Abstract
Light is a major environmental signal for entrainment of the circadian clock, but little is known about the intracellular phototransduction pathway triggered by light activation of the photoreceptive molecule(s) responsible for the phase shift of the clock in vertebrates. The chicken pineal gland and retina contain the autonomous circadian oscillators together with the photic entrainment pathway, and hence they represent useful experimental models for the clock system. Here we show the expression of G11α, an α subunit of heterotrimeric G-protein, in both tissues by cDNA cloning, Northern blot, and Western blot analyses. G11α immunoreactivity was colocalized with pinopsin in the chicken pineal cells and also with rhodopsin in the outer segments of retinal photoreceptor cells, suggesting functional coupling of G11α with opsins in the clock-containing photosensitive tissues. The physical interaction was examined by coimmunoprecipitation experiments, the results of which provided evidence for light- and GTP-dependent coupling between rhodopsin and G11α. To examine whether activation of endogenous G11 leads to a phase shift of the oscillator, Gq/11-coupled m1-type muscarinic acetylcholine receptor (mAChR) was ectopically expressed in the cultured pineal cells. Subsequent treatment of the cells with carbamylcholine (CCh), an agonist of mAChR, induced phase-dependent phase shifts of the melatonin rhythm in a manner very similar to the effect of light. In contrast, CCh treatment induced no measurable effect on the rhythm of nontransfected (control) cells or cells expressing Gi/o-coupled m2-type mAChR, indicating selectivity of the G-protein activation. Together, our results demonstrate the existence of a G11-mediated opsin-signaling pathway contributing to the photic entrainment of the circadian clock.
Keywords: G11, phototransduction, circadian rhythm, pinopsin, pineal gland, retina
Daily rhythms in biochemistry, physiology, and behavior of living organisms are driven by endogenous oscillators called circadian clocks (Pittendrigh, 1993). The circadian clock oscillates with a period of ∼24 hr under constant conditions, and it is entrained to the 24 hr cycle of a change in the ambient conditions (for example, the solar light/dark cycle) (Dunlap, 1999). The light-dependent phase control represents one of the most important properties of the biological clock, but little is known about the phototransduction mechanism responsible for photic entrainment in vertebrates (Zordan et al., 2001). Among vertebrate clock-containing cells, the chicken pineal cell, which is sensitive to light, is a prominent model for studying the photic entrainment mechanism, because the photo-entrainable clock machineries reside in individual cells and are well maintained in dispersed cell culture (Takahashi et al., 1989;Nakahara et al., 1997). The endogenous photoreceptive molecule pinopsin (Okano et al., 1994; Max et al., 1995) has been postulated to mediate the phase-shifting effect of light via a G-protein signaling pathway. We previously demonstrated light-dependent activation of rod-type transducin α subunit (Gt1α) expressed in the chicken pineal gland (Kasahara et al., 2000). It is unlikely, however, that pineal Gt1α plays a major role in photic entrainment, because the light-induced phase shift is unaffected by treatment of the pineal cells with pertussis toxin (PTX) (Zatz and Mullen, 1988), which blocks signaling of Gt1α by ADP ribosylation. Here we describe a novel phototransduction pathway mediated by a PTX-insensitive G-protein, G11, and discuss the biological significance of this signal transduction pathway.
MATERIALS AND METHODS
Animals. Animals were treated in accordance with the guidelines of The University of Tokyo. Newly hatched chicks were purchased from local suppliers and housed in a 12 hr light/dark cycle with a light intensity (at head level) of ∼300 lux.
Isolation of pineal G11α cDNA clone. A fragment of G11α was isolated from a chicken pineal cDNA library by PCR using degenerate primers (F1, 5′-GTITAYCARAAYATITTYACIGCIATGC-3′; R1, 5′-RTTYTGRAACCAIGGRTAIGTIATIATIG-3′). 5′ Rapid amplification of cDNA ends (RACE) was performed according to the circular or concatemeric RACE methodology (Maruyama et al., 1995) with chicken G11α-specific primers (F2, 5′-TGATTCAGCTAAATACTATCTCAGC-3′; R2, 5′-GAAAGTTGGTATTCTCTTCTTCTG-3′). 3′ RACE was performed using the chicken pineal cDNA library as a template with primers designed for chicken G11α (F2 primer) and for an arm of the λZAPII vector (M13Fw, 5′-CCCAGTCACGACGTTGTAAAACG-3′; M13Rv, 5′-GAGCGGATAACAATTTCACACAGG-3′). The full-length coding region of chicken pineal G11α cDNA was obtained by reverse transcription (RT)-PCR with the gene-specific primers cG11N (5′-AGATCTGACCATGACTCTGGAGTCCATG-3′) and cG11C (5′-GAATTCACCAACGGTGCCTCGC-3′).
