Abstract
The neurotrophin receptor p75 can induce apoptosis both in vitro and in vivo. The mechanisms by which p75 induces apoptosis have remained mostly unknown. Here, we report that p75 activates Rac GTPase, which in turn activates c-junN-terminal kinase (JNK), including an injury-specific JNK3, in an NGF-dependent manner. N17Rac blocks this JNK activation and subsequent NGF-dependent apoptosis, indicating that activation of Rac GTPase is required for JNK activation and apoptosis induced by p75. In addition, p75-mediated Rac activation is modulated by coactivation of Trk, identifying Rac GTPase as one of the key molecules whose activity is critical for cell survival and death in neurotrophin signaling. The crucial role of the JNK pathway in p75 signaling is further confirmed by the results that blocking p75 from signaling via the JNK pathway or suppressing the JNK activity itself led to inhibition of NGF-dependent death. Together, these results indicate that the apoptotic machinery of p75 comprises Rac GTPase and JNK.
Keywords: apoptosis, Rac GTPase, c-jun N-terminal kinase, signal transduction, p75, NGF
NGF belongs to a family of neurotrophins whose primary role is the promotion of neuronal survival and differentiation. NGF exerts its role by activating two distinct types of receptors, TrkA, a receptor-tyrosine kinase, and p75, a member of the tumor necrosis factor receptor family (Lewin and Barde, 1996;Carter and Lewin, 1997). The role of TrkA is undisputed as a survival-promoting receptor. The role of p75, however, still remains controversial, in part because it is associated with the promotion of both apoptosis and survival.
In the sympathetic system, p75 is involved in the death of superior cervical ganglion neurons during development, but whether p75 also plays a similar role in the adult is still unclear (Bamji et al., 1998;Brennan et al., 1999). In the CNS, the role of p75 similarly remains controversial, because the absence of p75 resulted contradictorily in an increase, a decrease, and no change in the total number of basal forebrain neurons (Yeo et al., 1997; Peterson et al., 1999; Ward and Hagg, 1999). The difficulty of discerning the role of p75 in these systems may continue, given the complexity of p75 action in the presence of resident TrkA. One consistent piece of evidence emerging in the literature from these data is that p75 can induce apoptosis when activated by a neurotrophin in the absence of the Trk specific to that neurotrophin (Dechant and Barde, 1997). Conversely, when coexpressed with Trk, p75 augments Trk function.
These dichotomous roles for p75 are reflected in the signaling pathways it activates. For its role in survival, p75 is known to activate NF-κB (Carter et al., 1996). For its role in apoptosis, it has been shown to activate c-jun N-terminal kinase (JNK) (Casaccia-Bonnefil et al., 1996; Yoon et al., 1998) and caspases (Gu et al., 1999) and to induce ceramide production (Dobrowsky et al., 1994). Of these apoptotic pathways, the JNK pathway has been shown to be necessary (Yoon et al., 1998), but the mechanisms whereby p75 activates the JNK pathway are mostly unknown.
In this report, we present data demonstrating that p75 activates Rac GTPase, which in turn activates JNK in an NGF-dependent manner. This activation is required for p75-mediated apoptosis. As an experimental system for studying the apoptotic pathway of p75 without the interference of TrkA, we chose to use primary oligodendrocytes. In oligodendrocytes, NGF induces cell death, and p75 activates all the known apoptotic pathways, such as JNK, caspases, and ceramide production (Casaccia-Bonnefil et al., 1996; Gu et al., 1999). Using oligodendrocytes, we demonstrate that activation of Rac is prolonged by NGF but not by BDNF or neurotrophin 3 (NT3). This prolonged activation of Rac correlates with the ability of these neurotrophins to induce apoptosis, suggesting that the kinetics of Rac activation may determine the fate of a cell.
MATERIALS AND METHODS
Primary rat cortical oligodendrocyte culture
Primary oligodendrocyte cultures were obtained as described previously (Yoon et al., 1998), except that the cells were subjected to an immunopanning procedure using Ran2 antibody to remove astrocytes and microglias after an overnight shakeoff. The purity of cultures was determined based on staining with antibodies against galactocerebroside (O1), myelin basic protein (MBP), and glial fibrillary acidic protein (GFAP). Staining procedures are described below. In these cultures, 47% of the cells were O1+/MBP+, 17% were O1+/MBP−, 28% were O1−/MBP+, and 6% were GFAP+. Of the O1+ cells, 88% were p75+, of the MBP+ cells, 80% were p75+, and none of the GFAP+ cells was p75+.
Primary mouse oligodendrocyte culture from the cortex
The p75 knock-out mice that carried the mutation in exon 3 of the p75 gene (Lee et al., 1992) and the wild-type mice were obtained from heterozygote mating as littermates. The mice were back-crossed to C57/BL6 for 10 generations to make them congenic. Their genotype was determined by PCR analyses of tail DNA according to the method ofBentley and Lee (2000). At postnatal days 16–18, the brain was dissected, a triturated cell suspension was loaded onto a 36% Percoll gradient, and oligodendrocytes were isolated after centrifugation at 10,000 × g according to the methods of Lubetzki et al. (1991) and Fuss et al. (2000). Isolated oligodendrocytes were resuspended in 10% FBS in DMEM and plated onto poly-d-lysine-coated four-well slide dishes at 0.1 × 106 per well. The following day, the medium was changed to a differentiation medium with no serum as described previously (Yoon et al., 1998). The culture was kept for 6 d before NGF was added at 100 ng/ml for the indicated time. In these cultures, 40% of the cells were MBP+ and 7% were GFAP+. Of the MBP+ cells, 70% were p75+; none of the GFAP+ cells was p75+.
Immunocytochemistry
The antibodies used for immunocytochemistry were 9651 (anti-p75;Huber and Chao, 1995), O1 (a generous gift from Dr. Patrick Wood, University of Miami, Miami, FL), anti-MBP (Roche Molecular Biochemicals, Indianapolis, IN), and anti-GFAP (Sigma, St. Louis, MO). For double staining of mouse oligodendrocytes for p75 and MBP, mouse oligodendrocytes were first stained live for p75 by incubating them for 1 hr at room temperature with 9651. To visualize the p75 stain, cells were washed twice with the serum-free media and incubated at 37°C for 1 hr with Cy3-conjugated anti-rabbit secondary antibody (Jackson ImmunoResearch, West Grove, PA). Cells were then fixed and double-stained for MBP. MBP staining was visualized using an anti-mouse secondary antibody conjugated to Alexa 488 (Molecular Probes, Eugene, OR). For double staining of rat oligodendrocytes with anti-75 and MBP or p75 and GFAP, cells were fixed and stained for p75 using 9651 without Triton X-100 and subsequently stained for MBP and GFAP after permeabilization with Triton X-100. For double staining of rat oligodendrocytes with O1 and p75, cells were stained live for O1 first, fixed, and stained for p75.
Generation of recombinant adenoviruses
Dominant negative JNK2 virus. The cDNA for a dominant negative (DN) mutant of JNK2 was isolated from SRα3-DN-JNK2 by digesting it with HindIII and SmaI and subcloned into a Track cytomegalovirus (CMV) shuttle vector (He et al., 1998) that was digested with HindIII and EcoRV. The DN-JNK2 mutant contains two point mutations, T183A and Y185F (Kallunki et al., 1994). The recombinant adenovirus construct was generated in RecA+ bacteria using Track CMV-DN-JNK2 and pAdEasy 1 according to the method of He et al. (1998). The virus was subsequently generated in 293 cells by transfection and further purified using two rounds of CsCl centrifugation. The CsCl present in the virus preparation was removed by dialysis.
DN-p75 virus. The construct comprises two domains: the extracellular and transmembrane (TM) domains are from rat p75, and the cytoplasmic domain is from human epidermal growth factor (EGF) receptor that was rendered kinase-dead. To join the two chimeric domains in-frame without altering any amino acid residues, a BstBI site was introduced at the junction between the p75 and EGF receptors. Introduction of the BstBI site results in the silent mutation of phenylalanine, the last amino acid in the p75 TM domain (TTC to TTT). The extracellular and TM domains of p75 were isolated by PCR using pcDNA3 hemagglutinin (HA)-p75 as a template (Khursigara et al., 1999). The sequence for the forward primer was GGGGTACCACCATGTCTGCACTTCTGATC, and the sequence for the reverse primer was gcttcga AAAGCAATATAGGCCAC (the underline represents the silent phenylalanine mutation, and the sequences in uppercase letters represent those in rat p75). The PCR fragment was first cloned into pCRII vector to generate pCRII-p75Ext/TM(Invitrogen, Carlsbad, CA) and sequenced in its entirety for any errors. The cytoplasmic domain of the human EGF receptor was isolated by PCR using the pCMV5-EGF receptor as a template (Yoon et al., 1997). The sequence for the forward primer was agcg ttCGAAGGCGCCACATC, and the sequence for the reverse primer was tcaTGCTCCAATAAATTCACT (the underline represents the BstBI site, and the sequence in italics represents the stop codon introduced at the 3′ end). The PCR fragment was first cloned into pT7Blue 3 (Novagen, Madison, WI) and later mutated at the ATP binding site (Lys to Phe) using primers CCCGTCGCTATC GCGGAATTAAGAGAA and TTCTCTTAATTCC GCGATAGCGACGGG (the underline represents the Lys-to-Ala mutation: AAG to GCG). The fragment was sequenced in its entirety for the presence of point mutations and for any PCR errors. To join the two domains, the cytoplasmic domain of the kinase-dead EGF receptor was first cut with XhoI, blunted with T4 DNA polymerase, and subsequently digested with BstBI. The digested fragment was ligated into the BstBI andEcoRV sites in the pCRII-p75Ext/TM. The resulting chimeric molecule has GCT TT TCGA AGC at the junction (the underline represents the silent mutation, and the italics represent the sequences from the EGF receptor). The chimera was cloned into the Track CMV shuttle vector using KpnI andHindIII sites. The virus was generated as described for the DN-JNK2.
