Abstract
An 82-year-old man with diabetes was admitted to the emergency department with a third-degree burn on his left leg. The deep swab specimen from his left leg was cultured on Sabouraud dextrose agar without cycloheximide and incubated at 25 °C for 5 days. On the basis of morphological characteristics and multigene phylogenetic analyses of the internal transcribed spacer region of ribosomal DNA and partial fragments of beta-tubulin and translation elongation factor 1-alpha, the causal agent of fungal skin infection was identified as Bisifusarium delphinoides, which was newly introduced by accommodating a Fusarium dimerum species complex. Thus, we describe here the first case of skin infection caused by B. delphinoides on a burn patient with diabetes mellitus based on morphological observation and molecular analysis.
Keywords: Antifungal susceptibility, FDSC, opportunistic pathogen, phylogenetic analysis
Fusarium, a well-known plant pathogen, has emerged as an important cause of invasive or disseminated infection in immunocompromised patients. Fusarium species, which are considered as the second most frequent mold in fungal infection, cause a broad spectrum of infections including keratitis, onychomycosis, endophthalmitis, and skin infection [1,2]. Especially, most of the skin infections were involved in immunocompromised patients [3]. The most common causative agent of fusariosis is Fusarium solani, followed by F. oxysporum and F. verticillioides. Fusarium dimerum species complex (FDSC) including F. dimerum, F. delphinoides, and F. nectrioides is rarely detected as a human pathogen [4,5]. Especially, F. delphinoides was very rarely reported as a human pathogen rather than F. dimerum within FDSC [6].
In the last few decades, as the number of people with diabetes increases, the case report of Fusarium infection associated with a diabetic patient is also increasing. Diabetes mellitus can increase the susceptibility to fungal infection due to the reduced response of T cells, neutrophil function, and disorders of humoral immunity [7]. In addition, Fusarium can rarely cause burn wound infection as an opportunistic pathogen [8,9]. Although common fungi observed in burn wound were species of Aspergillus, Mucor, and Candia, a small number of fungal infections caused by Fusarium species were frequently encountered in burn patients [10].
Recently, as the status of several genera in Nectriaceae were re-evaluated based on a multi-gene phylogenetic analysis, the new genus Bisifusarium was introduced by accommodating several species (e.g., Fusarium biseptatum, F. delphinoides, F. domesticum, and F. lunatum) which had been classified as FDSC [11]. In this study, we quote the new genus name of Bisifusarium and describe here the first case of skin infection caused by B. delphinoides in a burn patient with diabetic disease based on morphological observation and phylogenetic analysis.
Morphological characteristics were determined from isolates grown on synthetic nutrient-poor agar (SNA) and potato dextrose agar (PDA). Colony morphology was described using an isolate grown on SNA and PDA after 7 days. Fungal structures were mounted on a glass slide with Shear’s mounting fluid. An Olympus BX51 microscope (Olympus, Tokyo, Japan) was used to measure the morphological characteristics and a Zeiss AX10 microscope equipped with an AxioCam MRc5 was used for imaging. Thirty measurements for each structure were taken at 20× to 1000× magnification. To confirm the tentative identification based on morphological characteristics, molecular analysis was performed. A DNeasy Plant Mini Kit (Qiagen Inc., Valencia, CA) was used for extracting fungal genomic DNA. The internal transcribed spacer (ITS) region of rDNA, partial fragments of beta-tubulin (β-tubulin), and translation elongation factor 1-alpha (EF1-α) were amplified using the primer ITS1/ITS4 [12], T1/T22, and EF1/EF2 [13]. The PCR parameters for amplification were as follows: an initial denaturation at 94 °C for 2 min; 35 cycles of denaturation at 95 °C for 35 s, annealing at 52 °C for 50 s, and extension at 72 °C for 2 min, with a final extension at 72 °C for 6 min for ITS and EF1-α. The annealing temperature was changed to 58 °C for β-tubulin [6]. The PCR amplicons were visualized on a 1.5% agarose gels to confirm the target size and were purified using a QIAquick PCR purification Kit (Qiagen Inc.). The purified PCR products were sequenced directly using Bioneer Sequencing Service (Bioneer, Daejeon, Korea).
