Abstract
Nicotine, the neuroactive compound responsible for tobacco addiction, is primarily believed to have beneficial effects on the adult brain. However, in heavy smokers, abstinence from nicotine is accompanied by cognitive impairments that suggest adverse effects of nicotine on brain plasticity. For this reason, we studied changes in plasticity-related processes in the dentate gyrus (DG) of the hippocampal formation of animals trained to self-administer nicotine. The DG was chosen because it undergoes profound plastic rearrangements, many of which have been related to memory and learning performances. In this region, we examined the expression of the polysialylated (PSA) forms of neural cell adhesion molecule (NCAM), PSA-NCAM, neurogenesis, and cell death by measuring the number of pyknotic cells. It was found that nicotine self-administration profoundly decreased, in a dose-dependent manner, the expression of PSA-NCAM in the DG; a significant effect was observed at all the doses tested (0.02, 0.04, and 0.08 mg/kg per infusion). Neurogenesis was also decreased in the DG, but a significant effect was observed only for the two highest doses of nicotine. Finally, the same doses that decreased neurogenesis also increased cell death. These results raise an important additional concern for the health consequences of nicotine abuse and open new insight on the possible neural mechanisms of tobacco addiction.
Keywords: neurogenesis, PSA-NCAM, hippocampus, drug abuse, nicotine, tobacco abuse
Nicotine is the neuroactive compound that is considered to be responsible for the development and maintenance of tobacco addiction (Jaffe and Kanzler, 1979; Stolerman and Jarvis, 1995; Pontieri et al., 1996; Merlo Pich et al., 1997). Despite the abuse potential of nicotine, the acute effects of this drug on the adult brain are primarily considered beneficial and, in particular, neuroprotective. Nicotine derivatives have been proposed for the treatment of age-related brain pathologies (Arneric et al., 1995; Miñana et al., 1998) and as enhancers of cognitive performances (Everitt and Robbins, 1997; Changeux et al., 1998). In fact, in heavy smokers, abstinence from nicotine is characterized by a profound impairment of cognitive performances. This observation suggests that chronic exposure to nicotine might impair brain mechanisms related to learning and memory. Unfortunately, the potential biological bases of these effects of nicotine are unknown.
To address this question we studied changes in plasticity-related processes in the dentate gyrus (DG) of the hippocampal formation of animals trained to self-administer nicotine. Intravenous self-administration of drugs is considered the best experimental model of drug abuse and consists in reinforcing a behavioral response through a drug infusion. The DG was studied because it undergoes profound plastic rearrangements that have been related to learning and memory. Three parameters were studied in this region: (1) the expression of the polysialylated (PSA) forms of neural cell adhesion molecule (NCAM), PSA-NCAM; (2) neurogenesis, and (3) cellular death. NCAM is a cell adhesion protein in which polysialylation modifies the relative degree of overall membrane–membrane apposition between cells and facilitates cell migration and remodeling (Rougon, 1993). In the adult hippocampus, PSA-NCAM is expressed in newborn neurons and mossy fibers (Seki and Arai, 1993). Modifications of PSA-NCAM expression in mutant mice results in morphological modifications and impairment of cognitive function (Cremer et al., 2000) and perturbations of synaptic plasticity (Muller et al., 1996; Eckhardt et al., 2000). Neurogenesis, which defines the production of new neurons by active proliferation of progenitor cells, is maintained in the adult DG, and this phenomenon seems to play an important role in hippocampal-mediated learning (Kemperman et al., 1997; Gould et al., 1999a,b; Gross, 2000; Lemaire et al., 2000; Shors et al., 2001). To attest the specificity of the effect observed, these parameters were also analyzed in the subventricular zone (SVZ). The SVZ is the other brain region in which expression of PSA-NCAM and neurogenesis are maintained in the adult brain.
It was found that nicotine self-administration profoundly decreased the expression of PSA-NCAM and neurogenesis in the DG. In parallel, cell death was increased. In contrast, no significant effects were found in the SVZ. These results raise an important additional concern for the health consequences of nicotine abuse and open new insight on the possible neural mechanisms of tobacco addiction.
MATERIALS AND METHODS
Housing conditions. Male Sprague Dawley rats (Iffa-Credo, Lyon, France; 280–320 gm) were individually housed under a constant light/dark cycle (lights on at 8:00 A.M., off at 8:00 P.M.) and with ad libitum access to food and water.
Nicotine self-administration. The intravenous self-administration set up was similar to the one previously described (Deroche et al., 1997). Briefly, animals were placed daily for 1 hr in a self-administration chamber, where their chronically implanted intracardiac catheter was connected to a pump-driven syringe. Two holes, located on opposite sides of the self-administration chamber, were used as devices to record responding. The introduction of the animal's nose in one hole (active device) switched on the infusion pump, initiating the infusion of 20 μl of the nicotine solution over 1 sec. Each infusion was followed by a 60 sec time-out during which further nose pokes were recorded but did not result in additional infusions (inf). Nose pokes in the other hole (inactive device) had no scheduled consequences. A fixed ratio (FR) schedule was used. During the first five days of testing, an FR1 was applied (one response for one infusion), then the ratio was progressively increased over 6 d up to FR5 (five responses for one infusion) and was maintained at FR5 during the rest of the experiment. Experimental contingencies were controlled, and data was collected by a personal computer with Windows-compatible software (Imetronic, Bordeaux, France). Nicotine tartrate (Sigma, St. Louis, MO) was dissolved in NaCl 0.9%, and the pH was adjusted to 7.4 with NaOH. The concentrations of the nicotine solution are expressed as nicotine base. Four independent groups were tested over 42 d with the following doses of nicotine (0, 0.02, 0.04, and 0.08 mg/kg per infusion). These doses of nicotine were chosen because they were in the range of those previously reported to induce nicotine self-administration (Shoaib et al., 1997; Donny et al., 1998).
BrdU injections. To study neurogenesis, rats were treated with 5-bromo-2′-deoxyuridine (BrdU; Nowakowski et al., 1989). BrdU was dissolved in a phosphate buffer (0.1 m, pH 8.4) and injected intraperitoneally (50 mg/kg, i.p.) over 3 d at the end of the experiment (days 39, 40, 41). The first 2 d, the rats received one injection per day after the self-administration session. The third day, they received two injections, the first 12 hr before the self-administration session and the second immediately after. Animals were perfused 48 hr later.
