Abstract
Herbal dietary supplements have gained wide acceptance as alternatives to conventional therapeutic agents despite concerns regarding their efficacy and safety. In 2013, a spate of severe liver injuries across the United States was linked to the dietary supplement OxyELITE Pro-New Formula (OEP-NF), a multi-ingredient product marketed for weight loss and exercise performance enhancement. The principal goal of this study was to assess the hepatotoxic potential of OEP-NF in outbred and inbred mouse models. In an acute toxicity study, significant mortality was observed after administering 10X and 3X mouse-equivalent doses (MED) of OEP-NF, respectively. Increases in liver/body weight ratio, ALT and AST were observed in female B6C3F1 mice after gavaging 2X and 1.5X MED of OEP-NF. Similar findings were observed in a 90-day feeding study. These alterations were paralleled by altered expression of gene- and microRNA-signatures of hepatotoxicity, including Cd36, Nqo1, Aldoa, Txnrd1, Scd1 and Ccng1, as well as miR-192, miR-193a and miR-125b and were most pronounced in female B6C3F1 mice. Body weight loss, observed at week 1, was followed by weight gain throughout the feeding studies. These findings bolster safety and efficacy concerns for OEP-NF, and argue strongly for implementation of pre-market toxicity studies within the dietary supplement industry.
Keywords: Dietary supplements, Drug-induced liver injury, Hepatotoxicity, Phytochemicals
1. Introduction
Despite a general insufficiency of proof regarding their efficacy, multi-ingredient herbal dietary supplements (HDS) have gained wide-spread acceptance in the United States. In fact, the estimated number of HDS has risen from ~4000 in 1993 to ~55,000 in 2012 (Bailey et al., 2011; de Boer and Sherker, 2017), and that number is even greater today. Unlike conventional medications, HDS are not required to undergo premarket approval testing for safety or efficacy; thus, the toxicity potential of such products is not realized until after their ingestion by the consuming public. This regulatory shortcoming is especially concerning with regard to unusual and heretofore untested combinations of exotic botanical extracts or purified phytochemicals.
The association of acute liver injury with HDS usage, while uncommon, is not without precedent. As reported by the Drug-Induced Liver Injury Network (DILIN), about 20% of all drug-induced liver injuries (DILI) in 2013 were attributable to HDS (Navarro et al., 2014), which gave rise to a separate category known as HDS-induced liver injury or HILI (de Boer and Sherker, 2017). In 2013, a spate of severe liver injuries across the United States was linked to use of a new formulation of the dietary supplement OxyElite Pro (OEP-NF), a multi-ingredient product marketed for weight loss and exercise performance enhancement (Chatham-Stephens et al., 2017; Foley et al., 2014; Heidemann et al., 2016; Johnston et al., 2016; Roytman et al., 2014). Shortly thereafter, the FDA removed OEP-NF from the U.S. market, in part, because one of its constituents, aegeline, did not meet the qualifications of a “new dietary ingredient” and the product was deemed adulterated (U.S. Food and Drug Administration, 2013a,b).
Until the development and marketing of OEP-NF, consumers had never been exposed to the unique combination of its principal ingredients: caffeine, aegeline, higenamine, yohimbine and coclaurine. A review of the medical literature suggests that the hepatotoxic potential of each individual component is either relatively low (i.e., caffeine, higenamine) (Bloomer et al., 2015; Lo and Chen, 1997) or unknown (i.e, yohimbine, aegeline, coclaurine), although one study demonstrated that the plant source of aegeline, Aegle marmelos, was hepatotoxic when fed to mice (Arseculeratne et al., 1985), and another found that aegeline is cytotoxic to the HepG2 cell line (Mohammed et al., 2016). In addition, yohimbine can exacerbate lipopolysaccharide-induced liver injury (Chen et al., 2015), while chronic caffeine administration has been shown to intensify acute experimental hepatitis, possibly through its antagonism of adenosine 2A receptors (Ohta et al., 2007). Despite the equivocal findings related to OEP-NF’s individual components, the hepatotoxic potential of its combined ingredients was never determined prior to its marketing. However, two short term, small scale clinical studies of a combination of caffeine, higenamine and yohimbine, sponsored by the makers of OEP-NF, demonstrated no serious adverse effects, apart from elevations in heart rate and systolic blood pressure (Bloomer et al., 2015; Lee et al., 2013). Unfortunately, the phytochemical content of this three-ingredient formula was not independently verified by the investigators. This oversight is a common shortcoming when performing dietary supplement research, as the product’s label claim and its actual phytochemical content can be quite different, and without independent verification a study’s findings can be called into question (Gurley, 2012; Gurley et al., 2017).
As mentioned previously, no pre-market safety assessment of the combined ingredients in the lot of OEP-NF linked to the outbreaks of acute liver injury was ever conducted; therefore, the goal of this study was to assess the hepatotoxic potential of OEP-NF in mice. For this purpose, we employed a multi-strain mouse model incorporating B6C3F1 mice, an inbred strain used extensively in the preclinical assessments of hepatotoxicity and hepatocarcino-genesis, and an outbred CD-1 strain to address the idiosyncratic nature of OEP-NF toxicity. Both single-dose gavage studies to assess OEP-NF’s acute capacity for hepatotoxicity, as well as feeding studies of 28 and 90 days duration to evaluate the product’s sub-chronic hepatotoxic potential were performed (Avigan et al., 2016).
2. Materials and methods
2.1. Chemicals
Aegeline and higenamine were synthesized at the National Center for Natural Product Research (NCNPR) (University of Mississippi, University, MS) according to published methods (Albonico et al., 1967; Patra et al., 1981; Somanathan et al., 1983; Kamal et al., 2004; Pyo et al., 2008). Identity and purity was confirmed via published spectroscopic data. Caffeine and yohimbine were purchased from Sigma (St. Louis, MO, USA). Acetonitrile and formic acid were of HPLC grade and these, along with dimethylsulfoxide, were purchased from Fisher Scientific (Fair Lawn, NJ, USA). Water for the HPLC mobile phase was purified in a Milli-Q system (EMD Millipore, Temecula, CA, USA).
2.2. Phytochemical analysis
Phytochemical content of OEP-NF capsules was determined at the NCNPR via ultra-high performance liquid chromatography (UHPLC) with photodiode array detection. Briefly, the contents of OEP-NF capsules were extracted exhaustively in methanol. Five capsules were weighed, opened, and the contents emptied into a mortar and pestle then mixed and triturated. One hundred milligrams were then weighed and sonicated in 2.0 mL of methanol for 30 min followed by centrifugation for 30 min at 959×g. The supernatant was transferred to a 10-mL volumetric flask. The procedure was repeated four more times with 2.0 mL methanol and the respective supernatants were combined. The final volume was adjusted to 10 mL with methanol and mixed thoroughly. Prior to injection, an adequate volume (ca. 2 mL) was passed through a 0.45 μm polytetrafluoroethylene (PTFE) membrane filter. The first 1.0 mL was discarded and the remaining volume was collected in an LC sample vial. Dilutions were performed as required using methanol. If compounds were not detected in the 100-mg sample mass, the extraction was repeated using the average capsule content mass for the analysis and 2 μL aliquots were injected onto the UHPLC system. Phytochemical separations were performed on an Acquity UHPLC™ system (Waters Corp., Milford, MA, USA) equipped with the Waters Empower Software. Separation was achieved on an Acquity UHPLC™ BEH Shield RP18 (100 mm × 2.1 mm, i.d., 1.7 μm) column operated at 40 °C equipped with a 2 cm LC-18 guard column (Waters Corp., Milford, MA, USA) at a flow of 0.2 mL/min. A gradient separation was achieved with a mobile phase consisting of water (A) and acetonitrile (B), both containing 0.05% formic acid. The gradient elution was performed as follows: 97% A: 3% B isocratic for the first minute, followed by 75% B and over the next nine minutes, and up to 100% B for the last minute. Each run was followed by a 3-min wash with 100% acetonitrile and an equilibration period of 3.5 min. Higenamine was detected at 282 nm, while caffeine, yohimbine, and aegeline were detected at 272 nm. Individual stock solutions for each standard compound were prepared at a concentration of 1 mg/mL in methanol and calibration curves were prepared over a range of 1–200 μg/mL. The limits of detection of aegeline, caffeine, yohimbine and higenamine were 0.1 μg/mL, 0.1 μg/mL, 0.2 μg/mL and 0.25 μg/mL, respectively.
Additional OEP-NF extractions were performed at the University of Arkansas for Medical Sciences (UAMS) College of Pharmacy using both water and dimethylsulfoxide (DMSO) as extraction solvents. The contents of twelve OEP-NF capsules were transferred into six separate 20 mL round bottom, glass screw-cap tubes and extracted overnight with either 10 mL of water or 10 mL of DMSO using a rotational mixer (12 revolutions per min.). Tubes were centrifuged at 3300 rpm (Fisher Model 225 Centrific™ centrifuge, Fisher Scientific, Waltham, MA, USA) for one hour and supernatants were transferred to sterile 50 mL polypropylene tubes (Corning 430790, Corning, NY, USA). One mL aliquots of both water and DMSO extractions were sent to the NCNPR for phytochemical analysis. Combinations of the water/DMSO extractions were used for acute gavage administrations.
2.3. Animals
CD-1 mice of both sexes were purchased from Charles River Laboratories (Kingston, NY, USA). B6C3F1 mice of both sexes were purchased from Jackson Laboratories (Bar Harbor, ME, USA). All mice were 7–8 weeks of age at the time of purchase. All animals were housed at the UAMS Division of the Laboratory Animal Medicine (DLAM) facility. Mice were given one week for acclimation before the initiation of experiments. Food and water were provided ad libitum. Each animal was individually tagged with an ear tag. Animal body weights were measured and recorded weekly. All procedures were approved by the UAMS Institutional Animal Care and Use Committee (protocol number: AUP #3701).
2.4. Diets
Custom-made diets were purchased from Envigo (former Teklad Diets) (Madison, WI, USA). The following diets were used: Diet #8640 (vehicle); OEP-NF – 1X (this diet was formulated to deliver 110 mg/kg of total plant alkaloids per day per mouse); and OEP-NF – 3X (this diet was formulated to deliver 330 mg/kg of total plant alkaloids per day per mouse). These doses were based upon the product (lot# 421131) label recommendation (no more than 3 capsules daily) coupled with the total alkaloid (i.e., caffeine, aegeline, higenamine, yohimbine, coclaurine) content per capsule (209.5 mg) as determined by the NCNPR. Diet components were independently verified by Envigo before they were shipped to UAMS. Diets were stored in a cool dry place and a portion of the diet was provided to mice daily.
2.5. Animal gavage dosing
The maximum label-recommended human dose of OEP-NF is three capsules daily. Phytochemical analysis revealed that each capsule contained 209.45 mg of total plant alkaloids (i.e., caffeine, aegeline, higenamine, yohimbine, coclaurine). Based upon these findings, the maximum dose of total OEP-NF alkaloids for a 70-kg adult human is 8.98 mg/kg/day. Allometric scaling for mouse equivalent doses of total OEP-NF alkaloids were determined per the recommendation of Wojcikowski and Gobe (2014) which, in turn, is based upon the FDA Industry Guidance for Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Volunteers (U.S. Food and Drug Administration, 2005; Nair and Jacob, 2016). According to the scaling factor (12.3) commonly used for mice weighing between 11 and 34 mg, the mouse equivalent dose of total OEP-NF alkaloids was calculated to be 110 mg/kg/day. Gavage doses were assessed at the mouse equivalent dose (1X), as well as at three (3X) and ten times (10X) the mouse equivalent dose. This range of doses was well within the 100-fold uncertainty factor commonly recommended when designing HDS safety studies (Schilter et al., 2003).
Because aegeline is poorly extracted in water but readily recovered in DMSO, 1 mL each of the water and DMSO extractions were combined and then diluted with appropriate volumes of 50% DMSO/water such that a 300 μL gavage volume would deliver either the 1X, 3X, or 10X mouse equivalent doses of total OEP-NF alkaloids. Gavage doses were prepared daily, 1–2 h prior to administration. Gavage solutions were vortex-mixed immediately prior to administration in each mouse. The sub-chronic feeding phase of the study incorporated only the 1X and 3X mouse equivalent doses into the mouse chow.
2.6. Animal experiments
Animal experiments were performed in three stages.
Stage 1. Assessment of acute OEP toxicity.
An aqueous extract of the dietary supplement formulation was initially planned to be gavaged at three concentrations: mouse equivalent dose (110 mg/kg), 3X mouse equivalent dose (330 mg/kg) and 10X mouse equivalent dose (1100 mg/kg). As stated above, these doses are based upon total OEP-NF alkaloids. Gavage was performed by trained DLAM personnel administering a total volume of 300 μL. Due to the overt toxicity observed in male CD-1 mice 1 h after receiving the 10X dose, this dose was eliminated and a dosing range of 0.5–3X was adopted for further gavage studies. Male CD-1 mice receiving the 3X dose became lethargic after 3 h and, at the recommendation of the UAMS attending veterinarian, these animals were humanely euthanized 4 h after gavage. For consistency, all other mice receiving the 3X dose were euthanized at 4 h. All other animals were humanely euthanized 24 h after gavage. Livers, kidneys and hearts were harvested for future molecular and histopathological examination.
