Abstract
Background:
Substance use disorders (SUDs) and psychiatric disorders are common among people with HIV (PWH) and lead to poor outcomes. Yet these conditions often go unrecognized and untreated in primary care.
Methods:
The Promoting Access to Care Engagement (PACE) trial currently in process examines the impact of self-administered electronic screening for SUD risk, depression and anxiety in three large Kaiser Permanente Northern California primary care clinics serving over 5,000 PWH. Screening uses validated measures (Tobacco, Alcohol, Prescription medication, and other Substance use [TAPS]; and the Adult Outcomes Questionnaire [AOQ], which includes the Patient Health Questionnaire [PHQ-9] and Generalized Anxiety Disorder [GAD-2]) delivered via three modalities (secure messaging, tablets in waiting rooms, and desktop computers in exam rooms). Results are integrated automatically into the electronic health record. Based on screening results and physician referrals, behavioral health specialists embedded in primary care initiate motivational interviewing- and cognitive behavioral therapy-based brief treatment and link patients to addiction and psychiatry clinics as needed. Analyses examine implementation (screening and treatment rates) and effectiveness (SUD, depression and anxiety symptoms; HIV viral control) outcomes using a stepped-wedge design, with a 12-month intervention phase implemented sequentially in the clinics, and a 24-month usual care period prior to implementation in each clinic functioning as sequential observational phases for comparison. We also evaluate screening and treatment costs and implementation barriers and facilitators.
Discussion:
The study examines innovative, technology-facilitated strategies for improving assessment and treatment in primary care. Results may help to inform substance use, mental health, and HIV services.
Keywords: Drug use, opioid, cannabis, alcohol, depression, anxiety, suicidal ideation, HIV, primary care, motivational interviewing, cognitive behavioral therapy, patient portal
1. Introduction
Substance use disorders, depression, and anxiety are common among people with HIV (PWH) and add significant complexity to clinical care, resulting in poor HIV outcomes and higher mortality [1, 2]. Evidence-based substance use disorder (SUD), depression and anxiety interventions have been developed yet not effectively implemented in primary care settings serving PWH and other patient populations. For example, significant barriers to effective implementation including under-identification, limited intervention expertise, poor treatment fidelity, and clinic time constraints have been well documented [3–5].
SUDs have serious consequences for PWH, including poor antiretroviral therapy (ART) adherence [6–8], limiting HIV RNA control [9–11], and contributing to increased HIV transmission risk. However, substance use is often underreported to providers [12, 13], and even when recognized, providers often fail to advise cutting back or provide other interventions [14–16]. Yet when patients reduce use, HIV and mental health outcomes improve [17–19].
PWH also have a high burden of psychiatric problems, particularly depression [20–22], with prevalence ranging from 36% in a national sample of HIV-positive adults [23] to 53% in a primary care sample [24]. Anxiety among PWH also is very common but has been under-investigated relative to depression [25, 26]. As with SUDs, psychiatric disorders contribute to numerous medical comorbidities, poor ART adherence, faster HIV disease progression, higher cost of care, and higher mortality [27–30].
SUD and mental health screening in primary care is a key challenge for health care systems, and this difficulty impacts many other patient populations apart from PWH. For example, interviewer-administered surveys often lose fidelity due to wording changes when administered by clinical staff [31, 32]. Other screening barriers include insufficient staff training, reluctance to discuss sensitive topics, and limited provider time in the context of busy primary care clinics [32, 33]. If properly implemented, computerized self-administered screening can address these challenges, is highly acceptable to patients [34–40] and is cost effective.
In addition to screening, primary care-based intervention delivery and/or referral to specialty addiction and mental health treatment is necessary to improve care. Behavioral health specialists (BHSs) are trained to identify, triage and manage patients with behavioral health problems, including depression and substance use problems, but are underutilized [41–43]. BHSs can effectively deliver behavioral interventions such as motivational interviewing (MI) for SUDs and brief cognitive behavioral therapy (CBT) for depression and anxiety, and link patients to specialty SUD and mental health treatment as needed. Thus, BHSs can enhance care teams and help overcome behavioral health care implementation challenges that many clinics face.
The Promoting Access to Care Engagement (PACE) trial currently in process examines a novel intervention that combines routine electronic screening for SUD and mental health delivered to PWH every six months in 3 HIV primary care clinics in Kaiser Permanente Northern California (KPNC). Results are viewed by clinic staff including a BHS to deliver behavioral treatment. The study examines both implementation outcomes (e.g., screening and treatment rates), and effectiveness (e.g., substance use, mental health symptoms, and HIV viral control), to inform the field regarding strategies for primary care-based behavioral health treatment integration.
2. Methods
2.1. Specific aims.
Aim 1.
To evaluate the implementation of computerized SUD, depression and anxiety screening and BHS-delivered intervention in HIV primary care.
Aim 2.
To examine the effectiveness of computerized screening and BHS-delivered intervention in HIV primary care among patients with moderate or high SUD risk, depression and anxiety severity.
Aim 3.
To determine implementation costs and cost-effectiveness of screening and BHS-delivered intervention.
Aim 4.
Perform key informant interviews to evaluate provider- and clinic-level implementation barriers and facilitators to computerized screening and BHS-delivered intervention.
2.2. Study design overview
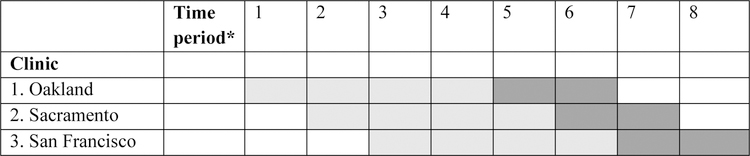
This hybrid design Type 2 intervention study [44] evaluates the implementation, effectiveness and cost of electronic screening and treatment for SUDs, depression and anxiety among PWH. The study is based in KPNC primary care clinics in Oakland, Sacramento and San Francisco that have an HIV care focus, and collectively serve over 5,000 PWH. The study team tracks scheduled appointments of PWH in these three clinics using the KPNC HIV registry and asks patients to complete an electronic screening instrument prior to their routine visits via secure messaging through the patient portal (www.kp.org) into the EHR. If screening is not completed in advance, patients are asked to complete it on a tablet in the waiting room or on a desktop computer in the exam room. The results are directly incorporated into the EHR and viewable by clinical staff including physicians and BHSs. Physicians review screening results and engage BHS support as needed, with screening results also informing BHS treatment sessions. The study employs a stepped wedge design [45], with implementation and effectiveness outcomes in the 12-month intervention phase compared with outcomes in the 24-month observational phase (i.e., usual care). Rollout of the study intervention (computerized screening + BHS-delivered treatment) occurs sequentially at the 3 clinics, allowing for refinement of procedures and accommodating implementation challenges at each site. The observational periods are also sequential (Figure 1). Study procedures were approved by the University of California, San Francisco and KPNC Institutional Review Boards.
Figure 1.
Stepped-wedge design to evaluate outcomes in the PACE Trial.
Notes: *Each time period represents six months. Observational period cells are light gray. Intervention period cells are dark gray. Rollout of the study intervention (computerized screening + BHS-delivered treatment) occurs sequentially at the 3 HIV primary care clinics, starting with Oakland. The observational periods are also sequential.
2.3. Study setting
KPNC is an integrated healthcare delivery system serving >4.3 million members at 21 hospital-based Medical Centers and >200 medical offices. This system provides comprehensive outpatient and inpatient care, including pharmacy and laboratory services. The study sites were selected because of the size of their HIV-positive patient populations, complementary demographics, and willingness of clinic directors to participate (Table 1).
Table 1.
