Abstract
Heterotromeric G-proteins of the Gq family are thought to transduce signals from group I metabotropic glutamate receptors (mGluRs) in central neurons. We investigated roles of this cascade in hippocampal long-term potentiation (LTP) by using null-mutant mice lacking the α subunit of Gq (Gαq) or G11 (Gα11). We found no obvious abnormalities in the morphology, layer structure, expression of NMDA receptors, and basic parameters of excitatory synaptic transmission in the hippocampus of Gαq mutant mice. We used theta burst stimulation (TBS) (3–10 burst trains at 5 Hz; each train consisted of five stimuli at 100 Hz) to induce LTP at Schaffer collateral to CA1 pyramidal cell synapses. Conventional TBS with 10 burst trains induced robust LTP in wild-type, Gαq mutant, and Gα11 mutant mice. Weak TBS with three burst trains consistently induced LTP in wild-type mice. In contrast, the same weak TBS was insufficient to induce LTP in Gαq and Gα11 mutant mice. In wild-type mice, the LTP by weak TBS was abolished by inhibiting group I mGluR or protein kinase C (PKC) but not by blocking muscarinic acetylcholine receptors. Prior activation of group I mGluR by an agonist significantly enhanced the LTP by weak TBS in wild-type mice. However, this priming effect was absent in Gαq mutant mice. These results indicate that the signaling from group I mGluR to PKC involving Gαq/Gα11 does not constitute the main pathway for LTP, but it secures LTP induction by lowering its threshold in the hippocampal area CA1.
Keywords: long-term potentiation, hippocampus, metabotropic glutamate receptor, G-protein, protein kinase C, mouse
Long-term potentiation (LTP) and long-term depression (LTD) in the hippocampal area CA1 are widely thought to be cellular bases for certain forms of learning and memory (Bliss and Collingridge, 1993; Bear and Malenka, 1994; Nicoll and Malenka, 1995; Bear and Abraham, 1996). Several previous studies suggest that metabotropic glutamate receptors (mGluRs) are involved in the processes of LTP or LTD in the CA1. The mGluRs consist of eight different subtypes, from mGluR1 to mGluR8, and are classified into groups I, II, and III (Nakanishi, 1994; Conn and Pin, 1997). The group I mGluR consists of mGluR1 and mGluR5 and couples to the Gq family heterotrimeric G-protein and phospholipase C (PLC)β, leading to triggering of inositol-1,4,5-trisphosphate (IP3)-mediated Ca2+release and to activation of protein kinase C (PKC) (Masu et al., 1991;Abe et al., 1992). Although several studies have addressed the involvement of group I mGluR in LTP or LTD in the CA1, the results remain controversial. For example, several researchers report that an antagonist of group I/II mGluRs, (R,S)-amino-methyl-4-carboxylphenylglycine (MCPG), inhibits LTP induction (Bashir et al., 1993; Riedel and Reymann, 1993;Bortolotto et al., 1994; Richter-Levin et al., 1994), whereas others found no effect (Chinestra et al., 1993; Manzoni et al., 1994; Selig et al., 1995; Thomas et al., 1995).
CA1 pyramidal cells strongly express mGluR5 that is densely concentrated at the perisynaptic region of the dendritic spines facing the excitatory synaptic terminals (Luján et al., 1996;Luján et al., 1997). In contrast, mGluR1 is not found on their dendrites (Shigemoto et al., 1997), indicating that mGluR5 is a major receptor at CA1 excitatory synapses that activates the Gq-PLCβ cascade. It is reported that the null-mutant mice lacking mGluR5 have a partial impairment in NMDA receptor-dependent LTP (Lu et al., 1997). Several previous studies have suggested an involvement of PKC in LTP. Postsynaptic activation of PKC is required for the induction of LTP (Wang and Feng, 1992). Tetanic stimulation that induces LTP increases the activation of PKC (Klann et al., 1993) and the phosphorylation of PKC substrates (Ramakers et al., 1999). Furthermore, LTP is impaired in PKCγ mutant mice (Abeliovich et al., 1993). However, maximal activation of postsynaptic G-protein does not occlude the induction of LTP in area CA1 (Goh and Pennefather, 1989; Katsuki et al., 1992).
These observations raise a question of whether and how the signaling from mGluR5 to PKC is involved in the induction of LTP in the area CA1. To address this issue, we used the null-mutant mice lacking the α subunit of Gq (Gαq) or G11 (Gα11) (Offermanns et al., 1997;Offermanns and Simon, 1998), members of the heterotrimeric Gq family that are shown to transduce signals from group I mGluR in central neurons (Masu et al., 1991; Abe et al., 1992). Our results suggest that the group I mGluR (presumably mGluR5) to PKC cascade does not constitute the main pathway for LTP at Schaffer collateral to CA1 pyramidal cell synapses, but that this cascade facilitates LTP induction by significantly lowering its threshold.
MATERIALS AND METHODS
Morphological and immunohistochemical studies. Under deep anesthesia by pentobarbital (100 mg/kg body weight, i.p.), wild-type and Gαq mutant mice were perfused transcardially with 4% paraformaldehyde in 0.1 m sodium phosphate buffer, pH 7.2. Parasagittal paraffin sections (5 μm in thickness) were prepared from fixed brains and processed for histological (hematoxylin) and immunohistochemical stainings. Before immunohistochemical incubations, sections were treated at 37°C for 10 min with 1 mg/ml pepsin (Dako, Carpinteria, CA) in 0.2N HCl, as reported previously (Watanabe et al., 1998). After blocking with 10% normal goat serum, sections were incubated with primary antibodies (0.5 μg/ml) against mouse NMDA receptor subunit GluRε1 (anti-GluRε1C), GluRε2 (anti-GluRε2N), or GluRξ1 (anti-GluRξ1C) (Watanabe et al., 1998; Yamada et al., 2001) at room temperature overnight, with biotinylated goat anti-rabbit IgG at room temperature for 2 hr, and with avidin–peroxidase complex at room temperature for 30 min, using a Histofine SAB-PO Kit (Nichirei Corp., Tokyo, Japan). The anti-GluRξ1C was directed against the C2 exon cassette. Immunoreaction was visualized with diaminobenzidine.
Hippocampal slice preparation. Hippocampal slices were prepared from Gαq mutant, Gα11 mutant, and wild-type mice using standard procedures (Manabe et al., 1993). In brief, young adult mice (6–12 weeks of age) were anesthetized and decapitated. Hippocampi were dissected free and cut with tissue slicer (VT1000S; Leica, Nussloch, Germany) in ice-cold medium composed of (in mm): 20 glucose, 120 choline-Cl, 3 KCl, 1.1 NaH2PO4, 8 MgCl2, and 26 NaHCO3, pH 7.4 (Tsubokawa et al., 2000). In the priming experiment, area CA3 was removed by a manual knife cut to prevent the slow onset potentiation induced by mGluR agonists (Bortolotto and Collingridge, 1992; Chinestra et al., 1993). Slices (300 and 500 μm thick; used for whole-cell recording and field recording, respectively) were transferred to an incubation chamber and allowed to recover for ≥1 hr before recording. During recording, a slice was placed into a submerged chamber and perfused continuously (1.5–2 ml/min, 30°C) with artificial CSF (ACSF) composed of (in mm): 20 glucose, 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 26 NaHCO3, 2 CaCl2, and 1 MgSO4, bubbled with 95% O2-5% CO2, pH 7.4.
Field recording. Field EPSPs (fEPSPs) were recorded by using an Axoclamp 2B amplifier (Axon Instruments, Foster City, CA) with glass electrodes (filled with ACSF; resistance, 3–6 MΩ) placed in the stratum radiatum of the area CA1. Field EPSPs were evoked by stimulating the Schaffer collateral/commissural pathway with short current pulses (50 μsec duration; 0.033 Hz) by using the Teflon-coated tungsten bipolar electrode that was placed 1 mm apart from the recording electrode. Protocols for the induction of LTP were theta burst stimulation (TBS) (3–10 trains/0.5 Hz; each train contains five pulses/100 Hz) for the CA1 region and tetanic stimulation (100 pulses/100 Hz) for the CA3 region.
Whole-cell recording and Ca2+imaging. Whole-cell recording was made from a visually identified pyramidal neuron under the upright microscope with a ×40 water immersion objective (Axioskop; Zeiss, Jena, Germany). The whole-cell voltage-clamp recording was performed using cesium-based solutions (for experiments in Fig. 2C,D) composed of (in mm): 120 CsCl, 20 TEA-Cl, 5 BAPTA, 10 HEPES, 5 MgCl2, 5N-(2,6-dimethylphenylcarbamoyl-methyl)triethylammonium bromide, 4 Na2-ATP, and 0.3 Na-GTP, pH 7.25 (adjusted with CsOH). The Ca2+imaging and whole-cell current-clamp recordings were made from a single neuron with a potassium-based solution (for experiments in Fig. 7) composed of (in mm): 120 K-gluconate, 20 KCl, 10 HEPES, 1 MgCl2, 4 Na2-ATP, and 0.3 Na-GTP, pH 7.25 (adjusted with KOH). A calcium indicator dye, fura-2 (200 μm), was loaded to the neuron through the whole-cell pipette, and the ratio of emission intensities at the two excitation wavelengths (340 and 380 nm) was measured using a cooled CCD camera system (IMAGO; T.I.L.L. Photonics GmbH, Martinsried, Germany).
