A basic feature of infection caused by Borrelia burgdorferi, the etiological agent of Lyme borreliosis, is that persistent infection is the rule in its many hosts. The ability to persist and evade host immune clearance poses a challenge to effective antimicrobial treatment. A link between therapy failure and the presence of persister cells has started to emerge.
KEYWORDS: Borrelia burgdorferi, antimicrobial tolerance, mouse model, persistence
ABSTRACT
A basic feature of infection caused by Borrelia burgdorferi, the etiological agent of Lyme borreliosis, is that persistent infection is the rule in its many hosts. The ability to persist and evade host immune clearance poses a challenge to effective antimicrobial treatment. A link between therapy failure and the presence of persister cells has started to emerge. There is growing experimental evidence that viable but noncultivable spirochetes persist following treatment with several different antimicrobial agents. The current study utilized the mouse model to evaluate if persistence occurs following antimicrobial treatment in disease-susceptible (C3H/HeJ [C3H]) and disease-resistant (C57BL/6 [B6]) mouse strains infected with B. burgdorferi strains N40 and B31 and to confirm the generality of this phenomenon, as well as to assess the persisters’ clinical relevance. The status of infection was evaluated at 12 and 18 months after treatment. The results demonstrated that persistent spirochetes remain viable for up to 18 months following treatment, as well as being noncultivable. The phenomenon of persistence in disease-susceptible C3H mice is equally evident in disease-resistant B6 mice and not unique to any particular B. burgdorferi strain. The results also demonstrate that, following antimicrobial treatment, both strains of B. burgdorferi, N40 and B31, lose one or more plasmids. The study demonstrated that noncultivable spirochetes can persist in a host following antimicrobial treatment for a long time but did not demonstrate their clinical relevance in a mouse model of chronic infection. The clinical relevance of persistent spirochetes beyond 18 months following antimicrobial treatment requires further studies in other animal models.
INTRODUCTION
Borrelia burgdorferi, the etiological agent of Lyme borreliosis, has become an important public health problem, and borreliosis has become the most prevalent tick-borne disease in the United States (1, 2). It occurs across much of the Northern Hemisphere, causing considerable morbidity and, in some cases, mortality in humans, domestic animals, and occasionally wildlife. It is the most frequently diagnosed tick-borne disease in the United States. According to the Centers for Disease Control and Prevention (CDC), it is estimated that there are more than 300,000 human cases of Lyme borreliosis annually (3), which is expecting to rise (4).
Untreated human Lyme borreliosis results in disease with a wide range of clinical symptoms and protean manifestation, depending on the stage of infection (5–9). Treatment of patients with diagnosed Lyme borreliosis with antimicrobial agents mainly resolves the objective clinical manifestations. Associated subjective symptoms often may persist for many weeks, or even months (10). However, a proportion of patients remain ill (11, 12), and delayed treatment is associated with negative clinical outcomes (13, 14). There are many reports that, despite resolution of the clinical signs of infection after treatment with antimicrobial agents, a minority of patients experience disabling subjective symptoms, a condition recognized by the term “post-Lyme disease syndrome” (PTLDS) (15–22).
Persistent Lyme borreliosis infection (without antimicrobials) has been experimentally demonstrated in various animal models by culture and PCR (23–30), as well as in reported confirmed spontaneous cases in humans based on culture (31–37) and PCR (38–41). A prolonged treatment regimen has been evaluated in patients with chronic Lyme disease, and treatment is often less effective (15, 42–44). The general findings of some of these studies were that a small population of persistent spirochetes that survived antimicrobial treatment are noncultivable but that B. burgdorferi-specific DNA (BbDNA) can be detected by PCR. The persistent spirochetes were primarily localized in collagenous tissues (17–21, 29, 45–54). There is also strong scientific evidence that uninfected ticks were able to acquire B. burgdorferi spirochetes that survived various antimicrobial treatments and transmit them to naive hosts after molting to the next stage (45–47, 50, 54).
The nature of the formation of persistent spirochetes is still unknown and is a matter of speculation. Several mechanisms have been proposed that involve suppression of the innate and adaptive immune systems, complement inhibition, induction of anti-inflammatory cytokines, formation of immune complexes, antigenic variation, and physical seclusion (27, 53, 55–60). Persistence of noncultivable and attenuated spirochetes has been demonstrated following antimicrobial treatment, and the phenomenon is likely explained by antimicrobial tolerance (61–63). B. burgdorferi has numerous linear and circular plasmids that are inclined to loss from a bacterial cell during long-term in vitro cultivation (64–66), which may correlate with loss of infectivity. It is likely that plasmid loss occurs during the course of infection and increases over time.
In our earlier studies, we detected populations of B. burgdorferi in tissues after antimicrobial therapy in mice (45–47, 50) and in rhesus macaques (53, 54) treated with ceftriaxone, doxycycline, or tigecycline at various intervals of infection, and tissues were tested at intervals after treatment. Specific BbDNA was consistently detected in tissues of mice as late as 12 months after treatment, but cu lture was consistently negative (46). Spirochetes could be visualized by immunohistochemistry in collagen-rich tissues of mice after treatment (46, 47). We have demonstrated that morphologically intact antimicrobial-tolerant persistent spirochetes could be acquired by larval ticks; that after a blood meal, the ticks molted into the next life stages, nymphs and adults, which remained BbDNA positive; that nymphs transmitted BbDNA to recipient immunocompromised mice with multiple tissues PCR positive, with no obvious inflammation observed; and that allografts from treated mice transplanted into recipient immunocompromised mice transferred BbDNA to the recipient mice (46). Transcriptional activity of BbDNA-positive tissues was detected for several target genes, suggesting their viability (47, 50). Furthermore, quantitative PCR (qPCR) indicated low levels of replication during the various stages (46, 47, 50). In the most recent study, the subpopulation of viable, antimicrobial-tolerant, but slowly dividing and persistent spirochetes of B. burgdorferi resurged in mice 12 months after treatment and redisseminated into multiple tissues. Isolation of these spirochetes has been unsuccessful due to their slow growth, low numbers, and probably changes in plasmid content. The noncultivable spirochetes in host tissues remained transcriptionally active, BbDNA was acquired by xenodiagnostic ticks, and spirochetal forms could be visualized within ticks and mouse tissues. A number of host cytokines were up- or downregulated in tissues of both saline- and antimicrobial-treated mice, indicating host response to the presence of such spirochetes, despite the lack of inflammation in tissues (47).
We hypothesize that a selected persistent B. burgdorferi population proliferates and incidentally generates an increasingly heterogeneous population of noncultivable, replicatively attenuated spirochetes. We propose that this may be due to a relatively large subpopulation of attenuated spirochetes as infection proceeds, which may lose one or more plasmids. Results obtained from this study demonstrated the generality of spirochete persistence following antimicrobial treatment by demonstrating the phenomenon in genetically susceptible C3H/HeJ (C3H) and resistant C57BL/6 (B6) mouse strains infected with B. burgdorferi strain N40 (C3H/N40 and B6/N40) compared to infection with strain B31 (C3H/B31 and B6/B31) for up to 18 months after treatment.
RESULTS
Pharmacokinetics.
Average serum concentrations of ceftriaxone were 18.625 μg/ml and 1.795 μg/ml at 2 and 4 h, respectively, but no inhibition was detected at 8 h. As expected, intraperitoneally administered ceftriaxone accumulated rapidly in circulation and then decreased gradually during the following 2 h. The serum ceftriaxone concentrations at 2 and 4 h after administration were at least 300- and 30-fold greater than the B. burgdorferi minimal bactericidal concentration (MBC) (obtained in our previous mouse studies), respectively. No differences were observed in serum ceftriaxone concentrations between different mouse strains (C3H and B6).
Persistence of B. burgdorferi N40 and B31 following antimicrobial treatment in C3H and B6 mice.
Mouse tissue samples—heart base, ventricular muscle, and tibiotarsal joint—were collected aseptically, immediately weighed, snap-frozen in liquid nitrogen, and stored at −80°C before total nucleic acid extraction. Quality control of qPCR was assessed by targeting the mouse gapDH gene. Spirochetal DNA was assessed by targeting flaB and ospA. Quantitative data were expressed as the number of DNA copies per milligram of tissue weight. Mouse gapDH qPCR was positive for all assessed samples (average quantification cycle [Cq] ± standard deviation [SD], 19.36 ± 4.69), confirming successful extraction of total nucleic acid.
Based upon flaB-ospA DNA qPCR on assessed tissue samples, all C3H and B6 mice inoculated with either B. burgdorferi strain N40 or B31 and treated with saline were qPCR positive at 12 and 18 months after treatment in most tissues. The data in Table 1 and Table 2 summarize positive or negative flaB-ospA DNA qPCR results. Among ceftriaxone-treated C3H and B6 mice infected with N40 or B31, 100% and 74% tested positive on flab-ospA DNA qPCR at tibiotarsal joints at 12 and 18 months after treatment, respectively. Sporadically positive heart base and ventricular muscle samples were detected in mice at 12 months after antimicrobial treatment (Table 1), but not in mice at 18 months (Table 2).
TABLE 1.
Analysis of flaB-ospA DNA in heart base, ventricular muscle, and tibiotarsus from B. burgdorferi-infected mice treated with ceftriaxone and saline solution and then necropsied 12 months after completion of treatment
| Mouse strain | Borrelia strain | Treatment | No. of samples positive/no. tested |
||
|---|---|---|---|---|---|
| Heart base | Ventricular muscle | Tibiotarsus | |||
| C3H | N40 | Ceftriaxone | 3/5 | 1/5 | 5/5 |
| C3H | B31 | Ceftriaxone | 2/5 | 0/5 | 5/5 |
| B6 | N40 | Ceftriaxone | 1/5 | 1/5 | 5/5 |
| B6 | B31 | Ceftriaxone | 1/5 | 0/5 | 5/5 |
| C3H | N40 | Saline | 4/5 | 5/5 | 3/5 |
| C3H | B31 | Saline | 5/5 | 5/5 | 3/5 |
| B6 | N40 | Saline | 4/5 | 5/5 | 2/5 |
| B6 | B31 | Saline | 1/3 | 3/3 | 2/3 |
TABLE 2.
Analysis of flaB-ospA DNA in heart base, ventricular muscle, and tibiotarsus from B. burgdorferi-infected mice treated with ceftriaxone and saline solution and then necropsied 18 months after completion of treatment
| Mouse strain | Borrelia strain | Treatment | No. of samples positive/no. tested |
||
|---|---|---|---|---|---|
| Heart base | Ventricular muscle | Tibiotarsus | |||
| C3H | N40 | Ceftriaxone | 0/4 | 0/4 | 1/4 |
| C3H | B31 | Ceftriaxone | 0/5 | 0/5 | 5/5 |
| B6 | N40 | Ceftriaxone | 0/5 | 0/5 | 4/5 |
| B6 | B31 | Ceftriaxone | 0/5 | 0/5 | 5/5 |
| C3H | N40 | Saline | 3/4 | 4/4 | 4/4 |
| C3H | B31 | Saline | 3/3 | 3/3 | 3/3 |
| B6 | N40 | Saline | 4/5 | 5/5 | 5/5 |
| B6 | B31 | Saline | 5/5 | 5/5 | 5/5 |
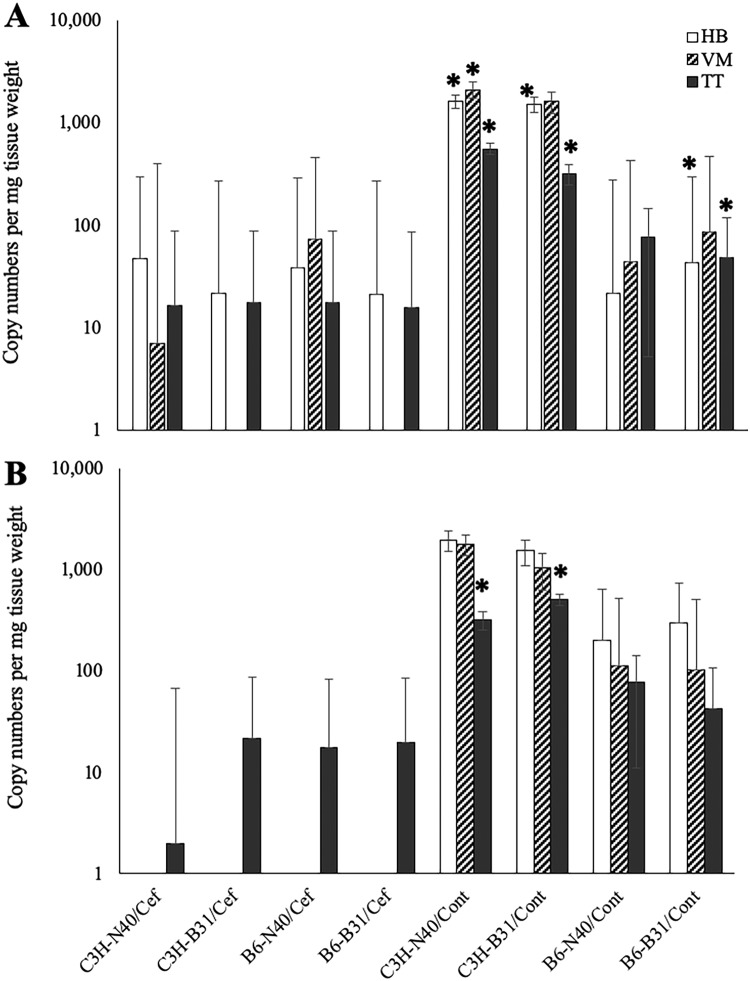
Quantification of each target gene in the assessed samples was accomplished by preparing plasmid standards in order to create absolute standard curves to determine the starting copy number. Mean DNA copy numbers of flaB-ospA genes in collected tissue samples among different mouse strains (C3H and B6) and different B. burgdorferi strains (N40 and B31) in ceftriaxone-treated groups at 12 months after treatment were nearly equivalent (mean ± SD, 2.97 × 101 ± 1.72 × 101). Among saline-treated groups, significantly higher DNA copy numbers of flaB-ospA were found in C3H mice infected with N40 and B31 than in B6 mice infected with N40 and B31 (P < 0.05) (Fig. 1A). Significantly higher copy numbers of flaB-ospA were found in tissue samples from saline-treated mice than in those from the ceftriaxone-treated mice (P < 0.05) (Fig. 1A). Thus, these results suggested persistence of spirochetes at 12 months following antimicrobial treatment in both C3H and B6 mice infected with both B. burgdorferi strains, N40 and B31, although all the mice remained culture negative. The qPCR results also show persistence of BbDNA after antimicrobial treatment at 12 months, remaining in the mice even after 18 months. Persistent BbDNA was detected exclusively in tibiotarsal joints of all treatment groups, with mean copy numbers of flaB-ospA per milligram of tissue that were nearly equivalent (mean ± SD, 1.52 × 101 ± 9.74 × 100) (Fig. 2). The qPCR results for saline-treated groups at 18 months were similar to the results for the same treatment groups at 12 months. flaB-ospA gene copies among the saline-treated groups at 18 months were lower in B6 mice infected with N40 and B31 than in C3H mice infected with N40 and B31, but not significantly. Mean flaB-ospA copy numbers in tibiotarsal joints at 18 months were significantly higher (P < 0.05) in saline-treated C3H mice infected with N40 and B31 than in the same groups of antimicrobial-treated mice (Fig. 1B).
