Abstract
Background:
Lead (Pb) exposure is associated with adverse neurological development. Most notably, it has been observed through externalizing behavior symptoms, as observed among Inuit children from northern Québec. Evidence for a persistent neurological impact of early Pb exposure later in life is however scarce. Pb exposure may initiate a developmental cascade that increases the risk of long-term behavior problems.
Objectives:
Testing for direct associations between childhood Pb concentrations and adolescent externalizing symptoms and substance use, as well as indirect associations through childhood behavior assessments.
Methods:
The study sample is a longitudinal cohort of Inuit children (n=212) followed since birth. Blood Pb concentrations were measured during childhood (median age = 11.4 years) and adolescence (median age=18.5 years). Externalizing/inattentive behavior were teacher-assessed through the Teacher Report Form and the Disruptive Behavior Disorders Rating Scale for children. At the adolescence follow-up, behavior problems were self-reported by filling Achenbach’s Youth Self-Report, the Barkley Adult ADHD-IV Rating Scale, and the Diagnostics Interview Schedule for Children. Adolescent substance use was also self-assessed through the DEP-ADO. Direct and indirect associations of child Pb concentrations with adolescent outcomes were tested through mediation models.
Results:
Child blood Pb concentrations were not directly associated with any adolescent outcomes. On the contrary, childhood Pb exposure was indirectly associated, through childhood externalizing behavior assessments, with adolescent externalizing behaviors, binge drinking, and cannabis use. These indirect associations held after controlling for adolescents’ concurrent Pb blood concentrations.
Discussion:
Our results highlight the indirect but lasting effects of child Pb exposure on adolescent behavior problems, and the importance of childhood externalizing behavior in this relationship. Adverse early-life environment put children on a riskier developmental trajectory, increasing their likelihood of lifelong psychological, social and health problems.
Keywords: lead, children and adolescents, externalizing behaviors, binge drinking, cannabis use
Introduction
Lead (Pb) exposure in children has been associated with negative neurological outcomes for more than a century (Turner, 1897). In Western countries, policies implemented since the 1970s were largely successful in reducing Pb exposure. However, certain groups and subpopulations remain highly exposed to Pb through diverse human-linked sources such as aged water pipes, dust and paint chips in older neighborhoods, or Pb-based ammunitions.
Pb exposure in children is of particular concern considering its known influence on developing neurological structures, which may lead to long-lasting cognitive and behavioral impairments (Téllez-Rojo et al., 2006). Several studies conducted in a variety of populations, such as major inner cities citizen (Chiodo et al., 2007, 2004; Needleman et al., 2002) and representative samples in the US (Braun et al., 2006; Froehlich et al., 2009), South Africa (Naicker et al., 2012), and Inuit children from northern Quebec (Boucher et al., 2012c), have reported cross-sectional associations between Pb exposure and numerous externalizing behavior or Attention Deficit Hyperactivity Disorder (ADHD) symptoms and diagnoses in childhood and adolescence. However, only a handful of longitudinal studies have documented these associations later in adolescence or early adulthood (Beckley et al., 2018; Burns et al., 1999; Dietrich et al., 2001; Winter and Sampson, 2017; Wright et al., 2008). Consequences of early Pb exposure throughout the lifespan still remain largely unknown, in part because of complex interactions with other determinants of neurodevelopment.
The long-term effects of early Pb exposure could be explained as a perturbation of early neurodevelopment that changes the context of later development (Bellinger et al., 2016). Pb exposure during childhood may not directly cause later behavioral and cognitive problems; it could instead contribute to putting exposed children on an adverse developmental path. For this reason, Bellinger et al. (2016) predict that although Pb exposure is directly associated with childhood behavioral and cognitive outcomes, its association with adulthood outcomes could be indirect. Thus, instead of a direct association between Pb exposure during childhood and later problem behaviors, we could expect a process mediated by the continuation of behavior problems during childhood into adolescence, corresponding to the concept of developmental cascade. In this framework, early Pb exposure would play a role in fostering and maintaining behavior problems from childhood to early adulthood, highlighting the importance of early-life environment.
In order to incorporate a developmental perspective into the study of effects of early neurotoxicant exposure, one should take into account the evolution of manifestations of neurodevelopmental perturbations throughout development. In adolescence and adulthood, externalizing behaviors manifest themselves as a spectrum of mental disorders and behaviors, such as substance use and abuse (Krueger et al., 2005), that children with behavior problems are more likely to develop later in life. A meta-analysis of longitudinal studies reported that adolescents and young adults who were diagnosed with ADHD as children were at an increased risk for alcohol use disorder and cannabis use (Lee et al., 2011). Childhood oppositional defiant/conduct disorder has also been prospectively associated with later substance use (Groenman et al., 2017). The only exploratory study, with a limited sample size, investigating the association between substance use and long-term Pb exposure did not find any significant association (Fishbein et al., 2008). The mechanisms linking childhood externalizing behaviors with adolescent or early adulthood substance use remain unclear. One potential mechanism explaining the increased risk of later substance use may be a vulnerability to disinhibitory behavior and impulse control(Mcgue et al., 2001). Accordingly, in this same cohort, our team has previously published significant results showing an association between child blood Pb concentrations and impairments in the child’s ability to correctly inhibit a response, resulting in increased impulsivity(Boucher et al., 2012a).
In a previous study by our group (Boucher et al., 2012b), we reported a cross-sectional association between Pb exposure and externalizing symptoms in a sample of Inuit children with a median age of 11.4 years. The goal of the present study is to expand these results, which includes a follow-up of the same participants into adolescence. The Inuit communities in northern Québec practice traditional activities central to their health and well-being, but some of the practices expose them to multiple environmental contaminants. The ingestion of Pb ammunition fragments in game meat is their primary source of Pb exposure (Lévesque et al., 2003). Alongside symptoms of externalizing and attention problems in adolescence, this study’s outcomes include substance use, a manifestation of externalizing problems in adolescence and adulthood that represent major public health concern in many indigenous communities across Canada (Firestone et al., 2015). Therefore, this paper will investigate two processes potentially leading to the association between child Pb exposure and adolescent externalizing behaviors/substance use: 1) direct association between child Pb concentrations and adolescent outcomes and 2) indirect association in which childhood behavior problems mediate the link between child Pb concentrations and adolescent outcomes, as expected in a developmental cascade framework.
Methods
Participants
The participants were Inuit children from the 14 coastal villages of Nunavik, in northern Québec. Between November 1993 and December 1996, 491 mothers were recruited as part of the Cord Blood Monitoring Program designed to document prenatal exposure to environmental contaminants and a range of nutrients in Arctic Québec (Dewailly et al., 1993). An additional 221 mothers were recruited between November 1995 and March 2002 as part of the National Institutes of Health (NIH) prospective infancy study (Jacobson et al., 2008; Muckle et al., 2001). At school age, a subsample of these children and their primary caregiver (n=294: 247 from the Cord Blood Monitoring Program and 47 from the NIH-infancy study) participated in the Nunavik Child Development Study (NCDS-childhood), a follow-up designed to examine effects of pre- and postnatal exposure to environmental contaminants on child behavior and cognitive abilities, which took place between September 2005 and February 2010. Recruitment methodologies for both the Cord Blood Monitoring Program and NCDS-childhood samples were reported in previous studies based on this cohort (Dallaire et al. 2014; Dewailly et al. 1993; Jacobson et al. 2008). The participants were met again during adolescence (NCDS-adolescence) between January 2013 and February 2016. Inclusion criteria for the NCDS-adolescence follow-up were participation in the previous two follow-ups, living in Nunavik, and ability to meet with the research team in one of Nunavik’s three main villages. Those who were identified as suffering from severe health or neurological problems unrelated to exposure at the NCDS-childhood interview (epilepsy n=2; head trauma n=1; meningitis n=1; multiple sclerosis n=1) were excluded from the NCDS-adolescence follow-up. An additional 49 children of the NCDS-childhood study were not eligible for the follow-up because they were either deceased, incarcerated, had moved away or were unreachable. An additional 28 adolescents declined to participate in the follow-up. Thus, a remaining 212 adolescents participated in the NCDS-adolescence study (Fig. 1). As compensation, the adolescent participants received an electronic device worth $50 USD.
Figure 1.
