Abstract
NMDA receptor currents desensitize in an agonist-dependent manner when either the glutamate or glycine agonist is subsaturating. This may result from a conformational change in the NMDA receptor protein that lowers glutamate and glycine binding site affinity induced by co-agonist binding, channel opening, or ion permeation. We have used whole-cell voltage clamp of cultured hippocampal neurons with agonist paired-pulse protocols to demonstrate that glutamate and glycine dissociate 7.9- and 6.8-fold slower in the absence of their respective co-agonists than when their co-agonists are present. Paired-pulse and desensitization protocols were used to show that co-agonist binding and channel opening are sufficient to cause a reduction in glycine affinity, but extracellular sodium or magnesium binding was required in addition to conformational changes leading to channel opening to reduce glutamate binding-site affinity. Use of cesium or potassium as the major extracellular cation prevented the reduction of glutamate affinity. In addition, the use of choline-, sodium-, or cesium-based intracellular solutions did not alter desensitization characteristics, indicating that the site responsible for reduction of glutamate affinity is not in the intracellular domain. The fact that the reduction of glutamate affinity is dependent on certain small extracellular cations whereas the reduction of glycine affinity is insensitive to such cations indicates that conformational changes induced by the binding of glutamate are not completely paralleled by the conformational changes induced by glycine. Although glutamate and glycine are essential co-agonists, these data suggest that they have differential roles in the process of NMDA receptor activation.
Keywords: glutamate, glycine, NMDA receptor, ion permeation, desensitization, channel gating, channel activation
Activation of NMDA receptors can cause both membrane depolarization and calcium influx into the postsynaptic neuron. Such a response can initiate changes in cellular physiology that may include induction of intracellular cascades, gene activation, and possible changes in synaptic strength (for review, seeOzawa et al., 1998; Dingledine et al., 1999). Among ligand-gated ion channels, NMDA receptors have a unique requirement for the binding of two different types of agonist, glutamate and glycine, for channel activation (Kleckner and Dingledine, 1988). Glutamate and glycine agonist binding sites have separate locations in the multimeric protein complex. Dose–response analysis of mutant NMDA channels suggests that the glycine binding site is located within the NR1 subunit (Hirai et al., 1996), and the glutamate binding site is located within the NR2 subunit (Laube et al., 1997; Anson et al., 1998).
In whole-cell voltage-clamp experiments, sustained glutamate and glycine agonist applications of constant concentration can result in a decay or “desensitization” of the receptor response. One type of NMDA receptor desensitization can be observed at saturating glutamate and glycine agonist concentrations and may be sensitive (Legendre et al., 1993; Medina et al., 1995; Krupp et al., 1996, 1999) or insensitive (Sather et al., 1990; Tong and Jahr, 1994) to the extracellular calcium concentration. A second type of NMDA receptor desensitization can be observed when NMDA receptors are activated by a nearly saturating pulse of glutamate agonist, NMDA, in the continual presence of a subsaturating background concentration of the co-agonist, glycine (Benveniste et al., 1990a; Lerma et al., 1990; Vyklicky et al., 1990). A third type of desensitization is also observed when channels are activated by a saturating pulse of glycine agonist,l-alanine, in the continual presence of a subsaturating background concentration of the co-agonist, glutamate (Nahum-Levy et al., 2001). For these latter two types of desensitization, the extent of desensitization is reduced as the concentration of the continually present “background” agonist increases. The extent of desensitization does not change once concentrations of the background agonist saturate. These results suggest that agonist binding affinities may be weakened as NMDA receptor channels are activated (Benveniste et al., 1990a; Vyklicky et al., 1990; Nahum-Levy et al., 2001).
High activity of glutamate and glycine transporters near the synaptic cleft of many excitatory synapses may reduce glutamate and glycine concentrations to subsaturating levels (Bergles et al., 1999;Gadea and Lopez-Colome, 2001), suggesting that affinity changes at the glutamate and glycine binding sites may play a role in NMDA receptor synaptic and extra-synaptic signaling. A weakening of agonist affinity implies that both high- and low-affinity binding states exist for both glutamate and glycine agonists and that the transitional trigger for the reduction in agonist affinity could depend on co-agonist binding, conformational changes that cause channel opening and ion binding, and permeation through the open NMDA channel. In this paper, we show that high-affinity binding states exist for glutamate and glycine when channels are not in an activated state and further that small cations influence glutamate but not glycine affinity.
MATERIALS AND METHODS
Dissociated neuronal cultures. Sprague Dawley postnatal day 1 rat pups were decapitated, and 14 hippocampal hemispheres were removed. The tissue was digested with papain (100 U; Sigma, St. Louis, MO) for 20 min, triturated to a single-cell suspension, and plated at a density of 150,000 cells per milliliter on a substrate of bovine collagen type IV and 100 μg/ml poly-l-lysine in 35 mm dishes. The culture medium consisted of Modified Eagle's Medium containing 5% horse serum (Biological Industries, Beit HaEmek, Israel), B-27 neuronal supplement (Invitrogen, Carlsbad, CA), 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mm glutamine.d-Glucose was supplemented to a final concentration of 6 gm/l. Cytosine-1-β-d-arabinofuranoside (5 μm) was added after ∼5 d to arrest glial cell division. All cultures were maintained at 36°C in humidified air containing 5% CO2.
Electrophysiology and agonist application. Whole-cell voltage-clamp experiments were conducted using an Axopatch 200A amplifier (Axon Instruments, Union City, CA) at room temperature between 1 and 2 weeks after neurons were plated. The holding potential was −60 mV unless indicated otherwise. Data were acquired with a Macintosh PPC 7600 computer equipped with an ITC-16 analog-to-digital converter (Instrutech Corp., Port Washington, NY) using a free Macintosh-based electrophysiological software package (Synapse, Synergistic Systems, Bromma, Sweden).
The standard extracellular control solution consisted of 160 mm NaCl, 2.5 mm KCl, 0.2 mmCaCl2, 10 mm glucose, 10 mm HEPES, 400 nm tetrodotoxin, 5 μm bicuculline methochloride, and 10 μg/ml phenol red and was adjusted to pH 7.3 and 325 mOsm. In some cases, 160 mm choline chloride, CsCl, or KCl was substituted for NaCl. Unless indicated otherwise, the standard intracellular solution consisted of 125 mm CsMeSO3, 15 mm CsCl, 0.5 mm CaCl2, 3 mm MgCl2, 5 mmCs4BAPTA, and 2 mmNa2ATP and was adjusted to pH 7.2 and 305 mOsm. In some cases, 140 mm NaCl replaced CsMeSO3 and CsCl, and BAPTA-free acid was used instead of its cesium salt and titrated with NaOH. In other cases, 140 mm choline chloride replaced CsMeSO3and CsCl, and BAPTA-free acid was used instead of its cesium salt and titrated with Trizma base; and Tris-ATP was used instead of its sodium salt.
Experimental solutions were perfused continuously over the whole neuron from a flow pipe consisting of an array of eight glass barrels (≈400 μm outer diameter) that was positioned ∼100 μm away from the neuronal soma as described previously (Nahum-Levy et al., 1999). The time constant for exchange between perfused solutions was measured as described previously (Vyklicky et al., 1990; Nahum-Levy et al., 2001) and is ∼10 msec.
Four different agonists were used in this study. In general for desensitization experiments, the background agonist present before NMDA receptor-channel activation had a relatively high affinity such that desensitization kinetics could be observed at subsaturating concentrations [for more details, see Nahum-Levy et al. (2001)]. Low-affinity agonists were used as the pulsing agonist because they can be applied at high concentration quickly, and NMDA receptor responses diminished rapidly on their removal. Glutamate, which has a steady-state EC50 of 1.7 μm and is saturating at 10 μm (Nahum-Levy et al., 2001), was the high-affinity glutamate agonist; NMDA, which has a steady-state EC50 of 36 μm and is saturating at 300 μm (Benveniste et al., 1990b), was the low-affinity glutamate agonist; glycine, which has a steady-state EC50 of 0.4 μm and is saturating at 10 μm (Vyklicky et al., 1990), was the high-affinity glycine agonist; and l-alanine, which has a steady-state EC50 of 35 μm and is saturating above 300 μm (Benveniste et al., 1990b), was the low-affinity glycine agonist. For agonist paired-pulse experiments, both the pulsing agonist and the agonist whose dissociation kinetics was being measured were applied at saturating concentrations to insure a high signal-to-noise ratio.
Two different treatments were used to reduce the level of NMDA receptor activation resulting from the application of glutamate agonists and low concentrations of endogenous glycine agonists present in our hippocampal cultures. For experiments in which 1 mml-alanine was the pulsing agonist, solutions lackingl-alanine contained 10 mm5-methyl-indole-2-carboxylic acid (5MeI2CA), a low-affinity glycine site competitive antagonist (Huettner, 1989). 5MeI2CA dissociates within the time for solution exchange around the neuron (Nahum-Levy et al., 2001). For some paired-pulse experiments in which glycine dissociation was evaluated, 0.5 μm 5,7-dichloro-kynurenic acid (5,7-diClKyn), a high-affinity glycine site competitive antagonist (McNamara et al., 1990), was included in all solutions to lower responses caused by endogenous glycine. In addition, during exogenous glycine application, the glycine concentration was raised to 100 μm to ensure saturation of glycine binding sites with glycine in the presence of 5,7-diClKyn.
RESULTS
Glutamate and glycine bind with high affinity in the absence of co-agonist
Previously, it has been estimated from analysis of desensitization experiments that a three- to eightfold difference exists between low- and high-affinity agonist binding states (Vyklicky et al., 1990;Nahum-Levy et al., 2001). The desensitization observed could result from (1) entrance of the NMDA receptor channel into a long-lived nonconducting state once agonist binds or (2) reduction of the binding affinity of the subsaturating background agonist once channels become activated such that desensitization reflects the re-equilibration of that agonist with a lower affinity state (Nahum-Levy et al., 2001). This second source of desensitization predicts that a high-affinity agonist binding state exists if only one type of agonist is bound and the channel is not activated.