Fig. 2.
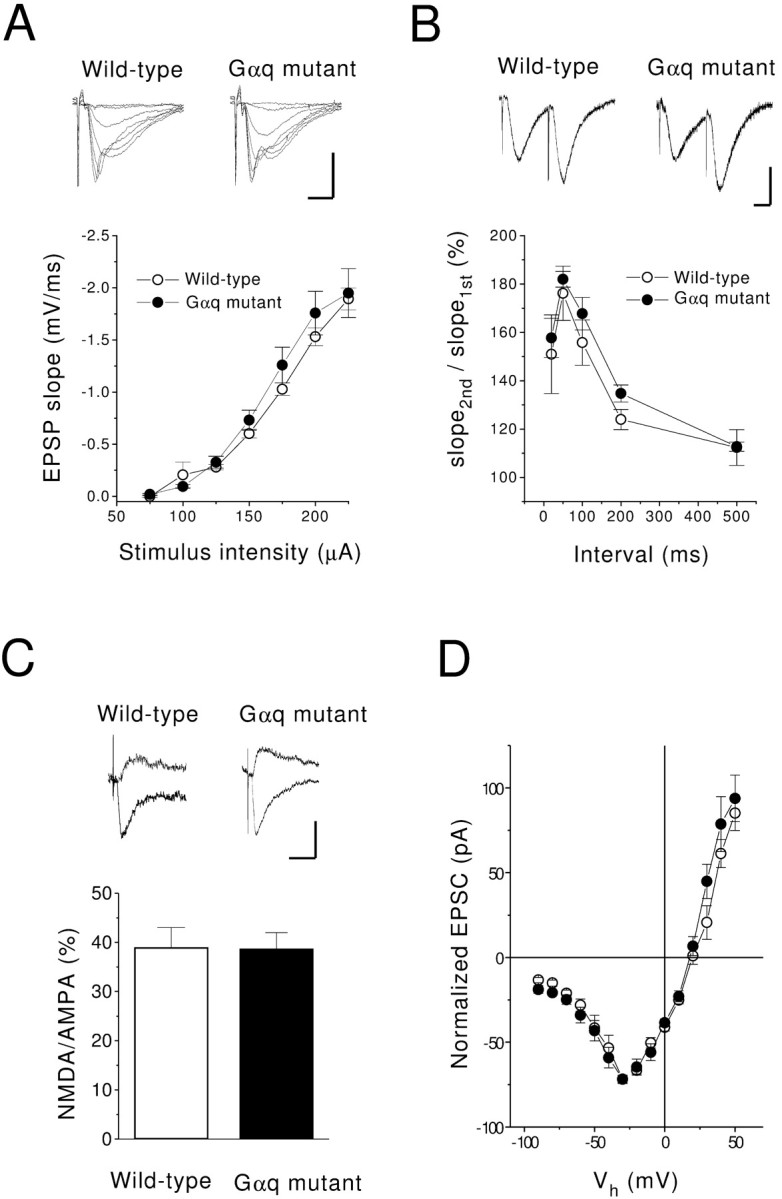
Basic properties of excitatory synaptic transmission of Gαq mutant are normal. A, The input–output relationships of fEPSPs in wild-type (○;n = 7) and Gαq mutant (●; n= 5) mice. The fEPSPs were evoked by single stimulation of the Schaffer collateral. Insets are typical responses. Calibration: 2 mV, 5 msec. B, Paired-pulse ratio of fEPSPs in wild-type (○; n = 5) and Gαq mutant (●;n = 5) mice. The initial slope of the fEPSP was measured to quantify the strength of the synaptic response. The ratio of the second to the first response is plotted against the interstimulus interval. Calibration: 1 mV, 20 msec. C, The ratio of amplitudes of the NMDA EPSC (recorded at a holding potential of +40 mV) to those of the AMPA EPSC (recorded at a holding potential of −70 mV) in wild-type (open column;n = 10) and Gαq mutant (filled column; n = 7) mice. The NMDA EPSC was recorded in the presence of CNQX (25 μm). Sample traces are shown in the top panel. Calibration: 20 pA, 20 msec.D, The current–voltage relationship of NMDA synaptic currents in wild-type (○; n = 8) and Gαq mutant (●; n = 6) mice. The amplitude of the EPSC is normalized to the value obtained at −30 mV.
Fig. 7.
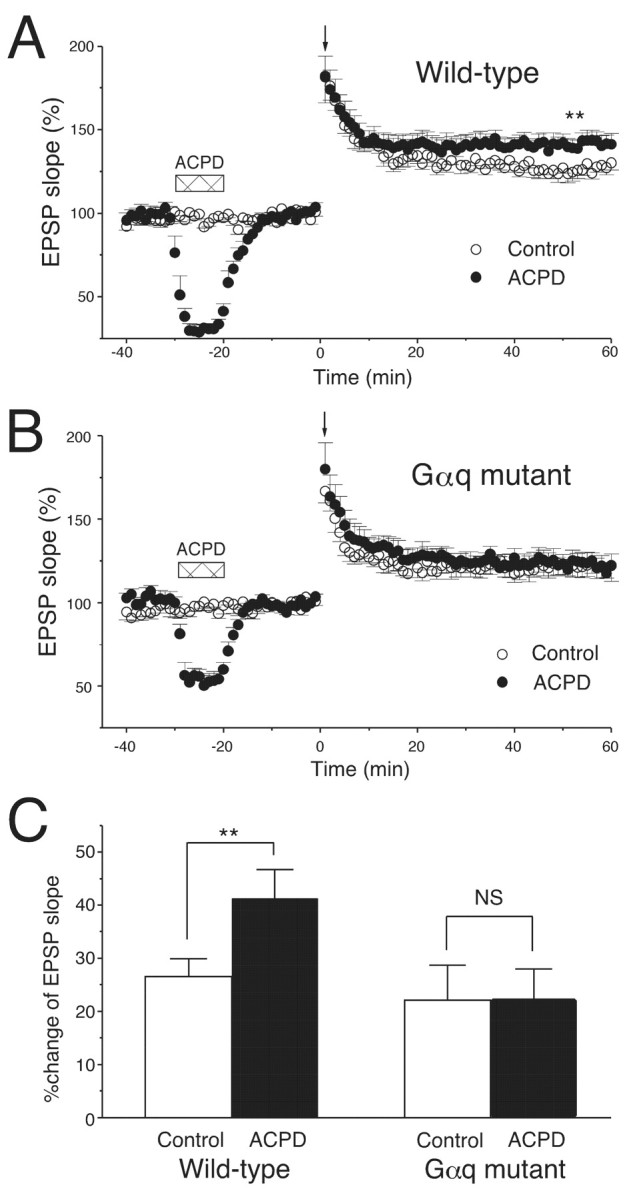
The ACPD-induced priming effect is deficient in Gαq mutant mice. A, Bath application of ACPD (20 μm, 10 min, horizontal bars) enhanced the subsequent LTP induced by the weak TBS of three trains (downward arrow) in wild-type mice (control,n = 6; ACPD, n = 7).B, A TBS of five trains (downward arrow) induced LTP in Gαq mutant mice (control, n = 6). Bath application of ACPD (20 μm, 10 min,horizontal bars) did not enhance LTP (ACPD,n = 6). C, The average increase in initial slopes of fEPSPs (50–60 min after TBS). ∗∗p < 0.01.
Data are expressed as mean ± SEM. Student's t test was used to determine whether there was a significant difference in the mean between the two sets of data.
Materials. Chelerythrine was obtained from Calbiochem (Nottingham, UK). MCPG, (±)-1-aminocyclopentane-trans-1,3-dicarboxylic acid (ACPD), and aminophosphonovalerate (AP-5) were obtained from Tocris Cookson (Bristol, UK). Fura-2 was obtained from Dojindo (Kumamoto, Japan). All other reagents were obtained from Wako Pure Chemicals Industries (Osaka, Japan).
RESULTS
Morphology and NMDA receptor expression of the Gαq mutant hippocampus
Gαq is expressed in CA1 pyramidal cells of the mouse hippocampus (Milligan, 1993; Friberg et al., 1998; Tanaka et al., 2000). We began by examining whether the genetic deletion of Gαq causes any change in the morphology of the hippocampus. As reported previously, the gross anatomy of the CNS was apparently normal in Gαq mutant mice (Offermanns et al., 1997; Hashimoto et al., 2000). The hippocampus of Gαq mutant mice at 2 months of age had similar morphology to that of wild-type mice at the same age (Fig.1A,E). The shape, size, cellular arrangement, and laminar organization of the CA1 and dentate gyrus were normal in hematoxylin staining (Fig.1A,E). We then assessed the expression and distribution of NMDA receptor subunits, because the decrease in NMDA receptor-mediated synaptic currents significantly attenuates the magnitude of LTP in the area CA1 (Sakimura et al., 1995; Kiyama et al., 1998). We examined immunohistochemical distribution of three NMDA receptor subunits, GluRε1 (Fig. 1B,F), GluRε2 (Fig. 1C,G), and GluRξ1 (Fig.1D,H). In both strains of mice, distinct laminar distribution was evident for each subunit, but no significant differences by the genotypes were noted in the distribution and intensity within the hippocampus. The highest immunostaining was detected in neuropils of the strata radiatum and oriens in the CA1 region. In contrast, cell bodies of pyramidal cells and stem apical dendrites were almost immunonegative. These morphological results suggest that the deletion of the Gαq gene caused no gross abnormalities in histology and NMDA receptor subunit expression.
