Abstract
Objective:
Zone model predictive control (MPC) has been proven to be an efficient approach to closed-loop insulin delivery in clinical studies. In this work, we aim to safely reduce mean glucose levels by proposing control penalty adaptation in the cost function of zone MPC.
Methods:
A zone MPC method with a dynamic cost function that updates its control penalty parameters in real time according to the predicted glucose and its rate of change is developed. The proposed method is evaluated on the entire 100-adult cohort of the FDA-accepted UVA/Padova T1DM simulator and compared with the zone MPC tested in an extended outpatient study.
Results:
For unannounced meals, the proposed method leads to statistically significant improvements in terms of mean glucose (153.8 mg/dL vs. 159.0 mg/dL; p < 0.001) and percentage time in [70, 180] mg/dL (70.5% vs. 66.3%; p < 0.001) without increasing the risk of hypoglycemia. Performance for announced meals is similar to that obtained without adaptation. The proposed method also behaves properly and safely for scenarios of moderate meal-bolus and basal rate mismatches, as well as simulated unannounced exercise. Advisory-mode analysis based on clinical data indicates that the method can reduce glucose levels through suggesting additional safe amounts of insulin on top of those suggested by the zone MPC used in the study.
Conclusion:
The proposed method leads to improved glucose control without increasing hypoglycemia risks.
Significance:
The results validate the feasibility of improving glucose regulation through glucose- and velocity-dependent control penalty adaptation in MPC design.
Index Terms: Artificial pancreas, Model predictive control, Adaptive controller tuning, Safety-critical control
I. Introduction
The development of continuous glucose monitoring (CGM) sensors and continuous subcutaneous insulin infusion pumps enables the design of artificial pancreas (AP) systems for patients with type 1 diabetes mellitus (T1DM). The overall goal is to achieve safe and satisfactory blood glucose regulation in terms of hyperglycemia and hypoglycemia prevention through designing closed-loop insulin delivery algorithms [1]–[3].
The authors’ group has been working on a zone model predictive control (MPC) approach to closed-loop insulin delivery [4]–[7], which has been evaluated in numerous clinical studies on more than 120 patients and 70,000 hours [8]–[12]. As euglycemia is defined as a range of blood glucose concentrations rather than a single value, a zone MPC penalizes the distance of glucose predictions from a carefully designed safe zone based on clinical requirements, which helps avoid unnecessary control moves and reduces glycemic variability and the risk of hypoglycemia. The original zone MPC for the AP was developed in Grosman et al. [4] utilizing an auto-regressive model with exogenous inputs, which was progressively extended to integrate a control-relevant state-space model [13] and a diurnal periodic target zone [5]. Asymmetric costs on insulin delivery above and below basal rate were further utilized in Gondhalekar et al. [6], to facilitate decoupled design for hyperglycemia and hypoglycemia. In a recent version of zone MPC [7], velocity-weighting and velocity-penalty were incorporated in the MPC cost function to reduce the likelihood of controller-induced hypoglycemia and achieve enhanced hyperglycemia correction. Although improved hemoglobin A1c was achieved in the corresponding clinical trial [12], improvements with average glucose were not obvious, which give rise to the need of developing further controller enhancements without weakening safety guarantees against hypoglycemia. In this work, we explore one promising approach by designing dynamic updating strategies for control penalty parameters.
The problem considered falls in the topic of adaptive AP design, for which a few different methods have been investigated in the literature. In earlier studies, basal rate and meal bolus adaptation were considered through run-to-run approaches utilizing sparse blood glucose (BG) measurements [14], [15]. The advent of CGM further enabled the utilization of advanced control approaches in adaptive AP design. For instance, a nonlinear adaptive MPC was proposed by Hovorka et al. [16] to maintain normoglycemia during fasting conditions using Bayesian model parameter estimation. El-Khatib et al. [17] employed a generalized predictive control (GPC) approach with a recursively updated subject model on a bi-hormone AP; a GPC approach was also explored in Turksoy et al. [18] to eliminate the need of meal or exercise announcements. A model predictive iterative learning control approach was proposed by Wang et al. [19] to adapt controller behavior on the basis of patient’s day-to-day lifestyle. Cameron et al. [20] developed a multiple model probabilistic predictive controller to obtain enhanced meal detection and prediction. In Toffanin et al. [21], a dynamic insulin-on-board approach was proposed to counteract the effect of insulin sensitivity circadian rhythm. Colmegna et al. [22] developed a switched linear parameter-varying approach to separately adjust controller behavior in hypoglycemia, hyperglycemia and euglycemia situations. Toffanin et al. [23] recently revisited the run-to-run approach to adapt the basal insulin delivery rate and carbohydrate-to-insulin ratio by taking into account the intraand inter-day insulin sensitivity variability. In Laguna Sanz et al. [24], a confidence index was incorporated in a zone MPC to allow controller parameter adaptation based on the accuracy of the prediction model in recent history. An MPC with event-triggered controller update strategies was proposed by Chakrabarty et al. [25] to reduce energy consumption of an embedded AP while maintaining satisfactory glucose regulation performance. A recent review of adaptive approaches to AP design can be found in Turksoy and Cinar [26].
The main difference of our work from existing adaptive MPC/GPC approaches in the literature is that a dynamic MPC cost function and a constant system model are utilized, instead of a constant cost function and a dynamically updated prediction model. Specifically, we propose an adaptive MPC cost function based on the values and change rates of glucose predictions, by exploiting the physiological properties of the insulin-glucose metabolic process. This approach is motivated by the multi-zone MPC design proposed in Grosman et al. [27]. However, the adapted controller parameters in Grosman et al. [27] were all kept piecewise constant according to the region in which the glucose level stayed, and more importantly, the effect of glucose trend on insulin dose calculation was not taken into account. To overcome these limitations, a continuous dependence of the control penalty parameters on both predicted values and trends of blood glucose is investigated in our work. A preliminary version of the design was reported in [28].
The performance of the proposed adaptive MPC is evaluated via extensive in silico tests on the 100-adult cohort of the US Food and Drug Administration (FDA) accepted Universities of Virginia (UVA)/Padova T1DM simulator [29] and comparative advisory-mode analysis based on the historical clinical data obtained in [12]. Compared with the zone MPC developed in [7], improved results are obtained by the proposed adaptive method in terms of mean glucose level and percentage time in the safe range without increasing the risk of hypoglycemia for both announced and unannounced meals. The robustness of the controller is evaluated utilizing a scenario-based approach [30] through considering different extreme situations; the proposed adaptive controller can behave properly in the scenarios of moderate meal-bolus mismatches, basal-rate mismatches and simulated unannounced exercise. In addition, through feeding the clinical data [12] obtained using the zone MPC in [7], we observe that the proposed method can help reduce glucose levels through suggesting additional but safe amounts of insulin.
