Abstract
Background
Several lipid‐lowering therapies reduce CRP (C‐reactive protein) independently of LDL‐C (low‐density lipoprotein cholesterol) reduction, but the association between CRP parameters and benefits from more‐intensive LDL‐C lowering is inconclusive. We aimed to determine whether the benefits of more‐ versus less‐intensive LDL‐C lowering on cardiovascular events related to baseline, achieved, or magnitude of reduction in CRP concentrations.
Methods and Results
PubMed, EMBASE, and Cochrane were searched through July 2, 2018. We included randomized controlled cardiovascular outcome trials of LDL‐C lowering with statins or ezetimibe. Two reviewers independently extracted study data and rated study quality. Data were analyzed using meta‐analysis and metaregression analysis. Rate ratios of mortality and cardiovascular outcomes associated with baseline, achieved, and magnitude reduction of CRP concentration were calculated. Twenty‐four trials were included, with 171 250 patients randomly assigned to more‐ or less‐intensive LDL‐C–lowering treatments. Median follow‐up duration was 4.2 years. More‐intensive LDL‐C lowering resulted in a significant reduction in incidences of all outcomes. Compared with less‐intensive LDL‐C lowering, more‐intensive LDL‐C lowering was associated with less reductions in myocardial infarction with a higher baseline CRP concentration (change in rate ratios per 1‐mg/L increase in log‐transformed CRP, 1.12 [95% CI, 1.04–1.22; P=0.007]), but not other outcomes. Similar risk reductions occurred for more‐ versus less‐intensive LDL‐C–lowering therapy regardless of the magnitude of CRP reduction or the achieved CRP level for all outcomes.
Conclusions
Baseline CRP concentrations might be associated with the benefits of LDL‐C lowering on myocardial infarction, but no other outcomes, whereas the achieved and magnitude of reduction in CRP did not seem to have an important association.
Keywords: cardiovascular outcomes, C‐reactive protein, LDL‐cholesterol, lipid lowering, meta‐analysis, randomized controlled trials
Subject Categories: Meta Analysis, Quality and Outcomes, Coronary Artery Disease, Lipids and Cholesterol
Clinical Perspective
What Is New?
Baseline CRP (C‐reactive protein) concentrations might be associated with the benefits of LDL‐C (low‐density lipoprotein cholesterol) lowering on myocardial infarction, but no other outcomes.
There appears to be similar risk reductions for more‐ versus less‐intensive LDL‐C–lowering therapy regardless of the magnitude of CRP reduction or the achieved CRP level for all outcomes, but with limited number of trials.
What Are the Clinical Implications?
More‐intensive LDL‐C lowering appeared to reduce the risk of myocardial infarction (but not other outcomes) to a lesser extent when baseline CRP levels were higher.
More‐intensive LDL‐C lowering was associated with similar risk reduction for mortality and other cardiovascular outcomes across baseline CRP concentrations.
The achieved and magnitude of reduction in CRP did not seem to have an important association with the benefits of LDL‐C lowering on all outcomes.
Introduction
LDL‐C (Low‐density lipoprotein cholesterol) and inflammation are important risk factors for cardiovascular disease. Lowering LDL‐C with statins or ezetimibe and inhibiting inflammation with canakinumab significantly reduce major cardiovascular events.1, 2, 3, 4 hsCRP (high‐sensitivity C‐reactive protein) is a predictor of cardiovascular disease and cardiovascular mortality as well as total cholesterol and blood pressure.5 Several lipid‐lowering therapies (ie, stains and ezetimibe) prove to reduce hsCRP independently of LDL‐C reduction.6 However, it is inconclusive whether benefits from LDL‐C lowering are associated with baseline CRP concentrations. Larger cardiovascular benefits were observed after statin therapy among patients with elevated baseline CRP concentrations in some trials,7 but not others.8, 9 Similarly, whether achieved and reduction of CRP concentrations would affect benefits from more‐intensive LDL‐C lowering is unknown. We sought to determine whether the benefits of LDL‐C–lowering therapy on cardiovascular events related to baseline, achieved, or magnitude of reduction in CRP concentrations.
Methods
The data that support the findings of this study are available from Dr Xin‐Lin Zhang upon reasonable request (xinlzhang0807@gmail.com). We conducted the meta‐analysis in accord with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guideline.
Data Sources and Searches
We searched PubMed, EMBASE, and the Cochrane Library from their inception through July 2, 2018. The following keywords were used: lipid lowering, statin, ezetimibe, low‐density lipoprotein cholesterol, randomized controlled trial, and individual drug names of statins. The search strategy is provided in Data S1. One reviewer (X.Z.) identified potential relevant citations from reference lists of the identified reports and relevant reviews.
Study Selection
Two reviewers (X.Z. and R.L.) independently evaluated the eligibility of studies. Discrepancies were resolved by discussion (W.X.). The main inclusion criteria were: (1) randomized controlled cardiovascular outcome trials involving human subjects; (2) evaluated any comparison of the following strategies: statins, ezetimibe, or placebo (therapy to lower LDL‐C versus no therapy or more‐ versus less‐intensive intervention); and (3) included a minimum of 500 patients and 40 clinical events and reported outcomes of interest with at least 6 months of follow‐up. We excluded trials investigating LDL‐C–lowering drugs other than statins and ezetimibe. Trials with PCSk9 (proprotein convertase subtilisin/kexin type 9) monoclonal antibodies were excluded because they do not affect CRP concentrations. We did not impose limitations on language, sex, or age.
Outcomes of Interest
Outcomes of interest were all‐cause and cardiovascular mortality, myocardial infarction, stroke, coronary revascularization, and major adverse cardiovascular events (MACEs).
Data Extraction and Assessment of Study Quality
Three investigators (X.Z., R.L., and W.X.) independently extracted data using a prespecified form. Median CRP and mean LDL‐C values were abstracted from each trial. Two reviewers (X.Z and W.X.) independently assessed risk of bias of each trial by using the Cochrane Collaboration's tool,10 which assessing random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other sources of bias. Consensus was achieved through referral to a third investigator (L.W.) in case of disagreement.
Data Synthesis and Statistical Analysis
To investigate the association between baseline CRP concentrations and risks of mortality and cardiovascular outcomes with more‐intensive LDL‐C lowering, random‐effects meta‐regression analysis was performed, with log‐transformed baseline CRP concentration as the covariate for the main model. Several other variables were added in the adjusted analyses, which included age, absolute magnitude of reduction in CRP concentrations (difference between achieved CRP concentrations in the more‐ and less‐intensive study arms), baseline LDL‐C, and absolute magnitude of reduction in LDL‐C concentrations. Baseline CRP concentrations were log‐transformed because their distributions were markedly skewed. Similar analyses were carried out for achieved and magnitude of reduction in CRP concentrations. Given that statins and ezetimibe differ in their effects on CRP concentrations, we performed sensitivity analyses restricted to statin trials. We also performed sensitivity analyses based on different study populations (primary or secondary prevention trials). To account for the variability in the length of follow‐up for each of these trials, we used rate ratios (RRs) with their corresponding 95% CIs adjusted for patient‐years as the statistic estimate.
Prespecified subgroup analyses were performed for all outcomes (see Data S1). A test for subgroup differences was performed across the examined subgroups with a χ2 test of interaction. Heterogeneity was assessed by the Cochran Q test and the I2 statistic. We examined potential publication bias by visually inspecting the asymmetry of the funnel plot and Begg's test. For the summary treatment effect estimate, a 2‐tailed P value <0.05 was considered statistically significant. Analyses were conducted with Stata software (version 12.0; StataCorp LP, College Station, TX) and Review Manager (version 5.3; Cochrane Collaboration).