Stages 2 and 3. Assessment of sub-acute and sub-chronic OEP-NF toxicity.
To assess the sub-acute and sub-chronic effects of OEP-NF, animals were fed the supplemented diet at the 1X (110 mg/kg/day) and 3X (330 mg/kg/day) doses for 4 and 13 weeks, respectively.
2.7. Blood sampling
To measure the effects of OEP-NF on liver enzymes (e.g., ALT, AST) and biomarkers (e.g., miR-122) characteristic for drug-induced liver injury, blood was collected at the end of each stage of the experiments described above. Blood was collected from the mandibular plexus using a GOLDENROD animal lancet (MEDIpoint, Inc., Mineola, NY, USA) by trained DLAM personnel into a 1.1 mL Z-gel microtube (Sarstedt, Newton USA). Tubes were kept on ice and centrifuged at 10,000 rpm for 20 min and the serum was immediately aliquoted and flash-frozen.
2.8. Histopathological assessment
Livers were excised and a 1 mm section was obtained from the left lateral lobe and another from the right medial lobe. The sections were fixed in 4% formalin for 24 h, then briefly rinsed in PBS and stored in 70% ethanol for 24 h. Livers were then processed at the UAMS Pathology Core Facility, stained with hematoxylin eosin and shipped to the Oklahoma Health Sciences Center (OHSC) for veterinary pathology assessment.
For histologic evaluation purposes, each liver was represented by two sections obtained from different locations within the liver. Each section was initially evaluated at magnifications of X40 and X100. The sections were then evaluated at X200 and X400 to better determine if significant changes were present and to check for the presence of mitotic figures and apoptotic bodies.
2.9. Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) measurement
Serum ALT and AST levels were detected using the activity colorimetric assays and quantitated by a standard curve. The assay kits were purchased from BioVision (Milpitas, CA, USA) (Cat. #K752–100 and K753–100 for ALT and AST, respectively). Serum samples from treatment and control groups were diluted in the assay buffer (1:2 or 2:5). Twenty microliter samples and standards were added to a 96-well plate. 100 μL reaction mix composed of 86 μL ALT (AST) Assay Buffer, 2 μL reconstituted ALT (AST) Enzyme mix, 2 μL OxiRed probe and 10 μL reconstituted ALT (AST) substrate were added to each well, as well as the standards and positive controls. The plate was shaken for 3 min to mix the reagents. The absorbance (OD) was read at 570 nm (T1) and then again at T2 after the plate incubation at 37 °C for 30–45 min and protected from light. ALT (AST) activity in serum was calculated using a standard curve and formula below:
where B is the glutamate amount calculated from the standard curve (in nmol); T1 is the time of the first reading (in min); T2 is the time of the second reading (in min); V is the original sample volume added into the reaction well (in mL).
2.10. miR-122 detection in serum
MicroRNAs (miRNAs) are a family of small non-coding RNAs that can affect xenobiotic metabolism and toxicity, and whose expression may be affected by certain drugs. They also play important roles in modulating inter-individual variability in drug metabolizing enzyme and transporter production (Koturbash et al., 2012, 2015). A miRNA important in hepatotoxicity is miR-122, the only liver-specific miRNA detectable in biological fluids following liver damage (Starkey Lewis et al., 2012). In this study, miR-122 levels were measured in serum by qRT-PCR. The standard was generated using synthetic miRNA 122–5p and added separately to RT reactions after serial dilution to generate a standard curve. The sample was diluted (1:10) in nuclease-free water treated with RNase inhibitor (0.4 U/μL). Reverse transcription reaction (RT), pre-amplification, and qRT-PCR were carried out for each sample or standard. (1) Two-step RT reaction was performed in a 15 μL-RT reaction using the TaqMan miRNA Reverse Transcription Kit (Life Technologies, Foster City, CA). The first step RT reaction was carried out using the following cycling conditions: denature at 94 °C for 10 min; then anneal at 75 °C for 2 min; 60 °C, 50 °C, 40 °C, 30 °C for 3 min, respectively; and hold at 4 °C. The second step was run at 16 °C and 42 °C for 30 min, respectively, then at 85 °C for 5 min, and hold at 4 °C. (2) Pre-Amplification reaction: The RT product needs to be pre-amplified prior to the real-time PCR step to potentially enhance sensitivity. The pre-amplification step was carried out in a 25 μL reaction comprised of 12.5 μL Taqman PreAmp Master Mix (2X), 0.0375 μL Taqman miRNA qPCR assay (20 X), 9.9625 μL water and 2.5 μL undiluted RT product. The Pre-Amp PCR reactions were carried out in the following cycling conditions: pre-denature at 95 °C for 10 min, then hold at 55 °C and 72 °C for 2 min, respectively; run 12 cycles at 95 °C for 15 Sec and 60 °C for 4 min, hold at 99.9 °C for 10 min, then hold at 4 °C. The pre-amplified PCR product was diluted in 35 μL water with the ratio of 1:8. (3) qRT-PCR reaction: The generation of a standard curve was carried out in parallel with qRT-PCR of samples to calculate the amount of the mir-122 in the samples. qRT-PCR reaction was run in 20 μL composed of 10 μL TaqMan Universal PCR Master Mix (2 X), 4 μL water, 1 μL Taqman miRNA qPCR assay (20 X) and 5 μL diluted Pre-Amp products on a ViiA7 qRT-PCR thermocycler at 95 °C for 10 min, 40 cycles at 95 °C for 15 Sec and 60 °C for 1 min and hold at 4 °C. The miRNA concentration was calculated based on the standard curve of the matching synthetic miRNA from the standard curve.
2.11. Gene expression array
Total RNA was extracted from flash frozen liver tissue using the RNeasy Mini Kit (Qiagen, Germantown, MD, USA). Following purification, 1000 ng was reverse transcribed with the High Capacity cDNA Reverse Transcription Kit (ThermoFisher, Waltham, MA, USA). The cDNA was diluted to 5 ng/μL and 105 μL was mixed with an equal volume of 2X TaqMan® Fast Advanced Master Mix. For real-time PCR, 100 μL of the mix was applied to each of two channels on a TaqMan Low Density Hepatotoxicity Array (TLDA) (Supplementary Table 1) (ThermoFisher, Waltham, MA, USA). Four biological samples were loaded onto each array with five samples analyzed per treatment group. Analysis was performed using the ExpressionSuite Software v1.1 (ThermoFisher, Waltham, MA, USA).
2.12. miRNA expression array
A panel of miRNAs whose expression was previously altered in response to hepatotoxicant exposure was utilized (Miousse et al., 2017). miRNAs were extracted from flash-frozen livers using the miRNeasy Kit (Qiagen, Germantown, MD, USA). RNA concentration was assessed by Nanodrop 2000 (Thermo Scientific, Waltham, MA, USA) and 1000 ng of RNA containing miRNAs was used for reverse transcription with the miScript reverse transcriptase and reverse transcription primers specific for nine miRNA targets and the internal reference U6). Transcript abundance was measured on a ViiA-7 instrument (Applied Biosystems, Foster City, CA, USA) and results calculated using the ΔΔ Ct method.
2.13. Statistical analyses
All statistical analyses were performed with the Graphpad Prism 6 software (Graphpad Software. San Diego, CA). Within gender and strain, treatment groups were compared with their respective untreated group using ANOVA followed by Dunnett’s multiple comparison test. In cases where the data was not normally distributed, a Dunn’s test was used instead.
3. Results
3.1. Analytical chemistry data
Phytochemical analysis of OEP-NF (lot #421131) capsules revealed the presence of caffeine (134.2 mg/cap.), aegeline (44.9 mg/cap.), higenamine (25.2 mg/cap), yohimbine (4.2 mg/cap), coclaurine (0.95 mg/cap.) and trace amounts of tannins (i.e., gallic acid and gallic acid derivatives). No characteristic marker phytochemicals were noted for extracts of either Bauhinia purpurea (e.g., isoquercetin, quercetin-3,4′-diglucoside, isorhamnetin-3,7-diglucoside, pelargonidin-3-triglucoside, quercetin 3-rutinoside-7-glucoside, kaempferol 3,4′-diglucoside) or Hemerocalis fulva (e.g., fulvanines A-E, kwansonine B-C). Besides yohimbine, no other characteristic marker phytochemicals for Pausinystalia johimbe extract (e.g., rauwolscine, β-yohimbine, corynanthine, dihydrositsirikine, pseudoyohimbine, yohimbic acid, alloyohimbine, hydroxyyohimbine) were detected. These findings indicate that neither of these botanical extracts were components of the formulation, despite label claims for each. In short, it appears that OEP-NF was a collection of synthetic alkaloids.
3.2. Acute effects of OEP-NF administration (4–24 h gavage study)
Gavaging CD-1 male mice with 10X MED resulted in 100% mortality within the first hour after OEP-NF administration. Two out of twelve CD-1 male mice gavaged with 3X MED were found dead by this time as well, and two others were moribund and were humanely euthanized along with the rest of the animals in this group at the request of the attending veterinarian. The observed overt toxicity led us to discontinue the 10X MED and the subsequent cohorts were gavaged within the range of 0.5–3X of the MED. All other mice gavaged with 3X MED formulation (i.e., CD-1 females) were humanely euthanized at 4 h so that biochemical findings would be consistent with CD-1 males. Mice receiving gavage doses less than 3X MED were euthanized at 24 h.
Despite the dosing reconsiderations noted above, two female and one male B6C3F1 mice receiving the 2X MED expired within 24 h.
3.2.1. Liver/body weight ratio and histopathological evaluation
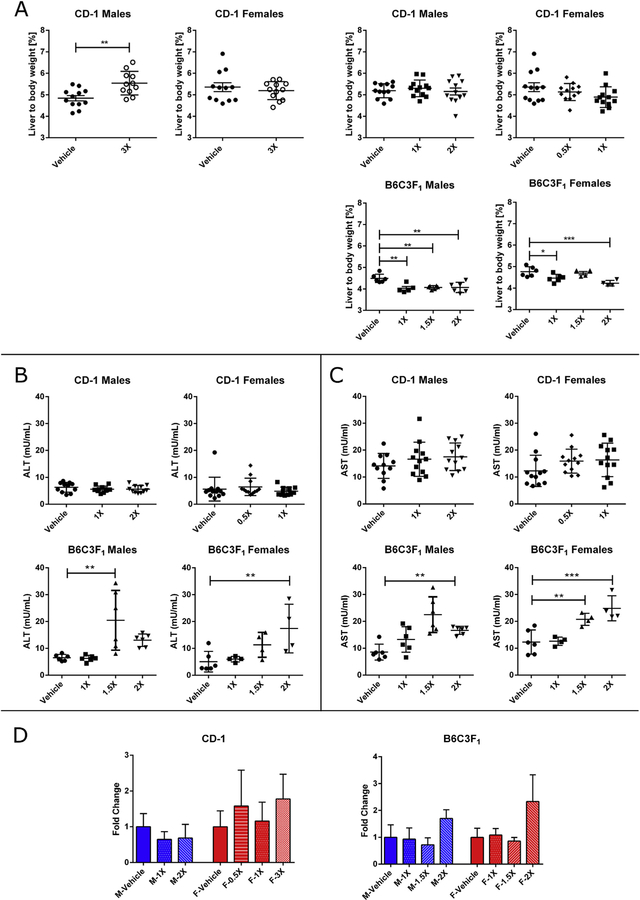
Significant increases in liver/body weight ratios at the 4 h time-point (+0.6%, p < 0.01) were noted for male CD-1 mice receiving the 3X MED. Gavaging both male and female B6C3F1 mice was associated with statistically significant decreases in the liver body/weight ratios for all gavage doses of OEP-NF (range 0.2–0.8%, p < 0.05) (Fig. 1A). At 24 h, no significant histopathological changes were identified in the livers of mice in any of the gavage groups.
Fig. 1.
Effects of OEP-NF gavaging in male and female CD-1 and B6C3F1 mice. A) Liver to body weight ratio in mice after gavaging with 3X MED (4 h time-points) and 0.5X-2X MED (24 h time-point); B) Levels of ALT, and C) Levels of AST in mice 24 h after gavaging; D) Levels of miR-122 in the serum of mice 24 h after gavage, data expressed as fold-change from respective control values. Mean ± SD. Asterisks “*” denotes significant (p < 0.05), “**” (p < 0.01), and “***” (p < 0.001) difference from control.
