). p62 binds both ubiquitin and Atg8 family proteins, thus regulating the formation of protein aggregates and their removal by autophagy. Subsequently, many more selective autophagy cargo receptors have been identified and can be largely classified into sequestosome-1–like receptors such as p62, NBR1, NDP52, OPTN, TAX1BP1, and TP53INP1, and mitophagy receptors including BNIP3, NIX, FUNDC1, and Bcl2-L-13 (6–13).
The sole yeast Atg8 gene has six mammalian homologs comprised of the MAP1LC3 (microtubule-associated protein-1 light chain 3) family (LC3A, LC3B, and LC3C, collectively LC3) and the GABA receptor–associated proteins (GABARAP, GABARAPL1, and GABARAPL2). All six mATG8s play crucial roles in autophagy, although the biological relevance for the expansion of Atg8 homologs in higher eukaryotes remains largely unknown. Structural analyses of mATG8-interacting proteins have demonstrated that selective autophagy receptors interact with two conserved hydrophobic pockets (termed the W-site and L-site) of mATG8 proteins through a linear motif called the LC3-interacting region (LIR).4 With a few specific exceptions, the LIR motif contains two conserved positions occupied by an aromatic residue and a hydrophobic residue separated by two variable amino acids, (W/F/Y)XX(I/L/V) (5, 14–17). More recently, studies have indicated that although the aromatic residue appears to be the most crucial binding determinant, both N-terminal and C-terminal residues of the core LIR motif may play a role in regulating binding affinity and selectivity between the mAtg8 proteins, a possibility that has not been fully explored in LIR-containing proteins (18, 19).
The interaction between mATG8 proteins and LIR-containing proteins has garnered increasing attention over recent years; however, we still lack a clear understanding of the critical features that define LIR peptide specificities toward each mATG8 homolog. Although peptide arrays have been conducted for individual LIRs, no studies have provided sensitive, quantitative, or comprehensive characterization of multiple LIRs across all Atg8 family members or peptide truncation studies to assess the importance of residues N- and C-terminal to the central core LIR motif. Here, we employed a competitive time-resolved (TR)–FRET assay using GST-tagged mATG8 proteins, FITC-labeled p62 LIR peptide, and a representative series of nonlabeled LIR peptides from 14 LIR-containing proteins. We found that the LIR domain peptide of TP53INP1 displays a high affinity for all six mATG8 proteins. Utilizing this peptide as a model, we sought to identify key residues that control selectivity toward Atg8 family members. Our results indicate that the N-terminal acidic residues to the central LIR motif contribute significantly to binding affinity for LC3A/B but less to Atg8, LC3C, and GABARAP family binding. Additionally, we identified the C-terminal cysteine residue of the TP53INP1 LIR as conferring high-affinity binding for all Atg8 family proteins. Finally, NMR analyses provide molecular insights to these findings. Collectively our results will facilitate the development of molecules that selectively disrupt specific mATG8–LIR interactions for dissecting the biological roles of the six mATG8 homologs and for therapeutic applications.
Results
TR-FRET is an effective method to screen LIR-containing peptides for their interaction with Atg8 homologs
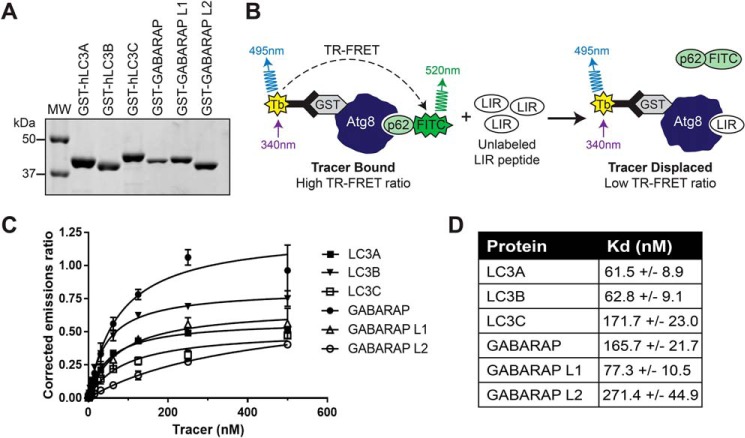
We first identified all known mammalian LIR peptide sequences and categorized them according to the aromatic residue in the central LIR position (F-type LIRs, W-type LIRs, Y-type LIRs, and atypical LIRs) (Table S1) and selected 14 representative peptides from across LIR classes. Using purified GST-tagged mATG8 proteins (Fig. 1A) and terbium-labeled anti-GST antibodies (LanthaScreen, Thermo Fisher), we performed TR-FRET (Fig. 1B) to determine the equilibrium dissociation constant, Kd, for protein interaction with a FITC-labeled 16-mer p62 LIR peptide (FITC-Ahx-SGGDDDWTHLSSKEVD; FITC-p62) (Fig. 1C). Consistent with the literature, all six mATG8 proteins interact with the p62 peptide with Kd in the range of 61–271 nm (Fig. 1D), thus establishing the TR-FRET assay as a sensitive and quantitative method to investigate the relative selectivity of LIR sequences toward Atg8 family proteins.
Figure 1.
TR-FRET is a sensitive method to assess LIR peptide binding to mATG8 proteins. A, verification of purified mATG8 proteins by SDS-PAGE and Coomassie Blue staining. B, schema of the TR-FRET–based competition assay. C, Kd determination of FITC-p62 binding to mATG8 proteins. The data are representative of three independent repeats. The error bars represent standard deviation from four technical replicates. D, Kd values of FITC-p62 binding to mATG8 proteins ± standard deviation from three independent experiments. MW, molecular weight.
