Abstract
Introduction: Compounds present in Cannabis sativa such as phytocannabinoids and terpenoids may act in concert to elicit therapeutic effects. Cannabinoids such as Δ9-tetrahydrocannabinol (Δ9-THC) directly activate cannabinoid receptor 1 (CB1) and cannabinoid receptor 2 (CB2); however, it is not known if terpenoids present in Cannabis also affect cannabinoid receptor signaling. Therefore, we examined six common terpenoids alone, and in combination with cannabinoid receptor agonists, on CB1 and CB2 signaling in vitro.
Materials and Methods: Potassium channel activity in AtT20 FlpIn cells transfected with human CB1 or CB2 receptors was measured in real time using FLIPR® membrane potential dye in a FlexStation 3 plate reader. Terpenoids were tested individually and in combination for periods up to 30 min. Endogenous somatostatin receptors served as a control for direct effects of drugs on potassium channels.
Results: α-Pinene, β-pinene, β-caryophyllene, linalool, limonene, and β-myrcene (up to 30–100 μM) did not change membrane potential in AtT20 cells expressing CB1 or CB2, or affect the response to a maximally effective concentration of the synthetic cannabinoid CP55,940. The presence of individual or a combination of terpenoids did not affect the hyperpolarization produced by Δ9-THC (10 μM): (CB1: control, 59%±7%; with terpenoids (10 μM each) 55%±4%; CB2: Δ9-THC 16%±5%, with terpenoids (10 μM each) 17%±4%). To investigate possible effect on desensitization of CB1 responses, all six terpenoids were added together with Δ9-THC and signaling measured continuously over 30 min. Terpenoids did not affect desensitization, after 30 min the control hyperpolarization recovered by 63%±6% in the presence of the terpenoids recovery was 61%±5%.
Discussion: None of the six of the most common terpenoids in Cannabis directly activated CB1 or CB2, or modulated the signaling of the phytocannabinoid agonist Δ9-THC. These results suggest that if a phytocannabinoid–terpenoid entourage effect exists, it is not at the CB1 or CB2 receptor level. It remains possible that terpenoids activate CB1 and CB2 signaling pathways that do not involve potassium channels; however, it seems more likely that they may act at different molecular target(s) in the neuronal circuits important for the behavioral effect of Cannabis.
Keywords: phytocannabinoid, cannabinoid receptor, terpenoid, entourage effect, THC, signaling
Introduction
An enduring notion in the medicinal Cannabis and cannabinoid field is that of entourage: the idea that use of the whole plant may exert substantially greater effects than the sum of its individual parts.1 Entourage is usually construed as a positive attribute, with the assumption that superior therapeutic actions, or a more favorable “high,” will be obtained from consuming the whole Cannabis plant rather than individual components such as Δ9-tetrahydrocannabinol (Δ9-THC). Somewhat surprisingly, the evidence for this widely cited notion is relatively sparse.
Cannabis contains ∼150 phytocannabinoids, the most common of which are Δ9-THC and cannabidiol (CBD), together with their acid precursors THCA and CBDA.2 Cannabis also contains a large number of monoterpene and sesquiterpene compounds (together called terpenoids), the most common of which include α-pinene, β-pinene, linalool, limonene and β-myrcene (monoterpenes) and β-caryophyllene and caryophyllene oxide (sesquiterpenes).3 Terpenoids are volatile compounds that are synthesized alongside phytocannabinoids mainly in the trichomes of the cannabis plant, and provide cannabis with its distinctive aroma and flavor.4 Terpenoids are often lost if the extraction process involves heating.5
The entourage concept applied to cannabis can encompass the potential for both cannabinoid–cannabinoid and cannabinoid–terpenoid interactions. With regard to the former, Δ9-THC-CBD synergy in producing analgesia was reported in an animal model of neuropathic pain6 while in humans, CBD has been proposed to ameliorate some of the adverse psychotomimetic and anxiogenic effects of Δ9-THC.7,8 This claim is controversial, however, with a number of contrary findings.9,10 CBD may modulate Δ9-THC effects at the receptor level acting as a CB1 negative allosteric modulator,11 providing some biological plausibility to a modulatory interaction.
Scientific evidence for cannabinoid–terpenoid interactions is essentially absent, and mostly comes from websites and dispensaries extolling the virtues of proprietary Cannabis chemical varieties, or chemovars.12,13 However, some terpenoids do have intrinsic psychoactive and physiological effects, and modulatory effects on Δ9-THC actions are not farfetched.1,14 For example, in studies with laboratory animals, limonene displayed anxiolytic effects, pinene increased gastrointestinal motility, linalool was sedative, anticonvulsant, and anxiolytic, while myrcene produced sedation, analgesia, and muscle relaxant effects (summarized in Russo and Marcu14). Lewis et al.13 reported that in a low terpenoids variety (1.1% terpenoids) myrcene concentration is 0.45%, while in a high variety (4.8% total) myrcene concentration is as high as 3.44%. Compelling evidence for cannabinoid–terpenoid interactions or synergy does not yet exist. A report on perceived efficacy of Cannabis for childhood epilepsy identified the presence of three predominant terpenoids (β-caryophyllene, β-myrcene, and α-pinene); however, when extracts perceived as “effective” were compared with “ineffective” extracts, differences in terpenoid profile/content were not significant.15
With so many bioactive components present in cannabis, the systematic, granular elucidation of possible entourage effects poses a substantial combinatorial puzzle and scientific challenge. As a preliminary approach to addressing this challenge, this study examined whether the effects of Δ9-THC on its cognate cannabinoid receptors (CB1 and CB2) would be modified in the presence of terpenoids that are commonly found in cannabis, either alone or in combination. The demonstration of such a receptor-level entourage effect might lead to predictions regarding functional cannabinoid–terpenoid interaction effects that could be tested in vivo.