Fig. 1.
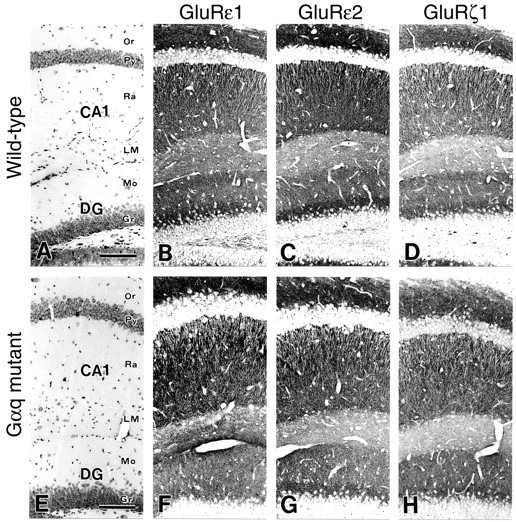
Histology and distributions of NMDA receptor subunits are normal in the Gαq mutant hippocampus.A–D, Wild-type mice; E–H, Gαq mutants. A, E, Hematoxylin staining. B, F, GluRε1 subunit. C, G, GluRε2 subunit.D, H, GluRξ1 subunit. CA1, CA1 region;DG, dentate gyrus; Gr, granule cell layer; LM, stratum lacunosum-moleculare;Mo, molecular layer; Or, stratum oriens;Py, pyramidal cell layer; Ra, stratum radiatum. Scale bars, 100 μm.
Excitatory synaptic transmission is normal in Gαq mutant hippocampus
We subsequently examined whether the basic property of excitatory synaptic transmission is normal in Gαq mutant mice. We recorded fEPSPs by stimulation of the Schaffer collateral–commissural pathway in hippocampal slices prepared from 6- to 11-week-old wild-type and mutant mice. We measured the initial slope of fEPSP to quantify the strength of the synaptic response. The input–output relationship in Gαq mutant mice was similar to that of wild-type mice (Fig.2A). In the area CA1, paired stimulation with short intervals usually causes paired-pulse facilitation (PPF). The extent of PPF of Gαq mutant mice was identical to that of wild-type mice, with an interpulse interval of 25–500 msec (Fig. 2B).
To evaluate the NMDA receptor functions of Gαq mutant mice, the ratio of the NMDA to AMPA receptor-mediated component of EPSC was measured with whole-cell recording techniques in the presence of a GABAA receptor antagonist, bicuculline (25 μm). AMPA receptor-mediated EPSCs were recorded at a membrane potential of −70 mV in a voltage-clamp mode. NMDA receptor-mediated EPSCs were then measured at the same stimulus strength in the presence of 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) (10 μm), a non-NMDA receptor antagonist, at +40 mV to relieve the voltage-dependent Mg2+ block of the NMDA receptor channel (Mayer et al., 1984; Nowak et al., 1984). The ratio of NMDA to AMPA EPSC amplitudes of Gαq mutant mice (38.7 ± 3.3%; n = 7) was identical to that of wild-type mice (39 ± 4.1%; n = 10) (Fig.2C). Furthermore, the current–voltage (I–V) relationships for the NMDA component of wild-type and Gαq mutant mice were almost identical (Fig.2D), suggesting that the voltage-dependent Mg2+ block of the NMDA receptor is not altered in Gαq mutant mice. These results indicate that excitatory synaptic transmission and functions of NMDA receptors are normal in CA1 pyramidal cells of Gαq mutant mice.
Elevated threshold for LTP induction in Gαq mutant hippocampus
We then examined whether LTP in the CA1 and the CA3 is altered in Gαq mutant mice. Conventional TBS applied to the Schaffer collateral–CA1 synapses (10 burst trains repeated at 5 Hz; each burst consisted of five stimuli at 100 Hz) induced a robust and stable LTP lasting >60 min in both Gαq mutant and wild-type mice (Fig.3A). The average increase in the fEPSP slope (50–60 min after TBS) was 40.8 ± 4.3% (n = 10) for wild-type mice and 35.4 ± 3.1% (n = 10) for Gαq mutant mice (Fig. 3A), showing no significant difference. Two excitatory synapses onto CA3 neurons, associational/commissural (A/C)–CA3 synapses and mossy fiber (mo)–CA3 synapses, display LTP with distinct mechanisms (Salin et al., 1996). Thus, we examined whether LTP at these two types of synapses is altered in Gαq mutant mice. Conventional tetanic stimulation (100 Hz for 1 sec) of the A/C–CA3 synapses induced robust and stable LTP in both Gαq mutant and wild-type mice (Fig. 3B). Furthermore, tetanic stimulation (100 Hz for 1 sec) of the mo–CA3 synapses also caused stable LTP in both strains of mice (Fig. 3C). The average fEPSP (the last 10 min of recording) at these two types of synapses showed no significant differences between the Gαq mutant and wild-type mice (Fig. 3B,C). These results indicate that LTP can be induced normally in both the CA1 and CA3 regions of Gαq mutant mice by conventional stimulation protocols. In the rest of the present study, we focused on the issue of whether and how the group I mGluR signaling involving Gαq contributes to LTP induction at the Schaffer collateral–CA1 synapses.
Fig. 3.
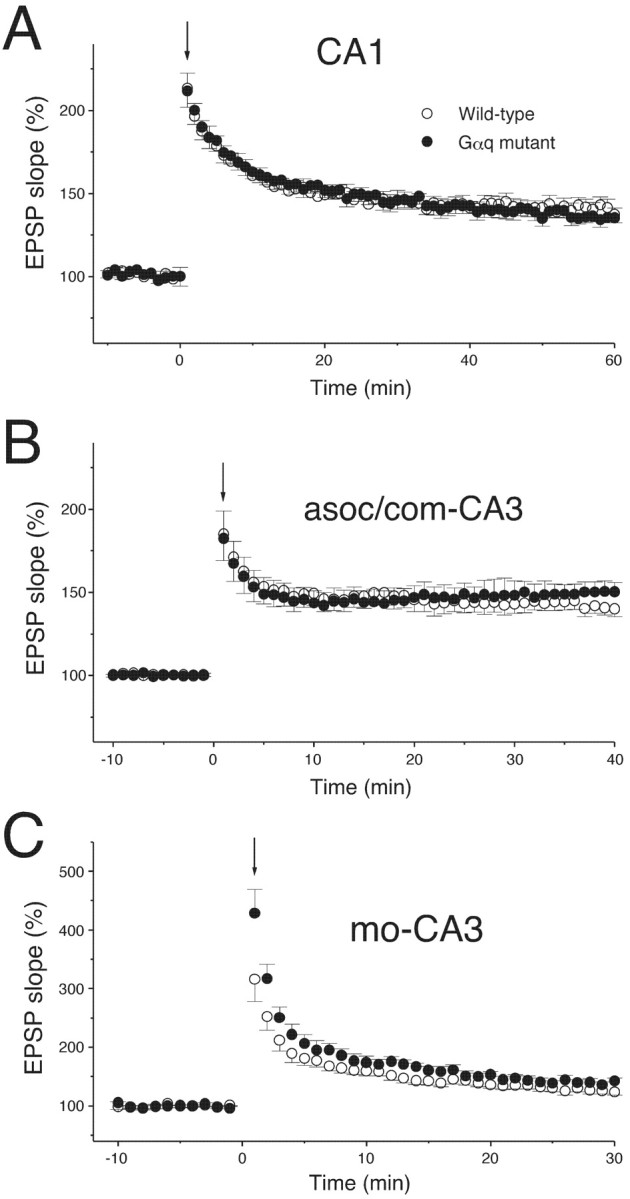
Normal LTP induced by conventional stimulation protocols in the Gαq mutant hippocampus.A, The averaged time course of LTP induced by 10 trains of TBS in the area CA1 of wild-type (n = 10) and Gαq mutant (n = 10) mice. Initial EPSP slopes were measured, and the values were normalized in each experiment using the averaged slope value measured during the control period (time, −10 to 0 min). The TBS was applied at time 0 (downward arrow). In the following figures, the averaged time course of LTP is illustrated similarly. B, The averaged time course of LTP induced by tetanic stimulation (100 Hz, 1 sec) at the associational/commissural (asoc/com)-CA3 synapses in wild-type (n = 10) and Gαq mutant (n = 8) mice. C, The averaged time course of LTP induced by tetanic stimulation (100 Hz, 1 sec) at the mo–CA3 synapses in wild-type (n = 9) and Gαq mutant (n = 8) mice. Records were taken in the presence of AP-5 (25 μm) to block NMDA receptors. Mossy fibers were stimulated via a bipolar electrode placed in the dentate hilus.
