Abstract
α7-Nicotinic acetylcholine receptors (nAChRs) are widely expressed in the vertebrate nervous system. α7-nAChR functions include postsynaptic transmission, modulating neurotransmitter release, reinforcing nicotine addiction, and a role in neurological disorders, such as schizophrenia and Alzheimer's disease. In chick parasympathetic ciliary ganglion (CG) neurons, α7-nAChRs are excluded from the synapse and localize perisynaptically. Despite their extrasynaptic distribution, the highly Ca2+-permeable α7-nAChRs have important synapse-related Ca2+-dependent signaling functions in the CG. We show here that the synaptic partners regulate α7-nAChR expression during synapse formation in embryonic CG neurons in situ. The absence of inputs and target tissues cause reductions in α7-nAChR mRNA and protein levels that primarily resemble those seen for synaptic α3-nAChRs. However, there is a difference in their regulation. α7-nAChR levels are downregulated by reduced activity, whereas α3-nAChR levels are not. We propose that the activity-dependent regulation of extrasynaptic α7-nAChR levels may be an important mechanism for postsynaptic CG neurons to detect changes in presynaptic activity levels and respond with Ca2+-dependent plasticity changes in gene expression.
Keywords: nicotinic acetylcholine receptor, nAChR, α7, α3, neuron-specific gene expression, synapse formation, innervation, target tissue interactions, induction, electrical activity, visual deprivation, ciliary ganglion, neuron
Synapses are essential for intercellular communication and rapid information processing in the nervous system. Given their central role in neural function, it is surprising that the mechanisms that regulate interneuronal synapse differentiation are still primarily undefined. Within a single neuron, multiple types of neurotransmitter receptors are active and targeted to discrete synaptic regions for proper function. The expression of receptors that concentrate at the postsynaptic membrane is regulated by both presynaptic inputs and retrograde signals from the target tissues (Levey et al., 1995; Broide et al., 1996; Levey and Jacob, 1996; Zhou et al., 1998, 2001; Devay et al., 1999). We define here the regulatory effects of synaptic partners on the expression of receptors that are excluded from the synapse and restricted to perisynaptic regions of the neuron surface membrane.
Nicotinic acetylcholine receptors (nAChRs) mediate fast excitatory synaptic transmission through the chick parasympathetic ciliary ganglion (CG). Chick CG neurons express two distinct types of nAChRs, α3-nAChRs and α7-nAChRs, that differ in their subunit composition and spatial distribution. α3-nAChRs, composed of α3, α5, and β4 (occasionally β2) subunits, are concentrated in the specialized postsynaptic membrane (Jacob et al., 1986; Loring and Zigmond, 1987;Vernallis et al., 1993). In contrast, α7-nAChRs, composed of α7 subunits, are excluded from the synapse at all ages ranging from early embryonic to reproductively mature adult CGs (Jacob and Berg, 1983;Loring et al., 1985; Vernallis et al., 1993; Conroy and Berg, 1995;Horch and Sargent, 1995; Shoop et al., 1999). The α7-nAChRs are restricted to perisynaptic regions in which they accumulate on the surface membrane of short dendrites that emerge from the postsynaptic neuron in the region of innervation. Presynaptically released acetylcholine activates both nAChR types, but their functional properties differ. Compared with α3-nAChRs, the α7-nAChRs have higher calcium permeability and faster activation and desensitization kinetics (Ullian et al., 1997; Chang and Berg, 1999). The different properties and spatial segregation of the two receptor types are likely to create functionally specialized synapse-associated microregions. Perisynaptic α7-nAChR activation is required for reliable synaptic transmission and synchronous firing at early embryonic ages (Chang and Berg, 1999). However, during synapse maturation at older embryonic ages, α7-nAChRs are not required for reliable synaptic transmission but have other synapse-related, Ca2+-dependent functions in CG neurons. Specifically, extrasynaptic α7-nAChRs mediate process remodeling, neuron survival, and Ca2+-dependent synaptically regulated changes in gene expression (Pugh and Berg, 1994;Pugh and Margiotta, 2000; Chang and Berg, 2001).
We report here the first in vivo analysis of the role of innervation and target tissues in regulating α7-nAChR expression during interneuronal synapse formation. We show that synaptic partners regulate the developmental expression of receptors that are excluded from the synapse. The absence of innervation and target tissues produce changes in extrasynaptic α7-nAChR subunit mRNA and protein levels that primarily parallel those seen for synaptic α3-nAChR subunits in embryonic chick CG neurons (Levey at al., 1995). However, there is a difference in the regulation of the two nicotinic receptor types. α7-nAChR levels are downregulated by visual deprivation–reduced activity, whereas α3-nAChR levels are not. Our findings suggest that α7-nAChRs have a unique synapse-related function as activity sensors in postsynaptic CG neurons.
MATERIALS AND METHODS
Chick embryos, staging, and surgical manipulations.White Leghorn embryonated chick eggs (Spafas, Norwich, CT) were maintained at 37°C in a forced-draft turning incubator until use. Embryos were staged according to the classification scheme of Hamburger and Hamilton (1951). Surgical micromanipulations to prevent preganglionic innervation or postganglionic target tissue interactions were performed as described in detail previously (Arenella et al., 1993; Dourado et al., 1994). Briefly, the sole source of presynaptic input, the accessory oculomotor nucleus, was ablated bilaterally at embryonic day 3.5 (E3.5) to E4, or the developing optic vesicle, which contains the target muscles, was unilaterally removed at E2. The surgeries are timed to precede synaptogenesis. To obtain ganglia deprived of both innervation and target tissues, the developing eye was removed at E2 and the preganglionic nucleus at E3.5–E4 in the same embryo (Levey et al., 1995). To ensure the complete removal of preganglionic and postganglionic tissues, ganglia were only dissected from embryos lacking visible preganglionic connections to the CG and residual eye structures. The complete removal of all preganglionic neurons and the absence of aberrant innervation from other sources or intraganglionic contacts were established by paraffin histological examination of the brain of operated embryos, by immunocytochemical labeling with monoclonal antibodies (mAbs) to synaptic vesicle antigens, and by ultrastructural analysis (Engisch and Fischbach, 1992;Arenella et al., 1993). CGs from normal-developing, operated and sham-operated embryos were dissected at selected stages of synapse formation and maturation, ranging from E5 to E15. CGs were immediately frozen in liquid nitrogen and stored at −80°C until use for reverse transcription (RT)-PCR or were fixed and processed for histochemical staining.
Quantitative RT-PCR. Quantitative RT-PCR was performed as described previously, with the following modifications to optimize amplification of α7 mRNA in individual CGs (Levey et al., 1995; Levey and Jacob, 1996). Briefly, total RNA was extracted from individual ganglia by the guanidinium isothiocyanate–hot phenol method (Feramisco et al., 1982) as modified by the addition of glycogen as carrier. Known concentrations of an α7-nAChR mutated cRNA internal standard, equivalent to the quantity of that transcript present in that age operated or control ganglion (as determined initially by competitive RT-PCR), were added to the ganglion at the start of RNA extraction. The use of serial dilutions of internal standard in competitive PCR demonstrated that PCR amplification of α7 is linear over the concentration range of α7 transcripts in operated and control CGs. α7 mutated standard was generated by site-directed mutagenesis with PCR (Higuchi et al., 1988) and resembles the region of the cellular α7 mRNA targeted for amplification with the exception of two base pair changes [at nucleotides (nt) 1170 and 1172] required to replace an existing TaqI restriction endonuclease site with a novelXbaI site. Mutated α7 product was subcloned in pCR vector (Invitrogen, San Diego, CA) and sequence verified (Sequenase version 2.0; Stratagene, La Jolla, CA). cRNA was generated by in vitro transcription (MEGA short script; Ambion, Austin, TX), and the concentrations were determined by OD260measurements. Ganglionic RNA and the mutated α7 internal standard cRNA were amplified by quantitative RT-PCR in the presence of [α32-P]dCTP using α7-specific primers. The α7 sense and antisense primers correspond to regions in exons 9 and 10 and flank the cytoplasmic domain between transmembrane regions III and IV (Couturier et al., 1990). The sequence of the forward primer is 5′ TGATTATTGTTGGCCTCTCTG (nt 906–926). The reverse primer is 5′ TGGTGCTGACATTAAGATGCC (nt 1457–1477). Briefly, single-stranded cDNA was synthesized by Moloney murine leukemia virus reverse transcriptase (MMLV-RT) (Promega, Madison, WI) and specific priming with the α7 reverse primer. The 20 μl reaction contained RNA from one CG, 10 μm antisense primer, 250 μm each dNTP, 0.5 U of RNasin (Promega), 0.1m dithiothreitol, 20 U of MMLV-RT, and buffer. The RNA and the antisense primer were heat denatured and then reverse transcribed for 1 hr at 42°C. Single-stranded cDNA was amplified by PCR in a programmable thermocycler (MJ Research, Watertown, MA) in a final volume of 20 μl. First, enzyme buffer, dH20, dNTPs, 1.5 mmMgCl2, 10 μm sense primer, and Ampliwax to reduce nonspecific priming (Stratagene) were heated at 80°C for 5 min and then allowed to cool. To the top of the wax layer, we added buffer, 1.0 U of TaqDNA polymerase (Promega), 10 nCi of [α32-P]dCTP (DuPont NEN, Boston, MA), and 2 μl of the RT product. The samples were heat denatured at 94°C for 2 min, amplified (30 cycles: denaturation at 94°C for 40 sec, primer annealing at 63°C for 1 min, and DNA extension at 72°C for 1.5 min), and incubated for a final 10 min at 72°C to complete extension. For comparison with α7, α3 transcript levels and cβ4-tubulin transcript levels, as a negative control, were also measured by quantitative RT-PCR with α3 and cβ4-tubulin mutated internal standard cRNAs, respectively, and specific primers as reported previously (Levey et al., 1995; Levey and Jacob, 1996), but using Ampliwax and the higher annealing temperature detailed above. Restriction enzyme mapping and gel electrophoresis were used to distinguish PCR products derived from the mutated standards and the ganglionic mRNAs. Fragments were transferred to a Zeta probe blotting membrane (Bio-Rad, Hercules, CA) and exposed x-ray film. The ratio of the ganglionic mRNA products and the mutated standard products was determined by densitometric scanning (PDI densitometer; PDI, Huntington Station, NY) of the resulting autoradiogram.
