Abstract
Background
The effectiveness of health checks aimed at the general population is disputable. However, it is not clear whether health checks aimed at certain groups at high risk may reduce adverse health behaviour and identify persons with metabolic risk factors and non-communicable diseases (NCDs).
Objectives
To assess the effect of general practice-based health checks on health behaviour and incidence on NCDs in individuals with low socioeconomic position.
Methods
Individuals with no formal education beyond lower secondary school and aged 45–64 years were randomly assigned to the intervention group of a preventive health check or to control group of usual care in a 1:1 allocation. Randomisation was stratified by gender and 5-year age group. Due to the real-life setting, blinding of participants was only possible in the control group. Effects were analysed as intention to treat (ITT) and per protocol. The trial was undertaken in 32 general practice units in Copenhagen, Denmark.
Intervention
Invitation to a prescheduled preventive health check from the general practitioner (GP) followed by a health consultation and an offer of follow-up with health risk behaviour change or preventive medical treatment, if necessary.
Primary outcome measures
Smoking status at 12-month follow-up. Secondary outcomes included status in other health behaviours such as alcohol consumption, physical activity and body mass index (measured by self-administered questionnaire), as well as incidence of metabolic risk factors and NCDs such as hypertension, hypercholesterolaemia, chronic obstructive pulmonary disease, diabetes mellitus, hypothyroidism, hyperthyroidism and depression (drawn from national healthcare registries).
Results
1104 participants were included in the study. For the primary outcome, 710 participants were included in the per protocol analysis, excluding individuals who did not attend the health check, and 1104 participants were included in the ITT analysis. At 12-month follow-up, 37% were daily smokers in the intervention group and 37% in the control group (ORs=0.99, 95% CI: 0.76 to 1.30). No difference in health behaviour nor in the incidence of metabolic risk factors and NCDs between the intervention and control group were found. Side effects were comparable across the two groups.
Conclusion
The lack of effectiveness may be due to low intensity of intervention, a high prevalence of metabolic risk factors and NCDs among the participants at baseline as well as a high number of contacts with the GPs in general or to the fact that general practices are not an effective setting for prevention.
Trial registration number
Keywords: general practitioner, health check, preventive medicine, social medicine, randomised controlled trial
Strengths and limitations of this study.
A major strength of this study is that it is a large-scale community-based health check intervention implemented in 32 general practice clinics and evaluated with long follow-up (1 year) and in a randomised controlled design.
The study targets both health behaviour changes and detection of non-communicable diseases (NCDs) and combines both patient-reported and register-based outcomes.
The patient-reported data were linked at the individual level with national health register and obtained information on NCDs, which ensured no loss to follow-up regarding this outcome.
The study focuses on individuals with low socioeconomic—an under-studied group in health check interventions.
The limitations of this study include the lack of data on smoking status in non-respondents and no access to primary care medical records with details on any condition not leading to hospital contact.
Introduction
There is a large body of evidence that developing non-communicable diseases (NCDs) is closely linked with modifiable health behaviours such as smoking, alcohol consumption, poor diet and physical inactivity as well as metabolic risk factors such as hypertension, lipid levels and blood glucose, and obesity.1 Furthermore, the occurrence of multiple of these adverse health behaviours is strongly associated with mortality2 but are difficult to modify.3 Health checks may identify individuals with adverse health behaviour and detect metabolic risk factors and NCDs at an early stage.4 To prevent NCDs or limit future harms from NCDs, health checks may provide an opportunity to motivate for behavioural change or to initiate appropriate preventive medical treatment. Benefits of general preventive health checks are, however, disputed. One Cochrane review, which included 14 trials on general health checks (n=533–57 460), concluded that health checks offered to the general population did not reduce morbidity or mortality beyond that of usual care.5 Most of the included trials, however, took place 20–30 years ago, prior to the introduction of much of the preventive medication in current use.6 A more recent meta-analysis, including six trials conducted in general practice (n=1442–7229), showed improvements in blood pressure, total cholesterol and body mass index (BMI), and reduced the proportion of patients remaining at high risk for NCDs.7 Among others, low socioeconomic position (SEP) has been shown to be associated with non-participation in health checks.5 8–11 Because of the lower participation among individuals of low SEP10 and a social gradient in modifiable adverse health behaviours and NCDs,1 12 13 it has been suggested that further research in the field of health check should put an extra effort into recruiting especially socioeconomically disadvantaged individuals.5 10
In Denmark, general practice and municipalities have a shared responsibility for preventive services aimed at the individual. General practitioners (GPs) assess patient health and implement disease-specific secondary prevention, the municipalities are tasked with primary prevention such as smoking cessation, alcohol treatment and other lifestyle-related services.14 GPs collaborate closely with municipal services and can refer to some services, for instance, lifestyle change programme at the municipality health centre.15 Visiting the GP is free of charge, and around 98% of the population is assigned to one specific GP.16
The ‘Check-In’ intervention was developed to test the effectiveness of a preventive health check in a randomised controlled trial (RCT) at general practice offered to individuals with low SEP as measured by short education. It was developed in response to health-behaviour models in which increased awareness about the causes, consequences and cures for a particular health behaviour or health problem is expected to increase the likelihood for change17 and in which knowledge is expected to lead to action.18 A preventive health check at the GP has the potential to confront the patient with a problem and provide feedback about both adverse health behaviour and the consequences of continuing the injurious behaviour. For example, poor lung function measure can demonstrate the health consequences of smoking and lead to a discussion about the adverse effects of smoking which may increase the chance for smoking cessation.
Short education was used as measure for low SEP as educational level captures the influence of resources on health and the knowledge and skills attained through education may affect an individual’s cognitive functioning, make individuals more receptive to health education messages or more able to communicate with and access appropriate health services.19
We set out to test if ‘Check-In’ results in lower prevalence of adverse health behaviour such as smoking, excessive alcohol consumption, physical inactivity and obesity, and to test if ‘Check-In’ results in more new hospital contacts and prescription medication for metabolic risk factors and NCDs such as hypertension, hypercholesterolaemia, chronic obstructive pulmonary disease (COPD), diabetes mellitus, hypothyroidism, hyperthyroidism and depression. In this article, we report on the effects of ‘Check-In’ at 12-month follow-up.
Methods and material
Trial design
‘Check-In’ was a two-arm 1:1 RCT conducted in Copenhagen, Denmark from January 2014 to September 2016.
