Abstract
The influenza A virus (IAV), a respiratory pathogen for humans, poses serious medical and economic challenges to global healthcare systems. The IAV genome, consisting of eight single-stranded viral RNA (vRNA) segments, is incorporated into virions by a complex process known as genome packaging. Specific RNA sequences within the vRNA segments serve as signals that are necessary for genome packaging. Even though efficient packaging is a prerequisite for viral infectivity, many of the mechanistic details about this process are still missing. In this review, we discuss the recent advances towards the understanding of IAV genome packaging and focus on the RNA features that play a role in this process.
Introduction
Influenza A virus (IAV), a member of the Orthomyxoviridae family, has a negative-sense, segmented, single-stranded RNA genome. It is composed of eight viral RNA (vRNA) segments that vary in length between 890 and 2341 nucleotides and each vRNA encodes for at least one viral protein. Depending on the strain, the IAV genome encodes for between 10–15 different viral proteins. IAV subtypes are classified based on the antigenicities of their two surface glycoproteins HA and NA. Currently, 18 different HA (H1–H18) and 11 NA (N1–N11) subtypes have been identified [9]. The majority of the IAV subtypes (H1–16 and N1–9) are found in aquatic bird species. H17N10 and H18N11 were isolated from bats in 2010. Even though aquatic birds serve as the natural reservoir, IAVs frequently infect other species including humans, pigs, horses, and wild mammals [10]. Subtypes H1N1 and H3N2 are currently endemic in the human population [11]. Avian IAV prefers to bind α2,3 linked sialic acids [12]. However, the majority of sialic acids on human upper respiratory track epithelial cells are α2,6 linked. Due to this, direct zoonosis of IAV between birds and humans is rare. Some avian strains that have been shown to infect humans and cause respiratory diseases includes H5N1, H6N1, H7N3, H7N7, H7N9, and H10N8[13–18]. In humans, influenza virus causes hundreds of thousands of deaths and millions of cases of severe illness each year, creating a significant economic burden to our health care systems. Moreover, IAV is capable of causing pandemics through a process called reassortment. Reassortment is the mixing of two or more strains of IAV to create new IAVs with novel traits. When the reassortment involves a human-adapted IAV and a novel HA surface protein, it gains the ability to evade pre-existing immunity and cause a global outbreak of IAV. In the last 100 years, IAV has caused four pandemics in 1918, 1957, 1968 and 2009 [19–22].
IAV genome packaging is a process by which individual vRNA segments are incorporated into virions. To overcome the complexity of packaging a segmented genome, IAVs efficiently utilize specific RNA sequence features encoded within its own genome as packaging signals. In this review, we summarize the current understanding of IAV genome packaging and highlight critical questions that remain to be answered.
vRNP complexes – the structural units of genome packaging
RNA segments of the IAV genome are incorporated into virions as viral ribonucleoprotein complexes (vRNP). Each vRNP consists of a single vRNA segment, numerous NPs that coat the vRNA and a heterotrimeric RNA-dependent RNA polymerase composed of PB2, PB1, and PA [23,24]. Each genome segment contains 5’ and 3’ terminal sequences that are highly conserved between segments as well as among other IAV strains. These terminal sequences are particularly important for genome replication and packaging. In vRNPs, the 5’ and 3’ ends of vRNAs partially base pair to form a panhandle structure [25–27]. Since this structure is specifically recognized by the viral polymerase complex, vRNAs that bear mutations in their terminal sequences fail to incorporate into vRNPs, and thus, into virions [28,29]. Other than the terminal regions of each segment, the rest of the vRNA is bound by NP and together they form a double helical vRNP structure that is closed by a loop-like structure at the opposite end of the panhandle structure (Fig. 1A) [30]. Mass spectrometry based analysis of purified vRNP units suggested that each NP covers ~26 bases of RNA sequence [31]. Baudin et al. studied the nucleoprotein-vRNA interaction by multiple enzymatic cleavage assays and chemical probing experiments [26]. They showed that NP binds the vRNA with no apparent sequence specificity and binding of NP can melt secondary RNA structures within the vRNA [26]. However, multiple recent studies have shown that binding of NP to vRNA is non-uniform, whereby NP-rich and NP-poor regions can be clearly distinguished within individual vRNAs [32–34]. The rationale for NP-poor or low-NP binding regions is not fully understood. One possibility is that the RNA forms secondary structures in these regions and these structures minimizes NP binding. This presence of double-stranded RNA and/or RNA structures in vRNP complexes is supported by the cleavage pattern of in vitro reconstituted NP-RNA -complexes with a ribonuclease that digests double stranded RNAs [35]. Alternatively, the low-NP binding regions might be involved in intra- or intersegment RNA-RNA interactions. Finally, it is also possible that these regions are occupied by a different protein.
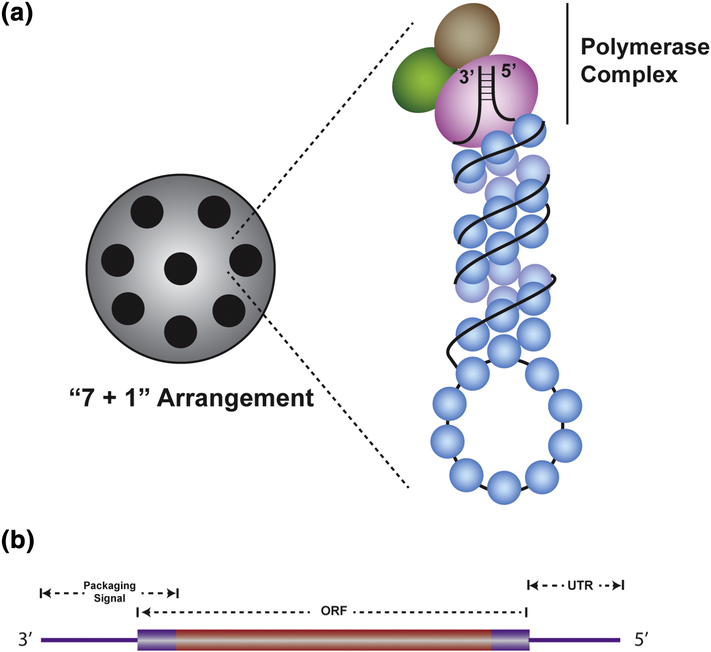
Figure 1. Structure of IAV vRNPs and vRNAs.
(A) Diagram showing the”7 + 1”arrangement of vRNPs inside a virion (left side). A schematic representation of a vRNP complex is shown with a tripartite viral polymerase complex at the top of the vRNP where it binds the panhandle structure formed by 5’ and 3’ UTRs (right side). Rest of the vRNA (black) is covered with multiple nucleoproteins (blue). (B) Schematic diagram showing the positions of packaging signals on a vRNA. The viral ORF is depicted as a rectangle that is flanked by UTRs, which are shown as lines. Typical packaging signals (violet) are situated at both ends of the vRNA consisting of UTRs and ends of the ORF.
The organization of the vRNPs inside the virion is not random. Electron micrographs and tomographs show that vRNPs are ordered in a specific pattern where a central vRNP is surrounded by the remaining seven vRNPs (Fig. 1A) [36,37]. Clearly, this unique “7 + 1” pattern is an outcome of a specific process that dictates the organization of vRNPs within virions. Interestingly, it has been shown that a seven-segment genome containing virion still retains its “7 + 1” pattern by acquiring host-derived 18S and 28S ribosomal RNAs as its eighth segment [38]. Even though the full length 18S rRNA was incorporated into these virions, the 28S rRNA was found to be divided into two fragments (2026 and 2313 nt). All three rRNA fragments are similar in size to the larger gene-segments of IAV suggesting that size compatibility is an important requirement for packaging. It is possible that, since IAV replication takes place in the nucleus, rRNAs, being the most abundant form of cellular RNAs, have a higher probability to be assembled into the “7 + 1” pattern as a substitute for missing vRNAs compared to other cellular RNAs. In the future, it may be worthwhile to determine whether rRNA incorporation is frequent in specific IAV strains that show altered packaging efficiency due to mutations within their vRNA. Interestingly, a recent study has shown that, similar to influenza A, both influenza C and D viruses preferentially package 8 vRNP segments in “7 + 1” pattern even though these viruses have seven segmented genomes [39]. Future studies will determine the position of these host derived RNAs in the “7 + 1” formation and perhaps shed more light on the origins of the spatial orientation of the IAV genome. It will also be interesting to know the genome architecture of related viruses with six gene-segments and determine the minimal number of vRNP complexes required for the “7 + 1” orientation.