Neuronal cell counts. Neuron numbers in normal developing ganglia were taken from Landmesser and Pilar (1974) and Furber et al. (1987). Neuron numbers in CGs from operated and sham-operated embryos at E8 and E12–E14 were determined as described previously (Levey et al., 1995). Briefly, ganglia were fixed, processed for paraffin histology, serially sectioned at 8 μm, and stained with toluidine blue (Arenella et al., 1993). All neurons possessing a nucleus with a distinct nucleolus were counted in each section of the ganglion. Cell counts were corrected for double counting by the method of Abercrombie (1946).
Histochemical staining procedures. α7-nAChR levels were examined in CG neurons of operated and control embryos by histochemical staining of frozen ganglion sections. CGs were dissected from normal, sham-operated and operated embryos at ages ranging from E5 to E18 (stages 25–44). Ganglia were lightly fixed with 0.25% paraformaldehyde in PBS under vacuum for 1 hr, embedded, and frozen sectioned as described previously (Jacob, 1991; Arenella et al., 1993). Sections (8-μm-thick) of age-matched CGs from operated and control embryos were mounted on the same slide and processed in parallel for comparison of α7-nAChR staining. The sections were rinsed in PBS containing 0.75% glycine to reduce nonspecific staining, incubated with biotinylated α-bungarotoxin (α-Bgt) (Molecular Probes, Eugene, OR) at 1:100 or 1:200 dilution in PBS for 1 hr, rinsed in PBS with 0.5m NaCl, and incubated for 40 min with an avidin–biotinylated horseradish peroxidase (HRP) complex (Vectastain Elite ABC kit; Vector Laboratories, Burlingame, CA) that was prepared in PBS according to the instructions of the manufacturer. Alternatively, some sections were incubated with biotinylated α-Bgt at 1:40 dilution and streptavidin–HRP conjugate (Vector Laboratories) at 1:20 dilution in PBS. The sections were then reacted for peroxidase activity and mounted as described previously (Jacob, 1991; Arenella et al., 1993) and viewed by bright-field microscopy with a Zeiss(Thornwood, NY) Axioskop microscope. To establish the specificity of α-Bgt staining, a few slides from each experiment were processed as described above, except that biotinylated α-Bgt was replaced with PBS.
For comparison with the α7-nAChR staining levels, alternate frozen ganglion sections from the operated, sham-operated and normal embryos were immunolabeled for three other neuron-specific components, synaptic vesicle protein SV2 and two microtubule-associated proteins, MAP1B and MAP2, as described previously (Arenella et al., 1993). The anti-SV2 mouse mAb to the synaptic vesicle transmembrane transporter (generously provided by Dr. Kathleen Buckley, Harvard Medical School, Boston, MA) was used at a 1:100 dilution in PBS. The anti-MAP1B-2 mouse mAb to MAP1B and the anti-MAP2 mouse mAb (the generous gift from Dr. Richard Vallee, Columbia University, New York, NY) were both used at a 1:200 dilution in PBS.
Visual deprivation. White Leghorn chicks were hatched in a darkened incubator in our laboratory, raised in complete darkness (dark reared) in temperature-controlled brooders, and force-fed as described previously (Gottlieb et al., 1987). All other newly hatched chicks were maintained on a 12 hr light/dark cycle (diurnally reared). In one group of the diurnally reared chicks, we covered one eye with an opaque black plastic dome-like goggle (light-tight occluder) to prevent light entry and visual function. The occluder (kindly provided by Dr. Josh Wallman of City College of New York, New York, NY) was glued to the circumorbital feathers and skin surrounding the eye (Wallman et al., 1978; Gottlieb et al., 1987; Shih et al., 1993). The CG from the contralateral untreated eye served as an internal control. To control for nonspecific effects of covering the eye, a clear occluder was applied over one eye in separate chicks. Treatments started at hatching and lasted for 1 or 2 weeks. In all conditions, food and water were available ad libitum.
nAChR assays. The total number of α7-nAChRs (surface plus internal) in CGs from visually deprived and control newly hatched chicks was determined. A filter assay was used to measure specific binding of 125I-α-Bgt to CG detergent extracts as described previously (Jacob and Berg, 1987). For comparison with α7-nAChRs, α3-nAChR levels were measured using125I-mAb-35 binding to CG detergent extracts and separation of the bound125I-mAb-35 by ion exchange chromatography on DEAE-cellulose (Jacob and Berg, 1987). Specific binding was calculated as the difference between total binding and nonspecific binding, in which nonspecific binding was determined by including a 40-fold excess of cold ligand in the binding reaction. The amount of specific binding per ganglion was normalized for the relative amount of total protein. Total ganglionic protein was measured in detergent extracts by the microtiter protein assay (Bio-Rad) as performed according to the instructions of the manufacturer.
RESULTS
α7 subunit mRNA levels increase dramatically during synaptogenesis
α7 transcript levels in individual chick CGs were established at landmark stages of synapse formation and maturation, ranging from E5 to E15 (Fig. 1). Innervation precedes target tissue synapse formation in the CG. Innervation begins at E4.5, and, by E8, functional chemical synapses are present on every neuron (Landmesser and Pilar, 1972; Jacob, 1991). From E8.5 to E14, CG neurons establish functional connections with their target cells, striated and smooth muscles in the eye (Meriney and Pilar, 1987; Pilar et al., 1987).
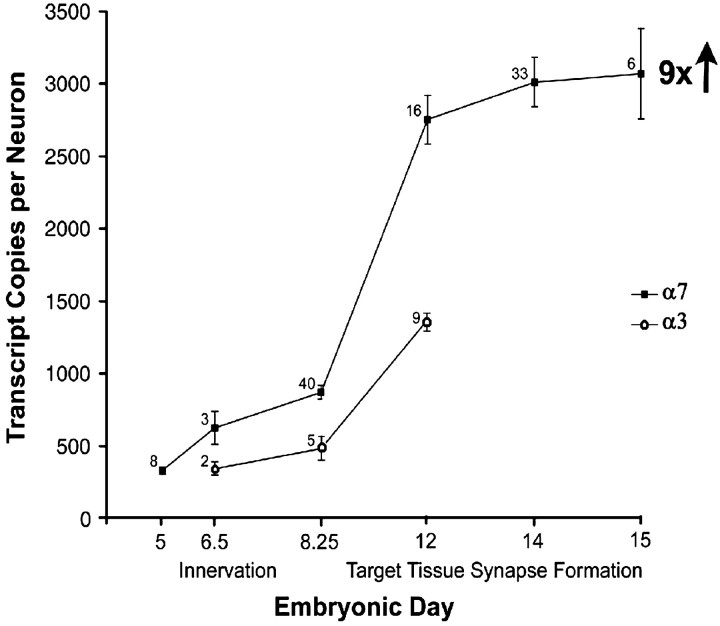
Fig. 1.