N17Rac1 virus. The fragment containing human Rac1 was prepared by PCR using SRα-N17Rac1 as a template. The PCR fragment was cloned into the pCRII vector (Invitrogen) and sequenced in its entirety for the presence of a point mutation (The to Asn) and for any PCR errors. The confirmed N17Rac1 was digested with XhoI andHindIII and introduced to the SalI andHindIII sites in the Track CMV vector. The recombinant adenovirus was generated as described for the DN-JNK2 virus.
Adenovirus infection
Mature oligodendrocytes were infected with recombinant adenoviruses at 100–150 pfu/cell. After 18–24 hr of infection, cells were treated with NGF for 4 hr, and lysates were prepared for immunoprecipitation/kinase (IP/K) assays and Western blot analyses as described below.
Quantification of apoptotic oligodendrocytes
Rat oligodendrocytes. After a 4 hr NGF treatment, cells were fixed with 3% paraformaldehyde, stained for terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick end labeling (TUNEL; tetra-methyl rhodamine red; Roche Molecular Biochemicals) and mounted with Vectashield containing 4,6-diamidino-2-phenylindole (DAPI) to label the nuclei (Vector Laboratories, Burlingame, CA). Cells expressing the mutant genes were identified by green fluorescent protein (GFP), because all the recombinant adenoviruses express GFP as well as the mutant genes. The apoptotic oligodendrocytes were quantified by counting TUNEL+ cells among GFP+ oligodendrocytes. The quantitation data are from two to four independent experiments, each with 200–300 cells counted for a total of 400–1200 cells.
Mouse oligodendrocytes. At indicated times after NGF treatment, cells were fixed and incubated with anti-MBP antibody. Cells were then stained for TUNEL and processed for visualization of MBP stain using an anti-mouse secondary antibody conjugated to Alexa 488 (Molecular Probes). For quantification of apoptotic cells after BDNF and NT3 treatment, the number of pyknotic cells among MBP+ cells was quantified. The quantitation data are from two to six independent experiments, each with 100–200 cells counted for a total of 200–1200 cells.
Immunoprecipitation
To detect the active form of TrkB and TrkC, oligodendrocytes were untreated or treated with BDNF or NT3 for 5 min, and the resulting lysates (1 mg) were subjected to immunoprecipitation using pan-Trk antibody (C14 and B3; Santa Cruz Biotechnologies, Santa Cruz, CA). The active forms of the receptors were detected using phospho-Trk-Y490 antibody (Cell Signaling Technology, Beverly, MA).
JNK kinase assay and Western blot analyses
The procedures for IP/K assays were identical to those described previously (Yoon et al., 1998). For IP/K assays with JNK2 and JNK3 antibodies 100–300 μg and for JNK1 antibodies 25–50 μg of lysates were used. For solid-phase kinase assays, bacterial cell lysates containing glutathione S-transferase (GST)-c-junwere first bound to glutathione beads in 50 mmNaCl, 5 mm MgCl2, 0.1 mm EDTA, 0.05% Triton X-100, and 20 mm HEPES, pH 8.0. After three washes of the beads in the binding buffer, oligodendrocyte lysates were added for a 2 hr binding at 4°C. This step was to bring down active JNKs that are known to bind its substrates (Derijard et al., 1994). The subsequent kinase reaction was the same as described previously (Yoon et al., 1998).
Western blot analyses were identical to what was described previously (Yoon et al., 1998)
Immunodepletion
The oligodendrocyte lysates were subjected to immunoprecipitation reactions twice using Affigel beads that were conjugated with anti-JNK1 (G151; PharMingen, San Diego, CA) and JNK2 antibodies (Santa Cruz Biotechnologies). To confirm the extent of depletion, ∼5 ng of 35S-labeled JNK1 and ∼5 ng of 35S-labeled JNK2 proteins were added to the oligodendrocyte lysates as tracers before immunodepletion. After immunodepletion, 10% of the total depleted or undepleted lysates were analyzed for the presence or absence of35S-labeled JNK1 and JNK2. The rest of the lysates were used for the solid-phase kinase assays.
In vitro translation of JNK1 and 2 proteins
The cDNAs of JNK1 and 2 were prepared by PCR and cloned into pCRII vector (Invitrogen) and subsequently sequenced in their entirety for any PCR errors. The JNK1 and 2 proteins were synthesized in vitro in the presence of [35S]methionine using the T7 transcription- and translation-coupled system (Promega, Madison, WI). The amount of each protein synthesized was estimated to be ∼200 ng/reaction at a 90% incorporation rate. As a tracer in immunodepletion, ∼5 ng of each protein was added to the oligodendrocyte lysates.
Rac activity assay
For samples loaded with GDP or GTPγS as specificity controls, lysates were incubated at 30°C for 15 min with either 100 μm GTPγS or 1 mm GDP in the presence of 10 mm EDTA. Samples were then placed on ice, and MgCl2 was added to a final concentration of 60 mm. GST-p21 binding domain (PBD)-Sepharose beads were added to the samples and rotated for 30 min at 4°C. The beads were washed, and Rac protein was detected using anti-Rac1 antibody (Upstate Biotechnology, Lake Placid, NY) as described previously (Benard et al., 1999).
For Rac activity assays using oligodendrocyte lysates, oligodendrocytes were either untreated or treated with neurotrophins at 100 ng/ml for 5 min and 1 and 4 hr. Dishes were then placed on ice, and cells were washed once with ice-cold PBS. After removal of PBS, cells were lysed in a lysis buffer, containing 25 mm HEPES, pH 7.5, 150 mm NaCl, 10 mm MgCl2, 1 mm EDTA, 10% glycerol, 1% NP-40, 0.25% sodium deoxycholate, 1 mm sodium orthovanadate, 25 mmNaF, 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 2 mmPMSF. Lysates were rotated for 5 min at 4°C and clarified by centrifugation at 14,000 rpm for 5 min at 4°C. Ten percent of the supernatant of each sample was saved for Western blot analysis as a control for the total Rac protein using anti-Rac1 antibody (Upstate Biotechnology), whereas the remainder was incubated with ∼30 μg of GST-PBD bound to glutathione-Sepharose beads. Lysates were rotated with beads for 1 hr at 4°C and washed three times with lysis buffer. Bound Rac-GTP protein was detected by Western blot analysis using anti-Rac1 antibody (Upstate Biotechnology).
Affinity cross-linking
Affinity cross-linking was performed as described using125I-NT3 as the ligand (Yoon et al., 1996), except that the full-length p75-NT3 complex was immunoprecipitated with 9992, an anti-p75 antibody, and the mutant p75-NT3 was immunoprecipitated with anti-HA antibody.
RESULTS
p75 protein is required for NGF-dependent apoptosis in oligodendrocytes
We asked whether oligodendrocytes undergo apoptosis in the absence of p75. For this, cortical oligodendrocytes were prepared from 16- to 18-d-old p75 knock-out mice and their wild-type littermates, and the responses of each to NGF were assessed. It should be stressed here that mouse oligodendrocytes were isolated by Percoll gradient immediately after dissection (Lubetzki et al., 1991; Fuss et al., 2000) and cultured in differentiation medium for 6 d before the analyses. This procedure differs from rat cultures, which are prepared by the shake-off method developed by McCarthy and de Vellis (1980). In the shake-off method, oligodendrocytes are expanded as a mixed culture during an 8–9 d incubation period, before they are isolated from astrocytes and microglia, and allowed to differentiate for 5–7 d. As shown in Figure 1A, MBP+ mouse oligodendrocytes express p75 in culture as do their rat counterparts. By the sixth day in culture, the proportion of MBP+ cells reached ∼40%, and the proportion of p75-expressing cells reached ∼70% among MBP+ cells. NGF induced apoptosis in these cultures based on TUNEL assays, the extent of which increased from 8–9% at 4 hr after NGF to 32–34% at 48 hr after NGF (Fig. 1C). In the knock-out mouse cultures, there was no increase in the number of TUNEL+ cells for the entire 48 hr period of NGF treatment (Fig. 1C). A representative picture of apoptotic cells bearing pyknotic nuclei is shown in Figure1B. These data therefore indicate that NGF-dependent death of oligodendrocytes requires the p75 protein.
