Abstract
Chronic myeloid leukemia (CML) is caused by BCRABL1 in a cell with the biological potential, intrinsic or acquired, to cause leukemia. This cell is commonly termed the CML leukemia stem cell (LSC). In humans a CML LSC is operationally-defined by ≥1 in vitro or in vivo assays of human leukemia cells transferred to immune-deficient mice. Results of these assays are sometimes discordant. There is also the unproved assumption that biological features of a CML LSC are stable. These considerations make accurate and precise identification of a CML LSC difficult or impossible. In this review, we consider biological features of CML LSCs defined by these assays. We also consider whether CML LSCs are susceptible to targeting by tyrosine kinase inhibitors (TKIs) and other drugs, and whether elimination of CML LSCs is needed to achieve therapy-free remission or cure CML.
Subject terms: Cancer stem cells, Cell signalling
Introduction
Chronic myeloid leukemia (CML) is a blood cancer caused by BCRABL1 in a cell with the biological ability, intrinsic or acquired, to cause leukemia [1]. BCRABL1 encodes a 210 KD chimeric protein (P210BCRABL1) with constitutive tyrosine-kinase activity [2, 3]. By various incompletely defined mechanisms, this abnormality results in expansion of the leukemia clone [4]. In chronic-phase CML, proliferation is regulated, such that the leukemia cells mature normally and respond appropriately to normal regulators, such as granulocyte-colony-stimulating and macrophage-colony-stimulating factors (G-CSF and G/M-CSF) and to infection [5]. There are simply too many of them. Also, in rare persons with cyclic neutropenia and CML levels of blood leukemia granulocytes also cycle indicating that they respond to normal regulation of granulocyte production [6]. Some data suggest the increased granulocyte mass typical of CML results from a few extra cell divisions within the hierarchy of granulopoiesis [5]. Untreated and/or absent effective therapy, chronic phase CML eventuates in uncontrolled proliferation, loss of differentiation and loss of response to normal control mechanisms. This phase of CML is termed acute phase or blast crisis and typically resembles acute myeloid leukemia (AML) or, less often, acute lymphoid leukemia (ALL). Acute phase is thought to result from additional genetic instability and acquisition of more mutations somehow caused by the activity of P210BCRABL1 [7].
Some persons appear to have a transition phase between chronic and acute phases termed as accelerated phase. Because of these diverse, arbitrarily defined, but not biologically based definitions of the accelerated phase, we consider CML a fundamentally biphasic disease. This is not a new concept. Many of the original CML chemotherapy trials done by CALGB divided the disease into chronic and non-chronic phases. There are many definitions of accelerated phase all of which are arbitrary, for example, defining accelerated phase by >10% blood or bone marrow blasts, >15% and >20% blood basophils, platelets <100 × 10E+9/L etc. means the same person can be in chronic phase in one study and accelerated phase in another. Then, there is the obvious problem of someone saying that 19% blasts are in the chronic phase and the rest of them with 20% in the accelerated phase. There is no biological basis for such an arbitrary boundary. Adding to this, the daily variability of blast percent in someone with CML who could be in chronic phase one day, accelerated phase the next and back to chronic phase the next. And there is the problem of precision. Surveying 100–200 blood cells to determine percent blasts has reasonably wide confidence intervals, which can easily span any arbitrary boundary like 20%. Then we add to this inter-observer and intra-observer variability. The same arbitrariness applies to using additional cytogenetic abnormalities to define accelerated phase. Sandberg et al. reported that they could detect cytogenetic abnormalities used by some to define accelerated phase in many newly-diagnosed persons with CML when they surveyed 100 s of metaphases [8]. These persons typically had clinical features of chronic phase and most remained in chronic phase for years, sometimes decades. This is not surprising given the long latency from the start of CML to its diagnosis (see below). Others reported some, but not all additional cytogenetic abnormalities used to define accelerated phase are not associated with an increased risk of dying from CML [9, 10]. Hehlmann and co-workers recently reported some additional chromosome abnormalities used to define accelerated phase do not correlate with an increased probability of death survival in persons with CML [11]. The sum of these considerations supports the concept of CML as a bi-phasic disease.
The cell in which BCRABL1 first occurs and which causes chronic phase CML is termed the CML leukemia stem cell (LSC). Some progeny of this cell may also have or acquire stem cell features including the biological ability to cause CML recurrence. As such there may eventually be more than 1 CML LSC in someone with CML, especially for a prolonged interval. However, there are several problems with this concept. First, there is substantial controversy over what feature(s) defines a stem cell. The most common definition is a cell which can continuously produce unaltered daughters, as well as daughter cells with different, more restricted properties. This contrasts with a progenitor cell, cells with proliferative capacity, which may or may not be committed to a lineage choice but are not terminally-differentiated. Adding to this complexity is the concept of precursor cells, cells which are usually, although not always, post-mitotic but have the capacity to assume one of several differentiated states. Whether CML starts in a stem, progenitor, or precursor cell is unknown and probably unknowable and unproveable. Although some may argue the presence of the Ph1-chromsome in other lineages such as B-cells proves CML must begin in a stem cell, we are not convinced considering the possibility BCRABL1 may confer on a more mature stem cell features via de-differentiation much like occurs with induced pluri-potent stem cells [12]
A second cause of controversy is the definition of a stem cell varies based on the field of study, the organism being studied, the assay and other considerations. For example, the phenotype of a stem cell may not be static but vary at different points in the cell-cycle [13]. The same changeability may apply to definitions based on gene-expression profiling. A third consideration is that a cell which is not initially a stem cell may become a stem cell because of mutational, environmental, and/or architectural events (the bone marrow microenvironment).
Tyrosine kinase-inhibitors (TKIs) which block the biochemical activity of P210BCRABL1 can cure persons with chronic phase CML [14]. Whether this cure is absolute, namely no residual leukemia cells are able to cause CML recurrence even in someone with an infinite lifespan (sometimes equated, without convincing data, as no residual CML LSCs) or operational (no recurrence of CML during a person’s remaining lifetime) is controversial and we cannot distinguish these cures with present technologies. Another controversial issue is whether operational cure implies no recurrence of CML whilst receiving or not receiving therapy. For example, is someone without recurrent CML whilst on TKI-therapy cured? Probably no. We would not consider someone with diabetes cured just because their disease is controlled by taking insulin. Regardless of these complexities, life-expectancy of persons with chronic phase CML successfully-treated with TKIs is like sex-matched and age-matched normals in some, but not all studies [15, 16]. Most deaths in successfully-treated persons with CML are from the causes other than leukemia such as cardio-vascular disease and new cancers. Considerable conceptual and experimental data suggest that although TKIs inhibit proliferation of the CML clone they do not target CML LSCs [17].
About one-half of persons with CML achieving a deep molecular remission for a few years [about one-third to one-half of the whole population] can discontinue TKI-therapy without leukemia recurrence for median observation intervals up to 7–8 years [18, 19], a condition termed therapy-free remission (TFR). How this occurs is controversial. In some persons in TFR a low-concentration BCRABL1 transcripts can be detected [20]. Whether this is important or convincing is difficult to know as there are several reports of detecting BCRABL1 transcripts in normals, presumably derived from cells without the biological capacity to cause CML [21, 22]. Another possibility is these transcripts are from the cells able to cause CML but do not do so within the observation interval, or perhaps within a person’s remaining lifetime. Stochastic considerations may also apply. Some studies report CML LSCs are present at diagnosis, during therapy and in persons who are in TFR. Other studies discussed the correlation between CML LSC detection and probability of achieving TFR, but this is controversial [23, 24].
Here, we review techniques to identify and quantify CML LSCs. We discuss possible mechanisms of resistance to TKIs and the potential influence of the bone marrow microenvironment on CML LSCs. These data may help develop strategies to target CML LSCs and perhaps cure more persons with CML.
Cell surface antigens
A major challenge in studying LSCs is identifying a possible unique cell surface antigen phenotype. CML LSCs are CD34-positive and CD38-negative but this phenotype is not exclusive to CML LSCs [25]. Consequently, a useful cell surface antigen target must be on CML LSCs, but not normal stem cells or more differentiated leukemia cells, or must have a different expression pattern, density, or distribution. It must also be stably expressed on the CML LSC surface; antigens whose expression might vary with the cell-cycle would be less or not useful. An example is Siglec-3 (CD33) purportedly on normal stem cells and CML LSCs but with greater antigen density on CML LSCs [26]. Landberg et al. reported a similar disparity for CD36 [27]. Discriminating normal stem cells from CML LSCs may be difficult. Many other antigens such as CD44, CD47, CD52 are reportedly present on CML LSCs but also on normal hematopoietic stem/progenitor cells [28]. Other, supposedly, differentially expressed antigens include CD25, CD26, and interleukin-1-receptor accessory protein (IL-1RAP) [23, 29–31]. CD25 (IL2Rα) is regulated by STAT5 activity and increased CD25 expression is reported to reduce proliferation capacity of CML LSCs [32]. Also binding to IL-1RAP, a co-receptor of IL-1, activates the NF-kβ and AKT signaling pathways which increase proliferation of CML LSCs [33]. Some data suggest CD25 and IL-1RAP expression are unique to CML LSCs in CD34-postive, CD38-negative population but not in the more mature CD34-positive and CD38-positive fraction [29–31, 34].