Histological procedure. Rats were deeply anesthetized with chloral hydrate (400 mg/kg, i.p.) and were perfused transcardially with 150 ml of PBS, pH 7.3, containing heparin (5 × 104 IU/ml), followed by 300 ml of 4% paraformaldehyde in 0.1m of phosphate buffer, pH 7.3. After a 24 hr post-fixation period of the brains in paraformaldehyde, 50 μm frontal sections were cut on a vibratome and collected in PBS (0.1m, pH 7.4). Free-floating sections were processed in a standard immunohistochemical procedure (Rodriguez et al., 1998). Adjacent sections were treated for PSA-NCAM immunoreactivity using a mouse anti-Men B monoclonal antibody (1:3000; Rougon et al., 1986). For BrdU immunohistochemistry, sections were treated with 2N HCl (30 min at 37°C), then rinsed in borate buffer for 5 min (0.1m, pH 8.4), and incubated with a mouse monoclonal anti-BrdU (1:200; Dako, Carpinteria, CA). After 72 hr of incubation, sections were incubated with a biotin-labeled rabbit anti-mouse IgM antibody (1:400; Dako) or a biotin-labeled horse anti-mouse IgG antibody (1:200; Vector Laboratories, Burlingame, CA). For each antibody, sections from all animals were processed in parallel, and immunoreactivities were visualized by the biotin–streptavidin technique (ABC kit; Dako) using 3,3′-diaminobenzidine as chromogen (10 min incubation). For counterstaining, mounted sections were soaked in a hematoxylin bath (Harris-type staining, RAL) for 3–5 min. After washing in a water bath (1–3 min), sections were incubated in acid-alcohol (HCl 1% in ethanol 70%) for 10 sec. The slides were then washed in a water bath (1–2 min), dehydrated, and coverslipped.
Quantitative evaluation of staining. The number of X-immunoreactive (IR) cells in the granule and subgranular layers of the dentate gyrus was estimated on counts made by systematic random sampling of every tenth section along the rostrocaudal axis of the hippocampal formation. On each thick section, all of the X-IR cells were counted inside a giant dissector, the volume of which was defined by the sectional profile of the granule and subgranule cell layers (measured with a Samba 2640 system; Alcatel system; TITN Answare, Grenoble, France) and by the height of the dissector, which did not extend to the topmost surface of the section. The counting was performed with a 100× lens at positions where labeled cells could be observed with lower-powered lenses. For each animal, the mean numerical density, Nv, of X-IR cells was calculated from the sum of the counts made within the disectors and the volume of the disectors. The total number of X-IR cells in the granule and subgranule layers,N, was then calculated by multiplying the numerical density of BrdU-IR cells, Nv, by the reference volume (in cubic millimeters), Vref N = Nv ×Vref. The Vref was estimated according to the Cavalieri method: Vref = a ×t × s, where a is the mean area of the granule and subgranule cell layers, t is the mean thickness of the vibratome section (17 ± 0.47 μm), ands the total number of sections through the reference volume. The number of pyknotic cells in the granule cell layer was determined on hematoxylin-counterstained sections that were used for the PSA-NCAM count. The number of BrdU-IR cells was determined on alternate sections. BrdU-IR cells were also counted within the dorsolateral corner of the subventricular zone (Wagner et al., 1999) (0.7 anterior to the bregma according to the atlas of Paxinos and Watson, 1982); its surface was measured, and results were expressed as the mean number of BrdU-IR cells per square millimeter.
Analysis of phenotype. To examine the phenotype of BrdU-IR cells, 3 of 10 series of sections were incubated with the BrdU antibody (1:800; Accurate Chemicals, Westbury, NY), which was revealed using a CY3-labeled anti-rat IgG antibody (1:400; Jackson ImmunoResearch, West Grove, PA). One of each series was respectively incubated with a GFAP polyclonal antibody (1:10,000; Dako) or with a mouse monoclonal anti-NeuN antibody (1:1000,Chemicon, Temecula, CA; Euromedex, Souffelweyersheim, France). Bound anti-GFAP polyclonal or anti-neuronal nuclei (NeuN) monoclonal antibodies were visualized with a Alexa 488-labeled goat anti-rabbit IgG antibody or Alexa 488-labeled goat anti-mouse IgG antibody (1:1000;Jackson ImmunoResearch). For double labeling, the percentage of BrdU-labeled cells that expressed NeuN or GFAP was determined by counts of labeled cells on a minimum of three sections throughout the dentate gyrus using a Leica fluorescent microscope. Approximately 35 labeled cells were examined for each animal. Immunofluorescent double-labeled cells were further examined using a Zeiss, (Oberkochen, Germany) Axiovert confocal microscope. For representative purposes, the images in Figure 2 were collected with simultaneous excitation by the laser lines for Alexa 488 and CY3. In contrast, determination of colocalization was performed by collecting each wavelength separately.
Fig. 2.
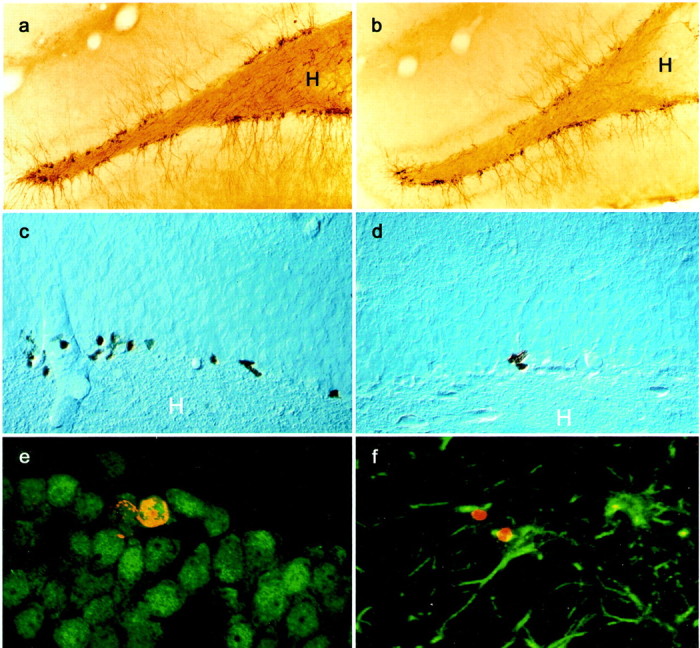
Illustration of PSA-NCAM- and BrdU-labeled cells in the dentate gyrus. Microphotography of PSA-NCAM staining in a control animal (a) and in an animal self-administering 0.04 mg/kg per infusion of nicotine (b). Microphotography of BrdU staining in a control animal (c) and in an animal self-administering 0.08 mg/kg per infusion of nicotine (d). Optical section (0.7 μm) obtained by confocal microscopy showing that BrdU-stained cells (red nuclear stain, CY3) were double-stained with the neuronal marker NeuN (green stain, Alexa 488) (e). In contrast, very few BrdU-stained cells (red nuclear stain) also expressed the astroglial marker GFAP (green stain, f). H,Hilus.