Materials and Methods
Cell culture
Experiments used mouse wild-type AtT20 FlpIn cells (AtT20-WT), or these cells stably transfected with human CB1 or CB2 receptors with 3×N-terminus hemagglutinin tags (AtT20-CB1 and AtT20-CB2, respectively).16 Cells were cultivated in Dulbecco's modified Eagle's medium (DMEM; Sigma-Aldrich) supplemented with 10% fetal bovine serum (FBS; Sigma/SAFC) and 100 U penicillin/100 μg streptomycin mL−1 (Gibco). Selection antibiotics were 80 μg mL−1 Zeocin (Invivogen) for AtT20-WT or 80 μg mL−1 hygromycin B Gold (Invivogen) for transfected cells.
Cells were grown in 75 mm2 flasks at 37°C/5% CO2 and passaged when 80–90% confluent. Assays were carried out on cells up to 20 passages in culture.
Potassium channel activity measurements
Changes in membrane potential were measured using the FLIPR® blue membrane potential dye (Molecular Devices) in a FlexStation 3, as outlined in Knapman 2013.17 Cells from a 90–100% confluent 75 mm2 flask were resuspended in Leibovitz's L-15 Medium (Gibco) supplemented with 1% FBS, 100 U penicillin/100 μg streptomycin mL−1, and glucose (15 mM) and plated in 96-well black-walled clear bottom microplates (Costar) in a volume of 90 μL per well. Cells were incubated overnight in humidified ambient air at 37°C incubator. Membrane potential dye, used at 50% of the manufacturer-recommended concentration, was resuspended in Hank's Balanced Salt Solution (HBSS) of composition (in mM): NaCl 145, HEPES 22, Na2HPO4 0.338, NaHCO3 4.17, KH2PO4 0.441, MgSO4 0.407, MgCl2 0.493, CaCl2 1.26, glucose 5.55 (pH 7.4, osmolarity 315±15). Dye was loaded onto each well (90 μL per well) and equilibrated at 37°C for ∼1 h before assay. Fluorescence was measured every 2 sec (λ excitation=530 nm, λ emission=565 nm, λ emission cutoff=550 nm). Assays were carried out at 37°C, and drugs were automatically added in volumes of 20 μL.
Determining the effects of terpenoids on acute hyperpolarization
Terpenoids were added after ∼60 sec of baseline recording and incubated for 5 min before cannabinoid (CP55,940 or Δ9-THC) addition. In AtT20-WT cells, somatostatin (SST) was added instead of cannabinoid.
Determining the effects of terpenoids on signaling desensitization
Homologous desensitization was measured by simultaneously adding Δ9-THC with terpenoids after 120 sec of baseline recording. Signaling desensitization was calculated as percentage decrease from peak Δ9-THC response after 30 min in drugs. SST (100 nM) was added 30 min after Δ9-THC addition to examine the potential effects of prolonged cannabinoid receptor activation on native SST receptors (heterologous desensitization). The SST response was compared between groups (with or without terpenoids).
Drug dilution
All drugs (except SST) were prepared in dimethyl sulfoxide (DMSO) and stored as frozen stocks at a concentration of 10–100 mM. Terpenoid stock solution concentrations were 100 mM, with the exception of β-myrcene (30 mM), which was insoluble at 100 mM. SST was dissolved in water. Fresh aliquots were used each day, with the drugs diluted in HBSS containing 0.1% bovine serum albumin (Sigma-Aldrich) immediately before the assay. The final concentration of DMSO in each well was 0.1–0.11%; this limited the maximum concentration of terpenoids able to be tested. A vehicle (HBSS plus solvent alone) well was included in each column of the 96-well plate, and the changes in fluorescence produced by vehicle alone were subtracted before determining the maximum hyperpolarization after each drug exposure.
Drugs and reagents
Δ9-THC was obtained from THCPharm (Frankfurt, Germany). Terpenoids were obtained from Sigma-Aldrich; (+)-α-pinene, (+)-β-pinene, (−)-β-caryophyllene, (+/−)-linalool, (R)-(+)-limonene, and β-myrcene. SST was obtained from Auspep and CP55,940 from Cayman. Unless otherwise indicated, the other chemicals and reagents were obtained from Sigma-Aldrich.
Data analysis
Each experiment was independently repeated at least five times, with two technical replicates in each determination. Data are expressed as a percentage change in the fluorescence compared with the predrug baseline (30 sec before drug addition) or as a percentage of 1 μM CP55,940 response. Graphs were plotted using Graphpad Prism 7.02, and scatter dot plots show means with standard error of the mean. Means were compared using unpaired Student's t-test or no matching one-way analysis of variance, followed by correction for multiple comparisons (Dunnett); and null hypothesis was rejected if p-value was <0.05 (p>0.05=not significant).
Results
Terpenoids in AtT20-WT cells
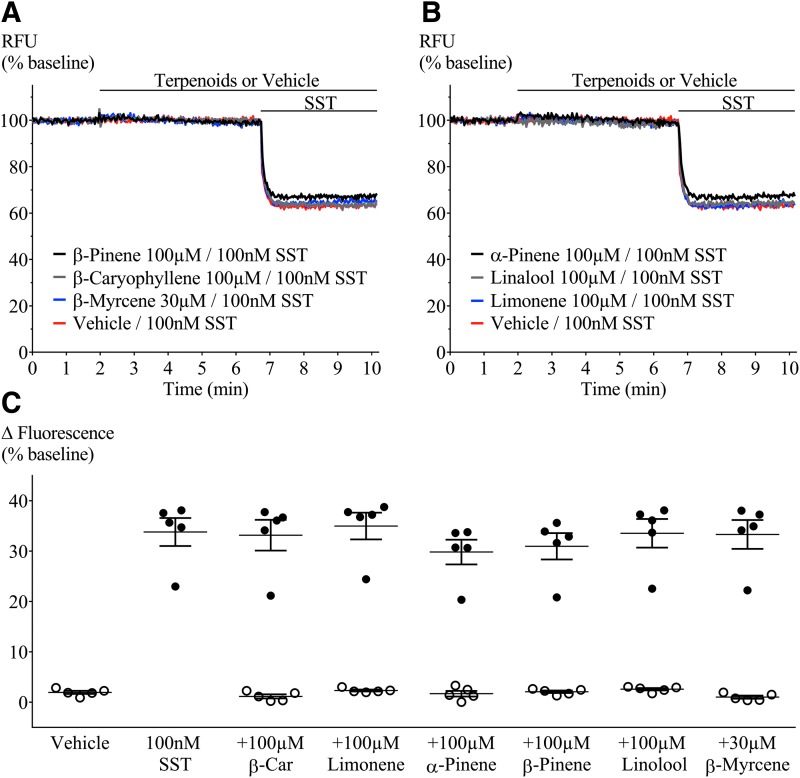
We first examined terpenoid action in nontransfected AtT20 cells. We used SST (100 nM) as a positive control because it hyperpolarizes AtT20-WT cells through activation of endogenous SST receptors (Fig. 1A, B).17,18 Addition of α-pinene, β-pinene, β-caryophyllene, linalool, limonene (100 μM), or β-myrcene (30 μM) did not affect the membrane potential of AtT20-WT cells (Fig. 1C, open circles). The presence of terpenoids (100 μM/30 μM) had no effect on the subsequent SST response (Fig. 1C).
