Abstract
In 1997, our laboratory used targeted gene disruption of the GH receptor (GHR) to generate GHR knockout (GHR−/−) mice, which have been used in >127 published studies to help elucidate GH’s numerous activities. However, because GH replacement studies cannot be performed using this line, a GH knockout mouse line via targeted disruption of the GH gene is needed. Therefore, we created and characterized GH gene–disrupted (GH−/−) mice. GH−/− mice have severely decreased IGF-1 levels, small body size, and altered body composition with increased adiposity. GH−/− mice are extremely insulin sensitive but glucose intolerant, with a dramatic reduction in pancreatic islet size. Importantly, disruption of the GH gene had profound and depot-specific effects on white adipose tissue (WAT). Subcutaneous WAT from male and female GH−/− mice have significantly larger adipocytes and reduced fibrosis, neither of which occurred in perigonadal WAT, suggesting that GH has a more pronounced effect on subcutaneous WAT. Comparisons of GH−/− mice to previously published data on GHR−/− mice show a remarkably similar phenotype. Finally, we demonstrate that GH−/− mice are responsive to GH treatment, as shown by changes to serum IGF-1 levels; body length, weight, and composition; and insulin sensitivity. This study not only provides characterization of the first mouse line with targeted mutation of the GH gene but also indicates that GH gene disruption dramatically influences fibrosis of subcutaneous WAT.
GH is a pleotropic hormone that plays a key role in numerous metabolic processes, including growth, lipid and glucose metabolism, body composition, reproduction, aging, and lifespan. GH is also a potent diabetogenic agent. In fact, studies as early as the 1930s showed that GH can block insulin action and cause insulin resistance in dogs treated with anterior pituitary extracts (1). Subsequent experiments in the 1960s by Rabinowitz et al. (2, 3) demonstrated that this activity is not limited to animals, because GH had strong diabetogenic activity in humans.
Because mouse GH is thought to bind exclusively to its cognate receptor, the GH receptor (GHR), GHR knockout (GHR−/−) mice were produced via targeted mutation of the GHR gene >20 years ago to provide researchers a mouse line that lacks GH action (4, 5). To date, these mice have been shipped to >16 countries and have been used in >127 published studies, producing many novel and clinically relevant discoveries that have greatly expanded our knowledge of GH. GHR−/− mice are completely insensitive to GH, resulting in dwarfism with elevated circulating GH and severely reduced IGF-1 levels (5). Most organs are decreased in mass in GHR−/− mice, suggesting GH plays an important role in organ growth. However, the brain is unchanged in size and is disproportionately larger when evaluated relative to body size, suggesting that brain growth (independent of potential effects on cognitive function) is less GH dependent than other tissues are (5, 6). GHR−/− mice are obese, with excess adiposity that results primarily from enlarged subcutaneous white adipose tissue (WAT) (7). Despite obesity, GHR−/− mice are extremely insulin sensitive, highlighting an important physiological transition that occurs when GH’s diabetogenic activity is removed. GHR−/− mice are also glucose intolerant, due to fewer and smaller pancreatic β cells (8). Perhaps the most intriguing finding in GHR−/− mice is that they are exceptionally long lived and hold the Methuselah mouse prize for “the world’s longest lived laboratory mouse” (5, 9). GHR−/− mice are resistant to certain types of cancer, including lymphoma, adenocarcinoma, prostate cancer, and mammary cancer, which contributes to the extreme longevity of these mice (10–12). Cancer resistance in the absence of GHR is also consistent with findings in humans; studies by multiple laboratories indicate that individuals with Laron syndrome are resistant to cancer (13–15).
Although GHR−/− mice are a valuable tool to help elucidate GH’s many activities, a GH knockout mouse line via targeted mutation of the GH gene would be critical for researchers in the field of endocrinology. First, because GHR−/− mice lack functional GHR and are GH resistant, one cannot assess the impact of GH replacement. Second, in mice (in contrast to humans, in whom GH binds and activates the GHR and prolactin receptor), it is assumed that 100% of GH activity is conveyed through the GHR and 100% of GHR signaling is activated by GH; therefore, the creation of a GH gene knockout-mouse line and comparison with previous findings in GHR−/− mice would help test this assumption, because any differences in phenotype would suggest GH or GHR may have other unknown interactions. Third, although several GH-deficient mouse lines exist, to our knowledge a GH knockout mouse line via targeted mutation of the GH gene has yet to be described in the literature. Therefore, in this study, we characterize a GH gene–disrupted [i.e., GH gene knockout (GH−/−)] mouse line to provide researchers with an additional tool for studying GH action and evaluating the impact of exogenous GH administration. Ultimately, this new mouse line offers versatility, which provides countless translational uses to further the field of GH research.
Methods
GH−/− mice
GH−/− mice were created from embryonic stem cell clone 10368A-F1 (allele name: Ghtm1(KOMP)Vlcg) generated by Regeneron Pharmaceuticals and made into live mice by the KOMP Repository (www.KOMP.org) and the Mouse Biology Program (www.mousebiology.org) at the University of California, Davis. Methods used to create the VelociGene targeted alleles have been published (16). Briefly, the mouse GH genomic sequence (Mouse Genome Informatics: 95707; Ensembl: ENSMUSG20713; National Center for Biotechnology Information: 14599) was removed using the VelociGene KOMP definitive null allele design, which involves removal of the entire gene coding region from the translational start to the stop codon and replacement with a ZEN-UB1 reporter/selection cassette (http://velocigene.com/komp/detail/10368). GH−/− mice were initially developed in a C57BL/6N background at University of California, Davis, and were backcrossed nine generations into a C57BL/6J background at Ohio University. Mice were housed three to four per cage and given ad libitum access to water and standard laboratory rodent chow, in which 60% of energy is from carbohydrates, 26% from protein, and 14% from fat (ProLab RMH 3000, Laboratory Supply, Fort Worth, TX). The cages were maintained in a temperature- and humidity-controlled room (22°C) and exposed to a 14-hour light, 10-hour dark cycle. All procedures were approved by the Ohio University Institutional Animal Care and Use Committee and fully complied with all federal, state, and local policies.
Body composition and length
Body composition over time was determined monthly starting at 1 month until 5 months of age, using a Bruker Minispec NMR analyzer (Bruker Corp., The Woodlands, TX), as previously described (17–19). Body length from the tip of the nose to the anus was determined at 6 months of age in euthanized mice just before dissection. A total of 37 mice, 8 to 11 mice per group, were used for body composition and length determination.
Serum measurements and glucose and insulin tolerance tests
For serum measurements, blood was collected at dissection in 6-month-old male and female mice after an overnight 12-hour fast. Serum levels of GH, IGF-1, insulin, c-peptide, and adiponectin were measured as described (18). Glucose tolerance tests (GTTs) and insulin tolerance tests (ITTs) were performed between 5 and 6 months of age, as previously described (17) with the following exceptions: for both tests, 6-hour–fasted mice received IP injections starting at ∼2:00 pm. For GTTs, 15% glucose at a dose of 1.5g/kg body weight was used. Because of extreme inulin sensitivity in GH−/− mice, a reduced dose of recombinant human insulin (Humulin-R; Eli Lilly & Co, Indianapolis, IN) was prepared by diluting Humulin-R (100 U/mL) in sterile 0.9% NaCl to 0.05 U/mL for male mice and 0.04 U/mL for female mice, resulting in a final dose of 0.5 U/kg body weight for male mice and 0.4 U/kg body weight for female mice. Levels of free fatty acids were measured using HR Series NEFA-HR (2) (catalog no. 999-34691, 995-34791, 991-34891, 993-35191, 276-76491; FUJIFILM, Wako Diagnostics USA Corp., Richmond, VA). All kits were completed according to the manufacturer’s instructions. A total of 37 mice, 8 to 11 mice per group, were used for serum measures, as well as GTTs and ITTs.