Statistical analysis. All data were analyzed by ANOVA using the Statistica software package. Newman–Keuls was used for post hoc comparison.
RESULTS
Nicotine self-administration
Four independent groups of animals were allowed to self-administer one of three unitary doses of nicotine (0.02, 0.04, and 0.08 mg/kg per infusion) or the vehicle solution (nicotine, 0.00 mg/kg per infusion). Infusions were delivered when the animal poked in one of two identical holes placed on the walls of the self-administration cage (active-device). Responding in the other hole (inactive-device) had no scheduled consequences.
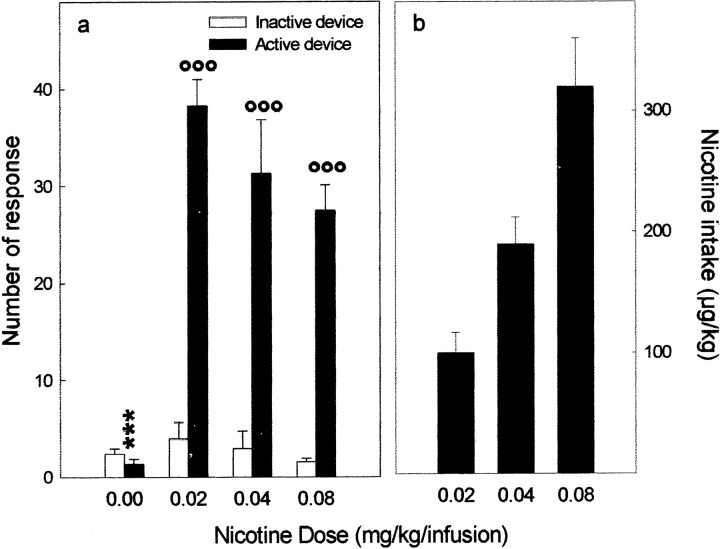
Nicotine induced self-administration at all doses tested (Fig.1). Animals showed a higher number of responses in the active device than in the inactive one (device effect,F(1,19) = 69.71; p < 0.0001), and this difference was dose dependent (dose × device interaction, F(3,19) = 17.72;p < 0.0001) (Fig. 1a). The daily intake of nicotine (Fig. 1b) was progressively higher across unitary doses (F(2,14) = 10.74;p < 0.01).
Fig. 1.
Intravenous nicotine self-administration. a, Mean ± SE of the daily number of responses in the device delivering the infusion of nicotine (active) and in the control device (inactive) calculated over the 42 d of testing. b, Mean ± SE of the daily intake of nicotine calculated over the 42 d of testing. Nicotine induced self-administration, as shown by the higher number of responses in the active than in the inactive device (°°°p < 0.001). In contrast, for animals having access only to vehicle (0.00 mg/kg per infusion dose), responding in the two devices did not differ and was lower than the one in the active device of all nicotine groups (***p< 0.001). The daily intake of nicotine increased across unitary doses.
Effect of nicotine self-administration on PSA-NCAM expression
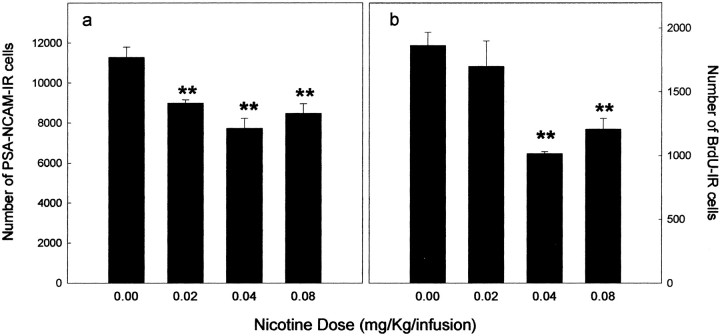
PSA-NCAM-IR cells were located in the deepest region of the granule cell layer at the interface of the hilus. Their dendrites radiated into the molecular layer and their axons innervated the CA3 subfield, the target of the mossy fibers (Fig.2a,b). Nicotine self-administration decreased PSA-NCAM expression in the dentate gyrus. Quantitative analysis (Fig.3a) revealed that nicotine decreased the number of PSA-NCAM-IR cells with respect to control (F(3,12) = 10.969; p< 0.001). This decrease reached 44% for the medium nicotine dose (0.04 mg/kg per infusion). These differences were not attributable to differences in the reference volume (0.00 mg/kg per infusion = 1.157 ± 0.058; 0.02 mg/kg per infusion = 1.028 ± 0.088; 0.04 mg/kg per infusion = 1.131 ± 0.201; 0.08 mg/kg per infusion = 1.155 ± 0.182;F(3,12) = 0.31; p > 0.81). Inspection of sections suggested that PSA-NCAM was not altered in the SVZ of nicotine-treated rats. However, the number of PSA-immunoreactive cells could not be counted in this structure because SVZ PSA-NCAM immunoreactive cells are organized in chains that form the rostral migratory stream.
Fig. 3.
Effect of nicotine self-administration on PSA-NCAM expression (a) and cell proliferation (b) in the granule cell layer. Mean ± SE of the number of cells per dentate gyrus in animals self-administering different unitary doses of nicotine. Self-administration of nicotine significantly reduced the number of PSA-NCAM- and BrdU-IR cells (**p < 0.01 in comparison with the control group).
