Abstract
One of the mitogen-activated protein kinases (MAPKs), c-Jun NH2-terminal protein kinase (JNK) plays an important role in regulating cell fate, such as proliferation, differentiation, development, transformation, and apoptosis. Its activity is induced through the interaction of MAPK kinase kinases (MAP3Ks), MAPK kinases (MAP2Ks), and various scaffolding proteins. Because of the importance of the JNK cascade to intracellular bioactivity, many studies have been conducted to reveal its precise intracellular functions and mechanisms, but its regulatory mechanisms remain elusive. In this review, we discuss the molecular characterization, activation process, and physiological functions of mitogen-activated protein kinase kinase 7 (MKK7), the MAP2K that most specifically controls the activity of JNK. Understanding the role of MKK7/JNK signaling in physiological conditions could spark new hypotheses for targeted anticancer therapies.
Keywords: apoptosis, cell proliferation, JNK, MAPK, MKK7
Introduction
The mitogen-activated protein kinase (MAPK) pathway is a major cell-mediated cascade that regulates processes such as cell growth, differentiation, stress response, survival, and cell death in response to endogenous stimuli such as growth factors, hormones, cytokines, mitogens, and stress.1–3 So far, four kinds of MAPK signaling processes have been found: extracellular signal-regulated kinase (ERK) 1/2, c-Jun N-terminal kinase (JNK), p38, and ERK5.4 These cascades constitute the ERK 1/2, JNK 1/2/3, p38 α/β/γ/δ, and ERK5 subfamilies of MAPK, respectively. These subfamilies respond to various extracellular stimuli, c-Fos, activating transcription factor-2, p53, ETS domain-containing protein-1, and c-Jun to regulate de novo gene expression and induction.5–8 Each subfamily generally comprises a signaling cascade consisting of MAPK kinase kinases (MAP3Ks), MAPK kinases (MAP2Ks), and MAPKs that are sequentially and selectively activated. So far, 20 MAP3Ks, 7 MAP2Ks, and 11 MAPKs have been identified.9,10 Among the MAPKs, the JNK cascade can be induced by environmental stresses such as heat shock, growth factor, and ultraviolet (UV) light.11,12 It regulates intracellular physiological functions such as cell death, growth, and differentiation. This process relies on activation through the serial phosphorylation of MAP3Ks (mixed-lineage protein kinase: MLK, apoptosis signal-regulating kinase, and transforming growth factor beta-activated kinase 1: TAK1), MAP2Ks (mitogen-activated protein kinase kinase 4: MKK4, and mitogen-activated protein kinase kinase 7: MKK7), and JNK (a MAPK).
Due to the importance of the JNK cascade to intracellular bioactivity, many studies have been conducted to elucidate its exact mechanisms.13–16 JNK activation relies on two upstream MAPKs with distinct JNK activation sites: tyrosine phosphorylation by MKK4 and threonine phosphorylation by MKK7. For instance, using genetically disrupted mouse embryonic fibroblasts (MEFs), it was found that axin-mediated JNK activation depends mainly on MKK7, and dishevelled-induced JNK activation depends almost equally on MKK4 and MKK7, whereas virus latent membrane protein-1-mediated JNK activation depends primarily on MKK4.17 JNK activity against stress responses such as UV irradiation, heat, and osmotic changes is significantly inhibited in MKK4 and MKK7 gene-deficient embryonic stem cells and MEFs, which confirms that MKK4 and MKK7 contribute to JNK activation.18,19 In MKK7-deficient cells, the activation of JNK by inflammatory cytokines such as tumor necrosis factor (TNF)-α and interleukin (IL)-1 was almost entirely lost, but it decreased only 50% in MKK4-deficient cells.20 Therefore, MKK4 is required for optimal JNK activation, but MKK7 is essential for JNK activation by pro-inflammatory cytokines. These findings underline that the different MKKs needed for JNK activation depend on many factors, such as the stimulus, different expression levels of MKK4 and MKK7, scaffolds, and other cell-type-specific regulators.21
Although various experiments using MKK4 and MKK7 deletions have been carried out to determine the activation and functional effects of JNK, further research is needed to fully elucidate how JNK regulates cell physiology. Herein, we review studies about the regulation of JNK signaling by MKK7, along with its relevance to cancer cell survival. We focus on MKK7 rather than MKK4 because MKK4 can also stimulate p38 MAPK activity,22 which requires more exploration because of p38’s functional role in cell survival.23–26 We focus on MKK7, an essential JNK activator, as a way to understand the JNK cascade in more detail, which will be helpful to subsequent researchers of the JNK cascade.
Molecular characterization of MKK7
MKK7, also known as signal regulatory protein kinase 2 (SEK2) and c-Jun N-terminal kinase kinase 2 (JNKK2), was first cloned using murine mRNA by researchers at Massachusetts Medical School in 1997. Primers for MKK7 were designed based on the coding sequence of the Drosophila JNK activation factor hemipterous, which is 70% analogous in amino acid sequence to the MKK7 Kinase domain.27,28 After the initial cloning of MKK7, this gene was identified on chromosomes of various species, such as humans, rats, zebrafish, horses, and chickens.29–32 Exons in the MKK7 gene undergo alternative splicing at the RNA level to form MKK7 isoforms, which have modifications in the N- and C-termini. The N-terminal-modified isoforms are identified by the Greek alphabet, and the C-terminal-modified isoforms are identified by numbers. To date, four variants in humans, six variants in mice, and two variants in rats have been found.29,32–34
MKK7 consists of three domains: the D (docking) domain, the Kinase domain, and the DVD (domain for versatile docking) domain. The D domain of MKK7, present at residues 22–81 and containing F-X or F-F-X2-ψ-X-ψ motifs (where F, X, and ψ stand for positively charged, intervening, and hydrophobic residues, respectively), is an essential part of binding JNK (Figure 1A). The D domain is correlated with the binding affinity and activity of MKK7–JNK.35 The Kinase domain, located at residues 120–380, involves a Ser–Xaa–Ala–Lys–Thr (S–X–A–K–T) kinase motif that is phosphorylated by upstream MKKKs.36 The DVD domain, located at residues 377–400, plays an important role in the docking of upstream MAP3Ks such as MLKs, ASKs, TAKs, and LZK (leucine zipper-bearing kinase).37–39
Figure 1.
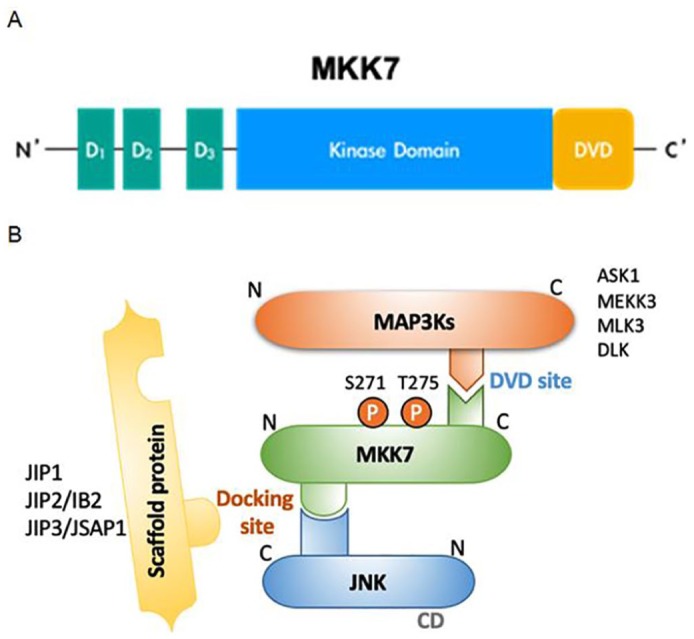
Scheme for the MKK7-dependent JNK pathway. (A) Domain mapping of the MKK7 protein. (B) Schematic illustration of the MKK-dependent JNK pathway, which is activated by the phosphorylation of two residues in the kinase/catalytic domain (Ser and Thr for MKK7; Thr and Tyr for JNK).
