Abstract
We report the intramolecular Tsuji–Trost reaction of Ugi adducts to give spiro-diketopiperazines in high yield and with high enantioselectivity. This approach allows the catalytic asymmetric construction of a broad range of these medicinally important heterocycles under mild conditions, in two steps from cheap, commercially available starting materials.
Introduction
Heterocyclic small molecules are of immense importance in drug discovery. In recent years, the focus has shifted from purely aromatic heterocycles to scaffolds with a higher fraction of sp3-hybridized atoms.1 Evidently, this is accompanied by a higher number of stereogenic centers. Consequently, new strategies that allow straightforward access to such scaffolds with full stereochemical control are of high and continuous interest. In this context, intramolecular transition metal-catalyzed allylation reactions such as the Tsuji–Trost reaction offer great opportunities, given the high level of stereocontrol and typically mild reaction conditions.2 While considerable progress has been made in applying this strategy to the synthesis of (hetero)cyclic molecules, we aim to expand the current state of the art to more challenging systems, such as precursors bearing diverse functionalities and/or not naturally predisposed to adopt a favorable conformation for cyclization.
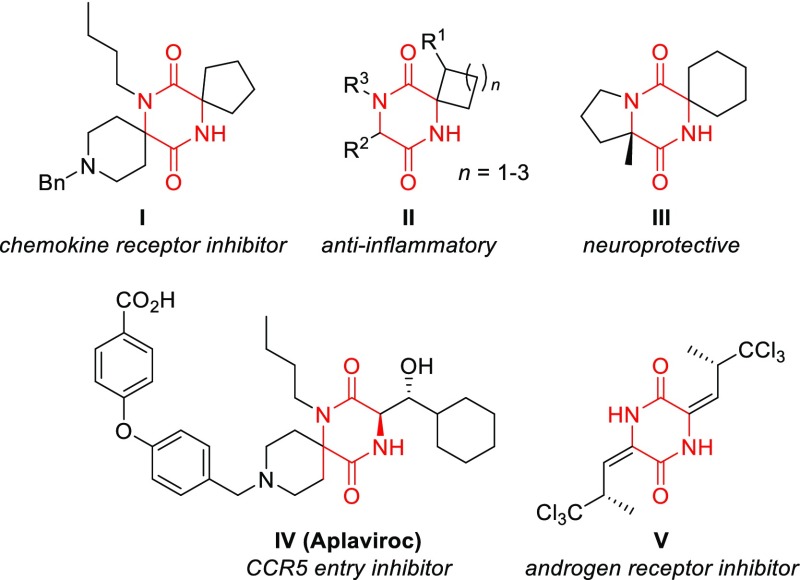
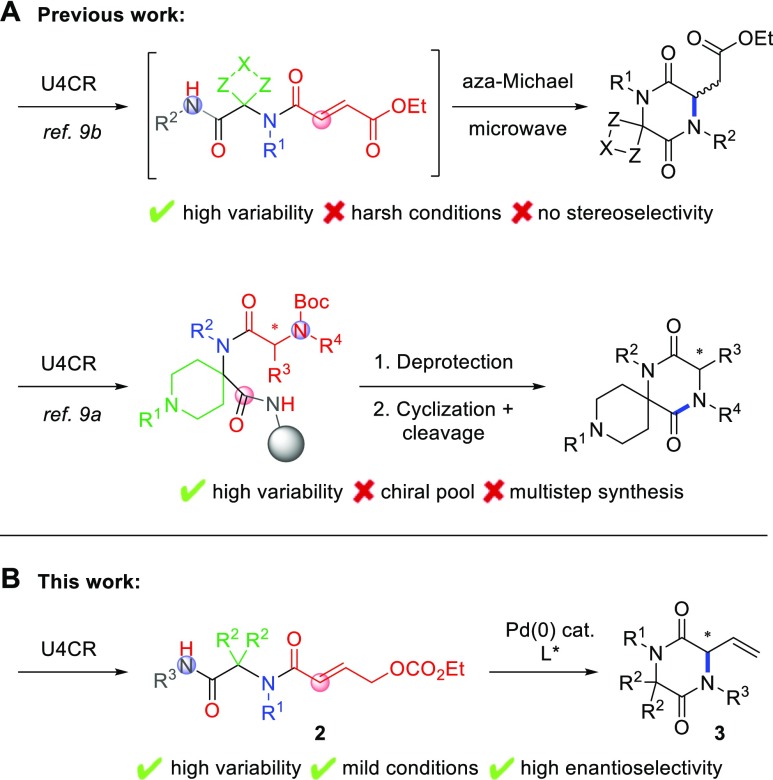
As an example, spiro-2,5-diketopiperazines3 (DKPs) display diverse biological activities (Figure 1), including neuroprotective properties,4 anti-inflammatory activity,5 and antiproliferative effects against drug-resistant human cancer cell lines.6 Despite these diverse medicinal properties, only few synthetic approaches to spiro-DKPs have been reported. Recent examples include Diels–Alder type reactions,7 intramolecular aminolysis,8 and post-Ugi cyclizations9,10 (Scheme 1A). Importantly, these methods invariably rely on chiral pool starting materials (mostly amino acids) as the source of chirality.3 Obviously, this leads to limited substituent variation, while the D-configured antipodes are often only available at considerably higher cost. Catalytic asymmetric methods that allow full stereochemical control in a late stage of the synthesis would greatly expand the range of accessible spiro-DKPs and thus their application in drug discovery.
Figure 1.
Bioactive compounds and natural products based on DKP scaffold.
Scheme 1. Synthesis of 2,5 DKPs by Post-Ugi Cyclization.
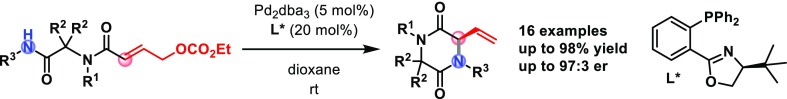
However, to the best of our knowledge, no catalytic asymmetric methods to prepare DKPs have been reported to date. In light of our interest in multicomponent reactions, palladium catalysis, and asymmetric synthesis, we envisioned the use of the versatile Ugi reaction to construct compounds 2 as substrates for an enantioselective intramolecular Tsuji–Trost reaction (Scheme 1B). Ugi adducts 2 can be regarded as challenging substrates for Tsuji–Trost cyclization, given their high degree of substitution, potentially unfavorable minimum energy conformation, and electron-deficient allylic system.11
On the other hand, strong bases such as LiHMDS, nBuLi, or NaH are usually required for the allylation of amides as a result of their low nucleophilicity in their neutral form.12 However, even a small excess of base could lead to racemization of the newly formed stereocenter (or isomerization of the alkene), while a substoichiometric amount of base would result in incomplete conversion. Thus, we decided to employ the ethyl carbonate as the leaving group in order to generate the base in situ in precisely stoichiometric amount.
Results and Discussion
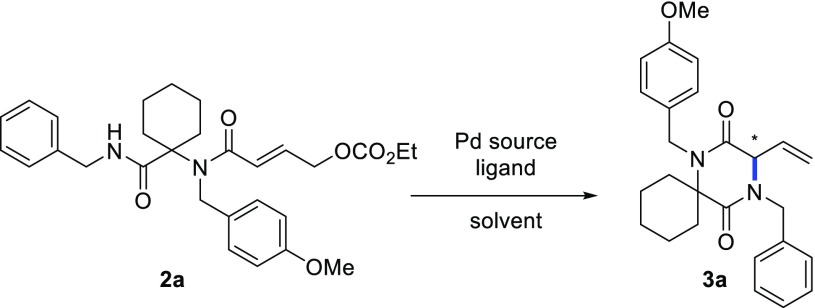
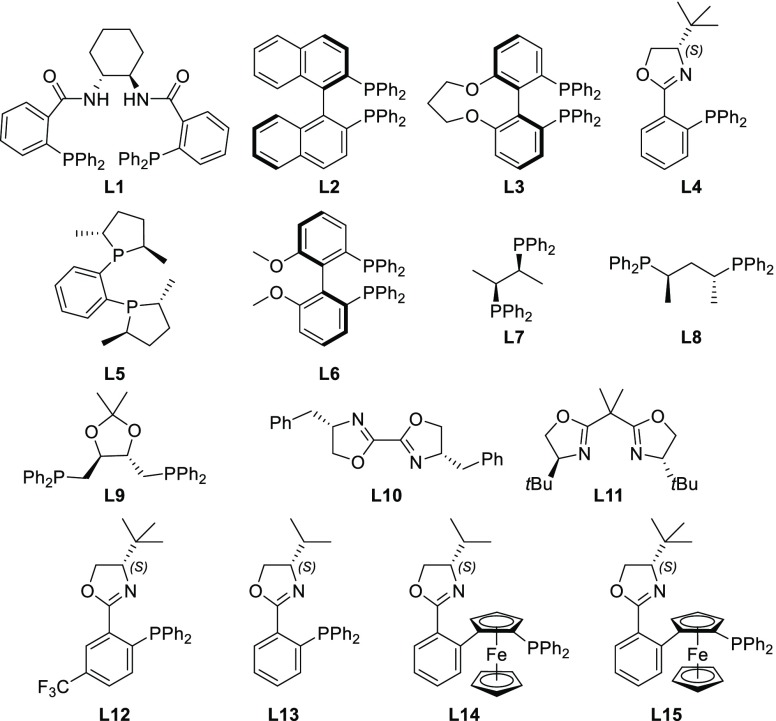
We began our investigation by treating the Ugi adduct 2aa with Pd2dba3 and dppe as the ligand in tetrahydrofuran (THF) at 50 °C. To our delight, the desired product 3a could be obtained in 86% yield after only 30 min (Table 1, entry 1). After screening various other palladium sources (Pd(PPh3)4, Pd(OAc)2, and [PdClallyl]2), it became evident that none could match the efficiency of Pd2dba3, which was thus selected for the screening of chiral ligands (Figure 2). Interestingly, reaction with the Trost ligand (L1) gave no conversion. Ligands L2, L5, and L7–9 showed poor enantioselectivity, despite the reasonable conversion (in case of L5 and L7–9). Higher stereoselectivity was observed with L3, L4, and L6, albeit with modest conversion. Remarkably, the enantioselectivity achieved with L2 was considerably lower than with L3 and L6, despite their similarity in terms of steric and electronic properties. Ligand L4(13) was selected for further optimization, combining the highest enantioselectivity with reasonable conversion. Having selected L4 as the best ligand, we performed the reaction at room temperature (entry 9), which led to a higher ee, although the reaction needed 24 h to reach completion and the yield dropped slightly. Switching the solvent to CH2Cl2 or toluene (entries 12 and 13) led to a decrease in enantioselectivity, whereas no reaction took place in dimethylformamide (DMF) (entry 14). On the other hand, using dioxane as the solvent gave the desired product in slightly better yield and enantioselectivity. Finally, we observed that the concentration plays a crucial role: running the reaction at higher dilution dramatically improved the yield as well as (to a minor degree) the enantioselectivity (entries 15–18), possibly as a result of the increased solubility of the palladium complex.14 Under the optimized conditions, we screened some additional ligands and conditions in an attempt to further improve the reaction outcome.
Table 1. Optimization of Reaction Conditions.
| entrya | ligand | solvent | T (°C) | yieldb (%) | erc |
|---|---|---|---|---|---|
| 1 | dpped | THF | 50 | 86 | |
| 2 | L1d | THF | 50 | ||
| 3 | L2d | THF | 50 | 35 | 58/42 |
| 4 | L3d | THF | 50 | 12 | 80/20 |
| 5 | L4e | THF | 50 | 46 | 88/12 |
| 6 | L5d | THF | 50 | 62 | 58/42 |
| 7 | L6d | THF | 50 | 17 | 82/18 |
| 8 | L7d | THF | 50 | 75 | 63/27 |
| 9 | L8d | THF | 50 | 72 | 60/40 |
| 10 | L9d | THF | 50 | 74 | 51/49 |
| 11 | L4e | THF | rt | 31 | 93/7 |
| 12 | L4e | CH2Cl2 | rt | 75 | 89/11 |
| 13 | L4e | PhMe | rt | 12 | 89/11 |
| 14 | L4e | DMF | rt | ||
| 15 | L4e | Diox | rt | 41 | 94/6 |
| 16f | L4e | Diox | rt | 53 | 95/5 |
| 17g | L4e | Diox | rt | 86 | 97/3 |
| 18h | L4e | Diox | rt | 58 | 97/3 |
| 19g | L10e | Diox | rt | ||
| 20g | L11e | Diox | rt | ||
| 21g | L12e | Diox | rt | 46 | 96/4 |
| 22g | L13e | Diox | rt | 49 | 71/29 |
| 23g | L14e | Diox | rt | 81 | 70/30 |
| 24g | L15e | Diox | rt | 88 | 95/5 |
Reaction conditions: 2aa (0.20 mmol), Pd2(dba)3 (0.01 mmol) in the indicated solvent (1 mL).
Isolated yield.
Determined by chiral HPLC.
0.02 mmol ligand.
0.04 mmol ligand.
0.05 M substrate concentration.
0.025 M substrate concentration.
0.01 M substrate concentration. Diox = 1,4-dioxane.
Figure 2.
Chiral ligands screened.
Bisoxazole ligands L10 and L11 did not promote the reaction. With ligand L12, which is highly similar to L4, the product was obtained in good stereoselectivity but with only moderate conversion. Ligand L15 performed similarly as L4, but gave slightly lower enantioselectivity. Substitution of the tBu group by an iPr group led to significant erosion of the stereoselectivity (entries 22 and 23). Finally, the use of Me and tBu carbonates proved less efficient, and the addition of commonly used halide additives (LiCl, nBu4NCl, nBu4NF) was found to completely inhibit the reaction (for details, see the Supporting Information).
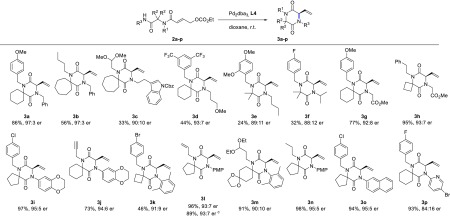
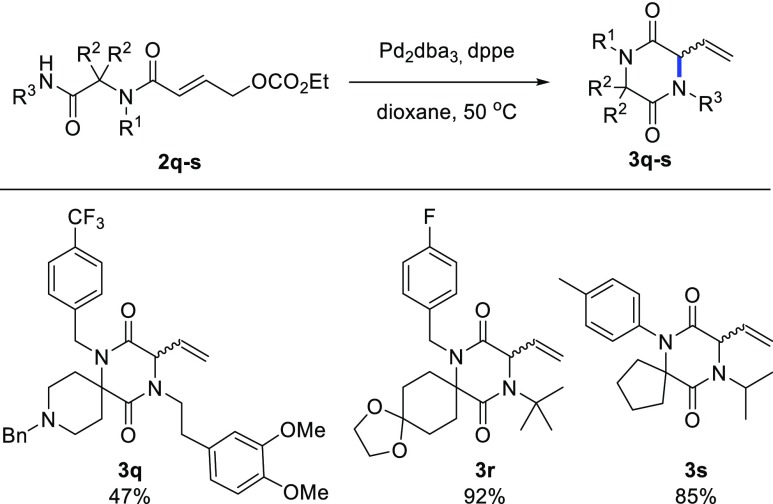
We then examined the scope of the reaction by subjecting various Ugi adducts 2 to the optimized reaction conditions (Scheme 2). We were delighted to observe that the reaction tolerates various spiro ring sizes, affording the desired DKPs smoothly for spiro-fused cyclohexanes and cyclopentanes (3a, 3d, 3g, 3i, 3j, 3l, 3n, 3o, and 3p) with generally high yield and enantioselectivity. In the case of spiro-cycloheptanes (3b–c), the yields are generally lower, possibly because of the increased flexibility of the system. To our delight, cyclobutane-containing substrates gave the corresponding DKP in excellent yield and enantioselectivity with a primary R3 substituent (3h), while the yield dropped with a sterically demanding R3 substituent (3k). The non-spiro products 3e,f were obtained in the lowest yields and selectivity, likely because of the reduced Thorpe–Ingold effect. Substrate 2q with a basic nitrogen atom in the spiro ring did not undergo the enantioselective cyclization, although the product 3q could be obtained as a racemate with the achiral catalyst (Scheme 3).
Scheme 2. Scope of the Reaction,
Reaction conditions: 2aa–o (0.20 mmol), Pd2(dba)3 (0.01 mmol). L4 (0.04 mmol) in dioxane (8 mL; 0.025 M) at rt.
Determined by chiral SFC analysis.
1.0 mmol scale reaction.
Scheme 3. Scope of the Racemic Tsuji–Trost Cyclization.
Reaction conditions: 2q–s (0.20 mmol), Pd2(dba)3 (0.01 mmol). dppe (0.04 mmol) in THF (2 mL) at 50 °C.
A wide variety of R3 substituents is tolerated in the reaction. In particular, aromatic and other electron-withdrawing substituents gave the highest yield and enantioselectivity (3g, 3h, 3j, 3l, 3m, 3n, and 3o). On the other hand, bulky electron-rich alkyl substituents led to lower yield, probably by increasing the pKa of the corresponding amide (3f). Primary alkyl substituents present an intermediate scenario, giving the products (3c, 3d, 3e, and 3f) in moderate yield. Reaction of tert-butyl amide 2r afforded the product only under the racemic conditions (Scheme 3, 3r). The lowest enantioselectivity was observed for 3o, bearing a pyridyl R3 substituent, probably because of the competing coordination of the Pd complex to the pyridine substituent, (partially) displacing the chiral oxazoline of L4. Aromatic R1 substituents are not tolerated; product 3s was only formed under the racemic conditions (Scheme 3). On the other hand, a broad range of (primary) aliphatic R1 substituents containing diverse functionalities (esters, amides, acetals, ethers, alkenes, alkynes, carbamates, and aromatic bromides) were shown to be compatible with the reaction, regardless of their steric and electronic properties.