Immunohistochemistry. The chicken pineal and retinal sections (10 μm thickness) were prepared as described previously (Kasahara et al., 2000). Bouin's-fixed pineal sections were incubated with a mixture of rabbit anti-Gq/11α (0.2 μg/ml; Santa Cruz Biotechnology, Santa Cruz, CA) and mouse anti-pinopsin (P9 antibody; 1 μg/ml) (Okano et al., 1997) antibodies. After washing with PBS, the sections were incubated with a mixture of FITC–anti-rabbit IgG antibody (10 μg/ml; Vector Laboratories, Burlingame, CA) and Texas Red–anti-mouse IgG antibody (10 μg/ml; Vector Laboratories), rinsed with PBS, and coverslipped with Vectashield mounting medium (Vector Laboratories). Paraformaldehyde-fixed retinal sections were incubated with a mixture of rabbit anti-Gq/11α (0.2 μg/ml) and mouse anti-rhodopsin (0.3 μg/ml) (T. Okano and Y. Fukada, unpublished observation) antibodies. For visualization of the retinal specimens, Alexa Fluor 488–anti-rabbit IgG antibody (1 μg/ml; Molecular Probes, Eugene, OR) and Alexa Fluor 568–anti-mouse IgG antibody (1 μg/ml; Molecular Probes) were used as secondary antibodies, and the sections were coverslipped with Vectashield containing 4′,6′-diamidino-2-phenylindole (DAPI) (1.5 μg/ml; Vector Laboratories).
Immunoprecipitation assay. Four chick retinas were isolated from dark-adapted (for at least 8 hr) animals (1–3 d of age) and homogenized in 900 μl of immunoprecipitation (IP) buffer (20 mm Tris-HCl, 120 mmNaCl, 6 mm MgCl2, 1 mm EDTA, 4 μg/ml aprotinin, and 4 μg/ml leupeptin, pH 7.4) containing 1% (w/v) 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate (CHAPS) under dim red light (λ > 670 nm) conditions. After centrifugation at 45,000 ×g for 30 min at 4°C, a reaction mixture (∼1 mg/ml protein) in IP buffer containing 100 μm (final) GDP, 1 mm GTP, and 0.1% (w/v) CHAPS was prepared from the supernatant. Aliquots (400 μl) of the reaction mixture were either irradiated for 5 min at 4°C with an orange light (λ > 560 nm) or kept in the dark, and then incubated with 5 μg of anti-Gt1α or anti-Gq/11α antibody (both from Santa Cruz Biotechnology) at 4°C for 1 hr. Rhodopsin–G-protein–antibody complex was precipitated with the aid of anti-rabbit IgG-Sepharose (Amersham Biosciences, Piscataway, NJ), and coimmunoprecipitated rhodopsin was detected by Western blotting using anti-chicken rhodopsin antibody. The blot was then stripped with stripping buffer [62.5 mm Tris-HCl, 2% (w/v) SDS, and 110 mm 2-mercaptoethanol, pH 6.8] at 50°C for 30 min and reprobed with a mixture of anti-Gt1α and anti-G11α antibodies (Santa Cruz Biotechnology).
Pineal cell culture and drug administration for transfection assay. Pineal glands were isolated from 1-d-old chicks. The pineal cells were dispersed by passing the tissue through a cell strainer (100 μm nylon mesh; Becton Dickinson, Franklin Lakes, NJ) using Medium 199 (Invitrogen, San Diego, CA) supplemented with 10 mm HEPES-NaOH, pH 7.4, 2.2 mg/ml NaHCO3, 100 U/ml penicillin, 100 μg/ml streptomycin, 250 μg/ml Fungizone, 2.5 μmarabinosylcytosine, and 10% fetal bovine serum. Arabinosylcytosine was used to suppress proliferation of non-neuronal cells such as fibroblasts, resulting in enrichment of the pinealocytes in the primary culture. The dispersed cells were cultured in 24-well cloning plates (∼6 × 106 cells/well; Greiner Labortechnik, Frickenhausen, Germany) at 39.5 ± 0.2°C under 95% air/5% CO2 in the 12 hr light/dark cycle. Cultured cells were transfected with pREP9 expression vector (Invitrogen) harboring porcine cDNA for m1 or m2 muscarinic acetylcholine receptors (mAChRs) (GenBank accession numbersX04413 or X04708) or with the vector not including any inserts. To increase transfection efficiency, transfection was performed twice (days 3 and 4), each for 12 hr, using a 1:1 (v/v) mixture of GeneFECTOR (Venn Nova, Pompano Beach, FL) and LipofectAMINE (Invitrogen) together with PLUS reagent (Invitrogen). The transfected cells were selected in medium containing 200 μg/ml G418 for 96 hr (days 5–8) and then cultured in medium containing 50 μg/ml G418 and 20 μm atropine until the end of the experiment. Atropine, an antagonist of all subtypes of mAChRs, was added to prevent internalization (sequestration) and agonist-independent spontaneous activation of the overexpressing mAChRs. On day 10, the cells were transferred to constant darkness and treated with atropine-free medium containing carbamylcholine (CCh; 100 μm) for 4 hr at the indicated time point. The medium was collected and replaced at 4 hr intervals, and the amount of secreted melatonin was measured using HPLC (Sanada et al., 2000).