Tissue collection
Six-month-old mice (male and female) were dissected starting at 9:00 am, after a 12-hour overnight fast. Heart, lung, kidney, spleen, brain, intestine, skeletal muscle (gastrocnemius, soleus, and quadriceps), retroperitoneal WAT, mesenteric WAT, and interscapular brown adipose tissue (BAT) were removed, weighed, frozen in liquid nitrogen, and stored at −80°C. Liver, pancreas, and inguinal subcutaneous and perigonadal WATs were removed and weighed; a portion was frozen in liquid nitrogen and stored at −80°C, while another portion was fixed for histology. A total of 37 mice, 8 to 11 mice per group, were used for tissue collection.
Tissue histology and analyses
For histological studies, portions of pancreatic tissue and subcutaneous and perigonadal WAT were prepared for histology by fixing the tissue in 10% formalin for 16 hours and embedding in paraffin. Histopathological analysis was carried out on 5-µm sections stained with hematoxylin and eosin. Sections were photographed using a Nikon Digital Sight DS-Fi1 camera (Nikon, Minato, Tokyo, Japan) and a Nikon Eclipse E600 microscope. Mean islet size and mean adipocyte size were determined using NIS-Elements software (Nikon). For determination of collagen content in adipose tissue, picrosirius red–stained area was determined, as previously described (20). Liver tissue was thawed and used for extraction and measurement of triacylglycerol levels, as described previously (21). For WAT, hydroxyproline content was assessed on frozen tissue as a secondary marker of fibrosis (22). A total of 37 mice, 8 to 11 mice per group, were used for histology and liver triacylglycerol measures.
GH treatment
For GH treatment studies, 5-week-old male and female GH−/− mice were treated with human GH (5 μg/g; Protein Laboratories Rehovot Ltd., Rehovot, Israel) or saline once daily for 6 days (n = 4 to 6 per group). This dose of GH was based on previous dosing studies in C57BL/6J mice required for observable changes to body composition and IGF-1 (23). Body composition was recorded daily, ITTs were performed on day 4 of GH treatment, and mice were dissected after a 12-hour overnight fast and after 6 days of treatment. Serum measurements, body composition, and insulin tolerance were determined using similar methods to those described for the 6-month-old cohorts. A total of 21 mice, four to six mice per group, were used for GH treatment studies.
Statistics
All values are given as mean ± SE. Statistics were performed using SPSS, version 14.0 (IBM, Armonk, NY). The two-tailed, unpaired Student t test was used to assess the significance of differences between the wild-type (WT) littermate controls and GH−/− mice for all blood measures, body length, islet size, organ sizes (including fat pads), liver triglyceride, adipocyte size, hydroxyproline, picrosirius-red staining (PSR), as well as individual time points in GTTs, and ITTs. For comparison of longitudinal data, including body weight, fat mass, percentage of fat mass, lean mass, and percentage of lean mass over time, repeated-measures ANOVA was used. For comparison of longitudinal data, Student t tests were also used to compare differences between genotype at individual time points. Differences were considered to be statistically significant when P < 0.05.
Results
GH gene disruption resulted in reduced GH and IGF-1 levels, reduced body size, and altered body composition
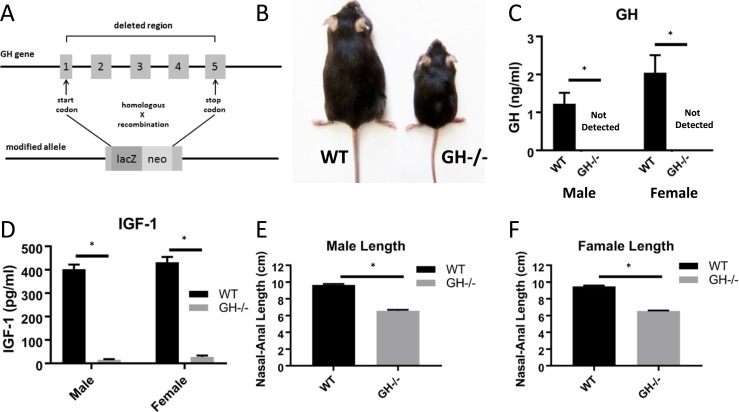
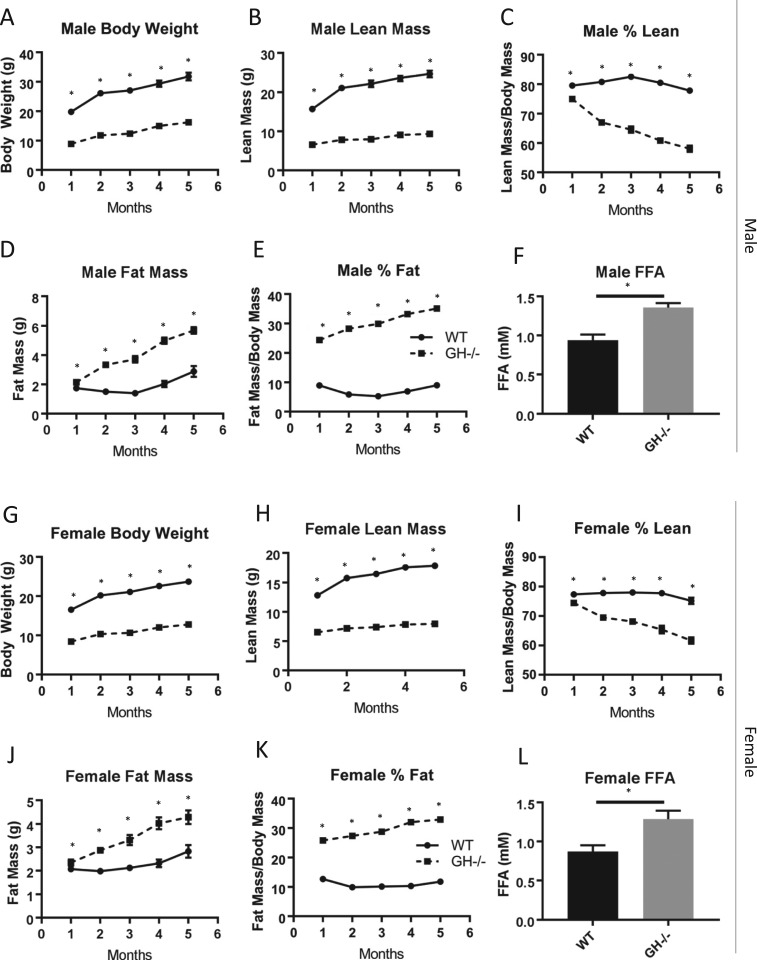
As expected, disruption of the GH gene (Fig. 1A) resulted in pronounced dwarfism; GH−/− mice were approximately half the size of WT littermate controls (Fig. 1B). The GH/IGF-1 axis was severely altered in GH−/− mice. Circulating GH levels (Fig. 1C) were undetectable in serum from male and female GH−/− mice and significantly decreased compared with WT controls (1.23 ± 0.28 ng/mL for male WT mice; 2.04 ± 0.29 ng/mL for female WT mice). Serum IGF-1 levels (Fig. 1D) were significantly reduced >90% in GH−/− mice compared with WT controls in males (15.8 ± 2.1 pg/mL vs 402 ± 20 pg/mL) and females (27.7 ± 5.7 pg/mL vs 431 ± 24 pg/mL). Disruption of the GH gene significantly reduced nasal-anal body length in male (6.58 ± 0.12 cm vs 9.67 ± 0.13 cm) and female (6.54 ± 0.07 cm vs 9.48 ± 0.12 cm) GH−/− mice compared with controls (Fig. 1E and 1F). Similarly, body composition over time was significantly altered in males (Fig. 2A–2E) and females (Fig. 2G–2K); body weight, lean mass, and percent lean mass were significantly decreased at all time points (determined monthly from 1 to 5 months of age), whereas fat mass and percent fat mass were significantly increased at all time points in GH−/− mice compared with WT controls. In agreement with increased body fat, male and female GH−/− mice had increased fasting serum free fatty acid levels (Fig. 2F and 2I).
