Abstract
Introduction
Delamination of the chondral surface of an osteochondral allograft (OCA) from the underlying cancellous bone has been described as a mode of failure after implantation in the knee. Our hypothesis was that increased storage time of the OCA is associated with increased risk of graft delamination after implantation.
Methods
Prospective data on 13 patients with evidence of OCA delamination identified on magnetic resonance imaging or during subsequent surgery from 2000 to 2015 were reviewed. A cohort of 33 patients without evidence of graft delamination were then matched to the delamination group based on recipient age, sex, body mass index (BMI), and chondral defect location. The matched cohort size was established based on a power calculation for determining differences in OCA storage times. All patients had a minimum 2-year follow-up.
Results
There was no difference in donor age, donor sex, and graft storage time between groups (30 vs. 31 days, P = 0.78). There were no differences between number of previous ipsilateral knee surgeries (1.8 vs. 0.84, P = 0.26), BMI (26.8 vs. 25.0 kg/m2, P = 0.31), total chondral defect size (6.5 vs. 5.8 cm2, P = 0.41) or preoperative Marx activity scores between groups.
Conclusion
There is no association between OCA storage time, activity level scores, or number of previous ipsilateral knee surgeries and graft delamination in our patient population. Further work is needed to identify the etiology for this mode of failure of OCAs.
Keywords: osteochondral defect, osteochondral allograft, delamination
Introduction
Full-thickness chondral and osteochondral injuries can lead to functional impairment and pain. Various treatment algorithms have been developed, and management largely depends on patient age and activity level as well as size and location of the lesion.1 Osteochondral allografts (OCAs) are a reliable technique for the treatment of large chondral or osteochondral defects. The use of matched OCAs eliminates donor site morbidity, provides immediate structural restoration of the articular surface, and allows for treatment of large lesions.2,3 Utilization of fresh OCA transplantation for the management of full-thickness chondral and osteochondral defects has demonstrated good long-term survival and clinical outcomes.4-11 Although graft failure rates are relatively low, many of those that do fail are characterized by delamination of the chondral surface of the plug from the underlying cancellous bone ( Figs. 1 and 2 ). This mechanism of failure has not been explicitly investigated or well described in the literature.12
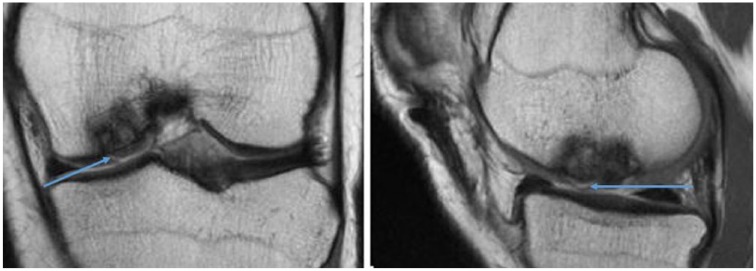
Figure 1.
Coronal and sagittal T2-weighted magnetic resonance imaging (MRI) of a left knee demonstrating subchondral incorporation of the graft but delamination of the chondral surface (blue arrows) of a medial femoral condyle osteochondral allograft (OCA) transplant.
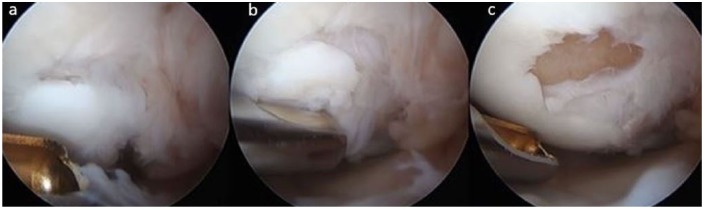
Figure 2.
Arthroscopic imaging of a left knee medial femoral condyle demonstrating (a) delamination of an osteochondral allograft (OCA) transplant, (b) debridement of the OCA delamination, and (c) exposed underlying subchondral bone.
A recent study performed in a canine model demonstrated that all successfully integrated OCAs had >70% viability of chondrocytes at time of implantation, and no graft with <70% chondrocyte viability had a successful outcome.13 Additional in vivo studies have demonstrated that diminished cellularity in OCA correlated negatively with cartilage structure and health.14 The effect of graft storage on chondrocyte viability has been extensively studied.13-17 Fresh human OCA tissue stored for more than 28 days demonstrates significant decreases in chondrocyte viability, viable cell density and metabolic activity.16 The effect these storage related graft changes has on clinical outcomes has not been defined. We postulate that prolonged storage time is associated with increased risk of OCA delamination after implantation by being associated with decreased cell viability, structural support and metabolic activity.