FIG. 1.
Terpenoid- and SST-mediated fluorescence change in AtT20-WT. Representative traces showing change in fluorescence signal after terpenoid and SST (100 nM) application. A decrease in signal corresponds to membrane hyperpolarization. Addition of terpenoids (A) β-pinene, β-caryophyllene, and β-myrcene; (B) α-pinene, linalool, and limonene did not change baseline fluorescence, while SST mediated a clear hyperpolarization. (C) Percentage change of fluorescence from baseline after each terpenoid (open circles) and SST (closed circles) application. Terpenoids were added at 2 min; 5 min before SST. When compared with positive (SST) or negative (vehicle) controls, none of the terpenoids tested affected baseline membrane potential or peak SST response. β-Car=β-caryophyllene. n=5, SEM, one-way ANOVA p>0.05. Drugs were added for the duration of the bar. ANOVA, analysis of variance; SEM, standard error of the mean; SST, somatostatin.
Terpenoids in AtT20-CB1 and -CB2 cells
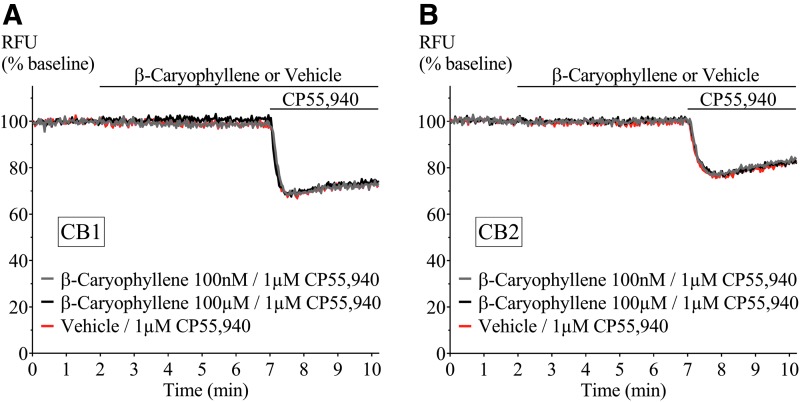
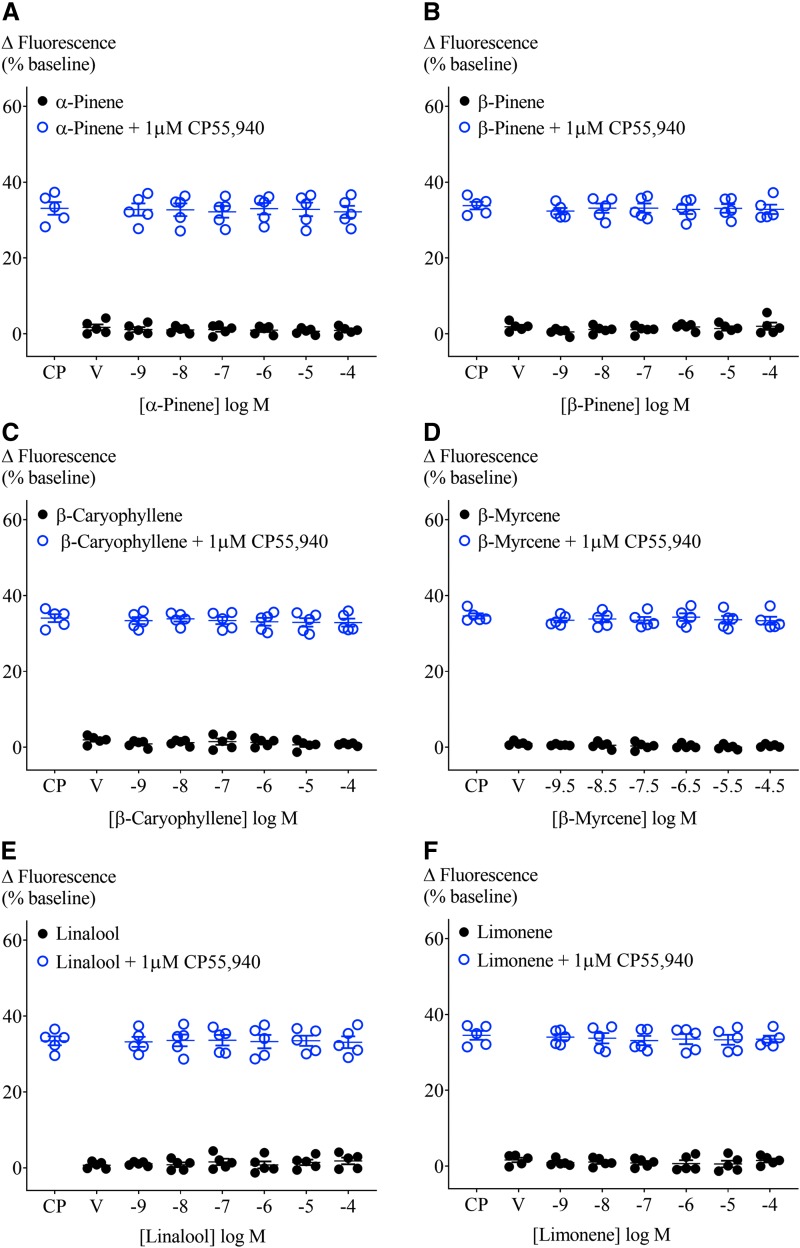
The absence of a terpenoid response in AtT20-WT cells enabled the study of their effect on membrane potential in AtT20 cells expressing human CB1 or CB2. We examined whether terpenoids (1 nM–100 μM, β-myrcene 300 pM–30 μM) hyperpolarized cells through these receptors and, in parallel, whether they affected a subsequent response to a maximally effective concentration of CP55,940 (1 μM; Fig. 2).16 A summary of the fluorescence change after terpenoid addition to AtT20-CB1 cells is shown in Figure 3 (closed circles). No difference between vehicle and terpenoids was observed. Further, none of the terpenoids changed the membrane potential of cells expressing CB2 (Supplementary Fig. S1). The change in fluorescence produced by the subsequent addition of the nonselective cannabinoid agonist CP55,940 (1 μM) was also unaffected in both AtT20-CB1 and -CB2 (Fig. 3 and Supplementary Fig. S1, open circles).