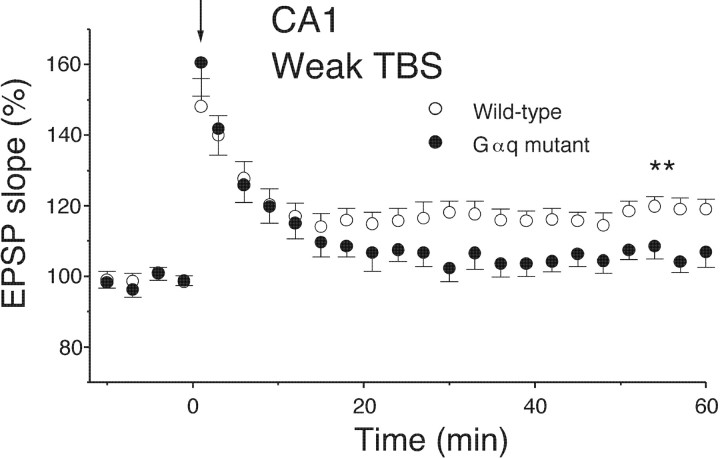
The lack of the effect of Gαq deletion on LTP induction (Fig.3A) could be attributable to the fact that the conventional TBS with 10 burst trains may be well above the threshold for LTP induction. To detect subtle differences that may exist between wild-type and Gαq mutant mice, we used a weaker TBS that consisted of three burst trains. This weak TBS applied to the Schaffer collateral produced a stable LTP in wild-type mice (Figs. 4, 5C) (19.8 ± 2.1%; n = 13; measured at 50–60 min after TBS). In contrast, the same TBS caused a short-term potentiation during the initial 10 min but failed to induce LTP in Gαq mutant mice (Figs. 4,5C) (6.3 ± 1.9%; n = 9; measured at 50–60 min after TBS). The difference between wild-type and mutant mice was significant (p < 0.01). These results indicate that the threshold for LTP induction is significantly elevated in the CA1 of Gαq mutant mice.
Fig. 4.
The threshold for LTP induction is elevated in the area CA1 of Gαq mutant mice. A weak TBS (3 trains) applied at time 0 (downward arrows) induced a clear LTP in wild-type (n = 13) but not Gαq mutant (n = 9) mice. ∗∗p < 0.01.
Fig. 5.
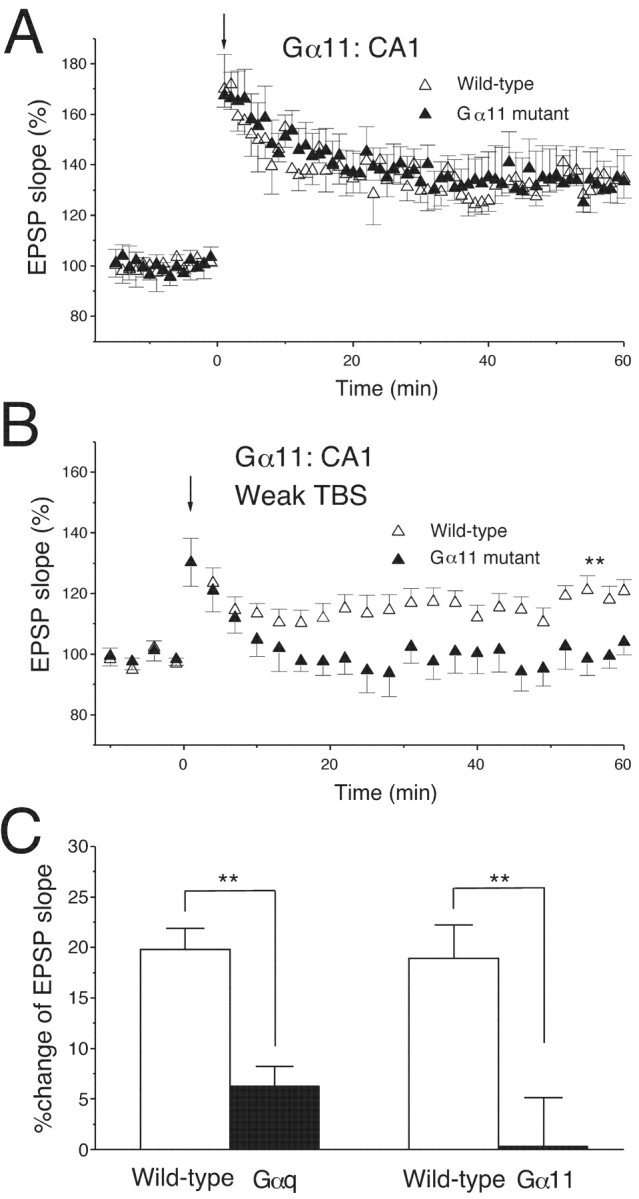
The threshold for LTP induction is elevated in the area CA1 of Gα11 mutant mice. A, A TBS with 10 burst trains (downward arrow) induced a robust and stable LTP in both wild-type (n = 7) and Gα11 mutant (n = 8) mice. B, A weak TBS of three trains (downward arrow) induced a clear LTP in wild-type (n = 10) but not Gα11 mutant (n = 10) mice; ∗∗p < 0.01.C, The average increase in initial slopes of fEPSPs (50–60 min after TBS); ∗∗p < 0.01.
Induction of LTP in the CA1 region is known to require elevation of [Ca2+]i in pyramidal cells. We examined whether there is any difference in Ca2+ transients during the weak TBS between the wild-type and Gαq mutant mice. We recorded the [Ca2+]i and EPSPs simultaneously from single CA1 pyramidal neurons under whole-cell current-clamp recording mode. The calcium indicator dye fura-2 was loaded to the neurons by diffusion from whole-cell recording pipettes, and the fluorescence ratio [Δ(F340/F380)] was measured at the soma and the dendrites in response to the weak TBS. We could not detect any significant difference in the Ca2+transients in the soma and dendrites between the two mouse strains (data not shown). These results suggest that the elevated threshold for LTP induction in the Gαq mutant hippocampus is not likely caused by the alteration in the Ca2+ transients during the weak TBS.
Another member of the Gq family, Gα11, is also expressed in CA1 pyramidal cells of the mouse hippocampus (Milligan, 1993; Friberg et al., 1998; Tanaka et al., 2000). Because both Gαq and Gα11 are thought to transduce signals from group I mGluR, it is possible that Gα11 has a facilitatory effect on LTP induction similar to Gαq. Therefore, we examined null-mutant mice lacking Gα11. The morphology of the hippocampus and other CNS regions appeared normal in Gα11 mutant mice. We found that the TBS with 10 burst trains induced robust and stable LTP in both Gα11 mutant and wild-type mice (Fig.5A). The average increase in the fEPSP slope (50–60 min after TBS) was 34.7 ± 7.7% (n = 7) for wild-type mice and 32 ± 12.7% (n = 8) for Gα11 mutant mice (Fig. 5A), showing no significant difference. In contrast, the weak TBS with three burst trains induced a clear LTP in wild-type mice but not Gα11 mutant mice (Fig. 5B). The average magnitude of LTP (50–60 min after TBS) was 18.9 ± 3.3% (n = 10) for wild-type mice and 0.3 ± 4.7% (n = 10) for Gα11 mutant mice (Fig. 5B,C), showing a significant difference (p < 0.01). These results indicate that Gαq and Gα11 have a similar facilitatory effect on LTP induction at Schaffer collateral–CA1 synapses.
mGluR signaling involving Gαq/Gα11 facilitates LTP induction
CA1 pyramidal cells express group I mGluRs (primarily mGluR5) (Lu et al., 1997) and muscarinic acetylcholine receptors (M1) (Tsubokawa and Ross, 1997; Tsubokawa et al., 2000) that functionally couple to Gαq/Gα11. To examine which type of the receptors exerts the facilitatory effect on LTP induction, an mGluR antagonist, MCPG, or a muscarinic receptor antagonist, atropine, was bath applied during weak TBS. In the presence of MCPG (500 μm), the weak TBS caused a short-term potentiation during the initial 10 min but failed to induce LTP (Fig.6A,C) (control: 18 ± 3.4%, n = 7; MCPG: 1.5 ± 2.3%,n = 6), which is very similar to the results seen in Gαq or Gα11 mutant mice (Figs. 4, 5B,C). In contrast, the same weak TBS readily induced LTP in the presence of atropine (1 μm) (Fig. 6B,C) (control: 18 ± 3.4%, n = 7; atropine: 17 ± 10.8%, n = 5). In addition, atropine significantly enhanced the short-term potentiation during the first 20 min after the weak TBS (Fig. 6B).
Fig. 6.