Characteristics of HIV patients in Kaiser Permanente Northern California clinics participating in the PACE Trial.
| Oakland | Sacramento | San Francisco | |
|---|---|---|---|
| Patients, n | 1,275 | 850 | 2,937 |
| Men (%) | 85 | 90 | 97 |
| Mean age in years | 51 | 50 | 53 |
| Race/ethnicity (%) | |||
| White | 36 | 57 | 57 |
| African-American | 35 | 15 | 9 |
| Hispanic | 14 | 13 | 16 |
| Asian | 6 | 5 | 8 |
| Other | 2 | 2 | 3 |
| Unknown | 7 | 8 | 7 |
| HIV risk (%) | |||
| Heterosexual | 20 | 16 | 4 |
| Injection drug use | 5 | 7 | 7 |
| Men who have sex with men | 67 | 67 | 76 |
| Other | 1 | 2 | <1 |
| Unknown | 8 | 8 | 12 |
| Prior AIDS diagnosis (%) | 52 | 56 | 59 |
| Receiving ART (%) | 94 | 92 | 94 |
| Mean CD4, cells/µl | 701 | 735 | 682 |
| HIV RNA<75 copies/ml, (%) | 87 | 86 | 87 |
Notes: Data are based on electronic health records as of 12/31/18. PACE = Promoting Access to Care Engagement. ART = antiretroviral therapy medication.
2.4. Conceptual model for evaluating implementation
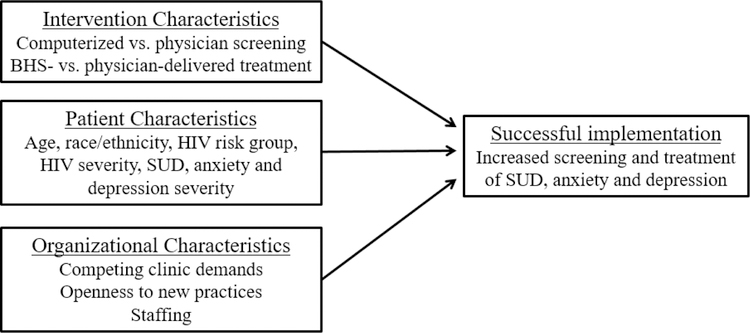
Prior studies indicate that key facilitators of successful primary care implementation include having a flexible treatment protocol, focusing on targeted problems and solutions that work within the time constraints of primary care, and being patient-centered [46, 47]. These features are consistent with the Practical, Robust Implementation and Sustainability Model (PRISM) [48], which holds that implementation of interventions in medical care settings is influenced by intervention, patient, and organizational characteristics. (Figure 2).
Figure 2.
Conceptual model of factors associated with successful intervention implementation in health care settings.
Notes: Conceptual model is based on the Practical, Robust Implementation and Sustainability Model (PRISM) [48]. BHS= behavioral health specialist; SUD = substance use disorder.
We anticipate that key intervention characteristics affecting intervention adoption are the mode of screening delivery and the accessibility and effectiveness of the intervention. Regarding patient characteristics, our work on automated mental health and substance use screening has found that older adults were less likely to complete a computerized intake system [49]. Among HIV patients in KPNC, patient use of the shared EHR was higher among those with medical need (i.e., recent CD4 decline or HIV RNA increase) [50]. Others have found that among PWH with psychiatric disorders, accessing psychiatric services was associated with older age, white race, and greater HIV severity [51]; and that among those with SUD, accessing treatment was associated with black race [51], lower CD4, not taking ART [52], and injection drug use [53].
Organizational characteristics also influence implementation [54, 55]. Measures of “resources for change” in primary care, including history of practice changes, structure of relationships with specialty care, and attitudes towards evidence-based treatment predict adoption of new primary care guidelines [56, 57]. We measure these factors in our model, including qualitative evaluation of competing priorities and quantitative measures of attitudes towards evidence-based practices and levels of behavioral health integration.
2.5. Computerized screening modalities
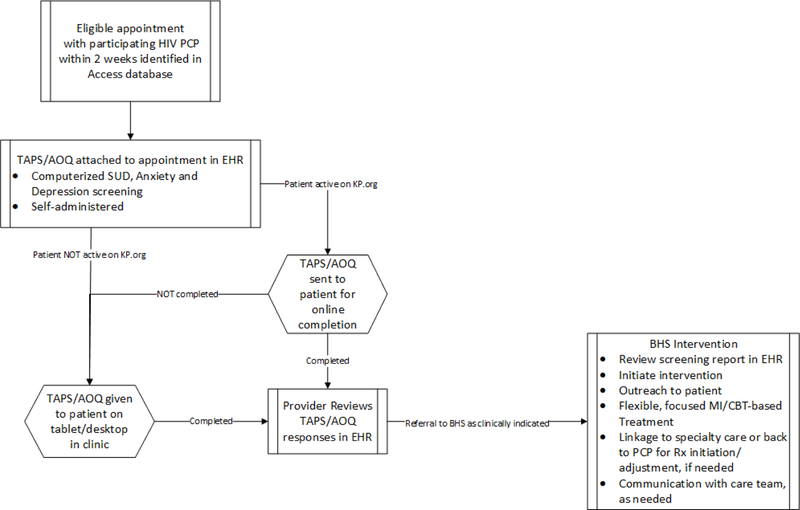
Three modalities are used to administer the screening questionnaire: 1) self-administration online via the electronic patient portal; 2) self-administration in clinic on a secure tablet provided by reception; 3) self-administration during the clinic visit via desktop computer. Our intent is to maximize online completion, with the second and third options serving as backup approaches for patients not completing the questionnaire prior to their visit. Electronic administration of questionnaires via waiting room tablets is a workflow widely used in KPNC primary care clinics. Study sites already utilizing tablets for other screening instruments are provided with additional tablets to ensure availability for PACE patients, and integrated administration of the screening instrument into existing workflows. Study sites not already using tablets are provided the necessary equipment.
Patients who have any upcoming in-person appointment with an HIV care provider are flagged to complete the questionnaire if they have not done so in the prior 6 months, and the questionnaire is attached to the appointment in the EHR. Patients who have activated their accounts in the KPNC electronic patient portal then receive a secure message via email up to two weeks prior to their visit, which includes a link to complete the questionnaire online. Patients who do not complete the questionnaire prior to their appointment have a flag in the EHR that alerts reception staff to provide them with a tablet at clinic registration. Patients unable to complete the questionnaire online or by tablet also have the opportunity to complete it via desktop computer in the exam room. Desktop administration uses EHR functionality that limits patient access to only the questionnaire. All three modalities automatically import patient responses directly into EHR and results are immediately available to clinicians (Figure 3).
Figure 3.
PACE intervention approach to computerized substance use, depression and anxiety screening and treatment in HIV primary care clinics.
Notes: TAPS/AOQ = Tobacco, Alcohol, Prescription Medication and other Substance Use/Adult Outcomes Questionnaire; PCP = primary care provider; BHS = behavioral health specialist; EHR = electronic health record. KP.org is Kaiser Permanente’s electronic patient portal.
2.6. Computerized patient self-administered questionnaire
SUD risk is measured using the Tobacco, Alcohol, Prescription medication, and other Substance use (TAPS) instrument [58, 59]. The Adult Outcomes Questionnaire (AOQ), initially developed by KPNC, includes the Patient Health Questionnaire (PHQ-9) [60] for depression and the Generalized Anxiety Disorder (GAD-2) [61] for anxiety. These measures are validated for electronic self-administration [62, 63]. In our study, these measures are combined as the TAPS/AOQ.
Prior to beginning the questionnaire, introductory text informs patients that TAPS/AOQ screening is part of research on ways to improve patient care, completion is optional, and their answers will be recorded in the EHR. Patients are directed to contact their primary care providers or KPNC Psychiatry if they experience any emotional distress when responding, and are provided a KPNC after-hours mental health phone number. Instructions indicate that in case of medical or psychiatric emergencies they should call 911 or go to their nearest hospital.
Automated TAPS/AOQ reporting is enabled via a questionnaire feature of the EHR. The generated reports, automatically displayed in a clinical progress note using a smart phrase in the EHR, include yes/no responses for self-reported use of tobacco, alcohol (4+ drinks/day for women, 5+ drinks/day for men), and 6 specific drug classes (including illicit and non-medical drug use) in the past year, follow-up questions on use in the prior 3 months, and a substance use risk level (low, medium and high) for each substance. Depression and anxiety scores are presented numerically. The intent of the report is to serve as a prompt in the EHR for medical providers to initiate a conversation and/or referral to the BHS, and a starting point for BHSs as they conduct further assessment and MI- and CBT-based treatment.
2.7. BHS-delivered intervention
BHSs are Masters-level licensed clinical social workers, marriage and family therapists or doctoral-level psychologists with experience in behavioral interventions. Currently BHSs provide care across medical centers. They often co-manage patients’ medical conditions through non-pharmacologic interventions, collaborate with primary care providers, and provide consultation in the areas of mental health, behavioral medicine, and health psychology.