Effect of nicotine self-administration on neurogenesis
Proliferation of progenitor cells in the dentate gyrus was studied by BrdU, a thymidine analog incorporated into genetic material during the synthetic DNA phase (S phase) of mitotic division. Animals were injected with BrdU during the last days of self-administration (days 39–41) and were killed 48 hr after the last injection.
Nicotine self-administration significantly decreased the number of BrdU-IR cells in the granule cell layer of the dentate gyrus (Fig.2c,d) in a dose-dependent manner (F(3,12) = 11.81; p < 0.001) (Fig. 3b). Indeed, neurogenesis was significantly decreased for the highest doses of nicotine (0.04 and 0.08 mg/kg per infusion), whereas it was not modified by the lower dose (0.2 mg/kg per infusion). These differences were not caused by differences in the reference volume (0.00 mg/kg per infusion = 1.145 ± 0.046; 0.02 mg/kg per infusion = 1.176 ± 0.071; 0.04 mg/kg per infusion = 1.161 ± 0.115; 0.08 mg/kg per infusion = 1.205 ± 0.119; F(3,12) = 0.102;p > 0.966).
To determine whether the effect of nicotine on the number of BrdU-IR cells was specific for the dentate gyrus, the number of BrdU-IR cells was also examined in the SVZ. Nicotine self-administration did not modify the number of BrdU-IR cells per square millimeter at any of the doses studied (0.00 mg/kg per infusion = 236,418.81 ± 28,061.75; 0.02 mg/kg per infusion = 245,298.41 ± 18,839.66; 0.04 mg/kg per infusion = 239,924.39 ± 24,480.30; 0.08 mg/kg per infusion = 234,547.03 ± 11,927.69;F(3,12) = 01.58; p > 0.24)
The phenotype of BrdU-stained cells was determined by immunofluorescent staining with the neuronal marker NeuN and the astroglial marker GFAP (Fig. 2e,f). In control animals ≈7% of BrdU-stained cells expressed the astroglial marker GFAP and ≈60% the neuronal marker NeuN (Table 1). The percentage of GFAP–BrdU and of NeuN–BrdU double-stained cells did not differ between experimental groups (Table 1) (all at p> 0.05). The ratio of BrdU-IR cells colabeled with GFAP or NeuN was multiplied with the total number of BrdU-labeled cells to give an estimate of the total number of BrdU-labeled astrocytes or the total number of BrdU-labeled neurons (Table 1). The extrapolated total number of BrdU-labeled astrocytes was not changed by nicotine self-administration (F(3,12) = 1.14;p > 0.37). In contrast, the total number of BrdU-labeled neurons was decreased dose dependently by nicotine self-administration (F(3,12) = 15.95; p < 0.01).
Table 1.
Effect of nicotine self-administration on the phenotype of the newly born cells in the dentate gyrus
| Groups | BrdU–GFAP (%) | BrdU–NeuN (%) | Total number of BrdU-labeled astrocytes | Total number of BrdU-labeled neurons |
|---|---|---|---|---|
| Control | 7.11 ± 0.67 | 63.83 ± 1.97 | 131.74 ± 18.46 | 1190.60 ± 73.15 |
| Nic 0.2 mg/kg/inf | 9.25 ± 2.65 | 58.92 ± 3.25 | 180.17 ± 63.29 | 993.29 ± 142.77 |
| Nic 0.4 mg/kg/inf | 8.84 ± 1.26 | 54.26 ± 2.06 | 96.69 ± 13.07 | 551.74 ± 29.151-165 |
| Nic 0.8 mg/kg/inf | 6.57 ± 3.34 | 53.05 ± 3.01 | 109.42 ± 37.41 | 633.95 ± 25.531-165 |
F1-165: p < 0.001 in comparison with the control group.
Effect of nicotine self-administration on cell death
We next evaluated the consequence of nicotine intake on cell death within the granule cell layer. The degenerating profiles, i.e., pyknotic cells, were characterized on counterstained sections by condensed chromatin (Fig.4a,b). Nicotine self-administration significantly increased the number of pyknotic cells in the granule cell layer of the dentate gyrus in a dose-dependent manner. Indeed, the number of pyknotic cells was increased for the highest doses of nicotine whereas it was not modified by the lower doses (F(3,12) = 9.026;p < 0.001). As described for the study of expression of PSA-NCAM, there was no difference in the reference volume between experimental groups.
Fig. 4.
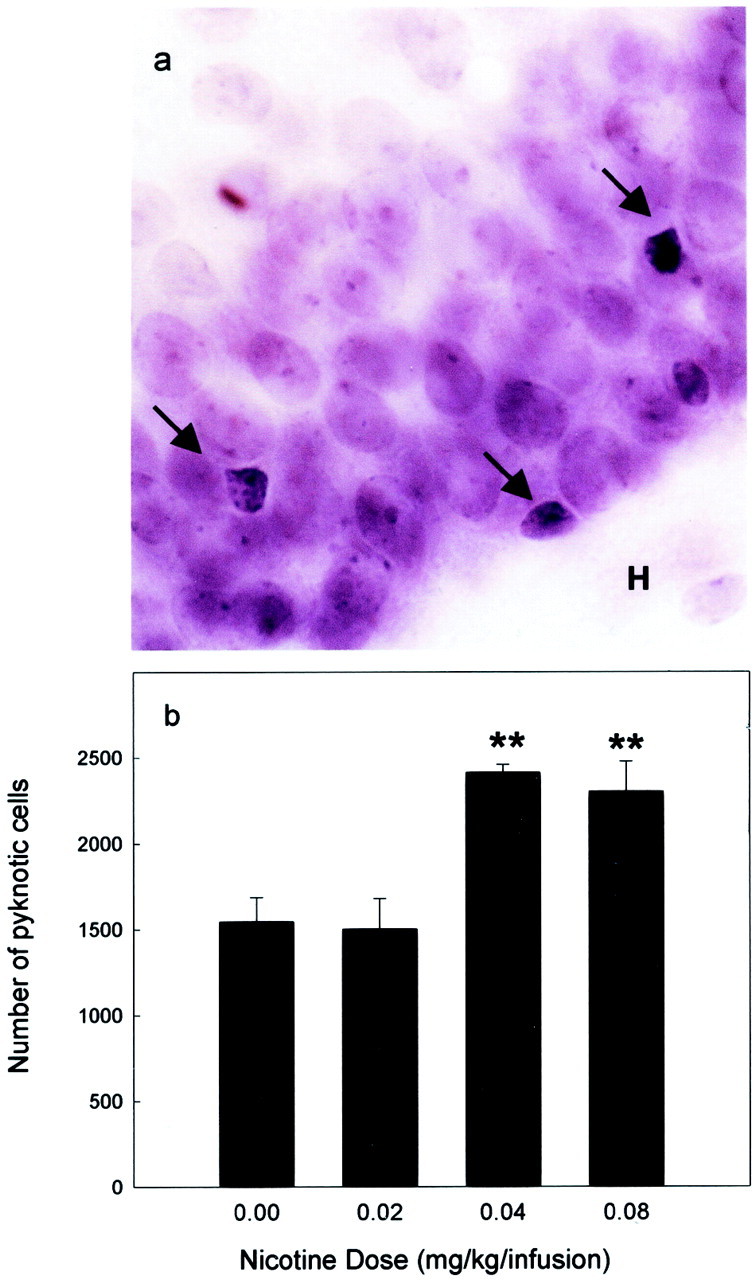
Effect of nicotine self-administration on pyknotic cells in the granule cell layer. Illustration of pyknotic cells in the dentate gyrus (a) and mean ± SE of the number of pyknotic cells per dentate gyrus in animals self-administering different unitary doses of nicotine (b). Self-administration of nicotine significantly increased the number of pyknotic cells (**p < 0.01 in comparison with the control group).H, Hilus.