Testing for changes in apparent agonist affinity in the absence of the respective co-agonist cannot be measured at equilibrium, because electrophysiological experiments cannot directly measure channel states other than those that allow ion conduction, and no NMDA receptor-mediated ion conduction can occur in the absence of either the glutamate or the glycine co-agonist (Kleckner and Dingledine, 1988). Yet, if solution switching is rapid in comparison to agonist dissociation kinetics, then paired-pulse experiments can be used to probe dissociation of one agonist in the absence of its co-agonist by pulsing with that co-agonist at various delay intervals after the agonist is removed.
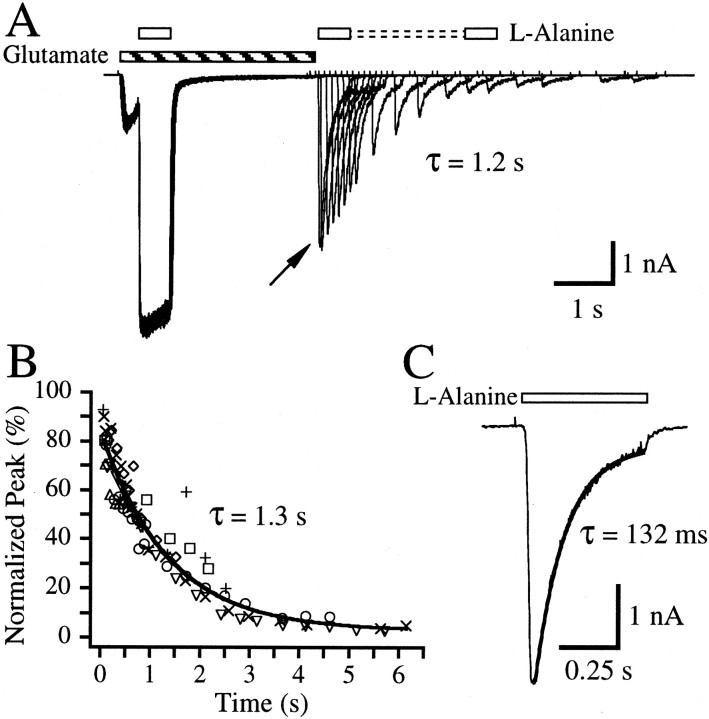
To determine whether a high-affinity glutamate binding state exists in the absence of a glycine co-agonist, a paired-pulse protocol was used as shown in Figure 1A. A control response was elicited by application of 10 μm glutamate and 1 mml-alanine. l-alanine was then removed, and after a 2.5 sec re-equilibration period, glutamate was removed. After a delay period of ∼25 msec, a test pulse ofl-alanine (in the absence of exogenously applied glutamate) revealed the population of channels still bound with glutamate. The experiment was then repeated at various delay periods (Fig. 1A). Analysis of the peak amplitudes resulting from the l-alanine test pulses yielded a single exponential decay time constant of 1.3 ± 0.1 sec (n = 7 cells), which reflects the apparent dissociation of glutamate in the absence of a glycine co-agonist (Fig.1B, Table 1). Current decay during the l-alanine test pulse should reflect the apparent glutamate dissociation rate when both types of agonist are bound (Fig. 1C). The exponential current decay time constant measured during the l-alanine test pulse of the first paired-pulse record was significantly faster than the time constant measured from the peak test pulse amplitudes in Figure 1B (Table 1). The average current decay time constant measured for the test l-alanine pulse of the first paired-pulse record was τ = 171.3 ± 38.1 msec (n = 13 cells). For comparison, apparent glutamate dissociation time constants were also measured by pulsing 10 μm glutamate in the continual presence of 1 mml-alanine. Decays of the glutamate-elicited current could only be measured with the sum of two exponentials (Table 1). The weighted average of these exponentials, τw = 221.5 ± 14.0 msec, approximates the τ measured from the test l-alanine pulse. The slower apparent dissociation of glutamate analyzed from the paired-pulse protocol suggests that glutamate is bound with higher affinity in the absence of the glycine co-agonist than whenl-alanine is present.
Fig. 1.
Glutamate dissociates slowly in the absence of a glycine co-agonist. A, Overlay of 18 paired-pulse records from a single hippocampal neuron. Inward currents were elicited when 10 μm glutamate (striped bar) was applied simultaneously with a 600 msec control pulse of 1 mml-alanine (open bars). A re-equilibration period of 2.5 sec followed the l-alanine control pulse after which the exogenous glutamate was removed. To reduce activation of NMDA receptors by glutamate and endogenous glycine, 10 mm 5MeI2CA was included in all solutions lacking l-alanine. An l-alanine test pulse then followed at various delay times after the removal of the glutamate. The current observed reflects receptors bound with l-alanine that still have glutamate bound. The τ value represents exponential analysis of the peak current amplitudes elicited by the test pulses with respect to time after glutamate removal for this cell.B, Plot of peak amplitudes of the l-alanine test pulse normalized to the amplitude measured near the end of thel-alanine control pulse plotted against the delay time between exogenous glutamate removal and the peak of the test pulse for each record. Peak measurements are indicative of the rate of glutamate dissociation in the absence of l-alanine. Eachsymbol type represents the data from a different cell. τ from the fit (line) of the data with a single exponential is noted on the graph. C, Enlargement of the current elicited during the l-alanine test pulse (open bar) from the first paired-pulse record shown inA (arrow), where the delay time between exogenous glutamate removal and the second l-alanine application was ∼25 msec. The τ value represents the single exponential decay time constant for this trace. Average fitted parameters are listed in Table 1.
Table 1.
Exponential decay times resulting from the removal of exogenous glutamate under various conditions
| Condition | Figure | τ1 (msec) | τ2(msec) | A1 (%) | A2(%) | τW (msec) | n cells |
|---|---|---|---|---|---|---|---|
| Paired-pulse protocol pulse withl-alanine | 1B | 1349.5 ± 131.5 | 7 | ||||
| Glutamate pulse in presence of l-alanine | NS | 106.6 ± 6.1 | 370.1 ± 11.0 | 60.3 ± 9.1 | 39.7 ± 9.1 | 221.5 | 5 |
| Decay during l-alanine test pulse in paired-pulse protocol | 1C | 171.3 ± 38.1 | 13 | ||||
| Paired-pulse protocol pulse with Na in presence of l-alanine | 3B | 61.3 ± 52.8 | 624.2 ± 147.2 | 39.3 ± 26.6 | 52.2 ± 2.4 | 382.5 | 7 |
| Paired-pulse protocol, 2 mm Mg present voltage jumps in presence ofl-alanine | 6B | 39.4 ± 12.1 | 631.4 ± 107.1 | 56.9 ± 18.1 | 32.3 ± 1.1 | 253.7 | 4 |
NS, Data not shown in figures.
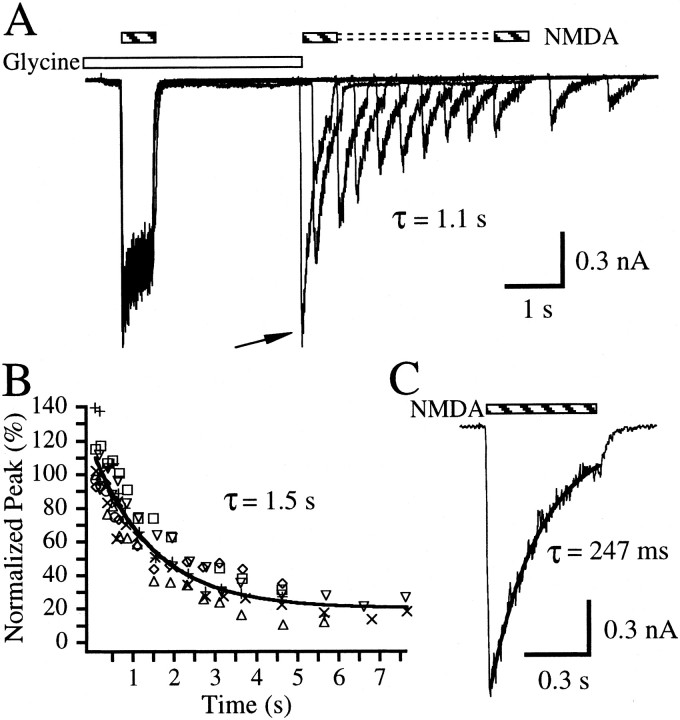
To determine whether a high-affinity glycine binding state exists in the absence of a glutamate co-agonist, the converse experiment was conducted (Fig. 2A). NMDA (300 μm), a low-affinity glutamate binding site agonist, was applied for 600 msec in the presence of 10 μm glycine. Exogenous glycine was removed after a re-equilibration period of 2.5 sec, and an NMDA test pulse was applied at various times after the removal of glycine. Analysis of the peak responses versus time after exogenous glycine removal yielded an exponential time constant of 0.9 ± 0.2 sec (n = 11 cells; data not shown). The peaks of the test pulses decayed to an offset of 46.1 ± 3.3%, indicating that peak responses to NMDA in the presence of endogenous glycine were significant. To lower the response resulting from the presence of endogenous glycine, 0.5 μm 5,7-diClKyn was added to all solutions. Paired-pulse experiments conducted in the presence of 5,7-diClKyn (Fig.2A) lowered the offset to 20.2 ± 3.3% and slowed the time constant measured from the plot of test pulse peak amplitudes (Fig. 2B) to 1.5 ± 0.2 sec (n = 7 cells,). The average decay time constant of current during the test pulse of NMDA in the paired-pulse protocol should reflect apparent dissociation of glycine in the presence of NMDA (Fig. 2C). This value was 219.7 ± 60.2 msec (n = 8 cells) and approximates earlier measurements of glycine apparent dissociation kinetics (Benveniste et al., 1990b;Priestley and Kemp, 1993, 1994). The current decay time constant during the NMDA test pulse of the first paired-pulse record (Fig.2C) is 6.8-fold faster than the exponential time constant measured from the plot of the peak amplitudes of the test pulses (Fig.2B). These results may indicate that glycine dissociates more rapidly in the presence of NMDA than when the glutamate co-agonist is absent and may suggest that glycine binds with higher affinity to its binding site in the absence of a glutamate co-agonist when compared with when NMDA is present.