Dipeptidyl peptidase-4, (DPP4; CD26) which cleaves diverse substrates such as chemokines and inhibits the stromal cell-derived factor-1 (SDF1; CXCL12). Cleaving the SDF1-CXCR4 axis by CD26 is implicated in releasing CML LSCs from the bone marrow into the blood and may be a marker of chronic phase CML LSCs [29, 35]. The concentration of CD26-positive CML LSCs in blood and bone marrow reportedly correlates with the white blood cells (WBC), but a relationship with response to TKI-therapy is unproved [29, 36]. CD26-positive LSCs are decreased after TKI treatment but following relapse or TKI-resistance number of CD26 expressing cells increases in the blood and bone marrow [29]. Similarly, Warfvinge et al. reported the concentration of CD26-postive LSCs correlates with TKI-resistance and identifies TKI-resistant sub-clones [37]. These authors also claim they can identify distinct sub-populations of CML LSCs based on single-cell gene expression patterns with CD26 expression restricted to resistant sub-clones [37]. The data we cite are complex and controversial. One possible profile of CML LSCs detected by flow cytometry could be cells which are Lin-negative, CD34-positive, CD38-negative/low, CD45RA-negative, KIT-negative, and CD26-positive [23, 37]. However, because we lack agreement on which biological assay defines a CML LSCs, it is presently impossible to know if this phenotype is correct. Newer studies suggest combining transcriptomics and proteomics data may be an effective approach in identifying antigens—qualitatively or quantitatively expressed on CML LSCs [38]. These data are displayed in Table 1.
Table 1.
Differential expression pattern of surface markers on normal stem cells and CML LSCs
Bone marrow microenvironment
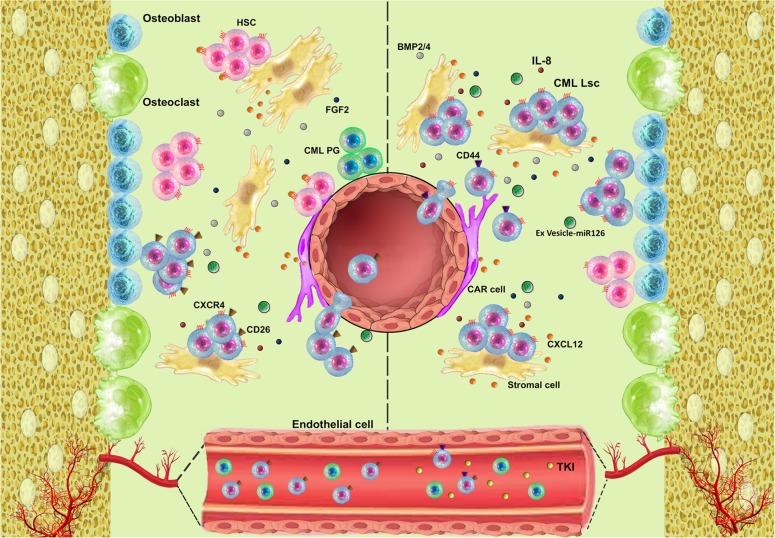
The bone marrow microenvironment is comprised of many cell-types including mesenchymal stromal cells, osteoblasts, osteoclasts, endothelial cells, and neural cells interact with normal haematopoietic stem cells through different molecules and signaling pathways [39–41]. Some data suggest levels of CXCR4, a chemokine receptor, correlate negatively with BCRABL1 transcription and translation [42]. Several studies report inhibiting the tyrosine kinase activity of P210BCRABL1 reduces CXCR4 protein levels which might favor release of CML LSCs from the bone marrow microenvironment into the blood. Why inhibiting P210BCRABL1 decreases CXCR4 levels is unknown. In contrast, increased levels of CXCR4 protein triggers homing of CML LSCs to the bone marrow microenvironment which induces quiescence and TKI-resistance [42, 43]. Other selectin-related ligands like CD44 are impliacted in homing of CML LSCs. This activity is different from normal stem cells which depend on β1-integrins like Very Late Antigens-4 (VLA4), Very Late Antigens-5 (VLA5) [44, 45], and are home to the bone marrow microenvironment. Secretion of granulocyte-colony stimulating factor (G-CSF), an antagonist of SDF1, by CML LSCs may facilitate their release into the blood [46]. Similarly, increased expression of CD26 on CML LSCs interrupts the SDF1-CXCR4 interaction which may also release CML LSCs into the blood [29].
Cross-talk between CML LSCs and the bone marrow microenvironment is mediated by diverse molecules via paracrine and autocrine mechanisms. Some data suggest CML LSCs secrete exosomes containing amphiregulin, which activates the epidermal growth factor (EGFR) pathway in mesenchymal stromal cells [47]. This interaction increases secretion of IL-8 facilitating adhesion of CML LSCs to mesenchymal stromal cells and favouring their survival [47]. Other data suggest several mechanisms combine to promote resistance of CML LSCs to TKI-therapy. For example, increased expression of BMPR1b in CML LSCs cells and activation via BMP2/4 by autocrine and paracrine mechanisms involving mesenchymal stromal cells increases the expression of TWIST1 facilitating resistance to TKIs [48–50]. Other studies report a mesenchymal stromal cell-mediated decrease of reactive oxygen species (ROS) concentrations in CML LSCs or production of FGF2 by mesenchymal stromal cell increases TKI-resistance of CML LSCs [51, 52]. These concepts are displayed in Fig. 1.
Fig. 1.
Structural and functional features of the bone marrow microenvironment. Before TKI-therapy downregulation of CXCR4 by P210BCRABL1 and increased expression of CD26 on CML LSCs causes them to exit the bone marrow and enter the blood. TKI-therapy reverses these effects causing CML LSCs are home to the bone marrow promoting their persistence
Effects of signaling pathways on CML LSCs
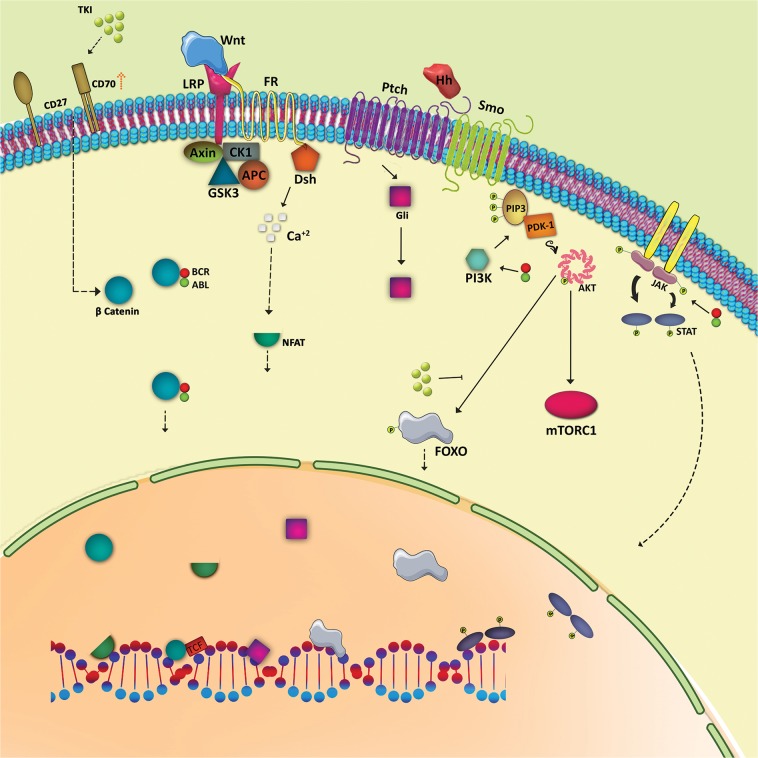
Mechanisms of resistance to TKIs include BCRABL1-dependent and BCRABL1-independent mechanisms [53–56] including mutations in the ATP-binding site or adjacent sites regulating the conformation of the dimeric P210BCRABL1 [57, 58] and the WNT/βcatenin, Hedgehog, PI3K/AKT and JAK/STAT signaling pathways.
β-catenin is important for self-renewal and long-term maintenance of normal stem cells and CML LSCs. Serial transplants of CML LSCs from β-catenin null mice into secondary recipients results in defects in self-renewal potential [59]. Also increased β-catenin expression is reported to correlate with advanced CML [60, 61]. Moreover, P210BCRABL1 interacts with β-catenin mediating its nuclear transition by stabilizing it [62]. One theory of TKI-resistance of CML LSCs is TKI-therapy amplifies CD70 expression by inhibiting miR29 which triggers WNT-signaling by activating CD27 [63]. Furthermore, interaction of mesenchymal stromal cells with CML LSCs via WNT/β-catenin signaling might cause TKI-resistance and increase proliferation of CML LSCs [64, 65]. Imatinib therapy increases activation of Nuclear Factor of Activated T-cells (NFAT), a transcription factor, by the non-canonical WNT signaling pathway resulting in increase pro-survival cytokines promoting imatinib resistance [66]. If so, therapy with a WNT/β-catenin-inhibitor and a TKI could eliminate CML LSCs [67, 68]. This remains to be proved.
Activation of Smoothened (Smo) by Ptch in the Hedgehog signaling pathway also activates Gli family transcription factors which stimulate transcription of target genes such as GLI1, PTCH1, BCL2, CYCLIN D, and MYC [69]. Chronic phase CML cells have high levels of mRNAs transcribed from genes in the Hedgehog signaling pathway suggesting a possible role in leukemia development [70]. Hedgehog signaling is activated in CML LSCs by upregulation of Smo persisting despite TKI-therapy suggesting this pathway is BCRABL1-independent [71]. Inhibiting Smo has no effect on normal HSCs but inhibits CML LSCs [71, 72]. One study reported exposing CML LSCs to cyclopamine, a Smo inhibitor, reduced their numbers and inhibited growth [73]. Another study reported PF-04449913, a Smo antagonist, causes cycling of CML LSCs and sensitizes them to TKIs [72].