Identification of the study population
All 126 general practices in four different suburbs of Copenhagen, Denmark, were invited by letter and phone to participate in the study. The recruitment of the GPs was, however, challenged due to a break down in the collective bargaining between the Danish Regions Salary and Rate Board and the Organisation of General Practitioners in late 2012.20 In all, ‘Check-In’ ended up having five rounds between January 2014 and September 2016.
GPs do not systematically register their patients’ educational level. Therefore, to identify the study population baseline questionnaires in Danish (including items about sex, date of birth, cohabitation status, highest educational level achieved, height and weight, smoking status, alcohol consumption, physical activity, diet, general self-efficacy, perceived stress and family disposition of NCDs) were sent out to all individuals aged 45–64 years, who lived in Copenhagen and who were on the participating GPs’ patient lists. The questionnaire was accompanied by a short letter from the GP and the research team describing that the questionnaire information would be entered into the electronic patient record at the GP, and thus could be used in future visits. Furthermore, it was explained that the questionnaire was part of a larger research project and that participation was voluntary and without negative consequences for the continuing doctor–patient relationship. At the end of the questionnaire, individuals were asked to indicate if they would consent to be contacted for participation in a future research project.
Eligible patients met the inclusion criteria which were no formal education beyond lower secondary school and consent to be contacted for research purpose. No exclusion criteria were implied.
Randomisation
Eligible patients were randomised in the Statistical Analysis Software by a data manager at the National Institute of Public Health to either ‘Check-In’ or usual care in a 1:1 allocation. The randomisation was stratified by gender and 5-year age group. Couples living together were allocated to the same group to avoid contamination.
Double-blinded, meaning that both patients and GPs were blinded to the allocation of group, would have been ideal21; nevertheless, due to real-life setting, blinding of participants was only possible in the control group and not in the intervention group and among GPs.
Interventions
‘Check-In’ group (intervention)
The intervention included (i) an invitation to a prescheduled health check, (ii) a health check at the GP and (iii) a health consultation at the GP which included an offer of further action if necessary.
Invitation
All participants allocated to the intervention group received a postal invitation to a prescheduled health check from their GP and the research team. Included with the invitation was a written description of the project. Furthermore, it was clarified that study participation was voluntary and that withdrawal could occur at any time. Three days before the prescheduled appointment, participants in the intervention group were reminded by phone by a member of the research team.
Health check
Before the health check, the GPs received results from the patient-reported questionnaire in the GPs electronic patient record in the form of an electronic data interchange message including summed scores and categorisation of items from the baseline questionnaire (see online supplementary file 1). The health check was free of charge and took place during the opening hour of the general practice clinic to which the patient was registered and was conducted by either the GP or other health staff at the clinic as per usual clinical practice. The health check consisted of measurements of weight and height, hip and waist circumference, cholesterol, glycated haemoglobin, thyroidal status and spirometry for smokers or former smokers. The health consultation with the purpose of review of results was scheduled at the health checks.
bmjopen-2019-029180supp001.pdf (32.5KB, pdf)
Health consultation
At the health consultation, the GP reviewed the results from the health check in combination with the summarised results of the questionnaire. Participants with abnormal screens, or health behaviour amenable to intervention at the health check, either follow the medical standards for general practice on procedures for diagnostics and treatment or received the offer of a referral to the municipality health centre for a lifestyle change programme. Furthermore, these participants were offered an additional health check scheduled 6 months after the first health check. Decisions for further action and reasons for referral or not were indicated by the GP in a project specified form.
Usual care (control)
Participants allocated to the control group received unrestricted usual care during the intervention period. The results from the questionnaires were entered in the GPs electronic patient record; however, no feedback was provided to the patients.
Measurement of health behaviour
Health behaviour was measured from a self-administrated questionnaire at baseline and at 12-month follow-up. The questionnaire contained information about sociodemographic characteristics (education level and cohabitation status), health-related quality of life (12-Item Short Form Health Survey),22 23 height and weight, smoking status, alcohol consumption, physical activity, diet, pulmonary symptoms, family dispositions of chronic diseases, general self-efficacy24 25 and stress (measured by Cohen’s 10-item Perceived Stress Scale (PSS)).26
The primary outcome was self-reported smoking status at 12-month follow-up. Questions included ‘Do you smoke?’, with the response categories ‘yes, daily’, ‘yes, I smoke occasional’, ‘no, I stopped less than 6 months ago’, ‘no, I stopped more than 6 months ago’, ‘no, I have never smoked’; dichotomised into ‘daily smokers’ versus ‘not daily smokers’ and ‘How much do you approximately smoke each day?’ used as continuous outcome.
Secondary outcomes included self-reported alcohol consumption, physical activity, BMI, self-efficacy and perceived stress. Alcohol consumption was measured as binge drinking (five or more units of alcohol on the same occasion) dichotomised into ‘weekly or more frequent’ versus ‘less than weekly’, and units of alcohol each day during the week used as a continuous outcome. Physical activity was measured from two questions on hours spend on exercise ‘making you short of breath’ during the week and everyday exercise, dichotomised into physical inactivity (yes, no), ‘yes’ defined as less than 150 min of moderate-intensity physical activity throughout the week, less than 75 min of vigorous-intensity physical activity throughout the week or an equivalent combination of moderate-intensity and vigorous-intensity activity and ‘no’ defined as more, as defined by WHO.27 BMI was generated from questions about height and weight and analysed as a dichotomised outcome into obese yes/no, ‘yes’ defined as BMI ≥30 and ‘no’ defined as BMI <3028 and as a continuous outcome (see online supplementary file 2). Stress during the past month was assessed by the PSS (score range 0–40).26 The person’s belief in their innate ability to achieve goals was assessed using general self-efficacy (score range 10–40).25 29
bmjopen-2019-029180supp002.pdf (71.8KB, pdf)
Measurement of metabolic risk factors and NCDs
Metabolic risk factors and NCDs were measured as any hospital contact and/or prescription medication for hypertension, hypercholesterolaemia, COPD, diabetes mellitus, thyroid disease and depression in the follow-up period. International Classification of Diseases, 10th revision codes and Anatomical Therapeutic Chemical Classification codes were specified for each of the conditions. The algorithm in the supplementary files gives an overview of the used definitions (see online supplementary file 3).
bmjopen-2019-029180supp003.pdf (33.5KB, pdf)
All citizens with a permanent residence in Denmark have a unique personal identification number, which makes it possible to link individual information from surveys to nation-wide administrative registries.30 For information about hospital contacts and discharge diagnoses, we linked to the Danish National Patient Register.31 The Danish National Prescription Registry32 was used to obtain information on dispensed prescription medications.