Results
Study Selection and Characteristics
The flow diagram of the study selection is shown in Figure S1. Twenty‐four trials were included in the meta‐analysis and metaregression analysis.3, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33 Twelve trials that were otherwise eligible were not included because CRP concentrations were not reported. All trials except 1 were multicenter studies. Statin monotherapy was used in 20 trials and statin and ezetimibe in 4 trials. Overall, 171 250 patients were randomly assigned to more‐ or less‐intensive LDL‐C–lowering treatments. Median follow‐up duration was 4.2 years (range, 1–11.5). Mean age of patients were 62.7 years, and 73.0% were men. The median baseline CRP concentration was 3.1 mg/L and ranged from 0.57 to 21.2 mg/L. Detailed characteristics of each trial are presented in Tables S1 through S3.
Risk of Bias in the Included Trials
Risk of bias for each trial is shown in Table S4. Most trials had blinded outcome adjudication and blinding of participants and personnel. Risk for attrition bias and reporting bias were generally low. Publication bias was detected for a number of outcomes, as revealed by visual inspection of the funnel plots and Begg's test (Figure S2).
All‐Cause Mortality
There were 8355 deaths among 83 209 patients randomly assigned to receive more‐intensive LDL‐C–lowering treatment and 8989 deaths among 83 018 patients assigned to less‐intensive LDL‐C–lowering treatment. Metaregression analysis showed that all‐cause mortality risk was not significantly different for each 1‐mg/L higher log‐transformed baseline CRP concentration between more‐ versus less‐intensive LDL‐C–lowering treatments (RR, 0.98; 95% CI, 0.91–1.05; P=0.512; Figure 1), with or without multivariable adjustment (Table). A similar observation was found for magnitude of reduction in CRP concentrations (RR, 0.98; 95% CI, 0.91–1.06; P=0.590; Figure S3). The overall risk reduction in all‐cause mortality with more‐ versus less‐intensive therapy across all trials was 0.91 (95% CI, 0.87–0.96) and were consistent across the range of baseline (Figure 2) and magnitude of reduction in CRP concentrations (Figure S4).
Figure 1.
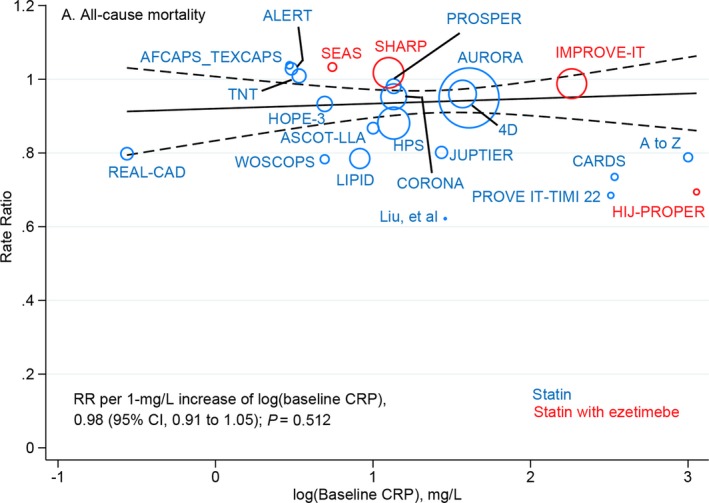
Meta‐regression analysis of all‐cause mortality rate ratios plotted against log‐transformed baseline CRP concentrations in the more‐intensive group. The size of the data marker is proportional to the weight in the metaregression. CRP indicates C‐reactive protein; RR, rate ratio.
Table 1.
Multivariable Metaregression Models for the Association of Each 1‐mg/L Increase in log(Baseline CRP Concentration), Magnitude of Reduction in CRP Concentration, Achieved CRP, and Mortality and Cardiovascular Outcomes
| Outcomes | No. of Trials | log(Baseline CRP) | Rate Ratio (95% CI) | |||
|---|---|---|---|---|---|---|
| log(Baseline CRP) Adjusted for Magnitude of Reduction in CRP | log(Baseline CRP) Adjusted for Magnitude of Reduction in CRP, Baseline LDL‐C, Magnitude of Reduction in LDL‐C and Age | Magnitude of Reduction in CRP | Achieved CRP | |||
| All‐cause mortality | 22 | 0.98 (0.91, 1.05) | 1.00 (0.92, 1.10) | 1.01 (0.90, 1.13) | 0.98 (0.91, 1.06) | 1.00 (0.96, 1.03) |
| Cardiovascular mortality | 22 | 1.01 (0.91, 1.12) | 1.02 (0.89, 1.16) | 1.03 (0.89, 1.19) | 0.97 (0.87, 1.08) | 1.00 (0.94, 1.05) |
| Myocardial infarction | 24 | 1.12 (1.04, 1.22) | 1.16 (1.05, 1.27) | 1.16 (1.02, 1.33) | 0.93 (0.84, 1.04) | 0.98 (0.93, 1.04) |
| Stroke | 24 | 0.94 (0.84, 1.05) | 0.96 (0.84, 1.09) | 0.96 (0.81, 1.13) | 0.90 (0.80, 1.01) | 0.97 (0.91, 1.03) |
| Coronary revascularization | 22 | 1.06 (1.00, 1.13) | 1.07 (0.99, 1.15) | 1.05 (0.96, 1.14) | 0.94 (0.84, 1.04) | 0.99 (0.94, 1.04) |
| MACE | 24 | 1.04 (0.98, 1.11) | 1.05 (0.96, 1.15) | 1.08 (0.97, 1.19) | 0.96 (0.89, 1.03) | 0.99 (0.95, 1.03) |
CRP indicates C‐reactive protein; LDL‐C, low‐density lipoprotein cholesterol; MACE, major adverse cardiovascular event.
Figure 2.
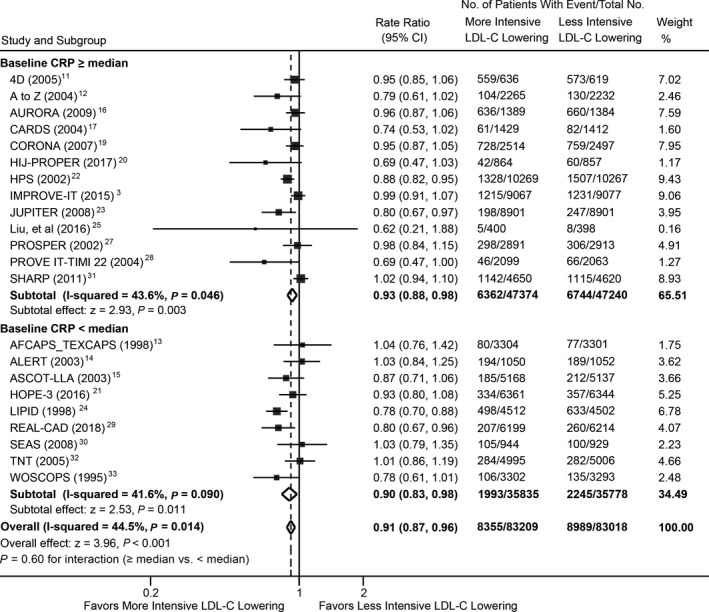
Meta‐analysis of all‐cause mortality stratified by baseline CRP concentrations between more‐ and less‐intensive lipid‐lowering group. CRP indicates C‐reactive protein; LDL‐C, low‐density lipoprotein cholesterol.