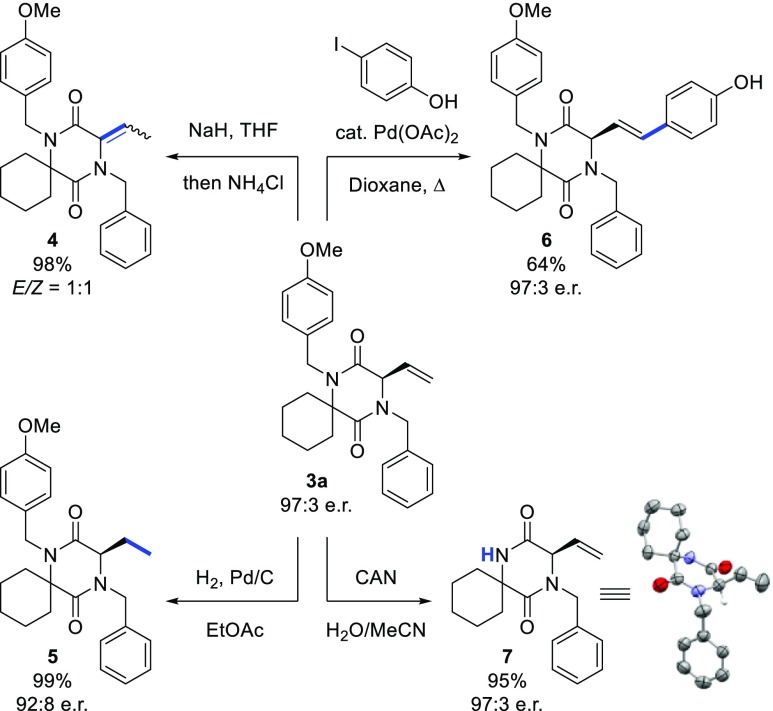
To further demonstrate the synthetic utility of our method, we isomerized the terminal vinyl group of 3a to give trisubstituted alkene 4 as a 1:1 mixture of E/Z isomers (Scheme 4). Catalytic hydrogenation of 3a afforded 5 bearing an ethyl side chain, leaving the two benzylic amides untouched. Furthermore, Heck coupling with the relatively challenging 4-iodophenol under harsh conditions afforded the corresponding alcohol 6 without loss of stereochemical information. Finally, we were able to selectively remove the PMB group of 3a by treatment with cerium ammonium nitrate (CAN) in H2O/MeCN to give the secondary amide 7. X-ray crystallographic analysis of 7 allowed us to unequivocally confirm the absolute configuration of the new stereocenter (R).
Scheme 4. Further Transformations of Spiro-DKP 3a.
The mechanism of DKP formation is proposed to proceed via the commonly accepted pathway for the Tsuji–Trost reaction, that is, via a π-allylpalladium intermediate and subsequent cross-coupling with the deprotonated secondary amide. As the pKa of such amides is considerably lower than the generally accepted cutoff value of 25, C–N bond formation likely proceeds via SN2-type substitution of the π-allylpalladium intermediate (“soft nucleophile mechanism”).15 The consistent performance of our reaction over various R3 substituents suggests that all reactions proceed via the same pathway. The regioselectivity is fully governed by the explicit E-geometry of the π-allylpalladium intermediate, considering that cyclization can be expected to outcompete allyl isomerization.15,16
Conclusions
In conclusion, we successfully developed the first method for the synthesis of enantioenriched DKPs based on asymmetric catalysis rather than chiral pool starting materials. This two-step method provides access to a wide range of highly functionalized (spiro-)DKPs with good to excellent enantioselectivity. Moreover, the mild reaction conditions tolerate the presence of a wide range of functional groups. Finally, various further transformations to extend the range of accessible products were demonstrated.
Experimental Section
General Information
Commercially available reagents were purchased from Sigma-Aldrich, Fischer, Strem Chemicals or Fluorochem and were used as purchased unless mentioned otherwise. Solvents were purchased from VWR Chemicals or Sigma-Aldrich and used without purification, unless stated otherwise. Anhydrous, air-free solvents were obtained from a PureSolv MD 5 solvent purification system. Infrared (IR) spectra were recorded neat using a Shimadzu FTIR-8400s spectrophotometer and wavelengths are reported in cm–1. Nuclear magnetic resonance (NMR) spectra were recorded on a Bruker AVANCE 600 (150.90 MHz for 13C), Bruker AVANCE 500 (125.78 MHz for 13C), Bruker AVANCE 400 (376.50 MHz for 19F), or Bruker AVANCE 300 using the residual CHCl3 as internal standard (1H: δ 7.26 ppm, 13C{1H}: δ 77.16 ppm). Chemical shifts (δ) are given in ppm and coupling constants (J) are quoted in hertz (Hz). Resonances are described as s (singlet), d (doublet), t (triplet), q (quartet), br (broad singlet), and m (multiplet) or combinations thereof. Electrospray ionization (ESI) high-resolution mass spectrometry was carried out using a Bruker micrOTOF-Q instrument in positive ion mode (capillary potential of 4500 V). Flash chromatography was performed on Silicycle Silica-P Flash Silica Gel (particle size 40–63 μm, pore diameter 60 Å) using the indicated eluent. Thin layer chromatography (TLC) was performed using TLC plates from Merck (SiO2, Kieselgel 60 F254 neutral, on aluminium with fluorescence indicator), and compounds were visualized by UV detection (254 nm) and/or KMnO4 stain. SFC-MS analysis was conducted using a Shimadzu Nexera SFC-MS equipped with a Nexera X2 SIL-30AC autosampler, Nexera UC LC-30AD SF CO2 pump, Nexera X2 LC-30AD liquid chromatograph, Nexera UC SFC-30A back pressure regulator, prominence SPD-M20A diode array detector, prominence CTO-20AC column oven, and CBM-20A system controller. Enantiomeric excess was determined by SFC-MS analysis using: Lux 3 μm Cellulose-1 column (cellulose tris(3,5-dimethylphenylcarbamate)) (column 1), Lux 3 μm Cellulose-2 column (cellulose tris(3-chloro-4-methylphenylcarbamate)) (column 2), Lux 3 μm Cellulose-3 column (cellulose tris(4-methylbenzoate), 150 × 4.6 mm) (column 3), and Lux 3 μm Cellulose-4 column (cellulose tris(4-chloro-3-methylphenyl-carbamate)) (column 4). A gradient of supercritical CO2 (A) and methanol (B) was used. Method 1 (column 1): 2% B/98% A to 30% B/70% A over the course of 4 min and was maintained at 30% B/70% A for 2 min (flow: 1.5 mL/min). Method 2 (column 1): 2% B/98% A to 25% B/75% A over the course of 6 min and was maintained at 25% B/75% A for 1 min (flow: 2 mL/min). Method 3 (column 2): 2% B/98% A to 30% B/70% A over the course of 4 min and was maintained at 30% B/70% A for 2 min (flow: 2 mL/min). Method 4 (column 3): 2% B/98% A to 25% B/75% A over the course of 5 min and was maintained at 25% B/75% A for 1 min (flow: 1 mL/min). Method 5 (column 3): 2% B/98% A to 30% B/70% A over the course of 4 min and was maintained at 30% B/70% A for 2 min (flow: 2 mL/min). Method 6 (column 3): 2% B/98% A to 30% B/70% A over the course of 15 min and was maintained at 30% B/70% A for 1 min (flow: 2 mL/min). Method 7 (column 4): 2% B/98% A to 25% B/75% A over the course of 5 min and was maintained at 25% B/75% A for 1 min (flow: 2 mL/min). The sample injection volume was 5 μL. Mass spectrometry analyses were performed using a Shimadzu LCMS-2020 mass spectrometer. The data were acquired in full-scan APCI mode (MS) from m/z 100 to 800 in positive ionization mode. Data were processed using Shimadzu Labsolutions 5.82. Specific rotations were measured with an automatic AA-10 polarimeter.
Procedure A: Synthesis of the Ugi Precursors (GP-A)
A solution of the corresponding aldehyde (5 mmol, 1 equiv) and amine (5 mmol, 1 equiv) in MeOH (1 M, 5 mL) was stirred for 30 min, then, the carboxylic acid 1 (871 mg, 5 mmol, 1 equiv) was added and, after 5 min, the corresponding isocyanide (5 mmol, 1 equiv) was added. The reaction mixture was stirred for 24 h, concentrated, and purified by silica gel column chromatography as described in the corresponding synthetic procedure.
Procedure B: Enantioselective Tsuji–Trost Cyclization (GP-B)
A solution of Pd2(dba)3 (9 mg, 0.01 mmol, 0.05 equiv) and L4 (16 mg, 0.04 mmol, 0.2 equiv) in dioxane (4 mL) was stirred at rt for 30 min, then, a solution of the corresponding Ugi precursor (0.2 mmol, 1 equiv) in dioxane (0.05 M, 4 mL) was added dropwise and stirred overnight. The reaction mixture was filtrated, concentrated, and purified by silica gel column chromatography as described in the corresponding synthetic procedure.
Procedure C: Racemic Tsuji–Trost Cyclization (GP-C)
A solution of Pd2(dba)3 (9 mg, 0.01 mmol, 0.05 equiv), dppe (8 mg, 0.02 mmol, 0.1 equiv), and the corresponding Ugi precursor (0.2 mmol, 1 equiv) in dioxane (0.2 M, 2 mL) was stirred at 50 °C in an oil bath until full conversion of the starting material (monitored by TLC). The reaction mixture was filtrated, concentrated, and purified by silica gel column chromatography as described in the corresponding synthetic procedure.
Procedure D: Synthesis of Carboxylic Acid (GP-D)
To a solution of the corresponding alcohol (18.303 g, 113.2 mmol, 1.00 equiv) in MeCN (1 M, 113.2 mL) were subsequently added CuBr (812 mg, 5.66 mmol, 0.05 equiv), 2,2′-bipyridine (884 mg, 5.66 mmol, 0.05 equiv), 6-tetramethylpiperidine-1-oxyl (884 mg, 5.66 mmol, 0.05 equiv), and 4-(dimethylamino)pyridine (2.074 g, 16.98 mmol, 0.15 equiv). An O2-balloon was fit to the flask, then the reaction mixture was degassed under vacuum, and the flask was backfilled with oxygen. This procedure was repeated three times, and the solution was stirred overnight. When the oxidation was complete (checked by TLC), the mixture was cooled to 0 °C and H2O2 (35%, 12.170 mL, 1.25 equiv), and a solution of KH2PO4 (6.932 g, 50.94 mmol, 0.45 equiv) and NaClO2 (20.476 g, 226.4 mmol, 2.00 equiv) in water (240 mL) was added dropwise. Stirring was continued for 24 h at room temperature.
After complete consumption of the aldehyde (checked by TLC), 1 M HCl was added, and the aqueous phase was extracted with EtOAc (3×). The combined organic phases were dried over Na2SO4 and filtered, and the solvent removed under vacuum.
E-4-((Ethoxycarbonyl)oxy)but-2-enoic Acid (1a)
It was prepared according to GP-D. Obtained as a pale oil that solidified when cooled to −20 °C (yield: 98%, 19.320 g, 110.936 mmol) and was used without further purification. 1H NMR (600 MHz, CDCl3): δ 12.30–10.95 (br, 1H), 7.04 (dt, J = 15.8, 4.3 Hz, 1H), 6.08 (dd, J = 15.8, 1.9 Hz, 1H), 4.82 (dd, J = 4.3, 1.9 Hz, 2H), 4.23 (q, J = 7.1 Hz, 2H), 1.32 (t, J = 7.1 Hz, 3H). 13C NMR{1H} (151 MHz, CDCl3): δ 171.1, 154.6, 143.4, 121.4, 65.4, 64.6, 14.2. IR (neat) νmax (cm–1): 3074, 2922, 1745, 1725, 1664, 1366, 1079, 1047. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C7H11O5, 175.0601; found, 175.0612.
E-4-((Methoxycarbonyl)oxy)but-2-enoic Acid (1b)
It was prepared according to GP-D on 5 mmol scale, obtained as a pale oil (yield: 97%, 777 mg, 4.85 mmol) and was used without further purification. 1H NMR (500 MHz, CDCl3): δ 10.29–9.10 (br, 1H), 7.03 (dt, J = 15.8, 4.3 Hz, 1H), 6.06 (d, J = 15.8 Hz, 1H), 4.82 (dd, J = 4.4, 2.0 Hz, 2H), 3.81 (s, 3H). 13C NMR (126 MHz, CDCl3): δ 171.1, 155.4, 143.4, 121.6, 65.8, 55.3. IR (neat) νmax (cm–1): 2964, 1745, 1682, 1655, 1437, 1252, 1205, 932, 908, 787. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C6H9O5, 161.0444; found, 161.0448.
E-4-((tert-Butoxycarbonyl)oxy)but-2-enoic Acid (1c)
It was prepared according to GP-D on 5 mmol scale, obtained as a pale oil that solidified when cooled to −20 °C (yield: 95%, 960 mg, 4.75 mmol), and was used without further purification. 1H NMR (500 MHz, CDCl3): δ 10.61–8.43 (br, 1H), 7.04 (dt, J = 15.7, 4.3 Hz, 1H), 6.06 (dt, J = 15.8, 2.0 Hz, 1H), 4.75 (dd, J = 4.4, 2.0 Hz, 2H), 1.49 (s, 9H). 13C NMR{1H} (126 MHz, CDCl3): δ 171.2, 153.0, 143.9, 121.4, 83.1, 64.8, 27.8 (3C). IR (neat) νmax (cm–1): 2982, 1742, 1701, 1369, 1273, 1252, 1155, 1121, 851, 756. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C9H15O5, 203.0914; found, 203.0910.
(E)-4-((1-(Benzylcarbamoyl)cyclohexyl)(4-methoxybenzyl)amino)-4-oxobut-2-en-1-yl Ethyl Carbonate (2aa)
It was prepared according to GP-A. Purification of the crude material by silica gel column chromatography (40% EtOAc/cHex) provided the title compound as a pale oil in 82% yield (2.085 g, 4.1 mmol). Rf = 0.31 (50% EtOAc/cHex). 1H NMR (600 MHz, CDCl3): δ 7.35–7.22 (m, 5H), 7.15 (d, J = 8.3 Hz, 2H), 6.86 (dt, J = 15.1, 5.0 Hz, 1H), 6.82 (d, J = 8.3 Hz, 3H), 6.36 (d, J = 15.1 Hz, 1H), 4.71 (d, J = 5.0 Hz, 2H), 4.62 (s, 2H), 4.40 (d, J = 5.5 Hz, 2H), 4.13 (q, J = 7.1 Hz, 2H), 3.78 (s, 3H), 2.44 (d, J = 12.5 Hz, 2H), 1.71 (td, J = 10.6, 5.4 Hz, 2H), 1.63–1.50 (m, 6H), 1.25 (t, J = 7.1 Hz, 3H). 13C NMR{1H} (151 MHz, CDCl3): δ 173.3, 168.4, 159.0, 154.7, 139.0, 138.7, 130.5, 128.7 (2C), 127.9 (2C), 127.6 (2C), 127.3 (2C), 124.7, 114.4, 66.5, 66.3, 64.4, 55.4, 47.9, 43.9, 33.1, 25.4 (2C), 23.0 (2C), 14.30. IR (neat) νmax (cm–1): 2366, 1745, 1670, 1510, 1355, 978, 704. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C29H36N2O6, 509.2646; found, 509.2624.
(E)-4-((1-(Benzylcarbamoyl)cyclohexyl)(4-methoxybenzyl)amino)-4-oxobut-2-en-1-yl Methyl Carbonate (2ab)
It was prepared according to GP-A. Purification of the crude material by silica gel column chromatography (40% EtOAc/cHex) provided the title compound as a pale oil in 88% yield (2.176 g, 4.4 mmol). Rf = 0.30 (50% EtOAc/cHex). 1H NMR (500 MHz, CDCl3): δ 7.31–7.28 (m, 2H), 7.27–7.24 (m, 3H), 7.15 (d, J = 8.7 Hz, 2H), 6.86 (dt, J = 15.1, 5.0 Hz, 1H), 6.82 (d, J = 8.6 Hz, 2H), 6.78–6.75 (m, 1H), 6.36 (dt, J = 15.1, 1.8 Hz, 1H), 4.71 (dd, J = 5.0, 1.8 Hz, 2H), 4.62 (s, 2H), 4.40 (d, J = 5.5 Hz, 2H), 3.78 (s, 3H), 3.72 (s, 3H), 2.43 (d, J = 13.2 Hz, 2H), 1.70 (ddd, J = 13.4, 10.7, 3.9 Hz, 2H), 1.64–1.48 (m, 6H). 13C NMR{1H} (126 MHz, CDCl3): δ 173.3, 168.3, 158.9, 155.3, 138.8, 138.6, 130.4, 128.7 (2C), 127.9 (2C), 127.6 (2C), 127.3, 124.7, 114.3 (2C), 66.5 (2C), 55.4, 55.1, 47.8, 43.9, 33.1 (2C), 25.4, 22.9 (2C). IR (neat) νmax (cm–1): 2932, 1749, 1663, 1610, 1512, 1445, 1244, 1173, 1030, 918, 727, 698. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C28H35N2O6, 495.2490; found, 495.2486.