3.2.2. Clinical biochemistry
No statistically significant changes in ALT or AST levels were observed in CD-1 male and female mice gavaged with OEP-NF. In B6C3F1 mice, gavaging with 1.5X and 2X MED resulted in statistically significant (p < 0.05–0.001) increases in the serum levels of both aminotransferases, the only exception being male mice receiving the 2X MED where the increase in ALT approached significance (p = 0.059) (Fig. 1B and C). Although, overall increases in ALT and AST were modest, clear dose-dependent trends were observed. Furthermore, compared to vehicle controls, some animals exhibited a 4–5 fold increase in serum aminotransferases. At the same time, no significant changes were observed in the levels of miR-122 in any of the experimental groups, although a greater than two-fold increase in miR-122 was observed in female B6C3F1 mice gavaged with 2X MED (p = 0.067) (Fig. 1D).
3.3. Sub-acute effects of OEP-NF (4-week feeding study)
3.3.1. Effects of OEP-NF on body weight dynamics
Differences in the initial body weights of experimental animals were observed as a result of the outbred nature of CD-1 mice, as well as sex-, and strain-differences. Therefore, body weight dynamics were evaluated as a percent change from the initial weight of each individual animal.
Administration of OEP for 4 weeks affected body weight dynamics in all mice in a strain-, sex-, and dose-dependent manner. Specifically, CD-1 mice lost body weight over the first week, but then gained weight over the next 3 weeks, although at a slower pace than controls. (Supplementary Fig. 1). No treatment-related declines in body weight were observed in B6C3F1 mice of either sex; however, similar to CD-1 mice, weight gain dynamics were slower when compared to controls.
3.3.2. Liver/body weight ratio and histopathological evaluation
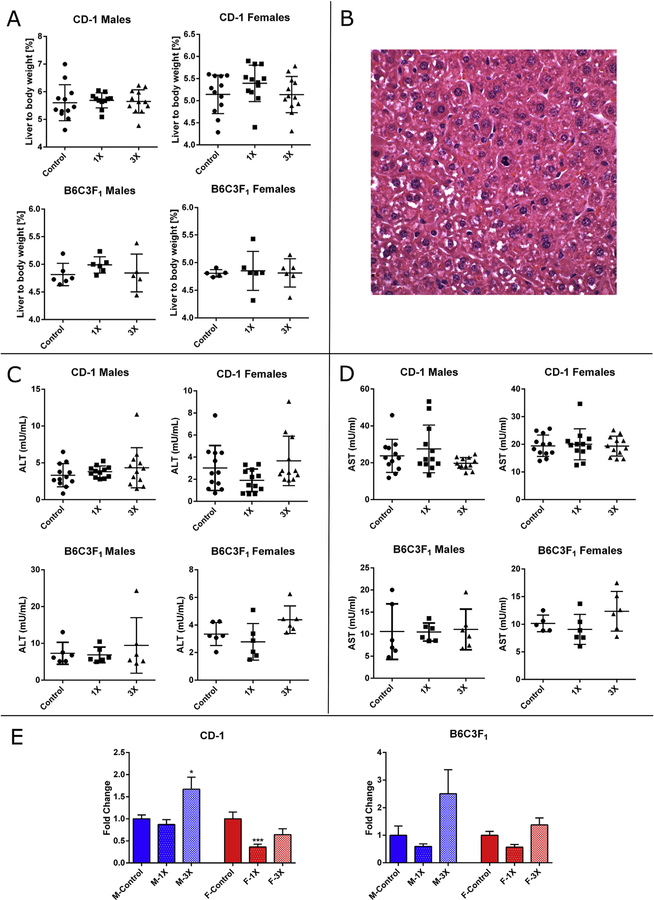
No significant changes in liver/body weight ratios were noted in mice fed OEP-NF for 4 weeks (Fig. 2A). At the end of the 4-week feeding study a number of animals exhibited modest to moderate histopathological changes, mostly in the form of multiple mitotic foci (Fig. 2B).
Fig. 2.
Effects of feeding mice with OEP-NF for 4 weeks. A) Liver to body weight ratio; B) Mitotic figures in the liver of a mouse (ID# 291) fed 1X OEP-NF (magnification 40×); C) Levels of ALT, and D) Levels of AST in the serum of mice; E) Levels of miR-122 in the serum of mice, data expressed as fold-change from respective control values. Mean ± SD. Asterisks “*” denotes significant (p < 0.05) difference from control.
3.3.3. Analysis of clinical biochemistry
Feeding OEP-NF for 4 weeks did not significantly affect serum ALT or AST (Fig. 2C and D). A statistically significant increase (p < 0.05) in the serum levels of miR-122 was observed in CD-1 males fed the 3X OEP-NF diet (Fig. 2E). Increases in serum miR-122 levels were also observed in male and female B6C3F1 mice fed the 3X diet, although these differences did not reach statistical significance.
3.3.4. Hepatotoxicity gene expression array
To explore potential molecular alterations associated with dietary administration of OEP-NF, we performed expression analysis of a panel of genes previously linked to hepatotoxicity (Aleksunes et al., 2006; Chen et al., 2017; Heise et al., 2015; Koturbash et al., 2011; Minami et al., 2005). At four weeks, 17 genes were identified that were significantly (±1.5-fold change from control, p < 0.05) dysregulated in at least two treatment groups (Table 1). The most pronounced changes were observed in female B6C3F1 mice in which 13 separate genes, including Nqo1, Cd36, and Lpl were differentially regulated.
Table 1.
List of dysregulated genes as a result of feeding mice with OEP for 4 or 13 weeks.
| 4 weeks | CD-1 | B6C3F1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Males | Females | Males | Females | |||||||||
| Control | 1X | 3X | Control | 1X | 3X | Control | 1X | 3X | Control | 1X | 3X | |
| Abcb4 | 1.00 (±0.16) | 1.12 (±0.12) | 1.05 (0.09) | 1.00 (±0.05) | 1.39 (±0.14) | 1.45 (0.19) | 1.00 (±0.07) | 1.18 (±0.07) | 1.53* (±0.17) | 1.00 (±0.11) | 1.52 (±0.04) | 1.58* (±0.20) |
| Abcc2 | 1.00 (±0.14) | 1.04 (±0.12) | 0.93 (0.12) | 1.00 (±0.04) | 1.56 (±0.28) | 1.65 (0.27) | 1.00 (±0.08) | 0.97 (±0.06) | 1.38** (±0.08) | 1.00 (±0.10) | 1.27 (±0.09) | 1.38* (±0.07) |
| Abcc3 | 1.00 (±0.13) | 0.84 (±0.06) | 1.34 (0.13) | 1.00 (±0.02) | 1.30 (±0.10) | 1.46 (0.29) | 1.00 (±0.18) | 1.02 (±0.18) | 2.38# (±0.42) | 1.00 (±0.15) | 1.31 (±0.17) | 1.84** (±0.13) |
| Aldoa | 1.00 (±0.17) | 0.98 (±0.08) | 1.06 (0.07) | 1.00 (±0.09) | 1.36 (±0.18) | 1.43 (0.02) | 1.00 (±0.03) | 1.47* (±0.15) | 1.66** (±0.14) | 1.00 (±0.08) | 1.58 (±0.22) | 1.55 (±0.24) |
| Avpr1a | 1.00 (±0.06) | 1.40* (±0.10) | 1.26 (0.13) | 1.00 (±0.06) | 0.94 (±0.12) | 0.75 (0.15) | 1.00 (±0.06) | 1.37 (±0.08) | 1.59# (±0.21) | 1.00 (±0.06) | 1.54 (±0.18) | 1.45 (±0.21) |
| Bhmt | 1.00 (±0.13) | 1.36 (±0.13) | 1.11 (0.39) | 1.00 (±0.22) | 1.12 (±0.14) | 0.98 (0.10) | 1.00 (±0.06) | 1.08 (±0.11) | 1.14 (±0.15) | 1.00 (±0.08) | 1.64 (±0.06) | 1.65 (±0.27) |
| Ccgn1 | 1.00 (±0.17) | 1.23 (±0.34) | 0.84 (0.08) | 1.00 (±0.15) | 1.34 (±0.18) | 1.13 (0.05) | 1.00 (±0.03) | 1.30 (±0.08) | 1.32* (±0.12) | 1.00 (±0.04) | 1.70* (±0.20) | 1.76* (±0.21) |
| Cd36 | 1.00 (±0.19) | 0.50 (±0.09) | 1.06 (0.23) | 1.00 (±0.36) | 1.50 (±0.30) | 2.98** (0.29) | 1.00 (±0.33) | 0.86 (±0.08) | 2.27 (±0.68) | 1.00 (±0.14) | 1.18 (±0.08) | 3.70# (±0.67) |
| Cdc14b | 1.00 (±0.16) | 1.41 (±0.15) | 1.40 (0.14) | 1.00 (±0.07) | 1.32 (±0.22) | 1.14 (0.14) | 1.00 (±0.05) | 1.02 (±0.10) | 1.72** (±0.14) | 1.00 (±0.14) | 1.73* (±0.13) | 1.87** (±0.19) |
| Cxcl12 | 1.00 (±0.06) | 0.80 (±0.08) | 0.67# (0.02) | 1.00 (±0.04) | 0.87 (±0.09) | 0.93 (0.06) | 1.00 (±0.12) | 0.86 (±0.04) | 1.08 (±0.06) | 1.00 (±0.09) | 1.40* (±0.09) | 1.08 (±0.10) |
| Cyp1a2 | 1.00 (±0.24) | 0.85 (±0.14) | 0.94 (0.07) | 1.00 (±0.10) | 1.36 (±0.22) | 1.98* (0.25) | 1.00 (±0.01) | 1.24 (±0.12) | 1.73## (±0.11) | 1.00 (±0.07) | 1.61* (±0.16) | 2.24*** (±0.16) |
| Fasn | 1.00 (±0.16) | 0.63 (±0.09) | 0.94 (0.12) | 1.00 (±0.37) | 0.65 (±0.38) | 0.61 (0.07) | 1.00 (±0.10) | 0.71 (±0.08) | 1.28 (±0.07) | 1.00 (±0.25) | 0.55 (±0.10) | 0.73 (±0.06) |
| Gclc | 1.00 (±0.12) | 1.48 (±0.21) | 1.79* (0.28) | 1.00 (±0.03) | 1.41* (±0.12) | 1.52* (0.14) | 1.00 (±0.04) | 1.06 (±0.11) | 1.83* (±0.37) | 1.00 (±0.10) | 1.40 (±0.22) | 1.63 (±0.24) |
| Gsr | 1.00 (±0.21) | 0.78 (±0.14) | 1.09 (0.14) | 1.00 (±0.07) | 1.00 (±0.18) | 1.43 (0.12) | 1.00 (±0.15) | 1.00 (±0.11) | 1.23 (±0.15) | 1.00 (±0.04) | 1.54 (±0.36) | 1.48 (±0.17) |
| Lpl | 1.00 (±0.17) | 1.15 (±0.32) | 1.34 (0.29) | 1.00 (±0.08) | 1.03 (±0.27) | 2.05 (0.57) | 1.00 (±0.15) | 1.23 (±0.19) | 1.50 (±0.09) | 1.00 (±0.09) | 1.67* (±0.24) | 2.06** (±0.08) |
| Lss | 1.00 (±0.11) | 0.55** (±0.06) | 1.01 (0.05) | 1.00 (±0.15) | 0.91 (±0.32) | 1.07 (0.13) | 1.00 (±0.11) | 0.90 (±0.06) | 1.58* (±0.18) | 1.00 (±0.08) | 1.11 (±0.04) | 1.21 (±0.17) |
| Mlxipl | 1.00 (±0.15) | 1.05 (±0.03) | 1.70# (0.25) | 1.00 (±0.13) | 1.06 (±0.30) | 0.87 (0.09) | 1.00 (±0.04) | 1.07 (±0.10) | 1.50** (±0.09) | 1.00 (±0.07) | 1.81# (±0.26) | 2.00# (±0.49) |
| Nqo1 | 1.00 (±0.17) | 1.22 (±0.28) | 1.76 (0.53) | 1.00 (±0.18) | 0.93 (±0.19) | 1.85* (0.27) | 1.00 (±0.07) | 1.09 (±0.05) | 2.55## (±0.41) | 1.00 (±0.17) | 1.79 (±0.10) | 2.40** (±0.33) |