CD, common docking domain; D, docking domain; DVD, domain of versatile docking; P, phosphorylation site.
The activation process for MKK7
MKK7 activity can be increased by either MKK7-autophosphorylation or phosphorylation of the Ser and Thr residues of the S-X-A-K-T motifs in the Kinase domain by upstream MAP3K1 (mitogen-activated protein kinase kinase kinase 1, MEKK1), MAP3K2 (MEKK2), or MAP3K11 (MLK3).40–42 Autophosphorylation occurs differently depending on the MKK7 isotype. For example, N-terminal-modified MKK7γ1 is phosphorylated only by MEKK1 without autophosphorylation, as opposed to MKK7β.34 In addition to being specifically activated by upstream kinases, binding between the MKK7 D and DVD domains and a substrate can change the structure of MKK7 to phosphorylate the Ser and Thr residues of the Kinase domain motif. Those reactions lead to the sequential binding and dissociation of MAP3K/MKK7/JNK complexes, which thus affects the signal amplification of the JNK pathway (Figure 1B).43,44
In addition to direct physical interaction with substrates, MKK7 activity is regulated by interaction with scaffold proteins, which play an important role in the assembly of MAP3K/MKK7/JNK complexes. Scaffold proteins do not have a direct catalytic function but play a crucial role in controlling the binding duration and signal intensity of MAP3K/MAP2K/MAPK complexes in the MAPK pathway.45,46 One hypothesis suggests that scaffold proteins are promoted so that limited MAPKs can balance their signaling relative to the number of MAP3Ks in mammalian cells.47 Several JNK signaling-related scaffold proteins have been identified and lead to cell proliferation, differentiation, and apoptosis. Those found so far are JNK-interacting protein (JIP) 1, JIP2, JNK/stress-activated protein kinase-associated protein 1 (JSAP1)/JIP3, JNK-associated leucine zipper protein (JLP)/JIP4, and the Plenty of SH3 (POSH) protein.48–50
JIP1 contains the JNK binding domain (JBD), SRC homology (SH3) domain, and the phosphotyrosine-binding (PTB) domain and affects JNK1 and JNK2 activation.51 JIP1 generally interacts with MAP3Ks such as MEKK3, MLK, DLK (dual leucine zipper-retaining kinase), and histidine protein kinase (HPK1), but MKK7 is the only MAP2K that interacts with JIP1. Therefore, simultaneous expression of JIP1, MLK, and MKK7 in the JNK signaling pathway enhances JNK activation.45,52 JIP2 contains the same domains (JBD, PTB, SH) as JIP1 and is found in several human tissues, including the brain, prostate, ovary, and pancreas. It interacts with both p38 and JNK. Like JIP1, JIP2 and JIP3 also interact with MKK7 and are involved in the formation of JNK signaling complexes.48,53,54 JIP3, identified through yeast hybrid screening as a binding partner of JNK-1, contains a leucine zipper domain rather than the SH3 domain found in other JIPs. JIP3 interacts with various MAP3Ks, such as MEKK1, MLK3, and ASK1, and also with the MAP2Ks MKK4 and MKK7. Although JIP3 is known to be associated with JNK1, JNK2, and JNK3, it has the highest affinity for JNK3.48,49,55
The POSH protein has a specific Rac1 binding site that can bind to GTP-bound active Rac1.50 It is also involved in regulating JNK signaling complexes of MAP3Ks/MAP2Ks/JNKs, similar to the JIPs.56 When the expression of POSH was inhibited in PC12 cells, apoptosis was inhibited,57 leading to the hypothesis that Rac1-mediated apoptosis occurs through an interaction with POSH.58 Recently, the possibility of synergy between POSH and the JIPs has been discovered, and that new aspect is being studied.59–61 It has also been shown that POSH plays a crucial role in neural development in the early embryonic stage and plays a role in immune response by regulating T cell function.62–65
Arrestins have also been reported as scaffolds for MAPK activation, although the mechanism by which they assemble MAPKs into a signaling complex remains unexplored. It is known that all four vertebrate arrestins can interact with JNK3, MKK4, and ASK1, but only arrestin-3 can mediate JNK3 activation.66 Initial studies demonstrated that only MKK4, not MKK7, could bind to arrestin-3,67 but later studies indicated that arrestin-3 could also interact with MKK7 and promote JNK3α2 phosphorylation.21,68 Notably, arrestin-3 binds MKK7 with a lower affinity than it does MKK4.21 Interestingly, JNK3α2 could both enhance the association between arrestin-3 and MKK4 and reduce arrestin-3 binding to MKK7.21 That finding also demonstrates how cooperative regulation of JNK3α2 by MKK4/MKK7 could be determined by the concentration of arrestin-3 needed to induce JNK3α2 phosphorylation, emphasizing the concentration-dependence of the scaffold effect.69 It is widely accepted that the formation of scaffold–kinase complexes contributes to the effective regulation of the specificity, efficiency, and amplitude of signal propagation.70 One recent study used the metaphor of a conveyor belt mechanism for JNK3 activation by scaffold proteins. Thus, an active JNK3 molecule becomes an inactive JNK3 by helping to build an arrestin-3/MKK4/MKK7 complex that causes signal amplification.68
Physiological roles of MKK7
According to several recent studies, MKK7 is involved in various biological responses through both JNK-dependent pathways and JNK-independent pathways. Here, we discuss the role of MKK7 in growth and development and the regulation of programmed cell death, paying particular attention to cancer cells.
Role of MKK7 in growth and development
MKK7 is reportedly essential for hepatocyte formation in embryonic development. The embryos of MKK7 knockout mice died between E11.5 and E13.5 due to immature hepatocyte formation.71 Similarly, primary MKK7–/– hepatoblasts showed defective cell proliferation, and the expression of the cyclin-dependent kinase 2 kinase associated with the G2/M phase cell cycle was inhibited.72 In addition, MKK7 has been shown to play an important role in molecular signaling for retinal development and retinal axonal damage. When retinal axonal damage occurred in MKK7-deficient mice, it caused optic nerve formation failure, irregular retinal axon trajectory, retinal thinning, retinal ganglion cell aggregation, and dendritic formation of dopaminergic amacrine cells.73
Role of MKK7 in programmed cell death and tumorigenesis response
Programmed cell death, defined as apoptosis, autophagy, and programmed necrosis, plays an important role in the development and maintenance of tissue homeostasis by balancing normal cell survival and death.74 JNK signaling pathways are associated with pro-apoptotic and anti-apoptotic processes in different cell types.75,76 Similarly, an MKK7-deficient model was used to show that MKK7 mediates apoptotic responses to a variety of stresses. For example, apoptosis was induced by stimulating mitochondrial antiviral signaling proteins (MAVS) in MKK7–/– MEF cells. Interestingly, the apoptosis induced by MAVS in MKK7–/– MEF cells was JNK2-dependent, not JNK1-dependent.77
Tumorigenesis is defined as a complex and dynamic process of initiation, progression, and metastasis.78 The JNK kinase signaling pathways have also been implicated in tumorigenesis79 through their regulation of cell survival, proliferation, differentiation, and metastasis in cancer cells. Although the exact mechanisms by which the MKK7-JNK signaling axis regulates tumorigenesis remain to be elucidated, many studies have been conducted to clarify the relationship between MKK7 and diverse cancer cells.