To ensure that only new contacts and/or prescription medication of metabolic risk factors and NCDs were included in the incidence analysis, register-based data on metabolic risk factors and NCDs were collected for a period of 15 years for diagnosis and 2 years for prescription medication before the baseline questionnaire were sent.
Furthermore, information on date of death was extracted from the Danish Register of Causes of Death.33 The Danish National Health Services Register was used for information on contacts with the general practice.16
Sample size consideration
The number of individuals to include in the ‘Check-In’ and usual care group were determined prior to data collection. Sample size calculation was performed to test the difference between two proportions.34 We expected a participation rate of 75% for the health check. Based on prevalence from the Danish National Health Survey from 2010, a daily smoking prevalence of 41% was assumed in the 45-year to 64-year-old individuals with basic education; of these we expected that 50% were motivated to quit smoking.35 High-standard smoking cessation courses have been shown to yield a cessation prevalence of 20%–30%.36 In the usual care group, we expected a cessation prevalence of 5%. Thus, we needed 150 daily smokers in each arm to detect a difference in quit rates of 15% with 80% power.
Statistical analysis
To compare health behaviour at 12-month follow-up in the ‘Check-In’ and usual care group, logistic regression modelling estimated intervention effectiveness on the binary outcomes daily smoking, binge drinking, obesity and physical inactivity. The model included the condition variable (‘Check-In’ vs usual care). For the continuous outcomes, cigarettes per day (among daily smokers), drinks per week (among those who drink alcohol) and BMI, median and IQR were estimated.
The analyses were performed (i) per protocol, excluding individuals who did not attend the health check and (ii) intention to treat (ITT). ITT analyses are recommended in the Consolidated Standards of Reporting Trials statement37 and implies that all randomised individuals are included in the analysis regardless of whether they attended the prescheduled health check or not. For the ITT analyses, we estimated missing data using multiple imputation.38 The imputation process for each outcome utilised participant’s sex, age, ethnicity, cohabitant and employment status, condition variable and baseline specific variable. Twenty imputations were undertaken for each imputation, estimate and pooled results from these were used. In general, missing at specific item responses was low at baseline (less than 5%)—except for drinks per week (7% missing) and self-efficacy (6% missing) (data not shown). Final levels of missing data on primary outcome were 1% at baseline and 25% at 12- month follow-up. Missing at follow-up was primary due to non-response.
To compare metabolic risk factors and NCDs at 12-month follow-up in the ‘Check-In’ and usual care group logistics regression were conducted for each of the outcomes hypertension, hypercholesterolaemia, COPD, diabetes mellitus, hypothyroidism and hyperthyroidism. Furthermore, the analyses were conducted for Any new chronic condition, defined as ‘yes’ if any new metabolic or NCDs were found in the follow-up period.
The analyses were performed both regarding all contacts/prescription medication (prevalence) and first contacts/prescriptions for metabolic risk factors and NCDs (incidence). The prevalence analysis included all individuals who had contact with the hospital and/or prescription medication for the metabolic risk factors and NCDs in the 12-month follow-up period. The incidence analyses excluded individuals who already had the specific metabolic risk factor or NCD at baseline. Information on metabolic risk factors and NCDs were obtained from registers and no missing occurred in these variables.
To evaluate the stability of our results, an interclass coefficient (ICC) was estimated within a two-level model with patients (level 1) nested within general practices (level 2), and all estimates were calculated in the model including the condition variable and general practices as random intercept, allowing for correlation between patients from the same general practice.39 Furthermore, sensitivity analysis including age and sex in the logistic regression were carried out.
Patient and public involvement
Patients were not formally involved in the development of the trial. The ‘Check-In’ intervention was, however, developed in close integration with GPs. Before ‘Check-In’ was rolled out in the bigger scale, the feasibility of the intervention was tested in a pilot study. In the pilot study, the questionnaire was tested among the target group by interviewing them after they filled it in and non-responders were contacted by phone to include their experiences and reasons to not answer. Participants will not be directly contacted with results. All findings, including null findings will be communicated to the public by use of press releases and a report in lay-language.
Results
Participant flow
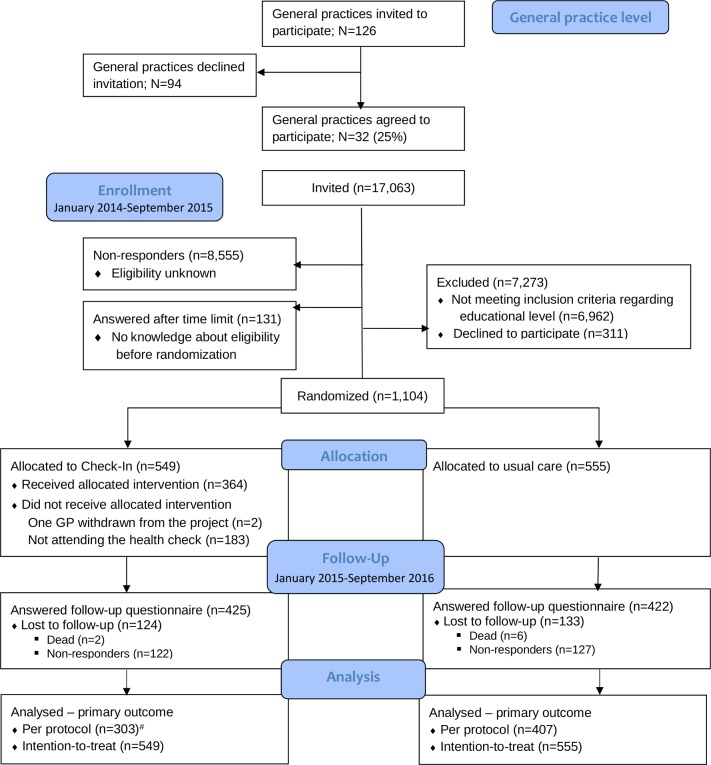
Of the 126 general practices invited, 32 clinics, including 56 GPs, agreed to participate (clinic level participation 25%). Figure 1 shows the flow diagram of ‘Check-In’. In total, 17 063 patients were mailed a baseline questionnaire. Of the 8508 (49%) who responded to the baseline questionnaire, 1104 met the inclusion criteria regarding level of education and marked that they could be contacted again (range per general practice clinic: 12–110 individuals; median=18). Of the 1104 participants, 549 were randomised to the ‘Check-In’ group and invited to the prescheduled health check, which 364 attended (attendance rate of 66%). Of the 1104 participants, 850 completed the follow-up questionnaire at 12 months (response rate of 77%). The number analysed for the ‘per protocol’ depended on the specific outcome—for the primary outcome daily smoking, this was 710, 303 for the ‘Check-In’ group and 407 for the usual care group. For the ITT analyses, the number of analysed equal the number allocated to ‘Check-In’ and usual care group, respectively (figure 1).