II. Problem Description
The base controller for this work is the periodic zone MPC with velocity-weighting and velocity-penalty developed in [7]. Driven by the arrival of glucose measurements, the controller runs in discrete time and calculates a numeric control law every 5 minutes. Specifically, at discrete time instant i, the relative dose correction uk of insulin infusion uabs,k from the basal rate ubasal (namely, uk ≔ uabs,k − ubasal) is obtained by solving a constrained optimization problem of the form:
| (1) |
with cost function
| (2) |
subject to system dynamics and safety constraints. In (2), and , which represent delivery rates above and below the basal rate ubasal, respectively; the separate weighting parameters and on and allow independent penalization of the costs of insulin delivery above and below basal rate. The detailed explanation of the other terms in (2) and the MPC algorithm can be found in the supplementary document of this paper and in [7].
In [6], [7], the control input penalties and ) were fixed to constant values. A natural question to ask is whether improved glucose regulation performance can be obtained by further adapting these parameters, which have a clear and direct relationship with the controller behavior - smaller values of and correspond to more aggressive controller activity and vice versa. In particular, we adjust the values of and according to the glucose state prediction yk (obtained via the prediction model of MPC) and glucose velocity
| (3) |
We implement this idea by designing - and -surfaces along continuum values of glucose prediction yk and velocity μk. Consequently, the MPC cost function is modified from its original form in (2) to
| (4) |
The objective of the design is to achieve enhanced control performance in terms of average blood glucose level and percent time in safe range [70, 180] mg/dL, without increasing risk of hypoglycemia. Note that and are calculated from the predicted glucose yk and velocity μk using the prediction model in the zone MPC.
III. Glucose and Velocity Dependent MPC Parameter Adaption
In this section, the proposed control penalty parameter adaptation approach is presented. We first present the detailed design and parameterization of and , and then provide discussions on the solution of the corresponding MPC optimization problem.
A. Design of
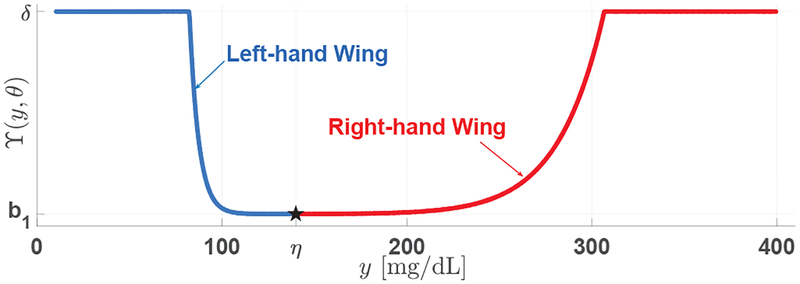
Before continuing, we introduce a basic function ϒ(y, θ) that is utilized to build . Specifically, ϒ(y, θ) is designed by connecting two exponential functions with saturation:
| (5) |
where θ ≔ [δ, a1, a2, b1, α, η,ℓ] is a septuple that parameterizes this “bowl-shaped” function. In particular, δ and b1 determine the maximum (saturation) and minimum values of the curve, respectively, a1 and α control the “steepness” of the “right-hand wing” (namely, y > η) exponential function, a2 decides the “steepness” of the “left-hand wing” (y ≤ η) exponential function, and η determines the connection point of the two exponential functions. An illustration of this function is provided in Fig. 1.
Fig. 1:
An illustration of the basic function defined in (5), composed of two exponential functions with saturation.
From (4), penalizes insulin infusion above the basal rate and is usually in effect when yk ≥ 80; because insulin delivery above basal rate is also the direct cause of low glucose values, determines both glucose regulation performance and hypoglycemia risk and therefore is the major focus of parameter adaptation. To separately consider the scenarios of ascending and descending glucose sequences, we parameterize according to the sign of predicted glucose velocity μk:
| (6) |
where Θ+ and Θ− are two vector-valued parameters that determine the relationship of and with μk and yk, respectively. In this work, and are both designed based on the basic function ϒ(·, ·), but are parameterized with different parameters Θ+ and Θ·− due to their different roles in glucose regulation.
1). Designing :
Specifically, we consider the following principles for the design of , which is related to increasing glucose predictions:
A1) given the same (non-negative) glucose velocity prediction μk, the aggressiveness of the controller (in terms of increasing insulin infusion above basal rate) should gradually decrease with the increase of yk when yk is above the normal range to avoid overdosing insulin (because a suitable amount of insulin may have been delivered during the period when the glucose is rising), and decrease with the decrease of yk when it is below its normal value;
A2) given the same glucose state prediction yk, the controller’s activeness in terms of increasing insulin infusion should decrease with the decrease of |μk|, namely, the absolute value of glucose velocity.
These principles reflect the clinical safety requirements in blood glucose regulation, while still providing an opportunity to enhance control performance by exploiting glucose state and velocity dependent controller adaptation. To implement principle A1, we propose upper and lower bounds and for as
| (7) |
| (8) |
by considering the two limiting cases μk = 0 and μk → ∞, respectively, where and are the parameter vectors. To address principle A2, we introduce an exponential decay function to allow the value of to flow monotonically between the upper and lower bounds for μk ∈ [0,∞):
| (9) |
where τ+ is a parameter that determines the decay rate and
| (10) |
2). Designing :
Following similar ideas, we consider the principles below to design :
B1) given a negative glucose velocity prediction μk < 0, the controller should decrease insulin infusion to avoid hypoglycemia, and should be even more cautious when the corresponding glucose prediction yk is either low or extremely high;
B2) given the same glucose prediction yk, the aggressiveness of the controller should decrease with the increase of the absolute value of the glucose velocity |μk|.
To implement principle B1, we introduce upper and lower bounds and for , which are defined as
| (11) |
| (12) |
by considering the two limiting cases μk → −∞ and μk = 0, respectively, where and are parameter vectors. To address principle B2, we similarly introduce an exponential decay function to allow the value of to flow monotonically between the upper and lower bounds for μk ∈ (−∞, 0):
| (13) |
where τ− determines the decay rate and
| (14) |
3). Parameter design:
A two-step procedure is performed to determine the parameters in Θ+ and Θ− for improved glucose regulation performance. In the first step, the parameters are iteratively tuned based on their roles using the 10-patient cohort of the UVA/Padova simulator [29] with the goal of achieving minimum average glucose values without increasing the risk of hypoglycemia, following a 24-hour in silico protocol with 3 unannounced meals of [50, 75, 75] g carbohydrate (CHO) at 08:00, 12:00 and 19:00, respectively. This step yields an optimal parameter pair , the approximate optimality of which is also verified through Genetic Algorithm and Pattern Search Algorithm by solving a corresponding constrained optimization problem. As the zone MPC [7] achieved satisfactory performance for announced meals, the parameters design was done using unannounced meals and evaluated on both announced and unannounced cases to ensure safe and effective design.