Fig. 1.

Oligodendrocytes from p75 null mice fail to die after NGF treatment. A, Mouse oligodendrocytes express p75 in culture, as do their rat counterparts. A representative picture shows a mouse oligodendrocyte culture taken from the wild-type mice at postnatal day 16 cortex. p75 expression on the cell surface was detected using 9651 anti-p75 antibody (red), and oligodendrocytes were identified by MBP stain (green). Scale bar, 8 μm. B, Oligodendrocytes fail to die in the absence of p75 when NGF is added. At 4–6 d after plating, mouse oligodendrocytes were treated with 100 ng/ml of NGF for 4 hr, fixed, and stained for MBP. Pyknotic cells among the MBP+ cells are indicated byarrows and also shown at higher magnification in theinset. Scale bar, 20 μm. C, Quantification of TUNEL+ cells among MBP+ cells. The quantitation data are from two to four independent experiments, each with 100–150 cells counted for a total of 200–600 cells. WT, Wild-type;KO, knock-out.
The ability to activate the JNK pathway is required for p75-mediated apoptosis in rat oligodendrocytes
NGF-dependent apoptosis in primary rat oligodendrocytes was inhibited when a p75-blocking antibody was used, suggesting that the apoptosis was mediated by NGF binding to p75 (Casaccia-Bonnefil et al., 1996). To further confirm that the apoptosis was indeed attributable to the action of p75, we sought to inhibit the signaling capacity of endogenous p75 using a mutant p75 that lacks its cytoplasmic signaling domain in adenovirus. The mutant p75 contains the extracellular and transmembrane domains of rat p75, but its cytoplasmic domain was replaced with that of the human EGF receptor (Fig.2A). The cytoplasmic domain of the EGF receptor was rendered inactive in its tyrosine kinase function by mutating its ATP-binding lysine residue to alanine. We expected the resulting construct to compete effectively for binding to NGF against the endogenous p75 but be incapable of its own signaling. To facilitate the detection of the chimeric receptor, an HA tag was placed at the N terminus of rat p75 cDNA after the signal peptide sequence (Khursigara et al., 1999). Because the adenovirus contains GFP under a separate promoter (Fig. 2A), all the infected cells also express GFP as well as the mutant p75 receptor (Fig.2E).
Fig. 2.

The signaling ability of p75 is required for NGF-dependent apoptosis in oligodendrocytes. A, Schematic diagram of the mutant-p75 lacking the cytoplasmic domain. The cytoplasmic domain of this mutant receptor was replaced with the corresponding domain of the kinase-dead EGF receptor. Thearrows indicate two independent CMV promoters, one directing expression of the mutant p75 and the other directing the expression of GFP. All the adenoviruses used in this study coexpress GFP. B, The mutant p75 binds 125I-NT3. Cos cells were infected with the full-length (FL) p75 or the mutant (Mut) p75 adenovirus and subjected to cross-linking with 125I-NT3. PC12 cells were used as a positive control (lane 1), and uninfected Cos cells were used as a negative control (lane 2). The FL-p75 was immunoprecipitated with 9992 antibody (lane 3), and the mutant p75 was immunoprecipitated with HA antibody (lane 4). C, The mutant p75 protects oligodendrocytes from NGF-dependent apoptosis. Oligodendrocytes were infected with GFP control or the mutant p75 adenovirus in four-well slide dishes for 24 hr at 150 pfu/cell. After 4 hr of NGF treatment, cells were stained for TUNEL. The number of TUNEL+cells was determined among GFP+ cells. The quantitation data are from three to five independent experiments, each with 200–300 cells counted for a total of 600–1500 cells.D, The mutant-p75 inhibits JNK activation in oligodendrocytes. Twenty-four hours after infection with the viruses, oligodendrocytes were treated with NGF at 100 ng/ml for 4 hr. The changes in JNK activity were measured by solid-phase kinase assays. The presence of the mutant-p75 was detected with anti-HA antibody, and the presence of the JNK protein was detected with anti-JNK antibody.E, Representative picture of oligodendrocytes quantified after infection with adenoviruses and NGF treatment. The cells expressing the mutant p75 were identified by GFP fluorescence, because the virus also expresses GFP as well as the mutant p75 cDNA. Thearrows indicate the TUNEL+ cells among GFP+ cells. Scale bars, 20 μm.EGFR-kin−, A kinase-dead EGF receptor;Ext, extracellular domain.
The mutant receptor was first tested for surface expression and its ability to bind neurotrophins in affinity cross-linking experiments using 125I-NT3 (Fig.2B). 125I-NT3 was chosen instead of 125I-NGF, because NT3 is more resistant than NGF to degradation after iodination. The mutant p75 yielded an NT3-receptor complex of ∼130 kDa in molecular weight, consistent with the increase in its size in chimeric form. This result suggests that the mutant p75 is expressed on the cell surface and binds neurotrophins as efficiently as the full-length p75.
The mutant p75 receptor was then introduced via infection into rat oligodendrocytes, and its effect on NGF-dependent apoptosis was assessed. The control cultures were infected with the GFP adenovirus. Twenty-four hours after infection, cells were treated with NGF for 4 hr at 100 ng/ml, and the extent of apoptosis was measured by counting the number of TUNEL+ oligodendrocytes among GFP+ infected cells. A representative picture is shown in Figure 2E. In cultures infected with the control GFP virus, the proportion of TUNEL+ cells increased sixfold to eightfold after a 4 hr NGF treatment. In the presence of the mutant p75, however, the number of TUNEL+oligodendrocytes remained at the basal level of 3–4%, (Fig.2C). This result suggests that the mutant p75 receptor inhibited the action of endogenous p75 in rat oligodendrocytes.
We have reported previously that inhibition of JNK activity by CEP-1347 resulted in rescue of rat oligodendrocytes from NGF-dependent apoptosis (Yoon et al., 1998). Because the mutant p75 receptor blocked NGF-dependent apoptosis, we asked whether the JNK activity was altered in the presence of the mutant p75. For this, cells were infected for 24 hr either with the control GFP or the mutant p75 receptor adenovirus, and the resulting lysates were subjected to a solid-phase JNK kinase assay to measure the total JNK activation (Derijard et al., 1994) (Fig. 2D). In control cultures, NGF addition led to an increase in total JNK activity, whereas activation of JNK was no longer observed in the presence of the mutant p75 (Fig. 2D). These data therefore indicate that not only the binding of p75 to NGF but also the ability of p75 to signal and activate JNK is necessary for its action in inducing apoptosis in oligodendrocytes.
p75 activates JNK1 and 3 in oligodendrocytes
JNK activity has been shown to increase as PC12 cells differentiate (Eilers et al., 1998) and to remain elevated after axotomy of adult dorsal root ganglion neurons (Kenney and Kocsis, 1998). Similarly, cerebellar granule neurons exhibit a high basal level of JNK activity (Coffey et al., 2000). These data suggest that JNK activity may be involved in axonal outgrowth or regeneration. In contrast, JNK activities have also been linked to apoptosis of a variety of neurons. The death of motor and sympathetic neurons as well as PC12 cells was prevented when JNK activity was inhibited (Xia et al., 1995; Eilers et al., 1998; Maroney et al., 1998, 1999).
This apparent dichotomy may be attributable to distinct actions among different JNK isoforms. An example is found in a recent report in which only JNK3, and not JNK1 or 2, was implicated in arsenite-induced apoptosis (Namgung and Xia, 2000). We therefore sought to identify the types of JNK activated by p75 in oligodendrocytes, which undergo apoptosis in an NGF-dependent manner. To address this question, the specificity of various JNK antibodies was first tested in 293 cells. 293 cells were transfected with HA-tagged JNK1 or 2 or Flag-tagged 3, and these JNK proteins were immunoprecipitated with commercial antibodies against JNK1–3. The presence of each transfected JNK protein in the immunoprecipitates was later detected using either HA or Flag antibodies in Western blot analyses. The JNK3 antibody (Upstate Biotechnology) immunoprecipitated both JNK1 and 3 efficiently (Fig.3A). JNK2 antibody (Santa Cruz Biotechnologies) immunoprecipitated JNK1 and 2 but not JNK3. For JNK1, we tested two different antibodies, C17 polyclonal (Santa Cruz Biotechnologies) and G151 monoclonal antibodies (PharMingen). The C17 JNK1 antibody brought down JNK1 and 3 but not JNK2, and the G151 JNK1 antibody immunoprecipitated JNK1 and 2 but not JNK3. These results are tabulated in Figure 3B.