Cardiovascular Mortality
Metaregression analysis showed that cardiovascular mortality risk was not significantly different for each 1‐mg/L higher log‐transformed baseline CRP concentration between more‐ versus less‐intensive LDL‐C–lowering treatments (RR, 1.01; 95% CI, 0.91–1.12; P=0.803; Figure 3), with or without multivariable adjustment (Table). A similar observation was found for magnitude of reduction in CRP concentrations (RR, 0.97; 95% CI, 0.87–1.08; P=0.542; Figure S5). The overall risk reduction in cardiovascular mortality with more‐ versus less‐intensive therapy across all trials was 0.84 (95% CI, 0.79–0.90) and was consistent across the range of baseline (Figure 4) and magnitude of reduction in CRP concentrations (Figure S6).
Figure 3.
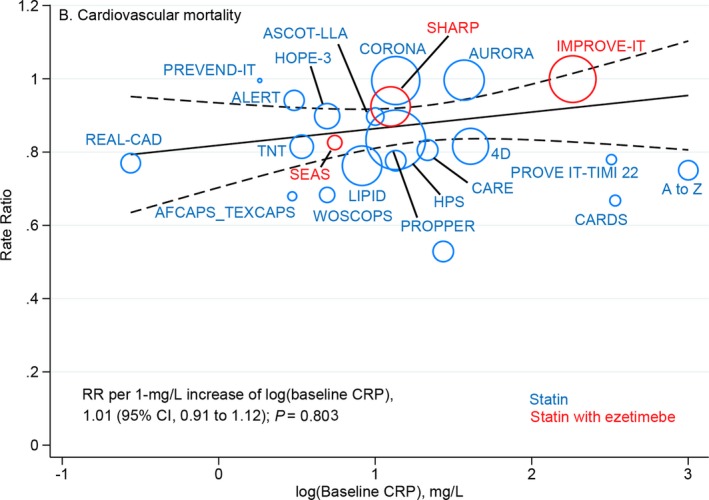
Meta‐regression analysis of cardiovascular mortality rate ratios plotted against log‐transformed baseline CRP concentrations in the more‐intensive group. The size of the data marker is proportional to the weight in the metaregression. CRP indicates C‐reactive protein; RR, rate ratio.
Figure 4.
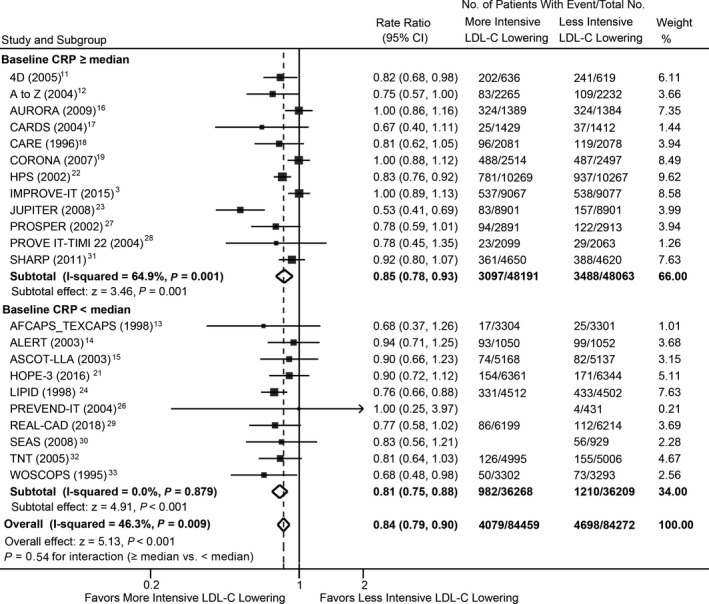
Meta‐analysis of cardiovascular mortality stratified by baseline CRP concentrations between more‐ and less‐intensive lipid‐lowering group. CRP indicates C‐reactive protein; LDL‐C, low‐density lipoprotein cholesterol.
Myocardial Infarction
Overall, 3745 of 85 723 patients receiving the more‐intensive LDL‐C–lowering strategy versus 4825 of 85 527 receiving the less‐intensive strategy experienced myocardial infarction. Metaregression showed that more‐ versus less‐intensive LDL‐C lowering was associated with a significant change in RR for myocardial infarction (RR, 1.12; 95% CI, 1.04–1.22; P=0.007) for each 1‐mg/L higher log‐transformed baseline CRP concentration (Figure 5), with or without multivariable adjustment (Table). The overall risk reduction in myocardial infarction associated with more‐ versus less‐intensive therapy across all trials was 0.75 (95% CI, 0.70–0.81), but varied by baseline CRP concentration (Figure 6). The RR was 0.79 (95% CI, 0.72–0.87) in trials with baseline CRP concentrations ≥2.7 mg/L (median) and 0.70 (95% CI, 0.65–0.76) in trials with baseline CRP concentrations <2.7 mg/L (P=0.060 for interaction). Metaregression analysis did not show a significant correlation between magnitude of reduction in CRP concentrations and risk of myocardial infarction (RR, 0.93; 95% CI, 0.84–1.04; P=0.19; Figure S7). The overall risk reduction in myocardial infarction with more‐ versus less‐intensive therapy was consistent across the range of magnitude of reduction in CRP concentrations (Figure S8).
Figure 5.
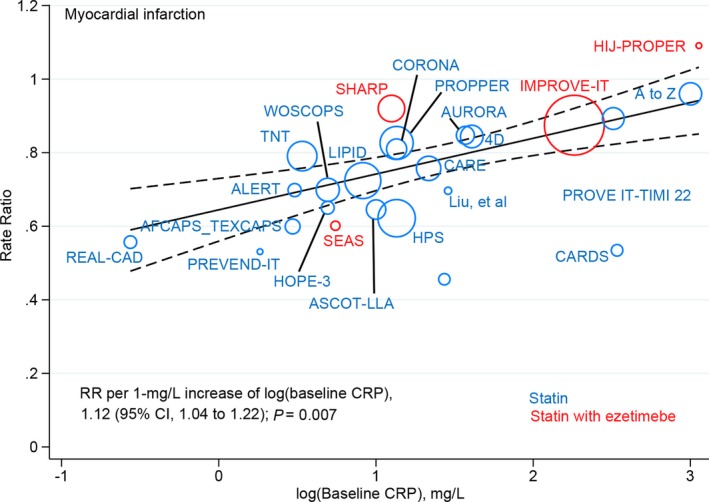
Meta‐regression analysis of myocardial infarction rate ratios plotted against log‐transformed baseline CRP concentrations in the more‐intensive group. The size of the data marker is proportional to the weight in the metaregression. CRP indicates C‐reactive protein; RR, rate ratio.
Figure 6.