The segmented nature of the IAV genome creates both opportunities and challenges for the virus. Perhaps the most significant opportunity is the ability to reassort with other strains of IAV to create new viruses with an expanded host range or evade pre-existing immunity. A segmented genome, in theory, also allows for more deleterious mutations, as these affect only a small portion of the entire genome (7–17%, based on the size of the gene-segment), and can be trans-complemented by other IAV virions. However, these benefits create a significant challenge for the virus, as it requires eight unique gene-segments to be packaged into a new virion to be fully infectious. Anything less than eight gene-segments results in a semi-infectious particle that cannot complete the virus life cycle and produce progeny virions. Semi-infectious and defective interfering particles increase the particle-to-pfu (plaque forming unit) ratio. Typically, stocks of IAV have a particle-to-pfu ratio of between 10:1 and 100:1, meaning that only 1–10% of the virus particles are capable of producing fully infectious progeny virions [40–43]. The rationale and significance of the high particle-to-pfu ratio is not completely understood, but likely involves errors in genome packaging or genome replication and could promote virus reassortment respectively. The rest of this review will focus on the current understanding of genome packaging and identify gaps in our knowledge.
Random vs specific packaging of IAV genome
Genome packaging is the process of incorporating all eight distinct vRNPs into the progeny virion. Two different models had been proposed regarding the mode of IAV genome packaging. According to the random packaging model, individual vRNA segments are separated from other cellular and non-genomic IAV RNAs by means of common vRNA features shared between all segments. However, in this model there is no selection process at play when a specific vRNA is incorporated into a virion. The probability of packaging eight different gene segments randomly is 1/416, meaning that in a viral population the vast majority of viral particles should be non-infectious or semi-infectious. This estimate does not match with experimentally determined average particle-to-pfu ratio of virus stocks suggesting that genome packaging is not a random process. Packaging of more than eight gene-segments can overcome this apparent discrepancy, however, this has not been observed in EM studies which showed that most progeny virions contain eight segments organized in a “7 + 1” pattern [37,44,45]. Earlier genetics based studies showed that progeny virions do not contain more than one allele of a specific gene-segment [46,47]. Consistent with this, heterozygous nine segment viruses are either genetically unstable or have to be maintained under strong selection [48,49].
The specific packaging model suggests that each of the eight vRNPs is selected specifically to ensure packaging of a complete and minimal set of vRNPs. In the past decades, numerous studies have provided evidence that packaging of eight IAV segments is a selective process. For example, Chou et al. studied the composition of viral RNAs at single-virus particle resolution using FISH [50]. They applied photo-bleaching analysis to determine the copy number of a specific vRNA inside one virion and found that each of the IAV vRNAs had a single copy incorporated into one virion. Moreover, radioactive labeling of vRNA and hybridization with segment specific RNA probes indicate that vRNAs are present in equimolar ratios inside purified virus particles [51,52]. In a different study, when the synthesis of a specific vRNA segment was reduced by mutagenesis, the equimolar ratio of vRNA segments inside virion did not change further suggesting that viral genome packaging is not a random process [46]. Taken together, these studies suggest that influenza virus genome packaging is a specific process.
Cis-acting packaging signals
The specific packaging of vRNA segments into progeny virus particles suggests that vRNA contains certain signals that mediate this process. Below is a description of known packaging signals, how they were discovered, and what they have revealed about the specificity of the genome packaging process.
Defective interfering (DI) RNAs played an important role in identifying the IAV genome packaging signals. DI RNAs are smaller forms of the influenza vRNA, which are generated by large internal deletions within vRNAs by an unknown mechanism. DI RNAs are produced during high-multiplicity infections and have the ability to compete with full length version of the vRNA from which they are derived from [53–55]. DI RNAs are most commonly derived from PB2, PB1 and PA segments of the IAV genome [56,57]. Several groups have characterized DI RNAs by sequencing and showed that these RNAs retain between 100 and 300 nucleotides from their terminal sequences [57–59]. These findings provided the first evidence that nucleotide sequences in the open reading frame of each segment were important for influenza genome packaging. The first molecular demonstration of segment-specific packaging signal inside the coding region of the vRNA was provided by Fujii et al [60]. Partial replacement of the NA ORF with a reporter gene can lead to its incorporation into IAV and the packaging efficiency of this reporter gene-segment can be estimated by infecting host cells and assaying for the reporter gene expression. This approach, in combination with incremental deletions of the 3’ and 5’ flanking sequences of the NA gene-segment, identified a signal within the NA vRNA that drove the incorporation of this reporter segment into virions. Since then, this approach has been successfully implemented for identification of the packaging signals in all eight vRNA segments [44,60–67]. More recently, Goto et al. used a reporter based reverse genetics system to further dissect the role of terminal sequences in vRNP packaging [68]. In this study, they showed that the terminal non-coding regions of the NP segment were sufficient for interacting with the IAV polymerase complex and for incorporating a reporter construct into virus-like particles (VLPs). However, a “bundling signal”, consisting of both the non-coding regions and partial terminal coding sequences, was required for the incorporation of a full set of vRNAs into VLPs. Aside from the packaging signals near the 3’ and 5’ termini of each segment, recent studies have identified other regions on the genome that play a key role in packaging of heterologous gene-segments. For example, competitive plasmid transfection experiments using A/Puerto Rico/8/1934 (PR8, H1N1) and A/Udorn/307/1972 (Udorn, H3N2) gene segments showed that the PB1 of the Udorn virus preferentially co-packaged with NA gene segment of the same H3N2 virus. The sequence region of PB1 that accounts for this co-selection falls within an internal coding region of PB1 [69]. Using chimeric PB1 constructs, this region was narrowed down to vRNA nucleotide position 1776–2070 of the PB1 gene which resides outside known terminal packaging signals [70].
Applying a bioinformatics-based approach, Gog et al. identified conserved codons among thousands of IAV genomes [71]. They hypothesized that codons falling within a functional RNA motif will be more conserved compared to other codons. Generally, these codons formed clusters near the terminal regions of each segment, likely representing the previously identified packaging signals. Introduction of one or more synonymous mutations within the identified terminal regions significantly reduced segment packaging in majority of cases suggesting that these sequences are important for genome packaging [71]. However, some of the clusters were positioned in the middle of coding sequences which represented RNA splice-sites (segments 7 and 8) or regions with overlapping codon usage (PB1–F2 in segment 2 and PA-X in segment 3). For other codons, the reason for conservation was not clear. Some of the conserved codons in PB2 segment identified by Gog et al. were evaluated for their efficiency as packaging signals by Marsh et al. using a different approach where the full-length PB2 segment was mutagenized (Marsh et al. 2008). They found that, indeed, mutations in the conserved codons that resides within the PB2 coding region negatively affects genome packaging. Of note, the effects of synonymous mutations in the terminal regions appear to be strain specific, as similar mutations in individual gene-segments (PB2, PB1, and PA) had no effect on the packaging of an H7N7 IAV (SC35M) [72]. Combined these and other studies have identified specific RNA elements in the 3’ and 5’ termini of vRNA which are important for genome packaging. The mechanism by which these elements mediate specific packaging are not fully understood. Based on existing literature (reviewed below), these signals are important in direct vRNA-vRNA interaction, albeit it is possible that these signals are required for RNA-protein interactions or mediate packaging through some unknown mechanism.