Fig. 3.
p75 activates JNK1 and 3. A, Specificity of antibodies used in IP/K assays. 293 cells were transfected with HA-JNK1, HA-JNK2, or Flag-JNK3 cDNAs. The lysates from each transfected dish were subjected to immunoprecipitation reactions using anti-JNK1 (C17, polyclonal; Santa Cruz Biotechnologies), anti-JNK1 (G151, monoclonal; PharMingen), anti-JNK2 (Santa Cruz Biotechnologies), and anti-JNK3 (Upstate Biotechnology) antibodies. The immunoprecipitated proteins were detected using either anti-HA (JNK1 and 2) or anti-Flag (JNK3) antibody. B, Summary of the data presented in Figure 4. C, The lysates from rat oligodendrocytes were subjected to IP/K assays using the four antibodies. p75 activates JNK1, based on C17 and G151 antibodies, and JNK3, based on C17 and JNK3 antibodies.
Each of these antibodies was then used in IP/K assays using lysates from oligodendrocytes that were treated with NGF for 4 hr at 100 ng/ml (Fig. 3C). p75 activated JNK1 based on G151 and C17 antibody data, but it did not activate JNK2 robustly. There appeared to be activation of JNK3 by p75 based on anti-JNK3 data, but the data were not conclusive, because JNK3 antibody detected both JNK1 and 3 efficiently (Fig. 3A). We therefore performed immunodepletion with oligodendrocyte lysates using the G151 JNK1 antibody and tested whether p75 still activated JNK on NGF treatment. To monitor the extent of depletion,35S-JNK1 and 2 were added to the lysates as tracers before depletion. As shown in the Figure4, top panel, there was robust JNK activation after depletion of JNK1 and 2 from oligodendrocyte extracts. On the basis of the tracers, there was very little JNK1 and 2 protein left after depletion (Fig. 4, bottom panel), suggesting that the JNK activation observed after immunodepletion represents activation of JNK3. We therefore conclude that p75 activates JNK1 and 3 in oligodendrocytes.
Fig. 4.
p75 activates JNK3 after depletion of JNK1 and 2. The oligodendrocyte lysates were subjected to immunodepletion to remove JNK1 and 2 using JNK1 and 2 antibodies. The depleted lysates were used in solid-phase kinase assays (top panel). The efficiency of immunodepletion is shown in the bottom panel. The extent of immunodepletion of JNK1 and 2 proteins was determined using 35S-JNK1 and JNK2 that were added together as a tracer to the oligodendrocyte lysates.
Activation of JNK is required for p75-mediated apoptosis
We have previously used an inhibitor of the JNK pathway, CEP-1347, to demonstrate that JNK activity was necessary for the death of oligodendrocytes (Yoon et al., 1998). Similarly, the mutant p75 receptor that is incapable of signaling via the JNK pathway inhibited NGF-dependent apoptosis in oligodendrocytes. With CEP-1347, however, the possibility exists that the drug may have affected other pathways that were not measured. With the mutant p75, it is also likely that another pathway that is required for survival, such as the NF-κB pathway, was concurrently blocked. Therefore, we tested whether direct inhibition of endogenous JNK could lead to suppression of p75-mediated apoptosis by introducing a DN JNK into oligodendrocytes. Because there is >70% sequence homology among the three JNK isoforms, it is likely that a DN mutant of one isoform will inhibit all three isoforms. We chose DN-JNK2 because it is regulated similarly to the other JNKs but has a higher affinity to c-jun than JNK1, and JNK2 activates the c-junpromoter, whereas JNK1 does not (Kallunki et al., 1994). The DN mutant contains two point mutations, T183A and Y185F, at the phosphorylation sites that are required for its activity (Kallunki et al., 1994).
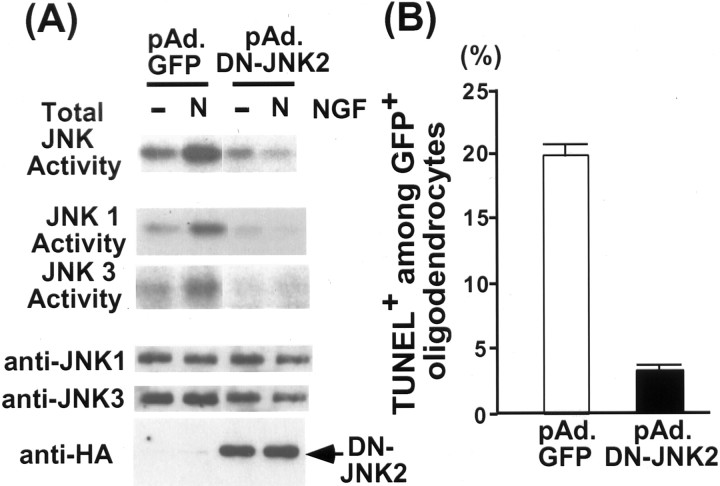
The DN-JNK2 virus was first tested for its action against JNK1 and 3 as well as for the total JNK activity in rat primary oligodendrocytes by infecting the cells with either the GFP control or the DN-JNK2 virus. Twenty-four hours after infection, cells were treated with NGF for 4 hr at 100 ng/ml, and the lysates were subjected to a solid-phase JNK assay to measure the total JNK activation (Fig.5A, top panel). NGF addition led to an increase in the total JNK activity in the control GFP virus-infected cells, whereas there was no increase in the DN-JNK2-infected cells. The DN-JNK2 was also effective against individual JNK1 and JNK3, as demonstrated in IP/K assays, using either JNK1 or JNK3 antibodies (Fig. 5A, middle panels). These results confirm that the mutant JNK2 was effective in inhibiting NGF-dependent JNK activation.
Fig. 5.
JNK activation is necessary for NGF-dependent apoptosis in oligodendrocytes. A, DN-JNK2 inhibits NGF-dependent activation of JNK in oligodendrocytes. Oligodendrocytes were infected with GFP control or DN-JNK2 adenovirus for 24 hr at 150 pfu/cell. Infected cells were untreated or treated with NGF for 4 hr, and the lysates were subjected to solid-phase kinase as well as IP/K assays. The presence of DN-JNK2 is shown in an HA Western blot, and the JNK protein is shown in a JNK Western blot. B, DN-JNK2 rescues oligodendrocytes from NGF-mediated apoptosis. The quantification procedure was identical to what was described in the legend of Figure 2.
To test whether the DN-JNK2 could rescue the death of oligodendrocytes, cell viability was determined by counting the number of TUNEL+ cells among the GFP+ oligodendrocytes. When NGF was added at 100 ng/ml for 4 hr, the number of TUNEL+ cells among GFP+ oligodendrocytes increased fourfold to fivefold in the GFP virus-infected cells, whereas it did not increase in the DN-JNK2 virus-infected cells (Fig. 5B). Therefore, these results together with the data from CEP-1347 and the data with the mutant p75 receptor indicate that JNK activation is indeed necessary for NGF-dependent apoptosis in oligodendrocytes.
Rac1 is an upstream regulator in p75-mediated JNK activation
p75 has been shown to activate the JNK pathway in several cell types other than oligodendrocytes. These include superior cervical ganglion (SCG) neurons (Bamji et al., 1998) and the Schwannoma cell line (Gentry et al., 2000). In SCG neurons (Mazzoni et al., 1999) and oligodendrocytes (Fig. 5), JNK activation is causally linked to p75-mediated apoptosis. The observation that p75 activates JNK in several different systems may imply that JNK activation by p75 is a central feature in p75 signaling. The mechanism by which p75 activates JNK is still unknown. Although nine different molecules are known to interact with p75 to date, none of them has been linked to the JNK pathway (Casademunt et al., 1999; Chittka and Chao, 1999; Irie et al., 1999; Khursigara et al., 1999; Yamashita et al., 1999; Ye et al., 1999;Salehi et al., 2000). We therefore sought to find an upstream regulator that may be most proximal to the receptor. A candidate molecule is Rac, a GTPase belonging to the Rho family of small G-proteins. Rac has been shown to function as the upstream regulator in the JNK pathway in cell lines as well as in neurons (Coso et al., 1995; Minden et al., 1995; Bazenet et al., 1998). In addition, p75 was recently reported to interact with another member of the Rho family of small G-proteins, RhoA (Yamashita et al., 1999). Binding of p75 to NGF led to suppression of Rho activity in 293 cells (Yamashita et al., 1999).