BHS intervention includes patient engagement and patient-centered goal setting (e.g., noting screening results but also focusing on patients’ priorities and concerns). BHSs offer individual MI/CBT sessions (by phone and in person) according to clinical assessment and patient preference, in line with organizational capacity and department guidelines. MI and CBT can be combined to address co-occurring mental health and substance problems [64]. Intervention has no specific time limits, but MI and brief CBT can be effectively delivered in less than 6 sessions [65, 66], and we will examine BHS utilization and time demands in our analysis. BHSs use MI to enhance motivation to initiate specialty SUD or psychiatry treatment within KPNC for higher severity patients, which is available for members in facilities nearby.
2.8. Clinician collaboration and training
The study team is meeting with clinic primary care providers and staff throughout the implementation phase to assist with planning, based on our prior work on computerized screening, e.g., workflow and staff training [49]. Each site is responsible for identifying a PACE “champion” who serves as a primary liaison between the clinical site and the study team, advising on adaptations necessary for local implementation, providing introductions for study team to clinic management and staff, and functioning as the “face of the study” in the clinic. The champions participate in frequent study team meetings to give them a thorough understanding of the project. Orientation for physicians and other providers (e.g., nurse practitioners) is provided by the PACE champion for each site and consists of two one-hour sessions. These trainings include overview and rationale for the project, workflows for each screening modality, use of the new screening measure (TAPS/AOQ), and information regarding how to locate TAPS/AOQ scores in the EHR.
The team also provides BHSs training on MI and CBT-based interventions for depression, anxiety and substance use problems. Training includes 10 hours of instruction and supervised practice of brief, solution-focused, MI/CBT interventions, similar to prior trials [65, 67]. BHS training also includes new technology and workflow (e.g., accessing TAPS/AOQ screening results in the EHR), interpretation and utilization of TAPS/AOQ scores, guidelines for when to help patients initiate specialty SUD and psychiatry care (e.g., based on severity, complexity, and patient goals), and follow-up (monitoring patient engagement and following up as needed with patient and care team to ensure linkage to treatment). Monthly consultation with authors DDS and ASL helps to reinforce key clinical skills and troubleshoot system and workflow challenges.
2.9. Observational phase
The 24 months prior to implementation at each study clinic is considered the observational phase, consisting of usual care. Hazardous drinking screening has been implemented systematically using NIAAA guidelines [68], along with standardized tobacco screening; both screenings are administered verbally by certified and licensed medical assistants during the rooming process, as part of Kaiser Permanente’s essential preventive health measures. Screening for other substances is not standardized and relies on physicians’ clinical practices. A combination of appointment codes and appropriate International Classification of Diseases, (ICD) 9th and 10th edition indicate screening, e.g., a depression diagnosis code is indicative that screening took place. Drug use data are captured in a section of the medical record where providers can enter information about a patient’s social history. Treatment for SUDs includes advice from primary care providers to reduce use and services in specialty addiction treatment clinics [69]. Treatments provided for depression and anxiety are based on current best practices for medication management and psychotherapy [70–72]. Mild to moderate depression/anxiety are often addressed in primary care with medication, whereas patients with more severe symptoms are usually referred to psychiatry clinics. We track these aspects of patient care in the EHR.
2.10. Data Sources
Study data are obtained from the EHR, HIV Registry, and informant interviews. The EHR database links information using unique member identifiers from multiple data sources including demographics, membership, ambulatory, inpatient, laboratory, patient questionnaire, and prescription data. The HIV Registry maintains up-to-date lists of all HIV patients, HIV transmission risk factors, dates of known HIV infection, AIDS diagnoses, and complete HIV-related lab and pharmacy data. Based on the HIV registry, a total of 9,418 HIV patients were current KPNC members as of December 2018, including 5,062 (54% of all KPNC HIV patients) from the three study clinics (Table 1). Informant interviews by the study team include physicians, medical assistants, BHSs and patients from each facility, to be conducted following implementation.
2.11. Measures
We include relevant patient demographic factors in all analyses, including age, sex, race/ethnicity, and HIV risk group. Insurance coverage-related variables include deductible level and Medicaid versus other source of coverage [73]. Other measures for each Aim are described below. See also Table 2.
Table 2.
PACE study measures by aim.
| Domain | Study Measure | Source | Aim 1* | Aim 2* | Aim 3* | Aim 4* |
|---|---|---|---|---|---|---|
| Demographics | HIV Risk Status | EHR | X | X | X | |
| Age | X | X | ||||
| Gender | X | X | ||||
| Race | X | X | ||||
| Neighborhood Deprivation Index | X | X | ||||
| Comorbidities | Charlson Score | EHR | X | X | X | |
| VACS Index Score | X | |||||
| HIV Viral Control | CD4 Value | HIV Registry | X | X | X | |
| Viral Load Value | X | X | X | |||
| AIDS Status | X | X | X | |||
| Length of Time with HIV | X | X | ||||
| HIV Care Retention | X | X | ||||
| AIDS-related Diagnoses | X | X | ||||
| ART Medication Adherence | X | X | X | |||
| Screening | SUD Screening (e.g., TAPS/AOQ, alcohol and drug screening by providers) | TAPS/AOQ and EHR | X | X | X | |
| Depression and Anxiety Screening (e.g., AOQ, psychiatric diagnoses) | X | X | X | |||
| Screening costs | Cost per patient | KPNC General ledger | X | |||
| Primary care treatment | Outpatient Care | EHR | X | X | ||
| Utilization—Visits | X | X | X | |||
| Brief Interventions | X | X | X | |||
| BHS-Delivered Intervention | ||||||
| Specialty care treatment | Psychiatry Visits | EHR | X | X | X | |
| SUD Clinic Visits | X | X | X | |||
| Substance use and mental health symptoms | SUD Symptoms | EHR | X | X | X | |
| Depression Symptoms (e.g., Alcohol/dependence/abuse, ICD-09/10 codes) | X | X | X | |||
| Anxiety Symptoms | X | X | X | |||
| Treatment | SUD/Depression/Anxiety/Smoking Treatment; Antidepressant Medications | EHR | X | X | X | |
| Provider Factors | Evidence-Based Practice Attitude Scale | Interview | X | |||
| Clinic Factors | Integrated Practice Assessment Tool | Interview | X | |||
| Barriers & Facilitators | Qualitative Interview Data | Interview | X | |||
Notes:
Aim 1: To evaluate the implementation of computerized SUD and depression screening and BHS-delivered intervention in HIV primary care.
Aim 2: To examine the effectiveness of computerized screening and BHS-delivered intervention in HIV primary care among patients with moderate or high SUD risk or depression severity.
Aim 3: To determine implementation costs and cost-effectiveness of screening and BHS-delivered intervention.
Aim 4: Perform key informant interviews to evaluate provider- and clinic-level implementation barriers and facilitators to computerized screening and BHS-delivered intervention.
PACE = Promoting Access to Care Engagement; EHR = electronic health record; SUD = substance use disorder; KPNC = Kaiser Permanente Northern California; ART = antiretroviral therapy; VACS = Veteran’s Aging Cohort Study; BHS = behavioral health specialist; AOQ = adult outcomes questionnaire.
2.11.1. SUD, depression and anxiety screening
During the observational phase, measures include EHR-based provider-documented screening for SUDs, depression and anxiety. Intervention phase screening includes patient self-administered screening using the TAPS/AOQ instrument offered to patients every six months and recorded in the EHR.
2.11.2. BHS-delivered treatment
During the observational and intervention phases, treatment is defined as one or more in-person or phone visits with a BHS. We also measure the number of phone and in-person visits.
2.11.3. SUD, depression and anxiety treatment initiation
We analyze SUD and psychiatry treatment initiation rates using electronic administrative data; i.e., whether the patient attended at least one in-person or phone visit with a BHS and/or in SUD or psychiatry treatment.
2.11.4. Psychotropic medication prescription rates
Prescription rates are extracted from the EHR and include standard antidepressant and anxiolytic medications based on best practices guidelines [74].