Packaging through vRNA-vRNA interactions
Evidence for vRNA-vRNA interactions mediating the packaging of vRNA segments into new virions came from a number of studies. 3-D structure constructed by scanning transmission EM data suggested the existence of frequent interactions between vRNPs that cover the whole length of vRNPs. Although the nature of these interactions remains to be investigated, small fiber-like appearance of these interactions resembles inter-RNA interactions. However, electron tomographs of vRNPs inside H3N2 virions reported a different pattern of interactions between them [73]. Contacts between vRNPs were observed near the packaging region and no interaction was observed outside the packaging region.
Several in vitro studies helped in identifying inter-RNA interactions between vRNA segments. Fournier et al. employed an electrophoretic mobility shift assay in order identify intramolecular interactions between vRNAs of human H3N2 in vitro [74]. They showed that, each vRNA segment interacts with at least one other vRNA segment. With a similar approach Gavazzi et al. identified interactions between vRNAs of an avian H5N2 strain [75]. They found that vRNAs of H5N2 form a network of interactions, however, this network, in its pattern of interactions, is different compared to the network described for H3N2 indicating that intermolecular interactions between segments are strain specific [75]. Interestingly, some of the identified vRNA-vRNA interactions for the avian H3N2 virus are positioned centrally in the vRNA molecule, in contrast to known packaging signals that are located at the 5’ and 3’ ends of the vRNA. The strongest interaction identified by the gel shift assays was an interaction between segments PB1 and NS [76]. This interaction has been shown to be required for optimal genome packaging since disruption of this interaction reduced the amount of specific vRNA segments in the viral particles and increased the number of defective viral particles. The interacting sequences in PB1 and NS can form stable stem-loops by themselves and they are proposed to interact with each other by forming a kissing loop complex. Importantly, this is the only inter-vRNA interaction that has been confirmed by trans-complementary mutations.
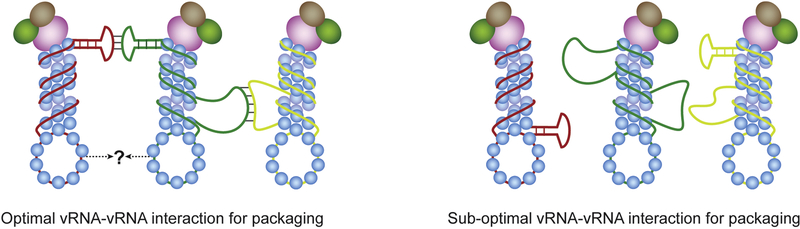
Recently, Dadonaite et al. employed a high-throughput sequencing based approach, called SPLASH (Sequencing of psoralen cross-linked, ligated, and selected hybrids), to identify intermolecular RNA interactions in virio [77]. They showed that most vRNA segments interact with multiple other segments and identified several regions within the viral genome that exhibit higher numbers of RNA-RNA interactions. The least number of interactions was identified for NA and NS segments, which is consistent with previous reports that these two segments have minimal effect on packaging of other vRNA segments. Additionally, synonymous mutations that disrupt potential RNA-RNA interactions affected viral genome packaging and virus growth. Exactly how these vRNA-vRNA interactions occur in the context of vRNP complexes is not understood. Small RNA structures are thought to be disrupted upon binding of NP and long vRNA-vRNA interactions (>20nt) appear less likely given the helical nature of vRNP complexes. One potential explanation for this discrepancy is the presence of NP-free RNA that can engage in vRNA-vRNA interactions. Perhaps the formation of stable secondary or tertiary RNA structures will prevent NP binding and make it available for gene-segment interactions (Fig. 2).
Figure 2. A model for vRNA-vRNA interactions and their consequence on IAV genome packaging.
Different vRNA segments are shown here as lines of different colors (red, green and yellow). Regions of vRNA that are not bound to nucleoprotein may form RNA structures, which mediate inter-vRNA interactions. It is also possible that vRNPs interact with each other by a still unknown mechanism, which is depicted here as a question mark. Closely related IAV strains have compatible vRNA-vRNA interaction profiles resulting in efficient genome packaging (left). For divergent IAV strains, dissimilarity in nucleotide sequences results in formation of RNA features that are not compatible for RNA-RNA interactions, and thus reduce genome packaging efficiency (right).
Several groups have employed bioinformatics based approaches to identify secondary structures within the vRNA segments [78–81]. Kobayashi et al. analyzed the M segment sequences of multiple IAV strains to identify conserved stem-loop structures [79]. They followed their in silico findings by introducing synonymous mutations that disrupt one of the predicted stem-loop structures, named SL3–10. They found that disruption of this structure negatively affects viral infectivity and produces more defective virus particles, indicating that this SL3–10 is important for packaging. Gultyaev et al. predicted RNA secondary structures within the NP segment using the RNAalifold software and did further analysis of structures that exhibit patterns of covariations [78]. The authors identified a pseudoknot structure within the NP packaging signal and showed that disruption of this structure leads to a decrease in virus titer and smaller plaque sizes. Notably, the functional importance of this pseudoknot structure were further corroborated by Williams et al. as the authors showed that this structure is involved in genome packaging [34]. Covariation analysis using thousands of IAV genomes available in public databases also enabled the identification of structural domains in the HA vRNA [81]. This analysis indicated that most of the predicted RNA structural domains are specific to individual strains. Several predicted RNA structures, particularly structures in the HA cleavage site region, are conserved among multiple HA subtypes. Interestingly, some RNA domains were identified that are not essential for viral replication, but contribute to virus fitness. These structures were proposed to be important of viral genome reassortment.
The formation of functional RNA secondary structures is linked to NP binding since it has been shown that small RNA structural motifs are disrupted upon binding of NP [26,82]. Therefore, to be functionally active in the genome packaging process, RNA structural domains may be required to be in an “NP-free state”. Based on this idea, Graham et al. employed a PAR-CLIP based approach to identify low-NP binding regions within all vRNAs [34]. Indeed, 24 vRNA regions identified in this study were enriched for predicted RNA secondary structures and some regions harbored sequences that were previously identified as genome packaging signals. Disruption of several predicted RNA structures by synonymous mutations resulted in packaging defects, whereas mutations restoring the disrupted RNA structures had no effect on packaging. To summarize, these studies confirm that the general architecture of a minimal packaging signal consists of UTRs at both ends of vRNAs as well as 9 to 80 nucleotides of their adjacent coding sequences (Fig. 1B). However additional signals are present in the genome that are required for optimal genome packaging [83]. Precisely how the 5’ and 3’ packaging signals function and their relationship to the internal packaging signals is not clear.
Interestingly, some of the regions that form inter-segment vRNA-vRNA interactions, identified by Dadonaite et al., overlap with low-NP binding regions that are predicted to form RNA secondary structures, whereas others did not [34]. One possibility is that RNA structural features may increase the specificity of the vRNA-vRNA interactions to promote the packaging of eight unique gene-segments, while minimizing the interactions between the homologous gene-segment. Indeed, analysis of potential complementary vRNA-vRNA interactions identified many intra-segment interactions, which in-theory could lead to the packaging of multiple copies of the same gene-segment. Alternatively, RNA structures determine the position and orientation of the packaging signals elsewhere on the gene-segment. Exactly how and where the vRNA-vRNA interactions occur in the genome will be the subject of future studies. It will also be interesting to determine the RNA structure and interaction profile of a diverse array of IAVs, including human, swine, and birds. Since the nucleotide sequences of these IAVs vary widely, these studies may help us to build better models for genome packaging and determine the likelihood of the emergence of reassortant IAV strains, including those with pandemic potential.
Genome packaging en route.
Inter-vRNA interactions are essential for genome packaging and productive infection of IAV. Whether the neo-synthesized vRNPs are transported outside the nucleus individually or as a bundle is not clear. A smFISH approach has revealed that vRNAs are detected in separate locations within the nucleus and remain separated immediately after export from the nucleus [84]. However, a four color FISH experiment that stained for PB2, PB1, PA and NP segments identified foci containing multiple vRNA segments near the external nuclear periphery, which indicates that vRNPs are exported from the nucleus as bundles[85]. Future studies will clarify the discrepancies between these studies and will provide a clearer picture for nuclear export of vRNPs.