Figure 1.
GH, IGF-1, and body length in GH−/− mice. (A) The entire GH coding sequence from the start to the stop codon was removed, as described in “Methods.” (B) Representative image of a WT littermate control next to a dwarf GH−/− mouse at 6 mo of age. (C, D) Fasting serum GH and IGF-1 levels at age 6 mo (n = 8 to 11). (E) Mean body lengths for males are shown (n = 8 to 11). (F) Mean body lengths for females are shown (n = 8 to 10). Vertical bars represent standard error. *P < 0.05, significantly different from WT controls.
Figure 2.
Body weight and composition in GH−/− mice. (A–F) Body weight, lean mass, percentage of lean mass, fat mass, percentage of fat mass, and 6-mo fasting free fatty acid (FFA) levels in males are shown (n = 8 to 11). (G–L) Body weight, lean mass, percentage of lean mass, fat mass, percentage of fat mass, and 6-mo fasting FFA levels in females are shown (n = 8 to 10). Vertical bars represent standard error. *P < 0.05, significantly different from WT controls.
GH−/− mice had markedly enhanced insulin sensitivity
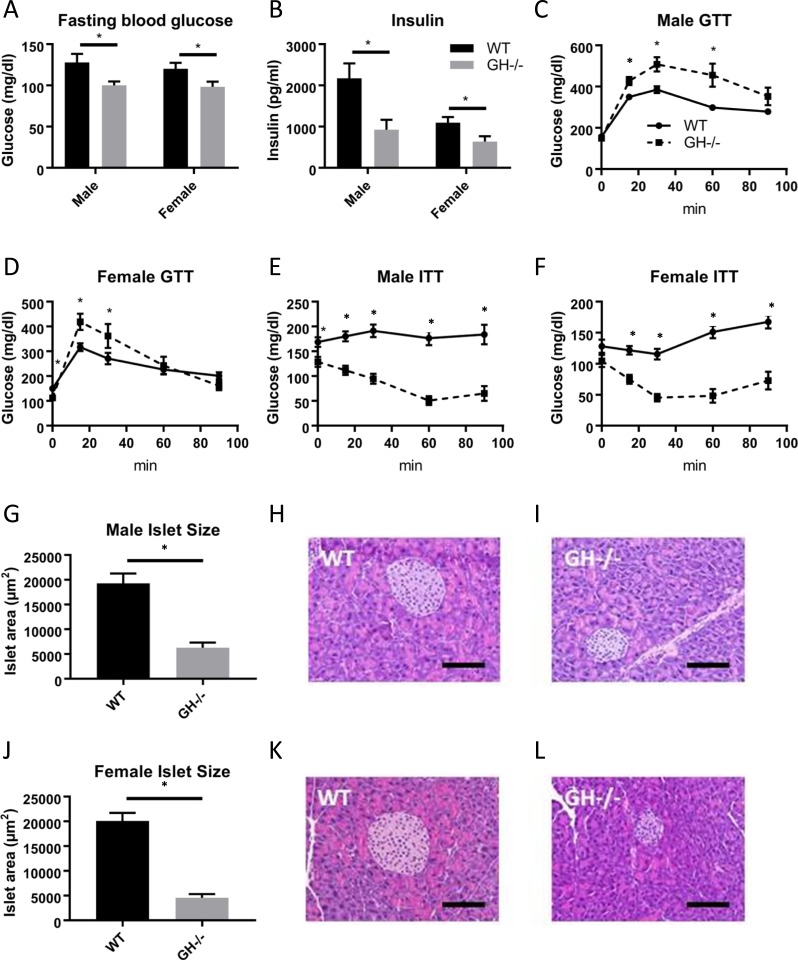
Measures of glucose homeostasis were assessed in GH−/− mice compared with WT controls (Fig. 3). Fasting blood glucose levels were significantly decreased in GH−/− mice compared with WT controls in both sexes (Fig. 3A). Similarly, fasting serum insulin levels were decreased in male and female GH−/− mice (Fig. 3B). GH−/− mice were significantly intolerant of an IP glucose load, with males showing a more pronounced intolerance than females (Fig. 3C and 3D). Male and female GH−/− mice demonstrated greatly enhanced insulin sensitivity compared with WT controls (Fig. 3E and 3F). It is important to note that GH−/− mice were extremely insulin sensitive; thus, ITTs were performed with much lower doses of insulin (0.5 U/kg body weight for males and 0.4 U/kg body weight for females) than commonly used in our laboratory (i.e., 0.75 to 2 U/kg). Because the GH−/− mice had impaired glucose tolerance, we evaluated pancreatic islet cell size. Mean islet size was significantly decreased for both male (Fig. 3G–3I) and female (Fig. 3J–3L) GH−/− mice compared with controls.
Figure 3.
Measures of glucose homeostasis in GH−/− mice. (A) Fasting blood glucose and (B) fasting serum insulin levels at age 6 mo (n = 8 to 11). GTTs in (C) males and (D) females at age 5 mo (n = 8 to 11). ITTs in (E) males and (F) females at age 5.5 mo (n = 8 to 11). Mean pancreatic islet cell size is shown for (G) males and (J) females. (H, I, K, L) Representative histological images. Hematoxylin and eosin–stained pancreatic islets are shown at ×20 magnification. Scale bars, 100 μm. Vertical bars represent standard error. *P < 0.05, significantly different from WT controls.
GH gene disruption affected organ size and hepatic triglyceride content
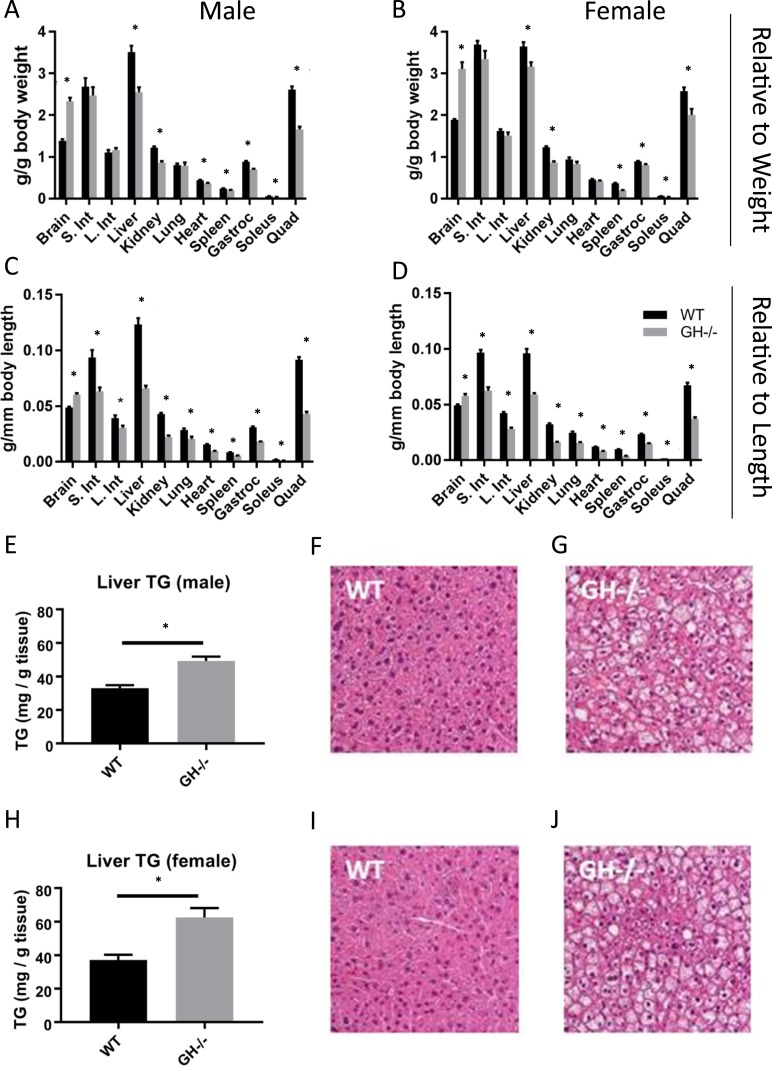
Relative organ size (organ mass divided by body mass) was altered in GH−/− mice in several tissues (Fig. 4). The masses of liver, kidney, heart, spleen, gastrocnemius, soleus, and quadriceps were significantly decreased in GH−/− mice compared with WT controls, whereas relative brain weight was significantly increased (Fig. 4A and 4B). Males and females showed similar results with the exception of heart, which was decreased in males only. When organ size was evaluated relative to body length, all tissues were significantly smaller except brain, which remained significantly larger (Fig. 4C and 4D). Additional analysis of hepatic tissue showed that liver triglyceride content was elevated in the absence of GH in both male and female GH−/− mice (Fig. 4E and 4H). This increase in hepatic triglyceride content was confirmed by hematoxylin and eosin–stained sections of hepatic tissue (Fig. 4F, 4G, 4I, 4J).