FIG. 2.
Representative traces of β-caryophyllene and CP55,940 in AtT20-CB1 and -CB2. Fluorescence was recorded for 10 min where β-caryophyllene (100 nM and 100 μM) was added at 2 min followed by incubation for 5 min, before 1 μM CP55,940 application. β-caryophyllene did not hyperpolarize (A) AtT20-CB1 and (B) AtT20-CB2 cells, or affect the response to CP55,940 (1 μM). Drugs were added for the duration of the bar. CB1, cannabinoid receptor 1, CB2, cannabinoid receptor 2.
FIG. 3.
Effect of terpenoids at varying concentrations on AtT20-CB1 membrane potential and on 1 μM CP55,940-induced hyperpolarization. Terpenoids (A) α-pinene, (B) β-pinene, (C) β-caryophyllene, (D) β-myrcene, (E) linalool, and (F) limonene were added to AtT20-CB1 cells and incubated for 5 min. Maximum fluorescence changes were not different from negative control (closed circles, n=5, SEM, one-way ANOVA p>0.05). CP55,940 (1 μM) addition to AtT20-CB1 cells induced fluorescence changes from 33.1%±1.7% to 34.6%±0.7%. Peak CP55,940 responses were not affected by the presence of terpenoids (open circles, n=5, SEM, one-way ANOVA p>0.05). V, vehicle.
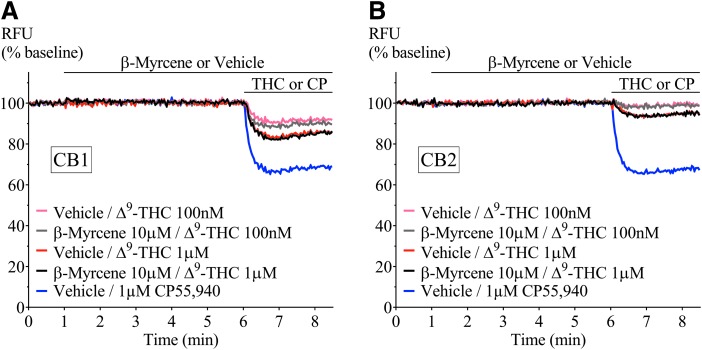
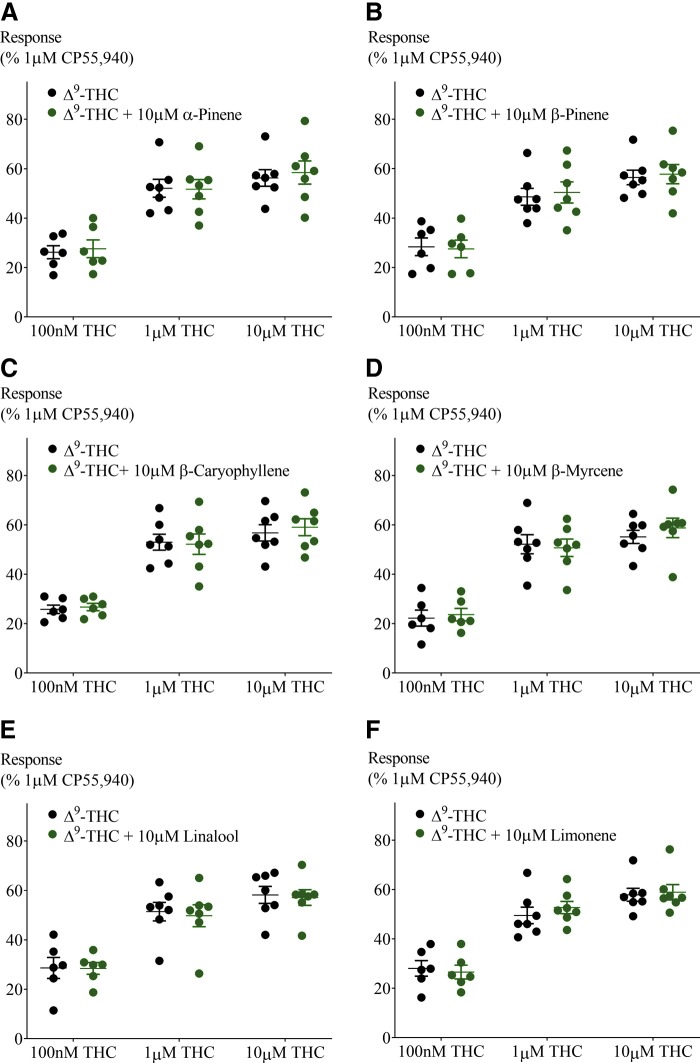
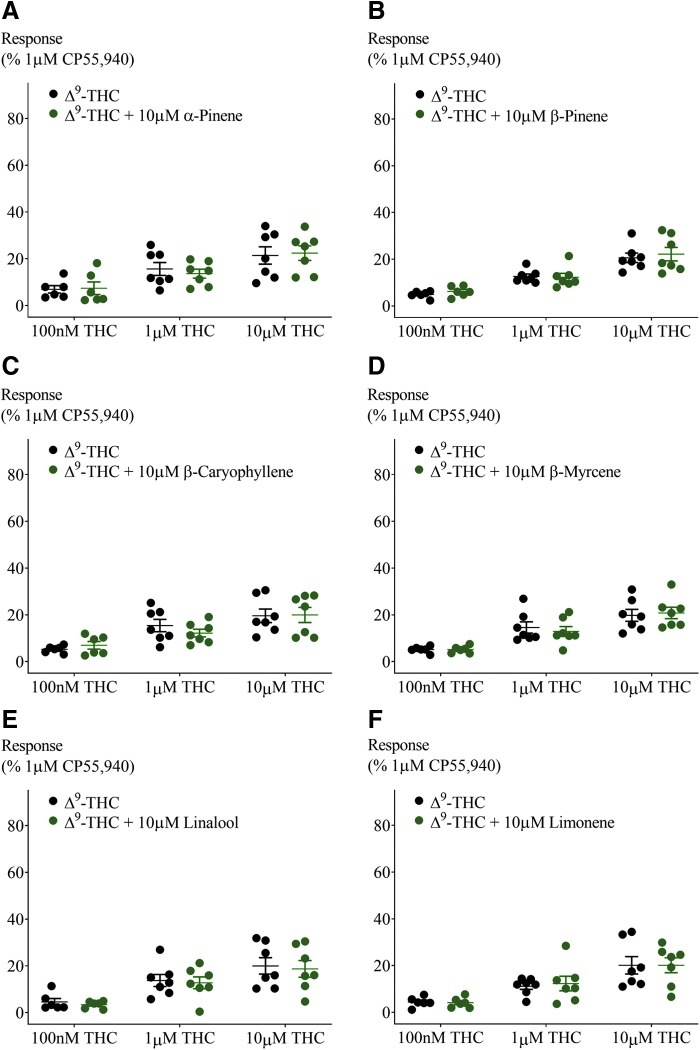
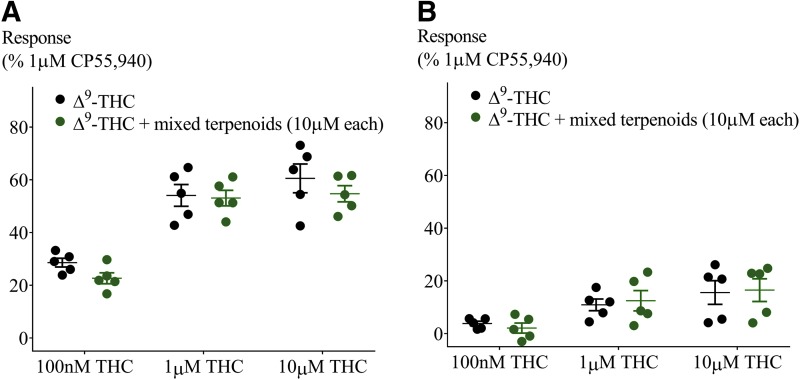
CP55,940 is a high-efficacy agonist of both CB1 and CB2 receptors.19 However, in Cannabis, Δ9-THC is the principle cannabinoid agonist, and it has a lower efficacy than CP55,940, which is apparent in the hyperpolarization assay as a lower maximal response.19 We next tested the effect of a low and high concentration of terpenoids (100 nM and 10 μM) on the hyperpolarization produced by three concentrations of Δ9-THC (100 nM, 1 and 10 