For phylogenetic analyses, all available ITS, β-tubulin, and EF1-α sequences of the species belonging to Fusarium dimerum species complex were retrieved from the NCBI GeneBank database. Consensus sequences were assembled using the SeqMan package of Lasergene software (DNAStar, Madison, WI) and each alignment of three loci was concatenated using Mesquite version 3.2 [14]. The sequences of Rectifusarium ventricosum, previously treated as F. ventricosum, were included to represent outgroup taxon. ML analysis was performed using RAxML 7.0.3 [15] as implemented in RAxML GUI 1.52 [16]. GTRCAT model was adjusted with 1000 bootstrap replicates. In vitro antifungal susceptibility of B. delphinoides was tested by a broth microdilution assay following the Clinical and Laboratory Standards Institute guidelines for filamentous fungi [17]. Amphotericin B, voriconazole, itraconazole, and ketoconazole were tested at final concentrations ranging from 0.03 to 16 µl/ml. Fluconazole and flucytosine (FC-5) were used at final concentrations ranging from 0.125 to 64 µl/ml. Candida parapsilosis ATCC22019 was used as quality control. The minimum inhibitory concentration (MIC) of the antifungal agents was determined at 48 h and the antifungal susceptibility tests were confirmed by performing twice.
An 82-year-old man with diabetes was admitted to the emergency department with a third-degree burn on his left leg at International St. Mary’s Hospital located in Incheon, Korea. With serial excision, he was treated with broad-spectrum antibiotics during hospitalization. On the 14th day of his stay, the anterior surface of the left leg with skin trauma of burn showed the painful ulcerous lesions with pus discharge. Laboratory investigations revealed leukocytosis (11.5 × 103/µl) and elevated ESR (52 mm/h). Other laboratory data such as serum electrolytes, kidney, and liver function tests were in the normal value range. On the biopsied skin specimen, hematoxylin and eosin staining revealed a vascular and fibroblastic proliferation in addition to chronic granulomatous infection. For the culture, the two separated deep swab samples were taken from the curettage of the ulcer lesion. On Gram staining from the ulcer lesion, it showed a filamentous fungus as the only detectable microorganism. After 5 days of incubation at 25 °C, the sample from the left leg also yielded a pure culture of a filamentous fungi which was consistent with the previous Gram stain observation. The morphology of the abundant conidia suggested Fusarium spp as a causative agent. We did not perform a blood culture because of the absence of fever or sign of dissemination to other tissues.
For the treatment, the guideline of Fusarium infection has not been clearly documented due to the lack of clinical data. For the localized superficial Fusarium infections, surgical debridement should be considered and combination therapy of antifungal agents and the debridement may be used. In our case, the patient was not in fever and it was considered as a possible localized infection rather than systematic disseminated infection. Therefore, the surgical debridement and excision of infected tissue were performed for the initial treatment without an antifungal treatment and clearing up of cutaneous lesion that he underwent after 6 weeks. The obtained isolate was deposited in the Korea Center for Disease Control and Prevention, the National Culture Collection for Pathogens (MFCCKHM00039).