Figure 4.
Effects of GH gene disruption on organ size, liver triglyceride content, and liver histology. Organ mass relative to body mass in 6-mo-old (A) males and (B) females (n = 8 to 11). Organ mass relative to body length in 6-mo-old (C) males and (D) females (n = 8 to 11). Triglyceride content and liver histology in liver tissue from 6-mo-old (E–G) males and (H–J) females (n = 8 to 11). Liver images are shown at ×400 magnification. Vertical bars represent standard error. *P < 0.05, significantly different from WT controls. Gastroc, gastrocnemius; Int, intestine; L, large; Quad, quadriceps; S, small; TG, triglyceride.
Subcutaneous but not perigonadal WAT was disproportionately enlarged, with increased adipocyte size and decreased markers of fibrosis
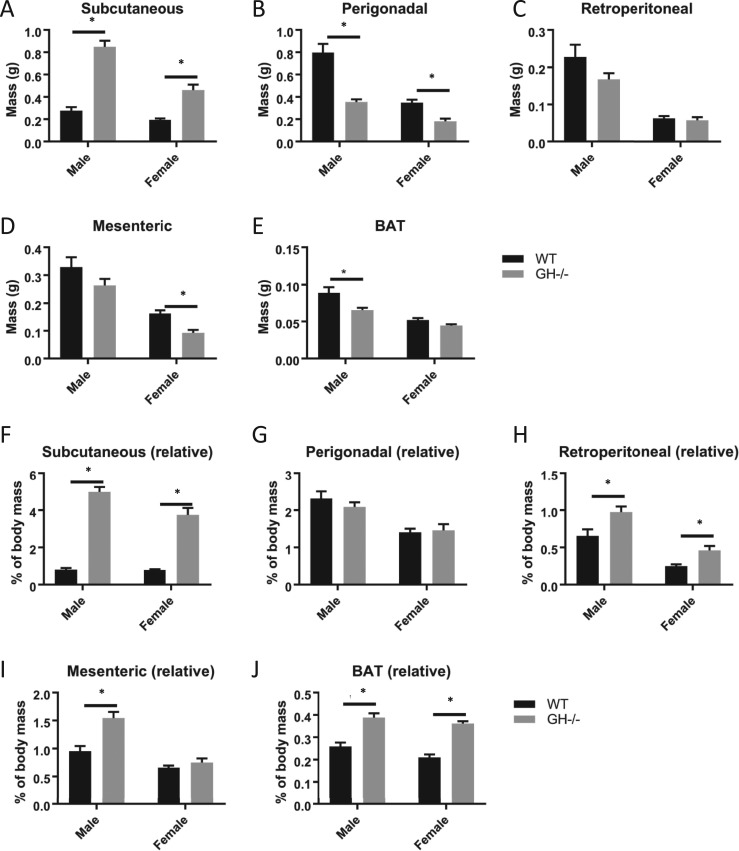
Four different WAT depots and the interscapular BAT depot were removed, and masses were recorded (Fig. 5). Absolute depot mass (Fig. 5A–5E) as well as depot mass relative to body mass (Fig. 5F–5J) are reported. The subcutaneous WAT depot was significantly enlarged regardless of analysis method (absolute and normalized to body weight) in male and female GH−/− mice compared with controls (Fig. 5A and 5F). Perigonadal mass was decreased in GH−/− mice compared with controls (Fig. 5B) but unchanged when normalized to body mass in both sexes (Fig. 5G). Retroperitoneal mass was unchanged in GH−/− mice (Fig. 5C) but increased when normalized to body mass in both sexes (Fig. 5H). Mesenteric mass was unchanged in male GH−/− mice but increased when normalized to body mass, whereas the mass of female mesenteric WAT was decreased in females but unchanged when normalized (Fig. 5D and 5I). Interscapular BAT mass was decreased in male and unchanged in female GH−/− mice compared with controls (Fig. 5E) but increased when normalized to body mass in both sexes (Fig. 5J).
Figure 5.
Depot-specific analysis of adipose tissue mass. (A–E) Raw adipose tissue depot mass and (F–J) depot mass relative to body mass are shown. All analyses were done on 6-mo-old mice (n = 8 to 11). Vertical bars represent standard error. *P < 0.05, significantly different from WT controls.
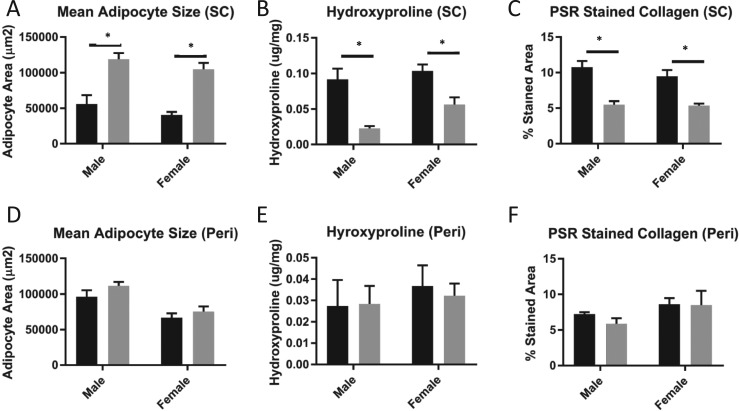
Cell size was analyzed in adipocytes from subcutaneous (Fig. 6A) and perigonadal (Fig. 6D) WAT. Adipocyte size in the subcutaneous depot was significantly increased in GH−/− mice for both sexes. Adipocytes from the perigonadal depot were unchanged in male and female GH−/− mice compared with controls. Hydroxyproline content was significantly decreased in subcutaneous WAT of GH−/− mice of both sexes compared with controls (Fig. 6B). Similarly, subcutaneous WAT of GH−/− mice had significantly decreased percent area of PSR for both sexes compared with controls (Fig. 6C). In perigonadal WAT, the hydroxyproline content as well as percent area of PSR were unchanged between GH−/− and controls in both sexes (Fig. 6E and 6F).
Figure 6.
Adipocyte size and level of fibrosis in subcutaneous (SC) vs perigonadal (Peri) WAT. Mean adipocyte size is shown for (A) SC and (D) Peri WAT depots. Hydroxyproline content is shown for (B) SC WAT and (E) Peri WAT. Percentage of stained PSR tissue area is shown for (C) SC WAT and (F) Peri WAT. Black bars represent control mice; gray bars represent GH−/− mice. All analyses were done on 6-mo-old mice (n = 8 to 11). Vertical bars represent standard error. *P < 0.05, significantly different from WT controls.
