Abstract
A 66-year-old woman presented with agrammatism and apraxia of speech, meeting criteria for non-fluent/agrammatic variant primary progressive aphasia (nfvPPA). However, three years later, she developed frontal/executive, short-term phonological memory, visuospatial, and visual memory deficits suggesting involvement of multiple brain networks. Multimodal neuroimaging showed damage of both fronto-striatal and posterior brain regions. She was found to have multiple pathological processes: corticobasal degeneration (CBD), Alzheimer’s disease (AD), and TAR DNA-binding protein (TDP)-43 type A. We hypothesize that cognitive and neuroimaging findings consistent with damage to multiple brain networks, each associated with vulnerability to certain molecular disease subtypes, could indicate mixed pathology.
Keywords: Non-fluent agrammatic primary progressive aphasia, syntax network, corticobasal degeneration, Alzheimer’s disease neuropathology, TDP-43 type A neuropathology
Introduction
Primary progressive aphasia (PPA) is a neurodegenerative disorder characterized by prominent and progressive speech and language deficits, initially without the involvement of other cognitive domains (Mesulam, 1982). There are three main variants that differ in their clinical presentation, pattern of brain atrophy, and most commonly associated neuropathology. Non-fluent agrammatic variant PPA (nfvPPA) is characterized by agrammatism and motor speech deficits (Gorno-Tempini et al., 2004; Grossman, 2012) and left inferior fronto-insular atrophy. Within the spectrum of nfvPPA, most individuals present with mixed apraxia of speech, dysarthria, and agrammatism. However, patients can also present mainly with motor speech symptoms, also called primary progressive apraxia of speech (PPAOS; Josephs et al., 2012). In either case, pathology is most often FTLD-tau (Josephs et al., 2006; Kertesz, McMonagle, Blair, Davidson, & Munoz, 2005; Spinelli et al., 2017). Semantic variant PPA (svPPA) is characterized by loss of object and word knowledge, bilateral anterior temporal atrophy (Hodges, Patterson, Oxbury, & Funnell, 1992), and most often TAR DNA-binding protein 43 (TDP-43) type C pathology (Davies et al., 2005; Mesulam et al., 2008; Snowden, Neary, & Mann, 2007). Logopenic variant PPA (lvPPA) is characterized by phonological deficits, left temporoparietal atrophy (Gorno-Tempini et al., 2004), and most often Alzheimer’s disease (AD) pathology or AD-positive biomarkers (Mesulam et al., 2008; Santos-Santos et al., 2018; Snowden et al., 2007; Spinelli et al., 2017). The association between clinico-anatomical syndromes and molecular pathology is related to the selective vulnerability of different brain networks to specific pathological processes (Seeley, Crawford, Zhou, Miller, & Greicius, 2009). Clinical-anatomical-pathological association is never absolute in any neurodegenerative disorder, and data from a recent clinico-pathological study in our cohort and a large multicenter analysis (Bergeron et al., 2018) showed that each syndrome is associated with each molecular change in about 85% of the cases (Spinelli etal., 2017). Furthermore, neurodegeneration has been shown to spread longitudinally along connected brain regions, thus determining predictable neuroanatomical and clinical longitudinal trajectories (Brambati et al., 2015; Lowe et al., 2018; Mandelli et al., 2016). While most PPA patients fit into one of these three main clinical syndromes, some patients fulfill the criteria for more than one variant or present with mixed or unexpected cognitive-anatomical deficits during the disease course. Since specific symptoms are related to damage to certain brain networks, mixed clinical syndromes relatively early in the disease course may be related to degeneration of multiple networks, which could occur in the presence of mixed pathology.
This report highlights a case which initially presented with a mainly agrammatic nfvPPA that developed unexpected clinical features about three years into the disease course. Multimodal MRI imaging showed involvement of multiple brain networks, molecular imaging showed amyloid positivity, and post-mortem pathological analyses revealed the presence of FTLD and AD pathology.
Clinical history
Ms. X was a right-handed former banker with a family history of Alzheimer’s disease who first noticed cognitive symptoms at age 63. She first noted difficulty with speech initiation after undergoing a minor varicose vein surgical procedure, during which she suffered an allergic reaction (˝throat swelling˝) to hydromorphone prescribed for post-operative pain. Her symptoms initially improved, but a few months later, she reported that she began to “mix up words” such as ˝he/she,˝ ˝yes/nő and ˝yesterday/tomorrow.˝ Shortly after, she began to notice difficulty reading and stopped writing in her journal due to trouble writing sentences. She noted difficulty with language comprehension, especially when she was trying to simultaneously pay attention to multiple conversations. Around this time, her husband also noted the language difficulties, and they sought out formal medical attention.
At age 64, she was evaluated by a neurologist and was found to have telegraphic speech, agrammatism, word-finding difficulty, and paraphasic errors. She underwent a stroke workup due to her report of sudden-onset symptoms, and her MRI revealed a small stroke versus a large perivascular space in the left basal ganglia. By age 65, she and her husband noted worsening language comprehension, especially of complex, multi-step sentences with complicated grammar. For example, her husband asked her to go to the grocery store to pick up vegetables, but she thought he had said that he would be responsible for this task. She had persistent difficulty understanding the books that she was reading and attempted to compensate by listening to the audiobook version at the same time.
Ms. X was evaluated at our center for the first time at age 66. She reported word-finding difficulties, word-finding pauses, impaired comprehension for sentences more than words, decreased speech output, and impaired reading, writing, and spelling. Notably, she did not report effortful, slurred, or distorted speech. She also denied difficulty with executive, visuospatial, motor, or sensory functioning, but she endorsed difficulty with ˝short-term˝ memory. In terms of behavior, she reported feeling anxious, agitated, and irritable about her language difficulties and was being treated with citalopram. Her husband also reported that she had become more irritable in general. For example, she uncharacteristically pulled him away to a different checkout line in a store after becoming impatient with the cashier in the original line. There were no hallucinations or delusions, changes in eating habits, fluctuations in weight, or difficulty with sleep. She remained independent in her activities of daily living and instrumental activities of daily living.