RESULTS
PTX-insensitive G11α colocalizes with opsins in the pineal gland and retina
To explore the possibility of a pinopsin-mediated pathway for photic entrainment, we first investigated whether specific G-protein(s) colocalize with pinopsin. We found that pinopsin-positive membrane structures of the pineal cells were immunolabeled with both anti-Gt1α antibody (Kasahara et al., 2000) and anti-Gq/11α antibody recognizing mammalian Gqα and G11α (Fig.1A–C) (see alsoMatsushita et al., 2000). In Western blot analysis of the chicken pineal and retinal homogenates, the anti-Gq/11α antibody detected a single band (42 kDa) with mobility identical to that of a band recognized by G11α-specific antibody (Fig. 1D). Together with the calculated molecular mass (41,959; see below), these observations suggest that G11α is expressed in the two tissues. In fact, cDNA for G11α but not for Gqα was detected by RT-PCR analysis performed with degenerate primers for amino acid sequences highly conserved among mammalian Gq-type α subunits. The entire coding sequence of pineal G11α was determined by 5′ and 3′ RACE. According to the deduced amino acid sequence, the Cys residue susceptible to ADP ribosylation by PTX is absent (Fig.1E), indicating that the protein is PTX insensitive. The chick retina contains a G11α transcript identical in size to the pineal transcript (Fig. 1F), and Gq/11α immunoreactivity was observed at the outer segment layer of the photoreceptor cells (Fig. 1G), being superimposable on rhodopsin immunoreactivity (Fig.1H,I). Together, these observations suggest a role for G11α in light-signaling processes, possibly as a mediator for the phase-shifting effect of light in the pineal gland and retina.
Fig. 1.
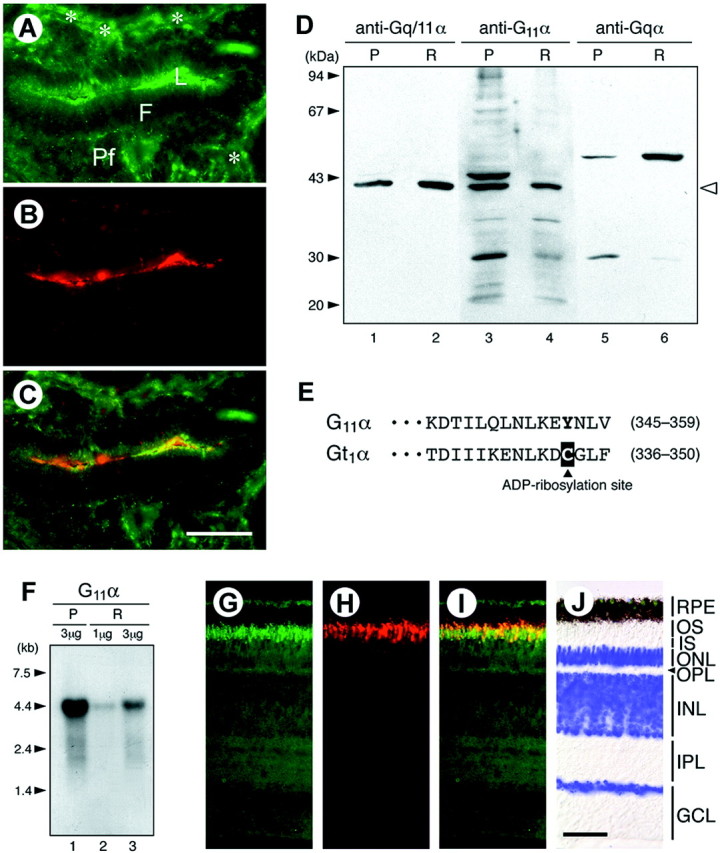
Chicken G11α in the pineal gland and retina. A–C, Localization of G11α and pinopsin immunoreactivities in the pineal gland. A chicken pineal section was double-labeled by antibodies specific for Gq/11α (A) and pinopsin (B). Yellow in the merged image (C) indicates colocalization. Anti-Gq/11α antibody stained the follicular lumen (L), the marginal region of the lumen, and the peripheral area of the follicle (asterisks).F, Follicular zone; Pf, parafollicular zone. Scale bar, 20 μm. D, Western blot analysis. The chicken pineal (P) membrane fraction (200 μg protein/lane; lanes 1, 3, and 5) and retinal (R) membrane fraction (80 μg/lane;lanes 2, 4, and 6) were subjected to SDS-PAGE. The gel was then electroblotted onto a polyvinylidene difluoride membrane, which was immunostained using the antibodies indicated (purchased from Santa Cruz Biotechnology). An open arrowhead (right) shows the position of G11α, and the observed Mr(∼42 kDa) agrees well with the molecular mass (41,959) calculated from the deduced amino acid sequence. Anti-Gqα antibody reacted with a band with an apparently higher molecular mass (∼50 kDa, lanes 5 and 6), but its identity remains unknown because it was not recognized by anti-Gq/11α (lanes 1 and2). E, The deduced amino acid sequences of the C-terminal regions of chicken G11α and Gt1α. A cysteine residue at the fourth position from the C termini of Gt1α (boxed inblack) is the site for PTX-catalyzed ADP ribosylation. The GenBank accession numbers of chicken G11α and Gt1α cDNAs are AF364326 and AF200338, respectively.F, Northern blot analysis. The chicken pineal (P) or retinal (R) poly(A)+ RNA (1 or 3 μg/lane) was subjected to electrophoresis, and blots were then hybridized with32P-random-labeled chicken G11α cDNA.G–J, Localization of G11α and rhodopsin immunoreactivities in the retina. A chicken retinal section was double-labeled with anti-Gq/11α (G) and anti-rhodopsin (H) antibodies, and the two images were merged (I). DAPI staining is shown in pseudocolor (blue) and overlaid on a Nomarski image (J). RPE, Retinal pigment epithelium; OS, outer segment;IS, inner segment; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer;GCL, ganglion cell layer. Scale bar, 50 μm.