α7 subunit transcript levels increase during preganglionic and postganglionic synapse formation and then plateau in embryonic CG neurons in situ. Absolute amounts of α7 subunit mRNAs were measured in individual ganglia at landmark stages of synaptogenesis by using quantitative RT-PCR with a mutated α7 cRNA internal standard. Values are normalized to the number of transcript copies per neuron to account for developmental changes in neuron number. Each value represents the mean ± SEM of the number of separate determinations indicated for each time point. For comparison, synaptic α3 subunit mRNA levels were measured at three key stages of preganglionic and postganglionic synapse formation. α7 transcript levels increase ninefold from E5 to E15 and are twofold greater than α3 mRNA levels at all stages of synaptogenesis examined.
The absolute levels of α7 mRNA per ganglion were measured using quantitative RT-PCR and known concentrations of a mutated α7 cRNA internal standard. The internal standard differed from the targeted α7 sequence by two base pair changes introduced to create a novel restriction enzyme site (XbaI) and remove an existing site (TaqI). α7 transcript levels were normalized to account for declines in CG neuron number attributable to naturally occurring cell death from E9 to E14, with greater declines occurring in CGs surgically deprived of synaptic interactions (see below) (Landmesser and Pilar, 1974; Furber et al., 1987; Levey et al., 1995).
α7 subunit mRNA levels per CG neuron increase ninefold during preganglionic and postganglionic synapse formation in situ, from E5 to E15 (Fig. 1). The greatest rise occurs between E8.25 and E12, during the time of target tissue innervation and maturational changes in the efficacy and morphology of preganglionic inputs, with calyces forming on the ciliary neurons (Landmesser and Pilar, 1972). Similar to the developmental increases in extrasynaptic α7 subunit mRNA, synaptic α3, β4, and α5 subunit transcript levels per CG neuron rise 7-, 5-, and 16-fold, respectively, over the same time course, from E4 to E15 (Fig. 1) (Corriveau and Berg, 1993; Levey and Jacob, 1996). α7 mRNA levels are twofold more abundant than α3 transcripts at all of the stages of synapse formation examined (Fig.1). A similar difference between α7 and α3 mRNA levels in embryonic CG neurons was observed by quantitative RNase protection assays (Corriveau and Berg, 1993). The temporal correlation of the increases in α7 subunit mRNA levels with preganglionic and postganglionic synapse formation suggests that innervation and target tissues may induce the rise.
α7 transcript levels are lower in the absence of synaptic partners
To determine the respective roles of innervation and target tissues in inducing the developmental increases in α7, we measured α7 subunit mRNA levels in CG neurons surgically deprived of these synaptic partners in situ. To prevent innervation, we bilaterally ablated the sole source of presynaptic inputs, the accessory oculomotor nucleus in the midbrain. To prevent target tissue interactions, we unilaterally removed the developing optic vesicle, with the contralateral ganglion serving as an internal control (Levey et al., 1995). Surgeries were performed before synaptogenesis and cause no direct damage to CG neurons or transection of their processes. Neuron numbers are reduced because of the removal of sources of trophic support (data not shown) (Landmesser and Pilar, 1974; Furber et al., 1987; Levey et al., 1995). Importantly, a stable population of neurons is retained in all operated conditions. These neurons are healthy based on ultrastructural and electrophysiological criteria and the demonstration of normal levels of specific mRNAs and proteins (Engisch and Fischbach 1990, 1992; Arenella et al., 1993; Dourado et al., 1994;Levey et al., 1995; Ikonomov et al., 1998). Input-deprived CG neurons form synapses on their target tissues, whereas innervation is established and maintained on target-deprived neurons (Landmesser and Pilar, 1974; Furber et al., 1987).
α7 subunit mRNA levels are decreased in CG neurons that have developed in the absence compared with the presence of synaptic partners in situ (Fig. 2, Table 1). In presynaptic input-deprived neurons, the number of α7 transcript copies is reduced to 70 and 80% of sham-operated control neuron values at E8 and E12–E14, respectively. Compared with input deprivation, there are greater decreases in α7 mRNA levels in CG neurons that developed in the absence of the target tissues. Specifically, in target-deprived neurons, α7 transcript levels are 50% of contralateral control neuron values at both E8 and E12–E14. E8 precedes the time of peripheral synapse formation, but axons from CG neurons are already in the vicinity of the developing target tissues (Meriney and Pilar, 1987;Pilar et al., 1987). Thus, the decrease in α7 transcripts in E8 target-deprived neurons suggests that soluble factors from the target muscles retrogradely influence α7 transcript levels before synaptic contact. Overall, both inputs and target tissues regulate α7 mRNA levels during synapse formation and maturation in CG neurons.
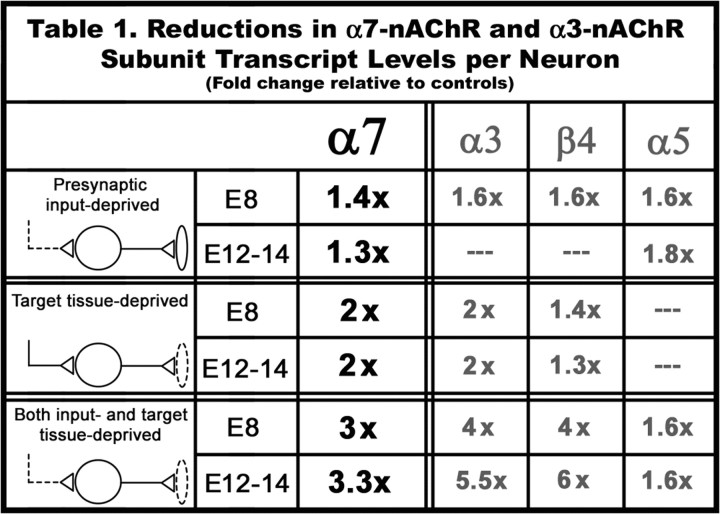
Comparison of the decreases in extrasynaptic α7 subunit mRNA levels relative to those seen for synaptic α3, β4, and α5 subunit mRNAs in CG neurons deprived of input, targets, and both synaptic partners. Data are expressed as the fold change in the absolute levels of nAChR subunit mRNA per neuron in the test condition relative to controls. For input-deprived CGs and both input- and target-deprived CGs, the sham-operated age-matched CGs are used as controls, whereas target-deprived CGs are compared with the contralateral control CG from the same embryo. The α7 mRNA data are from Figure 2, and the α3, β4, and α5 mRNA values are from Levey et al. (1995). —, Not significantly different from control values; Student'st test.
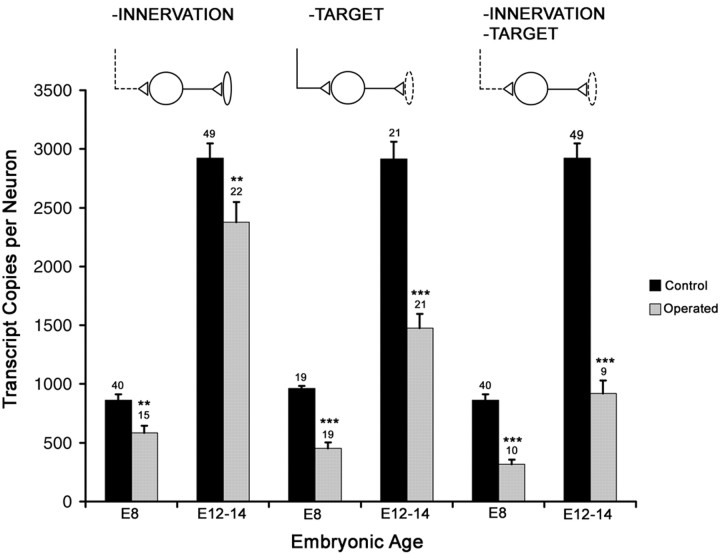
Fig. 2.
α7 transcript levels are reduced in CG neurons that have developed in the absence versus the presence of innervation, target tissues, or both synaptic partners. The absolute amounts of α7 subunit mRNA were determined in individual ganglia from operated (gray) and control (black) embryos at E8 and E12–E14 using quantitative RT-PCR. The values are expressed as the number of transcript copies per neuron to normalize for changes in neuron number after the surgeries. Results represent the mean ± SEM. The number of ganglia assayed is shown above eachbar. Ganglia deprived of inputs and both inputs and targets are compared with age-matched, sham-operated ganglia, whereas ganglia deprived of targets are compared with their contralateral control ganglia. Asterisks indicate statistically significant differences based on the Student's two-sidedt test; **p < 0.01; ***p < 0.001.
