To the Editor:
Systemic T and natural killer (NK) cell lymphomas (systemic TNKLs) are malignancies stemming from lymphocytes of T- and NK-cell lineage that preferentially occur in East Asians. Despite an aggressive nature and poor patient outcomes, their rarity and histological heterogeneity have limited the development of effective therapeutic options. Several subtypes have been described for TNKLs according to their cellular origin and site of occurrence: these include angioimmunoblastic T-cell lymphoma (lymph nodes) and extranodal NK/T-cell lymphoma (ENKTL) (nasal/paranasal sites of the head/neck). Some TNKLs, such as enteropathy-associated T-cell lymphoma or monomorphic epitheliotropic intestinal T-cell lymphoma (MEITL), are frequently found along the gastrointestinal (GI) tract and are known to be more aggressive, with patients experiencing bleak clinical outcomes [1]. While this might indicate a site-specific preference in the formation and progression of TNKLs, the molecular biology underlying such preference has not been fully elucidated. In this study, we sought to investigate genetic alterations in primary GI-TNKLs in a comparative manner to characterize the molecular features thereof and to gain insights into the site-specific tumorigenesis of TNKLs.
From Severance Hospital Cancer Registry data, 18 primary GI-TNKLs were collected: GI-TNKL was defined according to the definitions proposed by Lewin et al. and the fourth revision of World Health Organization classification [1, 2]. The 18 cases consisted of six MEITLs, six ENKTLs, three anaplastic large cell lymphomas, and three intestinal T-cell lymphomas not otherwise specified (ITCL-NOS). In addition, 28 cases of non-GI- TNKL were collected for comparative analysis. The complete list of samples, clinical/pathological features, and overall workflow for sample collection are described in the accompanying Supplementary Data (Supplementary Tables 1 and 2, Supplementary Figures 1–3, and Supplementary Appendix).
Initially, we conducted whole-exome sequencing (WES) analysis for six GI-TNKL samples (three MEITLs and three ENKTLs) to obtain a rough profile of somatic mutations. After assessment of the data quality (Supplementary Table 3), we applied the Genome Analysis Toolkit best practice pipeline on the WES data to discover somatic variants (Supplementary Figure 4 and Supplementary Appendix) and identified 230 genes with somatic mutations. We also conducted a literature survey to obtain additional gene variants: mutations in 187 lymphoma-related genes were reported in previous genomic studies (Supplementary Figure 5A). Finally, we constructed a targeted panel of 417 genes for deeper analysis of GI- and non-GI-TNKLs (Supplementary Table 4).
Using the targeted panel, we conducted deep targeted sequencing (~900×) for 46 TNKL samples (18 GI- and 28 non-GI-TNKLs) (Supplementary Figure 5B and Supplementary Appendix). At this stage, we applied more stringent filtering for genes from large public germline databases and frequent false positive genes (referred to as “blacklist” genes) (Supplementary Figure 4, Supplementary Table 5, and Supplementary Appendix). This variant analysis identified 880 nonsynonymous somatic mutations at 833 unique sites (19.1 total and 18.1 unique mutations per patient) (Supplementary Tables 6 and 7). We assumed that a high mutation load (8.02/Mb) resulted from the targeted sequencing. Mutation spectrums, base-substitution frequency (C > T enriched), and Ti/Tv ratio (~2.6) were similar between GI- and non-GI-TNKLs and reflected the typical characteristics of cancers (Supplementary Figures 6 and 7).
Inspecting the genetic landscape of nonsynonymous mutations (Fig. 1a), we discovered that TET2 was the most frequently mutated gene in all TNKL samples (15/46, 33%), followed by CSMD3 (10/46, 22%), CSMD2 (10/46, 20%), FSIP2 (10/46, 20%), and TP53 (9/46, 20%). Frequent indels, nonsense mutations, and splice site variants in TET2 (10/15, 66%) and TP53 (4/9, 44%) implied that mutations in these tumor suppressor genes may act in a loss-of-function manner. SETD2 mutations were also observed in 17% of samples, corresponding to previous reports [3].
Fig. 1.