Her symptoms continued to progress, and over the next year, Ms. X resorted to using nouns and then mostly hand motions for communication. For example, when describing a typical day, she said, “garden, swim class, Costco, Safeway, Trader Joe’s.” She stopped reading and watching movies because she had difficulty following the plots. She developed new behavioral symptoms including difficulty reading emotions. She became slightly disinhibited and overfamiliar in her interactions, hugging and kissing people that she did not know well. She was participating in fewer social activities, eating more sweets, and spending an excessive amount of time making “Wordles”, an online program that generates visual “word clouds” from the text that is provided by the user. She developed a tilted posture, which was causing back pain. She also reported deteriorating memory. She began to misplace objects such as her wedding ring and glasses, and she developed difficulty with calculations. She had previously been in charge of writing the checks for her church and could no longer do so, which was especially striking given her career as a banker. Due to worsening memory, a trial of Aricept was initiated. This was discontinued after a few months due to ineffectiveness and worsened agitation.
By age 68, she was experiencing increased behavioral difficulties including marked apathy, decreased range of emotional expression, and mild depression. She became more rigid about the color of her clothing and the types of food she would eat. She was adamant about carrying a full bottle of water and her eye-glasses case at all times. She was described as ˝losing her manners,˝ in that she began to eat with her fingers, would occasionally scream in stores or restaurants, and started to walk unclothed around her home. Her family noted that she was becoming stiffer in her movements, which contributed to poor balance and difficulty rising from a chair. The rigidity likely contributed to a fall, after which she was hospitalized with a fractured left patella. She developed difficulty swallowing. She could no longer dress herself properly or prepare meals.
Ms. X died at age 72, about 6 years after her first evaluation at our center and 9 years after her first symptoms.
Neurological examination
On examination at age 66, three years after symptom onset, she was noted to be alert and oriented with appropriate behavior. She had telegraphic, halting speech and spoke in 1–2 word phrases, using mostly nouns. Overall, she had decreased speech output. She had very mild apraxia of speech and phonemic paraphasias, but no dysarthria was noted. She was noted to have word-finding difficulty, word substitutions, trouble with repetition, and impaired comprehension of sentences. Writing was easier than speaking, but she was noted to be agrammatic with both. Cranial nerves were intact. She had normal muscle bulk. There was slightly increased left upper extremity tone with reinforcement and slowed foot taps on the left. Strength was full to confrontational testing. Sensory testing was normal. Reflexes were normal. Coordination was intact. She had a normal posture and gait with a normal arm swing. Toe, heel, and tandem walking were normal, and there was no retropulsion. In summary, she had prominent speech and language deficits, subtle left-sided upper motor neuron findings, and otherwise normal cranial nerve, motor, sensory, reflex, coordination, and gait examination.
On examination one year later, at age 67, she was noted to have minimal speech output. She responded to questions with one-word answers or hand motions. She had trouble following multiple-step commands. The remainder of her neurological exam was normal aside from increased right upper extremity tone with activation maneuvers. In summary, her examination was notable for severely reduced speech output and prominent language symptoms with mildly increased right upper extremity tone. She did not return for further in-person research visits after this examination at age 67. Therefore, the exact progression of her neurological deficits after this time was unclear.
Neuropsychological battery
Cognitive screening testing
She underwent neuropsychological testing at age 66 and one year later at age 67 (Table 1). This battery of memory, language, executive, and visuospatial tests has previously been described in detail (Kramer et al., 2003). For clarity, the language measures are reported in Table 2 in combination with supplemental language testing that is described in the subsequent section. At age 66, she scored 27/30 on the Mini-Mental Status Examination. Her most prominent deficits were in language and executive functioning. She had severe deficits in syntax, repetition (e.g., ˝pry the, the lin off˝ for pry the tin lid off), and verbal agility (e.g., ˝Methodist Espocoble˝ for Methodist Episcopal). The neuropsychologist noted that her poor score on verbal agility was related more to phonological paraphasias than speech distortions. Her scores on naming (Boston Naming Test), word comprehension (Peabody Picture Vocabulary Test), and reading were normal.
Table 1.
Summary of neuropsychological measures at initial presentation and 18 months later. All scores are reported as raw score, with bold, italicized text indicating that the patient performed at an impaired level relative to age-matched controls. n/a = not applicable (patient too impaired to participate in the task).
| Age 66 | Age 67 | |
|---|---|---|
| MMSE (30) | 27 | 12 |
| CDR Total (3) | 0.5 | 0.5 |
| Visuospatial Function: | ||
| Modified Rey-O Copy (17) | 13 | 13 |
| Polygon Copy (1) | 1 | 0 |
| VOSP Number Location (10) | 7 | n/a |
| Calculations (5) | 4 | 3 |
| Episodic Memory: | ||
| California Verbal Learning Test (9) | ||
| 4-trial total correct | 4, 6, 6, 7 | 3, 4, 6, 5 |
| 30-sec free recall | 7 | 5 |
| 10-min free recall | 6 | 5 |
| 10-min cued recall | 7 | 0 |
| 10-min recognition hits | 8 | 8 |
| 10-min recognition false positives | 0 | 7 |
| Modified Rey-O recall (17); recognition (y/n) | 5; no | 6; no |
| Attention/Working Memory: | ||
| Digits forward span (9) | 3 | 0 |
| Digits backward span (8) | 2 | 0 |
| Executive function: | ||
| Modified Trails Switching (secs) | 120 | 120 |
| Modified Trails Correct (14); # errors | 3; 2 | 2; 7 |
| CA Stroop Inhibition (# correct/60 secs) | 33 | n/a |
| Design Fluency Filled Dots (# designs) | 9 | 2 |
| D words/min | 5 | 2 |
| Abstract reasoning (6) | 1 | 0 |
Table 2.