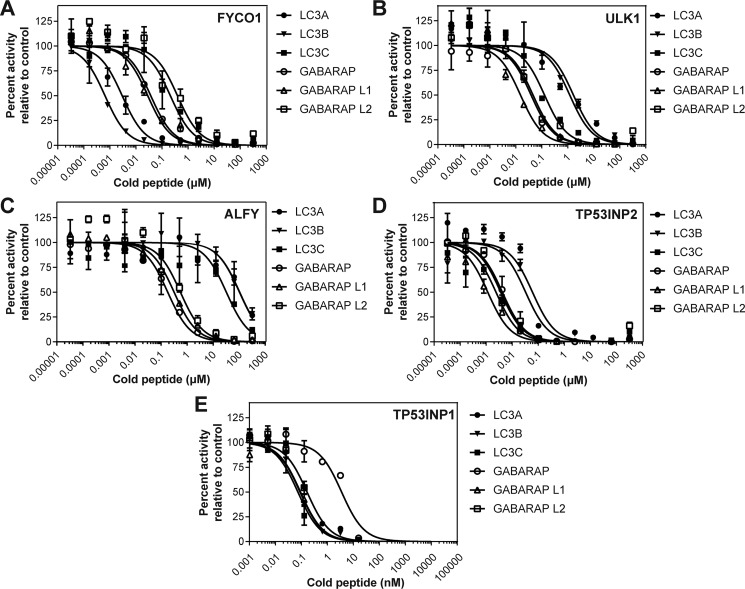
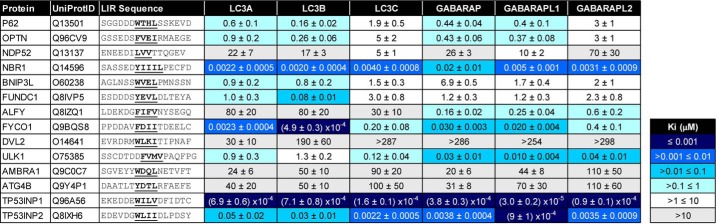
To use TR-FRET to screen 14 LIR-containing peptides, we employed a competition assay whereby unlabeled LIR peptides were used to compete with the FITC-p62 peptide for mATG8 binding (Fig. 2). In doing so, we then calculated the inhibitor constant, Ki, and determined binding affinity for a LIR peptide to each mATG8 protein. As shown in Table 1, we observed that the 14 selected LIR peptides demonstrate differential selectivity and affinity profiles toward each of the mATG8 proteins. Consistent with the literature, we observed that FYCO1 preferentially binds to LC3A and LC3B (20), whereas ULK1 (17), ALFY (18), and TP53INP2 (21) preferentially bind the GABARAP subfamily proteins relative to LC3A and LC3B (Table 1 and Fig. 2, A–D). Furthermore, the LIR domain of NDP52 preferentially binds to LC3C, although its binding is relatively weak for all mATG8 proteins, again consistent with previous reports (22, 23). Notably, of the 14 LIRs assessed in the competitive TR-FRET study, TP53INP1 demonstrated the most potent binding affinities to all mATG8 proteins (Table 1 and Fig. 2E). Therefore, we selected to utilize the TP53INP1 LIR peptide as a tool to investigate the selectivity of the LIR peptide sequence toward each mATG8 family member.
Figure 2.
Competitive TR-FRET identifies LIR peptides with differential binding to mATG8 proteins. Unlabeled LIR peptides were used to compete FITC-p62 binding to mATG8 proteins for FYCO1 (A), ULK1 (B), ALFY (C), TP53INP2 (D), and TP53INP1 (E). The data are representative of three independent experiments. The error bars represent standard deviation from two technical replicates.
Table 1.
TR-FRET profiling of 14 LIR peptides reveals differential binding affinities to mATG8 proteins
Unlabeled LIR peptides were screened by competitive TR-FRET using FITC-labeled p62 LIR peptide. Binding affinities are reported (Ki) and represent average data ± standard deviation from three independent experiments each with two technical replicates.
TP53INP1 LIR peptide truncation reveals key residues conferring mATG8-binding selectivity
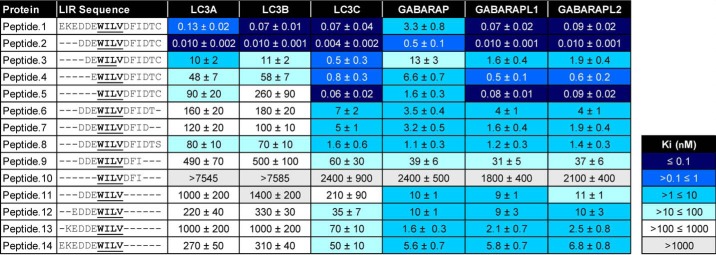
To examine the relative contribution of residues outside of the core LIR motif (WILV) to LIR binding affinity, we truncated the 16-mer TP53INP1 LIR peptide from the N or C terminus and determined each peptide's ability to displace the FITC-p62 peptide from the six mATG8 proteins (Table 2) or yeast Atg8 as a control (Table S2). Truncating the three N-terminal amino acids from positions 1–3 (with amino acid residues numbered 1–16 from the N terminus of peptide 1) increased the affinity of the LIR peptide for all mATG8 proteins ∼10-fold (peptide 1 versus peptide 2) (Table 2). However, further deletion of the N-terminal negatively charged residues at positions 4, 5, and 6 (peptides 3, 4 and 5, Table 2) gradually decreased the peptide binding affinity for LC3A and LC3B but had only modest effects on the binding to other members of the Atg8 family. Interestingly, removal of all six residues N-terminal to the core LIR motif had little effect on the overall affinity of the peptide for LC3C and the GABARAP subfamily (peptide 5 versus peptide 1). These results suggest that the presence of acidic residues N-terminal to the central LIR domain, although common in the majority of LIR-containing proteins, is more crucial for high-affinity binding to LC3A and LC3B than for interaction with LC3C and GABARAP, at least under these experimental conditions. Notably, the LIR binding profile of yeast Atg8 in these studies was more similar to that of LC3C compared with other Atg8 homologs.
Table 2.
TR-FRET using truncated TP53INP1 peptides demonstrates the contribution of specific amino acid residues to binding affinity with mATG8 proteins
Unlabeled TP53INP1 LIR peptides were screened by competitive TR-FRET using FITC-labeled p62 LIR peptide. Binding affinities are reported (Ki) and represent average data ± standard deviation from three independent experiments each with two technical replicates.