Genomic landscape of alterations in GI-TNKL and non-GI-TNKL. a Distribution of well-known somatic alterations in 46 GI-TNKL and non-GI-TNKL patients. Sites of origin (GI-TNKL = sky blue, non-GI-TNKL = yellow), stages (high = brown, low = pink), and EBV positivity (black) are displayed along the top. Individual patients and cancer type (GI-TNKL [G] or non-GI-TNKL [N]) are indicated at the bottom. Frequencies of the mutations in the study population are displayed on the left side of the table. Bars on the right show the total numbers of alterations in each gene. Mutation types are indicated in different colors: missense (red), indel (blue triangle), nonsense (green triangle), or splice site (black dot). b Alteration landscape showing recurrent distributions of mutations in key pathways discovered in GI-TNKL patients
We further analyzed the site-specificity of the detected mutations (Fig. 1b). While previously known mutant genes were highly present in non-GI-TNKLs (46, 32, and 25% for TET2, CSMD3, and CSMD2, respectively), we were able to find a few novel mutations enriched in GI-TNKLs that are important in three different biological functions: the voltage-gated potassium (Kv+) channel, the JAK-STAT pathway, and the immunoglobulin superfamily (see below).
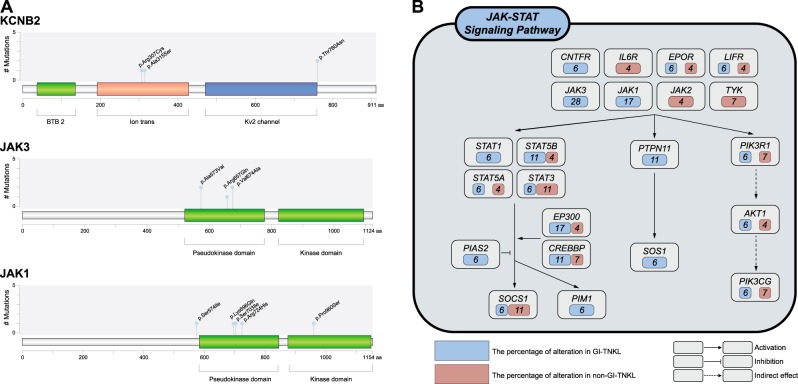
With regard to Kv+ channel pathway genes, eight mutations (seven missense and one nonsense single nucleotide variants) in KCNB2 (4/18, 22%), KCNH8 (2/18, 11%), and KCNJ12 (2/18, 11%) were discovered in six GI-TNKL samples. These genes encode Kv+ channel proteins that mediate transmembrane potassium transport in excitable membranes, which is primarily activated in brain and smooth muscle cells of the GI-tract [4]. Further analysis demonstrated that the KCNB2 mutations were located in the ion transport and Kv2 channel domains (Fig. 2a). In silico protein modeling and multiple sequence alignment predicted that one of the KCNB2 mutations (Arg307Cys) results in a critical defect in the function of voltage-gated channels (Supplementary Figures 8, 9 and Supplementary Appendix), while the others remained inconclusive. Compared to normal tonsil tissue, non-GI-TNKL tissue, and GI-TNKL tissue with wild-type KCNB2, GI-TNKL tissue with KCNB2 mutation exhibited lower levels of KCNB2 mRNA upon reverse transcription PCR, although statistical significance was not observed (Supplementary Figure 10 and Supplementary Appendix). Immunohistochemical protein expression was correlated with mRNA expression of KCNB2. Also, low expression of KCNB2 protein was found to be related with an inferior overall survival rate in systemic mature T- and NK-cell lymphomas regardless of GI or non-GI site (Supplementary Table 8, Supplementary Figure 11 and Supplementary Appendix).
Fig. 2.