In one recent study, five rare polymorphisms of MKK7 (p.Glu116Lys, p.Asn118Ser, p.Arg138Cys, p.Ala195Thr, and p.Leu259Phe) were analyzed in lung cancer patients. Among them, patients with the MKK7 p.Glu116Lys polymorphism had a significantly higher rate of lung cancer metastasis than the others. The p.Glu116Lys mutation affects the proliferation and metastasis of lung cancer cells by regulating a series of cancer-associated genes (upregulated: STC2, SLC1A3, MSMO1, BCL10, and HMGCR; downregulated: SAA1, SBK2, CDH5, COL4A2, and BCL9L).80
Because the liver is larger than other organs and has an abundant blood supply, cancer cells often induce metastasis to the liver through blood. Thus, the prognosis of patients with advanced colorectal cancer varies greatly depending on the presence or absence of liver metastasis. In colorectal cancer patients, a potent inhibitor of hepatic metastasis was found, miR-493. The amount of miR-493 expressed and the incidence of metastatic cancer are closely related. In colorectal cancer patients, miR-493 inhibited the expression of MKK7 by targeting its 3’- untranslated region. Inhibiting MKK7 expression with miR-493 significantly reduced the liver metastases of colon cancer cells. In other words, the occurrence of liver metastases from primary colorectal tumors is associated with elevated MKK7 levels.81
T-cell acute lymphoblastic leukemia (T-ALL) is a type of aggressive acute leukemia with a high recurrence rate. The incidence of T-ALL is about 15% of pediatric cases and 25% of adult cases.82 Although the incidence of pediatric T-ALL is lower than in adults, the recurrence rate of pediatric T-ALL is higher than that of adult T-ALL. In pediatric lymphoblastic leukemia, the gene for Kruppel-like factor 4 (KLF4), referred to as a zinc-finger transcription factor, is inhibited by DNA methylation.83 The loss of KLF4 in leukemic cells accelerated the development of T-ALL by enhancing the G1 to S phase transition. It is generally known that KLF4 regulates the JNK pathway by inhibiting the coding gene for MKK7. The absence of KLF4 in leukemic cells induces excessive MKK7 expression, which increases the proliferation of leukemic cells and eventually leads to T-ALL.84
Thus, MKK7 expression induces the differentiation and metastasis of cancer cells, thereby promoting tumor progression. However, it also plays a role in reducing the proliferation of cancer cells through functions such as apoptosis. For example, in hepatocellular carcinoma (HCC), the level of TIP41-like protein (TIPRL) correlates directly with the level of apoptosis, so high levels of TIPRL inhibit the expansion of cancer growth.85,86 TIPRL is a negative regulator of protein phosphatase 2A (PP2A), a serine/threonine phosphatase targeting the Raf, MEK, and protein kinase B signaling systems.87–90 In HCC, TIPRL and MKK7 competitively bind to PP2A to regulate cell apoptosis.86 In other words, PP2A binds to MKK7 to suppress MKK7 activity due to phosphorylation, thereby inhibiting the apoptosis reaction and promoting the proliferation of HCC. In another case, inactivation of MKK7 in KRasG12D-driven lung cancer increased tumorigenesis and reduced overall survival.79 That response was due to the abnormal role of p53, which is essential for tumor development and cell cycle arrest.91,92 Because the stability of p53 is obtained through the MKK7-mediated JNK pathway, the loss of MKK7 activity disrupts the stability of p53, so MKK7 is not acting directly as a cancer suppressant.79
Likewise, the ubiquitination-like post-translational neddylation of MKK7 in breast cancer cells inhibited its activity and positively affected cell proliferation.93 A direct association between MKK7 and a fragment of RAN-binding protein 2 (RanBP2), recognized as SUMO E3 ligase, was confirmed.93–95 RanBP2 knockdown inhibition of MKK7 neddylation in breast cancer cells maintained MKK7 activity, which reduced the proliferation of human breast cancer cells and impaired the epithelial–mesenchymal transition. Furthermore, the phenomena associated with RanBP2 knockdown were reduced by concomitant MKK7 knockdown.93
Regulatory functions of MKK7 in noncancerous cells have also been reported. Studies of primary MKK7–/– hepatoblasts, which have defective cell proliferation, suggest that the MKK7 signaling pathway is involved in cell proliferation through the regulation of Cdc2 expression.72 In contrast, MKK7–/– mast cells showed hyperproliferation of IL-3 and stem cell factor through the upregulation of cyclin D1 caused by decreased expression of JunB and the cell cycle inhibitor p16INK4a.30
MKK7 as a therapeutic target
In this section, we discuss several drug candidates that target MKK7, their modes of action, and their physiological relevance. The two main ways to selectively target MKK7 are developing a selective covalent inhibitor of MKK7 and targeting a specific protein–protein interaction of MKK7.
Covalent inhibition of MKK7
To date, selective and potent inhibitors of MKK7 have been poorly studied. Adequate selectivity is difficult to achieve because of the high structural homology among the MAP2Ks, particularly at the adenosine triphosphate (ATP) binding site, which is a common target binding region for kinase inhibitors.96 Thus, designing and optimizing an inhibitor of a single kinase at allosteric sites is challenging, although several strategies are available to enhance selectivity across the kinome, such as targeting poorly conserved residues. Research efforts to identify selective MKK7 inhibitors began when it was found that 5Z4Z-7-oxozeaenol (5Z7O), an irreversible inhibitor that covalently links to the cysteine residue at the gatekeeper-2 position (a residue adjacent to the DFG-motif in ERK, TAK1, and MAP2K1), could bind to the ATP sites of MKK7 in a mode that differs from the binding mode of ERK, TAK1, and MAP2K1.97 The unprecedented binding was possible because 5Z7O can covalently bind the Cys218 of MKK7, a residue that is not present in other MAP2Ks at a similar position,97 making it a distinct and advantageous point for developing selectivity across the kinome. More recently, it was discovered that the unprecedented binding to Cys218 is crucial for the auto-inhibition form of MKK7.96
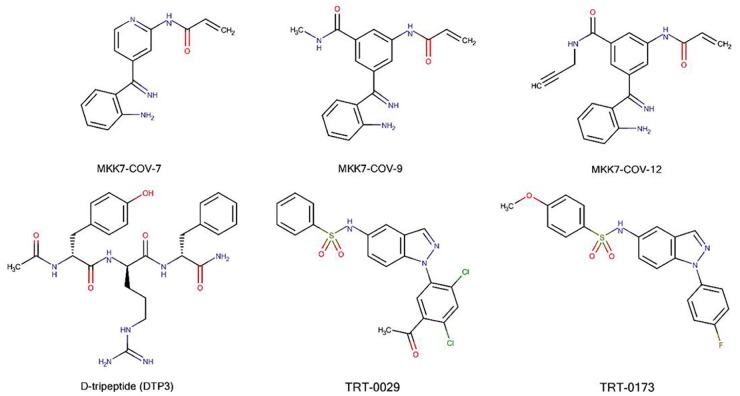
Shraga et al.98 systematically identified covalent inhibitors of MKK7 by covalently targeting this non-conserved cysteine using covalent docking, followed by hit optimization and multiple validations, such as genetic validation of on-target activity, assessment of its selectivity across the kinome and proteome, analysis of metabolic stability and, finally, trial on primary mouse B cells. The three best candidate inhibitors of MKK7 were (as indicated in the original manuscript) MKK7-COV-7, MKK7-COV-9, and MKK7-COV-12 (Figure 2).98 These three candidates inhibited around 90% of the response of primary B cells to lipopolysaccharide, which is a level similar to that of JNK-IN-8, a potent and specific JNK inhibitor.98 Although this interesting research has produced potential candidates for selective MKK7 inhibition and provided a starting point for further development, further validation and studies on in vivo systems are needed before any of the candidates can be used therapeutically.