Methods
A prospective registry was developed at our institution in 1999 to track patient outcomes after cartilage restoration procedures. An institutional review board approved the registry and all patients signed an informed consent form before participation. The registry data were recorded preoperatively and then followed prospectively up until 10 years postoperatively. The database was retrospectively reviewed.
Inclusion and Exclusion Criteria
Inclusion criteria included (1) symptomatic focal full-thickness cartilage lesions of the knee, (2) treatment with fresh OCA with available graft records, and (3) minimum of 2-year follow-up. Failure of the OCA was defined as a revision cartilage restoration procedure or conversion to either unicompartmental or total knee arthroplasty. Failures via delamination at the junction of the cartilage and subchondral bone of the allograft as determined by magnetic resonance imaging or during subsequent surgery were recorded ( Figs. 1 and 2 ). Exclusion criteria included (1) significant bone loss that required further bone grafting at the time of initial surgery, (2) inflammatory arthritis or autoimmune conditions, (3) prior or concomitant meniscal transplant, (4) generalized osteoarthritis, and (5) tibia OCA.
Patients
Of the 1,870 patients screened from 2000 to 2015, there were a total of 123 patients identified who fit the inclusion and exclusion criteria. The registry was used to identify which of these patients required revision surgery. Patients who required reoperation for any reason were closely scrutinized with regards to operative notes and magnetic resonance imaging (MRI) for evidence of delamination of the OCA cartilage off of the underlying bone by the primary author (RCR). When delamination was noted on MRI by the primary author, the diagnosis was confirmed with the read from a fellowship-trained musculoskeletal radiologist. As a result, 13 patients (11%) with failure of the allograft via delamination of the cartilage from the underlying subchondral bone were identified. Full graft records could not be identified for 1 patient, leaving 12 knees with complete data. Sample size calculations were then performed. Using a standard deviation in storage time of 4.1 days among all OCAs used for surgery in our database, at least 30 nonfailure controls were needed to detect a difference in storage time of 4 days or more between groups with at least a power of 0.80 and significance level of α = 0.05. Therefore, these failed knees were then matched to a cohort of 33 control knees who met all the same inclusion/exclusion criteria but did not exhibit graft failure. Matching was performed by hierarchical clustering based on age, sex, body mass index (BMI), and chondral defect location.
Demographic, preoperative, intraoperative, and postoperative data were collected for all 46 patients. Demographics included age, sex, and BMI. Preoperative data included number and type of prior ipsilateral knee surgeries, Marx activity scale scores, and International Knee Documentation Committee (IKDC) scores. Intraoperative data included laterality, chondral defect location, chondral defect size and depth, and concomitant procedures. Allograft information was also obtained including donor age, donor gender, and allograft storage time. Allograft storage time was defined with day 0 being the processing date. Postoperative data included reoperations and a minimum follow-up of 2 years.
Surgical Technique
All surgical procedures were performed by fellowship-trained orthopedic surgeons at a single institution with extensive experience in cartilage restoration procedures. Initially, an examination under anesthesia was performed followed by a diagnostic arthroscopy to assess the chondral lesion as well as the remainder of the articular surfaces, ligaments, and menisci.
Fresh, cold-stored OCAs were obtained from commercially available sources. American Association of Tissue Banks standards were used to screen and process the donor allografts.18 Grafts were transplanted between 20 to 35 days after processing depending on serological evaluation and patient availability, and implantation was performed via the dowel technique.6 A small parapatellar arthrotomy was used to expose the chondral defect which was then debrided to stable rim. Lesions were then sized and reamed to a bed of normal subchondral bone, typically 8 to 10 mm, and a graft is taken from the donor OCA to match the defect. Lesion depth is measured at 3 to 4 locations around the circumference of the defect, and the donor graft is prepared accordingly to match the depth. Grafts are then gently impacted into place for press-fit fixation. Grafts were either a single or dual circular dowel shape in most cases, depending on lesion size and shape. Inspection and palpation was performed to ensure the grafts were flush with surrounding native cartilage.