The PI3K signaling pathway is important in maintaining normal stem cells and CML LSCs. One study reported PI3K signaling upregulates in CML LSCs [74] and correlates with P210BCRABL1 levels [75]. Normally, AKT phosphorylates FOXO transcription factors where it is sequestered in the cytoplasm. TKI-therapy promotes FOXO nucleus re-localization and restores transcriptional activity. Levels of BCL6, ATM, and CDKN1C, believed to be important for survival of CML LSCs, are increased by FOXO expression [76]. Another study reported inhibition of mTORC1 has no effect on CML LSCs whereas inhibiting PI3K increases susceptibility of CML LSCs to TKI-mediated inhibition [77]. Other data suggest arachidonate-15 lipoxygenase (Alox15) is essential for the maintenance of LSCs in a mouse model of CML. Inhibiting Alox15 expression increases PTEN expression, a negative regulator of PI3K-AKT, and downregulates expression of β-catenin, PI3K, and AKT [78].
The JAK/STAT signaling pathway may also be important in CML [79]. BCRABL1 activates STAT1, STAT3, and STAT5 [80, 81]. Downstream oncogenic signaling and JAK2 knockdown reduce P210BCRABL1 levels in BCRABL1-transfected mouse myeloid cells expressing BCRABL1-positive cell lines, including BV173, KBM-7, and K562-R [82]. JAK2-inhibition causes apoptosis of imatinib resistant CD34-positive CML cells from persons in chronic and acute phases [82]. Combining imatinib and interferon-γ decreases STAT5 phosphorylation whilst increasing phosphorylation of STAT1. This increases LSC survival, likely by upregulating expression of BCL6 [83]. Ruxolitinib, a JAK2-inhibitor, with nilotinib may decrease CML LSCs whilst sparing normal stem cells [84]. This remains to be proved. Signaling pathways in CML LSC are displayed in Fig. 2.
Fig. 2.
Possible signaling pathways in CML LSCs. Binding of WNT to the frizzled receptor and LRP as co-receptor and activation of WNT/β-catenin is considered the normal signaling mechanism. However, stabilization and transduction of β-catenin into nucleus by P210BCRABL1 and stimulation of CD70-CD27 after TKI-therapy may be specific to CML LSCs. Activation of WNT/Ca2+/NFAT favors CML LSC resistance to imatinib. Attachment of Hedgehog signaling ligands to the Ptch receptor activates Gli family transcription factors. Induction of PI3K activity by P210BCRABL1 phosphorylates PIP2 resulting in recruitment of PDK1 which phosphorylates AKT activating mTORC1 and sequesters FOXO transcription factors. TKI-therapy enhances nucleus localization of FOXOs increasing survival of CML LSCs. Activation of JAK/STAT signaling by P210BCRABL1 also increases survival of CML LSCs
microRNAs and CML LSCs
Some data suggest microRNAs may be important in CML LSC self-renewal, maintenance, and perhaps TKI-resistance [85]. miR-126 is reported to regulate dormancy of normal stem cells and CML LSCs [86]. P210BCRABL1 phosphorylates SPRED1 which downregulates miR-126 resulting a loss of stemness. In contrast, bone marrow endosteal Sca-1 + endothelial cells secrete high amounts of miR-126 via extra-cellular vesicles. TKIs reverse miR-126 inhibition with miR-126 levels increased by TKI-therapy. Decreased miR-126 levels sensitize CML LSCs to TKI [86]. Increased JAK-STAT signaling mediated by P210BCRABL1 (discussed above) increases ADAR1 levels increasing metabolism of adenosine to inosine which regulates miRNA stability. ADAR1 impairs biogenesis of mir-let7, a miR precursor, increases self-renewal of CML LSCs [87]. Exposing CML LSCs to imatinib increases levels of miR-21 resulting in TKI-resistance [88]. Interruption of the PI3K/AKT signaling pathway depletes miR-21 by amplifying PDCD4 and PTEN restoring sensitivity of CML LSCs to TKIs [88]. Proliferation of CML LSCs by E2F1 is mediated by increased levels of miR183 [89]. Also, miR-30a levels are decreased after imatinib-therapy by a mechanism involving Beclin1 and ATG5, which favors LSC resistance to TKIs [90]. Other miRNAs unrelated to P210BCRABL1 may also operate [91]. Increases in miRs-29a and miRs-29a-660 which target TET2 and EPAS1 decrease miR-494 thereby increasing CML LSC TKI-resistance [91]. Whether long noncoding RNAs (lncRNAs) and circular RNAs (circRNAs) are important in CML LSC biology remains unstudied. These data are displayed in Table 2.
Table 2.
Expression pattern and role of relevant miroRNAs in CML LSCs
| MicroRNAs | Role | Expression | BCR-ABL dependency | Reference |
|---|---|---|---|---|
| MiR-126 | Dormancy | ↑ | + | [86] |
| MiR-let7 | Tumor suppressor | ↓ | + | [87] |
| MiR-21 | Drug resistance | ↑ | + | [88] |
| MiR-183 | Proliferation | ↑ | + | [89] |
| MiR-30a | Drug resistance | ↑ | + | [90] |
| MiR-29a | Drug resistance | ↑ | – | [91] |
| MiR-660 | Drug resistance | ↑ | – | [91] |
| MiR-494 | Tumor suppressor | ↓ | – | [91] |
Autophagy and CML LSCs
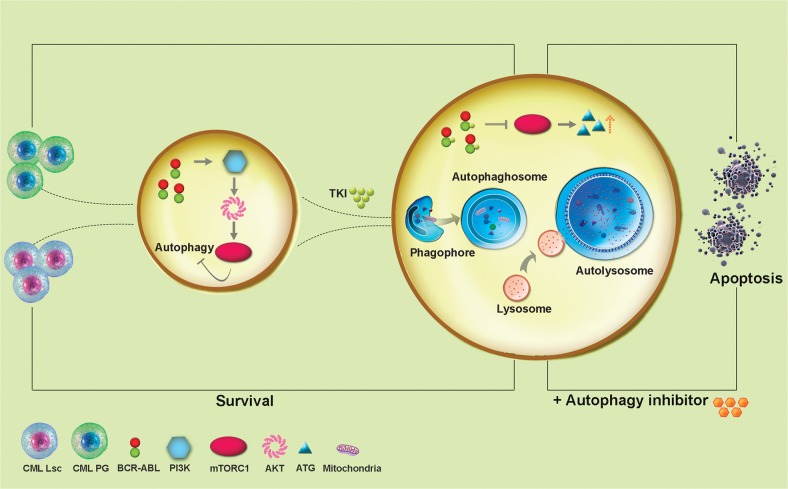
Several studies report complex, contradictory effects of TKI-therapy on autophagy in CML. For example, imatinib therapy increases intra-cellular levels of ATG4B which sensitizes CML LSCs cells to TKIs [92]. P210BCRABL1 increases levels of ATF5 via the PI3K/AKT pathway which upregulates mTORC1, an autophagy inhibitor. In contrast, inhibition of kinase activity of P210BCRABL1 by TKIs downregulates the PI3K/AKT pathway thereby increasing autophagy of CML progenitor cells but not CML LSCs [93–96]. Whether survival of CML LSCs can be overcome by adding other drugs is unknown [97]. In total, the role of autophagy in the biology of CML LSCs is complex and poorly understood. These concepts are displayed in Fig. 3.
Fig. 3.
Autophagy in CML. The interaction of P210BCRABL1 with PI3K-AKT inhibits autophagy. Contrariwise, TKIs inhibit kinase activity of P210BCRABL1 enhancing autophagy. These autophagy effects may kill CML progenitor cells but may preserve CML LSCs
Other molecules potentially involved in survival of CML LSCs
Blk
Concentrations of Blk, a tyrosine-kinase, are lower in CML LSCs compared with normal stem cells. Blk is reported to suppress CML LSCs by upregulating p27. However, P210BCRABL1 decreases Blk expression by modulating Pax5. Over-expression of Blk in CML LSCs inhibits self-renewal and increases apoptosis whereas Blk knock-down has no effect on normal stem cells [98].
EZH2
EZH2, part of the PRC2 complex, is an epigenetic repressor operating by tri-methylation of histone H3 (H3K27me3). EZH2 is upregulated in CML LSCs and is downregulated by TKI-therapy. Some data suggest inhibiting EZH2 increases the likelihood of eradicating CML LSCs with TKI-therapy whilst sparing normal stem cells [99] but this finding needs confirmation.
Fap-1
Fap-1 is a phosphatase which inhibits Fas-mediated apoptosis and stabilizes β-catenin by targeting Gsk3β, a β-catenin inhibitor. Increased Fap-1 levels are associated with persistence of CML LSCs. Inhibiting Fap-1 by Fap-1 blocking tripeptide in mice with a bone marrow transduced with a cDNA to human BCRABL1 increases response to TKIs and inhibits leukemia progression [100].
HIFs
Levels of hypoxia inducible factors (HIFs) increase in hypoxia conditions. This increase inhibits normal stem cell differentiation promoting a quiescent state [101]. HIF-1, a transcription factor, is important in regulating proliferation, maintenance, and survival of CML LSCs. Cheloni et al. reported acriflavine, a HIF-1 inhibitor, targets CML LSCs by reducing MYC and decreases stemness-related genes such as NANOG, SOX2, and OCT4 by decreasing HIF-2α. CML LSCs are more dependent on HIF-1 than normal HSCs. Consequently, combining a HIF-1 inhibitor with a TKI could potentially target CML LSC resident in hypoxic regions in the bone marrow microenvironment [102, 103]. Whether this is so requires confirmation.