FIG 1.
Copy numbers of B. burgdorferi DNA of flaB-ospA genes in heart base (HB), ventricular muscle (VM), and tibiotarsus (TT) of C3H and B6 mice infected with either strain N40 or B31 at 12 (A) and 18 (B) months after ceftriaxone and saline solution treatment. *, significantly higher copy number (P < 0.05). The error bars indicate SD.
FIG 2.
Dark-field images of B. burgdorferi spirochetes cultured from mouse tissues necropsied 18 months after completion of treatment. Magnification, ×400. (A) Note the numerous spirochetes from ear culture of a saline solution-treated C3H mouse infected with strain N40. (B) Ear culture of a ceftriaxone-treated B6 mouse infected with strain B31. (C) Front joint of a ceftriaxone-treated B6 mouse infected with strain N40.
Analysis of B. burgdorferi flaB and 16S rRNA gene transcriptional activity revealed that most of the saline-treated C3H and B6 mice infected with the N40 or B31 strain were positive. The transcriptional activities of flaB in samples from antimicrobial-treated mice (C3H and B6) necropsied at 12 and 18 months after treatment were 52% and 35%, respectively, while the 16S rRNA gene was constitutively detected in all samples. The results indicate viability among the persisting spirochetes.
Confirmation of spirochete noncultivability at 12 and 18 months following antimicrobial treatment.
The infection status of mice at 12 months after antimicrobial treatment was assessed by culture of the inoculation site and the urinary bladder. For culture, we used a modified Barbour-Stoenner-Kelly II (BSK-II) medium. The sensitivity of BSK-II medium for detection of viable spirochetes was verified by serial 10-fold dilutions of B. burgdorferi N40 and B31 cultures, as described previously (46). The culture results revealed that all the saline-treated mice were positive, with significant numbers of spirochetes detected (Table 3). When mice treated with ceftriaxone were evaluated 12 months after completion of treatment, none of the tissues were culture positive. In a single culture, two spirochetes were observed, but we were unable to subculture them (Table 3).
TABLE 3.
Culture results for individual mice treated with ceftriaxone and saline solution commencing 4 weeks after inoculation and then subjected to necropsy 12 months after completion of treatment
| Mouse strain | Borrelia strain | Treatment | No. of samples positive/no. tested |
|
|---|---|---|---|---|
| Inoculation site | Urinary bladder | |||
| C3H | N40 | Ceftriaxone | 0/5 | 0/5 |
| C3H | B31 | Ceftriaxone | 0/4 | 0/4 |
| B6 | N40 | Ceftriaxone | 0/5 | 1/5a |
| B6 | B31 | Ceftriaxone | 0/5 | 0/5 |
| C3H | N40 | Saline | 5/5 | 5/5 |
| C3H | B31 | Saline | 5/5 | 5/5 |
| B6 | N40 | Saline | 5/5 | 5/5 |
| B6 | B31 | Saline | 3/3 | 3/3 |
Two spirochetes were observed, but we were unable to subculture them.
For the first time point of this study (12 months), a limited number of sample sites (urinary bladder and inoculation site) were cultured. These are not optimal sites for culture after antimicrobial treatment, since persisting spirochetes tend to be localized in heart base and joint tissues. To determine if resurgent spirochetes regain cultivability 18 months after treatment, multiple sample types were collected, such as joint, ear, quadriceps muscle, spleen, urinary bladder, and inoculation site tissues. Heart tissue was not available for culture as it was for PCR and histology. To facilitate cultivation, several media have been introduced, such as BSK-II medium, modified Kelly-Pettenkofer (MKP) medium, BSK-II plus agarose, and BSK-II plus carbohydrates. The inoculated cultures were incubated for 50 days at 33°C and examined weekly. The culture tubes were gently but thoroughly stirred, and a drop of culture was examined by dark-field microscopy. The culture results revealed that all the saline-treated mice were positive, with significant numbers of motile spirochetes detected (Table 4 and Fig. 2A) in the majority of tissue samples cultured. When mice treated with ceftriaxone were evaluated, one ear tissue sample from a B6 mouse infected with B31 (Fig. 2B) and one front joint tissue sample from a B6 mouse infected with N40 (Fig. 2C) were culture positive (Table 4). Tissues were cultured in MKP and BSK-II plus carbohydrates media, respectively. After 4 weeks of incubation, spirochetes in culture medium from ceftriaxone-treated mice appeared nonmotile with irregular curves, so we were unable to subculture them. These results confirm our previous findings that antimicrobial-treated spirochetes are not cultivable. We hypothesized that noncultivability is probably a result of spirochetes being genetically impaired after treatment with antimicrobials.
TABLE 4.
Culture results for individual mice treated with ceftriaxone and saline solution commencing 4 weeks after inoculation and then subjected to necropsy 18 months after completion of treatment
| Mouse strain | Borrelia strain | Treatment | No. of samples positive/no. testeda
|
|||||
|---|---|---|---|---|---|---|---|---|
| IS | Ear | UB | QM | FJ | Sp | |||
| C3H | N40 | Ceftriaxone | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 |
| C3H | B31 | Ceftriaxone | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| B6 | N40 | Ceftriaxone | 0/5 | 0/5 | 0/5 | 0/5 | 1/5b | 0/5 |
| B6 | B31 | Ceftriaxone | 0/5 | 1/5b | 0/5 | 0/5 | 0/5 | 0/5 |
| C3H | N40 | Saline | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 | 3/4 |
| C3H | B31 | Saline | 3/3 | 1/3 | 2/3 | 2/3 | 3/3 | 1/3 |
| B6 | N40 | Saline | 5/5 | 4/5 | 1/5 | 3/5 | 3/5 | 2/5 |
| B6 | B31 | Saline | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 |
IS, inoculation site; UB, urinary bladder; QM, quadriceps muscle; FJ, front joint; Sp, spleen.
Several spirochetes were observed, but we were unable to subculture them.
Persistence of B. burgdorferi DNA in xenodiagnostic ticks that fed upon antimicrobial-treated mice.
Several days prior to necropsy, each mouse was infested with approximately 40 larval Ixodes scapularis ticks, derived from a single population of laboratory-reared pathogen-free larvae. The ticks were allowed to feed to repletion. The engorged larvae were collected and allowed to molt into nymphs and harden, thereby allowing postfeeding amplification of spirochete populations and minimization of inhibitory products (blood) for PCR. Samples of 5 saline-treated and 10 ceftriaxone-treated randomly selected nymphal ticks from each tick cohort from each mouse were collected. The ticks were tested for the presence of B. burgdorferi flaB-ospA DNA by qPCR. The cohorts of nymphs from each mouse treated with saline solution at 12 and 18 months were all flaB-ospA PCR positive (Table 5). The mean copy numbers of flaB-ospA DNA ranged from 389 to 13,100 (mean ± SD, 3,120 ± 3,790) and from 97 to 11,950 (mean ± SD, 2,340 ± 2,820) copies per tick at 12 months and 18 months, respectively. In contrast, 9 of 20 and 3 of 19 ceftriaxone-treated mice had 1 flaB-ospA PCR-positive tick each among the 10 ticks tested from each mouse at 12 and 18 months, respectively. The mean copy numbers of flaB-ospA DNA ranged from 238 to 1,090 (mean ± SD, 596 ± 249) and from 69 to 714 (mean ± SD, 473 ± 352) copies per tick at 12 months and 18 months, respectively (Table 5).
TABLE 5.
Summary results of xenodiagnosis testing for mice treated with ceftriaxone and saline solution 4 weeks after infection and then infested with larval ticks 12 and 18 months after completion of treatment
| Mouse strain | Borrelia strain | Treatment |
No. of mice positive/no. tested (xenoDxa) |
|
|---|---|---|---|---|
| 12 mo | 18 mo | |||
| C3H | N40 | Ceftriaxone | 3/5 | 1/4 |
| C3H | B31 | Ceftriaxone | 2/5 | 0/0 |
| B6 | N40 | Ceftriaxone | 2/5 | 2/5 |
| B6 | B31 | Ceftriaxone | 2/5 | 0/0 |
| C3H | N40 | Saline | 5/5 | 4/4 |
| C3H | B31 | Saline | 5/5 | 3/3 |
| B6 | N40 | Saline | 5/5 | 5/5 |
| B6 | B31 | Saline | 3/3 | 5/5 |
xenoDx, xenodiagnosis.
Histopathology of mouse tissues following antimicrobial treatment.