μM). Application of Δ9-THC, after 5 min of individual terpenoid application, produced a fluorescence change (Fig. 4) that was not significantly different from that produced by Δ9-THC alone in both AtT20-CB1 and -CB2 cells (10 μM Δ9-THC, Figs. 5 and 6; 100 nM Δ9-THC, Supplementary Figs. S2 and S3). To explore the possibility of an emergent entourage effect, we combined all six terpenoids (10 μM each) and tested the effect of the mixture on the Δ9-THC-induced hyperpolarization. Similar to individually tested terpenoids, the effects of Δ9-THC were not changed by the mixture (Fig. 7).
FIG. 4.
Representative traces of β-myrcene and Δ9-THC in (A) AtT20-CB1 and (B) AtT20-CB2. Fluorescence change mediated by two submaximal concentrations of Δ9-THC (100 nM and 1 μM) in the presence of β-myrcene (10 μM). Terpenoid was added at 1 min and incubated for 5 min before Δ9-THC application. CP55,940 added as positive control. Drugs were added for the duration of the bar. Δ9-THC, Δ9-tetrahydrocannabinol.
FIG. 5.
Effect of 10 μM terpenoids on Δ9-THC-induced hyperpolarization in AtT20-CB1. Terpenoids tested were (A) α-pinene, (B) β-pinene, (C) β-caryophyllene, (D) β-myrcene, (E) linalool, and (F) limonene. Response to Δ9-THC at two submaximal and one maximal concentration (n=6–7, SEM, unpaired t-test p>0.13). Data presented as % of maximum CP55,940 (1 μM) response.
FIG. 6.
Effect of 10 μM terpenoids on Δ9-THC-induced hyperpolarization in AtT20-CB2. Terpenoids tested were (A) α-pinene, (B) β-pinene, (C) β-caryophyllene, (D) β-myrcene, (E) linalool, and (F) limonene. Response to Δ9-THC at two submaximal and one maximal concentration (n=6–7, SEM, unpaired t-test p>0.26). Data presented as % of maximum CP55,940 (1 μM) response.
FIG. 7.