Patients followed the same postoperative rehabilitation protocol. They were touch down weightbearing in a hinged brace for 1 week, followed by progression to full weightbearing as tolerated. Immediate full range of motion was permitted and assisted by using a continuous passive motion (CPM) machine. The hinged knee brace was continued for a minimum of 2 weeks postoperatively, with the timing of discontinuation dependent on return of quadriceps strength and control. A supervised physical therapy program was undertaken for all patients postoperatively. The therapy regimen focused on restoration of normal gait, return of quadriceps function, and performance of sport-specific skills. Return to sport was initiated on an individual basis, typically starting with a running program at 6 months postoperatively. Sport-specific training and unrestricted activities were then progressed thereafter depending on return of lower extremity strength and coordination. Return to sport was typically permitted at 6 to 12 months following OCA.
Assessment of Outcomes
All complications and reoperations after the index OCA were recorded. Reoperation included operations such as arthroscopic removal of loose bodies, lysis of adhesions, debridement, and hardware removal. Allograft failure was defined as removal or revision of the OCA, or conversion to either a unicompartmental or total knee arthroplasty.
Statistical Analysis
Comparisons of baseline patient characteristics between the delamination and control groups were performed with nonparametric Mann-Whitney tests for continuous variables and chi-square or Fisher exact tests for categorical variables. Two-tailed tests were used for all statistical analysis with a P value set at 0.05.
Results
Thirteen patients were identified on MRI and/or at the time of revision surgery to have delamination of OCA cartilage from the underlying cancellous bone from 2000 to 2015. Mean time to failure was 39 months (range 3-144 months). Patients had a variety of symptoms that ultimately led to revision, including: mechanical symptoms (3/13), recurrent effusions (3/13), pain (9/13), or instability (2/13). Concomitant procedures were performed in 38% of the OCA delamination cohort ( Table 1 ). For all analyzed patients, donor OCA was obtained from 3 companies. In the delamination group, 8 patients received a Musculoskeletal Transplant Foundation (MTF) allograft, 4 received a Joint Restoration Foundation (JRF) allograft, and 1 received a CryoLife, Inc. allograft. In the control group, 30 patients received an MTF allograft, and 3 received a JRF allograft.
Table 1.
Characteristics of Osteochondral Allograft Delamination Group.
| Time to revision, years (range) | 3.24 (0.25-12) |
| Symptoms leading to revision, n (%) | |
| Recurrent effusions | 3 (23.0) |
| Pain | 9 (69.0) |
| Instability | 2 (15.0) |
| Mechanical | 3 (23.0) |
| Concomitant procedures, n | |
| None | 8 (62.0) |
| ACL reconstruction | 1 (7.7) |
| ACL reconstruction + meniscal repair | 1 (7.7) |
| Revision ACL reconstruction | 1 (7.7) |
| Partial meniscectomy | 1 (7.7) |
| HTO + partial meniscectomy | 1 (7.7) |
ACL = anterior cruciate ligament; HTO = high tibial osteotomy.
Age, gender, BMI, and laterality of the patients were similar between the 2 groups. There was no difference between the groups in number of prior ipsilateral knee surgeries (P = 0.26), total chondral defect size (P = 0.41), or preoperative Marx activity scores (P = 0.216). The delamination group did have a lower preoperative IKDC score than the control group (P < 0.01) ( Table 2 ).
Table 2.
Patient and Surgery Characteristics between Study Groups.a
| OCA Delamination (n = 13) | OCA Matches (n = 33) | P | |
|---|---|---|---|
| Patient characteristics | |||
| Age, years | 32 (16-53) | 36 (14-57) | 0.38 |
| Sex (male/female), n | 10/3 | 23/10 | 0.66 |
| Body mass index, kg/m2 | 26.8 (18.0-39.1) | 25.0 (15.8-36.2) | 0.310 |
| Laterality (right/left), n | 7/6 | 17/16 | 0.89 |
| No. of prior surgeries | 1.8 (0-10) | 0.84 (0-3, n = 31) | 0.26 |
| Preoperative Marx activity score | 4.45 (0-16, n = 11) | 7.23 (0-16, n = 26) | 0.216 |
| Preoperative IKDC score | 42.7 (29.9-52.8, n = 9) | 54.0 (28.7-87.3, n = 26) | 0.00848 |
| Allograft characteristics | |||
| Allograft donor age, years | 27 (15-42) | 29 (17-41) | 0.54 |
| Allograft donor gender (male/female) | 10/3 | 27/6 | 0.71 |
| Allograft storage timeb | 30 (n = 12) | 31 | 0.78 |
| No. of plugs used | 1.8 | 1.3 | 0.056 |
| Average plug size, mm | 21 | 22 | 0.76 |
| Lesion characteristics | |||
| OCA location, n | |||
| MFC | 5 | 7 | |
| LFC | 5 | 16 | |
| Trochlea | 1 | 7 | |
| Patella | 0 | 0 | |
| MFC + LFC | 1 | 0 | |
| MFC + trochlea | 1 | 3 | |
| Chondral defect area, cm2 | 5.31 (1.0-9.5) | 5.50 (0.75-15.25) | 0.809 |
OCA = osteochondral allograft; MFC = medial femoral condyle; LFC = lateral femoral condyle; IKDC = International Knee Documentation Committee.
