Abstract
Objectives: To investigate the immunohistochemical features of different stages of BK virus allograft nephropathy (BKVN) and further elucidate the underlying immunological mechanism involved in the evolution of BKVN.
Methods: Fifty-two renal transplant recipients with biopsy proven BKVN were retrospectively selected. According to the third edition of the American Society of Transplantation Infection guidelines, 10 patients were categorized as having mild BKVN (stage A), 25 were moderate (stage B) and 17 were severe (stage C). The differential infiltrations of CD3+ (T lymphocytes), CD4+ (helper T lymphocytes), CD8+ (cytotoxic T lymphocytes), CD20+ (B lymphocytes), CD68+ (macrophages) and CD138+ (plasma cells) cells and the expression of interleukin-2 receptor (IL-2R) and human leukocyte antigen DR (HLA-DR) were compared among the three groups.
Results: CD3+, CD4+, CD8+, CD20+, CD138+ and CD68+ cells infiltrations, IL-2R and HLA-DR expression were positive in the BKVN patients. Moreover, with increasing stages of BKVN, the numbers of positively stained inflammatory cells and the expression of IL-2R were significantly increased in the severe group compared to the mild group, whereas no statistically significant differences were observed with regard to HLA-DR expression. Eosinophil and neutrophil infiltration could also be observed in moderate to advanced BKVN.
Conclusion: Renal allograft damage caused by BKVN involved T lymphocyte-, B lymphocyte- and mononuclear macrophage-mediated immune responses. Inflammatory cell infiltrations in the renal allograft were probably the driving force for BKVN progression. Additionally, eosinophils and neutrophils may be involved in the pathophysiological mechanism of BKVN.
Keywords: Renal transplantation, BK virus allograft nephropathy, renal pathology, immunopathology, infection
Introduction
With the widespread use of potent and newer immunosuppressive drugs, the incidence of BK virus (BKV) infection after renal transplantation is rising [1–3]. BKV allograft nephropathy (BKVN) has been one of the major causes of allograft dysfunction and even graft loss [1,4]. Unfortunately, the precise pathophysiological mechanism of BKVN remains unclear, and there is still a lack of specific and effective therapeutic interventions. The differential diagnosis between BKVN and T cell-mediated rejection is notoriously difficult, but the associated treatments are diametrically opposed. Reducing immunosuppression is successful in the former, but potentially deleterious in the latter [5]. Furthermore, during the treatment of BKVN, secondary acute rejection frequently ensues after the reduction in immunosuppressive agents [6,7]. Therefore, early effective diagnosis and accurate staging of BKVN are essential to the timely adjustment of immunosuppressive agents, which help to delay graft function deterioration and improve long-term allograft survival.
Kidney allograft biopsy remains the gold standard for diagnosing BKVN based on molecular, cytological and histological examinations [8]. Although the characteristics of infiltrating cells in patients with BKVN have been previously investigated [9–12], the influence of inflammatory cell infiltration on BKVN progression remains unknown. Therefore, we aimed to elucidate the underlying immunological mechanism involved in the evolution of BKVN by retrospectively reviewing the immunohistochemical phenotypes of infiltrating cells and the expression of interleukin-2 receptor (IL-2R) and human leukocyte antigen (HLA-DR) in patients with biopsy-proven BKVN.
Materials and methods
Patients
Fifty-two patients diagnosed with BKVN by renal allograft biopsy between June 2008 and July 2016 at Jinling Hospital (Nanjing, China), Nanjing University School of Medicine, were retrospectively identified. Patients with other concomitant pathological changes, such as rejection or calcineurin inhibitor toxicity, were excluded from further analysis. Informed consent was obtained from all patients, and the Human Subjects Committee of Jinling Hospital (Nanjing, China), Nanjing University School of Medicine, approved the study protocols.
Diagnostic criteria for BKVN
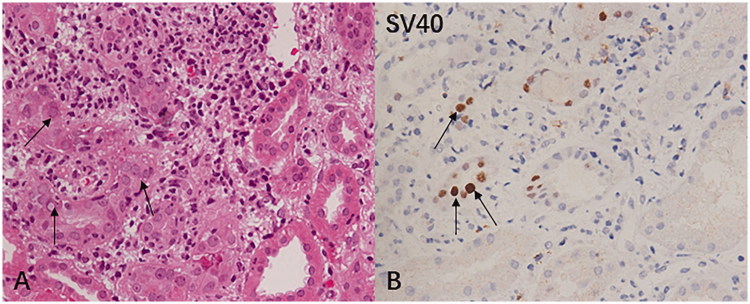
The diagnosis of BKVN is based on detecting signs of viral cytopathic changes, including intranuclear viral inclusions in renal tubular epithelial cells and/or Bowman’s capsule epithelial cells, accompanied by tubular epithelial cell necrosis and denudation of basement membranes, as well as tubule interstitial infiltrates and tubulitis. Positive SV40 staining is used to confirm the diagnosis of BKVN (Figure 1) [13,14].