Testing the “Entourage effect.” Effect of combination of six terpenoids at 10 μM each on Δ9-THC-induced hyperpolarization in (A) AtT20-CB1 and (B) AtT20-CB2. Response to Δ9-THC at two submaximal and one maximal concentration (n=5, SEM, unpaired t-test p>0.13). Data presented as % of maximum CP55,940 (1 μM) response.
Terpenoids and desensitization in AtT20-CB1
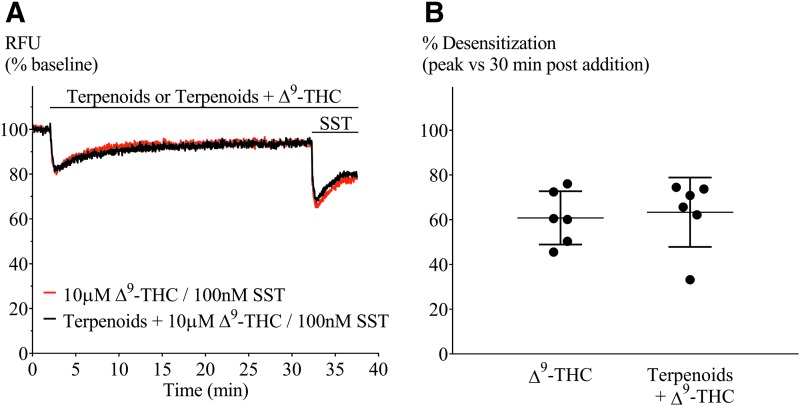
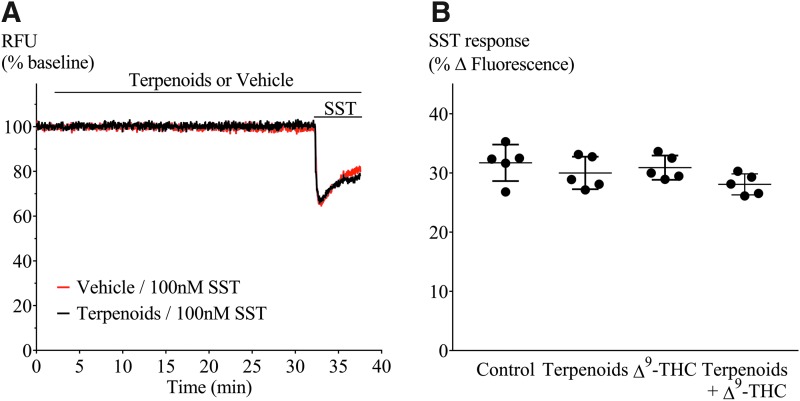
We have previously reported desensitization cannabinoid-mediated cellular hyperpolarization in AtT20 cells expressing rat or human CB1 receptors,20,21 and we found that this reversal of CP55,940-induced hyperpolarization was accelerated by negative allosteric modulators such as ORG27569 and PSNCBAM-1. Therefore, we tested whether terpenoids may act in a similar way to ORG27569 and other negative allosteric modulators, altering desensitization time course. We used Δ9-THC instead of CP55,940, as Δ9-THC is the main phytocannabinoid agonist. Prolonged application of Δ9-THC (10 μM) produced a hyperpolarization that reversed substantially over 30 min. Representative traces for this experiment are illustrated in Figure 8A. We measured the peak response to Δ9-THC and the signal remaining 30 min after agonist exposure, and quantified desensitization as a percentage decline in the peak response. The Δ9-THC (10 μM) signal desensitized by 63%±6%, in the presence of the terpenoid mix desensitization, was 61%±5% (Fig. 8B). Thus, terpenoids did not interfere with desensitization of CB1 signaling produced by Δ9-THC. We also assessed the capacity of Δ9-THC alone, terpenoids alone (10 μM each), or terpenoids combined with Δ9-THC to affect SST receptor signaling in AtT20-CB1 cells (heterologous desensitization). SST (100 nM) was applied 30 min after first drug application (Figs. 8A and 9A), and the hyperpolarization produced by SST after Δ9-THC, terpenoids alone, or Δ9-THC with terpenoids was not significantly different from that produced by SST alone (p>0.05, Fig. 9B).
FIG. 8.
Terpenoids on Δ9-THC-mediated desensitization in AtT20-CB1. (A) Representative traces of hyperpolarization and signal desensitization mediated by Δ9-THC alone (10 μM, black) or with terpenoids (10 μM each, red). Cells were then challenged with SST (100 nM) after 30 min to examine heterologous desensitization. (B) Percentage desensitization after 30 min exposure to Δ9-THC alone (10 μM) or in the presence of terpenoids (10 μM each), compared with peak fluorescence response. Terpenoids did not affect Δ9-THC-mediated desensitization (n=5, SEM, unpaired t-test p=0.76). Drugs were added for the duration of the bar.
FIG. 9.
SST challenge of AtT20-CB1 cells to investigate heterologous desensitization. (A) Representative traces of cells preincubated with (black) or without (red) terpenoids for 30 min before SST (100 nM) challenge. (B) Comparison of peak hyperpolarization (% fluorescence change) obtained after SST (100 nM) challenge (n=5, one-way ANOVA p>0.05). Drugs were added for the duration of the bar.
Discussion
The principal finding of this study is that agonist activation of CB1 and CB2 receptors is not obviously altered by any or all of the six major terpenoids from Cannabis sativa. The terpenoids tested did not activate CB1 or CB2 by themselves, nor did they modify the signaling of the high-efficacy agonist CP55,940 or the lower efficacy agonist Δ9-THC. In particular, Δ9-THC effects would be expected to be very sensitive to the presence of drugs that inhibited (or enhanced) signaling at the receptor. There are no spare receptors for Δ9-THC in this assay, and changes in ligand binding would be directly reflected as a change in the maximum response. The lack of effect of terpenoids on the response to the synthetic cannabinoid CP55,940 indicates that terpenoids do not interfere with maximal cannabinoid receptor-mediated hyperpolarization, suggesting no direct modulation of the potassium channel response. This was confirmed by the lack of effect of terpenoids on the response to SST.