We first tested whether p75 modulates Rac activity in oligodendrocytes in an NGF-dependent manner, using the GST-PBD of PAK 1. PAK 1 binds Rac only when Rac is bound to GTP via PBD. The activated, GTP-bound Rac can then be detected in Western blot analyses after pull-down assays using the GST-PBD (Benard et al., 1999). The specificity of this assay was tested using the unhydrolyzable GTP analog GTP-γS or GDP. As shown in Figure6A, the GST-PBD pulled down only the GTP-γS-bound Rac and not the GDP-bound Rac. Using the assay, we examined whether p75 can modulate Rac activity in oligodendrocytes. In oligodendrocytes, NGF induced Rac activation starting 5 min after NGF addition at 100 ng/ml, and its activation lasted for 4 hr after NGF treatment (Fig. 6B). In contrast to NGF, BDNF failed to activate Rac, whereas NT3 addition led to transient activation of Rac. We next asked whether the different temporal pattern of activation of Rac observed with the different neurotrophins correlates with their ability to activate JNK. NGF addition led to robust JNK activation starting 1 hr after NGF, lasting up to 4 hr, whereas BDNF did not during a 4 hr treatment (Fig.7A). There was a small increase in JNK activity after a 4 hr NT3 treatment, but this activation was not sufficient to lead to apoptosis, because only NGF could induce apoptosis in oligodendrocytes, whereas BDNF or NT3 could not (Fig. 7B). These results suggest that prolonged Rac activation by p75 may be one of the key steps required for NGF-dependent apoptosis.
Fig. 6.
p75 activates Rac1 in an NGF-dependent manner.A, Specificity of the pull-down Rac assay. The lysates from 293 cells were incubated with either 1 mm GDP or 0.1 mm GTPγS and subjected to a pull-down assay using GST-PBD. The bound Rac protein was detected in a Western blot analysis with anti-Rac1 antibody. B, NGF addition led to a prolonged activation of Rac1 in oligodendrocytes. Oligodendrocytes were treated for the indicated time with NGF, BDNF, or NT3 at 100 ng/ml. The lysates were subjected to Rac activity assays. Note that BDNF does not activate Rac1, whereas NT3 activates it transiently.
Fig. 7.

Trk activation intercepts p75-mediated JNK activity at or upstream of Rac GTPase. A, Temporal course of JNK activation by neurotrophins. Rat oligodendrocytes were treated with neurotrophins for the indicated time at 100 ng/ml. The lysates were subjected to solid-phase kinase assays. B, Only NGF is capable of inducing cell death among oligodendrocytes. Mouse oligodendrocytes were treated with 100 ng/ml NGF, BDNF, or NT3 for 4–5 hr, and the number of pyknotic cells was counted among MBP+ cells. The quantitation data are from three independent experiments, each with 100–200 cells counted for a total of 300–600 cells. C, Rat oligodendrocytes express TrkB and TrkC. Rat oligodendrocytes were untreated or treated with 100 ng/ml BDNF or NT3 for 5 min. The activated receptors were detected using phospho-TrkY490 antibody. Active TrkA from PC12 cells was used as a positive control (lanes 4, 5).
It was reported recently that NGF induced Rac activation in PC12 cells (Yamaguchi et al., 2001; Yasui et al., 2001). In those studies, Rac activation subsided 3 min after NGF treatment. These results suggest that in rat oligodendrocyte cultures, transience or absence of Rac activation, with NT3 or BDNF, respectively, is attributable to concomitant activation of resident TrkB and TrkC. The rat oligodendrocytes expressed TrkB and TrkC, because BDNF and NT3 treatment resulted in tyrosine phosphorylation of the receptors, based on Western blot analyses with the phospho-TrkY490 antibody (Fig.7C). We have reported previously that coactivation of TrkA and p75 after introduction of ectopic TrkA into rat oligodendrocytes led to suppression of JNK (Yoon et al., 1998). Our data here with BDNF and NT3, in addition to the reports by Yasui et al. (2001) andYamaguchi et al. (2001), indicate that one of the key points where Trk activation intercepts p75 signaling is at or upstream of Rac.
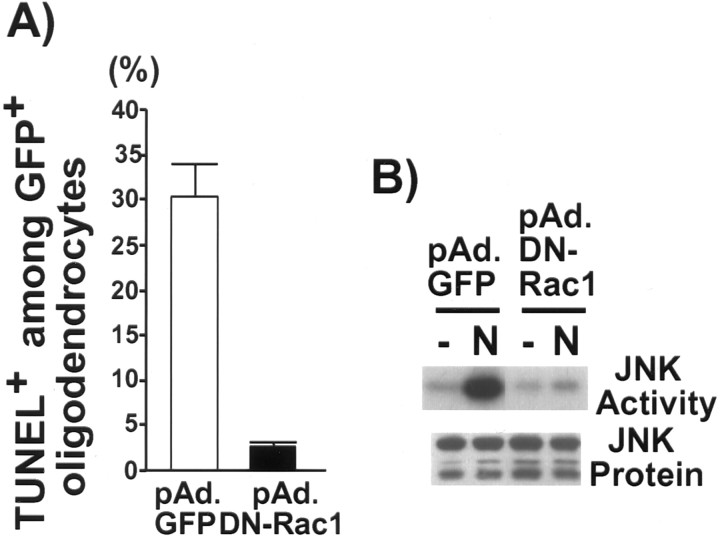
Because temporal activation of Rac correlated with cell death in oligodendrocytes, we next investigated whether Rac activation was required for NGF-dependent apoptosis. For this purpose, we generated an adenovirus carrying the cDNA for the DN-Rac1. The DN-Rac1 contains a mutation at residue 17 (threonine to asparagine). After infection with either GFP or the DN-Rac1 virus, cell viability was determined by counting the number of TUNEL+ cells among the GFP+ oligodendrocytes after a 4 hr NGF treatment at 100 ng/ml. In the presence of the DN-Rac1, the number of apoptotic oligodendrocytes was reduced compared with the control GFP virus-infected cells after NGF treatment (Fig.8A). To test whether Rac activation was required for NGF-dependent JNK activity, the lysates from either GFP or the DN-Rac1 virus-infected cultures were subjected to solid-phase kinase assays. In oligodendrocytes infected with the control GFP virus, there was activation of JNK on NGF addition, but in cells infected with DN-Rac1, JNK activation was no longer observed (Fig. 8B). These data, therefore, indicate that p75 uses Rac1 to activate JNK, and Rac activation is an obligatory step in the NGF-dependent apoptotic machinery activated by p75. This Rac activation is under competitive regulation by Trk signaling, thereby governing cell survival and death.
Fig. 8.
Rac1 is the upstream regulator of the p75-mediated JNK pathway. A, DN-Rac1 protects oligodendrocytes from NGF-dependent apoptosis. The procedure is identical to the one described in the legend of Figure 2. B, DN-Rac1 inhibits NGF-dependent JNK activation. Oligodendrocytes were infected with either GFP or DN-Rac1 for 24 hr and either left untreated or treated with 100 ng/ml NGF for 4 hr. The resulting lysates were used in solid-phase kinase assays.
DISCUSSION
In this report, we present the data that p75 activates Rac GTPase in an NGF-dependent manner. This Rac activation is causally linked to apoptotic action by p75, because N17Rac1 inhibits NGF-dependent JNK activation, which in turn leads to suppression of apoptosis. Coactivation of Trk modulates p75-mediated Rac activation, thereby identifying Rac GTPase as one of the key molecules whose activity is critical for cell survival and death in neurotrophin signaling. The crucial role of the JNK pathway in p75 signaling is further confirmed by the fact that blocking p75 from signaling via the JNK pathway or suppressing the JNK activity itself led to inhibition of NGF-dependent death. We also report that p75 activates JNK3, an injury-specific JNK, in a system in which it induces apoptosis.
p75 is required for NGF-dependent death of oligodendrocytes in culture
We demonstrate here that mouse oligodendrocytes expressed p75 as early as the second day in culture, and they did not die in the absence of p75 when NGF was added (Fig. 1). In addition, blocking the signaling ability of p75 in rat oligodendrocytes also resulted in reversal of the death effect of NGF (Fig. 2). These results directly confirm the apoptotic role of p75 in oligodendrocytes. It is not clear, however, whether p75 plays a similar role in oligodendrocyte biology in vivo. The current literature strongly indicates that p75 is induced among oligodendrocytes by an injury or disease state. For instance, in the white matter plaques of multiple sclerosis (MS) patients, p75 was induced among oligodendrocytes and their precursors (Dowling et al., 1999; Chang et al., 2000). Direct infliction of injuries to the white matter in the cortex and the spinal cord can also induce p75 expression among oligodendrocytes (S. O. Yoon, J. Bresnahan, and M. Beattie, unpublished data). This correlation between p75 expression in vitro and in vivo suggests that culture conditions can model injury or stress situations these cells encounter in vivo. Our data demonstrating that p75 activates an injury-specific JNK3 in cultured oligodendrocytes also support this notion (Fig. 4).