Figure 1.
(A) Light microscopy image of BK virus renal allograft nephropathy. The histological manifestations are characterized by nuclear inclusion bodies in tubular epithelial cells (arrow indicates the hematoxylin-eosin-stained paraffin section, 400×). (B) Immunostaining of BK virus-infected cells with anti-SV40 large T antigen antibodies showing the nuclei of renal tubular epithelial cells have a transparent center and thorn-shaped periphery (arrow indicates the immunohistochemical staining, 400×).
Classification of BKVN
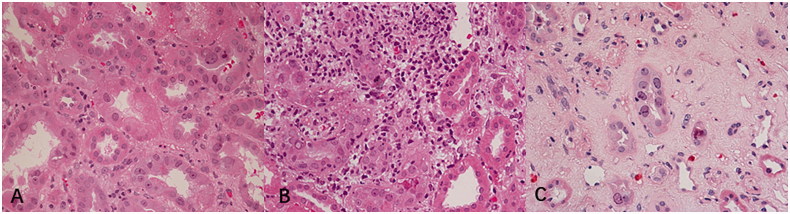
According to the third edition of the American Society of Transplantation Infection guidelines [13], BKVN patients were divided into three groups: a mild group of 10 patients (stage A, viral cytopathic changes in almost normal renal parenchyma), moderate group of 25 patients (stage B, more severe cellular damage with a combination of viral cytopathic changes and focal/multifocal areas of tubular atrophy, and/or interstitial fibrosis), and severe group of 17 patients (stage C, extensive tubular atrophy and interstitial fibrosis; Figure 2).
Figure 2.
Histological patterns of BK virus renal allograft nephropathy (hematoxylin-eosin-stained paraffin section, 400×). (A) Stage A: viral cytopathic changes in nearly normal renal parenchyma, (B) Stage B: more severe cellular damage with a combination of viral cytopathic changes and focal/multifocal areas of tubular atrophy and/or interstitial fibrosis, and (C) Stage C: extensive tubular atrophy and interstitial fibrosis.
Renal biopsies
Biopsies were performed upon protocol or clinical indication under the guidance of ultrasonography. Haematoxylin-eosin (HE), periodic acid Schiff (PAS), periodic acid-silver methenamine (PASM) and Masson trichrome staining were routinely performed. Fresh-frozen tissue was analyzed by immunofluorescence microscopy using a conventional panel of antibodies against Immunoglobulin A (IgA), IgG, IgM, C3, C1q and C4d. C4d staining was detected by an indirect immunofluorescence technique with a primary affinity-purified mouse anti-human monoclonal antibody (Quidel, San Diego, CA) and a fluorescein isothiocyanate-labelled affinity-purified secondary rabbit anti-mouse IgG antibody (Dako, Glostrup, Denmark). Positive C4d staining was defined as bright linear staining along the capillary basement membranes that involved more than ten percent of the sampled capillaries according to the Banff Meeting guidelines [15].