PML
PML (promyelocyte leukemia protein) forms PML-nuclear bodies (PML-NBs) which are involved in multiple genome maintenance pathways including the DNA damage response and repair, telomere homeostasis, and p53-associated apoptosis. PML-NBs also play a role in repairing DNA double-strand breaks [DSBs] by homologous recombination [104]. Upregulation of PML in CML LSCs prevents cycling thereby increasing resistance to TKIs. Targeting PML in acute promyelocytic leukemia (APL) with all-trans retinoic acid and/or arsenic trioxide results in PML degradation and triggers cycling of the quiescent APL LSCs. This strategy could help eradicate CML LSCs by restoring TKI-sensitivity [105]. Interestingly, various forms of arsenic were used to treat persons with CML in the early 20th century. There are several ongoing and complete clinical trials of arsenic trioxide and TKIs in persons with CML (https://clinicaltrials.gov/ct2/results?cond=Chronic+Myeloid+Leukemia&term=arsenic&cntry=&state=&city=&dist=)
PP2A
Protein phosphatase 2A (PP2A), a serine/threonine phosphatase, is involved in the β-catenin pathway, programmed cell death, and cell cycle progression [106]. Decreasing PP2A in CML LSCs stimulates self-renewal [107]. The several isoforms of PP2A have diverse stimulatory and inhibitory effects on cancer cells [106]. Recently, Lai et al. reported combining a PP2A-inhibitor with a TKI suppresses CML [107, 108].
ALOX5
ALOX5 encodes 5-lipoxygenase (5-LO) which converts arachidonic acid into leukotrienes and is involved in inflammation and cancer development [109]. ALOX5 is important for induction of CML in mice [110]. Inhibiting ALOX5 with zileuton selectively reduces survival of CML LSCs compared with normal mouse stem cells [110]. In contrast to mice, humans have low ALOX5 expression and zileuton is inactive [111]. These data suggest targeting ALOX5 is probably not an effective strategy in humans.
SIRT1
Sirtuin 1 (SIRT1), a histone deacetylase, regulates gene expression, metabolic activity, and aging [112]. SIRT1 over-expression in CML LSCs deacetylates many transcription factors including P53, Ku70, and FOXO1 promoting drug resistance and survival of CML LSCs [113, 114]. Targeting SIRT1 in CML LSCs enhances acetylation of P53 increasing apoptosis [115]. This approach is untested in humans.
BCL2
Levels of BCL2-related anti-apoptotic proteins are reported to be expressed at higher levels in CML LSCs compared with normal stem cells [116] and is further increased in acute phase. Some data suggest P210BCRABL1 activates downstream signaling pathways such as PI3K and JAK/STAT resulting in TKI-resistance [117]. Some data suggest inhibiting BCL2 using subutoclax increases killing of CML LSCs by TKIs [116]. Another study in transgenic mice reported acute phase CML LSCs express high levels of BCL-xl, MCL-1, and BCL2. Venetoclax, another BCL2-inhibitor, combined with a TKI reduces the serial re-engraftment capacity of CML LSCs in mice compared with a TKI alone [118]. Clinical trials of venetoclax and a TKI in chronic (NCT02689440) and acute phase CML (NCT03576547) are beginning.
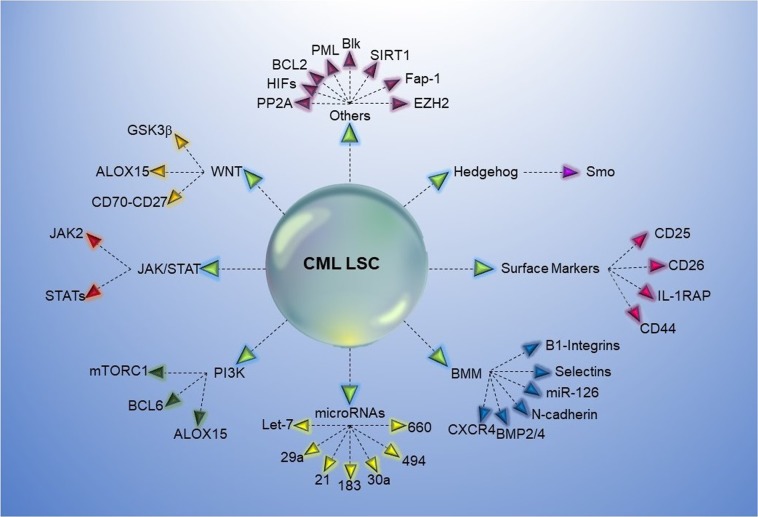
All potential molecules for targeting CML LSC are displayed in Fig. 4.
Fig. 4.
Potential molecules and pathways to target CML LSCs
Metabolomics of CML LSCs
Metabolic re-programming of CML LSCs in the hypoxic bone marrow microenvironment is a proposed mechanism of BCRABL1-independent TKI-resistance of CML LSCs. The concentration of intra-cellular dipeptide amino acids is increased in CML LSCs compared with normal stem cells. This effect is thought to be the results of upregulation of SLC15A2, a peptide transporter. Dipeptides activate the p38MAPK pathway resulting in phosphorylation of Smad3, important in maintaining CML LSCs [119].
Prostaglandin E1 (PGE1) an E2 (PGE2) are derived from di-homo-γ-linolenic acid [120]. PGE2 promotes β-catenin signaling in CML LSCs. In contrast, PGE1s via its interaction with E-type prostanoid receptor 4 (EP4) suppresses the self-renewal of CML LSCs and inhibits their engraftment in immune deficient mice [121].
CML LSCs have increased lipolysis and fatty acid oxidation with increased levels of glycerol-3-phosphate, carnitine, and acylcarnitine derivatives and decreased free fatty acids compared with differentiated CML cells. Consequently, inhibiting this pathway could be a therapy target [122, 123]. CML LSCs also have increased mitochondrial oxidative phosphorylation mediated by upregulation of genes involved in oxidative metabolism [122]. Single-cell transcriptome data suggest over-expression of oxidative phosphorylation and glycolysis-associated genes in CML LSCs compared with normal stem cells [124].
Under physiological hypoxic conditions (PO2 < 32 mmHg), HIF-1α signaling is crucial for the survival of CML LSCs treated with TKIs [125]. Short-term culture of CML LSCs under hypoxic conditions induces upregulation of genes involved in carbohydrate metabolism. These data suggest HIF-1α-driven glycolytic adaptation to support energy production [125].
In summary, there are some differences in the metabolomics of CML LSCs compared with normal stem cells. However, these pathways are poorly-defined and there are no data targeting the metabolism of CML LSCs alone or with TKIs is a reasonable therapy target.
Is targeting CML LSCs clinically important?
Above we discuss detailed studies of CML LSCs including their conclusions and limitations. Despite this extraordinary interest in CML LSCs—the question arises: is any of this research clinically important and do we have a correct definition of what is a CML LSC?
Data from several clinical trials indicate it’s possible to stop TKI-therapy in about one-half of persons with CML achieving a deep molecular response for an interval without CML recurrence for an extended subsequent interval, a situation referred to as therapy-free remission (TFR) [19, 126, 127]. Exactly what duration of deep molecular response is associated with the highest probability of TFR which is controversial. It is unlikely any specific duration is best. More likely, different durations of DMR will be associated with different probabilities of achieving TFR. However, these data coming from cohort studies, are complicated by guarantee time bias and will have wide 95% confidence intervals, limiting utility as applied to individuals [128]. That longer deep molecular remission intervals are associated with higher probabilities of TFR is a self-fulfilling prophesy which remains to be proved in a randomized clinical trial.
Considerable data indicate most if not all persons in TFR have some or even many residual CML LSCs as currently-defined including detection of BCRABL1 transcripts with sensitive technique such as digital polymerase chain reaction [23, 24]. These data support two conclusions: (1) it may not be necessary to eradicate all CML LSCs to achieve TFR in at least some people with CML; and (2) some or perhaps all of what we currently define as CML LSCs are resistant to killing by TKIs and can persist in persons in deep molecular remission including those able to stop TKI-therapy without CML recurrence. It is also possible that our definition[s] of a CML LSC is wrong and the cells we are measuring as CML LSCs are not.
What is the nature of the residual CML cells in persons in TFR? Are they really CML LSCs or CML progenitor cells with residual clonogenic activity or which have acquired stemness and revert to being LSCs? Why do some persons with deep molecular response who stop TKI therapy cause CML recurrence and in others not? Does everyone stopping TKI-therapy have residual CML LSCs? Could leukemia recurrence be a stochastic event or will everyone have leukemia recurrence if followed for enough interval? The latter theory is consistent with radiation-induced CML in the A-bomb survivors with median latencies for males and females of 13 and 17 years after exposure [16]. Or does the immune system keep residual CML LSCs in check as some have suggested? [129, 130]. Here, we must contend with contradictory data. There is a strong anti-CML-effect detected after allogeneic haematopoietic cell transplants [131]. Whether this anti-leukemia effect is distinct from graft-versus-host disease (GvHD) is controversial and little anti-leukemia effect occurs after transplants from genetically-identical twins [131]. It is also noteworthy that neither the CML is increased in persons with inherited, congenital, or acquired immune deficiency diseases nor in immune-suppressed recipients of solid organ transplant [132].
Conclusion
Identifying and understanding the biology of CML LSCs is an interesting and important area for scientific research. There are considerable data in this regard including >1500 articles published since 2000. Some of these provide valuable insights into the biology of CML LSCs, some are of questionable value and others are contradictory and/or mis-leading. There are important biological insights in many of these typescripts but an over-riding question is what the clinical importance of this research? For example, some people claim the cure of CML with TKIs would not be possible without a knowledge of CML LSCs. No scientific data support for this claim. The success of TKI-therapy hinged on measuring BCRABL1 transcript levels, not quantifying CML LSCs. These transcript are probably not transcribed by CML LSCs. Perhaps, if we could accurately and precisely identify and quantify CML LSCs, we might be able to cure more persons with CML. Time will tell if the investment in studying CML LSCs will be of clinical value.
Acknowledgements
Prof. Andreas Hochhaus [Univ. Jena] kindly reviewed the typescript and made helpful suggestions. Supported by AIRC [Italian Association for Cancer Research] investigator grant to GS. Ali Anjam-Najmedini prepared the figures. RPG acknowledges support from the National Institute of Health Research [NIHR] Biomedical Research Centre funding scheme.