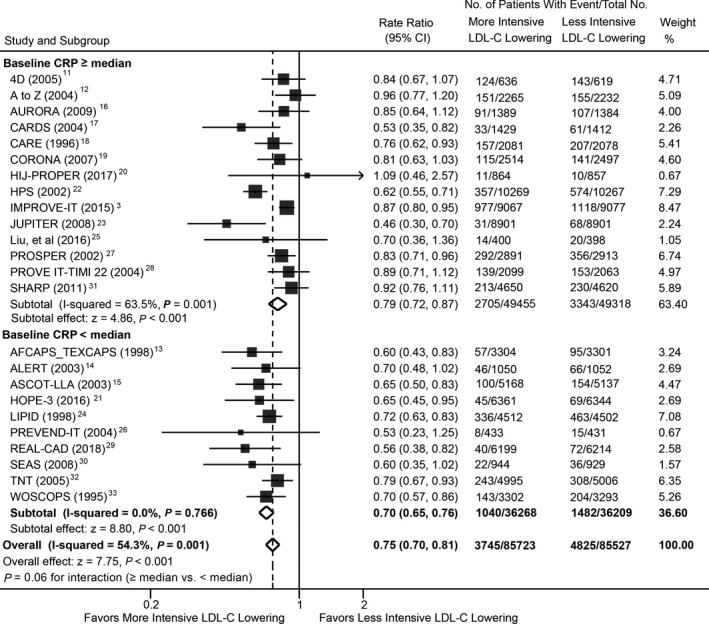
Meta‐analysis of myocardial infarction stratified by baseline CRP concentrations between more‐ and less‐intensive lipid‐lowering group. CRP indicates C‐reactive protein; LDL‐C, low‐density lipoprotein cholesterol.
Stroke
Metaregression analysis showed that stroke risk was not significantly different for each 1‐mg/L higher log‐transformed baseline CRP concentration between more‐ versus less‐intensive LDL‐C–lowering treatments (RR, 0.94; 95% CI, 0.84–1.05; P=0.253; Figure S9), with or without multivariable adjustment (Table). A similar observation was found for magnitude of reduction in CRP concentrations (RR, 0.90; 95% CI, 0.80–1.01; P=0.084; Figure S10). The overall risk reduction in stroke with more‐ versus less‐intensive therapy across all trials was consistent across the range of baseline (Figure S11) and magnitude of reduction in CRP concentrations (Figure S12).
Coronary Revascularization
For each 1‐mg/L higher log‐transformed baseline CRP concentration, more‐ versus less‐intensive LDL‐C lowering was associated with a modest change in RRs for coronary revascularization (RR, 1.06; 95% CI, 1.00–1.13; P=0.062; Figure S13), which became nonsignificant after multivariable adjustment (Table). Metaregression analysis did not show a significant correlation between magnitude of reduction in CRP concentrations and risk of revascularization (RR, 0.94; 95% CI, 0.84–1.04; P=0.181; Figure S14). The overall risk reduction in coronary revascularization with more‐ versus less‐intensive therapy across all trials was consistent across the range of baseline (Figure S15) and magnitude of reduction in CRP concentrations (Figure S16).
Major Adverse Cardiovascular Events
Metaregression analysis showed that MACE risk was not significantly different for each 1‐mg/L higher log‐transformed baseline CRP concentration between more‐ versus less‐intensive LDL‐C–lowering treatments (RR, 1.04; 95% CI, 0.98–1.11; P=0.182; Figure S17), with or without multivariable adjustment (Table). A similar observation was found for magnitude of reduction in CRP concentrations (RR, 0.96; 95% CI, 0.89–1.03; P=0.252; Figure S18). The overall risk reduction in MACE with more‐ versus less‐intensive therapy across all trials was consistent across the range of baseline (Figure S19) and magnitude of reduction in CRP concentrations (Figure S20).
Additional Analyses
Analyses excluding trials with heart failure or chronic kidney disease requiring hemodialysis, trials with less than 1000 patients, or trials published before 2000 yielded similar results (Table S5), as were analyses stratified by types of intervention in the more‐intensive LDL‐C–lowering treatment (Table S6), types of treatment in the less‐intensive LDL‐C–lowering treatment (Table S7), and type of population (Table S8). Consistent with previous studies, a lack of significant reduction in all‐cause and cardiovascular mortality was observed in statin with ezetimibe trials (Table S6).
Metaregression analysis restricted to statin trials confirmed that more‐ versus less‐intensive LDL‐C lowering was associated with a significant change in RRs for myocardial infarction, but no other outcomes of interest (Table S9). For each 1‐mg/L higher log‐transformed baseline CRP concentration, more‐ versus less‐intensive LDL‐C lowering was associated with a significant change in RRs for myocardial infarction (RR, 1.12; 95% CI, 1.03–1.21; P=0.011) in secondary prevention trials (Table S10; Figure S21), but not in primary prevention trials (Table S11). Metaregression and meta‐analysis of mortality and cardiovascular outcomes found no association with achieved CRP concentrations (Table; Figures S22 through S27).
Discussion
In this meta‐analysis and metaregression analysis of 24 trials involving >170 000 patients and ≈24 000 clinical events, more‐intensive LDL‐C lowering appeared to reduce the risk of myocardial infarction to a lesser extent when baseline CRP levels were higher, but was associated with similar risk reduction for mortality and other cardiovascular outcomes across baseline CRP concentrations. Similar risk reductions occurred for more‐ versus less‐intensive LDL‐C–lowering therapy regardless of the magnitude of CRP reduction or the achieved CRP level for all outcomes.
Plasma CRP concentrations is a predictor of cardiovascular risk independent of other risk factors.1 Although a causal role of CRP for atherosclerosis and ischemic vascular disease is not supported by previous studies,34 there is potential in using CRP concentration as a marker for benefit from LDL‐C–lowering therapy. In the AFCAPS/TexCAPS (Air Force/Texas Coronary Atherosclerosis Prevention) trial, patients with an elevated baseline CRP concentration benefited markedly from lovastatin, whereas those with a low baseline CRP level had no cardiovascular benefit.7 However, others have not shown such an association both in primary and secondary prevention trials.8 Our present metaregression analyses demonstrated no association between baseline CRP concentrations with mortality outcomes following LDL‐C lowering, which, to the best of our knowledge, has not been evaluated in randomized trials because of the rarity of mortality outcomes. It is worth noting that a significant association between baseline CRP concentrations and risks for myocardial infarction was evident, with a less‐robust benefit for more‐intensive LDL‐C lowering in patients who had higher baseline CRP concentrations. In line with our finding, post‐hoc analyses of the JUPITER (the JUPITER trial from the US Food and Drug Administration) trial from the US Food and Drug Administration revealed an inverse relationship between baseline hsCRP concentrations and clinical response to statin therapy.35 Subjects with baseline hsCRP above the median cut point of 4.2 mg/L had lower relative risk reduction with statin therapy than those with hsCRP <4.2 mg/L (relative risk reduction, 29% versus 58%).35 The very recently published St. Francis Heart Study also reported a trend toward less benefit in patients with higher baseline hsCRP.36
Several trials suggest that achieving lower CRP concentrations might be associated with better outcomes for patients being treated with statins.37, 38, 39, 40, 41 In the PROVE IT‐TIMI 22 (Pravastatin or Atorvastatin Evaluation and Infection Therapy–Thrombolysis In Myocardial Infarction 22) trial, patients who achieved CRP concentrations of <2 mg/L after statin therapy had a lower rate of cardiovascular events than those who did not.38 A similarly negative association was detected in the REVERSAL (Reversal of Atherosclerosis with Aggressive Lipid Lowering),39 A‐to‐Z (Aggrastat‐to‐Zocor),40 and the JUPITER41 trials. Fueling this debate, trials including the ASCOT‐LLA (Anglo‐Scandinavian Cardiac Outcome Trial–Lipid Lowering Arm),42 the CARDS (Collaborative Atorvastatin Diabetes Study),43 and TNT (Treating New Targets)44 studies showed no association between achieved hsCRP concentrations and magnitude of statin efficacy in the prevention of cardiovascular events. Our meta‐analysis and metaregression analysis do not lend support to the hypotheses that the beneficial effects of LDL‐C–lowering therapy are affected by achieved CRP concentrations, in contrast with those found with achieved LDL‐C concentrations.45, 46
The REVERSAL trial demonstrates that magnitude of reduction in CRP concentrations is significantly correlated with rate of progression of atherosclerosis (determined with intravascular ultrasonography).39 The JUPITER trial also shows an association with magnitude of cardiovascular benefit of statin therapy.41 However, evidence remains scare given that the vast majority of trials did not report these relationship data. Our metaregression analysis revealed no significant correlation between magnitude of reduction in CRP concentrations and benefit from LDL‐C–lowering therapy, which needs to be confirmed in large, prospective trials in the future.