Immunohistochemical staining
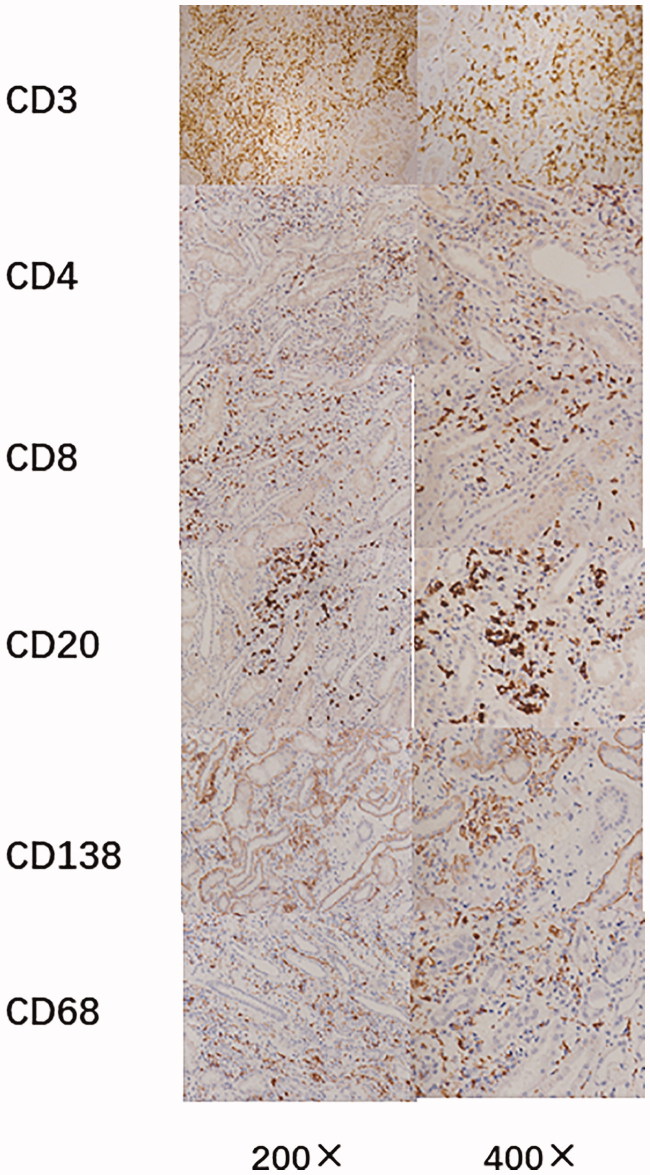
Formalin-fixed, paraffin-embedded renal biopsy sections were deparaffinized in xylene for 20 min and rehydrated in a graded ethanol series (100–70%) for 8 min. Endogenous peroxidase were deactivated with 3% hydrogen peroxide for 5–510 min at room temperature after deparaffinization. Antigen retrieval was performed with a sodium citrate buffer (10 mM, pH = 6) for 10 min. For the detection of infiltrating cell subpopulations, CD3 (T lymphocytes), CD4 (cytotoxic T lymphocytes), CD8 (suppressor T lymphocytes), CD20 (B lymphocytes), CD68 (macrophages) and CD138 (plasma cells) were regularly detected with mouse monoclonal antibodies as follows: anti-CD3 (CD3-PS1, Gene Tech, Shanghai, China), anti-CD4 (CD4-1F6, Gene Tech, Shanghai, China), anti-CD8 (C8/144B, Dako, Glostrup, Denmark), anti-CD20 (L26, Dako), anti-CD68 (KPI, Dako) and anti-CD138 (MI 15, Dako; Figure 3). In addition, IL-2R expression was detected by an anti-IL-2R mouse monoclonal antibody (ACT-1, Dako) and HLA-DR staining was evaluated using an anti-HLA-DR monoclonal antibody (TAL.1B5, Dako) by visually assessing the approximate proportion of positively stained tubules. Immunohistochemical staining for BKV antigens was performed using an anti-BKV large T antigen mouse monoclonal antibody (BK-T (0).1, Chemicon International, Billerica, MA).
Figure 3.
Immunohistochemical characterization of the inflammatory infiltrates in BK virus renal allograft nephropathy.
All immunohistochemical sections were scanned by using a full-slice digital scanner (Aperio ScanScope XT Turbo Scanner, Leica, Germany). The counting of immunohistochemically positive cells, including IL-2R-expressing cells in the unscarred cortical parenchyma was performed by the Aperio scanner and expressed as the total number of positive cells per square millimeter, while the expression of tubular HLA-DR was assessed as the overall percentage of positive tubular cross-sections in the entire biopsy sample.
Statistical analysis
Statistical analyses were conducted using SPSS (v25.0, SPSS, Chicago, IL) software. Categorical variables were expressed as percentages and compared using Fisher’s exact test with the Bonferroni correction for p values among the groups. Continuous variables with normal distribution are presented as the mean ± standard deviation and compared using one-way analysis of variance (ANOVA) followed by post-hoc pairwise comparisons using least significant difference (LSD) tests or a nonparametric method, as appropriate. p < 0.05 was accepted as statistically significant.
Results
Baseline patient characteristics
The baseline patient characteristics at biopsy are listed in Table 1. Among the 52 patients, 37 were male and 15 were female. The average age and serum creatinine (Scr) level of the patients at biopsy was 37.1 ± 9.9 years and 2.77 ± 1.20 mg/dL, respectively and the time of BKVN diagnosis was 21.6 ± 19.7 months after transplantation. Noticeably, the differences in the Scr level among the three groups were statistically significant (p < 0.05). Additionally, the BKV viral load in the plasma and urine before graft biopsy of all the patients was positive; however, there were no significant differences among the groups. The post-transplant maintenance immunosuppressive protocols of the patients mainly consisted of tacrolimus (Tac) or cyclosporine A (CyA), mycophenolate mofetil (MMF) and prednisone (Pred) at the time of biopsy. All patients were negative for donor-specific antibodies (DSA) in the blood.