| Pla2g12a | 1.00 (±0.12) | 0.87 (±0.07) | 0.99 (0.22) | 1.00 (±0.13) | 1.41 (±0.09) | 1.51 (0.22) | 1.00 (±0.09) | 1.35 (±0.16) | 1.54* (±0.11) | 1.00 (±0.07) | 1.87*** (±0.06) | 1.51** (±0.13) |
| pygl | 1.00 (±0.14) | 1.20 (±0.14) | 1.04 (0.15) | 1.00 (±0.16) | 0.93 (±0.23) | 0.86 (0.05) | 1.00 (±0.06) | 1.36 (±0.12) | 1.69 (±0.25) | 1.00 (±0.10) | 1.10 (±0.16) | 1.16 (±0.17) |
| Scd1 | 1.00 (±0.33) | 0.67 (±0.17) | 1.55 (0.45) | 1.00 (±0.69) | 0.68 (±0.36) | 0.83 (0.23) | 1.00 (±0.16) | 0.67 (±0.14) | 0.99 (±0.15) | 1.00 (±0.13) | 0.77 (±0.24) | 0.85 (±0.07) |
| Thrsp | 1.00 (±0.25) | 0.85 (±0.21) | 0.81 (0.18) | 1.00 (±0.36) | 0.22 (±0.07) | 0.89 (0.33) | 1.00 (±0.13) | 0.62 (±0.10) | 0.88 (±0.07) | 1.00 (±0.12) | 0.83 (±0.36) | 0.50 (±0.10) |
| Tmem2 | 1.00 (±0.07) | 0.89 (±0.06) | 1.01 (0.07) | 1.00 (±0.06) | 1.14 (±0.16) | 1.20 (0.10) | 1.00 (±0.08) | 0.92 (±0.04) | 1.23 (±0.10) | 1.00 (±0.06) | 1.38* (±0.06) | 1.52** (±0.11) |
| Txnrd1 | 1.00 (±0.21) | 0.89 (±0.09) | 1.02 (0.12) | 1.00 (±0.03) | 1.01 (±0.13) | 1.40 (0.30) | 1.00 (±0.08) | 1.12 (±0.09) | 1.63 (±0.19) | 1.00 (±0.15) | 1.36 (±0.19) | 1.67 (±0.27) |
| 13 weeks | CD-1 | B6C3F1 | ||||||||||
| Males | Females | Males | Females | |||||||||
| Control | 1X | 3X | Control | 1X | 3X | Control | 1X | 3X | Control | 1X | 3X | |
| Abcb4 | 1.00 (±0.13) | 0.96 (±0.06) | 1.29 (0.12) | 1.00 (±0.09) | 1.31 (±0.22) | 1.33 (0.17) | 1.00 (±0.06) | 0.89 (±0.12) | 1.26 (±0.09) | 1.00 (±0.12) | 1.11 (±0.16) | 1.07 (±0.12) |
| Abcc2 | 1.00 (±0.08) | 1.37 (±0.20) | 1.37 (0.14) | 1.00 (±0.04) | 1.17 (±0.13) | 1.30 (0.10) | 1.00 (±0.10) | 0.81 (±0.09) | 1.12 (±0.12) | 1.00 (±0.06) | 1.17 (±0.12) | 1.05 (±0.05) |
| Abcc3 | 1.00 (±0.27) | 0.61 (±0.02) | 1.66 (0.07) | 1.00 (±0.09) | 1.57 (±0.25) | 2.07 (0.27) | 1.00 (±0.09) | 0.78 (±0.05) | 1.22 (±0.10) | 1.00 (±0.11) | 1.05 (±0.11) | 1.70 (±0.21) |
| Aldoa | 1.00 (±0.18) | 1.18 (±0.11) | 1.39 (0.14) | 1.00 (±0.06) | 1.21* (±0.04) | 1.54*** (0.05) | 1.00 (±0.04) | 1.33 (±0.08) | 1.57** (±0.13) | 1.00 (±0.12) | 1.12 (±0.15) | 1.17 (±0.12) |
| Avpr1a | 1.00 (±0.20) | 1.54 (±0.25) | 1.28 (0.15) | 1.00 (±0.07) | 1.09 (±0.13) | 0.98 (0.15) | 1.00 (±0.10) | 1.06 (±0.11) | 1.14 (±0.07) | 1.00 (±0.11) | 0.82 (±0.10) | 1.30 (±0.12) |
| Bhmt | 1.00 (±0.08) | 0.87 (±0.02) | 0.38# (0.04) | 1.00 (±0.05) | 0.82 (±0.11) | 0.55* (0.10) | 1.00 (±0.06) | 1.00 (±0.15) | 0.81 (±0.09) | 1.00 (±0.15) | 0.80 (±0.13) | 0.79 (±0.15) |
| Ccgn1 | 1.00 (±0.15) | 1.78 (±0.59) | 1.00 (0.08) | 1.00 (±0.10) | 0.95 (±0.09) | 1.08 (0.07) | 1.00 (±0.12) | 0.92 (±0.01) | 0.96 (±0.07) | 1.00 (±0.06) | 1.19 (±0.15) | 1.04 (±0.04) |
| Cd36 | 1.00 (±0.16) | 0.65 (±0.14) | 2.56 (0.96) | 1.00 (±0.22) | 1.07 (±0.55) | 2.52* (0.48) | 1.00 (±0.21) | 0.57 (±0.14) | 0.74 (±0.15) | 1.00 (±0.11) | 1.23 (±0.16) | 3.66* (±0.50) |
| Cdc14b | 1.00 (±0.17) | 0.68 (±0.12) | 1.14 (0.04) | 1.00 (±0.05) | 0.93 (±0.05) | 0.74 (0.06) | 1.00 (±0.13) | 0.62 (±0.15) | 1.10 (±0.20) | 1.00 (±0.10) | 0.99 (±0.12) | 1.17 (±0.10) |
| Cxcl12 | 1.00 (±0.13) | 0.99 (±0.13) | 1.05 (0.06) | 1.00 (±0.11) | 1.09 (±0.09) | 1.28 (0.12) | 1.00 (±0.10) | 0.82 (±0.07) | 1.10 (±0.09) | 1.00 (±0.10) | 0.90 (±0.09) | 0.82 (±0.06) |
| Cyp1a2 | 1.00 (±0.14) | 1.15 (±0.12) | 1.34 (0.07) | 1.00 (±0.22) | 1.16 (±0.17) | 1.68 (0.09) | 1.00 (±0.12) | 0.97 (±0.16) | 1.52 (±0.16) | 1.00 (±0.10) | 1.42 (±0.24) | 1.31 (±0.12) |
| Fasn | 1.00 (±0.06) | 0.49** (±0.12) | 0.40*** (0.04) | 1.00 (±0.18) | 0.64 (±0.05) | 0.81 (0.11) | 1.00 (±0.16) | 0.36# (±0.03) | 0.37# (±0.04) | 1.00 (±0.08) | 0.75 (±0.02) | 0.85 (±0.20) |
| Gclc | 1.00 (±0.12) | 0.86 (±0.13) | 1.75 (0.14) | 1.00 (±0.11) | 0.96 (±0.09) | 1.30 (0.22) | 1.00 (±0.17) | 0.93 (±0.11) | 1.02 (±0.03) | 1.00 (±0.13) | 1.04 (±0.13) | 1.47 (±0.14) |
| Gsr | 1.00 (±0.09) | 0.93 (±0.09) | 1.17 (0.05) | 1.00 (±0.11) | 0.81 (±0.05) | 1.26 (0.10) | 1.00 (±0.04) | 1.06 (±0.08) | 1.28* (±0.07) | 1.00 (±0.07) | 1.14 (±0.08) | 1.41** (±0.07) |
| Lpl | 1.00 (±0.16) | 1.67 (±0.33) | 1.99* (0.17) | 1.00 (±0.15) | 1.56 (±0.42) | 1.00 (0.16) | 1.00 (±0.11) | 1.37 (±0.16) | 2.82## (±0.72) | 1.00 (±0.10) | 1.26 (±0.23) | 2.12** (±0.23) |
| Lss | 1.00 (±0.12) | 0.94 (±0.18) | 0.92 (0.06) | 1.00 (±0.12) | 0.85 (±0.04) | 1.37 (0.13) | 1.00 (±0.12) | 0.70 (±0.04) | 0.66 (±0.03) | 1.00 (±0.09) | 0.65 (±0.15) | 0.70 (±0.12) |
| Mlxipl | 1.00 (±0.09) | 0.92 (±0.12) | 1.03 (0.11) | 1.00 (±0.14) | 0.87 (±0.10) | 0.73 (0.06) | 1.00 (±0.13) | 0.66 (±0.10) | 0.96 (±0.05) | 1.00 (±0.05) | 1.27 (±0.25) | 1.13 (±0.11) |
| 4 weeks | CD-1 | B6C3F1 | ||||||||||
| Males | Females | Males | Females | |||||||||
| Control | 1X | 3X | Control | 1X | 3X | Control | 1X | 3X | Control | 1X | 3X | |
| Nqo1 | 1.00 (±0.20) | 0.96 (±0.13) | 2.68*** (0.22) | 1.00 (±0.11) | 0.99 (±0.21) | 1.86* (0.24) | 1.00 (±0.22) | 0.66 (±0.06) | 1.59* (±0.05) | 1.00 (±0.07) | 1.57** (±0.12) | 2.28*** (±0.13) |
| Pla2g12a | 1.00 (±0.21) | 1.57 (±0.31) | 1.81 (0.17) | 1.00 (±0.13) | 1.37 (±0.06) | 1.60 (0.22) | 1.00 (±0.12) | 1.02 (±0.11) | 1.41 (±0.17) | 1.00 (±0.15) | 1.15 (±0.19) | 0.88 (±0.08) |
| Pygl | 1.00 (±0.09) | 0.92 (±0.18) | 0.70 (0.07) | 1.00 (±0.10) | 0.95 (±0.16) | 0.79 (0.08) | 1.00 (±0.08) | 0.84 (±0.13) | 0.68 (±0.02) | 1.00 (±0.14) | 0.96 (±0.05) | 0.91 (±0.04) |
| Scd1 | 1.00 (±0.10) | 0.33** (±0.08) | 0.44** (0.11) | 1.00 (±0.08) | 0.44* (±0.14) | 0.52* (0.14) | 1.00 (±0.09) | 0.46** (±0.12) | 0.26*** (±0.05) | 1.00 (±0.30) | 0.83 (±0.15) | 1.08 (±0.28) |
| Thrsp | 1.00 (±0.21) | 0.31* (±0.09) | 0.17** (0.04) | 1.00 (±0.23) | 0.51 (±0.10) | 0.39 (0.15) | 1.00 (±0.27) | 0.28 (±0.05) | 0.14** (±0.01) | 1.00 (±0.26) | 0.78 (±0.18) | 0.36 (±0.10) |
| Tmem2 | 1.00 (±0.08) | 0.97 (±0.20) | 0.93 (0.04) | 1.00 (±0.15) | 0.92 (±0.06) | 1.12 (0.17) | 1.00 (±0.08) | 0.81 (±0.04) | 0.97 (±0.10) | 1.00 (±0.11) | 1.00 (±0.06) | 1.26 (±0.17) |
| Txnrd1 | 1.00 (±0.14) | 1.40* (±0.10) | 1.79** (0.13) | 1.00 (±0.05) | 1.11 (±0.11) | 1.68*** (0.06) | 1.00 (±0.11) | 1.35 (±0.11) | 1.26 (±0.13) | 1.00 (±0.08) | 1.20 (±0.18) | 1.17 (±0.09) |
Values are mean (±SEM). Significant values are presented in bold with asterisks (*) for parametric analysis and number signs (#) for non-parametric analysis (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001).
3.3.5. Hepatotoxicity miRNA expression panel
As a result of the high inter-individual variability in miRNA expression, no statistically significant changes were observed in the expression of miRNAs related to hepatotoxicity after 4 weeks of OEP-NF exposure. However, dose-dependent trends in the expression of miR-122, miR-29a, and miR-101a were observed in the livers of female B6C3F1 mice (Supplementary Fig. 2).
3.4. Analysis of OEP hepatotoxicity at a 13-week time-point
3.4.1. Body weight dynamics
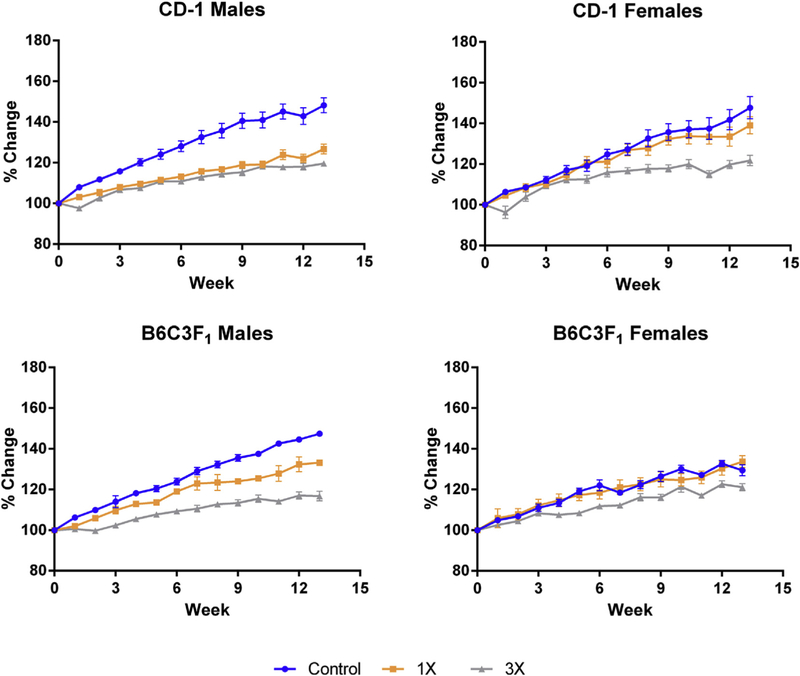
Despite continuous dietary administration of OEP-NF, a purported weight-loss supplement, all animals in all treatment groups gained weight over the course of 13 weeks. The rate of weight gain was slowest in the CD-1 males on both the 1X and 3X diets with total body weight increases of 21% and 16%, respectively. For female mice on the 1X diet, the rate of weight gain was no different than that for controls, whereas female mice on 3X dose gained weight a slower rate than controls or females on the 1X dose (Fig. 3).