Fig. 2.
Glycine dissociates slowly in the absence of a glutamate co-agonist. A, Overlay of 12 paired-pulse records from a single hippocampal neuron. Inward currents were elicited when 100 μm glycine (open bar) was applied simultaneously with a 600 msec control pulse of 300 μmNMDA (striped bar). 5,7DiClKyn (0.5 μm) was present continuously throughout the experiment to limit activation by NMDA with endogenous glycine. A re-equilibration period of 2.5 sec followed the NMDA control pulse, after which the exogenous glycine was removed. An NMDA test pulse then followed at various delay times after the removal of the glutamate. The current observed reflects receptors bound with NMDA that still have glycine bound. The τ value represents exponential analysis of the peak current amplitudes elicited by the test pulses with respect to time after glycine removal for this cell.B, Plot of peak amplitudes of the NMDA test pulse normalized to the amplitude measured near the end of the NMDA control pulse plotted against the delay time between exogenous glycine removal and the peak of the test pulse for each record. Peak measurements are indicative of the rate of glycine dissociation in the absence of NMDA. Each symbol type represents the data from a different cell. Line indicates a fit to a single exponential + offset with a measured time constant, τ, indicated on thegraph. C, Enlargement of current elicited during the NMDA test pulse (striped bar) from the first paired-pulse record shown in A (arrow), where the delay time between exogenous glycine removal and the NMDA test pulse was ∼25 msec. The measured single exponential time constant for this trace (τ) is noted on thegraph.
Permeant ions may reduce the affinity for glutamate but not for glycine
Figures 1 and 2 suggest that agonist binding is in an apparent high-affinity state in the absence of co-agonist when the channel is in a nonactivated state, and agonist binding is in an apparent low-affinity state when the co-agonist is bound and the channel is activated. We measure receptor activation by current flow through open channels. This depends on (1) glutamate and glycine agonist binding, (2) conformational changes in the protein allowing channel opening once the agonists are bound, and (3) ion permeation through the open channel. Each of these three events could trigger a change in agonist binding from a high- to a low-affinity state.
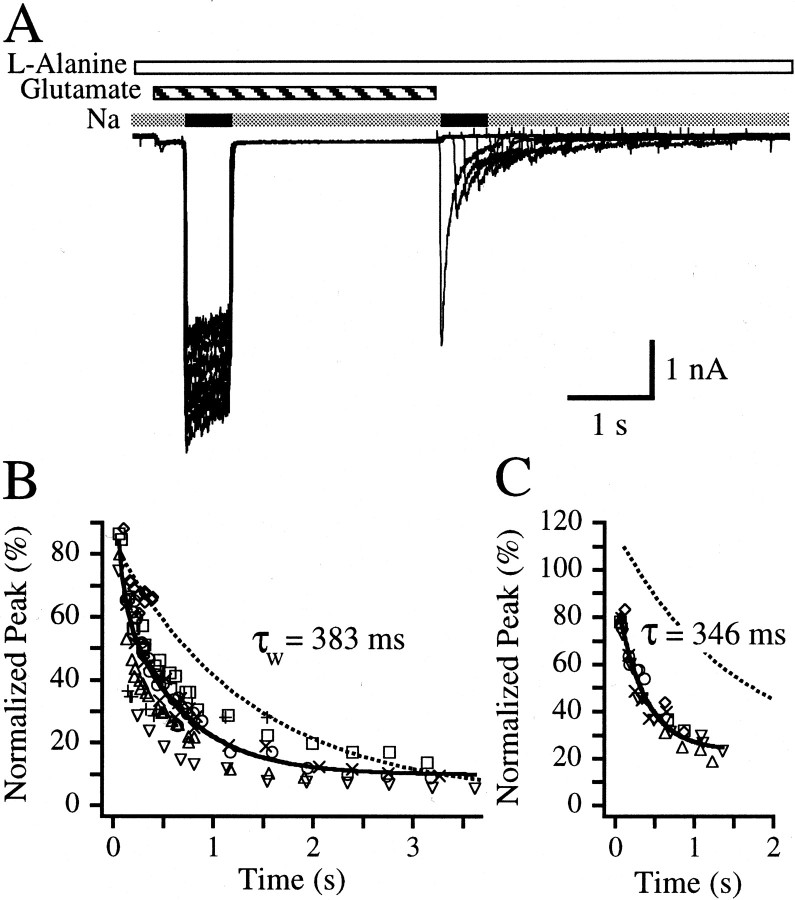
To examine whether ion permeation through open NMDA channels leads to a transition in glutamate apparent affinity from a high- to low-affinity state, paired-pulse experiments were conducted in which neurons were bathed with 10 μm glutamate and 1 mml-alanine in the presence of nonpermeating 160 mm choline chloride. The control pulse was initiated by switching the choline chloride to NaCl for 600 msec after which choline again replaced sodium as the main extracellular cation. After a 2.5 sec re-equilibration time in the presence of glutamate,l-alanine, and choline chloride, exogenous glutamate was removed while l-alanine remained. Test pulses of NaCl were elicited at various delay times after the removal of glutamate (Fig.3A). A plot of the peak amplitudes of the second sodium pulse as a function of the delay time between glutamate removal and the peak of the second sodium pulse may serve as a measure of glutamate apparent dissociation from the NMDA receptor in the absence of permeant ions but in the presence of co-agonist l-alanine (Fig. 3B). This plot could not be well fit with a single exponential (Fig.3B). Thus, these data were fit with a sum of two exponentials, and the parameters are presented in Table 1. The weighted average for apparent glutamate dissociation in the absence of permeant ions (τw = 382.5 msec) is considerably faster than the time constant measured for glutamate apparent dissociation in the absence of l-alanine (Fig.1B, Table 1). Yet, this weighted average is also 1.7-fold slower than the weighted average measured for glutamate apparent dissociation in the presence of co-agonist,l-alanine (Table 1) and 2.4-fold slower than the single exponential time constant measured for the current decay from the test pulse of the first record of this paired-pulse experiment (159.9 ± 49.6 msec; n = 8 cells). The slower apparent dissociation of glutamate in the presence ofl-alanine and extracellular choline in comparison to when l-alanine is present with extracellular sodium suggests that glutamate may bind with a higher apparent affinity when permeant ions are absent in comparison to when they are present.
Fig. 3.
Glutamate but not glycine dissociation is slowed in the absence of ion permeation through NMDA channels.A, Overlay of 12 paired-pulse records from a single hippocampal neuron. Inward currents were elicited when a sodium-based extracellular solution (black bars) replaced a choline-based extracellular solution (gray bars) in the presence of 10 μm glutamate (striped bar). l-alanine (1 mm) (open bar) was present continuously throughout the experiment. The initial sodium-based control pulse was 600 msec followed by a re-equilibration period of 2.5 sec in choline-based extracellular solution after which the exogenous glutamate was removed. A test pulse of sodium-based extracellular solution then followed at various delay times after the removal of the glutamate. The current observed reflects receptors bound with l-alanine that still have glutamate bound. B, Plot of peak current amplitudes elicited from the sodium-based extracellular solution test pulse normalized to the amplitude measured near the end of the sodium-based extracellular solution control pulse plotted against the delay time between exogenous glutamate removal and the peak of the test pulse for each record. Peak measurements are indicative of the rate of glutamate dissociation in the presence of l-alanine but in the absence of permeant ions. Each symbol type represents the data from a different cell. The continuous line indicates a fit to a sum of two exponentials with the weighted average time constant (τw) noted on the graph. The fit obtained from Figure 1B is shown for comparison (dotted line). The fitted parameters are listed in Table 1.C, Summary of results from the converse experiment testing the rate of glycine dissociation in the absence of permeant ions. NMDA (300 μm) was present continuously. In the presence of 10 μm glycine, a sodium-based extracellular solution replaced a choline-based extracellular solution for 600 msec, creating a control pulse of inward current. After return to the choline-based extracellular solution, the system was allowed to re-equilibrate for 2.5 sec, after which exogenous glycine was removed. This was followed by a test pulse of sodium-based extracellular solution at various delay times after glycine removal. Note that 5,7DiClKyn was not used in this experiment. The figure represents the plot of peak amplitudes of the sodium-based extracellular solution test pulse normalized to the amplitude measured near the end of the sodium-based extracellular solution control pulse plotted against the delay time between exogenous glycine removal and the peak of the test pulse for each record. Peak measurements are indicative of the rate of glycine dissociation in the presence of NMDA but in the absence of permeant ions. Each symbol type represents the data from a different cell. The continuous line indicates a fit to one exponential + offset with the time constant, τ, listed on thegraph. The fit obtained from Figure 2B is shown for comparison (dotted line).
Our extracellular solutions routinely contained 0.2 mmcalcium. We have conducted paired-pulse experiments like those depicted in Figure 3A in which calcium was omitted from the extracellular solution and 10 mm EGTA was added. Under these conditions, the decay of peak amplitudes elicited by the test sodium pulse with respect to the time after glutamate removal yielded a single exponential time constant of 561.2 ± 156.2 msec (n = 5 cells). This is consistent with our results obtained under routine conditions (Fig. 3, Table 1), suggesting that 0.2 mm calcium does not cause the transition of the glutamate site to a low-affinity state.