(E)-4-((1-(Benzylcarbamoyl)cyclohexyl)(4-methoxybenzyl)amino)-4-oxobut-2-en-1-yl tert-Butyl Carbonate (2ac)
It was prepared according to GP-A. Purification of the crude material by silica gel column chromatography (40% EtOAc/cHex) provided the title compound as a pale oil in 78% yield (2.093 g, 3.9 mmol). Rf = 0.35 (50% EtOAc/cHex). 1H NMR (500 MHz, CDCl3): δ 7.33–7.28 (m, 2H), 7.27–7.24 (m, 3H), 7.15 (d, J = 8.7 Hz, 2H), 6.87 (dt, J = 15.1, 4.9 Hz, 1H), 6.83–6.79 (m, 3H), 6.34 (dt, J = 15.1, 1.8 Hz, 1H), 4.65 (dd, J = 4.9, 1.8 Hz, 2H), 4.61 (s, 2H), 4.39 (d, J = 5.5 Hz, 2H), 3.77 (s, 3H), 2.46–2.40 (m, 2H), 1.74–1.67 (m, 2H), 1.62–1.51 (m, 6H), 1.41 (s, 9H). 13C NMR{1H} (126 MHz, CDCl3): δ 173.3, 168.5, 158.9, 153.0, 139.6, 138.7, 130.4, 128.7 (2C), 127.9 (2C), 127.6 (2C), 127.3, 124.3, 114.3 (2C), 82.7, 66.4, 65.5, 55.3, 47.9, 43.9, 33.1 (2C), 27.7 (3C), 25.4, 23.0 (2C). IR (neat) νmax (cm–1): 2934, 1742, 1661, 1512, 1275, 1246, 1157, 1119, 727, 698. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C31H41N2O6, 537.2959; found, 537.2961.
Benzyl (E)-3-(2-(1-(N-(2,2-Dimethoxyethyl)-4-((ethoxy carbonyl)oxy)but-2-enamido)cycloheptane-1-carboxamido)ethyl)-1H-indole-1-carboxylate (2b)
It was prepared according to GP-A. Purification of the crude material by silica gel column chromatography (25% EtOAc/cHex) provided the title compound as a pale oil in 74% yield (2.508 g, 3.7 mmol). Rf = 0.33 (50% EtOAc/cHex). 1H NMR (600 MHz, CDCl3): δ 8.20–8.05 (m, 1H), 7.54 (d, J = 7.7 Hz, 1H), 7.50–7.44 (m, 2H), 7.42–7.31 (m, 5H), 7.29 (t, J = 7.8 Hz, 1H), 7.23 (t, J = 7.5 Hz, 1H), 6.76 (dt, J = 15.3, 4.8 Hz, 1H), 6.39 (d, J = 15.3 Hz, 1H), 5.42 (s, 2H), 4.69 (d, J = 4.8 Hz, 2H), 4.62–4.48 (m, 1H), 4.18 (q, J = 7.1 Hz, 2H), 3.62–3.54 (m, 2H), 3.51 (q, J = 6.7 Hz, 2H), 3.22 (s, 6H), 2.85 (t, J = 6.9 Hz, 2H), 2.44–2.26 (m, 2H), 1.95–1.79 (m, 2H), 1.73–1.63 (m, 2H), 1.56–1.40 (m, 6H), 1.27 (t, J = 7.2 Hz, 3H). 13C NMR{1H} (151 MHz, CDCl3): δ 154.7, 150.8, 138.2, 136.5, 135.7, 135.2, 130.4, 128.8 (2C), 128.7, 128.5 (2C), 127.3, 124.8, 123.8, 123.0, 122.8, 119.1, 119.0, 115.3, 69.2, 68.7, 66.4, 66.2, 64.4, 55.3 (2C), 46.3 (2C), 39.3, 30.4 (2C), 25.2 (2C), 24.1 (2C), 14.3. IR (neat) νmax (cm–1): 2925, 1736, 1666, 1454, 1396, 1354, 1244, 1180, 1122, 1088, 1049, 1016, 731, 698. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C37H48N3O9, 678.3385; found, 678.3357.
(E)-4-((1-(Benzylcarbamoyl)cycloheptyl)(butyl)amino)-4-oxobut-2-en-1-yl Ethyl Carbonate (2c)
It was prepared according to GP-A. Purification of the crude material by silica gel column chromatography (25% EtOAc/cHex) provided the title compound as a white solid in 78% yield (1.789 g, 3.9 mmol). Rf = 0.27 (50% EtOAc/cHex). 1H NMR (600 MHz, CDCl3): δ 7.41–7.14 (m, 5H), 6.85 (dt, J = 15.1, 4.8 Hz, 1H), 6.44 (dt, J = 15.1, 1.9 Hz, 1H), 6.16 (t, J = 5.6 Hz, 1H), 4.78 (dd, J = 4.8, 1.9 Hz, 2H), 4.43 (d, J = 5.6 Hz, 2H), 4.23 (q, J = 7.1 Hz, 2H), 3.51–3.28 (m, 2H), 2.43 (ddd, J = 15.2, 9.4, 1.6 Hz, 2H), 2.09–1.89 (m, 2H), 1.76–1.68 (m, 2H), 1.67–1.47 (m, 8H), 1.38–1.25 (m, 5H), 0.95 (t, J = 7.4 Hz, 3H). 13C NMR{1H} (151 MHz, CDCl3) 175.1, 166.9, 154.8, 138.9, 138.4, 128.6 (2C), 127.9 (2C), 127.3, 123.6, 69.4, 66.3, 64.4, 44.8, 43.9 (2C), 35.7, 34.1, 30.2 (2C), 23.8 (2C), 20.3, 14.4, 13.7. IR (neat) νmax (cm–1): 3321, 2930, 1744, 1670, 1603, 1526, 1423, 1250, 1217, 1188, 997, 957, 793, 716, 644. HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C26H38N2NaO5, 481.2673; found, 481.2660.
(E)-4-((3,5-Bis(trifluoromethyl)benzyl)(1-((3-methoxy propyl)carbamoyl)cyclohexyl)amino)-4-oxobut-2-en-1-yl Ethyl Carbonate (2d)
It was prepared according to GP-A. Purification of the crude material by silica gel column chromatography (50% EtOAc/cHex) provided the title compound as a white solid in 67% yield (2.063 g, 3.8 mmol). Rf = 0.13 (50% EtOAc/cHex). 1H NMR (600 MHz, CDCl3): δ 7.84 (s, 2H), 7.78 (s, 1H), 6.93 (t, 1H), 6.85 (dt, J = 15.1, 4.8 Hz, 1H), 6.17 (d, J = 15.1 Hz, 1H), 4.80 (s, 2H), 4.66 (d, J = 4.8 Hz, 2H), 4.08 (q, J = 7.1 Hz, 2H), 3.47 (t, J = 5.7 Hz, 2H), 3.41–3.33 (m, 2H), 3.29 (s, 3H), 2.43–2.35 (m, 2H), 1.82–1.73 (m, 2H), 1.62–1.53 (m, 8H), 1.20 (t, J = 7.2 Hz, 3H). 13C NMR{1H} (151 MHz, CDCl3): δ 172.8, 168.2, 154.5, 142.0, 139.6, 132.1 (q, J = 33.4 Hz, 2C), 126.6, 123.7 (2C), 123.2 (q, J = 274.8, 2C), 121.4, 72.1, 66.3, 65.9, 64.3, 58.8, 47.7, 38.6, 33.2, 28.8 (2C), 25.3, 22.8 (2C), 14.1. 19F NMR{1H} (376 MHz, CDCl3): δ −62.9. IR (neat) νmax (cm–1): 2932, 1749, 1663, 1653, 1620, 1377, 1348, 1277, 1254, 1169, 1128, 1003, 995, 906, 791, 733, 706, 681, 409. HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C27H34F6N2NaO6, 619.2213, found, 619.2189.
(E)-4-((1-(Butylamino)-2-methyl-1-oxopropan-2-yl)(2,4-dimethoxybenzyl)amino)-4-oxobut-2-en-1-yl Ethyl Carbonate (2e)
It was prepared according to GP-A. Purification of the crude material by silica gel column chromatography (60% EtOAc/cHex) provided the title compound as a white solid in 54% yield (1.254 g, 2.7 mmol). Rf = 0.13 (50% EtOAc/cHex). 1H NMR (600 MHz, CDCl3): δ 7.50 (d, J = 8.4 Hz, 1H), 6.84 (dt, J = 15.2, 5.2 Hz, 1H), 6.49 (dd, J = 8.4, 2.4 Hz, 1H), 6.42 (d, J = 2.4 Hz, 1H), 6.23 (dt, J = 15.2, 1.7 Hz, 1H), 5.77 (t, J = 5.7 Hz, 1H), 4.64 (dd, J = 5.2, 1.8 Hz, 2H), 4.51 (s, 2H), 4.08 (q, J = 7.1 Hz, 2H), 3.78 (s, 3H), 3.76 (s, 3H), 3.23 (td, J = 7.3, 5.6 Hz, 2H), 1.50–1.42 (m, 2H), 1.39 (s, 6H), 1.33–1.26 (m, 2H), 1.20 (t, J = 7.1 Hz, 3H), 0.88 (t, J = 7.4 Hz, 3H). 13C NMR{1H} (151 MHz, CDCl3): δ 174.8, 167.0, 160.2, 156.9, 154.6, 138.5, 128.4, 123.8, 118.9, 104.2, 98.4, 66.3, 64.2, 62.5, 55.4, 55.2, 42.5, 39.6, 31.5, 24.1 (2C), 20.2, 14.2, 13.8. IR (neat) νmax (cm–1): 3283, 2962, 2934, 1744, 1643, 1616, 1508, 1412, 1373, 1248, 1209, 1198, 1175, 1159, 1115, 1045, 1036, 1007, 989, 960, 928, 791. HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C24H36N2NaO7, 487.2415; found, 487.2396.
(E)-Ethyl (4-((4-Fluorobenzyl)(1-(isopropylamino)-2-methyl-1-oxopropan-2-yl)amino)-4-oxobut-2-en-1-yl)carbonate (2f)
It was prepared according to GP-A. Purification of the crude material by silica gel column chromatography (60% EtOAc/cHex) provided the title compound as a white solid in 47% yield (0.960 g, 2.35 mmol). Rf = 0.10 (50% EtOAc/cHex). 1H NMR (600 MHz, CDCl3): δ 7.44–7.39 (m, 2H), 7.05 (t, J = 8.6 Hz, 2H), 6.88 (dt, J = 15.2 Hz, J = 4.7, 1H), 6.24 (d, J = 15.2 Hz, 1H), 5.49 (d, J = 7.8 Hz, 1H), 4.68 (d, J = 4.7 Hz, 2H), 4.64 (s, 2H), 4.11 (q, J = 7.1 Hz, 2H), 4.05 (m, 1H), 1.42 (s, 6H), 1.23 (t, J = 7.1 Hz, 3H), 1.14 (d, J = 7.8 Hz, 6H). 13C NMR{1H} (151 MHz, CDCl3): δ 173.8, 167.6, 167.0, 162.2 (d, J = 245.8 Hz), 154.7, 139.2, 134.4, 128.0, 123.3 (2C), 115.9 (d, J = 21.5 Hz, 2C), 66.2, 64.4, 62.6, 47.0, 41.7 (2C), 22.7 (2C), 14.3. 19F NMR{1H} (376 MHz, CDCl3): δ −115.3. IR (neat) νmax (cm–1): 3323, 2974, 1749, 1651, 1599, 1526, 1508, 1383, 1364, 1252, 1227, 1186, 1173, 1155, 1097, 1057, 1034, 827, 791, 500. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C21H30FN2O5, 409.2133; found, 409.2125.
Methyl (E)-(1-(4-((Ethoxycarbonyl)oxy)-N-(4-methoxybenzyl)but-2-enamido)cyclohexane-1-carbonyl)glycinate (2g)
It was prepared according to GP-A. Purification of the crude material by silica gel column chromatography (60% EtOAc/cHex) provided the title compound as an orange oil in 79% yield (1.937 g, 3.95 mmol). Rf = 0.11 (50% EtOAc/cHex). 1H NMR (600 MHz, CDCl3): δ 7.22 (d, J = 8.6 Hz, 2H), 6.95–6.83 (m, 4H), 6.35 (d, J = 15.1 Hz, 1H), 4.70 (dd, J = 4.9, 1.5 Hz, 2H), 4.60 (s, 2H), 4.11 (q, J = 7.1 Hz, 2H), 4.00 (d, J = 5.0 Hz, 2H), 3.79 (s, 3H), 3.73 (s, 3H), 2.43–2.36 (m, 2H), 1.69–1.50 (m, 8H), 1.23 (t, J = 7.1 Hz, 3H). 13C NMR{1H} (151 MHz, CDCl3): δ 173.7, 170.7, 168.4, 159.0, 154.7, 139.1, 130.4 (2C), 127.5, 124.5, 114.4 (2C), 66.3, 66.2, 64.4, 55.4, 52.3, 47.8, 41.6 (2C), 32.9, 25.4, 22.8 (2C), 14.3. IR (neat) νmax (cm–1): 2932, 1744, 1664, 1612, 1512, 1401, 1364, 1244, 1202, 1173, 1028, 1007, 991, 789. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C25H34N2O8, 491.2388; found, 491.2390.
Methyl (E)-(1-(4-((Ethoxycarbonyl)oxy)-N-phenethylbut-2-enamido)cyclobutane-1-carbonyl)glycinate (2h)
It was prepared according to GP-A. Purification of the crude material by silica gel column chromatography (25% EtOAc/cHex) provided the title compound as a yellow oil in 84% yield (1.875 g, 4.2 mmol). Rf = 0.28 (50% EtOAc/cHex). 1H NMR (600 MHz, CDCl3): δ 8.04 (t, J = 5.8 Hz, 1H), 7.34–7.22 (m, 2H), 7.22–7.16 (m, 1H), 7.14 (d, J = 7.5 Hz, 2H), 6.88 (dt, J = 15.1, 4.7 Hz, 1H), 6.49 (d, J = 15.1 Hz, 1H), 4.78 (dd, J = 4.7, 2.0 Hz, 2H), 4.21 (q, J = 7.1 Hz, 2H), 4.00 (d, J = 5.8 Hz, 2H), 3.68 (s, 3H), 3.45 (t, J = 8.4 Hz, 2H), 2.88–2.63 (m, 4H), 2.28–2.21 (m, 2H), 1.82–1.66 (m, 2H), 1.31 (t, J = 7.1 Hz, 3H). 13C NMR{1H} (151 MHz, CDCl3): δ 174.3, 170.1, 166.7, 154.7, 138.9, 138.0, 128.7 (2C), 128.6 (2C), 126.7, 121.9, 66.0, 65.3, 64.4, 52.1, 47.2, 41.3 (2C), 36.7, 31.8, 14.7, 14.3. IR (neat) νmax (cm–1): 3321, 2930, 1744, 1670, 1655, 1597, 1528, 1508, 1423, 1377, 1252, 1190, 999, 793, 717, 644, 451, 407. HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C23H30N2NaO7, 469.1945; found, 469.1941.
(E)-4-((4-Chlorobenzyl)(1-((2,3-dihydrobenzo[b][1,4]dioxin-6-yl)carbamoyl)cyclopentyl)amino)-4-oxobut-2-en-1-yl Ethyl Carbonate (2i)
It was prepared according to GP-A. Purification of the crude material by silica gel column chromatography (35% EtOAc/cHex) provided the title compound as a pale oil in 76% yield (2.063 g, 3.8 mmol). Rf = 0.41 (50% EtOAc/cHex). 1H NMR (600 MHz, CDCl3): δ 9.37 (s, 1H), 7.30 (d, J = 8.3 Hz, 2H), 7.15 (d, J = 8.3 Hz, 2H), 7.10 (d, J = 2.5 Hz, 1H), 6.95 (dt, J = 15.1, 4.7 Hz, 1H), 6.84 (d, J = 8.8 Hz, 1H), 6.75 (d, J = 8.7 Hz, 1H), 6.19 (d, J = 15.0 Hz, 1H), 4.68 (dd, J = 4.7, 1.9 Hz, 2H), 4.62 (s, 2H), 4.20–4.17 (m, 4H), 4.07 (q, J = 7.1 Hz, 2H), 2.81–2.75 (m, 2H), 1.86–1.80 (m, 2H), 1.69–1.62 (m, 4H), 1.21 (t, J = 7.1 Hz, 3H). 13C NMR{1H} (151 MHz, CDCl3): δ 170.8, 169.2, 154.5, 143.4, 140.4, 140.1, 136.7, 133.2, 132.1, 129.1 (2C), 127.3 (2C), 123.0, 117.0, 113.4, 109.5, 74.6, 65.9, 64.4, 64.3, 64.3, 50.5, 36.0 (2C), 22.8 (2C), 14.1. IR (neat) νmax (cm–1): 2328, 1659, 1502, 1414, 1256, 1244, 1203, 1190, 1067, 1014, 982, 812, 783, 608, 482, 451, 411. HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C28H31ClN2NaO7, 565.1712; found, 565.1687.