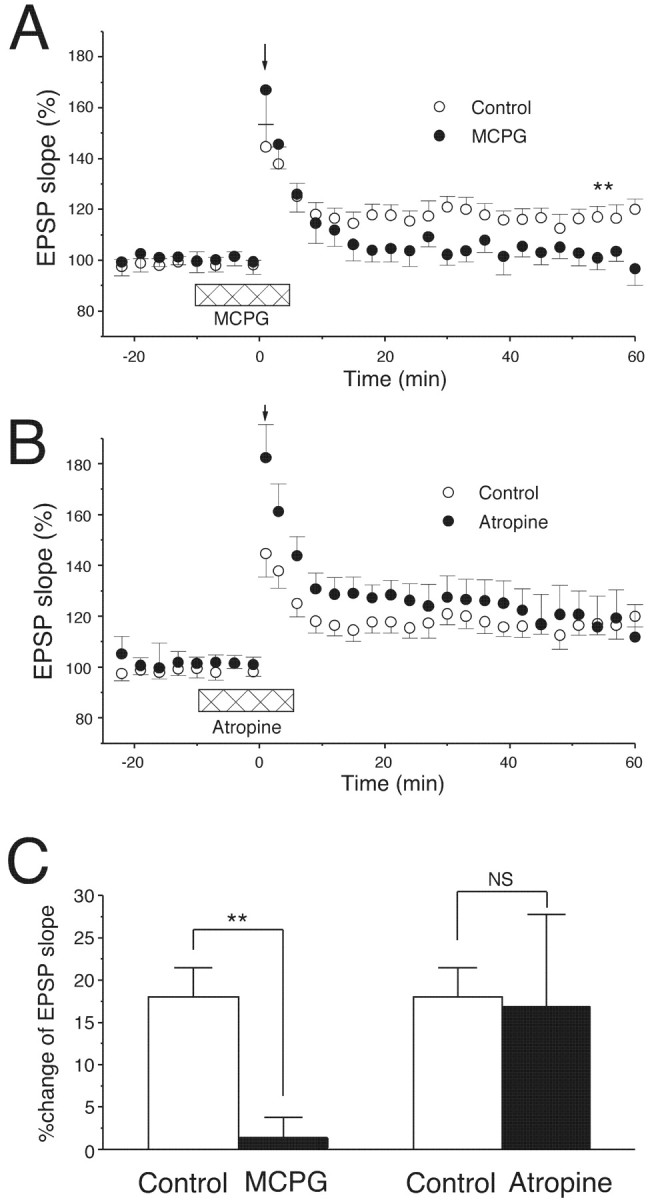
LTP induced by the weak TBS in the area CA1 is abolished by MCPG but not by atropine. A, The weak TBS (downward arrow) induced LTP in the area CA1 in control saline (n = 7). MCPG bath applied before and during the TBS (500 μm, horizontal bar) abolished LTP (n = 6); ∗∗p < 0.01. B, Atropine (1 μm, horizontal bar) bath applied before and during the TBS (downward arrow) enhanced post-tetanic potentiation but did not affect the level of LTP. n= 7 for control; n = 5 for atropine.C, The average increase in initial slopes of fEPSPs (50–60 min after TBS); ∗∗p < 0.01.
It has been reported previously that previous activation of mGluRs by bath-applied ACPD facilitates the induction of LTP (Cohen and Abraham, 1996). Because ACPD is an agonist for both group I and II mGluRs, this priming effect may be attributable to activation of group I mGluR in CA1 pyramidal cells. Thus, we examined whether the priming effect by ACPD is altered in Gαq mutant mice. In this experiment, we applied TBS with five burst trains to Gαq mutant pyramidal cells to induce LTP comparable with that induced in wild-type pyramidal cells by TBS with three burst trains. The mean magnitude of LTP in wild-type mice was 22.1 ± 6.6% (Fig.7A,C) (control,n = 6) and that in Gαq mutant mice was 26.6 ± 3.3% (Fig. 7B,C) (Gαq mutant, n = 6). Bath-applied ACPD (20 μm) caused transient depression of fEPSP in both genotypes (Fig. 7A,B, ACPD). However, the priming effect was clearly observed in wild-type mice (41.3 ± 5.4%) (Fig. 7A,C, ACPD; n = 7) but was deficient in Gαq mutant mice (22.4 ± 5.6%) (Fig.7B,C, ACPD; n = 6).
A PKC inhibitor abolishes LTP by weak TBS
Because the group I mGluR signaling pathway including Gq leads to activation of PKC, we assessed whether the facilitatory effect on LTP induction involves PKC. We tested whether a PKC inhibitor, chelerythrine (2 μm), affects the LTP by weak TBS. In wild-type mice, a TBS with three burst trains readily induced LTP in the control saline (Fig.8A,C) (control: 23.1 ± 5.7%; n = 9). Bath-applied chelerythrine (2 μm) effectively blocked LTP (Fig.8A,C) (chelerythrine: 5.5 ± 5.8%;n = 9; ∗∗p < 0.01). In Gαq mutant mice, a TBS with five burst trains induced LTP in the control saline (Fig. 8B,C) (control: 26.5 ± 10.8%;n = 8) that was comparable with the LTP in wild-type mice (Fig. 8A,C, control). However, bath-applied chelerythrine (2 μm) had no blocking effect on LTP in Gαq mutant mice (Fig. 8B,C) (chelerythrine: 20.1 ± 6.2%; n = 9). These results indicate that the facilitatory effect on LTP induction by the Gαq cascade involves activation of PKC.
Fig. 8.
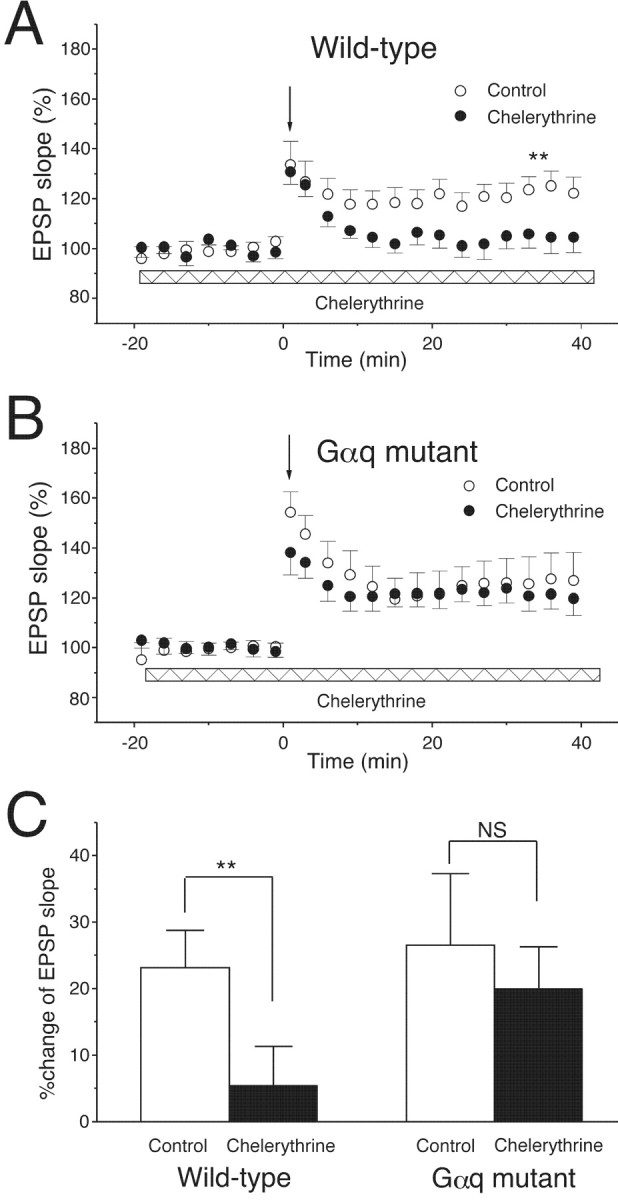
LTP induced by the weak TBS is abolished by a PKC inhibitor, chelerythrine. A, In wild-type mice, a PKC inhibitor, chelerythrine (2 μm, bath applied during the recoding period indicated with the horizontal bar), abolished the LTP induced by the TBS of three trains (downward arrow) in normal control saline (control, n = 9; chelerythrine, n = 9; ∗∗p < 0.01). B, In Gαq mutant mice, chelerythrine had no blocking effect on the LTP induced by the TBS of five trains (downward arrow) (control,n = 8; chelerythrine, n = 9).C, The average increase in initial slopes of fEPSPs (50–60 min after TBS); ∗∗p < 0.01.
DISCUSSION
We show that the threshold for LTP induction in the CA1 is significantly elevated in null-mutant mice lacking Gαq or Gα11. Full-size LTP can be induced in the area CA1 of both Gαq and Gα11 mutant mice with the TBS of 10 burst trains, a widely used LTP induction protocol. In contrast, a weak TBS that consistently induced LTP in wild-type mice was subthreshold for LTP induction in Gαq and Gα11 mutant mice. The LTP by the weak TBS was abolished by inhibiting group I mGluR or PKC but not by blocking muscarinic acetylcholine receptors. Prior activation of group I mGluR by an agonist significantly enhanced the LTP by weak TBS in wild-type mice but not Gαq mutant mice. These results suggest that the signaling from group I mGluR to PKC involving Gαq/Gα11 facilitates LTP induction by lowering its threshold in the hippocampal area CA1.
Role of signal transduction pathway via Gαq/Gα11 in LTP induction
Gαq and Gα11 are the major Gq family isoforms in the adult brain (Strathmann and Simon, 1990; Simon et al., 1991) and are wildly distributed in the dendritic spines of pyramidal neurons in area CA1 (Mailleux et al., 1992; Tanaka et al., 2000). We could not evaluate the effect of total deletion of the Gq family on LTP, because Gαq and Gα11 double knock-out mice are embryonic lethal (Offermanns et al., 1998). Because deletion of either Gαq or Gα11 resulted in elevation of the threshold for LTP induction to the same extent, both isoforms appear to contribute to the facilitation of LTP induction. A previous study indicates that Gαq- or Gα11-deficient mice do not exhibit a compensatory upregulation of the other isoform (Kleppisch et al., 2001). This might be a reason that Gαq and Gα11 cannot be mutually complementary in facilitating LTP induction. In contrast to the weak TBS, a conventional LTP induction protocol, such as TBS with 10 burst trains, can produce full-size LTP in both Gαq- and Gα11-deficient mice that is indistinguishable from LTP in wild-type mice. In other studies, postsynaptic injection of GTPγS, a nonhydrolyzable guanine nucleotide analog, does not occlude LTP in area CA1 (Goh and Pennefather, 1989; Katsuki et al., 1992). These results suggest that the signal transduction pathway involving Gαq or Gα11 is not essential but has a significant modulatory effect in the induction of LTP.