Fig. 3.
Body weight dynamics in mice fed OEP for 13 weeks. Data presented as a percent-change from control values at Week 0.
3.4.2. Liver/body weight ratio and histopathological evaluation
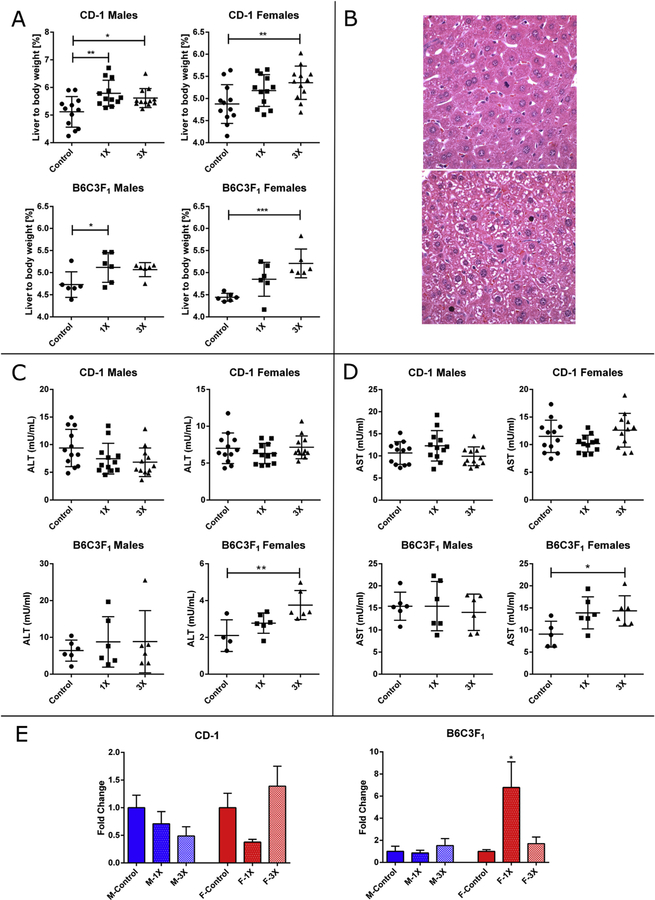
Administration of OEP-NF for 13 weeks resulted in significant (p < 0.05) increases in liver/body weight ratios for CD-1 male mice on both 1X and 3X doses, whereas female CD-1 and B6C3F1 mice exhibited significant increases only on the high dose (Fig. 4A). There was a trend toward an increase in the liver/body weight ratio in B6C3F1 male mice, however it lacked significance. The only notable histopathological findings was the appearance of multiple mitotic foci primarily in mice fed OEP-NF (Fig. 4B).
Fig. 4.
Effects of feeding mice with OEP-NF for 13 weeks. A) Liver to body weight ratio; B) Mitotic figures in the livers of mice (ID# 259 and 332) fed OEP-NF (magnification 40×); C) Levels of ALT, and D) Levels of AST in the serum of mice; E) Levels of miR-122 in the serum of mice, data expressed as fold-change from respective control values. Mean ± SD. Asterisks “*” denotes significant (p < 0.05), “**” (p < 0.01), and “***” (p < 0.001) difference from control.
3.4.3. Analysis of clinical biochemistry
Statistically significant increases in both aminotransferases (p < 0.01, ALT; p < 0.05, AST) were noted in the serum of female B6C3F1 mice (Fig. 4C and D). No significant changes in ALT or AST were observed in the serum of other animals. Statistically significant increases in serum miR-122 levels were observed in B6C3F1 female mice fed low-dose OEP-NF (6.2-fold, p < 0.05). Increases in miR-122 were also observed in the serum of CD-1 female mice, as well as B6C3F1 male and female mice fed high-dose OEP, but these trends did not reach statistical significance. (Fig. 4E).
3.4.4. Hepatotoxicity gene expression array
Administration of OEP-NF for 13 weeks also revealed that 12 genes associated with hepatotoxicity were significantly dysregulated in at least two groups, compared to control mice (Table 1). Interestingly, there were 9 genes that were commonly affected at both 4- and 13-week time-points, including Nqo1 and Lpl.
3.4.5. Hepatotoxicity miRNA expression panel
Analysis of microRNA expression revealed that the expression of various miRNAs was down-regulated at the 3X dose. Statistically significant reductions in miR-21 were noted for CD-1 females; whereas significant reductions were noted in miR-192, mir101a, B6C3F1 mice (Supplementary Fig. 3).
3.5. Identification if individual responders to OEP-NF toxicity
During the data analysis, our attention was attracted to a number of outliers – mice that exhibited responses substantially different from the mean values of their respective groups. Given the idiosyncratic nature of OEP-NF hepatotoxicity in humans, we hypothesized that some mice exhibit individual susceptibility to diet-fed OEP-NF. Therefore, we created datasets that included the records of each individual “outlier” response and linked them to individually tagged mice. Only animals with endpoint values greater than 2 SEM from the control group mean were selected for inclusion into Table 2. In total, 39 animals displayed an “outlier” response for at least 1 endpoint. Among them, 2 animals were from the vehicle-fed mice, 15 received 1X MED, and 22 received 3X MED. Interestingly, a number of mice in the study (e.g., female CD-1 mouse #332: refer to Table 2) exhibited a spectrum of findings that included increased liver/body weight ratio, elevated serum levels of ALT, AST and miR-122, the appearance of hepatocellular mitotic foci, as well as altered gene expression profiles, all of which render these animals as genuine responders to OEP-NF. These findings also speak to the idiosyncratic nature of OEP-NF toxicity, in that select individuals, particularly those among the outbred CD-1 strain, appear more susceptible to the product than others.
Table 2.
List of animals that exhibited outlier responses as a result of feeding mice with OEP for 4 or 13 weeks.
| Animal # | Strain | Sex | Time-point | Diet | L/BW | ALT | AST | miR-122 | Pathology |
|---|---|---|---|---|---|---|---|---|---|
| 200 | CD-1 | M | 4 wks | Vehicle | ↑ | ||||
| 212 | CD-1 | M | 4 wks | 1X | ↑ | ||||
| 223 | CD-1 | M | 4 wks | 1X | ↑ | ||||
| 233 | CD-1 | M | 4 wks | 3X | ↑ | ↑ | |||
| 283 | CD-1 | F | 4 wks | Vehicle | ↑ | ||||
| 285 | CD-1 | F | 4 wks | 1X | ↑ | ||||
| 307 | CD-1 | F | 4 wks | 3X | ↑ | ↑ | |||
| 358 | B6C3F1 | M | 4 wks | 3X | ↑ | ↑ | ↑ | ||
| 359 | B6C3F1 | M | 4 wks | 3X | ↑ | ||||
| 387 | B6C3F1 | F | 4 wks | 1X | ↑ | ||||
| 388 | B6C3F1 | F | 4 wks | 1X | ↑ | ↑ | |||
| 392 | B6C3F1 | F | 4 wks | 3X | ↑ | ↑ | |||
| 393 | B6C3F1 | F | 4 wks | 3X | ↑ | ↑ | |||
| 395 | B6C3F1 | F | 4 wks | 3X | ↑ | ||||
| 248 | CD-1 | M | 13 wks | 1X | ↑ | ↑ | |||
| 258 | CD-1 | M | 13 wks | 1X | ↑ | ↑ | |||
| 259 | CD-1 | M | 13 wks | 1X | ↑ | ↑ | Mild mitotic changes | ||
| 268 | CD-1 | M | 13 wks | 3X | ↑ | ||||
| 332 | CD-1 | F | 13 wks | 3X | ↑ | ↑ | ↑ | ↑ | Mild mitotic changes |
| 336 | CD-1 | F | 13 wks | 3X | ↑ | ↑ | |||
| 338 | CD-1 | F | 13 wks | 3X | ↑ | ↑ | |||
| 340 | CD-1 | F | 13 wks | 3X | ↑ | ↑ | |||
| 341 | CD-1 | F | 13 wks | 3X | ↑ | ↑ | |||
| 371 | B6C3F1 | M | 13 wks | 1X | ↑ | ↑ | ↑ | ↑ | |
| 372 | B6C3F1 | M | 13 wks | 1X | ↑ | ↑ | ↑ | ↑ | |
| 375 | B6C3F1 | M | 13 wks | 3X | ↑ | ↑ | Mild mitotic changes | ||
| 376 | B6C3F1 | M | 13 wks | 3X | ↑ | ↑ | ↑ | ||
| 415 | B6C3F1 | M | 13 wks | 3X | ↑ | ↑ | Mild mitotic changes | ||
| 402 | B6C3F1 | F | 13 wks | 1X | ↑ | ↑ | |||
| 403 | B6C3F1 | F | 13 wks | 1X | ↑ | ↑ | |||
| 404 | B6C3F1 | F | 13 wks | 1X | ↑ | ||||
| 406 | B6C3F1 | F | 13 wks | 1X | ↑ | ↑ | ↑ | ||
| 407 | B6C3F1 | F | 13 wks | 1X | ↑ | ||||
| 408 | B6C3F1 | F | 13 wks | 3X | ↑ | ↑ | ↑ | ||
| 409 | B6C3F1 | F | 13 wks | 3X | ↑ | ||||
| 410 | B6C3F1 | F | 13 wks | 3X | ↑ | ||||
| 411 | B6C3F1 | F | 13 wks | 3X | ↑ | ||||
| 412 | B6C3F1 | F | 13 wks | 3X | ↑ | ↑ | ↑ | ||
| 413 | B6C3F1 | F | 13 wks | 3X | ↑ |
M – male mice, F – female mice; 4 wks – animals that received vehicle or OEP-NF-containing diet for 4 weeks; 13 weeks - animals that received vehicle or OEP-NF-containing diet for 4 weeks; 1X – animals received 1x MED OEP-NF with the chow; 3X - animals received 3x MED OEP-NF with the chow; L/BW – liver to body weight ratio; ALT – alanine aminotransferase, AST – aspartate amino transferase. Only animals with endpoint values greater than 2 SEM from the control group mean were selected for inclusion into the table. Mice highlighted in grey exhibited a “responder” phenotype for 2 or more evaluated parameters.
3.6. Other non-liver-specific effects of OEP-NF
Feeding mice with OEP-NF was also associated with other, non-liver-specific effects. Among the most pronounced was a kidney/body weight ratio increase observed in CD-1 male mice at both 4- and 13-week time points (Supplementary Fig. 4). Other noteworthy observations were associated with affected behavior characterized by food-wasting, “asocial” activity, and unusual barbering patterns.
4. Discussion
It has been reported that about 50% of the adult population in the United States have used at least one HDS in the past month and these numbers continue to grow (Bailey et al., 2011). On the other hand, a significant increase in HDS-induced liver injury, from 7% to nearly 20% of all cases of liver injury has been observed in the last decade (Bailey et al., 2011; de Boer and Sherker, 2017). Of particular concern are dietary supplements marketed as weight-loss aids and performance enhancers, a number of which were suspected in causing liver injury and subsequently removed from the market (Bonkovsky, 2006; Church et al., 2015; Fong et al., 2010; Kanda et al., 2003; Stickel et al., 2009). The most recent suspect in this controversy involved OxyELITE Pro, a dietary supplement whose previous formulation was removed from the market due to serious adverse health effects related to its combination of purportedly natural stimulants and sympathomimetics, including 1,3-dimethylamylamine (DMAA) (U.S. Food and Drug Administration, 2013a,b). As it turned out, DMAA was not of natural origin but rather a drug developed by Eli Lilly more than 70 years ago, which was removed from the market in the 1983 (Zhang et al., 2012). In its new formula, DMAA was replaced by aegeline, a phytochemical extracted from the leaves of the bael tree (Aegle marmelos). However, shortly after OEP-NF was introduced onto the market, a series of liver injuries was linked to its usage, with some cases requiring liver transplantation or resulting in death (Chatham-Stephens et al., 2017; Foley et al., 2014; Heidemann et al., 2016; Johnston et al., 2016; Roytman et al., 2014).
A paucity of scientific data exists regarding the safety of A. marmelos, and while extracts of its various plant parts may not appear hepatotoxic in some rodent models (Khan and Sultana, 2009; Singh et al., 2000; Veerappan et al., 2007), others have demonstrated significant liver injury (Arseculeratne et al., 1985). These equivocal findings for whole plant extracts are not exculpatory for synthetic aegeline, especially when combined with the other synthetic ingredients within OEP-NF. In fact, a recent in vitro screening found that purified aegeline was cytotoxic to HepG2 cells (Mohammed et al., 2016), and that when administered to rats aegeline was rapidly absorbed and concentrated in the liver (Manda et al., 2017).