GH stimulated growth and abrogated the enhanced insulin sensitivity in GH−/− mice
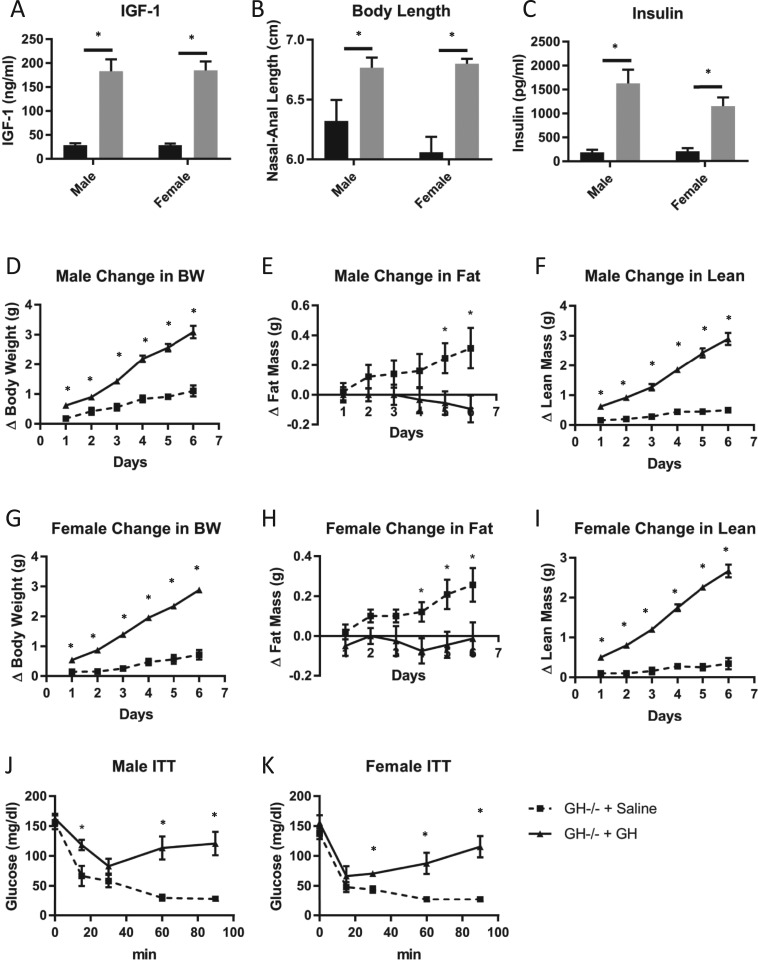
To ensure that GH−/− mice were responsive to GH, young (5-week-old) GH−/− mice were either treated with GH for 6 days or were not. Serum IGF-1 levels (Fig. 7A) and nasal-anal body length (Fig. 7B) were significantly increased in male and female GH−/− mice treated with GH compared with GH−/− mice treated with saline. Body composition analysis (Fig. 7D–7I) showed that GH treatment increased body weight (Fig. 7D and 7G) and lean body mass (Fig. 7F and 7I) while decreasing fat mass (Fig. 7E and 7H) in male and female GH−/− mice. Furthermore, GH treatment increased fasting insulin levels (Fig. 7C) and decreased insulin sensitivity (Fig. 7J and 7K) in male and female GH−/− mice compared with saline-treated controls.
Figure 7.
Effects of GH vs saline treatment in GH−/− mice. Levels of (A) plasma IGF-1, (B) body length, and (C) fasting plasma insulin are shown for both sexes. (A–C) GH−/− control mice receiving saline injections are represented by black bars; GH−/− mice receiving GH are represented by gray bars. Measures of body composition for (D–F) males and (G–I) females are shown and include change in (D, G) body weight (BW), (E, H) fat mass, and (F, I) lean mass. Insulin tolerance of (J) male and (K) female mice is shown. (D–K) Saline-treated GH−/− control mice are represented by square boxes and dashed lines; GH-treated GH−/− mice are represented by circles and solid lines. Vertical bars represent standard error. All analyses were performed on mice from 5 to 6 wk of age (n = 4 to 6). * P < 0.05, significantly different from WT controls.
Discussion
In this study, we report initial characterization of a mouse line to have targeted disruption of the GH gene. To more thoroughly evaluate the effects of GH gene disruption, male and female mice were studied. GH−/− mice have severely decreased IGF-1 levels, small body size, and altered body composition with an increased percentage of fat mass and decreased percentage of lean mass. GH−/− mice are extremely insulin sensitive, with decreased fasting blood glucose, insulin, and c-peptide levels. Despite extreme insulin sensitivity, GH−/− mice are glucose intolerant. Histological analyses of pancreatic islets suggest that decreased islet size likely contributes to the inability to deal with the exogenous glucose load. Livers of GH−/− mice were disproportionally smaller and had increased triglyceride content. Depot-specific analysis of WAT in these mice showed that increased adiposity was mainly due to a prominent increase in the subcutaneous depot, showing that GH action on WAT is depot dependent. Moreover, subcutaneous WAT had significantly reduced markers of fibrosis, because both hydroxyproline and PSR were decreased specifically in the subcutaneous but not perigonadal WAT depot. This may be partially explained by adipocyte size, because adipocytes that make up the subcutaneous WAT depot have a greater enlargement than adipocytes that make up the perigonadal WAT depot after GH gene disruption; however, adipocyte size alone does fully account for the reductions observed. Importantly, GH−/− mice were responsive to GH treatment: 6 days of injections in 5-week-old mice significantly increased serum IGF-1 levels, body length and weight, and lean mass, and significantly decreased fat mass. GH treatment also abrogated the enhanced insulin sensitivity and low insulin levels found in these mice.
Although several GH-deficient mouse lines exist, a GH knockout mouse line via targeted mutation of the GH gene has yet to be described in the literature, to our knowledge. For example, several mouse lines are available with mutations or gene deletions to components of the GH regulatory pathways, including the lit/lit mouse line, which is caused by a mutation to the GHRH receptor (24) and GHRH−/− mice, which lack the GHRH gene (25). However, despite severe reductions in GH secretion, the GH gene remains intact in these lines, and pituitary GH production is not completely eliminated in these mice. Furthermore, because both of these lines rely on disruption of hypothalamic/pituitary control of GH, we would also expect that extrapituitary GH remains fully intact. This is important, considering that GH gene expression is not confined to the pituitary gland; extrapituitary GH expression occurs in neural, immune, reproductive, alimentary, and respiratory tissues, and in the integumentary, muscular, skeletal, and cardiovascular systems (26–28). In addition, two commonly used GH-deficient mouse lines are the Snell and Ames dwarf mice. Both these mouse lines lack pituitary GH, due to mutations to genes involved in pituitary development, namely the Pit1 and Prop1 genes, respectively (29). However, these mouse lines also lack prolactin and TSH, making GH-specific interpretations from these mice challenging. For these reasons, the extensive use of the GHR−/− mouse line in the GH field is not surprising, because they are completely GH insensitive and thus unaffected by pituitary or extrapituitary GH. However, because GHR−/− mice lack the GHR and are GH resistant, they cannot be used in studies where GH replacement is needed. Therefore, the combination of completely lacking GH action (similar to GHR−/− mice) but still being responsive to GH (similar to Ames, Snell, lit/lit, and GHRH−/− mice) make the GH−/− a unique mouse line. Accordingly, this new mouse line has great potential for clinical impact and utility, with countless translational uses for further studies.