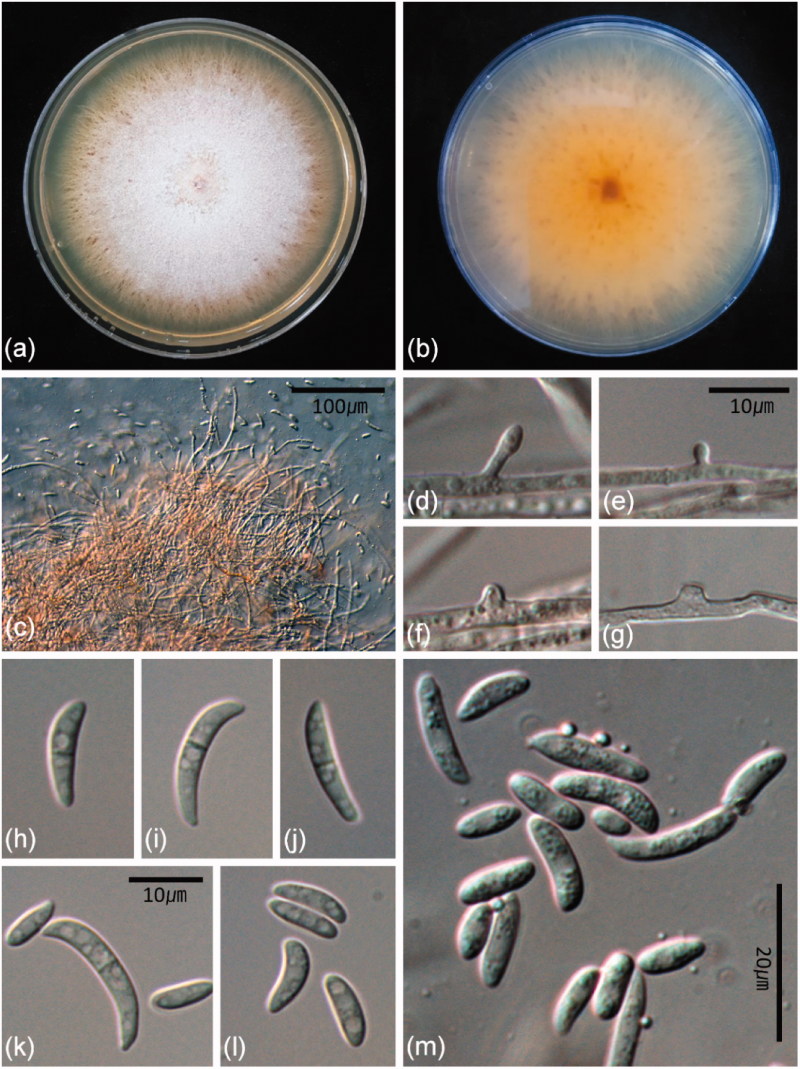
The fungal colonies moderately fast growing on PDA initially appeared white, but later turned pale orange due to sporulation and aerial mycelia was sparsely developed (Figure 1(a)). Colony reverse on PDA was pale orange with red-brown and rust-brown speckles (Figure 1(b)). Conidiophores were later phialidic pegs arising from superficial or submerged hyphae. Simple conidiophores were monophialidic, solitary, or aggregated when forming terminally or laterally on hyphae. Pionnotal sporodochia were poorly developed with densely arranged phialides (Figure 1(c)). Monophialides formed terminally or laterally along hyphae were cylindrical and slightly tapering toward the tip or flask-shaped with the widest point (Figures 1(d–g)). Polyphialides were absent. Macroconidia were formed abundantly in masses on poorly or well-developed sporodochia, mostly 1-septate, rarely 2-septate, curved to lunated, and 10–21 × 2.5–4 µm. Microconidia were mostly aseptate, ellipsoidal, allantoid, broadly lunate, similar shape but smaller than macroconidia, mostly formed by monophialidic conidiophores and later phialidic pegs and 4.5–9.5 × 2–3 µm (Figure 1(h–m)). The morphological and cultural characteristics were consistent with those of Fusarium delphinoides of which current name is Bisifusarium delphinoides [6].
Figure 1.
Morphological characteristics of Bisifusarium delphinoides. (a) One-week-old colony grown on potato dextrose agar (PDA); (b) one-week-old reverse colony on PDA; (c) Sporodochia; (d–g) lateral phialidic pegs; (h–m) macroconidia and microconidia.