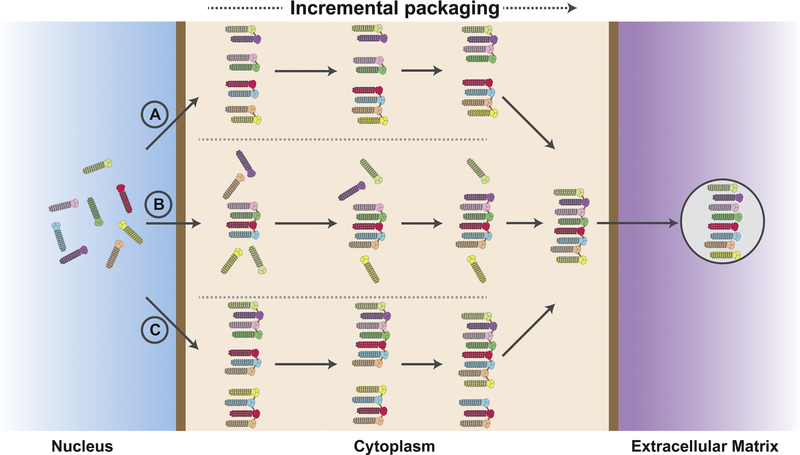
Rab11, a small GTP binding protein required for viral budding, has been shown to be associated with vRNPs at all stages of cytoplasmic transport of vRNPs towards the plasma membrane [86,87]. Currently, two distinct models have been proposed that elucidate how vRNPs are transported to the plasma membrane. According to the first model, vRNPs associate with Rab11-positive recycling endosomes near the microtubule organizing center and are carried through the cytoplasm using microtubules [88,89]. The second model proposes that after exiting from the nucleus, vRNPs associate with a remodeled endoplasmic reticulum, from which, vRNPs are transported by Rab11-dependent irregularly coated vesicles (ICVs) [90]. It is currently not clear at which stage of cytoplasmic transport the packaging of vRNPs initiates. It is possible that a limited number of vRNPs can interact with each other during earlier steps of transport to the plasma membrane. These interactions may form a core complex of vRNPs that goes through subsequent assembly steps en route to the plasma membrane (Fig. 3). This hierarchical model for viral genome packaging is further corroborated by studies that show that some vRNA segments play a more important role than other segments during the packaging process [64,91]. It is worth noting that co-localization of vRNPs detected by FISH does not necessarily confirm physical interaction between vRNPs due to the lack of sufficient resolution provided by FISH. It is possible that the co-localization signals simply reflect close proximity of vRNPs within the cytoplasm. Moreover, one or more cellular proteins might facilitate the packaging of vRNPs without the need of any physical interaction among vRNPs at all. Even though the model of stepwise packaging is plausible, critical questions regarding this process remains to be answered. Is there a specific vRNP that preferentially form the initial vRNP sub-bundle? Do the initial interactions between two specific vRNPs remain persistent all the way until virion release, or do they change as the vRNPs progress through the sequential packaging process while more vRNPs are added?
Figure 3. A hierarchical model for IAV genome packaging during trafficking through the cytoplasm.
Here, eight different vRNPs are shown in different colors. Upon export from the nucleus, multiple vRNPs may interact with each other to form a “core complex” for packaging. However, the identity of the initial “core complex” and the sequence of vRNP association are not clear. Three different models for vRNP transport and bundling through the cytoplasm are shown here as A, B and C. In model A, multiple “core complex” form after exit from the nucleus and these clusters associate with each other as they move towards the plasma membrane. In model B, one major type of core-complex forms after nuclear exit. As the vRNPs move towards the plasma membrane rest of the vRNPs associate with the core complex one by one before being released as progeny virions. In model C, multiple vRNP pre-complex that are formed after nuclear exit can interact with each other and exchange vRNPs among themselves.
After infecting a new cell, vRNPs need to be successfully transported from the endosome to the nucleus. Loss of any vRNP before nuclear import will lead to replication of an incomplete set of vRNAs inside the nucleus, and ultimately, to an unproductive viral infection. Moreover, it has been shown that some host factors, including Mx1 and PLSCR1, exert their antiviral activity by inhibiting nuclear import [92,93]. One study investigated the movement of single vRNPs across the cytoplasm in the early stage of infection by fluorescence microscopy [94]. The authors compared the diffusion coefficients of vRNPs that were estimated from single-particle trajectories with a simulated distribution and concluded that vRNPs are transported to the nuclear envelope by diffusion. In a different study, Chou et al. used a single-molecule FISH (smFISH) based approach to study whether vRNPs stay together or transport individually to the nucleus [84]. They show that vRNPs from the incoming virion travel together until they reach the nucleus suggesting that loss of vRNPs prior to nuclear entry is not a major event. However, these findings contradict with a recent study that found that vRNPs from an individual virion separate into distinct units after uncoating at the late endosome [95]. Critical questions remain about the nature of molecular interactions that keep the vRNPs together upon cell entry. Do the genome packaging signals play a role in holding the vRNPs together or is there a protein component that transports packaged vRNPs towards the nucleus? How do the bundled vRNPs dissociate from each other inside the nucleus for replication and transcription? Future studies will shed light on these issues.
Genome packaging dictates IAV genome reassortment
The segmented nature of the IAV genome and its ability to reassort is evolutionarily advantageous for the virus as it supports the generation of novel strains of IAV that can evade pre-existing immunity or infect new host species. Reassortment can also play a role in elimination of deleterious mutations [96]. Still, a multipartite genome adds to the complexity of genome packaging. All eight vRNA segments must be functionally intact for a productive infection [30]. Thus, virus particles that fail to incorporate all eight vRNA segments will not replicate and produce viable progeny. The significance of genome packaging on the outcome of reassortment is not known. When multiple viruses co-infect a cell, genome packaging will dictate which set of eight unique vRNAs will be incorporated into progeny virions, and thus determines the genetic architecture of the reassortant virus. The rules that govern whether two IAVs will reassort and which vRNAs from the parental strains will be selected for packaging are poorly understood. Data suggests that the compatibility of packaging signals is a prerequisite for reassortment. Essere et al. showed that HA segment of a H5N2 strain was unable to enter H3N2 background upon co-infection. However, a recombinant version of the H5N2 HA segment, which contains the packaging signal of H3N2 HA segment, readily incorporates into H3N2 background [97]. Similarly, Gilbertson et al. showed preferential packaging of the N3 subtype in the presence of the PB1 gene of a H3N2 virus [70]. Presumably, when two divergent IAV strains co-infect a cell, incompatible packaging signals on the heterologous segments creates a barrier for reassortment that may lead to poor reassortment or no reassortment at all. How similar or divergent the packaging signals must be in order to affect the packaging process is not known.
Conclusions
In summary, packaging of all eight IAV vRNA segment into progeny virus is a complex process and a complete understanding of this process is still missing. Closely related IAV strains reassort frequently and the rate of reassortment decreases as the strains become more divergent [98,99]. One of the reasons for this decrease in reassortment rate is clearly the incompatibility between viral proteins [100]. However, as more and more evidence indicates that inter-vRNA interactions are important for proper genome packaging, it is critical to evaluate their contribution in genome reassortment.
Highlights:
Genome packaging of the Influenza A virus is a complex process.
Packaging signals within the viral RNA dictate Influenza A virus genome packaging
Inter viral RNA interactions are important for Influenza A virus genome packaging.