The effect of truncating residues C-terminal to the core LIR motif on peptide binding toward all mAtg8 family proteins is listed in Table 2. Removal of the C-terminal cysteine (position 16; peptide 2 versus peptide 6) resulted in a large loss of affinity for all proteins, with the most significant shifts observed for LC3A and LC3B and more moderate effects on the GABARAP subfamily. When the C-terminal cysteine was substituted by a serine residue (peptide 2 versus peptide 8), there was little recovery of LIR binding affinity to any Atg8 family member, indicating that the cysteine residue in this position likely plays a crucial role in regulating both binding affinity and specificity. Of note, control experiments using an alternative protein (GST–Mcl-1) in the competition assay and performing the assay in the presence of 2 mm DTT to disrupt any potential disulfide bonds concluded that the cysteine was not affecting the integrity of the assay or resulting in nonspecific binding (data not shown). Following the loss of the C-terminal cysteine, we observed that further removal of the threonine residue in position 15 (peptide 7) had only a modest effect on peptide binding, whereas subsequent removal of the aspartic acid residue in position 14 substantially reduced the binding affinity toward all proteins (peptide 9). The contribution of the aspartic acid residue in position 14 to mATG8 binding affinity is consistent with literature demonstrating that the FYCO1 LIR is C-terminally extended to contain a glutamic acid residue in the equivalent position that contributes to LC3B binding (19).
We next examined the effect of truncating both N- and C-terminal residues to the core LIR motif on peptide binding to mATG8 proteins. Consistent with our results using peptides 2–5, removal of the N-terminal acidic residues from C-terminal truncated peptide 9 (peptide 10), severely reduced binding to all proteins. Notably, peptide binding to the GABARAP subfamily of proteins can be achieved in the absence of any residues C-terminal of the central LIR motif (peptide 11), whereas this is not the case for other mATG8 proteins. When additional N-terminal residues are included in peptides 12–14, they had little effect on binding affinity toward the GABARAP proteins but modestly restored binding for the other Atg8 family members. Overall, our data suggest that the GABARAP subfamily, as well as LC3C and yeast Atg8, can tolerate amino acid truncations to a much higher degree than LC3A/B, which display a much stricter sequence requirement for high-affinity binding.
NMR chemical shift perturbation experiments provide molecular insights into the binding selectivity of TP53INP1 LIR peptides to Atg8 family proteins
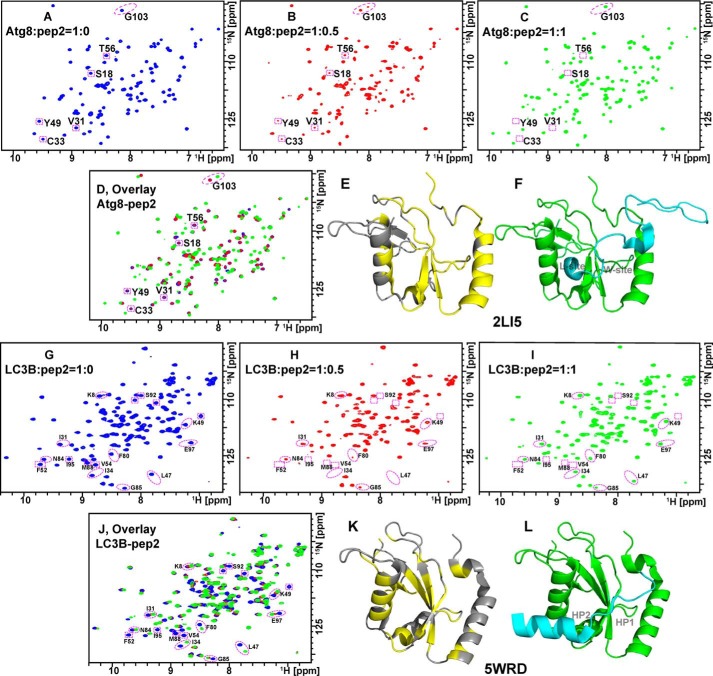
As described above, our TR-FRET measurements indicated that the binding of LC3A/B to TP53INP1 LIR peptides is more sensitive to truncations and substitutions of N- and C-terminal residues than the binding of LC3C, Atg8, and GABARAP subfamily proteins. To understand the molecular basis for this difference, we performed solution NMR chemical shift perturbation (CSP) experiments using LC3B and Atg8 as models. When 15N-labeled Atg8 (Fig. 3, A–D) or 15N-labeled LC3B (Fig. 3, G–J) was titrated with TP53INP1 LIR peptide 2, a new set of resonances appeared, whereas some resonances disappeared in the 2D 15N-1H HSQC spectra. The new resonances correspond to the bound state of the protein/peptide 2 complex and characteristically reflect the high-affinity binding of peptide 2 to Atg8 or LC3B. Using published NMR chemical shift assignments (14, 25–28), we identified some of the perturbed residues and colored them yellow on the Atg8 and LC3B structures in Fig. 3 (E and K), respectively. The LIR motif interacts with two conserved hydrophobic pockets termed the W-site and L-site, which are highly conserved among Atg8 family proteins. To identify W- and L-sites in the Atg8 protein structure, we utilized the NMR structure of a complex formed between Atg8 and an Atg7 peptide corresponding to amino acids 601–630 (Atg7C30, Protein Data Bank code 2LI5; Fig. 3F). Here, Phe-619 of Atg7 interacts with the W-site of Atg8 (composed of the side chains of Glu-17, Ile-21, Pro-30, Ile-32, Lys-48, Leu-50, and Phe-104) (26), whereas Ile-629 and Ala-630 residues of Atg7 occupied the L-site of Atg8 (composed of the side chains of Tyr-49, Val-51, Pro-52, Leu-55, Phe-60, and Val-63). Notably, many of the residues in these sites overlap with resonances of Atg8 that are shifted upon the addition of peptide 2 (Fig. 3D). Similar examination of the published crystal structure of murine LC3B in complex with FYCO1 LIR peptide (Protein Data Bank code 5WRD; Fig. 3L) shows two hydrophobic pockets: HP1 (formed by residues Val-20, Ile-23, Pro-32, Leu-53, and Phe-108) and HP2 (formed by residues Phe-52, Val-54, Pro-55, Val-58, Leu-63 and Ile-66) (29). Consistently, resonances of LC3B that were shifted upon addition of peptide 2 (Fig. 3J) overlap with residues in these sites. Collectively, our NMR data indicate that the TP53INP1 LIR peptide 2 generally interacts with the same regions in Atg8 and LC3B that are expected based on the literature, including the highly conserved hydrophobic W- and L-sites.
Figure 3.