A previous study showed that β-caryophyllene is a CB2 agonist.22 However, we were unable to detect any effect of β-caryophyllene on CB2 signaling in this study. The reasons for this are unclear, but the efficacy of β-caryophyllene has not been defined in cellular assays and may be lower than that of Δ9-THC. The CB2 response to even high concentrations of Δ9-THC in our assay is small, suggesting that productive coupling of CB2 to endogenous potassium channels in AtT20 cells requires high-efficacy agonists. The affinity of β-caryophyllene for CB2 (155 nM) has been determined in membranes from HEK293 cells heterologously expressing CB2,22 but is not known in intact cells. Its EC50 for inhibition of forskolin-induced adenylyl cyclase in CHO-K1 expressing CB2 was ∼2 μM,22 suggesting a low functional affinity, which may not be sufficient to significantly affect the rapid response to the higher affinity agonist Δ9-THC.
The role of terpenoids in cannabis-induced analgesia in rats was recently evaluated by Harris et al.23 They tested THC, isolated terpenoids, extract without terpenoids, and full extract, and suggested that the analgesic effect of cannabis is mainly due to THC presence and proposed that terpenoids do not contribute to cannabis-mediated analgesia. These findings support our results, and interestingly their extract had a very high percentage of β-caryophyllene.
Positive and negative allosteric modulators have been reported for CB1,24,25 and the effects of several negative allosteric modulators have been defined in the hyperpolarization assay used here.20 Both PSNCBAM-1 and ORG27569 enhanced CP55,940 signal desensitization, while PSNCBAM-1 also inhibited the initial CP55,940 hyperpolarization. Coapplication of the terpenoids with Δ9-THC failed to affect the peak response, or the degree of tachyphylaxis observed over a 30-min exposure to drug, suggesting that they are not acting as allosteric modulators of this CB1 signaling pathway.
Limitations
A limitation of this study is that we only examined CB1 and CB2 signaling through one pathway, involving Gi/o. The hyperpolarization of the AtT20 cells likely represents G-protein-mediated activation of inwardly rectifying potassium channels (GIRK), as previously described for CB1 and other GPCR in these cells as well as in several different neurons.26–28 Cannabinoid receptors couple to multiple G proteins as well as signaling through other pathways such as those dependent on arrestins, and it is possible that entourage effects of terpenoids are mediated through modulation of a subset of the cannabinoid receptor signaling repertoire.26 CB1 and CB2 receptors can be activated in a ligand-biased manner—the phenomenon where a drug preferentially activates a subset of the signaling pathways that the receptor can access.29 In general, this bias has been best defined for G protein coupling versus activation of arrestin-mediated signaling, but to our knowledge there are no examples of cannabinoid ligands only affecting arrestin-mediated signaling.19,30 It remains possible that terpenoids have such an absolute bias, but this would be unprecedented, and in any case recruitment of arrestin would be expected to produce enhanced desensitization of the CB1 responses to prolonged agonist exposure.20,29 Any subtle change to receptor signaling should be clear with use of the low-efficacy agonist Δ9-THC.
Overall, our data suggest that it is unlikely that the terpenoids studied here affect Δ9-THC interactions with cannabinoid receptors. However, this is not a definitive rebuttal of the entourage effect. Our study cannot address the possibility of entourage effects emerging through effects of terpenoids on cannabinoid metabolism and distribution as well as interaction with other G-protein-coupled receptors, ligand-gated ion channels, signaling cascades present on the same cells that express cannabinoid receptors, or on other cells up or downstream of the cannabinoid receptor expressing cells. There are many other ways that these molecules could interact with cannabinoids to influence the overall therapeutic and subjective outcomes of cannabis administration, and it should be acknowledged that Δ9-THC influences signaling at a wide variety of other noncannabinoid receptor targets (see Banister et al.31 for a review). Terpenoids may even have primary effects on distinct functional modules that together with cannabinoid receptor-modulated pathways are ultimately integrated into a behavioral or physiological output. So the quest for entourage does not end here; in many ways, it has only just begun.
Supplementary Material
Acknowledgments
S.S. is a recipient of the Macquarie University Research Excellence Scholarship for International Students. We thank Lambert Initiative for Cannabinoid Therapeutics for funding this project.
Abbreviations Used
- Δ9-THC
Δ9-tetrahydrocannabinol
- β-Car
β-caryophyllene
- ANOVA
analysis of variance
- CBD
cannabidiol
- DMEM
Dulbecco's modified Eagle's medium
- DMSO
dimethyl sulfoxide
- FBS
fetal bovine serum
- HBSS
Hank's Balanced Salt Solution
- RFU
relative fluorescence units
- SEM
standard error of the mean
- SST
somatostatin
- V
vehicle
Author Disclosure Statement
M.S., S.S., and M.C. have no competing financial interests to disclose. I.S.M. currently acts as a consultant to Kinoxis Therapeutics. J.C.A. has acted as a consultant to the World Health Organization in the last 12 months in its review of cannabis and the cannabinoids. I.S.M. and J.C.A. are inventors on several patents involving cannabinoid therapeutics.
Supplementary Material
Cite this article as: Santiago M, Sachdev S, Arnold JC, McGregor IS, Connor M (2019) Absence of entourage: terpenoids commonly found in Cannabis sativa do not modulate the functional activity of Δ9-THC at human CB1 and CB2 receptors, Cannabis and Cannabinoid Research 4:3, 165–176, DOI: 10.1089/can.2019.0016.