DISCUSSION
The results of the present experiments show that nicotine intake has major effects on the adult hippocampus. In the adult dentate gyrus it induces: (1) a decrease in PSA-NCAM expression, (2) a downregulation of neurogenesis as revealed by the number of BrdU-labeled cells, and (3) an increase in cell death as measured by the number of pyknotic cells. A significant effect of nicotine on all of these parameters was observed for a daily intake of this drug at doses that ranged between 180 and 320 μg/kg. A recent report (Shoaib and Stolerman, 1999) indicates that these doses of nicotine produce plasma levels of the drug that are in the range of those observed in smokers. Consequently, these observations suggest that nicotine abuse can have adverse consequences in the adult brain.
The decrease in hippocampal PSA-NCAM and neurogenesis induced by nicotine could have important functional consequences. The DG is one of the few regions of the adult brain maintaining the expression of PSA-NCAM and producing de novo neurons throughout life (Altman, 1962; Kiss and Rougon, 1997). Polysialylation of NCAM in PSA-NCAM has been hypothesized to play a role in the selective stabilization of synaptic contacts that are transiently produced during memory (Rose, 1995). Supporting this hypothesis, it has been shown that: (1) PSA-NCAM is transiently increased as a consequence of learning processes (Murphy et al., 1996; O'Connell et al., 1997;Murphy and Regan, 1999), (2) administration of endoneuraminidase NE (endoN), an enzyme that removes PSA by cleaving 2–8 linked polysialic acid, diminishes performance in a water maze (Becker et al., 1996), (3) treatments of hippocampal slices with endoN block the induction of LTP (Becker et al., 1996; Muller et al., 1996; Cremer et al., 1998). Neurogenesis also seems important for hippocampal-mediated learning. Survival of the newly born cells and cell proliferation are increased by spatial learning (Gould et al., 1999a,b; Lemaire et al., 2000). Conversely, a decrease in neurogenesis has been associated with decreased learning (Lemaire et al., 2000). Finally and more importantly, an elegant recent study has recently shown that blockade of neurogenesis causes memory impairments in hippocampal-dependent learning (Shors et al., 2001). Nicotine self-administration also induced cell death as shown by the increase in the number of pyknotic cells. Part of these cells were very likely newborn or progenitor cells because they were localized in the inner part of the granule cell layer, at the interface of the hilus. However, part of the pyknotic cells were probably more mature neurons because they were localized in the deepest part of the granule cell layer.
Because PSA-NCAM is expressed by newborn cells, it could be questioned whether the decrease in PSA-NCAM observed here results form the decrease in neurogenesis. The observation that PSA-NCAM is significantly decreased for a dose of nicotine (0.02 mg/kg per infusion) lower than the one necessary for decreasing neurogenesis indicates that changes in PSA-NCAM are independent and/or primary to alterations in neurogenesis. In fact, is very likely that the decrease in PSA-NCAM influences the levels of neurogenesis. Thus, the expression of PSA-NCAM is necessary for the migration of the newly born cells (Hu et al., 1996). An alteration of migration is consistent with the death of the newly born cells, which cannot establish appropriate synaptic contacts without migrating.
Neurogenesis is a phenomenon that consists of three major phases: proliferation, migration, and differentiation. The last two phases being ultimately necessary for the survival of the newly born cell. As discussed above, nicotine, by decreasing PSA-NCAM, could impair cell migration and consequently cell survival. However, the protocol of BrdU injections used in this study does not allow to unequivocally distinguish between changes in cell proliferation and survival. Consequently, an effect of nicotine at other levels cannot be excluded. It is noteworthy, in this context, that several reports indicate that nicotine can have toxic effect on immature cells. It has been shownin vitro (Berger et al., 1998) that nicotine induces apoptotic cell death of immortalized hippocampal progenitor cells. It has also been shown in vivo that during the early stages of development, nicotine can induce the death of progenitor cells (Berger et al., 1998; Roy et al., 1998) and produce behavioral impairments in the offspring of mothers exposed to nicotine during pregnancy (Sexton et al., 1990; Olds et al., 1994).
It could be argued that the observed decrease in the number of BrdU-labeled cells could be simply an artifact caused by a decrease in BrdU availability induced by nicotine. This possibility seems unlikely on the basis of two observations. First, nicotine self-administration had no significant effects on cell proliferation in the SVZ, which should be modified if the effects of nicotine were nonspecifically mediated by changes in bioavailability of BrdU. Second, nicotine also induced an increase in the number of pyknotic cells, which is an independent measure of cell death and is not influenced by BrdU bioavailability. The differential effect of nicotine in the dentate gyrus and in the SVZ could seem surprising. However, it has been shown before that proliferation and differentiation of newly born cells in these two areas can be regulated independently. For example, aging, learning, and glucocorticoid hormones modify neurogenesis in the dentate gyrus but not in the SVZ (Kuhn et al., 1996; Rodriguez et coll., 1998; Gould et al., 1999a; Montaron et al., 1999). Conversely, epidermal growth factor and fibroblast growth factor 2 increase cell proliferation in the SVZ but not in the dentate gyrus (Kuhn et al., 1997; Wagner et al., 1999).