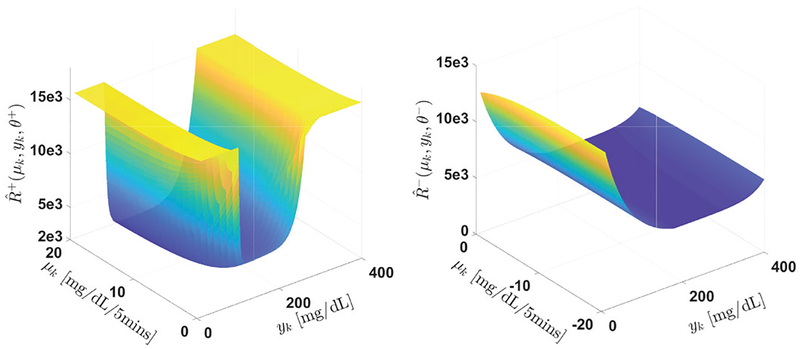
In the second step, the robustness of the controller is evaluated by considering scenarios of different measurement noises, basal rate mismatches, simulated exercise using undetected insulin delivery, and over/underestimated meal boluses. The effect of different meal sizes is also considered by testing a scenario of fasting (no meals), a snack scenario with [10, 30, 20] g CHO and a large meal scenario with [50, 100, 80] g CHO with/without meal announcements. The parameters (Θ+, Θ−) are then fine tuned on the basis of to ensure increased risk of hypoglycemia is caused by the obtained (Θ+, Θ−) compared with the original zone MPC and minimal performance degradation in terms of average glucose level is incurred compared with that of the pair The value of the obtained parameters are provided in TABLE I, and an illustration of the relationship of and with glucose state yk and glucose velocity μk is provided in Fig. 2. Considering the computation burden in this off-line parameter optimization procedure, the 10-patient cohort is used here as a “training dataset”. To ensure the performance of the designed parameters, the 100-patient cohort of the simulator is used as a “test dataset” to evaluate the performance of the design in Section IV.
TABLE I:
Parameters for Θ+ and Θ−
| Parameters for Θ+ |
6,500 |
0.14 |
0.32 |
5,500 |
0.75 |
130 |
τ+ 0.20 |
|
15,500 |
0.11 |
0.20 |
2,000 |
0.75 |
0.20 |
||
| Parameters for Θ− |
1,000,000 |
0.03 |
0.02 |
5,000 |
1 |
180 |
τ− 0.20 |
|
1,000,000 |
0.03 |
0.02 |
4,910 |
1 |
180 |
Fig. 2:
Relationship of and with μk and yk.
B. Design of
affects insulin infusion below the basal rate, which critically determines controller actions when the glucose concentration drifts toward or falls into the hypoglycemia region (yk < 70 mg/dL). The proposed approach to designing in Section III-A equally applies to . To maintain minimal complexity of the controller, however, we resort to a simple and effective design here considering the different role of . As mentioned in Section II, in (2) is usually set to a small value to ensure that proper pump suspensions are performed to avoid hypoglycemia events. To enhance this safety concern, it suffices to ignore the dependency of on μk and consider the following simple yk-dependent multi-zone parameter adaptation formula:
| (15) |
where a short-hand notation is used instead of . The implication of this design is that when the glucose prediction yk is conspicuously low, an active pump suspension strategy will be enforced, regardless of the glucose velocity μk.
IV. In Silico Performance Analysis
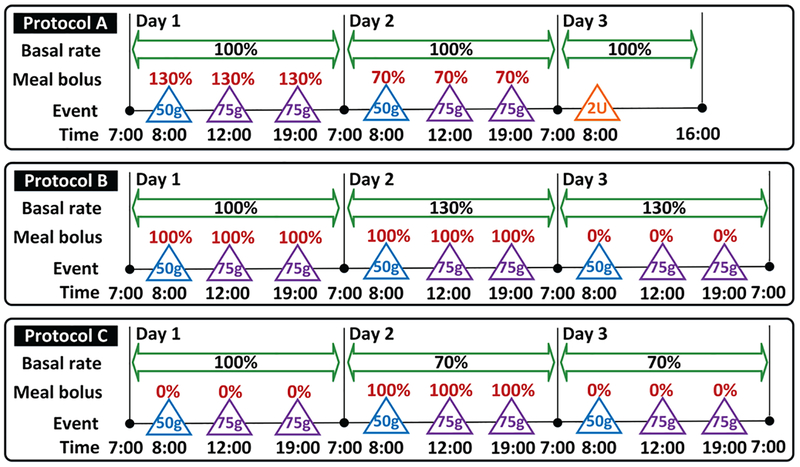
The proposed controller adaptation method is evaluated on the 100-adult cohort of the FDA accepted UVA/Padova T1DM simulator [29]. Three protocols (namely, Protocols A-C below) are designed to evaluate the performance of glucose regulation under various scenarios (see Fig. 3):
Fig. 3:
List of protocols used for performance evaluation of the proposed controller. The blue and purple triangles denote meals of 50 g and 75 g CHO, respectively. The orange triangle denotes unannounced insulin bolus of 2 units. Basal rate is provided in terms of percentages of the default value. Meal boluses are described in terms of the percentages of the actual meal sizes.
Protocol A is a 57-hour 6-meal protocol starting from 7:00 on Day 1. On Day 1 and Day 2, breakfast (50 g CHO), lunch (75 g CHO) and dinner (75 g CHO) are consumed at 8:00, 13:00 and 19:00, respectively. All the meals are announced but the meal boluses are calculated according to 130% and 70% of the actual meal sizes on Day 1 and Day 2, respectively, to evaluate the robustness of the controller with respect to over- and under-bolused meals. No meal is given on Day 3, but a 2-unit unannounced insulin bolus is applied at 8:00 AM to simulate the effect of exercise.
Protocol B is composed of 72 hours and 9-meals starting from 7:00 on Day 1. On Days 1–3, breakfast (50 g CHO), lunch (75 g CHO) and dinner (75 g CHO) are consumed at 08:00, 13:00 and 19:00, respectively. On Days 1–2, the meals are announced and 100% bolused, while meals are unannounced on Day 3. On Days 2–3, the basal rates of all subjects are increased by 30% of their normal values to evaluate controller’s behavior under overestimated basal rates for announced and unannounced meals.
Protocol C is also composed of 72 hours and 9-meals starting from 7:00 on Day 1. On Days 1–3, breakfast (50 g CHO), lunch (75 g CHO) and dinner (75 g CHO) are still consumed at 8:00, 13:00 and 19:00, respectively. Meals are unannounced on Day 1 and Day 3, but are 100% bolused on Day 2. On Days 2–3, the basal rates of all subjects are decreased by 30% of their normal values to evaluate controller’s behavior under underestimated basal rates given announced and unannounced meals.