Although previous LDL‐C–lowering trials with stains or ezetimibe reduce CRP concentrations, the concomitant reduction of LDL‐C makes it difficult to conclude a causal role of inflammation in atherothrombotic events. The recently published CANTOS (Canakinumab Anti‐inflammatory Thrombosis Outcomes Study) trial, which enrolled 10 061 patients with previous myocardial infarction and an hsCRP level of ≥2 mg/L, is a proof‐of‐concept trial directly testing the inflammatory hypothesis of atherothrombosis.4 Canakinumab confers a significant 15% reduction in MACEs without altering the lipid profile, supporting that reducing inflammation per se could reduce vascular risk.4 Of note, a CRP concentration <2 mg/dL after the first dose of cankinumab was associated with greater relative reduction in MACE risk.47 Canakinumab's reduction in atherothrombotic events involves inhibition of interleukin‐6, indicating that treatments targeting downstream from interleukin‐1β merit evaluation for cardiovascular benefits.48 However, whether the cardiovascular benefits of canakinumab will translate to other targeted anti‐inflammatory treatments that reduce CRP remains to be determined. If confirmed, whether these benefits relate to baseline, achieved, or reduction of CRP concentrations also requires investigation.
Limitations
Our study has several limitations. First, our analysis was based on trial‐level data rather than patient‐level data. Metaregression analyses might be subject to risk of aggregation bias because they attempt to make inferences about individuals using study‐level information.49 Second, a number of LDL‐C–lowering cardiovascular trials did not report CRP data (especially achieved CRP concentrations), which might contribute to the publication bias detected in several analyses. The inclusion of these trials, if CRP data are reported, might erase the publication bias and considerably improve the statistical power and improve strength of evidence of our analysis. Third, considerable heterogeneity was detected in several analyses, which may be attributed to the differences in patient characteristics not evaluated in our study given that no characteristics tested appeared to affect the results. Fourth, the inclusion criteria in these trials varied; these differences in selection will play out in the baseline risk and the magnitude of absolute risk reduction achieved. Fifth, the definitions of some outcomes, such as MACE and myocardial infarction, were not completely consistent across trials, and a considerable part of trials did not report outcome definition; it is unclear whether this variation could affect our results. Finally, the study enrollment included in the analysis extended from 1995 to 2018, during which background therapy and cardiovascular event rates have changed.
Conclusions
In this metaregression and meta‐analysis, more‐intensive LDL‐C lowering might have reduced the risk of myocardial infarction to a lesser extent when baseline CRP levels were higher, but was associated with similar risk reduction for mortality and other cardiovascular outcomes across baseline CRP concentrations. Similar risk reductions occurred for more‐ versus less‐intensive LDL‐C–lowering therapy regardless of the magnitude of CRP reduction or the achieved CRP level for all outcomes.
Sources of Funding
This study was supported by the National Natural Science Foundation of China (No. 81600312) and Fund for Distinguished Young Scholars of Nanjing (JQX15002). The funders had no role in the study design, data collection and analysis, writing of the report, and decision to submit the article for publication.
Disclosures
None.
Supporting information
Data S1. Supplemental methods.
Table S1. Study and Patient Baseline Characteristics
Table S2. Study Characteristics of the Included Randomized Trials
Table S3. Inclusion and Exclusion criteria of Included Randomized Controlled Trials
Table S4. Listing of Potential Sources of Bias
Table S5. Meta‐Analysis Excluding Trials With Potential Bias
Table S6. Sensitivity Analysis Stratified for Agent Used in the More‐Intensive Treatment Group
Table S7. Sensitivity Analysis Stratified for the Type of Treatment in the Less‐Intensive Group
Table S8. Sensitivity Analysis Stratified for the Type of Population
Table S9. Multivariable Metaregression Models for the Association of Each 1‐mg/L Reduction in log(baseline CRP Concentration), Magnitude of Reduction in CRP Concentration, and Mortality and Cardiovascular Outcomes in Statin Trials
Table S10. Multivariable Metaregression Models for the Association of Each 1‐mg/L Reduction in log(baseline CRP Concentration), Magnitude of Reduction in CRP Concentration, and Mortality and Cardiovascular Outcomes in Secondary Prevention Trials*
Table S11. Multivariable Metaregression Models for the Association of Each 1‐mg/L Reduction in log(baseline CRP Concentration), Magnitude of Reduction in CRP Concentration, and Mortality and Cardiovascular Outcomes in Primary Prevention Trials*
Figure S1. Identification and selection of randomized clinical trials evaluating the effect of low‐density lipoprotein cholesterol–lowering therapy on cardiovascular outcomes.
Figure S2. Publication bias. (A) All‐cause mortality; (B) cardiovascular mortality; (C) myocardial infarction; (D) stroke; (E) coronary revascularization; and (F) MACE.
Figure S3. Metaregression analysis of all‐cause mortality rate ratio plotted against magnitude of reduction in CRP concentrations between more‐ and less‐intensive lipid‐lowering group.
Figure S4. Meta‐analysis of all‐cause mortality stratified by magnitude of reduction in CRP concentrations between more‐ and less‐intensive lipid‐lowering group.
Figure S5. Metaregression analysis of cardiovascular mortality rate ratio plotted against magnitude of reduction in CRP concentrations between more‐ and less‐intensive lipid‐lowering group.
Figure S6. Meta‐analysis of cardiovascular mortality stratified by magnitude of reduction in CRP concentrations between more‐ and less‐intensive lipid‐lowering group.
Figure S7. Metaregression analysis of myocardial infarction rate ratio plotted against magnitude of reduction in CRP concentrations between more‐ and less‐intensive lipid‐lowering group.
Figure S8. Meta‐analysis of myocardial infarction stratified by magnitude of reduction in CRP concentrations between more‐ and less‐intensive lipid‐lowering group.
Figure S9. Metaregression analysis of stroke rate ratio plotted against log(baseline CRP concentrations) in the more‐intensive group.
Figure S10. Metaregression analysis of stroke‐rate ratio plotted against magnitude of reduction in CRP concentrations between more‐ and less‐intensive lipid‐lowering group.
Figure S11. Meta‐analysis of stroke stratified by baseline CRP concentrations.
Figure S12. Meta‐analysis of stroke stratified by magnitude of reduction in CRP concentrations between more‐ and less‐intensive lipid‐lowering group.
Figure S13. Metaregression analysis of coronary revascularization‐rate ratio plotted against log(baseline CRP concentrations) in the more‐intensive group.
Figure S14. Metaregression analysis of coronary revascularization‐rate ratio plotted against magnitude of reduction in CRP concentrations between more‐ and less‐intensive lipid‐lowering group.