Figure 2.
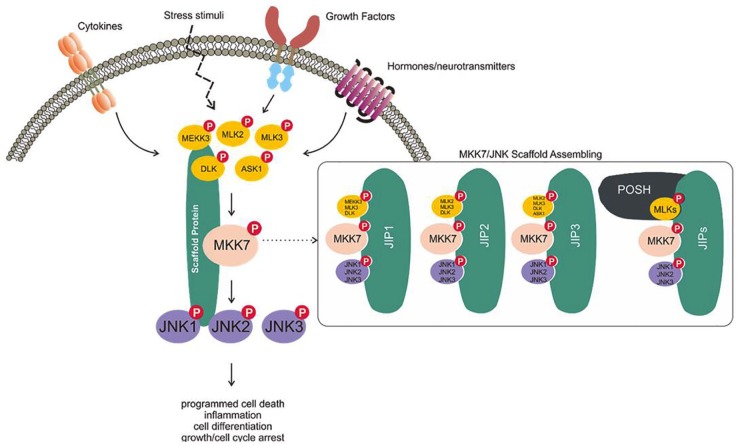
Putative scheme for the MKK7/JNK signaling pathway. The MKK7/JNK signaling axis can be triggered by cytokines, stress stimuli, growth factors, and hormones/neurotransmitters. This stimulation promotes the incorporation of an MKK7/JNK scaffold that mediates the phosphorylation of MAP3Ks (such as MEKK3, MLK2, MLK3, DLK, and ASK1), phosphorylates MKK7, and then phosphorylates the JNK kinases. These signaling cascades have been implicated in regulating various physiological functions, including programmed cell death, inflammation, cell differentiation, and growth/cell cycle arrest.
Targeting an MKK7 binding partner (protein–protein interactions)
Targeting a specific protein–protein interaction of MKK7 might resolve the risk of non-selective inhibition or the toxicity common to ATP analogue inhibitors, and compounds targeting the protein–protein interactions of MKK7 have indeed been reported. Growth arrest and DNA damage-inducible beta (GADD45β), a small acidic protein whose expression denotes aggressive disease in multiple myeloma (MM),99 has been reported to physically interact with MKK7.100 Enzymatic inhibition of MKK7 happens when GADD45β is able to interfere with the access of ATP to the catalytic pocket of MKK7. Tornatore et al.99 have identified a peptide-based structure, D-tripeptide (DTP3) (Figure 3), that can specifically target the GADD45β–MKK7 interaction. DTP3 works by binding to MKK7 with a high affinity, producing a conformational change that facilitates the displacement of GADD45β.99,100 The interface models between the GADD45β–MKK7 and MKK7–DTP3 complexes demonstrate that the interactions of GADD45β and DTP3 with MKK7 are mutually exclusive. As reported by Rega et al.100 ‘DTP3 interacts with two spatially adjacent outer MKK7 region that form a shallow pocket located proximally to the ATP pocket; while in the presence of GADD45β, the DTP3-binding region is partly occupied by loop 2 of the GADD45β.’
Figure 3.
Chemical structure of compounds targeting MKK7/JNK-signaling.
Regarding its therapeutic relevance, constitutive NF-κB signaling promotes survival in cancers, including MM.99 Therefore, a therapeutic strategy that can selectively target NF-κB is highly desired. One way to achieve such specificity is by targeting NF-κB target genes that contribute to the anti-apoptotic mechanism of cancer cells. GADD45β, a transcriptional target of NF-κB, also mediates MKK7/JNK signaling inhibition, which contributes to survival in MM conditions.99 MKK7 is an upstream activator of pro-apoptotic JNK kinases, so inhibition of this signaling axis with a GADD45β interaction can also inhibit apoptosis.99,101,102 DTP3 has a high therapeutic index in vitro and displays potent activity against MM in vivo by selectively inhibiting the NF-κB survival pathway for MM.99 The current understanding of DTP3 as a selective anticancer agent is limited to MM; therefore, further research should be conducted in other types of cancer or other pathological conditions associated with aberrant expression of GADD45β, such as several types of lymphoma, HCC,103 pituitary gonadotrope tumors,104 and colorectal cancer.105,106
Using a similar mechanism, cellular caspase 8 (FLICE)-like inhibitory protein (c-FLIPL) can also interact directly with MKK7, disrupting the interactions between MKK7 and MAP3Ks and inhibiting prolonged activation of the MKK7-JNK pathway.107 Despite reports that some chemical agents can alter c-FLIP expression in ways relevant to cancer therapy,108 selective and potent agents that specifically target the cFLIPL–MKK7 interaction remain to be elucidated. Moreover, the correlation and coordination of cFLIPL and GADD45β in controlling MKK7 remain largely unknown. Cordycepin has been showed to inhibit TNF-α-mediated NF-κB/GADD45 signaling by upregulating MKK7-JNK signaling activation through the inhibition of c-FLIPL expression,109 although the target specificity still needs to be validated.
The TOR signaling pathway regulator-like protein contributes to TNF-related apoptosis-inducing ligand (TRAIL) resistance by forming an MKK7-PP2Ac-TIPRL complex.110,111 Binding of TIPRL to MKK7 and PP2Ac restricts the prolonged activation of MKK7 by tethering the PP2Ac phosphatase, which in turn facilitates MKK7 dephosphorylation, suppressing the JNK/caspase axis and inhibiting TRAIL-induced cell death in TRAIL-resistant cancer.111 Yoon et al.110 elucidated MKK7-TIPRL interaction inhibitors using high-throughput enzyme-linked immunosorbent assay screening followed by hit-to-lead optimization, and they reported two promising compounds, TRT-0029 and TRT-0173 (as designated in the original manuscript) (Figure 3). These indazole-based compounds act as TRAIL sensitizers. In a combination treatment with TRAIL, these compounds enhanced TRAIL-induced apoptosis in in vitro systems of Huh7 cells and suppressed tumor growth in vivo in a mouse xenograft model.110
Other protein binding partners worth mentioning are the receptor for activated C kinase 1 (RACK1), the small-GTPase Rac1, and Ras-association domain family 7 (RASSF7). RACK1 has been implicated in regulating the activity of the JNK pathway. Although the molecular mechanism by which RACK1 regulates the JNK pathway could be cell context-dependent, it has been reported that RACK1 can interact directly with MKK7 in in vitro and in vivo systems, aiding the binding of MKK7 to MAP3Ks, which in turn enhances MKK7/JNK activity in HCC.112 Rac1 is involved in the activation of the MKK7-JNK signaling pathway, which induces Atg5 expression and consequently autophagic cell death in response to oncogenic H-ras.113 Meanwhile, RASSF7 associates specifically with the phosphorylated form of MKK7, maintaining MKK7’s phosphorylated state even in the absence of stress stimuli (independent of MAP3Ks activation).114 Takahashi et al. 114 proposed that RASSF7 could impose a rigid interaction with phosphorylated MKK7 that would likely cause a decrease in phospho-MKK7’s ability to contact subsequent substrates, such as JNK or a phosphatase. As a result, the RASSF7–MKK7 interaction contributes to anti-apoptotic regulation by inhibiting JNK phosphorylation.114 As RASSF7 and phospho-MKK7 accumulate (which can also be accelerated by stress stimuli), RASSF7 tends to degrade, and subsequent JNK-mediated apoptosis can proceed.114 Growing evidence demonstrates the important role of this protein in both tumorigenesis and cell death responses. Chemical agents that regulate Rac1 with respect to cancer angiogenesis and metastasis have been reported elsewhere,115 but a chemical agent with a selective mechanism targeting Rac1/MKK7/JNK has not been found. Furthermore, no studies have reported chemical compounds that regulate the interaction between RACK1 or RASSF7 and MKK7.