Figure 1.
ConsolidatedStandards of Reporting Trials flow diagram showing recruitment of general practices and patients in Check-In. #In the per protocol analyses are only included individuals who responded the questionnaire and who followed the ‘treatment’ for the allocated group (for individuals allocated to intervention, this meant attending the health check and responding to the questionnaire; for individuals allocated to usual care, this meant responding to the questionnaire). Hence, of the 425 responders in the intervention group, 303 individuals attended the health check and could be included in the per protocol analyse. Of the 422 responders in the usual care group, 407 answered the questions regarding the smoking status and could be included in the per protocol analyse.
Baseline characteristics
Table 1 shows the baseline characteristics for the ‘Check-In’ and usual care group. The average age was 54 years, about half were men and more than 40% were unemployed or on social security. About 41% reported daily smoking and 17% in the ‘Check-In’ group and 20% in the usual care group reported ‘binge drinking at least weekly’. Median BMI was 26, with 20% obese in ‘Check-In’ group and 23% in usual care group at baseline. Overall, 61% in the ‘Check-In’ and 65% in the usual care group, respectively, had at least one NCD and 18% had ≥3 NCDs in the two groups. Around 88% had contact with their GP within the last year. The baseline characteristics were well balanced between the ‘Check-In’ and usual care group (table 1).
Table 1.
Baseline characteristics for participants with low socioeconomic position allocated to a preventive health check at the general practitioner (‘Check-In’) or to usual care
| ‘Check-In’ group (n=549) | Usual care group (n=555) | |
| Demographic and socioeconomic characteristics | ||
| Age, years (median (IQR2; IQR3)) | 54 (49; 59) | 54 (49; 59) |
| Men | 282 (51) | 293 (53) |
| Danish/other Western ethnic background | 437 (79) | 446 (81) |
| Married/cohabitant | 279 (51) | 270 (49) |
| Children living at home | 137 (25) | 134 (24) |
| Employment status | ||
| Employed | 269 (49) | 266 (48) |
| Unemployed/social security | 224 (41) | 239 (43) |
| Retired/other | 56 (10) | 49 (9) |
| Health behaviour | ||
| Cigarette smoking | ||
| Daily smoker | 228 (42) | 225 (41) |
| Not daily smoker | 312 (58) | 326 (59) |
| Cigarettes/day*, (median (IQR2; IQR3)) | 18 (10; 20) | 20 (10; 20) |
| Current non-drinkers | 171 (32) | 174 (32) |
| Drinks/week†, (median (IQR2; IQR3)) | 6 (3; 15) | 6 (2; 16) |
| Binge drinking at least weekly | 94 (17) | 110 (20) |
| Physical inactivity‡ | 268 (49) | 286 (52) |
| BMI (kg/m2), (median (IQR2; IQR3)) | 25.9 (23.1; 29.1) | 26.2 (23.4; 29.7) |
| Obese (BMI ≥30 kg/m2) | 104 (20) | 124 (23) |
| Self-rated bad to very bad health | 213 (39) | 225 (41) |
| Self-efficacy, (median (IQR2; IQR3)) | 29 (24; 33) | 29 (24; 33) |
| Morbidity and contact with GP | ||
| Non-communicable diseases | ||
| Any chronic condition | 337 (61) | 359 (65) |
| Hypertension | 118 (22) | 133 (24) |
| Hypercholesterolaemia | 97 (18) | 99 (18) |
| COPD | 124 (23) | 127 (23) |
| Diabetes mellitus | 175 (32) | 191 (34) |
| Hypothyroidism | 55 (10) | 44 (8) |
| Hyperthyroidism | 24 (4) | 22 (4) |
| Depression | 79 (15) | 81 (15) |
| Number of non-communicable diseases | ||
| 0 | 212 (39) | 196 (35) |
| 1 | 147 (27) | 149 (27) |
| 2 | 93 (17) | 112 (20) |
| ≥3 | 97 (18) | 98 (18) |
| Contact with GP within the last year | 495 (90) | 480 (87) |
| Number of contacts with the GP within the last year§, (median (IQR2; IQR3)) | 7 (4; 13) | 8 (4; 14) |
Values are number (percentages) unless stated otherwise.
*Among daily smokers.
†Among those who drink alcohol.
‡Less than 150 min of moderate-intensity physical activity.
§Among those who visit their GP within the last year.
COPD, chronic obstructive pulmonary disease; GP, General practitioner.
Effectiveness of ‘Check-In’ on daily smoking and other health behaviour
After 12 months of follow-up, no difference was found between the ‘Check-In’ and usual care group on daily smoking (ITT: ORs=0.99; 95% CI: 0.76 to 1.30), binge drinking (ITT: OR=0.82; 95% CI: 0.59 to 1.14), physical inactivity (ITT: OR=0.97; 95% CI: 0.74 to 1.27) or obesity (ITT: OR=0.90; 95% CI: 0.67 to 1.21) (table 2)—this was seen in both the per protocol and ITT analysis. No differences were found for comparison of number of cigarettes/day (ITT: Coefficient (coef.)=0; 95% CI: −2.9 to 2.9)), units of alcohol/day (ITT: coef.=0; 95% CI: −1.7 to 1.8) and BMI (ITT: coef.=−0.5; 95% CI: −1.2 to 0.1) (table 3) indicating no effect of ‘Check-In’ on daily smoking or other health behaviour. Furthermore, no difference between the two groups were found regarding self-efficacy where both groups had a median at 29 (IQR for ‘Check-In’ 25,33; IQR for usual care 24,34) (data not shown).
Table 2.