Day 1 of Protocols B and C is used to evaluate the nominal scenarios of fully announced and unannounced meals, and Days 2–3 in these two protocols simulate the scenarios of over- and under-estimated meal boluses; Days 1–2 in Protocol A evaluate the effect of meal bolus mismatches, while Day 3 studies the effect of exercise. The proposed adaptive zone MPC with parameter setting in TABLE I is evaluated using these protocols, and the zone MPC with velocity-weighting and velocity-penalty [7] is used for the “control-group” comparisons, the results of which will be denoted as “control” in the tables and plots. The three protocols are simulated 10 times using all 100 virtual subjects and additive CGM noises with random seeds 1 through 10, which result in 1000 simulations for each protocol and each controller. The obtained results are summarized in Figs. 4–6, where the mean curves together with the 5%, 25%, 75% and 95% quartile curves are presented; a comparison of the statistics is provided in Tables II–IV, where the data for day & night and night time (24:00–06:00) are reported separately. The performance metrics are selected according to [31], [32], where details of the definitions can be found.
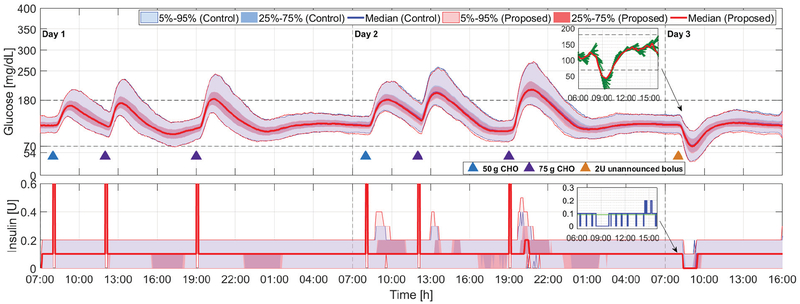
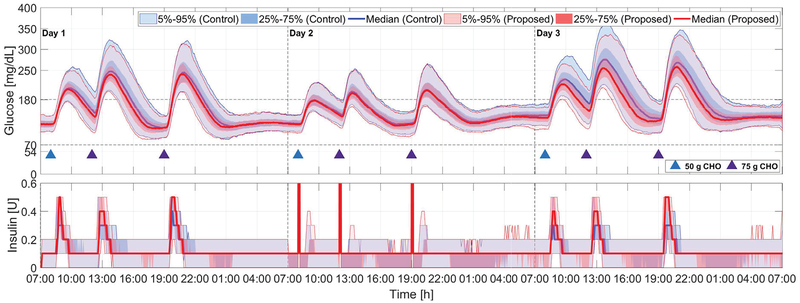
Fig. 4:
Comparison of the proposed method (red curves and light red areas) with the zone-MPC developed in [7] (blue curves and light blue areas) for Protocol A; see Section IV and Fig. 3. Blue and purple triangles denote meals of 50 g and 75 g CHO, respectively, and the orange triangle denotes a 2-unit unannounced insulin bolus. Snapshots of the glucose and insulin profile of a particular in silico subject on Day 3 obtained using the proposed adaptation method are also provided, where in the glucose panel the red curve denotes the CGM readings and the green curves denote the glucose predictions at each sampling instant, in the insulin panel the blue curve denotes insulin delivered at each time instant and the green line denotes basal rate.
Fig. 6:
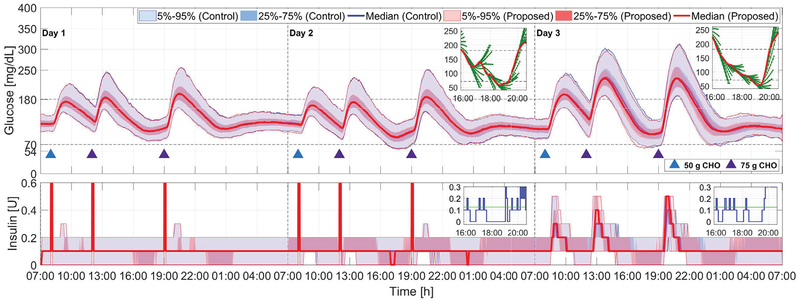
Comparison of the proposed method (red curves and light red areas) with the zone-MPC developed in [7] (blue curves and light blue areas) for Protocol C; see Section IV and Fig. 3. Blue and purple triangles denote meals of 50 g and 75 g CHO, respectively.
TABLE II:
Glycemic metrics comparing the proposed method with the zone MPC developed in [7] (Protocol A)
| Protocol A | Day 1 | Day 2 | Day 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Metrics | Control | Proposed | p value | Control | Proposed | p value | Control | Proposed | p value |
| Day & night | |||||||||
| % time <54 mg/dL | 0.0 (0.0) | 0.0 (0.0) | 0.047* | 0.0 (0.0) | 0.0 (0.0) | 0.581 | 0.0 (2.0) | 0.0 (3.7) | <0.001* |
| % time <70 mg/dL | 0.0 (0.0) | 0.0 (0.0) | 0.037* | 0.0 (0.0) | 0.0 (0.0) | <0.001* | 4.1 (11.1) | 5.2 (11.7) | <0.001* |
| % time 70–180 mg/dL | 95.5 (8.7) | 95.4 (8.8) | 0.679 | 81.7 (13.4) | 83.1 (12.6) | <0.001* | 95.8 (11.1) | 94.8 (11.7) | <0.001* |
| % time >250 mg/dL | 0.0 (0.0) | 0.0 (0.0) | 0.885 | 0.0 (0.0) | 0.0 (0.0) | <0.001* | 0.0 (0.0) | 0.0 (0.0) | 1.000 |
| Mean BG (mg/dL) | 129.3 (9.2) | 129.2 (8.8) | <0.001* | 143.6 (8.8) | 141.8 (8.4) | <0.001* | 114.6 (7.4) | 113.7 (7.6) | <0.001* |
| SD BG (mg/dL) | 26.4 (9.2) | 26.4 (9.4) | 0.635 | 33.9 (10.7) | 33.8 (10.6) | 0.005* | 20.4 (11.5) | 20.7 (11.6) | <0.001* |
| Night time (0–6 AM) | |||||||||
| % time <54 mg/dL | 0.0 (0.0) | 0.0 (0.0) | 1.000 | 0.0 (0.0) | 0.0 (0.0) | 1.000 | - | - | - |
| % time < 70 mg/dL | 0.0 (0.0) | 0.0 (0.0) | 0.090 | 0.0 (0.0) | 0.0 (0.0) | 0.014* | - | - | - |
| % time 70–180 mg/dL | 100.0 (0.0) | 100.0 (0.0) | 0.341 | 100.0 (0.0) | 100.0 (0.0) | 0.024* | - | - | - |
| % time >250 mg/dL | 0.0 (0.0) | 0.0 (0.0) | 1.000 | 0.0 (0.0) | 0.0 (0.0) | 1.000 | - | - | - |
| Mean BG (mg/dL) | 118 (9.2) | 118.2 (8.7) | 0.004* | 118.4 (8.4) | 118.2 (8.4) | <0.001* | - | - | - |
| SD BG (mg/dL) | 12.9 (6.8) | 13.1 (7.0) | <0.001* | 12.3 (6.3) | 12.5 (6.6) | <0.001* | - | - | - |
| Mean BG at 7 AM (mg/dL) | 122.0 (14.0) | 121.0 (16.0) | <0.001* | 122.0 (16.0) | 121.0 (16.0) | <0.001* | - | - | - |
Data in this table are shown as median (inter quartile range). Statistical significance is assessed by Wilcoxon signed-rank test. Statistically significant (p < 0.05) changes are highlighted in bold with asterisks.