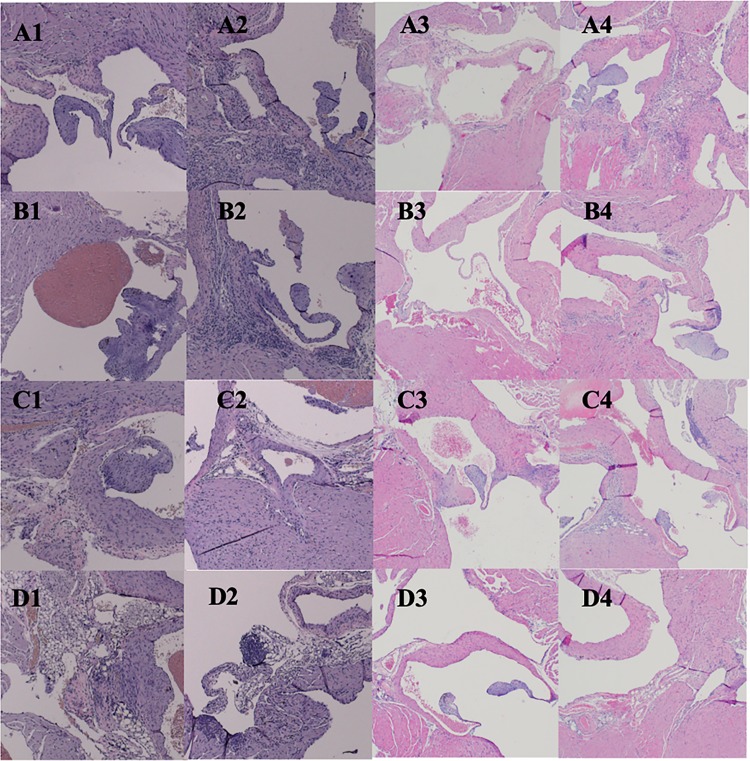
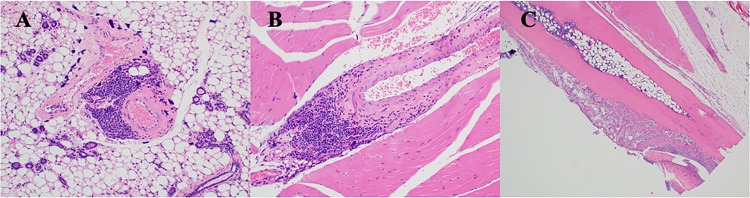
Here, we report on the general features of histopathology. Legs were examined for evidence of arthritis and synovitis and hearts for neutrophil and macrophage infiltration. At 12 months after treatment, inflammatory scores were greatest and were more robust for myocarditis in control C3H/N40 and C3H/B31 mice than in the antimicrobial treatment groups (Fig. 3). Tibiotarsal arthritis was mild, characterized predominantly by synovial hyperplasia and hypertrophy with joint or tendon sheath effusion in both antimicrobial-treated and saline-treated mice at 12 months. There were statistically significant differences (Kruskal-Wallis test; P = 0.0227) between groups based on the myocarditis scores. The antimicrobial-treated C3H/B31 mice had statistically significantly lower scores than the saline-treated C3H/B31 mice (Dunn's multiple-comparison test; P = 0.043). No statistically significant differences (Kruskal-Wallis test; P = 0.1187) were observed between groups based on tibiotarsal arthritis scores (Table 6). At 18 months, inflammation scores were greatest for myocarditis in the saline-treated groups: C3H/N40, C3H/B31, and B6/N40 mice (Fig. 3). Chronic periarteritis and tenosynovitis were observed in a few saline-treated C3H/B31 and B6/N40 mice, respectively, a pathohistological finding frequently observed in chronically borrelia-infected mice (Fig. 4). Osteoarthritis was very mild and was considered a background, age-related finding. There were statistically significant differences (Kruskal-Wallis test; P = 0.0019) between groups based on the myocarditis scores. The C3H/N40 and C3H/B31 saline-treated groups had statistically significantly higher scores than the C3H/B31 antimicrobial-treated group (Dunn's multiple-comparison test; P = 0.046 and P = 0.007). No statistically significant differences (Kruskal-Wallis test; P = 0.1431) were observed between groups based on the tibiotarsal arthritis scores. Statistical analyses were performed using GraphPad Prism v. 7.03 (Table 6).
FIG 3.
Comparisons of carditis severity between ceftriaxone-treated and saline-treated C3H and B6 mice infected with either B. burgdorferi strain N40 or B31 necropsied at 12 and 18 months after treatment. (A1 to A4) C3H/N40. (B1 to B4) C3H/B31. (C1 to C4) B6/N40. (D1 to D4) B6/B31. (Column 1) Ceftriaxone treated/12 months. (Column 2) Saline treated/12 months. (Column 3) Ceftriaxone treated/18 months. (Column 4) Saline treated/18 months.
TABLE 6.
Prevalence and severity of carditis and arthritis in different mouse strains infected with two different B. burgdorferi strains 12 and 18 months after treatment with ceftriaxone or sham treatment with saline solution
| Mouse strain | Borrelia strain | Treatment/mo after treatment | Carditis prevalencea | Mean carditis score ± SDb | Arthritis prevalencea | Mean arthritis score ± SDc |
|---|---|---|---|---|---|---|
| C3H | N40 | Ceftriaxone/12 | 2/5 | 0.40 ± 0.55 | 4/5 | 0.88 ± 0.45 |
| C3H | B31 | Ceftriaxone/12 | 0/4 | 0 | 0/4 | 0 |
| B6 | N40 | Ceftriaxone/12 | 3/5 | 0.60 ± 0.55 | 2/5 | 0.40 ± 0.55 |
| B6 | B31 | Ceftriaxone/12 | 3/5 | 0.60 ± 0.55 | 0/5 | 0 |
| C3H | N40 | Ceftriaxone/18 | 2/4 | 0.50 ± 0.58 | 2/4 | 0.50 ± 0.58 |
| C3H | B31 | Ceftriaxone/18 | 0/5 | 0 | 0/5 | 0 |
| B6 | N40 | Ceftriaxone/18 | 2/5 | 0.40 ± 0.55 | 2/5 | 0.40 ± 0.55 |
| B6 | B31 | Ceftriaxone/18 | 2/5 | 0.40 ± 0.55 | 0/5 | 0 |
| C3H | N40 | Saline/12 | 5/5 | 1.60 ± 0.89 | 4/5 | 0.80 ± 0.45 |
| C3H | B31 | Saline/12 | 5/5 | 2.00 ± 1.00 | 4/5 | 0.80 ± 0.45 |
| B6 | N40 | Saline/12 | 3/5 | 0.60 ± 0.55 | 4/5 | 0.80 ± 0.45 |
| B6 | B31 | Saline/12 | 3/3 | 1.33 ± 0.58 | 2/3 | 0.67 ± 0.58 |
| C3H | N40 | Saline/18 | 4/4 | 1.50 ± 0.58 | 4/4 | 1.00 ± 0.00 |
| C3H | B31 | Saline/18 | 3/3 | 2.00 ± 0.00 | 1/3 | 0.33 ± 0.58 |
| B6 | N40 | Saline/18 | 5/5 | 1.20 ± 0.45 | 3/5 | 0.60 ± 0.55 |
| B6 | B31 | Saline/18 | 4/5 | 0.67 ± 0.58 | 1/5 | 0.33 ± 0.58 |
Number of hearts or joints with inflammation/total number of hearts or joints examined. One tibiotarsus from each mouse was examined.
The severity of carditis was scored on a scale from 0 to 3.
The severity of arthritis was scored on a scale from 0 to 1.
FIG 4.
(A and B) Chronic periarteritis observed in a C3H mouse infected with B. burgdorferi B31 and then treated with saline solution and necropsied 18 months after treatment. (C) Tenosynovitis observed in a B6 mouse infected with B. burgdorferi N40 and then treated with saline solution and necropsied 18 months after treatment.
B. burgdorferi plasmid content in mouse tissues.
In our previous work, we conducted experiments utilizing primarily strain N40, so the designed qPCR assays were based on its genome sequence. For analysis of plasmid presence in tissue samples from C3H and B6 mice infected with B. burgdorferi strain B31, fewer genes were targeted. A comparison of the plasmid profiles, sizes, and GC contents of strains N40 and B31 appeared to show major structural differences, so only four designed qPCR assays targeting bptA, arp, ospA, and erp23 of strain N40 are homologous to those of strain B31.
Among 12-month and 18-month samples, all the gene targets were detected in tissue samples from saline-treated C3H and B6 mice infected with either B. burgdorferi strain N40 or B31 (Tables 7 and 8). As controls for plasmid content we used cultured B. burgdorferi N40 and B31, confirming detection of all the targeted genes. The B. burgdorferi strain N40 genomes in tissue samples with high BbDNA copy numbers from 12 antimicrobial-treated mice were uniformly missing bptA (lp25), erp22 (cp32-9), and erp23 (cp34-2) and variably missing arp (lp28-5), eppA (cp9), and ospC (cp26), whereas ospA (lp54) was detected in all examined tissue samples (Table 7). All the assessed tissue samples from mice infected with strain B31 were uniformly missing bptA (lp25) and arp (lp28-1) and variably missing erp23 (cp32-7). As in tissue samples from mice infected with strain N40, ospA (lp54) was detected in all the samples examined (Table 8). The data suggest loss of linear and circular plasmids in spirochetes persisting at 12 and 18 months after treatment with antimicrobials. No difference was observed between different B. burgdorferi strains (B31 and N40) or in genetically different strains of mice that are disease susceptible (C3H) and disease resistant (B6). Most notable was the uniform absence of lp25 in both B. burgdorferi strains N40 and B31. The results also demonstrated amplification of multiple gene targets, thereby verifying the specificity of residual-BbDNA results (Tables 7 and 8).
TABLE 7.
Analysis of plasmid presence in heart base, ventricular muscle, and tibiotarsus of mice infected with B. burgdorferi strain N40 and then treated with ceftriaxone or sham treated with saline solution
| Mouse strain | Treatment/time (h) | Sample typea | Plasmid presenceb
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Chrom flaB | lp25 bptA | lp28-5 arp | lp54 ospA | cp9 eppA | cp26 ospC | cp32-9 erp22 | cp34-2 erp23 | |||
| C3H | Saline/12 | HB | + | + | + | + | + | + | + | + |
| B6 | Saline/12 | TT | + | + | + | + | + | + | + | + |
| C3H | Saline/18 | HB | + | + | + | + | + | + | + | + |
| B6 | Saline/18 | VM | + | + | + | + | + | + | + | + |
| C3H | Ceftriaxone/12 | HB | + | − | − | + | − | − | − | − |
| C3H | Ceftriaxone/12 | TT | + | − | − | + | − | + | − | − |
| C3H | Ceftriaxone/12 | TT | + | − | − | + | − | − | − | − |
| B6 | Ceftriaxone/12 | TT | + | − | + | + | − | − | − | − |
| B6 | Ceftriaxone/12 | HB | + | − | − | + | − | − | − | − |
| B6 | Ceftriaxone/12 | TT | + | − | − | + | − | − | − | − |
| C3H | Ceftriaxone/18 | TT | + | − | − | + | − | − | − | − |
| C3H | Ceftriaxone/18 | TT | + | − | − | + | − | − | − | − |
| C3H | Ceftriaxone/18 | TT | + | − | + | + | + | + | − | − |
| B6 | Ceftriaxone/18 | TT | + | − | − | + | − | − | − | − |
| B6 | Ceftriaxone/18 | TT | + | − | − | + | − | − | − | − |
| B6 | Ceftriaxone/18 | TT | + | − | − | + | − | − | − | − |
HB, heart base; VM, ventricular muscle; TT, tibiotarsus.
Chrom, chromosomal; +, present; −, absent.
TABLE 8.
Analysis of plasmid presence in heart base, ventricular muscle, and tibiotarsus of mice infected with B. burgdorferi strain B31 and then treated with ceftriaxone or sham treated with saline solution
| Mouse strain | Treatment/time (h) | Sample typea | Plasmid presenceb
|
||||
|---|---|---|---|---|---|---|---|
| Chrom flaB | lp25 bptA | lp28-1 arp | lp54 ospA | cp32-7 erp23 | |||
| C3H | Saline/12 | VM | + | + | + | + | + |
| B6 | Saline/12 | VM | + | + | + | + | + |
| C3H | Saline/18 | HB | + | + | + | + | + |
| B6 | Saline/18 | HB | + | + | + | + | + |
| C3H | Ceftriaxone/12 | TT | + | − | − | + | − |
| C3H | Ceftriaxone/12 | HB | + | − | − | + | − |
| C3H | Ceftriaxone/12 | TT | + | − | − | + | − |
| B6 | Ceftriaxone/12 | TT | + | − | − | + | + |
| B6 | Ceftriaxone/12 | HB | + | − | − | + | − |
| B6 | Ceftriaxone/12 | TT | + | − | − | + | − |
| C3H | Ceftriaxone/18 | TT | + | − | − | + | − |
| C3H | Ceftriaxone/18 | TT | + | − | − | + | − |
| C3H | Ceftriaxone/18 | TT | + | − | − | + | − |
| B6 | Ceftriaxone/18 | TT | + | − | − | + | − |
| B6 | Ceftriaxone/18 | TT | + | − | − | + | − |
| B6 | Ceftriaxone/18 | TT | + | − | − | + | − |
HB, heart base; VM, ventricular muscle; TT, tibiotarsus.
Chrom, chromosomal; +, present; −, absent.
DISCUSSION
The most commonly prescribed antimicrobial agents for the treatment of human Lyme borreliosis, such as penicillin, amoxicillin, cefotaxime, cefuroxime, ceftriaxone, doxycycline, and erythromycin, have been shown to be effective against B. burgdorferi (67, 68). It is important to mention that treatment during early infection is more effective in clearing the pathogen than treatment during later infection in humans (69) and in the mouse model (46).