G11α functionally interacts with chicken rhodopsin
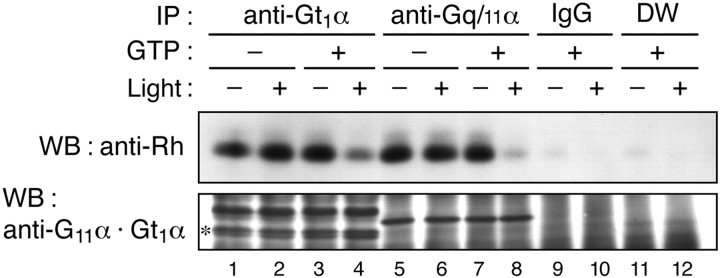
An activated (GTP-bound) form of Gq/11α stimulates phospholipase C-β (PLC-β) in various types of cells (Berridge, 1993). Consistently, retinal phosphatidylinositol (PI) turnover is stimulated by light in the photoreceptor outer segments of the rat, chicken, and frog (Ghalayini and Anderson, 1984; Hayashi and Amakawa, 1985; Brown et al., 1987; Millar et al., 1988), but no direct evidence has been presented for light-dependent functional coupling between vertebrate opsins and Gq/11-type G-protein (Peng et al., 1997). To explore this further, the physical interaction of G11α with rhodopsin was examined by an immunoprecipitation technique. Detergent (CHAPS)-solubilized extract was prepared from dark-adapted chick retinas, and after either no treatment or treatment by light irradiation (λ > 560 nm), the extract was subjected to immunoprecipitation with anti-Gα antibody in the presence or absence of GTP, followed by detection of coprecipitated rhodopsin. The well established interaction of rhodopsin with transducin (Gt1α) was used as a control (Fig. 2, lanes 1–4), in which rhodopsin was coimmunoprecipitated regardless of the light condition in the absence of GTP and the amount of coimmunoprecipitated rhodopsin was substantially reduced by light irradiation in the presence of GTP (Fig. 2, lane 4). A similar or more pronounced change was observed for G11α (Fig.2, lanes 5–8), demonstrating that G11α is associated with rhodopsin in the dark and dissociated from rhodopsin in response to light in a GTP-dependent manner.
Fig. 2.
Coimmunoprecipitation of rhodopsin with G-protein α subunits from chicken retinal membrane extract. Before immunoprecipitation, the chicken retinal extract in 0.1% CHAPS was incubated at 4°C in the presence or absence of GTP (1 mm, final) and with or without orange light irradiation for 5 min. The extract was then incubated with either anti-Gt1α antibody (lanes 1–4), anti-Gq/11α antibody (lanes 5–8), affinity-purified rabbit IgG (lanes 9 and 10), or no IgG [distilled water (DW; lanes 11 and 12)] for immunoprecipitation, and coprecipitated rhodopsin was detected by Western blotting (WB) with anti-chicken rhodopsin antibody (top panel). The same blot was reprobed with a mixture of anti-G11α and anti-Gt1α antibodies (bottom panel). Anasterisk marks the position of Gt1α. The data are representative of three independent experiments with similar results.