Chemical shift perturbation analyses demonstrate TP53INP1 LIR binding to similar regions in Atg8 and LC3B proteins. 15N-1H HSQC spectra of 15N-labeled Atg8 and LC3B titrated with peptide 2 in a molar ratio of 0 (A and G, blue), 0.5 (B and H, red), and 1 (C and I, green). D and J show their overlaid spectra, respectively. Select perturbed residues of Atg8 and LC3B upon addition of peptide 2 are circled in red in A–D and G–J and colored yellow onto cartoon structural models of Atg8 (E, Protein Data Bank code 2LI5) and LC3B (K, Protein Data Bank code 5WRD). F and L are for Atg8 in complex with Atg7C30 (cyan) and LC3B in complex with FYCO1 LIR (cyan), respectively.
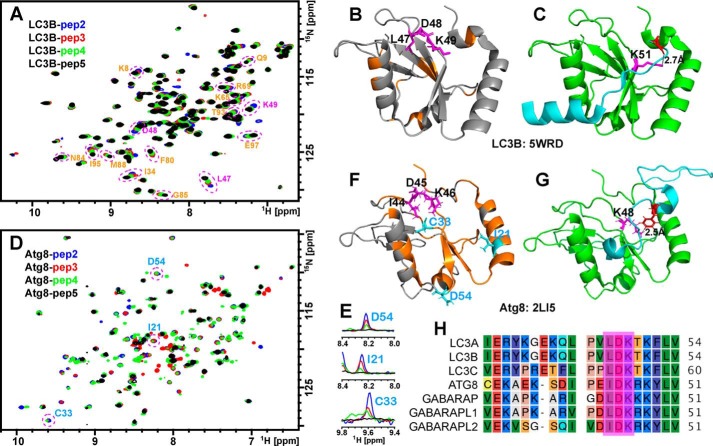
We next examined the CSPs of LC3B and Atg8 in the presence of N-terminal truncated TP53INP1 LIR peptides. Fig. 4A shows an overlay of the HSQC spectra of 15N-labeled LC3B in the presence of peptides 2, 3, 4, and 5. Upon the progressive removal of N-terminal residues from peptides 2 to 5, multiple resonances of LC3B (labeled in orange in Fig. 4A) shift toward their positions in free LC3B. Notably, the perturbed residues include a loop that harbors Leu-47, Asp-48, and Lys-49 (Fig. 4B, magenta), which suggests that this region likely directly interacts with the N-terminal residues of TP53INP1 LIR peptides. This is consistent with crystal structures in which the corresponding residue (Asp) from the N terminus of the FYCO1 LIR peptide (Fig. 4C, red) forms a strong hydrogen bond with Lys-51 of mouse LC3B (Fig. 4C, magenta) (29). Abolishing this interaction by removing the N-terminal residues of the LIR peptide decreases its binding affinity to LC3B, as reported by our TR-FRET measurements. However, when the N-terminal truncated peptides were titrated to 15N-labeled Atg8, their perturbations on the protein were quite different from that of LC3B. As shown in Fig. 4D, upon progressive removal of N-terminal residues from peptides 2 to 5, many resonances in Atg8 are broadened and eventually go beyond detection in the presence of peptide 5 (Fig. 4, D and E). Those perturbed resonances include residues Ile-44, Asp-45, and Lys-46 (Fig. 4F), which correspond to residues Leu-47, Asp-48, and Lys-49 in LC3B (Fig. 4H). This is consistent with NMR structures demonstrating that the N-terminal residue Phe-619 of Atg7C30 is tightly associated with Lys-48 of Atg8 (Fig. 4G) (26). However, additional residues such as Cys-33, Asp-54, and Ile-21 are also perturbed upon LIR peptide binding and appear scattered in the Atg8 structure (Fig. 4F). This pattern is likely due to the allosteric effect of peptide binding. Our CSP data suggest that unlike LC3B, Atg8 is able to accommodate peptides regardless of the presence or absence of additional acidic Asp or Glu residues N-terminal to the central LIR domain. As a result, Atg8 becomes less sensitive to truncations of the N-terminal residues of TP53INP1 LIR peptides, as seen by our TR-FRET measurements.
Figure 4.
Atg8 is less sensitive to N-terminal TP53INP1 peptide truncations relative to LC3B. A and D, overlays of 2D 15N-1H HSQC spectra of 15N-labeled LC3B (A) and Atg8 (D) titrated with peptides 2 (blue), 3 (red), 4 (green), and 5 (black). B and F, select perturbed residues are circled and mapped (orange, magenta, or cyan) on the structural models of LC3B (B, Protein Data Bank code 5WRD) and Atg8 (F, 2LI5). C, structural model of LC3B (green) in complex with FYCO1 LIR (cyan, Protein Data Bank code 5WRD). The distance between the side chain Nζ from Lys-51 (magenta) of LC3B and the backbone carbonyl oxygen from Asp (red) of FYCO1 LIR peptide is indicated. E, 1D slices highlighting broadenings of Atg8 resonances upon additions of N-terminal deleted peptides. G, structural model of Atg8 (green) in complex with Atg7C30 (cyan, 2LI5). The distance between the side chain Nζ from K48 (magenta) of Atg8 and the side chain Hζ from Phe (red) of Atg7C30 is indicated. H, sequence alignment of the conserved LDK regions for Atg8 and mATG8.