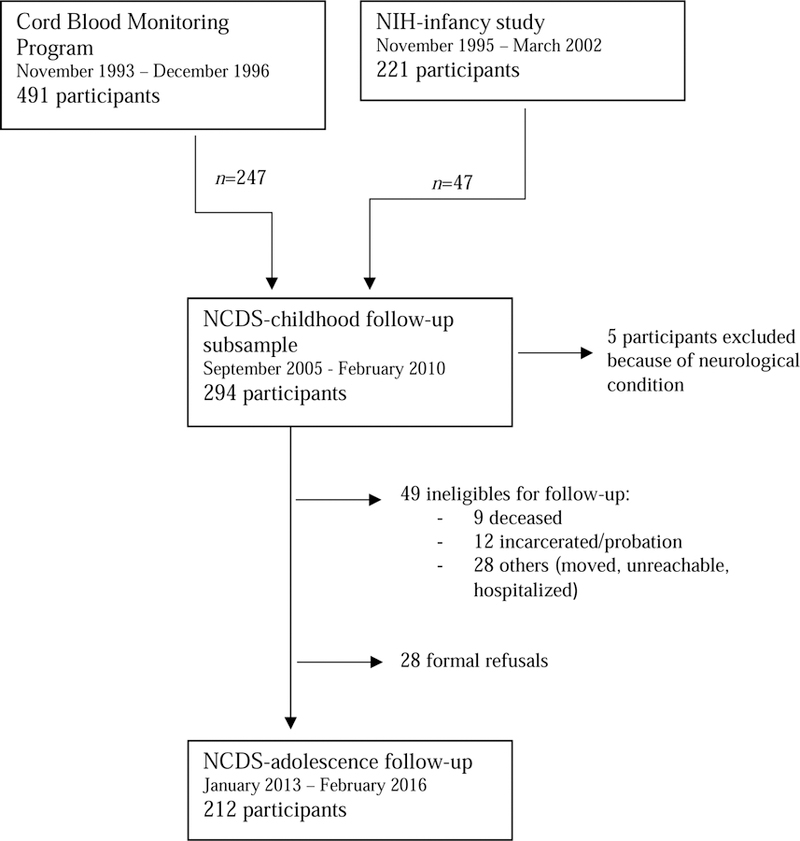
Flow chart for recruitment and follow-up of study participants from November 1993 to February 2016 including reasons and number of excluded participants through follow-ups.</p/ss>Note: NIH, National Institutes of Health; NCDS, Nunavik Child Development Study.
The interviews and cognitive/behavioral assessments for the child and adolescent follow-ups were conducted in the three largest villages of the region. Participants from smaller communities were transported by plane to meet with the research team. Written informed consent was provided by the biological mother at recruitment, the primary caregiver at the child follow-up, and the participants themselves at the adolescent follow-up. The children also gave verbal consent at the childhood follow-up. The Université Laval and Wayne State University ethics committees approved the Infancy and NCDS-childhood follow-ups, and the ethics committee of the Centre de recherche du CHU de Québec-Université Laval approved the consent and study procedures at the adolescence.
Biological samples
Contaminants and nutrients, including mercury (Hg), Pb and, docosahexaenoic acid (DHA, an omega-3 fatty acid) were measured in umbilical cord blood samples at birth (30 mL) and in venous blood samples of children (20 mL) and adolescents (30 mL). Analyses were performed at the Centre de Toxicologie, Institut National de Santé Publique du Québec (Québec, Canada) for all cord, child, and adolescent contaminant concentrations. Cord and child omega-3 fatty acid composition of plasma phospholipids were analyzed at the University of Guelph Lipid Analytical Laboratory (Guelph, Canada) and at the CHU de Québec for adolescent blood (see Supplemental Material for details of blood samples analytical procedures).
Child behavior assessment
Child externalizing.
The Child Behavior Checklist (CBCL) from the Achenbach System of Empirically Based Assessments was used to evaluate externalizing behavior during childhood (Achenbach and Rescorla, 2001). Each participant’s classroom teacher assessed symptoms through the Teacher Report Form obtained from the research team via the school principal. The Teacher Report Form includes 112 items rated from 0 to 2 (0 = not true, 1 = somewhat or sometimes true, 2 = very true or often true). It allows for computing various syndrome scores by summing the scores of specific items. The externalizing problems score was obtained by combining both the aggressive behavior and the rule-breaking behavior scores (sum of 32 of the 112 items). The Teacher Report Form had never been used with the Inuit population before the NCDS-childhood study (Boucher et al. 2012), but internal consistency of the externalizing subscale was high (αordinal = 0.97). No normative data was available for Inuit children, thus raw scores were used in all statistical analyses as in the childhood study.
Child hyperactivity-impulsivity.
The Disruptive Behavior Disorders Rating Scale (DBD), designed to be completed by parents and teachers, provides the information necessary for clinical diagnoses of disruptive behaviors in children (Pelham et al., 1992). The questionnaire is composed of 45 behavioral descriptors rated on a four-point frequency scale (‘never’, ‘sometimes’, ‘often’, ‘very often’) based on the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV). A symptom was considered present if reported as ‘often’ or ‘very often’ by the participant’s classroom teacher, and the symptom score (continuous) was computed as the count of hyperactivity-impulsivity symptoms. This assessment tool has never been validated in the Inuit population; however, internal consistency was high (αordinal = 0.92). Even though Boucher et al. (2012) used the diagnostic/dichotomous scores, we used the continuous scores to maximize statistical power in the context of a mediation model.
Child oppositional defiant and conduct disorder (OD/CD).
The DBD was also used to evaluate symptoms of oppositional defiant disorder and conduct disorder in children. The DBD has two separate scales for oppositional defiant disorder (8 items) and conduct disorder (15 items; Pelham et al. 1992). A symptom was considered present if reported as ‘often’ or ‘very often’ by the participant’s classroom teacher. Because most (88%) children identified as CD were also identified as OD, children meeting criteria for either of these diagnoses were grouped together in the statistical analyse (Boucher et al., 2012c). Internal consistency was high (αordinal = 0.95) and the continuous score was retained in analyses.
Adolescent behavior assessment
Adolescent externalizing.
The Youth Self-Report 2001-revision (YSR) from the Achenbach System of Empirically Based Assessments was used to evaluate externalizing behavior during adolescence (Achenbach and Rescorla, 2001). This screening tool is intended for use with youths up to 18 years of age. The interviewer asked the participant to rate the frequency of their symptoms from 0 to 2 (0 = not true, 1 = somewhat or sometimes true, 2 = very true or often true) for each of the 112 items included in the self-report form. The externalizing problems score was obtained by combining the aggressive behavior and the rule-breaking behavior scores (sum of 32 of the 112 items). The YSR has never been used in the Inuit population before, but internal consistency of the externalizing subscale was high (αordinal = 0.88). No normative data was available for Inuit adolescents, thus raw score were used in all statistical analyses.
Adolescent hyperactivity-impulsivity.
The Barkley Adult ADHD-IV Rating Scale (BAARS) is a questionnaire based on the DSM-IV-TR diagnostic criterion for ADHD, which also assesses ADHD symptoms and subtypes (inattention, hyperactivity-impulsivity) and total ADHD in American adults aged 18 to 81 years of age (Barkley, 2011). The subtype hyperactivity-impulsivity was used for its similarity with the childhood measure of hyperactivity-impulsivity. The interviewer asked participants to rate how often they exhibited each symptom (‘never’, ‘sometimes’, ‘often’, ‘very often’). A symptom was considered present if reported as present ‘often’ or ‘very often’, and a symptom score was computed as the count of symptoms present for hyperactivity-impulsivity items (5 and 4 items, respectively). The BAARS has never been validated in the Inuit population, but the internal consistency of the selected subscale was acceptable (αordinal = 0.76).
Adolescent conduct disorder.
The Diagnostic Interview Schedule for Children (DISC-IV) is a structured diagnostic instrument originally developed to assess 34 psychiatric disorders in epidemiological studies on children and adolescents. Designed to collect information corresponding to the DSM-IV diagnostic criterion, it has since been used in clinical studies and service settings (Shaffer et al., 2000). The eight-item subsection of the DISC-IV on conduct disorder was included in the adolescent follow-up. We included it in our analyses for its similarity with the childhood measure of OD/CD. The participants were asked if the behavior described in each item applied to them (yes/no). The count of items present was used as an adolescent conduct disorder score. Neither the DISC-IV nor any subsection were validated in the Inuit population, but internal consistency in our sample was moderate (αordinal = 0.77).
Adolescent substance use.
The DEP-ADO 3.2 is a substance use evaluation grid originally developed and tested for use among Quebec’s French-speaking adolescents (12 to 18 years old; Landry et al. 2004). The seven-question grid evaluates frequency, intensity and age of initiation of alcohol and drug use. Additional items were added to our questionnaire to describe the drinking environment (during a meal, at a party, etc.) and motives. Questions about age of substance use initiation were added at the third of four data collection trips, therefore age at first use data was available only for half of the participants. The binge drinking score (continuous) was defined as the number of days, in the last year, where more than five/eight alcohol beverages were consumed in one occasion (for women/men). The cannabis use frequency was modeled at four levels: never, ≤once a month, 1–6 times per week and daily.