Transient activation of pineal G11-mediated pathway phase-shifts the circadian oscillator
We then addressed the question of whether G11 signaling inputs to the circadian oscillator in cultured chicken pineal cells. To activate G11without stimulating the other (redundant) photic pathway(s), we adopted transient expression of a Gq/11-coupled receptor, m1 mAChR, the activity of which can be controlled pharmacologically (Nakamura et al., 1995) in the dark. A primary culture of pineal cells was transfected with an expression plasmid containing cDNA for m1 mAChR or for m2 mAChR (as a control for the Gi/o-coupled receptor) (Nakamura et al., 1995), and transfected cells were selected by incubation in the presence of G418. Although G418 treatment modified the clock oscillation (i.e., the phase of the melatonin rhythm was delayed) as observed previously (Okano et al., 2001), the clock oscillated autonomously and was reset by light in a phase-dependent manner (Fig.3C,D). We also confirmed the daily fluctuation in the mRNA levels of the clock genes (cPer2, cBmal1, and cBmal2) (Okano et al., 2001) in the G418-treated pineal cells by RT-PCR assay (T. Kasahara, Okano, and Fukada, unpublished data). After entrainment of the transfected cells to the light/dark cycle, cells were transferred to constant darkness and treated for 4 hr with 100 μm CCh, an agonist of all subtypes of mAChRs. In the m1 mAChR-expressing cells, the transient CCh treatment induced a clear phase shift of the melatonin rhythm (Fig. 3). The direction of the phase shift, either delay or advance, was dependent on the phase of CCh treatment, and, importantly, the CCh-induced phase-dependent phase shift was very similar to that elicited by 4 hr of exposure to light (Fig. 3E,F). In sharp contrast, CCh treatment had little or no effect on the melatonin rhythm of m2 mAChR-expressing cells and of control vector-transfected cells (Fig. 3). We conclude that the phase-shifting effect of light on the pineal circadian oscillator was mimicked by selective activation of the Gq/11-coupled receptor, most likely via endogenous G11, but not by activation of Gi/o-coupled receptors.
Fig. 3.
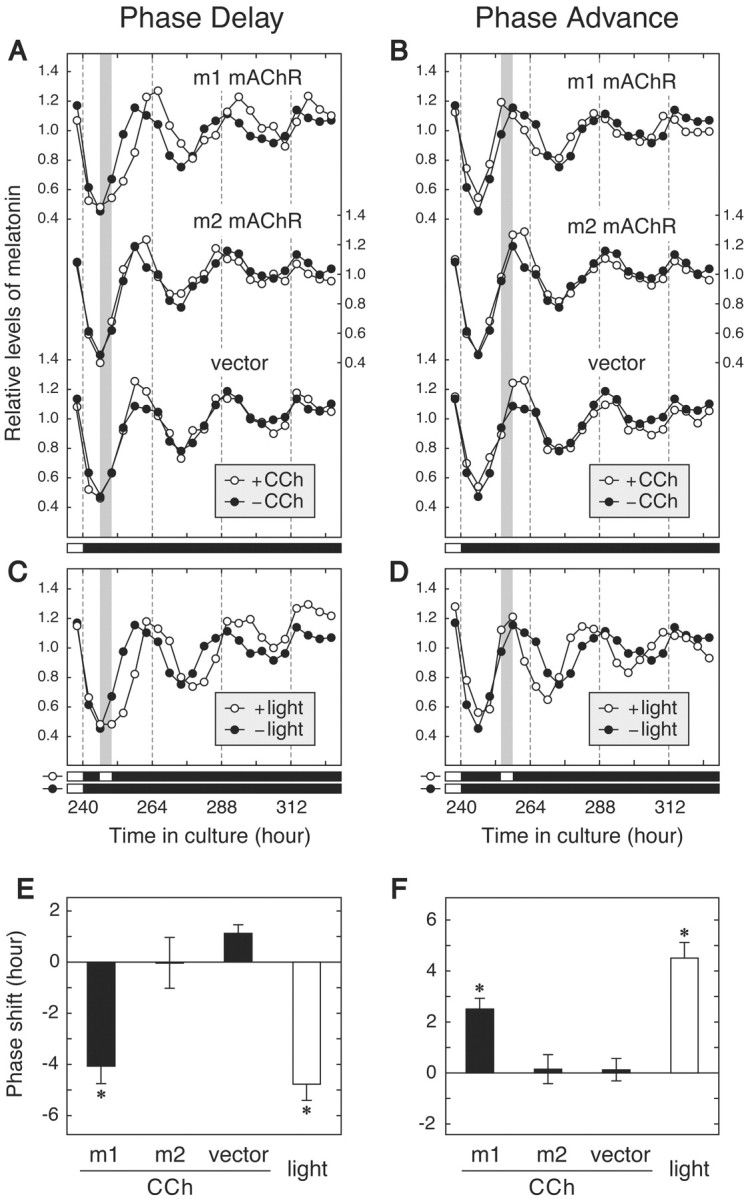
Melatonin rhythm of chicken pineal cells transfected with m1 or m2 mAChR. A primary culture of pineal cells was transfected with pREP9 vector harboring cDNA for m1 or m2 mAChR or with control vector, and the transfected cells were treated in the dark with (○) or without (●) CCh (100 μm) for 4 hr at either time (hour) 246–250 (A) or time 254–258 (B). The middle panels show the melatonin rhythm of the cells exposed to white fluorescent light (∼300 lux) for 4 hr at time (hour) 246–250 (C) or time 254–258 (D). A gray bar in each panel indicates the period of CCh treatment (A, B) or light expossure (C, D). These melatonin levels were the means of three independent cell cultures. E, F, The phase shift induced by treatment at time (hour) 246–250 (E) or time 254–258 (F) was calculated according to the method of Zatz et al. (1994). Phase delays and advances are plotted ± SEM as negative and positive values, respectively.Asterisks indicate a significant phase shift relative to that observed in the cells transfected with control vector (p < 0.025; one-tailed Student'st test). A similar result was obtained in the other experiment using two independent wells of the cell culture.