(E)-4-((1-((2,3-Dihydrobenzo[b][1,4]dioxin-6-yl)carbamoyl)cyclohexyl)(prop-2-yn-1-yl)amino)-4-oxobut-2-en-1-yl Ethyl Carbonate (2j)
It was prepared according to GP-A. Purification of the crude material by silica gel column chromatography (40% EtOAc/cHex) provided the title compound as a pale solid in 77% yield (1.812 g, 3.85 mmol). Rf = 0.51 (50% EtOAc/cHex). 1H NMR (600 MHz, CDCl3): δ 8.48 (s, 1H), 7.07 (d, J = 2.5 Hz, 1H), 6.91–6.80 (m, 2H), 6.72 (d, J = 8.7 Hz, 1H), 6.55 (d, J = 15.2 Hz, 1H), 4.76 (dd, J = 4.8, 1.9 Hz, 2H), 4.31–4.02 (m, 8H), 2.43 (t, J = 2.4 Hz, 1H), 2.38–2.24 (m, 2H), 2.17–2.06 (m, 2H), 1.74–1.64 (m, 2H), 1.59–1.37 (m, 4H), 1.29 (t, J = 7.2 Hz, 3H). 13C NMR{1H} (151 MHz, CDCl3): δ 171.5, 168.5, 154.7, 143.4, 140.2, 139.6, 132.0, 123.6, 117.0, 113.7, 109.8, 80.0, 73.8, 66.9, 66.1, 64.4, 64.4, 64.3, 35.0, 32.9 (2C), 25.3, 22.7 (2C), 14.3. IR (neat) νmax (cm–1): 3288, 2934, 1744, 1664, 1504, 1406, 1379, 1300, 1254, 1240, 1202, 1173, 1067, 885, 802, 791, 737. HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C25H30N2NaO7, 493.1945; found, 493.1935.
(E)-4-((4-Bromobenzyl)(1-((2,6-dimethylphenyl)carbamoyl)cyclobutyl)amino)-4-oxobut-2-en-1-yl Ethyl Carbonate (2k)
It was prepared according to GP-A. Purification of the crude material by silica gel column chromatography (40% EtOAc/cHex) provided the title compound as a white solid in 62% yield (1.685 g, 3.1 mmol). Rf = 0.53 (50% EtOAc/cHex). 1H NMR (600 MHz, CDCl3): δ 8.94 (s, 1H), 7.47 (d, J = 8.2 Hz, 2H), 7.12 (d, J = 8.2 Hz, 2H), 7.06–6.98 (m, 3H), 6.94 (dt, J = 15.1, 4.5 Hz, 1H), 6.16 (d, J = 15.1 Hz, 1H), 4.66 (d, J = 4.5 Hz, 2H), 4.46 (s, 2H), 4.05 (q, J = 7.1 Hz, 2H), 2.83 (s, 2H), 2.31 (s, 2H), 2.12 (s, 6H), 1.85–1.68 (m, 2H), 1.19 (t, J = 7.2 Hz, 3H). 13C NMR{1H} (151 MHz, CDCl3): δ 170.9, 167.1, 154.2, 140.4, 136.7, 134.7 (2C), 134.0, 131.8 (2C), 127.9 (2C), 127.5 (2C), 126.7, 121.6, 121.2, 66.1, 65.6, 64.1, 48.3, 18.1 (2C), 14.7, 14.0. Two secondary carbons of the cyclobutane are not visible. IR (neat) νmax (cm–1): 2957, 2336, 1745, 1664, 1489, 1462, 1396, 1379, 1248, 11 981, 1009, 787, 770, 480. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C27H32BrN2O5, 543.1489; found, 543.1462.
(E)-4-(Allyl(1-((4-methoxyphenyl)carbamoyl)cyclopentyl)amino)-4-oxobut-1-en-1-yl Ethyl Carbonate (2l)
It was prepared according to GP-A. Purification of the crude material by silica gel column chromatography (30% EtOAc/cHex) provided the title compound as a pale solid in 66% yield (1.421 g, 3.3 mmol). Rf = 0.43 (50% EtOAc/cHex). 1H NMR (600 MHz, CDCl3): δ 9.47 (s, 1H), 7.41 (d, J = 9.0 Hz, 2H), 6.92 (dt, J = 15.1, 4.8 Hz, 1H), 6.83 (d, J = 9.0 Hz, 2H), 6.38 (dt, J = 15.1, 1.8 Hz, 1H), 5.91 (ddt, J = 17.1, 10.4, 4.4 Hz, 1H), 5.29 (d, J = 10.4 Hz, 1H), 5.25 (d, J = 17.1 Hz, 1H), 4.76 (dd, J = 4.8, 1.9 Hz, 2H), 4.20 (q, J = 7.1 Hz, 2H), 4.06–3.96 (m, 2H), 3.77 (s, 3H), 2.94–2.76 (m, 2H), 1.99–1.90 (m, 2H), 1.77–1.65 (m, 4H), 1.30 (t, J = 7.1 Hz, 3H). 13C NMR{1H} (151 MHz, CDCl3): δ 171.3, 169.3, 156.2, 154.8, 139.5, 134.9, 131.8, 123.9, 121.6 (2C), 117.4, 114.2 (2C), 74.6, 66.2, 64.5, 55.6, 50.0, 36.0 (2C), 23.0 (2C), 14.4. IR (neat) νmax (cm–1): 3340, 2957, 1742, 1663, 1624, 1510, 1408, 1396, 1257, 1244, 1227, 1202, 1169, 1034, 993, 976, 959, 920, 829, 789, 523, 419. HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C23H30N2NaO6, 453.1996; found, 453.1989.
(E)-4-((3,3-Diethoxypropyl)(8-((2,6-dimethylphenyl)carbamoyl)-1,4-dioxaspiro[4.5]decan-8-yl)amino)-4-oxobut-2-en-1-yl Ethyl Carbonate (2m)
It was prepared according to GP-A. Purification of the crude material by silica gel column chromatography (50% EtOAc/cHex) provided the title compound as a pale solid in 58% yield (1.713 g, 2.9 mmol). Rf = 0.17 (50% EtOAc/cHex). 1H NMR (600 MHz, CDCl3): δ 8.47 (s, 1H), 7.02–6.84 (m, 3H), 6.77 (dt, J = 15.1, 4.4 Hz, 1H), 6.59 (dt, J = 15.1, 2.2 Hz, 1H), 4.69 (t, J = 4.4, 2.2 Hz, 2H), 4.41 (t, J = 4.7 Hz, 1H), 4.12 (q, J = 7.2 Hz, 2H), 3.84 (s, 4H), 3.62–3.49 (m, 4H), 3.44–3.30 (m, 2H), 2.52–2.38 (m, 2H), 2.31 (t, J = 11.0 Hz, 2H), 2.09 (s, 6H), 1.96–1.80 (m, 4H), 1.62–1.52 (m, 2H), 1.22 (t, J = 7.1 Hz, 3H), 1.08 (t, J = 7.1 Hz, 6H). 13C NMR{1H} (151 MHz, CDCl3): δ 171.4, 168.6, 154.4, 138.4, 134.9 (2C), 134.1, 127.9 (2C), 126.4, 124.0, 107.5, 100.7, 65.9, 65.1, 64.1, 64.0 (2C), 61.9 (2C), 41.1, 35.2 (2C), 31.3 (2C), 30.3, 18.6 (2C), 15.0 (2C), 14.0. IR (neat) νmax (cm–1): 3339, 2976, 2935, 1744, 1684, 1621, 1516, 1366, 1248, 1230, 1198, 1171, 1144, 1109, 1094, 1070, 1038, 991, 959, 947, 926, 899, 889, 866, 852, 793, 775. HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C31H46N2NaO9, 613.3096; found, 613.3074.
(E)-Ethyl (4-((1-((4-Methoxyphenyl)carbamoyl)cyclopentyl)(propyl)amino)-4-oxobut-2-en-1-yl)carbonate (2n)
It was prepared according to GP-A. Purification of the crude material by silica gel column chromatography (30% EtOAc/cHex) provided the title compound as a white solid in 63% yield (1.362 g, 3.15 mmol). Rf = 0.44 (50% EtOAc/cHex). 1H NMR (600 MHz, CDCl3): δ 9.60 (s, 1H), 7.41 (d, J = 9.0 Hz, 2H), 6.91 (dd, J = 13.5, 4.7 Hz, 1H), 6.83 (d, J = 9.0 Hz, 2H), 6.47 (d, J = 13.5 Hz, 1H), 4.80 (d, J = 4.7 Hz, 2H), 4.23 (q, J = 7.1 Hz, 2H), 3.78 (s, 3H), 3.38 (m, 2H), 2.88–2.75 (m, 2H), 1.95–1.82 (m, 2H), 1.80–1.68 (m, 4H), 1.68–1.55 (m, 2H), 1.32 (t, J = 7.1 Hz, 3H), 0.85 (t, J = 7.4 Hz, 3H). 13C NMR{1H} (151 MHz, CDCl3): δ 172.2, 169.2, 156.3, 154.9, 139.0, 132.0, 124.0, 121.7 (2C), 114.3 (2C), 74.2, 66.3, 64.6, 55.7, 49.6, 36.5, 24.4 (2C), 23.0 (2C), 14.5, 11.4. IR (neat) νmax (cm–1): 2935, 1745, 1664, 1620, 1514, 1448, 1418, 1252, 1234, 1198, 1155, 1140, 1026, 789, 700. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C23H33N2O6, 433.2333; found, 433.2345.
(E)-4-((4-Chlorobenzyl)(1-(naphthalen-2-ylcarbamoyl)cyclopentyl)amino)-4-oxobut-2-en-1-yl Ethyl Carbonate (2o)
It was prepared according to GP-A. Purification of the crude material by silica gel column chromatography (40% EtOAc/cHex) provided the title compound as an orange solid in 68% yield (1.819 g, 3.4 mmol). Rf = 0.55 (50% EtOAc/cHex). 1H NMR (600 MHz, CDCl3): δ 9.86 (s, 1H), 8.19 (s, 1H), 7.88–7.72 (m, 3H), 7.48–7.36 (m, 3H), 7.33 (d, J = 8.4 Hz, 2H), 7.18 (d, J = 8.0 Hz, 2H), 7.04 (dt, J = 15.1, 4.7 Hz, 1H), 6.25 (dt, J = 15.1, 1.8 Hz, 1H), 4.73 (dd, J = 4.7, 1.9 Hz, 2H), 4.67 (s, 2H), 4.11 (q, J = 7.1 Hz, 2H), 2.90 (d, J = 13.3 Hz, 2H), 1.97–1.86 (m, 2H), 1.76–1.67 (m, 4H), 1.24 (t, J = 7.1 Hz, 3H). 13C NMR{1H} (151 MHz, CDCl3): δ 171.4, 169.6, 154.6, 140.8, 136.7, 135.9, 134.0, 133.5, 130.6, 129.3 (2C), 128.7, 127.7 (2C), 127.6, 127.5, 126.5, 124.9, 123.2, 120.2, 116.5, 75.0, 66.0, 64.5, 50.8, 36.1 (2C), 22.9 (2C), 14.2. IR (neat) νmax (cm–1): 3244, 2957, 2326, 1744, 1663, 1524, 1491, 1429, 1400, 1354, 1256, 1236, 1215, 1194, 1094, 814, 787, 731, 474. HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C30H31ClN2NaO5, 557.1814; found, 557.1788.
(E)-4-((1-((5-Bromopyridin-2-yl)carbamoyl)cyclohexyl)(4-fluorobenzyl)amino)-4-oxobut-2-en-1-yl Ethyl Carbonate (2p)
It was prepared according to GP-A. Purification of the crude material by silica gel column chromatography (30% EtOAc/cHex) provided the title compound as a pale solid in 46% yield (1.294 g, 2.3 mmol). Rf = 0.36 (50% EtOAc/cHex). 1H NMR (600 MHz, CDCl3): δ 8.96 (s, 1H), 8.26 (d, J = 2.5 Hz, 1H), 8.00 (d, J = 8.9 Hz, 1H), 7.69 (dd, J = 8.8, 2.5 Hz, 1H), 7.24 (dd, J = 8.5, 5.3 Hz, 2H), 6.99 (t, J = 8.6 Hz, 2H), 6.94 (dt, J = 15.1, 4.6 Hz, 1H), 6.37 (dt, J = 15.1, 1.9 Hz, 1H), 4.70 (dd, J = 4.7, 1.9 Hz, 2H), 4.66 (s, 2H), 4.10 (q, J = 7.1 Hz, 2H), 2.43–2.32 (m, 2H), 1.84–1.75 (m, 2H), 1.70–1.51 (m, 6H), 1.21 (t, J = 7.1 Hz, 3H). 13C NMR{1H} (151 MHz, CDCl3): δ 171.7, 168.6, 162.1 (d, J = 246.7 Hz), 154.5, 148.7, 140.4, 140.4, 133.5 (d, J = 3.1 Hz), 128.2 (d, J = 8.0 Hz, 2C), 123.2, 115.9 (d, J = 21.6 Hz, 2C), 115.1, 114.2, 66.7, 66.0, 64.4, 47.8, 32.6 (2C), 25.3, 22.6 (2C), 14.2. 19F NMR{1H} (376 MHz, CDCl3): δ −114.5. IR (neat) νmax (cm–1): 2934, 2330, 1745, 1502, 1452, 1369, 1288, 1256, 1223, 1190, 1157, 1128, 1092, 1001, 824, 791, 737, 631, 515. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C26H30BrFN3O5, 562.1347; found, 562.1326.
(E)-4-((1-Benzyl-4-((3,4-dimethoxyphenethyl)carbamoyl)piperidin-4-yl)(4-(trifluoromethyl)benzyl)amino)-4-oxobut-2-en-1-yl Ethyl Carbonate (2q)
It was prepared according to GP-A. Purification of the crude material by silica gel column chromatography (80% EtOAc/cHex) provided the title compound as a pale oil in 49% yield (1.743 g, 2.45 mmol). Rf = 0.10 (90% EtOAc/cHex). 1H NMR (600 MHz, CDCl3): δ 7.58 (d, J = 8.1 Hz, 2H), 7.33 (d, J = 8.1 Hz, 2H), 7.31–7.16 (m, 5H), 6.87–6.81 (m, 2H), 6.78 (m, 2H), 6.74 (d, J = 7.81 Hz, 1H), 6.10 (d, J = 15.1 Hz, 1H), 4.68 (d, J = 4.7, 2H), 4.58 (s, 2H), 4.04 (q, J = 7.1 Hz, 2H), 3.86 (s, 3H), 3.82 (s, 3H), 3.56 (q, J = 6.8 Hz, 2H), 3.31 (s, 2H), 2.78 (t, J = 6.8 Hz, 2H), 2.62–2.58 (m, 2H), 2.56–2.50 (m, 2H), 2.06 (t, J = 11.6 Hz, 2H), 1.75 (m, 2H), 1.16 (t, J = 7.1 Hz, 3H). 13C NMR{1H} (151 MHz, CDCl3): δ 171.7, 168.7, 154.5, 149.1, 147.7, 142.6, 140.0, 138.0, 131.4, 129.7 (q, J = 32.5 Hz), 129.1 (2C), 128.2 (2C), 127.1, 126.3 (2C), 125.9 (d, J = 3.7 Hz, 2C), 124.0 (d, J = 272.0 Hz), 123.6, 120.8, 111.9, 111.2, 65.9, 64.9, 64.3, 62.7, 55.8, 55.8, 50.3 (2C), 48.3, 40.4, 35.0, 32.8 (2C), 14.1. 19F NMR{1H} (376 MHz, CDCl3): δ −62.4. IR (neat) νmax (cm–1): 2915, 1649, 1323, 1279, 1261, 1238, 1159, 1119, 1067, 1028, 1016, 737, 700. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C38H45F3N3O7, 712.3204; found, 712.3232.
(E)-4-((8-(tert-Butylcarbamoyl)-1,4-dioxaspiro[4.5]decan-8-yl)(4-fluorobenzyl)amino)-4-oxobut-2-en-1-yl Ethyl Carbonate (2r)
It was prepared according to GP-A. Purification of the crude material by silica gel column chromatography (50% EtOAc/cHex) provided the title compound as a white solid in 74% yield (1.926 g, 3.7 mmol). Rf = 0.25 (50% EtOAc/cHex). 1H NMR (600 MHz, CDCl3): δ 7.19 (dd, J = 8.1, 5.3 Hz, 2H), 6.96 (t, J = 8.3 Hz, 2H), 6.80 (dt, J = 15.1, 4.7 Hz, 1H), 6.40–6.30 (br, 1H), 6.26 (d, J = 15.1 Hz, 1H), 4.63 (d, J = 4.7 Hz, 2H), 4.55 (s, 2H), 4.04 (q, J = 7.1 Hz, 2H), 3.80 (s, 4H), 2.35–2.24 (m, 2H), 1.96–1.83 (m, 2H), 1.82–1.69 (m, 2H), 1.56–1.46 (m, 2H), 1.19 (s, 9H), 1.14 (t, J = 7.1 Hz, 3H). 13C NMR{1H} (151 MHz, CDCl3): δ 171.1, 168.2, 161.7 (d, J = 246.0 Hz), 154.3, 138.9, 134.1, 127.8 (d, J = 7.7 Hz, 2C), 123.9, 115.5 (d, J = 21.5 Hz, 2C), 107.3, 65.7 (2C), 65.6, 64.0, 63.9, 50.7 (2C), 47.3, 31.3 (2C), 30.3, 28.2 (3C), 13.9. 19F NMR{1H} (376 MHz, CDCl3): δ −116.0. IR (neat) νmax (cm–1): 3357, 2296, 1753, 1664, 1616, 1508, 1410, 1367, 1246, 1219, 1190, 1105, 1095, 1040, 951, 895, 862, 814, 791. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C27H38FN2O7, 521.2658; found, 521.2641.