KCNB2, JAK3, and JAK1 lollipop plot and the JAK-STAT signaling pathway. a KCNB2, JAK3, and JAK1 lollipop plots. The diagrams represent the protein domains of KCNB2, JAK3, and JAK1 genes. Mutations are plotted along the x-axis, and the numbers of the mutations presented along the y-axis. Blue dots indicate individual missense mutations. b The schematic diagram represents genomic alterations found in the JAK-STAT signaling pathway. The blue boxes (GI-TNKL cases) and red boxes (non-GI-TNKL) indicate the variant frequencies of mutations found in the pathway. Each arrow represents the type of interaction
Associations between altered regulation of the Kv+ channel and cancer development have been continuously reported, such as mutations of KCNH1 in breast cancer and acute myeloid leukemia [5, 6], of KCNH2 in glioblastoma [7], and of KCNA3 in pancreatic cancer [8]. In T cells, aberrations of Kv+ channels have been shown to induce changes in intracellular K+ and Ca+ concentrations and to impair T-cell receptor-driven Akt-mTOR signaling and Ca2+ signaling pathways, thereby triggering T-cell activation [9]. In addition, such aberrations may induce a blockade of T-cell effector function by eliciting an ionic checkpoint, which results in immune suppression within the tumor microenvironment [9]. Thus, we conjectured that the newly found mutations in the Kv+ channel family genes might be a potential driving mechanism of T-cell lymphoma and cancer immunity. Moreover, we suspect that the site-specificity of the mutations may mirror differences in the tumor microenvironment, considering the known functional roles of Kv+ channels in the GI tract, including electrolyte and substrate transport, cell migration, cell proliferation, and apoptosis [10].
The JAK-STAT pathway is a well-known mutation target in systematic TNKLs [11–14]. In the present study, mutations in the JAK-STAT pathway showed GI specificity by presenting only in JAK3 (5/18, 28%) and JAK1 (3/18, 17%). Most previous studies have reported JAK2 mutations only. The newly found JAK3 and JAK1 mutations were located in the pseudokinase and kinase domains (Fig. 2a). Further, we found more GI-specific mutations in the downstream genes of JAK-STAT pathways, including STAT1, PTPN11, and SOS1, which might indicate GI-TNKL-specific aberrations in the pathway (Fig. 2b). Although further study should follow, these findings suggest that differences in the tumor microenvironment might be related with the vulnerability of GI-TNKLs to genetic mutations in JAK3 and JAK1.
Mutations in IGSF9 (1/18, 6%) and IGSF10 (4/18, 22%), members of the immunoglobulin superfamily, were found exclusively in GI-TNKLs. So far, no strong associations have been reported between immunoglobulin function and TNKLs, except one recent study that showed a recurrent IGSF10 mutation in familial gastric and colorectal cancer [15]. We expect that further in-depth studies can test the vulnerability of resident cells, such as mucosa epithelial cells and immune T cells, in the milieu of the GI-tract to IGSF10 mutations.
Finally, we conducted direct Sanger sequencing to confirm the presence of mutations in KCNB2, JAK3, and JAK1 (Supplementary Table 9 and Supplementary Appendix). In seven cases in which genomic DNA were available, nine mutations were validated (Supplementary Figure 12 and Supplementary Appendix).
In conclusion, we noted characteristic mutations of KCNB2, as well as JAK3, JAK1, and IGSF10, in GI-TNKLs. Although more comprehensive work is needed, the present findings provide some insights into understanding the physiological and pathological links between these genes and GI-TNKL genesis and into the potential for targeted therapy against tumor cells and the tumor microenvironment associated therewith.
Supplementary information
Acknowledgements
The study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (NRF-2015R1D1A1A09059399) (SOY). This work was supported by the Bio-Synergy Research Project (NRF-2017M3A9C4092978) of the Ministry of Science, ICT (SK).
Author contributions
Contribution: SOY, SK, and GL conceived and designed the study, analyzed data, and drafted the paper; SOY, SK, GL, and HJR performed most of the research, analyzed the data, and edited the paper; JWC, HK, WIY, ISY, and M-kS analyzed the data.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Sangwoo Kim, Phone: +82-10-3407-9861, Email: swkim@yuhs.ac.
Sun Och Yoon, Phone: +82-2-2228-1763, Email: soyoon@yuhs.ac.
Supplementary information
The online version of this article (10.1038/s41375-018-0309-4) contains supplementary material, which is available to authorized users.
References
- 1.Swerdlow SHCE, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues—WHO Classification of Tumours, revised 4th ed, Vol. 2. International Agency for Research on Cancer; Lyon 2017.