What is the consequence of p75 induction in an injury or a disease state? On the basis of our culture data, one might suggest that p75 plays a proapoptotic role, perhaps serving to eliminate diseased cell populations. In support of this notion, Dowling et al. (1999) reported that 47% of TUNEL-positive oligodendrocytes expressed p75 in MS lesions. Contrary to these data, Chang et al. (2000) found no TUNEL-positive cells among NG2+oligodendrocyte precursors in MS lesions. These data may be interpreted as indicating that p75 plays an opposing, antiapoptotic role in developing oligodendrocytes when the greater population is injured and in this way may contribute to regeneration. Alternatively, p75 may still be involved in inducing apoptosis in NG2+ oligodendrocytes, but its expression is not sufficient to induce apoptosis. It is quite possible that induction of p75 is one of many factors required for these cells to undergo apoptosis but not sufficient to induce death in vivo. In support of this notion, Ladiwala et al. (1998) reported that although human oligodendrocytes do express p75 in culture, they failed to die when NGF was added. NGF did not induce the JNK pathway in human oligodendrocytes, indicating that some component of the p75-mediated JNK signaling pathway is not present in these cells, or its activation was somehow inhibited. Analyses of p75 knock-out mice in an injury or disease paradigm will help answer the question of whether the induced expression of p75 among oligodendrocytes in vivopresages their fate as it does in culture.
P75 activates JNK1 and JNK3
Our results demonstrate that p75 activates JNK1 in addition to JNK3, and their activation is required for NGF-dependent apoptosis. The involvement of JNK1 in apoptosis of oligodendrocytes is different from that revealed by the data for cortical cultures, in which JNK1 was not implicated in arsenite-induced apoptosis (Namgung and Xia, 2000). In cortical neurons, the basal level of JNK1 activity was high, suggesting that JNK1 may be involved in differentiation (Namgung and Xia, 2000). JNK activity has been shown to increase as PC12 cells or cerebellar granule neurons differentiate, although the specific JNK isoform responsible for this increase was not determined (Yao et al., 1997;Eilers et al., 1998; Coffey et al., 2000). This dual role of JNK has also been demonstrated in the analyses of JNK1 and 2 double-knock-out mice. Loss of JNK1 and 2 resulted in increased apoptosis in the forebrain region, whereas it inhibited apoptosis in the hindbrain area during embryonic development (Kuan et al., 1999). In oligodendrocytes in culture, JNK1 plays a role in inducing cell death, because total JNK activity as well as JNK1 activity was suppressed with the mutant p75, the DN-JNK2, and N17Rac (Figs. 2, 5, 8).
Although JNK1 and 2 may play diverse roles, it appears that the role of JNK3 is in inducing apoptosis after injuries to the nervous system (Yang et al., 1997; Namgung and Xia, 2000). It is highly relevant in this regard that p75 activates an injury-specific JNK3 in a system in which it induces cell death (Fig. 4). It is well documented in the literature that p75 is robustly induced in neurons, Schwann cells, and oligodendrocytes after injuries (Taniuchi et al., 1986; Koliatsos et al., 1991; Hayes et al., 1992; Roux et al., 1999). For instance, p75 has been reported to be induced in dying neurons after a seizure (Roux et al., 1999) and ischemia (Park et al., 2000) and in cortical neurons after experimental allergic encephalomyelitis (Calza et al., 1997;Nataf et al., 1998). It remains to be seen whether JNK3 activation is indeed required for p75-mediated apoptosis in vivo.
P75 activates Rac GTPase persistently
We demonstrated that p75 activates Rac GTPase in an NGF-dependent manner (Fig. 6). These results differ from those for RhoA activity, in which NGF binding to p75 led to suppression rather than activation (Yamashita et al., 1999). The suppression of RhoA activity by p75 was implicated in the promotion of process outgrowth in ciliary neurons (Yamashita et al., 1999). In oligodendrocytes that undergo apoptosis in an NGF-dependent manner, Rac activation is causally linked to apoptosis (Fig. 8). As a member of the Rho family of small G-proteins, Rac plays a role in cytoskeletal reorganization as well as being the upstream regulator in the JNK pathway (Coso et al., 1995; Minden et al., 1995). In NGF signaling, Rac activity is distinctly involved in activation of the JNK pathway, as we demonstrated by the effect of DN-Rac1 in oligodendrocytes. The dual roles for Rac have been more clearly demonstrated by a single point mutation (tyrosine at residue 40 to cysteine) that resulted in the loss of JNK activation but a continued effect on cytoskeletal reorganization (Lamarche et al., 1996). These results suggest that distinct effector molecules are involved in eliciting these diverse, Rac-mediated effects, although it is not known what regulates the process that determines whether Rac activation would lead to cell death or to cytoskeletal reorganization.
Our data suggest that the kinetics of Rac activation may be a determinant in this process. In oligodendrocytes, NGF induced long-term Rac activation, lasting up to 4 hr after NGF (Fig.6B). In PC12 cells in which NGF activated Rac transiently (Yasui et al., 2001), this activation was linked to neurite extension and differentiation, rather than cell death (Yamaguchi et al., 2001). Similarly to the data from PC12 cells, NT3 activated Rac only transiently in oligodendrocytes that expressed TrkC (Fig.6B) and did not induce cell death (Fig.7B). These results may be interpreted as suggesting that the kinetics of Rac activation determine whether Rac plays a role in apoptosis or in differentiation in neurotrophin signaling. This is reminiscent of the data that prolonged ERK activation correlated with differentiation, whereas transient ERK activation correlated with proliferation in PC12 cells (Qui and Green, 1992). On the basis of the correlation that we observed with NGF, between apoptosis and prolonged Rac activation, we hypothesize that additional effector molecules may be recruited to Rac-GTP at later points in NGF induction. Recruitment of these effector molecules may also be responsible for the differences in temporal regulation of Rac GTPase, perhaps by providing stabilizing scaffolds.
Although NT3 activated Rac transiently and induced JNK, it did not induce apoptosis in oligodendrocytes (Figs. 6, 7). This NT3 response in oligodendrocytes is similar to the NGF response in PC12 cells. In PC12 cells, NGF induced JNK (Minden et al., 1994; Eilers et al., 1998) as well as transient Rac activation (Yamaguchi et al., 2001; Yasui et al., 2001). We interpret these data as suggesting that JNK activation may not be sufficient to induce apoptosis in oligodendrocytes, although it is indeed necessary, because blocking JNK activity prevented NGF-dependent apoptosis. To induce apoptosis, p75 may need to activate a proapoptotic pathway(s) other than the JNK pathway or to suppress an antiapoptotic pathway(s). Alternatively, TrkC may activate a pathway that intercepts p75 signaling downstream from JNK in oligodendrocytes.
In conclusion, we report that p75 activates Rac GTPase persistently, and this activation is essential for p75 to induce JNK and apoptosis. Simultaneous activation of Trk counteracts apoptotic action by p75 by modulating the kinetics of p75-mediated Rac activation.
Footnotes
This project was supported by grants to S.O.Y. from the American Cancer Society, Whitehall Foundation, Ohio Cancer Research Associate, National Alliance for Research on Schizophrenia and Depression (Maltz Family Foundation), and National Institutes of Health (Grant RO1 NS39472-01). We thank Drs. Tong-Chuan He and Bert Vogelstein for providing adenoviral constructs, Dr. Michael Karin for the DN-JNK2 cDNA, Dr. Audrey Minden for the N17Rac1 cDNA, Dr. Gary Bokoch for GST-PBD, and Dr. Patrick Wood for the monoclonal line for O1. We especially thank Dr. Lino Tessarollo for providing breeding pairs of p75 knock-out and wild-type mice. We also thank Drs. James K. T. Wang, Bruce Carter, Pilar Perez, John Oberdick, Moses Chao, and Pappachan Kolattukudy for providing advice and reading this manuscript.
Correspondence should be addressed to Sung Ok Yoon, Neurobiotech Center and Department of Neuroscience, Ohio State University, 184 Rightmire Hall, 1060 Carmack Road, Columbus, OH 43210. E-mail: yoon.84@osu.edu.