While A. marmelos extracts have been used as traditional Asian medicines, an absence of historical records documenting its toxicity does not exclude the potential for aegeline to cause liver injury or other negative health effects when consumed in a cocktail of other drugs/phytochemicals not present in A. marmelos. From a historical perspective, pyrrolizidine alkaloids derived from Senecio spp. provide a useful example. The so-called “bush tea” brewed from Senecio was used in Jamaican medicine for centuries, but only in the 20th century was it linked to a commonly-observed hepatic sinusoidal obstruction syndrome and cirrhosis (Bras et al., 1954). When it became evident that the aegeline present in OEP-NF was not part of an A. marmelos extract, but chemically synthesized and imported to the U.S., questions arose regarding the toxicological contribution of potential contaminants either from synthetic intermediates, non-naturally occurring enantiomers, or both (Navarro et al., 2017).
While the pharmacological and toxicological profile of aegeline is virtually unknown, contributions from other components within OEP-NF cannot be dismissed. It is our contention that toxic manifestations of OEP-NF ingestion are attributable to the complete formulation and not a single phytochemical entity. Pharmacodynamic interactions among the various constituents are also likely contributing factors. For example, caffeine, a major constituent of OEP-NF, is recognized for its ability to augment the toxicity of other stimulants and sympathomimetics (Brown et al., 1991; Derlet et al., 1992; Haller et al., 2004; McNamara et al., 2006). Combinations of caffeine and ephedrine alkaloids can produce a host of cardiovascular and central nervous system pathologies that are more remarkable than those observed after administration of each individual component (Brown et al., 2012; Dunnick et al., 2007). Such findings reinforced the FDA’s decision to remove of Ephedra-containing dietary supplements from the U.S. market in 2004 (Anonymous, 2004). Today, many Ephedra-free formulations, like OEP-NF, which combine various caffeine sources with other natural sympathomimetics, are also plagued by adverse health effects, especially when taken in conjunction with vigorous exercise (Gurley et al., 2015). In the case of OEP-NF, caffeine is known to intensify the hemodynamic response to the α2-antagonist yohimbine (Waluga et al., 1998), thus its augmentation of the sympathomimetic activities of higenamine and coclaurine is not unexpected (Lee et al., 2013). Supplement formulas containing caffeine and yohimbine are particularly concerning since Yohimbe-containing products have been associated with a significantly greater proportion of severe outcomes reported to the California Poison Control System (Kearney et al., 2010). Moreover, caffeine and yohimbine were also present in Hydroxycut, another weight loss product removed from the market in 2009 following reports of liver injury (Avigan et al., 2016; Fong et al., 2010). Finally, caffeine has been shown to exacerbate acute inflammatory liver injury (Ohta et al., 2007) while yohimbine’s α2-antagonism aggravates lipopolysaccharide-induced liver injury (Chen et al., 2015).
Pharmacokinetic interactions among OEP-NF ingredients must also be taken into consideration. From that perspective, it is worth noting that aegeline, higenamine, yohimbine, and coclaurine are all CYP3A4 substrates and yohimbine, higenamine, and coclaurine are also CYP2D6 substrates, with the latter two also being CYP2D6 inhibitors (Le Corre et al., 2004; Liu and Santillo, 2016; Manda et al., 2016). Moreover, CYP2D6 is highly polymorphic and individuals exhibiting a CYP2D6 “poor metabolizer” phenotype may be more susceptible to the effects of OEP-NF (Ingelman-Sundberg, 2005). Given that almost 50% of Asians exhibit a CYP2D6 poor metabolizer phenotype (Ingelman-Sundberg, 2005), it is interesting to note that most of those with severe liver injuries linked to OEP-NF use in Hawaii were of Asian descent (Johnston et al., 2016; Roytman et al., 2014). This observation also underscores the impact individual pharmacogenetic differences can have on dietary supplement safety and efficacy.
In this study, we investigated the hepatotoxic potential of OEP-NF in acute and sub-chronic protocols using both inbred and outbred mouse strains. Gavaging mice with 10X MED resulted in overt toxicity and 100% mortality within an hour, an effect most likely due to the high doses of caffeine and other OEP-NF ingredients. While the death of some of mice gavaged with 3X MED may have also been related to the combined cardiovascular effects of OEP-NF components, a hepatotoxic element cannot be ruled out, since an increase in liver/body weight ratio was observed in these mice. There were also significant increases in serum aminotransferases even at 1.5X MED, which contained individual quantities of caffeine, yohimbine, and higenamine well below the reported LD50 values.
Perhaps of greater concern than the mortality associated with the 3X and 10X MED is the narrow safety margin implied by these findings. For a dietary supplement to produce adverse health effects at 10X the maximum daily label recommended dose, while concerning, is not unexpected; however, serious toxicity at doses just 3X greater than the maximum daily label recommended dose gives cause for great concern. That the OEP-NF warning language takes up almost half of the product label is a good indicator of the potential problems to be expected. Partial statements such as “Under no circumstances should initial serving size be exceeded or the warning on this bottle ignored; ” “Do not exceed 3 capsules in any 24 h period; ” or “Consult with your physician before using this product, especially if you are using any prescription or over the counter medication or if you have any pre-existing medical condition including but not limited to high or low blood pressure, cardiac arrhythmia, stroke, heart, liver, kidney, or thyroid disease, seizure disorder, psychiatric disease, diabetes, difficulty urinating due to prostate enlargement or if you are taking a MOAI (monoamine oxidase inhibitor) or any other medication,” are tacit admissions to OEP-NF’s questionable safety.
In the feeding phase of the experiment, no marked histomorphological or biochemical changes were observed after 4 weeks. However, at the 13-week time-point, hepatotoxic effects of OEP-NF were evidenced by increases in liver/body weight ratios and in serum levels of ALT, AST and miR-122 of female B6C3F1 mice. The magnitude of those changes, while not as dramatic as those observed following oral gavage, are indicative of mild liver injury after 13 weeks of OEP-NF exposure in the diet.
To further explore the molecular mechanisms underlying the OEP-NF-induced feeding effects, we performed an expression analysis of select genes and miRNAs commonly associated with hepatotoxicity. A number of critical genes were affected in response to OEP-NF ingestion both at 3X and 1X MED. Most notably, gene expression analysis revealed that OEP-NF ingestion similarly affected a number of genes that are also altered by potent liver toxicants like acetaminophen (acetyl-para-aminophenol; APAP), bromobenzene, carbon tetrachloride, dimethyl nitrosamine and thioacetamide (Minami et al., 2005). These genes included aldolase A, fructosebisphosphate (Aldoa); thioredoxin reductase 1 (Txnrd1); stearoyl-CoA desaturase-1 (Scd1); and cyclin G1 (Ccng1), just to name a few.
One of the genes most strongly affected by OEP-NF was Cd36. Expression of Cd36 was increased 2-to-4-fold in the livers of CD-1 and B6C3F1 mice at both 4 and 13-week time-points. Cd36 is an adhesion molecule involved in hemostasis, thrombosis, inflammation, atherogenesis and lipid metabolism (Daviet and McGregor, 1997; Schneiderhan et al., 2001). As a recognized transcriptional target of Ahr, Cd36 is required for Ahr-induced hepatosteatosis (Lee et al., 2010). Up-regulation of Cd36 leads to increased fatty acid uptake from the bloodstream into the liver (Lee et al., 2010; Schneiderhan et al., 2001). This gene was previously reported to be up-regulated after 4 weeks of exposure to the hepatotoxic triazole fungicides - cyproconazole, epoxiconazole and prochloraz (Heise et al., 2015). Interestingly, the lipogenesis-associated gene Cd36 has also been shown to play an important role in promoting caffeine’s ability to reduce hepatic lipid accumulation (Zheng et al., 2015). Yet, in the study by Zheng et al., the hepatoprotective effect of caffeine was mediated by down-regulation of Cd36, while caffeine-containing OEP-NF caused substantial up-regulation of Cd36. This paradoxical finding highlights another important message regarding multi-ingredient HDS: in the context of a complex mixture, phytochemicals may exhibit effects contradictory to those observed when administered alone. Interestingly, other plant alkaloids appear to induce liver steatosis via Cd36-mediated mechanisms. For instance, Choi and colleagues reported that berberine, an isoquinoline alkaloid with anti-microbial and anti-diarrheal activities, caused hepatic lipid accumulation by activating Cd36 in the mouse model (Choi et al., 2017).
Another gene that was significantly up-regulated in response to OEP-NF in all mice independently of the strain or sex was the nicotinamide adenine dinucleotide phosphate: quinone oxidoreductase-1 (Nqo1). This gene, usually poorly expressed in normal liver, is a potent scavenger of the superoxide anion, and serves as part of the antioxidant defense system; it also plays an important role in metabolism (Palming et al., 2007; Siegel and Ross, 2000; Strassburg et al., 2002). NQO1 was shown to be induced in cases of APAP overdose, as well as in primary biliary cirrhosis (Aleksunes et al., 2006). Palming and colleagues also report that elevated levels of NQO1 correlate with several clinical markers of liver dysfunction (Palming et al., 2007). In the same study, the authors also noted that NQO1 was reduced during diet-induced weight loss e a result opposite to that observed with OEP-NF. In a more relevant comparison, it was shown that usnic acid – a component in several previously banned weight-loss HDS linked to hepatotoxicity – caused up-regulation of NQO1 in human HepG2 cells (Chen et al., 2017). Similarly, another known hepatotoxicant, Mequindox, caused up-regulation of NQO1 in parallel with a modest increase in ALT and AST, an effect similar to that observed in our study (Liu et al., 2017). It is generally accepted that up-regulation of this cytoprotective enzyme is an adaptive stress response to limit further disease progression (Aleksunes et al., 2006).
Altogether, around two dozen genes were significantly dysregulated (at least 1.5-fold from control) in two or more treatment groups. Importantly, those genes whose expression was dysregulated are associated with multiple cellular or biochemical pathways, including, but not limited to: xenobiotic metabolism, cholestasis, oxidative stress response, fatty acid metabolism, hepatocarcinogenesis and necrosis. These effects were observed at the 4- and 13-week time-points, in both male and female CD-1 and B6C3F1 strains; however, the most substantial effects were observed in B6C3F1 female mice, congruent with other observed adverse effects, discussed above.
Feeding mice with OEP-NF also affected the expression of a number of miRNAs that have been associated with other liver toxicants. There was a clear dose-dependent response, in that feeding mice a 3X MED of OEP-NF caused substantial reductions in the expression of different miRNAs, where the most robust and significant effects were observed for miR-101a, miR-192, miR-193a and miR-125b. Taken together, the observed changes in gene and miRNA expression suggest that OEP-NF induces substantial molecular changes within the livers of experimental mice.
It can be argued that up-regulation of various oxidative stress genes can be advantageous by inducing the expression of redox enzymes within the liver. Many phytochemicals can indeed increase oxidative enzyme expression through their ability to activate the Keap1/Nrf2 pathway (Qin and Hou, 2016; Stefanson and Bakovic, 2014). Once activated, the transcription factor Nrf2 binds to the antioxidant response element in the hepatocyte nucleus to promote expression of a plethora of antioxidant enzymes. In fact, Nrf2 activators have been investigated as potential therapeutic agents, although the development of some has been stopped due to liver injury concerns (Zoja et al., 2013). Despite their purported benefits, excessive exposure or prolonged use of natural Nrf2 activators may become pro-oxidant or give rise to a state of reductive stress, both of which can be detrimental to the hepatocyte (Brewer et al., 2013; Dodson et al., 2015). Only recently has this redox paradox garnered attention as a pathological consequence of Nrf2 activation, and may explain certain types of herbal hepatotoxicity (Lei et al., 2016). For example, oleanolic acid is a potent phytochemical Nrf2 activator that acts as a hepatoprotectant at low concentrations but becomes hepatotoxic at higher doses (Liu et al., 2013). Other phytochemicals or botanicals that display hormesis with regard to antioxidant properties at low doses and pro-oxidant effects at higher doses include quercetin (Vargas and Burd, 2010), rhubarb (Wang et al., 2011), epigallocatechin gallate (EGCG) (Calabrese et al., 2012) and various other polyphenolics (Skibola and Smith, 2000). Because the phytochemical content of HDS is often much greater than that found in the plant itself, pro-oxidant or reductive stress effects may be a more common problem with these products than heretofore anticipated.
This comprehensive and integrative pre-clinical assessment reports two major outcomes of administration of OEP-NF in the mouse model. First, the dietary supplement OEP-NF is clearly liver-reactive, exhibiting a number of molecular and biochemical responses e from a modest increase in liver/body weight ratios and elevations in serum aminotransferase levels to marked dysregulation of genes involved in numerous detoxification and pathological pathways. These effects were cumulative and were most strongly pronounced after 13 weeks of ingestion. These findings suggest that administration of OEP-NF for periods longer than 13 weeks increase the risk for progressive liver injury.