2.11.5. Antiretroviral (ART) medication adherence
ART adherence is defined using the Medication Possession Ratio (MPR) [75, 76] for each ART prescribed [77]. MPR is calculated using a numerator of days’ supply dispensed from first fill to end of the interval (i.e., 12 months), and a denominator of total days between first fill to end of the interval, with values ranging from 0% to 100%. Mean refill adherence is calculated across individual ARTs dispensed in the interval [76, 78].
2.11.6. HIV RNA response
HIV RNA control is defined as undetectable HIV RNA levels below 75 copies/ml.
2.11.7. Retention in care
We use the Institute of Medicine’s encounter-based definition of retention, defined as ≥2 HIV primary care visits within a 12-month period, ≥90 days apart [79].
2.11.8. VACS Index Score
The VACS score has been shown to predict mortality [80]. It incorporates 7 routinely collected clinical variables, including age, CD4, HIV RNA, hemoglobin, fibrosis index 4 (FIB-4), Hepatitis C, and estimated glomerular filtration rate. The FIB-4 index incorporates age, platelets, and two liver function test results, including aspartate aminotransferase and alanine aminotransferase. The VACS Index 2.0, adding albumin, WBC, and BMI to version 1.0 and using continuous variables, provides improved mortality risk discrimination [81].
2.11.9. Substance use, depression and anxiety
The TAPS assesses frequency (never, less than monthly, monthly, weekly, daily or almost daily) of past-year substance use for 4 broad categories of substances (tobacco, alcohol, prescription medications used other than as prescribed, and other drugs) and provides a risk score for each specific substance used in the past 3 months: 1) tobacco, 2) alcohol, 3) opioid pain medication misuse, 4) anxiety/sleep medication misuse, 5) stimulant medication misuse, 6) cannabis, 7) illicit stimulants (cocaine, crack and methamphetamine), and 8) heroin [58]. We added items about other drug use and injection drug use. We use the PHQ-9[60] for depression and GAD-2[61] for anxiety, measured continuously.
2.11.10. Screening costs
These costs include equipment cost of the tablet computers and the labor costs for programming each screening modality (patient portal, tablet, and desktop), as well as time spent on instrument programming and the development of tracking systems, derived from the KPNC general ledger. Self-administered computerized screening entails no additional labor cost.
2.11.11. BHS training costs
Training consists of approximately 10 hours of BHS staff time and includes MI/CBT training, BHS consultation with the research team.
2.11.12. Intervention Costs
We include costs associated with clinic staff meetings and training to orient providers to the project and BHS role. BHS-delivered intervention may be done over the phone or in person. Intervention time is based on appointment codes to determine the total cost of BHS interventions with HIV-positive patients during observation versus intervention phase. The cost will be estimated by the average time spent in delivering the intervention x wage per minute of staff performing the intervention.
2.11.13. The Evidence-Based Practice Attitude Scale (EBPAS)
This is a 36-item scale developed to measure provider attitudes in the context of mental health care [57], and has strong psychometric properties [82]. The EBPAS yields an overall score and 12 subscales related to the importance of using evidence-based interventions, e.g., MI and CBT, perceived administrative support, and potential barriers to intervention delivery.
2.11.14. The Integrated Practice Assessment Tool (IPAT)
This 8-item instrument is used to assess levels of integration of behavioral health in the context of primary care, based on 6 levels of care integration [83]. The IPAT will measure the degree to which BHSs are effectively integrated into the clinics.
2.11.15. Qualitative interviews
Interviews with 15 patients, 15 providers, as well as technology partners are conducted over the phone or in person. Patients and providers are asked to share their experiences with electronic screening, the degree to which providers incorporate results into patient visits, perceptions regarding working with BHSs, barriers and facilitators to screening and treatment, benefits and challenges of the three different screening modalities, and perceptions regarding acceptability and sustainability of computerized screening and BHS intervention. Technology partners are asked about their perceptions regarding work with the study team in developing systems related to TAPS/AOQ delivery and result reporting. Interviews are recorded and transcribed.
2.11.16. Data analysis
The study’s primary aims are to examine the implementation, effectiveness and cost of computerized screening and BHS-delivered intervention. HIV, SUD, depression and anxiety outcomes will be compared between the observational and intervention phase, including short-term (i.e., within 12-month intervention phase) and long-term (i.e., within 1–2 years after initial intervention phase) outcomes. As described below, we will also employ two complementary study designs: 1) a repeated cross-section design with a different mix of individuals in each cluster (clinic) for examining implementation outcomes (e.g., Aim 1: screening rate) and 2) a cohort design for examining effectiveness in outcomes over time within the same individuals (e.g., Aim 2: retention in HIV care). Data analysis for both the cross-sectional and cohort approaches will use the random effects modeling framework which accounts for correlation between individuals within clusters and over time and is generalizable to non-normal distributions [84]. These models may be estimated using SAS PROC MIXED or PROC NLMIXED for normal or non-normal distributions allowing for correlation between observations and variable cluster sizes. We will assess the incremental cost-effectiveness ratio (ICER) as the additional cost per unit of outcome (e.g., 1% increase in mean ART adherence) between the observation and intervention phases. A cost-effectiveness acceptability curve will be obtained from the distribution of the ICER which will be constructed using the bootstrap method based on the variances of estimates of costs and effectiveness [85]. ICER for screening and BHS intervention will be calculated separately.
We will integrate qualitative and quantitative findings using a triangulation approach to identify varying levels of agreement between study components [86], as in our prior work [73]. The qualitative analysis will note recurring implementation-related themes, comparing interviewees in different clinics on demographics, position and knowledge of and attitudes about integration of behavioral health into HIV primary care. Analysis also will note recurring patient experience-related themes (e.g., screening modalities, medical provider/BHS use of screening results, willingness to engage with a BHS).
Analyses will include all PWH who are members of the 3 clinics during the study time period (~5,000). We anticipate adequate statistical power. For example, assuming an overall screening rate of 85% of whom 25% screen positive and one third of whom are severe enough to initiate specialty SUD treatment, we will have a power of .97 to detect a 12% difference in the treatment initiation rate between the observation and intervention phases. Hypothesis tests involving closed cohort designs (i.e., repeated measures on the same individual as in Aim 2) will have greater power than cross-sectional designs with the same sample size. For example, we will have > .99 power to detect a difference of .15 standard deviations in ART adherence between initial and follow-up screenings.
3. Results
The study is currently in process, with outcomes anticipated to be available in 2021. Results below describe key factors affecting development of screening instruments, refinement of study procedures in conjunction with IRB modifications, and clinical concerns addressed in collaboration with clinic leaders prior to launching the intervention phase of the study.
3.1. Technological considerations
The study team worked closely with KPNC Information Technology groups to integrate screening into the EHR; ensure that the TAPS/AOQ could be effectively delivered across three modalities each with different technical demands; create and distribute job aids (i.e., training guides) to clinicians and reception staff describing how to administer the survey and access results in structured clinical flowsheets and notes; as well as provide ongoing support and troubleshooting.
We also worked directly with the KPNC Quality and Operations Support (QOS) group, which served as a liaison with EHR developers during questionnaire development. In order to have operational leadership support to pilot the questionnaire in the EHR, a business case was developed to justify the use of resources and document how functionality would be affected and improved. Once approved, the research team met weekly with QOS to develop and test the questionnaire itself and refine associated functions and tools. One challenge was identifying a SUD screening instrument that was thorough, brief enough for patients to complete routinely, and technologically feasible. For example, the team needed to select an instrument to accommodate Epic® limitations (e.g., branching capabilities), and then adapt it to be Epic®-compatible.
3.2. Microsoft Access® database
An important initial challenge was how to minimize the burden of TAPS/AOQ administration on clinic workflows. Study staff worked with clinical leads to identify criteria for appropriate patient appointments with which to link a questionnaire (e.g., excluding telephone/video visits, and visits with non-HIV providers) and timing the administration based on a combination of factors (upcoming appointment with participating provider, no questionnaire completed in past 6 months). Even within an integrated health care system such as KPNC, the three individual clinic sites have different appointment codes, adding to the complexity of administering automated screening, and necessitating development of an Access® database for the study that draws on EHR data. By using the tracking system to support to clinicians and clinic staff, the research team reduced the impact on clinic workflows.