Effectiveness of ‘Check-In’ on smoking status (primary outcome) and other health behaviour at 12-month follow-up measured as dichotomised outcomes
| Dichotomies outcomes | n (%) | Effectiveness (‘Check-In’ vs usual care) | ||
| ‘Check-In’ group (n=549) | Usual care group (n=555) | OR (95% CI) | P value | |
| Primary outcome | ||||
| Daily smokers | ||||
| Per protocol, n=710 | 94 (31) | 147 (36) | 0.80 (0.58 to 1.09) | 0.16 |
| ITT; multiple imputation | 203 (37) | 205 (37) | 0.99 (0.76 to 1.30) | 0.95 |
| Secondary outcomes | ||||
| Binge drinking ≥weekly | ||||
| Per protocol, n=718 | 55 (18) | 84 (20) | 0.87 (0.60 to 1.27) | 0.48 |
| ITT; multiple imputation | 98 (18) | 116 (21) | 0.82 (0.59 to 1.14) | 0.24 |
| Physical inactivity (<150 min/week) | ||||
| Per protocol, n=721 | 132 (43) | 186 (45) | 0.92 (0.68 to 1.23) | 0.56 |
| ITT; multiple imputation | 252 (46) | 260 (47) | 0.97 (0.74 to 1.27) | 0.84 |
| Obese (BMI ≥30 kg/m2) | ||||
| Per protocol, n=684 | 68 (23) | 90 (23) | 1.01 (0.71 to 1.45) | 0.95 |
| ITT; multiple imputation | 131 (24) | 122 (22) | 0.90 (0.67 to 1.21) | 0.93 |
Values are number (percentages), ORs and p values for the intervention effectiveness. The analyses are performed as per protocol and ITT with multiple imputation.
BMI, body mass index; ITT, intention to treat.
Table 3.
Effectiveness of ‘Check-In’ on health behaviour measured as continuous outcomes at 12-month follow-up measured as continuous outcomes
| Continuous outcomes | Median (IQR2;IQR3) | Effectiveness (‘Check-In’ vs usual care) | P value | |
| ‘Check-In’ group (n=549) | Usual care group (n=555) | Coef. (95% CI) | Median regression* | |
| Cigarettes/day† | ||||
| Per protocol, n=239 | 17 (14; 20) | 15 (10; 20) | 2 (−4.7 to 8.7) | 0.35 |
| ITT; multiple imputation | 15 (7; 20) | 15 (7; 20) | 0 (−2.9 to 2.9) | 0.99 |
| Drinks/week‡ | ||||
| Per protocol, n=419 | 7 (4; 19) | 8 (4; 17) | −1 (−2.8 to 0.8) | 0.38 |
| ITT; multiple imputation | 7 (4; 17) | 7 (4; 15) | 0 (−1.7 to 1.8) | 0.95 |
| BMI | ||||
| Per protocol, n=684 | 25.9 (23.5; 29.7) | 26.4 (23.8; 29.6) | −0.5 (−1.2 to 0.2) | 0.19 |
| ITT; multiple imputation | 25.9 (23.2; 29.4) | 26.4 (23.6 :29.8) | −0.5 (−1.2 to 0.1) | 0.11 |
The analyses are performed as per protocol and ITT with multiple imputation.
*Median regression estimates the median of the dependent variable.
†Among daily smokers.
‡Among those who drink alcohol.
BMI, body mass index; ITT, intention to treat.
Effectiveness of ‘Check-In’ on detection of metabolic risk factors and NCDs
At 12-month follow-up, we found a difference in incidence of depression in the ‘Check-In’ group when compared with usual care (OR=2.90; 95% CI: 1.34 to 6.29) and a tendency for COPD (OR=1.44; 95% CI: 0.83 to 2.50) (table 4). No differences between the ‘Check-In’ and usual care groups were observed for incidence in hypertension, hypercholesterolaemia and diabetes mellitus. The estimates for hypothyroidism and hyperthyroidism have not been reported for ethical reasons as there were too few cases to report (table 4).
Table 4.
Effectiveness of ‘Check-In’ on incidence of COPD, diabetes mellitus, disorder of the thyroid gland, hypertension and hypercholesterolaemia
| n (%) | Effectiveness (‘Check-In’ vs Usual care) | |||
| ‘Check-In’ group | Usual care group | OR (95% CI) | P value | |
| Any new chronic condition* | ||||
| Per protocol, n=919 | 82 (23) | 120 (22) | 1.05 (0.77 to 1.45) | 0.75 |
| ITT, n=1104 | 125 (23) | 120 (22) | 1.07 (0.80 to 1.42) | 0.65 |
| Hypertension | ||||
| Per protocol, n=704 | 40 (14) | 60 (14) | 1.01 (0.66 to 1.56) | 0.96 |
| ITT, n=856 | 55 (13) | 60 (14) | 0.88 (0.60 to 1.31) | 0.54 |
| Hypercholesterolaemia | ||||
| Per protocol, n=752 | 13 (4) | 20 (4) | 1.00 (0.49 to 2.05) | 0.99 |
| ITT, n=908 | 18 (4) | 20 (4) | 0.90 (0.47 to 1.73) | 0.76 |
| COPD | ||||
| Per protocol, n=711 | 19 (7) | 23 (5) | 1.24 (0.66 to 2.31) | 0.51 |
| ITT, n=844 | 32 (8) | 23 (5) | 1.44 (0.83 to 2.50) | 0.2 |
| Diabetes mellitus | ||||
| Per protocol, n=604 | 8 (3) | 15 (4) | 0.74 (0.31 to 1.76) | 0.49 |
| ITT, n=720 | 14 (4) | 15 (4) | 0.89 (0.42 to 1.87) | 0.76 |
| Hypothyroidism† | ||||
| Per protocol, n=919 | – | – | – | – |
| ITT, n=840 | – | – | – | – |
| Hyperthyroidism† | ||||
| Per protocol, n=878 | – | – | – | – |
| ITT, n=1051 | – | – | – | – |
| Depression | ||||
| Per protocol, n=789 | 12 (4) | 9 (2) | 2.05 (0.85 to 4.91) | 0.11 |
| ITT, n=944 | 25 (5) | 9 (2) | 2.90 (1.34 to 6.29) | 0.007 |
The analyses are performed per protocol and as ITT.
*Hypertension if no hypertension at baseline, hypercholesterolaemia if no hypercholesterolaemia at baseline, COPD if no COPD at baseline, diabetes if no diabetes at baseline, hypothyroidism if no hypothyroidism at baseline, hyperthyroidism if no hyperthyroidism at baseline or depression if no depression at baseline.