Compelling evidence suggests that this signaling axis is crucial to tumorigenesis. MKK7/JNK axis signaling has been intriguingly implicated in both positively and negatively regulating apoptosis and other types of cell death, independent of the cellular context. Therefore, a better understanding of cellular-context specificity and subsequent regulatory mechanisms is still needed, along with a consideration of the intricate interactions between MKK7 and various cascade components. Some selective inhibitors of this signaling axis have been elucidated, along with their modes of action (Figure 4); nevertheless, chemical agents targeting MKK7-binding partner proteins could be an option for providing specificity and delineating context-dependent regulation of JNK signaling. Because JNK signaling is not limited to regulating apoptosis/tumorigenesis, confirmatory studies of these candidate inhibitors in multiple pathological conditions must be included in future studies. Based on our observations here, several potential targets for MKK7/JNK signaling have not been adequately studied. Therefore, many paths are open for future studies.
Figure 4.
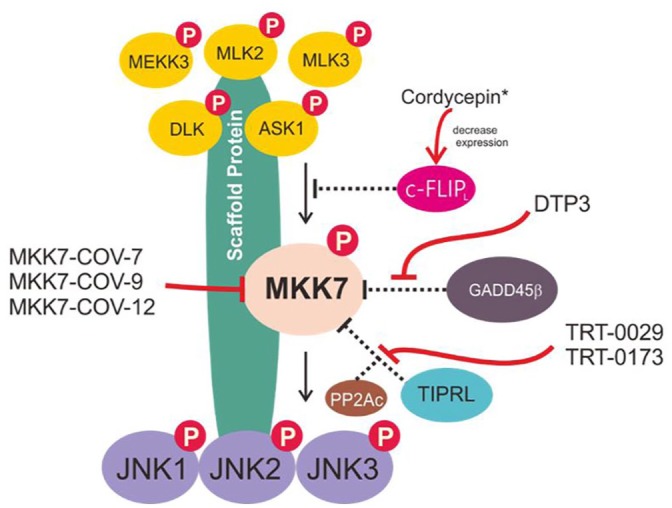
Mode of action of selective chemical agents targeting MKK7. (*) need to be further validated for selectivity.
Conclusion
The JNK cascade is a major MAPK signaling response to many extracellular stimuli. JNK cascades can be activated transiently or continuously by various kinases, scaffolding proteins, and phosphatases. Of the kinases, MKK7 is the MAP2K most essential to regulating the activity of the JNK cascade. Many in vitro and in vivo studies have reported the importance of MKK7 to diverse intracellular functions, such as cell growth, proliferation, senescence, differentiation, transformation, cell cycle regulation, and tumor metabolism. The loss of MKK7 function in cells and mice disrupts many key processes needed to maintain organic homeostasis, such as apoptosis, cell formation and development, and tumorigenesis. However, it is difficult to judge the exact role of MKK7 because its physiological and pathological functions are highly contradictory.
Therefore, further studies are needed to elucidate the detailed molecular mechanisms of various scaffold proteins that interact with MKK7 to regulate cell survival and proliferation. Defining the role of MKK7 in tumorigenesis will have a profound effect on future cancer prevention and treatment strategies.
Footnotes
Author contributions: Jae Gwang Park, Nur Aziz, and Jae Youl Cho designed the study, interpreted the data, and wrote and revised the manuscript.
Author note: Jae Gwang Park is also affiliated to Division of Translational Science, Research Institute, National Cancer Center, Goyang 10408, Republic of Korea.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by BK21 PLUS and the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (2017R1A6A1A03015642), Korea.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iDs: Nur Aziz  https://orcid.org/0000-0001-7696-1025
https://orcid.org/0000-0001-7696-1025
Jae Youl Cho  https://orcid.org/0000-0001-8141-9927
https://orcid.org/0000-0001-8141-9927
Contributor Information
Jae Gwang Park, Department of Integrative Biotechnology, Sungkyunkwan University, Suwon, Republic of Korea.
Nur Aziz, Department of Integrative Biotechnology, Sungkyunkwan University, Suwon, Republic of Korea.
Jae Youl Cho, Department of Integrative Biotechnology, Sungkyunkwan University, 2066 Seobu-ro, Suwon 16419, Republic of Korea.
References
- 1. Seger R, Krebs EG. The MAPK signaling cascade. FASEB J 1995; 9: 726–735. [PubMed] [Google Scholar]
- 2. Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res 2002; 12: 9–18. [DOI] [PubMed] [Google Scholar]
- 3. Junttila MR, Li SP, Westermarck J. Phosphatase-mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. FASEB J 2008; 22: 954–965. [DOI] [PubMed] [Google Scholar]
- 4. Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002; 298: 1911–1912. [DOI] [PubMed] [Google Scholar]
- 5. Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell 2000; 103: 239–252. [DOI] [PubMed] [Google Scholar]
- 6. Ono K, Han J. The p38 signal transduction pathway activation and function. Cell Signal 2000; 12: 1–13. [DOI] [PubMed] [Google Scholar]
- 7. Roskoski R., Jr. ERK1/2 MAP kinases: structure, function, and regulation. Pharmacol Res 2012; 66: 105–143. [DOI] [PubMed] [Google Scholar]
- 8. Wang X, Tournier C. Regulation of cellular functions by the ERK5 signalling pathway. Cell Signal 2006; 18: 753–760. [DOI] [PubMed] [Google Scholar]
- 9. Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature 2001; 410: 37–40. [DOI] [PubMed] [Google Scholar]
- 10. Qi M, Elion EA. MAP kinase pathways. J Cell Sci 2005; 118: 3569–3572. [DOI] [PubMed] [Google Scholar]
- 11. Lee JO, Kim E, Kim JH, et al. Antimelanogenesis and skin-protective activities of Panax ginseng calyx ethanol extract. J Ginseng Res 2018; 42: 389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim E, Kim D, Yoo S, et al. The skin protective effects of compound K, a metabolite of ginsenoside Rb1 from Panax ginseng. J Ginseng Res 2018; 42: 218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gilot D, Loyer P, Corlu A, et al. Liver protection from apoptosis requires both blockage of initiator caspase activities and inhibition of ASK-1/JNK pathway via glutathione S-transferase regulation. J Biol Chem 2002; 277: 49220–49229. [DOI] [PubMed] [Google Scholar]
- 14. Haeusgen W, Herdegen T, Waetzig V. The bottleneck of JNK signaling: molecular and functional characteristics of MKK4 and MKK7. Eur J Cell Biol 2011; 90: 536–544. [DOI] [PubMed] [Google Scholar]
- 15. Seit-Nebi A, Cheng W, Xu H, et al. MLK4 has negative effect on TLR4 signaling. Cell Mol Immunol 2012; 9: 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yamamoto K, Ichijo H, Korsmeyer SJ. BCL-2 is phosphorylated and inactivated by an ASK1/Jun N-terminal protein kinase pathway normally activated at G2/M. Mol Cell Biol 1999; 19: 8469–8478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zou H, Li Q, Lin SC, et al. Differential requirement of MKK4 and MKK7 in JNK activation by distinct scaffold proteins. FEBS Lett 2007; 581: 196–202. [DOI] [PubMed] [Google Scholar]
- 18. Kishimoto H, Nakagawa K, Watanabe T, et al. Different properties of SEK1 and MKK7 in dual phosphorylation of stress-induced activated protein kinase SAPK/JNK in embryonic stem cells. J Biol Chem 2003; 278: 16595–16601. [DOI] [PubMed] [Google Scholar]