DISCUSSION
Since the establishment of the central role of cGMP as an intracellular messenger in the vertebrate visual transduction pathway (Yau, 1994), no definitive role with biological significance has been assigned to light-induced retinal PI turnover (Ghalayini and Anderson, 1984; Hayashi and Amakawa, 1985; Brown et al., 1987; Millar et al., 1988), although G11α and PLC-β4 were found in bovine retinal photoreceptor cells (Peng et al., 1997). Inhibition of calcium mobilization was observed to block the phase-shifting effect of light in chicken pineal cells, and conversely, induction of calcium mobilization was observed to phase-shift the circadian oscillator in a similar manner to photic stimulation (Zatz and Heath, 1995). Based on the results presented in this study, together with structural and functional similarities between the photosensitive retinal and pineal cells (Korf, 1994), we speculate that photic entrainment of the circadian clock in the two cells is triggered by an opsin-G11 pathway accompanying PI turnover and subsequent calcium mobilization. A similar G11-mediated pathway may also contribute to phototransduction in mammalian retinal ganglion cells, which have been shown to express melanopsin in recent studies (Berson et al., 2002;Hattar et al., 2002).
The opsin-G11 pathway is novel in vertebrate photoreceptive cells, but it is analogous to the early steps in fruitfly visual transduction [i.e., rhodopsin–DGQα (Gq/11α homolog)–NorpA (PLC-β4 homolog)] (Strathmann and Simon, 1990; Berridge, 1993; Ferreira et al., 1993; Lee et al., 1993; Zuker, 1996). In this species, the rhodopsin–DGQα–NorpA phototransduction pathway also plays a role in entrainment in a redundant manner together with pathways present in the extra-retinal tissues (the peripheral tissues with CRY photoreceptor and the Hofbauer-Buchner eyelet with unknown photoreceptor) (Stanewsky et al., 1998; Helfrich-Forster et al., 2001). Similar redundancy in the vertebrate photic entrainment system has been suggested by several loss-of-function studies (Selby et al., 2000; Foster and Helfrich-Forster, 2001). Hence, we used an alternative strategy in this study to show the contribution of the G11-mediated pathway. It will now be necessary to re-evaluate negative results in loss-of-function studies on the photic entrainment system such as those observed with inhibitors and mutant mice (Zatz, 1994; Ikeda et al., 2000; Selby et al., 2000; Lucas et al., 2001).
Footnotes
This work was supported in part by grants-in-aid from the Japanese Ministry of Education, Culture, Sports, Science, and Technology. T.K. was supported by a research fellowship of the Japan Society for the Promotion of Science for Young Scientists. We thank T. Hirota for assistance with this experiment and for critical reading of our manuscript and Dr. T. I. Webb for preparing this manuscript.
Correspondence should be addressed to Y. Fukada, Department of Biophysics and Biochemistry, Graduate School of Science, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan. E-mail:sfukada@mail.ecc.u-tokyo.ac.jp.
T. Haga's present address: Institute for Biomolecular Science, Faculty of Science, Gakushuin University, 1-5-1 Mejiro, Toshima-ku, Tokyo 171-8588, Japan.
T. Kasahara's present address: Laboratory for Molecular Dynamics of Mental Disorders, RIKEN Brain Science Institute, 2-1 Hirosawa, Wako-shi, Saitama 351-0198, Japan.