- 2.Lewin KJ, Ranchod M, Dorfman RF. Lymphomas of the gastrointestinal tract: a study of 117 cases presenting with gastrointestinal disease. Cancer. 1978;42:693–707. doi: 10.1002/1097-0142(197808)42:2<693::AID-CNCR2820420241>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 3.Roberti A, Dobay MP, Bisig B, Vallois D, Boéchat C, Lanitis E, et al. Type II enteropathy-associated T-cell lymphoma features a unique genomic profile with highly recurrent SETD2 alterations. Nat Commun. 2016;7:12602. doi: 10.1038/ncomms12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vogalis F. Potassium channels in gastrointestinal smooth muscle. J Auton Pharmacol. 2000;20:207–19. doi: 10.1046/j.1365-2680.2000.00183.x. [DOI] [PubMed] [Google Scholar]
- 5.Hammadi M, Chopin V, Matifat F, Dhennin‐Duthille I, Chasseraud M, Sevestre H, et al. Human ether à‐gogo K+ channel 1 (hEag1) regulates MDA‐MB‐231 breast cancer cell migration through Orai1‐dependent calcium entry. J Cell Physiol. 2012;227:3837–46. doi: 10.1002/jcp.24095. [DOI] [PubMed] [Google Scholar]
- 6.Agarwal JR, Griesinger F, Stühmer W, Pardo LA. The potassium channel Ether a go-go is a novel prognostic factor with functional relevance in acute myeloid leukemia. Mol Cancer. 2010;9:18. doi: 10.1186/1476-4598-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masi A, Becchetti A, Restano-Cassulini R, Polvani S, Hofmann G, Buccoliero A, et al. hERG1 channels are overexpressed in glioblastoma multiforme and modulate VEGF secretion in glioblastoma cell lines. Br J Cancer. 2005;93:781. doi: 10.1038/sj.bjc.6602775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brevet M, Fucks D, Chatelain D, Regimbeau JM, Delcenserie R, Sevestre H, et al. Deregulation of 2 potassium channels in pancreas adenocarcinomas: implication of KV1. 3 gene promoter methylation. Pancreas. 2009;38:649–54. doi: 10.1097/MPA.0b013e3181a56ebf. [DOI] [PubMed] [Google Scholar]
- 9.Eil R, Vodnala SK, Clever D, Klebanoff CA, Sukumar M, Pan JH, et al. Ionic immune suppression within the tumour microenvironment limits T cell effector function. Nature. 2016;537:539. doi: 10.1038/nature19364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Grady SM, Lee SY. Molecular diversity and function of voltage-gated (Kv) potassium channels in epithelial cells. Int J Biochem Cell Biol. 2005;37:1578–94. doi: 10.1016/j.biocel.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Kücük C, Jiang B, Hu X, Zhang W, Chan JK, Xiao W, et al. Activating mutations of STAT5B and STAT3 in lymphomas derived from γδ-T or NK cells. Nat Commun. 2015;6:6025. doi: 10.1038/ncomms7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nairismägi M, Tan J, Lim J, Nagarajan S, Ng C, Rajasegaran V, et al. JAK-STAT and G-protein-coupled receptor signaling pathways are frequently altered in epitheliotropic intestinal T-cell lymphoma. Leukemia. 2016;30:1311. doi: 10.1038/leu.2016.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koo GC, Tan SY, Tang T, Poon SL, Allen GE, Tan L, et al. Janus kinase 3–activating mutations identified in natural killer/T-cell Lymphoma. Cancer Discov. 2012. [DOI] [PubMed]
- 14.Nicolae A, Xi L, Pham TH, Pham TA, Navarro W, Meeker HG, et al. Mutations in the JAK/STAT and RAS signaling pathways are common in intestinal T-cell lymphomas. Leukemia. 2016;30:2245. doi: 10.1038/leu.2016.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thutkawkorapin J, Picelli S, Kontham V, Liu T, Nilsson D, Lindblom A. Exome sequencing in one family with gastric-and rectal cancer. BMC Genet. 2016;17:41. doi: 10.1186/s12863-016-0351-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.