TABLE IV:
Glycemic metrics comparing the proposed method with the zone MPC developed in [7] (Protocol C)
| Protocol C | Day 1 | Day 2 | Day 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Metrics | Control | Proposed | p value | Control | Proposed | p value | Control | Proposed | p value |
| Day & night | |||||||||
| % time <54 mg/dL | 0.0 (0.0) | 0.0 (0.0) | 0.064 | 0.0 (0.0) | 0.0 (0.0) | 1.000 | 0.0 (0.0) | 0.0 (0.0) | 0.500 |
| % time <70 mg/dL | 0.0 (0.0) | 0.0 (0.0) | 0.397 | 0.0 (0.0) | 0.0 (0.0) | 0.096 | 0.0 (0.0) | 0.0 (0.0) | 0.001* |
| % time 70–180 mg/dL | 66.3 (14.3) | 70.5 (11.5) | <0.001* | 84.7 (13.6) | 85.8 (12.6) | <0.001* | 56.1 (21.1) | 64.8 (18.0) | <0.001* |
| % time >250 mg/dL | 3.6 (10.8) | 1.9 (7.1) | <0.001* | 0.0 (0.0) | 0.0 (0.0) | <0.001* | 12.2 (17.2) | 5.9 (13.4) | <0.001* |
| Mean BG (mg/dL) | 159.0 (18.1) | 153.8 (13.5) | <0.001* | 148.9 (10.3) | 146.1 (9.6) | <0.001* | 180.6 (31.6) | 166.4 (21.9) | <0.001* |
| SD BG (mg/dL) | 47.7 (13.8) | 45.6 (12.7) | <0.001* | 27.7 (10.5) | 28.4 (10.6) | <0.001* | 49.6 (14.5) | 45.3 (13.3) | <0.001* |
| Night time (0–6 AM) | |||||||||
| % time <54 mg/dL | 0.0 (0.0) | 0.0 (0.0) | 0.219 | 0.0 (0.0) | 0.0 (0.0) | 1.000 | 0.0 (0.0) | 0.0 (0.0) | 1.000 |
| % time <70 mg/dL | 0.0 (0.0) | 0.0 (0.0) | 0.010* | 0.0 (0.0) | 0.0 (0.0) | 1.000 | 0.0 (0.0) | 0.0 (0.0) | 0.008* |
| % time 70–180 mg/dL | 100.0 (0.0) | 100.0 (0.0) | <0.001* | 100.0 (0.0) | 100.0 (0.0) | <0.001* | 100.0 (7.5) | 100.0 (0.0) | <0.001* |
| % time >250 mg/dL | 0.0 (0.0) | 0.0 (0.0) | 1.000 | 0.0 (0.0) | 0.0 (0.0) | 1.000 | 0.0 (0.0) | 0.0 (0.0) | 0.143 |
| Mean BG (mg/dL) | 120.6 (9.0) | 119.7 (8.2) | <0.001* | 133.1 (10.8) | 131.0 (9.8) | <0.001* | 138.0 (17.8) | 134.3 (12.6) | <0.001* |
| SD BG (mg/dL) | 13.5 (7.3) | 13.0 (7.3) | <0.001* | 12.5 (5.7) | 12.9 (6.2) | <0.001* | 14.6 (8.3) | 13.9 (6.9) | <0.001* |
| Mean BG at 7 AM (mg/dL) | 122.0 (15.0) | 121.0 (14.0) | <0.001* | 140.5 (19.0) | 136.0 (21.0) | <0.001* | 140.5 (25.0) | 135.0 (26.0) | <0.001* |
Data in this table are shown as median (inter quartile range). Statistical significance is assessed by Wilcoxon signed-rank test. Statistically significant (p < 0.05) changes are highlighted in bold with asterisks.
A. Announced meals
The scenario of fully announced meals is considered on Day 1 of Protocol B for the original zone MPC and the proposed adaptive zone MPC (see Fig. 5 and TABLE III). Satisfactory performance for hypoglycemia prevention measured by percent time < 70 mg/dL and percent time < 54 mg/dL (severe hypoglycemia [32]) are observed for both controllers; in particular, a comparison of the values of these two performance metrics indicates that the proposed parameter adaptation approach does not introduce increased risk for hypoglycemia (percent time < 70 mg/dL, 0.0% vs. 0.0%, p < 0.001). As was indicated in [7], the glucose response is dominated by meal boluses for fully bolused meals and the effect of closed-loop control in terms of improvements in glucose regulation is concealed, given the fact that the original zone MPC is able to achieve good control performance for announced meals [6]. Consequently, the proposed adaptive approach only leads to a small performance improvement in terms of mean glucose (135.9 mg/dL vs. 136.5 mg/dL; p < 0.001) with very close percent time in the euglycemic range [70, 180] mg/dL (90.6% vs. 90.5%; p < 0.001). This discussion is consistent with the quartile curves in Fig. 5. Finally, we note that the controllers result in very close and satisfactory glycemic control performance for night time (24:00 – 06:00 h).
Fig. 5:
Comparison of the proposed method (red curves and light red areas) with the original zone-MPC developed in [7] (blue curves and light blue areas) for Protocol B; see Section IV and Fig. 3. Blue and purple triangles denote meals of 50 g and 75 g CHO, respectively. Snapshots of the glucose and insulin profile of a particular in silico subject obtained using the proposed adaptation method are also provided; keys are the same as the snapshots in Fig. 4.