Summary of additional language measures at initial presentation and 18 months later. All scores are reported as raw score. n/a = not applicable (patient too impaired to participate in the task).
| Age 66 | Age 67 | |
|---|---|---|
| Language | ||
| Boston Naming Test (15) | 13 | 6 |
| Pyramid/Palm Trees: Pictures (52) | 49 | 45 |
| PPVT-R word comprehension (16) | 14 | 10 |
| Repetition (5) | 1 | 0 |
| Animals/min | 12 | 5 |
| Syntax comprehension (5) | 0 | 2 |
| Verbal agility (6) | 3 | 0 |
| Western Aphasia Battery | ||
| Information Content (10) | 9 | 4 |
| Fluency (10) | 4 | 2 |
| Picture Description: oral (10) | 6 | n/a |
| Auditory Word Recognition (60) | 59 | 35 |
| Sequential Command (60) | 57 | 29 |
| Repetition (100) | 72 | 28 |
| Motor Speech Evaluation | ||
| Dysarthria rating (7) | 0 | 1 |
| Apraxia of speech rating (7) | 2 | 5 |
| Other | ||
| Goodglass Expressive Syntax (22) | 2 | n/a |
| CYCLE Syntactic Comprehension (55) | n/a | 20 |
On tests of executive functioning, her digit span was 3 forward and 2 backward. She was unable to complete a test of set-shifting (CA Trails Switching) in the allotted 120 s. Design fluency was normal. However, her verbal fluency was impaired with 5 D words and 12 animals. Her performance on tests of abstract reasoning was also impaired.
In addition to the severe deficits in language and executive functioning, she also had deficits in visual memory and visuos-patial functioning, despite the lack of history of functional deficits in these domains. She had impaired scores on the Benson figure recall, a test of visual memory. Her scores in the visuospatial domain were mixed, showing impairment on the Visual Object and Space Perception Battery (VOSP) but normal polygon copy, Benson Figure Copy, and calculation testing. Her scores on verbal memory testing (California Verbal Learning Test-MS) were within the normal range.
On repeat neuropsychological testing at age 67, performed 18 months after her first testing, her Mini-mental State Examination (MMSE) dropped to 12/30. She was noted to be mildly stimulus-bound and moderately perseverative. She developed mild deficits in verbal memory in addition to the visual memory deficits. Her performance in language, executive, and visuospatial domains worsened.
Language testing
She underwent extensive language testing at both time points with a trained speech-language pathologist (Table 2). At age 66, she was noted to have effortful speech with changes in prosody and grammatical errors. She used simple sentence structures and tended to use nouns. She scored poorly on the Goodglass Expressive Syntax task, a test of syntax production (Goodglass, Gleason, Bernholtz, & Hyde, 1972). Her score on a syntax comprehension task was also impaired, although her score on the WAB sequential commands was normal. This score is generally used as a measure of sentence comprehension, but it is less sensitive at detecting changes in syntax, which may explain the discordance in these two scores. She had mild apraxia of speech and phonological paraphasias, which also likely contributed to difficulty with repetition. However, no dysarthria was noted.
These deficits are illustrated in her description of the picnic scene from the Western Aphasia Battery: “The picnic... basket, and the men, and the book. And the woman has...a wine [laughs], and a pismi-, piskit, picnic basket and a, and the, and the phone. Radio. And uh. and a, a blanket. And...a thongs off. The man.”
In contrast to the significant difficulties with grammar, apraxia of speech, repetition, and phonological paraphasias, she had preservation of single word comprehension, confrontation naming, and semantics.
At age 67, she had worsening in most language domains, including on tasks of fluency, syntax production and comprehension, word and sentence comprehension, confrontation naming, and repetition. She had worsening AOS and developed subtle dysarthria. Her performance on the Pyramid and Palm Trees Test, a test of nonverbal semantic processing, was spared.
Genetic testing
She underwent genetic testing for research purposes, which was negative for C9ORF72 expansion or MAPT, GRN, or FUS mutations. Her apolipoprotein E genotype was E3/E4.
Neuroimaging
Structural brain MRI without contrast at age 66 revealed atrophy in insular, inferior frontal, posterior temporal, medial frontal, dorsolateral and inferior parietal regions, left worse than right. There were minimal white matter hyperintensities on T2 FLAIR. Repeat brain MRI without contrast (14 months later) revealed the progression of left-greater-than-right atrophy (Figure 1).
Figure 1.
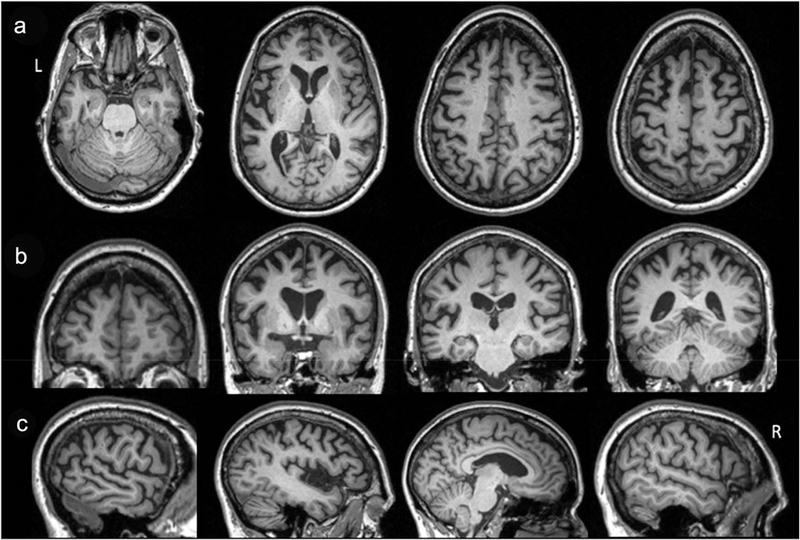
Axial (a), coronal (b), and sagittal (c) T1-imaging at age 67 demonstrate predominantly left-sided atrophy of the insular, inferior frontal, medial frontal, dorsolateral and parietal regions on a background of mild global atrophy
Pathological predictions
Overall, considering Mrs. X’s history and main clinical and neuropsychological findings, the multidisciplinary team of clinicians established that she met criteria for imaging-supported nonfluent/agrammatic variant PPA (Gorno-Tempini et al., 2011). This diagnosis was based on main complaints of early and predominant language and speech symptoms, specifically agrammatism, apraxia of speech, and impaired comprehension of syntactically complex sentences with spared single-word comprehension and object knowledge, in the context of predominant left inferior frontal atrophy. She did not meet the criteria for lvPPA because she showed prominent agrammatism and signs of apraxia of speech. Nevertheless, the presence of language-based executive deficits on testing and general extrapyramidal motor syndrome was not surprising after a few years of clinical course and was consistent with a fronto-insular-striatal anatomical syndrome and FTLD-tau pathology (Santos-Santos, 2018). Therefore, the team’s main pathology prediction (before PET-PIB findings were reviewed) was still FTLD-4R tau, most likely CBD. PSP-S symptoms, such as prominent dysarthria or early falls, were absent early in the disease course. However, atypical/mixed features were noted in her clinical and neuroimaging presentation: (A) Agrammatism and executive deficits seemed particularly severe in comparison with motor speech deficits; (B) Frequent phonological paraphasias and short-term phonological memory deficits were noted; and (C) visuospatial and visual memory deficits were present on formal testing. These unusual clinical features were supported by some posterior temporoparietal atrophy on structural MRI. Thus, while her clinical picture was thought to be mostly consistent with an underlying CBD pathology, the team considered AD pathology or co-pathology, although these were lower on the differential.