The changes in hippocampal plasticity observed in our experimental conditions could be relevant to nicotine abuse. Indeed, in dependent smokers, cognitive performances fluctuate between two opposite poles: an impaired state during abstinence and a normal-enhanced state immediately after nicotine intake (Snyder and Henningfield, 1989;Snyder et al., 1989; Foulds et al., 1996). This fluctuation has been proposed as one of the mechanisms maintaining tobacco addiction (Mangam and Golding, 1978; Heishman et al., 1994). The basic idea being that smokers seek nicotine to relieve the cognitive impairment experienced during abstinence. The effect of nicotine on hippocampal plasticity could contribute to the progressive development of cognitive deficits observed in smokers and consequently contribute to maintaining tobacco addiction. It is noteworthy that cognitive deficits in smokers are long-lasting, especially for tasks involving working memory (Snyder et al., 1989), a cognitive function in which the hippocampal formation is primarily involved (Everitt and Robbins, 1997).
More generally, our results join recent evidence highlighting the potential role of hippocampal plasticity in drug abuse. Indeed, other drugs of abuse such as morphine, heroin, and alcohol induce structural changes in the hippocampal formation (Paula-Barbosa et al., 1993;Lukoyanov et al., 2000), alter long term potentiation (Carlen and Wilkinson, 1987), and decrease neurogenesis (Eisch et al., 2000; Nixon and Crews, 2001). These neuronal changes may be associated with permanent functional alteration in learning (Gould et al., 1999a,b;Lemaire et al., 2000; Shors et al., 2001), behavioral inhibition (Gould and Cameron, 1997), and ultimately modify drug-directed behaviors. This idea is supported by a recent report (Vorel et al., 2001) showing that electrical stimulation of the glutamatergic efferent pathway of the hippocampal formation to the nucleus accumbens reinstates cocaine-seeking behavior. Because the hippocampal formation subserves contextual learning, reinstatement of cocaine seeking after hippocampal stimulation suggests that memory traces of the association between a context and the availability of cocaine could be stored in this brain region.
In conclusion, our results demonstrate that nicotine self-administration reduces the expression of PSA-NCAM and neurogenesis, whereas it increases cell death in the adult hippocampal formation. These results suggest that nicotine abuse can have adverse consequences in the adult brain, raising an additional concern about the consequences of tobacco smoking. Furthermore, they also provide new insights on biological adaptations occurring during nicotine intake that could be relevant for the development of nicotine abuse.
Footnotes
This work was supported by Institut National de la Santé et de la Recherche Médicale, University of Bordeaux II, Institut Fédératif de Recherche number 8. Walter Adriani was supported by a “Marie Curie” Fellowship. This work was also supported by grants from Pôle Médicament Aquitaine, Ministère de La Recherche, and European Community Grant EC QRT 1999-02187. We are grateful to C. Brechenmacher for her help with the confocal analysis. The technical help of M. C. Donat and J. M. Claustrat is acknowledged.
Correspondence should be addressed to Pier Vincenzo Piazza, Laboratoire de Psychobiologie des Comportements Adaptatifs, Institut National de la Santé et de la Recherche Médicale U259, Université de Bordeaux II, Domaine de Carreire, Rue Camille Saint-Saëns, 33077 Bordeaux cedex, France.
E-mail: pier-vincenzo.piazza@bordeaux.inserm.fr.
REFERENCES
- 1.Altman J. Are new neurons formed in the brain of adult mammals? Science. 1962;135:1127–1128. doi: 10.1126/science.135.3509.1127. [DOI] [PubMed] [Google Scholar]
- 2.Arneric SP, Sullivan JP, Decker MW, Brioni JD, Bannon AW, Briggs CA, Donnelly-Roberts D, Radek RJ, Marsh KC, Kyncl J, Williams M, Buccafusco JJ. Alzheimer's disease and associative disorders, Vol 9. Lippincott-Raven; Philadelphia: 1995. Potential treatment of Alzheimer's disease using cholinergic channel activators (ChCAs) with cognitive enhancement, anxiolytic-like and cytoprotective properties. pp. 50–61. [DOI] [PubMed] [Google Scholar]
- 3.Becker CG, Artola A, Gerardy-Schahn R, Becker T, Welzl H, Schachner M. The polysialic acid modification of the neural cell adhesion molecule is involved in spatial learning and hippocampal long-term potentiation. J Neurosci Res. 1996;45:143–152. doi: 10.1002/(SICI)1097-4547(19960715)45:2<143::AID-JNR6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 4.Berger F, Gage FH, Vijayaraghavan S. Nicotine receptor-induced apoptotic cell death of hippocampal progenitor cells. J Neurosci. 1998;18:6871–6881. doi: 10.1523/JNEUROSCI.18-17-06871.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlen PL, Wilkinson A. Reversibility of alcohol-related brain damage: clinical and experimental observations. Acta Med Scand [Suppl] 1987;717:19–26. doi: 10.1111/j.0954-6820.1987.tb13038.x. [DOI] [PubMed] [Google Scholar]
- 6.Changeux JP, Bertrand D, Corringer PJ, Dehaene S, Edelstein S, Léna C, Le Novère N, Marubio L, Picciotto M, Zoli M. Brain nicotinic receptors: structure and regulation, role in learning and reinforcement. Brain Res Rev. 1998;26:198–216. doi: 10.1016/s0165-0173(97)00040-4. [DOI] [PubMed] [Google Scholar]
- 7.Cremer H, Chazal G, Carleton A, Goridis C, Vincent JD, Lledo PM. Long-term but not short-term plasticity at mossy fiber synapses is impaired in neural cell adhesion molecule-deficient mice. Proc Natl Sci USA. 1998;95:13242–13247. doi: 10.1073/pnas.95.22.13242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cremer H, Chazal G, Lledo PM, Rougon G, Montaron MF, Mayo W, Le Moal M, Abrous DN. PSA-NCAM: an important regulator of hippocampal plasticity. Int J Dev Neurosci. 2000;18:213–220. doi: 10.1016/s0736-5748(99)00090-8. [DOI] [PubMed] [Google Scholar]