In contrast to human GH, which binds to both the GH and prolactin receptors, mouse GH is thought to be exclusive to the GHR. To help test this assumption, there is value in comparing our findings in GH−/− mice with previous findings in GHR−/− mice. Phenotypic differences between the two lines would suggest that GH and the GHR might not be completely exclusive to one another. To facilitate this comparison, we crossed our GH−/− mouse line into the same background strain (C57BL/6J) as our GHR−/− mice. Overall, GH−/− mice have a remarkably similar phenotype to GHR−/− mice, despite underlying genetic differences (i.e., GH vs GHR gene disruption) between the two mouse lines (Table 1). The only striking difference is that GH levels in GHR−/− mice are significantly increased, whereas circulating GH is absent in GH−/− mice. However, this is expected, because the GHR−/− mouse line was generated by disrupting the GH receptor/binding protein gene, resulting in GH insensitivity and a phenotype similar to the clinical condition of Laron syndrome (4, 5). Regardless, both mouse lines have a significant reduction in IGF-1 levels (4), suggesting the observed similarities in phenotype are due to an absence in GH action. GH−/− mice have significantly decreased body length and weight, similar to well-documented findings in the GHR−/− mice (4, 7, 30, 31). Both mouse lines have a similar pattern of body composition, with a reduction in percent lean mass and significant increase in percent body fat (7, 30–34). Visceral organs (e.g., kidney, heart, spleen) and skeletal muscle are reduced in mass proportional to the reduction in body mass, whereas the relative brain weight is significantly increased and liver weight is disproportionately decreased (6). GH−/− mice have elevated levels of liver triglycerides, which is consistent with other reports in mouse lines with reduced GH action (e.g., GHR−/− and Ames mice) as well as individuals with Laron syndrome (35–38). However, the increase in liver triglycerides in GHR−/− mice may be age dependent, because liver triglyceride levels are not different from those of controls at advanced age (2 years) (30). GH−/− mice have a similar glucose homeostatic profile to GHR−/− mice. Both mouse lines have reduced fasting glucose and serum insulin levels (8, 31, 39). GH−/− mice are glucose intolerant but have enhanced insulin sensitivity, well-known characteristics of the GHR−/− mouse line documented throughout their lifespan (5, 8, 39, 40). Likewise, GH−/− and GHR−/− mice are glucose intolerant, in part due to the significantly smaller pancreatic islets in both mouse lines (8). Although not assessed for GH−/− mice, GHR−/− mice also have reduced insulin content within the β cells (8), which may explain the duality of impaired glucose tolerance and enhanced insulin sensitivity. Although the findings are relatively similar between the GH−/− mice and GHR−/− mice, it is important to note inconsistencies in the ages and sex used for these experiments. That is, the pancreatic islet cell size measurements have only been reported in 2-month-old male GHR−/− mice (8) compared with the 6-month-old male and female GH−/− mice used in this study. The GTTs and ITTs previously have been conducted exclusively in male GHR−/− mice, whereas this study examined both sexes of GH−/− mice. Furthermore, there are sex-dependent differences seen in glucose homeostasis in the GH−/− mice, with a more pronounced phenotype in males, highlighting the importance of examining the phenotype in both sexes.
Table 1.
Phenotypic Comparison of GH−/− and GHR−/− Mice
| GH−/− | GHR−/−a | |
|---|---|---|
| Human disorder equivalent | IGHD type IA | Laron syndrome |
| Gene disrupted | Gh | Ghr |
| GH | Not detected | ↑ |
| IGF-1 | ↓ | ↓ |
| Fasting blood glucose levels | ↓ | ↓ |
| Fasting insulin levels | ↓ | ↓ |
| ITT result | ↑ | ↑ |
| GTT result | ↓ | ↓ |
| Pancreatic islet size | ↓ | ↓ |
| Adiposity (% body fat) | ↑ | ↑ |
| FFA levels | ↑ | ↑ |
| Liver triglyceride levels | ↑ | ↑ |
| WAT depot most enlarged | SC | SC |
| Adipocyte size | ||
| SC | ↑ | ↑ |
| Visceral (perigonadal) | ↔ | ↔ |
| Lifespan | ? | ↑ |
Abbreviations: ↑ increased; ↓ decreased; ↔ unchanged; ?, unknown; FFA, free fatty acid; IGHD, isolated GH deficiency; SC, subcutaneous.
aData from GHR−/− mice were reviewed in List et al. (5).
GH has a lipolytic effect on adipose tissue that is depot dependent (23, 41, 42). As such, increased WAT accumulation, with preferential enlargement of the subcutaneous depots, has been well documented in mouse lines with decreased GH action, including GHR−/− mice (30, 31, 42), GHR-antagonist transgenic mice (41, 43), Ames dwarf mice (44), and adult specific GHR−/− mice (45). A similar trend of increased, subcutaneous WAT accumulation has also been reported in two separate, fat-specific GHRKO mouse lines, FaGHRKO and AdGHRKO mice (17, 46). Based on these results, it is not surprising that an increase in WAT predominantly in the subcutaneous region was also observed in our GH−/− mice. Likewise, in mice with reduced GH action, most other depots are proportional to the dwarf size of the animal, which was also observed in the GH−/− mice. With the increase in subcutaneous WAT mass, GH−/− mice exhibit an increase in subcutaneous adipocyte size, again a pattern similar to that of other mice with reduced GH action (42) and similar to mice with adipocyte-specific reduction in GH action (46). The underlying mechanisms contributing to the depot-specific role of GH on WAT remains poorly understood. However, depot differences have been reported for mice with modified GH action in IGF-1R expression (47), insulin receptor expression (47, 48), fibrosis (20, 46), proliferation and differentiation of preadipocytes (49–51), and cellular senescence (52–54). Importantly, the global trend we observed with GH−/− mice in terms of mass, adipocyte size, and fibrosis matches what would be expected based on other mice with decreased GH action; however, these mice also provide the added benefit of being able to determine what would happen to WAT with acute GH treatment in future studies. WAT fibrosis has emerged as an important contributor to health of the tissue, with increases associated with tissue hypoxia and the metabolic complications associated with obesity (55, 56). In this study we evaluated the effects of a complete loss of GH action on fibrosis in WAT. Subcutaneous WAT from male and female GH−/− mice has significantly decreased collagen deposition, indicative of reduced fibrosis. Moreover, the reduction in fibrosis occurred specifically in the subcutaneous and not the perigonadal WAT depot in both sexes. As stated previously, this may be partially explained by increased adipocyte size; however, adipocyte size alone does not fully account for the reductions observed. Finally, it is important to point out that there is potential concern for housing temperature in mouse studies, especially with dwarf mice. Because the vast majority of mouse studies are conducted at room temperature (∼22°C) and thermoneutrality for mice is ∼30°C to 32°C, there are potential confounding effects with mice of different sizes, such as increased metabolic rates required to maintain basal body temperature.
In conclusion, GH−/− mice exhibit a remarkably similar phenotype in body size, composition, glucose homeostasis, and WAT changes compared with GHR−/− mice, suggesting that in mice, GH signals largely, if not exclusively, through the GHR. Moreover, we found that reduction in fibrosis occurred specifically in the subcutaneous and not the perigonadal WAT depot in both sexes. Combined data from this and previous studies strongly suggest that removal of GH action reduces fibrosis in WAT. This effect appears to be specific to the subcutaneous WAT depot and is not sex dependent. Importantly, GH−/− mice are responsive to GH treatment, as shown by changes to serum IGF-1, body length, body weight, and lean and fat mass. Thus, results of this study not only provide insight into GH’s effect on glucose metabolism and adipose tissue biology but also provide useful characterization of the first mouse line with specific targeted mutation of the GH gene, which makes available an important new tool to the field of endocrinology: the GH knockout mouse.