Research neuroimaging
A series of analyses were performed at age 67 (time point 2) for research purposes, and the results are summarized in this section.
Voxel-based analysis of T1 structural imaging
Tl-weighted images from Ms. X at age 67 and a group of healthy controls (n = 150) were processed using optimized standard methods. Briefly, preprocessing involved T1 image segmentation into grey matter, white matter, and cerebrospinal fluid and spatial normalization into MNI space using the statistical parametric mapping toolbox (SPM12) (www.fil.ion.ucl.ac.uk/spm/software/spm12) using Matlab 2017 (http:// www.mathworks.com). To optimize intersubject registration, a custom template was created from 150 healthy control participants using the Diffeomorphic Anatomical Registration through Exponentiated Lie algebra (DARTEL) toolbox. Spatially normalized, segmented, and modulated grey matter images were smoothed using an 8 mm FWHM isotropic Gaussian kernel. A W-score map of our patient was obtained by calculating the Z-score maps adjusted for age and total intracranial volume (TIV). Negative W-scores represent below-average volume. W-scores less than –2.0 (2 standard deviations below the mean) are shown in Figure 2.
Figure 2.
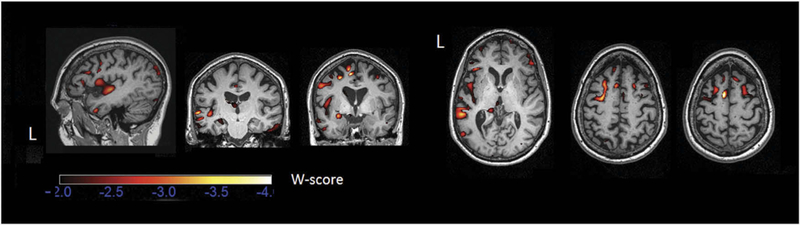
Atrophic brain regions in Ms. X’s T1-weighted structural MRI scan at age 67. Atrophy map was created by calculating the patient’s W-score map compared to a group of controls (n = 150) adjusted for age and total intracranial volume (TIV).
PET metabolic and molecular imaging
FDG-PET and PET-PiB imaging were obtained at age 67 as previously described (Lehmann et al., 2013; Villeneuve et al., 2015). These scans were obtained for research purposes only and were not used for clinical diagnosis. On FDG-PET, there was hypometabolism in the left operculum that extended into the perisylvian area and into the superior temporal gyrus (Figure 3). On PET-PiB, there was evidence of amyloid deposition in the precuneus area. Overall, this scan was read at the time of the research visit as equivocal for amyloid binding (Figure 3). Retrospectively, a “PiB index” was derived from the native-space distribution volume ratio (DVR) image by averaging the weighted mean value from Freesurfer-derived regions of interest in frontal, temporal, parietal and posterior cingulate cortex using the Desikan-Killiany atlas. The patient’s PiB index was 1.16, above the threshold of 1.065 (Villeneuve et al., 2015); therefore, this scan is now read as an early positive amyloid scan.
Figure 3.
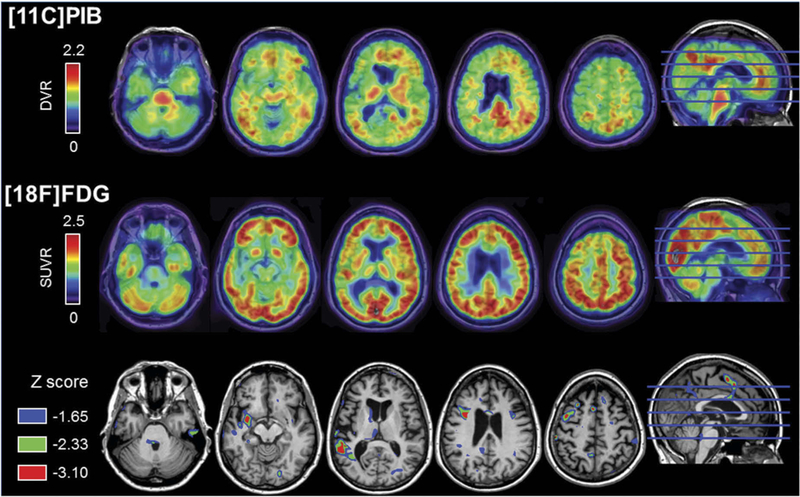
Top row depicts the patient’s PiB-PET distribution volume ratio (DVR) image, which was initially read as equivocal for amyloid binding at the time of Ms. X’s research visit. However, this is now visually read as an early positive scan. Cortical binding can be observed mainly in the precuneus and anterior cingulate cortex. Middle row shows the patient’s FDG-PET standardized uptake value ratio (SUVR). Bottom row illustrates the comparison of the patient’s FDG-SUVR to a control group of clinically normal age-matched women (n = 13, age at FDG: 66.6 ± 2.7). Colored areas correspond to voxels where the patients’ SUVR value was significantly lower than the controls (corresponding to uncorrected one-tailed p < 0.05, 0.01, and 0.001), confirming a left-asymmetric pattern hypometabolism in left operculum and superior temporal gyrus regions
Diffusion-weighted imaging
Diffusion-weighted images were acquired in order to dissect in-vivo the principal tracts usually affected in the variants of PPA. Tractography was performed as previously described (Mandelli et al., 2014) and showed white matter disruption of the left arcuate and left superior longitudinal fasciculus (Figure 4(a)). Similarly, there was also white matter disruption in the component of the aslant tract connecting BA44 to the supplementary motor area (SMA), but there was the preservation of the portion connecting ventral BA6 to the SMA relative to other nfvPPA patients and controls (n = 20) (Figure 4(b)).