Covariates
Paths in the mediation analysis (exposure to mediator, mediator to outcome, direct and indirect effect) are modeled by two regression analyses. Therefore, for each regression model, a different set of confounding variables was investigated. An a priori list of candidate variables was built for each relation based on previous knowledge and the Inuit population’s specificities, including child age and sex (Claycomb et al., 2004), socioeconomic status (SES) (Froehlich et al., 2007), age of biological mother at delivery (Claycomb et al., 2004), maternal tobacco use during pregnancy (Huang et al., 2018), birth weight (Lim et al., 2018), gestational age (Bhutta et al., 2002), adoption status (Decaluwe et al., 2015), and house crowding (Solarig and Mare, 2012).
Confounders in the prediction of the child behavior scores.
The same confounding variables as those selected in Boucher et al. (2012) were included in the prediction of childhood behaviors (mediators) from childhood Pb exposure: child age, sex, and birth weight, and childhood socioeconomic status, age of biological mother at delivery, maternal tobacco use during pregnancy, and cord blood Hg
Confounders in the prediction of adolescent outcomes.
Age at adolescent testing, sex, and SES of principal care provider at the adolescence follow-up were treated as obligatory covariates. Adolescent schooling level was not considered because of its presence in the hypothetical causal pathway.
The potential covariates considered for the association between child blood Pb concentrations and adolescent outcomes, as well as for the association between child behavior (mediators) and adolescent outcomes were the same. They included a) participant characteristics: birth weight, adoption status (yes/no), and suicidal thoughts in adolescence (yes/no); b) maternal and family characteristics: gestational age, breast feeding duration, primary caregiver education level, age and parity of biological mother at delivery, marital status (single or not),maternal non verbal reasoning ability at child follow-up (Raven et al., 1992), and childhood and adolescent domestic crowding (> 1 person per room; yes/no); c) contaminants and nutrients: cord, child and adolescent blood Hg concentrations, and DHA expressed in percentage by weight of total fatty acids in plasma phospholipids. Blood lead concentrations measured in cord blood and at adolescent follow-up were also considered.
Statistical analyses
Descriptive statistics, t-test, Wilcoxon tests and Pearson’s Chi-squared tests were computed with the R 3.5.0 software (R Core Team, 2018). The psych and GPArotation packages were used to obtain ordinal alphas, Pearson correlations and confidence intervals (Bernaards and Jennrich, 2005; Revelle, 2018). The RVAideMemoire package was used to compute Spearman correlations and confidence intervals (Hervé, 2018). The BaylorEdPsych package was used to perform missing data pattern analysis and test the missing completely at random (MCAR) assumption (Beaujean, 2012).
Assumptions.
Continuous variables’ distributions were visually checked for normality and the following variables were log2-transformed: contaminant variables, mother’s age at delivery, child externalizing problems scores and number of binge drinking days in the last year. We analysed the missing value patterns to assess potential bias. The most common missing data were adolescent domestic crowding (5.2%, n=11), binge drinking (4.7%, n=10), cannabis use (4.2%, n=9) and pregnancy tobacco use (2.8%, n=6). Little’s MCAR test indicated no systematic bias arising from missingness (χ2 = 308.81, df = 324, p = 0.72; Little 1988).
Bivariate associations.
We computed either Pearson or Spearman correlations depending on the distribution of the variables to document associations between mediators and outcomes (Table 2). Correlations were also performed to document associations between adolescent blood Pb concentrations and adolescent outcomes.
Table 2–
Pearson and Spearman correlation coeffici ents (95% Confidence Intervals) between mediators and outcomes
| Childhood |
Adolescence |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Externalizing a | Hyperactivity-impulsivitya | OD/CD a | Blood Pb | Externalizingb | Hyperactivity-impulsivityb | Conduct disorderb | Binge drinkingb | Cannabis useb | |
| Childhood | |||||||||
| Blood Pb | 0.26 (0.12, 0.39) | 0.21sp (0.07, 0.35) | 0.20sp (0.06, 0.32) | 0.40 (0.28, 0.51) | 0.08 (−0.04, 0.22) | 0.10 (−0.04, 0.23) | 0.08sp (−0.06, 0.21) | −0.03 (−0.17,0.08) | 0.12sp (−0.02, 0.25) |
| Externalizing a | 1 | 0.73sp (0.66, 0.79) | 0.82sp (0.77, 0.87) | 0.25 (0.13, 0.36) | 0.20 (0.009, 0.32) | −0.003 (−0.13, 0.14) | 0.09sp (−0.05, 0.22) | 0.14 (−0.02, 0.28) | 0.20sp (0.06, 0.33) |
| Hyperactivity-impulsivity a | - | 1 | 0.65sp (0.56, 0.72) | 0.18sp (0.04, 0.30) | 0.11sp (−0.02, 0.25) | −0.01sp (−0.16, 0.14) | 0.07sp (−0.07, 0.21) | 0.05sp (−0.09, 0.19) | 0.10sp (−0.04, 0.23) |
| OD/CD a | - | - | 1 | 0.21sp (0.08, 0.33) | 0.19 sp (0.05, 0.32) | −0.04 sp (−0.17, 0.10) | 0.08 sp (−0.05, 0.22) | 0.10 sp (−0.04, 0.24) | 0.23 sp (0.08, 0.36) |
| Adolescence | |||||||||
| Blood Pb | - | - | - | 1 | 0.01 (−0.12, 0.15) | −0.04 (−0.17, 0.09) | 0.05sp (−0.08, 0.19) | 0.16 (0.03, 0.30) | 0.13sp (−0.02, 0.27) |
| Externalizing b | - | - | - | - | 1 | 0.44 (0.32, 0.57) | 0.34sp (0.21, 0.45) | 0.22 (0.10, 0.34) | 0.32sp (0.19, 0.43) |
| Hyperactivity-impulsivity b | - | - | - | - | - | 1 | 0.16sp (0.03, 0.29) | 0.10 (−0.04, 0.24) | −0.02sp (−0.15,0.12) |
| Conduct disorder b | - | - | - | - | - | - | 1 | 0.09sp (−0.05, 0.23) | 0.18sp (0.05, 0.31) |
| Binge drinking b | - | - | - | - | - | - | - | 1 | 0.17sp (0.03, 0.30) |
Sample size for individual comparisons varies between 195 and 212 due to missing values.
Note: OD/CD, Oppositional defiant/conduct disorder
teachers assessment
self-reported with assistance of interviewer, sp, Spearman coefficient.
Confounders selection.
Linear and ordinal regressions were performed prior to mediation analysis to evaluate the final inclusion of confounding variables in models. Covariates were included in a forward selection process: variables associated (p < 0.20) with both the mediator and the outcome were entered in decreasing order of association with the outcome and were retained if they altered the association by ± 10% (Rothman et al., 2008). When the correlation between two a priori potential confounders was greater than ρ=0.4 we only retained the one with the strongest association with the outcome. This selection process of confounding variables followed the same principles and criteria previously used by Boucher et al. (2012).
Mediation analyses.
Mediation analyses were conducted to examine the direct and indirect association between child Pb exposure and adolescent externalizing behaviors/substance use by testing three different childhood behaviors scores as mediators based on the results of Boucher et al. (2012; Figure 2). Six mediation analyses were conducted with the three childhood behavior scores as mediators (externalizing, hyperactive-impulsive and OD/CD) to predict their corresponding adolescent behavioral outcomes. Three more were conducted to predict adolescent substance use outcomes (binge drinking and cannabis use) through the same mediators (Figure 2). The parameters were estimated using structural equation modeling in Mplus 8.1 (Muthén and Muthén, 2017).
Figure 2.
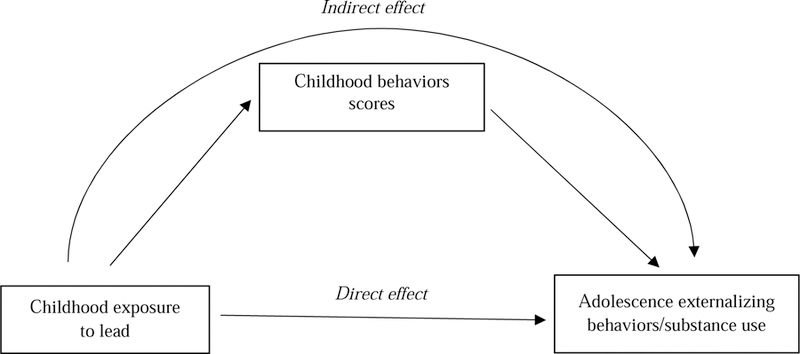
Path diagram showing the associations tested in mediation analyses. Direct effect refer to the association from child blood lead concentrations and externalizing behavior/substance use in the adolescence follow-up. Indirect effect refer to the association of child blood lead concentrations on adolescent’s externalizing behaviors/substance use outcomes through childhood behaviors scores.