Compliance with ethical standards
Conflict of interest
RPG is a part-time employee of Celgene Corp. The remaining authors declare that they have no conflict of interest.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Goldman JM, Melo JV. Targeting the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1084–6. doi: 10.1056/NEJM200104053441409. [DOI] [PubMed] [Google Scholar]
- 2.Konopka JB, Watanabe SM, Witte ON. An alteration of the human c-abl protein in K562 leukemia cells unmasks associated tyrosine kinase activity. Cell. 1984;37:1035–42. doi: 10.1016/0092-8674(84)90438-0. [DOI] [PubMed] [Google Scholar]
- 3.Ren R. Mechanisms of BCR-ABL in the pathogenesis of chronic myelogenous leukaemia. Nat Rev Cancer. 2005;5:172–83. doi: 10.1038/nrc1567. [DOI] [PubMed] [Google Scholar]
- 4.Cilloni D, Saglio G. Molecular pathways: BCR-ABL. Clin Cancer Res. 2012;18:930–7. doi: 10.1158/1078-0432.CCR-10-1613. [DOI] [PubMed] [Google Scholar]
- 5.Clarkson B, Strife A, Wisniewski D, Lambek CL, Liu C. Chronic myelogenous leukemia as a paradigm of early cancer and possible curative strategies. Leukemia. 2003;17:1211–62. doi: 10.1038/sj.leu.2402912. [DOI] [PubMed] [Google Scholar]
- 6.Gluckman E, Socie G, Yver A, Esperou H, Devergie A, Stern A. Transient cyclic neutropenia following GM-CSF in a patient with chronic granulocytic leukemia transplanted with HLA-identical T cell-depleted donor bone marrow. Bone Marrow Transplant. 1989;4:591–2. [PubMed] [Google Scholar]
- 7.Perrotti D, Jamieson C, Goldman J, Skorski T. Chronic myeloid leukemia: mechanisms of blastic transformation. J Clin Invest. 2010;120:2254–64. doi: 10.1172/JCI41246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sonta SI, Sandberg AA. Chromosomes and causation of human cancer and leukemia: XXVIII. Value of detailed chromosome studies on large numbers of cells in CML. Am J Hematol. 1977;3:121–6. doi: 10.1002/ajh.2830030202. [DOI] [PubMed] [Google Scholar]
- 9.Wiernik PH, Baig MA, Lee SH, Dutcher JP, Paietta E, Racevskis J. Survival more than 19 years after the diagnosis of accelerated phase of chronic myelocytic leukemia. Clin Adv Hematol Oncol. 2011;9:242–8. [PubMed] [Google Scholar]
- 10.Parant M, Parant F, Chedid L, Yapo A, Petit JF, Lederer E. Fate of the synthetic immunoadjuvant, muramyl dipeptide (14C-labelled) in the mouse. Int J Immunopharmacol. 1979;1:35–41. doi: 10.1016/0192-0561(79)90028-6. [DOI] [PubMed] [Google Scholar]
- 11.Hehlmann R, Lauseker M, Saussele S, Pfirrmann M, Krause S, Kolb HJ, et al. Assessment of imatinib as first-line treatment of chronic myeloid leukemia: 10-year survival results of the randomized CML study IV and impact of non-CML determinants. Leukemia. 2017;31:2398–406. doi: 10.1038/leu.2017.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi Y, Inoue H, Wu JC, Yamanaka S. Induced pluripotent stem cell technology: a decade of progress. Nat Rev Drug Discov. 2017;16:115–30. doi: 10.1038/nrd.2016.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quesenberry PJ, Dooner MS, Goldberg LR, Aliotta JM, Pereira M, Amaral A, et al. A new stem cell biology: the continuum and microvesicles. Trans Am Clin Clim Assoc. 2012;123:152–66. [PMC free article] [PubMed] [Google Scholar]
- 14.Savona MR, Saglio G. Identifying the time to change BCR-ABL inhibitor therapy in patients with chronic myeloid leukemia. Acta Haematol. 2013;130:268–78. doi: 10.1159/000353163. [DOI] [PubMed] [Google Scholar]
- 15.Hehlmann R, Muller MC, Lauseker M, Hanfstein B, Fabarius A, Schreiber A, et al. Deep molecular response is reached by the majority of patients treated with imatinib, predicts survival, and is achieved more quickly by optimized high-dose imatinib: results from the randomized CML-study IV. J Clin Oncol. 2014;32:415–23. doi: 10.1200/JCO.2013.49.9020. [DOI] [PubMed] [Google Scholar]
- 16.Radivoyevitch T, Jankovic GM, Tiu RV, Saunthararajah Y, Jackson RC, Hlatky LR, et al. Sex differences in the incidence of chronic myeloid leukemia. Radiat Environ Biophys. 2014;53:55–63. doi: 10.1007/s00411-013-0507-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graham SM, Jorgensen HG, Allan E, Pearson C, Alcorn MJ, Richmond L, et al. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood. 2002;99:319–25. doi: 10.1182/blood.V99.1.319. [DOI] [PubMed] [Google Scholar]
- 18.Etienne G, Guilhot J, Rea D, Rigal-Huguet F, Nicolini F, Charbonnier A, et al. Long-Term Follow-Up of the French Stop Imatinib (STIM1) Study in Patients With Chronic Myeloid Leukemia. J Clin Oncol. 2017;35:298–305. doi: 10.1200/JCO.2016.68.2914. [DOI] [PubMed] [Google Scholar]
- 19.Ross DM, Masszi T, Gomez Casares MT, Hellmann A, Stentoft J, Conneally E, et al. Durable treatment-free remission in patients with chronic myeloid leukemia in chronic phase following frontline nilotinib: 96-week update of the ENESTfreedom study. J Cancer Res Clin Oncol. 2018;144:945–54. doi: 10.1007/s00432-018-2604-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goh HG, Lin M, Fukushima T, Saglio G, Kim D, Choi SY, et al. Sensitive quantitation of minimal residual disease in chronic myeloid leukemia using nanofluidic digital polymerase chain reaction assay. Leuk Lymphoma. 2011;52:896–904. doi: 10.3109/10428194.2011.555569. [DOI] [PubMed] [Google Scholar]
- 21.Biernaux C, Loos M, Sels A, Huez G, Stryckmans P. Detection of major bcr-abl gene expression at a very low level in blood cells of some healthy individuals. Blood. 1995;86:3118–22. [PubMed] [Google Scholar]
- 22.Boquett JA, Alves JR, de Oliveira CE. Analysis of BCR/ABL transcripts in healthy individuals. Genet Mol Res. 2013;12:4967–71. doi: 10.4238/2013.October.24.8. [DOI] [PubMed] [Google Scholar]
- 23.Bocchia M, Sicuranza A, Abruzzese E, Iurlo A, Sirianni S, Gozzini A, et al. Residual Peripheral Blood CD26(+) Leukemic Stem Cells in Chronic Myeloid Leukemia Patients During TKI Therapy and During Treatment-Free Remission. Front Oncol. 2018;8:194. doi: 10.3389/fonc.2018.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cui J, Zhu Z, Liu S, Li Q, Meng L, Cheng H, et al. Monitoring of leukemia stem cells in chronic myeloid leukemia patients. Leuk Lymphoma. 2018;59:2264–6. doi: 10.1080/10428194.2017.1421755. [DOI] [PubMed] [Google Scholar]
- 25.Eisterer W, Jiang X, Christ O, Glimm H, Lee KH, Pang E, et al. Different subsets of primary chronic myeloid leukemia stem cells engraft immunodeficient mice and produce a model of the human disease. Leukemia. 2005;19:435–41. doi: 10.1038/sj.leu.2403649. [DOI] [PubMed] [Google Scholar]
- 26.Herrmann H, Cerny-Reiterer S, Gleixner KV, Blatt K, Herndlhofer S, Rabitsch W, et al. CD34(+)/CD38(-) stem cells in chronic myeloid leukemia express Siglec-3 (CD33) and are responsive to the CD33-targeting drug gemtuzumab/ozogamicin. Haematologica. 2012;97:219–26. doi: 10.3324/haematol.2010.035006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Landberg N, von Palffy S, Askmyr M, Lilljebjorn H, Sanden C, Rissler M, et al. CD36 defines primitive chronic myeloid leukemia cells less responsive to imatinib but vulnerable to antibody-based therapeutic targeting. Haematologica. 2018;103:447–55. doi: 10.3324/haematol.2017.169946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valent P, Sadovnik I, Racil Z, Herrmann H, Blatt K, Cerny-Reiterer S, et al. DPPIV (CD26) as a novel stem cell marker in Ph+chronic myeloid leukaemia. Eur J Clin Invest. 2014;44:1239–45. doi: 10.1111/eci.12368. [DOI] [PubMed] [Google Scholar]
- 29.Herrmann H, Sadovnik I, Cerny-Reiterer S, Rulicke T, Stefanzl G, Willmann M, et al. Dipeptidylpeptidase IV (CD26) defines leukemic stem cells (LSC) in chronic myeloid leukemia. Blood. 2014;123:3951–62. doi: 10.1182/blood-2013-10-536078. [DOI] [PubMed] [Google Scholar]
- 30.Landberg N, Hansen N, Askmyr M, Agerstam H, Lassen C, Rissler M, et al. IL1RAP expression as a measure of leukemic stem cell burden at diagnosis of chronic myeloid leukemia predicts therapy outcome. Leukemia. 2016;30:253–7. doi: 10.1038/leu.2015.135. [DOI] [PubMed] [Google Scholar]
- 31.Sadovnik I, Herrmann H, Eisenwort G, Blatt K, Hoermann G, Mueller N, et al. Expression of CD25 on leukemic stem cells in BCR-ABL1(+) CML: potential diagnostic value and functional implications. Exp Hematol. 2017;51:17–24. doi: 10.1016/j.exphem.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sadovnik I, Hoelbl-Kovacic A, Herrmann H, Eisenwort G, Cerny-Reiterer S, Warsch W, et al. Identification of CD25 as STAT5-Dependent Growth Regulator of Leukemic Stem Cells in Ph+CML. Clin Cancer Res. 2016;22:2051–61. doi: 10.1158/1078-0432.CCR-15-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agerstam H, Hansen N, von Palffy S, Sanden C, Reckzeh K, Karlsson C, et al. IL1RAP antibodies block IL-1-induced expansion of candidate CML stem cells and mediate cell killing in xenograft models. Blood. 2016;128:2683–93. doi: 10.1182/blood-2015-11-679985. [DOI] [PubMed] [Google Scholar]