Values are reported as the mean with the range in parentheses unless otherwise indicated.
Defined with day 0 being the processing date.
Among the allografts transplanted, there was no difference in donor age (P = 0.54), sex (P = 0.66), or storage time (P = 0.78). The average age for the allograft donors was 27 years in the delamination group and 29 years in the control group. Average storage time was 30 days in the delamination group and 31 days in the control group. Both groups had a preponderance of male allograft donors. The delamination group used an overage of 1.8 OCA plugs compared with 1.3 in the control group (P = 0.056). The average size of each plug was 21 mm in the delamination group and 22 mm in the control group (P = 0.76) ( Table 2 ).
Regarding the chondral defect location, in the OCA delamination group, 38% (5/13) were medial femoral condyle (MFC) lesions, 38% (5/13) were lateral femoral condyle (LFC) lesions and there was one each for trochlea (7%), MFC + LFC (7%), and MFC + trochlea (7%) lesion. In the OCA-matched group, 48% (16/33) were LFC, 21% (7/33) were MFC, 21% (7/33) were trochlea, and 9% (3/33) were MFC + trochlea. Moreover, grafts were considered condyle-matched if the donor hemicondyle allograft was the same condyle (medial or lateral) as the location of the treated chondral defect. In the delamination group, the graft was condyle-matched to the recipient for 54% (7/13) of knees compared to 67% (22/33) of knees in the control group (P = 0.45). The chondral defect was Outerbridge grade IV in all cases and the size average was 5.32 cm2 for the delamination group and 5.50 cm2 for the control group (P = 0.809) ( Table 2 ).
Discussion
The principal finding of this study rejects the hypothesis that longer storage time of OCA would be associated with OCA delamination after implantation into the knee. Additionally, there was no association between other donor characteristics, host characteristics, or number of prior ipsilateral knee surgeries and risk of OCA delamination. Furthermore, there was no association between condyle-matching and risk of OCA delamination.
Our hypothesis was based on in vitro studies that demonstrated chondrocyte viability decreased as storage time increased.13,16 There has only been one prior study of two cases describing delamination of the donor cartilage from the underlying subchondral surface, but this occurred in decellularized osteochondral allograft plugs, not fresh OCAs.12 The role chondrocyte viability plays in delamination has not been defined, but given the results of our study, it may not play a significant role in this particular type of failure. Rather than chondrocyte viability, delamination may be the result of the different mechanical properties between the chondral and osseous portion of the graft. Those grafts that have a mismatch in osseous topography exhibit higher shear forces at the chondral-bone interface.19 This occurs intraoperatively because the cartilage thickness varies depending on the location within the knee and this chondral topography is another area where mismatch can occur.
Another possible mechanism of delamination could relate to immunogenicity. There is an immunologic response to allograft chondrocytes; however, humoral antibodies have a difficult time penetrating an intact cartilage matrix to be sensitized to foreign chondrocytes.20 In an animal study, grafts with an intact articular surface demonstrated no significant antibodies or leukocyte migration, but when isolated chondrocytes were injected, they invoked significant immune response.20 There can be an immunologic response at the bone-bone interface due to the expression of antigenic proteins by the osseous portion of the donor graft.21 While the role immunogenicity plays in osteochondral delamination is unknown, it is possible that the immune response occurring at the interface contributes to microinstability and increases the risk of delamination.