To test for additive effects, both innervation and target tissue interactions were prevented from forming in single embryos. α7 transcript levels per neuron are lowest in CGs from these double-operated embryos, being decreased to 30–35% of control neuron values at both E8 and E12–E14 (Fig. 2, Table 1). Input and target tissue interactions have additive effects because α7 mRNA levels are significantly lower in CGs from double-operated embryos compared with the levels in CGs deprived of input alone and target alone (for input-deprived CGs, p < 0.005 for E8 andp < 0.001 for E12–E13; for target-deprived CGs,p < 0.05 for E8 and p < 0.01 for E12–E13; Student's t test). In the absence of both synaptic partners, α7 transcript levels remain low relative to control values but increase threefold over time in situ, from E8 to E14 (Fig. 2), indicating that other regulatory influences, in addition to inputs and target tissues, induce α7 expression. As independent confirmation of the quantitative RT-PCR results, Northern blot analyses show similar declines in α7 mRNA levels for all operated conditions when the data are normalized per neuron number (data not shown).
The declines in α7 transcript levels in the absence of inputs and target tissues are specific. mRNA levels for cβ4-tubulin, a neuron-specific form of β-tubulin (Sullivan et al., 1986; Lee et al., 1990), are not altered over the same time course in CGs from operated embryos, resembling our previous findings for cβ4-tubulin (data not shown) (Levey et al., 1995; Ikonomov et al., 1998).
These regulatory changes in extrasynaptic α7 subunit transcript levels closely resemble the declines in synaptic α3-nAChR subunit mRNA levels in CGs from operated embryos (Levey et al., 1995). Transcripts encoding subunits of the extrasynaptic nAChRs and synaptic nAChRs, α7, and α3, β4, and α5, respectively, are reduced to a similar extent, 1.4- to 2-fold, in the absence of inputs and targets. The only exception is that α5 mRNA levels are not significantly altered in target-deprived embryonic CG neurons but are decreased by input deprivation (Table 1) (Levey et al., 1995). Thus, the developmental expression of extrasynaptic α7-nAChR and synaptic α3-nAChR subunit mRNAs is primarily regulated in parallel by synaptic interactions.
Altogether, the data demonstrate that innervation and target tissues induce increases in perisynaptic α7 subunit mRNA levels during synapse formation in embryonic chick CG neurons in situ. Retrograde signals from the target tissues have the greater regulatory effects. The additive effects of removing both inputs and targets suggest that the two different synaptic partners provide distinct regulatory signals.
α7-nAChR protein levels are reduced in the absence of synaptic partners
For comparison with α7 transcripts, we examined the regulatory changes in α7-nAChR protein levels after the surgical manipulations. Qualitative changes in α7-nAChR levels were detected by histochemical staining of frozen ganglionic sections using α-Bgt that specifically binds to α7 subunits in chick CG neurons and HRP for visualization (Jacob and Berg, 1983; Smith et al., 1985; Conroy and Berg, 1995). The α7-nAChR staining is primarily intracellular and associated with organelles that function in the synthesis, processing, and transport of integral plasma membrane proteins (Carbonetto and Fambrough, 1979;Jacob et al., 1986). In comparison, surface α7-nAChRs are not readily detected in the thin cryostat sections.
In neurons deprived of synaptic partners, less intense α7-nAChR staining is present in the somata relative to age-matched control neurons, suggesting a decline in the number of α7-nAChRs in the internal biosynthetic pool (Fig. 3). Compared with the declines in input-deprived neurons (Fig.3B), greater decreases in α7-nAChR protein levels are seen in the absence of target tissues (Fig. 3D). The lowest α7-nAChR levels are present in neurons deprived of both inputs and targets (Fig. 3F). The declines are specific: there are no detectable differences in the relative levels of soma immunoreactivity for three other neuron-specific components, the synaptic vesicle protein SV2 and two microtubule-associated proteins MAP1B and MAP2 in synaptic partner-deprived CG neurons compared with control neurons (Fig. 3G,H) (Arenella et al., 1993). In summary, the histochemical staining data demonstrate qualitative reductions in α7-nAChR protein levels that resemble the quantitative changes in α7 mRNA levels in CG neurons from operated embryos. The correlation suggests that the regulation of α7-nAChR expression may occur at the level of gene transcription.
Fig. 3.
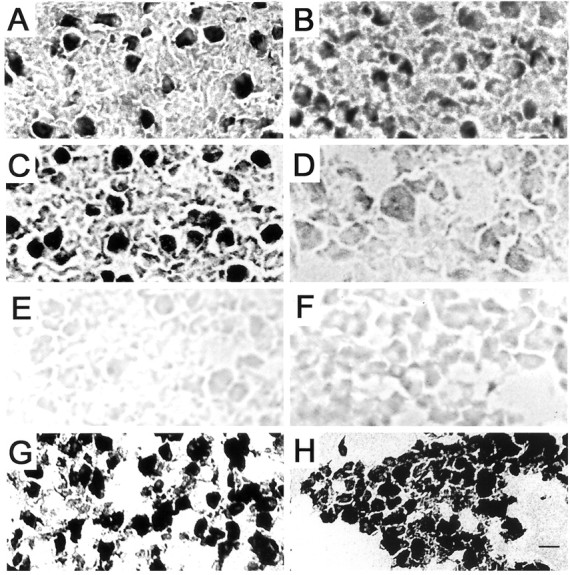
α7-nAChR staining is specifically reduced in CG neurons deprived of synaptic partners compared with control neurons. Cryostat sections of CGs from operated and control embryos at E12–E14 were incubated with biotinylated α-Bgt followed by streptavidin–HRP. The sections were then reacted for peroxidase activity and examined by bright-field microscopy. A, Sham-operated E14 CG; B, input-deprived E14 CG;C, contralateral control E12 CG; D, target tissue-deprived E12 CG; E, staining control E14 CG; F, both input- and target-deprived E12 CG. Most of the neuronal somata are intensely stained in the unoperated control ganglion sections (A, C). The interiors of the somata are filled with HRP reaction product deposits, with the exception of the nuclei, which, when visible, are not stained above background levels (E). In contrast, the majority of the neuronal somata are moderately stained in the input-deprived ganglion section (B), only lightly stained in the target-deprived ganglion section (D), and just slightly stained above background levels in the input- and target-deprived ganglion section (F). Specific α-Bgt staining is demonstrated by the absence of HRP reaction product in the sham-operated E14 ganglion section that was incubated with PBS in place of biotinylated α-Bgt (C). In contrast to the declines in α7-nAChRs, similar relative levels of MAP2 immunolabeling are present in the input- and target-deprived E8 ganglion section (H) and the sham-operated E8 ganglion section (G). Intense MAP2 immunoperoxidase labeling fills the soma and dendrites of the developing CG neurons in G and H. The decreases in α7-nAChR levels are specific. Scale bar:A–F, 30 μm; G, H, 35 μm.
Reduced activity specifically lowers α7-nAChR, but not α3-nAChR, levels
To identify the regulatory signals provided by synaptic partners, we examined the effects of activity on α7-nAChR protein levels in CGs neurons of newly hatched chicks. We used visual deprivation to greatly reduce activity in the retinal input pathway to the CG without directly damaging the intercellular connections (Shih et al., 1993: Pendrak et al., 1995). Visual deprivation was achieved by either dark rearing of chicks immediately after hatching in a darkened incubator or application of a light-tight occluder over one eye, with the contralateral uncovered eye serving as an internal control. These approaches prevent light entry and greatly reduce activity in the retina that drives the central visual pathway that innervates the CG neurons (Gamlin et al., 1982, 1984; Gamlin and Reiner, 1991). Previous reports show that visual deprivation (monocular occlusion or lid suture) significantly reduces by 28% the activity of choline acetyltransferase (ChAT), the acetylcholine biosynthetic enzyme, in the CG (Pendrak et al., 1995).
Visual deprivation decreases the total number of α7-nAChRs per ganglion as detected by specific125I-αBgt binding in ganglionic detergent extracts and normalizing for protein. Dark rearing for 1 week reduces α7-nAChR levels per ganglion to 75% of control diurnally reared chick CG values (p < 0.05; Student'st test) (Fig. 4). No additional decline is observed at 2 weeks of dark rearing. Similarly, light-tight patching one eye for 1 week reduces α7-nAChRs to 85% of the contralateral control CG value, a small but significant decrease (p < 0.025; Student's paired ttest) (Fig. 4). Compared with dark rearing, monocular occlusion is less effective at reducing activity because there are some bilateral afferent projections in the central visual pathway to the CG (Gamlin et al., 1982; Gamlin and Reiner, 1991). As a negative control, covering the eye with a clear occluder for 1 week, which permits light entry and visual function, has no significant effect on α7-nAChR levels (Fig.4). The declines in α7-nAChRs observed after visual deprivation represent reductions in the number of receptors rather than decreases in the affinity of receptor for the toxin probe as determined by increasing the concentration of125I-αBgt threefold in the standard binding assay (data not shown). In all of these experiments, the number of α7-nAChRs was calculated assuming, for convenience, a 1:1 stoichiometry of α-Bgt bound to receptor. The number of α-Bgt binding sites per receptor is not known (Chen and Patrick, 1997;Rangwala et al., 1997). However, the exact stoichiometry is not important for the present studies, which depend only on a comparison of the relative amounts of α7-nAChRs.