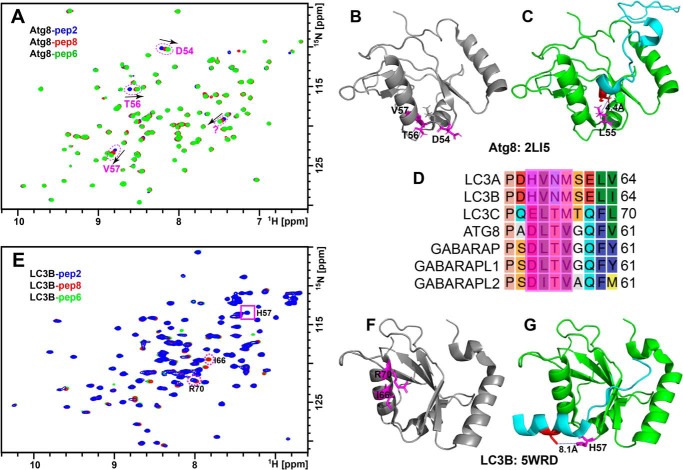
We also examined the role of the C-terminal Cys residue of the TP53INP1 LIR peptide in its interaction with LC3B and Atg8 proteins. Fig. 5A shows an overlay of HSQC spectra of 15N-labeled Atg8 with peptides 2, 6, and 8. Deletion of the C-terminal Cys residue or mutation to Ser shifted the resonances of Asp-54, Thr-56, and Val-57 of Atg8 but induced little or no change in other resonances (Fig. 5, A and B). This is consistent with the NMR structure of the Atg8–Atg7C30 complex in which the C-terminal Ala (Fig. 5C, red), corresponding to the last Cys from our peptide, is close to the loop region of 54–57 (Fig. 5C, magenta). However, peptides lacking the C-terminal Cys show different effects on LC3B. As seen in Fig. 5E, the most perturbed residues of LC3B by peptides 8 and 6 are Ile-66 and Arg-70, whereas few or no additional perturbations are observed for other resonances including His-57, which corresponds to Asp-54 from Atg8. These data suggest that the C-terminal Cys residue directly interacts with a nonconserved loop region of Atg8 that includes residues 54–57 but interacts with a different region of LC3B (Fig. 5, D–F). This is consistent with crystal structures in which the corresponding C-terminal Cys of the FYCO1 LIR peptide (Fig. 5G, red) is more than 8 Å away from His-57 of mouse LC3B (Fig. 5G, magenta) (29). As a result, mutation or deletion of this Cys residue should yield different consequences for Atg8 and LC3B, as observed in our TR-FRET measurements.
Figure 5.
The C-terminal cysteine of the TP53INP1 peptide interacts with a nonconserved loop region in Atg8 but not LC3B. A and E, overlays of 2D 15N-1H HSQC spectra of 15N-labeled Atg8 (A) and LC3B (E) titrated with peptide 2 (blue), peptide 8 (red), and peptide 6 (green). B, C, F, and G, select perturbed residues are circled and mapped in magenta on the structural model of Atg8 (B, Protein Data Bank code 2LI5) in complex with Atg7C30 (cyan in C) and LC3B (F, Protein Data Bank code 5WRD) in complex with FYCO1 LIR (cyan in G), respectively. C and G, the distance between the side chain Cδ from Leu-55 (magenta, C) of Atg8 and the Cβ from Ala (red, C) of Atg7C30 and between the side chain Nϵ from His-57 (magenta, G) of LC3B and the Sγ from Cys (red, G) of FYCO1 LIR are indicated. D, sequence alignment for the nonconserved DLTV regions for Atg8 and mATG8.
Discussion
Our study is the first of its kind to use highly sensitive TR-FRET to quantitatively measure the interaction of LIR-containing peptides with Atg8 family proteins. Our findings underscore the importance of both N- and C-terminal residues outside the central core LIR motif in contributing to the binding affinity and selectivity of LIR peptides to Atg8 homolog proteins. We reveal that both N- and C-terminal acidic residues, as well as the C-terminal Cys residue of the TP53INP1 LIR peptide, are required for its high-affinity binding to LC3A and LC3B, whereas binding of LIR peptides to GABARAP subfamily proteins may be facilitated by residues either N-terminal or C-terminal to the core motif. Interestingly, LC3C demonstrates sequence requirements for LIR binding between that of LC3A/B and the GABARAP proteins, more often reflecting a binding pattern most similar to Atg8. For LC3C and Atg8, the aspartic acid residues C-terminal to the core LIR motif are crucial to high-affinity binding, whereas the N-terminal acidic residues of the LIR peptide significantly contribute to binding affinity for LC3A and LC3B. These data support the notion that the most important LIR residues for binding affinity are the acidic residues, as has been previously reported (16, 19).
Although TR-FRET provides a direct measurement of the binding affinity of Atg8 family proteins for LIR peptides, NMR studies provide molecular insights into protein–LIR binding, in particular amino acid residues directly involved in the protein–peptide interactions. Notably, the Kd values for the FITC-p62 peptide using TR-FRET (Fig. 1D) are greater than the Ki values for the unlabeled p62 peptide (Table 1), which is probably, at least in part, because of the different assay formats. Our NMR CSP data suggest that the N-terminal residues of the TP53INP1 LIR peptide interact with a loop that harbors Leu-47, Asp-48, and Lys-49 residues of LC3B. Although these three residues are conserved in the Atg8 family (Fig. 4H), residues that precede and follow LDK are not. The residue preceding LDK is a hydrophobic residue, Pro or Val, in the LC3 subfamily, but it is an acidic residue, Asp or Glu, in Atg8 and the GABARAP subfamily. The residue following LDK is Thr in the LC3 subfamily but is a positively charged residue, Arg or Lys, in Atg8 and the GABARAP subfamily proteins. These differences likely affect their interactions with the negatively charged N terminus of the TP53INP1 LIR peptide. For the C-terminal Cys, our NMR studies suggest that it interacts with a region that includes residues D54 to V57 of Atg8. Although this region is conserved in Atg8, LC3C, and the GABARAP subfamily, it is not conserved in LC3A and LC3B proteins (Fig. 5D). This result provides an explanation of our TR-FRET findings that LC3C and GABARAP subfamily differ from LC3A and LC3B proteins in their sensitivity to the truncation or substitution of the LIR C-terminal Cys residue.
Numerous previous studies have noted the importance of negatively charged (acidic) residues in close proximity to the core LIR motif in improving the strength of binding interaction between LIRs and mATG8 proteins (9, 16, 19). However, what is previously unappreciated is that the presence of negatively charged residues at either the N or C terminus, but not necessarily both, is sufficient for high-affinity binding of the GABARAP subfamily proteins, whereas LC3A and LC3B appear to benefit from negatively charged residues in both the N- and C-terminal locations. Others have reported a GABARAP interaction motif and determined that amino acid alterations in the central LIR motif (WXXV) can increase binding to the GABARAP subfamily relative to LC3; however, residues external to the central LIR motif were not explored; thus our findings complement and enhance existing knowledge regarding peptide interaction with the GABARAP family of mATG8 proteins (30).