- 19. Yang D, Tournier C, Wysk M, et al. Targeted disruption of the MKK4 gene causes embryonic death, inhibition of c-Jun NH2-terminal kinase activation, and defects in AP-1 transcriptional activity. Proc Natl Acad Sci U S A 1997; 94: 3004–3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tournier C, Dong C, Turner TK, et al. MKK7 is an essential component of the JNK signal transduction pathway activated by proinflammatory cytokines. Genes Dev 2001; 15: 1419–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhan X, Kaoud TS, Kook S, et al. JNK3 enzyme binding to arrestin-3 differentially affects the recruitment of upstream mitogen-activated protein (MAP) kinase kinases. J Biol Chem 2013; 288: 28535–28547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang X, Destrument A, Tournier C. Physiological roles of MKK4 and MKK7: insights from animal models. Biochim Biophys Mol Cell Res 2007; 1773: 1349–1357. [DOI] [PubMed] [Google Scholar]
- 23. Koul HK, Pal M, Koul S. Role of p38 MAP kinase signal transduction in solid tumors. Genes Cancer 2013; 4: 342–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Phong MS, Van Horn RD, Li S, et al. p38 mitogen-activated protein kinase promotes cell survival in response to DNA damage but is not required for the G(2) DNA damage checkpoint in human cancer cells. Mol Cell Biol 2010; 30: 3816–3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wada M, Canals D, Adada M, et al. P38 delta MAPK promotes breast cancer progression and lung metastasis by enhancing cell proliferation and cell detachment. Oncogene 2017; 36: 6649–6657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Igea A, Nebreda AR. The stress kinase p38α as a target for cancer therapy. Cancer Res 2015; 75: 3997–4002. [DOI] [PubMed] [Google Scholar]
- 27. Tournier C, Whitmarsh AJ, Cavanagh J, et al. Mitogen-activated protein kinase kinase 7 is an activator of the c-Jun NH2-terminal kinase. Proc Natl Acad Sci U S A 1997; 94: 7337–7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Holland PM, Suzanne M, Campbell JS, et al. MKK7 is a stress-activated mitogen-activated protein kinase kinase functionally related to hemipterous. J Biol Chem 1997; 272: 24994–24998. [DOI] [PubMed] [Google Scholar]
- 29. Lu X, Nemoto S, Lin A. Identification of c-Jun NH2-terminal protein kinase (JNK)-activating kinase 2 as an activator of JNK but not p38. J Biol Chem 1997; 272: 24751–24754. [DOI] [PubMed] [Google Scholar]
- 30. Haeusgen W, Herdegen T, Waetzig V. Specific regulation of JNK signalling by the novel rat MKK7γ1 isoform. Cell Signal 2010; 22: 1761–1772. [DOI] [PubMed] [Google Scholar]
- 31. Gaudet P, Livstone MS, Lewis SE, et al. Phylogenetic-based propagation of functional annotations within the Gene Ontology consortium. Brief Bioinform 2011; 12: 449–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yao Z, Diener K, Wang XS, et al. Activation of stress-activated protein kinases/c-Jun N-terminal protein kinases (SAPKs/JNKs) by a novel mitogen-activated protein kinase kinase (MKK7). J Biol Chem 1997; 272: 32378–32383. [DOI] [PubMed] [Google Scholar]
- 33. Wu Z, Wu J, Jacinto E, et al. Molecular cloning and characterization of human JNKK2, a novel Jun NH2-terminal kinase-specific kinase. Mol Cell Biol 1997; 17: 7407–7416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Michael L, Swantek J, Robinson MJ. Cloning and expression of human mitogen-activated protein kinase kinase 7γ1. Biochem Biophys Res Commun 2006; 341: 679–683. [DOI] [PubMed] [Google Scholar]
- 35. Ho DT, Bardwell AJ, Grewal S, et al. Interacting JNK-docking sites in MKK7 promote binding and activation of JNK mitogen-activated protein kinases. J Biol Chem 2006; 281: 13169–13179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Widmann C, Gibson S, Jarpe MB, et al. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev 1999; 79: 143–180. [DOI] [PubMed] [Google Scholar]
- 37. Takekawa M, Tatebayashi K, Saito H. Conserved docking site is essential for activation of mammalian MAP kinase kinases by specific MAP kinase kinase kinases. Mol Cell 2005; 18: 295–306. [DOI] [PubMed] [Google Scholar]
- 38. Deacon K, Blank JL. MEK kinase 3 directly activates MKK6 and MKK7, specific activators of the p38 and c-Jun NH2-terminal kinases. J Biol Chem 1999; 274: 16604–16610. [DOI] [PubMed] [Google Scholar]
- 39. Gallo KA, Johnson GL. Signalling: mixed-lineage kinase control of JNK and p38 MAPK pathways. Nat Rev Mol Cell Biol 2002; 3: 663. [DOI] [PubMed] [Google Scholar]
- 40. Karandikar M, Xu S, Cobb MH. MEKK1 binds raf-1 and the ERK2 cascade components. J Biol Chem 2000; 275: 40120–40127. [DOI] [PubMed] [Google Scholar]
- 41. Zhang H, Wu W, Du Y, et al. Hsp90/p50cdc37 is required for mixed-lineage kinase (MLK) 3 signaling. J Biol Chem 2004; 279: 19457–19463. [DOI] [PubMed] [Google Scholar]
- 42. Huang J, Tu Z, Lee FS. Mutations in protein kinase subdomain X differentially affect MEKK2 and MEKK1 activity. Biochem Biophys Res Commun 2003; 303: 532–540. [DOI] [PubMed] [Google Scholar]
- 43. Cheng J, Yang J, Xia Y, et al. Synergistic interaction of MEK kinase 2, c-Jun N-terminal kinase (JNK) kinase 2, and JNK1 results in efficient and specific JNK1 activation. Mol Cell Biol 2000; 20: 2334–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xia Y, Wu Z, Su B, et al. JNKK1 organizes a MAP kinase module through specific and sequential interactions with upstream and downstream components mediated by its amino-terminal extension. Genes Dev 1998; 12: 3369–3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Whitmarsh AJ, Davis RJ. Structural organization of MAP-kinase signaling modules by scaffold proteins in yeast and mammals. Trends Biochem Sci 1998; 23: 481–485. [DOI] [PubMed] [Google Scholar]
- 46. Morrison DK, Davis RJ. Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu Rev Cell Dev Biol 2003; 19: 91–118. [DOI] [PubMed] [Google Scholar]
- 47. Uhlik MT, Abell AN, Cuevas BD, et al. Wiring diagrams of MAPK regulation by MEKK1, 2, and 3. Biochem Cell Biol 2004; 82: 658–663. [DOI] [PubMed] [Google Scholar]
- 48. Yasuda J, Whitmarsh AJ, Cavanagh J, et al. The JIP group of mitogen-activated protein kinase scaffold proteins. Mol Cell Biol 1999; 19: 7245–7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ito M, Yoshioka K, Akechi M, et al. JSAP1, a novel jun N-terminal protein kinase (JNK)-binding protein that functions as a Scaffold factor in the JNK signaling pathway. Mol Cell Biol 1999; 19: 7539–7548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tapon N, Nagata Ki, Lamarche N, et al. A new Rac target POSH is an SH3-containing scaffold protein involved in the JNK and NF-κB signalling pathways. EMBO J 1998; 17: 1395–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dickens M, Rogers JS, Cavanagh J, et al. A cytoplasmic inhibitor of the JNK signal transduction pathway. Science 1997; 277: 693–696. [DOI] [PubMed] [Google Scholar]