- 34.Zhao K, Yin LL, Zhao DM, Pan B, Chen W, Cao J, et al. IL1RAP as a surface marker for leukemia stem cells is related to clinical phase of chronic myeloid leukemia patients. Int J Clin Exp Med. 2014;7:4787–98. [PMC free article] [PubMed] [Google Scholar]
- 35.Matteucci E, Giampietro O. Dipeptidyl peptidase-4 (CD26): knowing the function before inhibiting the enzyme. Curr Med Chem. 2009;16:2943–51. doi: 10.2174/092986709788803114. [DOI] [PubMed] [Google Scholar]
- 36.Culen M, Borsky M, Nemethova V, Razga F, Smejkal J, Jurcek T, et al. Quantitative assessment of the CD26+leukemic stem cell compartment in chronic myeloid leukemia: patient-subgroups, prognostic impact, and technical aspects. Oncotarget. 2016;7:33016–24. doi: 10.18632/oncotarget.9108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Warfvinge R, Geironson L, Sommarin MNE, Lang S, Karlsson C, Roschupkina T, et al. Single-cell molecular analysis defines therapy response and immunophenotype of stem cell subpopulations in CML. Blood. 2017;129:2384–94. doi: 10.1182/blood-2016-07-728873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonardi F, Fusetti F, Deelen P, van Gosliga D, Vellenga E, Schuringa JJ. A proteomics and transcriptomics approach to identify leukemic stem cell (LSC) markers. Mol Cell Proteom. 2013;12:626–37. doi: 10.1074/mcp.M112.021931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006;6:93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- 40.Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–34. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Houshmand M, Soleimani M, Atashi A, Saglio G, Abdollahi M, Nikougoftar Zarif M. Mimicking the Acute Myeloid Leukemia Niche for Molecular Study and Drug Screening. Tissue Eng Part C Methods. 2017;23:72–85. doi: 10.1089/ten.tec.2016.0404. [DOI] [PubMed] [Google Scholar]
- 42.Jin L, Tabe Y, Konoplev S, Xu Y, Leysath CE, Lu H, et al. CXCR4 up-regulation by imatinib induces chronic myelogenous leukemia (CML) cell migration to bone marrow stroma and promotes survival of quiescent CML cells. Mol Cancer Ther. 2008;7:48–58. doi: 10.1158/1535-7163.MCT-07-0042. [DOI] [PubMed] [Google Scholar]
- 43.Weisberg E, Azab AK, Manley PW, Kung AL, Christie AL, Bronson R, et al. Inhibition of CXCR4 in CML cells disrupts their interaction with the bone marrow microenvironment and sensitizes them to nilotinib. Leukemia. 2012;26:985–90. doi: 10.1038/leu.2011.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bhatia R, McCarthy JB, Verfaillie CM. Interferon-alpha restores normal beta 1 integrin-mediated inhibition of hematopoietic progenitor proliferation by the marrow microenvironment in chronic myelogenous leukemia. Blood. 1996;87:3883–91. [PubMed] [Google Scholar]
- 45.Krause DS, Lazarides K, Lewis JB, von Andrian UH, Van Etten RA. Selectins and their ligands are required for homing and engraftment of BCR-ABL1+leukemic stem cells in the bone marrow niche. Blood. 2014;123:1361–71. doi: 10.1182/blood-2013-11-538694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang B, Ho YW, Huang Q, Maeda T, Lin A, Lee SU, et al. Altered microenvironmental regulation of leukemic and normal stem cells in chronic myelogenous leukemia. Cancer Cell. 2012;21:577–92. doi: 10.1016/j.ccr.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Corrado C, Saieva L, Raimondo S, Santoro A, De Leo G, Alessandro R. Chronic myelogenous leukaemia exosomes modulate bone marrow microenvironment through activation of epidermal growth factor receptor. J Cell Mol Med. 2016;20:1829–39. doi: 10.1111/jcmm.12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang RN, Green J, Wang Z, Deng Y, Qiao M, Peabody M, et al. Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes Dis. 2014;1:87–105. doi: 10.1016/j.gendis.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grockowiak E, Laperrousaz B, Jeanpierre S, Voeltzel T, Guyot B, Gobert S, et al. Immature CML cells implement a BMP autocrine loop to escape TKI treatment. Blood. 2017;130:2860–71. doi: 10.1182/blood-2017-08-801019. [DOI] [PubMed] [Google Scholar]
- 50.Houshmand M, Circosta P, Saglio G. Immature CML cells implement a BMP autocrine loop to escape TKI treatment. Transl Cancer Res. 2018;7:S722–S5.
- 51.Traer E, Javidi-Sharifi N, Agarwal A, Dunlap J, English I, Martinez J, et al. Ponatinib overcomes FGF2-mediated resistance in CML patients without kinase domain mutations. Blood. 2014;123:1516–24. doi: 10.1182/blood-2013-07-518381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bourgeais J, Ishac N, Medrzycki M, Brachet-Botineau M, Desbourdes L, Gouilleux-Gruart V, et al. Oncogenic STAT5 signaling promotes oxidative stress in chronic myeloid leukemia cells by repressing antioxidant defenses. Oncotarget. 2017;8:41876–89. doi: 10.18632/oncotarget.11480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thomas J, Wang L, Clark RE, Pirmohamed M. Active transport of imatinib into and out of cells: implications for drug resistance. Blood. 2004;104:3739–45. doi: 10.1182/blood-2003-12-4276. [DOI] [PubMed] [Google Scholar]
- 54.Soverini S, Hochhaus A, Nicolini FE, Gruber F, Lange T, Saglio G, et al. BCR-ABL kinase domain mutation analysis in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors: recommendations from an expert panel on behalf of European LeukemiaNet. Blood. 2011;118:1208–15. doi: 10.1182/blood-2010-12-326405. [DOI] [PubMed] [Google Scholar]
- 55.Barnes DJ, Palaiologou D, Panousopoulou E, Schultheis B, Yong AS, Wong A, et al. Bcr-Abl expression levels determine the rate of development of resistance to imatinib mesylate in chronic myeloid leukemia. Cancer Res. 2005;65:8912–9. doi: 10.1158/0008-5472.CAN-05-0076. [DOI] [PubMed] [Google Scholar]
- 56.Corbin AS, Agarwal A, Loriaux M, Cortes J, Deininger MW, Druker BJ. Human chronic myeloid leukemia stem cells are insensitive to imatinib despite inhibition of BCR-ABL activity. J Clin Invest. 2011;121:396–409. doi: 10.1172/JCI35721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim T, Tyndel MS, Kim HJ, Ahn JS, Choi SH, Park HJ, et al. Spectrum of somatic mutation dynamics in chronic myeloid leukemia following tyrosine kinase inhibitor therapy. Blood. 2017;129:38–47. doi: 10.1182/blood-2016-04-708560. [DOI] [PubMed] [Google Scholar]
- 58.Mahon FX, Belloc F, Lagarde V, Chollet C, Moreau-Gaudry F, Reiffers J, et al. MDR1 gene overexpression confers resistance to imatinib mesylate in leukemia cell line models. Blood. 2003;101:2368–73. doi: 10.1182/blood.V101.6.2368. [DOI] [PubMed] [Google Scholar]
- 59.Zhao C, Blum J, Chen A, Kwon HY, Jung SH, Cook JM, et al. Loss of beta-catenin impairs the renewal of normal and CML stem cells in vivo. Cancer Cell. 2007;12:528–41. doi: 10.1016/j.ccr.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jamieson CH, Ailles LE, Dylla SJ, Muijtjens M, Jones C, Zehnder JL, et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med. 2004;351:657–67. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- 61.Minami Y, Stuart SA, Ikawa T, Jiang Y, Banno A, Hunton IC, et al. BCR-ABL-transformed GMP as myeloid leukemic stem cells. Proc Natl Acad Sci USA. 2008;105:17967–72. doi: 10.1073/pnas.0808303105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Coluccia AM, Vacca A, Dunach M, Mologni L, Redaelli S, Bustos VH, et al. Bcr-Abl stabilizes beta-catenin in chronic myeloid leukemia through its tyrosine phosphorylation. EMBO J. 2007;26:1456–66. doi: 10.1038/sj.emboj.7601485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Riether C, Schurch CM, Flury C, Hinterbrandner M, Druck L, Huguenin AL, et al. Tyrosine kinase inhibitor-induced CD70 expression mediates drug resistance in leukemia stem cells by activating Wnt signaling. Sci Transl Med. 2015;7:298ra119. doi: 10.1126/scitranslmed.aab1740. [DOI] [PubMed] [Google Scholar]
- 64.Zhang B, Li M, McDonald T, Holyoake TL, Moon RT, Campana D, et al. Microenvironmental protection of CML stem and progenitor cells from tyrosine kinase inhibitors through N-cadherin and Wnt-beta-catenin signaling. Blood. 2013;121:1824–38. doi: 10.1182/blood-2012-02-412890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu N, Zang S, Liu Y, Wang Y, Li W, Liu Q, et al. FZD7 regulates BMSCs-mediated protection of CML cells. Oncotarget. 2016;7:6175–87. doi: 10.18632/oncotarget.6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gregory MA, Phang TL, Neviani P, Alvarez-Calderon F, Eide CA, O’Hare T, et al. Wnt/Ca2+/NFAT signaling maintains survival of Ph+leukemia cells upon inhibition of Bcr-Abl. Cancer Cell. 2010;18:74–87. doi: 10.1016/j.ccr.2010.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou H, Mak PY, Mu H, Mak DH, Zeng Z, Cortes J, et al. Combined inhibition of beta-catenin and Bcr-Abl synergistically targets tyrosine kinase inhibitor-resistant blast crisis chronic myeloid leukemia blasts and progenitors in vitro and in vivo. Leukemia. 2017;31:2065–74. doi: 10.1038/leu.2017.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hu Y, Chen Y, Douglas L, Li S. beta-Catenin is essential for survival of leukemic stem cells insensitive to kinase inhibition in mice with BCR-ABL-induced chronic myeloid leukemia. Leukemia. 2009;23:109–16. doi: 10.1038/leu.2008.262. [DOI] [PubMed] [Google Scholar]