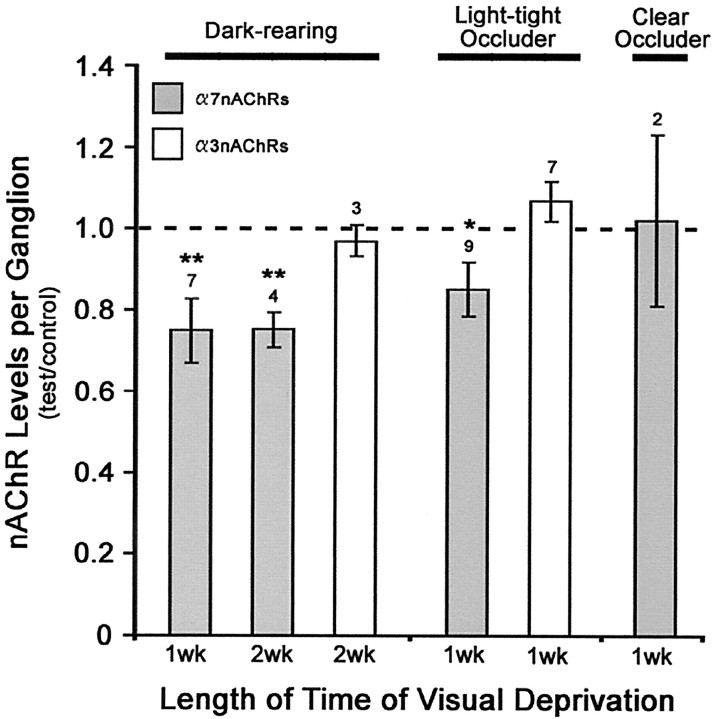
Fig. 4.
Visual deprivation downregulates α7-nAChR levels, but not α3-nAChRs, in newly hatched chick CG neurons. Visual deprivation was used to reduce activity in retinal inputs to CG neurons. Chicks were either dark reared immediately after hatching, or a light-tight occluder was applied over one eye, with the contralateral uncovered eye serving as an internal control. Dark-reared chick CGs are compared with diurnally reared chick CGs at matched ages. To control for nonspecific effects of covering the eye with a plastic goggle, a clear occluder was applied on separate chicks. The total number of α7-nAChRs per ganglion was determined by the specific binding of125I-α-Bgt in ganglionic detergent extracts. Results represent the mean ± SEM of the specific activity (the number of binding sites per ganglionic protein) of the test, with both the mean and SEM values normalized to the mean of the appropriate control. The number of separate determinations is indicated above eachbar. The hatched line represents the control diurnally reared chick CG value. For comparison with α7-nAChRs, the total number of α3-nAChRs was assayed in separate ganglia using 125I-mAb-35. α7-nAChR levels are reduced in visually deprived chick CGs, whereas α3-nAChR levels are not.Asterisks indicate statistically significant differences in the raw data of Bgt-specific activities in test versus control CGs based on the Student's t test for dark-reared chicks and the Student's paired t test for monocular occluded chicks; **p < 0.05 and *p < 0.025, respectively.
In contrast to the declines in α7-nAChRs seen with visual deprivation, α3-nAChR levels are not altered. Neither dark rearing nor monocular occlusion produce a significant change in the total number of α3-nAChRs per ganglion as detected by specific125I-mAb-35 binding in ganglionic detergent extracts and normalizing for protein (Fig. 4). These unexpected results suggest that reduced activity regulates the levels of extrasynaptic α7-nAChRs but has no effect on synaptic α3-nAChR levels, in CG neurons in situ.
DISCUSSION
Two major findings are reported here. One, although α7-nAChRs are excluded from the synapse, their developmental expression is regulated by innervation and target tissue interactions in embryonic chick CG neurons in situ. Two, reduced activity downregulates perisynaptic α7-nAChR levels but not synaptic α3-nAChRs. Innervation and target tissue interactions both induce increases in α7 transcript and protein levels during synapse formation in the CG, with retrograde signals from the target tissues having the greater inductive effect. The absence of synaptic partners causes changes in perisynaptic α7-nAChR expression that primarily parallel that seen for synaptic α3-nAChRs (Table 1). However, there are some differences in their regulation. Most striking, reduced activity has unique effects on α7-nAChR levels. These results establish a difference in the signals that mediate the regulatory effects of synaptic partners on the two nicotinic receptor types. The parallel expression of α7-nAChRs and α3-nAChRs, mediated at least in part by different regulatory signals, suggests that perisynaptic α7-nAChRs are likely to have important and distinct synapse-related functions in vertebrate autonomic neurons.
The present study establishes the parallel regulation of the subunits of perisynaptic α7-nAChRs and synaptic α3-nAChRs, with one exception. Innervation induces increases in α7 mRNA that closely resemble in time course and extent those seen for all of the α3-nAChR subunit mRNAs, α3, β4, and α5, suggesting that innervation coordinately regulates the developmental expression of perisynaptic and synaptic nAChRs (this study; Levey et al., 1995). In contrast, the target tissues have some differential regulatory effects. α7 mRNA levels are upregulated in a manner resembling α3 and β4, whereas α5 mRNA is not affected by target tissue interactions in embryonic CG neurons (this study; Levey et al., 1995). The parallel regulation of perisynaptic α7 and synaptic α3 and β4, but not α5, is interesting. For α3-nAChR complexes, α3 is the essential subunit, being required for surface expression of functional α3-nAChRs and their targeting to the synapse (De Koninck and Cooper, 1995; Levey et al., 1995; Williams et al., 1998; Xu et al., 1999a). The β4 subunit is required for α3-nAChR complex assembly (Xu et al., 1999b). In contrast, α5 is needed for the formation of high-conductance α3-nAChR channels and appears to be a developmentally late component of the α3-nAChR complex in CG neurons (Margiotta and Gurantz, 1989;Levey et al., 1995; Ramirez-Latorre et al., 1996; Wang et al., 1996). For perisynaptic α7-nAChRs, α7 is the critical ligand-binding subunit, and, in many neural tissues, α7-nAChRs are homopentamers (Chen and Patrick, 1997; Drisdel and Green, 2000). In summary, our results show that the transcript levels of α7 and α3, the key subunits of the perisynaptic and synaptic nAChR complexes, respectively, are regulated in parallel during synapse formation in chick CG neurons.
At mature ages, perisynaptic α7-nAChR expression continues to be regulated by innervation and target tissue interactions. In newly hatched chickens, the disruption of functional synaptic connections (preganglionic denervation or postganglionic axotomy) causes specific declines in α7-nAChR protein levels (Jacob and Berg, 1987;Zhou et al., 2001). Again, perisynaptic α7-nAChR and synaptic α3-nAChR levels are regulated similarly at these later ages but, in this case, with distinct time courses. After denervation and axotomy, the decreases in α7-nAChRs are steeper and more rapid than those seen for α3-nAChRs (Jacob and Berg, 1987). Altogether, this study and our previous work demonstrate that signals from both presynaptic inputs and target tissues are required for the initial induction and the maintenance of mature levels of α7-nAChR expression in CG neuronsin situ.