Group I mGluR is linked to the Gαq/Gα11 to PKC signaling cascade
In the area CA1, mGluR5 is highly expressed and enriched at the perisynaptic site of the postsynaptic membrane (Nusser et al., 1994; Luján et al., 1996, 1997). Immunoreactivity of Gαq/Gα11 is observed in postsynaptic extrajunctional membrane and colocalized with mGluR5 in the neuropil of hippocampal pyramidal neurons (Tanaka et al., 2000). Mutant mice lacking mGluR5 reportedly exhibit partial reduction of LTP in CA1 (Liu and Simon, 1996). It has been shown previously that mGluR antagonists have inhibitory effects on LTP in certain experimental situations (Bashir et al., 1993; Riedel and Reymann, 1993; Bortolotto et al., 1994; Richter-Levin et al., 1994), especially in those studies using weak stimulation protocols (Riedel et al., 1996; Wilsch et al., 1998; Balschun et al., 1999). In the present study, we show that LTP induced by weak TBS was abolished by an mGluR antagonist, MCPG. Together, it is most likely that group I mGluR is the major Gq-coupled receptor that is activated during the induction of LTP in area CA1.
The heterotrimeric Gq family couples to a wide variety of seven transmembrane receptors other than group I mGluR (Exton, 1996). These include muscarinic (M1, M3, and M5) receptors (Wess et al., 1995), dopamine (D2, D3, and D5) receptors, α1-adrenergic receptors (Docherty, 1998), and serotonin (5-HT1A, 5-HT1c, and 5-HT2) receptors (Hoyer and Martin, 1997). Possible involvement of these receptors in LTP has been investigated. Activation of muscarinic M1 and M3 receptors has no effect on the LTP in area CA1, whereas the agonist of the M2 receptor, which couples to Gi/o, enhances the level of LTP (Auerbach and Segal, 1994; Kaneko et al., 1997). Cholinergic denervation does not attenuate the magnitude of LTP and short-term potentiation induced by weak stimulation protocol (Jouvenceau et al., 1996). Dopamine D2 antagonists prevent the maintenance of LTP (Frey et al., 1990), but the role of D2 receptors in the induction of LTP is not clear. Noradrenaline has little effect on LTP in CA1 and does not enhance the level of LTP induced by weak TBS protocols (Katsuki et al., 1997). Application of serotonin decreases the magnitude of LTP (Staubli and Otaky, 1994), and depletion of serotonin does not reduce LTP (Stanton and Sarvey, 1985). The selective 5-HT2Aantagonist facilitates the LTP in the area CA1 (Wang and Arvanov, 1998). Mutant mice lacking 5-HT2C exhibit normal LTP of Schaffer collateral–CA1 and mo–CA3 synapses (Tecott et al., 1998). In the present study, we show that LTP induced by weak TBS was not abolished by a muscarinic acetylcholine receptor antagonist, atropine. These results suggest that these Gq-coupled receptors, other than group I mGluR, do not play significant modulatory roles in LTP.
What could be the reasons for the lack of the facilitatory effect of muscarinic receptors? A recent study indicates that spatial proximity of G-protein-coupled receptors and their transducing molecules in “signaling microdomains” is critical for the specificity and sensitivity of cellular responses (Delmas et al., 2002). In many neurons, it is known that B2 bradykinin receptors (B2Rs) but not M1 muscarinic receptors mobilize Ca2+ from internal stores, although both B2Rs and M1 receptors couple to the same transducing proteins (Gq-PLCβ) (Cruzblanca et al., 1998). Delmas et al. (2002) demonstrated that in sympathetic neurons, only IP3 formed by B2Rs has the ability to activate IP3 receptors, although both B2Rs and M1 receptors rapidly produce IP3 and diacylglycerol. This exclusive coupling results from spatially restricted complexes linking B2Rs to IP3 receptors. It is possible that group I mGluR may constitute signaling microdomains in CA1 pyramidal cells for the facilitation of LTP induction, whereas muscarinic receptors might be spatially apart and cannot participate in the microdomains.
We show that a PKC inhibitor, chelerythrine, abolished LTP induced by weak TBS in the wild-type mice. However, a slightly stronger TBS can induce LTP in Gαq-deficient pyramidal cells that was comparable with LTP in wild-type pyramidal cells but insensitive to chelerythrine. This result suggests that PKC activation can facilitate LTP induction but is not essential for it. Together, we conclude that in CA1 pyramidal cells, the group I mGluRs couple to Gαq/11 to the PKC signaling cascade and exert facilitatory effects on LTP.
Other effects of group I mGluR to Gαq/Gα11 signaling on synaptic plasticity
We show that the priming effect of bath-applied ACPD on subsequent induction of LTP is absent in Gαq mutant mice. A previous study shows the involvement of PLC in the priming effect in area CA1 (Cohen et al., 1998). These results indicate that the priming effect of ACPD is through the group I mGluR–Gαq/Gα11–PLC signaling cascade. The priming effect lasts ≥20 min after the washout of ACPD. This indicates that activation of group I mGluR does not itself cause potentiation of synaptic responses but can prepare a condition in which LTP can be induced with weaker stimulation than control condition. Such a phenomenon has been referred to as “metaplasticity” (Abraham and Bear, 1996). At the behavioral level, this mechanism could facilitate learning and memory in response to certain stimuli that have been applied repeatedly to the animal in a certain time window.
It was reported recently that Gαq mutant mice lack mGluR-dependent LTD in the hippocampal CA1 region (Kleppisch et al., 2001). We also found that LTD induced by low-frequency stimulation (1 Hz, 15 min) is deficient in Gαq mutant mice (M. Miura, S. Offermanns, M. I. Simon, and M. Kano, unpublished observations). Interestingly, Kleppisch et al. (2001) report that mGluR-dependent LTD is normal in Gα11 mutant mice. These results indicate that the activation of the group I mGluR–Gαq cascade can induce LTD instead of LTP when a different stimulation protocol is adopted. It remains to be elucidated how the switchover between LTP and LTD is regulated in CA1 pyramidal cells. The results suggest that the group I mGluR–Gαq signaling cascade is involved in both establishment of LTD and facilitation of LTP induction and thus plays an important role in the modulation of synaptic strength in the hippocampal area CA1.
Footnotes
This work was supported in part by grants-in-aid for Scientific Research (M.M., M.W., and M.K.) and Special Coordination Funds for Promoting Science and Technology (M.W. and M.K.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and by grants from the Novartis Foundation (M.K.) and the Cell Science Research Foundation (M.K.).
Correspondence should be addressed to Masanobu Kano, Department of Cellular Neurophysiology, Graduate School of Medical Science, Kanazawa University, 13-1 Takara-machi, Kanazawa 920-8640, Japan. E-mail:mkano@med.kanazawa-u.ac.jp.
M. Miura's present address: Department of Autonomic Nervous System, Tokyo Metropolitan Institute of Gerontology, Sakae-cho, Itabashi 173-0015, Japan.