TABLE III:
Glycemic metrics comparing the proposed method with the zone MPC developed in [7] (Protocol B)
| Protocol B | Day 1 | Day 2 | Day 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Metrics | Control | Proposed | p value | Control | Proposed | p value | Control | Proposed | p value |
| Day & night | |||||||||
| % time <54 mg/dL | 0.0 (0.0) | 0.0 (0.0) | 0.012* | 0.0 (0.0) | 0.0 (0.0) | <0.001* | 0.0 (0.0) | 0.0 (0.0) | 0.491 |
| % time <70 mg/dL | 0.0 (0.0) | 0.0 (0.0) | <0.001* | 0.2 (2.7) | 0.0 (2.4) | <0.001* | 0.0 (1.5) | 0.0 (2.1) | 0.024* |
| % time 70–180 mg/dL | 90.5 (12.1) | 90.6 (11.7) | <0.001* | 93.3 (11.4) | 93.3 (11.5) | 1.000 | 71.8 (12.8) | 73.6 (11.7) | <0.001* |
| % time >250 mg/dL | 0.0 (0.0) | 0.0 (0.0) | 0.412 | 0.0 (0.0) | 0.0 (0.0) | 0.008* | 0 (5.8) | 0 (4.7) | <0.001* |
| Mean BG (mg/dL) | 136.5 (8.4) | 135.9 (8.0) | <0.001* | 126.0 (9.2) | 126.5 (9.3) | <0.001* | 145.8 (13.8) | 143.6 (12.7) | <0.001* |
| SD BG (mg/dL) | 29.4 (9.6) | 29.5 (9.7) | 0.155 | 30.3 (10.2) | 30.3 (10.4) | <0.001* | 47.8 (14.0) | 46.6 (12.6) | <0.001* |
| Night time (0–6 AM) | |||||||||
| % time <54 mg/dL | 0.0 (0.0) | 0.0 (0.0) | 1.000 | 0.0 (0.0) | 0.0 (0.0) | 0.152 | 0.0 (0.0) | 0.0 (0.0) | 0.011* |
| % time <70 mg/dL | 0.0 (0.0) | 0.0 (0.0) | 0.007* | 0.0 (0.0) | 0.0 (0.0) | 0.004* | 0.0 (0.0) | 0.0 (0.0) | 0.627 |
| % time 70–180 mg/dL | 100.0 (0.0) | 100.0 (0.0) | 0.144 | 100.0 (0.0) | 100.0 (0.0) | 0.032* | 100.0 (0.0) | 100.0 (0.0) | 0.292 |
| % time >250 mg/dL | 0.0 (0.0) | 0.0 (0.0) | 1.000 | 0.0 (0.0) | 0.0 (0.0) | 1.000 | 0.0 (0.0) | 0.0 (0.0) | 1.000 |
| Mean BG (mg/dL) | 117.9 (8.3) | 117.9 (8.0) | 0.106 | 107.7 (8.1) | 108.9 (7.9) | <0.001* | 108.8 (8.0) | 109.6 (8.0) | <0.001* |
| SD BG (mg/dL) | 12.3 (6.6) | 12.5 (6.7) | <0.001* | 14.0 (7.1) | 14.1 (7.6) | <0.001* | 14.8 (7.9) | 14.8 (8.1) | 0.776 |
| Mean BG at 7 AM (mg/dL) | 122.0 (14.0) | 121.0 (14.5) | <0.001* | 107.0 (18.0) | 108.0 (17.0) | <0.001* | 107.0 (24.0) | 108.0 (24.0) | <0.001* |
Data in this table are shown as median (inter quartile range). Statistical significance is assessed by Wilcoxon signed-rank test. Statistically significant (p < 0.05) changes are highlighted in bold with asterisks.
B. Unannouced meals
Performance comparison for the scenario of unannounced meals is considered on Day 1 of Protocol C (see Fig. 6 and TABLE IV). In this scenario, the proposed adaptation approach is observed to achieve improved glucose regulation performance compared with the original zone MPC in terms of percent time in the safe range (70.5% vs. 66.3%; p < 0.001), percent time > 250 mg/dL (1.9% vs. 3.6%; p < 0.001), mean glucose (153.8 mg/dL vs. 159.0 mg/dL; p < 0.001). The performance improvement is achieved without increasing the risk of hyperglycemia, measured by percent time < 70 mg/dL (0.0% vs. 0.0%, p = 0.397) and percent time < 54 mg/dL (0.0% vs. 0.0%, p = 0.064). The underlying reason, as observed from Fig. 5, is that the proposed adaptation approach encourages reasonably more active insulin infusion when the glucose concentration is rapidly increasing and is able to safely turn off insulin infusion when the glucose stops to increase or decreases, due to the dynamic glucoseand velocity-dependent choice of and . Both controllers achieve satisfactory glycemic control performance for night time (24:00 – 06:00 h), as is also observed in the mean BG at 07:00 h (121.0 mg/dL vs. 122.0 mg/dL; p < 0.001). Combined with the observations in the previous subsection, the proposed method is able to achieve improved glucose control for unannounced meals while maintaining safety and performance for announced meals.
C. Moderate meal-bolus mismatch
Days 1–2 in Protocol A are utilized to evaluate the case that subjects provide modest over or under-estimates of meal sizes, which is less extreme but more realistic for meal announcements. The data are reported in Fig. 4 and TABLE II (Days 1–2). When meal boluses with sizes larger than required are provided, we observe in Fig. 4 (Day 1) that both controllers are able to maintain their outputs around basal rates and perform pump suspension if low glucose predictions occur (e.g., around 17:00 and 23:00 on Day 1 in Fig. 4). On the other hand, the proposed adaptation method compensates conservative meal boluses more actively (see Fig. 4 (Day 2)), and thus obtains slightly improved glucose regulation performance in terms of mean glucose (141.8 mg/dL vs. 143.6 mg/dL; p < 0.001) and percent time in the safe range (83.1% vs. 81.7%; p < 0.001). The improvements are small because of the small difference (70%) in the underestimated meal bolus from the exact bolus (100%). Clinically significant differences in hypoglycemia risk are not observed.
D. Simulated exercise response
Day 3 in Protocol A is utilized to evaluate the behavior of the controller under an unannounced insulin bolus (2 units), which emulates the effect of exercises that increase insulin sensitivity. As the subjects are fasting when the announced bolus is infused, hypoglycemia is inevitable in this scenario; but we observe that both controllers are vigilant in suspending insulin infusion when the risks of hypoglycemia are discerned from the glucose predictions (see the steep decrease in insulin delivery before the glucose readings drop below 70 mg/dL around 8:00–9:00 on Day 3 in Fig. 4), which indicates that the proposed adaptation approach is able to maintain appropriate controller behavior under unannounced exercise events and severe hypoglycemia episodes. This point can also be observed in the glucose and insulin example of an in silico subject on Day 3 obtained using the proposed adaptation method (see Fig. 4), where the controller is able to turn off insulin when the glucose predictions become low to ensure safety. Note that the proposed method yields slightly increased percent time < 70 mg/dL (5.2%, vs. 4.1%, p < 0.001); however, the increase is relatively small and expected with increased responsiveness of the controller.
E. Basal rate mismatch
Days 2–3 in Protocols B and C are utilized to analyze the sensitivity of the controller with respect to inaccurately estimated basal rates. Concretely, the basal rates are intentionally increased by 30% on Day 2–3 of Protocol B to simulate the effect of overestimated basal rates with announced and unannounced meals, which invariably increase the risk of hypoglycemia. From Table III and Fig. 5 (Days 2–3), we observe that the proposed method does not increase the risk of hypoglycemia compared with the original zone MPC. The snapshots of an in silico subject in Fig. 5 further confirm that the zone MPC behaves properly with the proposed parameter adaptation approach.