In this study, we used ceftriaxone, the half-life of which in mouse serum ranges from 1 h (70) to 1.35 h (71) or 1.41 h (72). Its antimicrobial activity and pharmacokinetic (PK)/pharmacodynamic (PD) properties display time-dependent killing (percent T > MIC) and moderate persistent effect, which were indications for multiple daily dosages (72–74). Time-dependent killing is considered a main parameter determining antimicrobial efficacy (73). The MICs and minimal bactericidal concentrations (MBCs) for ceftriaxone against both B. burgdorferi N40 and B31 were evaluated previously in our laboratory (46, 50) and were in accordance with those in similar published studies (75, 76). Studies with ceftriaxone in mice have been challenging because the pharmacodynamics (T > MIC) of ceftriaxone do not parallel those in humans (77). However, the ceftriaxone treatment regimen used (twice daily for 5 days, followed by once daily for 23 days) in our study transiently achieves more than adequate serum concentrations, >300-fold greater than the MBC. Cremieux et al. (78) demonstrated significant bacterial killing when ceftriaxone concentrations exceeded the MBC. The magnitude of bacterial regrowth after the effects of antimicrobials wane and before the next dose is administered depends on the doubling time of the bacteria (79), which is 5 to 12 h for B. burgdorferi (80, 81). Several publications reported clearance of bacteria with short doubling times (20 to 60 min) from infected mice after two daily doses (71), and even a single daily dose (70, 72), of ceftriaxone. Wu et al. (82) and Pavia and Wormser (83) reported B. burgdorferi clearance from infected mice after administrating ceftriaxone twice or once a day for 5 days, respectively. These reports confirm that the ceftriaxone treatment regimen used in our study was adequate.
Over the past several years, there have been numerous well-supported reports of Borrelia detection after completion of antimicrobial therapy (46, 50, 53, 60, 84, 85). The prevalent findings of all these studies were detection of B. burgdorferi by PCR, but not by culture. In BbDNA-positive tissues from antimicrobial-treated mice, morphologically intact spirochetes were observed by immunohistochemistry. Ticks could acquire the spirochetes and transmit them to recipient mice. Transcriptional activity of mouse tissues treated with antimicrobials was detected for numerous targeted B. burgdorferi genes (86).
In the current study, we evaluated persistence at 12 and 18 months after ceftriaxone treatment in two strains of mice that are disease susceptible (C3H) and disease resistant (B6). We recognize that inbred mice do not represent genetically heterogeneous humans, but the use of two genetically and phenotypically disparate mouse strains confirmed the generality of persistence following antimicrobial treatment. Disease severity and tissue spirochete burdens vary among mouse strains (87), although the 50% infective doses (ID50s) among the mice are identical (88). C3H/HeJ mice are susceptible to infection by Gram-negative bacteria as a result of spontaneous mutation in the Tlr4 gene (89). It has been demonstrated that C3H mice develop more severe arthritis and carditis following B. burgdorferi infection than B6 mice (90). B6 mice harbor tissue spirochete burdens equivalent to those of C3H mice, become persistently infected, and have normal macrophage function (89).
Our results have shown that, indeed, persistence in fully immunocompetent B6 mice is similar to that in disease-susceptible C3H mice, which confirms the generality of persistence in a mouse model not being affected by the genetic and immunological makeup of the mammalian host. These results confirm our previous finding (47) that a subpopulation of viable, antimicrobial-tolerant, but slowly dividing spirochetes of B. burgdorferi persists in mice 12 months after treatment and is redisseminated into multiple tissues. Importantly, the copy numbers of spirochetal DNA in tissue samples from antimicrobial-treated disease-resistant B6 mice were nearly equivalent to those in disease-susceptible C3H mice. However, significantly higher DNA copy numbers of spirochetal DNA were determined in saline-treated groups of C3H mice infected with either N40 or B31 than in antimicrobial-treated mice (C3H and B6). This finding is in concordance with the results published by Armstrong et al. (91) showing that C3H mice develop more severe disease than B6 mice, with greater spirochete numbers and later clearance.
Despite the fact that tissues of treated mice remained BbDNA PCR positive at 12 months with significant copy numbers of the targeted genes, culture was consistently negative. Actually, in a single cultured urinary bladder, we observed two spirochetes for up to 3 weeks of incubation. On the other hand, all saline-treated mice were culture positive. Our studies (46, 47, 50) and those of others (45, 49, 92) in mice, dogs (29, 52), and nonhuman primates (53, 54) have all reached similar conclusions: spirochetes persist in tissues after antimicrobial treatment but, paradoxically, cannot be cultured. It has been suggested that the persisting remnants of B. burgdorferi in the tissues of infected mice after antimicrobial treatment are DNA or DNA-containing structures rather than live bacteria (77, 85, 93). In their in vitro study, Iyer et al. (94) could not successfully subculture spirochetes after exposure to ceftriaxone. However, BbDNA was detected by PCR for up to 56 days in aliquots from both ceftriaxone-treated and untreated cultures. Pavia and Wormser (83) demonstrated that B. burgdorferi could not be cultured from experimentally infected mice after ceftriaxone treatment with only 5 daily doses. The treatment regimen used in the study was judged based on the absence of a positive culture.
It has been stated that “unless proven otherwise, culture should be regarded as the gold standard to address viability of B. burgdorferi” (95, 96). Culture may indeed be a gold standard when it is positive, but it is often not. It is apparent that not all isolates or strains can be easily cultured, and this is especially apparent during long-term infection. It is becoming increasingly clear that in B. burgdorferi-infected and antimicrobial-treated animals, spirochetes are noncultivable but viable, as has been demonstrated by xenodiagnoses (46, 47, 50, 54), immunohistology (46, 47, 84), and transcriptional activity of mRNA (47, 50, 54). Here, we demonstrated that B. burgdorferi spirochetes that survived antimicrobial treatment in disease-susceptible C3H mice, as well as in disease-resistant B6 mice, could be acquired by larval ticks. BbDNA was detected in nymphs after molting. In addition, transcription of the chromosomal B. burgdorferi 16S rRNA and flaB genes was detected in treated mice, indicating that spirochetes are metabolically active and alive 12 and 18 months after treatment. The data obtained in this study indicate that in a significant number of culture-negative tissue samples following antimicrobial treatment, complementary methods in diagnostic microbiology should be considered. The clinical significance and the prognostic value of these findings have to be more deeply investigated. It was shown that dead bacterial DNA can be detected for up to 4 to 5 months after antimicrobial treatment (97), as extracellular DNA is very prone to degradation (98–100). Lazarus et al. (101) demonstrated that BbDNA could not be detected in skin tissues of mice that received killed spirochetes 8 h after injection. Bacterial mRNAs, on the other hand, have very short half-lives, as they are degraded by exonucleases very quickly. The half-life ranges from a few minutes to several hours, depending on the bacterial strain (102–104). These findings support the notion that the noncultivability of antimicrobial-tolerant and persistent spirochetes does not negate their viability.
Our previous studies in mice (46, 47, 50) and those of others (49, 92) are all based on B. burgdorferi strain N40. Although N40 probably represents B. burgdorferi, the generality of long-term spirochete persistence following antimicrobial treatment should be evaluated with other B. burgdorferi strains. Therefore, we used B. burgdorferi strain B31 and confirmed persistence at 12 months after antimicrobial treatment in both C3H and B6 mice. Our results indicate that disseminated spirochetes of two different B. burgdorferi strains can persist in mice at 12 and 18 months following antimicrobial treatment. Noncultivable spirochetes persisted in mice (105), dogs (29), and nonhuman primates (54) inoculated with alternate strains of B. burgdorferi, so we have confirmed that persistence is not unique to N40.
An additional long interval, holding mice up to 18 months, facilitated several observations regarding persistent antimicrobial-tolerant spirochetes. Within the observation period, there was molecular evidence of BbDNA in 79% of all assessed mice following antimicrobial treatment. Spirochetal DNA was exclusively detected in the tibiotarsal joint, but not in heart base and ventricular muscle. Copy numbers of BbDNA were not significantly different between treated C3H and B6 mice infected with either N40 or B31. Interestingly, BbDNA copy numbers in the tibiotarsal joints of all the antimicrobial-treated mice at 18 months were equivalent to those at 12 months, suggesting that antimicrobial-tolerant spirochetes were persistent exclusively in connective tissue. One of the proposed mechanisms of B. burgdorferi immune evasion and persistence is sequestration in collagen-rich connective tissue, which makes them less accessible to cells and molecules of the host’s immune system (55, 106). It has been suggested that joint tissue is the niche of B. burgdorferi persistence in mice following antimicrobial treatment (49). There is scientific evidence that tissues with greater decorin expression levels, such as joint tissue, harbor the greatest spirochete loads during chronic infection (107). The findings of our study reinforce the notion of persistence of viable spirochetes following antimicrobial treatment and further implicate connective tissue as a privileged site that may contribute to treatment failure. Although spirochetes persisted for 12 months after antimicrobial treatment, overt disease was not present. Also, despite spirochete presence, postmortem gross signs of disease were not observed in the assessed tissues (heart and joint) of treated mice at 18 months. The resolution of arthritis and carditis in animal models of Lyme borreliosis has been shown to be mediated by the acquired humoral immune response of the host (108). Under these conditions, anatomically defined inflammation resolves, but infection persists, reinforcing the motion that inflammation does not inevitably correlate with the presence of spirochetes (108–110).
Spirochetal RNA was detected in the joint tissue of C3H and B6 mice infected with either N40 or B31 at 18 months following treatment, suggesting its viability. Interestingly, xenodiagnosis was positive in only 3 of 19 treated animals. We speculate that the inability of ticks to acquire spirochetes from all the treated mice could be ascribed to the clearance of spirochetes from heart tissue. Migration of spirochetes from joints to tick attachment sites, that is, usually the head and/or a dorsal part of the mouse body, is farther than from the heart. It was also suggested that the efficiency of spirochete acquisition from a host by a tick depends on the intensity and duration of infection, and it is significantly less during chronic infection (111). However, we recovered a few slow-growing spirochetes by culture from joint tissues of two mice, utilizing several specially prepared media that were graciously provided by Monica E. Embers. We were unable to propagate the recovered spirochetes. Obviously, the media used were unable to support the prolonged growth of slow-growing spirochetes.
Several studies have shown that B. burgdorferi is inclined to plasmid loss (64, 66, 81, 112–114). In addition to a complete loss, there is a report of a partial plasmid loss of the vls gene cassette (115). Several plasmids were found to be associated with spirochete infectivity (65). Plasmid loss was reported to impact infectivity (66, 116), and spirochetes became more vulnerable to host-mediated clearance (117). Plasmid profile analysis of tissue samples from antimicrobial-treated C3H and B6 mice infected with either B. burgdorferi N40 or B31 revealed loss of several plasmids in persisting spirochetes at 12 and 18 months after antimicrobial treatment. A correlation between plasmid loss and persistence of antimicrobial-tolerant spirochetes is not clear and will require further investigation.
This study has shown that following antimicrobial treatment, both strains of B. burgdorferi, N40 and B31, produce a progressively diverse population of noncultivable and persistent spirochetes. The persistent spirochetes divide and grow slowly, which probably results in their tolerance for the effects of antimicrobial agents, as well as being noncultivable, confirming our previous findings (47). Antibacterial resistance has not been observed for B. burgdorferi, so the phenomenon of persistence of noncultivable spirochetes following antimicrobial treatment has been explained by antimicrobial tolerance (63). Unlike antimicrobial resistance, antimicrobial tolerance, for all classes of antimicrobials, fails to completely eliminate nondividing or slowly dividing subpopulations of a broad array of bacteria and fungi (62, 118). Persistence is difficult to study, as persisters are phenotypic variants of actively dividing cells produced in the population at low abundance during the late exponential phase of growth (118–121). The mechanisms of B. burgdorferi persistence, especially after antimicrobial treatment, is still to be determined. Several mechanisms have been proposed, such as gene modulation as a result of the stringent response (122), active immune suppression and evasion (55), metabolic alteration of guanosine pentaphosphate [(p)ppGpp] and probably the dksA gene (123, 124), and frequency of environmental changes (123). Further studies are required to establish the roles of those mechanisms in persistence.
The clinical relevance of microbial persistence has been demonstrated for Burkholderia pseudomallei (125), Escherichia coli (126), Pseudomonas aeruginosa (127), Candida albicans (128), Streptococcus pneumoniae (129), Staphylococcus aureus (126, 130), Mycobacterium tuberculosis (131, 132), Legionella spp. (133, 134), and Salmonella enterica (135). The persistent cells have transient tolerance for antimicrobial agents, and their ability to revert to a sensitive state, in which cells rapidly divide, makes them important in chronic infections (136). There are two major consequences of the presence of persister cells: (i) the continuous presence of viable cells during consecutive rounds of antimicrobial treatment contributes to the emergence of antimicrobial resistance and (ii) experimental evidence indicates that prolonged exposure to antimicrobial agents leads to the selection of highly persistent mutants (137, 138). The infectious life cycle relevance of attenuated B. burgdorferi spirochetes is probably inconsequential, while their clinical relevance in mice was a subject of this study. The study demonstrated that noncultivable spirochetes can persist in mice following antimicrobial treatment for up to 18 months but did not demonstrate their clinical relevance. The relatively short mouse life span (18 to 24 months) is a limiting factor in studying the persistence and resurgence of antimicrobial-tolerant B. burgdorferi beyond 18 months after treatment. The phenomenon of resurgence observed in mice at 12 months after antimicrobial treatment (47) resembles recurrent Lyme borreliosis observed in humans (139–141). Therefore, the clinical relevance of persistent spirochetes and their resurgence beyond 18 months following antimicrobial treatment compels further studies utilizing other animal models.