Table 1.
The clinical characteristic of patients.
| Characteristic | Total | Mild group | Moderate group | Severe group |
p Value |
||
|---|---|---|---|---|---|---|---|
| p12 | p13 | p23 | |||||
| Number | 52 | 10 | 25 | 17 | – | – | – |
| Male, n (%) | 37 (71.2%) | 8 (80%) | 17 (68%) | 12 (70.6%) | NS | NS | NS |
| Age (years) | 37.1 ± 9.9 | 33.9 ± 8.9 | 37.3 ± 11.6 | 38.6 ± 7.5 | NS | NS | NS |
| Scr at time of biopsy(mg/dL) | 2.77 ± 1.20 | 2.11 ± 0.80 | 2.63 ± 1.08 | 3.37 ± 1.34 | NS | 0.001 | 0.042 |
| BKV viral load before graft biopsy, Log10 (copies/mL) | |||||||
| Urine | 9.84 ± 1.21 | 9.40 ± 1.04 | 10.08 ± 1.35 | 9.77 ± 1.11 | NS | NS | NS |
| Plasma | 4.28 ± 1.07 | 4.20 ± 1.02 | 4.48 ± 1.25 | 4.07 ± 1.04 | NS | NS | NS |
| The time of biopsy from renal transplantation (months) | 21.6 ± 19.7 | 19.1 ± 16.8 | 19.0 ± 14.2 | 26.8 ± 27.2 | NS | NS | NS |
| Immunosuppression at time of biopsy | |||||||
| Tac + MMF + Pred, n (%) | 48 (92.3%) | 9 (90.0%) | 23 (92.0%) | 16 (94.1%) | NS | NS | NS |
| CyA + MMF + Pred, n (%) | 2(3.8%) | 0 | 1 (4.0%) | 1 (5.9%) | NS | NS | NS |
| Others, n (%) | 2(3.8%) | 1 (10.0%) | 1 (4.0%) | 0 | NS | NS | NS |
BKV: BK virus; CyA: cyclosporine; MMF: mycophenolate mofetil; NS: not significant statistically; Pred: prednisone; p12: p values for mild and moderate group; p13: p values for mild and severe group; p23: p values for moderate and severe group; Scr: serum creatinine; Tac: tacrolimus.
Comparison of the inflammatory infiltrates among the different groups of BKVN patients
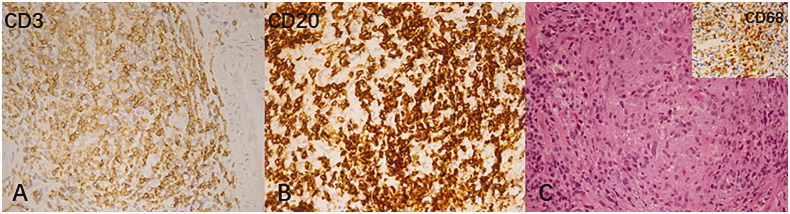
The numbers of CD3+, CD4+, CD8+, CD20+, CD68+ and CD138+ cells at different stages of BKVN are shown in Table 2. The expression of these infiltrating cells increased with the progression of BKVN (Figure 3). Infiltrating cells were mainly concentrated in atrophic renal tubules, especially those with viral inclusion bodies in both the cortex and medulla. Furthermore, the numbers of CD3+, CD4+, CD8+, CD20+, CD68+ and CD138+ cells in the severe group were significantly higher than those in the mild group (p < 0.05). Lymphoid and macrophage aggregates as well as granulomas were observed in some BKVN patients (Figure 4). Additionally, eosinophil and neutrophil infiltrations could also be found in moderate to severe BKVN patients (Table 3).
Table 2.