REFERENCES
- 1.Bamji SX, Majdan M, Pozniak CD, Belliveau DJ, Aloyz R, Kohn J, Causing CG, Miller FD. The p75 neurotrophin receptor mediates neuronal apoptosis and is essential for naturally occurring sympathetic neuron death. J Cell Biol. 1998;140:911–923. doi: 10.1083/jcb.140.4.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bazenet CE, Mota MA, Rubin LL. The small GTP-binding protein Cdc42 is required for nerve growth factor withdrawal-induced neuronal death. Proc Natl Acad Sci USA. 1998;95:3984–3989. doi: 10.1073/pnas.95.7.3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benard V, Bohl BP, Bokoch GM. Characterization of rac and cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J Biol Chem. 1999;274:13198–13204. doi: 10.1074/jbc.274.19.13198. [DOI] [PubMed] [Google Scholar]
- 4.Bentley CA, Lee KF. p75 is important for axon growth, Schwann cell migration during development. J Neurosci. 2000;20:7706–7715. doi: 10.1523/JNEUROSCI.20-20-07706.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brennan C, Rivas-Plata K, Landis SC. The p75 neurotrophin receptor influences NT-3 responsiveness of sympathetic neurons in vivo. Nat Neurosci. 1999;2:699–705. doi: 10.1038/11158. [DOI] [PubMed] [Google Scholar]
- 6.Calza L, Giardino L, Pozza M, Micera A, Aloe L. Time-course changes of nerve growth factor, corticotropin-releasing hormone, and nitric oxide synthase isoforms and their possible role in the development of inflammatory response in experimental allergic encephalomyelitis. Proc Natl Acad Sci USA. 1997;94:3368–3373. doi: 10.1073/pnas.94.7.3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carter BD, Lewin GR. Neurotrophins live or let die: does p75NTR decide? Neuron. 1997;18:187–190. doi: 10.1016/s0896-6273(00)80259-7. [DOI] [PubMed] [Google Scholar]
- 8.Carter BD, Kaltschmidt C, Kaltschmidt B, Offenhauser N, Bohm-Matthaei R, Baeuerle PA, Barde YA. Selective activation of NF-kappa B by nerve growth factor through the neurotrophin receptor p75 [see comments]. Science. 1996;272:542–545. doi: 10.1126/science.272.5261.542. [DOI] [PubMed] [Google Scholar]
- 9.Casaccia-Bonnefil P, Carter BD, Dobrowsky RT, Chao MV. Death of oligodendrocytes mediated by the interaction of nerve growth factor with its receptor p75. Nature. 1996;383:716–719. doi: 10.1038/383716a0. [DOI] [PubMed] [Google Scholar]
- 10.Casademunt E, Carter BD, Benzel I, Frade JM, Dechant G, Barde YA. The zinc finger protein NRIF interacts with the neurotrophin receptor p75(NTR) and participates in programmed cell death. EMBO J. 1999;18:6050–6061. doi: 10.1093/emboj/18.21.6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang A, Nishiyama A, Peterson J, Prineas J, Trapp BD. NG2-positive oligodendrocyte progenitor cells in adult human brain, multiple sclerosis lesions. J Neurosci. 2000;20:6404–6412. doi: 10.1523/JNEUROSCI.20-17-06404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chittka A, Chao MV. Identification of a zinc finger protein whose subcellular distribution is regulated by serum and nerve growth factor. Proc Natl Acad Sci USA. 1999;96:10705–10710. doi: 10.1073/pnas.96.19.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coffey ET, Hongisto V, Dickens M, Davis RJ, Courtney MJ. Dual roles for c-Jun N-terminal kinase in developmental and stress responses in cerebellar granule neurons. J Neurosci. 2000;20:7602–7613. doi: 10.1523/JNEUROSCI.20-20-07602.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coso OA, Chiarielo M, Yu J-C, Teramoto H, Crespo P, Xu N, Miki T, Gutkind JS. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell. 1995;81:1137–1146. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- 15.Dechant G, Barde YA. Signalling through the neurotrophin receptor p75NTR. Curr Opin Neurobiol. 1997;7:413–418. doi: 10.1016/s0959-4388(97)80071-2. [DOI] [PubMed] [Google Scholar]
- 16.Derijard B, Hibi M, Wu I-H, Barrett T, Su B, Deng T, Karin M, Davis R. JNK1: a protein kinase stimulated by UV light and Ha-ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 17.Dobrowsky RT, Werner MH, Castellino AM, Chao MV, Hannun YA. Activation of the sphingomyelin cycle through the low-affinity neurotrophin receptor. Science. 1994;265:1596–1599. doi: 10.1126/science.8079174. [DOI] [PubMed] [Google Scholar]
- 18.Dowling P, Ming X, Raval S, Husar W, Casaccia-Bonnefil P, Chao M, Cook S, Blumberg B. Up-regulated p75NTR neurotrophin receptor on glial cells in MS plaques [see comments]. Neurology. 1999;53:1676–1682. doi: 10.1212/wnl.53.8.1676. [DOI] [PubMed] [Google Scholar]
- 19.Eilers A, Whitfield J, Babij C, Rubin LL, Ham J. Role of the Jun kinase pathway in the regulation of c-Jun expression and apoptosis in sympathetic neurons. J Neurosci. 1998;18:1713–1724. doi: 10.1523/JNEUROSCI.18-05-01713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuss B, Mallon B, Phan T, Ohlemeyer C, Kirchhoff F, Nishiyama A, Macklin WB. Purification and analysis of in vivo-differentiated oligodendrocytes expressing the green fluorescent protein. Dev Biol. 2000;218:259–274. doi: 10.1006/dbio.1999.9574. [DOI] [PubMed] [Google Scholar]
- 21.Gentry JJ, Casaccia-Bonnefil P, Carter BD. Nerve growth factor activation of nuclear factor kappaB through its p75 receptor is an anti-apoptotic signal in RN22 Schwannoma cells. J Biol Chem. 2000;275:7558–7565. doi: 10.1074/jbc.275.11.7558. [DOI] [PubMed] [Google Scholar]
- 22.Gu C, Casaccia-Bonnefil P, Srinivasan A, Chao MV. Oligodendrocyte apoptosis mediated by caspase activation. J Neurosci. 1999;19:3043–3049. doi: 10.1523/JNEUROSCI.19-08-03043.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayes RC, Wiley RG, Armstrong DM. Induction of nerve growth factor receptor (p75NGFr) mRNA within hypoglossal motoneurons following axonal injury. Brain Res Mol Brain Res. 1992;15:291–297. doi: 10.1016/0169-328x(92)90120-z. [DOI] [PubMed] [Google Scholar]
- 24.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huber LJ, Chao MV. Mesenchymal and neuronal cell expression of the p75 neurotrophin receptor gene occur by different mechanisms. Dev Biol. 1995;167:227–238. doi: 10.1006/dbio.1995.1019. [DOI] [PubMed] [Google Scholar]
- 26.Irie S, Hachiya T, Rabizadeh S, Maruyama W, Mukai J, Li Y, Reed JC, Bredesen DE, Sato TA. Functional interaction of Fas-associated phosphatase-1 (FAP-1) with p75(NTR) and their effect on NF-kappaB activation. FEBS Lett. 1999;460:191–198. doi: 10.1016/s0014-5793(99)01324-1. [DOI] [PubMed] [Google Scholar]
- 27.Kallunki T, Su B, Tsigelny I, Sluss HK, Derijard B, Moore G, Davis R, Karin M. JNK2 contains a specificity-determining region responsible for efficient c-Jun binding and phosphorylation. Genes Dev. 1994;8:2996–3007. doi: 10.1101/gad.8.24.2996. [DOI] [PubMed] [Google Scholar]
- 28.Kenney AM, Kocsis JD. Perpheral axotomy induces long-term c-Jun amino-terminal kinase-1 activation and activator protein-1 binding activity by c-Jun and junD in adult rat dorsal root ganglia in vivo. J Neurosci. 1998;18:1318–1328. doi: 10.1523/JNEUROSCI.18-04-01318.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khursigara G, Orlinick JR, Chao MV. Association of the p75 neurotrophin receptor with TRAF6. J Biol Chem. 1999;274:2597–2600. doi: 10.1074/jbc.274.5.2597. [DOI] [PubMed] [Google Scholar]
- 30.Koliatsos VE, Crawford TO, Price DL. Axotomy induces nerve growth factor receptor immunoreactivity in spinal motor neurons. Brain Res. 1991;549:297–304. doi: 10.1016/0006-8993(91)90471-7. [DOI] [PubMed] [Google Scholar]
- 31.Kuan CY, Yang DD, Samanta Roy DR, Davis RJ, Rakic P, Flavell RA. The Jnk1 and Jnk2 protein kinases are required for regional specific apoptosis during early brain development. Neuron. 1999;22:667–676. doi: 10.1016/s0896-6273(00)80727-8. [DOI] [PubMed] [Google Scholar]