†Too few in each group to report for ethical reasons.
COPD, chronic obstructive pulmonary disease; ITT, intention to treat.
We found no differences between the Check-In and the usual care group in prevalence of hypertension, hypercholesterolaemia, COPD, diabetes mellitus, hypothyroidism, hyperthyroidism or depression (see online supplementary file 4)—this was seen in both the per protocol and ITT analysis.
bmjopen-2019-029180supp004.pdf (54.3KB, pdf)
Stability of our results and sensitivity analysis
The ICC was low (ICC=0.008) indicating that patients within the same general practice were not clustered, and all estimates from multi-level analyses (data not shown) showed no different in estimates compared with estimates from logistics regression. The adjusted sensitivity analysis did not affect the estimates (data not shown). In addition, the baseline characteristics for those lost to follow-up and those not lost to follow-up were comparable; however, the proportion of daily smokers and physical inactive were higher among those lost to follow-up compared with those not lost to follow-up (see online supplementary file 5).
bmjopen-2019-029180supp005.pdf (36.9KB, pdf)
Potential side effects
To evaluate potential side effects of ‘Check-In’, we analysed perceived level of stress for the ‘Check-In’ and usual care group at 12-month follow-up. We found no difference between the two groups; both groups had a median at 16 on the perceived level of stress scale (IQR for ‘Check-In’ 11,20; IQR for usual care 11,21) (data not shown).
Discussion
In this RCT, we found no effect of an intervention of GPs invited individuals with low SEP to a prescheduled preventive health check. We found no differences in smoking status, alcohol consumption, physical inactivity, BMI or in the prevalence of metabolic risk factors and NCDs at 12-month follow-up between the ‘Check-In’ group and usual care group. We did, however, find a difference in incidence of depression, as measured by first prescription of antidepressant medication between the ‘Check-In’ and the usual care group at 12-month follow-up.
The baseline characteristics showed that more than 40% of the participants were daily smokers (table 1) as compared with 17% in the general Danish population.40 This indicated that we did reach a group with a more adverse health behaviour profile than the general population. However, the intensity of the intervention might have been too low to achieve sufficient change of adverse health behaviour among individuals with low SEP, which may have contributed to the lack of measurable behavioural change in ‘Check-In’. In a previous Danish study of health checks, a significant higher smoking abstinence rate was found in a high-intensity intervention group compared with usual care.41 The high-intensity intervention included a consultation based on motivational interviewing, complementary samples of nicotine products, a self-help pamphlet and the offer of participation in six smoking cessation group counselling sessions over a period of 5 months.41 Moreover, higher SEP was a predictor of successful smoking cessation.41 In contrast, ‘Check-In’ relied on the behaviour change services offered by the municipality since 2007.15 42 The idea in ‘Check-In’ was that patients with adverse health behaviour amenable to intervention at the health check should be offered a referral to the municipality health centre for a free lifestyle change programme. However, project data indicated that the opportunity of a referral may have been under-utilised as some of the patients rejected a referral to the municipality, and in some cases, the GP considered a referral to be irrelevant. The result was a low level of intensity of the part of the intervention targeting adverse health behaviour.
Our results are, however, in line with the results from another study focusing at patients with high risk of cardiovascular disease, as they found no differences in the proportion of non-smoking among patients in the intervention compared with usual-care group.43
The lack of effectiveness of ‘Check-In’ regarding more new hospital contacts and prescription medication for metabolic risk factors and NCDs can be ascribed to the fact that more than 60% of individuals included in the study were known with one or more NCDs at baseline. Most had visited their GP within the last year with a median number of contacts to the GP of 7 and 8 in the ‘Check-In’ and usual care group, respectively (table 1). Patients with a known NCD may, as such most likely, already be in some kind of scheduled treatment at their GP. This illustrates that in terms of health, it is indeed a high-risk group participating in ‘Check-In’, but the intervention may not in absolute numbers have picked up many individuals undiagnosed with metabolic risk factors or NCDs, although we did see that there were more persons who initiated treatment with antidepressants in the ‘Check-In’ group compared with the usual care group. This is in line with another Danish study.44 Even so, we cannot completely rule out that the effectiveness regarding depression was due to chance because of the small sample size in ‘Check-In’.
Strengths and weaknesses
One strength of the study was that the randomisation resulted in two balanced groups at baseline and minimised the influence of known and unknown confounding in the comparison of the ‘Check-In’ and the usual care group. Another strength was the use of both patient-reported outcomes and register-based outcomes, where the use of register-based data allowed us to follow all individuals in the study independent of attendance and respond to follow-up questionnaire. A third strength in ‘Check-In’ was the real-life setting, where the health checks were carried out at the general practice clinics to which the patients were registered. Previous trials testing the effectiveness of preventive health check have been criticised for designing a special unit to deliver the health check.6 In ‘Check-In’, it was an assumption that GPs may be in a better position to deliver preventive health services than other health professionals and can offer professional advice accounting for the patients’ state of health in order to encourage compliance.45
A potential limitation in the study was contamination between groups, which potentially occurred if patients in the usual care group had treatment beyond usual care, for example, a health check in the intervention period or if GPs because of the project had more awareness of the preventive work such as smoking cessation when seeing patients allocated to usual care regarding other health issues. However, the risk of contamination is low because GPs did not know who were allocated to the usual care group and couples living together were allocated to same group. If contamination had occurred, the observed effectiveness of the intervention is most likely conservative. Another limitation is the lack of data on smoking status in non-respondents and that we had no access to GP chart notes—any condition not leading to hospital contact are not registered. However, our inclusion of prescription medication should ensure the capture of conditions only managed in general practice.
Furthermore, our sample calculations were based on several assumptions which can be discussed. The assumption that half are motivated to quit smoking can seem high and cannot be verified in the design. This assumption is, however, supported by the literature where 63% of daily smokers in Denmark with no education beyond lower secondary school are found to be motivated to quit smoking.40 Moreover, the 10% a priori loss to follow-up was conservative when compared with the fact that the actually loss was 24%. Nevertheless, in total, 228 and 225 daily smokers were enrolled in the ‘Check-In’ intervention and control group, respectively, which exceeded the sample size calculations that indicated that we needed 150 daily smokers in each group. This indicates that despite a higher loss to follow-up than expected the sample was most likely large enough to detect had there been any effect of ‘Check-In’ regarding adverse health behaviour. It can be argued that the GPs who participated in ‘Check-In’ were especially motivated; hence, if no effect on health behaviour and detection of metabolic risk factors and NCDs are found with these GPs it is plausible to say that no effect will be found if the intervention were rolled out to all GPs. However, further studies are needed to understand non-participants and to understand the process after a preventive health at the GP.