Inflammatory cellular constituents in different groups of BKVN.
| Total (n = 52) | Mild group (n = 10) | Moderate group (n = 25) | Severe group (n = 17) |
p Value |
|||
|---|---|---|---|---|---|---|---|
| p12 | p13 | p23 | |||||
| CD3 (cells/mm2) | 645.08 ± 320.88 | 455.75 ± 265.78 | 600.55 ± 245.37 | 816.21 ± 356.11 | NS | 0.002 | 0.023 |
| CD4 (cells/mm2) | 375.00 ± 192.63 | 254.17 ± 152.63 | 353.50 ± 146.38 | 476.21 ± 216.44 | NS | 0.001 | 0.031 |
| CD8 (cells/mm2) | 289.47 ± 137.00 | 226.00 ± 134.83 | 268.45 ± 103.94 | 353.89 ± 151.35 | NS | 0.010 | 0.040 |
| CD20 (cells/mm2) | 299.96 ± 189.20 | 175.33 ± 110.11 | 301.59 ± 141.94 | 376.79 ± 236.45 | NS | 0.003 | NS |
| CD138 (cells/mm2) | 115.88 ± 103.26 | 25.92 ± 24.39 | 70.00 ± 45.35 | 190.63 ± 150.43 | NS | <0.001 | <0.001 |
| CD68 (cells/mm2) | 760.02 ± 292.25 | 449.92 ± 163.10 | 754.36 ± 170.45 | 962.42 ± 298.66 | <0.001 | <0.001 | 0.005 |
| T cell-rich aggregates, n (%) | 24 (46.2%) | 4 (40%) | 11(44%) | 9 (52.9%) | NS | NS | NS |
| B cell-rich aggregates, n (%) | 21 (40.4%) | 3 (30%) | 9 (36%) | 9 (52.9%) | NS | NS | NS |
| Macrophage aggregates (CD68), n (%) | 15 (28.8%) | 0 | 7 (28%) | 8 (47.1%) | NS | 0.012 | NS |
BKVN: BK virus allograft nephropathy; NS: not significant statistically; p12: p values for mild and moderate group; p13: p values for mild and severe group; p23: p values for moderate and severe group.
Figure 4.
Inflammatory changes in BK virus renal allograft nephropathy. (A,B) T and B lymphocyte aggregates were highlighted with staining for CD3 and CD20, respectively (immunohistochemical staining, 400×) and (C) Syncytial granulomatous inflammation with clusters of macrophages was noted (hematoxylin-eosin-stained paraffin section, 400×). Inset (C): clusters of macrophages were highlighted with staining for CD68 stain (immunohistochemical staining, 400×).
Table 3.
The other inflammatory reaction and the expression of IL-2R and HLA-DR in different groups of BKVN.
| Total (n = 52) | Mild group (n = 10) | Moderate group (n = 25) | Severe group (n = 17) |
p Value |
|||
|---|---|---|---|---|---|---|---|
| p12 | p13 | p23 | |||||
| Granulomatous inflammation, n (%) | 1 (1.9%) | 0 | 0 | 1 (5.9%) | – | NS | NS |
| Eosinophil infiltration, n (%) | 4 (7.7%) | 0 | 3 (12%) | 1 (5.9%) | NS | NS | NS |
| Neutrophil clusters, n (%) | 16 (30.8%) | 0 | 10 (40%) | 6 (35.3%) | 0.034 | NS | NS |
| IL-2R (cells/mm2) | 140.87 ± 88.09 | 93.01 ± 92.33 | 141.18 ± 48.79 | 171.16 ± 108.69 | NS | 0.015 | NS |
| HLA-DR (%) | 6.85 ± 12.72 | 4.50 ± 8.32 | 5.40 ± 10.60 | 10.35 ± 16.98 | NS | NS | NS |
BKVN: BK virus allograft nephropathy; IL-2R: interleukin-2 receptors; HLA: human leukocyte antigen; NS: not significant statistically; p12: p values for mild and moderate group; p13: p values for mild and severe group; p23: p values for moderate and severe group.
The expression patterns of IL-2R and HLA-DR in different groups of BKVN patients
As shown in Table 3, the expression of IL-2R in renal allografts and HLA-DR in renal tubular epithelial cells also increased with the progression of BKVN. The expression of IL-2R in the severe group was significantly higher than that in the mild group (p = 0.015), whereas there were no significant differences in the expression of HLA-DR among the three groups (p > 0.05).
C4d deposition in different groups of BKVN patients
C4d focal deposition was noted in 2/10 patients (20%) in the mild group, 1/25 (4%) in the moderate group and 0/17 (0%) in the severe group.
