Abstract
Intro:
Dedifferentiated/undifferentiated endometrial carcinomas (DDEC/UEC) are aggressive endometrial cancers with frequent genomic inactivation of core components of switch/sucrose non-fermentable (SWI/SNF) complex proteins. Claudin-4, an epithelial intercellular tight junction protein was recently found to be expressed in SWI/SNF-deficient undifferentiated carcinomas but not SWI/SNF-deficient sarcomas. The aim of this study is to examine claudin-4 expression in UEC/DDEC and other high-grade uterine carcinomas.
Materials and Methods:
We examined claudin-4 expression by immunohistochemistry (clone 3E2C1) on tissue microarrays that contained 44 UEC/DDEC (24 SWI/SNF-deficient), 50 carcinosarcomas, 164 grade 3 endometrioid carcinomas, 57 serous carcinomas and 20 clear cell carcinomas. Tumors with less than 5% claudin-4 expression were considered to be negative.
Results:
Nearly all SWI/SNF-deficient, and most SWI/SNF proficient, UEC/DDEC showed a complete absence of claudin-4 expression in the undifferentiated tumor, while the differentiated component in DDEC showed consistent and diffuse claudin-4 expression. Only one SWI/SNF-deficient DDEC showed focal expression of claudin-4 in the undifferentiated component compared to diffuse expression in the corresponding differentiated component. Claudin-4 expression was consistently absent in the sarcomatous component of carcinosarcoma and it was absent in a 24% of grade 3 endometrioid carcinomas and serous carcinomas.
Conclusion:
Claudin-4 expression can be absent or very focal in a subset of high-grade endometrial carcinomas and is almost always absent in the undifferentiated area of SWI/SNF-deficient UEC/DDEC, despite the apparent epithelial origin in the case of DDEC. Claudin-4 expression therefore cannot be used to infer mesenchymal or epithelial tumor origin in the endometrium. The consistent loss or downregulation of claudin-4, a tight junction protein, in SWI/SNF-deficient UEC/DDEC further supports the undifferentiated nature of these tumors.
Keywords: Endometrial cancer, endometrioid adenocarcinoma, undifferentiated carcinoma, uterine sarcomas, claudin-4, SWI/SNF proteins
INTRODUCTION:
Dedifferentiated endometrial carcinomas (DDEC) and undifferentiated endometrial carcinomas (UEC) are among the most aggressive histologic types of endometrial cancer, with a reported outcome that is worse than FIGO grade 3 endometrioid endometrial carcinomas.1, 2 The undifferentiated carcinomas in UEC and the undifferentiated component of DDEC are similar histologically. It consists of a sheet-like proliferation of monomorphic medium-sized cells with no apparent epithelial differentiation histologically and the tumors cells frequently appear discohesive in nature, imparting rhabdoid morphology in some cases.3 Immunophenotypically, the undifferentiated carcinoma shows significantly diminished to completely absent expression of epithelial markers (i.e. cytokeratins and epithelial membrane antigen) and Mullerian epithelial transcriptional factors including PAX8 and estrogen receptor (ER), in contrast to the typically intact expression of these proteins in the corresponding differentiated carcinoma component in DDEC. Furthermore, the majority of DDEC/UEC are deficient in the expression of mismatch repair (MMR) proteins. We recently identified frequent genomic inactivation of certain core components of switch/sucrose non-fermentable (SWI/SNF) complex proteins that are associated with histologic dedifferentiation.1, 4 More specifically, there were three mutually exclusive patterns of genomic inactivation that results in 1) loss of BRG1 (encoded by SMARCA4) expression, 2) loss of INI1 (encoded by SMARCB1) expression, or 3) concurrent losses of ARID1A and ARID1B expression in the undifferentiated tumor. This is analogous to lung carcinoma and sinonasal carcinoma5–7, where SWI/SNF inactivation has been reported and is associated with undifferentiated histology as well as aggressive clinical behavior. Therapeutically, there is accumulating preclinical and early clinical evidence to suggest that tumors that are SWI/SNF-deficient may be more responsive to drugs that target chromatin remodeling.8–10
Claudin-4 is a transmembrane protein involved in the formation of intercellular tight junction which is important in mediating cell adhesion, proliferation and differentiation.11, 12 It is expressed in a variety of normal epithelial tissues and its expression is also seen in many different types of epithelial malignancy.13–15 A number of studies have implicated increased claudin-4 expression with decreased survival, particularly in breast cancer and gastric cancer.16–18 Overexpression of claudin-4 in breast cancer and gastric cancer cell lines has been shown to increase cell proliferation and migration in vitro19, 20. In endometrial cancer, Pan et al have observed consistent claudin-4 expression in a series of predominantly low-grade endometrioid-type carcinoma (n=30, with 23 tumors being FIGO grade 1 or 2).21
Schaefer et al recently examined the expression of claudin-4 in a large number of epithelial and mesenchymal malignancies that included SWI/SNF-deficient tumors such as epithelioid sarcoma, INI1-deficient sinonasal carcinomas and BRG1-deficient small cell carcinoma hypercalcemic type of the ovary and the results suggested a potential utility for claudin-4 in differentiating between tumors of epithelial versus mesenchymal origin.22 The study showed that the great majority of sarcomas with epithelioid morphology or SWI/SNF deficiency lacks claudin-4 expression while the great majority of undifferentiated carcinomas including tumors that are SWI/SNF-deficient shows claudin-4 expression. This study only examined one DDEC for which claudin-4 staining was not specifically reported.