Within the mediation models, linear regressions were used to model continuous outcomes, whereas probit regression was used for the ordinal outcome (cannabis use). The product of coefficients method was used to estimate the indirect effect in all models (MacKinnon et al., 2007). Full information maximum likelihood (FIML) or weighted least squares means variance adjusted (WLSMV, for the ordinal outcome) estimators were used to minimise the exclusion of participants because of missing values (Enders and Bandalos, 2001). The assumption of missing at random (for FIML) and missing at random with respect to independent variables (for WLSMV) were both met since our data were considered missing completely at random based on Little’s MCAR test (Asparouhov and Muthén, 2010). The bias-corrected and accelerated 95% confidence intervals (95% CI) were estimated by bootstrap (10 000 resamples; Hayes & Scharkow, 2013). Non-parametric bootstrap methods were preferred to protect against the effects of departure from the normality assumption underlying parametric confidence intervals (Carpenter and Bithell, 2000). Model fit was assessed via three fit statistics: the model chi-square (χ2), the comparative fit index (CFI) and the root mean square error of approximation (RMSEA; Hooper, Coughlan, & Mullen, 2008).
Results
Descriptive analyses
Sample characteristics.
Participants ranged in age from 9–14 years (median 11.37 years) at the childhood follow-up visit and between 16–22 years at the adolescent follow-up (median 18.48 years; see Table 1). A third of the participants were still in school at the adolescent follow-up, whereas more than 35% were fully employed. The median blood Pb concentrations were higher during childhood (2.07 µg/dL) than at the adolescence (1.52 µg/dL). The majority of participants showed few symptoms of externalizing behaviors at both the childhood and adolescent follow-ups. Substance use was very common among adolescents, with over 40% of participants drinking alcohol at least weekly and more than half using cannabis at least weekly. Tobacco was the most widespread substance used, with nearly 8 of 10 participants (79.50%) smoking daily.
Table 1–
Sample characteristics
| Variables | N | Mean ± SD or n (%) | Median | Range |
|---|---|---|---|---|
| Family/birth characteristics | ||||
| Marital status (% married or living with someone) | 211 | 156 (73.90) | ||
| Parity before child birth | 212 | 1.97 ± 1.79 | 2.00 | 0.00 – 8.00 |
| Maternal tobacco smoking pregnancy (% yes) | 206 | 177 (85.92) | ||
| Maternal age at delivery (years) | 212 | 23.69 ± 5.67 | 22.25 | 15.00 – 42.00 |
| Gestational age (weeks) | 212 | 39.20 ± 1.44 | 39.00 | 36.00 – 44.00 |
| Birth weight (kg) | 212 | 3.48 ± 0.46 | 3.50 | 2.44 – 4.74 |
| Child sex (% girls) | 212 | 118 (55.66) | ||
| Breast-feeding status (% yes) | 207 | |||
| None | 52 (25.62) | |||
| 0 < 3 months | 36 (17.73) | |||
| 3 < 6 months | 24 (11.82) | |||
| ≥ 6 months | 91 (44.83) | |||
| Cord blood Pb, geomean ± GSD (µg/dL) | 204 | 3.80 ± 1.84 | 3.73 | 0.83 – 17.80 |
| Cord blood Hg, geomean ± GSD (µg/dL) | 204 | 1.52 ± 2.15 | 1.53 | 0.18 – 9.93 |
| Cord blood DHA (% total fatty acids) | 203 | 1.60 ± 1.29 | 3.44 | 1.12 – 7.73 |
| Childhood characteristics | ||||
| Age (years) | 212 | 11.34 ± 0.71 | 11.37 | 9.32 – 13.97 |
| Adoption status (% adopted) | 212 | 33 (15.57) | ||
| Primary caregiver education (years completed) | 211 | 8.45 ± 2.61 | 9.00 | 0.00 – 16.00 |
| SES scorea | 212 | 28.59 ± 11.38 | 28.25 | 8.00 – 66.00 |
| Behaviors (teachers assessment) | ||||
| Externalizing - CBCL | 205 | 14.23 ± 12.78 | 11.00 | 0.00 – 53.00 |
| Hyperactivity-impulsivity - DBD | 207 | 1.72 ± 2.51 | 0.00 | 0.00 – 9.00 |
| Oppositional defiant/conduct disorder - DBD | 207 | 2.33 ± 3.37 | 1.00 | 0.00 – 16.00 |
| Blood Pb, geomean ± GSD (µg/dL) | 210 | 2.34 ± 1.86 | 2.07 | 0.54 – 12.83 |
| Blood Hg, geomean ± GSD (µg/dL) | 210 | 3.36 ± 2.56 | 3.41 | 0.05 – 28.02 |
| Blood DHA (% total fatty acids) | 209 | 2.40 ± 0.97 | 2.21 | 0.60 – 4.96 |
| Adolescent characteristics | ||||
| Age (years) | 212 | 18.47 ± 1.11 | 18.48 | 16.01 – 21.88 |
| Main language at home (% mainly Inuktitut) | 210 | 200 (95.24) | ||
| SES principal provider | 207 | 28.59 ± 13.01 | 28.00 | 8.00 – 61.00 |
| In a relationship (% yes) | 209 | 95 (45.45) | ||
| Education | 210 | 3.00 | 0.00 – 5.00 | |
| Secondary 1 or less | 46 (21.9) | |||
| Secondary 2 or 3 | 105 (50.0) | |||
| Secondary 4 or higher | 59 (28.1) | |||
| Occupational status (% yes) | 210 | |||
| Working | 75 (35.71) | |||
| School | 34 (16.19) | |||
| Both | 37 (17.62) | |||
| None | 64 (30.48) | |||
| Behaviors (self-reported) | ||||
| Externalizing - CBCL | 210 | 15.80 ± 7.46 | 15.00 | 0.00 – 40.00 |
| Hyperactivity-impulsivity - BAARS | 203 | 1.59 ± 1.52 | 1.00 | 0.00 – 6.00 |
| Conduct disorder - DISC | 212 | 0.94 ± 1.21 | 1.00 | 0.00 – 5.00 |
| Substance use | ||||
| Age started drugs regular basis (years) | 127 | 14.72 ± 1.93 | 15.00 | 7.00 – 19.00 |
| Age started alcohol regular basis (years) | 106 | 15.76 ± 1.63 | 16.00 | 10.00 – 19.00 |
| Cannabis use (%, last 12 months) | 203 | |||
| Never | 63 (31.03) | |||
| ≤ 1 / month | 34 (16.75) | |||
| 1–6 times / week | 42 (20.69) | |||
| Everyday | 64 (31.53) | |||
| Tobacco smoking (%, last 12 months) | 200 | |||
| None | 24 (12.00) | |||
| Occasional | 17 (8.50) | |||
| Daily | 159 (79.50) | |||
| Alcohol use (% last 12 months) | 203 | 177 (87.19) | ||
| Binge drinking days (number in last year)b | 176 | 36.40 ± 47.16 | 12.00 | 0.00 – 250.00 |
| Blood Pb, geomean ± GSD (µg/dL) | 212 | 1.63 ± 2.00 | 1.52 | 0.35 – 18.13 |
| Blood Hg, geomean ± GSD (µg/dL) | 212 | 3.82 ± 2.76 | 4.21 | 0.14 – 36.11 |
| Blood DHA (% total fatty acids) | 212 | 3.35 ± 1.21 | 3.32 | 0.60 – 7.12 |
Note: Pb, lead; Hg, mercury; DHA, docosahexaenoic acid; GSD, geomean standard deviation; CBCL, Child Behavior Checklist; DBD, Disruptive Behavior Disorders Rating Scale; BAARS, Barkley Adult ADHD-IV Rating Scale; DISC, Diagnostic Interview Schedule for Children.
Assessed on the Hollingshead Index, which represents a weighted score of parental occupation (type of work) and education level (Hollingshead, 1975).
Binge drinking, among drinkers, corresponds to consumption of ≥ 5 standard drinks per occasion for women and 8 for men; 1 standard drink ~ 0.5 oz absolute alcohol (~ 350 mL of beer (12 oz), 175 mL of wine (6 oz), or 44 mL of liquor (1.5 oz)).
Of the 294 participants at the childhood follow-up, 212 participated in the adolescent follow-up (72%). Men were more likely to be lost at follow-up (64.6% vs. 44.3%, p=0.003). Participants with higher scores on the OD/CD scale were more likely to be lost at follow-up (mean of 2.33 for participants at the adolescent follow-up and of 3.73 for those lost).