Previous studies have demonstrated an association between OCA failure (not delamination specifically) and number of prior ipsilateral knee surgeries, as well as increased BMI.11 Our study did not find an association between number of prior ipsilateral knee surgeries or increased BMI and delamination as the particular mechanism of failure. We also examined preoperative activity scales for associations with increased risk of OCA delamination. We found that patients who had OCA delamination had lower IKDC scores but similar Marx activity scores. Patient activity scores and BMI are often used as a surrogate for increased joint reactive forces in the knee. However, this is the first study to begin examining for potential factors associated with OCA delamination. To further investigate the etiology of OCA delamination, we will need more long-term clinical data and further basic science investigation. Examination of the interface between the chondral surface and underlying subchondral bone with electron microscopy and biomechanical studies that measure the shear resistance at this interface are warranted.
This study has several limitations. Storage time was used as an indirect measure of chondrocyte viability; whereas, tissue biopsies of the OCA at the time of implantation would have provided more precise information about the possible role of chondrocyte viability in this mode of failure. The technical goal is to achieve a <1-mm step-off between the native cartilage and the OCA; however, there may be a >1-mm step-off and the benefits of completely flush articular congruity must be weighed against the known chondrocyte cell death associated with increased impaction.22 An elevated step-off of greater than 1 mm creates significantly greater contact pressure of the OCA.23,24 Despite using inspection and palpation to ensure adequate fit of the grafts, we are unable to objectively examine the role of graft step-off, since we did not specifically measure and record any step-off between graft and host cartilage intraoperatively. Additionally, immediate postoperative MRIs, which can evaluate the magnitude of articular incongruency, were not obtained on most patients since this was not the standard of care. Therefore, we are unable to assess the role step-off may play in this mode of failure. An additional limitation is that we had incomplete preoperative scores for both groups, which may introduce bias. A power analysis was performed for storage time but not for other graft or patient characteristics, and therefore, the study may have been underpowered to detect differences in those criteria. Furthermore, the delamination group was compared to successful OCA determined at minimum 2-year follow up, which may be insufficient to detect all cases of delamination, particularly given the wide range of delamination failure (3-144 months) in our population. Ultimately, the etiology of failure of OCA by delamination may be multifactorial and difficult to assess. We would need many more delamination failures to be able to assess multiple factors at once.
Conclusion
This is the first study to our knowledge that examines risk factors for OCA delamination and found there was no association between prolonged graft storage time and increased risk of delamination at minimum 2-year follow-up.
Footnotes
Acknowledgments and Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: The Hospital for Special Surgery Institutional Review Board approved the registry (IRB #25023).
Informed Consent: All patients signed an informed consent form before participation.
Trial Registration: Not applicable.
References
- 1. Cole BJ, Pascual-Garrido C, Grumet RC. Surgical management of articular cartilage defects in the knee. J Bone Joint Surg Am. 2009;91(7):1778-90. [PubMed] [Google Scholar]
- 2. Meyers MH, Akeson W, Convery FR. Resurfacing of the knee with fresh osteochondral allograft. J Bone Joint Surg Am. 1989;71(5):704-13. [PubMed] [Google Scholar]
- 3. Camp CL, Stuart MJ, Krych AJ. Current concepts of articular cartilage restoration techniques in the knee. Sports Health. 2014;6(3):265-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. LaPrade RF, Botker J, Herzog M, Agel J. Refrigerated osteoarticular allografts to treat articular cartilage defects of the femoral condyles. A prospective outcomes study. J Bone Joint Surg Am. 2009;91(4):805-11. [DOI] [PubMed] [Google Scholar]
- 5. Raz G, Safir OA, Backstein DJ, Lee PT, Gross AE. Distal femoral fresh osteochondral allografts: Follow-up at a mean of twenty-two years. J Bone Joint Surg Am. 2014;96(13):1101-7. [DOI] [PubMed] [Google Scholar]