The second noteworthy observation is identification of individual mice that exhibited a spectrum of affected endpoints despite a lack of statistical significance in the group mean for those parameters. This finding speaks towards the potential idiosyncratic nature of OEP-NF-induced liver toxicity observed in humans.
Finally, it must be emphasized that while individual compounds of a given HDS formulation may be safe/non-toxic, atypical combinations of ingredients, especially those with little to no data regarding their pharmacology, toxicology, or drug interaction potential (i.e., aegeline), may produce serious adverse health effects. The potent mixture of plant alkaloids present in the OEP-NF formulation gave rise to a spectrum of adverse cardiovascular, neurological, and hepatotoxic effects in inbred and outbred mouse strains. While these findings were not unexpected at higher gavage doses, those emanating from allometrically scaled doses mimicking a 1–3X human equivalent are disconcerting and indicate that this and similar products pose significant health risks. These risks are likely amplified in individuals whose pharmacogenetic profile may lead to altered pharmacokinetics and pharmacodynamics of the phytochemical cocktail. Our findings underscore the need for dietary supplement companies to conduct integrative pre-clinical toxicological studies of multi-ingredient HDS, especially those marketed for weight-loss and/or exercise performance enhancement.
Supplementary Material
Acknowledgements and disclaimer
The authors are thankful to Dr. Christy Simecka, Robin Mulkey, and Bridgette Engie for excellent animal care at the UAMS Animal Facility. The authors would like to thank Dr. Saqlain Haider (NCNPR) and Dr. Amar Chittiboyina (NCNPR) for synthesis of aegeline and higenamine. The authors are also thankful to Dr. Ramesh Khanal of Envigo for his invaluable help and expertise in diet preparation, to Julia Tobacyk for technical assistance, and to Christopher Fettes for editorial assistance. B.J.G and I.K. (UAMS), and M.A.E. (PSI) are serving as expert witnesses for the United States Department of Justice.
Funding
This work was supported by the United States Department of Justice [contract # 6L-CIV02–0850].
Abbreviations:
- ALT
alanine aminotransferase
- APAP
acetaminophen
- AST
aspartate aminotransferase
- DILI
drug-induced liver injury
- DILIN
Drug-Induced Liver Injury Network
- DMAA
1,3-dimethylamylamine
- DMSO
Dimethyl sulfoxide
- HDS
herbal and dietary supplements
- HILI
Herbal-induced liver injury
- MED
mouse equivalent dose
- miRNA
microRNA
- OEP-NF
OxyELITE Pro New Formula
- qRT-PCR
quantitative Real-Time Polymerase Chain Reaction
- TLDA
TaqMan Low Density Arrays
- UHPLC
ultra-high performance liquid chromatography
Footnotes
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.fct.2017.08.025.
Transparency document
Transparency document related to this article can be found online at http://dx.doi.org/10.1016/j.fct.2017.08.025.
References
- Albonico SM, Kuck AM, Deulofeu V, 1967. Tembamide from Fagara hiemalis. J. Chem. Soc C 14, 1327–1328. [Google Scholar]
- Aleksunes LM, Goedken M, Manautou JE, 2006. Up-regulation of NAD(P)H quinone oxidoreductase 1 during human liver injury. World J. Gastroenterol 12, 1937–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anonymous, 2004. Final rule declaring dietary supplements containing ephedrine alkaloids adulterated because they present an unreasonable risk. Fed. Reg 69, 6787–6854. [PubMed] [Google Scholar]
- Arseculeratne SN, Gunatilaka AL, Panabokke RG, 1985. Studies on medicinal plants of Sri Lanka. Part 14: toxicity of some traditional medicinal herbs. J. Ethnopharmacol 13, 323–335. [DOI] [PubMed] [Google Scholar]
- Avigan MI, Mozersky RP, Seeff LB, 2016. Scientific and Regulatory Perspectives in Herbal and dietary supplement associated hepatotoxicity in the United States. Int. J. Mol. Sci 17, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey RL, Gahche JJ, Lentino CV, Dwyer JT, Engel JS, Thomas PR, Betz JM, Sempos CT, Picciano MF, 2011. Dietary supplement use in the United States, 2003–2006. J. Nutr 141, 261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomer R, Schriefer J, Gunnels T, 2015. Clinical safety assessment of oral higenamine supplementation in healthy, young men. Hum. Exp. Toxicol 34, 935–945. [DOI] [PubMed] [Google Scholar]
- Bonkovsky HL, 2006. Hepatotoxicity associated with supplements containing Chinese green tea (Camellia sinensis). Ann. Intern. Med 144, 68–71. [DOI] [PubMed] [Google Scholar]
- Bras G, Jelliffe DB, Stuart KL, 1954. Veno-occlusive disease of liver with nonportal type of cirrhosis, occurring in Jamaica. Ama Arch. Pathol 57, 285–300. [PubMed] [Google Scholar]
- Brewer AC, Mustafi SB, Murray TVA, Rajasekaran NS, Benjamin IJ, 2013. Reductive stress linked to small HSPs, G6PD, and Nrf2 pathways in heart disease. Antioxid. Redox Signal 18, 1114–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown NJ, Ryder D, Branch RA, 1991. A pharmacodynamic inter- action between caffeine and phenylpropanolamine. Clin. Pharmacol. Ther 50, 363–371. [DOI] [PubMed] [Google Scholar]
- Brown CE, Trauth SE, Grippo RS, Gurley BJ, Grippo AA, 2012. Combined effects of ephedrine- containing dietary supplements, caf- feine, and nicotine on morphology and ultrastructure of rat hearts. J. Caffeine Res 2, 123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese V, Cornelius C, Dinkova-Kostova AT, Iavicoli I, Di Paola R, Koverech A, Cuzzocrea S, Rizzarelli E, Calabrese EJ, 2012. Cellular stress responses, hormetic phyto- chemicals and vitagenes in aging and longevity. Biochim. Biophys. Acta 1822, 753–783. [DOI] [PubMed] [Google Scholar]
- Chatham-Stephens K, Taylor E, Chang A, Peterson A, Daniel J, Martin C, Deuster P, Noe R, Kieszak S, Schier J, Klontz K, Lewis L, 2017. Hepatotoxicity associated with weight loss or sports dietary supplements, including OxyELITE Pro (TM) - United States, 2013. Drug Test. Anal 9, 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JH, Yu GF, Jin SY, Zhang WH, Lei DX, Zhou SL, Song XR, 2015. Activation of alpha 2 adrenoceptor attenuates lipopolysaccharide-induced hepatic injury. Int. J. Clin. Exp. Pathol 8, 10752–10759. [PMC free article] [PubMed] [Google Scholar]
- Chen S, Zhang Z, Qing T, Ren Z, Yu D, Couch L, Ning B, Mei N, Shi L, Tolleson WH, 2017. Activation of the Nrf2 signaling pathway in usnic acid-induced toxicity in HepG2 cells. Arch. Toxicol 91, 1293–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YJ, Lee KY, Jung SH, Kim HS, Shim GY, Kim MG, Oh YK, Oh SH, Jun DW, Lee BH, 2017. Activation of AMPK by berberine induces hepatic lipid accumulation by upregulation of fatty acid translocase CD36 in mice (vol 316, pg 74, 2017). Toxicol. Appl. Pharm 320, 73. [DOI] [PubMed] [Google Scholar]
- Church RJ, Gatti DM, Urban TJ, Long N, Yang X, Shi Q, Eaddy JS, Mosedale M, Ballard S, Churchill GA, Navarro V, Watkins PB, Threadgill DW, Harrill AH, 2015. Sensitivity to hepatotoxicity due to epigallocatechin gallate is affected by genetic background in diversity outbred mice. Food Chem. Toxicol 76, 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daviet L, McGregor JL, 1997. Vascular biology of CD36: roles of this new adhesion molecule family in different disease states. Thromb. Haemost 78, 65–69. [PubMed] [Google Scholar]
- de Boer YS, Sherker AH, 2017. Herbal and dietary supplement-induced liver injury. Clin. Liver Dis 21, 135.-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derlet RW, Tseng JC, Albertson TE, 1992. Potentiation of cocaine and d-amphetamine toxicity with caffeine. Am. J. Emerg. Med 10, 211–216. [DOI] [PubMed] [Google Scholar]
- Dodson M, Redmann M, Rajasekaran NS, Darley-Usmar V, Zhang J, 2015. KEAP1-NRF2 signalling and autophagy in protection against oxidative and reductive proteotoxicity. Biochem. J 469, 347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnick JK, Kissling G, Gerken DK, Vallant MA, Nyska A, 2007. Cardiotoxicity of Ma Huang/caffeine or ephedrine/caffeine in a rodent model system. Toxicol. Path 35, 657–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley S, Butlin E, Shields W, Lacey B, 2014. Experience with OxyELITE pro and acute liver injury in active duty service members. Dig. Dis. Sci 59, 3117–3121. [DOI] [PubMed] [Google Scholar]
- Fong TL, Klontz KC, Canas-Coto A, Casper SJ, Durazo FA, Davern TJ, Hayashi P, Lee WM, Seeff LB, 2010. Hepatotoxicity due to Hydroxycut: a case series. Am. J. Gastroenterol 105, 1561–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurley BJ, 2012. Pharmacokinetic herb-drug interactions (Part 1): origins, mechanisms, and the impact of botanical dietary supplements. Planta Med. 78, 1478–1489. [DOI] [PubMed] [Google Scholar]
- Gurley BJ, Steelman SC, Thomas SL, 2015. Multi-ingredient, caffeine-containing dietary supplements: history, safety, and efficacy. Clin. Ther 37, 275–301. [DOI] [PubMed] [Google Scholar]
- Gurley BJ, Markowitz JS, Williams DK, Barone GW, 2017. Practical considerations when designing and conducting clinical pharmacokinetic herb–drug interaction studies. Int. J. Pharmacokinet 2, 57–69. [Google Scholar]
- Haller CA, Peyton J, Benowitz NL, 2004. Enhanced stimulant and metabolic effects of combined ephedrine and caffeine. Clin. Pharmacol. Ther 75, 259–273. [DOI] [PubMed] [Google Scholar]
- Heidemann LA, Navarro VJ, Ahmad J, Hayashi PH, Stolz A, Kleiner DE, Fontana RJ, 2016. Severe acute hepatocellular injury attributed to OxyELITE Pro: a case series. Dig. Dis. Sci 61, 2741–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heise T, Schmidt F, Knebel C, Rieke S, Haider W, Pfeil R, Kneuer C, Niemann L, Marx-Stoelting P, 2015. Hepatotoxic effects of (tri)azole fungicides in a broad dose range. Arch. Toxicol 89, 2105–2117. [DOI] [PubMed] [Google Scholar]
- Ingelman-Sundberg M, 2005. Genetic polymorphism of cytochrome P450 2D6 (CYP2D6): clinical consequences, evolutionary aspects and functional diversity. Pharmacogenomics J. 5, 6–13. [DOI] [PubMed] [Google Scholar]
- Johnston DI, Chang A, Viray M, Chatham-Stephens K, He H, Taylor E, Wong LL, Schier J, Martin C, Fabricant D, Salter M, Lewis L, Park SY, 2016. Hepatotoxicity associated with the dietary supplement OxyELITE Pro (TM) Hawaii, 2013. Drug Test. Anal 8, 319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal A, Shaik AA, Sandbhor M, Malik MS, 2004. Chemoenzymatic synthesis of (R)- and (S)-tembamide, aegeline and denopamine by a one-pot lipase resolution protocol. Tetrahedron Asymmetry 15, 3939–3944. [Google Scholar]
- Kanda T, Yokosuka O, Okada O, Suzuki Y, Saisho H, 2003. Severe hepatotoxicity associated with Chinese diet product ‘Onshidou-Genbi-Kounou’. J. Gastroenterol. Hepatol 18, 354–355. [DOI] [PubMed] [Google Scholar]
- Kearney T, Tu N, Haller C, 2010. Adverse drug events associated with yohimbine-containing products: a retrospective review of the California Poison Control System reported cases. Ann. Pharmacother 44, 1022–1029. [DOI] [PubMed] [Google Scholar]
- Khan TH, Sultana S, 2009. Antioxidant and hepatoprotective potential of Aegle marmelos Correa. against CCl4-induced oxidative stress and early tumor events. J. Enzym. Inhib. Med. Ch 24, 320–327. [DOI] [PubMed] [Google Scholar]