- 52. Nihalani D, Meyer D, Pajni S, et al. Mixed lineage kinase-dependent JNK activation is governed by interactions of scaffold protein JIP with MAPK module components. EMBO J 2001; 20: 3447–3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Negri S, Oberson A, Steinmann M, et al. cDNA cloning and mapping of a novel islet-brain/JNK-interacting protein. Genomics 2000; 64: 324–330. [DOI] [PubMed] [Google Scholar]
- 54. Schoorlemmer J, Goldfarb M. Fibroblast growth factor homologous factors and the islet brain-2 scaffold protein regulate activation of a stress-activated protein kinase. J Biol Chem 2002; 277: 49111–49119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kelkar N, Gupta S, Dickens M, et al. Interaction of a mitogen-activated protein kinase signaling module with the neuronal protein JIP3. Mol Cell Biol 2000; 20: 1030–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Figueroa C, Tarras S, Taylor J, et al. Akt2 negatively regulates assembly of the POSH-MLK-JNK signaling complex. J Biol Chem 2003; 278: 47922–47927. [DOI] [PubMed] [Google Scholar]
- 57. Wilhelm M, Kukekov NV, Schmit TL, et al. Sh3rf2/POSHER protein promotes cell survival by ring-mediated proteasomal degradation of the c-Jun N-terminal kinase scaffold POSH (Plenty of SH3s) protein. J Biol Chem 2012; 287: 2247–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhang QG, Han D, Xu J, et al. Ischemic preconditioning negatively regulates plenty of SH3s–mixed lineage kinase 3–Rac1 complex and c-Jun N-terminal kinase 3 signaling via activation of Akt. Neuroscience 2006; 143: 431–444. [DOI] [PubMed] [Google Scholar]
- 59. Kukekov NV, Xu Z, Greene LA. Direct interaction of the molecular scaffolds POSH and JIP is required for apoptotic activation of JNKs. J Biol Chem 2006; 281: 15517–15524. [DOI] [PubMed] [Google Scholar]
- 60. Yarza R, Vela S, Solas M, et al. c-Jun N-terminal kinase (JNK) signaling as a therapeutic target for Alzheimer’s disease. Front Pharmacol 2016; 6: 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Blessing NA, Brockman AL, Chadee DN. The E3 ligase CHIP mediates ubiquitination and degradation of mixed-lineage kinase 3. Mol Cell Biol 2014; 34: 3132–3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tsuda M, Langmann C, Harden N, et al. The RING-finger scaffold protein Plenty of SH3s targets TAK1 to control immunity signalling in Drosophila. EMBO Rep 2005; 6: 1082–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kim GH, Park E, Han JK. The assembly of POSH-JNK regulates Xenopus anterior neural development. Dev Biol 2005; 286: 256–269. [DOI] [PubMed] [Google Scholar]
- 64. Zhang F, Yu J, Yang T, et al. A novel c-Jun N-terminal kinase (JNK) signaling complex involved in neuronal migration during brain development. J Biol Chem 2016; 291: 11466–11475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cunningham CA. The scaffold protein POSH regulates T lymphocyte function. PhD Thesis, University of Missouri-Columbia, 2015. [Google Scholar]
- 66. Song X, Coffa S, Fu H, et al. How does arrestin assemble MAPKs into a signaling complex? J Biol Chem 2009; 284: 685–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. McDonald PH, Chow CW, Miller WE, et al. β-Arrestin 2: a receptor-regulated MAPK scaffold for the activation of JNK3. Science 2000; 290: 1574–1577. [DOI] [PubMed] [Google Scholar]
- 68. Perry NA, Kaoud TS, Ortega OO, et al. Arrestin-3 scaffolding of the JNK3 cascade suggests a mechanism for signal amplification. Proc Natl Acad Sci U S A 2019; 116: 810–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gurevich VV, Gurevich EV, Uversky VN. Arrestins: structural disorder creates rich functionality. Protein Cell 2018; 9: 986–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Levchenko A, Bruck J, Sternberg PW. Scaffold proteins may biphasically affect the levels of mitogen-activated protein kinase signaling and reduce its threshold properties. Proc Natl Acad Sci U S A 2000; 97: 5818–5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Nishina H, Vaz C, Billia P, et al. Defective liver formation and liver cell apoptosis in mice lacking the stress signaling kinase SEK1/MKK4. Development 1999; 126: 505–516. [DOI] [PubMed] [Google Scholar]
- 72. Wada T, Joza N, Hai-ying MC, et al. MKK7 couples stress signalling to G2/M cell-cycle progression and cellular senescence. Nat Cell Biol 2004; 6: 215–226. [DOI] [PubMed] [Google Scholar]
- 73. Syc-Mazurek SB, Rausch RL, Fernandes KA, et al. Mkk4 and Mkk7 are important for retinal development and axonal injury-induced retinal ganglion cell death. Cell Death Dis 2018; 9: 1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144: 646–674. [DOI] [PubMed] [Google Scholar]
- 75. Xia Z, Dickens M, Raingeaud J, et al. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 1995; 270: 1326–1331. [DOI] [PubMed] [Google Scholar]
- 76. Berberich I, Shu G, Siebelt F, et al. Cross-linking CD40 on B cells preferentially induces stress-activated protein kinases rather than mitogen-activated protein kinases. EMBO J 1996; 15: 92–101. [PMC free article] [PubMed] [Google Scholar]
- 77. Huang Y, Liu H, Li S, et al. MAVS-MKK7-JNK2 defines a novel apoptotic signaling pathway during viral infection. PLoS Pathog 2014; 10: e1004020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wang M, Zhao J, Zhang L, et al. Role of tumor microenvironment in tumorigenesis. J Cancer 2017; 8: 761–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Schramek D, Kotsinas A, Meixner A, et al. The stress kinase MKK7 couples oncogenic stress to p53 stability and tumor suppression. Nat Genet 2011; 43: 212–219. [DOI] [PubMed] [Google Scholar]
- 80. Qiu F, Yang L, Lu X, et al. The MKK7 p. Glu116Lys rare variant serves as a predictor for lung cancer risk and prognosis in Chinese. PLOS Genet 2016; 12: e1005955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sakai H, Sato A, Aihara Y, et al. MKK 7 mediates miR-493-dependent suppression of liver metastasis of colon cancer cells. Cancer Sci 2014; 105: 425–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Chiaretti S, Foà R. T-cell acute lymphoblastic leukemia. Haematologica 2009; 94: 160–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Chowdhury SK, Liu W, Zi M, et al. Stress-activated kinase MKK7 governs epigenetics of cardiac pepolarization for arrhythmia prevention. Circulation. Epub ahead of print 29 November 2016. DOI: 10.1161/CIRCULATIONAHA.116.022941. [DOI] [PubMed] [Google Scholar]