MATERIALS AND METHODS
Mouse infections.
All experiments involving vertebrate animals were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of California Davis (UCD). All the animals were purchased from The Jackson Laboratory, Bar Harbor, ME, and were cared for by staff from Teaching and Research Animal Care Services (TRACS) at UCD. The mice were maintained in an isolated room within filter top cages and were provided food and water ad libitum. The animals used in this study were C3H and B6 specific-pathogen-free 5-week-old female mice and were maintained in cohorts of 5 mice per cage under identical husbandry conditions in the same animal room. Euthanasia was performed by rapid carbon dioxide narcosis in accordance with guidelines of the American Veterinary Medical Association (AVMA) (142). C3H and B6 mice were randomly divided into two equal groups. One group of C3H and B6 mice were infected with B. burgdorferi N40 and another with B. burgdorferi B31 (Table 9). Each mouse was infected by syringe inoculation of 104 B. burgdorferi spirochetes at the mid-log phase in 0.1 ml of BSK-II medium intradermally at the dorsal thoracic midline.
TABLE 9.
Experimental design for eight treatment groups
| Group | B. burgdorferi strain | Mouse strain | Treatment | No. of mice treated for: |
Total no. of mice (n) | |
|---|---|---|---|---|---|---|
| 12 mo | 18 mo | |||||
| 1 | N40 | C3H | Ceftriaxone | 5 | 5 | 10 |
| 2 | B31 | C3H | Ceftriaxone | 5 | 5 | 10 |
| 3 | N40 | B6 | Ceftriaxone | 5 | 5 | 10 |
| 4 | B31 | B6 | Ceftriaxone | 5 | 5 | 10 |
| 5 | N40 | C3H | Saline | 5 | 5 | 10 |
| 6 | B31 | C3H | Saline | 5 | 5 | 10 |
| 7 | N40 | B6 | Saline | 5 | 5 | 10 |
| 8 | B31 | B6 | Saline | 5 | 5 | 10 |
B. burgdorferi culturing.
Two low-passage clonal strains of B. burgdorferi sensu stricto were used. B. burgdorferi strains N40 and B31 were cloned by 3-fold limiting dilution in vitro and passage in mice to prove infectivity and pathogenicity, as described previously (24). Frozen aliquots of clonal strains N40 and B31 were thawed and cultured in modified BSK-II medium (81) at 33°C. Spirochetes were assessed for viability and enumerated by dark-field microscopy using a Petroff-Hausser bacterial counting chamber (Hausser Scientific, Horsham, PA) immediately prior to use and diluted to appropriate concentrations in BSK-II medium. The mice were inoculated subdermally on the dorsal thoracic midline with 104 mid-log-phase spirochetes in 0.1 ml of BSK-II medium. The status of infection with cultivable B. burgdorferi was determined by culture of the urinary bladder and the subinoculation site (deep dermis) at 12 and 18 months after completion of antimicrobial treatment. Tissues were collected from mice aseptically at necropsy and then cultured in medium without antibiotic, as described previously (143).
To determine if persistent spirochetes regain cultivability at 18 months after treatment and to increase their detectability, multiple tissue sites were collected and cultured in four different media. In addition to the urinary bladder and inoculation site, we also collected front joint, ear, quadriceps muscle, and spleen tissues. Heart tissue was not available for culture as it was for PCR and histology. To facilitate cultivation, several media have been introduced, such as BSK-II medium, MKP medium, BSK-II plus agarose, and BSK-II plus carbohydrates (cordially provided by Monica E. Embers of Tulane National Primate Research Center, Covington, LA). However, these media differ in their potentials to support the growth of borrelia.
Xenodiagnosis.
Laboratory-reared, pathogen-free I. scapularis larvae were provided by Melissa J. Caimano, Department of Molecular Biology and Biophysics, University of Connecticut Health, Farmington, CT. All the larvae were derived from single gravid ticks for each study. Around 40 larval ticks were placed on each mouse 1 week prior to necropsy, allowed to feed to repletion, collected, and then allowed to molt and harden into nymphs. Cohorts of ticks collected from each mouse were maintained separately, so that ticks within each cohort could be tested individually or in groups by qPCR. The nymphal ticks were frozen in liquid nitrogen and ground with a mortar and pestle, and DNA was extracted for PCR analysis.
Antimicrobial treatment and monitoring.
Treatment with ceftriaxone was initiated during the early immune/dissemination stage at 4 weeks postinfection with B. burgdorferi in immunocompetent C3H and B6 mice to mimic the stages found in human disease. The experimental design for this experiment involved eight treatment groups, as depicted in Table 9. The treatment regimen used in the study was published previously (45, 46). A ceftriaxone dosage of 16 mg/kg of body weight in 500 μl of 0.9% normal saline was administered intraperitoneally to the mice twice daily for 5 days, followed by a once-daily dose for 23 days. The serum concentrations of ceftriaxone in mice were measured on the day of treatment by bleeding the mice at 0, 2, 4, and 8 h after treatment. Serum samples were separated from whole blood and stored at −80°C until analysis. A previously described agar diffusion assay method (144) was utilized to determine the serum concentration of ceftriaxone.
Tissue processing for molecular analysis.
Mouse tissue samples for molecular analysis were processed as previously described (47, 145), with slight modifications. Briefly, samples of the heart base, ventricular myocardium (the heart was bisected through the atria and ventricles, with one-half of the heart base and ventricles used for nucleic acid extraction and the other half processed for histology), and the left tibiotarsal joint were collected from each mouse. The samples were collected aseptically, immediately weighed, snap-frozen in liquid nitrogen, and stored at −80°C before nucleic acid extraction. Total nucleic acid was extracted with a QIAcube HT system (Qiagen, Valencia, CA) according to the manufacturer’s instructions for tissue or insects. The system enables semiautomated high-throughput nucleic acid purification, yielding high-quality pathogen nucleic acids free of contaminants and inhibitors. The copy number of each B. burgdorferi target gene was expressed per milligram of tissue weight.
Molecular quantitative analysis.
Because low DNA/RNA copy numbers were expected in tissues from antimicrobial-treated mice, each sample was subjected to preamplification. We used the B. burgdorferi outer surface protein (ospA) and flagellin (flaB) primers and a mouse glyceraldehyde-3-phosphate dehydrogenase (gapDH) assay primer, standardized and optimized for real-time qPCR, as described previously (146). The preamplified products were diluted at a ratio of 1:10 and used as templates for qPCR analysis. To assess the transcriptional activity of the B. burgdorferi flaB and 16S rRNA genes, first-strand cDNA was synthesized from total RNA using a QuantiTect reverse transcription kit (Qiagen) in 50-μl reaction mixtures, as described previously (146). To increase the fidelity, efficiency, and yield of cDNA, Advantage 2 polymerase mix (Clontech Laboratories) was used, according to the manufacturer’s instructions.
Two specific primers and one internal fluorescence-labeled probe were designed with Primer Express software (ThermoFisher Scientific), as described previously (147). The amplification efficiency (E) of all assays was calculated from the slope of a standard curve as described in detail previously (47). To determine the copy numbers of DNA target genes, plasmid standards were prepared in order to create absolute standard curves, as described previously (147). Based on the amplification efficiencies, the detection limits were <10 copies of DNA per reaction, and the analytical sensitivity for each target gene was in the range from <10 to 109 copies. All samples were assayed in duplicate with positive and negative controls by qPCR optimized assays and analyzed for the presence of mouse gapDH in order to determine the efficiency of the nucleic acid extraction and amplification and as an indicator of inhibition. Amplification, data acquisition, and data analysis were performed on a 7900HT Fast real-time PCR system (ThermoFisher Scientific). The thermal-cycling conditions were described previously (148).
Histology.
Formalin-fixed rear legs (demineralized in 15% formic acid) and hearts were routinely processed for histology, embedded in paraffin, and sectioned at 5 μm. The sections were stained with hematoxylin and eosin. All tissues were evaluated by a board-certified veterinary anatomical pathologist. The legs were examined for evidence of arthritis and synovitis involving the knee and tibiotarsal joints, as characterized by examination of infiltration of neutrophils, synovial proliferation, and exudation of fibrin into joint or tendon sheath lumina, as well as neutrophil and macrophage infiltration of tissues at the base of the heart (myocarditis). All determinations were made in a blinded fashion.
Statistics.
Statistical analysis of qPCR data between antimicrobial-treated and sham-treated mice at different time points was performed using an independent-samples t test or one-way analysis of variance, followed by multiple pairwise comparisons by Tukey’s honestly significant difference (HSD) test (SPSS 16.0 for Mac; SPSS Inc., Chicago, IL). Differences were considered significant at a P value of ≤0.5. To statistically evaluate the severity of inflammation in collected tissue between antimicrobial-treated and sham-treated mice, a Kruskal-Wallis test was performed using GraphPad Prism v. 7.03.
ACKNOWLEDGMENTS
We declare that no competing interests exist.
This work was supported by the Bay Area Lyme Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The technical assistance of Kim Olsen and Cara Wademan was essential to this study and is greatly appreciated. We thank Melissa J. Caimano for providing I. scapularis larval ticks and Monica E. Embers for providing culture media.