Figure S15. Meta‐analysis of coronary revascularization stratified by baseline CRP concentrations.
Figure S16. Meta‐analysis of coronary revascularization stratified by magnitude of reduction in CRP concentrations between more‐ and less‐intensive lipid‐lowering group.
Figure S17. Metaregression analysis of mace rate ratio plotted against log(baseline CRP concentrations) in the more‐intensive group.
Figure S18. Metaregression analysis of MACE‐rate ratio plotted against magnitude of reduction in CRP concentrations between more‐ and less‐intensive lipid‐lowering group.
Figure S19. Meta‐analysis of MACE stratified by baseline CRP concentrations.
Figure S20. Meta‐analysis of MACE stratified by magnitude of reduction in CRP concentrations between more‐ and less‐intensive lipid‐lowering group.
Figure S21. Metaregression analysis of myocardial‐infarction–rate ratio plotted against log(baseline CRP concentrations) in the secondary prevention trials.
Figure S22. Meta‐analysis of all‐cause mortality stratified by the achieved CRP concentrations.
Figure S23. Meta‐analysis of cardiovascular mortality stratified by the achieved CRP concentrations.
Figure S24. Meta‐analysis of myocardial infarction stratified by the achieved CRP concentrations.
Figure S25. Meta‐analysis of stroke stratified by the achieved CRP concentrations.
Figure S26. Meta‐analysis of coronary revascularization stratified by the achieved CRP concentrations.
Figure S27. Meta‐analysis of MACE stratified by the achieved CRP concentrations.
(J Am Heart Assoc. 2019;8:e012428 DOI: 10.1161/JAHA.119.012428.)
Contributor Information
Wei Xu, Email: 13390900868@163.com.
Biao Xu, Email: xubiao62@nju.edu.cn.
References
- 1. Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta‐analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;18:1713–1722. [DOI] [PubMed] [Google Scholar]
- 3. Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, De Ferrari GM, Ruzyllo W, De Lucca P, Im K, Bohula EA, Reist C, Wiviott SD, Tershakovec AM, Musliner TA, Braunwald E, Califf RM. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397. [DOI] [PubMed] [Google Scholar]
- 4. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein J, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida‐Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi P, Troquay R, Libby P, Glynn RJ. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 5. Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, Danesh J. C‐reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta‐analysis. Lancet. 2010;375:132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jialal I, Stein D, Balis D, Grundy SM, Adams‐Huet B, Devaraj S. Effect of hydroxymethyl glutaryl coenzyme a reductase inhibitor therapy on high sensitive C‐reactive protein levels. Circulation. 2001;103:1933–1935. [DOI] [PubMed] [Google Scholar]
- 7. Ridker PM, Rifai N, Clearfield M, Downs JR, Weis SE, Miles JS, Gotto AJ. Measurement of C‐reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med. 2001;344:1959–1965. [DOI] [PubMed] [Google Scholar]
- 8. Sattar N, Murray HM, McConnachie A, Blauw GJ, Bollen EL, Buckley BM, Cobbe SM, Ford I, Gaw A, Hyland M, Jukema JW, Kamper AM, Macfarlane PW, Murphy MB, Packard CJ, Perry IJ, Stott DJ, Sweeney BJ, Twomey C, Westendorp RG, Shepherd J. C‐reactive protein and prediction of coronary heart disease and global vascular events in the Prospective Study of Pravastatin in the Elderly at Risk (PROSPER). Circulation. 2007;115:981–989. [DOI] [PubMed] [Google Scholar]
- 9. Jonathan E, Derrick B, Emma L, Sarah P, John D, Jane A, Rory C. C‐reactive protein concentration and the vascular benefits of statin therapy: an analysis of 20,536 patients in the Heart Protection Study. Lancet. 2011;377:469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Higgins JPT, Altman DG, Sterne JAC. Assessing risk of bias in included studies. In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration; 2011. Accessed at: http://handbook ‐5‐1.cochrane.org/.
- 11. Wanner C, Krane V, Marz W, Olschewski M, Mann JF, Ruf G, Ritz E. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238–248. [DOI] [PubMed] [Google Scholar]
- 12. de Lemos JA, Blazing MA, Wiviott SD, Lewis EF, Fox KA, White HD, Rouleau JL, Pedersen TR, Gardner LH, Mukherjee R, Ramsey KE, Palmisano J, Bilheimer DW, Pfeffer MA, Califf RM, Braunwald E. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307–1316. [DOI] [PubMed] [Google Scholar]
- 13. Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, Beere PA, Langendorfer A, Stein EA, Kruyer W, Gotto AJ. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279:1615–1622. [DOI] [PubMed] [Google Scholar]
- 14. Holdaas H, Fellström B, Jardine AG, Holme I, Nyberg G, Fauchald P, Grönhagen‐Riska C, Madsen S, Neumayer HH, Cole E, Maes B, Ambühl P, Olsson AG, Hartmann A, Solbu DO, Pedersen TR. Effect of fluvastatin on cardiac outcomes in renal transplant recipients: a multicentre, randomised, placebo‐controlled trial. Lancet. 2003;361:2024–2031. [DOI] [PubMed] [Google Scholar]
- 15. Sever PS, Dahlöf B, Poulter NR, Wedel H, Beevers G, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O'Brien E, Ostergren J. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower‐than‐average cholesterol concentrations, in the Anglo‐Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm (ASCOT‐LLA): a multicentre randomised controlled trial. Lancet. 2003;361:1149–1158. [DOI] [PubMed] [Google Scholar]
- 16. Fellström BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J, Chae DW, Chevaile A, Cobbe SM, Grönhagen‐Riska C, De Lima JJ, Lins R, Mayer G, McMahon AW, Parving HH, Remuzzi G, Samuelsson O, Sonkodi S, Sci D, Süleymanlar G, Tsakiris D, Tesar V, Todorov V, Wiecek A, Wüthrich RP, Gottlow M, Johnsson E, Zannad F. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360:1395–1407. [DOI] [PubMed] [Google Scholar]
- 17. Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, Thomason MJ, Mackness MI, Charlton‐Menys V, Fuller JH. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo‐controlled trial. Lancet. 2004;364:685–696. [DOI] [PubMed] [Google Scholar]
- 18. Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, Brown L, Warnica JW, Arnold JM, Wun CC, Davis BR, Braunwald E. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335:1001–1009. [DOI] [PubMed] [Google Scholar]
- 19. Kjekshus J, Apetrei E, Barrios V, Böhm M, Cleland JG, Cornel JH, Dunselman P, Fonseca C, Goudev A, Grande P, Gullestad L, Hjalmarson A, Hradec J, Jánosi A, Kamenský G, Komajda M, Korewicki J, Kuusi T, Mach F, Mareev V, McMurray JJ, Ranjith N, Schaufelberger M, Vanhaecke J, van Veldhuisen DJ, Waagstein F, Wedel H, Wikstrand J. Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357:2248–2261. [DOI] [PubMed] [Google Scholar]