On Days 2–3 of Protocol C, the basal rates are reduced by 30% on Day 2–3 of Protocol B to simulate the effect of overestimated basal rates with announced and unannounced meals, which leads to lifted glucose profiles due to decreased “open-loop” insulin delivery. From Table IV and Fig. 6 (Days 2–3), we observe that the proposed approach compensates for the lack of basal insulin in a more active fashion, which leads to increased percentage time in the euglycemic range [70, 180] mg/dL (85.8% vs. 84.7%; p < 0.001) and mean glucose (146.1 mg/dL vs. 148.9 mg/dL; p < 0.001) for announced meals, and significant performance improvements in terms of percentage time in the euglycemic range (64.8% vs. 56.1%; p < 0.001) and mean glucose (166.4 mg/dL vs. 180.6 mg/dL; p < 0.001) for unannounced meals. This indicates the proposed approach is less sensitive to underestimated basal rates compared with the original zone MPC.
F. Comparison with existing results
We further compare the results obtained by the proposed method with existing in silico and experimental results in the literature, obtained using previous zone MPC controller schemes [8], [12], [24]. The results are summarized in Table V. For fully announced meals (100% meal bolus), the result obtained is close to that of a zone MPC with trust index proposed in [24], although the results in [24] were obtained using the 10-subject cohort of the T1DM simulator with a different size of dinner (100 g vs. 75 g). From Table V, we observe that patients usually have higher average glucose values and lower percentage time in range [70, 180] mg/dL with higher frequency of hypoglycemia in clinical studies, which justifies the need to reduce hyperglycemia while preventing the occurrence of hypoglycemia. By adjusting the control penalties adaptively according to the predicted glucose and rate of change, the proposed method provides one feasible and robust solution, as indicated by the in silico results in this section.
TABLE V:
Performance comparison with previous studies in the literature using zone MPC scheme.
| Mean BG (mg/dL) | % time 70–180 mg/dL | % time <70 mg/dL | |
|---|---|---|---|
| Proposed (100% meal bolus) | 135.9 (8.0) | 90.6 (11.7) | 0.0 (0.0) |
| Proposed (70% meal bolus) | 141.8 (8.4) | 83.1 (12.6) | 0.0 (0.0) |
| Proposed (0% meal bolus) | 153.8 (13.5) | 70.5 (11.5) | 0.0 (0.0) |
| Laguna Sanz et al. [24] in silico (100% meal bolus) | 134.0 〈6.0〉 | 91 〈5.6〉 | 0.0 〈0.0〉 |
| Dassau et al. [8] outpatient (overnight) | 141 [130–158] | 79.1 [60.4–86.1] | 1.37 [0–2.84] |
| Dassau et al. [12] outpatient (week 12) | 152.5 〈20.7〉 | 69.5 〈20.2〉 | 1.9 〈2.0〉 |
G. Control penalty adaptation
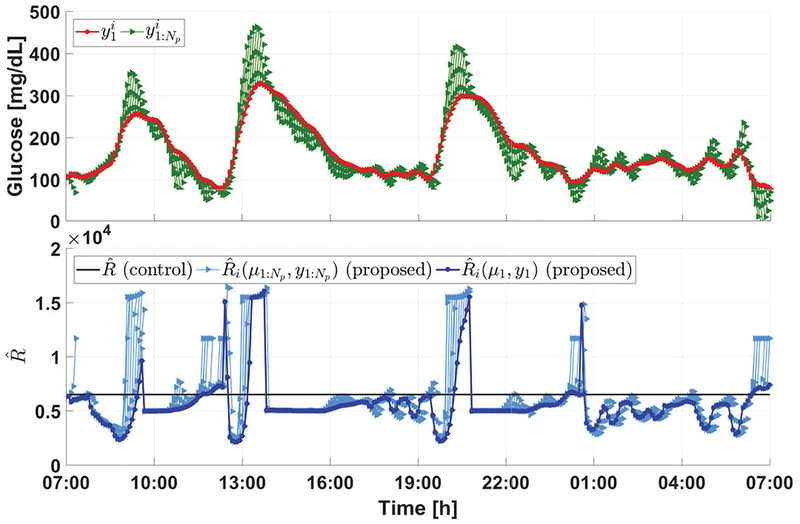
Finally, we note that the performance improvement of the proposed method is achieved through the adaptation of control penalty parameters. To illustrate this point, the relationship between and for an in silico subject on Day 1 of Protocol C (which correspond to 288 controller update instants) is provided in Fig. 7. The superscript i here is used to represent the dependence of the variables on time. Compared with the constant choice of in [7], we observe that the proposed parameter adaptation law turns off insulin infusion by choosing relatively larger values of when the glucose predictions are relatively low, or extremely high, or decreasing, but allows active insulin infusion by choosing comparably small values only when the glucose predictions are steeply increasing above the nominal glucose range. This further explains the mechanism of the proposed adaptive approach to alleviate hyperglycemia without increasing hypoglycemia risks.
Fig. 7:
Illustration of control penalty adaptation. In the upper panel, the red curve shows the trend of first element in the glucose prediction sequence with respect to discrete time i, while the green curves denote the sequences of . Note that the red and green curves denotes glucose predictions, which are different from actual CGM measurements. In the lower panel, the dark blue curve shows the trend of with respect to discrete time i, the light blue curves denote the sequences of , and the black line indicates the constant adopted in [7]. The superscript i used here indicates the dependence of a variable on time.
V. Advisory-mode comparisons using clinical data
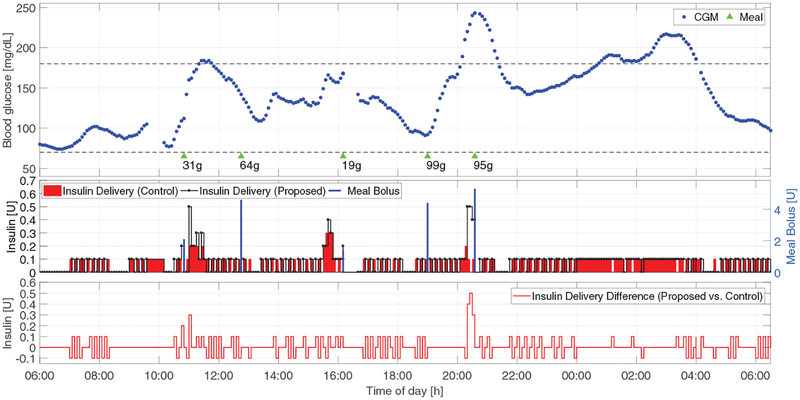
In this section, the historical clinical data obtained using the zone-MPC developed in [7] (the corresponding clinical results were published in [12]) are re-run using the proposed method to obtain the corresponding insulin command trajectory, assuming the controller received exactly the same input data. This is the so-called advisory-mode comparison [33] and allows dose-to-dose evaluation of the safety and effectiveness of the proposed method by feeding the identical glucose data obtained in the clinical trial to the controller.