Discussion
Since the development and widespread use of reliable and newer immunosuppressive drugs, BKV infection has become common after renal transplantation and BKVN has become an important cause of graft loss [2,3,16]. The reported incidence of biopsy proven BKVN varies from 1 to 10% [1,13] with subsequent graft loss in 10 to 80% of cases [17]. BKVN is characterized by the presence of viral cytopathic changes in the epithelium of the renal tubules and urothelial lining, as well as tubulointerstitial inflammation and tubular atrophy/interstitial fibrosis [18]. Infected cells have an enlarged nucleus with a gelatinous basophilic inclusion resulting from the accumulation of newly formed virions. Immunohistochemistry has been widely adopted for the diagnosis of viral infections in immunocompromised patients. Previous studies have shown that the infiltrating cells in BKVN predominantly comprise mononuclear cells, including lymphocytes and macrophages [11,14,19–21]. Nevertheless, the precise pathophysiological mechanisms of BKVN remain unclear. To our knowledge, this is the first study to investigate the immunohistochemical features of different stages of BKVN based on the American Society of Transplantation Infection guidelines [13].
In agreement with prior studies [11,14,19,21,22], our study showed that the infiltration of CD3+, CD4+, CD8+, CD20+, CD138+ and CD68+ cells as well as IL-2R and HLA-DR expression increased with the progression of BKVN. Noticeably, the increases in the numbers of CD3+, CD4+, CD8+, CD20+, CD138+ and CD68+ cells were significant, indicating that these cells may play important roles in BKVN progression.
CD3+ T cells are mainly composed of CD4+ and CD8+ T cells. These cells are the main source of IL-2R secretion. Studies have shown that CD3+ T cells play crucial roles in the initiation and progression of BKVN and that both CD8+ and CD4+ T cells are involved in the recognition and clearance of BKV [19,23]. Moreover, it has been confirmed that CD4+ T cells have a specific polyfunctional antiviral effect on BKV infection [4,14,24]. Large T antigen preferentially stimulates CD8+ T cells, while the virion protein 1 of BKV preferentially stimulates CD4+ T cells [20]. CD4+ T cells can control BKV infection by secreting interferon-γ (IFN-γ), tumor necrosis factor-alpha and IL-2 [24]. In this study, with BKVN development, CD4+ and CD8+ T cells numbers in the kidney allografts increased. Additionally, this study highlighted that IL-2R expression was also correlated with BKVN progression, supporting the conclusion that the T cell-mediated immune response plays a pivotal role in BKVN.
Recent studies have demonstrated that the percentage of B lymphocytes in BKVN increases significantly [10,11,21], favoring the involvement of humoral immunity in the pathogenesis of BKVN, but the precise immunopathological mechanisms remain unclear. Part of the virus could increase B lymphocyte counts by secreting a B cell-activating factor that is mediated by NF-kB signaling pathways [25–29]. Nevertheless, it remains unknown whether BKV causes allograft damage by a similar mechanism. We also found a marked increase in CD20+ and plasma (CD138+) cell numbers with concordant BKVN progression, which was consistent with previous investigations [11,21]. These observations suggested that humoral immunity may be involved in the immunological reaction against BKV. Nevertheless, in our study, there was no significant difference regarding the expression of HLA-DR among the groups. Furthermore, only three of the 52 patients had focal positive C4d expression in renal allograft peritubular capillaries and DSA levels in the blood were also negative without histological evidence of antibody-mediated rejection [15]. Therefore, we speculated that there was a difference in activated B lymphocyte function between BKVN and antibody-mediated rejection.
There is currently no reported mechanism for the significant increase in CD68+ cell infiltration in BKVN. We assumed that BKV can activate macrophages in a manner similar to other viruses via the CCL2/CCR2 pathway that mediates monocyte migration to an inflamed site [30,31]. Additionally, the encroachment of BKV directly upon renal tubular epithelial cells may be another possible explanation. Furthermore, Braga et al. [32] found that the factors released after BKV infection could activate macrophages through Toll-like receptors and the MyD88 signaling pathway to promote renal allograft fibrosis.
We observed eosinophil and neutrophil infiltration in moderate to advanced BKVN, consistent with previous research. Eosinophils and neutrophils are multifunctional pro-inflammatory leukocytes that are involved in the pathogenesis of immune disorders through releasing cytokines, chemokines and toxic cytoplasmic granule constituents [33,34]. Some studies have indicated that interstitial eosinophil and neutrophil infiltration in renal allografts is significantly associated with acute renal allograft rejection [35–37]. Nevertheless, the role of eosinophil and neutrophil infiltration in the pathophysiology of BKVN is unclear.
Virus-specific T cells can recognize cells presenting viral epitopes on the cell surface via major histocompatibility complex (MHC) molecules [38]. Classically, CD8+ T cells recognize and destroy infected cells displaying viral peptides presented by MHC class I molecules, whereas CD4+ T cells scan viral epitopes presented by MHC class II molecules on antigen-presenting cells such as macrophages, B lymphocytes and dendritic cells. On the other hand, activated T lymphocytes regulate the proliferation, differentiation and immune function of B lymphocytes and macrophages by secreting a variety of cytokines to control viral infection, such as IL-2 and IFN-γ. Therefore, T lymphocyte-, B lymphocyte- and mononuclear macrophage-mediated immune responses are involved during BKV infection in renal allografts and these immune components interact intimately.