- 20. Hagiwara N, Kawada‐Watanabe E, Koyanagi R, Arashi H, Yamaguchi J, Nakao K, Tobaru T, Tanaka H, Oka T, Endoh Y, Saito K, Uchida T, Matsui K, Ogawa H. Low‐density lipoprotein cholesterol targeting with pitavastatin + ezetimibe for patients with acute coronary syndrome and dyslipidaemia: the HIJ‐PROPER study, a prospective, open‐label, randomized trial. Eur Heart J. 2017;38:2264–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yusuf S, Bosch J, Dagenais G, Zhu J, Xavier D, Liu L, Pais P, López‐Jaramillo P, Leiter LA, Dans A, Avezum A, Piegas LS, Parkhomenko A, Keltai K, Keltai M, Sliwa K, Peters RJ, Held C, Chazova I, Yusoff K, Lewis BS, Jansky P, Khunti K, Toff WD, Reid CM, Varigos J, Sanchez‐Vallejo G, McKelvie R, Pogue J, Jung H, Gao P, Diaz R, Lonn E. Cholesterol lowering in intermediate‐risk persons without cardiovascular disease. N Engl J Med. 2016;374:2021–2031. [DOI] [PubMed] [Google Scholar]
- 22. Heart Protection Study Collaborative Group . MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high‐risk individuals: a randomised placebo‐controlled trial. Lancet. 2002;360:7–22.12114036 [Google Scholar]
- 23. Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AJ, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Rosuvastatin to prevent vascular events in men and women with elevated C‐reactive protein. N Engl J Med. 2008;359:2195–2207. [DOI] [PubMed] [Google Scholar]
- 24. Long‐Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group . Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339:1349–1357. [DOI] [PubMed] [Google Scholar]
- 25. Liu Z, Joerg H, Hao H, Xu J, Hu S, Li B, Sang C, Xia J, Chu Y, Xu D. Efficacy of high‐intensity atorvastatin for Asian patients undergoing percutaneous coronary intervention. Ann Pharmacother. 2016;50:725–733. [DOI] [PubMed] [Google Scholar]
- 26. Asselbergs FW, Diercks GF, Hillege HL, van Boven AJ, Janssen WM, Voors AA, de Zeeuw D, de Jong PE, van Veldhuisen DJ, van Gilst WH. Effects of fosinopril and pravastatin on cardiovascular events in subjects with microalbuminuria. Circulation. 2004;110:2809–2816. [DOI] [PubMed] [Google Scholar]
- 27. Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Buckley BM, Cobbe SM, Ford I, Gaw A, Hyland M, Jukema JW, Kamper AM, Macfarlane PW, Meinders AE, Norrie J, Packard CJ, Perry IJ, Stott DJ, Sweeney BJ, Twomey C, Westendorp RG. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623–1630. [DOI] [PubMed] [Google Scholar]
- 28. Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, Joyal SV, Hill KA, Pfeffer MA, Skene AM. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504. [DOI] [PubMed] [Google Scholar]
- 29. Taguchi I, Iimuro S, Iwata H, Takashima H, Abe M, Amiya E, Ogawa T, Ozaki Y, Sakuma I, Nakagawa Y, Hibi K, Hiro T, Fukumoto Y, Hokimoto S, Miyauchi K, Yamazaki T, Ito H, Otsuji Y, Kimura K, Takahashi J, Hirayama A, Yokoi H, Kitagawa K, Urabe T, Okada Y, Terayama Y, Toyoda K, Nagao T, Matsumoto M, Ohashi Y, Kaneko T, Fujita R, Ohtsu H, Ogawa H, Daida H, Shimokawa H, Saito Y, Kimura T, Inoue T, Matsuzaki M, Nagai R. High‐dose versus low‐dose pitavastatin in japanese patients with stable coronary artery disease (REAL‐CAD): a randomized superiority trial. Circulation. 2018;137:1997–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rossebo AB, Pedersen TR, Boman K, Brudi P, Chambers JB, Egstrup K, Gerdts E, Gohlke‐Barwolf C, Holme I, Kesaniemi YA, Malbecq W, Nienaber CA, Ray S, Skjaerpe T, Wachtell K, Willenheimer R. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359:1343–1356. [DOI] [PubMed] [Google Scholar]
- 31. Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, Wanner C, Krane V, Cass A, Craig J, Neal B, Jiang L, Hooi LS, Levin A, Agodoa L, Gaziano M, Kasiske B, Walker R, Massy ZA, Feldt‐Rasmussen B, Krairittichai U, Ophascharoensuk V, Fellstrom B, Holdaas H, Tesar V, Wiecek A, Grobbee D, de Zeeuw D, Gronhagen‐Riska C, Dasgupta T, Lewis D, Herrington W, Mafham M, Majoni W, Wallendszus K, Grimm R, Pedersen T, Tobert J, Armitage J, Baxter A, Bray C, Chen Y, Chen Z, Hill M, Knott C, Parish S, Simpson D, Sleight P, Young A, Collins R. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo‐controlled trial. Lancet. 2011;377:2181–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart JC, Gotto AM, Greten H, Kastelein JJ, Shepherd J, Wenger NK. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–1435. [DOI] [PubMed] [Google Scholar]
- 33. Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, McKillop JH, Packard CJ. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333:1301–1307. [DOI] [PubMed] [Google Scholar]
- 34. Lane T, Wassef N, Poole S, Mistry Y, Lachmann HJ, Gillmore JD, Hawkins PN, Pepys MB. Infusion of pharmaceutical‐grade natural human C‐reactive protein is not proinflammatory in healthy adult human volunteers. Circ Res. 2014;114:672–676. [DOI] [PubMed] [Google Scholar]
- 35. Kaul S, Morrissey RP, Diamond GA. By Jove! What is a clinician to make of JUPITER? Arch Intern Med. 2010;170:1073–1077. [DOI] [PubMed] [Google Scholar]
- 36. Blaha MJ, Nasir K, Budoff MJ, Dardari ZA, Blumenthal RS, Pollack S, Reichek N, Guerci AD. Impact of C‐reactive protein and coronary artery calcium on benefit observed with atorvastatin. J Am Coll Cardiol. 2018;71:2487–2488. [DOI] [PubMed] [Google Scholar]
- 37. Braunwald E. Creating controversy where none exists: the important role of C‐reactive protein in the CARE, AFCAPS/TexCAPS, PROVE IT, REVERSAL, A to Z, JUPITER, HEART PROTECTION, and ASCOT trials. Eur Heart J. 2012;33:430–432. [DOI] [PubMed] [Google Scholar]
- 38. Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, Pfeffer MA, Braunwald E. C‐reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–28. [DOI] [PubMed] [Google Scholar]
- 39. Nissen SE, Tuzcu EM, Schoenhagen P, Crowe T, Sasiela WJ, Tsai J, Orazem J, Magorien RD, O'Shaughnessy C, Ganz P. Statin therapy, LDL cholesterol, C‐reactive protein, and coronary artery disease. N Engl J Med. 2005;352:29–38. [DOI] [PubMed] [Google Scholar]
- 40. Morrow DA, de Lemos JA, Sabatine MS, Wiviott SD, Blazing MA, Shui A, Rifai N, Califf RM, Braunwald E. Clinical relevance of C‐reactive protein during follow‐up of patients with acute coronary syndromes in the Aggrastat‐to‐Zocor Trial. Circulation. 2006;114:281–288. [DOI] [PubMed] [Google Scholar]
- 41. Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AJ, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, Macfadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Reduction in C‐reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet. 2009;373:1175–1182. [DOI] [PubMed] [Google Scholar]
- 42. Sever PS, Poulter NR, Chang CL, Thom SA, Hughes AD, Welsh P, Sattar N. Evaluation of C‐reactive protein before and on‐treatment as a predictor of benefit of atorvastatin: a cohort analysis from the Anglo‐Scandinavian Cardiac Outcomes Trial lipid‐lowering arm. J Am Coll Cardiol. 2013;62:717–729. [DOI] [PubMed] [Google Scholar]