The influence of extracellular choline on glycine apparent dissociation was also examined in an analogous paired-pulse experiment. Neurons were bathed with 300 μm NMDA and 10 μm glycine in the presence of choline chloride. Control currents were elicited by replacing the choline chloride with NaCl for 600 msec. Exogenous glycine was removed 2.5 sec after the end of the 160 mmNaCl control pulse. Test pulses of sodium were then elicited at various delay times after the removal of exogenous glycine. A plot of the peaks of the second sodium pulse as a function of the delay time between glycine removal and the peak of the normalized second sodium pulse serves as a measurement of glycine apparent dissociation from the NMDA receptor in the absence of permeant ions (Fig. 3C). A single exponential decay time constant of 346.1 ± 49.0 msec was measured from six cells. Analysis of the current decay from the test pulse from the first paired-pulse record yielded a τ = 381.7 ± 126.1 msec (n = 7 cells), indicating that there is no difference between glycine apparent dissociation when NMDA is present in choline or sodium-based extracellular solutions.
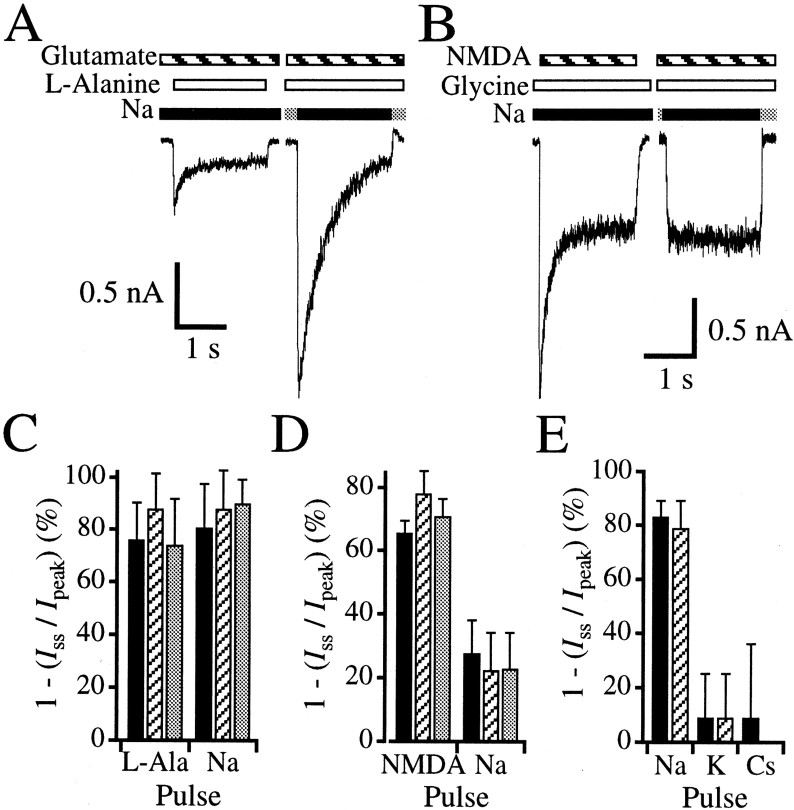
Changes in extracellular sodium concentration can cause changes in electrogenic transporter and exchanger activity that could influence peak current amplitudes in our paired-pulse assay. Potential effects resulting from electrogenic activity could be removed by conducting experiments in the presence and absence of glutamate binding site competitive antagonist, 2-amino-5-phosphonopentanoic acid (AP5). By subtracting the traces acquired in the presence of 200 μmAP5 from those acquired in the absence of AP5, NMDA receptor-mediated currents could be isolated from other electrogenic activity. However, acquisition of agonist dissociation data with paired-pulse protocols in the presence and absence of AP5 required a large number of traces in the same cell. This was technically very difficult. Therefore, we designed a desensitization protocol in which desensitization is modulated by subsaturating concentrations of either glutamate or glycine (Fig. 4). This type of desensitization results from agonist re-equilibration that takes place after a decrease in agonist apparent affinity on channel activation (Nahum-Levy et al., 2001).
Fig. 4.
Ion permeation can cause glutamate- but not glycine-sensitive desensitization. A, Assay for glutamate-sensitive desensitization in a hippocampal neuron. Theleft trace shows current activated by a pulse of 1 mml-alanine (open bars) with a background concentration of 0.1 μm glutamate (striped bars) in a 160 mm sodium-based extracellular solution (black bars). The inward current that was elicited peaks and desensitizes 69.0%. The right trace shows current activated by switching a 160 mmcholine-based extracellular solution (gray bars) for a 160 mm sodium-based solution (black bar) in the continual presence of 1 mml-alanine and 0.1 μm glutamate in the same cell. The peak amplitude of this current is 382% of the peak amplitude of the left trace. The current in the right panel desensitizes 92.4% and reaches a steady-state level similar to that of the left trace. Note that all solutions not containing l-alanine contained 10 mm 5MeI2CA to limit activation of NMDA receptors by glutamate with endogenous glycine. B, Assay for glycine-sensitive desensitization in a hippocampal neuron. Theleft trace shows current activated by a pulse of 1 mm NMDA (striped bars) with a background concentration of 0.1 μm glycine (open bars) in a 160 mm sodium-based extracellular solution (black bars). The inward current that was elicited peaks and desensitizes 66.1%. The right traceshows current activated by switching a 160 mm choline-based extracellular solution (gray bars) for a 160 mm sodium-based solution in the continual presence of 1 mm NMDA and 0.1 μm glycine in the same cell. Although the steady-state levels are similar in both theleft and right traces, no peak is observed by current elicited by permeant ions (right panel). To isolate NMDA receptor-mediated currents from other electrogenic activity, the protocols depicted in Aand B were acquired in the presence of AP5 and subtracted from those acquired in the absence of AP5. C, Summary of the degree of glutamate-sensitive desensitization for three different intracellular solutions in which cesium (black bars), sodium (striped bars), or choline (gray bars) is the major cation. The left three bars summarize results from currents elicited by a 1 mml-alanine test pulse (L-Ala) in the continual presence of 0.1 μm glutamate in a sodium-based extracellular solution (A, left panel). The right three bars summarize results from currents elicited by the switch from a choline-based extracellular to a sodium-based extracellular solution (Na) in the continual presence of 0.1 μmglutamate and 1 mml-alanine (A,right panel). D, Summary of the degree of glycine-sensitive desensitization for three different intracellular solutions in which cesium (black bars), sodium (striped bars), or choline (gray bars) is the major cation. The left three barssummarize results from currents elicited by a 1 mm NMDA test pulse (NMDA), in the continual presence of 0.1 μm glycine in a sodium-based extracellular solution (B, left panel). The right three bars summarize results from currents elicited by the switch from a choline-based extracellular to a sodium-based extracellular solution (Na), in the continual presence of 0.1 μm glycine and 1 mm NMDA (B, right panel).E, Summary of the degree of desensitization elicited by changes of various extracellu- lar solutions containing 0.1 μm glutamate and 1 mml-alanine using a cesium-based intracellular solution. Currents were elicited by a switch from either a choline-based (black bars) or sucrose-based (striped bars) extracellular solution to solutions in which the major extracellular permeant cation was sodium (Na), potassium (K), or cesium (Cs). Note that only the switch to a sodium-based extracellular solution exhibits high degrees of desensitization.
When incubating neurons with a subsaturating background concentration of glutamate (0.1 μm) and pulsing with 1 mml-alanine in an extracellular solution containing sodium as the predominating cation, a peak current is observed (Fig.4A, left panel) that desensitizes 76.1 ± 14.3% (n = 13 cells). This desensitization is similar to that observed previously for subsaturating concentrations of glutamate and is diminished with increasing background concentrations of glutamate (Nahum-Levy et al., 2001). To determine whether the decrease in agonist apparent affinity results from the presence of permeant ions, a similar desensitization protocol was constructed in which the same neuron was simultaneously incubated with 1 mml-alanine and 0.1 μmglutamate in a choline-based extracellular solution. When sodium rapidly replaced choline as the predominating cation, a peak current was observed (Fig. 4A, right panel) that was 363.9 ± 220.7% (n = 10 cells) of the peak current elicited by a 1 mml-alanine pulse in the continual presence of 0.1 μm glutamate in extracellular sodium (Fig.4A, left panel). The current elicited with the sodium pulse desensitized 80.6 ± 16.9% (n = 13 cells) to a steady-state level (Fig.4A, right panel), which was similar to the steady-state current elicited by thel-alanine pulse on the same cell (Fig.4A, left panel). The observation of a peak elicited by the pulse of sodium ions suggests that immediately before this pulse, the glutamate site was in a high-affinity state. Therefore, these results confirm the results from the paired-pulse protocols in Figure 3, A and B, and suggest that the glutamate site switches from a high- to a low-affinity site when extracellular sodium is present.
A similar strategy was used to test whether ion permeation could cause a decrease in glycine binding site apparent affinity resulting in glycine-sensitive desensitization. Figure 4B(left panel) shows that when 1 mm NMDA is pulsed in the presence of a background concentration of subsaturating glycine (0.1 μm) in a sodium-based extracellular solution, a peak current is observed that desensitizes 65.7 ± 3.9% (n = 12 cells) in accordance with previous observations (Benveniste et al., 1990a;Vyklicky et al., 1990; Nahum-Levy et al., 2001). The sensitivity of glycine apparent affinity to permeant ions was tested by continually incubating the same neuron with 1 mm NMDA and 0.1 μm glycine and eliciting current by switching from a choline-based extracellular solution to a sodium-based extracellular solution. In contrast to the results obtained for the glutamate binding site, no initial peak current was observed (Fig.4B, right panel). Instead, the response to the sodium pulse was relatively square with a steady-state current that was similar to the steady-state current observed in the desensitizing trace elicited by the NMDA pulse (Fig.4B). These results complement results obtained from glycine apparent dissociation experiments in Figure 3C, where no significant differences were observed between the time constants of glycine apparent dissociation in the presence of extracellular sodium or choline. Together these results suggest that the transition from the high to low apparent affinity state for glycine binding occurs regardless of the predominant extracellular cation.