To better understand its expression in DDEC/UEC, we examined claudin-4 expression by immunohistochemistry in a series of DDEC/UEC (including both SWI/SNF-deficient and SWI/SNF-intact tumors) as well as a series of high-grade endometrial epithelial and/or mesenchymal neoplasms.
MATERIALS AND METHODS:
Study sample:
This study examined a total of 366 tumors, with 44 UEC/DDEC (24 SWI/SNF-deficient), 50 carcinosarcomas, 164 grade 3 endometrioid carcinomas, 57 serous carcinomas and 20 clear cell carcinomas and 31 uterine sarcomas (leiomyosarcomas, rhabdomyosarcomas, adenosarcomas, endometrial stromal sarcomas and undifferentiated uterine sarcomas). These tumors were evaluated in a series of tissue microarrays (TMA), with each tumor represented in duplicate cores (0.6mm). Additional cores were used to represent the different components of DDEC (differentiated and undifferentiated components) and carcinosarcoma (carcinomatous and sarcomatous components) in these TMAs.1 The study was approved by the Institutional Review Board.
Immunohistochemistry:
Immunohistochemistry for claudin-4 was performed using the clone 3E2C1 (mouse monoclonal, Invitrogen, Carlsbad, CA, USA) at 1/300 dilution in Da Vinci Green diluent at room temperature for 37 minutes. Slides were then washed and incubated with Mach2 Mouse-HRP polymer for 32 minutes at room temperature and detected with IP DAB chromogen for 5 minutes. Nuclei were counterstained with a 1/10 dilution of CAT hematoxylin then slides were again washed, air dried and coverslipped manually. Mismatch repair immunohistochemistry was performed with the stainings scored as previously described.23 The slides were incubated with MLH1 (DAKO clone ES05 1:100), MSH2 (NCL clone 25D12 prediluted), MSH6 (BD Bioscience 44/MSH6 1:2000), PMS2 (BD Bioscience A16–4 1:100) and processed using the Leica Bond Max platform (Leica Microsystems, Wetzlar, Germany) as per manufacturer’s protocol with proprietary reagents.”
Claudin-4 staining was scored by two pathologists (BTC and CHL) and membranous staining was evaluated. Differences in scoring were further reviewed by the two pathologists to reach a consensus score. This included two cases with discrepant scoring results that crossed the cut-off threshold (for claudin-4 positivity) described below, where consensus scores were reached upon further review. Tumor cells were scored based on their staining intensity (0: no staining, 1: weak membranous staining, 2: moderate membranous staining and 3: strong membranous staining) and percentage of tumor cell staining broken down in 6 increments (0: no staining, 1: <5%, 2: 5–24%, 3: 25–49%, 4: 50–74%, 5: 75–100%). Positive claudin-4 immunostaining was defined by at least weak intensity staining in over 5% of the tumor cells and this was the same threshold used by previous study.22 The different histologic components of DDEC and carcinosarcoma were scored separately. The claudin-4 scoring results of UEC/DDEC cases were confirmed on whole section.