Acknowledgements
The production of this review was funded in part by the National Institutes of Health (NIH) grant R01-AI139251 (M.S.D and A.C.M.B.) and the Children’s Discovery Institute PDII2018702 (A.C.M.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Te Velthuis AJW, Fodor E, Influenza virus RNA polymerase: Insights into the mechanisms of viral RNA synthesis, Nat. Rev. Microbiol 14 (2016) 479–493. doi: 10.1038/nrmicro.2016.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Portela A, Digard P, The influenza virus nucleoprotein: A multifunctional RNA-binding protein pivotal to virus replication, J. Gen. Virol 83 (2002) 723–734. doi: 10.1099/0022-1317-83-4-723. [DOI] [PubMed] [Google Scholar]
- [3].Dou D, Revol R, Östbye H, Wang H, Daniels R, Influenza A virus cell entry, replication, virion assembly and movement, Front. Immunol 9 (2018) 1–17. doi: 10.3389/fimmu.2018.01581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Pinto LH, Lamb RA, The M2 proton channels of influenza A and B viruses, J. Biol. Chem 281 (2005) R500020200. doi: 10.1002/9781118636817.ch6. [DOI] [PubMed] [Google Scholar]
- [5].Martin K, Heleniust A, Nuclear transport of influenza virus ribonucleoproteins: The viral matrix protein (M1) promotes export and inhibits import, Cell. 67 (1991) 117–130. doi: 10.1016/0092-8674(91)90576-K. [DOI] [PubMed] [Google Scholar]
- [6].Gómez-Puertas P, Albo C, Pérez-Pastrana E, Vivo A, Portela A, Influenza Virus Matrix Protein Is the Major Driving Force in Virus Budding, J. Virol 74 (2000) 11538–11547. doi: 10.1128/JVI.74.24.11538-11547.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ayllon J, García-Sastre A, The NS1 protein: a multitasking virulence factor., Curr. Top. Microbiol. Immunol 386 (2015) 73–107. doi: 10.1007/82_2014_400. [DOI] [PubMed] [Google Scholar]
- [8].O’Neill RE, Talon J, Palese P, The influenza virus NEP (NS2 protein) mediates the nuclear export of viral ribonucleoproteins, EMBO J. 17 (1998) 288–296. doi: 10.1093/emboj/17.1.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tong S, Zhu X, Li Y, Shi M, Zhang J, Bourgeois M, Yang H, Chen X, Recuenco S, Gomez J, Chen LM, Johnson A, Tao Y, Dreyfus C, Yu W, McBride R, Carney PJ, Gilbert AT, Chang J, Guo Z, Davis CT, Paulson JC, Stevens J, Rupprecht CE, Holmes EC, Wilson IA, Donis RO, New World Bats Harbor Diverse Influenza A Viruses, PLoS Pathog. 9 (2013). doi: 10.1371/journal.ppat.1003657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yoon S-W, Webby RJ, Webster RG, Evolution and ecology of influenza A viruses., Curr. Top. Microbiol. Immunol 385 (2014) 359–375. doi: 10.1007/82_2014_396. [DOI] [PubMed] [Google Scholar]
- [11].Hay AJ, Gregory V, Douglas AR, Yi PL, The evolution of human influenza viruses, Philos. Trans. R. Soc. B Biol. Sci 356 (2001) 1861–1870. doi: 10.1098/rstb.2001.0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rogers GN, Paulson JC, Daniels RS, Skehel JJ, Wilson IA, Wiley DC, Single amino acid substitutions in influenza haemagglutinin change receptor binding specificity., Nature. 304 (1983) 76–78. [DOI] [PubMed] [Google Scholar]
- [13].Fouchier RAM, Schneeberger PM, Rozendaal FW, Broekman JM, Kemink SAG, Munster V, Kuiken T, Rimmelzwaan GF, Schutten M, Van Doornum GJJ, Koch G, Bosman A, Koopmans M, Osterhaus ADME, Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome., Proc. Natl. Acad. Sci. U. S. A 101 (2004) 1356–61. doi: 10.1073/pnas.0308352100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gao R, Cao B, Hu Y, Feng Z, Wang D, Hu W, Chen J, Jie Z, Qiu H, Xu K, Xu X, Lu H, Zhu W, Gao Z, Xiang N, Shen Y, He Z, Gu Y, Zhang Z, Yang Y, Zhao X, Zhou L, Li X, Zou S, Zhang Y, Li X, Yang L, Guo J, Dong J, Li Q, Dong L, Zhu Y, Bai T, Wang S, Hao P, Yang W, Zhang Y, Han J, Yu H, Li D, Gao GF, Wu G, Wang Y, Yuan Z, Shu Y, Human Infection with a Novel Avian-Origin Influenza A (H7N9) Virus, N. Engl. J. Med 368 (2013) 1888–1897. doi: 10.1056/NEJMoa1304459. [DOI] [PubMed] [Google Scholar]
- [15].Hirst M, Astell CR, Griffith M, Coughlin SM, Moksa M, Zeng T, Smailus DE, Holt RA, Jones S, Marra MA, Petric M, Krajden M, Lawrence D, Mak A, Chow R, Skowronski DM, Tweed SA, Goh SH, Brunham RC, Robinson J, Bowes V, Sojonky K, Byrne SK, Li Y, Kobasa D, Booth T, Paetzel M, Novel avian influenza H7N3 strain outbreak, British Columbia, Emerg. Infect. Dis 10 (2004) 2192–2195. doi: 10.3201/eid1012.040743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Koopmans M, Wilbrink B, Conyn M, Natrop G, Van Der Nat H, Vennema H, Meijer A, Van Steenbergen J, Fouchier R, Osterhaus A, Bosman A, Transmission of H7N7 avian influenza A virus to human beings during a large outbreak in commercial poultry farms in the Netherlands, Lancet. 363 (2004) 587–593. doi: 10.1016/S0140-6736(04)15589-X. [DOI] [PubMed] [Google Scholar]
- [17].Tweed SA, Skowronski DM, David ST, Larder A, Petric M, Lees W, Li Y, Katz J, Krajden M, Tellier R, Halpert C, Hirst M, Astell C, Lawrence D, Mak A, Human illness from avian influenza H7N3, British Columbia., Emerg. Infect. Dis 10 (2004) 2196–9. doi: 10.3201/eid1012.040961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wei SH, Yang JR, Wu HS, Chang MC, Lin JS, Lin CY, Liu YL, Lo YC, Yang CH, Chuang JH, Lin MC, Chung WC, Liao CH, Lee MS, Huang WT, Chen PJ, Liu MT, Chang FY, Human infection with avian influenza A H6N1 virus: An epidemiological analysis, Lancet Respir. Med 1 (2013) 771–778. doi: 10.1016/S2213-2600(13)70221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Johnson NPAS, Mueller J, Updating the accounts: global mortality of the 1918–1920 “Spanish” influenza pandemic., Bull. Hist. Med 76 (2002) 105–15. doi: 10.1353/bhm.2002.0022. [DOI] [PubMed] [Google Scholar]
- [20].Viboud C, Simonsen L, Fuentes R, Flores J, Miller MA, Chowell G, Global mortality impact of the 1957–1959 influenza pandemic, J. Infect. Dis 212 (2016) 738–745. doi: 10.1093/infdis/jiv534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cockburn WC, Delon PJ, Ferreira W, Origin and progress of the 1968–69 Hong Kong influenza epidemic., Bull. World Health Organ 41 (1969) 345–348. doi: 10.1641/0006-3568(2004)054[0066:TVOMCF]2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Simonsen L, Spreeuwenberg P, Lustig R, Taylor RJ, Fleming DM, Kroneman M, Van Kerkhove MD, Mounts AW, Paget WJ, Echenique H, Savy V, Muscatello D, MacIntyre CR, Dwyer DE, Azziz-Baumgartner E, Homaira N, Moura FEA, Schuck C, Akwar H, Schanzer D, Fuentes R, Olea A, Sotomayor V, Feng L, Yu H, Mazick A, Mølbak K, Nielsen J, Carrat F, Lemaitre M, Buchholz U, Schweiger B, Höhle M, Vesenbeckh S, Cowling B, Leung G, Tsang T, Chuang SK, Bromberg M, Kaufman Z, Sugaya N, Oka Ezoe K, Hayashi S, Matsuda M, Lopez-Gatell H, Alpuche-Aranda C, Noyola D, Chowell G, van Asten L, Meijer A, van den Wijngaard K, van der Sande M, Baker M, Zhang J, Benavides JG, Munayco C, Laguna-Torres A, Rabczenko D, Wojtyniak B, Park SH, Lee YK, Zolotusca L, Popovici O, Popescu R, Ang LW, Cutter J, Lin R, Ma S, Chen M, Lee VJ, Prosenc K, Socan M, Cohen C, Larrauri A, de Mateo S, Méndez LS, Sanz CD, Andrews N, Green HK, Pebody R, Saei A, Shay D, Viboud C, Global Mortality Estimates for the 2009 Influenza Pandemic from the GLaMOR Project: A Modeling Study, PLoS Med. 10 (2013). doi: 10.1371/journal.pmed.1001558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Arranz R, Coloma R, Chichón FJ, Conesa JJ, Carrascosa JL, Valpuesta JM, Ortín J, Martín-Benito J, The structure of native influenza virion ribonucleoproteins., Science. 338 (2012) 1634–7. doi: 10.1126/science.1228172. [DOI] [PubMed] [Google Scholar]