Recently, two papers have reported Atg8/LC3/GABARAP sensors based on LIR peptides (21, 23). By using the sensors, Lee et al. (21) identified TP53INP2 as a LIR-containing protein that can efficiently bind all mATG8 proteins. It is interesting to note that the authors observed similar results with TP53INP1 GFP-tagged LIR peptide, supporting our findings. Also, consistent with our study and previous literature (19, 20), Lee et al. (21) identified that FYCO1 can be used as a probe for LC3A and LC3B. Although our current study does not extend to functional evaluation, the consistency of our findings with those of published reports using other methods adds confidence in the ability of TR-FRET to sensitively detect LIR-LC3 interactions and relative selectivity between homolog proteins. Further validation of our findings in cells will provide greater understanding of each Atg8 family member in a biological context. As exemplified by a recent study by Li et al. (31), specific LIR peptides can be used to study autophagy manipulation in cells and organisms, as well as uncover potential therapeutic targets. Collectively, our results and TR-FRET assay will facilitate the development of molecules that selectively disrupt mATG8–LIR interactions for dissecting the biological roles of the six mATG8 homologs and for therapeutic applications.
Experimental procedures
Expression and purification of mATG proteins
pETM30 vectors for the expression of GST tagged LC3A, LC3B, LC3C, GABARAP, GABARAPL1, and GABARAPL2 were a gift from Felix Randow at the MRC Laboratory of Molecular Biology (22). Vector for N-terminal His-tagged Atg8 expression (pOPC-His-TEV-Atg8) is a gift from Sascha Martens at the University of Vienna (32). Briefly, plasmids were transformed into BL21 Escherichia coli, and single colonies were isolated. Following sequence confirmation, 1 liter of transformed E. coli with an A600 of ∼0.7 was induced to express GST fusion proteins using 0.05 mm isopropyl-1-thio-β-galactopyranoside and incubated at 25 °C. Following an 8-h incubation, bacteria was pelleted by centrifugation at 12,000 rpm, 4 °C. 15N-Labeled proteins (Atg8 and LC3B) were prepared by culturing cells in M9 medium using 15NH4Cl as sole nitrogen source. When cells were grown to an A600 of 0.8, isopropyl-1-thio-β-galactopyranoside was added to a final concentration of 0.5 mm for protein expression. After induction at 25 °C for 8 h, the cells were collected. The bacteria pellet was resuspended in lysis buffer (1× PBS, pH 7.4, 1% Triton X-100, 2 mm DTT, 1 mm phenylmethylsulfonyl fluoride) for 1 h at 4 °C with gentle agitation, followed by sonication on ice for 4 × 30 s with a duty cycle of 3 s on and 7 s off. The lysate was centrifuged for 20 min at 4 °C at 12,000 rpm, and the supernatant was collected. Supernatant was then filtered through a 0.45-μm filter four times before purification using an AKTAPrime plus FLPC (GE Healthcare) with superloop and a GSTrapp FF or HiTrap HP 5-ml column (GE Healthcare, Amersham Biosciences). Fractions containing GST-tagged mATG8 protein were combined, buffer-exchanged into PBS containing 2 mm DTT using Slide-A-Lyzer dialysis cassettes (Thermo-Fisher), and concentrated using Amicon ultracentrifugal filter units (Millipore). For NMR experiments, His tag from Atg8 and GST tag from LC3B were removed using TEV protease (32). After cleavage, the reaction samples were concentrated and applied to 16/60 Superdex 75 size exclusion column in 50 mm HEPES, pH 7.5, 150 mm NaCl, 1 mm DTT. Fractions containing target protein were concentrated and exchanged into 20 mm PBS buffer of pH 6.8, 150 mm NaCl, 2 mm DTT through Amicon ultracentrifugal filter units (Millipore). The resulting purified protein was verified and quantified via NuPAGEBis-Tris gels or Western blotting using an anti-GST antibody.
TR-FRET Kd determination
We employed LanthaScreen technology from Thermo Fisher following the manufacturer's recommendations. Assay conditions were optimized for black low-volume 384-well plates (Corning) to 5 nm GST-mATG8 protein, 2 nm Tb-labeled anti-GST antibody, 20 nm p62 FITC-labeled peptide. The reaction volumes were 15 μl with samples diluted in LanthaScreen assay buffer. Assay plates were mixed on an orbital shaker for 2 min before being incubated at room temperature for 1 h. Peptide titration in the absence of mATG8 protein was used as a negative control. TR-FRET was measured using the Clariostar multimode plate reader (BMG Labtech) using a 100-μs delay and 200-μs integration time (excitation filter EX TR, emission filters 520-10 and 490-10, and dichroic mirror LP TR). All peptides were synthesized by United Peptide (Herndon, VA). Kd was calculated using GraphPad Prism following normalization to peptide-only control.
TR-FRET competition assays
For competition assays, TR-FRET conditions remained as detailed above but conducted in the presence of a serial dilution of unlabeled peptide. Briefly, 5-fold dilutions of unlabeled peptides were made resulting in a final screening concentration ranging from 320 μm to 30 pm for screening multiple LIRs and from 10 μm to 1 pm for TP53INP1 studies. 5 μl of each dilution was added in duplicate or quadruplicate to the assay plate before 5 μl of protein was added at the required concentration. Finally, FITC-p62 peptide and Tb-labeled anti-GST antibody was added on the final step in a 5-μl volume. IC50 values were calculated through nonlinear mixed-effects regression with normalized data assuming variable slope with repeated measurements. Ki values were determined by the Cheng–Prusoff equation (24).
NMR sample preparation and NMR experiments
15N-Labeled protein of 0.1 mm in 20 mm phosphate buffer (pH 6.8) with 150 mm NaCl, 2 mm DTT, and 4% (v/v) D2O was used for NMR measurement. LIR peptides were prepared in DMSO with a stock concentration of 20 mm (200 times of protein). Protein solution was titrated with LIR peptides in a protein/peptide ratio of 1:0, 1:0.5, 1:1, and 1:2. NMR experiments were carried out at 25 °C on a Bruker 600 MHz spectrometer. Two-dimensional 15N-1H HSQC spectra were acquired with a 1H spectral width of 9615.385 Hz and a 15N width of 1763.551 Hz. The matrix size was 2048 by 256 complex points. Typically, 16 scans/complex t1 increment were accumulated with a total measurement time of ∼2 h. NMR spectra were processed by Topspin2.1 and NMRPipe and analyzed by Topspin2.1 and NMRView. Resonance assignments were obtained based on NMR data deposited at the Biological Magnetic Resonance Data Bank.