RESULTS:
The results of claudin-4 immunohistochemistry are summarized in Table S1. We studied 44 DDEC/UEC (20 DDEC and 24 UEC) where 24 tumors were SWI/SNF-deficient. The differentiated components in all DDEC examined were low-grade endometrioid in type and were all positive for claudin-4, most of which showing expression in over 75% of tumor cells (Figure 1). Nearly all SWI/SNF-deficient UEC/DDEC showed a complete absence of claudin-4 expression in the undifferentiated tumor (Figure 1A-D). Only 1 of 24 SWI/SNF-deficient DDEC/UEC (BRG1-deficient) was positive for claudin-4, and this tumor showed patchy expression (20%) of claudin-4 in the undifferentiated component but diffuse expression in the corresponding differentiated component. In addition, 4 cases showed very focal expression of claudin-4 in the undifferentiated component (in 1–2% of the tumor cells) and these cases were considered negative for claudin-4 as they were well below the 5% cut-off threshold used by prior and current studies. While claudin-4 expression in SWI/SNF-deficient undifferentiated tumor typically occurred as scattered single cell staining in which the cells expressing claudin-4 appear to be morphologically indistinguishable from cells lacking claudin-4 expression, its expression in one case appeared to occur focally in small nests of tumor cells that possessed a greater amount of cytoplasm (Figure 1E-F). Among the 20 SWI/SNF-intact DDEC/UEC, 4 tumors were positive for claudin-4 expression in the undifferentiated area (ranging from 30 to 80%). With regards to MMR protein expression status, 2 of 24 (8%) MMR-deficient DDEC/UEC were positive for claudin-4 while 3 of 19 (16%) MMR-intact were positive for claudin-4 in the undifferentiated tumor.
Figure 1:
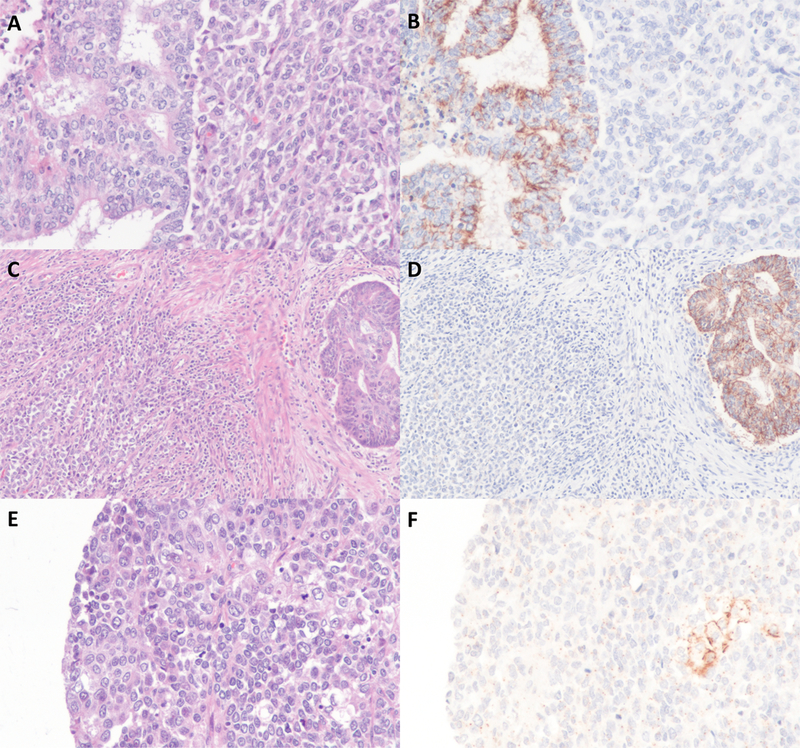
Claudin-4 immunohistochemistry DDEC/UEC. A-D) Two cases of SWI/SNF-deficient DDEC showing absent claudin-4 expression in undifferentiated component and diffuse claudin-4 expression in the differentiated component. E-F) A case of SWI/SNF-deficient UEC showing focal expression of claudin-4.
Among other high-grade endometrial carcinomas surveyed, claudin-4 expression was observed in 76% of FIGO grade 3 endometrioid carcinomas, 74% of serous carcinomas and 55% of clear cell carcinomas. In uterine carcinosarcomas, claudin-4 expression was found in 92% of the cases, but its expression was restricted to only the carcinomatous component in the positive cases whereas the corresponding sarcomatous components were consistently negative for claudin-4. The extent of claudin-4 expression in the carcinomatous component of uterine carcinosarcoma was however highly variable, ranging from 5 to 100%. In uterine adenosarcoma, claudin-4 was expressed in the benign epithelial elements in the tumor but was consistently absent in the mesenchymal/sarcoma component. There was no claudin-4 expression observed in the pure uterine sarcomas (leiomyosarcomas, rhabdomyosarcomas, endometrial stromal sarcomas and undifferentiated sarcomas) examined (Figure 2).