REFERENCES
- 1.Abe T, Sugihara H, Nawa H, Shigemoto R, Mizuno N, Nakanishi S. Molecular characterization of a novel metabotropic glutamate receptor mGluR5 coupled to inositol phosphate/Ca2+ signal transduction. J Biol Chem. 1992;267:13361–13368. [PubMed] [Google Scholar]
- 2.Abeliovich A, Chen C, Goda Y, Silvia AJ, Stevens CF, Tonegawa S. Modified hippocampal long-term potentiation in PKCγ mutant mice. Cell. 1993;75:1253–1262. doi: 10.1016/0092-8674(93)90613-u. [DOI] [PubMed] [Google Scholar]
- 3.Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- 4.Auerbach JM, Segal M. A novel cholinergic induction of long-term potentiation in rat hippocampus. J Neurophysiol. 1994;72:2034–2040. doi: 10.1152/jn.1994.72.4.2034. [DOI] [PubMed] [Google Scholar]
- 5.Balschun D, Manahan-Vaughan D, Wagner T, Behnisch T, Reymann KG, Wetzel W. A specific role for group I mGluRs in hippocampal LTP and hippocampus-dependent spatial learning. Learn Mem. 1999;6:138–152. [PMC free article] [PubMed] [Google Scholar]
- 6.Bashir ZI, Bortolotto ZA, Davies CH, Berretta N, Irving AJ, Seal AJ, Henley JM, Jane DE, Watkins JC, Collingridge GL. Induction of LTP in the hippocampus needs synaptic activation of glutamate metabotropic receptors. Nature. 1993;363:347–350. doi: 10.1038/363347a0. [DOI] [PubMed] [Google Scholar]
- 7.Bear MF, Abraham WC. Long-term depression in hippocampus. Annu Rev Neurosci. 1996;19:437–462. doi: 10.1146/annurev.ne.19.030196.002253. [DOI] [PubMed] [Google Scholar]
- 8.Bear MF, Malenka RC. Synaptic plasticity: LTP and LTD. Curr Opin Neurobiol. 1994;4:389–399. doi: 10.1016/0959-4388(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 9.Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 10.Bortolotto ZA, Collingridge GL. Activation of glutamate metabotropic receptors induces long-term potentiation. Eur J Pharmacol. 1992;214:297–298. doi: 10.1016/0014-2999(92)90135-q. [DOI] [PubMed] [Google Scholar]
- 11.Bortolotto ZA, Bashir ZI, Davies CH, Collingridge GL. A molecular switch activated by metabotropic glutamate receptors regulates induction of long-term potentiation. Nature. 1994;368:740–743. doi: 10.1038/368740a0. [DOI] [PubMed] [Google Scholar]
- 12.Chinestra P, Aniksztejn L, Diabira D, Ben-Ari Y. (RS)-alpha-methyl-4-carboxyphenylglycine neither prevents induction of LTP nor antagonizes metabotropic glutamate receptors in CA1 hippocampal neurons. J Neurophysiol. 1993;70:2684–2689. doi: 10.1152/jn.1993.70.6.2684. [DOI] [PubMed] [Google Scholar]
- 13.Cohen AS, Abraham WC. Facilitation of long-term potentiation by prior activation of metabotropic glutamate receptors. J Neurophysiol. 1996;76:953–962. doi: 10.1152/jn.1996.76.2.953. [DOI] [PubMed] [Google Scholar]
- 14.Cohen AS, Raymond CR, Abraham WC. Priming of long-term potentiation induced by activation of metabotropic glutamate receptors coupled to phospholipase C. Hippocampus. 1998;8:160–170. doi: 10.1002/(SICI)1098-1063(1998)8:2<160::AID-HIPO8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 15.Conn PJ, Pin J-P. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- 16.Cruzblanca H, Koh DS, Hille B. Bradykinin inhibits M current via phospholipase C and Ca2+ stores in rat sympathetic neurons. Proc Natl Acad Sci USA. 1998;95:7151–7156. doi: 10.1073/pnas.95.12.7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delmas P, Wanaverbecq N, Abogadie FC, Mistry M, Brown DA. Signaling microdomains define the specificity of receptor-mediated InsP3 pathways in neurons. Neuron. 2002;24:209–220. doi: 10.1016/s0896-6273(02)00641-4. [DOI] [PubMed] [Google Scholar]
- 18.Docherty JR. Subtypes of functional alpha1- and alpha2-adrenoceptors. Eur J Pharmacol. 1998;361:1–15. doi: 10.1016/s0014-2999(98)00682-7. [DOI] [PubMed] [Google Scholar]
- 19.Exton JH. Regulation of phosphoinositide phospholipases by hormones, neurotransmitters, and other agonists linked to G-proteins. Annu Rev Pharmacol Toxicol. 1996;36:481–509. doi: 10.1146/annurev.pa.36.040196.002405. [DOI] [PubMed] [Google Scholar]
- 20.Frey U, Schroeder H, Matthies H. Dopaminergic antagonists prevent long-term maintenance of posttetanic LTP in the CA1 region of rat hippocampal slices. Brain Res. 1990;522:69–75. doi: 10.1016/0006-8993(90)91578-5. [DOI] [PubMed] [Google Scholar]
- 21.Friberg IK, Young AB, Standaert DG. Differential localization of the mRNAs for the pertussis toxin insensitive G-protein alpha sub-units Gq, G11, and Gz in the rat brain, and regulation of their expression after striatal deafferentation. Brain Res Mol Brain Res. 1998;54:298–310. doi: 10.1016/s0169-328x(97)00346-x. [DOI] [PubMed] [Google Scholar]
- 22.Goh JW, Pennefather PS. A pertussis toxin-sensitive G protein in hippocampal long-term potentiation. Science. 1989;244:980–983. doi: 10.1126/science.2543072. [DOI] [PubMed] [Google Scholar]
- 23.Hashimoto K, Watanabe M, Kurihara H, Offermanns S, Jiang H, Wu Y, Jun K, Shin HS, Inoue Y, Wu D, Simon MI, Kano M. Climbing fiber synapse elimination during postnatal cerebellar development requires signal transduction involving Gαq and phospholipase C β4. Prog Brain Res. 2000;124:31–48. doi: 10.1016/S0079-6123(00)24006-5. [DOI] [PubMed] [Google Scholar]
- 24.Hoyer D, Martin GR. 5-HT receptor classification and nomenclature: towards a harmonization with the human genome. Neuropharmacology. 1997;36:419–428. doi: 10.1016/s0028-3908(97)00036-1. [DOI] [PubMed] [Google Scholar]
- 25.Jouvenceau A, Billard JM, Lamour Y, Dutar P. Persistence of CA1 hippocampal LTP after selective cholinergic denervation. NeuroReport. 1996;7:948–952. doi: 10.1097/00001756-199603220-00024. [DOI] [PubMed] [Google Scholar]
- 26.Kaneko S, Maeda T, Satoh M. Cognitive enhancers and hippocampal long-term potentiation in vitro. Behav Brain Res. 1997;83:45–49. doi: 10.1016/s0166-4328(97)86044-5. [DOI] [PubMed] [Google Scholar]
- 27.Katsuki H, Kaneko S, Satoh M. Involvement of postsynaptic G proteins in hippocampal long-term potentiation. Brain Res. 1992;581:108–114. doi: 10.1016/0006-8993(92)90349-e. [DOI] [PubMed] [Google Scholar]
- 28.Katsuki H, Izumi Y, Zorumski CF. Noradrenergic regulation of synaptic plasticity in the hippocampal CA1 region. J Neurophysiol. 1997;77:3013–3020. doi: 10.1152/jn.1997.77.6.3013. [DOI] [PubMed] [Google Scholar]
- 29.Kiyama Y, Manabe T, Sakimura K, Kawakami F, Mori H, Mishina M. Increased thresholds for long-term potentiation and contextual learning in mice lacking the NMDA-type glutamate receptor ε1 subunit. J Neurosci. 1998;18:6704–6712. doi: 10.1523/JNEUROSCI.18-17-06704.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klann E, Chen SJ, Sweatt JD. Mechanism of protein kinase C activation during the induction and maintenance of long-term potentiation probed using a selective peptide substrate. Proc Natl Acad Sci USA. 1993;90:8337–8341. doi: 10.1073/pnas.90.18.8337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kleppisch T, Voigt V, Allmann R, Offermanns S. Gαq-deficient mice lack metabotropic glutamate receptor-dependent long-term depression but show normal long-term potentiation in the hippocampal CA1 region. J Neurosci. 2001;21:4943–4948. doi: 10.1523/JNEUROSCI.21-14-04943.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu M, Simon MI. Regulation by cAMP-dependent protein kinase of a G-protein-mediated phospholipase C. Nature. 1996;382:83–87. doi: 10.1038/382083a0. [DOI] [PubMed] [Google Scholar]
- 33.Lu YM, Jia Z, Janus C, Henderson JT, Gerlai R, Wojtowicz JM, Roder JC. Mice lacking metabotropic glutamate receptor 5 show impaired learning and reduced CA1 long-term potentiation (LTP) but normal CA3 LTP. J Neurosci. 1997;17:5196–5205. doi: 10.1523/JNEUROSCI.17-13-05196.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luján R, Nusser Z, Roberts JDB, Shigemoto R, Somogyi P. Perisynaptic location of metabotropic glutamate receptors mGluR1 and mGluR5 on dendrites and dendritic spines in the rat hippocampus. Eur J Neurosci. 1996;8:1488–1500. doi: 10.1111/j.1460-9568.1996.tb01611.x. [DOI] [PubMed] [Google Scholar]
- 35.Luján R, Roberts JDB, Shigemoto R, Ohishi H, Somogyi P. Differential plasma membrane distribution of metabotropic glutamate receptor mGluR1α, mGluR2 and mGluR5, relative to neurotransmitter release sites. J Chem Neuroanat. 1997;13:219–241. doi: 10.1016/s0891-0618(97)00051-3. [DOI] [PubMed] [Google Scholar]
- 36.Mailleux P, Mitchell F, Vanderhaeghen JJ, Milligan G, Erneux C. Immunohistochemical distribution of neurons containing the G-proteins Gq alpha/G11 alpha in the adult rat brain. Neuroscience. 1992;51:311–316. doi: 10.1016/0306-4522(92)90317-u. [DOI] [PubMed] [Google Scholar]