Acknowledgments
Financial Support: E.O.L. was supported by Ohio University Baker and OURC grants and a Grant for Growth Innovation from Merck KGaA, Darmstadt, Germany. J.J.K., E.O.L., and D.E.B. were supported by the National Institutes of Health (Grant R01AG059779). J.J.K. was also supported by the State of Ohio’s Eminent Scholar Program, which includes a gift from Milton and Lawrence Goll; the AMVETS; and the Diabetes Institute at Ohio University.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- BAT
brown adipose tissue
- GHR
GH receptor
- GHR−/−
GH receptor knockout
- GTT
glucose tolerance test
- ITT
insulin tolerance test
- PSR
picrosirius-red staining
- WAT
white adipose tissue
- WT
wild-type
References and Notes
- 1. Houssay B. The hypophysis and metabolism. N Engl J Med. 1936;214(20):961–971. [Google Scholar]
- 2. Rabinowitz D, Klassen GA, Zierler KL. Effect of human growth hormone on muscle and adipose tissue metabolism in the forearm of man. J Clin Invest. 1965;44(1):51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rabinowitz D, Zierler KL. A metabolic regulating device based on the actions of human growth hormone and of insulin, singly and together, on the human forearm. Nature. 1963;199(4896):913–915. [DOI] [PubMed] [Google Scholar]
- 4. Zhou Y, Xu BC, Maheshwari HG, He L, Reed M, Lozykowski M, Okada S, Cataldo L, Coschigamo K, Wagner TE, Baumann G, Kopchick JJ. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse). Proc Natl Acad Sci USA. 1997;94(24):13215–13220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. List EO, Sackmann-Sala L, Berryman DE, Funk K, Kelder B, Gosney ES, Okada S, Ding J, Cruz-Topete D, Kopchick JJ. Endocrine parameters and phenotypes of the growth hormone receptor gene disrupted (GHR-/-) mouse. Endocr Rev. 2011;32(3):356–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. List EO, Coschigano KT, Kopchick JJ. Growth hormone receptor/binding protein (GHR/BP) knockout mice: a 3-year update. Mol Genet Metab. 2001;73(1):1–10. [DOI] [PubMed] [Google Scholar]
- 7. Berryman DE, List EO, Coschigano KT, Behar K, Kim JK, Kopchick JJ. Comparing adiposity profiles in three mouse models with altered GH signaling. Growth Horm IGF Res. 2004;14(4):309–318. [DOI] [PubMed] [Google Scholar]
- 8. Liu JL, Coschigano KT, Robertson K, Lipsett M, Guo Y, Kopchick JJ, Kumar U, Liu YL. Disruption of growth hormone receptor gene causes diminished pancreatic islet size and increased insulin sensitivity in mice. Am J Physiol Endocrinol Metab. 2004;287(3):E405–E413. [DOI] [PubMed] [Google Scholar]
- 9. Pilcher HR. Money for old mice. Competition seeks world's longest-lasting mouse. Nature News. 2003;22:1–2. [Google Scholar]
- 10. Ikeno Y, Hubbard GB, Lee S, Cortez LA, Lew CM, Webb CR, Berryman DE, List EO, Kopchick JJ, Bartke A. Reduced incidence and delayed occurrence of fatal neoplastic diseases in growth hormone receptor/binding protein knockout mice. J Gerontol A Biol Sci Med Sci. 2009;64(5):522–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang Z, Prins GS, Coschigano KT, Kopchick JJ, Green JE, Ray VH, Hedayat S, Christov KT, Unterman TG, Swanson SM. Disruption of growth hormone signaling retards early stages of prostate carcinogenesis in the C3(1)/T antigen mouse. Endocrinology. 2005;146(12):5188–5196. [DOI] [PubMed] [Google Scholar]
- 12. Zhang X, Mehta RG, Lantvit DD, Coschigano KT, Kopchick JJ, Green JE, Hedayat S, Christov KT, Ray VH, Unterman TG, Swanson SM. Inhibition of estrogen-independent mammary carcinogenesis by disruption of growth hormone signaling. Carcinogenesis. 2007;28(1):143–150. [DOI] [PubMed] [Google Scholar]
- 13. Shevah O, Laron Z. Patients with congenital deficiency of IGF-I seem protected from the development of malignancies: a preliminary report. Growth Horm IGF Res. 2007;17:54–57. [DOI] [PubMed] [Google Scholar]
- 14. Steuerman R, Shevah O, Laron Z. Congenital IGF1 deficiency tends to confer protection against post-natal development of malignancies. Eur J Endocrinol. 2011;164(4):485–489. [DOI] [PubMed] [Google Scholar]
- 15. Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, Wei M, Madia F, Cheng CW, Hwang D, Martin-Montalvo A, Saavedra J, Ingles S, de Cabo R, Cohen P, Longo VD. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci Transl Med. 2011;3(70):70ra13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Valenzuela DM, Murphy AJ, Frendewey D, Gale NW, Economides AN, Auerbach W, Poueymirou WT, Adams NC, Rojas J, Yasenchak J, Chernomorsky R, Boucher M, Elsasser AL, Esau L, Zheng J, Griffiths JA, Wang X, Su H, Xue Y, Dominguez MG, Noguera I, Torres R, Macdonald LE, Stewart AF, DeChiara TM, Yancopoulos GD. High-throughput engineering of the mouse genome coupled with high-resolution expression analysis. Nat Biotechnol. 2003;21(6):652–659. [DOI] [PubMed] [Google Scholar]
- 17. List EO, Berryman DE, Funk K, Gosney ES, Jara A, Kelder B, Wang X, Kutz L, Troike K, Lozier N, Mikula V, Lubbers ER, Zhang H, Vesel C, Junnila RK, Frank SJ, Masternak MM, Bartke A, Kopchick JJ. The role of GH in adipose tissue: lessons from adipose-specific GH receptor gene-disrupted mice. Mol Endocrinol. 2013;27(3):524–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. List EO, Berryman DE, Funk K, Jara A, Kelder B, Wang F, Stout MB, Zhi X, Sun L, White TA, LeBrasseur NK, Pirtskhalava T, Tchkonia T, Jensen EA, Zhang W, Masternak MM, Kirkland JL, Miller RA, Bartke A, Kopchick JJ. Liver-specific GH receptor gene-disrupted (LiGHRKO) mice have decreased endocrine IGF-I, increased local IGF-I, and altered body size, body composition, and adipokine profiles. Endocrinology. 2014;155(5):1793–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. List EO, Berryman DE, Ikeno Y, Hubbard GB, Funk K, Comisford R, Young JA, Stout MB, Tchkonia T, Masternak MM, Bartke A, Kirkland JL, Miller RA, Kopchick JJ. Removal of growth hormone receptor (GHR) in muscle of male mice replicates some of the health benefits seen in global GHR-/- mice. Aging (Albany NY). 2015;7(7):500–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Householder LA, Comisford R, Duran-Ortiz S, Lee K, Troike K, Wilson C, Jara A, Harberson M, List EO, Kopchick JJ, Berryman DE. Increased fibrosis: a novel means by which GH influences white adipose tissue function. Growth Horm IGF Res. 2018;39:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tchoukalova YD, Koutsari C, Votruba SB, Tchkonia T, Giorgadze N, Thomou T, Kirkland JL, Jensen MD. Sex- and depot-dependent differences in adipogenesis in normal-weight humans. Obesity (Silver Spring). 2010;18(10):1875–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Woessner JF., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93(2):440–447. [DOI] [PubMed] [Google Scholar]
- 23. List EO, Palmer AJ, Berryman DE, Bower B, Kelder B, Kopchick JJ. Growth hormone improves body composition, fasting blood glucose, glucose tolerance and liver triacylglycerol in a mouse model of diet-induced obesity and type 2 diabetes. Diabetologia. 2009;52(8):1647–1655. [DOI] [PubMed] [Google Scholar]
- 24. Flurkey K, Papaconstantinou J, Miller RA, Harrison DE. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc Natl Acad Sci USA. 2001;98(12):6736–6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alba M, Salvatori R. A mouse with targeted ablation of the growth hormone-releasing hormone gene: a new model of isolated growth hormone deficiency. Endocrinology. 2004;145(9):4134–4143. [DOI] [PubMed] [Google Scholar]
- 26. Harvey S, Johnson CD, Sanders EJ. Extra-pituitary growth hormone in peripheral tissues of early chick embryos. J Endocrinol. 2000;166(3):489–502. [DOI] [PubMed] [Google Scholar]
- 27. Harvey S. Extrapituitary growth hormone. Endocrine. 2010;38(3):335–359. [DOI] [PubMed] [Google Scholar]