- 84. Shen Y, Park C, Suppipat K, et al. Inactivation of KLF4 promotes T-cell acute lymphoblastic leukemia and activates the MAP2K7 pathway. Leukemia 2017; 31: 1314–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Liu E, Knutzen CA, Krauss S, et al. Control of mTORC1 signaling by the Opitz syndrome protein MID1. Proc Natl Acad Sci U S A 2011; 108: 8680–8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Song IS, Jun SY, Na HJ, et al. Inhibition of MKK7–JNK by the TOR signaling pathway regulator-like protein contributes to resistance of HCC cells to TRAIL-induced apoptosis. Gastroenterology 2012; 143: 1341–1351. [DOI] [PubMed] [Google Scholar]
- 87. Yang X, Zhang Y, Liu H, et al. Cancerous inhibitor of PP2A silencing inhibits proliferation and promotes apoptosis in human multiple myeloma cells. BioMed Res Int 2016; 2016: 6864135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Garcia A, Cayla X, Guergnon J, et al. Serine/threonine protein phosphatases PP1 and PP2A are key players in apoptosis. Biochimie 2003; 85: 721–726. [DOI] [PubMed] [Google Scholar]
- 89. Chen S, Kong H, Lu X, et al. Pseudotargeted metabolomics method and its application in serum biomarker discovery for hepatocellular carcinoma based on ultra high-performance liquid chromatography/triple quadrupole mass spectrometry. Anal Chem 2013; 85: 8326–8333. [DOI] [PubMed] [Google Scholar]
- 90. Nakashima A, Tanimura-Ito K, Oshiro N, et al. A positive role of mammalian Tip41-like protein, TIPRL, in the amino-acid dependent mTORC1-signaling pathway through interaction with PP2A. FEBS Lett 2013; 587: 2924–2929. [DOI] [PubMed] [Google Scholar]
- 91. Miyashita T, Krajewski S, Krajewska M, et al. Tumor suppressor p53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene 1994; 9: 1799–1805. [PubMed] [Google Scholar]
- 92. Agarwal ML, Agarwal A, Taylor WR, et al. p53 controls both the G2/M and the G1 cell cycle checkpoints and mediates reversible growth arrest in human fibroblasts. Proc Natl Acad Sci U S A 1995; 92: 8493–8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zhu T, Wang J, Pei Y, et al. Neddylation controls basal MKK7 kinase activity in breast cancer cells. Oncogene 2016; 35: 2624–2633. [DOI] [PubMed] [Google Scholar]
- 94. Pichler A, Gast A, Seeler JS, et al. The nucleoporin RanBP2 has SUMO1 E3 ligase activity. Cell 2002; 108: 109–120. [DOI] [PubMed] [Google Scholar]
- 95. Bandyopadhyay S, Chiang CY, Srivastava J, et al. A human MAP kinase interactome. Nat Methods 2010; 7: 801–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Sogabe Y, Hashimoto T, Matsumoto T, et al. A crucial role of Cys218 in configuring an unprecedented auto-inhibition form of MAP2K7. Biochem Biophys Res Commun 2016; 473: 476–481. [DOI] [PubMed] [Google Scholar]
- 97. Sogabe Y, Matsumoto T, Hashimoto T, et al. 5Z-7-Oxozeaenol covalently binds to MAP2K7 at Cys218 in an unprecedented manner. Bioorg Med Chem Lett 2015; 25: 593–596. [DOI] [PubMed] [Google Scholar]
- 98. Shraga A, Olshvang E, Davidzohn N, et al. Covalent docking identifies a potent and selective MKK7 inhibitor. Cell Chem Biol 2019; 26: 98–108.e105. [DOI] [PubMed] [Google Scholar]
- 99. Tornatore L, Sandomenico A, Raimondo D, et al. Cancer-selective targeting of the NF-kappaB survival pathway with GADD45beta/MKK7 inhibitors. Cancer Cell 2014; 26: 495–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Rega C, Russo R, Focà A, et al. Probing the interaction interface of the GADD45β/MKK7 and MKK7/DTP3 complexes by chemical cross-linking mass spectrometry. Int J Biol Macromol 2018; 114: 114–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Tamura RE, de Vasconcellos JF, Sarkar D, et al. GADD45 proteins: central players in tumorigenesis. Curr Mol Med 2012; 12: 634–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Papa S, Zazzeroni F, Bubici C, et al. Gadd45 beta mediates the NF-kappa B suppression of JNK signalling by targeting MKK7/JNKK2. Nat Cell Biol 2004; 6: 146–153. [DOI] [PubMed] [Google Scholar]
- 103. Ou DL, Shen YC, Yu SL, et al. Induction of DNA damage-inducible gene GADD45beta contributes to sorafenib-induced apoptosis in hepatocellular carcinoma cells. Cancer Res 2010; 70: 9309–9318. [DOI] [PubMed] [Google Scholar]
- 104. Michaelis KA, Knox AJ, Xu M, et al. Identification of growth arrest and DNA-damage-inducible gene beta (GADD45beta) as a novel tumor suppressor in pituitary gonadotrope tumors. Endocrinology 2011; 152: 3603–3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Wang L, Xiao X, Li D, et al. Abnormal expression of GADD45B in human colorectal carcinoma. J Transl Med 2012; 10: 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Zhao Z, Gao Y, Guan X, et al. GADD45B as a prognostic and predictive biomarker in stage II colorectal cancer. Genes 2018; 9: E361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Nakajima A, Komazawa-Sakon S, Takekawa M, et al. An antiapoptotic protein, c-FLIPL, directly binds to MKK7 and inhibits the JNK pathway. EMBO J 2006; 25: 5549–5559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Safa AR, Pollok KE. Targeting the anti-apoptotic protein c-FLIP for cancer therapy. Cancers 2011; 3: 1639–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Hwang IH, Oh SY, Jang HJ, et al. Cordycepin promotes apoptosis in renal carcinoma cells by activating the MKK7-JNK signaling pathway through inhibition of c-FLIPL expression. PLoS One 2017; 12: e0186489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Yoon JY, Lee JJ, Gu S, et al. Novel indazole-based small compounds enhance TRAIL-induced apoptosis by inhibiting the MKK7-TIPRL interaction in hepatocellular carcinoma. Oncotarget 2017; 8: 112610–112622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Song IS, Jun SY, Na HJ, et al. Inhibition of MKK7–JNK by the TOR signaling pathway regulator-like protein contributes to resistance of HCC cells to TRAIL-induced apoptosis. Gastroenterology 2012; 143: 1341–1351. [DOI] [PubMed] [Google Scholar]
- 112. Guo Y, Wang W, Wang J, et al. Receptor for activated C kinase 1 promotes hepatocellular carcinoma growth by enhancing mitogen-activated protein kinase kinase 7 activity. Hepatology 2013; 57: 140–151. [DOI] [PubMed] [Google Scholar]
- 113. Byun JY, Yoon CH, An S, et al. The Rac1/MKK7/JNK pathway signals upregulation of Atg5 and subsequent autophagic cell death in response to oncogenic Ras. Carcinogenesis 2009; 30: 1880–1888. [DOI] [PubMed] [Google Scholar]
- 114. Takahashi S, Ebihara A, Kajiho H, et al. RASSF7 negatively regulates pro-apoptotic JNK signaling by inhibiting the activity of phosphorylated-MKK7. Cell Death Differ 2011; 18: 645–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Bid HK, Roberts RD, Manchanda PK, et al. RAC1: an emerging therapeutic option for targeting cancer angiogenesis and metastasis. Mol Cancer Ther 2013; 12: 1925–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]