Figure 4.
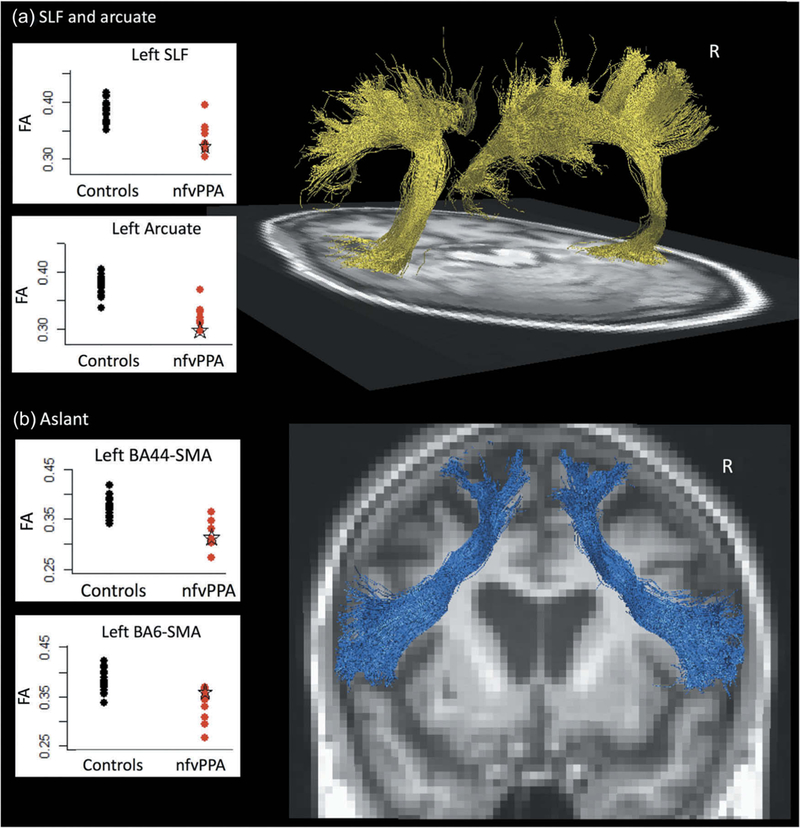
Tractography and average fractional anisotropy (FA) of patient’s (a) superior longitudinal fasciculus and arcuate tracts and (b) aslant tract at age 67. Black dots represent a cohort of 21 controls, and red dots represent a cohort of 9 nfvPPA patients, with the patient’s results indicated by a green star.
Neuropathology
The fresh brain was collected at 7 h after death and weighed 1005 g. Both hemispheres, entire brainstem, right cerebellum, and partially dissected spinal cord were analyzed. The pattern and regions of gross atrophy were well correlated with the clinical features (Figure 5). The most severe atrophy was found in classic language areas, including left-greater-than-right frontal operculum and adjacent anterior temporal and frontal regions. However, the areas of atrophy also included the hippocampus, entorhinal cortex, posterior cingulate, and superior/middle temporal gyrus.
Figure 5.
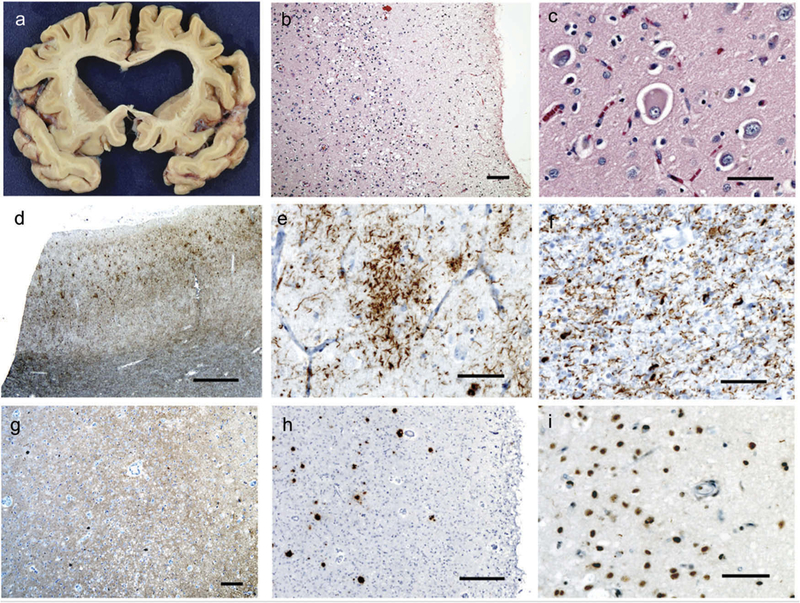
Neuropathology. On gross pathology (a), there was diffuse cortical atrophy, most prominent in the insula, inferior frontal gyrus, and superior temporal gyrus with associated ex-vacuo dilatation of the lateral ventricles. On hematoxylin and eosin staining, there were nonspecific signs of neurodegeneration, including superficial astrogliosis and microvacuolation (b). Balloon cells were frequently identified in the cortex (c). Immunohistochemical staining for phosphorylated tau protein demonstrated abundant deposition, including prominent white matter staining in addition to the cytoplasmic accumulations noted in the cortex (d), frequent astrocytic plaques (f), and numerous white matter threads and coiled bodies (f), consistent with corticobasal degeneration. A 3-repeat tau immunostain was notable for scattered neurofibrillary tangles in the cortex (g), and frequent beta-amyloid-positive plaques were also present (h), indicating the presence of Alzheimer’s disease neuropathology. Finally, TDP-43-positive cytoplasmic inclusions and threads consistent with a TDP-43-type A pattern of FTLD were also identified (i). Scale bars are as follows: B – 100 microns, C – 50 microns, D – 1 mm, E – 50 microns, F – 50 microns, G – 100 microns, H – 250 microns, I – 50 microns.