The main limitation of this study is that renal allograft biopsies were performed for-cause instead of per-protocol. Serial biopsies from patients with BKVN help to define the histological evolution of BKVN and provide insight into the underlying mechanism.
In conclusion, we observed renal allograft damage caused by BKVN involving T lymphocyte-, B lymphocyte- and mononuclear macrophage-mediated immune responses. Inflammatory cell infiltration into the renal allografts was probably the driving force for BKVN progression. Eosinophil and neutrophil involvement in the pathophysiological mechanisms of BKVN requires further study.
Funding Statement
This study was funded by the National Natural Science Foundation and National Key Research and Development Program of China.
Disclosure statement
No potential conflicts of interest were reported by the authors.
References
- 1.Ramos E, Drachenberg CB, Wali R, et al. The decade of polyomavirus BK-associated nephropathy: state of affairs. Transplantation. 2009;87:621–630. [DOI] [PubMed] [Google Scholar]
- 2.Pham PT, Schaenman J, Pham PC. BK virus infection following kidney transplantation: an overview of risk factors, screening strategies, and therapeutic interventions. Curr Opin Organ Transplant. 2014;19:401–412. [DOI] [PubMed] [Google Scholar]
- 3.Manitpisitkul W, Drachenberg C, Ramos E, et al. Maintenance immunosuppressive agents as risk factors for BK virus nephropathy: a case-control study. Transplantation. 2009;88:83–88. [DOI] [PubMed] [Google Scholar]
- 4.Lamarche C, Orio J, Collette S, et al. BK polyomavirus and the transplanted kidney: immunopathology and therapeutic approaches. Transplantation. 2016;100:2276–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saad ER, Bresnahan BA, Cohen EP, et al. Successful treatment of BK viremia using reduction in immunosuppression without antiviral therapy. Transplantation. 2008;85:850–854. [DOI] [PubMed] [Google Scholar]
- 6.Drachenberg CB, Papadimitriou JC, Chaudhry MR, et al. Histological evolution of BK virus-associated nephropathy: importance of integrating clinical and pathological findings. Am J Transplant. 2017;17:2078–2091. [DOI] [PubMed] [Google Scholar]
- 7.Nankivell BJ, Renthawa J, Sharma RN, et al. BK virus nephropathy: histological evolution by sequential pathology. Am J Transplant. 2017;17:2065–2077. [DOI] [PubMed] [Google Scholar]
- 8.Hirsch HH, Knowles W, Dickenmann M, et al. Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N Engl J Med. 2002;347:488–496. [DOI] [PubMed] [Google Scholar]
- 9.Drachenberg CB, Papadimitriou JC, Ramos E. Histologic versus molecular diagnosis of BK polyomavirus-associated nephropathy: a shifting paradigm. CJASN. 2006;1:374–379. [DOI] [PubMed] [Google Scholar]
- 10.Drachenberg CB, Papadimitriou JC, Hirsch HH, et al. Histological patterns of polyomavirus nephropathy: correlation with graft outcome and viral load. Am J Transplant. 2004;4:2082–2092. [DOI] [PubMed] [Google Scholar]
- 11.Buettner M, Xu H, Böhme R, et al. Predominance of TH2 cells and plasma cells in polyoma virus nephropathy: a role for humoral immunity. Hum Pathol. 2012;43:1453–1462. [DOI] [PubMed] [Google Scholar]
- 12.Sawinski D, Goral S. BK virus infection: an update on diagnosis and treatment. Nephrol Dial Transplant. 2015;30:209–217. [DOI] [PubMed] [Google Scholar]
- 13.Hirsch HH, Randhawa P. BK polyomavirus in solid organ transplantation. Am J Transplant. 2013;13(Suppl 4):179–188. [DOI] [PubMed] [Google Scholar]
- 14.Dekeyser M, François H, Beaudreuil S, et al. Polyomavirus-specific cellular immunity: from BK-virus-specific cellular immunity to BK-virus-associated nephropathy. Front Immunol. 2015;6:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haas M, Loupy A, Lefaucheur C, et al. The Banff 2017 kidney meeting report: revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant. 2018;18:293–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El AM, Abd ES, Saadi G, et al. Prevalence of polyoma BK virus infection among living-donor renal transplant recipients. Transpl Infect Dis. 2016;18:529–537. [DOI] [PubMed] [Google Scholar]