- 69.Hanna A, Shevde LA. Hedgehog signaling: modulation of cancer properies and tumor mircroenvironment. Mol Cancer. 2016;15:24. doi: 10.1186/s12943-016-0509-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Su W, Meng F, Huang L, Zheng M, Liu W, Sun H. Sonic hedgehog maintains survival and growth of chronic myeloid leukemia progenitor cells through beta-catenin signaling. Exp Hematol. 2012;40:418–27. doi: 10.1016/j.exphem.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 71.Dierks C, Beigi R, Guo GR, Zirlik K, Stegert MR, Manley P, et al. Expansion of Bcr-Abl-positive leukemic stem cells is dependent on Hedgehog pathway activation. Cancer Cell. 2008;14:238–49. doi: 10.1016/j.ccr.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 72.Sadarangani A, Pineda G, Lennon KM, Chun HJ, Shih A, Schairer AE, et al. GLI2 inhibition abrogates human leukemia stem cell dormancy. J Transl Med. 2015;13:98. doi: 10.1186/s12967-015-0453-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhao C, Chen A, Jamieson CH, Fereshteh M, Abrahamsson A, Blum J, et al. Hedgehog signalling is essential for maintenance of cancer stem cells in myeloid leukaemia. Nature. 2009;458:776–9. doi: 10.1038/nature07737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Diaz-Blanco E, Bruns I, Neumann F, Fischer JC, Graef T, Rosskopf M, et al. Molecular signature of CD34(+) hematopoietic stem and progenitor cells of patients with CML in chronic phase. Leukemia. 2007;21:494–504. doi: 10.1038/sj.leu.2404549. [DOI] [PubMed] [Google Scholar]
- 75.Cokic VP, Mojsilovic S, Jaukovic A, Kraguljac-Kurtovic N, Mojsilovic S, Sefer D, et al. Gene expression profile of circulating CD34(+) cells and granulocytes in chronic myeloid leukemia. Blood Cells Mol Dis. 2015;55:373–81. doi: 10.1016/j.bcmd.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pellicano F, Scott MT, Helgason GV, Hopcroft LE, Allan EK, Aspinall-O’Dea M, et al. The antiproliferative activity of kinase inhibitors in chronic myeloid leukemia cells is mediated by FOXO transcription factors. Stem Cells. 2014;32:2324–37. doi: 10.1002/stem.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Airiau K, Mahon FX, Josselin M, Jeanneteau M, Belloc F. PI3K/mTOR pathway inhibitors sensitize chronic myeloid leukemia stem cells to nilotinib and restore the response of progenitors to nilotinib in the presence of stem cell factor. Cell Death Dis. 2013;4:e827. doi: 10.1038/cddis.2013.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen Y, Peng C, Abraham SA, Shan Y, Guo Z, Desouza N, et al. Arachidonate 15-lipoxygenase is required for chronic myeloid leukemia stem cell survival. J Clin Invest. 2014;124:3847–62. doi: 10.1172/JCI66129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Warsch W, Walz C, Sexl V. JAK of all trades: JAK2-STAT5 as novel therapeutic targets in BCR-ABL1+chronic myeloid leukemia. Blood. 2013;122:2167–75. doi: 10.1182/blood-2013-02-485573. [DOI] [PubMed] [Google Scholar]
- 80.Chai SK, Nichols GL, Rothman P. Constitutive activation of JAKs and STATs in BCR-Abl-expressing cell lines and peripheral blood cells derived from leukemic patients. J Immunol. 1997;159:4720–8. [PubMed] [Google Scholar]
- 81.Xie S, Wang Y, Liu J, Sun T, Wilson MB, Smithgall TE, et al. Involvement of Jak2 tyrosine phosphorylation in Bcr-Abl transformation. Oncogene. 2001;20:6188–95. doi: 10.1038/sj.onc.1204834. [DOI] [PubMed] [Google Scholar]
- 82.Samanta A, Perazzona B, Chakraborty S, Sun X, Modi H, Bhatia R, et al. Janus kinase 2 regulates Bcr-Abl signaling in chronic myeloid leukemia. Leukemia. 2011;25:463–72. doi: 10.1038/leu.2010.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Madapura HS, Nagy N, Ujvari D, Kallas T, Krohnke MCL, Amu S, et al. Interferon gamma is a STAT1-dependent direct inducer of BCL6 expression in imatinib-treated chronic myeloid leukemia cells. Oncogene. 2017;36:4619–28. doi: 10.1038/onc.2017.85. [DOI] [PubMed] [Google Scholar]
- 84.Gallipoli P, Cook A, Rhodes S, Hopcroft L, Wheadon H, Whetton AD, et al. JAK2/STAT5 inhibition by nilotinib with ruxolitinib contributes to the elimination of CML CD34+cells in vitro and in vivo. Blood. 2014;124:1492–501. doi: 10.1182/blood-2013-12-545640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Agatheeswaran S, Pattnayak NC, Chakraborty S. Identification and functional characterization of the miRNA-gene regulatory network in chronic myeloid leukemia lineage negative cells. Sci Rep. 2016;6:32493. doi: 10.1038/srep32493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang B, Nguyen LXT, Li L, Zhao D, Kumar B, Wu H, et al. Bone marrow niche trafficking of miR-126 controls the self-renewal of leukemia stem cells in chronic myelogenous leukemia. Nat Med. 2018;24:450–62. doi: 10.1038/nm.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zipeto MA, Court AC, Sadarangani A, Delos Santos NP, Balaian L, Chun HJ, et al. ADAR1 activation drives leukemia stem cell self-renewal by impairing Let-7 biogenesis. Cell Stem Cell. 2016;19:177–91. doi: 10.1016/j.stem.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang WZ, Pu QH, Lin XH, Liu MY, Wu LR, Wu QQ, et al. Silencing of miR-21 sensitizes CML CD34+stem/progenitor cells to imatinib-induced apoptosis by blocking PI3K/AKT pathway. Leuk Res. 2015;39:1117–24. doi: 10.1016/j.leukres.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 89.Pellicano F, Park L, Hopcroft LEM, Shah MM, Jackson L, Scott MT, et al. hsa-mir183/EGR1-mediated regulation of E2F1 is required for CML stem/progenitor cell survival. Blood. 2018;131:1532–44. doi: 10.1182/blood-2017-05-783845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yu Y, Yang L, Zhao M, Zhu S, Kang R, Vernon P, et al. Targeting microRNA-30a-mediated autophagy enhances imatinib activity against human chronic myeloid leukemia cells. Leukemia. 2012;26:1752–60. doi: 10.1038/leu.2012.65. [DOI] [PubMed] [Google Scholar]
- 91.Salati S, Salvestrini V, Carretta C, Genovese E, Rontauroli S, Zini R, et al. Deregulated expression of miR-29a-3p, miR-494-3p and miR-660-5p affects sensitivity to tyrosine kinase inhibitors in CML leukemic stem cells. Oncotarget. 2017;8:49451–69. doi: 10.18632/oncotarget.17706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rothe K, Lin H, Lin KB, Leung A, Wang HM, Malekesmaeili M, et al. The core autophagy protein ATG4B is a potential biomarker and therapeutic target in CML stem/progenitor cells. Blood. 2014;123:3622–34. doi: 10.1182/blood-2013-07-516807. [DOI] [PubMed] [Google Scholar]
- 93.Sheng Z, Ma L, Sun JE, Zhu LJ, Green MR. BCR-ABL suppresses autophagy through ATF5-mediated regulation of mTOR transcription. Blood. 2011;118:2840–8. doi: 10.1182/blood-2010-12-322537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Elzinga BM, Nyhan MJ, Crowley LC, O’Donovan TR, Cahill MR, McKenna SL. Induction of autophagy by Imatinib sequesters Bcr-Abl in autophagosomes and down-regulates Bcr-Abl protein. Am J Hematol. 2013;88:455–62. doi: 10.1002/ajh.23428. [DOI] [PubMed] [Google Scholar]
- 95.Eisenberg-Lerner A, Bialik S, Simon HU, Kimchi A. Life and death partners: apoptosis, autophagy and the cross-talk between them. Cell Death Differ. 2009;16:966–75. doi: 10.1038/cdd.2009.33. [DOI] [PubMed] [Google Scholar]