There were numerous findings on pathology (Figure 5). H&E staining was significant for superficial vacuolation and astrogliosis throughout cortical and subcortical regions, with the most prominent findings mirroring the previously mentioned areas.
Immunohistochemistry against phosphorylated tau protein (CP-13) showed unequivocal evidence of corticobasal degeneration, with frequent ballooned neurons, astrocytic plaques, and teeming white > grey matter threads and coiled bodies in most regions examined, especially in the insula, inferior frontal gyrus (IFG), middle frontal gyrus (MFG), inferior temporal gyrus, amygdala, superior and middle temporal gyrus, and precentral gyrus, areas that are implicated in the clinical symptoms of primary progressive aphasia.
Immunohistochemistry against 3-repeat tau (RD3) and against beta-amyloid (4G8) was also positive, indicating the presence of Alzheimer’s disease. The beta-amyloid-positive plaques were found throughout the neocortex, including the angular gyrus, and spread as far as the basal ganglia, indicative of Thal Amyloid Plaque Phase 3. 3-repeat tau-positive neurofibrillary tangles were identified in large numbers within the temporal lobe and in small numbers in the inferior frontal and angular gyrus, consistent with Braak stage 4.
Immunohistochemistry against TDP-43 also revealed interesting findings with frequent cytoplasmic inclusions, neuritic threads, and loss of nuclear staining identified most prominently in the IFG, MFG, and precentral gyrus as well as in limbic/para-limbic areas. The pattern of staining along with the superficial accentuation was consistent with a TDP-43 type A FTLD pathology. TDP-43 was also noted to lace the astrocytic tau-positive plaques and was present in oligodendrocytes, as can be seen in CBD. The distribution of TDP-43 pathology in this patient suggests that it may have contributed to the clinical deficits.
Finally, mild arteriolosclerosis was incidentally noted and thought to have contributed little, if at all, to the patient’s clinical deficits.
Discussion
Ms. X’s clinical syndrome met root diagnosis of PPA as language was her main and most disabling complaint for three years. She met nfvPPA criteria, showing frank agrammatism and mild apraxia of speech with impaired syntax processing and spared single-word comprehension and semantics. Consistently, the prediction was that her main pathological diagnosis was CBD. However, Ms. X’s clinical course and neuroimaging features were somewhat atypical and indicated multiple affected neural networks and thus likely mixed pathology. We argue that the presence of clinical and neuroimaging features indicating the involvement of brain networks associated with susceptibility to different pathologies might predict multiple molecular etiologies.
At the time of her visit to our center, three years into the disease course, formal testing showed difficulties in visual memory and visuospatial functioning. Functionally, neither the patient nor her family reported difficulties with navigation or other spatial tasks. Patients with nfvPPA may have visuospatial dysfunction on testing, which is thought to be related to executive dysfunction, but deficits in visual memory are unusual in cases that are not mixed pathology. (Ramanan et al., 2016; Watson et al., 2018). These visual memory deficits point towards early right hippocampal involvement (Shin, Park, Park, Seol, & Kwon, 2006; Soininen et al., 1994), which is more commonly seen in AD. Therefore, in the case of Ms. X, this pattern of testing that involved visual memory dysfunction in addition to the expected visuospatial/executive dysfunction hinted at potential mixed pathology, particularly AD pathology.
In addition, Ms. X’s language examination showed some features of lvPPA, such as impaired repetition and phonological errors. However, she did not meet the criteria for lvPPA because she showed frank agrammatism and AOS. Impaired repetition performance was not only caused by grammatical errors, as frank phonological errors were noted (e.g., “Pry the, the lin off” for Pry the tin lid off, and “Methodist Espocoble for Methodist Episcopal). Since phonological processing is a dorsal pathway task, phonological impairment can be seen in both nfvPPA and lvPPA (Wilson et al., 2010b; Henry et al., 2016). However, it is uncommon for nfvPPA patients to have phonological paraphasias without clear, evident, accompanying apraxia of speech (Croot, Ballard, Leyton, & Hodges, 2012), and therefore the presence of phonological paraphasias in Ms. X’s case was curious in the setting of only mild apraxia of speech at the first visit. Given the severity of her repetition deficits even at her year one visit, it seems plausible that the sound substitution errors were not only motorical in nature but at least in part related to phonological loop impairment, pointing to involvement of left temporoparietal regions (Gorno-Tempini et al., 2008), which are more commonly involved in AD pathology.
PET-PIB findings were not known at the time of clinical diagnosis but in retrospect could have also pointed towards mixed pathology. In fact, recent evidence from our center, as well as a multicenter study, showed that patients who meet criteria for nfvPPA or svPPA but also show positive AD bio-markers in-vivo often showed FTLD and AD mixed pathology post-mortem (Santos-Santos et al., 2018). The presence of AD and FTLD-4R tau co-pathology has been frequently reported across frontotemporal dementia syndromes and warrants further investigation (Ossenkoppele et al., 2015).