Figure 2:
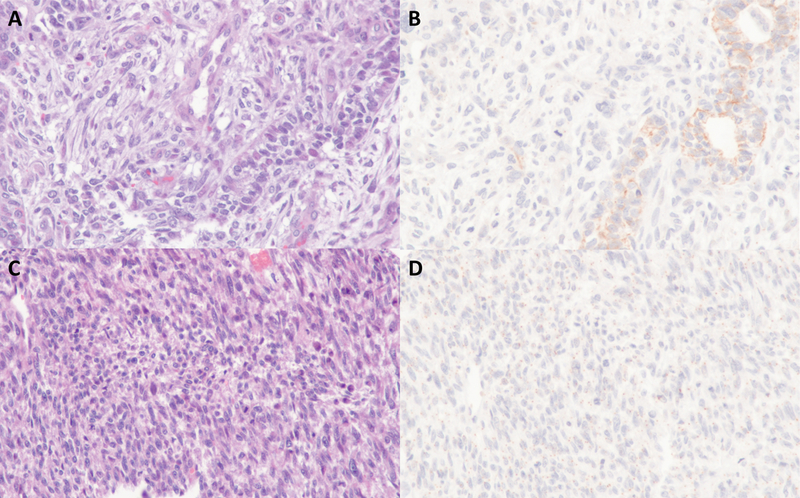
Representative examples of claudin-4 expression in a carcinosarcoma (A-B) and a leiomyosarcoma (C-D).
DISCUSSION:
We confirmed in this study frequent expression of claudin-4 in several histotypes of endometrial carcinomas, but the percentage of tumors showing claudin-4 expression is variable across the different histotypes. Most notably, in the case of SWI/SNF-deficient DDEC, while the low-grade endometrioid carcinoma component consistently exhibited claudin-4 expression, the corresponding undifferentiated carcinoma component in the great majority of cases lacked claudin-4 expression, despite apparent epithelial origin. In addition, all SWI/SNF-deficient UEC examined also lacked claudin-4 expression. In the cases of SWI/SNF-intact DDEC/UEC, the undifferentiated carcinoma in the majority of cases also lacks claudin-4 expression. These findings clearly demonstrate that claudin-4 expression is frequently lost in the process of cellular dedifferentiation in endometrial carcinoma, either through core SWI/SNF complex protein inactivation or other mechanisms that are currently undefined. This observation is in keeping with our proposed mechanism of dedifferentiation in endometrial cancer where a significant perturbation such as genomic inactivation of core SWI/SNF protein(s) prevents Mullerian epithelial differentiation, hence arresting the tumor cells in a primitive cellular state that is reflected histologically and immunophenotypically as an undifferentiated tumor. This phenomenon is best exemplified in the case of SWI/SNF-deficient DDEC, in which the undifferentiated carcinoma arises from the typically low-grade endometrioid carcinoma component.1, 4 The genomic inactivation of core SWI/SNF complex proteins only occurs in the undifferentiated carcinoma component, and it is associated with loss or markedly diminished expression of epithelial markers such as cytokeratins and epithelial membrane antigen (EMA), and for PAX8 and ER. While claudin-4 expression overexpression has been implicated as a negative prognostic and a pro-tumorigenic factor in a number of cancer types, we speculate that in the case of DDEC/UEC, the loss or diminished claudin-4 expression primarily reflects the undifferentiated state of the tumor cells and it is this arrest at a primitive cellular state that underlies the aggressive clinical behavior of these tumors. Furthermore, a comparable majority of MMR-deficient DDEC/UEC and MMR-intact DDEC/UEC displayed absent claudin-4 expression, suggesting that the absence of claudin-4 expression is not related to the hypermutating nature of MMR-deficient tumor.
In contrast to SWI/SNF-deficient DDEC/UEC where the undifferentiated tumor was consistently negative for claudin-4, the undifferentiated tumor in a small subset of SWI/SNF-intact DDEC/UEC was positive for claudin-4. While the reason underlying this difference is unknown as the mechanisms of dedifferentiation in these SWI/SNF-intact tumors remain undefined, it is plausible that the claudin-4 positive tumors may represent very poorly differentiated examples of FIGO grade 3 endometrioid carcinoma that were morphologically inseparable from DDEC/UEC despite centralized review. Further studies are needed to gain insights into the genetic basis underlying the undifferentiated morphology in SWI/SNF-intact DDEC/UEC.