- 6. Williams RJ, 3rd, Ranawat AS, Potter HG, Carter T, Warren RF. Fresh stored allografts for the treatment of osteochondral defects of the knee. J Bone Joint Surg Am. 2007;89(4):718-26. [DOI] [PubMed] [Google Scholar]
- 7. Gross A, Kim W, Las Heras F, Backstein D, Safir O, Pritzker K. Fresh osteochondral allografts for posttraumatic knee defects: long-term followup. Clin Orthop Relat Res. 2008;466(8):1863-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McCulloch PC, Kang RW, Sobhy MH, Hayden JK, Cole BJ. Prospective evaluation of prolonged fresh osteochondral allograft transplantation of the femoral condyle: minimum 2-year follow-up. Am J Sports Med. 2007;35(3):411-20. [DOI] [PubMed] [Google Scholar]
- 9. Levy YD, Görtz S, Pulido PA, McCauley JC, Bugbee WD. Do fresh osteochondral allografts successfully treat femoral condyle lesions? Clin Orthop Relat Res. 2013;471(1):231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Krych AJ, Robertson CM, Williams RJ, 3rd; Cartilage Study Group. Return to athletic activity after osteochondral allograft transplantation in the knee. Am J Sports Med. 2012;40(5):1053-9. [DOI] [PubMed] [Google Scholar]
- 11. Frank RM, Lee S, Levy D, Poland S, Smith M, Scalise N, et al. Osteochondral allograft transplantation of the knee: analysis of failures at 5 years. Am J Sports Med. 2017;45(4):864-74. [DOI] [PubMed] [Google Scholar]
- 12. Degen RM, Tetreault D, Mahony GT, Williams RJ. Acute delamination of commercially available decellularized osteochondral allograft plugs: a report of two cases. Cartilage. 2016;7(4):316-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cook JL, Stannard JP, Stoker AM, Bozynski CC, Kuroki K, Cook CR, et al. Importance of donor chondrocyte viability for osteochondral allografts. Am J Sports Med. 2016;44(5):1260-8. [DOI] [PubMed] [Google Scholar]
- 14. Pallante AL, Chen AC, Ball ST, Amiel D, Masuda K, Sah RL, et al. The in vivo performance of osteochondral allografts in the goat is diminished with extended storage and decreased cartilage cellularity. Am J Sports Med. 2012;40(8):1814-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bugbee WD, Pallante-Kichura AL, Görtz S, Amiel D, Sah R. Osteochondral allograft transplantation in cartilage repair: graft storage paradigm, translational models, and clinical applications. J Orthop Res. 2016;34(1):31-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Williams SK, Amiel D, Ball ST, Allen RT, Wong VW, Chen AC, et al. Prolonged storage effects on the articular cartilage of fresh human osteochondral allografts. J Bone Joint Surg Am. 2003;85(11):2111-20. [DOI] [PubMed] [Google Scholar]
- 17. Allen RT, Robertson CM, Pennock AT, Bugbee WD, Harwood FL, Wong VW, et al. Analysis of stored osteochondral allografts at the time of surgical implantation. Am J Sports Med. 2005;33(10):1479-84. [DOI] [PubMed] [Google Scholar]
- 18. McAllister DR, Joyce MJ, Mann BJ, Vangsness CT., Jr Allograft update: the current status of tissue regulation, procurement, processing, and sterilization. Am J Sports Med. 2007;35(12):2148-58. [DOI] [PubMed] [Google Scholar]
- 19. von Rechenberg B, Akens MK, Nadler D, Bittmann P, Zlinszky K, Kutter A, et al. Changes in subchondral bone in cartilage resurfacing—an experimental study in sheep using different types of osteochondral grafts. Osteoarthritis Cartilage. 2003;11(4):265-77. [DOI] [PubMed] [Google Scholar]
- 20. Langer F, Gross AE. Immunogenicity of allograft articular cartilage. J Bone Joint Surg Am. 1974;56(2):297-304. [PubMed] [Google Scholar]
- 21. Stevenson S, Shaffer JW, Goldberg VM. The humoral response to vascular and nonvascular allografts of bone. Clin Orthop Relat Res. 1996;(326):86-95. [DOI] [PubMed] [Google Scholar]
- 22. Borazjani BH, Chen AC, Bae WC, Patil S, Sah RL, Firestein GS, et al. Effect of impact on chondrocyte viability during insertion of human osteochondral grafts. J Bone Joint Surg Am. 2006;88(9):1934-43. [DOI] [PubMed] [Google Scholar]
- 23. Koh JL, Wirsing K, Lautenschlager E, Zhang LO. The effect of graft height mismatch on contact pressure following osteochondral grafting. Am J Sports Med. 2004;32(2):317-20. [DOI] [PubMed] [Google Scholar]
- 24. Pearce SG, Hurtig MB, Clarnette R, Kalra M, Cowan B, Miniaci A. An investigation of 2 techniques for optimizing joint surface congruency using multiple cylindrical osteochondral autografts. Arthroscopy. 2001;17(1):50-5. [DOI] [PubMed] [Google Scholar]