REFERENCES
- 1.Mead PS. 2015. Epidemiology of Lyme disease. Infect Dis Clin North Am 29:187–210. doi: 10.1016/j.idc.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Adrion ER, Aucott J, Lemke KW, Weiner JP. 2015. Health care costs, utilization and patterns of care following Lyme disease. PLoS One 10:e0116767. doi: 10.1371/journal.pone.0116767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nelson CA, Saha S, Kugeler KJ, Delorey MJ, Shankar MB, Hinckley AF, Mead PS. 2015. Incidence of clinician-diagnosed Lyme disease, United States, 2005-2010. Emerg Infect Dis 21:1625–1631. doi: 10.3201/eid2109.150417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eisen RJ, Eisen L, Beard CB. 2016. County-scale distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the continental United States. J Med Entomol 53:349–386. doi: 10.1093/jme/tjv237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steere AC, Sikand VK. 2003. The presenting manifestations of Lyme disease and the outcomes of treatment. N Engl J Med 348:2472–2474. doi: 10.1056/NEJM200306123482423. [DOI] [PubMed] [Google Scholar]
- 6.Tibbles CD, Edlow JA. 2007. Does this patient have erythema migrans? JAMA 297:2617–2627. doi: 10.1001/jama.297.23.2617. [DOI] [PubMed] [Google Scholar]
- 7.Stinco G, Ruscio M, Bergamo S, Trotter D, Patrone P. 2014. Clinical features of 705 Borrelia burgdorferi seropositive patients in an endemic area of northern Italy. Sci World J 2014:1. doi: 10.1155/2014/414505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strle F, Wormser GP, Mead P, Dhaduvai K, Longo MV, Adenikinju O, Soman S, Tefera Y, Maraspin V, Lotrič-Furlan S, Ogrinc K, Cimperman J, Ružić-Sabljić E, Stupica D. 2013. Gender disparity between cutaneous and non-cutaneous manifestations of Lyme borreliosis. PLoS One 8:e64110. doi: 10.1371/journal.pone.0064110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steere AC, Dhar A, Hernandez J, Fischer PA, Sikand VK, Schoen RT, Nowakowski J, McHugh G, Persing DH. 2003. Systemic symptoms without erythema migrans as the presenting picture of early Lyme disease. Am J Med 114:58–62. doi: 10.1016/s0002-9343(02)01440-7. [DOI] [PubMed] [Google Scholar]
- 10.Baker PJ, Wormser GP. 2017. The clinical relevance of studies on Borrelia burgdorferi persisters. Am J Med doi: 10.1016/j.amjmed.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 11.Aucott JN. 2015. Posttreatment Lyme disease syndrome. Infect Dis Clin North Am 29:309–323. doi: 10.1016/j.idc.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 12.Aucott JN, Crowder LA, Kortte KB. 2013. Development of a foundation for a case definition of post-treatment Lyme disease syndrome. Int J Infect Dis 17:e443–e449. doi: 10.1016/j.ijid.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Asch ES, Bujak DI, Weiss M, Peterson MG, Weinstein A. 1994. Lyme disease: an infectious and postinfectious syndrome. J Rheumatol 21:454–461. [PubMed] [Google Scholar]
- 14.Shadick NA, Phillips CB, Logigian EL, Steere AC, Kaplan RF, Berardi VP, Duray PH, Larson MG, Wright EA, Ginsburg KS, Katz JN, Liang MH. 1994. The long-term clinical outcomes of Lyme disease. A population-based retrospective cohort study. Ann Intern Med 121:560–567. doi: 10.7326/0003-4819-121-8-199410150-00002. [DOI] [PubMed] [Google Scholar]
- 15.Feder HM Jr, Johnson BJ, O'Connell S, Shapiro ED, Steere AC, Wormser GP, Ad Hoc International Lyme Disease Group, Agger WA, Artsob H, Auwaerter P, Dumler JS, Bakken JS, Bockenstedt LK, Green J, Dattwyler RJ, Munoz J, Nadelman RB, Schwartz I, Draper T, McSweegan E, Halperin JJ, Klempner MS, Krause PJ, Mead P, Morshed M, Porwancher R, Radolf JD, Smith RP Jr, Sood S, Weinstein A, Wong SJ, Zemel L. 2007. A critical appraisal of “chronic Lyme disease”. N Engl J Med 357:1422–1430. doi: 10.1056/NEJMra072023. [DOI] [PubMed] [Google Scholar]
- 16.Marques A, Telford SR III, Turk SP, Chung E, Williams C, Dardick K, Krause PJ, Brandeburg C, Crowder CD, Carolan HE, Eshoo MW, Shaw PA, Hu LT. 2014. Xenodiagnosis to detect Borrelia burgdorferi infection: a first-in-human study. Clin Infect Dis 58:937–945. doi: 10.1093/cid/cit939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Breier F, Khanakah G, Stanek G, Kunz G, Aberer E, Schmidt B, Tappeiner G. 2001. Isolation and polymerase chain reaction typing of Borrelia afzelii from a skin lesion in a seronegative patient with generalized ulcerating bullous lichen sclerosus et atrophicus. Br J Dermatol 144:387–392. doi: 10.1046/j.1365-2133.2001.04034.x. [DOI] [PubMed] [Google Scholar]
- 18.Honegr K, Hulinska D, Beran J, Dostal V, Havlasova J, Cermakova Z. 2004. Long term and repeated electron microscopy and PCR detection of Borrelia burgdorferi sensu lato after an antibiotic treatment. Cent Eur J Public Health 12:6–11. [PubMed] [Google Scholar]
- 19.Hunfeld KP, Ruzic-Sabljic E, Norris DE, Kraiczy P, Strle F. 2005. In vitro susceptibility testing of Borrelia burgdorferi sensu lato isolates cultured from patients with erythema migrans before and after antimicrobial chemotherapy. Antimicrob Agents Chemother 49:1294–1301. doi: 10.1128/AAC.49.4.1294-1301.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oksi J, Marjamaki M, Nikoskelainen J, Viljanen MK. 1999. Borrelia burgdorferi detected by culture and PCR in clinical relapse of disseminated Lyme borreliosis. Ann Med 31:225–232. doi: 10.3109/07853899909115982. [DOI] [PubMed] [Google Scholar]
- 21.Priem S, Burmester GR, Kamradt T, Wolbart K, Rittig MG, Krause A. 1998. Detection of Borrelia burgdorferi by polymerase chain reaction in synovial membrane, but not in synovial fluid from patients with persisting Lyme arthritis after antibiotic therapy. Ann Rheum Dis 57:118–121. doi: 10.1136/ard.57.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Middelveen MJ, Sapi E, Burke J, Filush KR, Franco A, Fesler MC, Stricker RB. 2018. Persistent borrelia infection in patients with ongoing symptoms of Lyme disease. Healthcare (Basel) 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwan TG, Burgdorfer W, Schrumpf ME, Karstens RH. 1988. The urinary bladder, a consistent source of Borrelia burgdorferi in experimentally infected white-footed mice (Peromyscus leucopus). J Clin Microbiol 26:893–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barthold SW, de Souza MS, Janotka JL, Smith AL, Persing DH. 1993. Chronic Lyme borreliosis in the laboratory mouse. Am J Pathol 143:959–971. [PMC free article] [PubMed] [Google Scholar]
- 25.Moody KD, Barthold SW, Terwilliger GA, Beck DS, Hansen GM, Jacoby RO. 1990. Experimental chronic Lyme borreliosis in Lewis rats. Am J Trop Med Hyg 42:165–174. doi: 10.4269/ajtmh.1990.42.165. [DOI] [PubMed] [Google Scholar]
- 26.Goodman JL, Jurkovich P, Kodner C, Johnson RC. 1991. Persistent cardiac and urinary tract infections with Borrelia burgdorferi in experimentally infected Syrian hamsters. J Clin Microbiol 29:894–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Preac Mursic V, Patsouris E, Wilske B, Reinhardt S, Gross B, Mehraein P. 1990. Persistence of Borrelia burgdorferi and histopathological alterations in experimentally infected animals. A comparison with histopathological findings in human Lyme disease. Infection 18:332–341. doi: 10.1007/BF01646399. [DOI] [PubMed] [Google Scholar]
- 28.Sonnesyn SW, Manivel JC, Johnson RC, Goodman JL. 1993. A guinea pig model for Lyme disease. Infect Immun 61:4777–4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Straubinger RK, Summers BA, Chang YF, Appel MJ. 1997. Persistence of Borrelia burgdorferi in experimentally infected dogs after antibiotic treatment. J Clin Microbiol 35:111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roberts ED, Bohm RP Jr, Cogswell FB, Lanners HN, Lowrie RC Jr, Povinelli L, Piesman J, Philipp MT. 1995. Chronic Lyme disease in the rhesus monkey. Lab Invest 72:146–160. [PubMed] [Google Scholar]
- 31.Asbrink E, Hovmark A. 1985. Successful cultivation of spirochetes from skin lesions of patients with erythema chronicum migrans Afzelius and acrodermatitis chronica atrophicans. Acta Pathol Microbiol Immunol Scand B 93:161–163. [DOI] [PubMed] [Google Scholar]
- 32.Kuiper H, van Dam AP, Spanjaard L, de Jongh BM, Widjojokusumo A, Ramselaar TC, Cairo I, Vos K, Dankert J. 1994. Isolation of Borrelia burgdorferi from biopsy specimens taken from healthy-looking skin of patients with Lyme borreliosis. J Clin Microbiol 32:715–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maraspin V, Ogrinc K, Ružić-Sabljić E, Lotrič-Furlan S, Strle F. 2011. Isolation of Borrelia burgdorferi sensu lato from blood of adult patients with borrelial lymphocytoma, Lyme neuroborreliosis, Lyme arthritis and acrodermatitis chronica atrophicans. Infection 39:35–40. doi: 10.1007/s15010-010-0062-8. [DOI] [PubMed] [Google Scholar]
- 34.Miklossy J, Khalili K, Gern L, Ericson RL, Darekar P, Bolle L, Hurlimann J, Paster BJ. 2005. Borrelia burgdorferi persists in the brain in chronic Lyme neuroborreliosis and may be associated with Alzheimer disease. J Alzheimers Dis 6:639–649. doi: 10.3233/JAD-2004-6608. [DOI] [PubMed] [Google Scholar]
- 35.Snydman DR, Schenkein DP, Berardi VP, Lastavica CC, Pariser KM. 1986. Borrelia burgdorferi in joint fluid in chronic Lyme arthritis. Ann Intern Med 104:798–800. doi: 10.7326/0003-4819-104-6-798. [DOI] [PubMed] [Google Scholar]
- 36.Stanek G, Klein J, Bittner R, Glogar D. 1990. Isolation of Borrelia burgdorferi from the myocardium of a patient with longstanding cardiomyopathy. N Engl J Med 322:249–252. doi: 10.1056/NEJM199001253220407. [DOI] [PubMed] [Google Scholar]
- 37.Strle F, Cheng Y, Cimperman J, Maraspin V, Lotric-Furlan S, Nelson JA, Picken MM, Ruzic-Sabljic E, Picken RN. 1995. Persistence of Borrelia burgdorferi sensu lato in resolved erythema migrans lesions. Clin Infect Dis 21:380–389. doi: 10.1093/clinids/21.2.380. [DOI] [PubMed] [Google Scholar]
- 38.Bradley JF, Johnson RC, Goodman JL. 1994. The persistence of spirochetal nucleic acids in active Lyme arthritis. Ann Intern Med 120:487–489. doi: 10.7326/0003-4819-120-6-199403150-00007. [DOI] [PubMed] [Google Scholar]
- 39.Moter SE, Hofmann H, Wallich R, Simon MM, Kramer MD. 1994. Detection of Borrelia burgdorferi sensu lato in lesional skin of patients with erythema migrans and acrodermatitis chronica atrophicans by ospA-specific PCR. J Clin Microbiol 32:2980–2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nocton JJ, Dressler F, Rutledge BJ, Rys PN, Persing DH, Steere AC. 1994. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N Engl J Med 330:229–234. doi: 10.1056/NEJM199401273300401. [DOI] [PubMed] [Google Scholar]
- 41.von Stedingk LV, Olsson I, Hanson HS, Asbrink E, Hovmark A. 1995. Polymerase chain reaction for detection of Borrelia burgdorferi DNA in skin lesions of early and late Lyme borreliosis. Eur J Clin Microbiol Infect Dis 14:1–5. doi: 10.1007/BF02112610. [DOI] [PubMed] [Google Scholar]
- 42.Bockenstedt LK, Radolf JD. 2014. Xenodiagnosis for posttreatment Lyme disease syndrome: resolving the conundrum or adding to it? Clin Infect Dis 58:946–948. doi: 10.1093/cid/cit942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marques A. 2008. Chronic Lyme disease: a review. Infect Dis Clin North Am 22:341–360. doi: 10.1016/j.idc.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gaubitz M, Dressler F, Huppertz HI, Krause A, Kommission Pharmakotherapie der DGRb. 2014. Diagnosis and treatment of Lyme arthritis. Recommendations of the Pharmacotherapy Commission of the Deutsche Gesellschaft fur Rheumatologie (German Society for Rheumatology). Z Rheumatol 73:469–474. doi: 10.1007/s00393-014-1370-7. [DOI] [PubMed] [Google Scholar]
- 45.Bockenstedt LK, Mao J, Hodzic E, Barthold SW, Fish D. 2002. Detection of attenuated, noninfectious spirochetes in Borrelia burgdorferi-infected mice after antibiotic treatment. J Infect Dis 186:1430–1437. doi: 10.1086/345284. [DOI] [PubMed] [Google Scholar]
- 46.Hodzic E, Feng S, Holden K, Freet KJ, Barthold SW. 2008. Persistence of Borrelia burgdorferi following antibiotic treatment in mice. Antimicrob Agents Chemother 52:1728–1736. doi: 10.1128/AAC.01050-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hodzic E, Imai D, Feng S, Barthold SW. 2014. Resurgence of persisting non-cultivable Borrelia burgdorferi following antibiotic treatment in mice. PLoS One 9:e86907. doi: 10.1371/journal.pone.0086907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barthold SW, Cadavid D, Philipp MT. 2010. Animal models of borreliosis, p 353–405. In Samuels DS, Radolf JD (ed), Borrelia molecular biology, host interaction and pathogenesis. Caister Academic Press, Norfolk, United Kingdom. [Google Scholar]
- 49.Yrjanainen H, Hytonen J, Hartiala P, Oksi J, Viljanen MK. 2010. Persistence of borrelial DNA in the joints of Borrelia burgdorferi-infected mice after ceftriaxone treatment. Apmis 118:665–673. doi: 10.1111/j.1600-0463.2010.02615.x. [DOI] [PubMed] [Google Scholar]
- 50.Barthold SW, Hodzic E, Imai DM, Feng S, Yang X, Luft BJ. 2010. Ineffectiveness of tigecycline against persistent Borrelia burgdorferi. Antimicrob Agents Chemother 54:643–651. doi: 10.1128/AAC.00788-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Straubinger RK, Straubinger AF, Summers BA, Jacobson RH. 2000. Status of Borrelia burgdorferi infection after antibiotic treatment and the effects of corticosteroids: an experimental study. J Infect Dis 181:1069–1081. doi: 10.1086/315340. [DOI] [PubMed] [Google Scholar]
- 52.Straubinger RK, Straubinger AF, Summers BA, Jacobson RH, Erb HN. 1998. Clinical manifestations, pathogenesis, and effect of antibiotic treatment on Lyme borreliosis in dogs. Wien Klin Wochenschr 110:874–881. [PubMed] [Google Scholar]