- 9.Deroche V, Marinelli M, Le Moal M, Piazza PV. Glucocorticoids and behavioral effects of psychostimulants. II: cocaine intravenous self-administration and reinstatement depend on glucocorticoid levels. J Pharmacol Exp Ther. 1997;281:1401–1407. [PubMed] [Google Scholar]
- 10.Donny EC, Caggiula AR, Mielke MM, Jacobs KS, Rose C, Sved AF. Acquisition of nicotine self-administration in rats: the effects of dose, feeding schedule, and drug contingency. Psychopharmacology. 1998;136:83–90. doi: 10.1007/s002130050542. [DOI] [PubMed] [Google Scholar]
- 11.Eckhardt M, Bukalo O, Chazal G, Wang L, Goridis C, Schachner M, Gerardy-Schahn R, Cremer H, Dityatev A. Mice deficient in the polysialyltransferase ST8SiaIV/PST-1 allow discrimination of the roles of neural cell adhesion molecule protein and polysialic acid in neural development and synaptic plasticity. J Neurosci. 2000;20:5234–5344. doi: 10.1523/JNEUROSCI.20-14-05234.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisch AJ, Barrot M, Schad CA, Self DW, Nestler EJ. Opiates inhibit neurogenesis in the adult rat hippocampus. Proc Natl Acad Sci USA. 2000;97:7579–7584. doi: 10.1073/pnas.120552597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Annu Rev Psychol. 1997;48:649–684. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- 14.Foulds J, Stapleton J, Swettenham J, Bell N, McSorley K, Russel MAH. Cognitive performance effects of subcutaneous nicotine in smokers and never-smokers. Psychopharmacology. 1996;127:31–38. doi: 10.1007/BF02805972. [DOI] [PubMed] [Google Scholar]
- 15.Gould E, Cameron HA. Early NMDA receptor blockade impairs defensive behavior and increases cell proliferation in the dentate gyrus of developing rats. Behav Neurosci. 1997;111:49–56. doi: 10.1037//0735-7044.111.1.49. [DOI] [PubMed] [Google Scholar]
- 16.Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999a;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- 17.Gould E, Tanapat P, Hastings NB, Shors JT. Neurogenesis in adulthood: a possible role in learning. Trends Cogn Sci. 1999b;3:186–191. doi: 10.1016/s1364-6613(99)01310-8. [DOI] [PubMed] [Google Scholar]
- 18.Gross GC. Neurogenesis in the adult brain: death of a dogma. Nat Rev Neurosci. 2000;1:67–72. doi: 10.1038/35036235. [DOI] [PubMed] [Google Scholar]
- 19.Heishman SJ, Tayolor RC, Henningfield JE. Nicotine and smoking: a review of effects on human performance. Exp Clin Psychopharmacol. 1994;2:345–395. [Google Scholar]
- 20.Hu H, Tomasiewicz H, Magnuson T, Rutishauser U. The role of polysialic acid in migration of olfactory bulb interneuron precursors in the subventricular zone. Neuron. 1996;16:735–743. doi: 10.1016/s0896-6273(00)80094-x. [DOI] [PubMed] [Google Scholar]
- 21.Jaffe JH, Kanzler M. Smoking as an addictive disorder. NIDA Res Monogr. 1979;23:4–23. [PubMed] [Google Scholar]
- 22.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 23.Kiss JZ, Rougon G. Cell biology of polysialic acid. Curr Opin Neurobiol. 1997;7:640–646. doi: 10.1016/s0959-4388(97)80083-9. [DOI] [PubMed] [Google Scholar]
- 24.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuhn HG, Winkler J, Kempermann G, Thal LJ, Gage FH. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J Neurosci. 1997;17:5820–5829. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lemaire V, Koehl M, Le Moal M, Abrous DN. Prenatal produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci USA. 2000;97:11032–11037. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lukoyanov NV, Brandao F, Cadete-Leite A, Madeira MD, Paula-Barbosa MM. Synaptic reorganization in the hippocampal formation of alcohol-fed rats may compensate for functional deficits related to neuronal loss. Alcohol. 2000;20:139–148. doi: 10.1016/s0741-8329(99)00069-5. [DOI] [PubMed] [Google Scholar]
- 28.Mangam GL, Golding J. An enhancement model of smoking maintenance? In: Thorn RE, editor. Smoking behavior: physiological and psychological influences. Churchill Livingston; Edinburgh: 1978. pp. 87–114. [Google Scholar]
- 29.Merlo Pich E, Pagliusi SR, Tessari M, Talbot-Ayer D, van Huijsduijen RH, Chiamulera C. Common neuronal substrate for the addictive properties of nicotine and cocaine. Science. 1997;275:83–86. doi: 10.1126/science.275.5296.83. [DOI] [PubMed] [Google Scholar]
- 30.Miñana MD, Montoliu C, Llansola M, Grisolia S, Felipo V. Nicotine prevents glutamate induced proteolysis of the microtubule-associated protein MAP-2 and glutamate neurotoxicity in primary cultures of cerebellar neurons. Neuropharmacology. 1998;37:847–857. doi: 10.1016/s0028-3908(98)00074-4. [DOI] [PubMed] [Google Scholar]
- 31.Montaron MF, Petry KG, Rodriguez JJ, Marinelli M, Aurousseau C, Rougon G, Le Moal M, Abrous DN. Increase in neurogenesis but not PSA-NCAM expression in aged rats after adrenalectomy. Eur J Neurosci. 1999;11:1479–1485. doi: 10.1046/j.1460-9568.1999.00579.x. [DOI] [PubMed] [Google Scholar]
- 32.Muller D, Wang C, Skibo G, Toni N, Cremer H, Calaora V, Rougon G, Kiss JZ. PSA-NCAM is required for activity-induced synaptic plasticity. Neuron. 1996;17:413–422. doi: 10.1016/s0896-6273(00)80174-9. [DOI] [PubMed] [Google Scholar]
- 33.Murphy KJ, Regan CM. Sequential training in separate paradigms impairs second task consolidation and learning-associated modulations of hippocampal NCAM polysialylation. Neurobiol Learn Mem. 1999;72:28–38. doi: 10.1006/nlme.1998.3894. [DOI] [PubMed] [Google Scholar]
- 34.Murphy KJ, O'Connell AW, Regan CM. Repetitive and transient increases in hippocampal neural cell adhesion molecule polysialylation state following multitrial spatial training. J Neurochem. 1996;67:1268–1274. doi: 10.1046/j.1471-4159.1996.67031268.x. [DOI] [PubMed] [Google Scholar]
- 35.Nixon K, Crews FT (2001) Decreased neurogenesis following an alcohol binge. Thirty-First Annual Meeting of the Society for Neuroscience, San Diego, CA, November.