- [24].Moeller A, Kirchdoerfer RN, Potter CS, Carragher B, Wilson IA, Organization of the influenza virus replication machinery, Science (80-.). 338 (2012) 1631–1634. doi: 10.1126/science.1227270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hsu MT, Parvin JD, Gupta S, Krystal M, Palese P, Genomic RNAs of influenza viruses are held in a circular conformation in virions and in infected cells by a terminal panhandle., Proc. Natl. Acad. Sci. U. S. A 84 (1987) 8140–4. doi: 10.1073/pnas.84.22.8140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Baudin F, Bach C, Cusack S, Ruigrok RW, Structure of influenza virus RNP. I. Influenza virus nucleoprotein melts secondary structure in panhandle RNA and exposes the bases to the solvent., EMBO J. 13 (1994) 3158–65. doi: 10.1002/j.1460-2075.1994.tb06614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Pflug A, Guilligay D, Reich S, Cusack S, Structure of influenza A polymerase bound to the viral RNA promoter, Nature. 516 (2014) 355–360. doi: 10.1038/nature14008. [DOI] [PubMed] [Google Scholar]
- [28].Luytjes W, Krystal M, Enami M, Parvin JD, Palese P, Amplification, expression, and packaging of a foreign gene by influenza virus, Cell. 59 (1989) 1107–1113. doi: 10.1016/0092-8674(89)90766-6. [DOI] [PubMed] [Google Scholar]
- [29].Neumann G, Hobom G, Mutational analysis of influenza virus promoter elements in vivo, J. Gen. Virol 76 (1995) 1709–1717. doi: 10.1099/0022-1317-76-7-1709. [DOI] [PubMed] [Google Scholar]
- [30].Eisfeld AJ, Neumann G, Kawaoka Y, At the centre: Influenza A virus ribonucleoproteins, Nat. Rev. Microbiol 13 (2015) 28–41. doi: 10.1038/nrmicro3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hutchinson EC, Charles PD, Hester SS, Thomas B, Trudgian D, Martínez-Alonso M, Fodor E, Conserved and host-specific features of influenza virion architecture, Nat. Commun 5 (2015). doi: 10.1038/ncomms5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lee N, Le Sage V, Nanni AV, Snyder DJ, Cooper VS, Lakdawala S, Genome-wide analysis of influenza viral RNA and nucleoprotein association, Nucleic Acids Res. 45 (2017) 8968–8977. doi: 10.1093/nar/gkx584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Le Sage V, Nanni A, Bhagwat A, Snyder D, Cooper V, Lakdawala S, Lee N, Non-Uniform and Non-Random Binding of Nucleoprotein to Influenza A and B Viral RNA, Viruses. 10 (2018) 522. doi: 10.3390/v10100522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Williams GD, Townsend D, Wylie KM, Kim PJ, Amarasinghe GK, Kutluay SB, Boon ACM, Nucleotide resolution mapping of influenza A virus nucleoprotein-RNA interactions reveals RNA features required for replication, Nat. Commun 9 (2018) 465. doi: 10.1038/s41467-018-02886-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Yamanaka K, Ishihama A, Nagata K, Reconstitution of influenza virus RNA-nucleoprotein complexes structurally resembling native viral ribonucleoprotein cores, J. Biol. Chem 265 (1990) 11151–11155. [PubMed] [Google Scholar]
- [36].Noda T, Sagara H, Yen A, Takada A, Kida H, Cheng RH, Kawaoka Y, Architecture of ribonucleoprotein complexes in influenza A virus particles, Nature. 439 (2006) 490–492. doi: 10.1038/nature04378. [DOI] [PubMed] [Google Scholar]
- [37].Noda T, Sugita Y, Aoyama K, Hirase A, Kawakami E, Miyazawa A, Sagara H, Kawaoka Y, Three-dimensional analysis of ribonucleoprotein complexes in influenza A virus, Nat. Commun 3 (2012) 636–639. doi: 10.1038/ncomms1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Noda T, Murakami S, Nakatsu S, Imai H, Muramoto Y, Shindo K, Sagara H, Kawaoka Y, Importance of the 1+7 configuration of ribonucleoprotein complexes for influenza A virus genome packaging, Nat. Commun 9 (2018) 1–10. doi: 10.1038/s41467-017-02517-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Nakatsu S, Murakami S, Shindo K, Horimoto T, Sagara H, Noda T, Kawaoka Y, Influenza C and D Viruses Package Eight Organized Ribonucleoprotein Complexes., J. Virol 92 (2018) 1–9. doi: 10.1128/JVI.02084-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].DONALD HB, ISAACS A, Counts of influenza virus particles., J. Gen. Microbiol 10 (1954) 457–64. doi: 10.1099/00221287-10-3-457. [DOI] [PubMed] [Google Scholar]
- [41].Schulze IT, The structure of influenza virus. II. A model based on the morphology and composition of subviral particles, Virology. 47 (1972) 181–196. doi: 10.1016/0042-6822(72)90251-6. [DOI] [PubMed] [Google Scholar]
- [42].Noton SL, Simpson-Holley M, Medcalf E, Wise HM, Hutchinson EC, McCauley JW, Digard P, Studies of an Influenza A Virus Temperature-Sensitive Mutant Identify a Late Role for NP in the Formation of Infectious Virions, J. Virol 83 (2009) 562–571. doi: 10.1128/JVI.01424-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Vahey MD, Fletcher DA, Low fidelity assembly of influenza A virus promotes escape from host cells, Cell. 176 (2018) 281–294.e19. doi: 10.1016/j.cell.2018.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Fujii K, Fujii Y, Noda T, Muramoto Y, Watanabe T, Takada A, Goto H, Horimoto T, Kawaoka Y, Importance of both the coding and the segment-specific noncoding regions of the influenza A virus NS segment for its efficient incorporation into virions, J. Virol 79 (2005) 3766–3774. doi: 10.1128/JVI.79.6.3766-3774.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Nakatsu S, Sagara H, Sakai-Tagawa Y, Sugaya N, Noda T, Kawaoka Y, Complete and incomplete genome packaging of influenza A and B viruses, MBio. 7 (2016) 1–7. doi: 10.1128/mBio.01248-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Laver WG, Downie JC, Influenza virus recombination. I. Matrix protein markers and segregation during mixed infections., Virology. 70 (1976) 105–17. doi: 10.1099/0022-1317-76-12-3211. [DOI] [PubMed] [Google Scholar]
- [47].Nakajima K, Sugiura A, Three-factor cross of influenza virus, Virology. 81 (1977) 486–489. doi: 10.1016/0042-6822(77)90165-9. [DOI] [PubMed] [Google Scholar]
- [48].Scholtissek C, Rohde W, Harms E, Rott R, Orlich M, Boschek CB, A possible partial heterozygote of an influenza A virus., Virology. 89 (1978) 506–16. http://www.ncbi.nlm.nih.gov/pubmed/716216. [DOI] [PubMed] [Google Scholar]
- [49].Enami M, Sharma G, Benham C, Palese P, An influenza virus containing nine different RNA segments, Virology. 185 (1991) 291–298. doi: 10.1016/0042-6822(91)90776-8. [DOI] [PubMed] [Google Scholar]