- 53.Embers ME, Barthold SW, Borda JT, Bowers L, Doyle L, Hodzic E, Jacobs MB, Hasenkampf NR, Martin DS, Narasimhan S, Phillippi-Falkenstein KM, Purcell JE, Ratterree MS, Philipp MT. 2012. Persistence of Borrelia burgdorferi in rhesus macaques following antibiotic treatment of disseminated infection. PLoS One 7:e29914. doi: 10.1371/journal.pone.0029914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Embers ME, Hasenkampf NR, Jacobs MB, Tardo AC, Crossland NA, Doyle-Meyers LA, Philipp MT, Hodzic E. 2017. Variable manifestations, diverse seroreactivity and post-treatment persistence in nonhuman primates exposed to Borrelia burgdorferi by tick feeding. PLoS One 12:e0189071. doi: 10.1371/journal.pone.0189071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Embers ME, Ramamoorthy R, Philipp MT. 2004. Survival strategies of Borrelia burgdorferi, the etiologic agent of Lyme disease. Microbes Infect 6:312–318. doi: 10.1016/j.micinf.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 56.Tilly K, Rosa PA, Stewart PE. 2008. Biology of infection with Borrelia burgdorferi. Infect Dis Clin North Am 22:217–234. doi: 10.1016/j.idc.2007.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tracy KE, Baumgarth N. 2017. Borrelia burgdorferi manipulates innate and adaptive immunity to establish persistence in rodent reservoir hosts. Front Immunol 8:116. doi: 10.3389/fimmu.2017.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Anguita J, Hedrick MN, Fikrig E. 2003. Adaptation of Borrelia burgdorferi in the tick and the mammalian host. FEMS Microbiol Rev 27:493–504. doi: 10.1016/S0168-6445(03)00036-6. [DOI] [PubMed] [Google Scholar]
- 59.Smith AJ, Oertle J, Prato P. 2014. Chronic Lyme disease: persistent clinical symptoms related to immune evasion, antibiotic resistance and various defense mechanisms of Borrelia burgdorferi. OJMM 4:252–260. doi: 10.4236/ojmm.2014.44029. [DOI] [Google Scholar]
- 60.Straubinger RK, Straubinger AF, Summers BA, Erb HN, Harter L, Appel MJ. 1998. Borrelia burgdorferi induces the production and release of proinflammatory cytokines in canine synovial explant cultures. Infect Immun 66:247–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lewis K. 2010. Persister cells. Annu Rev Microbiol 64:357–372. doi: 10.1146/annurev.micro.112408.134306. [DOI] [PubMed] [Google Scholar]
- 62.Lewis K. 2008. Multidrug tolerance of biofilms and persister cells. Curr Top Microbiol Immunol 322:107–131. [DOI] [PubMed] [Google Scholar]
- 63.Sharma B, Brown AV, Matluck NE, Hu LT, Lewis K. 2015. Borrelia burgdorferi, the causative agent of Lyme disease, forms drug-tolerant persister cells. Antimicrob Agents Chemother 59:4616–4624. doi: 10.1128/AAC.00864-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Biškup UG, Strle F, Ružić-Sabljić E. 2011. Loss of plasmids of Borrelia burgdorferi sensu lato during prolonged in vitro cultivation. Plasmid 66:1–6. doi: 10.1016/j.plasmid.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 65.Purser JE, Norris SJ. 2000. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc Natl Acad Sci U S A 97:13865–13870. doi: 10.1073/pnas.97.25.13865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schwan TG, Burgdorfer W, Garon CF. 1988. Changes in infectivity and plasmid profile of the Lyme disease spirochete, Borrelia burgdorferi, as a result of in vitro cultivation. Infect Immun 56:1831–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arvikar SL, Steere AC. 2015. Diagnosis and treatment of Lyme arthritis. Infect Dis Clin North Am 29:269–280. doi: 10.1016/j.idc.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wormser GP, Nadelman RB, Dattwyler RJ, Dennis DT, Shapiro ED, Steere AC, Rush TJ, Rahn DW, Coyle PK, Persing DH, Fish D, Luft BJ. 2000. Practice guidelines for the treatment of Lyme disease. Clin Infect Dis 31(Suppl 1):1–14. doi: 10.1086/512462. [DOI] [PubMed] [Google Scholar]
- 69.Maloney EL. 2016. Controversies in persistent (chronic) Lyme disease. J Infus Nurs 39:369–375. doi: 10.1097/NAN.0000000000000195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Connolly KL, Eakin AE, Gomez C, Osborn BL, Unemo M, Jerse AE. 2019. Pharmacokinetic data are predictive of in vivo efficacy for cefixime and ceftriaxone against susceptible and resistant Neisseria gonorrhoeae strains in the gonorrhea mouse model. Antimicrob Agents Chemother 63:e01644-18. doi: 10.1128/AAC.01644-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sauve C, Azoulay-Dupuis E, Moine P, Darras-Joly C, Rieux V, Carbon C, Bédos JP. 1996. Efficacies of cefotaxime and ceftriaxone in a mouse model of pneumonia induced by two penicillin- and cephalosporin-resistant strains of Streptococcus pneumoniae. Antimicrob Agents Chemother 40:2829–2834. doi: 10.1128/AAC.40.12.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moine P, Vallee E, Azoulay-Dupuis E, Bourget P, Bedos JP, Bauchet J, Pocidalo JJ. 1994. In vivo efficacy of a broad-spectrum cephalosporin, ceftriaxone, against penicillin-susceptible and -resistant strains of Streptococcus pneumoniae in a mouse pneumonia model. Antimicrob Agents Chemother 38:1953–1958. doi: 10.1128/AAC.38.9.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhao M, Lepak AJ, Andes DR. 2016. Animal models in the pharmacokinetic/pharmacodynamic evaluation of antimicrobial agents. Bioorg Med Chem 24:6390–6400. doi: 10.1016/j.bmc.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 74.Turnidge JD. 1998. The pharmacodynamics of beta-lactams. Clin Infect Dis 27:10–22. doi: 10.1086/514622. [DOI] [PubMed] [Google Scholar]
- 75.Murgia R, Marchetti F, Cinco M. 1999. Comparative bacteriostatic and bactericidal activities of cefodizime against Borrelia burgdorferi sensu lato. Antimicrob Agents Chemother 43:3030–3032. doi: 10.1128/AAC.43.12.3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sicklinger M, Wienecke R, Neubert U. 2003. In vitro susceptibility testing of four antibiotics against Borrelia burgdorferi: a comparison of results for the three genospecies Borrelia afzelii, Borrelia garinii, and Borrelia burgdorferi sensu stricto. J Clin Microbiol 41:1791–1793. doi: 10.1128/jcm.41.4.1791-1793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wormser GP, Schwartz I. 2009. Antibiotic treatment of animals infected with Borrelia burgdorferi. Clin Microbiol Rev 22:387–395. doi: 10.1128/CMR.00004-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cremieux AC, Maziere B, Vallois JM, Ottaviani M, Bouvet A, Pocidalo JJ, Carbon C. 1991. Ceftriaxone diffusion into cardiac fibrin vegetation. Qualitative and quantitative evaluation by autoradiography. Fundam Clin Pharmacol 5:53–60. doi: 10.1111/j.1472-8206.1991.tb00701.x. [DOI] [PubMed] [Google Scholar]
- 79.Levison ME, Levison JH. 2009. Pharmacokinetics and pharmacodynamics of antibacterial agents. Infect Dis Clin North Am 23:791–815. doi: 10.1016/j.idc.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bugrysheva JV, Bryksin AV, Godfrey HP, Cabello FC. 2005. Borrelia burgdorferi rel is responsible for generation of guanosine-3'-diphosphate-5'-triphosphate and growth control. Infect Immun 73:4972–4981. doi: 10.1128/IAI.73.8.4972-4981.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barbour AG. 1984. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med 57:521–525. [PMC free article] [PubMed] [Google Scholar]
- 82.Wu X, Sharma B, Niles S, O'Connor K, Schilling R, Matluck N, D'Onofrio A, Hu LT, Lewis K. 2018. Identifying vancomycin as an effective antibiotic for killing Borrelia burgdorferi. Antimicrob Agents Chemother 62:e01201-18. doi: 10.1128/AAC.01201-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pavia CS, Wormser GP. 2014. Culture of the entire mouse to determine whether cultivable Borrelia burgdorferi persists in infected mice treated with a five-day course of ceftriaxone. Antimicrob Agents Chemother 58:6701–6703. doi: 10.1128/AAC.03751-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Crossland NA, Alvarez X, Embers ME. 2018. Late disseminated Lyme disease: associated pathology and spirochete persistence posttreatment in rhesus macaques. Am J Pathol 188:672–682. doi: 10.1016/j.ajpath.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Salo J, Jaatinen A, Soderstrom M, Viljanen MK, Hytonen J. 2015. Decorin binding proteins of Borrelia burgdorferi promote arthritis development and joint specific post-treatment DNA persistence in mice. PLoS One 10:e0121512. doi: 10.1371/journal.pone.0121512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hodzic E. 2015. Lyme borreliosis: is there a preexisting (natural) variation in antimicrobial susceptibility among Borrelia burgdorferi strains? Bosn J Basic Med Sci 15:1–13. doi: 10.17305/bjbms.2015.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ma Y, Seiler KP, Eichwald EJ, Weis JH, Teuscher C, Weis JJ. 1998. Distinct characteristics of resistance to Borrelia burgdorferi-induced arthritis in C57BL/6N mice. Infect Immun 66:161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Barthold SW. 1991. Infectivity of Borrelia burgdorferi relative to route of inoculation and genotype in laboratory mice. J Infect Dis 163:419–420. doi: 10.1093/infdis/163.2.419. [DOI] [PubMed] [Google Scholar]
- 89.Montgomery RR, Booth CJ, Wang X, Blaho VA, Malawista SE, Brown CR. 2007. Recruitment of macrophages and polymorphonuclear leukocytes in Lyme carditis. Infect Immun 75:613–620. doi: 10.1128/IAI.00685-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bramwell KK, Teuscher C, Weis JJ. 2014. Forward genetic approaches for elucidation of novel regulators of Lyme arthritis severity. Front Cell Infect Microbiol 4:76. doi: 10.3389/fcimb.2014.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Armstrong AL, Barthold SW, Persing DH, Beck DS. 1992. Carditis in Lyme disease susceptible and resistant strains of laboratory mice infected with Borrelia burgdorferi. Am J Trop Med Hyg 47:249–258. doi: 10.4269/ajtmh.1992.47.249. [DOI] [PubMed] [Google Scholar]
- 92.Yrjanainen H, Hytonen J, Soderstrom KO, Oksi J, Hartiala K, Viljanen MK. 2006. Persistent joint swelling and Borrelia-specific antibodies in Borrelia garinii-infected mice after eradication of vegetative spirochetes with antibiotic treatment. Microbes Infect 8:2044–2051. doi: 10.1016/j.micinf.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 93.Wormser GP, O'Connell S, Pachner AR, Schwartz I, Shapiro ED, Stanek G, Strle F. 2018. Critical analysis of a doxycycline treatment trial of rhesus macaques infected with Borrelia burgdorferi. Diagn Microbiol Infect Dis 92:183–188. doi: 10.1016/j.diagmicrobio.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Iyer R, Mukherjee P, Wang K, Simons J, Wormser GP, Schwartz I. 2013. Detection of Borrelia burgdorferi nucleic acids after antibiotic treatment does not confirm viability. J Clin Microbiol 51:857–862. doi: 10.1128/JCM.02785-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, Krause PJ, Bakken JS, Strle F, Stanek G, Bockenstedt L, Fish D, Dumler JS, Nadelman RB. 2006. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 43:1089–1134. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]
- 96.Varde S, Wormser GP, Nowakowski J, Nadelman RB, Bittker S, Cooper D, Schwartz I. 1999. Lyme disease: disparity between culture and polymerase chain reaction detection of Borrelia burgdorferi after exposure to ceftriaxone in vitro. Conn Med 63:589–591. [PubMed] [Google Scholar]
- 97.Choe H, Inaba Y, Kobayashi N, Ike H, Iwamoto N, Ishida T, Yukizawa H, Fujimaki T, Tezuka H, Hirata Y, Saito T. 2010. How long can dead bacterial DNA be detected by real-time PCR?, p 2144 Proceedings by University of Pennsylvania Orthopaedic Journal. 56th Annual Meeting of the Orthopaedic Research Society, New Orleans, LA. [Google Scholar]
- 98.Burger J, Hummel S, Herrmann B, Henke W. 1999. DNA preservation: a microsatellite-DNA study on ancient skeletal remains. Electrophoresis 20:1722–1728. doi:. [DOI] [PubMed] [Google Scholar]
- 99.Lindahl T. 1993. Instability and decay of the primary structure of DNA. Nature 362:709–715. doi: 10.1038/362709a0. [DOI] [PubMed] [Google Scholar]
- 100.Lo YM, Zhang J, Leung TN, Lau TK, Chang AM, Hjelm NM. 1999. Rapid clearance of fetal DNA from maternal plasma. Am J Hum Genet 64:218–224. doi: 10.1086/302205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lazarus JJ, McCarter AL, Neifer-Sadhwani K, Wooten RM. 2012. ELISA-based measurement of antibody responses and PCR-based detection profiles can distinguish between active infection and early clearance of Borrelia burgdorferi. Clin Dev Immunol 2012:138069. doi: 10.1155/2012/138069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Laalami S, Zig L, Putzer H. 2014. Initiation of mRNA decay in bacteria. Cell Mol Life Sci 71:1799–1828. doi: 10.1007/s00018-013-1472-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hu Y, Mangan JA, Dhillon J, Sole KM, Mitchison DA, Butcher PD, Coates AR. 2000. Detection of mRNA transcripts and active transcription in persistent Mycobacterium tuberculosis induced by exposure to rifampin or pyrazinamide. J Bacteriol 182:6358–6365. doi: 10.1128/jb.182.22.6358-6365.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cangelosi GA, Meschke JS. 2014. Dead or alive: molecular assessment of microbial viability. Appl Environ Microbiol 80:5884–5891. doi: 10.1128/AEM.01763-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Feng J, Li T, Zhang Y. 2018. Biofilm structures of Borrelia burgdorferi not only display more tolerance to Lyme antibiotics but also cause more severe pathology in a mouse arthritis model: implications for understanding persistence, PTLDS, and treatment failure. bioRxiv https://www.biorxiv.org/content/10.1101/440461v1.