Author contributions
J. M. A. and Y. Y. data curation; J. M. A., Y. Y., M. T. G., S. Z., M. M. Y., Y. T., F. T., and H.-G. W. formal analysis; J. M. A. validation; J. M. A., Y. Y., M. T. G., Q. Liu, M. M. Y., and Y. T. investigation; J. M. A., Y. Y., and Y. T. methodology; J. M. A. and Y. Y. writing-original draft; Y. Y., M. M. Y., Y. T., F. T., and H.-G. W. writing-review and editing; Q. Lin, F. T., and H.-G. W. conceptualization; Q. Lin, F. T., and H.-G. W. supervision; F. T. and H.-G. W. resources; F. T. and H.-G. W. funding acquisition.
Supplementary Material
Acknowledgments
We thank the Randow and Martens labs for providing the vectors for mATG8 expression. We also thank James Dreer for technical assistance with protein purification.
This work was supported in part by National Institutes of Health Grants CA222349 and GM127954 (to H.-G. W.) and GM105963 and GM127730 (to F. T.), Hyundai Hope on Wheels, the Lois High Berstler Research Endowment Fund, and the Four Diamonds Fund of the Pennsylvania State University. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains Tables S1 and S2.
- LIR
- LC3-interacting region
- TR
- time-resolved
- CSP
- chemical shift perturbation
- HSQC
- heteronuclear single quantum coherence.
References
- 1. Xie Z., and Klionsky D. J. (2007) Autophagosome formation: core machinery and adaptations. Nat. Cell Biol. 9, 1102–1109 10.1038/ncb1007-1102 [DOI] [PubMed] [Google Scholar]
- 2. Mancias J. D., and Kimmelman A. C. (2016) Mechanisms of selective autophagy in normal physiology and cancer. J. Mol. Biol. 428, 1659–1680 10.1016/j.jmb.2016.02.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bjørkøy G., Lamark T., Brech A., Outzen H., Perander M., Overvatn A., Stenmark H., and Johansen T. (2005) p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J. Cell Biol. 171, 603–614 10.1083/jcb.200507002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Komatsu M., Waguri S., Koike M., Sou Y. S., Ueno T., Hara T., Mizushima N., Iwata J., Ezaki J., Murata S., Hamazaki J., Nishito Y., Iemura S., Natsume T., Yanagawa T., et al. (2007) Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell 131, 1149–1163 10.1016/j.cell.2007.10.035 [DOI] [PubMed] [Google Scholar]
- 5. Pankiv S., Clausen T. H., Lamark T., Brech A., Bruun J. A., Outzen H., Øvervatn A., Bjørkøy G., and Johansen T. (2007) p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J. Biol. Chem. 282, 24131–24145 10.1074/jbc.M702824200 [DOI] [PubMed] [Google Scholar]
- 6. Birgisdottir Å. B., Lamark T., and Johansen T. (2013) The LIR motif: crucial for selective autophagy. J. Cell Sci. 126, 3237–3247 [DOI] [PubMed] [Google Scholar]
- 7. Kirkin V., Lamark T., Sou Y. S., Bjørkøy G., Nunn J. L., Bruun J. A., Shvets E., McEwan D. G., Clausen T. H., Wild P., Bilusic I., Theurillat J. P., Øvervatn A., Ishii T., Elazar Z., et al. (2009) A role for NBR1 in autophagosomal degradation of ubiquitinated substrates. Mol. Cell 33, 505–516 10.1016/j.molcel.2009.01.020 [DOI] [PubMed] [Google Scholar]
- 8. Thurston T. L., Ryzhakov G., Bloor S., von Muhlinen N., and Randow F. (2009) The TBK1 adaptor and autophagy receptor NDP52 restricts the proliferation of ubiquitin-coated bacteria. Nat. Immunol. 10, 1215–1221 10.1038/ni.1800 [DOI] [PubMed] [Google Scholar]
- 9. Wild P., Farhan H., McEwan D. G., Wagner S., Rogov V. V., Brady N. R., Richter B., Korac J., Waidmann O., Choudhary C., Dötsch V., Bumann D., and Dikic I. (2011) Phosphorylation of the autophagy receptor optineurin restricts Salmonella growth. Science 333, 228–233 10.1126/science.1205405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hanna R. A., Quinsay M. N., Orogo A. M., Giang K., Rikka S., and Gustafsson Å. B. (2012) Microtubule-associated protein 1 light chain 3 (LC3) interacts with Bnip3 protein to selectively remove endoplasmic reticulum and mitochondria via autophagy. J. Biol. Chem. 287, 19094–19104 10.1074/jbc.M111.322933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Novak I., Kirkin V., McEwan D. G., Zhang J., Wild P., Rozenknop A., Rogov V., Löhr F., Popovic D., Occhipinti A., Reichert A. S., Terzic J., Dötsch V., Ney P. A., and Dikic I. (2010) Nix is a selective autophagy receptor for mitochondrial clearance. EMBO Rep. 11, 45–51 10.1038/embor.2009.256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu L., Feng D., Chen G., Chen M., Zheng Q., Song P., Ma Q., Zhu C., Wang R., Qi W., Huang L., Xue P., Li B., Wang X., Jin H., et al. (2012) Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat. Cell Biol. 14, 177–185 10.1038/ncb2422 [DOI] [PubMed] [Google Scholar]
- 13. Murakawa T., Yamaguchi O., Hashimoto A., Hikoso S., Takeda T., Oka T., Yasui H., Ueda H., Akazawa Y., Nakayama H., Taneike M., Misaka T., Omiya S., Shah A. M., Yamamoto A., et al. (2015) Bcl-2-like protein 13 is a mammalian Atg32 homologue that mediates mitophagy and mitochondrial fragmentation. Nat. Commun. 6, 7527 10.1038/ncomms8527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Noda N. N., Kumeta H., Nakatogawa H., Satoo K., Adachi W., Ishii J., Fujioka Y., Ohsumi Y., and Inagaki F. (2008) Structural basis of target recognition by Atg8/LC3 during selective autophagy. Genes Cells 13, 1211–1218 10.1111/j.1365-2443.2008.01238.x [DOI] [PubMed] [Google Scholar]