3.3. Online HIV reporting tool
The KPNC Division of Research has actively maintained the KPNC HIV registry since 1988 to identify cases of HIV as well as provide data and reports to support clinical case management, quality of care assessments, and research studies. The HIV Registry is updated monthly with newly identified cases, and registry staff maintain a web-based reporting tool used by HIV case managers and clinicians to access up-to-date data on HIV patients at their respective KPNC medical center. The application (internally known as iHIV) includes >80 custom reports, such as lists of HIV patients sorted by provider with information on demographic factors (e.g., age, race, sex) or clinical factors (last CD4 or HIV RNA test results). The research team worked with KPNC HIV registry staff to develop a new report, updated daily, for TAPS/AOQ responses. The report displays summary level patient TAPS/AOQ responses that can be sorted and filtered to better identify patients who require clinical follow-up. These customized reports can then be exported to Excel or other software.
3.4. Institutional Review Board
The study team spent considerable time developing study materials and protocols that were both feasible within the constraints of the EHR functionality and were acceptable to the KPNC Institutional Review Board (IRB). Two issues of concern involved (1) adequate informed consent and HIPAA authorization given delivery of the survey using existing health system tools (i.e., patient portal and clinic tablets) and (2) provisions for any emotional distress that may arise from the screening tool including how to track patients who endorse suicidal ideation. A strong case was made to the IRB that mental health and substance use screening is a central part of HIV care, and that the study innovation was in the method and not the content of screening.
Based on these discussions with the IRB and the submission of additional modifications, a waiver of signed informed consent and signed HIPAA authorization was granted. IRB-approved language was provided both electronically in the patient secure messaging, and on a paper information sheet given to patients along with the tablets, to inform them of the purpose of screening, that completion is voluntary and part of a research study, that clinical staff and researchers would see the responses, and that mental health resources are available to them if they experienced distress.
3.5. Clinician concerns regarding use of screening results
Planning for use of a new instrument to assess SUDs, the TAPS, included provider education about the instrument’s interpretation and implications for clinical follow-up. Providers were instructed that any SUD risk score >0 merits discussion with the patient, consideration of patient presentation and history, and potentially referral to the BHS or specialty addiction care. Additional clinician concerns included limited knowledge about and access to personalized SUD specialty treatment referrals, even though KPNC is an integrated health system where most patients’ coverage includes access to such treatment. When necessary, the study team identified specialty department liaisons and facilitated personalized introductions for BHSs and PACE Champions.
Rollout of the computerized AOQ to the general HIV population also prompted clinician questions. Although the AOQ has been used widely in KPNC for several years, including for HIV patients with new diagnoses of major depression, provider familiarity with the instrument varied. Clinicians were provided guidance about existing KPNC protocols for interpreting AOQ scores and responding with appropriate clinical interventions. Additional physician concerns about expanded use of the AOQ included increase in clinician or staff time needed to review a greater volume of incoming patient responses, especially in cases where the questionnaire is completed up to 2 weeks prior to a visit.
The study team assisted clinicians in identifying self-reported suicidal ideation in the AOQ (any thoughts of self-harm in the prior two weeks). In addition to training on KPNC protocols for interpreting and responding to online AOQ scores, and information about existing ways that the region has addressed liability concerns, the study team offered additional assistance in identifying endorsement of thoughts of self-harm during the study. Specifically, the study team built into the tracking database an alert for suicidal ideation endorsed prior to the visit and a protocol whereby study staff alerted providers via EHR staff message once suicidal ideation was identified (which varies based on EHR and tracking system update schedules and staff hours).
4. Discussion
Previous studies have identified SUD and mental health as substantial problems among PWH that are not often effectively identified and treated in the context of primary care services. The research team developed a novel approach to improve care integration for this clinic population. This hybrid design intervention study is currently in process and evaluates both implementation and effectiveness. Findings will inform the field regarding: 1) how self-administered computerized SUD, depression and anxiety screening and behavioral interventions can be effectively implemented in HIV primary care; 2) which patient and organizational characteristics influence screening and intervention delivery; 3) to what extent implementation of screening and intervention impacts HIV care, SUD, depression and anxiety outcomes; and 4) financial costs of implementation. The study addresses high-priority HIV research topics since effective treatment of SUD and psychiatric disorders would improve retention in care, alleviate HIV treatment disparities, and reduce SUD- and mental health-related medical comorbidity [87].
The study addresses challenging aspects of integrating computerized patient questionnaires into health care settings. The three screening modalities we employ (secure messaging, tablet-based administration in clinic waiting rooms, and desktop computer administration in exam rooms) each have advantages and disadvantages. It often is advantageous to have patients respond to health surveys prior to their visit, e.g., via secure messaging, to give providers clinical information in advance and to maximize efficiency of clinic visits and front desk workflows. Yet these procedures necessitate additional tracking systems and procedures to deliver questionnaires and monitor responses, and patients without internet access may not be reached. The electronic screening tools developed by the research team aim to provide maximum flexibility for the clinics and to reach the largest number of patients either at or before routine visits.
Given the complexity of these challenges, the study team is supporting the clinical sites by using the KPNC HIV registry in combination with appointment databases to identify eligible patients, deliver questionnaires, and monitor patient responses. Although the study team has worked closely with the clinics in the mechanics of the screening process, the team is leaving details regarding utilization of screening results up to clinic staff including clinic leads, clinic champions, and BHS staff. It is not known whether clinics will be able to sustain these same procedures over time, given the staffing commitment that would be required (e.g., dedicated clinic staff that would track upcoming visits, attach questionnaires to visits in the EHR, and monitor responses). However, we anticipate that if the study shows that these systems can be well-integrated into patient care and have a measurable impact on patient outcomes at a manageable cost, this may help motivate a commitment to adapting and sustaining these processes after the study has ended.
4.1. Strengths and Limitations
Given the population of insured HIV patients, generalizability to public systems or resource-constrained environments that do not have access to an EHR or a BHS may be limited. In addition, the population mostly consists of white race/ethnicity and men who have sex with men, although this reflects the demographics of reported AIDS cases in California [88]. Nevertheless, our large cohort size, and selection of clinics with a higher prevalence of minorities and women helps maximize generalizability to groups less represented. Finally, with the ACA and expansion of Medicare, KPNC has seen significant increases in enrollment of PWH [89]. As California’s benchmark ACA Insurance Exchange plan, other health systems are emulating KPNC’s integrated care model, and KPNC’s impact on HIV care and prevention is large [79, 90].
We will compare baseline measures of participants with and without missing data to detect systematic pattern of missingness in TAPS/AOQ completion (including patient demographic characteristics and medical comorbidities, provider demographics and years of experience, and clinic location associated with missingness). We will also test the assumption of missing at random (MAR) using a pattern-mixture modeling approach [91]. To account for such missing data, we will use modern missing data techniques, with emphasis on multiple imputation methods [92–94] that provide a useful umbrella for handling different forms of missing data, including coarse-measured [95] or mis-measured [96] covariates. These will be used to adjust for some covariates in cross-sectional effectiveness outcome comparisons because participants are not randomized, and because some individuals may be part of one cohort but not the other (e.g., due to patients entering or leaving the health plan over the course of the study).
4.2. Conclusion
Identification and treatment of SUDs and psychiatric disorders is a significant challenge for primary care HIV clinics. The PACE study will examine the impact of self-administered electronic screening for SUD risk, depression and anxiety in three large KPNC primary care clinics serving over 5,000 patients with HIV. Based on screening results and physician referrals, behavioral health specialists embedded in primary care initiate MI- and CBT-based brief treatment, and link patients to addiction medicine and psychiatry clinics as needed. Analyses will examine implementation (e.g., screening and treatment rates) and effectiveness outcomes (e.g., SUD, depression and anxiety symptoms; and HIV viral control). We also will evaluate screening and treatment costs and implementation barriers and facilitators. Results will help to inform effective strategies for improving substance use, mental health, and HIV clinical outcomes, as well as broader integration of SUD screening and treatment in primary care settings.
Acknowledgments:
We wish to thank Sujaya Parthasarathy for assistance in study design, and Courtney Ellis and Gina Smith-Anderson for assistance in project management. We thank Gary Zin, Nancy Facher, and Nicholas Shapiro at Kaiser Permanente Quality and Operations Support for technical assistance; and the clinic staff at the Kaiser Permanente primary care HIV clinics in Oakland, Sacramento, and San Francisco. We thank Agatha Hinman for help in preparing the manuscript.