References
- 1. Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol. 2011;163:1344–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hanuš LO, Meyer SM, Muñoz E, et al. . Phytocannabinoids: a unified critical inventory. Nat Prod Rep. 2016;33:1357–1392 [DOI] [PubMed] [Google Scholar]
- 3. Ibrahim EA, Wang M, Radwan MM, et al. . Analysis of Terpenes in Cannabis sativa L. Using GC/MS: Method Development, Validation, and Application. Planta Med. 2019;85:431–438 [DOI] [PubMed] [Google Scholar]
- 4. Pertwee RG. Handbook of cannabis. 1st ed. Oxford University Press: Oxford, United Kingdom, 2014 [Google Scholar]
- 5. Sexton M, Shelton K, Haley P, et al. . Evaluation of Cannabinoid and terpenoid content: Cannabis flower compared to supercritical CO2 concentrate. Planta Med. 2018;84:234–241 [DOI] [PubMed] [Google Scholar]
- 6. Casey SL, Atwal N, Vaughan CW. Cannabis constituent synergy in a mouse neuropathic pain model. Pain. 2017;158:2452–2460 [DOI] [PubMed] [Google Scholar]
- 7. Englund A, Freeman TP, Murray RM, et al. . Can we make cannabis safer? Lancet Psychiatry. 2017;4:643–648 [DOI] [PubMed] [Google Scholar]
- 8. Russo E, Guy GW. A tale of two cannabinoids: the therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Med Hypotheses. 2006;66:234–246 [DOI] [PubMed] [Google Scholar]
- 9. Haney M, Malcolm RJ, Babalonis S, et al. . Oral Cannabidiol does not alter the subjective, reinforcing or cardiovascular effects of smoked Cannabis. Neuropsychopharmacology. 2016;41:1974–1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ilan AB, Gevins A, Coleman M, et al. . Neurophysiological and subjective profile of marijuana with varying concentrations of cannabinoids. Behav Pharmacol. 2005;16:487–496 [DOI] [PubMed] [Google Scholar]
- 11. Laprairie RB, Bagher AM, Kelly ME, et al. . Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br J Pharmacol. 2015;172:4790–4805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Russo EB. The case for the entourage effect and conventional breeding of clinical Cannabis: no “Strain,” no gain. Front Plant Sci. 2018;9:1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lewis MA, Russo EB, Smith KM. Pharmacological foundations of Cannabis chemovars. Planta Med. 2018;84:225–233 [DOI] [PubMed] [Google Scholar]
- 14. Russo EB, Marcu J. Cannabis pharmacology: the usual suspects and a few promising leads. Adv Pharmacol. 2017;80:67–134 [DOI] [PubMed] [Google Scholar]
- 15. Suraev A, Lintzeris N, Stuart J, et al. . Composition and use of Cannabis extracts for childhood epilepsy in the Australian community. Sci Rep. 2018;8:10154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Banister SD, Longworth M, Kevin R, et al. . Pharmacology of valinate and tert-leucinate synthetic Cannabinoids 5F-AMBICA, 5F-AMB, 5F-ADB, AMB-FUBINACA, MDMB-FUBINACA, MDMB-CHMICA, and their analogues. ACS Chem Neurosci. 2016;7:1241–1254 [DOI] [PubMed] [Google Scholar]
- 17. Knapman A, Santiago M, Du YP, et al. . A continuous, fluorescence-based assay of mu-opioid receptor activation in AtT-20 cells. J Biomol Screen. 2013;18:269–276 [DOI] [PubMed] [Google Scholar]
- 18. Gunther T, Culler M, Schulz S. Research resource: real-time analysis of somatostatin and dopamine receptor signaling in pituitary cells using a fluorescence-based membrane potential assay. Mol Endocrinol. 2016;30:479–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Soethoudt M, Grether U, Fingerle J, et al. . Cannabinoid CB2 receptor ligand profiling reveals biased signalling and off-target activity. Nat Commun. 2017;8:13958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cawston EE, Redmond WJ, Breen CM, et al. . Real-time characterization of cannabinoid receptor 1 (CB1) allosteric modulators reveals novel mechanism of action. Br J Pharmacol. 2013;170:893–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cawston EE, Connor M, Di Marzo V, et al. . Distinct temporal fingerprint for cyclic adenosine monophosphate (cAMP) signaling of indole-2-carboxamides as allosteric modulators of the Cannabinoid receptors. J Med Chem. 2015;58:5979–5988 [DOI] [PubMed] [Google Scholar]
- 22. Gertsch J, Leonti M, Raduner S, et al. . Beta-caryophyllene is a dietary cannabinoid. Proc Natl Acad Sci U S A. 2008;105:9099–9104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harris HM, Rousseau MA, Wanas AS, et al. . Role of cannabinoids and terpenes in cannabis-mediated analgesia in rats. Cannabis and Cannabinoid Research. 2019;4:177–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Price MR, Baillie GL, Thomas A, et al. . Allosteric modulation of the cannabinoid CB1 receptor. Mol Pharmacol. 2005;68:1484–1495 [DOI] [PubMed] [Google Scholar]
- 25. Ignatowska-Jankowska BM, Baillie GL, Kinsey S, et al. . A Cannabinoid CB1 receptor-positive allosteric modulator reduces neuropathic pain in the mouse with no psychoactive effects. Neuropsychopharmacology. 2015;40:2948–2959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bacci A, Huguenard JR, Prince DA. Long-lasting self-inhibition of neocortical interneurons mediated by endocannabinoids. Nature. 2004;431:312–316 [DOI] [PubMed] [Google Scholar]
- 27. Marinelli S, Pacioni S, Cannich A, et al. . Self-modulation of neocortical pyramidal neurons by endocannabinoids. Nat Neurosci. 2009;12:1488–1490 [DOI] [PubMed] [Google Scholar]
- 28. Felder CC, Joyce KE, Briley EM, et al. . Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Mol Pharmacol. 1995;48:443–450 [PubMed] [Google Scholar]
- 29. Ibsen MS, Connor M, Glass M. Cannabinoid CB1 and CB2 receptor signaling and bias. Cannabis Cannabinoid Res. 2017;2:48–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Atwood BK, Wager-Miller J, Haskins C, et al. . Functional selectivity in CB(2) cannabinoid receptor signaling and regulation: implications for the therapeutic potential of CB(2) ligands. Mol Pharmacol. 2012;81:250–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Banister SD, Arnold JC, Connor M, et al. . Dark classics in chemical neuroscience: delta(9)-tetrahydrocannabinol. ACS Chem Neurosci. 2019;10:2160–2175 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.