Reduction in glutamate affinity by permeant ions is sodium specific
We can use the desensitization protocol with different intracellular and extracellular solutions to determine whether the transition from high to low apparent affinity glutamate or glycine binding states is specific to a particular monovalent cation. In addition, such experiments might indicate whether the cation modulation site is located in the intracellular or extracellular domains. Experiments similar to those done with a cesium-based intracellular solution (Fig. 4A,B), were conducted with sodium-based and choline-based intracellular solutions. Peak responses elicited by the switch from a choline-based to a sodium-based extracellular solution in the presence of a background concentration of 0.1 μm glutamate (Fig.4A, right panel) were 248.0 ± 88.0 and 229.3 ± 71.6% of the peak elicited by anl-alanine pulse (Fig. 4A,left panel) for experiments done with sodium-based or choline-based intracellular solutions, respectively. These responses desensitized to similar degrees regardless of the intracellular solution used (Fig. 4C). The extent of desensitization observed when a background concentration of 0.1 μm glycine was used was also not dependent on whether a sodium-based or choline-based intracellular solution was used (Fig. 4D).
We also checked the specificity of extracellular cations in their ability to reduce glutamate apparent affinity. Using a desensitization protocol, extracellular solutions were switched from a choline-based extracellular solution to a sodium-, potassium-, or cesium-based extracellular solution in the continual presence of 0.1 μm glutamate and 1 mml-alanine. High degrees of desensitization (>75%) were observed only when the main extracellular cation was sodium (Fig. 4E), suggesting that reduction of the glutamate apparent affinity may be sodium specific.
Extracellular sucrose was also substituted for the choline cation to reduce ion permeation in some experiments. Degrees of desensitization for solution switches from sucrose to sodium or potassium were similar to their choline counterparts (Fig. 4E), indicating that reversion of the glutamate site to a high apparent affinity state does not result from an interaction of choline with open NMDA channels.
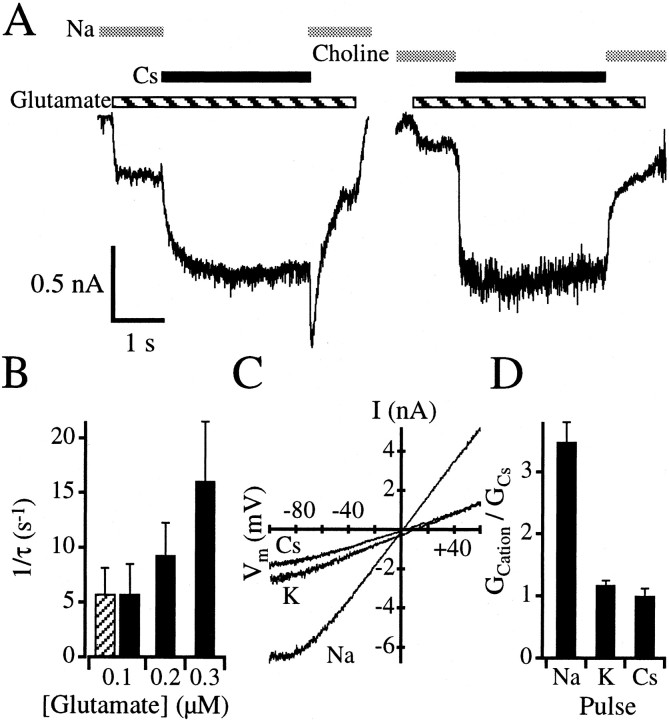
Extracellular cesium and potassium do not cause large degrees of desensitization when switching from a choline-based extracellular solution in the continual presence of 0.1 μm glutamate and 1 mml-alanine (Figs. 4E,5A, right panel), suggesting that the glutamate binding site remains in an apparent high-affinity state when extracellular cesium and potassium are the main extracellular cations. This enabled us to determine whether re-equilibration with the high apparent affinity state of the glutamate binding site could be observed. Neurons were incubated with 0.1 μmglutamate and 1 mml-alanine in a sodium-based extracellular solution. This should cause the glutamate binding site of NMDA receptors to revert to their low apparent affinity state. Indeed, only a minimal amount of inward current is initially observed in Figure5A (left panel), because 0.1 μm glutamate is well below the steady-state EC50 for glutamate (Nahum-Levy et al., 2001). After switching to a cesium-based extracellular solution, inward current increased slowly. The exponential rate constant for the increase in current was 222.6 ± 115.6 msec (n = 15 cells) when 0.1 μm glutamate was used but decreased to 68.1 ± 22.2 msec when 0.3 μm glutamate was used (n = 7 cells). Figure 5B shows that the current relaxation rate increases with increasing glutamate concentration. Similarly, the switch from a sodium-based extracellular solution to a potassium-based extracellular solution in the presence of 0.1 μm glutamate and 1 mml-alanine caused an increase in current with a time constant of 202.2 ± 85.0 msec (n = 5 cells). These results would be expected if the increase in current reflected the re-equilibration of glutamate with its high apparent affinity receptor binding state.
Fig. 5.
Extracellular sodium reduces glutamate affinity.A, A neuron was incubated in either a sodium-based (left panel) or choline-based (right panel) extracellular solution (gray bars) in the continual presence of 1 mml-alanine. A minimal amount of inward current was elicited after addition of 0.1 μm glutamate (striped bar). After 1 sec, the extracellular solution was replaced with a cesium-based solution (black bar) containing the same agonist concentrations for 3 sec. Inward current increased rapidly for the choline-to-cesium solution transition and did not desensitize appreciably (right panel). In contrast, the sodium-to-cesium solution transition caused a slow increase in inward current until the same steady state was reached (left panel). After changing the cesium-based solution back to sodium (left panel), a rapid increase in inward current was observed that then desensitized. Shown are records in which the leak current has been subtracted by repeating each protocol in the presence of 200 μm AP5. B, Plot of current relaxation rates with increasing concentrations of glutamate in the presence of extracellular cesium (black bars) or potassium (striped bar) measured from experiments like those in A (left panel). Changes in current result from glutamate re-equilibration with a high-affinity receptor state after the switch from a sodium-based extracellular solution to either a cesium-based or potassium-based extracellular solution. C, I–V relationship for NMDA receptor-mediated currents elicited by 10 μm glutamate and 1 mml-alanine in the presence of a sodium-based (Na), potassium-based (K), or cesium-based (Cs) extracellular solution. Currents depicted have been leak subtracted by eliciting voltage ramps in the presence of AP5 and in the absence of glutamate. D, Relative conductance of NMDA receptors in different extracellular solutions from whole-cell recordings. In each cell, I–V relationships were measured for either sodium-based and cesium-based extracellular solutions or potassium-based and cesium-based extracellular solutions. The reversal potential was determined for each condition, in each cell, and the conductance at −30 and +30 mV was calculated and averaged to provide an average conductance value (GCation) for each extracellular cation tested. Relative conductances were determined by dividingGCation by the average conductance value determined for cesium (GCs) for each cell. Results indicate that the relative conductances for cesium and potassium are similar, but the relative conductance measured in a sodium-based extracellular solution is more than threefold larger.
After returning to a sodium-based extracellular solution from either a potassium-based or cesium-based extracellular solution, currents subsequently desensitized with a time course reminiscent of that observed for choline-based to sodium-based extracellular solution changes (Fig. 4A, right panel). This desensitization further indicates that the glutamate binding site reverted to a high apparent affinity state in the presence of extracellular potassium or cesium.
It should also be noted that the switch from a cesium-based or potassium-based extracellular solution to one in which the major cation is sodium induces a rapid rise in inward current (Fig. 5A). Steady-state NMDA receptor-mediated currents in the presence of either extracellular potassium or cesium were 45.7 ± 10.5% (n = 5 cells) or 42.7 ± 14.7% (n= 8 cells) of their peak currents in the presence of the sodium-based extracellular solution (Fig. 5A, left panel). This change in current amplitude may reflect differences between the permeabilities of the different extracellular monovalent cations or may indicate that channel gating changes when sodium is present. Current–voltage (I–V) relationships were measured for NMDA receptor channels activated with 10 μm glutamate and 1 mml-alanine in which the intracellular solution was cesium-based and the extracellular solution was sodium-, potassium-, or cesium-based. Comparison of the relative amplitudes of NMDA currents in the presence of different extracellular solutions revealed that currents measured when using a sodium-based extracellular solution were more than threefold larger than when potassium or cesium extracellular solutions were used (Fig. 5C). Outward rectification observed at hyperpolarized potentials probably results from contamination of solutions with micromolar amounts of magnesium.
Reversal potentials for NMDA currents elicited in extracellular sodium-based and cesium-based solutions were 0.7 ± 3.0 mV (n = 9 cells) and 1.5 ± 3.7 mV (n= 13 cells), respectively, indicating a similarity in the extracellular cation relative permeabilities. The potassium-based extracellular solution yielded NMDA receptor-mediated currents with a slightly higher reversal potential of 9.1 ± 3.2 mV (n = 9 cells). We normalized for any differences in driving force by making conductance calculations at ±30 mV. These potentials were chosen because they are in the linear part of the I–Vcurve. Conductances measured for NMDA receptor mediated currents at ±30 mV in either sodium-based or potassium-based extracellular solutions were then averaged and normalized to similar measurements made during perfusion with the cesium-based extracellular solution on the same cell. Figure 5D indicates that cesium and potassium had similar overall conductances despite the differences observed in their reversal potentials (Fig. 5C). In contrast, the relative conductance of the population of NMDA channels was 3.5-fold higher in a sodium-based extracellular solution in comparison with the cesium-based control. The differences in relative conductance despite the similarity in reversal potentials for sodium-based and cesium-based extracellular solutions suggests that the permeability of sodium and cesium through NMDA channels is similar but that changes in channel gating occur.