Funding:
The study is supported by the National Institute on Drug Abuse (R01 DA043139). Dr. Satre is also supported by a career development award from the National Institute on Alcohol Abuse and Alcoholism (K24 AA025703) and by the Dolby Family Center for Mood Disorders at the University of California, San Francisco.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial Registration:
References
- [1].DeLorenze GN, Satre DD, Quesenberry CP, Tsai AL, Weisner CM, Mortality after diagnosis of psychiatric disorders and co-occurring substance use disorders among HIV-infected patients, AIDS Patient Care STDS 24 (2010) 705–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].DeLorenze GN, Weisner C, Tsai AL, Satre DD, Quesenberry CP Jr., Excess mortality among HIV-infected patients diagnosed with substance use dependence or abuse receiving care in a fully integrated medical care program, Alcohol. Clin. Exp. Res 35 (2011) 203–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Teague GB, Mueser KT, Rapp CA, Advances in fidelity measurement for mental health services research: four measures, Psychiatr. Serv 63 (2012) 765–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Webster-Stratton CH, Reid MJ, Marsenich L, Improving therapist fidelity during implementation of evidence-based practices: Incredible years program, Psychiatr. Serv 65 (2014) 789–95. [DOI] [PubMed] [Google Scholar]
- [5].Hall K, Staiger PK, Simpson A, Best D, Lubman DI, After 30 years of dissemination, have we achieved sustained practice change in motivational interviewing?, Addiction 111 (2016) 1144–50. [DOI] [PubMed] [Google Scholar]
- [6].Peretti-Watel P, Spire B, Lert F, Obadia Y, Vespa Group, Drug use patterns and adherence to treatment among HIV-positive patients: evidence from a large sample of French outpatients (ANRS-EN12-VESPA 2003), Drug Alcohol Depend 82 Suppl 1 (2006) S71–9. [DOI] [PubMed] [Google Scholar]
- [7].Azar MM, Springer SA, Meyer JP, Altice FL, A systematic review of the impact of alcohol use disorders on HIV treatment outcomes, adherence to antiretroviral therapy and health care utilization, Drug Alcohol Depend 112 (2010) 178–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chander G, Lau B, Moore RD, Hazardous alcohol use: a risk factor for non-adherence and lack of suppression in HIV infection, J. Acquir. Immune Defic. Syndr 43 (2006) 411–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chander G, Addressing alcohol use in HIV-infected persons, Top. Antivir. Med 19 (2011) 143–7. [PMC free article] [PubMed] [Google Scholar]
- [10].Shacham E, Agbebi A, Stamm K, Overton ET, Alcohol consumption is associated with poor health in HIV clinic patient population: a behavioral surveillance study, AIDS Behav 15 (2011) 209–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Williams EC, McGinnis KA, Edelman EJ, Matson TE, Gordon AJ, Marshall BDL, et al. , Level of alcohol use associated with HIV care continuum targets in a national U.S. sample of persons living with HIV receiving healthcare, AIDS Behav 23 (2019) 140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hormes JM, Gerhardstein KR, Griffin PT, Under-reporting of alcohol and substance use versus other psychiatric symptoms in individuals living with HIV, AIDS Care 24 (2011) 420–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Roux P, Cohen J, Lascoux-Combe C, Sogni P, Winnock M, Salmon-Ceron D, et al. , Determinants of the underreporting of alcohol consumption by HIV/HCV co-infected patients during face-to-face medical interviews: the role of the physician, Drug Alcohol Depend 116 (2011) 228–32. [DOI] [PubMed] [Google Scholar]
- [14].Strauss SM, Tiburcio NJ, Munoz-Plaza C, Gwadz M, Lunievicz J, Osborne A, et al. , HIV care providers’ implementation of routine alcohol reduction support for their patients, AIDS Patient Care STDS 23 (2009) 211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Korthuis PT, Josephs JS, Fleishman JA, Hellinger J, Himelhoch S, Chander G, et al. , Substance abuse treatment in human immunodeficiency virus: the role of patient-provider discussions, J. Subst. Abuse Treat 35 (2008) 294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Metsch LR, Pereyra M, Colfax G, Dawson-Rose C, Cardenas G, McKirnan D, et al. , HIV-positive patients’ discussion of alcohol use with their HIV primary care providers, Drug Alcohol Depend 95 (2008) 37–44. [DOI] [PubMed] [Google Scholar]
- [17].Lucas GM, Gebo KA, Chaisson RE, Moore RD, Longitudinal assessment of the effects of drug and alcohol abuse on HIV-1 treatment outcomes in an urban clinic, AIDS 16 (2002) 767–74. [DOI] [PubMed] [Google Scholar]
- [18].Delaney JA, Nance RM, Whitney BM, Altice FL, Dong X, Trejo MEP, et al. , Brief report: Reduced use of illicit substances, even without abstinence, is associated with improved depressive symptoms among people living with HIV, J Acquir Immune Defic Syndr 79 (2018) 283–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bahorik AL, Leibowitz A, Sterling SA, Travis A, Weisner C, Satre DD, The role of hazardous drinking reductions in predicting depression and anxiety symptom improvement among psychiatry patients: A longitudinal study, J. Affect. Disord 206 (2016) 169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lopes M, Olfson M, Rabkin J, Hasin DS, Alegria AA, Lin KH, et al. , Gender, HIV status, and psychiatric disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions, J. Clin. Psychiatry 73 (2011) 384–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nurutdinova D, Chrusciel T, Zeringue A, Scherrer JF, Al-Aly Z, McDonald JR, et al. , Mental health disorders and the risk of AIDS-defining illness and death in HIV-infected veterans, AIDS 26 (2012) 229–34. [DOI] [PubMed] [Google Scholar]
- [22].Pence BW, Miller WC, Whetten K, Eron JJ, Gaynes BN, Prevalence of DSM-IV-defined mood, anxiety, and substance use disorders in an HIV clinic in the Southeastern United States, J. Acquir. Immune Defic. Syndr 42 (2006) 298–306. [DOI] [PubMed] [Google Scholar]
- [23].Bing EG, Burnam MA, Longshore D, Fleishman JA, Sherbourne CD, London AS, et al. , Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States, Arch. Gen. Psychiatry 58 (2001) 721–8. [DOI] [PubMed] [Google Scholar]
- [24].Fairfield KM, Libman H, Davis RB, Eisenberg DM, Beckett A, Phillips RS, Brief communication: detecting depression: providing high quality primary care for HIV-infected patients, Am. J. Med. Qual 16 (2001) 71–4. [DOI] [PubMed] [Google Scholar]
- [25].Brandt C, Zvolensky MJ, Woods SP, Gonzalez A, Safren SA, O’Cleirigh CM, Anxiety symptoms and disorders among adults living with HIV and AIDS: A critical review and integrative synthesis of the empirical literature, Clin. Psychol. Rev 51 (2017) 164–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Heywood W, Lyons A, HIV and elevated mental health problems: diagnostic, treatment, and risk patterns for symptoms of depression, anxiety, and stress in a national community-based cohort of gay men living with HIV, AIDS Behav 20 (2016) 1632–45. [DOI] [PubMed] [Google Scholar]
- [27].Antelman G, Kaaya S, Wei R, Mbwambo J, Msamanga GI, Fawzi WW, et al. , Depressive symptoms increase risk of HIV disease progression and mortality among women in Tanzania, J. Acquir. Immune Defic. Syndr 44 (2007) 470–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ickovics JR, Hamburger ME, Vlahov D, Schoenbaum EE, Schuman P, Boland RJ, et al. , Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: longitudinal analysis from the HIV Epidemiology Research Study, JAMA 285 (2001) 1466–74. [DOI] [PubMed] [Google Scholar]