- [50].Chou Y. -y., Vafabakhsh R, Doganay S, Gao Q, Ha T, Palese P, One influenza virus particle packages eight unique viral RNAs as shown by FISH analysis, Proc. Natl. Acad. Sci 109 (2012) 9101–9106. doi: 10.2139/ssrn.1985979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].McGeoch D, Fellner P, Newton C, Influenza virus genome consists of eight distinct RNA species., Proc. Natl. Acad. Sci 73 (1976) 3045–3049. doi: 10.1073/pnas.73.9.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Hatada E, Hasegawa M, Mukaigawa J, Shimizu K, Fukuda R, Control of influenza virus gene expression: Quantitative analysis of each viral RNA species in infected cells, J. Biochem 105 (1989) 537–546. doi: 10.1093/oxfordjournals.jbchem.a122702. [DOI] [PubMed] [Google Scholar]
- [53].Von Magnus P, Incomplete forms of influenza virus., Adv. Virus Res 2 (1954) 59–79. [DOI] [PubMed] [Google Scholar]
- [54].Odagiri T, Tashiro M, Segment-specific noncoding sequences of the influenza virus genome RNA are involved in the specific competition between defective interfering RNA and its progenitor RNA segment at the virion assembly step., J. Virol 71 (1997) 2138–45. doi: 10.1063/1.5004040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Duhaut SD, McCauley JW, Defective RNAs inhibit the assembly of influenza virus genome segments in a segment-specific manner, Virology. 216 (1996) 326–337. doi: 10.1006/viro.1996.0068. [DOI] [PubMed] [Google Scholar]
- [56].Davis AR, Hiti AL, Nayak DP, Influenza defective interfering viral RNA is formed by internal deletion of genomic RNA, Proc Natl Acad Sci U S A. 77 (1980) 215–219. doi: 10.1073/pnas.77.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Jennings PA, Finch JT, Winter G, Robertson JS, Does the higher order structure of the influenza virus ribonucleoprotein guide sequence rearrangements in influenza viral RNA?, Cell. 34 (1983) 619–627. doi: 10.1016/0092-8674(83)90394-X. [DOI] [PubMed] [Google Scholar]
- [58].Duhaut SD, Dimmock NJ, Heterologous protection of mice from a lethal human H1N1 influenza A virus infection by H3N8 equine defective interfering virus: Comparison of defective RNA sequences isolated from the DI inoculum and mouse lung, Virology. 248 (1998) 241–253. doi: 10.1006/viro.1998.9267. [DOI] [PubMed] [Google Scholar]
- [59].Noble S, Dimmock NJ, Characterization of putative defective interfering (DI) A/WSN RNAs isolated from the lungs of mice protected from an otherwise lethal respiratory infection with influenza virus A/WSN (H1N1): A subset of the inoculum DI RNAs, Virology. 210 (1995) 9–19. doi: 10.1006/viro.1995.1312. [DOI] [PubMed] [Google Scholar]
- [60].Fujii Y, Goto H, Watanabe T, Yoshida T, Kawaoka Y, Selective incorporation of influenza virus RNA segments into virions, Proc. Natl. Acad. Sci 100 (2003) 2002–2007. doi: 10.1073/pnas.0437772100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Dos Santos Afonso E, Escriou N, Leclercq I, Van Der Werf S, Naffakh N, The generation of recombinant influenza A viruses expressing a PB2 fusion protein requires the conservation of a packaging signal overlapping the coding and noncoding regions at the 5′ end of the PB2 segment, Virology. 341 (2005) 34–46. doi: 10.1016/j.virol.2005.06.040. [DOI] [PubMed] [Google Scholar]
- [62].Liang Y, Hong Y, Parslow TG, Liang Y, Hong Y, Parslow TG, cis -Acting Packaging Signals in the Influenza Virus PB1, PB2, and PA Genomic RNA Segments cis -Acting Packaging Signals in the Influenza Virus PB1, PB2, and PA Genomic RNA Segments, J. Virol 79 (2005) 10348–10355. doi: 10.1128/JVI.79.16.10348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Marsh GA, Hatami R, Palese P, Specific Residues of the Influenza A Virus Hemagglutinin Viral RNA Are Important for Efficient Packaging into Budding Virions, J. Virol 81 (2007) 9727–9736. doi: 10.1128/JVI.01144-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Muramoto Y, Takada A, Fujii K, Noda T, Iwatsuki-Horimoto K, Watanabe S, Horimoto T, Kida H, Kawaoka Y, Hierarchy among viral RNA (vRNA) segments in their role in vRNA incorporation into influenza A virions., J. Virol 80 (2006) 2318–25. doi: 10.1128/JVI.80.5.2318-2325.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Ozawa M, Fujii K, Muramoto Y, Yamada S, Yamayoshi S, Takada A, Goto H, Horimoto T, Kawaoka Y, Contributions of Two Nuclear Localization Signals of Influenza A Virus Nucleoprotein to Viral Replication, J. Virol 81 (2007) 30–41. doi: 10.1128/JVI.01434-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Ozawa M, Maeda J, Iwatsuki-Horimoto K, Watanabe S, Goto H, Horimoto T, Kawaoka Y, Nucleotide Sequence Requirements at the 5’ End of the Influenza A Virus M RNA Segment for Efficient Virus Replication, J. Virol 83 (2009) 3384–3388. doi: 10.1128/JVI.02513-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Watanabe T, Watanabe S, Noda T, Fujii Y, Kawaoka Y, Exploitation of Nucleic Acid Packaging Signals To Generate a Novel Influenza Virus-Based Vector Stably Expressing Two Foreign Genes, J. Virol 77 (2003) 10575–10583. doi: 10.1128/JVI.77.19.10575-10583.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Goto H, Muramoto Y, Noda T, Kawaoka Y, The Genome-Packaging Signal of the Influenza A Virus Genome Comprises a Genome Incorporation Signal and a Genome-Bundling Signal, J. Virol 87 (2013) 11316–11322. doi: 10.1128/JVI.01301-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Cobbin JCA, Ong C, Verity E, Gilbertson BP, Rockman SP, Brown L, Influenza Virus PB1 and Neuraminidase Gene Segments Can Cosegregate during Vaccine Reassortment Driven by Interactions in the PB1 Coding Region, J. Virol 88 (2014) 8971–8980. doi: 10.1128/JVI.01022-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Gilbertson B, Zheng T, Gerber M, Printz-Schweigert A, Ong C, Marquet R, Isel C, Rockman S, Brown L, Influenza NA and PB1 gene segments interact during the formation of viral progeny: Localization of the binding region within the PB1 gene, Viruses. 8 (2016) 1–17. doi: 10.3390/v8080238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Gog JR, Dos Santos Afonso E, Dalton RM, Leclercq I, Tiley L, Elton D, von Kirchbach JC, Naffakh N, Escriou N, Digard P, Codon conservation in the influenza A virus genome defines RNA packaging signals, Nucleic Acids Res. 35 (2007) 1897–1907. doi: 10.1093/nar/gkm087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Bolte H, Rosu ME, Hagelauer E, García-Sastre A, Schwemmle M, Packaging of the influenza A virus genome is governed by a plastic network of RNA/protein interactions., J. Virol (2018). doi: 10.1128/JVI.01861-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Fournier E, Moules V, Essere B, Paillart JC, Sirbat JD, Isel C, Cavalier A, Rolland JP, Thomas D, Lina B, Marquet R, A supramolecular assembly formed by influenza A virus genomic RNA segments, Nucleic Acids Res. 40 (2012) 2197–2209. doi: 10.1093/nar/gkr985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Fournier E, Moules V, Essere B, Paillart JC, Sirbat JD, Cavalier A, Rolland JP, Thomas D, Lina B, Isel C, Marquet R, Interaction network linking the human H3N2 influenza A virus genomic RNA segments, Vaccine. 30 (2012) 7359–7367. doi: 10.1016/j.vaccine.2012.09.079. [DOI] [PubMed] [Google Scholar]