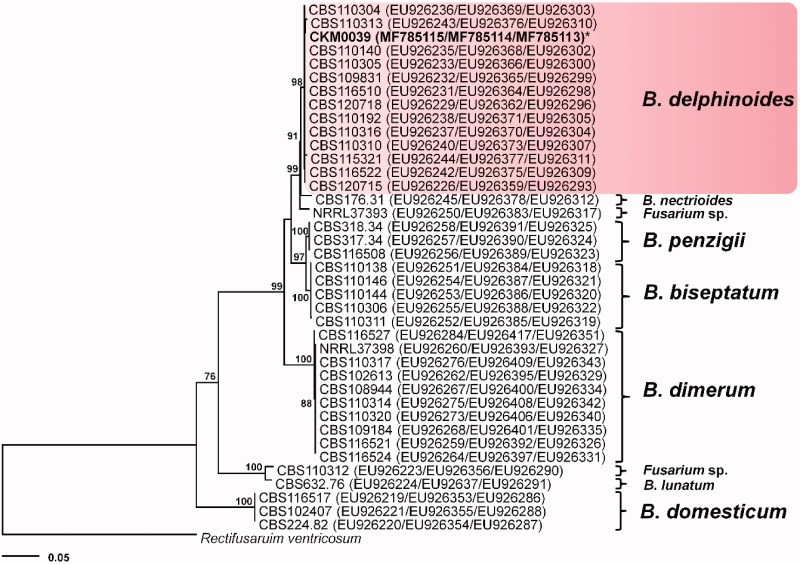
The resulting 522-bp ITS, 1265-bp β-tubulin, and 637 bp-bp EF1-α sequences were deposited in GenBank (Accession Nos. MF785113 to MF785115). A GenBank BLAST search using the ITS, β-tubulin, and EF1-α revealed that the sequences of the isolate have 99–100% similarity with those of F. delphinoides (EU926231 for ITS, EU926376 for β-tubulin, EU926298 for EF1-α). The phylogenetic trees constructed using a combined dataset of ITS, β-tubulin, and EF1-α alignments revealed that B. delphinoides was separated from other B. dimerum species complex (Figure 2). The strain was susceptible to amphotericin B (MIC = 2) and voriconazole (MIC = 1) and resistance to itraconazole (MIC > 16), ketoconazole (MIC = 16), fluconazole (MIC > 64), and flucytosine (MIC > 64). In vitro antifungal susceptibility pattern showed that voriconazole was the most active drug candidate against B. delphinoides.
Figure 2.
Phylogenetic tree inferred from the maximum likelihood method for Bisifusarium delphinoides and Fusarium dimerum species complex based on the multigene dataset (ITS, β-tubulin, EF1-α). The numbers above the nodes are the bootstrap values obtained from 1000 replicates. The isolate used in the present study is indicated by an asterisk.
Fusarium dimerum species complex (FDSC) was separated into at least 12 lineages including F. dimerum sensu stricto, F. delphinoides, F. nectrioides, and F. penzigii, which have caused the fungal infection [6]. The members of FDSC have been more often related to eye and disseminated infections than onychomycoses [5]. Especially, F. delphinoides occurred in soil and dead plant and was rarely reported as an agent of trauma-related eye infections. However, there is no documented report of F. delphinoides (currently Bisifusarium delphinoides) as a causative agent of skin fungal infection. Recently, the new genus Bisifusarium was introduced by accommodating FDSC and F. delphinoides was reclassified as B. delphinoides. A well-supported clade of Bisifusarium was formed and separated from the clade representing the genus Fusarium in the multi-gene phylogenetic analysis [11]. In addition, Diepenningen et al. [5] had already suggested that FDSC was necessary to be reallocated into new genera according to a narrow definition of the genus Fusarium. Until now, this genus name has not yet been used in clinical isolates. To our knowledge, this is the first report to mention B. delphinoides in medical mycology.
The etymology of Bisifusarium referred to 2-celled macroconidia and this genus was established based on this morphological character. At maturity, the members of the FDSC have a low number of macroconidial septa (less than 3-septate), which has been a distinguishable character to reclassify the FDSC into taxonomic groups [6]. In this study, 2-septate macroconidia which is a typical characteristic of Bisifusarium was observed (Figure 1(h–k)). However, the majority of Fusarium species complex cannot be identified into species level only with traditional morphological observation because 33–50% of Fusarium isolates could be erroneously misidentified [18]. Furthermore, Fusarium species cannot be distinguished from other frequent opportunistic mold Aspergillus species based on histopathological examination from the clinical specimens because the structure of septate fungal hyphae with acute angle branching of Fusarium is remarkably similar to that of Aspergillus [3,19]. Therefore, it is necessary to identify a clinical isolate with combining morphological observation and molecular analysis.