Magnesium binding also causes a reduction in glutamate affinity
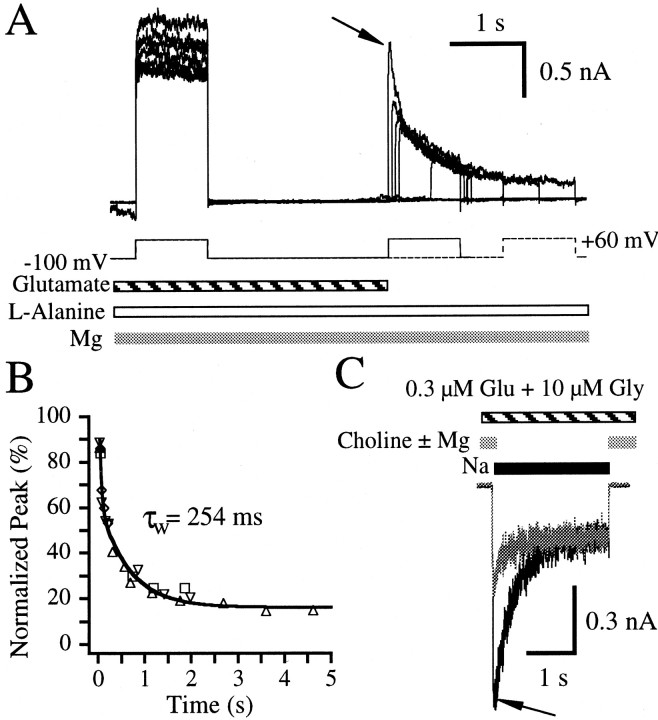
The data presented thus far are consistent with the possibility that the cation binding site responsible for the reduction of glutamate binding site apparent affinity may also be one of the sites that is involved in ion permeation. Magnesium is known to block ion permeation by binding within the channel pore. Because the key residue required for magnesium binding within the pore also significantly affects permeation properties (Burnashev et al., 1992; Kawajiri and Dingledine, 1993), magnesium binding within the pore may also trigger a reduction in glutamate apparent affinity. To test this, paired-pulse experiments were conducted in which 10 μm glutamate, 1 mml-alanine, and 2 mm magnesium were applied to a cell held at −100 mV in the presence of a choline-based extracellular solution. Outward currents were evoked on relief of magnesium block by stepping the holding potential to +60 mV (Fig.6A). Subsequent test voltage pulses were elicited at various delay times after the removal of exogenous glutamate. Outward peak amplitudes from the test voltage steps elicited after the removal of exogenous glutamate coincided with the decrease in current from the test pulse of the first paired-pulse record (Fig. 6A, arrow). Because the decay of the test pulse of the first paired-pulse record may be indicative of dissociation of glutamate from its binding site in a low-affinity state, overlap of this decay with the peak amplitudes from the first five records of the paired-pulse protocol suggests that glutamate apparent dissociation during the paired-pulse protocol is from glutamate binding sites in a low-affinity state. This is confirmed by exponential analysis of the peak amplitudes of the test pulses as a function of time since exogenous glutamate removal (Fig.6B), which has a time constant comparable to that of glutamate apparent dissociation in the presence ofl-alanine (Table 1). Because extracellular choline does not cause glutamate affinity to enter a low-affinity state (Figs. 3A, 4A), the 2 mm magnesium that is bound at hyperpolarized potentials is probably responsible for transition of the glutamate binding site into a low-affinity state.
Fig. 6.
Magnesium binding reduces glutamate affinity.A, Overlay of seven paired-pulse records from a single hippocampal neuron. The cell was incubated with 10 μmglutamate (striped bar), 1 mml-alanine (open bar), and 2 mmmagnesium (gray bar) in a choline-based extracellular solution at a holding potential of −100 mV. Outward currents were elicited by relieving magnesium block with a control voltage pulse to a holding potential to +60 mV for 1 sec. This was followed by a re-equilibration period of 2.5 sec at a holding potential of −100 mV, after which the exogenous glutamate was removed. A 1 sec voltage test pulse to +60 mV then followed at various delay times. The current observed reflects receptors bound with l-alanine that still have glutamate bound. The arrow indicates the test pulse of the first record. B, Peak current amplitudes elicited by the voltage test pulse to +60 mV were normalized to the steady-state current amplitude elicited by the voltage control pulse from the same trace and plotted against the delay time between exogenous glutamate removal and the peak of the test pulse for each record. Peak measurements are indicative of the rate of glutamate dissociation in the presence of l-alanine and magnesium but in the absence of permeant ions. Each symbol type represents the data from a different cell. Lineindicates a fit to a sum of two exponentials with the weighted average time constant (τw) noted on thegraph. The fitted parameters are listed in Table 1.C, A desensitizing current (black trace) was elicited after switching from a choline-based (gray bars) to a sodium-based (black bar) extracellular solution during a continuous incubation with 0.3 μm glutamate and 10 μm glycine. When 2 mm magnesium was added to the choline-based control solution, the peak current was only 46.6% of the peak current observed in the absence of magnesium. In addition, currents desensitized 47.3 and 75.4% in the presence and absence of magnesium in the control solution. The arrow indicates the point of a 23 msec rise time delay from 10% of the amplitude of the peak current in the absence of magnesium.
To confirm that magnesium binding can cause the glutamate binding site to revert to a low-affinity state, a desensitization protocol was designed in which a subsaturating concentration of glutamate (0.3 μm) and 10 μm glycine were applied to a neuron in a choline-based extracellular solution in the presence or absence of 2 mm magnesium at a holding potential of −60 mV (Fig. 6C). While keeping agonist concentrations constant, the choline-based extracellular solution was switched to a sodium-based extracellular solution that was devoid of exogenous magnesium. The sodium-induced currents elicited after incubation in the choline-based extracellular solution lacking magnesium peaked and desensitized 71.4 ± 6.1% (n = 5 cells). In contrast, the sodium-induced currents elicited after the incubation in the choline solution containing magnesium produced a peak which was only 41.9 ± 6.5% of the peak produced when magnesium was absent from the control. These currents subsequently desensitized 43.9 ± 6.5% (n = 5 cells) to the same steady state (Fig.6C). The 10–80% rise times of the peak currents were 22.6 ± 6.4 and 11.5 ± 1.5 msec for currents in which the control solution contained or lacked magnesium, respectively. Although the current rise time is slower when magnesium is present in the control solution, this cannot account for the difference in peak amplitudes observed. The amplitude of the current without magnesium in the control would be only slightly reduced if the 10–80% rise time were slowed to 23 msec (Fig. 6C, arrow). This indicates that something other than slow recovery from magnesium block of NMDA channels accounts for the striking peak amplitude reduction when magnesium is present in the control solution and suggests that magnesium binding causes a reduction of glutamate apparent affinity.
DISCUSSION
Glutamate and glycine binding affinities systematically change during NMDA receptor activation
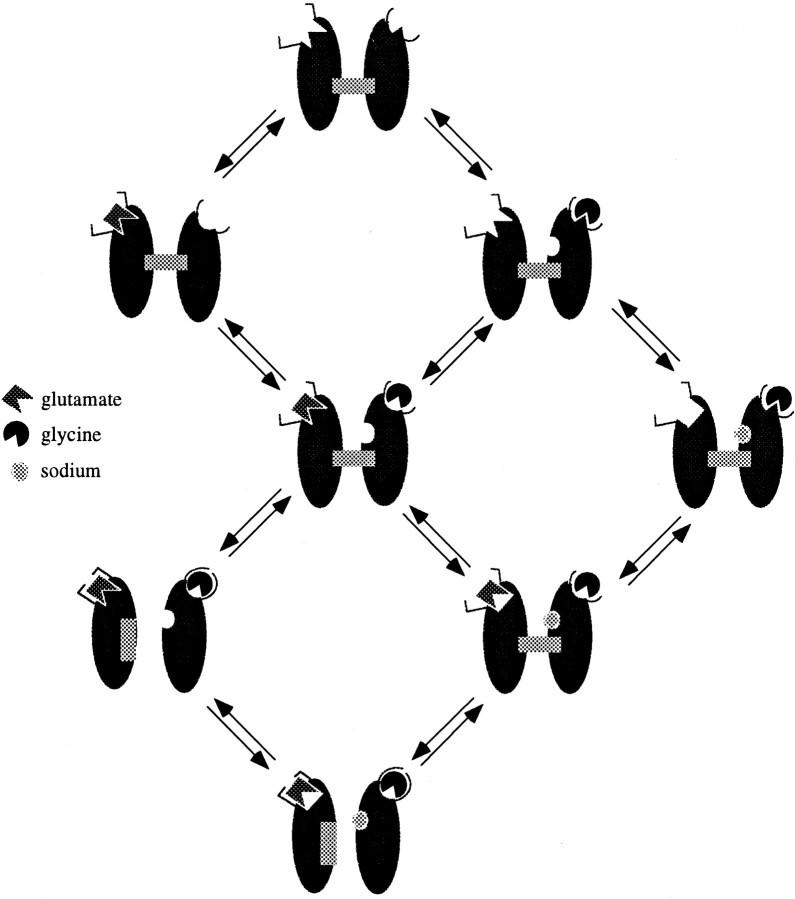
A plausible model for NMDA channel activation should contain at least three different states for the binding of each agonist (Fig.7). The existence of an apparent high-affinity glutamate binding state in the absence of a glycine co-agonist has been confirmed by paired-pulse experiments that showed an apparent dissociation constant for glutamate that is 7.9-fold slower in the absence of l-alanine than in its presence (Fig. 1). Similar experiments also showed that the apparent dissociation time constant for glycine in the absence of NMDA was 6.8-fold slower than when NMDA was present and the channel was activated (Fig. 2). These results confirm earlier desensitization studies (Benveniste et al., 1990a; Vyklicky et al., 1990; Nahum-Levy et al., 2001), which suggested that when glutamate or glycine alone is bound, the receptor binding site for that agonist is in an apparent high-affinity state (Fig.7).