REFERENCES
- 1.Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 2.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 3.Brown JE, Blazynski C, Cohen AI. Light induces a rapid and transient increase in inositol-trisphosphate in toad rod outer segments. Biochem Biophys Res Commun. 1987;146:1392–1396. doi: 10.1016/0006-291x(87)90804-7. [DOI] [PubMed] [Google Scholar]
- 4.Dunlap JC. Molecular bases for circadian clocks. Cell. 1999;96:271–290. doi: 10.1016/s0092-8674(00)80566-8. [DOI] [PubMed] [Google Scholar]
- 5.Ferreira PA, Shortridge RD, Pak WL. Distinctive subtypes of bovine phospholipase C that have preferential expression in the retina and high homology to the norpA gene product of Drosophila. Proc Natl Acad Sci USA. 1993;90:6042–6046. doi: 10.1073/pnas.90.13.6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foster RG, Helfrich-Forster C. The regulation of circadian clocks by light in fruitflies and mice. Philos Trans R Soc Lond B Biol Sci. 2001;356:1779–1789. doi: 10.1098/rstb.2001.0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghalayini A, Anderson RE. Phosphatidylinositol 4,5-bisphosphate: light-mediated breakdown in the vertebrate retina. Biochem Biophys Res Commun. 1984;124:503–506. doi: 10.1016/0006-291x(84)91582-1. [DOI] [PubMed] [Google Scholar]
- 8.Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayashi F, Amakawa T. Light-mediated breakdown of phosphatidylinositol-4,5-bisphosphate in isolated rod outer segments of frog photoreceptor. Biochem Biophys Res Commun. 1985;128:954–959. doi: 10.1016/0006-291x(85)90139-1. [DOI] [PubMed] [Google Scholar]
- 10.Helfrich-Forster C, Winter C, Hofbauer A, Hall JC, Stanewsky R. The circadian clock of fruit flies is blind after elimination of all known photoreceptors. Neuron. 2001;30:249–261. doi: 10.1016/s0896-6273(01)00277-x. [DOI] [PubMed] [Google Scholar]
- 11.Ikeda M, Sugiyama T, Suzuki K, Moriya T, Shibata S, Katsuki M, Allen CN, Yoshioka T. PLC β4-independent Ca2+ rise via muscarinic receptors in the mouse suprachiasmatic nucleus. NeuroReport. 2000;11:907–912. doi: 10.1097/00001756-200004070-00002. [DOI] [PubMed] [Google Scholar]
- 12.Kasahara T, Okano T, Yoshikawa T, Yamazaki K, Fukada Y. Rod-type transducin α-subunit mediates a phototransduction pathway in the chicken pineal gland. J Neurochem. 2000;75:217–224. doi: 10.1046/j.1471-4159.2000.0750217.x. [DOI] [PubMed] [Google Scholar]
- 13.Korf HW. The pineal organ as a component of the biological clock: phylogenetic and ontogenetic considerations. Ann NY Acad Sci. 1994;719:13–42. doi: 10.1111/j.1749-6632.1994.tb56818.x. [DOI] [PubMed] [Google Scholar]
- 14.Lee CW, Park DJ, Lee KH, Kim CG, Rhee SG. Purification, molecular cloning, and sequencing of phospholipase C-β4. J Biol Chem. 1993;268:21318–21327. [PubMed] [Google Scholar]
- 15.Lucas RJ, Freedman MS, Lupi D, Munoz M, David-Gray ZK, Foster RG. Identifying the photoreceptive inputs to the mammalian circadian system using transgenic and retinally degenerate mice. Behav Brain Res. 2001;125:97–102. doi: 10.1016/s0166-4328(01)00274-1. [DOI] [PubMed] [Google Scholar]
- 16.Maruyama IN, Rakow TL, Maruyama HI. cRACE: a simple method for identification of the 5′ end of mRNAs. Nucleic Acids Res. 1995;23:3796–3797. doi: 10.1093/nar/23.18.3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsushita A, Yoshikawa T, Okano T, Kasahara T, Fukada Y. Colocalization of pinopsin with two types of G-protein α-subunits in the chicken pineal gland. Cell Tissue Res. 2000;299:245–251. doi: 10.1007/s004419900145. [DOI] [PubMed] [Google Scholar]
- 18.Max M, McKinnon PJ, Seidenman KJ, Barrett RK, Applebury ML, Takahashi JS, Margolskee RF. Pineal opsin: a nonvisual opsin expressed in chick pineal. Science. 1995;267:1502–1506. doi: 10.1126/science.7878470. [DOI] [PubMed] [Google Scholar]
- 19.Millar FA, Fisher SC, Muir CA, Edwards E, Hawthorne JN. Polyphosphoinositide hydrolysis in response to light stimulation of rat and chick retina and retinal rod outer segments. Biochim Biophys Acta. 1988;970:205–211. doi: 10.1016/0167-4889(88)90180-2. [DOI] [PubMed] [Google Scholar]
- 20.Nakahara K, Murakami N, Nasu T, Kuroda H, Murakami T. Individual pineal cells in chick possess photoreceptive, circadian clock, and melatonin-synthesizing capacities in vitro. Brain Res. 1997;774:242–245. doi: 10.1016/s0006-8993(97)81713-1. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura F, Kato M, Kameyama K, Nukada T, Haga T, Kato H, Takenawa T, Kikkawa U. Characterization of Gq family G proteins GL1α (G14α), GL2α (G11α), and Gqα expressed in the baculovirus-insect cell system. J Biol Chem. 1995;270:6246–6253. doi: 10.1074/jbc.270.11.6246. [DOI] [PubMed] [Google Scholar]