Ms. X showed early, severe agrammatism with only mild motor speech deficits. She also showed quite severe executive deficits. This pattern is uncommon in nfvPPA patients, who, at least in our cohort, most often present with predominant, or equally severe, motor speech impairment (Mandelli et al., 2016; Ogar, Dronkers, Brambati, Miller, & Gorno-Tempini, 2007). This could have been related to the pattern of anatomical damage within and across language- and speech-related cortical regions and white matter tract damage and pathology. Motor speech deficits in nfvPPA have been associated with fronto-insular/premotor cortical damage but most specifically are thought to be related to the severity of white matter damage in intra-frontal white matter tracts (aslant and cortico-striatal damage). On the other hand, task- related fMRI studies (Antonenko et al., 2013) in healthy controls and nfvPPA patients (Wilson et al., 2010a) have shown that grammar is sustained by a left inferior and middle frontal and posterior temporal network of cortical regions that are connected functionally and structurally through the arcuate fasciculus (AF) and superior longitudinal fasciculus (SLF) (Catani et al., 2013; Mandelli et al., 2016; Whitwell, Anderson, Scahill, Rossor, & Fox, 2004; Wilson et al., 2010a; Wilson et al., 2011; Wilson, Galantucci, Tartaglia, & Gorno-Tempini, 2012). While the epicenter of nfvPPA is most often the IFG, the disease eventually spreads through the speech production network to several cortical regions, including middle frontal gyrus and posterior regions, through these white matter tracts. Diffusion tensor imaging (DTI) in the case of Ms. X showed greater white matter damage in the left AF, SLF and BA44-SMA portion of the frontal aslant tract (Figure 4). Therefore, we can speculate that the clinical picture of severe agrammatism with relatively spared speech praxis in Ms. X was caused by prominent damage to these three areas with a relatively preserved volume of the ventral BA6-SMA portion of the aslant tract.
The presence of both FTLD-tau and AD pathology could have been suspected based on previous studies and clinical and neuroimaging findings. However, the additional presence of FTLD- TDP-A was surprising. In particular, FTLD-TDP-A pathology was reported as severe in the middle frontal gyrus and might have contributed to the significant grammatical and executive deficits. Ms. X’s network involved in grammatical processing was perhaps rendered even more vulnerable to breakdown due to the multiple pathological processes that together were targeting both the frontal and posterior cortical regions that are important for syntax, further impacting her syntactic processing.
Language domains such as syntax, speech production, and phonological processing involve various cortical regions and white matter tracts; deficits in each domain should, therefore, point towards disease in each individual network. Since these distinct neural networks show susceptibility to different underlying disease processes, we speculate that the presence of deficits in several cognitive domains and their associated neural networks may be related to mixed neuropathology.
Acknowledgments
Funding
This work was funded by the National Institutes of Health (MLGT, NINDS R01 NS050915), (MLGT, NIDCD K24 DC015544), (BM, NIA P50 AG023501), (BM, NIA P01 AG019724), (BM, Alzheimer’s Disease Center of California (03-75271 DHS/ADP/ARCC));Alzheimer’s Disease Research Center of California [03-75271];National Institute of Neurological Disorders and Stroke [R01 NS050915];National Institute on Deafness and Other Communication Disorders [K24 DC015544,R01 DC016291-01];National Institute on Aging [P01 AG019724,P50 AG023501].
Footnotes
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Antonenko D, Brauer J, Meinzer M, Fengler A, Kerti L, Friederici AD, & Floel A (2013). Functional and structural syntax networks in aging. Neuroimage, 83, 513–523. [DOI] [PubMed] [Google Scholar]
- Bergeron D, Gorno-Tempini ML, Rabinovici GD, Santos-Santos MA, Seeley W, Miller BL, ... Ossenkoppele R (2018). Prevalence of amy-loid-ß pathology in distinct variants of primary progressive aphasia. Annals of Neurology, 84, 729–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambati SM, Amici S, Racine CA, Neuhaus J, Miller Z, Ogar J, ... Gorno-Tempini ML (2015). Longitudinal gray matter contraction in three variants of primary progressive aphasia: A tenser-based morphometry study. Neuroimage: Clinical, 8, 345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Mesulam MM, Jakobsen E, Malik F, Martersteck A, Wieneke C, & Rogalski. (2013). A novel frontal pathway underlies verbal fluency in primary progressive aphasia. Brain, 136, 2619–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croot K, Ballard K, Leyton CE, & Hodges JR (2012). Apraxia of speech and phonological errors in the diagnosis of nonfluent/agrammatic and logopenic variants of primary progressive aphasia. Journal of Speech, Language, and Hearing Research, 55, S1562–S1572. [DOI] [PubMed] [Google Scholar]
- Davies RR, Hodges JR, Kril JJ, Patterson K, Halliday GM, & Xuereb JH (2005). The pathological basis of semantic dementia. Brain, 128, 1984–1995. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Gleason JB, Bernholtz NA, & Hyde MR (1972). Some linguistic structures in the speech of a Broca’s aphasic. Cortex, 8,191–212. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Brambati SM, Ginex V, Ogar J, Dronkers NF, Marcone A, ... Miller BL. (2008). The logopenic/phonological variant of primary progressive aphasia. Neurology, 71, 1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, ... Miller BL (2004). Cognition and anatomy in three variants of primary progressive aphasia. Annals of Neurology, 55, 335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, ... Grossman M (2011). Classification of primary progressive aphasia and its variants. Neurology, 76, 1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M (2012). The non-fluent/agrammatic variant of primary progressive aphasia. Lancet Neurology, 11, 545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry ML, Wilson SM, Babiak MC, Mandelli ML, Beeson PM, Miller ZA, & Gorno-Tempini ML (2016). Phonological processing in primary progressive aphasia. Journal of Cognitive Neuroscience, 28, 210–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges JR, Patterson K, Oxbury S, & Funnell E (1992). Semantic dementia. Progressive fluent aphasia with temporal lobe atrophy. Brain, 115, 1783–1806. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Master AV, ... Whitwell JL (2012). Characterizing a neurodegenerative syndrome: Primary progressive apraxia of speech. Brain, 135, 1522–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Whitwell JL, Layton KF, Parisi JE,... Petersen RC (2006). Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain, 129,1385–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A, McMonagle P, Blair M, Davidson W, & Munoz DG (2005). The evolution and pathology of frontotemporal dementia. Brain, 128, 1996–2005. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Jurik J, Sha SJ, Rankin KP, Rosen HJ, Johnson JK, & Miller BL (2003). Distinctive neuropsychological patterns in fronto-temporal dementia, semantic dementia, and Alzheimer disease. Cognitive and Behavioral Neurology, 16, 211–218. [DOI] [PubMed] [Google Scholar]
- Lehmann M, Ghosh PM, Madison C, Laforce R, Corbetta-Rastelli C, Weiner MW, & Rabinovici GD (2013). Diverging patterns of amyloid deposition and hypometabolism in clinical variants of probable Alzheimer’s disease. Brain, 136, 844–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe VJ, Wiste HJ, Senjem ML, Weigand SD, Therneau TM, Boeve BF,... Jack CR (2018). Widespread brain tau and its association with ageing, Braak stage and Alzheimer’s dementia. Brain, 141, 271–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelli ML, Caverzasi E, Binney RJ, Henry ML, Lobach I, Block N, ... Gorno-Tempini ML (2014). Frontal white matter tracts sustaining speech production in primary progressive aphasia. Journal of Neuroscience, 34, 9754–9767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelli ML, Vilaplana E, Brown JA, Hubbard HI, Binney RJ, Attygalle S,... Gorno-Tempini ML (2016). Healthy brain connectivity predicts atrophy progression in non-fluent variant of primary progressive aphasia. Brain, 139, 2778–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM (1982). Slowly progressive aphasia without generalized dementia. Annals of Neurology, 11, 592–598. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Wicklund A, Johnson N, Rogalski E, Leger GC, Rademaker A, ... Bigio EH (2008). Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Annals of Neurology, 63, 709–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogar JM, Dronkers NF, Brambati SM, Miller BL, & Gorno-Tempini ML (2007). Progressive nonfluent aphasia and its characteristic motor speech deficits. Alzheimer Disease & Associated Disorders, 21, S23–30. [DOI] [PubMed] [Google Scholar]
- Ossenkoppele R, Jansen WJ, Rabinovici GD, Knol DL, van der Flier WM, van Berckel BN, & Brooks DJ (2015). Prevalence of amyloid PET positivity in dementia syndromes: A meta-analysis. JAMA, 313, 1939–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanan S, Flanagan E, Leyton CE, Villemagne VL, Rowe CC, Hodges JR, & Hornberger M (2016). Non-verbal episodic memory deficits in primary progressive aphasias are highly predictive of underlying amyloid pathology. Journal of Alzheimers Disease, 51, 367–376. [DOI] [PubMed] [Google Scholar]
- Santos-Santos MA, Rabinovici GD, Iaccarino L, Ayakta N, Tammewar G, Lobach I, ... Gorno-Tempini ML (2018). Rates of amyloid imaging positivity in patients with primary progressive aphasia. JAMA Neurology. doi: 10.1001/jamaneurol.2017.4309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Crawford RK, Zhou J, Miller BL, & Greicius MD (2009). Neurodegenerative diseases target large-scale human brain networks. Neuron, 62, 42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin MS, Park SY, Park SR, Seol SH, & Kwon JS (2006). Clinical and empirical applications of the rey-osterrieth complex figure test. Nature Protocols, 1, 892–899. [DOI] [PubMed] [Google Scholar]
- Snowden J, Neary D, & Mann D (2007). Frontotemporal lobar degeneration: Clinical and pathological relationships. Acta Neuropathologica, 114, 31–38. [DOI] [PubMed] [Google Scholar]
- Soininen HS, Partanen K, Pitkanen A, Vainio P, Hanninen T, Hallikainen M, ... Riekkinen PJ (1994). Volumetric MRI analysis of the amygdala and the hippocampus in subjects with age-associated memory impairment: Correlation to visual and verbal memory. Neurology, 44, 1660–1668. [DOI] [PubMed] [Google Scholar]
- Spinelli E, Mandelli ML, Miller ZA, Santos-Santos MA, Wilson SM, Agosta F,... Gorno-Tempini ML (2017). Typical and atypical pathology in primary progressive aphasia variants. Annals of Neurology, 81, 430–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve S, Rabinovici GD, Cohn-Sheehy BI, Madison C, Ayakta N, Ghosh PM, ... Jagust W (2015). Existing Pittsburgh Compound-B positron emission tomography thresholds are too high: Statistical and pathological evaluation. Brain, 138, 2020–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CL, Possin K, Allen IE, Hubbard HI, Meyer M, Welch AE, & Gorno-Tempini ML (2018). Visuospatial functioning in the primary progressive aphasias. Journal of the International Neuropsychological Society, 24, 259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell J, Anderson VM, Scahill RI, Rossor MN, & Fox NC (2004). Longitudinal patterns of regional change on volumetric MRI in frontotemporal lobar degeneration. Dementia and Geriatric Cognitive Disorders, 17, 307–310. [DOI] [PubMed] [Google Scholar]
- Wilson SM, Dronkers NF, Ogar JM, Jang J, Growdon ME, Agosta F, & Gorno-Tempini,. (2010a). Neural correlates of syntactic processing in the nonfluent variant of primary progressive aphasia. Journal of Neuroscience, 30, 16845–16854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Galantucci S, Tartaglia MC, & Gorno-Tempini ML (2012). The neural basis of syntactic deficits in primary progressive aphasia. Brain and Language, 122, 190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Galantucci S, Tartaglia MC, Rising K, Patterson DK, Henry ML, & Gorno-Tempini ML (2011). Syntactic processing depends on dorsal language tracts. Neuron, 72, 397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Henry ML, Besbris M, Ogar JM, Dronkers NF, Jarrold W, & Gorno-Tempini ML (2010b). Connected speech production in three variants of primary progressive aphasia. Brain, 133, 2069–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]