(E)-Ethyl (4-((1-(Isopropylcarbamoyl)cyclopentyl)(p-tolyl)amino)-4-oxobut-2-en-1-yl)carbonate (2s)
It was prepared according to GP-A. Purification of the crude material by silica gel column chromatography (50% EtOAc/cHex) provided the title compound as a pale solid in 67% yield (1.395 g, 3.35 mmol). Rf = 0.27 (50% EtOAc/cHex). 1H NMR (600 MHz, CDCl3): δ 7.14 (d, J = 8.3 Hz, 2H), 7.07 (d, J = 8.3 Hz, 2H), 6.72 (dt, J = 15.3, 5.1 Hz, 1H), 6.43 (d, J = 7.5 Hz, 1H), 5.68 (d, J = 15.3 Hz, 1H), 4.52 (d, J = 5.1 Hz, 2H), 4.05 (m, 3H), 2.33 (s, 3H), 2.30–2.20 (m, 2H), 1.77–1.66 (m, 2H), 1.58–1.50 (m, 4H), 1.19 (t, J = 7.1 Hz, 3H), 1.13 (d, J = 7.5 Hz, 6H). 13C NMR{1H} (151 MHz, CDCl3): δ 173.0, 166.1, 154.5, 138.4, 137.4, 137.1, 129.9 (2C), 129.8 (2C), 124.4, 74.0, 66.1, 64.1, 41.5, 36.8 (2C), 23.2 (2C), 22.3 (2C), 21.1, 14.2. IR (neat) νmax (cm–1): 3346, 2943, 1744, 1655, 1510, 1375, 1244, 1227, 1169, 1036, 966, 829, 791, 685, 525. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C23H33N2O5, 417.2384; found, 417.2381.
(R)-4-Benzyl-1-(4-methoxybenzyl)-3-vinyl-1,4-diazaspiro[5.5]undecane-2,5-dione (3a)
It was prepared according to GP-B using 2a. The crude material was purified by silica gel column chromatography (20% EtOAc/cHex) provided the title compound as a colorless oil in 86% yield (71.9 mg, 0.17 mmol). Rf = 0.36 (30% EtOAc/cHex). 1H NMR (600 MHz, CDCl3): δ 7.34 (t, J = 7.0 Hz, 2H), 7.29 (t, J = 7.4 Hz, 1H), 7.25–7.21 (m, 2H), 7.10 (d, J = 8.3 Hz, 2H), 6.82 (d, J = 8.3 Hz, 2H), 5.98 (ddd, J = 16.9, 10.3, 5.5 Hz, 1H), 5.50–5.42 (m, 2H), 5.36 (d, J = 16.9 Hz, 1H), 4.94 (d, J = 15.8 Hz, 1H), 4.57 (d, J = 5.6 Hz, 1H), 4.41 (d, J = 15.8 Hz, 1H), 3.84 (d, J = 14.8 Hz, 1H), 3.78 (s, 3H), 2.42 (dddd, J = 17.5, 13.1, 8.7, 4.3 Hz, 1H), 2.05–1.99 (m, 1H), 1.96–1.92 (m, 1H), 1.80 (td, J = 12.9, 5.2 Hz, 1H), 1.74–1.53 (m, 5H), 1.10 (tdd, J = 12.9, 9.0, 3.3 Hz, 1H). 13C NMR{1H} (151 MHz, CDCl3): δ 169.7, 167.2, 158.7, 136.1, 133.2, 130.4, 129.0 (2C), 128.2 (2C), 128.0 (3C), 119.0, 114.0 (2C), 63.1, 61.8, 55.4, 47.6, 45.0, 36.0, 33.3, 24.6, 23.5, 22.3. IR (neat) νmax (cm–1): 2926, 2851, 1649, 1512, 1452, 1410, 1354, 1300, 1242, 1175, 1032, 802, 731, 698, 625, 419. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C26H31N2O3, 419.2329; found, 419.2343. [α]D20 +100.0 (c = 0.2, CHCl3). SFC-MS (method 5) er: 97:3; tret (major) = 3.875 min (96.9%), tret (minor) = 4.067 min (3.1%).
Benzyl-(R)-3-(2-(1-(2,2-dimethoxyethyl)-2,5-dioxo-3-vinyl-1,4-diazaspiro[5.6]dodecan-4-yl)ethyl)-1H-indole-1-carboxylate (3b)
It was prepared according to GP-B using 2b. The crude material was purified by silica gel column chromatography (30% EtOAc/cHex) provided the title compound as a pale oil in 33% yield (37.8 mg, 0.07 mmol). Rf = 0.22 (30% EtOAc/cHex). 1H NMR (600 MHz, CDCl3): δ 8.16 (s, 1H), 7.59 (d, J = 7.7 Hz, 1H), 7.47 (d, J = 7.2 Hz, 2H), 7.45–7.36 (m, 4H), 7.32 (t, J = 7.6 Hz, 1H), 7.26 (t, J = 7.2 Hz, 1H), 5.84 (ddd, J = 16.8, 10.2, 6.2 Hz, 1H), 5.42 (s, 2H), 5.35 (d, J = 10.8 Hz, 1H), 5.25 (d, J = 17.1 Hz, 1H), 4.67 (t, J = 5.0 Hz, 1H), 4.41 (d, J = 6.1 Hz, 1H), 4.09 (dt, J = 14.6, 7.4 Hz, 1H), 3.54 (dd, J = 13.9, 4.6 Hz, 1H), 3.42 (s, 3H), 3.40–3.35 (m, 4H), 3.17 (dt, J = 13.5, 7.8 Hz, 1H), 2.98 (t, J = 7.7 Hz, 2H), 2.67 (dd, J = 15.7, 11.1 Hz, 1H), 2.18 (dd, J = 15.9, 7.8 Hz, 1H), 2.05–1.99 (m, 2H), 1.88 (q, J = 7.2 Hz, 1H), 1.74–1.61 (m, 4H), 1.55–1.49 (m, 2H), 1.37–1.31 (m, 1H). 13C NMR{1H} (151 MHz, CDCl3) 169.6, 166.2, 150.8, 135.1, 133.4, 133.3, 130.4, 128.9 (2C), 128.8, 128.6 (2C), 124.9, 123.1, 119.5, 119.1, 118.3, 115.5, 103.0, 76.9, 68.7, 67.9, 63.8, 56.0, 55.9, 47.3, 46.4, 39.7, 36.5, 31.5, 31.3, 25.7, 23.6, 22.9. IR (neat) νmax (cm–1): 2934, 1736, 1666, 1454, 1396, 1354, 1244, 1180, 1122, 1088, 1049, 1016, 731, 698. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C34H42N3O6, 588.3068; found, 588.3048. [α]D20 +40.0 (c = 0.3, CHCl3). SFC-MS (method 1) er: 90:10; tret (major) = 5.446 min (89.7%), tret (minor) = 6.092 min (10.3%).
(R)-4-Benzyl-1-butyl-3-vinyl-1,4-diazaspiro[5.6]dodecane-2,5-dione (3c)
It was prepared according to GP-B using 2c. The crude material was purified by silica gel column chromatography (30% EtOAc/cHex) provided the title compound as a colorless oil in 56% yield (41.3 mg, 0.11 mmol). Rf = 0.31 (30% EtOAc/cHex). 1H NMR (600 MHz, CDCl3): δ 7.41–7.26 (m, 3H), 7.25–7.11 (m, 2H), 5.89 (ddd, J = 17.1, 10.3, 6.0 Hz, 1H), 5.46 (d, J = 14.7 Hz, 1H), 5.42 (dd, J = 10.2, 1.5 Hz, 1H), 5.33 (dd, J = 17.1, 1.5 Hz, 1H), 4.43 (d, J = 6.0 Hz, 1H), 3.83 (d, J = 14.7 Hz, 1H), 3.51 (ddd, J = 13.5, 11.1, 5.1 Hz, 1H), 3.25 (ddd, J = 13.5, 11.2, 4.9 Hz, 1H), 2.89–2.72 (m, 1H), 2.12 (dd, J = 14.7, 9.0 Hz, 1H), 2.08–1.88 (m, 3H), 1.77 (q, J = 7.6 Hz, 2H), 1.71–1.53 (m, 6H), 1.48–1.23 (m, 3H), 0.94 (t, J = 7.4 Hz, 3H). 13C NMR{1H} (151 MHz, CDCl3): δ 170.0, 165.0, 136.0, 133.3, 128.9 (2C), 128.3 (2C), 127.9, 119.4, 67.6, 61.8, 47.9, 44.2, 40.10, 37.2, 31.6, 31.5, 31.4, 25.9, 23.7, 20.7, 13.8. IR (neat) νmax (cm–1): 2926, 1649, 1452, 1416, 1400, 1393, 1358, 1302, 1250, 1205, 727, 698, 419. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C23H33N2O2, 369.2537; found, 369.2542. [α]D20 +62.0 (c = 1.0, CHCl3). SFC-MS (method 4) er: 97:3; tret (major) = 2.451 min (96.6%), tret (minor) = 2.586 min (3.4%).
(R)-1-(3,5-Bis(trifluoromethyl)benzyl)-4-(3-methoxypropyl)-3-vinyl-1,4-diazaspiro[5.5]undecane-2,5-dione (3d)
It was prepared according to GP-B using 2d. The crude material was purified by silica gel column chromatography (30% EtOAc/cHex) provided the title compound as a colorless oil in 44% yield (35.3 mg, 0.09 mmol). Rf = 0.39 (40% EtOAc/cHex). 1H NMR (600 MHz, CDCl3): δ 7.75 (s, 1H), 7.62 (s, 2H), 5.98 (ddd, J = 17.2, 10.4, 4.9 Hz, 1H), 5.41 (dd, J = 10.4, 1.9 Hz, 1H), 5.31 (dd, J = 17.2, 1.9 Hz, 1H), 5.15 (d, J = 16.6 Hz, 1H), 4.75 (dt, J = 5.0, 1.9 Hz, 1H), 4.43 (d, J = 16.6 Hz, 1H), 4.09–3.98 (m, 1H), 3.48–3.37 (m, 2H), 3.30 (s, 3H), 2.93 (dt, J = 13.6, 7.4 Hz, 1H), 2.49–2.32 (m, 1H), 2.06–1.91 (m, 2H), 1.90–1.80 (m, 2H), 1.75–1.66 (m, 2H), 1.64–1.47 (m, 4H), 1.10 (qt, J = 13.8, 4.4 Hz, 1H). 13C NMR{1H} (151 MHz, CDCl3): δ 169.0, 167.6, 141.1, 133.6, 132.0 (q, J = 33.3 Hz, 2C), 126.6 (q, J = 3 Hz, 2C), 123.3 (d, J = 272.8 Hz, 2C), 121.3 (h, J = 3.7 Hz) 118.6, 69.9, 63.3, 63.1, 58.7, 45.1, 43.9, 35.3, 33.7, 27.3, 24.5, 23.4, 22.0. 19F NMR{1H} (376 MHz, CDCl3): δ −62.9. IR (neat) νmax (cm–1): 2932, 1745, 1649, 1281, 1240, 1175, 1165, 1121, 1101, 878, 702, 681, 413. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C24H29F6N2O3, 507.2077; found, 507.2092. [α]D20 +48.0 (c = 1.0, CHCl3). SFC-MS (method 7) er: 93:7; tret (minor) = 3.926 min (6.9%), tret (major) = 4.063 min (93.1%).
(R)-1-Butyl-4-(2,4-dimethoxybenzyl)-3,3-dimethyl-6-vinyl Piperazine-2,5-dione (3e)
It was prepared according to GP-B using 2e. The crude material was purified by silica gel column chromatography (30% EtOAc/cHex) provided the title compound as a pale solid in 24% yield (18.0 mg, 0.05 mmol). Rf = 0.37 (40% EtOAc/cHex). 1H NMR (600 MHz, CDCl3): δ 7.00 (d, J = 8.6 Hz, 1H), 6.49–6.35 (m, 2H), 5.97 (ddd, J = 17.2, 10.3, 5.3 Hz, 1H), 5.40 (dd, J = 10.4, 1.7 Hz, 1H), 5.32 (dd, J = 17.2, 1.7 Hz, 1H), 4.69 (d, J = 16.1 Hz, 1H), 4.65 (dt, J = 5.4, 1.8 Hz, 1H), 4.54 (d, J = 16.1 Hz, 1H), 4.02 (ddd, J = 13.5, 8.9, 6.7 Hz, 1H), 3.81 (s, 3H), 3.77 (s, 3H), 2.77 (ddd, J = 13.5, 8.7, 5.9 Hz, 1H), 1.61–1.52 (m, 2H), 1.48 (s, 3H), 1.37 (s, 3H), 1.36–1.31 (m, 2H), 0.93 (t, J = 7.4 Hz, 3H). 13C NMR{1H} (151 MHz, CDCl3): δ 170.0, 165.7, 160.0, 157.2, 133.7, 128.4, 118.6, 118.6, 104.4, 98.4, 62.9, 61.9, 55.5, 55.4, 45.3, 39.9, 29.4, 27.1, 25.6, 20.2, 13.9. IR (neat) νmax (cm–1): 2934, 1651, 1612, 1512, 1412, 1244, 1175, 1034, 733, 700. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C21H31N2O4, 375.2278; found, 375.2283. [α]D20 +90.0 (c = 0.2, CHCl3). SFC-MS (method 1) er: 12:88; tret (minor) = 5.687 min (11.8%), tret (major) = 6.215 min (88.2%).
(R)-4-(4-Fluorobenzyl)-1-isopropyl-3,3-dimethyl-6-vinyl Piperazine-2,5-dione (3f)
It was prepared according to GP-B using 2f. The crude material was purified by silica gel column chromatography (30% EtOAc/cHex) provided the title compound as a colorless oil in 32% yield (20.4 mg, 0.06 mmol). Rf = 0.22 (30% EtOAc/cHex). 1H NMR (600 MHz, CDCl3): δ 7.18 (dd, J = 8.6, 5.4 Hz, 2H), 6.95 (t, J = 8.7 Hz, 2H), 5.91 (ddd, J = 17.1, 10.3, 6.1 Hz, 1H), 5.49 (dd, J = 17.1, 1.5 Hz, 1H), 5.38 (dd, J = 10.4, 1.6 Hz, 1H), 4.71 (d, J = 15.6 Hz, 1H), 4.67 (dt, J = 6.1, 1.6 Hz, 1H), 4.51 (d, J = 15.6 Hz, 1H), 4.37 (hept, J = 6.9 Hz, 1H), 1.48 (s, 3H), 1.34 (s, 3H), 1.26 (d, J = 6.9 Hz, 3H), 1.22 (d, J = 6.9 Hz, 3H). 13C NMR{1H} (151 MHz, CDCl3): δ 169.2, 166.3, 161.7 (d, J = 245.5 Hz), 135.6, 134.0 (d, J = 3.2 Hz), 128.9 (d, J = 8.1 Hz), 119.5, 115.5 (d, J = 21.5 Hz), 62.0, 60.3, 48.5, 45.3, 27.6, 26.2, 20.4, 20.0. 19F NMR{1H} (376 MHz, CDCl3): δ −115.5. IR (neat) νmax (cm–1): 2926, 2851, 1652, 1508, 1412, 1354, 1221, 1194, 1157, 829, 698, 419. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C18H24FN2O2, 319.1816; found, 319.1832. [α]D20 +16.0 (c = 0.5, CHCl3). SFC-MS (method 3) er: 88:12; tret (major) = 3.419 min (87.8%), tret (minor) = 3.545 min (12.2%).
Methyl (R)-2-(1-(4-Methoxybenzyl)-2,5-dioxo-3-vinyl-1,4-diazaspiro[5.5]undecan-4-yl)acetate (3g)
It was prepared according to GP-B using 2g. The crude material was purified by silica gel column chromatography (30% EtOAc/cHex) provided the title compound as a pale oil in 77% yield (62.78 mg, 0.15 mmol). Rf = 0.16 (30% EtOAc/cHex). 1H NMR (600 MHz, CDCl3): δ 7.15 (d, J = 8.6 Hz, 2H), 6.83 (d, J = 8.6 Hz, 2H), 6.02 (m, 1H), 5.43 (d, J = 10.3 Hz, 1H), 5.39 (d, J = 17.1 Hz, 1H), 4.92 (d, J = 15.8 Hz, 1H), 4.69 (d, J = 6.1 Hz, 1H), 4.49 (d, J = 15.8 Hz, 1H), 4.42 (d, J = 17.1 Hz, 1H), 3.78 (s, 3H), 3.77–3.74 (m, 4H), 2.24–2.14 (m, 1H), 1.99–1.92 (m, 2H), 1.76–1.53 (m, 6H), 1.10–1.01 (m, 1H). 13C NMR{1H} (151 MHz, CDCl3): δ 170.3, 169.2, 167.1, 158.9, 133.2, 130.6, 128.2 (2), 119.9, 114.3 (2), 64.6, 63.1, 55.6, 52.8, 47.0, 45.3, 36.2, 33.0, 24.8, 23.4, 22.6. IR (neat) νmax (cm–1): 2929, 1747, 1649, 1512, 1427, 1412, 1400, 1263, 1244, 1209, 1176, 1032, 1014, 804. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C22H29N2O5, 401.2071; found, 401.2084. [α]D20 +30.0 (c = 1.0, CHCl3). SFC-MS (method 4) er: 92:8; tret (major) = 3.718 min (92.0%), tret (minor) = 3.971 min (8.0%).