- 22.Okano T, Yoshizawa T, Fukada Y. Pinopsin is a chicken pineal photoreceptive molecule. Nature. 1994;372:94–97. doi: 10.1038/372094a0. [DOI] [PubMed] [Google Scholar]
- 23.Okano T, Takanaka Y, Nakamura A, Hirunagi K, Adachi A, Ebihara S, Fukada Y. Immunocytochemical identification of pinopsin in pineal glands of chicken and pigeon. Brain Res Mol Brain Res. 1997;50:190–196. doi: 10.1016/s0169-328x(97)00184-8. [DOI] [PubMed] [Google Scholar]
- 24.Okano T, Yamamoto K, Okano K, Hirota T, Kasahara T, Sasaki M, Takanaka Y, Fukada Y. Chicken pineal clock genes: implication of BMAL2 as a bidirectional regulator in circadian clock oscillation. Genes Cells. 2001;6:825–836. doi: 10.1046/j.1365-2443.2001.00462.x. [DOI] [PubMed] [Google Scholar]
- 25.Peng YW, Rhee SG, Yu WP, Ho YK, Schoen T, Chader GJ, Yau KW. Identification of components of a phosphoinositide signaling pathway in retinal rod outer segments. Proc Natl Acad Sci USA. 1997;94:1995–2000. doi: 10.1073/pnas.94.5.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pittendrigh CS. Temporal organization: reflections of a Darwinian clock-watcher. Annu Rev Physiol. 1993;55:16–54. doi: 10.1146/annurev.ph.55.030193.000313. [DOI] [PubMed] [Google Scholar]
- 27.Sanada K, Hayashi Y, Harada Y, Okano T, Fukada Y. Role of circadian activation of mitogen-activated protein kinase in chick pineal clock oscillation. J Neurosci. 2000;20:986–991. doi: 10.1523/JNEUROSCI.20-03-00986.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Selby CP, Thompson C, Schmitz TM, Van Gelder RN, Sancar A. Functional redundancy of cryptochromes and classical photoreceptors for nonvisual ocular photoreception in mice. Proc Natl Acad Sci USA. 2000;97:14697–14702. doi: 10.1073/pnas.260498597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stanewsky R, Kaneko M, Emery P, Beretta B, Wager-Smith K, Kay SA, Rosbash M, Hall JC. The cryb mutation identifies cryptochrome as a circadian photoreceptor in Drosophila. Cell. 1998;95:681–692. doi: 10.1016/s0092-8674(00)81638-4. [DOI] [PubMed] [Google Scholar]
- 30.Strathmann M, Simon MI. G protein diversity: a distinct class of α subunits is present in vertebrates and invertebrates. Proc Natl Acad Sci USA. 1990;87:9113–9117. doi: 10.1073/pnas.87.23.9113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahashi JS, Murakami N, Nikaido SS, Pratt BL, Robertson LM. The avian pineal, a vertebrate model system of the circadian oscillator: cellular regulation of circadian rhythms by light, second messengers, and macromolecular synthesis. Recent Prog Horm Res. 1989;45:279–348. doi: 10.1016/b978-0-12-571145-6.50010-8. [DOI] [PubMed] [Google Scholar]
- 32.Yau KW. Phototransduction mechanism in retinal rods and cones. Invest Ophthalmol Vis Sci. 1994;35:9–32. [PubMed] [Google Scholar]
- 33.Zatz M. Photoendocrine transduction in cultured chick pineal cells. IV. What do vitamin A depletion and retinaldehyde addition do to the effects of light on the melatonin rhythm? J Neurochem. 1994;62:2001–2011. doi: 10.1046/j.1471-4159.1994.62052001.x. [DOI] [PubMed] [Google Scholar]
- 34.Zatz M, Heath JR., III Calcium and photoentrainment in chick pineal cells revisited: effects of caffeine, thapsigargin, EGTA, and light on the melatonin rhythm. J Neurochem. 1995;65:1332–1341. doi: 10.1046/j.1471-4159.1995.65031332.x. [DOI] [PubMed] [Google Scholar]
- 35.Zatz M, Mullen DA. Two mechanisms of photoendocrine transduction in cultured chick pineal cells: pertussis toxin blocks the acute but not the phase-shifting effects of light on the melatonin rhythm. Brain Res. 1988;453:63–71. doi: 10.1016/0006-8993(88)90143-6. [DOI] [PubMed] [Google Scholar]
- 36.Zatz M, Lange GD, Rollag MD. What does changing the temperature do to the melatonin rhythm in cultured chick pineal cells? Am J Physiol. 1994;266:R50–R58. doi: 10.1152/ajpregu.1994.266.1.R50. [DOI] [PubMed] [Google Scholar]
- 37.Zordan MA, Rosato E, Piccin A, Foster R. Photic entrainment of the circadian clock: from Drosophila to mammals. Semin Cell Dev Biol. 2001;12:317–328. doi: 10.1006/scdb.2001.0259. [DOI] [PubMed] [Google Scholar]
- 38.Zuker CS. The biology of vision of Drosophila. Proc Natl Acad Sci USA. 1996;93:571–576. doi: 10.1073/pnas.93.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]