Although ITS region is used for DNA-based barcoding, this region of Fusarium species is too conserved for species level discrimination of species of Fusarium. A multi-locus sequence typing (MLST) approach has been recommended for accurate species determination [20]. Also, as the number of the newly identified and uncommon fungal pathogen is increasing, the MLST analysis has become essential for reliable fungal identification. In addition, nomenclature systems have been suggested by the MLST analysis with using 2–5 genomic loci for identifying a number of Fusarium species complex [21]. In this study, MLST analysis using 3 loci showed that B. delphinodies was clearly separated from other species in Fusarium dimerum species complex. As Fusarium species are known to be relatively resistant to most antifungals and their different susceptibility is largely depending on species of Fusarium, accurate identification is important for the appropriate antifungal treatment. Several studies on antifungal susceptibility of Fusarium suggested that voriconazole is suggested to be efficient in treating disseminated Fusarium infection [22,23], which is consistent with our results. As there has been no standardized treatment of invasive fusariosis, antifungal susceptibility data of Fusarium species should be accumulated throughout the case reports.
There have been several case reports of Fusarium infection associated with diabetes mellitus. Fusarium osteomyelitis was reported on the foot of a patient with diabetes mellitus in the USA [24]. Fusarium solani was isolated from chronic diabetic ulcer in India and Fusarium endophthalmitis in a case of diabetes mellitus was reported in India [19,25]. Fusarium species was documented as a causative factor in diabetic foot infection in Turkey [26]. These suggest that diabetes mellitus can be a determinant of the Fusarium infection. Moreover, a burn wound is alsoa risk factor of Fusarium infection. A few case reports of Fusarium species related to severely burnt patients have been published [9,10]. Thus, a combination of diabetes mellitus and the burn wound is probably responsible for the emergence of B. delphinoides as a fungal pathogen in human skin. Bisifusarium delphinoides was mostly isolated from soil, dead, or living plant material and sometimes from human corneal ulcers, once from the human peritoneal fluid. Clinical isolates of B. delphinoides were recorded in the USA, South Africa, and Argentina [6]. There has been no record of B. delphinoides on human skin. Therefore, this is the first case report of B. delphinoides isolated from human skin in a burn patient with diabetes mellitus.
Funding Statement
This subject is supported by Korea Center for Disease Control and Prevention, the National Culture Collection for Pathogens with a funding assistance program of Specialized Pathogen Resource Banks [SPRB-2018-02].
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.Nucci M, Anaissie E. Fusarium infections in immunocompromised patients. Clin Microbiol Rev. 2007;20:695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collado C, Medina L, Zorraquino A, et al. Cutaneous fusariosis by a species of the Fusarium dimerum species complex in a patient with acute myeloblastic leukemia. Rev Iberoam Micol. 2013;30:119–121. [DOI] [PubMed] [Google Scholar]
- 3.Nucci M, Anaissie E. Cutaneous infection by Fusarium species in healthy and immunocompromised hosts: implications for diagnosis and management. Clin Infect Dis. 2002;35:909–920. [DOI] [PubMed] [Google Scholar]
- 4.Ricna D, Lengerova M, Palackova M, et al. Disseminated fusariosis by Fusarium proliferatum in a patient with aplastic anaemia receiving primary posaconazole prophylaxis-case report and review of the literature. Mycoses. 2016;59:48–55. [DOI] [PubMed] [Google Scholar]
- 5.Diepeningen AD, Al-Hatmi AMS, Brankovics B, et al. Taxonomy and clinical spectra of Fusarium species: where do we stand in 2014? Curr Clin Micro Rpt. 2014;1:10–18. [Google Scholar]