Fig. 7.
Model of NMDA receptor activation. Glutamate and glycine binding sites exist in a high-affinity state before any ligand binding. High-affinity states are represented by binding sites with cusps. After binding of glutamate, a conformation change occurs to convert the glycine binding site to low affinity (semicircle without cusp). Note thatarrows in this drawing may indicate more than one mechanistic step and thus may appear to violate the law of microscopic reversibility. Exposure of the small cation modulatory site requires the binding of glycine but may also require the binding of glutamate. Sodium or magnesium can then freely bind, causing the glutamate binding site to revert to a low-affinity state (square withoutcusp). The exact location of the small cation modulatory site should also not be inferred from the drawing. With both glutamate and glycine bound, the channel can now undergo a transition to an open state. Once in the open state, both glutamate and glycine become irreversibly bound.
The conditions required for the reduction of glutamate apparent affinity are somewhat different from those that are required for the reduction of glycine apparent affinity. The process of agonist binding and possibly channel opening is sufficient to cause the transition of glycine binding to an apparent low-affinity state (Figs. 3C,4B), whereas the reduction in glutamate apparent affinity also requires the presence of extracellular magnesium or sodium (Figs. 3A,B,4A). When the NMDA channel enters the open state, both glutamate and glycine agonists may enter a third binding state (Fig. 7). Studies conducted with voltage-dependent open channel blocker, 9-aminoacridine, suggest that glutamate and glycine are “locked on” to their binding sites when NMDA channels are in the open state (Benveniste and Mayer, 1995). Binding domain closure is also supported by structural studies of the glutamate binding core of AMPA channels (Armstrong and Gouaux, 2000).
Nature of the small cation modulatory site that reduces glutamate affinity
Data presented in this paper suggest that a reduction in glutamate apparent affinity requires the binding of the glycine co-agonist and small extracellular cations. Glutamate appears to dissociate slowly from its binding site in the absence of l-alanine (Fig. 1) and in the absence of sodium (Figs.3A,B). Desensitization assays suggest that glutamate starts to re-equilibrate with its binding site in a low-affinity state only on addition ofl-alanine, although extracellular sodium was already present (Fig. 4A, left panel). Yet, re-equilibration of glutamate with its low-affinity binding site occurs on addition of extracellular sodium when l-alanine is already present (Fig.4A, right panel). Together, these results suggest that dissociation of glutamate from its binding site in a low-affinity state can occur only after a binding site for small cations becomes exposed. These experiments cannot differentiate whether exposure of the cation binding site requires the binding of glycine alone or the binding of both glutamate and glycine agonists (Fig. 7).
The degree of current desensitization measured in the presence of subsaturating glutamate (Figs.4A,C) did not depend on the major monovalent cation used in the intracellular solution, suggesting that the small cation modulatory site that reduces glutamate affinity is not in the intracellular domain of the protein. However, desensitization under subsaturating glutamate conditions was dependent on the major monovalent extracellular cation. The desensitization observed on switching from a choline-based extracellular solution to a sodium-based extracellular solution (Fig. 4A, right panel) suggests that glutamate binding was in a high-affinity state in the choline-based extracellular solution before sodium exposure.
Although desensitization was observed after switching from choline to sodium in the presence of 0.1 μm glutamate and 1 mml-alanine, switching the extracellular solution from choline to either cesium or potassium did not cause desensitization (Fig. 5A, right panel). Furthermore, the switch from sodium to either potassium or cesium caused an increase in NMDA receptor responses, the kinetics of which became more rapid as glutamate concentration was raised (Fig.5A,B). This suggests that although glutamate and glycine agonists are bound, the presence of extracellular sodium causes a weakening of glutamate affinity, whereas extracellular cesium or potassium allows glutamate binding to remain in a high apparent affinity state.
In these same experiments (Fig. 5A), peak currents elicited in the presence of extracellular sodium were larger than the maximal currents elicited in the presence of cesium or potassium. Under saturating agonist conditions, the relative whole-cell conductance observed with a sodium-based extracellular solution was 3.5-fold higher relative to that measured in a cesium-based extracellular solution (Fig. 5C,D). This difference does not result from differences in permeation properties for cesium or sodium as was shown for NR1 N598Q-NR2A recombinant channels (Schneggenburger and Ascher, 1997), because reversal potentials were comparable for all three extracellular cations. However, channel open probability has been shown to increase after intracellular sodium is raised (Yu and Salter, 1998). Thus, the larger relative whole-cell conductance observed in the presence of extracellular sodium (Fig. 5C,D) could possibly result from an increase in open probability induced by elevated intracellular sodium. The Nernst equation (Nernst, 1888) predicts that the reversal potential for sodium should be approximately +90 mV under the conditions of the experiment (intracellular sodium ∼4 mm; extracellular sodium = 160 mm), which suggests that sodium influx may be significant at all tested potentials. Alternatively, the larger relative whole-cell conductance observed in the presence of extracellular sodium (Fig. 5D) may result from changes in channel gating induced by sodium binding to an extracellular site on the NMDA receptor. In any case, there is a correlation between the relative conductances of specific cations permeating NMDA channels (Fig. 5D) and the ability of those cations to cause glutamate-dependent desensitization (Fig. 5A), suggesting that a sodium-induced change in channel gating may be related to a sodium-induced reduction in glutamate affinity.
It is possible that sodium, cesium, and potassium may generally influence glutamate apparent affinity at an extracellular location not involved with glutamate-dependent desensitization. We tested for this by measuring outward current decays at a holding potential of +60 mV in response to a 10 μm glutamate pulse in the presence of 1 mml-alanine. The experiment was done with cesium-, potassium-, or sodium-based extracellular solutions with a sodium-based intracellular solution. The single exponential current decay time constants were similar (555.9 ± 138.8, 444.2 ± 72.4, and 523.8 ± 51.0 msec for cesium-, potassium-, and sodium-based extracellular solutions, respectively; n = 3–6 cells for each condition). This indicates that when sodium was the primary monovalent cation flowing outward through the channel, changes in the primary extracellular monovalent ion did not cause an allosteric effect that influenced apparent glutamate dissociation.
Finally, channels exposed to magnesium have a glutamate apparent dissociation rate similar to that of apparent low-affinity dissociation (Fig. 6A, Table 1). In addition, the degree of glutamate-dependent desensitization observed on switching from a choline-based to a sodium-based extracellular solution was reduced when magnesium was present in the choline-based solution (Fig.6C). This also suggests that glutamate equilibrates with binding site in a low-affinity state in the presence of magnesium. High concentrations of magnesium can potentiate NMDA receptor responses, raise glycine apparent affinity, reduce single-channel current amplitudes, and cause voltage-dependent block of the pore (Paoletti et al., 1995). Although magnesium interactions with the NMDA receptor are complex, the possibility exists that extracellular magnesium weakens glutamate affinity by a mechanism similar to that of extracellular sodium. Both sodium and magnesium associate with the ion pore and inner and outer vestibules (Antonov et al., 1998; Antonov and Johnson, 1999;Zhu and Auerbach, 2001a,b). In addition, extracellular sodium and magnesium potentiate NMDA receptor responses in a voltage-independent manner (Fig. 5C) (Paoletti et al., 1995). We are currently investigating these possibilities.
The fact that sodium and magnesium may permit a reduction of glutamate binding site apparent affinity, whereas potassium and cesium cannot, may indicate that access to the small cation modulatory site might be governed by atomic size. Magnesium and sodium have empirical atomic radii of 1.5 and 1.8 Å, respectively, whereas potassium and cesium have much larger radii of 2.2 and 2.6 Å, respectively.
Physiological implications
Recent evidence suggests that powerful glycine uptake in the synaptic cleft reduces glycine concentrations such that <50% of NMDA channels may be activated during miniature EPSCs at some synapses (Berger et al., 1998). Glutamate concentrations after synaptic vesicle release also may be subsaturating (Bergles et al., 1999; Mainen et al., 1999). The apparent high affinity of agonist binding sites of the NMDA receptor may serve to maximize NMDA responses under subsaturating agonist conditions, yet repetitive glutamate release at ∼50–100 Hz might cause the postsynaptic glutamate binding sites to become saturated and thus insensitive to continued repetitive stimuli. The six- to eightfold lowering of glutamate and glycine affinity during the NMDA activation process may provide a mechanism by which these channels can maintain high sensitivity to agonists before activation but can dissociate more rapidly to facilitate clearing of agonists from the synaptic cleft.
R.N.-L. and E.T. contributed equally to this work.
This research was supported by Grant 96-00245 from the United States–Israel Binational Science Foundation, Jerusalem, Israel, and Grant 572/99-16.0 from the Israel Science Foundation. We thank Professor Bernard Attali and Dr. Kathryn Partin for comments on this work.
Correspondence should be addressed to Dr. Morris Benveniste, Department of Physiology and Pharmacology, Sackler School of Medicine, Tel Aviv University, Ramat Aviv, 69978 Israel. E-mail:morrisb@post.tau.ac.il.