Correlations between externalizing symptoms/behaviors.
Associations between mediators and outcomes are shown in Table 2. All three childhood behavior scores were highly correlated (all correlation coefficients ≥ 0.65). The childhood externalizing problem and OD/CD scores were both significantly but weakly related to adolescent externalizing problem scores and cannabis use. Adolescent externalizing problem score was associated with all the adolescent behaviors; whereas child hyperactivity-impulsivity and the adolescent hyperactivity-impulsivity scores were poorly correlated. The child OD/CD score was poorly associated with adolescent conduct disorder score. Cannabis use was moderately related to adolescent conduct disorder score and binge drinking.
Correlations between blood Pb and adolescent behaviors scores.
Associations between blood Pb concentrations and adolescent outcomes are summarized in Table 2. No significant association was observed between child blood Pb concentrations and adolescent outcomes nor between adolescent blood Pb concentrations and adolescent externalizing or hyperactivity-impulsivity symptoms, conduct disorder, or cannabis use. By contrast, significant associations were observed between adolescent blood Pb concentrations and binge drinking.
Mediation analyses
Associations between child Pb and adolescent behavior scores.
We investigated the effect of child blood Pb concentrations on adolescent behavior scores, directly and indirectly through their corresponding child behavior scores (Table 3). No direct association was observed between child Pb concentrations and any of the adolescent behavior scores. However, a significant indirect association was observed between child blood Pb concentrations and adolescent externalizing problem through child externalizing problem scores [0.32 (95% CI: 0.08, 0.72)]. Thus, a 2-fold increase in blood Pb concentrations units explained on average an increase of 0.25 symptoms on the adolescent externalizing problem score. No indirect association was observed with the adolescent hyperactivity-impulsivity or with adolescent conduct disorder scores. Fit statistics were good for the adolescent externalizing problem and hyperactivity-impulsivity models and acceptable for the adolescent conduct disorder model.
Table 3-.
Mediation analysis of childhood Pb exposure on adolescent externalizing behaviors, using childhood behavior scores as mediator. Regression coefficients (95% CI) and fit indices (n=212).
| Adolescent | |||
|---|---|---|---|
| Mediator | Externalizing Estimate (95% CI) |
Hyperactivity-impulsivity Estimate (95% CI) |
Conduct disorder Estimate (95% CI) |
| Child externalizing (log2-transformed) | |||
| Child Pb → Child externalizinga | 0.42 (0.15, 0.69) | ||
| Child externalizing → Adolescent externalizingb | 0.77 (0.15, 1.36) | ||
| Direct effect | 0.61 (−0.63, 1.96) | ||
| Indirect effect | 0.32 (0.08, 0.72) | ||
| χ2(df), p-value | 10.24(8), 0.25 | ||
| CFI | 0.92 | ||
| RMSEA (90% CI) | 0.04 (0.00, 0.09) | ||
| Child hyperactivity-impulsivity | |||
| Child Pb → Child hyperactivity-impulsivitya | 0.58 (0.17, 0.99) | ||
| Child hyperactivity-impulsivity → Adolescent hyperactivity-impulsivityc | −0.03 (−0.12, 0.06) | ||
| Direct effect | 0.11 (−0.14, 0.37) | ||
| Indirect effect | −0.02 (−0.09, 0.03) | ||
| χ2(df), p-value | 5.01(8), 0.75 | ||
| CFI | 1.00 | ||
| RMSEA (90% CI) | <0.01 (0.00, 0.06) | ||
| Child oppositional defiant/conduct disorder | |||
| Child Pb → Child OD/CDa | 0.67 (0.11, 1.25) | ||
| Child OD/CD → Adolescent conduct disorderd | 0.02 (−0.03, 0.08) | ||
| Direct effect | 0.02 (−0.20, 0.21) | ||
| Indirect effect | 0.02 (−0.01, 0.07) | ||
| χ2(df), p-value | 7.29(7), 0.40 | ||
| CFI | 0.98 | ||
| RMSEA (90% CI) | 0.01 (0.002, 0.09) | ||
Note : CI, Confidence interval; χ2(df), chi square (degrees of freedom); CFI, comparative fit index; RMSEA, root mean square error of approximation; OD/CD, Oppositional defiant/conduct disorder.
Adjusted for child age and sex, SES, age of the biological mother at delivery, maternal tobacco smoking during pregnancy, and birth weight. Models with child hyperactivity-impulsivity and child OD/CD as mediator were also adjusted for cord Hg (log2-transformed).
Adjusted for adolescent age and sex, provider SES, and child blood Hg (log2-transformed).
Adjusted for adolescent age and sex, provider SES, age of the biological mother at delivery and education level of the primary caregiver at child follow-up .
Adjusted for adolescent age and sex, provider SES, age of the biological mother at delivery, maternal tobacco use during pregnancy, and house crowding at adolescence follow-up.
Association between child Pb and adolescent substance use.
We examined the effect of child blood Pb concentrations on adolescent substance use, directly and indirectly through child behavior scores (Table 4). No direct association was observed between child Pb exposure and any of the adolescent substance use. Significant indirect associations were observed between child blood Pb concentrations and both binge drinking and cannabis use through child externalizing problem scores [0.09 (95% CI: 0.02, 0.23)] and [0.05 (95% CI: 0.002, 0.14)], respectively. A 2-fold increase in blood Pb concentrations explains on average an increase of 0.06 binge drinking episode per year. There is no straight-forward way to assess the effect size of an ordinal regression included in a mediation model. The 0.04 indirect association estimate in the cannabis use model can be interpreted as the probability to move from an inferior category of cannabis use frequency to a superior one for each 2-fold increase in blood Pb concentrations units. Fit statistics were acceptable for binge drinking models and good for cannabis use models.
Table 4-.
Mediation analysis of childhood Pb exposure on adolescent substance use, using childhood behavior scores as mediator. Regression coefficients (95% CI) and fit indices (n=212).
|
Adolescent |
||
|---|---|---|
| Mediator | Binge drinking (log2-transformed) Estimate (95% CI) |
Cannabis use Estimate (95% CI) |
| Child externalizing (log2-transformed) | ||
| Child Pb → Child externalizinga | 0.41 (0.14, 0.69) | 0.45 (0.01, 0.85) |
| Child externalizing → Adolescent substance useb | 0.22 (0.02, 0.43) | 0.11 (0.01, 0.20) |
| Direct effect | −0.23 (−0.65,0.21) | 0.04 (−0.24, 0.31) |
| Indirect effect | 0.09 (0.02, 0.23) | 0.05 (0.002, 0.14) |
| χ2(df), p-value | 12.39(9), 0.19 | 16.69(11), 0.12 |
| CFI | 0.92 | 0.98 |
| RMSEA (90% CI) | 0.04 (0.00, 0.09) | 0.05 (0.00, 0.10) |
| Child hyperactivity-impulsivity | ||
| Child Pb → Child hyperactivity-impulsivitya | 0.57 (0.16, 0.99) | 0.59 (0.09, 1.06) |
| Child hyperactivity-impulsivity → Adolescent substance usec | −0.001 (−0.14,0.14) | 0.03 (−0.04, 0.09) |
| Direct effect | −0.15 (−0.58, 0.31) | 0.10 (−0.15, 0.40) |
| Indirect effect | 0.001 (−0.09, 0.09) | 0.02 (−0.02, 0.08) |
| χ2(df), p-value | 13.06(10), 0.22 | 9.73.(11), 0.56 |
| CFI | 0.90 | 1.00 |
| RMSEA (90% CI) | 0.04 (0.00, 0.09) | 0.00 (0.00, 0.07) |
| Child oppositional defiant/conduct disorder | ||
| Child Pb → Child OD/CDa | 0.66 (0.11, 1.25) | 0.68 (−0.02, 1.33) |
| Child OD/CD → Adolescent substance used | 0.04 (−0.07, 0.15) | 0.04 (−0.01, 0.08) |
| Direct effect | −0.17 (−0.59, 0.28) | 0.09 (−0.18, 0.38) |
| Indirect effect | 0.02 (−0.04, 0.15) | 0.02 (−0.002, 0.08) |
| χ2(df), p-value | 20.05(12), 0.07 | 11.09(10), 0.35 |
| CFI | 0.74 | 0.99 |
| RMSEA(90% CI) | 0.06 (0.00, 0.10) | 0.02 (0.00, 0.08) |
Note : CI, Confidence interval; χ2(df), chi square (degrees of freedom); CFI, comparative fit index; RMSEA, root mean square error of approximation; OD/CD, Oppositional defiant/conduct disorder.
Adjusted for child age and sex, SES, age of the biological mother at delivery, maternal tobacco use during pregnancy, and birth weight. Models with child hyperactivity-impulsivity and child OD/CD as mediator were also adjusted for cord Hg (log2-transformed).