The regulatory signals that mediate the effects of innervation and target tissues on α7-nAChR expression are as yet primarily undefined. We focused on the role of electrical activity as a potential regulatory signal. Precedence demonstrates that electrical activity regulates neural expression of other neurotransmitter receptor types (Philpot et al., 2001; Kilman et al., 2002). We used visual deprivation to greatly reduce activity in the retinal inputs to the CG neurons via multisynaptic central visual pathways (Gamlin et al., 1982, 1984;Gamlin and Reiner, 1991). Precedence shows that visual deprivation significantly reduces the levels of particular synapse-related proteins in the CG. Specifically, the activity of ChAT, the acetylcholine biosynthetic enzyme, is reduced by 28% (Pendrak et al., 1995). We show here that visual deprivation selectively regulates perisynaptic α7-nAChR levels, but not synaptic α3-nAChRs, in CG neurons of newly hatched chicks. Similar to these in vivo effects of visual deprivation, in vitro studies show that activity (membrane depolarization) specifically influences α7, but not α3, nAChR transcript and protein levels in neonatal rat sympathetic neurons (De Koninck and Cooper, 1995). α7-nAChR levels in the rodent somatosensory cortex are also regulated by sensory deprivation, the removal of all vibrissa on one side of the face (Bina et al., 1998). In the chick CG, the declines in α7-nAChR protein levels caused by visual deprivation in vivo are modest (1.3-fold) but significant. In comparison, there are greater declines in α7-nAChR protein levels (twofold) after the disruption of functional connections (preganglionic denervation) in newly hatched chick CGs (Jacob and Berg, 1987). It should be noted that visual deprivation is likely to reduce, but not eliminate, synaptic activation of CG neurons (Jackson, 1983). The effects of reduced activity may be direct or indirect, possibly involving other regulatory signals (Loeb et al., 2002). Thus, factors other than activity may mediate the regulatory effects of visual deprivation on α7-nAChR levels. Intercellular signals, such as specific factors, influence α7-nAChR levels in neurons. Neuregulin (the neural-specific cysteine-rich domain-containing isoform) is a presynaptic input-derived soluble factor that increases α7-nAChR levels in sympathetic neurons in vitro (Yang et al., 1998;Liu et al., 2001). Neuregulin splice variants are expressed in presynaptic inputs to the CG (Corfas et al., 1995). They are also likely expressed in CG neurons, suggesting the possible contribution of autocrine and paracrine actions of this soluble factor (Corfas et al., 1995; Sandrock et al., 1995). In addition, two distinct target tissue-derived factors have been isolated that influence α7-nAChR levels in opposite ways (Nishi, 1994; Finn and Nishi, 1996). Ciliary neurotrophic factor, a trophic factor, specifically downregulates α7-nAChRs, whereas a soluble component of 50 kDa increases α7 levels in CG neurons in vitro (Halvorsen and Berg, 1989;Halvorsen et al., 1991). In the present study, we show additive effects of inputs and target tissues on α7-nAChR levels, suggesting that the two different synaptic partners may provide distinct regulatory signals. Overall, the combinatorial regulatory effects of activity and multiple factors are likely to govern α7 subunit levels during synapse formation and maturation in chick autonomic neurons in situ.
Our findings that innervation and target tissue interactions induce increases in α7-nAChR expression during synapse differentiation suggest that the perisynaptic receptors may have synapse-related functions. Recent electrophysiological studies show that α7-nAChR activation is required for reliable synaptic transmission in the early embryonic chick CG (Chang and Berg, 1999). Greater activity may stabilize the newly formed synaptic connections, resulting in the uptake of essential trophic factors and survival of the neurons during the critical developmental period of synapse formation and elimination (Meriney et al., 1987: Maderdrut et al., 1988; Pugh and Margiotta, 2000). However, during synapse maturation at older embryonic ages, α7-nAChR activation is no longer required for reliable synaptic transmission (Chang and Berg, 1999). Other synapse-related functions are likely as suggested by the α7-nAChR abundant surface expression, spatial segregation, and distinct functional properties relative to α3-nAChRs on CG neurons. In particular, α7-nAChRs have a higher Ca2+ permeability and faster kinetics of activation and desensitization (Zhang et al., 1996; Ullian et al., 1997). Our findings that visual deprivation causes declines in α7-nAChRs, but not α3-nAChRs, suggest that α3-nAChRs are required to maintain synaptic transmission, whereas α7-nAChRs are activity sensors in the postsynaptic neuron. A recent report demonstrates that nicotinic signaling to CG neurons can induce Ca2+-dependent plasticity changes (prolonged activation of phosphorylated cAMP response element-binding protein and gene expression) under certain conditions (Chang and Berg, 2001). Activation of nAChRs is required, whereas voltage-gated Ca2+ channels are silent, with intracellular Ca2+ levels being the critical determinant. In particular, the amounts and the temporal and spatial patterns of internal Ca2+elevations are important. Activity-dependent changes in α7-nAChR levels are likely to affect the critical variable of Ca2+ influx in CG neurons. We propose that α7-nAChRs have the unique function of activity sensors in CG neurons. The activity-dependent regulation of extrasynaptic α7-nAChRs may be an important mechanism for CG neurons to detect alterations in presynaptic activity levels and respond by Ca2+-dependent plasticity changes in gene expression.
In summary, our data demonstrate that, within single CG neurons, the coexpression of extrasynaptic α7-nAChRs and synaptic α3-nAChRs is induced by innervation and target tissue interactions, with some differences in the underlying regulatory signals. The coexpression of the two nicotinic receptor subtypes is important for optimal synaptic signaling and plasticity in neural circuits of the vertebrate autonomic nervous system.
Footnotes
This work was supported by National Institutes of Health Grant 21725 (M.H.J.). We thank Marjory Levey for designing and testing the primers, Ogi Ikonomov for optimizing PCR conditions, Rachel Blitzblau and Brian Williams for generous help in preparing the figures, and Madelaine Rosenberg for insightful comments on this manuscript.
Correspondence should be addressed to Dr. Michele Jacob, Department of Neuroscience, Tufts University, Sackler School of Biomedical Sciences, 136 Harrison Avenue, Boston, MA 02111. E-mail:michele.jacob@tufts.edu.
C. L. Brumwell's present address: University of Massachusetts Medical Center, Department of Biochemistry and Molecular Pharmacology, Biotech 4, Worcester, MA 01605.
J. L. Johnson's present address: Department of Anesthesia, Tri-City Medical Center, 4002 Vista Way, Oceanside, CA 92056.
REFERENCES
- 1.Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- 2.Arenella LS, Oliva JM, Jacob MH. Reduced levels of acetylcholine receptor expression in chick ciliary ganglion neurons developing in the absence of innervation. J Neurosci. 1993;13:4525–4537. doi: 10.1523/JNEUROSCI.13-10-04525.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bina KG, Park M, O'Dowd DK. Regulation of α7 nicotinic acetylcholine receptors in mouse somatosensory cortex following whisker removal at birth. J Comp Neurol. 1998;397:1–9. [PubMed] [Google Scholar]
- 4.Broide RS, Robertson RT, Leslie FM. Regulation of alpha7 nicotinic acetylcholine receptors in the developing rat somatosensory cortex by thalamocortical afferents. J Neurosci. 1996;16:2956–2971. doi: 10.1523/JNEUROSCI.16-09-02956.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carbonetto S, Fambrough DM. Synthesis, insertion into the plasma membrane, and turnover of alpha-bungarotoxin receptors in chick sympathetic neurons. J Cell Biol. 1979;81:555–569. doi: 10.1083/jcb.81.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang KT, Berg DK. Nicotinic acetylcholine receptors containing alpha7 subunits are required for reliable synaptic transmission in situ. J Neurosci. 1999;19:3701–3710. doi: 10.1523/JNEUROSCI.19-10-03701.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang KT, Berg DK. Voltage-gated channels block nicotinic regulation of CREB phosphorylation and gene expression in neurons. Neuron. 2001;32:855–865. doi: 10.1016/s0896-6273(01)00516-5. [DOI] [PubMed] [Google Scholar]