- 32.Ladiwala U, Lachance C, Simoneau SJ, Bhakar A, Barker PA, Antel JP. p75 neurotrophin receptor expression on adult human oligodendrocytes: signaling without cell death in response to NGF. J Neurosci. 1998;18:1297–1304. doi: 10.1523/JNEUROSCI.18-04-01297.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lamarche N, Tapon N, Stowers L, Burbelo PD, Aspenstrom P, Bridges T, Chant J, Hall A. Rac and Cdc42 induce actin polymerization and G1 cell cycle progression independently of p65PAK and the JNK/SAPK MAP kinase cascade. Cell. 1996;87:519–529. doi: 10.1016/s0092-8674(00)81371-9. [DOI] [PubMed] [Google Scholar]
- 34.Lee KF, Li E, Huber LJ, Landis SC, Sharpe AH, Chao MV, Jaenisch R. Targeted mutation of the gene encoding the low affinity NGF receptor p75 leads to deficits in the peripheral sensory nervous system. Cell. 1992;69:737–749. doi: 10.1016/0092-8674(92)90286-l. [DOI] [PubMed] [Google Scholar]
- 35.Lewin GR, Barde YA. Physiology of the neurotrophins. Annu Rev Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- 36.Lubetzki C, Goujet-Zalc C, Gansmuller A, Monge M, Brillat A, Zalc B. Morphological, biochemical, and functional characterization of bulk isolated glial progenitor cells. J Neurochem. 1991;56:671–680. doi: 10.1111/j.1471-4159.1991.tb08202.x. [DOI] [PubMed] [Google Scholar]
- 37.Maroney AC, Glicksman MA, Basma AN, Walton KM, Knight E, Jr, Murphy CA, Bartlett BA, Finn JP, Angeles T, Matsuda Y, Neff NT, Dionne CA. Motoneuron apoptosis is blocked by CEP-1347 (KT 7515), a novel inhibitor of the JNK signaling pathway. J Neurosci. 1998;18:104–111. doi: 10.1523/JNEUROSCI.18-01-00104.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maroney AC, Finn JP, Bozyczko-Coyne D, O'Kane TM, Neff NT, Tolkovsky AM, Park DS, Yan CY, Troy CM, Greene LA. CEP-1347 (KT7515), an inhibitor of JNK activation, rescues sympathetic neurons and neuronally differentiated PC12 cells from death evoked by three distinct insults. J Neurochem. 1999;73:1901–1912. [PubMed] [Google Scholar]
- 39.Mazzoni IE, Said FA, Aloyz R, Miller FD, Kaplan D. Ras regulates sympathetic neuron survival by suppressing the p53-mediated cell death pathway. J Neurosci. 1999;19:9716–9727. doi: 10.1523/JNEUROSCI.19-22-09716.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCarthy KD, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Minden A, Lin A, McMahon M, Lange-Carter C, Derijard B, Davis RJ, Johnson GL, Karin M. Differential activation of ERK and JNK mitogen-activated protein kinases by Raf-1 and MEKK. Science. 1994;266:1719–1723. doi: 10.1126/science.7992057. [DOI] [PubMed] [Google Scholar]
- 42.Minden A, Lin A, Claret F-X, Abo A, Karin M. Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and CdC42Hs. Cell. 1995;81:1147–1157. doi: 10.1016/s0092-8674(05)80019-4. [DOI] [PubMed] [Google Scholar]
- 43.Namgung U, Xia Z. Arsenite-induced apoptosis in cortical neurons is mediated by c-Jun N-terminal protein kinase 3 and p38 mitogen-activated protein kinase. J Neurosci. 2000;20:6442–6451. doi: 10.1523/JNEUROSCI.20-17-06442.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nataf S, Naveilhan P, Sindji L, Darcy F, Brachet P, Montero-Menei CN. Low affinity NGF receptor expression in the central nervous system during experimental allergic encephalomyelitis. J Neurosci Res. 1998;52:83–92. doi: 10.1002/(SICI)1097-4547(19980401)52:1<83::AID-JNR8>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 45.Park JA, Lee JY, Sato TA, Koh JY. Co-induction of p75NTR, p75NTR-associated death executor in neurons after zinc exposure in cortical culture or transient ischemia in the rat. J Neurosci. 2000;20:9096–9103. doi: 10.1523/JNEUROSCI.20-24-09096.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peterson DA, Dickinson-Anson HA, Leppert JT, Lee KF, Gage FH. Central neuronal loss and behavioral impairment in mice lacking neurotrophin receptor p75. J Comp Neurol. 1999;404:1–20. doi: 10.1002/(sici)1096-9861(19990201)404:1<1::aid-cne1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 47.Qui MS, Green SH. PC12 cell neuronal differentiation is associated with prolonged p21ras activity and consequent prolonged ERK activity. Neuron. 1992;9:705–717. doi: 10.1016/0896-6273(92)90033-a. [DOI] [PubMed] [Google Scholar]
- 48.Roux PP, Colicos MA, Barker PA, Kennedy TE. p75 neurotrophin receptor expression is induced in apoptotic neurons after seizure. J Neurosci. 1999;19:6887–6896. doi: 10.1523/JNEUROSCI.19-16-06887.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salehi AH, Roux PP, Kubu CJ, Zeindler C, Bhakar A, Tannis LL, Verdi JM, Barker PA. NRAGE, a novel MAGE protein, interacts with the p75 neurotrophin receptor, facilitates nerve growth factor-dependent apoptosis. Neuron. 2000;27:279–288. doi: 10.1016/s0896-6273(00)00036-2. [DOI] [PubMed] [Google Scholar]
- 50.Taniuchi M, Clark HB, Johnson EM. Induction of nerve growth factor receptor in Schwann cells after axotomy. Proc Natl Acad Sci USA. 1986;83:4094–4098. doi: 10.1073/pnas.83.11.4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ward NL, Hagg T. p75(NGFR) and cholinergic neurons in the developing forebrain: a re-examination. Brain Res Dev Brain Res. 1999;118:79–91. doi: 10.1016/s0165-3806(99)00133-9. [DOI] [PubMed] [Google Scholar]
- 52.Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 53.Yamaguchi Y, Katoh H, Yasui H, Mori K, Negishi M. RhoA inhibits the nerve growth factor-induced Rac1 activation through a Rho-associated kinase-dependent pathway. J Biol Chem. 2001;276:18977–18983. doi: 10.1074/jbc.M100254200. [DOI] [PubMed] [Google Scholar]
- 54.Yamashita T, Tucker KL, Barde YA. Neurotrophin binding to the p75 receptor modulates Rho activity and axonal outgrowth. Neuron. 1999;24:585–593. doi: 10.1016/s0896-6273(00)81114-9. [DOI] [PubMed] [Google Scholar]
- 55.Yang DD, Kuan CY, Whitmarsh AJ, Rincon M, Zheng TS, Davis RJ, Rakic P, Flavell RA. Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature. 1997;389:865–870. doi: 10.1038/39899. [DOI] [PubMed] [Google Scholar]
- 56.Yao R, Yoshihara M, Osada H. Specific activation of a c-Jun NH2-terminal kinase isoform and induction of neurite outgrowth in PC-12 cells by staurosporine. J Biol Chem. 1997;272:18261–18266. doi: 10.1074/jbc.272.29.18261. [DOI] [PubMed] [Google Scholar]
- 57.Yasui H, Katoh H, Yamaguchi Y, Aoki J, Fujita H, Mori K, Negishi M. Differential responses to nerve growth factor and epidermal growth factor in neurite outgrowth of PC12 cells are determined by Rac1 activation systems. J Biol Chem. 2001;276:15298–15305. doi: 10.1074/jbc.M008546200. [DOI] [PubMed] [Google Scholar]
- 58.Ye X, Mehlen P, Rabizadeh S, VanArsdale T, Zhang H, Shin H, Wang JJ, Leo E, Zapata J, Hauser CA, Reed JC, Bredesen DE. TRAF family proteins interact with the common neurotrophin receptor and modulate apoptosis induction. J Biol Chem. 1999;274:30202–30208. doi: 10.1074/jbc.274.42.30202. [DOI] [PubMed] [Google Scholar]
- 59.Yeo TT, Chua-Couzens J, Butcher LL, Bredesen DE, Cooper JD, Valletta JS, Mobley WC, Longo FM. Absence of p75NTR causes increased basal forebrain cholinergic neuron size, choline acetyltransferase activity, and target innervation. J Neurosci. 1997;17:7594–7605. doi: 10.1523/JNEUROSCI.17-20-07594.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoon SO, Lois C, Alvirez M, Alvarez-Buylla A, Falck-Pedersen E, Chao MV. Adenovirus-mediated gene delivery into neuronal precursors of the adult mouse brain. Proc Natl Acad Sci USA. 1996;93:11974–11979. doi: 10.1073/pnas.93.21.11974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoon SO, Soltoff SP, Chao MV. A dominant role of the juxtamembrane region of the TrkA nerve growth factor receptor during neuronal cell differentiation. J Biol Chem. 1997;272:23231–23238. doi: 10.1074/jbc.272.37.23231. [DOI] [PubMed] [Google Scholar]
- 62.Yoon SO, Casaccia-Bonnefil P, Carter BD, Chao MV. Competitive signaling between TrkA and p75 determines cell survival. J Neurosci. 1998;18:3273–3281. doi: 10.1523/JNEUROSCI.18-09-03273.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]