Methyl (R)-2-(6,9-Dioxo-5-phenethyl-7-vinyl-5,8-diazaspiro[3.5]nonan-8-yl)acetate (3h)
It was prepared according to GP-B using 2h. The crude material was purified by silica gel column chromatography (50% EtOAc/cHex) provided the title compound as a pale oil in 95% yield (67.7 mg, 0.19 mmol). Rf = 0.05 (30% EtOAc/cHex). 1H NMR (600 MHz, CDCl3): δ 7.35–7.27 (m, 2H), 7.25–7.19 (m, 3H), 5.82 (ddd, J = 16.9, 10.2, 6.6 Hz, 1H), 5.40–5.25 (m, 2H), 4.58–4.46 (m, 2H), 3.98 (ddd, J = 13.7, 10.4, 5.4 Hz, 1H), 3.82–3.71 (m, 4H), 3.56 (ddd, J = 13.7, 10.2, 5.8 Hz, 1H), 2.97 (ddd, J = 13.2, 10.2, 5.4 Hz, 1H), 2.87–2.75 (m, 2H), 2.52–2.43 (m, 2H), 2.36–2.27 (m, 1H), 2.13–2.00 (m, 1H), 1.89–1.78 (m, 1H). 13C NMR{1H} (151 MHz, CDCl3) 169.4, 168.8, 164.9, 138.5, 132.4, 128.9 (2C), 128.7 (2C), 126.7, 119.9, 64.3, 62.3, 52.5, 46.4, 45.3, 35.6, 34.4, 30.5, 14.5. IR (neat) νmax (cm–1): 2953, 1749, 1655, 1454, 1412, 1402, 1283, 1257, 1207, 1178, 1148, 748, 700, 505. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C20H25N2O4, 357.1809; found, 357.1815. [α]D20 +12.0 (c = 1.0, CHCl3). SFC-MS (method 1) er: 88:12; tret (minor) = 5.673 min (12.3%), tret (major) = 6.190 min (87.7%).
(R)-6-(4-Chlorobenzyl)-9-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-8-vinyl-6,9-diazaspiro[4.5]decane-7,10-dione (3i)
It was prepared according to GP-B using 2i. The crude material was purified by silica gel column chromatography (30% EtOAc/cHex) provided the title compound as a brown oil in 97% yield (87.9 mg, 0.19 mmol). Rf = 0.18 (30% EtOAc/cHex). 1H NMR (600 MHz, CDCl3): δ 7.29 (d, J = 8.4 Hz, 2H), 7.17 (d, J = 8.4 Hz, 2H), 6.86 (d, J = 8.6 Hz, 1H), 6.82 (d, J = 2.5 Hz, 1H), 6.75 (dd, J = 8.6, 2.5 Hz, 1H), 5.95 (ddd, J = 16.9, 10.3, 6.3 Hz, 1H), 5.42–5.34 (m, 2H), 4.98 (d, J = 16.0 Hz, 1H), 4.92 (dt, J = 6.3, 1.5 Hz, 1H), 4.25 (d, J = 16.0 Hz, 1H), 4.23 (s, 4H), 2.57–2.51 (m, 1H), 2.34 (ddd, J = 12.8, 7.2, 4.7 Hz, 1H), 1.97–1.71 (m, 6H). 13C NMR{1H} (151 MHz, CDCl3): δ 170.7, 166.4, 143.7, 143.0, 136.5, 133.3, 132.9 (2C), 128.9 (2C), 127.8 (2C), 120.4, 120.0, 117.6, 116.2, 71.0, 66.3, 64.3, 64.3, 46.5, 41.5, 35.8, 27.0, 26.4. IR (neat) νmax (cm–1): 3292, 2932, 1744, 1664, 1504, 1402, 1302, 1254, 1240, 1202, 1175, 1067, 885, 800, 791, 734, 652, 420. HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C25H25ClN2NaO4, 475.1395; found, 475.1387. [α]D20 +22.0 (c = 1.0, CHCl3). SFC-MS (method 2): er: 95:5; tret (major) = 6.118 min (95.1%), tret (minor) = 6.417 min (4.9%).
It was also prepared according to GP-B from 430.5 mg (1.00 mmol) of 2i, providing the title compound in 89% yield (303.0 mg, 0.89 mmol).
(R)-4-(2,3-Dihydrobenzo[b][1,4]dioxin-6-yl)-1-(prop-2-yn-1-yl)-3-vinyl-1,4-diazaspiro[5.5]undecane-2,5-dione (3j)
It was prepared according to GP-B using 2j. The crude material was purified by silica gel column chromatography (40% EtOAc/cHex), providing the title compound as a pale oil in 73% yield (55.5 mg, 0.15 mmol). Rf = 0.16 (30% EtOAc/cHex). 1H NMR (600 MHz, CDCl3): δ 6.87 (d, J = 8.5 Hz, 1H), 6.74 (d, J = 2.5 Hz, 1H), 6.69 (dd, J = 8.6, 2.5 Hz, 1H), 5.91 (ddd, J = 16.7, 10.3, 5.9 Hz, 1H), 5.35 (d, J = 17.1 Hz, 1H), 5.32 (dd, J = 10.4, 1.6 Hz, 1H), 4.90 (d, J = 5.9 Hz, 1H), 4.41 (dd, J = 17.6, 2.4 Hz, 1H), 4.30–4.22 (m, 5H), 2.41 (tdd, J = 13.0, 8.8, 4.6 Hz, 1H), 2.26 (t, J = 2.5 Hz, 1H), 2.23–2.19 (m, 1H), 2.14–2.10 (m, 1H), 2.01 (td, J = 12.8, 5.0 Hz, 1H), 1.91 (td, J = 12.9, 4.8 Hz, 1H), 1.76–1.68 (m, 4H), 1.22 (dd, J = 10.6, 6.4 Hz, 1H). 13C NMR{1H} (151 MHz, CDCl3): δ 169.0, 166.5, 143.9, 143.1, 133.1, 133.1, 120.5, 120.2, 117.9, 116.7, 80.0, 71.8, 65.9, 64.4, 64.4, 63.1, 35.8, 32.8, 31.5, 24.6, 23.2, 22.3. IR (neat) νmax (cm–1): 2932, 1647, 1504, 1404, 1308, 1292, 1279, 1261, 1242, 1213, 1065, 887, 746, 663, 621, 409. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C22H25N2O4, 381.1809; found, 381.1813. [α]D20 +10.0 (c = 1.0, CHCl3). SFC-MS (method 2) er: 94:6; tret (minor) = 4.943 min (5.8%), tret (major) = 5.505 min (94.2%).
(R)-5-(4-Bromobenzyl)-8-(2,6-dimethylphenyl)-7-vinyl-5,8-diazaspiro[3.5]nonane-6,9-dione (3k)
It was prepared according to GP-B using 2k. The crude material was purified by silica gel column chromatography (20% EtOAc/cHex), providing the title compound as a pale oil in 46% yield (41.7 mg, 0.09 mmol). Rf = 0.19 (30% EtOAc/cHex). 1H NMR (600 MHz, CDCl3): δ 7.46 (d, J = 8.4 Hz, 2H), 7.17 (t, J = 7.5 Hz, 1H), 7.14–7.05 (m, 4H), 5.82 (ddd, J = 17.0, 9.9, 8.4 Hz, 1H), 5.32–5.20 (m, 2H), 5.09 (d, J = 16.0 Hz, 1H), 4.74 (d, J = 16.0 Hz, 1H), 4.59 (d, J = 8.4 Hz, 1H), 2.87–2.78 (m, 1H), 2.69 (ddt, J = 12.3, 8.0, 3.7 Hz, 1H), 2.51–2.42 (m, 2H), 2.22 (s, 3H), 2.20 (s, 3H), 2.09–2.00 (m, 1H), 1.85–1.78 (m, 1H). 13C NMR{1H} (151 MHz, CDCl3): δ 167.3, 167.1, 137.1, 136.9, 134.9, 132.0 (2C), 131.1, 129.2, 128.8, 128.5 (2C), 128.4, 121.9 (2C), 121.3, 66.3, 63.1, 45.8, 33.9, 30.4, 18.7, 18.1, 14.8. IR (neat) νmax (cm–1): 2957, 1663, 1487, 1398, 1306, 1284, 1221, 1161, 1009, 924, 771, 731, 473. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C24H26BrN2O2, 453.1172; found, 453.1162. [α]D20 −38.0 (c = 2.0, CHCl3). SFC-MS (method 2) er: 91:9; tret (major) = 4.955 min (91.1%), tret (minor) = 5.126 min (8.9%).
(R)-6-Allyl-9-(4-methoxyphenyl)-8-vinyl-6,9-diazaspiro[4.5]decane-7,10-dione (3l)
It was prepared according to GP-B using 2l. The crude material was purified by silica gel column chromatography (30% EtOAc/cHex), providing the title compound as a yellow oil in 96% yield (65.4 mg, 0.19 mmol). Rf = 0.17 (30% EtOAc/cHex). 1 mmol scale: prepared according to GP-B using 2l. The crude material was purified by silica gel column chromatography (30% EtOAc/cHex) providing the title compound as a yellow oil in 89% yield (303.0 mg, 0.89 mmol). 1H NMR (600 MHz, CDCl3): δ 7.17 (d, J = 8.9 Hz, 2H), 6.89 (d, J = 8.9 Hz, 2H), 6.02–5.83 (m, 2H), 5.37–5.29 (m, 2H), 5.25–5.09 (m, 2H), 4.83 (d, J = 6.5 Hz, 1H), 4.35–4.24 (m, 1H), 3.79 (s, 3H), 3.77–3.65 (m, 1H), 2.61 (ddd, J = 14.3, 8.4, 7.2 Hz, 1H), 2.36–2.30 (m, 1H), 2.13–2.07 (m, 1H), 2.01–1.95 (m, 1H), 1.94–1.68 (m, 4H). 13C NMR{1H} (151 MHz, CDCl3): δ 171.0, 165.4, 158.6, 134.0, 133.6, 132.5, 128.2 (2C), 120.2, 116.6, 114.5 (2C), 70.8, 66.4, 55.5, 46.4, 41.6, 36.0, 27.2, 26.6. IR (neat) νmax (cm–1): 2957, 1655, 1508, 1425, 1404, 1298, 1279, 1238, 1180, 1134, 1030, 926, 827, 532. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C20H25N2O3, 341.1860; found, 341.1864. [α]D20 −14.0 (c = 1.0, CHCl3). SFC-MS (method 4) er: 93:7; tret (minor) = 4.244 min (7.3%), tret (major) = 4.479 min (92.7%).
(R)-9-(3,3-Diethoxypropyl)-12-(2,6-dimethylphenyl)-11-vinyl-1,4-dioxa-9,12-diazadispiro[4.2.58.25]pentadecane-10,13-dione (3m)
It was prepared according to GP-B using 2m. The crude material was purified by silica gel column chromatography (50% EtOAc/cHex), providing the title compound as a colorless oil in 91% yield (91.1 mg, 0.18 mmol). Rf = 0.14 (40% EtOAc/cHex). 1H NMR (600 MHz, CDCl3): δ 7.11 (t, J = 7.5 Hz, 1H), 7.08–6.98 (m, 2H), 5.79 (ddd, J = 16.8, 10.0, 8.3 Hz, 1H), 5.19 (d, J = 9.9 Hz, 1H), 5.11 (d, J = 16.8 Hz, 1H), 4.60 (t, J = 5.6 Hz, 1H), 4.53 (d, J = 8.3 Hz, 1H), 3.93–3.87 (m, 4H), 3.77–3.64 (m, 3H), 3.55–3.48 (m, 2H), 3.42 (ddd, J = 13.8, 10.8, 4.9 Hz, 1H), 2.54 (td, J = 13.2, 4.9 Hz, 1H), 2.33–2.24 (m, 2H), 2.18 (td, J = 13.4, 4.1 Hz, 1H), 2.13 (s, 3H) 2.12 (s, 3H), 2.10–2.04 (m, 3H), 1.88 (dtd, J = 13.2, 6.0, 3.0 Hz, 1H), 1.71–1.65 (m, 2H), 1.20 (q, J = 7.1 Hz, 6H). 13C NMR{1H} (151 MHz, CDCl3): δ 167.6, 166.2, 136.6 (2C), 134.3, 129.1, 128.7, 128.3, 121.8, 107.6, 101.0, 65.1, 64.5, 64.2, 61.8, 61.7, 61.1, 39.4, 33.9, 32.7, 31.8, 31.2, 30.7, 26.9, 18.5, 17.7, 15.3 (2C). IR (neat) νmax (cm–1): 2934, 2874, 1655, 1416, 1371, 1277, 1167, 1121, 1103, 1090, 1051, 1036, 930, 903, 783. HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C28H40N2NaO6, 523.2779; found, 523.2748. [α]D20 −124.0 (c = 1.0, CHCl3). SFC-MS (method 1) er: 90:10; tret (minor) = 5.059 min (9.6%), tret (major) = 6.027 min (90.4%).
(R)-9-(4-Methoxyphenyl)-6-propyl-8-vinyl-6,9-diazaspiro[4.5]decane-7,10-dione (3n)
It was prepared according to GP-B using 2n. The crude material was purified by silica gel column chromatography (30% EtOAc/cHex), providing the title compound as a colorless oil in 98% yield (67.1 mg, 0.20 mmol). Rf = 0.23 (30% EtOAc/cHex). 1H NMR (600 MHz, CDCl3): δ 7.17 (d, J = 8.9 Hz, 2H), 6.90 (d, J = 8.9 Hz, 2H), 5.90 (ddd, J = 16.9, 10.3, 6.5 Hz, 1H), 5.38–5.27 (m, 2H), 4.80 (d, J = 6.5 Hz, 1H), 3.80 (s, 3H), 3.53 (ddd, J = 13.4, 11.2, 5.2 Hz, 1H), 3.00 (ddd, J = 13.4, 11.3, 4.9 Hz, 1H), 2.64 (tt, J = 9.8, 4.8 Hz, 1H), 2.35–2.29 (m, 1H), 2.06–1.97 (m, 2H), 1.93–1.77 (m, 5H), 1.67–1.56 (m, 1H), 0.95 (t, J = 7.4 Hz, 3H). 13C NMR{1H} (151 MHz, CDCl3): δ 171.1, 165.6, 158.7, 133.7, 132.6, 128.2 (2C), 120.1, 114.5 (2C), 70.7, 66.5, 55.6, 46.4, 41.6, 36.1, 27.2, 26.5, 22.8, 11.7. IR (neat) νmax (cm–1): 2959, 2934, 1647, 1512, 1420, 1300, 1240, 1034, 928, 831, 808, 563, 527. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C20H27N2O3, 343.2016; found, 343.2033. [α]D20 −34.0 (c = 1.0, CHCl3). SFC-MS (method 5) er: 95:5; tret (major) = 3.701 min (95.1%), tret (minor) = 3.945 min (4.9%).
(R)-6-(4-Chlorobenzyl)-9-(naphthalen-2-yl)-8-vinyl-6,9-diazaspiro[4.5]decane-7,10-dione (3o)
It was prepared according to GP-B using 2o. The crude material was purified by silica gel column chromatography (20% EtOAc/cHex), providing the title compound as a pale oil in 94% yield (83.7 mg, 0.19 mmol). Rf = 0.36 (30% EtOAc/cHex). 1H NMR (600 MHz, CDCl3): δ 7.89 (d, J = 8.8 Hz, 1H), 7.86–7.81 (m, 2H), 7.79 (d, J = 2.1 Hz, 1H), 7.54–7.49 (m, 2H), 7.45 (dd, J = 8.7, 2.2 Hz, 1H), 7.33 (d, J = 8.5 Hz, 2H), 7.23 (d, J = 8.5 Hz, 2H), 6.07 (ddd, J = 17.1, 10.3, 6.1 Hz, 1H), 5.43 (dd, J = 17.1, 1.5 Hz, 1H), 5.39 (dd, J = 10.3, 1.5 Hz, 1H), 5.18 (dt, J = 6.1, 1.6 Hz, 1H), 5.05 (d, J = 15.8 Hz, 1H), 4.29 (d, J = 15.9 Hz, 1H), 2.66–2.59 (m, 1H), 2.46 (ddd, J = 13.4, 7.3, 4.7 Hz, 1H), 2.06–1.92 (m, 3H), 1.92–1.86 (m, 1H), 1.85–1.76 (m, 2H). 13C NMR{1H} (151 MHz, CDCl3): δ 171.0, 166.4, 137.3, 136.5, 133.5, 133.4, 133.0, 132.3, 129.1, 128.9 (2C), 128.0, 127.9 (2C), 127.7, 126.6, 126.6, 125.1, 124.7, 120.4, 71.2, 66.3, 46.6, 41.6, 36.0, 27.1, 26.5. IR (neat) νmax (cm–1): 2957, 1649, 1491, 1398, 1308, 1296, 1273, 1227, 1092, 1014, 808, 793, 748, 733, 476. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C27H26ClN2O2, 445.1677; found, 445.1680. [α]D20 +33.0 (c = 2.0, CHCl3). SFC-MS (method 6) er: 95:5; tret (major) = 11.817 min (95.2%), tret (minor) = 13.022 min (4.8%).