- 15. Noda N. N., Ohsumi Y., and Inagaki F. (2010) Atg8-family interacting motif crucial for selective autophagy. FEBS Lett. 584, 1379–1385 10.1016/j.febslet.2010.01.018 [DOI] [PubMed] [Google Scholar]
- 16. Ichimura Y., Kumanomidou T., Sou Y. S., Mizushima T., Ezaki J., Ueno T., Kominami E., Yamane T., Tanaka K., and Komatsu M. (2008) Structural basis for sorting mechanism of p62 in selective autophagy. J. Biol. Chem. 283, 22847–22857 10.1074/jbc.M802182200 [DOI] [PubMed] [Google Scholar]
- 17. Alemu E. A., Lamark T., Torgersen K. M., Birgisdottir A. B., Larsen K. B., Jain A., Olsvik H., Øvervatn A., Kirkin V., and Johansen T. (2012) ATG8 family proteins act as scaffolds for assembly of the ULK complex: sequence requirements for LC3-interacting region (LIR) motifs. J. Biol. Chem. 287, 39275–39290 10.1074/jbc.M112.378109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lystad A. H., Ichimura Y., Takagi K., Yang Y., Pankiv S., Kanegae Y., Kageyama S., Suzuki M., Saito I., Mizushima T., Komatsu M., and Simonsen A. (2014) Structural determinants in GABARAP required for the selective binding and recruitment of ALFY to LC3B-positive structures. EMBO Rep. 15, 557–565 10.1002/embr.201338003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Olsvik H. L., Lamark T., Takagi K., Larsen K. B., Evjen G., Øvervatn A., Mizushima T., and Johansen T. (2015) FYCO1 contains a C-terminally extended, LC3A/B-preferring LC3-interacting region (LIR) motif required for efficient maturation of autophagosomes during basal autophagy. J. Biol. Chem. 290, 29361–29374 10.1074/jbc.M115.686915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cheng X., Wang Y., Gong Y., Li F., Guo Y., Hu S., Liu J., and Pan L. (2016) Structural basis of FYCO1 and MAP1LC3A interaction reveals a novel binding mode for Atg8-family proteins. Autophagy 12, 1330–1339 10.1080/15548627.2016.1185590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee Y. K., Jun Y. W., Choi H. E., Huh Y. H., Kaang B. K., Jang D. J., and Lee J. A. (2017) Development of LC3/GABARAP sensors containing a LIR and a hydrophobic domain to monitor autophagy. EMBO J. 36, 1100–1116 10.15252/embj.201696315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. von Muhlinen N., Akutsu M., Ravenhill B. J., Foeglein Á., Bloor S., Rutherford T. J., Freund S. M., Komander D., and Randow F. (2012) LC3C, bound selectively by a noncanonical LIR motif in NDP52, is required for antibacterial autophagy. Mol. Cell 48, 329–342 10.1016/j.molcel.2012.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stolz A., Putyrski M., Kutle I., Huber J., Wang C., Major V., Sidhu S. S., Youle R. J., Rogov V. V., Dötsch V., Ernst A., and Dikic I. (2017) Fluorescence-based ATG8 sensors monitor localization and function of LC3/GABARAP proteins. EMBO J. 36, 549–564 10.15252/embj.201695063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cheng Y., and Prusoff W. H. (1973) Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 22, 3099–3108 10.1016/0006-2952(73)90196-2 [DOI] [PubMed] [Google Scholar]
- 25. Kumeta H., Watanabe M., Nakatogawa H., Yamaguchi M., Ogura K., Adachi W., Fujioka Y., Noda N. N., Ohsumi Y., and Inagaki F. (2010) The NMR structure of the autophagy-related protein Atg8. J. Biomol. NMR 47, 237–241 10.1007/s10858-010-9420-1 [DOI] [PubMed] [Google Scholar]
- 26. Noda N. N., Satoo K., Fujioka Y., Kumeta H., Ogura K., Nakatogawa H., Ohsumi Y., and Inagaki F. (2011) Structural basis of Atg8 activation by a homodimeric E1, Atg7. Mol. Cell 44, 462–475 10.1016/j.molcel.2011.08.035 [DOI] [PubMed] [Google Scholar]
- 27. Fumagalli F., Noack J., Bergmann T. J., Cebollero E., Pisoni G. B., Fasana E., Fregno I., Galli C., Loi M., Soldà T., D'Antuono R., Raimondi A., Jung M., Melnyk A., Schorr S., et al. (2016) Translocon component Sec62 acts in endoplasmic reticulum turnover during stress recovery. Nat. Cell Biol. 18, 1173–1184 10.1038/ncb3423 [DOI] [PubMed] [Google Scholar]
- 28. Kuang Y., Ma K., Zhou C., Ding P., Zhu Y., Chen Q., and Xia B. (2016) Structural basis for the phosphorylation of FUNDC1 LIR as a molecular switch of mitophagy. Autophagy 12, 2363–2373 10.1080/15548627.2016.1238552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sakurai S., Tomita T., Shimizu T., and Ohto U. (2017) The crystal structure of mouse LC3B in complex with the FYCO1 LIR reveals the importance of the flanking region of the LIR motif. Acta Crystallogr. F Struct. Biol. Commun. 73, 130–137 10.1107/S2053230X17001911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rogov V. V., Stolz A., Ravichandran A. C., Rios-Szwed D. O., Suzuki H., Kniss A., Löhr F., Wakatsuki S., Dötsch V., Dikic I., Dobson R. C., and McEwan D. G. (2017) Structural and functional analysis of the GABARAP interaction motif (GIM). EMBO Rep. 18, 1382–1396 10.15252/embr.201643587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li J., Zhu R., Chen K., Zheng H., Zhao H., Yuan C., Zhang H., Wang C., and Zhang M. (2018) Potent and specific Atg8-targeting autophagy inhibitory peptides from giant ankyrins. Nat. Chem. Biol. 14, 778–787 10.1038/s41589-018-0082-8 [DOI] [PubMed] [Google Scholar]
- 32. Zens B., Sawa-Makarska J., and Martens S. (2015) In vitro systems for Atg8 lipidation. Methods 75, 37–43 10.1016/j.ymeth.2014.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.