REFERENCES
- 1.Anson LC, Chen PE, Wyllie DJA, Colquhoun D, Schoepfer R. Identification of amino acid residues of the NR2A subunit that control glutamate potency in recombinant NR1/NR2A NMDA receptors. J Neurosci. 1998;18:581–589. doi: 10.1523/JNEUROSCI.18-02-00581.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antonov SM, Johnson JW. Permeant ion regulation of N-methyl-d-aspartate receptor channel block by Mg(2+). Proc Natl Acad Sci USA. 1999;96:14571–14576. doi: 10.1073/pnas.96.25.14571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antonov SM, Gmiro VE, Johnson JW. Binding sites for permeant ions in the channel of NMDA receptors and their effects on channel block. Nat Neurosci. 1998;1:451–461. doi: 10.1038/2167. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong N, Gouaux E. Mechanisms for activation and antagonism of an AMPA-sensitive glutamate receptor: crystal structures of the GluR2 ligand binding core. Neuron. 2000;28:165–181. doi: 10.1016/s0896-6273(00)00094-5. [DOI] [PubMed] [Google Scholar]
- 5.Benveniste M, Mayer ML. Trapping of glutamate and glycine during open channel block of rat hippocampal neuron NMDA receptors by 9-aminoacridine. J Physiol (Lond) 1995;483:367–384. doi: 10.1113/jphysiol.1995.sp020591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benveniste M, Clements J, Vyklicky L, Jr, Mayer ML. A kinetic analysis of the modulation of N-methyl-d-aspartic acid receptors by glycine in mouse cultured hippocampal neurones. J Physiol (Lond) 1990a;428:333–357. doi: 10.1113/jphysiol.1990.sp018215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benveniste M, Mienville JM, Sernagor E, Mayer ML. Concentration-jump experiments with NMDA antagonists in mouse cultured hippocampal neurons. J Neurophysiol. 1990b;63:1373–1384. doi: 10.1152/jn.1990.63.6.1373. [DOI] [PubMed] [Google Scholar]
- 8.Berger AJ, Dieudonne S, Ascher P. Glycine uptake governs glycine site occupancy at NMDA receptors of excitatory synapses. J Neurophysiol. 1998;80:3336–3340. doi: 10.1152/jn.1998.80.6.3336. [DOI] [PubMed] [Google Scholar]
- 9.Bergles DE, Diamond JS, Jahr CE. Clearance of glutamate inside the synapse and beyond. Curr Opin Neurobiol. 1999;9:293–298. doi: 10.1016/s0959-4388(99)80043-9. [DOI] [PubMed] [Google Scholar]
- 10.Burnashev N, Schoepfer R, Monyer H, Ruppersberg JP, Gunther W, Seeburg PH, Sakmann B. Control by asparagine residues of calcium permeability and magnesium blockade in the NMDA receptor. Science. 1992;257:1415–1419. doi: 10.1126/science.1382314. [DOI] [PubMed] [Google Scholar]
- 11.Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- 12.Gadea A, Lopez-Colome AM. Glial transporters for glutamate, glycine, and GABA III. Glycine transporters. J Neurosci Res. 2001;64:218–222. doi: 10.1002/jnr.1069. [DOI] [PubMed] [Google Scholar]
- 13.Hirai H, Kirsch J, Laube B, Betz H, Kuhse J. The glycine binding site of the N-methyl-d-aspartate receptor subunit NR1: identification of novel determinants of co-agonist potentiation in the extracellular M3–M4 loop region. Proc Natl Acad Sci USA. 1996;93:6031–6036. doi: 10.1073/pnas.93.12.6031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huettner JE. Indole-2-carboxylic acid: a competitive antagonist of potentiation by glycine at the NMDA receptor. Science. 1989;243:1611–1613. doi: 10.1126/science.2467381. [DOI] [PubMed] [Google Scholar]
- 15.Kawajiri S, Dingledine R. Multiple structural determinants of voltage-dependent magnesium block in recombinant NMDA receptors. Neuropharmacology. 1993;32:1203–1211. doi: 10.1016/0028-3908(93)90014-t. [DOI] [PubMed] [Google Scholar]
- 16.Kleckner NW, Dingledine R. Requirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes. Science. 1988;241:835–837. doi: 10.1126/science.2841759. [DOI] [PubMed] [Google Scholar]
- 17.Krupp JJ, Vissel B, Heinemann SF, Westbrook GL. Calcium-dependent inactivation of recombinant N-methyl-d-aspartate receptors is NR2 subunit specific. Mol Pharmacol. 1996;50:1680–1688. [PubMed] [Google Scholar]
- 18.Krupp JJ, Vissel B, Thomas CG, Heinemann SF, Westbrook GL. Interactions of calmodulin and α-actinin with the NR1 subunit modulate Ca2+-dependent inactivation of NMDA receptors. J Neurosci. 1999;19:1165–1178. doi: 10.1523/JNEUROSCI.19-04-01165.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laube B, Hirai H, Sturgess M, Betz H, Kuhse J. Molecular determinants of agonist discrimination by NMDA receptor subunits: analysis of the glutamate binding site on the NR2B subunit. Neuron. 1997;18:493–503. doi: 10.1016/s0896-6273(00)81249-0. [DOI] [PubMed] [Google Scholar]
- 20.Legendre P, Rosenmund C, Westbrook GL. Inactivation of NMDA channels in cultured hippocampal neurons by intracellular calcium. J Neurosci. 1993;13:674–684. doi: 10.1523/JNEUROSCI.13-02-00674.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lerma J, Zukin RS, Bennett MV. Glycine decreases desensitization of N-methyl-d-aspartate (NMDA) receptors expressed in Xenopus oocytes and is required for NMDA responses. Proc Natl Acad Sci USA. 1990;87:2354–2358. doi: 10.1073/pnas.87.6.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mainen ZF, Malinow R, Svoboda K. Synaptic calcium transients in single spines indicate that NMDA receptors are not saturated. Nature. 1999;399:151–155. doi: 10.1038/20187. [DOI] [PubMed] [Google Scholar]
- 23.McNamara D, Smith EC, Calligaro DO, O'Malley PJ, McQuaid LA, Dingledine R. 5,7-Dichlorokynurenic acid, a potent and selective competitive antagonist of the glycine site on NMDA receptors. Neurosci Lett. 1990;120:17–20. doi: 10.1016/0304-3940(90)90157-5. [DOI] [PubMed] [Google Scholar]
- 24.Medina I, Filippova N, Charton G, Rougeole S, Ben-Ari Y, Khrestchatisky M, Bregestovski P. Calcium-dependent inactivation of heteromeric NMDA receptor-channels expressed in human embryonic kidney cells. J Physiol (Lond) 1995;482:567–573. doi: 10.1113/jphysiol.1995.sp020540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nahum-Levy R, Fossom LH, Skolnick P, Benveniste M. Putative partial agonist 1-aminocyclopropanecarboxylic acid acts concurrently as a glycine-site agonist and a glutamate-site antagonist at N-methyl-d-aspartate receptors. Mol Pharmacol. 1999;56:1207–1218. doi: 10.1124/mol.56.6.1207. [DOI] [PubMed] [Google Scholar]
- 26.Nahum-Levy R, Lipinski D, Shavit S, Benveniste M. Desensitization of NMDA receptor channels is modulated by glutamate agonists. Biophys J. 2001;80:2152–2166. doi: 10.1016/S0006-3495(01)76188-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nernst W. Zur Kinetick der in Lösung befindlichen Körper: Theorie der Diffusion. Z Phys Chem. 1888;2:613–637. [Google Scholar]
- 28.Ozawa S, Kamiya H, Tsuzuki K. Glutamate receptors in the mammalian central nervous system. Prog Neurobiol. 1998;54:581–618. doi: 10.1016/s0301-0082(97)00085-3. [DOI] [PubMed] [Google Scholar]
- 29.Paoletti P, Neyton J, Ascher P. Glycine-independent and subunit-specific potentiation of NMDA responses by extracellular Mg2+. Neuron. 1995;15:1109–1120. doi: 10.1016/0896-6273(95)90099-3. [DOI] [PubMed] [Google Scholar]
- 30.Priestley T, Kemp JA. Agonist response kinetics of N-methyl-d-aspartate receptors in neurons cultured from rat cerebral cortex and cerebellum: evidence for receptor heterogeneity. Mol Pharmacol. 1993;44:1252–1257. [PubMed] [Google Scholar]
- 31.Priestley T, Kemp JA. Kinetic study of the interactions between the glutamate and glycine recognition sites on the N-methyl-d-aspartic acid receptor complex. Mol Pharmacol. 1994;46:1191–1196. [PubMed] [Google Scholar]
- 32.Sather W, Johnson JW, Henderson G, Ascher P. Glycine-insensitive desensitization of NMDA responses in cultured mouse embryonic neurons. Neuron. 1990;4:725–731. doi: 10.1016/0896-6273(90)90198-o. [DOI] [PubMed] [Google Scholar]
- 33.Schneggenburger R, Ascher P. Coupling of permeation and gating in an NMDA-channel pore mutant. Neuron. 1997;18:167–177. doi: 10.1016/s0896-6273(01)80055-6. [DOI] [PubMed] [Google Scholar]
- 34.Tong G, Jahr CE. Regulation of glycine-insensitive desensitization of the NMDA receptor in outside-out patches. J Neurophysiol. 1994;72:754–761. doi: 10.1152/jn.1994.72.2.754. [DOI] [PubMed] [Google Scholar]
- 35.Vyklicky L, Jr, Benveniste M, Mayer ML. Modulation of N-methyl-d-aspartic acid receptor desensitization by glycine in mouse cultured hippocampal neurones. J Physiol (Lond) 1990;428:313–331. doi: 10.1113/jphysiol.1990.sp018214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu XM, Salter MW. Gain control of NMDA-receptor currents by intracellular sodium. Nature. 1998;396:469–474. doi: 10.1038/24877. [DOI] [PubMed] [Google Scholar]
- 37.Zhu Y, Auerbach A. Na+ Occupancy and Mg2+ block of the N-methyl-d-aspartate receptor channel. J Gen Physiol. 2001a;117:275–286. doi: 10.1085/jgp.117.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu Y, Auerbach A. K+ occupancy of the N-methyl-d-aspartate receptor channel probed by Mg2+ block. J Gen Physiol. 2001b;117:287–298. doi: 10.1085/jgp.117.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]