(R)-4-(5-Bromopyridin-2-yl)-1-(4-fluorobenzyl)-3-vinyl-1,4-diazaspiro[5.5]undecane-2,5-dione (3p)
It was prepared according to GP-B using 2p. The crude material was purified by silica gel column chromatography (20% EtOAc/cHex), providing the title compound as a pale oil in 93% yield (87.9 mg, 0.19 mmol). Rf = 0.39 (30% EtOAc/cHex). 1H NMR (600 MHz, CDCl3): δ 8.46 (d, J = 2.4 Hz, 1H), 7.81 (dd, J = 8.8, 2.5 Hz, 1H), 7.77 (d, J = 8.7 Hz, 1H), 7.22 (dd, J = 8.6, 5.4 Hz, 2H), 6.98 (t, J = 8.7 Hz, 2H), 6.23 (dt, J = 4.4, 2.4 Hz, 1H), 6.08 (ddd, J = 17.3, 10.6, 4.2 Hz, 1H), 5.23 (dd, J = 10.6, 2.4 Hz, 1H), 5.15 (dd, J = 17.3, 2.4 Hz, 1H), 5.02 (d, J = 16.0 Hz, 1H), 4.47 (d, J = 16.0 Hz, 1H), 2.41–2.30 (m, 1H), 2.26–2.19 (m, 1H), 1.96–1.91 (m, 1H), 1.77 (dt, J = 13.0, 6.5 Hz, 1H), 1.73–1.49 (m, 5H), 1.13–1.04 (m, 1H). 13C NMR{1H} (151 MHz, CDCl3): δ 169.6, 167.5, 161.9 (d, J = 245.4 Hz), 150.2, 148.9, 140.0, 134.1, 134.0 (d, J = 3.2 Hz), 128.6 (d, J = 8.0 Hz, 2C), 121.9, 117.5, 117.1, 115.5 (d, J = 21.5 Hz, 2C), 63.9, 60.4, 44.9, 35.7, 33.0, 24.5, 23.3, 22.2. 19F NMR{1H} (376 MHz, CDCl3): δ −115.5. IR (neat) νmax (cm–1): 2934, 1680, 1643, 1508, 1454, 1394, 1366, 1263, 1221, 1140, 1095, 814, 731, 411. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C23H24BrFN3O2, 472.1030; found, 472.1040. [α]D20 +40.0 (c = 2.0, CHCl3). SFC-MS (method 2) er: 84:16; tret (major) = 4.565 min (84.2%), tret (minor) = 4.796 min (15.8%).
9-Benzyl-4-(3,4-dimethoxyphenethyl)-1-(4-(trifluoromethyl)benzyl)-3-vinyl-1,4,9-triazaspiro[5.5]undecane-2,5-dione (3q)
It was prepared according to GP-C using 2q. The crude material was purified by silica gel column chromatography (80% EtOAc/cHex), providing the title compound as pale solid in 47% yield (58.4 mg, 0.09 mmol). Rf = 0.16 (80% EtOAc/cHex). 1H NMR (600 MHz, CDCl3): δ 7.57 (d, J = 8.1 Hz, 2H), 7.35–7.17 (m, 7H), 6.83–6.66 (m, 3H), 5.92 (ddd, J = 17.2, 10.3, 5.1 Hz, 1H), 5.39 (dd, J = 10.4, 1.9 Hz, 1H), 5.27 (dd, J = 17.2, 1.8 Hz, 1H), 5.13 (d, J = 16.5 Hz, 1H), 4.52–4.49 (m, 1H), 4.40 (d, J = 16.6 Hz, 1H), 4.31–4.23 (m, 1H), 3.92–3.79 (m, 7H), 3.51 (t, J = 12.2 Hz, 2H), 3.07–2.96 (m, 2H), 2.91–2.85 (m, 2H), 2.74–2.70 (m, 2H), 2.47–2.38 (m, 1H), 2.02–1.91 (m, 2H), 1.65 (d, J = 13.5 Hz, 1H). 13C NMR{1H} (151 MHz, CDCl3): δ 169.1, 166.8, 149.2, 148.0, 142.2, 130.5, 133.3, 129.5 (q J = 32.4 Hz), 129.3 (2C) 129.3, 128.4 (2C), 126.7 (2C), 125.7 (q, J = 3.6 Hz 2C), 124.2 (q, J = 272.0 Hz), 120.9, 118.7, 112.2, 111.4, 70.0, 63.0, 62.8, 61.0, 56.0, 56.0, 51.1, 49.0, 47.0, 45.3, 33.0, 29.8; 1 quaternary aromatic C is not visible. 19F NMR{1H} (376 MHz, CDCl3): δ −62.0. IR (neat) νmax (cm–1): 2932, 1649, 1323, 1279, 1261, 1238, 1159, 1119, 1067, 1028, 1016, 737, 700. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C35H39F3N3O4, 622.2814; found, 622.2827.
12-(tert-Butyl)-9-(4-fluorobenzyl)-11-vinyl-1,4-dioxa-9,12-diazadispiro[4.2.58.25]pentadecane-10,13-dione (3r)
It was prepared according to GP-C using 2r. The crude material was purified by silica gel column chromatography (30% EtOAc/cHex), providing the title compound as a white solid in 92% yield (72.2 mg, 0.18 mmol). Rf = 0.30 (30% EtOAc/cHex). 1H NMR (600 MHz, CDCl3): δ 7.17 (dd, J = 8.5, 5.4 Hz, 2H), 6.97 (t, J = 8.7 Hz, 2H), 5.97 (ddd, J = 17.2, 10.4, 5.4 Hz, 1H), 5.49 (dd, J = 17.2, 1.7 Hz, 1H), 5.37 (dd, J = 10.5, 1.8 Hz, 1H), 4.98–4.84 (m, 2H), 4.31 (d, J = 16.0 Hz, 1H), 3.96–3.84 (m, 4H), 2.66 (td, J = 13.1, 5.4 Hz, 1H), 2.09–1.96 (m, 4H), 1.81–1.73 (m, 1H), 1.58 (dq, J = 10.5, 2.7 Hz, 2H), 1.48 (s, 9H). 13C NMR{1H} (151 MHz, CDCl3): δ 170.1, 167.7, 161.9 (d, J = 245.0 Hz), 137.2, 134.0 (d, J = 3.1 Hz), 128.3 (d, J = 7.6 Hz, 2C), 118.8, 115.4 (d, J = 21.5 Hz, 2C), 107.9, 64.5, 64.2, 62.3, 60.8, 58.7, 45.3, 32.2, 32.2, 32.0, 30.8, 28.4 (3C). 19F NMR{1H} (376 MHz, CDCl3): δ −116.0. IR (neat) νmax (cm–1): 2939, 1651, 1508, 1400, 1221, 1196, 1178, 1157, 1097, 1034, 928, 808, 486, 465, 413. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C24H32FN2O4, 431.2341; found, 431.2341.
9-Isopropyl-6-(p-tolyl)-8-vinyl-6,9-diazaspiro[4.5]decane-7,10-dione (3s)
It was prepared according to GP-C using 2s. The crude material was purified by silica gel column chromatography (20% EtOAc/cHex), providing the title compound as a pale solid in 85% yield (55.52 mg, 0.17 mmol). Rf = 0.25 (30% EtOAc/cHex). 1H NMR (600 MHz, CDCl3): δ 7.21 (d, J = 8.0 Hz, 2H), 6.98 (d, J = 8.0 Hz, 2H), 6.02 (ddd, J = 17.2, 10.4, 5.6 Hz, 1H), 5.53 (dd, J = 17.2, 1.6 Hz, 1H), 5.39 (dd, J = 10.4, 1.7 Hz, 1H), 4.71 (dt, J = 5.7, 1.8 Hz, 1H), 4.55 (hept, J = 6.9 Hz, 1H), 2.50–2.42 (m, 1H), 2.36 (s, 3H), 2.27 (ddd, J = 14.3, 8.0, 6.6 Hz, 1H), 2.15 (ddd, J = 13.9, 8.2, 6.1 Hz, 1H), 1.76–1.64 (m, 3H), 1.56 (dt, J = 8.6, 6.2 Hz, 1H), 1.29 (d, J = 6.8 Hz, 3H), 1.25 (d, J = 6.9 Hz, 3H), 1.23–1.12 (m, 1H). 13C NMR{1H} (151 MHz, CDCl3): δ 170.6, 167.2, 138.4, 135.9, 135.6, 130.2 (4C), 119.2, 71.2, 60.4, 47.8, 42.6, 36.1, 26.4, 26.0, 21.3, 20.6, 20.1. IR (neat) νmax (cm–1): 2942, 1649, 1510, 1393, 1362, 1215, 1188, 949, 802. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C20H27N2O2, 327.2067; found, 327.2065.
Synthesis of (E/Z)-4-Benzyl-3-ethylidene-1-(4-methoxybenzyl)-1,4-diazaspiro[5.5]undecane-2,5-dione (4a/4b)
To a solution of 3a (84 mg, 0.2 mmol, 1 equiv) in THF (0.033 M, 6.66 mL) at 0 °C was added NaH (0.22 mmol, 1.1 equiv), which then was allowed to reach rt and stirred for 30 min. The reaction mixture was quenched with a saturated solution of NH4Cl (2 mL) and extracted with ethyl acetate (2 × 5 mL), the combined organic layers were dried with Na2SO4, and the solvent was removed under reduced pressure. The reaction mixture was purified by silica gel column chromatography (25% EtOAc/cHex), affording the product as a colorless solid, as a 1/1 mixture of isomers in 98% yield. The absolute configurations of the two isomers have been assigned with NOESY experiments.
(E)-4-Benzyl-3-ethylidene-1-(4-methoxybenzyl)-1,4-diaza Spiro[5.5]undecane-2,5-dione (4a)
Rf = 0.45 (30% EtOAc/cHex). 1H NMR (600 MHz, CDCl3): δ 7.33 (dd, J = 8.2, 7.0 Hz, 2H), 7.29–7.22 (m, 1H), 7.21–7.13 (m, 4H), 6.83 (d, J = 8.6 Hz, 2H), 5.72 (q, J = 7.4 Hz, 1H), 4.91 (s, 2H), 4.76 (s, 2H), 3.79 (s, 3H), 2.21–2.15 (m, 2H), 2.12 (d, J = 7.4 Hz, 3H), 1.81 (qt, J = 11.8, 3.2 Hz, 2H), 1.71–1.50 (m, 5H), 1.11–1.02 (m, 1H). 13C NMR{1H} (151 MHz, CDCl3): δ 168.6, 163.5, 158.7, 137.4, 132.8, 130.9, 128.9 (2C), 128.5 (2C), 127.4, 126.3 (2C), 121.0, 114.1 (2C), 62.7, 55.4, 49.4, 43.9, 33.7 (2C), 25.1, 23.3 (2C), 14.1. IR (neat) νmax (cm–1) (1/1 mixture of isomers): 2934, 1672, 1630, 1612, 1387, 1356, 1304, 1242, 810, 727, 700, 407. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C18H23N2O2, 299.1754; found, 299.1759.
(Z)-4-Benzyl-3-ethylidene-1-(4-methoxybenzyl)-1,4-diaza Spiro[5.5]undecane-2,5-dione (4b)
Rf = 0.30 (30% EtOAc/cHex). 1H NMR (600 MHz, CDCl3): δ 7.31 (dd, J = 8.1, 6.7 Hz, 2H), 7.26 (d, J = 3.5 Hz, 1H), 7.21–7.17 (m, 2H), 7.06 (d, J = 8.7 Hz, 2H), 6.78 (d, J = 8.7 Hz, 2H), 6.36 (q, J = 7.7 Hz, 1H), 4.88 (s, 2H), 4.71 (s, 2H), 3.78 (s, 3H), 2.18–2.08 (m, 2H), 1.84 (d, J = 7.7 Hz, 3H), 1.81–1.71 (m, 2H), 1.70–1.50 (m, 6H). 13C NMR{1H} (151 MHz, CDCl3): δ 170.1, 165.4, 158.7, 137.2, 134.8, 131.0, 128.7 (2C), 128.4 (2C), 127.5, 126.8 (2C), 120.4, 114.0 (2C), 63.1, 55.4, 50.1, 44.1, 33.5 (2C), 25.1, 23.6 (2C), 14.2.
Synthesis of (R)-4-Benzyl-3-ethyl-1-(4-methoxybenzyl)-1,4-diazaspiro[5.5]undecane-2,5-dione (5)
To a solution of 3a (84 mg, 0.2 mmol, 1 equiv) in EtOAc (4 mL), 10% Pd/C was added (21 mg). The reaction flask was filled with H2 and then was degassed under vacuum, and the flask was backfilled with H2. The procedure was repeated three times, and then the reaction mixture was stirred overnight. The crude was filtrated through a Celite pad, and the solvent was removed under reduced pressure, affording the product without further purification as a white solid in 99% yield (83 mg). Rf = 0.36 (30% EtOAc/cHex). 1H NMR (600 MHz, CDCl3): δ 7.39–7.31 (m, 2H), 7.31–7.28 (m, 1H), 7.25–7.20 (m, 2H), 7.12 (d, J = 8.6 Hz, 2H), 6.83 (d, J = 8.7 Hz, 2H), 5.39 (d, J = 14.9 Hz, 1H), 4.76 (d, J = 15.7 Hz, 1H), 4.56 (d, J = 15.7 Hz, 1H), 3.97 (d, J = 14.9 Hz, 1H), 3.92 (dd, J = 6.6, 3.5 Hz, 1H), 3.78 (s, 3H), 2.24 (qt, J = 13.0, 4.3 Hz, 1H), 2.12–1.90 (m, 4H), 1.89–1.84 (m, 1H), 1.81–1.72 (m, 3H), 1.69–1.57 (m, 2H), 1.12 (qt, J = 13.2, 4.0 Hz, 1H), 0.97 (t, J = 7.5 Hz, 3H). 13C NMR{1H} (151 MHz, CDCl3): δ 169.8, 168.1, 158.6, 136.3, 130.6, 129.0 (2C), 128.0 (2C), 127.9, 127.9 (2C), 114.0 (2C), 62.7, 59.2, 55.4, 46.9, 45.3, 35.2, 34.4, 25.6, 24.5, 23.1, 22.7, 9.9. IR (neat) νmax (cm–1): 2934, 1636, 1614, 1514, 1456, 1427, 1418, 1273, 1250, 1175, 1129, 1040, 797, 739, 702, 600, 492. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C26H33N2O3, 421.2486; found, 421.2495. [α]D20 +72.0 (c = 1.0, CHCl3). SFC-MS (method 5) er: 91:9; tret (major) = 4.161 min (90.6%), tret (minor) = 4.490 min (9.4%).
Synthesis of (R,E)-4-Benzyl-3-(4-hydroxystyryl)-1-(4-methoxy benzyl)-1,4-diazaspiro[5.5]undecane-2,5-dione (6)
To a solution of 3a (84 mg, 0.2 mmol, 1.0 equiv), 4-iodophenol (88 mg, 0.4 mmol, 2.0 equiv), palladium(II) acetate (9 mg, 0.04 mmol, 0.2 equiv), and triethyl phosphite (7 μL, 0.04 mmol, 0.20 equiv) in 1,4-dioxane (3.6 mL) was added N,N-diisopropylethylamine (70 μL, 0.4 mmol, 2.0 equiv) and refluxed overnight. The reaction mixture was diluted with DCM (8 mL), washed with 1 M HCl (4 mL), dried over Na2SO4, and concentrated under reduced pressure. The crude was purified by silica gel column chromatography (30% EtOAc/cHex), affording the product as a pale solid, in 64% yield (65 mg).
Rf = 0.22 (30% EtOAc/cHex). 1H NMR (600 MHz, CDCl3): δ 7.52–7.44 (m, 1H), 7.37–7.33 (m, 2H), 7.33–7.28 (m, 1H), 7.27–7.23 (m, 2H), 7.12 (d, J = 8.4 Hz, 2H), 7.02 (d, J = 8.6 Hz, 2H), 6.83 (d, J = 8.7 Hz, 2H), 6.70 (d, J = 8.5 Hz, 2H), 6.46 (d, J = 15.8 Hz, 1H), 5.95 (dd, J = 15.8, 6.7 Hz, 1H), 5.43 (d, J = 14.8 Hz, 1H), 4.97 (d, J = 15.9 Hz, 1H), 4.69 (d, J = 6.7 Hz, 1H), 4.48 (d, J = 15.9 Hz, 1H), 3.96 (d, J = 14.8 Hz, 1H), 3.78 (s, 3H), 2.41 (qt, J = 12.8, 4.2 Hz, 1H), 2.10–2.04 (m, 1H), 2.02–1.95 (m, 1H), 1.87–1.60 (m, 6H), 1.13 (qt, J = 12.4, 3.2 Hz, 1H). 13C NMR{1H} (151 MHz, CDCl3): δ 169.5, 168.1, 158.8, 157.1, 136.1, 134.9, 130.0, 129.1 (2C), 128.3 (2C), 128.2 (2C), 128.0, 127.9 (2C), 127.5, 120.4, 116.0 (2C), 114.2 (2C), 63.3, 61.7, 55.4, 47.6, 45.5, 36.2, 33.4, 24.6, 23.4, 22.4. IR (neat) νmax (cm–1): 2934, 1657, 1632, 1609, 1510, 1414, 1261, 1244, 1171, 908, 802, 725, 698, 519, 444, 434. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C32H35N2O4, 511.2591; found, 511.2573. [α]D20 +20.0 (c = 0.3, CHCl3). SFC-MS (method 5) er: 96:4; tret (major) = 5.514 min (95.7%), tret (minor) = 5.806 min (4.3%).