- 106.Zambrano MC, Beklemisheva AA, Bryksin AV, Newman SA, Cabello FC. 2004. Borrelia burgdorferi binds to, invades, and colonizes native type I collagen lattices. Infect Immun 72:3138–3146. doi: 10.1128/IAI.72.6.3138-3146.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Imai DM, Feng S, Hodzic E, Barthold SW. 2013. Dynamics of connective-tissue localization during chronic Borrelia burgdorferi infection. Lab Invest 93:900–910. doi: 10.1038/labinvest.2013.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Barthold SW, deSouza M, Feng S. 1996. Serum-mediated resolution of Lyme arthritis in mice. Lab Invest 74:57–67. [PubMed] [Google Scholar]
- 109.Barthold SW, Hodzic E, Tunev S, Feng S. 2006. Antibody-mediated disease remission in the mouse model of Lyme borreliosis. Infect Immun 74:4817–4825. doi: 10.1128/IAI.00469-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Barthold SW, Feng S, Bockenstedt LK, Fikrig E, Feen K. 1997. Protective and arthritis-resolving activity in sera of mice infected with Borrelia burgdorferi. Clin Infect Dis 25:S9–S17. doi: 10.1086/516166. [DOI] [PubMed] [Google Scholar]
- 111.Shih CM, Liu LP, Spielman A. 1995. Differential spirochetal infectivities to vector ticks of mice chronically infected by the agent of Lyme disease. J Clin Microbiol 33:3164–3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Norris SJ, Howell JK, Odeh EA, Lin T, Gao L, Edmondson DG. 2011. High-throughput plasmid content analysis of Borrelia burgdorferi B31 by using Luminex multiplex technology. Appl Environ Microbiol 77:1483–1492. doi: 10.1128/AEM.01877-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Moody KD, Barthold SW, Terwilliger GA. 1990. Lyme borreliosis in laboratory animals: effect of host species and in vitro passage of Borrelia burgdorferi. Am J Trop Med Hyg 43:87–92. doi: 10.4269/ajtmh.1990.43.87. [DOI] [PubMed] [Google Scholar]
- 114.Casjens SR, Mongodin EF, Qiu WG, Luft BJ, Schutzer SE, Gilcrease EB, Huang WM, Vujadinovic M, Aron JK, Vargas LC, Freeman S, Radune D, Weidman JF, Dimitrov GI, Khouri HM, Sosa JE, Halpin RA, Dunn JJ, Fraser CM. 2012. Genome stability of Lyme disease spirochetes: comparative genomics of Borrelia burgdorferi plasmids. PLoS One 7:e33280. doi: 10.1371/journal.pone.0033280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Glockner G, Schulte-Spechtel U, Schilhabel M, Felder M, Suhnel J, Wilske B, Platzer M. 2006. Comparative genome analysis: selection pressure on the Borrelia vls cassettes is essential for infectivity. BMC Genomics 7:211. doi: 10.1186/1471-2164-7-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Purser JE, Lawrenz MB, Caimano MJ, Howell JK, Radolf JD, Norris SJ. 2003. A plasmid-encoded nicotinamidase (PncA) is essential for infectivity of Borrelia burgdorferi in a mammalian host. Mol Microbiol 48:753–764. doi: 10.1046/j.1365-2958.2003.03452.x. [DOI] [PubMed] [Google Scholar]
- 117.Grimm D, Eggers CH, Caimano MJ, Tilly K, Stewart PE, Elias AF, Radolf JD, Rosa PA. 2004. Experimental assessment of the roles of linear plasmids lp25 and lp28-1 of Borrelia burgdorferi throughout the infectious cycle. Infect Immun 72:5938–5946. doi: 10.1128/IAI.72.10.5938-5946.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lewis K. 2007. Persister cells, dormancy and infectious disease. Nat Rev Microbiol 5:48–56. doi: 10.1038/nrmicro1557. [DOI] [PubMed] [Google Scholar]
- 119.Balaban NQ, Merrin J, Chait R, Kowalik L, Leibler S. 2004. Bacterial persistence as a phenotypic switch. Science 305:1622–1625. doi: 10.1126/science.1099390. [DOI] [PubMed] [Google Scholar]
- 120.Gefen O, Balaban NQ. 2009. The importance of being persistent: heterogeneity of bacterial populations under antibiotic stress. FEMS Microbiol Rev 33:704–717. doi: 10.1111/j.1574-6976.2008.00156.x. [DOI] [PubMed] [Google Scholar]
- 121.Keren I, Kaldalu N, Spoering A, Wang Y, Lewis K. 2004. Persister cells and tolerance to antimicrobials. FEMS Microbiol Lett 230:13–18. doi: 10.1016/S0378-1097(03)00856-5. [DOI] [PubMed] [Google Scholar]
- 122.Feng J, Shi W, Zhang S, Zhang Y. 2015. Persister mechanisms in Borrelia burgdorferi: implications for improved intervention. Emerg Microbes Infect 4:e51. doi: 10.1038/emi.2015.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cabello FC, Godfrey HP, Bugrysheva JV, Newman SA. 2017. Sleeper cells: the stringent response and persistence in the Borreliella (Borrelia) burgdorferi enzootic cycle. Environ Microbiol 19:3846–3862. doi: 10.1111/1462-2920.13897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Boyle WK, Groshong AM, Drecktrah D, Boylan JA, Gherardini FC, Blevins JS, Samuels DS, Bourret TJ. 2019. DksA controls the response of the Lyme disease spirochete Borrelia burgdorferi to starvation. J Bacteriol 201:e00582-18. doi: 10.1128/JB.00582-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hamad MA, Austin CR, Stewart AL, Higgins M, Vazquez-Torres A, Voskuil MI. 2011. Adaptation and antibiotic tolerance of anaerobic Burkholderia pseudomallei. Antimicrob Agents Chemother 55:3313–3323. doi: 10.1128/AAC.00953-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Allison KR, Brynildsen MP, Collins JJ. 2011. Metabolite-enabled eradication of bacterial persisters by aminoglycosides. Nature 473:216–220. doi: 10.1038/nature10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mulcahy LR, Burns JL, Lory S, Lewis K. 2010. Emergence of Pseudomonas aeruginosa strains producing high levels of persister cells in patients with cystic fibrosis. J Bacteriol 192:6191–6199. doi: 10.1128/JB.01651-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lafleur MD, Qi Q, Lewis K. 2010. Patients with long-term oral carriage harbor high-persister mutants of Candida albicans. Antimicrob Agents Chemother 54:39–44. doi: 10.1128/AAC.00860-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Colijn C, Cohen T, Fraser C, Hanage W, Goldstein E, Givon-Lavi N, Dagan R, Lipsitch M. 2010. What is the mechanism for persistent coexistence of drug-susceptible and drug-resistant strains of Streptococcus pneumoniae?. J R Soc Interface 7:905–919. doi: 10.1098/rsif.2009.0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dhar N, McKinney JD. 2007. Microbial phenotypic heterogeneity and antibiotic tolerance. Curr Opin Microbiol 10:30–38. doi: 10.1016/j.mib.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 131.Adams KN, Takaki K, Connolly LE, Wiedenhoft H, Winglee K, Humbert O, Edelstein PH, Cosma CL, Ramakrishnan L. 2011. Drug tolerance in replicating mycobacteria mediated by a macrophage-induced efflux mechanism. Cell 145:39–53. doi: 10.1016/j.cell.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Boxall AB. 2004. The environmental side effects of medication. EMBO Rep 5:1110–1116. doi: 10.1038/sj.embor.7400307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.O'Reilly KM, Urban MA, Barriero T, Betts RF, Trawick DR. 2005. Persistent culture-positive Legionella infection in an immunocompromised host. Clin Infect Dis 40:e87-9. doi: 10.1086/429826. [DOI] [PubMed] [Google Scholar]
- 134.Schindel C, Siepmann U, Han S, Ullmann AJ, Mayer E, Fischer T, Maeurer M. 2000. Persistent Legionella infection in a patient after bone marrow transplantation. J Clin Microbiol 38:4294–4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Boumart Z, Roche SM, Lalande F, Virlogeux-Payant I, Hennequet-Antier C, Menanteau P, Gabriel I, Weill FX, Velge P, Chemaly M. 2012. Heterogeneity of persistence of Salmonella enterica serotype Senftenberg strains could explain the emergence of this serotype in poultry flocks. PLoS One 7:e35782. doi: 10.1371/journal.pone.0035782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dawson CC, Intapa C, Jabra-Rizk MA. 2011. Persisters: survival at the cellular level. PLoS Pathog 7:e1002121. doi: 10.1371/journal.ppat.1002121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fauvart M, De Groote VN, Michiels J. 2011. Role of persister cells in chronic infections: clinical relevance and perspectives on anti-persister therapies. J Med Microbiol 60:699–709. doi: 10.1099/jmm.0.030932-0. [DOI] [PubMed] [Google Scholar]
- 138.Levin BR, Rozen DE. 2006. Non-inherited antibiotic resistance. Nat Rev Microbiol 4:556–562. doi: 10.1038/nrmicro1445. [DOI] [PubMed] [Google Scholar]
- 139.Svecova D, Gavornik P. 2008. Recurrent erythema migrans as a persistent infection. Epidemiol Mikrobiol Imunol 57:97–100. [PubMed] [Google Scholar]
- 140.Steere AC, Bartenhagen NH, Craft JE, Hutchinson GJ, Newman JH, Rahn DW, Sigal LH, Spieler PN, Stenn KS, Malawista SE. 1983. The early clinical manifestations of Lyme disease. Ann Intern Med 99:76–82. doi: 10.7326/0003-4819-99-1-76. [DOI] [PubMed] [Google Scholar]
- 141.Rebman AW, Bechtold KT, Yang T, Mihm EA, Soloski MJ, Novak CB, Aucott JN. 2017. The clinical, symptom, and quality-of-life characterization of a well-defined group of patients with posttreatment Lyme disease syndrome. Front Med 4:224. doi: 10.3389/fmed.2017.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.American Veterinary Medical Association. 2013. AVMA guidelines for the euthanasia of animals, 2013 ed American Veterinary Medical Association, Schumberg, IL. [Google Scholar]
- 143.Barthold SW, deSouza MS, Janotka JL, Smith AL, Persing DH. 1993. Chronic Lyme borreliosis in the laboratory mouse. Am J Pathol 143:951–971. [PMC free article] [PubMed] [Google Scholar]
- 144.Moody KD, Adams RL, Barthold SW. 1994. Effectiveness of antimicrobial treatment against Borrelia burgdorferi infection in mice. Antimicrob Agents Chemother 38:1567–1572. doi: 10.1128/aac.38.7.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hodzic E, Feng S, Freet KJ, Barthold SW. 2003. Borrelia burgdorferi population dynamics and prototype gene expression during infection of immunocompetent and immunodeficient mice. Infect Immun 71:5042–5055. doi: 10.1128/iai.71.9.5042-5055.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hodzic E, Feng S, Barthold SW. 2013. Assessment of transcriptional activity of Borrelia burgdorferi and host cytokine genes during early and late infection in a mouse model. Vector Borne Zoonotic Dis 13:694–711. doi: 10.1089/vbz.2012.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hodzic E, Feng S, Freet KJ, Borjesson DL, Barthold SW. 2002. Borrelia burgdorferi population kinetics and selected gene expression at the host-vector interface. Infect Immun 70:3382–3388. doi: 10.1128/iai.70.7.3382-3388.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Osman F, Hodzic E, Kwon SJ, Wang J, Vidalakis G. 2015. Development and validation of a multiplex reverse transcription quantitative PCR (RT-qPCR) assay for the rapid detection of Citrus tristeza virus, Citrus psorosis virus, and Citrus leaf blotch virus. J Virol Methods 220:64–75. doi: 10.1016/j.jviromet.2015.04.013. [DOI] [PubMed] [Google Scholar]