- [29].Hessol NA, Kalinowski A, Benning L, Mullen J, Young M, Palella F, et al. , Mortality among participants in the Multicenter AIDS Cohort Study and the Women’s Interagency HIV Study, Clin. Infect. Dis 44 (2007) 287–94. [DOI] [PubMed] [Google Scholar]
- [30].French AL, Gawel SH, Hershow R, Benning L, Hessol NA, Levine AM, et al. , Trends in mortality and causes of death among women with HIV in the United States: a 10-year study, J. Acquir. Immune Defic. Syndr 51 (2009) 399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bradley KA, Lapham GT, Hawkins EJ, Achtmeyer CE, Williams EC, Thomas RM, et al. , Quality concerns with routine alcohol screening in VA clinical settings, J. Gen. Intern. Med 26 (2011) 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Williams EC, Achtmeyer CE, Thomas RM, Grossbard JR, Lapham GT, Chavez LJ, et al. , Factors underlying quality problems with alcohol screening prompted by a clinical reminder in primary care: a multi-site qualitative study, J. Gen. Intern. Med 30 (2015) 1125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Johnson M, Jackson R, Guillaume L, Meier P, Goyder E, Barriers and facilitators to implementing screening and brief intervention for alcohol misuse: a systematic review of qualitative evidence, J Public Health (Oxf) 33 (2011) 412–21. [DOI] [PubMed] [Google Scholar]
- [34].Kobak KA, Taylor LH, Dottl SL, Greist JH, Jefferson JW, Burroughs D, et al. , Computerized screening for psychiatric disorders in an outpatient community mental health clinic, Psychiatr. Serv 48 (1997) 1048–57. [DOI] [PubMed] [Google Scholar]
- [35].Hess R, Santucci A, McTigue K, Fischer G, Kapoor W, Patient difficulty using tablet computers to screen in primary care, J. Gen. Intern. Med 23 (2008) 476–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bruckel J, Wagle N, O’Brien C, Elias J, McKenna S, Meyers P, et al. , Feasibility of a tablet computer system to collect patient-reported symptom severity in patients undergoing diagnostic coronary angiography, Crit. Pathw. Cardiol 14 (2015) 139–45. [DOI] [PubMed] [Google Scholar]
- [37].Cifuentes M, Davis M, Fernald D, Gunn R, Dickinson P, Cohen DJ, Electronic health record challenges, workarounds, and solutions observed in practices integrating behavioral health and primary care, J. Am. Board Fam. Med 28 Suppl 1 (2015) S63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lofland JH, Schaffer M, Goldfarb N, Evaluating health-related quality of life: cost comparison of computerized touch-screen technology and traditional paper systems, Pharmacotherapy 20 (2000) 1390–5. [DOI] [PubMed] [Google Scholar]
- [39].Turner-Bowker DM, Saris-Baglama RN, DeRosa MA, Giovannetti ER, Jensen RE, Wu AW, A computerized adaptive version of the SF-36 is feasible for clinic and Internet administration in adults with HIV, AIDS Care 24 (2012) 886–96. [DOI] [PubMed] [Google Scholar]
- [40].McNeely J, Kumar PC, Rieckmann T, Sedlander E, Farkas S, Chollak C, et al. , Barriers and facilitators affecting the implementation of substance use screening in primary care clinics: a qualitative study of patients, providers, and staff, Addict. Sci. Clin. Pract 13 (2018) 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Taylor SL, Burnam MA, Sherbourne C, Andersen R, Cunningham WE, The relationship between type of mental health provider and met and unmet mental health needs in a nationally representative sample of HIV-positive patients, J Behav Health Serv Res 31 (2004) 149–63. [DOI] [PubMed] [Google Scholar]
- [42].Bazemore A, Wingrove P, Peterson L, Petterson S, The Diversity of Providers on the Family Medicine Team, J. Am. Board Fam. Med 29 (2016) 8–9. [DOI] [PubMed] [Google Scholar]
- [43].Miller-Matero LR, Dubaybo F, Ziadni MS, Feit R, Kvamme R, Eshelman A, et al. , Embedding a psychologist into primary care increases access to behavioral health services, J. Prim. Care Community Health 6 (2015) 100–4. [DOI] [PubMed] [Google Scholar]
- [44].Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C, Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact, Med Care 50 (2012) 217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Brown CA, Lilford RJ, The stepped wedge trial design: a systematic review, BMC Med. Res. Methodol 6 (2006) 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Cully JA, Armento ME, Mott J, Nadorff MR, Naik AD, Stanley MA, et al. , Brief cognitive behavioral therapy in primary care: a hybrid type 2 patient-randomized effectiveness-implementation design, Implement. Sci 7 (2012) 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Martin LA, Mignogna J, Mott J, Hundt N, Kauth M, Kunik M, et al. , Implementing brief Cognitive Behavioral Therapy (CBT) in primary care: clinicians’ experiences from the field, 2015. https://higherlogicdownload.s3.amazonaws.com/ACADEMYHEALTH/0f965b51-b3f9-4e21-9aa4-384a66a6fbbd/UploadedImages/Martin-DI%20conference%20presentation%20FINAL%20without%20notes.pdf.
- [48].Feldstein AC, Glasgow RE, A practical, robust implementation and sustainability model (PRISM) for integrating research findings into practice, Jt Comm J Qual Patient Saf 34 (2008) 228–43. [DOI] [PubMed] [Google Scholar]
- [49].Satre DD, Wolfe W, Eisendrath S, Weisner C, Computerized screening for alcohol and drug use among adults seeking outpatient psychiatric services, Psychiatr. Serv 59 (2008) 441–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ralston JD, Silverberg MJ, Grothaus L, Leyden WA, Ross T, Stewart C, et al. , Use of web-based shared medical records among patients with HIV, Am. J. Manag. Care 19 (2013) e114–24. [PMC free article] [PubMed] [Google Scholar]
- [51].Burnam MA, Bing EG, Morton SC, Sherbourne C, Fleishman JA, London AS, et al. , Use of mental health and substance abuse treatment services among adults with HIV in the United States, Arch. Gen. Psychiatry 58 (2001) 729–36. [DOI] [PubMed] [Google Scholar]
- [52].Orwat J, Saitz R, Tompkins CP, Cheng DM, Dentato MP, Samet JH, Substance abuse treatment utilization among adults living with HIV/AIDS and alcohol or drug problems, J. Subst. Abuse Treat 41 (2011) 233–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Pisu M, Cloud G, Austin S, Raper JL, Stewart KE, Schumacher JE, Substance abuse treatment in an urban HIV clinic: who enrolls and what are the benefits?, AIDS Care 22 (2010) 348–54. [DOI] [PubMed] [Google Scholar]
- [54].Guerrero EG, Heslin KC, Chang E, Fenwick K, Yano E, Organizational correlates of implementation of colocation of mental health and primary care in the Veterans Health Administration, Adm. Policy Ment. Health 42 (2015) 420–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Reddy A, Shea JA, Canamucio A, Werner RM, The effect of organizational climate on patient-centered medical home implementation, Am. J. Med. Qual 30 (2015) 309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ohman-Strickland PA, John Orzano A, Nutting PA, Perry Dickinson W, Scott-Cawiezell J, Hahn K, et al. , Measuring organizational attributes of primary care practices: development of a new instrument, Health Serv. Res 42 (2007) 1257–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Aarons GA, Mental health provider attitudes toward adoption of evidence-based practice: the Evidence-Based Practice Attitude Scale (EBPAS), Ment. Health Serv. Res 6 (2004) 61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].McNeely J, Wu LT, Subramaniam G, Sharma G, Cathers LA, Svikis D, et al. , Performance of the Tobacco, Alcohol, Prescription Medication, and Other Substance Use (TAPS) tool for substance use screening in primary care patients, Ann. Intern. Med 165 (2016) 690–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].National Institute on Drug Abuse, TAPS: Tobacco, alcohol, prescription medication, and other substance use tool https://www.drugabuse.gov/taps/#/.