- 28. Daude N, Lee I, Kim TK, Janus C, Glaves JP, Gapeshina H, Yang J, Sykes BD, Carlson GA, Hood LE, Westaway D. A common phenotype polymorphism in mammalian brains defined by concomitant production of prolactin and growth hormone. PLoS One. 2016;11(2):e0149410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bartke A, Westbrook R. Metabolic characteristics of long-lived mice. Front Genet. 2012;3:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Berryman DE, List EO, Palmer AJ, Chung MY, Wright-Piekarski J, Lubbers E, O’Connor P, Okada S, Kopchick JJ. Two-year body composition analyses of long-lived GHR null mice. J Gerontol A Biol Sci Med Sci. 2010;65(1):31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sackmann-Sala L, Berryman DE, Lubbers ER, Zhang H, Vesel CB, Troike KM, Gosney ES, List EO, Kopchick JJ. Age-related and depot-specific changes in white adipose tissue of growth hormone receptor-null mice. J Gerontol A Biol Sci Med Sci. 2014;69(1):34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bonkowski MS, Pamenter RW, Rocha JS, Masternak MM, Panici JA, Bartke A. Long-lived growth hormone receptor knockout mice show a delay in age-related changes of body composition and bone characteristics. J Gerontol A Biol Sci Med Sci. 2006;61(6):562–567. [DOI] [PubMed] [Google Scholar]
- 33. Berryman DE, List EO, Kohn DT, Coschigano KT, Seeley RJ, Kopchick JJ. Effect of growth hormone on susceptibility to diet-induced obesity. Endocrinology. 2006;147(6):2801–2808. [DOI] [PubMed] [Google Scholar]
- 34. Egecioglu E, Bjursell M, Ljungberg A, Dickson SL, Kopchick JJ, Bergström G, Svensson L, Oscarsson J, Törnell J, Bohlooly-Y M. Growth hormone receptor deficiency results in blunted ghrelin feeding response, obesity, and hypolipidemia in mice. Am J Physiol Endocrinol Metab. 2006;290(2):E317–E325. [DOI] [PubMed] [Google Scholar]
- 35. Stiedl P, McMahon R, Blaas L, Stanek V, Svinka J, Grabner B, Zollner G, Kessler SM, Claudel T, Müller M, Mikulits W, Bilban M, Esterbauer H, Eferl R, Haybaeck J, Trauner M, Casanova E. Growth hormone resistance exacerbates cholestasis-induced murine liver fibrosis. Hepatology. 2015;61(2):613–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bartke A, Peluso MR, Moretz N, Wright C, Bonkowski M, Winters TA, Shanahan MF, Kopchick JJ, Banz WJ. Effects of soy-derived diets on plasma and liver lipids, glucose tolerance, and longevity in normal, long-lived and short-lived mice. Horm Metab Res. 2004;36(8):550–558. [DOI] [PubMed] [Google Scholar]
- 37. Wang Z, Al-Regaiey KA, Masternak MM, Bartke A. Adipocytokines and lipid levels in Ames dwarf and calorie-restricted mice. J Gerontol A Biol Sci Med Sci. 2006;61(4):323–331. [DOI] [PubMed] [Google Scholar]
- 38. Laron Z, Ginsberg S, Webb M. Nonalcoholic fatty liver in patients with Laron syndrome and GH gene deletion - preliminary report. Growth Horm IGF Res. 2008;18(5):434–438. [DOI] [PubMed] [Google Scholar]
- 39. Coschigano KT, Holland AN, Riders ME, List EO, Flyvbjerg A, Kopchick JJ. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology. 2003;144(9):3799–3810. [DOI] [PubMed] [Google Scholar]
- 40. Lubbers ER, List EO, Jara A, Sackman-Sala L, Cordoba-Chacon J, Gahete MD, Kineman RD, Boparai R, Bartke A, Kopchick JJ, Berryman DE. Adiponectin in mice with altered GH action: links to insulin sensitivity and longevity? J Endocrinol. 2013;216(3):363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Berryman DE, List EO, Coschigano KT, Behar K, Kim JK, Kopchick JJ. Comparing adiposity profiles in three mouse models with altered GH signaling. Growth Horm IGF Res. 2004;14:309–318. [DOI] [PubMed] [Google Scholar]
- 42. Berryman DE, List EO. Growth hormone’s effect on adipose tissue: quality versus quantity. Int J Mol Sci. 2017;18(8):e1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Berryman DE, Lubbers ER, Magon V, List EO, Kopchick JJ. A dwarf mouse model with decreased GH/IGF-1 activity that does not experience life-span extension: potential impact of increased adiposity, leptin, and insulin with advancing age. J Gerontol A Biol Sci Med Sci. 2014;69(2):131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Heiman ML, Tinsley FC, Mattison JA, Hauck S, Bartke A. Body composition of prolactin-, growth hormone, and thyrotropin-deficient Ames dwarf mice. Endocrine. 2003;20(1-2):149–154. [DOI] [PubMed] [Google Scholar]
- 45. Junnila RK, Duran-Ortiz S, Suer O, Sustarsic EG, Berryman DE, List EO, Kopchick JJ. Disruption of the GH receptor gene in adult mice increases maximal lifespan in females. Endocrinology. 2016;157(12):4502–4513. [DOI] [PubMed] [Google Scholar]
- 46. List EO, Berryman DE, Buchman M, Parker C, Funk K, Bell S, Duran-Ortiz S, Qian Y, Young JA, Wilson C, Slyby J, McKenna S, Jensen EA, Kopchick JJ. Adipocyte-specific GH receptor-null (AdGHRKO) mice have enhanced insulin sensitivity with reduced liver triglycerides. Endocrinology. 2019;160(1):68–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hjortebjerg R, Berryman DE, Comisford R, Frank SJ, List EO, Bjerre M, Frystyk J, Kopchick JJ. Insulin, IGF-1, and GH receptors are altered in an adipose tissue depot-specific manner in male mice with modified GH action. Endocrinology. 2017;158(5):1406–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bennis MT, Schneider A, Victoria B, Do A, Wiesenborn DS, Spinel L, Gesing A, Kopchick JJ, Siddiqi SA, Masternak MM. The role of transplanted visceral fat from the long-lived growth hormone receptor knockout mice on insulin signaling. Geroscience. 2017;39(1):51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nam SY, Lobie PE. The mechanism of effect of growth hormone on preadipocyte and adipocyte function. Obes Rev. 2000;1(2):73–86. [DOI] [PubMed] [Google Scholar]
- 50. Kelder B, Berryman DE, Clark R, Li A, List EO, Kopchick JJ. CIDE-A gene expression is decreased in white adipose tissue of growth hormone receptor/binding protein gene disrupted mice and with high-fat feeding of normal mice. Growth Horm IGF Res. 2007;17(4):346–351. [DOI] [PubMed] [Google Scholar]
- 51. Olarescu NC, Berryman DE, Householder LA, Lubbers ER, List EO, Benencia F, Kopchick JJ, Bollerslev J. GH action influences adipogenesis of mouse adipose tissue-derived mesenchymal stem cells. J Endocrinol. 2015;226(1):13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stout MB, Tchkonia T, Pirtskhalava T, Palmer AK, List EO, Berryman DE, Lubbers ER, Escande C, Spong A, Masternak MM, Oberg AL, LeBrasseur NK, Miller RA, Kopchick JJ, Bartke A, Kirkland JL. Growth hormone action predicts age-related white adipose tissue dysfunction and senescent cell burden in mice. Aging (Albany NY). 2014;6(7):575–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Comisford R, Lubbers ER, Householder LA, Suer O, Tchkonia T, Kirkland JL, List EO, Kopchick JJ, Berryman DE. Growth hormone receptor antagonist transgenic mice have increased subcutaneous adipose tissue mass, altered glucose homeostasis and no change in white adipose tissue cellular senescence. Gerontology. 2016;62(2):163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Spadaro O, Goldberg EL, Camell CD, Youm YH, Kopchick JJ, Nguyen KY, Bartke A, Sun LY, Dixit VD. Growth hormone receptor deficiency protects against age-related NLRP3 inflammasome activation and immune senescence. Cell Reports. 2016;14(7):1571–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sun K, Tordjman J, Clément K, Scherer PE. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013;18(4):470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Crewe C, An YA, Scherer PE. The ominous triad of adipose tissue dysfunction: inflammation, fibrosis, and impaired angiogenesis. J Clin Invest. 2017;127(1):74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]