Both models adjusted for adolescent age and sex, provider SES, and adolescent blood Pb (log2-transformed). Model with binge drinking as outcome was also adjusted for birth weight, adoption status, and house crowding at adolescence follow-up. Model with cannabis use as outcome was also adjusted for breast feeding duration.
Both models adjusted for adolescent age and sex, provider SES, and adolescent blood Pb(log2-transformed). Model with binge drinking as outcome was also adjusted for birth weight, adoption status and house crowding at adolescence follow-up. Model with cannabis use as outcome was also adjusted for educational level of the primary caregiver at child follow-up and child blood Hg.
Both models adjusted for adolescent age and sex, provider SES, and adolescent blood Pb (log2-transformed). Model with binge drinking as outcome was adjusted for adoption status and house crowding at adolescence follow-up. Model with cannabis use as outcome was also adjusted for educational level of the primary caregiver at child follow-up
Discussion
The main objective of this study was to test the developmental cascade linking child blood Pb concentrations with adolescent behavior problems/substance use through behavior problems assessed in childhood. Child blood Pb concentrations were associated with behavioral challenges at the same age as previously reported by Boucher et al. (2012), and these problems, in turn, predicted externalizing behaviors and substance use in adolescence. Direct associations between child blood Pb concentrations and any of the adolescent behavior assessments or with substance use were not significant. Thus, child Pb concentrations were associated with some problem outcomes in adolescence but only indirectly through child externalizing problem score. No indirect associations linked childhood Pb concentrations with adolescent outcomes through child hyperactivity-impulsivity or conduct disorder scores. While the presence of a direct effect was previously considered as the first step in a mediation analysis, it is now recognized that some relationships may be only indirect through a mediator. This is particularly relevant in the longitudinal investigation of externalizing behavior given the persistence from childhood to adolescence/adulthood of behaviors reported in other cohorts (Reef et al., 2011).
The indirect associations observed in our study between child Pb concentrations and adolescent outcomes are consistent with the developmental cascades hypothesized by Bellinger et al. (2016). In a developmental framework, such a cascade would start with an early-life neurotoxic exposure affecting some child characteristics, which in turn modifies the neurocognitive development, and consequently cause lifelong impairments. In this context, an intermediary endpoint is considered as a mediating factor between the child’s prior exposure and later end-points (Bellinger et al., 2016). The developmental cascades framework predicts direct paths between early exposure and childhood outcomes and indirect paths between the exposure and later impairments, as observed in the present study with externalizing problem score, binge drinking and cannabis use. The underlying mechanisms explaining the persistence of externalizing behaviors (and manifestation of substance use) in adolescence remain unclear. In a previous study, our team reported a significant association between child Pb exposure and increased impulsivity showed by impairments in child’s ability to correctly inhibit a response (Boucher at al. 2012b). These results were consistent with the proposition McGue and colleagues (2001) that a vulnerability to disinhibitory behavior and impulse control might explain those associations.
The few studies that investigated the effect of child blood Pb concentrations on adolescent behavior problems only reported total effects without decomposing them in direct and indirect paths. One such study reported that an increase of 1 µg/dL of the average child blood Pb concentrations was associated with an increase of 0.06 standard deviation on the adolescent impulsivity symptoms score (Winter and Sampson, 2017). In our study, no significant direct or indirect association was observed between child blood Pb on the adolescent hyperactivity-impulsivity scale. The inclusion of adolescent blood Pb concentrations did not modifying the relationship between child Pb concentrations and adolescent outcomes even if it was associated with substance use outcomes. However, we observed indirect associations – through child externalizing behaviors – showing that a 2-fold increase in blood Pb concentrations can explain 0.25 symptoms on the adolescent externalizing score and an increase of 0.06 binge drinking episode. As pointed out by Bellinger et al. (2016), longitudinal studies are able to provide a more complete picture of the burden of child Pb exposure on later impairments if direct/indirect effects are considered instead of the traditional approach of only considering the total effect. The small magnitude of the associations we reported was expected given that adolescent behavior problems are complex outcomes with multiple predictors; we would not expect a single contaminant exposure to explain a large proportion of these behaviors, particularly in Inuit communities where historical and social determinants, coupled with limited educational and social services, may play a predominant role.
Strengths
The major strength of this paper is its longitudinal design, which documented exposure and behavior beyond the critical child developmental period and allowed the use of mediation analyses as a novel analytic approach in the investigation of Pb exposure on future impairments, yet well-grounded in Bellinger et al.’s (2016) developmental cascade framework. Second, externalizing behaviors at the childhood and adolescent follow-up were assessed by different raters, namely the school teachers and the participants themselves, ensuring independence of behavioral measures. Additionally, a longitudinal design reduces the potential reverse causation between externalizing behaviors and substance use; the overwhelming majority of our participants started to consume alcohol or drugs regularly after the childhood assessment of externalizing behaviors.
Limitations
One of the key limitations of our study is its relatively small sample size. The social precariousness in Inuit communities may explain why many participants were not eligible for the adolescence follow-up. We had a retention rate of 72% between the child and adolescent follow-up. Most participants ineligible to participate to the adolescent follow-up were unreachable because of incarceration, hospitalization or death. All of these are known to be related to gender and oppositional defiant/conduct disorder, which may explain differences in the adolescent follow-up sample (Begg et al., 1999; Malakieh, 2018; Mordre et al., 2011; Sorenson, 2011). Furthermore, because of limited statistical power, we were not able to conduct sex effect modification analyses even though externalizing behaviors and substance use manifestations are likely to differ between boys and girls (Hammerslag and Gulley, 2016). Additionally, nine mediation models were tested, which raises the risk of type I error. However, it is important to note that significant results were not randomly distributed: the indirect associations were consistently present in models mediated by externalizing behaviors. Next, our sample was comprised of Inuit children from northern Québec, who are more exposed to Pb than children from the general Canadian population (2.34 µg/dL compared to 0.8–0.9 µg/dL, respectively; Health Canada 2010). This and the major historical, cultural and societal differences of Inuit population with the southern Canadian population, mean that the generalization of our results is not straightforward. We have consider many potential covariates measured at different follow-ups, but it is always possible that some were omitted. Also, lead exposure is usually highest around age 1 to 3, while our measure of Pb concentration was taken a later age. Using early-life Pb exposure might have shown stronger associations. Still we were able to observed significant association between later childhood exposure and behavioral outcomes. Further cohort studies on neurobehavioral outcomes should measure blood Pb concentrations at an earlier age, during the peak of exposition. Finally, the behavior measurement tools used in this study were all developed for, validated in non-indigenous populations, and administered by non-Inuit interviewers. Therefore, they may not fully capture behaviors that are considered as problematic by the Inuit themselves. However, internal consistencies were adequate in our sample (ordinal alphas higher than 0.76).
Conclusion
Children exposed to Pb have a higher rate of developmental problems, but uncertainties remain about what role Pb exposure might play in the relation between childhood developmental problems and persisting impairments. Our study highlights the indirect but persistent effects of child Pb exposure on adolescent behavior problems and the importance of childhood externalizing behavior in this relationship; these early-life impairments may put children on a impeded developmental trajectory, increasing their likelihood of lifelong psychological, social and health problems.. Despite the small contribution of Pb exposure in the prediction of adolescent behavior problems, our results offer a new perspective in the investigation of the Pb burden on human capital. Further studies should document the long-term effect of Pb exposure and other neurotoxicants during adolescence or early adulthood by modelling developmental processes reflecting the risk of developmental cascades instead of documenting mere associations.
Supplementary Material
Highlights.
Child Pb associated with adolescent externalizing behavior and substance use
These associations mediated by child externalizing behaviors
Early-life impairments may put children on a riskier developmental trajectory
Acknowledgements
The authors gratefully thank the Nunavik population, all the people involved in this study and the Nunavik Nutrition and Health Committee for their useful comments on this manuscript.
This longitudinal research was funded by the Institute of Indigenous People’s Health from the Canadian Institutes of Health Research (NRF 130242), NIH/National Institute of Environmental Health Sciences (R01-ES007902); the Northern Contaminants Program from Government Canada; the Lycaki-Young, Sr., Fund from the State of Michigan.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare they have no actual or potential competing financial interests.