Conclusion
This study suggests that a systematic offer of a preventive health check at the general practice aimed at individuals with low SEP has no effect on adverse health behaviour or incidence on metabolic risk factors or NCDs compared with usual care. The explanations can be low intensity of intervention, a high prevalence of metabolic risk factors and NCDs among the participants at baseline, a high number of contacts with the GP in general or that general practices are not an effective setting for primary prevention.
Supplementary Material
Acknowledgments
The authors would like to acknowledge all participants taking part in the ‘Check-In’ trial – both GPs and patients. Thanks to Annette Kjær Ersbøll for supervision on the sample size calculations and Bjarne Laursen for supervision on the data management and for generated the randomisation.
Footnotes
Contributors: NK-L participated in the design of the study and its coordination and drafted the manuscript. JST participated in the design of the study. NK-L and JST performed the statistical analysis. MB-J participated in the design of the study and its coordination. JT participated in the design of the study. LBL participated in the design of the study. MG participated in the design of the study. CJ participated in the design of the study. SOD participated in the design of the study and its coordination and supervised the statistical analysis. All authors read and approved the final manuscript.
Funding: The project was funded by the Danish Cancer Society. The Danish Cancer Society had no influence on design, analysis and interpretation of the results.
Competing interests: None declared.
Patient consent for publication: Obtained.
Ethics approval: ‘Check-In’ was developed to examine primary prevention aspects beyond existing standard clinical practice, thus no persons were refused access to standard clinical care. Participation was voluntary, and our information material highlighted the option to withdraw without further explanation and without consequences for any treatment or other contact with the GP. Personal identification is encrypted, and data are kept in accordance with the requirements of the Danish Data Protection Agency. The trial was notified to the Danish Data Protection Agency (permission 2015-57-0008, Acadre no. 16/100534) and the National Committee on Health Research Ethics was notified of the project. However, according to the Act on Research Ethics Review of Health Research Projects (section 14.2), projects like ‘Check-In’ do not need ethical approval from a Research Ethics Board (Protocol no.: H-1-2013-FSP). The trial is registered at ClinicalTrials.gov (Early detection of and intervention towards chronic diseases; ID NCT01979107; October 25, 2013).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data may be obtained from a third party and are not publicly available.
References
- 1. World Health Organization Global status report on noncommunicable diseases 2014. World Health Organization, 2014. [Google Scholar]
- 2. Van Dam RM, Li T, Spiegelman D. Combined impact of lifestyle factors on mortality: prospective cohort study in US women. BMJ 2008;337:a1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alageel S, Gulliford MC, McDermott L, et al. . Multiple health behaviour change interventions for primary prevention of cardiovascular disease in primary care: systematic review and meta-analysis. BMJ Open 2017;7:e015375 10.1136/bmjopen-2016-015375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wilson JM, Jungner YG. [Principles and practice of mass screening for disease]. Bol Oficina Sanit Panam 1968;65:281–393. [PubMed] [Google Scholar]
- 5. Krogsbøll LT, Jørgensen KJ, Grønhøj Larsen C, et al. . General health checks in adults for reducing morbidity and mortality from disease. Cochrane Database Syst Rev 2012;10 10.1002/14651858.CD009009.pub2 [DOI] [PubMed] [Google Scholar]
- 6. Sox HC. The health checkup: was it ever effective? Could it be effective? JAMA 2013;309:2496–7. 10.1001/jama.2013.5040 [DOI] [PubMed] [Google Scholar]
- 7. Si S, Moss JR, Sullivan TR, et al. . Effectiveness of general practice-based health checks: a systematic review and meta-analysis. Br J Gen Pract 2014;64:e47–53. 10.3399/bjgp14X676456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bjerregaard A-L, Maindal HT, Bruun NH, et al. . Patterns of attendance to health checks in a municipality setting: the Danish ‘Check Your Health Preventive Program’. Prev Med Rep 2017;5:175–82. 10.1016/j.pmedr.2016.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Petter J, Reitsma-van Rooijen MM, Korevaar JC, et al. . Willingness to participate in prevention programs for cardiometabolic diseases. BMC Public Health 2015;15:44 10.1186/s12889-015-1379-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bender AM, Jørgensen T, Helbech B, et al. . Socioeconomic position and participation in baseline and follow-up visits: the Inter99 study. Eur J Prev Cardiol 2014;21:899–905. 10.1177/2047487312472076 [DOI] [PubMed] [Google Scholar]
- 11. Dryden R, Williams B, McCowan C, et al. . What do we know about who does and does not attend general health checks? Findings from a narrative scoping review. BMC Public Health 2012;12:723 10.1186/1471-2458-12-723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marmot M, Bell R, society F. Fair Society, healthy lives. Public Health 2012;126(Suppl 1):S4–10. 10.1016/j.puhe.2012.05.014 [DOI] [PubMed] [Google Scholar]
- 13. Olsen GS, Holm A-SS, Jørgensen T, et al. . Distribution of ideal cardiovascular health by educational levels from 1978 to 2006: a time trend study from the capital region of Denmark. Eur J Prev Cardiol 2014;21:1145–52. 10.1177/2047487313485513 [DOI] [PubMed] [Google Scholar]
- 14. Pedersen KM, Søndergaard J. Det samarbejdende hospital: Almen praksis og kommuner [The collaborative hospital: General practice and municipalities] : Fremtidens Hospital [The hospital of the future]. København: Munksgaard, 2014: 115–26. [Google Scholar]
- 15. Pedersen KM, Andersen JS, Sondergaard J. General practice and primary health care in Denmark. J Am Board Fam Med 2012;25(Suppl 1):S34–8. 10.3122/jabfm.2012.02.110216 [DOI] [PubMed] [Google Scholar]
- 16. Andersen JS, Olivarius NDF, Krasnik A. The Danish National health service register. Scand J Public Health 2011;39(7 Suppl):34–7. 10.1177/1403494810394718 [DOI] [PubMed] [Google Scholar]
- 17. Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol 1983;51:390–5. 10.1037/0022-006X.51.3.390 [DOI] [PubMed] [Google Scholar]
- 18. Champion VL SC. The Health Belief Model In: Glanz K, Rimer BK, Viswanath K, eds Health behavior and health education, 2008: 45–66. [Google Scholar]
- 19. Galobardes B, Shaw M, Lawlor DA, et al. . Indicators of socioeconomic position (Part 1). J Epidemiol Community Health 2006;60:7–12. 10.1136/jech.2004.023531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nexøe J. Developing Danish general practice. Scand J Prim Health Care 2013;31:129–30. 10.3109/02813432.2013.815989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rothman KJ, Greenland S, Lash TL. Types of Epidemiologic Studies : Modern epidemiology. 3rd edn Philadelphia, USA: Lippincott Williams &Wilkins, 2008: 87–110. [Google Scholar]
- 22. Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. MedCare 1992;30:473–83. [PubMed] [Google Scholar]
- 23. Jenkinson C, Layte R, Jenkinson D, et al. . A shorter form health survey: can the SF-12 replicate results from the SF-36 in longitudinal studies? J Public Health 1997;19:179–86. 10.1093/oxfordjournals.pubmed.a024606 [DOI] [PubMed] [Google Scholar]
- 24. Bandura A. Self-Efficacy: the exercise of control. 1997 New York: W.H. Freeman, 1997. [Google Scholar]
- 25. Mikkelsen EG, Schwarzer R, Jerusalem M. Danish version of the general self-efficacy scale. Available: http://userpage.fu-berlin.de/health/danish.htm1999
- 26. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav 1983;24:385–96. 10.2307/2136404 [DOI] [PubMed] [Google Scholar]
- 27. World Health Organization Recommended levels of physical activity for adults aged 18 - 64 years. Physical Activity and Adults. World Health Organization. Available: www.who.int [Accessed 23 Oct 2017].