- 8.Chen D, Patrick JW. The alpha-bungarotoxin-binding nicotinic acetylcholine receptor from rat brain contains only the alpha7 subunit. Biol Chem. 1997;272:24024–24029. doi: 10.1074/jbc.272.38.24024. [DOI] [PubMed] [Google Scholar]
- 9.Conroy WG, Berg DK. Neurons can maintain multiple classes of nicotinic acetylcholine receptors distinguished by different subunit compositions. J Biol Chem. 1995;270:4424–4431. doi: 10.1074/jbc.270.9.4424. [DOI] [PubMed] [Google Scholar]
- 10.Corfas G, Rosen KM, Aratake H, Krauss R, Fischbach GD. Differential expression of ARIA isoforms in the rat brain. Neuron. 1995;14:103–115. doi: 10.1016/0896-6273(95)90244-9. [DOI] [PubMed] [Google Scholar]
- 11.Corriveau RA, Berg DK. Coexpression of multiple acetylcholine receptor genes in neurons: quantification of transcripts during development. J Neurosci. 1993;13:2662–2671. doi: 10.1523/JNEUROSCI.13-06-02662.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Couturier S, Bertrand D, Matter J-M, Hernandez M-C, Bertrand S, Millar N, Valera S, Barkas T, Ballivet M. A neuronal nicotinic acetylcholine receptor subunit (α7) is developmentally regulated and forms a homo-oligomeric channel blocked by α-Btx. Neuron. 1990;5:847–856. doi: 10.1016/0896-6273(90)90344-f. [DOI] [PubMed] [Google Scholar]
- 13.De Koninck P, Cooper E. Differential regulation of neuronal nicotinic ACh receptor subunit genes in cultured neonatal rat sympathetic neurons: specific induction of α7 by membrane depolarization through a Ca2+/calmodulin-dependent kinase pathway. J Neurosci. 1995;15:7966–7978. doi: 10.1523/JNEUROSCI.15-12-07966.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Devay P, McGehee DS, Yu CR, Role LW. Target-specific control of nicotinic receptor expression at developing interneuronal synapses in chick. Nat Neurosci. 1999;2:528–534. doi: 10.1038/9183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dourado MM, Brumwell C, Wisgirda ME, Jacob MH, Dryer SE. Target tissues and innervation regulate the characteristics of K+ currents in chick ciliary ganglion neurons developing in situ. J Neurosci. 1994;14:3156–3165. doi: 10.1523/JNEUROSCI.14-05-03156.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drisdel RC, Green WN. Neuronal α-bungarotoxin receptors are α7 subunit homomers. J Neurosci. 2000;20:133–139. doi: 10.1523/JNEUROSCI.20-01-00133.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engisch KL, Fischbach GD. The development of ACH- and GABA-activated currents in normal and target-deprived embryonic chick ciliary ganglia. Dev Biol. 1990;139:417–426. doi: 10.1016/0012-1606(90)90310-f. [DOI] [PubMed] [Google Scholar]
- 18.Engisch KL, Fischbach GD. The development of ACh- and GABA-activated currents in embryonic chick ciliary ganglion neurons in the absence of innervation in vivo. J Neurosci. 1992;12:1115–1125. doi: 10.1523/JNEUROSCI.12-03-01115.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feramisco JR, Smart JE, Burridge K, Helfman DM, Thomas GP. Co-existence of vinculin and a vinculin-like protein of higher molecular weight in smooth muscle. J Biol Chem. 1982;257:11024–11031. [PubMed] [Google Scholar]
- 20.Finn TP, Nishi R. Expression of a chicken ciliary neurotrophic factor in targets of ciliary ganglion neurons during and after the cell-death phase. J Comp Neurol. 1996;366:559–571. doi: 10.1002/(SICI)1096-9861(19960318)366:4<559::AID-CNE1>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 21.Furber S, Oppenheim RW, Prevette D. Naturally occurring neuron death in the ciliary ganglion of the chick embryo following removal of preganglionic input: evidence for the role of afferents in ganglion cell survival. J Neurosci. 1987;7:1816–1832. doi: 10.1523/JNEUROSCI.07-06-01816.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gamlin PD, Reiner A. The Edinger-Westphal nucleus: sources of input influencing accommodation, pupilloconstriction, and choroidal blood flow. J Comp Neurol. 1991;306:425–438. doi: 10.1002/cne.903060307. [DOI] [PubMed] [Google Scholar]
- 23.Gamlin PD, Reiner A, Karten H. Substance P-containing neurons of the avian suprachiasmatic nucleus project directly to the nucleus of Edinger-Westphal. Proc Natl Acad Sci USA. 1982;79:3891–3895. doi: 10.1073/pnas.79.12.3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gamlin PD, Reiner A, Erichsen JT, Karten HJ, Cohen DH. The neural substrate for the pupillary light reflex in the pigeon (Columba livia). J Comp Neurol. 1984;226:523–543. doi: 10.1002/cne.902260407. [DOI] [PubMed] [Google Scholar]
- 25.Gottlieb MD, Fugate-Wentzek LA, Wallman J. Different visual deprivations produce different ametropias and different eye shapes. Invest Ophthalmol Vis Sci. 1987;28:1225–1235. [PubMed] [Google Scholar]
- 26.Halvorsen SW, Berg DK. Specific down-regulation of the α-bungarotoxin binding component on chick autonomic neurons by ciliary neuronotrophic factor. J Neurosci. 1989;9:3673–3680. doi: 10.1523/JNEUROSCI.09-10-03673.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halvorsen SW, Schmid HA, McEachern AE, Berg DK. Regulation of acetylcholine receptors on chick ciliary ganglion neurons by components from the synaptic target tissue. J Neurosci. 1991;11:2177–2186. doi: 10.1523/JNEUROSCI.11-07-02177.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–82. [PubMed] [Google Scholar]
- 29.Higuchi R, Krummel B, Saiki RK. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 1988;16:7351–7367. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horch HL, Sargent PB. Perisynaptic surface distribution of multiple classes of nicotinic acetylcholine receptors on neurons in the chicken ciliary ganglion. J Neurosci. 1995;15:7778–7795. doi: 10.1523/JNEUROSCI.15-12-07778.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ikonomov OC, Kulesa MC, Shisheva AC, Jacob MH. Innervation and target tissue interactions induce Rab-GDP dissociation inhibitor (GDI) expression during peripheral synapse formation in developing chick ciliary ganglion neurons in situ. J Neurosci. 1998;18:6331–6339. doi: 10.1523/JNEUROSCI.18-16-06331.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jackson PC. Reduced activity during development delays the normal rearrangement of synapses in the rabbit ciliary ganglion. J Physiol (Lond) 1983;345:319–327. doi: 10.1113/jphysiol.1983.sp014980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacob MH. Acetylcholine receptor expression in developing chick ciliary ganglion neurons. J Neurosci. 1991;11:1701–1712. doi: 10.1523/JNEUROSCI.11-06-01701.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacob MH, Berg DK. The ultrastructural localization of α-bungarotoxin binding sites in relation to synapses on chick ciliary ganglion neurons. J Neurosci. 1983;3:260–271. doi: 10.1523/JNEUROSCI.03-02-00260.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jacob MH, Berg DK. Effects of preganglionic denervation and postganglionic axotomy on acetylcholine receptors in the chick ciliary ganglion. J Cell Biol. 1987;105:1847–1854. doi: 10.1083/jcb.105.4.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacob MH, Lindstrom JM, Berg DK. Surface and intracellular distribution of a putative neuronal nicotinic acetylcholine receptor. J Cell Biol. 1986;103:205–214. doi: 10.1083/jcb.103.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kilman V, van Rossum MC, Turrigiano GG. Activity deprivation reduces miniature IPSC amplitude by decreasing the number of postsynaptic GABAA receptors clustered at neocortical synapses. J Neurosci. 2002;22:1328–1337. doi: 10.1523/JNEUROSCI.22-04-01328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Landmesser L, Pilar G. The onset and development of transmission in the chick ciliary ganglion. J Physiol (Lond) 1972;222:691–713. doi: 10.1113/jphysiol.1972.sp009822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Landmesser L, Pilar G. Synaptic transmission and cell death during normal ganglionic development. J Physiol (Lond) 1974;241:737–749. doi: 10.1113/jphysiol.1974.sp010681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee MK, Tuttle JB, Rebhun LI, Cleveland DW, Frankfurter A. The expression and posttranslational modification of a neuron-specific beta-tubulin isotype during chick embryogenesis. Cell Motil Cytoskeleton. 1990;17:118–132. doi: 10.1002/cm.970170207. [DOI] [PubMed] [Google Scholar]
- 41.Levey MS, Jacob MH. Changes in the regulatory effects of cell–cell interactions on neuronal AChR subunit transcript levels after synapse formation. J Neurosci. 1996;16:6878–6885. doi: 10.1523/JNEUROSCI.16-21-06878.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levey MS, Brumwell CL, Dryer SE, Jacob MH. Innervation and target tissue interactions differentially regulate acetylcholine receptor subunit mRNA levels in developing neurons in situ. Neuron. 1995;14:153–162. doi: 10.1016/0896-6273(95)90249-x. [DOI] [PubMed] [Google Scholar]