In addition to DDEC/UEC, other high-grade endometrial carcinomas can also show an absence of claudin-4 expression, with negative claudin-4 staining seen in about 50% of clear cell carcinomas, 25% of serous carcinomas and 25% of FIGO grade 3 endometrioid carcinomas. The clinical and biological significance of loss of claudin-4 expression in these endometrial carcinomas is unknown. Diagnostically, these findings show that absent claudin-4 expression is not specific to DDEC/UEC. However, as with other epithelial keratin markers and mullerian epithelial differentiation markers (i.e. ER and PAX8), the finding of diffuse claudin-4 expression in a tumor would argue against the diagnosis of undifferentiated carcinoma, similar to the presence of diffuse ER, PAX8, pankeratin and/or EMA staining.24 There was also consistent absence of claudin-4 expression in the sarcomatous component of uterine carcinosarcoma and adenosarcoma, as well as in pure uterine sarcomas such as leiomyosarcoma and endometrial stromal sarcoma.
Schaefer et al recently reported that 80% of undifferentiated carcinomas including tumors that were SWI/SNF-deficient retained claudin-4 expression.22 This earlier study however focused on undifferentiated carcinoma from other anatomical sites (bone and soft tissue, ovary, nose and sinuses) and the difference in our findings likely reflects biologic differences between undifferentiated carcinomas of the endometrium versus undifferentiated carcinomas from other sites. However, given that SWI/SNF-deficient DDEC/UEC frequently lacks claudin-4 expression despite the apparent epithelial origin especially in the case of DDEC, it is clear that the absence of claudin-4 expression cannot be used to infer mesenchymal origin in an endometrial malignancy. On that note, even though BRG1-deficient small cell carcinoma – hypercalcemic type of the ovary consistently lacks claudin-4 expression, the lack of claudin-4 expression alone does not necessarily indicate a non-epithelial origin of this tumor.
In summary, claudin-4 expression is frequently absent in DDEC/UEC, particularly in cases that are SWI/SNF-deficient and this further reflects its primitive undifferentiated state. While the absence of claudin-4 expression in an undifferentiated tumor can be used to support a diagnosis of DDEC/UEC, it is not specific as claudin-4 expression can be absent in a subset of other high-grade endometrial carcinomas, and correlation with histologic and other immunophenotypic features is important.
Supplementary Material
Acknowledgements:
BT-C and CHL: Design the research study, analyzed the data and wrote the paper. RAS, CJRS and MR: Provided clinical information and case materials, and also contributed to study design and revision of the draft manuscript.
Sources of support/funding: This study is supported in part by research funds from Cancer Research Society of Canada (20313), Royal Alexandra Hospital foundation, Alberta Cancer Foundation, Calgary Laboratory Services Internal Research Competition (RS14–513). Dr. Soslow is supported in part by the MSK Cancer Center Support Grant P30 CA008748.
Footnotes
Disclosure: The study authors have no conflicts of interest to declare.