- Koturbash I, Scherhag A, Sorrentino J, Sexton K, Bodnar W, Swenberg JA, Beland FA, de Villena FPM, Rusyn I, Pogribny IP, 2011. Epigenetic mechanisms of mouse interstrain variability in genotoxicity of the environmental toxicant 1,3-butadiene. Toxicol. Sci 122, 448–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koturbash I, Beland FA, Pogribny IP, 2012. Role of microRNAs in the regulation of drug metabolizing genes and the response to environmental toxicants. Expert Opin. Drug Metab. Toxicol 8, 597–606. [DOI] [PubMed] [Google Scholar]
- Koturbash I, Tolleson W, Guo L, Yu D, Chen S, Hong H, Mattes W, Ning B, 2015. MicroRNAs as pharmacogenomic biomarkers for drug efficacy and drug safety assessment. Biomark. Med 9, 1153–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Corre P, Parmer RJ, Kailasam MT, Kennedy BP, Skaar TP, Ho H, Leverge R, Smith DW, Ziegler MG, Insel PA, Schork NJ, Flockhart DA, O’Connor DT, 2004. Human sympathetic activation by α2-adrenergic blockade with yohimbine: bimodal, epistatic influence of cytochrome P450-mediated drug metabolism. Clin. Pharmacol. Ther 76, 139–153. [DOI] [PubMed] [Google Scholar]
- Lee JH, Wada T, Febbraio M, He JH, Matsubara T, Lee MJ, Gonzalez FJ, Xie W, 2010. A novel role for the dioxin receptor in fatty acid metabolism and hepatic steatosis. Gastroenterology 139, 653–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SR, Schriefer JM, Gunnels TA, Harvey IC, Bloomer RJ, 2013. Acute oral intake of a higenamine-based dietary supplement increases circulating free fatty acids and energy expenditure in human subjects. Lipids Health Dis. 12, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei XG, Zhu J-H, Cheng W-H, Bao Y, Ho Y-S, Reddi AR, Holmgren A, Arnér ESJ, 2016. Paradoxical roles of antioxidant enzymes: basic mechanisms and health implications. Physiol. Rev 96, 307–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Santillo MF, 2016. CYtochrome P450 2D6 and 3A4 enzyme inhibition by amine stimulants in dietary supplements. Drug Test. Anal 8, 307–310. [DOI] [PubMed] [Google Scholar]
- Liu J, Lu Y-F, Zhang Y, Wu KC, Fan F, Klaassen CD, 2013. Oleanolic acid alters bile acid metabolism and produces cholestatic liver injury in mice. Toxicol. Appl. Pharmacol 272, 816–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QY, Lei ZX, Huang AX, Wu QH, Xie SY, Awais I, Dai MH, Wang X, Yuan ZH, 2017. Toxic metabolites, MAPK and Nrf2/Keap1 signaling pathways involved in oxidative toxicity in mice liver after chronic exposure to Mequindox. Sci. Rep-Uk 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo CF, Chen CM, 1997. Acute toxicity of higenamine in mice. Planta Med. 63, 95–96. [DOI] [PubMed] [Google Scholar]
- Manda VK, Avula B, Chittiboyina AG, Khan IA, Walker LA, Khan SI, 2016. Inhibition of CYP3A4 and CYP1A2 by Aegle marmelos and its constituents. Xenobiotica 46, 117–125. [DOI] [PubMed] [Google Scholar]
- Manda V, Avula B, Mir TM, Ashfaq MK, Khan IA, Khan SI, 2017. Pharmacokinetics of aegeline after oral administration in a mouse model In: Poster Presentation, International Conference on the Science of Botanicals; Oxford, MS, April 4–7, 2017 http://www.oxfordicsb.org/images/PDF/17th/2017_ICSB_Poster_Abstracts.pdf. [Google Scholar]
- McNamara R, Aoife K, O’Neill B, Harkin A, 2006. Caffeine promotes hyperthermia and serotonergic loss following co-administration of the substituted amphetamines, MDMA (“Ecstasy”) and MDA (“Love”). Neuropharmacology 50, 69–80. [DOI] [PubMed] [Google Scholar]
- Minami K, Saito T, Narahara M, Tomita H, Kato H, Sugiyama H, Katoh M, Nakajima M, Yokoi T, 2005. Relationship between hepatic gene expression profiles and hepatotoxicity in five typical hepatotoxicant-administered rats. Toxicol. Sci 87, 296–305. [DOI] [PubMed] [Google Scholar]
- Miousse IR, Murphy LA, Lin H, Schisler MR, Sun J, Chalbot MC, Sura R, Johnson K, LeBaron MJ, Kavouras IG, Schnackenberg LK, Beger RD, Rasoulpour RJ, Koturbash I, 2017. Dose-response analysis of epigenetic, metabolic, and apical endpoints after short-term exposure to experimental hepatotoxicants. Food Chem. Toxicol 17, 30241–30247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed MMD, Ibrahim NA, El-Sakhawy FS, Mohamed KM, Deabes DAH, 2016. Two new cytotoxic furoquinoline alkaloids isolated from Aegle marmelos (Linn.) Correa. Nat. Prod. Res 30, 2559–2566. [DOI] [PubMed] [Google Scholar]
- Nair AB, Jacob S, 2016. A simple practice guide for dose conversion between animals and humans. J. Basic. Clin. Pharma 7, 27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro VJ, Barnhart H, Bonkovsky HL, Davern T, Fontana RJ, Grant L, Reddy KR, Seeff LB, Serrano J, Sherker AH, Stolz A, Talwalkar J, Vega M, Vuppalanchi R, 2014. Liver injury from herbals and dietary supplements in the US drug-induced liver injury Network. Hepatology 60, 1399–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro VJ, Khan I, Bjornsson E, Seeff LB, Serrano J, Hoofnagle JH, 2017. Liver Injury from herbal and dietary supplements. Hepatology 65, 363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta A, Lukashev D, Jackson EK, Fredholm BB, Sitkovsky M, 2007. 1,3,7-trimethylxanthine (caffeine) may exacerbate acute inflammatory liver injury by weakening the physiological immunosuppressive mechanism. J. Immunol 179, 7431–7438. [DOI] [PubMed] [Google Scholar]
- Palming J, Sjoholm K, Jernas M, Lystig TC, Gummesson A, Romeo S, Lonn L, Carlsson B, Carlsson LMS, 2007. The expression of NAD(P)H : quinone oxidoreductase 1 is high in human adipose tissue, reduced by weight loss, and correlates with adiposity, insulin sensitivity, and markers of liver dysfunction. J. Clin. Endocr. Metab 92, 2346–2352. [DOI] [PubMed] [Google Scholar]
- Patra A, Mitra AK, Ghosh A, Mukhopadhyay PK, 1981. Carbon-13 NMR spectra of tembamide, aegeline, and related amides. Org. Magn. Reson 16, 65–67. [Google Scholar]
- Pyo MK, Lee D-H, Kim D-H, Lee J-H, Moon J-C, Chang KC, Yun-Choi HS, 2008. Enantioselective synthesis of (R)-(+)- and (S)-(−)-higenamine and their analogs with effects on platelet aggregation and experimental animal model of disseminated intravascular coagulation. Bioorg. Med. Chem. Lett 18, 4110–4114. [DOI] [PubMed] [Google Scholar]
- Qin S, Hou D-X, 2016. Multiple regulations of Keap1/Nrf2 system by dietary phytochemicals. Mol. Nutr. Food Res 60, 1731–1755. [DOI] [PubMed] [Google Scholar]
- Roytman MM, Porzgen P, Lee CL, Huddleston L, Kuo TT, Bryant-Greenwood P, Wong LL, Tsai N, 2014. Outbreak of severe hepatitis linked to weight-loss supplement OxyELITE Pro. Am. J. Gastroenterol 109, 1296–1298. [DOI] [PubMed] [Google Scholar]
- Schilter B, Andersson C, Anton R, Constable A, Kleiner J, O’Brien J, Renwick AG, Korver O, Smit F, Walker R, 2003. Guidance for the safety assessment of botanicals and botanical preparations for use in food and food supplements. Food Chem. Toxicol 41, 1625–1649. [DOI] [PubMed] [Google Scholar]
- Schneiderhan W, Schmid-Kotsas A, Zhao JS, Grunert A, Nussler A, Weidenbach H, Menke A, Schmid RM, Adler G, Bachem MG, 2001. Oxidized low-density lipoproteins bind to the scavenger receptor, CD36, of hepatic stellate cells and stimulate extracellular matrix synthesis. Hepatology 34, 729–737. [DOI] [PubMed] [Google Scholar]
- Siegel D, Ross D, 2000. Immunodetection of NAD (P) H: quinone oxidoreductase 1 (NQO1) in human tissues. Free Radic. Biol. Med 29, 246–253. [DOI] [PubMed] [Google Scholar]
- Singh RP, Banerjee S, Rao AR, 2000. Effect of Aegle marmelos on biotransformation enzyme systems and protection against free-radical-mediated damage in mice. J. Pharm. Pharmacol 52, 991–1000. [DOI] [PubMed] [Google Scholar]
- Skibola CF, Smith MT, 2000. Potential health impacts of excessive flavonoid intake. Free Radic. Biol. Med 29, 375–383. [DOI] [PubMed] [Google Scholar]
- Somanathan R, Aguilar HR, Ventura GR, Smith KM, 1983. Syntheses of natural hydroxyamides using trimethylsilyl cyanide. Synth. Commun 13, 273–280. [Google Scholar]
- Starkey Lewis PJ, Merz M, Couttet P, Grenet O, Dear J, Antoine DJ, Goldring C, Park BK, Moggs JG, 2012. Serum microRNA biomarkers for drug-induced liver injury. Clin. Pharmacol. Ther 92, 291–293. [DOI] [PubMed] [Google Scholar]
- Stefanson AL, Bakovic M, 2014. Dietary regulation of Keap1/Nrf2/ARE pathway: focus on plant-derived compounds and trace minerals. Nutrients 6, 3777–3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickel F, Droz S, Patsenker E, Bogli-Stuber K, Aebi B, Leib SL, 2009. Severe hepatotoxicity following ingestion of Herbalife (R) nutritional supplements contaminated with Bacillus subtilis. J. Hepatol 50, 111–117. [DOI] [PubMed] [Google Scholar]
- Strassburg A, Strassburg CP, Manns MP, Tukey RH, 2002. Differential gene expression of NAD(P)H : quinone oxidoreductase and NRH: quinone oxidoreductase in human hepatocellular and biliary tissue. Mol. Pharmacol 61, 320–325. [DOI] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration, Nov. 18, 2013a. OxyElite Pro Supplements Recalled. https://www.fda.gov/forconsumers/consumerupdates/ucm374742.htm. (Accessed 25 May 2017).
- U.S. Food and Drug Administration, 2005. Guidance for Industry: Estimating the Maximum Safe Starting Dose in Initial Clinicla Trials for Therapeutics in Adult Healthy Volunteers. http://www.fda.gov/cder/guidance/index.htm. (Accessed 20 September 2016).
- U.S. Food and Drug Administration, July 16, 2013b. DMAA in Dietary Supplements. Food and Drug Administration. https://www.fda.gov/food/dietarysupplements/productsingredients/ucm346576.htm. (Accessed 7 October 2017).
- Vargas AJ, Burd R, 2010. Hormesis and synergy: pathways and mechanisms of quercetin in cancer prevention and management. Nutr. Rev 68, 418–428. [DOI] [PubMed] [Google Scholar]
- Veerappan A, Miyazaki S, Kadarkaraisamy M, Ranganathan D, 2007. Acute and subacute toxicity studies of Aegle mamrelos Corr., an Indian medicinal plant. Phytomedicine 14, 209–215. [DOI] [PubMed] [Google Scholar]
- Waluga M, Janusz M, Karpel E, Hartleb M, Nowak A, 1998. Cardiovascular effects of ephedrine, caffeine and yohimbine measured by thoracic electrical impedance in obese women. Clin. Physiol 18, 69–76. [DOI] [PubMed] [Google Scholar]
- Wang J-B, Zhao H-P, Zhao Y-L, Jin C, Liu D-J, Kong W-J, Fang F, Zhang L, Wang H-J, Xiao X-H, 2011. Hepatotoxicity or hepatoprotection? Pattern recognition for the paradoxical effect of the Chinese herb Rheum palmatum L. in treating rat liver injury. PLoS One 6, e24498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcikowski K, Gobe G, 2014. Animal studies on medicinal herbs: predictability, dose conversion and potential value. Phytother. Res 28, 22–27. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Woods RM, Breitbach ZS, Armstrong DW, 2012. 1,3-dimethylamylamine (DMAA) in supplements and geranium products: natural or synthetic? Drug. Test. Anal 4, 986–990. [DOI] [PubMed] [Google Scholar]
- Zheng XC, Dai WC, Chen XH, Wang KY, Zhang WQ, Liu L, Hou JL, 2015. Caffeine reduces hepatic lipid accumulation through regulation of lipogenesis and ER stress in zebrafish larvae. J. Biomed. Sci 22, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoja C, Corna D, Nava V, Locatelli M, Abbate M, Gaspari F, Carrara F, Sangalli F, Remuzzi G, Benigni A, 2013. Analogs of bardoxolone methyl worsen diabetic nephropathy in rats with additional adverse effects. Am. J. Physiol. Ren. Physiol 304, F808–F819. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.