- 43.Liu Y, Ford B, Mann MA, Fischbach GD. Neuregulins increase α7 nicotinic acetylcholine receptors and enhance excitatory synaptic transmission in GABAergic interneurons of the hippocampus. J Neurosci. 2001;21:5660–5669. doi: 10.1523/JNEUROSCI.21-15-05660.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loeb JA, Hmadcha A, Fischbach GD, Land SJ, Zakarian VL. Neuregulin expression at neuromuscular synapses is modulated by synaptic activity and neurotrophic factors. J Neurosci. 2002;22:2206–2214. doi: 10.1523/JNEUROSCI.22-06-02206.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Loring RH, Zigmond RE. Ultrastructural distribution of 125I-toxin F binding sites on chick ciliary neurons: synaptic localization of a toxin that blocks ganglionic nicotinic receptors. J Neurosci. 1987;7:2153–2162. doi: 10.1523/JNEUROSCI.07-07-02153.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Loring RH, Dahm LM, Zigmond RE. Localization of alpha-bungarotoxin binding sites in the ciliary ganglion of the embryonic chick: an autoradiographic study at the light and electron microscopic level. Neuroscience. 1985;14:645–660. doi: 10.1016/0306-4522(85)90316-1. [DOI] [PubMed] [Google Scholar]
- 47.Maderdrut JL, Oppenheim RW, Prevette D. Enhancement of naturally occurring cell death in the sympathetic and parasympathetic ganglia of the chicken embryo following blockade of ganglionic transmission. Brain Res. 1988;444:189–194. doi: 10.1016/0006-8993(88)90928-6. [DOI] [PubMed] [Google Scholar]
- 48.Margiotta JF, Gurantz D. Changes in the number, function, and regulation of nicotinic acetylcholine receptors during neuronal development. Dev Biol. 1989;135:326–339. doi: 10.1016/0012-1606(89)90183-8. [DOI] [PubMed] [Google Scholar]
- 49.Meriney SD, Pilar G. Cholinergic innervation of the smooth muscle cells in the choroid coat of the chick eye and its development. J Neurosci. 1987;7:3827–3839. doi: 10.1523/JNEUROSCI.07-12-03827.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meriney SD, Pilar G, Ogawa M, Nunez R. Differential neuronal survival in the avian ciliary ganglion after chronic acetylcholine receptor blockade. J Neurosci. 1987;7:3840–3849. doi: 10.1523/JNEUROSCI.07-12-03840.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nishi R. Target-derived molecules that influence the development of neurons in the avian ciliary ganglion. J Neurobiol. 1994;25:612–619. doi: 10.1002/neu.480250604. [DOI] [PubMed] [Google Scholar]
- 52.Pendrak K, Lin T, Stone RA. Ciliary ganglion choline acetyltransferase activity in avian macrophthalmos. Exp Eye Res. 1995;60:237–243. doi: 10.1016/s0014-4835(05)80106-x. [DOI] [PubMed] [Google Scholar]
- 53.Philpot BD, Sekhar AK, Shouval HZ, Bear MF. Visual experience and deprivation bidirectionally modify the composition and function of NMDA receptors in visual cortex. Neuron. 2001;29:157–169. doi: 10.1016/s0896-6273(01)00187-8. [DOI] [PubMed] [Google Scholar]
- 54.Pilar G, Nunez R, McLennan IS, Meriney SD. Muscarinic and nicotinic synaptic activation of the developing chicken iris. J Neurosci. 1987;7:3813–3826. doi: 10.1523/JNEUROSCI.07-12-03813.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pugh PC, Berg DK. Neuronal acetylcholine receptors that bind α-bungarotoxin mediate neurite retraction in a calcium-dependent manner. J Neurosci. 1994;14:889–896. doi: 10.1523/JNEUROSCI.14-02-00889.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pugh PC, Margiotta JF. Nicotinic acetylcholine receptor agonists promote survival and reduce apoptosis of chick ciliary ganglion neurons. Mol Cell Neurosci. 2000;15:113–122. doi: 10.1006/mcne.1999.0810. [DOI] [PubMed] [Google Scholar]
- 57.Ramirez-Latorre J, Yu CR, Qu X, Perin F, Karlin A, Role L. Functional contributions of alpha5 subunit to neuronal acetylcholine receptor channels. Nature. 1996;380:347–351. doi: 10.1038/380347a0. [DOI] [PubMed] [Google Scholar]
- 58.Rangwala F, Drisdel RC, Rakhilin S, Ko E, Atluri P, Harkins AB, Fox AP, Salman SS, Green WN. Neuronal alpha-bungarotoxin receptors differ structurally from other nicotinic acetylcholine receptors. J Neurosci. 1997;17:8201–8212. doi: 10.1523/JNEUROSCI.17-21-08201.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sandrock AW, Jr, Goodearl AD, Yin QW, Chang D, Fischbach GD. ARIA is concentrated in nerve terminals at neuromuscular junctions and at other synapses. J Neurosci. 1995;15:6124–6136. doi: 10.1523/JNEUROSCI.15-09-06124.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shih YF, Fitzgerald ME, Norton TT, Gamlin PD, Hodos W, Reiner A. Reduction in choroidal blood flow occurs in chicks wearing goggles that induce eye growth toward myopia. Curr Eye Res. 1993;12:219–227. doi: 10.3109/02713689308999467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shoop RD, Martone ME, Yamada N, Ellisman MH, Berg DK. Neuronal acetylcholine receptors with α-7 subunits are concentrated on somatic spines for synaptic signaling in embryonic chick ciliary ganglia. J Neurosci. 1999;19:692–704. doi: 10.1523/JNEUROSCI.19-02-00692.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith MA, Stollberg J, Lindstrom JM, Berg DK. Characterization of a component in chick ciliary ganglia that cross-reacts with monoclonal antibodies to muscle and electric organ acetylcholine receptor. J Neurosci. 1985;5:2726–2731. doi: 10.1523/JNEUROSCI.05-10-02726.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sullivan KF, Havercroft JC, Machlin PS, Cleveland DW. Sequence and expression of the chicken beta 5- and beta 4-tubulin genes define a pair of divergent beta-tubulins with complementary patterns of expression. Mol Cell Biol. 1986;6:4409–4418. doi: 10.1128/mcb.6.12.4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ullian EM, McIntosh JM, Sargent PB. Rapid synaptic transmission in the avian ciliary ganglion is mediated by two distinct classes of nicotinic receptors. J Neurosci. 1997;17:7210–7219. doi: 10.1523/JNEUROSCI.17-19-07210.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vernallis AB, Conroy WG, Berg DK. Neurons assemble acetylcholine receptors with as many as three kinds of subunits while maintaining subunit segregation among receptor subtypes. Neuron. 1993;10:451–464. doi: 10.1016/0896-6273(93)90333-m. [DOI] [PubMed] [Google Scholar]
- 66.Wallman J, Ledoux C, Friedman MB. Simple devices for restricting the visual fields of birds. Behav Res Methods Instrum. 1978;10:401–403. [Google Scholar]
- 67.Wang F, Gerzanich V, Wells GB, Anand R, Peng X, Keyser K, Lindstrom J. Assembly of human neuronal nicotinic receptor alpha5 subunits with alpha3, beta2, and beta4 subunits. J Biol Chem. 1996;271:17656–17665. doi: 10.1074/jbc.271.30.17656. [DOI] [PubMed] [Google Scholar]
- 68.Williams BM, Temburni MK, Levey MS, Bertrand S, Bertrand D, Jacob MH. The long internal loop of the α3 subunit targets nAChRs to subdomains within individual synapses in vivo. Nat Neurosci. 1998;1:557–562. doi: 10.1038/2792. [DOI] [PubMed] [Google Scholar]
- 69.Xu W, Gelber S, Orr-Urtreger A, Armstrong D, Lewis RA, Ou C, Patrick J, Role L, de Biasi M, Beaudet AM. Megacystis, mydriasis and ion channel deficits in mice lacking the α3 neuronal nicotinic acetyl choline receptor. Proc Natl Acad Sci USA. 1999a;96:5746–5751. doi: 10.1073/pnas.96.10.5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xu W, Orr-Urtreger A, Nigro F, Gelber S, Sutcliffe CB, Armstrong D, Patrick JW, Role LW, Beaudet AL, De Biasi M. Multiorgan autonomic dysfunction in mice lacking the β2 and the β4 subunits of neuronal nicotinic acetylcholine receptors. J Neurosci. 1999b;19:9298–9305. doi: 10.1523/JNEUROSCI.19-21-09298.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang X, Kuo Y, Devay P, Yu C, Role L. A cysteine-rich isoform of neuregulin controls the level of expression of neuronal nicotinic receptor channels during synaptogenesis. Neuron. 1998;20:255–270. doi: 10.1016/s0896-6273(00)80454-7. [DOI] [PubMed] [Google Scholar]
- 72.Zhang ZW, Coggan JS, Berg DK. Synaptic currents generated by neuronal acetylcholine receptors sensitive to alpha-bungarotoxin. Neuron. 1996;17:1231–1240. doi: 10.1016/s0896-6273(00)80253-6. [DOI] [PubMed] [Google Scholar]
- 73.Zhou Y, Deneris E, Zigmond RE. Differential regulation of levels of nicotinic receptor subunit transcripts in adult sympathetic neurons after axotomy. J Neurobiol. 1998;34:164–178. doi: 10.1002/(sici)1097-4695(19980205)34:2<164::aid-neu6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 74.Zhou Y, Deneris E, Zigmond RE. Nicotinic acetylcholine receptor subunit proteins alpha7 and beta4 decrease in the superior cervical ganglion after axotomy. J Neurobiol. 2001;46:178–192. doi: 10.1002/1097-4695(20010215)46:3<178::aid-neu1001>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]