References
- Achenbach TM, Rescorla LA, 2001. Manual for the ASEBA School-Age Forms & Profiles University of Vermont, Research Centre for Children, Youth, & Families, Burlington, VT. [Google Scholar]
- Asparouhov T, Muthén B, 2010. Weighted least squares estimation with missing data Muthén & Muthén, Los Angeles. [Google Scholar]
- Barkley RA, 2011. Barkley Adult ADHD Rating Scale-IV (BAARS-IV) The Guilford Press, New York. [Google Scholar]
- Beaujean AA, 2012. BaylorEdPsych: R Package for Baylor University Educational Psychology Quantitative Courses. R package version 0.5
- Beckley AL, Caspi A, Broadbent J, Harrington H, Houts RM, Poulton R, Ramrakha S, Reuben A, Moffitt TE, 2018. Association of childhood blood lead levels with criminal offending. JAMA Pediatr 172, 166–173. 10.1001/jamapediatrics.2017.4005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg DJ, Langley JD, Williams SM, 1999. A longitudinal study of lifestyle factors as predictors of injuries and crashes among young adults. Accid. Anal. Prev 31, 1–11. [DOI] [PubMed] [Google Scholar]
- Bellinger DC, Matthews-bellinger JA, Kordas K, 2016. A developmental perspective on early-life exposure to neurotoxicants. Environ. Int 94, 103–112. 10.1016/j.envint.2016.05.014 [DOI] [PubMed] [Google Scholar]
- Bernaards CA, Jennrich RI, 2005. Gradient Projection Algorithms and Software for ArbitraryRotation Criteria in Factor Analysis. Educ. Psychol. Mes 65, 676–696. [Google Scholar]
- Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJS, 2002. Cognitive and Behavioral Outcomes of School-Aged Children Who Were Born Preterm. JAMA - J. Am. Med. Assoc 288. [DOI] [PubMed] [Google Scholar]
- Boucher O, Burden MJ, Muckle G, Saint-Amour D, Ayotte P, Dewailly É, Nelson CA, Jacobson SW, Jacobson JL, 2012a. Response inhibition and error monitoring during a visual go/no-go task in inuit children exposed to lead, polychlorinated biphenyls, and methylmercury. Environ. Health Perspect 120, 608 10.1289/ehp.1103828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher O, Jacobson SW, Plusquellec P, Dewailly E, Ayotte P, Forget-Dubois N, Jacobson JL, Muckle G, 2012b. Prenatal methylmercury, postnatal lead exposure, and evidence of attention deficit/hyperactivity disorder among Inuit children in Arctic Québec. Environ. Health Perspect 120, 1456 10.1289/ehp.1204976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher O, Jacobson SW, Plusquellec P, Dewailly É, Ayotte P, Forget-Dubois N, Jacobson JL, Muckle G, 2012c. Prenatal methylmercury, postnatal lead exposure, and evidence of attention deficit/hyperactivity disorder among Inuit children in Arctic Québec. Environ. Health Perspect 120, 1456–1461. 10.1289/ehp.1204976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kahn RS, Froehlich T, Auinger P, Lanphear BP, 2006. Exposures to Environmental Toxicants and Attention Deficit Hyperactivity Disorder in U.S. Children. Environ. Health Perspect 114, 1904–1909. 10.1289/ehp.9478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns J, Baghurst P, Sawyer MG, McMichael AJ, Tong S-L, 1999. Lifetime Low-Level Exposure to Environmental Lead and Children ‘ s Emotional and Behavioral Development at Ages 11–13 Years. Public Health 149, 740–749. [DOI] [PubMed] [Google Scholar]
- Carpenter J, Bithell J, 2000. Bootstrap confidence intervals: when, which, what? A practical guide for medical statisticians. Stat. Med 19, 1141–1164. [DOI] [PubMed] [Google Scholar]
- Chiodo LM, Covington C, Sokol RJ, Hannigan JH, Jannise J, Ager J, Greenwald M, Delaney-Black V, 2007. Blood lead levels and specific attention effects in young children. Neurotoxicol. Teratol 29, 538–546. 10.1016/j.ntt.2007.04.001 [DOI] [PubMed] [Google Scholar]
- Chiodo LM, Jacobson SW, Jacobson JL, 2004. Neurodevelopmental effects of postnatal lead exposure at very low levels. Neurotoxicol. Teratol 26, 359–371. 10.1016/j.ntt.2004.01.010 [DOI] [PubMed] [Google Scholar]
- Claycomb CD, Ryan JJ, Miller LJ, Schnakenberg-Ott SD, 2004. Relationships among attention deficit hyperactivity disorder, induced labor, and selected physiological and demographic variables. J. Clin. Psychol 60, 689–693. 10.1002/jclp.10238 [DOI] [PubMed] [Google Scholar]
- Dallaire R, Dewailly É, Ayotte P, Forget-Dubois N, Jacobson SW, Jacobson JL, Muckle G, 2014. Growth in Inuit children exposed to polychlorinated biphenyls and lead during fetal development and childhood. Environ. Res 134, 17–23. 10.1016/j.envres.2014.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaluwe B, Jacobson SW, Poirier M-A, Forget-Dubois N, Jacobson JL, Muckle G, 2015. Impact of Inuit customary adoption on behavioral problems in school-age Inuit children. Am J Orthopsychiatry 85, 250–258. 10.1037/ort0000071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewailly E, Bruneau S, Ayotte P, Laliberté C, Gingras S, Bélanger D, Ferron L, 1993. Health status at birth of inuit newborn prenatally exposed to organochlorines. Chemosphere 27, 359–366. 10.1016/0045-6535(93)90313-T [DOI] [Google Scholar]
- Dietrich KN, Douglas RM, Succop PA, Berger OG, Bornschein RL, 2001. Early exposure to lead and juvenile delinquency. Neurotoxicol. Teratol 23, 511–518. 10.1016/S0892-0362(01)00184-2 [DOI] [PubMed] [Google Scholar]
- Enders CK, Bandalos DL, 2001. The Relative Performance of Full Information Maximum Likelihood Estimation for Missing Data in Structural Equation Models. Struct. Equ. Model. A Multidiscip. J 8, 430–457. 10.1207/S15328007SEM0803_5 [DOI] [Google Scholar]
- Firestone M, Tyndall M, Fischer B, 2015. Substance Use and Related Harms among Aboriginal People in Canada: A Comprehensive Review. J. Health Care Poor Underserved 26, 1110–1131. 10.1353/hpu.2015.0108 [DOI] [PubMed] [Google Scholar]
- Fishbein DH, Todd AC, Ricketts EP, Semba RD, 2008. Relationship between lead exposure, cognitive function, and drug addiction: Pilot study and research agenda. Environ. Res 108, 315–319. 10.1016/j.envres.2008.07.012 [DOI] [PubMed] [Google Scholar]
- Froehlich TE, Lanphear BP, Auinger P, Hornung R, Epstein JN, Braun J, Kahn RS, 2009. Association of Tobacco and Lead Exposures With Attention-Deficit/Hyperactivity Disorder. Pediatrics 124, e1054–e1063. 10.1542/peds.2009-0738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich TE, Lanphear BP, Epstein JN, Barbaresi WJ, Katusic SK, Kahn RS, Objective:, 2007. Prevalence, recognition, and treatment of attention-deficit/hyperactivity disorder in a national sample of US children. Arch. Pediatr. Adolesc. Med 161, 857–864. https://doi.org/10.1001/archpedi.161.9.857 LK - https://doi.org/10.1001/archpedi.161.9.857http://vu.on.worldcat.org/atoztitles/link?sid=EMBASE&issn=10724710&id=doi:10.1001%2Farchpedi.161.9.857&atitle=Prevalence%2C+recognition%2C+and+treatment+of+attention-deficit%2Fhyperactivity+disorder+in+a+national+sample+of+US+children&stitle=Arch.+Pediatr.+Adolesc.+Med.&title=Archives+of+Pediatrics+and+Adolescent+Medicine&volume=161&issue=9&spage=857&epage=864&aulast=Froehlich&aufirst=Tanya+E.&auinit=T.E.&aufull=Froehlich+T.E.&coden=APAME&isbn=&pages=857-864&date LK - http://vu.on.worldcat.org/atoztitles/link?sid=EMBASE&issn=10724710&id=doi:10.1001%2Farchpedi.161.9.857&atitle=Prevalence%2C+recognition%2C+and+treatment+of+attention-deficit%2Fhyperactivity+disorder+in+a+national+sample+of+US+children&stitle=Arch.+Pediatr.+Adolesc.+Med.&title=Archives+of+Pediatrics+and+Adolescent+Medicine&volume=161&issue=9&spage=857&epage=864&aulast=Froehlich&aufirst=Tanya+E.&auinit=T.E.&aufull=Froehlich+T.E.&coden=APAME&isbn=&pages=857-864&date [DOI] [PubMed] [Google Scholar]
- Groenman AP, Janssen TWP, Oosterlaan J, 2017. Childhood Psychiatric Disorders as Risk Factor for Subsequent Substance Abuse: A Meta-Analysis. J. Am. Acad. Child Adolesc. Psychiatry 56, 556–569. 10.1016/j.jaac.2017.05.004 [DOI] [PubMed] [Google Scholar]