- 37.Manabe T, Wyllie DJ, Perkel DJ, Nicoll RA. Modulation of synaptic transmission and long-term potentiation: effects on paired pulse facilitation and EPSC variance in the CA1 region of the hippocampus. J Neurophysiol. 1993;70:1451–1459. doi: 10.1152/jn.1993.70.4.1451. [DOI] [PubMed] [Google Scholar]
- 38.Manzoni OJ, Weisskopf MG, Nicoll RA. MCPG antagonizes metabotropic glutamate receptors but not long-term potentiation in the hippocampus. Eur J Neurosci. 1994;6:1050–1054. doi: 10.1111/j.1460-9568.1994.tb00599.x. [DOI] [PubMed] [Google Scholar]
- 39.Masu M, Tanabe Y, Tsuchida K, Shigemoto R, Nakanishi S. Sequence and expression of a metabotropic glutamate receptor. Nature. 1991;349:760–765. doi: 10.1038/349760a0. [DOI] [PubMed] [Google Scholar]
- 40.Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- 41.Milligan G. Regional distribution and quantitative measurement of the phosphoinositidase C-linked guanine nucleotide binding proteins G11 alpha and Gq alpha in rat brain. J Neurochem. 1993;61:845–851. doi: 10.1111/j.1471-4159.1993.tb03595.x. [DOI] [PubMed] [Google Scholar]
- 42.Nakanishi S. Metabotropic glutamate receptors: synaptic transmission, modulation, and plasticity. Neuron. 1994;13:1031–1037. doi: 10.1016/0896-6273(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 43.Nicoll RA, Malenka RC. Contrasting properties of two forms of long-term potentiation in the hippocampus. Nature. 1995;377:115–118. doi: 10.1038/377115a0. [DOI] [PubMed] [Google Scholar]
- 44.Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- 45.Nusser Z, Mulvihill E, Streit P, Somogyi P. Subsynaptic segregation of metabotropic and ionotropic glutamate receptors as revealed by immunogold localization. Neuroscience. 1994;61:421–427. doi: 10.1016/0306-4522(94)90421-9. [DOI] [PubMed] [Google Scholar]
- 46.Offermanns S, Simon MI. Genetic analysis of mammalian G-protein signalling. Oncogene. 1998;17:1375–1381. doi: 10.1038/sj.onc.1202173. [DOI] [PubMed] [Google Scholar]
- 47.Offermanns S, Hashimoto K, Watanabe M, Sun W, Kurihara H, Thompson RF, Inoue Y, Kano M, Simon MI. Impaired motor coordination and persistent multiple climbing fiber innervation of cerebellar Purkinje cells in mice lacking Gαq. Proc Natl Acad Sci USA. 1997;94:14089–14094. doi: 10.1073/pnas.94.25.14089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Offermanns S, Zhao LP, Gohla A, Sarosi I, Simon MI, Wilkie TM. Embryonic cardiomyocyte hypoplasia and craniofacial defects in G alpha q/G alpha 11-mutant mice. EMBO J. 1998;17:4304–4312. doi: 10.1093/emboj/17.15.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramakers GM, McNamara RK, Lenox RH, De Graan PN. Differential changes in the phosphorylation of the protein kinase C substrates myristoylated alanine-rich C kinase substrate and growth-associated protein-43/B-50 following Schaffer collateral long-term potentiation and long-term depression. J Neurochem. 1999;73:2175–2183. [PubMed] [Google Scholar]
- 50.Richter-Levin G, Errington ML, Maegawa H, Bliss TVP. Activation of metabotropic glutamate receptors is necessary for long-term potentiation in the dentate gyrus and for spatial learning. Neuropharmacology. 1994;33:853–857. doi: 10.1016/0028-3908(94)90181-3. [DOI] [PubMed] [Google Scholar]
- 51.Riedel G, Reymann KG. An antagonist of the metabotropic glutamate receptor prevents LTP in the dentate gyrus of freely moving rats. Neuropharmacology. 1993;32:929–931. doi: 10.1016/0028-3908(93)90149-w. [DOI] [PubMed] [Google Scholar]
- 52.Riedel G, Wetzel W, Reymann KG. Comparing the role of metabotropic glutamate receptors in long-term potentiation and in learning and memory. Prog Neuropsychopharmacol Biol Psychiatry. 1996;20:761–789. doi: 10.1016/0278-5846(96)00058-9. [DOI] [PubMed] [Google Scholar]
- 53.Sakimura K, Kutsuwada T, Ito I, Manabe T, Takayama C, Kushiya E, Yagi T, Aizawa S, Inoue Y, Sugiyama H, Mishina M. Reduced hippocampal LTP and spatial learning in mice lacking NMDA receptor epsilon 1 subunit. Nature. 1995;373:151–155. doi: 10.1038/373151a0. [DOI] [PubMed] [Google Scholar]
- 54.Salin PA, Scanziani M, Malenka RC, Nicoll RA. Distinct short-term plasticity at two excitatory synapses in the hippocampus. Proc Natl Acad Sci USA. 1996;93:13304–13309. doi: 10.1073/pnas.93.23.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Selig DK, Lee HK, Bear MF, Malenka RC. Reexamination of the effects of MCPG on hippocampal LTP, LTD, and depotentiation. J Neurophysiol. 1995;74:1075–1082. doi: 10.1152/jn.1995.74.3.1075. [DOI] [PubMed] [Google Scholar]
- 56.Shigemoto R, Kinoshita A, Wada E, Nomura S, Ohishi H, Takada M, Flor PJ, Neki A, Abe T, Nakanishi S, Mizuno N. Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J Neurosci. 1997;17:7503–7522. doi: 10.1523/JNEUROSCI.17-19-07503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simon MI, Strathmann MP, Gautam N. Diversity of G proteins in signal transduction. Science. 1991;252:802–808. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- 58.Stanton PK, Sarvey JM. Depletion of norepinephrine, but not serotonin, reduces long-term potentiation in the dentate gyrus of rat hippocampal slices. J Neurosci. 1985;5:2169–2176. doi: 10.1523/JNEUROSCI.05-08-02169.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Staubli U, Otaky N. Serotonin controls the magnitude of LTP induced by theta bursts via an action on NMDA-receptor-mediated responses. Brain Res. 1994;643:10–16. doi: 10.1016/0006-8993(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 60.Strathmann MP, Simon MI. G protein diversity: a distinct class of α subunits is present in vertebrates and invertebrates. Proc Natl Acad Sci USA. 1990;87:9113–9117. doi: 10.1073/pnas.87.23.9113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tanaka J, Nakagawa S, Kushiya E, Yamasaki M, Fukaya M, Iwanaga T, Simon MI, Sakimura K, Kano M, Watanabe M. Gq protein α subunits Gαq and Gα11 are localized at postsynaptic extra-junctional membrane of cerebellar Purkinje cells and hippocampal pyramidal cells. Eur J Neurosci. 2000;12:781–792. doi: 10.1046/j.1460-9568.2000.00959.x. [DOI] [PubMed] [Google Scholar]
- 62.Tecott LH, Logue SF, Wehner JM, Kauer JA. Perturbed dentate gyrus function in serotonin 5-HT2C receptor mutant mice. Proc Natl Acad Sci USA. 1998;95:15026–15031. doi: 10.1073/pnas.95.25.15026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thomas CP, Dunn MJ, Mattera R. Ca2+ signalling in K562 human erythroleukaemia cells: effect of dimethyl sulphoxide and role of G-proteins in thrombin- and thromboxane A2-activated pathways. Biochem J. 1995;312:151–158. doi: 10.1042/bj3120151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsubokawa H, Ross WN. Muscarinic modulation of spike backpropagation in the apical dendrites of hippocampal CA1 pyramidal neurons. J Neurosci. 1997;17:5782–5791. doi: 10.1523/JNEUROSCI.17-15-05782.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tsubokawa H, Offermanns S, Simon MI, Kano M. Calcium-dependent persistent facilitation of spike backpropagation in the CA1 pyramidal neurons. J Neurosci. 2000;20:4878–4884. doi: 10.1523/JNEUROSCI.20-13-04878.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang JH, Feng DP. Postsynaptic protein kinase C essential to induction and maintenance of long-term potentiation in the hippocampal CA1 region. Proc Natl Acad Sci USA. 1992;89:2576–2580. doi: 10.1073/pnas.89.7.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang RY, Arvanov VL. M100907, a highly selective 5-HT2A receptor antagonist and a potential atypical antipsychotic drug, facilitates induction of long-term potentiation in area CA1 of the rat hippocampal slice. Brain Res. 1998;779:309–313. doi: 10.1016/s0006-8993(97)01174-8. [DOI] [PubMed] [Google Scholar]
- 68.Watanabe M, Fukaya M, Sakimura K, Manabe T, Mishina M, Inoue Y. Selective scarcity of NMDA receptor channel subunits in the stratum lucidum (mossy fibre-recipient layer) of the mouse hippocampal CA3 subfield. Eur J Neurosci. 1998;10:478–487. doi: 10.1046/j.1460-9568.1998.00063.x. [DOI] [PubMed] [Google Scholar]
- 69.Wess J, Blin N, Mutschler E, Bluml K. Muscarinic acetylcholine receptors: structural basis of ligand binding and G protein coupling. Life Sci. 1995;56:915–922. doi: 10.1016/0024-3205(95)00028-5. [DOI] [PubMed] [Google Scholar]
- 70.Wilsch VW, Behnisch T, Jager T, Reymann KG, Balschun D. When are class I metabotropic glutamate receptors necessary for long-term potentiation? J Neurosci. 1998;18:6071–6080. doi: 10.1523/JNEUROSCI.18-16-06071.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yamada K, Fukaya M, Shimizu H, Sakimura K, Watanabe M. NMDA receptor subunits GluRε1, GluRε3, and GluRζ1 are enriched at the mossy fiber-granule cell synapse in the adult mouse cerebellum. Eur J Neurosci. 2001;13:2025–2036. doi: 10.1046/j.0953-816x.2001.01580.x. [DOI] [PubMed] [Google Scholar]