- 36.Nowakowski RS, Lewin SB, Miller MW. Bromodeoxyuridine immunohistochemical determination of the lengths of the cell cycle and the DNA-synthetic phase for an anatomically defined population. J Neurocytol. 1989;18:311–318. doi: 10.1007/BF01190834. [DOI] [PubMed] [Google Scholar]
- 37.O'Connell AW, Fox B, Barry T, Murphy KJ, Fichera G, Foley AG, Kelly J, Regan CM. Spatial learning activated neural cell adhesion molecule polysialylation in a corticohippocampal pathway within the medial temporal lobe. J Neurochem. 1997;38:2538–2546. doi: 10.1046/j.1471-4159.1997.68062538.x. [DOI] [PubMed] [Google Scholar]
- 38.Olds DL, Henderson CR, Tatelbaum R. Intellectual impairment in children of women who smoke cigarettes during pregnancy. Pediatrics. 1994;93:221–227. [PubMed] [Google Scholar]
- 39.Paula-Barbosa MM, Brabdai F, Madeira MD, Cadete-Letite A. Structural changes in the hippocampal formation after long-term alcohol consumption and withdrawal in the rat. Addiction. 1993;88:237–247. doi: 10.1111/j.1360-0443.1993.tb00807.x. [DOI] [PubMed] [Google Scholar]
- 40.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic; Sidney: 1982. [DOI] [PubMed] [Google Scholar]
- 41.Pontieri FE, Tanda G, Orzi F, Di Chiara G. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature. 1996;382:255–257. doi: 10.1038/382255a0. [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez JJ, Montaron MF, Petry KG, Aurousseau C, Marinelli M, Premier S, Rougon G, Le Moal M, Abrous DN. Complex regulation of PSA-NCAM expression by glucocorticoids in the rat hippocampus. Eur J Neurosci. 1998;10:2994–3006. doi: 10.1046/j.1460-9568.1998.00316.x. [DOI] [PubMed] [Google Scholar]
- 43.Rose SPR. Cell-adhesion molecules, glucocorticoids and long-term-memory formation. Trends Neurosci. 1995;18:502–506. doi: 10.1016/0166-2236(95)92774-k. [DOI] [PubMed] [Google Scholar]
- 44.Rougon G. Structure, metabolism and cell biology of polysialic acids. Eur J Cell Biol. 1993;61:197–207. [PubMed] [Google Scholar]
- 45.Rougon G, Dubois C, Buckley N, Magnani JL, Zollinger W. A monoclonal antibody against meningococcus group B polysaccharides distinguishes embryonic from adult N-CAM. J Cell Biol. 1986;103:2429–2437. doi: 10.1083/jcb.103.6.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roy TS, Andrews JE, Seidler FJ, Slotkin TA. Nicotine evokes cell death in embryonic rat brain during neurulation. J Pharmacol Exp Ther. 1998;287:1136–1144. [PubMed] [Google Scholar]
- 47.Seki T, Arai Y. Highly polysialylated neural cell adhesion molecule (NCAM-H) is expressed by newly generated granule cells in the dentate gyrus of the adult rat. J Neurosci. 1993;13:2351–2369. doi: 10.1523/JNEUROSCI.13-06-02351.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sexton M, Fox NL, Hebel R. Prenatal exposure to tobacco: II. Effects on cognitive functioning at age three. Int J Epidemiol. 1990;19:72–77. doi: 10.1093/ije/19.1.72. [DOI] [PubMed] [Google Scholar]
- 49.Shoaib M, Stolerman IP. Plasma nicotine and cotinine levels following intravenous nicotine self-administration in rats. Psychopharmacology. 1999;143:318–321. doi: 10.1007/s002130050954. [DOI] [PubMed] [Google Scholar]
- 50.Shoaib M, Schindler CW, Goldeberg SR. Nicotine self-administration in rats: strain and nicotine pre-exposure effects on acquisition. Psychopharmacology. 1997;29:35–45. doi: 10.1007/s002130050159. [DOI] [PubMed] [Google Scholar]
- 51.Shors JT, Miesegaes G, Beylin A, Zao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–375. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- 52.Snyder FR, Henningfield JE. Effects of nicotine administration following 12h of tobacco deprivation: assessment on computerized performance tasks. Psychopharmacology. 1989;97:17–22. doi: 10.1007/BF00443406. [DOI] [PubMed] [Google Scholar]
- 53.Snyder FR, Davis FC, Henningfield JE. The tobacco withdrawal syndrome: performance decrements assessed on a computerized test battery. Drug Alcohol Depend. 1989;23:259–266. doi: 10.1016/0376-8716(89)90090-2. [DOI] [PubMed] [Google Scholar]
- 54.Stolerman IP, Jarvis MJ. The scientific case that nicotine is addictive. Psychopharmacology. 1995;117:2–10. doi: 10.1007/BF02245088. [DOI] [PubMed] [Google Scholar]
- 55.Vorel SR, Liu X, Hayes RJ, Spector JA, Gardner EL. Relapse to cocaine-seeking after hippocampal theta burst stimulation. Science. 2001;292:1175–1178. doi: 10.1126/science.1058043. [DOI] [PubMed] [Google Scholar]
- 56.Wagner JP, Black IB, DiCicco-Bloom E. Stimulation of neonatal and adult brain neurogenesis by subcutaneous injection of basic fibroblast growth factor. J Neurosci. 1999;19:6006–6016. doi: 10.1523/JNEUROSCI.19-14-06006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]