REFERENCES:
- 1.Karnezis AN, Hoang LN, Coatham M et al. Loss of switch/sucrose non-fermenting complex protein expression is associated with dedifferentiation in endometrial carcinomas. Mod Pathol 2016;29;302–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coatham M, Li X, Karnezis AN et al. Concurrent arid1a and arid1b inactivation in endometrial and ovarian dedifferentiated carcinomas. Mod Pathol 2016;29;1586–1593. [DOI] [PubMed] [Google Scholar]
- 3.Altrabulsi B, Malpica A, Deavers MT, Bodurka DC, Broaddus R, Silva EG. Undifferentiated carcinoma of the endometrium. Am J Surg Pathol 2005;29;1316–1321. [DOI] [PubMed] [Google Scholar]
- 4.Kuhn E, Ayhan A, Bahadirli-Talbott A, Zhao C, Shih Ie M. Molecular characterization of undifferentiated carcinoma associated with endometrioid carcinoma. Am J Surg Pathol 2014;38;660–665. [DOI] [PubMed] [Google Scholar]
- 5.Agaimy A, Fuchs F, Moskalev EA, Sirbu H, Hartmann A, Haller F. Smarca4-deficient pulmonary adenocarcinoma: Clinicopathological, immunohistochemical, and molecular characteristics of a novel aggressive neoplasm with a consistent ttf1neg/ck7pos/heppar-1pos immunophenotype. Virchows Arch 2017;471;599–609. [DOI] [PubMed] [Google Scholar]
- 6.Agaimy A, Koch M, Lell M et al. Smarcb1(ini1)-deficient sinonasal basaloid carcinoma: A novel member of the expanding family of smarcb1-deficient neoplasms. Am J Surg Pathol 2014;38;1274–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agaimy A, Weichert W. Smarca4-deficient sinonasal carcinoma. Head Neck Pathol 2017;11;541–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Filippakopoulos P, Qi J, Picaud S et al. Selective inhibition of bet bromodomains. Nature 2010;468;1067–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helming KC, Wang X, Roberts CW. Vulnerabilities of mutant swi/snf complexes in cancer. Cancer Cell 2014;26;309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oike T, Ogiwara H, Tominaga Y et al. A synthetic lethality-based strategy to treat cancers harboring a genetic deficiency in the chromatin remodeling factor brg1. Cancer Res 2013;73;5508–5518. [DOI] [PubMed] [Google Scholar]
- 11.Beeman N, Webb PG, Baumgartner HK. Occludin is required for apoptosis when claudin-claudin interactions are disrupted. Cell Death Dis 2012;3;e273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Itallie C, Rahner C, Anderson JM. Regulated expression of claudin-4 decreases paracellular conductance through a selective decrease in sodium permeability. J Clin Invest 2001;107;1319–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding L, Lu Z, Lu Q, Chen YH. The claudin family of proteins in human malignancy: A clinical perspective. Cancer Manag Res 2013;5;367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holczbauer A, Gyongyosi B, Lotz G et al. Distinct claudin expression profiles of hepatocellular carcinoma and metastatic colorectal and pancreatic carcinomas. J Histochem Cytochem 2013;61;294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu S, Singh K, Mangray S et al. Claudin expression in high-grade invasive ductal carcinoma of the breast: Correlation with the molecular subtype. Mod Pathol 2013;26;485–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lanigan F, McKiernan E, Brennan DJ et al. Increased claudin-4 expression is associated with poor prognosis and high tumour grade in breast cancer. Int J Cancer 2009;124;2088–2097. [DOI] [PubMed] [Google Scholar]
- 17.Liu JX, Wei ZY, Chen JS, Lu HC, Hao L, Li WJ. Prognostic and clinical significance of claudin-4 in gastric cancer: A meta-analysis. World J Surg Oncol 2015;13;207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin de la Fuente L, Malander S, Hartman L et al. Claudin-4 expression is associated with survival in ovarian cancer but not with chemotherapy response. Int J Gynecol Pathol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hwang TL, Changchien TT, Wang CC, Wu CM. Claudin-4 expression in gastric cancer cells enhances the invasion and is associated with the increased level of matrix metalloproteinase-2 and −9 expression. Oncol Lett 2014;8;1367–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma X, Miao H, Jing B et al. Claudin-4 controls the proliferation, apoptosis, migration and in vivo growth of mcf-7 breast cancer cells. Oncol Rep 2015;34;681–690. [DOI] [PubMed] [Google Scholar]
- 21.Pan XY, Wang B, Che YC, Weng ZP, Dai HY, Peng W. Expression of claudin-3 and claudin-4 in normal, hyperplastic, and malignant endometrial tissue. Int J Gynecol Cancer 2007;17;233–241. [DOI] [PubMed] [Google Scholar]
- 22.Schaefer IM, Agaimy A, Fletcher CD, Hornick JL. Claudin-4 expression distinguishes swi/snf complex-deficient undifferentiated carcinomas from sarcomas. Mod Pathol 2017;30;539–548. [DOI] [PubMed] [Google Scholar]
- 23.Hoang LN, Ali RH, Lau S, Gilks CB, Lee CH. Immunohistochemical survey of mismatch repair protein expression in uterine sarcomas and carcinosarcomas. Int J Gynecol Pathol 2014;33;483–491. [DOI] [PubMed] [Google Scholar]
- 24.Hoang LN, Lee YS, Karnezis AN et al. Immunophenotypic features of dedifferentiated endometrial carcinoma - insights from brg1/ini1-deficient tumours. Histopathology 2016;69;560–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