- 43. Soedamah‐Muthu SS, Livingstone SJ, Charlton‐Menys V, Betteridge DJ, Hitman GA, Neil HA, Bao W, DeMicco DA, Preston GM, Fuller JH, Stehouwer CD, Schalkwijk CG, Durrington PN, Colhoun HM. Effect of atorvastatin on C‐reactive protein and benefits for cardiovascular disease in patients with type 2 diabetes: analyses from the Collaborative Atorvastatin Diabetes Trial. Diabetologia. 2015;58:1494–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Arsenault BJ, Barter P, DeMicco DA, Bao W, Preston GM, LaRosa JC, Grundy SM, Deedwania P, Greten H, Wenger NK, Shepherd J, Waters DD, Kastelein JJ. Prediction of cardiovascular events in statin‐treated stable coronary patients of the treating to new targets randomized controlled trial by lipid and non‐lipid biomarkers. PLoS One. 2014;9:e114519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Navarese EP, Robinson JG, Kowalewski M, Kolodziejczak M, Andreotti F, Bliden K, Tantry U, Kubica J, Raggi P, Gurbel PA. Association between baseline LDL‐C level and total and cardiovascular mortality after LDL‐C lowering: a systematic review and meta‐analysis. JAMA. 2018;319:1566–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Silverman MG, Ference BA, Im K, Wiviott SD, Giugliano RP, Grundy SM, Braunwald E, Sabatine MS. Association between lowering LDL‐C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta‐analysis. JAMA. 2016;316:1289–1297. [DOI] [PubMed] [Google Scholar]
- 47. Ridker PM, MacFadyen JG, Everett BM, Libby P, Thuren T, Glynn RJ. Relationship of C‐reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet. 2018;391:319–328. [DOI] [PubMed] [Google Scholar]
- 48. Ridker PM, Libby P, MacFadyen JG, Thuren T, Ballantyne C, Fonseca F, Koenig W, Shimokawa H, Everett BM, Glynn RJ. Modulation of the interleukin‐6 signalling pathway and incidence rates of atherosclerotic events and all‐cause mortality: analyses from the Canakinumab Anti‐Inflammatory Thrombosis Outcomes Study (CANTOS). Eur Heart J. 2018;39:3499–3507. [DOI] [PubMed] [Google Scholar]
- 49. Thompson SG, Higgins JP. How should meta‐regression analyses be undertaken and interpreted? Stat Med. 2002;21:1559–1573. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplemental methods.
Table S1. Study and Patient Baseline Characteristics
Table S2. Study Characteristics of the Included Randomized Trials
Table S3. Inclusion and Exclusion criteria of Included Randomized Controlled Trials
Table S4. Listing of Potential Sources of Bias
Table S5. Meta‐Analysis Excluding Trials With Potential Bias
Table S6. Sensitivity Analysis Stratified for Agent Used in the More‐Intensive Treatment Group
Table S7. Sensitivity Analysis Stratified for the Type of Treatment in the Less‐Intensive Group
Table S8. Sensitivity Analysis Stratified for the Type of Population
Table S9. Multivariable Metaregression Models for the Association of Each 1‐mg/L Reduction in log(baseline CRP Concentration), Magnitude of Reduction in CRP Concentration, and Mortality and Cardiovascular Outcomes in Statin Trials
Table S10. Multivariable Metaregression Models for the Association of Each 1‐mg/L Reduction in log(baseline CRP Concentration), Magnitude of Reduction in CRP Concentration, and Mortality and Cardiovascular Outcomes in Secondary Prevention Trials*
Table S11. Multivariable Metaregression Models for the Association of Each 1‐mg/L Reduction in log(baseline CRP Concentration), Magnitude of Reduction in CRP Concentration, and Mortality and Cardiovascular Outcomes in Primary Prevention Trials*
Figure S1. Identification and selection of randomized clinical trials evaluating the effect of low‐density lipoprotein cholesterol–lowering therapy on cardiovascular outcomes.
Figure S2. Publication bias. (A) All‐cause mortality; (B) cardiovascular mortality; (C) myocardial infarction; (D) stroke; (E) coronary revascularization; and (F) MACE.
Figure S3. Metaregression analysis of all‐cause mortality rate ratio plotted against magnitude of reduction in CRP concentrations between more‐ and less‐intensive lipid‐lowering group.
Figure S4. Meta‐analysis of all‐cause mortality stratified by magnitude of reduction in CRP concentrations between more‐ and less‐intensive lipid‐lowering group.
Figure S5. Metaregression analysis of cardiovascular mortality rate ratio plotted against magnitude of reduction in CRP concentrations between more‐ and less‐intensive lipid‐lowering group.
Figure S6. Meta‐analysis of cardiovascular mortality stratified by magnitude of reduction in CRP concentrations between more‐ and less‐intensive lipid‐lowering group.
Figure S7. Metaregression analysis of myocardial infarction rate ratio plotted against magnitude of reduction in CRP concentrations between more‐ and less‐intensive lipid‐lowering group.
Figure S8. Meta‐analysis of myocardial infarction stratified by magnitude of reduction in CRP concentrations between more‐ and less‐intensive lipid‐lowering group.
Figure S9. Metaregression analysis of stroke rate ratio plotted against log(baseline CRP concentrations) in the more‐intensive group.
Figure S10. Metaregression analysis of stroke‐rate ratio plotted against magnitude of reduction in CRP concentrations between more‐ and less‐intensive lipid‐lowering group.
Figure S11. Meta‐analysis of stroke stratified by baseline CRP concentrations.
Figure S12. Meta‐analysis of stroke stratified by magnitude of reduction in CRP concentrations between more‐ and less‐intensive lipid‐lowering group.
Figure S13. Metaregression analysis of coronary revascularization‐rate ratio plotted against log(baseline CRP concentrations) in the more‐intensive group.
Figure S14. Metaregression analysis of coronary revascularization‐rate ratio plotted against magnitude of reduction in CRP concentrations between more‐ and less‐intensive lipid‐lowering group.
Figure S15. Meta‐analysis of coronary revascularization stratified by baseline CRP concentrations.
Figure S16. Meta‐analysis of coronary revascularization stratified by magnitude of reduction in CRP concentrations between more‐ and less‐intensive lipid‐lowering group.
Figure S17. Metaregression analysis of mace rate ratio plotted against log(baseline CRP concentrations) in the more‐intensive group.
Figure S18. Metaregression analysis of MACE‐rate ratio plotted against magnitude of reduction in CRP concentrations between more‐ and less‐intensive lipid‐lowering group.
Figure S19. Meta‐analysis of MACE stratified by baseline CRP concentrations.
Figure S20. Meta‐analysis of MACE stratified by magnitude of reduction in CRP concentrations between more‐ and less‐intensive lipid‐lowering group.
Figure S21. Metaregression analysis of myocardial‐infarction–rate ratio plotted against log(baseline CRP concentrations) in the secondary prevention trials.
Figure S22. Meta‐analysis of all‐cause mortality stratified by the achieved CRP concentrations.
Figure S23. Meta‐analysis of cardiovascular mortality stratified by the achieved CRP concentrations.
Figure S24. Meta‐analysis of myocardial infarction stratified by the achieved CRP concentrations.
Figure S25. Meta‐analysis of stroke stratified by the achieved CRP concentrations.
Figure S26. Meta‐analysis of coronary revascularization stratified by the achieved CRP concentrations.
Figure S27. Meta‐analysis of MACE stratified by the achieved CRP concentrations.