- 28. World Health Organization Obesity and overweight. World Health Organization, 2017. Available: www.who.int [Accessed Oct 2017].
- 29. Bandura A. Self-Efficacy: toward a unifying theory of behavioral change. Psychol Rev 1977;84:191–215. 10.1037/0033-295X.84.2.191 [DOI] [PubMed] [Google Scholar]
- 30. Pedersen CB. The Danish civil registration system. Scand J Public Health 2011;39(7 Suppl):22–5. 10.1177/1403494810387965 [DOI] [PubMed] [Google Scholar]
- 31. Lynge E, Sandegaard JL, Rebolj M. The Danish national patient register. Scand J Public Health 2011;39(7_suppl):30–3. 10.1177/1403494811401482 [DOI] [PubMed] [Google Scholar]
- 32. Kildemoes HW, Sørensen HT, Hallas J. The Danish national prescription registry. Scand J Public Health 2011;39(7 Suppl):38–41. 10.1177/1403494810394717 [DOI] [PubMed] [Google Scholar]
- 33. Helweg-Larsen K. The Danish register of causes of death. Scand J Public Health 2011;39(7_suppl):26–9. 10.1177/1403494811399958 [DOI] [PubMed] [Google Scholar]
- 34. Houe H, Ersbøll AK, Toft N. Sample size and sampling methods : Houe H, Ersbøll AK, Toft N, Introduction to veterinary epidemiology. Biofolia, 2004. [Google Scholar]
- 35. Danskernes Sundhed - Tal fra Den Nationale Sundhedsprofil [the Danes' health - Numbers from the National health profile], 2013. Statens Institut for Folkesundhed, Syddansk Universitet & Sundhedsstyrelsen [the National Institute of Public Health, Southern University of Denmark & Danish Health Authority]. Available: www.danskernessundhed.dk
- 36. Pisinger CH. Behandling af tobaksafhængighed - Anbefalinger til en styrket klinisk praksis [Treatment for tobacco dependence - Recommendations for practice, 2011. [Google Scholar]
- 37. Moher D, Hopewell S, Schulz KF, et al. . CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c869 10.1136/bmj.c869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Spratt M, Carpenter J, Sterne JAC, et al. . Strategies for multiple imputation in longitudinal studies. Am J Epidemiol 2010;172:478–87. 10.1093/aje/kwq137 [DOI] [PubMed] [Google Scholar]
- 39. Merlo J, et al. A brief conceptual tutorial of multilevel analysis in social epidemiology: using measures of clustering in multilevel logistic regression to investigate contextual phenomena. J Epidemiol Community Health 2006;60:290–7. 10.1136/jech.2004.029454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jensen HAR, Davidsen M, Ekholm O, et al. . Danskernes sundhed - Den nationale sundhedsprofil 2017 [the Danes' health - The national health profile]. Sundhedsstyrelsen 2018. [Google Scholar]
- 41. Pisinger C, Vestbo J, Borch-Johnsen K, et al. . Smoking cessation intervention in a large randomised population-based study. The Inter99 study. Prev Med 2005;40:285–92. 10.1016/j.ypmed.2004.06.001 [DOI] [PubMed] [Google Scholar]
- 42. Andersen PT, Jensen J-J. Healthcare reform in Denmark. Scand J Public Health 2010;38:246–52. 10.1177/1403494809350521 [DOI] [PubMed] [Google Scholar]
- 43. Wood DA, Kotseva K, Connolly S, et al. . Nurse-coordinated multidisciplinary, family-based cardiovascular disease prevention programme (EUROACTION) for patients with coronary heart disease and asymptomatic individuals at high risk of cardiovascular disease: a paired, cluster-randomised controlled trial. Lancet 2008;371:1999–2012. 10.1016/S0140-6736(08)60868-5 [DOI] [PubMed] [Google Scholar]
- 44. Geyti C, Maindal HT, Dalsgaard E-M, et al. . Mental health assessment in health checks of participants aged 30–49 years: a large-scale cohort study. Prev Med Rep 2018;9:72–9. 10.1016/j.pmedr.2017.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Devroey D, Coigniez P, Vandevoorde J, et al. . Prevention and follow-up of cardiovascular disease among patients without a personal GP. Fam Pract 2003;20:420–4. 10.1093/fampra/cmg415 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-029180supp001.pdf (32.5KB, pdf)
bmjopen-2019-029180supp002.pdf (71.8KB, pdf)
bmjopen-2019-029180supp003.pdf (33.5KB, pdf)
bmjopen-2019-029180supp004.pdf (54.3KB, pdf)
bmjopen-2019-029180supp005.pdf (36.9KB, pdf)