- 17.Costa C, Cavallo R. Polyomavirus-associated nephropathy. World J Transplant. 2012;2:84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drachenberg CB, Papadimitriou JC. Polyomavirus-associated nephropathy: update in diagnosis. Transplant Infect Dis. 2006;8:68–75. [DOI] [PubMed] [Google Scholar]
- 19.Comoli P, Cioni M, Basso S, et al. Immunity to polyomavirus BK infection: immune monitoring to regulate the balance between risk of BKV nephropathy and induction of alloimmunity. Clin Dev Immunol. 2013;2013:256923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Binggeli S, Egli A, Schaub S, et al. Polyomavirus BK-specific cellular immune response to VP1 and large T-antigen in kidney transplant recipients. Am J Transplant. 2007;7:1131–1139. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Sun Q, Chen J, et al. Immunophenotyping in BK virus allograft nephropathy distinct from acute rejection. Clin Dev Immunol. 2013;2013:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mannon RB, Hoffmann SC, Kampen RL, et al. Molecular evaluation of BK polyomavirus nephropathy. Am J Transplant. 2005;5:2883–2893. [DOI] [PubMed] [Google Scholar]
- 23.Comoli P, Hirsch HH, Ginevri F. Cellular immune responses to BK virus. Curr Opin Organ Transplant. 2008;13:569–574. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt T, Adam C, Hirsch HH, et al. BK polyomavirus-specific cellular immune responses are age-dependent and strongly correlate with phases of virus replication. Am J Transplant. 2014;14:1334–1345. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez B, Valdez H, Freimuth W, et al. Plasma levels of B-lymphocyte stimulator increase with HIV disease progression. AIDS. 2003;17:1983–1985. [DOI] [PubMed] [Google Scholar]
- 26.Toubi E, Gordon S, Kessel A, et al. Elevated serum B-Lymphocyte activating factor (BAFF) in chronic hepatitis C virus infection: association with autoimmunity. J Autoimmun. 2006;27:134–139. [DOI] [PubMed] [Google Scholar]
- 27.Dalrymple NA, Mackow ER. Endothelial cells elicit immune-enhancing responses to dengue virus infection. J Virol. 2012;86:6408–6415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vincent FB, Saulep-Easton D, Figgett WA, et al. The BAFF/APRIL system: emerging functions beyond B cell biology and autoimmunity. Cytokine Growth Factor Rev. 2013;24:203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu B, Zhang B, Wang L, et al. Hepatitis B virus e antigen regulates monocyte function and promotes B lymphocyte activation. Viral Immunol. 2017;30:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bose S, Cho J. Role of chemokine CCL2 and its receptor CCR2 in neurodegenerative diseases. Arch Pharm Res. 2013;36:1039–1050. [DOI] [PubMed] [Google Scholar]
- 31.Covino DA, Sabbatucci M, Fantuzzi L. The CCL2/CCR2 axis in the pathogenesis of HIV-1 infection: a new cellular target for therapy. Curr Drug Targets. 2016;17:76–110. [DOI] [PubMed] [Google Scholar]
- 32.Braga TT, Correa-Costa M, Guise YF, et al. MyD88 signaling pathway is involved in renal fibrosis by favoring a TH2 immune response and activating alternative M2 macrophages. Mol Med. 2012;18:1231–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gleich GJ, Adolphson CR. The eosinophilic leukocyte: structure and function. Adv Immunol. 1986;39:177–253. [DOI] [PubMed] [Google Scholar]
- 34.Naumenko V, Turk M, Jenne CN, et al. Neutrophils in viral infection. Cell Tissue Res. 2018;371:505–516. [DOI] [PubMed] [Google Scholar]
- 35.Weir MR, Hall-Craggs M, Shen SY, et al. The prognostic value of the eosinophil in acute renal allograft rejection. Transplantation. 1986;41:709–712. [DOI] [PubMed] [Google Scholar]
- 36.Saisu K, Morozumi K, Suzuki K, et al. Significance of interstitial lesions as the early indicator for acute vascular rejection in human renal allografts. Clin Transplant. 1999;13:17–23. [PubMed] [Google Scholar]
- 37.Wen T, Rothenberg ME. The regulatory function of eosinophils. Microbiol Spectr. 2016;4:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sester M, Leboeuf C, Schmidt T, et al. The “ABC” of virus-specific T cell immunity in solid organ transplantation. Am J Transplant. 2016;16:1697–1706. [DOI] [PubMed] [Google Scholar]