- 6.Schroers HJ, O’Donnell K, Lamprecht SC, et al. Taxonomy and phylogeny of the Fusarium dimerum species group. Mycologia. 2009;101:44–70. [DOI] [PubMed] [Google Scholar]
- 7.Casqueiro J, Casqueiro J, Alves C. Infections in patients with diabetes mellitus: a review of pathgenesis. Indian J Endocr Metab. 2012;16:S27–S36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Capoor MR, Sarabahi S, Tiwari VK, et al. Fungal infections in burns: diagnosis and management. Indian J Plast Surg. 2010;43:S37–S42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosanova MT, Brizuela M, Villasboas M, et al. Fusarium spp. infections in a pediatric burn unit: nine years of experience. Braz J Infect Dis. 2016;20:389–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barbara AL. Fusarium infections in burn patients: a case report and review of the literature. J Burn Care Rehabil. 2003;24:285–288. [DOI] [PubMed] [Google Scholar]
- 11.Lombard L, Merwe NA, Groenewald JZ, et al. Generic concepts in Nectriaceae. Stud Mycol. 2015;80:189–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White TJ, Bruns T, Lee S, et al. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. San Diego, CA, USA; Academic Press; 1990. p. 315–322. [Google Scholar]
- 13.O’Donnell K, Kistler HC, Cigelnik E, et al. Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. Proc Natl Acad Sci USA. 1998;95:2044–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maddison WP, Maddison DR Mesquite: a modular system for evolutionary analysis version 3.5; 2018. [cited 2018 May 9]. Available from: http://mesquiteproject.org.
- 15.Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. [DOI] [PubMed] [Google Scholar]
- 16.Silvestro D, Michalak I. RaxmlGUI: a graphical front-end for RAxML. Org Divers Evol. 2012;12:335–337. [Google Scholar]
- 17.CLSI Reference method for broth dilution antifungal susceptibility testing of filamentous fungi: approved standard CLSI document M38-A2. Wayne: Clinical and Laboratory Standards Institute; 2008. [Google Scholar]
- 18.Tortorano AM, Prigitano A, Dho G, et al. Species distribution and in vitro antifungal susceptibility patterns of 75 clinical isolates of Fusarium spp. from Northern Italy. Antimicrob Agents Chemother. 2008;52:2683–2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pai R, Boloor R, Shreevidya K, et al. Fusarium solani: an emerging fungus in chronic diabetic ulcer. J Lab Physicians. 2010;2:37–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Donnell K, Sutton DA, Rinaldi MG, et al. Novel multilocus sequence typing scheme reveals high genetic diversity of human pathogenic members of the Fusarium incarnatum-F. equiseti and F. chlamydosporum species complexes within the United State. J Clin Microbiol. 2009;47:3851–3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang H, Xiao M, Kong F, et al. Accurate and practical identification of 20 Fusarium species by seven-locus sequence analysis and reverse line blot hybridization and an in vitro antifungal susceptibility study. J Clin Microbiol. 2011;49:1890–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sagnelli C, Fumagalli L, Prigitano A, et al. Successful voriconazole therapy of disseminated Fusarium verticillioides infection in an immunocompromised patient receiving chemotherapy. J Antimicrob Chemother. 2006;57:796–798. [DOI] [PubMed] [Google Scholar]
- 23.Labois A, Gray C, Lepretre S. Successful treatment of disseminated fusariosis with voriconazole in an acute lymphoblastic leukaemia patient. Mycoses. 2011;55:8–11. [DOI] [PubMed] [Google Scholar]
- 24.Bader M, Jafri AK, Krueger T, et al. Fusarium osteomyelitis of the foot in a patient with diabetes mellitus. Scand J Infect Dis. 2003;35:895–896. [DOI] [PubMed] [Google Scholar]
- 25.Balamurugan S, Khodifad A. Endogenous Fusarium endophthalmitis in diabetes mellitus. Case Rep Ophthalmol Med. 2016; 2016:6736413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozisik H, Cetinkalp S, Erdem HA, et al. Fusarium: a rare factor in diabetic foot infection. J Microbiol Infect Dis. 2017;7:42–45. [Google Scholar]