- Hammerslag LR, Gulley JM, 2016. Sex differences in behavior and neural development and their role in adolescent vulnerability to substance use. Behav. Brain Res 298, 15–26. 10.1016/j.bbr.2015.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF, Scharkow M, 2013. The Relative Trustworthiness of Inferential Tests of the Indirect Effect in Statistical Mediation Analysis: Does Method Really Matter? Psychol. Sci 24, 1918–1927. 10.1177/0956797613480187 [DOI] [PubMed] [Google Scholar]
- Health Canada, 2010. Report on Human Biomonitoring of Environmental Chemicals in Canada - Results of the Canadian Health Measures Survey Cycle 1 (2007–2009) Ottawa. [Google Scholar]
- Hervé M, 2018. RVAideMemoire: Testing and Plotting Procedures for Biostatistics. R Packag. version 0.9-69-03
- Hollingshead A, 1975. Four Factor Index of Social Status Yale University Department of Sociology, New Haven. [Google Scholar]
- Hooper D, Coughlan J, Mullen M, 2008. Structural Equation Modelling: Guidelines for Determining Model Fit. Electron. J. Buisness Res 6, 53–60. [Google Scholar]
- Huang L, Wang Y, Zhang L, Zheng Z, 2018. Maternal Smoking and Attention-Deficit / Hyperactivity Disorder in Offspring : A Meta-analysis. Pediatrics 141. [DOI] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW, Muckle G, Kaplan-Estrin M, Ayotte P, Dewailly É, 2008. Beneficial Effects of a Polyunsaturated Fatty Acid on Infant Development : Evidence from the Inuit of Artic Quebec. J. Pediatr 152, 356–364. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Markon KE, Patrick CJ, Iacono WG, 2005. Externalizing psychopathology in adulthood: A dimensional-spectrum conceptualization and its implications for DSM-V. J. Abnorm. Psychol 114, 537–550. 10.1037/0021-843X.114.4.537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry M, Tremblay J, Guyon L, Bergeron J, Brunelle N, 2004. La Grille de dépistage de la consommation problématique d’alcool et de drogues chez les adolescents et les adolescentes (DEP-ADO) : développement et qualités psychométriques. Drog. santé société 3, 20–37. 10.7202/010517ar [DOI] [Google Scholar]
- Lee SS, Humphreys KL, Flory K, Liu R, Glass K, 2011. Prospective association of childhood attention-deficit/hyperactivity disorder (ADHD) and substance use and abuse/dependence: A meta-analytic review. Clin. Psychol. Rev 31, 328–341. 10.1016/j.cpr.2011.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévesque B, Duchesne J-F, Gariépy C, Rhainds M, Dumas P, Scheuhammer AM, Proulx J-F, Déry S, Muckle G, Dallaire F, Dewailly É, 2003. Monitoring of umbilical cord blood lead levels and sources assessment among the Inuit. Occup. Environ. Med 60, 693 10.1136/oem.60.9.693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KX, Liu C-Y, Schoeler T, Cecil CAM, Barker ED, Viding E, Greven CU, Pingault J-B, 2018. The role of birth weight on the causal pathway to child and adolescent ADHD symptomatology: a population-based twin differences longitudinal design. J. Child Psychol. Psychiatry 59, 1036–1043. 10.1111/jcpp.12949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little RJA, 1988. A Test of Missing Completely at Random for Multivariate Data with Missing Values. J. Am. Stat. Assoc 83, 1198–1202. 10.1080/01621459.1988.10478722 [DOI] [Google Scholar]
- MacKinnon DP, Lockwood CM, Brown CH, Wang W, Hoffman JM, 2007. The intermediate endpoint effect in logistic and probit regression. Clin. Trials 4, 499–513. 10.1177/1740774507083434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malakieh J, 2018. Adult and youth correctional statistics, 2016/2017
- Mcgue M, Iacono WG, Legrand LN, Malone S, Elkins I, 2001. Origins and Consequences of Age at First Drink. I. Associations With Substance□Use Disorders, Disinhibitory Behavior and Psychopathology, and P3 Amplitude. Alcohol. Clin. Exp. Res 25, 1156–1165. 10.1111/j.1530-0277.2001.tb02330.x [DOI] [PubMed] [Google Scholar]
- Mordre M, Groholt B, Kjelsberg E, Sandstad B, Myhre AM, 2011. The impact of ADHD and conduct disorder in childhood on adult delinquency: A 30 years follow-up study using official crime records. BMC Psychiatry 11, 57 10.1186/1471-244X-11-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muckle G, Ayotte P, Dewailly E, Jacobson SW, Jacobson JL, 2001. Prenatal Exposure of the Northern Quebec Inuit Infants to Environmental Contaminants. Environ. Health Perspect 109, 1291–1299. 10.2307/3454753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO, 2017. Mplus User’s Guide, Eighth Edi. ed. Los Angeles, CA. [Google Scholar]
- Naicker N, Richter L, Mathee A, Becker P, Norris SA, 2012. Environmental lead exposure and socio-behavioural adjustment in the early teens: The birth to twenty cohort. Sci. Total Environ 414, 120–125. 10.1016/j.scitotenv.2011.11.013 [DOI] [PubMed] [Google Scholar]
- Needleman HL, McFarland C, Ness RB, Fienberg SE, Tobin MJ, 2002. Bone lead levels in adjudicated delinquents: A case control study. Neurotoxicol. Teratol 24, 711–717. 10.1016/S0892-0362(02)00269-6 [DOI] [PubMed] [Google Scholar]
- Pelham WE, Gnagy EM, Greenslade KE, Milich R, 1992. Teacher Ratings of DSM-III-R Symptoms for the Disruptive Behavior Disorders. J. Am. Acad. Child Adolesc. Psychiatry 31, 210–218. 10.1097/00004583-199203000-00006 [DOI] [PubMed] [Google Scholar]
- R Core Team, 2018. R: A Language and Environment for Statistical Computing
- Raven JC, Court J, Raven J, 1992. Manual for Raven’s Progressive Matrices and Vocabulary Scales: Standard Progressive Matrices, 192nd ed. Oxford. [Google Scholar]
- Reef J, Diamantopoulou S, Van Meurs I, Verhulst FC, Van Der Ende J, 2011. Developmental trajectories of child to adolescent externalizing behavior and adult DSM-IV disorder: Results of a 24-year longitudinal study. Soc. Psychiatry Psychiatr. Epidemiol 46, 1233–1241. 10.1007/s00127-010-0297-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revelle W, 2018. psych: Procedures for Personality and Psychological Research, Northwestern University Northwest. Univ; Evanston, Illinois, USA. [Google Scholar]
- Rothman KJ, Greenland S, Lash TL, 2008. Modern Epidemiology, 3rd ed. Lippincott Williams & Wilkins, Philadelphia. [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME, 2000. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, Differences From Previous Versions, and Reliability of Some Common Diagnoses. J. Am. Acad. Child Adolesc. Psychiatry 39, 28–38. 10.1097/00004583-200001000-00014 [DOI] [PubMed] [Google Scholar]
- Solarig CD, Mare RD, 2012. HOUSING CROWDING EFFECTS ON CHILDREN’S WELLBEING. Soc Sci Res 41, 464–476. 10.1016/j.ssresearch.2011.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorenson SB, 2011. Gender disparities in injury mortality: consistent, persistent, and larger than you’d think. Am. J. Public Health 101 Suppl, S353–S358. 10.2105/AJPH.2010.300029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Téllez-Rojo MM, Bellinger DC, Arroyo-Quiroz C, Lamadrid-Figueroa H, Mercado-García A, Schnaas-Arrieta L, Wright RO, Hernández-Avila M, Hu H, 2006. Longitudinal associations between blood lead concentrations lower than 10 microg/dL and neurobehavioral development in environmentally exposed children in Mexico City. Pediatrics 118, e323–e330. 10.1542/peds.2005-3123 [DOI] [PubMed] [Google Scholar]
- Turner A, 1897. Lead poisoning among Queensland Children. Australas. Med. Gaz 16, 475–479. [Google Scholar]
- Winter AS, Sampson RJ, 2017. From lead exposure in early childhood to adolescent health: A chicago birth cohort. Am. J. Public Health 107, 1496–1501. 10.2105/AJPH.2017.303903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JP, Dietrich KN, Ris MD, Hornung RW, Wessel SD, Lanphear BP, Ho M, Rae MN, 2008. Association of Prenatal and Childhood Blood Lead Concentrations with Criminal Arrests in Early Adulthood. PLoS Med 5, 732–740. 10.1371/journal.pmed.0050101 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