- [60].Kroenke K, Spitzer RL, Williams JB, The PHQ-9: validity of a brief depression severity measure, J. Gen. Intern. Med 16 (2001) 606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Plummer F, Manea L, Trepel D, McMillan D, Screening for anxiety disorders with the GAD-7 and GAD-2: a systematic review and diagnostic metaanalysis, Gen. Hosp. Psychiatry 39 (2016) 24–31. [DOI] [PubMed] [Google Scholar]
- [62].Spangenberg L, Glaesmer H, Boecker M, Forkmann T, Differences in Patient Health Questionnaire and Aachen Depression Item Bank scores between tablet versus paper-and-pencil administration, Qual. Life Res 24 (2015) 3023–32. [DOI] [PubMed] [Google Scholar]
- [63].Grunauer M, Schrock D, Fabara E, Jimenez G, Miller A, Lai Z, et al. , Tablet-based screening of depressive symptoms in quito, ecuador: efficiency in primary care, Int J Family Med 2014 (2014) 845397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Baker AL, Kavanagh DJ, Kay-Lambkin FJ, Hunt SA, Lewin TJ, Carr VJ, et al. , Randomized controlled trial of MICBT for co-existing alcohol misuse and depression: outcomes to 36-months, J. Subst. Abuse Treat 46 (2014) 281–90. [DOI] [PubMed] [Google Scholar]
- [65].Satre DD, Leibowitz A, Sterling SA, Lu Y, Travis A, Weisner C, A randomized clinical trial of Motivational Interviewing to reduce alcohol and drug use among patients with depression, J Consult Clin Psychol 84 (2016) 571–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Cape J, Whittington C, Buszewicz M, Wallace P, Underwood L, Brief psychological therapies for anxiety and depression in primary care: meta-analysis and meta-regression, BMC Med 8 (2010) 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Satre DD, Leibowitz AS, Leyden W, Catz SL, Hare CB, Jang H, et al. , Interventions to reduce unhealthy alcohol use among primary care patients with HIV: the Health and Motivation Randomized Clinical Trial, J. Gen. Intern. Med (2019). [DOI] [PMC free article] [PubMed]
- [68].Mertens JR, Chi FW, Weisner CM, Satre DD, Ross TB, Allen S, et al. , Physician versus non-physician delivery of alcohol screening, brief intervention and referral to treatment in adult primary care: The ADVISe cluster randomized controlled implementation trial, Addict. Sci. Clin. Pract 10 (2015) 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Satre DD, Mertens JR, Arean PA, Weisner C, Five-year alcohol and drug treatment outcomes of older adults versus middle-aged and younger adults in a managed care program, Addiction 99 (2004) 1286–97. [DOI] [PubMed] [Google Scholar]
- [70].Kaiser Permanente Northern California Dual Diagnosis Best Practices Committee. Recommendations for improving treatment of co-occurring disorders Oakland, Ca: Kaiser Permanente Medical Care Program,2007. [Google Scholar]
- [71].Kaiser Permanente Care Management Institute. Diagnosis and treatment of depression in adults: 2012 clinical practice guideline Oakland, CA: Kaiser Permanente Care Management Institute,2012. [Google Scholar]
- [72].Kendall T, Cape J, Chan M, Taylor C, Guideline Development G, Management of generalised anxiety disorder in adults: summary of NICE guidance, BMJ 342 (2011) c7460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Satre DD, Altschuler A, Parthasarathy S, Silverberg MJ, Volberding P, Campbell CI, Implementation and operational research: Affordable Care Act implementation in a California health care system leads to growth in HIV-positive patient enrollment and changes in patient characteristics, J. Acquir. Immune Defic. Syndr 73 (2016) e76–e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Kaiser Permanente Medical Care Program Adult depression clinical practice guideline. Approved by the National Guideline Directors February 2012 Oakland, CA: Care Management Institute, 2012. [Google Scholar]
- [75].Andrade SE, Kahler KH, Frech F, Chan KA, Methods for evaluation of medication adherence and persistence using automated databases, Pharmacoepidemiol Drug Saf 15 (2006) 565–74; discussion 575–7. [DOI] [PubMed] [Google Scholar]
- [76].Steiner JF, Prochazka AV, The assessment of refill compliance using pharmacy records: methods, validity, and applications, J. Clin. Epidemiol 50 (1997) 105–16. [DOI] [PubMed] [Google Scholar]
- [77].Grossberg R, Gross R, Use of pharmacy refill data as a measure of antiretroviral adherence, Curr. HIV/AIDS Rep 4 (2007) 187–91. [DOI] [PubMed] [Google Scholar]
- [78].Choudhry NK, Shrank WH, Levin RL, Lee JL, Jan SA, Brookhart MA, et al. , Measuring concurrent adherence to multiple related medications, Am J Manag Care 15 (2009) 457–64. [PMC free article] [PubMed] [Google Scholar]
- [79].Institute of Medicine, Monitoring HIV Care in the United States: Indicators and Data Systems, National Academy Press, Washington, DC, 2012. [PubMed] [Google Scholar]
- [80].Justice AC, Freiberg MS, Tracy R, Kuller L, Tate JP, Goetz MB, et al. , Does an index composed of clinical data reflect effects of inflammation, coagulation, and monocyte activation on mortality among those aging with HIV?, Clin. Infect. Dis 54 (2012) 984–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Tate JP, Sterne JAC, Justice AC, Veterans Aging Cohort S, The C Antiretroviral Therapy Cohort, Improved discrimination of mortality with Veterans Aging Cohort Study (VACS) Index 2.0 in HIV-positive individuals, AIDS (2019).
- [82].Rye M, Torres EM, Friborg O, Skre I, Aarons GA, The Evidence-Based Practice Attitude Scale-36 (EBPAS-36): A brief and pragmatic measure of attitudes to evidence-based practice validated in US and Norwegian samples, Implement Sci 12 (2017) 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Waxmonsky J, Auxier A, Wise Romero P, Heath B, IPAT: Integrated Practice Tool, 2014. http://www.integration.samhsa.gov/operations-administration/IPAT_v_2.0_FINAL.pdf.
- [84].Hussey MA, Hughes JP, Design and analysis of stepped wedge cluster randomized trials, Contemp. Clin. Trials 28 (2007) 182–91. [DOI] [PubMed] [Google Scholar]
- [85].Weisner C, Mertens J, Parthasarathy S, Moore C, Lu Y, Integrating primary medical care with addiction treatment: a randomized controlled trial, JAMA 286 (2001) 1715–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].O’Cathain A, Murphy E, Nicholl J, Three techniques for integrating data in mixed methods studies, BMJ 341 (2010) c4587. [DOI] [PubMed] [Google Scholar]
- [87].National Institutes of Health, Office of AIDS Research. NIH HIV/AIDS Research Priorities and Guidelines for Determining AIDS Funding Notice Number: NOT-OD-15-137 Washington, DC: National Institutes of Health and Office of AIDS Research, 2015. [Google Scholar]
- [88].California Department of Public Health, California HIV Surveillance Report - 2014, 2016 https://www.cdph.ca.gov/Programs/CID/DOA/CDPH%20Document%20Library/California%20HIV%20Surveillance%20Report%20-%202014_ADA.pdf.
- [89].Satre DD, Parthasarathy S, Altschuler A, Silverberg MJ, Storholm E, Campbell CI, Demographic, insurance, and health characteristics of newly enrolled HIV patients after implementation of the Affordable Care Act in California, Am. J. Public Health 106 (2016) 1211–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Volk JE, Marcus JL, Phengrasamy T, Blechinger D, Nguyen DP, Follansbee S, et al. , No new HIV infections with increasing use of HIV preexposure prophylaxis in a clinical practice setting, Clin. Infect. Dis 61 (2015) 1601–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Hedeker D, Gibbons RD, Longitudinal Data Analysis, John Wiley & Sons, Hoboken, NJ, 2006. [Google Scholar]
- [92].Rubin DB, Multiple Imputation for Nonresponse in Surveys, Wiley & Sons, Hoboken, NJ, 2004. [Google Scholar]
- [93].Wang Y, Taylor JMG, Jointly modeling longitudinal and event time data with application to Acquired Immunodeficiency Syndrome, J. Am. Stat. Assoc 96 (2001) 895–905. [Google Scholar]
- [94].Wulfsohn MS, Tsiatis AA, A joint model for survival and longitudinal data measured with error, Biometrics 53 (1997) 330–9. [PubMed] [Google Scholar]
- [95].Heitjan DF, Rubin DB, Inference from coarse data via multiple imputation with application to age heaping, J. Am. Stat. Assoc 85 (1990) 304–314. [Google Scholar]
- [96].Cole SR, Chu H, Greenland S, Multiple-imputation for measurement-error correction, Int. J. Epidemiol 35 (2006) 1074–81. [DOI] [PubMed] [Google Scholar]