Synthesis of (R)-4-Benzyl-3-vinyl-1,4-diazaspiro[5.5]undecane-2,5-dione (7)
To a solution of 3a (84 mg, 0.2 mmol, 1 equiv) in MeCN (0.2 mL) was added a solution of CAN (329 mg, 0.6 mmol, 3 equiv) in H2O (0.2 mL), and it was stirred at room temperature for 3 h. The reaction mixture was extracted with DMC (3 × 2 mL), the combined organic layers were dried with Na2SO4, and the solvent was removed under reduced pressure. The crude was purified by silica gel column chromatography (40% EtOAc/cHex), affording the product as a white solid, in 95% yield (57 mg).
mp 138.9 °C. Rf = 0.23 (50% EtOAc/cHex). 1H NMR (600 MHz, CDCl3): δ 7.36–7.27 (m, 3H), 7.21–7.14 (m, 2H), 6.79 (s, 1H), 5.87 (ddd, J = 17.1, 10.2, 6.0 Hz, 1H), 5.51 (d, J = 14.8 Hz, 1H), 5.44 (dd, J = 10.3, 1.5 Hz, 1H), 5.35 (dd, J = 17.1, 1.5 Hz, 1H), 4.36 (d, J = 6.1 Hz, 1H), 3.75 (d, J = 14.8 Hz, 1H), 2.31 (td, J = 13.6, 4.4 Hz, 1H), 1.91 (td, J = 13.1, 3.9 Hz, 1H), 1.83–1.74 (m, 2H), 1.72–1.64 (m, 3H), 1.46–1.30 (m, 3H). 13C NMR {1H} (151 MHz, CDCl3): δ 170.0, 165.9, 135.8, 132.6, 129.0 (2C), 128.3 (2C), 128.1, 119.8, 61.8, 58.3, 47.5, 36.9, 34.5, 24.6, 20.6, 20.6. IR (neat) νmax (cm–1): 2930, 1659, 1649, 1427, 1290, 1269, 1240, 1167, 939, 824, 816, 750, 731, 700, 434. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C18H23N2O2, 299.1754; found, 299.1759. [α]D20 +44.0 (c = 0.5, CHCl3). SFC-MS (method 1) er: 96:4; tret (major) = 5.754 min (96.1%), tret (minor) = 6.266 min (3.9%).
Acknowledgments
This work was supported by the Netherlands Organisation for Scientific Research (NWO). We thank Ellymay Goossens for the synthesis of some racemic standards, Elwin Janssen for NMR support, and Daniel Preschel for HRMS measurements (all VUA). We also thank Kristof Van Hecke (Ghent University) for providing diffractometer time, and the Hercules Foundation (project AUGE/11/029 “3D-SPACE: 3D Structural Platform Aiming for Chemical Excellence”) for funding of the diffractometer.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.joc.9b01994.
Accession Codes
CCDC 1902629 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
The authors declare no competing financial interest.
Supplementary Material
References
- a Lovering F.; Bikker J.; Humblet C. Escape from flatland: increasing saturation as an approach to improving clinical success. J. Med. Chem. 2009, 52, 6752. 10.1021/jm901241e. [DOI] [PubMed] [Google Scholar]; b Lovering F. Escape from Flatland 2: complexity and promiscuity. MedChemComm 2013, 4, 515. 10.1039/c2md20347b. [DOI] [Google Scholar]; c Walters W. P.; Green J.; Weiss J. R.; Murcko M. A. What do medicinal chemists actually make? A 50-year retrospective. J. Med. Chem. 2011, 54, 6405. 10.1021/jm200504p. [DOI] [PubMed] [Google Scholar]
- For recent examples, see:; a Gao R.-D.; Ding L.; Zheng C.; Dai L.-X.; You S.-L. Palladium(0)-Catalyzed Intermolecular Asymmetric Allylic Dearomatization of Polycyclic Indoles. Org. Lett. 2018, 20, 748. 10.1021/acs.orglett.7b03887. [DOI] [PubMed] [Google Scholar]; b Hethcox J. C.; Shockley S. E.; Stoltz B. M. Enantioselective Synthesis of Vicinal All-Carbon Quaternary Centers via Iridium-Catalyzed Allylic Alkylation. Angew. Chem., Int. Ed. 2018, 57, 8664. 10.1002/anie.201804820. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Braun J.; Ariëns M. I.; Matsuo B. T.; de Vries S.; van Wordragen E. D. H.; Ellenbroek B. D.; Vande Velde C. M. L.; Orru R. V. A.; Ruijter E. Stereoselective Synthesis of Fused Vinylcyclopropanes by Intramolecular Tsuji–Trost Cascade Cyclization. Org. Lett. 2018, 20, 6611. 10.1021/acs.orglett.8b02232. [DOI] [PMC free article] [PubMed] [Google Scholar]; Related iridium-catalyzed reactions:; d Yang Z.-P.; Wu Q.-F.; You S.-L. Direct asymmetric dearomatization of pyridines and pyrazines by iridium-catalyzed allylic amination reactions. Angew. Chem., Int. Ed. 2014, 53, 6986. 10.1002/anie.201404286. [DOI] [PubMed] [Google Scholar]; e Yang Z.-P.; Zheng C.; Huang L.; Qian C.; You S.-L. Iridium-Catalyzed Intramolecular Asymmetric Allylic Dearomatization Reaction of Benzoxazoles, Benzothiazoles, and Benzimidazoles. Angew. Chem., Int. Ed. 2017, 56, 1530. 10.1002/anie.201611056. [DOI] [PubMed] [Google Scholar]; f Yang Z.-P.; Jiang R.; Zheng C.; You S.-L. Iridium-Catalyzed Intramolecular Asymmetric Allylic Alkylation of Hydroxyquinolines: Simultaneous Weakening of the Aromaticity of Two Consecutive Aromatic Rings. J. Am. Chem. Soc. 2018, 140, 3114. 10.1021/jacs.8b00136. [DOI] [PubMed] [Google Scholar]; g Sandmeier T.; Krautwald S.; Carreira E. M. Stereoselective Synthesis of Piperidines by Iridium-Catalyzed Cyclocondensation. Angew. Chem., Int. Ed. 2017, 56, 11515. 10.1002/anie.201706374. [DOI] [PubMed] [Google Scholar]; h Review:Cheng Q.; Tu H.-F.; Zheng C.; Qu J.-P.; Helmchen G.; You S.-L. Iridium-Catalyzed Asymmetric Allylic Substitution Reactions. Chem. Rev. 2019, 119, 1855. 10.1021/acs.chemrev.8b00506. [DOI] [PubMed] [Google Scholar]; i Trost B. M.; Sacchi K. L.; Schroeder G. M.; Asakawa N. Intramolecular Palladium-Catalyzed Allylic Alkylation: Enantio- and Diastereoselective Synthesis of [2.2.2] Bicycles. Org. Lett. 2002, 4, 3427. 10.1021/ol0265766. [DOI] [PubMed] [Google Scholar]; j Bandini M.; Melloni A.; Piccinelli F.; Sinisi R.; Tommasi S.; Umani-Ronchi A. Highly Enantioselective Synthesis of Tetrahydro-β-Carbolines and Tetrahydro-γ-Carbolines Via Pd-Catalyzed Intramolecular Allylic Alkylation. J. Am. Chem. Soc. 2006, 128, 1424. 10.1021/ja054109o. [DOI] [PubMed] [Google Scholar]
- For a review of synthesis and biological activity of DKPs, see:Martins M. B.; Carvalho I. Diketopiperazines: Biological Activity and Synthesis. Tetrahedron 2007, 63, 9923–9932. 10.1016/j.tet.2007.04.105. [DOI] [Google Scholar]
- See, e.g.: Brimble M. A.; Guan J.; Sieg F.. Neuroprotective Bicyclic Compounds and Methods for Their Use. WO2005023815A2, 2005.
- a Faden A. I.; Knoblach S. M.; Cernak I.; Fan L.; Vink R.; Araldi G. L.; Fricke S. T.; Roth B. L.; Kozikowski A. P. Novel diketopiperazine enhances motor and cognitive recovery after traumatic brain injury in rats and shows neuroprotection in vitro and in vivo. J. Cereb. Blood Flow Metab. 2003, 23, 342–354. 10.1097/01.wcb.0000046143.31247.fd. [DOI] [PubMed] [Google Scholar]; b Habashita H.; Takaoka Y.; Shibayama S.. Spiroheterocyclic derivative compounds and drugs comprising the compounds as the active ingredient. WO2004035581A1, 2004.
- Gomez-Monterrey I.; Campiglia P.; Carotenuto A.; Califano D.; Pisano C.; Vesci L.; Lama T.; Bertamino A.; Sala M.; di Bosco A. M.; Grieco E.; Novellino E. Design, Synthesis, and Cytotoxic Evaluation of a New Series of 3-Substituted Spiro[(dihydropyrazine-2,5-dione)-6,3′-(2′,3′-dihydrothieno[2, 3-b]naphtho-4′,9′-dione)] Derivatives. J. Med. Chem. 2007, 50, 1787–1798. 10.1021/jm0612158. [DOI] [PubMed] [Google Scholar]; Gomez-Monterrey I. Spiro[(dihydropyrazin-2,5-dione)-6,3′-(2′,3′-dihydrothieno[2,3-b]naphtho-4′,9′-dione)]-Based Cytotoxic Agents: Structure–Activity Relationship Studies on the Substituent at N4-Position of the Diketopiperazine Domain. J. Med. Chem. 2008, 51, 2924–2932. 10.1021/jm7013056. [DOI] [PubMed] [Google Scholar]
- Kuster G. J. T.; van Berkom L. W. A.; Kalmoua M.; van Loevezijn A.; Sliedregt L. A. J. M.; van Steen B. J.; Kruse C. G.; Scheeren H. W.; Scheeren H. W. Synthesis of spirohydantoins and spiro-2, 5-diketopiperazines via resin-bound cyclic α, α-disubstituted α-amino esters. J. Comb. Chem. 2006, 8, 85. 10.1021/cc050072s. [DOI] [PubMed] [Google Scholar]
- Levins C. G.; Schafmeister C. E. The Synthesis of Functionalized Nanoscale Molecular Rods of Defined Length. J. Am. Chem. Soc. 2003, 125, 4702. 10.1021/ja0293958. [DOI] [PubMed] [Google Scholar]
- a Habashita H.; Kokubo M.; Hamano S.-I.; Hamanaka N.; Toda M.; Shibayama S.; Tada H.; Sagawa K.; Fukushima D.; Maeda K.; Mitsuya H. Design, Synthesis, and Biological Evaluation of the Combinatorial Library with a New Spirodiketopiperazine Scaffold. Discovery of Novel Potent and Selective Low-Molecular-Weight CCR5 Antagonists. J. Med. Chem. 2006, 49, 4140. 10.1021/jm060051s. [DOI] [PubMed] [Google Scholar]; b Santra S.; Andreana P. R. A Rapid, One-Pot, Microwave-Influenced Synthesis of Spiro-2,5-diketopiperazines via a Cascade Ugi/6-Exo-Trig Aza-Michael Reaction. J. Org. Chem. 2011, 76, 2261. 10.1021/jo102305q. [DOI] [PubMed] [Google Scholar]
- For recent examples of Ugi/cyclization sequences, see:; a Zidan A.; Garrec J.; Cordier M.; El-Naggar A. M.; Abd El-Sattar N. E. A.; Ali A. K.; Hassan M. A.; El Kaim L. β-Lactam Synthesis through Diodomethane Addition to Amide Dianions. Angew. Chem., Int. Ed. 2017, 56, 12179. 10.1002/anie.201706315. [DOI] [PubMed] [Google Scholar]; b He Y.; Li Z.; Robeyns K.; Van Meervelt L.; Van der Eycken E. V. A Gold-Catalyzed Domino Cyclization Enabling Rapid Construction of Diverse Polyheterocyclic Frameworks. Angew. Chem., Int. Ed. 2018, 57, 272. 10.1002/anie.201710592. [DOI] [PubMed] [Google Scholar]; c Zidan A.; Cordier M.; El-Naggar A. M.; Abd El-Sattar N. E. A.; Hassan M. A.; Ali A. K.; El Kaïm L. Propargylation of Ugi Amide Dianion: An Entry into Pyrrolidinone and Benzoindolizidine Alkaloid Analogues. Org. Lett. 2018, 20, 2568. 10.1021/acs.orglett.8b00687. [DOI] [PubMed] [Google Scholar]; d For a related Ugi/Tsuji–Trost approach, see:Spallarossa M.; Banfi L.; Basso A.; Moni L.; Riva R. Access to Polycyclic Alkaloid-Like Structures by Coupling the Passerini and Ugi Reactions with Two Sequential Metal-Catalyzed Cyclizations. Adv. Synth. Catal. 2016, 358, 2940. 10.1002/adsc.201600638. [DOI] [Google Scholar]
- Carbonyl-conjugated allylic systems are generally poor substrates for transition metal-catalyzed allylation; see, e.g.:He Z.-T.; Hartwig J. F. Enantioselective α-functionalizations of ketones via allylic substitution of silyl enol ethers. Nat. Chem. 2019, 11, 177. 10.1038/s41557-018-0165-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Shintani R.; Moriya K.; Hayashi T. Guiding the nitrogen nucleophile to the middle: palladium-catalyzed decarboxylative cyclopropanation of 2-alkylidenetrimethy-lene carbonates with isocyanates. Chem. Commun. 2011, 47, 3057. 10.1039/c0cc05308b. [DOI] [PubMed] [Google Scholar]; b Terauchi J.; Curran D. P. N-Allylation of anilides with chiral palladium catalysts: the first catalytic asymmetric synthesis of axially chiral anilides. Tetrahedron: Asymmetry 2003, 14, 587. 10.1016/s0957-4166(03)00041-7. [DOI] [Google Scholar]; c Boutier A.; Kammerer-Pentier C.; Krause N.; Prestat G.; Poli G. Pd-Catalyzed Asymmetric Synthesis of N-Allenyl Amides and Their Au-Catalyzed Cycloisomerizative Hydroalkylation: A New Route Toward Enantioenriched Pyrrolidones. Chem.—Eur. J. 2012, 18, 3840. 10.1002/chem.201103902. [DOI] [PubMed] [Google Scholar]
- a Helmchen G.; Pfaltz A. Phosphinooxazolines A New Class of Versatile, Modular P,N-Ligands for Asymmetric Catalysis. Acc. Chem. Res. 2000, 33, 336. 10.1021/ar9900865. [DOI] [PubMed] [Google Scholar]; b Tani K.; Behenna D. C.; McFadden R. M.; Stoltz B. M. A Facile and Modular Synthesis of Phosphinooxazoline Ligands. Org. Lett. 2007, 9, 2529. 10.1021/ol070884s. [DOI] [PubMed] [Google Scholar]
- Unusually high dilution has been previously reported to be required with this particular catalyst/ligand combination; see, e.g.:; a Sun A. W.; Hess S. N.; Stoltz B. M. Enantioselective synthesis of gem-disubstituted NBoc diazaheterocycles via decarboxylative asymmetric allylic alkylation. Chem. Sci. 2019, 10, 788. 10.1039/c8sc03967d. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Numajiri Y.; Jiménez-Osés G.; Wang B.; Houk K. N.; Stoltz B. M. Enantioselective Synthesis of Dialkylated N-Heterocycles by Palladium-Catalyzed Allylic Alkylation. Org. Lett. 2015, 17, 1082. 10.1021/ol503425t. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Liu W.-B.; Reeves C. M.; Virgil S. C.; Stoltz B. M. Construction of Vicinal Tertiary and All-Carbon Quaternary Stereocenters via Ir-Catalyzed Regio-, Diastereo-, and Enantioselective Allylic Alkylation and Applications in Sequential Pd Catalysis. J. Am. Chem. Soc. 2013, 135, 10626. 10.1021/ja4052075. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Reeves C. M.; Eidamshaus C.; Kim J.; Stoltz B. M. Enantioselective Construction of α-Quaternary Cyclobutanones by Catalytic Asymmetric Allylic Alkylation. Angew. Chem., Int. Ed. 2013, 52, 6718. 10.1002/anie.201301815. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Korch K. M.; Eidamshaus C.; Behenna D. C.; Nam S.; Horne D.; Stoltz B. M. Enantioselective Synthesis of a-Secondary and α-Tertiary Piperazin-2-ones and Piperazines by Catalytic Asymmetric Allylic Alkylation. Angew. Chem., Int. Ed. 2015, 54, 179. 10.1002/anie.201408609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For an in-depth discussion, see:Trost B. M.; Van Vranken D. L. Asymmetric transition metal-catalyzed allylic alkylations. Chem. Rev. 1996, 96, 395. 10.1021/cr9409804. [DOI] [PubMed] [Google Scholar]
- Our previous findings suggest that E-to-Z isomerization of π-allylpalladium intermediates is outcompeted by intramolecular cross-coupling even at 80 °C; see ref (2c).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.