- [75].Gavazzi C, Isel C, Fournier E, Moules V, Cavalier A, Thomas D, Lina B, Marquet R, An in vitro network of intermolecular interactions between viral RNA segments of an avian H5N2 influenza A virus: Comparison with a human H3N2 virus, Nucleic Acids Res. 41 (2013) 1241–1254. doi: 10.1093/nar/gks1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Gavazzi C, Yver M, Isel C, Smyth RP, Rosa-Calatrava M, Lina B, Moules V, Marquet R, A functional sequence-specific interaction between influenza A virus genomic RNA segments, Proc. Natl. Acad. Sci 110 (2013) 16604–16609. doi: 10.1073/pnas.1314419110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Bernadeta Dadonaite1 AL, Barilaite1 Egle, Fodor1 Ervin, Bauer DLV, The structure of the influenza A virus genome, BioRxiv. (2017). http://www.albayan.ae. [Google Scholar]
- [78].Gultyaev AP, Tsyganov-Bodounov A, Spronken MIJ, Van Der Kooij S, Fouchier RAM, Olsthoorn RCL, RNA structural constraints in the evolution of the influenza A virus genome NP segment, RNA Biol. 11 (2014) 942–952. doi: 10.4161/rna.29730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Kobayashi Y, Dadonaite B, van Doremalen N, Suzuki Y, Barclay WS, Pybus OG, Computational and molecular analysis of conserved influenza A virus RNA secondary structures involved in infectious virion production, RNA Biol. 13 (2016) 883–894. doi: 10.1080/15476286.2016.1208331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Moss WN, Priore SF, Turner DH, Identification of potential conserved RNA secondary structure throughout influenza A coding regions., RNA. 17 (2011) 991–1011. doi: 10.1261/rna.2619511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Gultyaev AP, Spronken MI, Richard M, Schrauwen EJA, Olsthoorn RCL, Fouchier RAM, Subtype-specific structural constraints in the evolution of influenza A virus hemagglutinin genes., Sci. Rep 6 (2016) 38892. doi: 10.1038/srep38892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Klumpp K, Ruigrok RWH, Baudin F, Roles of the influenza virus polymerase and nucleoprotein in forming a functional RNP structure, EMBO J. 16 (1997) 1248–1257. doi: 10.1093/emboj/16.6.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Gerber M, Isel C, Moules V, Marquet R, Selective packaging of the influenza A genome and consequences for genetic reassortment, Trends Microbiol. 22 (2014) 446–455. doi: 10.1016/j.tim.2014.04.001. [DOI] [PubMed] [Google Scholar]
- [84].ying Chou Y, Heaton NS, Gao Q, Palese P, Singer R, Lionnet T, Colocalization of Different Influenza Viral RNA Segments in the Cytoplasm before Viral Budding as Shown by Single-molecule Sensitivity FISH Analysis, PLoS Pathog. 9 (2013). doi: 10.1371/journal.ppat.1003358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Lakdawala S, Wu Y, Wawrzusin P, Kabat J, Broadbent AJ, Lamirande EW, Fodor E, Altan-Bonnet N, Shroff H, Subbarao K, Influenza A Virus Assembly Intermediates Fuse in the Cytoplasm, PLoS Pathog. 10 (2014). doi: 10.1371/journal.ppat.1003971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Bruce EA, Digard P, Stuart AD, The Rab11 Pathway Is Required for Influenza A Virus Budding and Filament Formation, J. Virol 84 (2010) 5848–5859. doi: 10.1128/JVI.00307-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Eisfeld AJ, Kawakami E, Watanabe T, Neumann G, Kawaoka Y, RAB11A Is Essential for Transport of the Influenza Virus Genome to the Plasma Membrane, J. Virol 85 (2011) 6117–6126. doi: 10.1128/JVI.00378-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Amorim MJ, Bruce EA, Read EKC, Foeglein A, Mahen R, Stuart AD, Digard P, A Rab11- and Microtubule-Dependent Mechanism for Cytoplasmic Transport of Influenza A Virus Viral RNA, J. Virol 85 (2011) 4143–4156. doi: 10.1128/JVI.02606-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Momose F, Sekimoto T, Ohkura T, Jo S, Kawaguchi A, Nagata K, Morikawa Y, Apical transport of influenza A virus ribonucleoprotein requires Rab11-positive recycling endosome, PLoS One. 6 (2011) 1–15. doi: 10.1371/journal.pone.0021123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].De Castro Martin IF, Fournier G, Sachse M, Pizarro-Cerda J, Risco C, Naffakh N, Influenza virus genome reaches the plasma membrane via a modified endoplasmic reticulum and Rab11-dependent vesicles, Nat. Commun 8 (2017). doi: 10.1038/s41467-017-01557-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Gao Q, Chou Y-Y, Doganay S, Vafabakhsh R, Ha T, Palese P, The Influenza A Virus PB2, PA, NP, and M Segments Play a Pivotal Role during Genome Packaging, J. Virol 86 (2012) 7043–7051. doi: 10.1128/JVI.00662-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Luo W, Zhang J, Liang L, Wang G, Li Q, Zhu P, Zhou Y, Li J, Zhao Y, Sun N, Huang S, Zhou C, Chang Y, Cui P, Chen P, Jiang Y, Deng G, Bu Z, Li C, Jiang L, Chen H, Phospholipid scramblase 1 interacts with influenza A virus NP, impairing its nuclear import and thereby suppressing virus replication, PLoS Pathog. 14 (2018) 1–26. doi: 10.1371/journal.ppat.1006851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Götz V, Magar L, Dornfeld D, Giese S, Pohlmann A, Höper D, Kong BW, Jans DA, Beer M, Haller O, Schwemmle M, Influenza A viruses escape from MxA restriction at the expense of efficient nuclear vRNP import, Sci. Rep 6 (2016) 1–15. doi: 10.1038/srep23138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Babcock HP, Chen C, Zhuang X, Using single-particle tracking to study nuclear trafficking of viral genes., Biophys. J 87 (2004) 2749–2758. doi: 10.1529/biophysj.104.042234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Qin C, Li W, Li Q, Yin W, Zhang X, Zhang Z, Zhang X-E, Cui Z, Real-time dissection of dynamic uncoating of individual influenza viruses, Proc. Natl. Acad. Sci (2019) 201812632. doi: 10.1073/pnas.1812632116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Westgeest KB, Russell CA, Lin X, Spronken MIJ, Bestebroer TM, Bahl J, Van Beek R, Skepner E, Halpin RA, De Jong JC, Rimmelzwaan GF, Osterhaus ADME, Smith DJ, Wentworth DE, Fouchier RAM, Graaf D, Genomewide Analysis of Reassortment and Evolution of Human Influenza A (H3N2) Viruses Circulating between 1968 and 2011, 88 (2014) 2844–2857. doi: 10.1128/JVI.02163-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Essere B, Yver M, Gavazzi C, Terrier O, Isel C, Fournier E, Giroux F, Textoris J, Julien T, Socratous C, Rosa-Calatrava M, Lina B, Marquet R, Moules V, Critical role of segment-specific packaging signals in genetic reassortment of influenza A viruses, Proc. Natl. Acad. Sci 110 (2013) E3840–E3848. doi: 10.1073/pnas.1308649110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Lubeck MD, Palese P, Schulman JL, Nonranom association of parental genes in influenza A virus recombinants, Virology. 95 (1979) 269–274. doi: 10.1016/0042-6822(79)90430-6. [DOI] [PubMed] [Google Scholar]
- [99].Greenbaum BD, Li OTW, Poon LLM, Levine AJ, Rabadan R, Viral reassortment as an information exchange between viral segments, Proc. Natl. Acad. Sci 109 (2012) 3341–3346. doi: 10.1073/pnas.1113300109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].White MC, Lowen AC, Implications of segment mismatch for influenza A virus evolution, J. Gen. Virol 99 (2018) 3–16. doi: 10.1099/jgv.0.000989. [DOI] [PMC free article] [PubMed] [Google Scholar]