- 96.Calabretta B, Salomoni P. Inhibition of autophagy: a new strategy to enhance sensitivity of chronic myeloid leukemia stem cells to tyrosine kinase inhibitors. Leuk Lymphoma. 2011;52(Suppl 1):54–9. doi: 10.3109/10428194.2010.546913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bellodi C, Lidonnici MR, Hamilton A, Helgason GV, Soliera AR, Ronchetti M, et al. Targeting autophagy potentiates tyrosine kinase inhibitor-induced cell death in Philadelphia chromosome-positive cells, including primary CML stem cells. J Clin Invest. 2009;119:1109–23. doi: 10.1172/JCI35660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang H, Peng C, Hu Y, Li H, Sheng Z, Chen Y, et al. The Blk pathway functions as a tumor suppressor in chronic myeloid leukemia stem cells. Nat Genet. 2012;44:861–71. doi: 10.1038/ng.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Scott MT, Korfi K, Saffrey P, Hopcroft LE, Kinstrie R, Pellicano F, et al. Epigenetic reprogramming sensitizes CML stem cells to combined EZH2 and tyrosine kinase inhibition. Cancer Discov. 2016;6:1248–57. doi: 10.1158/2159-8290.CD-16-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Huang W, Luan CH, Hjort EE, Bei L, Mishra R, Sakamoto KM, et al. The role of Fas-associated phosphatase 1 in leukemia stem cell persistence during tyrosine kinase inhibitor treatment of chronic myeloid leukemia. Leukemia. 2016;30:1502–9. doi: 10.1038/leu.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li L, Bhatia R. Stem cell quiescence. Clin Cancer Res. 2011;17:4936–41. doi: 10.1158/1078-0432.CCR-10-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cheloni G, Tanturli M, Tusa I, Ho DeSouza N, Shan Y, Gozzini A, et al. Targeting chronic myeloid leukemia stem cells with the hypoxia-inducible factor inhibitor acriflavine. Blood. 2017;130:655–65. doi: 10.1182/blood-2016-10-745588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang H, Li H, Xi HS, Li S. HIF1alpha is required for survival maintenance of chronic myeloid leukemia stem cells. Blood. 2012;119:2595–607. doi: 10.1182/blood-2011-10-387381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.de Lallemand-Breitenbach V. The H. PML nuclear bodies. Cold Spring Harb Perspect Biol. 2010;2:a000661. doi: 10.1101/cshperspect.a000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ito K, Bernardi R, Morotti A, Matsuoka S, Saglio G, Ikeda Y, et al. PML targeting eradicates quiescent leukaemia-initiating cells. Nature. 2008;453:1072–8. doi: 10.1038/nature07016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hong CS, Ho W, Zhang C, Yang C, Elder JB, Zhuang Z. LB100, a small molecule inhibitor of PP2A with potent chemo- and radio-sensitizing potential. Cancer Biol Ther. 2015;16:821–33. doi: 10.1080/15384047.2015.1040961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Neviani P, Harb JG, Oaks JJ, Santhanam R, Walker CJ, Ellis JJ, et al. PP2A-activating drugs selectively eradicate TKI-resistant chronic myeloid leukemic stem cells. J Clin Invest. 2013;123:4144–57. doi: 10.1172/JCI68951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lai D, Chen M, Su J, Liu X, Rothe K, Hu K, et al. PP2A inhibition sensitizes cancer stem cells to ABL tyrosine kinase inhibitors in BCR-ABL(+) human leukemia. Sci Transl Med. 2018;10. pii: eaan8735. 10.1126/scitranslmed.aan8735. [DOI] [PubMed]
- 109.Massoumi R, Sjolander A. The role of leukotriene receptor signaling in inflammation and cancer. Sci World J. 2007;7:1413–21. doi: 10.1100/tsw.2007.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen Y, Hu Y, Zhang H, Peng C, Li S. Loss of the Alox5 gene impairs leukemia stem cells and prevents chronic myeloid leukemia. Nat Genet. 2009;41:783–92. doi: 10.1038/ng.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dolinska M, Piccini A, Wong WM, Gelali E, Johansson AS, Klang J, et al. Leukotriene signaling via ALOX5 and cysteinyl leukotriene receptor 1 is dispensable for in vitro growth of CD34(+)CD38(-) stem and progenitor cells in chronic myeloid leukemia. Biochem Biophys Res Commun. 2017;490:378–84. doi: 10.1016/j.bbrc.2017.06.051. [DOI] [PubMed] [Google Scholar]
- 112.Rahman S, Islam R. Mammalian Sirt1: insights on its biological functions. Cell Commun Signal. 2011;9:11. doi: 10.1186/1478-811X-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yuan H, Wang Z, Li L, Zhang H, Modi H, Horne D, et al. Activation of stress response gene SIRT1 by BCR-ABL promotes leukemogenesis. Blood. 2012;119:1904–14. doi: 10.1182/blood-2011-06-361691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang Z, Yuan H, Roth M, Stark JM, Bhatia R, Chen WY. SIRT1 deacetylase promotes acquisition of genetic mutations for drug resistance in CML cells. Oncogene. 2013;32:589–98. doi: 10.1038/onc.2012.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li L, Wang L, Li L, Wang Z, Ho Y, McDonald T, et al. Activation of p53 by SIRT1 inhibition enhances elimination of CML leukemia stem cells in combination with imatinib. Cancer Cell. 2012;21:266–81. doi: 10.1016/j.ccr.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Goff DJ, Court Recart A, Sadarangani A, Chun HJ, Barrett CL, Krajewska M, et al. A Pan-BCL2 inhibitor renders bone-marrow-resident human leukemia stem cells sensitive to tyrosine kinase inhibition. Cell Stem Cell. 2013;12:316–28. doi: 10.1016/j.stem.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tzifi F, Economopoulou C, Gourgiotis D, Ardavanis A, Papageorgiou S, Scorilas A. The role of BCL2 family of apoptosis regulator proteins in acute and chronic leukemias. Adv Hematol. 2012;2012:524308. doi: 10.1155/2012/524308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Carter BZ, Mak PY, Mu H, Zhou H, Mak DH, Schober W, et al. Combined targeting of BCL-2 and BCR-ABL tyrosine kinase eradicates chronic myeloid leukemia stem cells. Sci Transl Med. 2016;8:355ra117. doi: 10.1126/scitranslmed.aag1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Naka K, Jomen Y, Ishihara K, Kim J, Ishimoto T, Bae EJ, et al. Dipeptide species regulate p38MAPK-Smad3 signalling to maintain chronic myelogenous leukaemia stem cells. Nat Commun. 2015;6:8039. doi: 10.1038/ncomms9039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang X, Lin H, Gu Y. Multiple roles of dihomo-gamma-linolenic acid against proliferation diseases. Lipids Health Dis. 2012;11:25. doi: 10.1186/1476-511X-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li F, He B, Ma X, Yu S, Bhave RR, Lentz SR, et al. Prostaglandin E1 and ITs Analog Misoprostol Inhibit Human Cml Stem Cell Self-renewal Via EP4 receptor activation and repression of AP-1. Cell Stem Cell. 2017;21:359–73. doi: 10.1016/j.stem.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kuntz EM, Baquero P, Michie AM, Dunn K, Tardito S, Holyoake TL, et al. Targeting mitochondrial oxidative phosphorylation eradicates therapy-resistant chronic myeloid leukemia stem cells. Nat Med. 2017;23:1234–40. doi: 10.1038/nm.4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ye H, Adane B, Khan N, Sullivan T, Minhajuddin M, Gasparetto M, et al. Leukemic stem cells evade chemotherapy by metabolic adaptation to an adipose tissue niche. Cell Stem Cell. 2016;19:23–37. doi: 10.1016/j.stem.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Giustacchini A, Thongjuea S, Barkas N, Woll PS, Povinelli BJ, Booth CAG, et al. Single-cell transcriptomics uncovers distinct molecular signatures of stem cells in chronic myeloid leukemia. Nat Med. 2017;23:692–702. doi: 10.1038/nm.4336. [DOI] [PubMed] [Google Scholar]
- 125.Ng KP, Manjeri A, Lee KL, Huang W, Tan SY, Chuah CT, et al. Physiologic hypoxia promotes maintenance of CML stem cells despite effective BCR-ABL1 inhibition. Blood. 2014;123:3316–26. doi: 10.1182/blood-2013-07-511907. [DOI] [PubMed] [Google Scholar]
- 126.Hochhaus A, Masszi T, Giles FJ, Radich JP, Ross DM, Gomez Casares MT, et al. Treatment-free remission following frontline nilotinib in patients with chronic myeloid leukemia in chronic phase: results from the ENESTfreedom study. Leukemia. 2017;31:1525–31. doi: 10.1038/leu.2017.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Legros L, Nicolini FE, Etienne G, Rousselot P, Rea D, Giraudier S, et al. Second tyrosine kinase inhibitor discontinuation attempt in patients with chronic myeloid leukemia. Cancer. 2017;123:4403–10. doi: 10.1002/cncr.30885. [DOI] [PubMed] [Google Scholar]
- 128.Giobbie-Hurder A, Gelber RD, Regan MM. Challenges of guarantee-time bias. J Clin Oncol. 2013;31:2963–9. doi: 10.1200/JCO.2013.49.5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jaiswal S, Chao MP, Majeti R, Weissman IL. Macrophages as mediators of tumor immunosurveillance. Trends Immunol. 2010;31:212–9. doi: 10.1016/j.it.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tarafdar A, Hopcroft LE, Gallipoli P, Pellicano F, Cassels J, Hair A, et al. CML cells actively evade host immune surveillance through cytokine-mediated downregulation of MHC-II expression. Blood. 2017;129:199–208. doi: 10.1182/blood-2016-09-742049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gale RP, Horowitz MM, Ash RC, Champlin RE, Goldman JM, Rimm AA, et al. Identical-twin bone marrow transplants for leukemia. Ann Intern Med. 1994;120:646–52. doi: 10.7326/0003-4819-120-8-199404150-00004. [DOI] [PubMed] [Google Scholar]
- 132.Gale RP, Opelz G. Is there immune surveillance against chronic myeloid leukaemia? Possibly, but not much. Leuk Res. 2017;57:109–11. doi: 10.1016/j.leukres.2017.03.003. [DOI] [PubMed] [Google Scholar]