The results are provided in Fig. 8. Compared with the approach in [7], the proposed controller is more active when the glucose level is escaping the safe euglycemic range from above, but is able to resemble almost-identical safe insulin commands when the glucose level is low or decreasing. As an example, we observe that driven by the rapidly increasing CGM values, the proposed controller would suggest (approximately) additional 1.2 units of insulin between 20:00 and 21:00, which would decrease the glucose levels after the 95 g meal to a safe but lower range, and thus further help reduce the hyperglycemia that happened between 01:00 and 04:00. This explains how the proposed method can obtain improved glucose regulation with lower average glucose levels in real-life scenarios, which fulfills our motivation of alleviating the high-average glucose effect introduced by the zone MPC [7] used in [12] (see Section I). Note that both the glucose response and the insulin command trajectory would be different had the proposed control law actually been employed.
Fig. 8:
Advisory-mode comparison of insulin command sequences of the proposed method (black curve in the second panel) with the zone-MPC developed in [7] (red bars in the second panel) based on clinical data from [12] (Clinical trial reg. no. NCT02705053, clinicaltrials.gov). Meals are denoted by green triangles with sizes below them. The difference of insulin delivery between the proposed method and that introduced in [7] is plotted in the third panel.
VI. Conclusions
In this work, a control penalty adaptation approach is developed for zone MPC of AP based on the predicted glucose and its rate of change. The obtained adaptive controller is able to actively perform insulin infusion when blood glucose is rapidly increasing above the nominal value, but cautiously reduces or suspends insulin infusion when glucose rate of change is positively small or negative, and when low glucose values are predicted. The effectiveness and robustness of the proposed method is evaluated on the 100-adult cohort of the FDA-approved UVA/Padova simulator through comparisons with the original zone MPC developed in [7]. Although the approach is developed for the zone MPC, it is rich enough to be generalized to other MPC formulations for AP (e.g., the enhanced set-point MPC with asymmetric exponential and quadratic costs [34]).
Supplementary Material
Acknowledgement
Access to the full version of the UVA/Padova metabolic simulator was provided by an agreement with Prof. C. Cobelli (University of Padova) and Prof. B. P. Kovatchev (UVA) for research purposes.
This work was supported by the National Institutes of Health under Grants DP3DK104057 and UC4DK108483.
Contributor Information
Dawei Shi, Email: daweishi@seas.harvard.edu.
Eyal Dassau, Email: dassau@seas.harvard.edu.
Francis J. Doyle, III, Email: frank_doyle@seas.harvard.edu.
References
- [1].Doyle FJ III, et al. , “Closed-loop artificial pancreas systems: Engineering the algorithms,” Diabetes Care, vol. 37, no. 5, pp. 1191–1197, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Thabit H and Hovorka R, “Coming of age: the artificial pancreas for type 1 diabetes,” Diabetologia, vol. 59, no. 9, pp. 1795–1805, September 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Haidar A, “The artificial pancreas: How closed-loop control is revolutionizing diabetes,” IEEE Control Systems, vol. 36, no. 5, pp. 28–47, October 2016. [Google Scholar]
- [4].Grosman B, et al. , “Zone model predictive control: A strategy to minimize hyper- and hypoglycemic events,” J. Diabetes Sci. Technol, vol. 4, no. 4, pp. 961–975, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gondhalekar R, et al. , “Periodic-zone model predictive control for diurnal closed-loop operation of an artificial pancreas,” J. Diabetes Sci. Technol, vol. 7, no. 6, pp. 1446–1460, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gondhalekar R, et al. , “Periodic zone-MPC with asymmetric costs for outpatient-ready safety of an artificial pancreas to treat type 1 diabetes,” Automatica, vol. 71, pp. 237–246, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gondhalekar R, et al. , “Velocity-weighting & velocity-penalty MPC of an artificial pancreas: Improved safety & performance,” Automatica, vol. 91, pp. 105–117, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Dassau E, et al. , “Adjustment of open-loop settings to improve closed-loop results in type 1 diabetes: A multicenter randomized trial,” J. Clin. Endocrinol. Metab, vol. 100, no. 10, pp. 3878–3886, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Huyett LM, et al. , “Outpatient closed-loop control with unannounced moderate exercise in adolescents using zone model predictive control,” Diabetes Technol. Ther, vol. 19, no. 6, pp. 3878–3886, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Forlenza GP, et al. , “Application of zone model predictive control artificial pancreas during extended use of infusion set and sensor: A randomized crossover-controlled home-use trial,” Diabetes Care, vol. 40, no. 8, pp. 1096–1102, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dassau E, et al. , “Intraperitoneal insulin delivery provides superior glycemic regulation to subcutaneous insulin delivery in model predictive control-based fully-automated artificial pancreas in patients with type 1 diabetes: A pilot study,” Diabetes, Obesity and Metabolism, vol. 19, no. 12, pp. 1698–1705, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Dassau E, et al. , “Twelve week 24/7 ambulatory artificial pancreas with weekly adaptation of insulin delivery settings: Effect on hemoglobin A1c and hypoglycemia,” Diabetes Care, vol. 40, no. 12, pp. 1719–1726, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].van Heusden K, et al. , “Control-relevant models for glucose control using a priori patient characteristics,” IEEE Trans. Biomed. Eng, vol. 59, no. 7, pp. 1839–1849, July 2012. [DOI] [PubMed] [Google Scholar]
- [14].Palerm CC, et al. , “A run-to-run framework for prandial insulin dosing: handling real-life uncertainty,” Int. J. Robust. Nonlin, vol. 17, no. 13, pp. 1194–1213, 2007. [Google Scholar]
- [15].Palerm C, et al. , “A run-to-run control strategy to adjust basal insulin infusion rates in type 1 diabetes,” J. Process Control, vol. 18, no. 3, pp. 258–265, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hovorka R, et al. , “Nonlinear model predictive control of glucose concentration in subjects with type 1 diabetes,” Physiol. Meas, vol. 25, no. 4, pp. 905–920, 2004. [DOI] [PubMed] [Google Scholar]
- [17].El-Khatib FH, et al. , “Adaptive closed-loop control provides blood-glucose regulation using dual subcutaneous insulin and glucagon infusion in diabetic swine,” J. Diabetes Sci. Technol, vol. 1, no. 2, pp. 181–192, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Turksoy K, et al. , “Multivariable adaptive identification and control for artificial pancreas systems,” IEEE Trans. Biomed. Eng, vol. 61, no. 3, pp. 883–891, 2014. [DOI] [PubMed] [Google Scholar]
- [19].Wang Y, et al. , “Closed-loop control of artificial pancreatic β-cell in type 1 diabetes mellitus using model predictive iterative learning control,” IEEE Trans. Biomed. Eng, vol. 57, no. 2, pp. 211–219, 2010. [DOI] [PubMed] [Google Scholar]
- [20].Cameron F, et al. , “Extended multiple model prediction with application to blood glucose regulation,” J. Process Control, vol. 22, no. 8, pp. 1422–1432, 2012. [Google Scholar]
- [21].Toffanin C, et al. , “Dynamic insulin on board: Incorporation of circadian insulin sensitivity variation,” J. Diabetes Sci. Technol, vol. 7, no. 4, pp. 928–940, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Colmegna PH, et al. , “Switched LPV glucose control in type 1 diabetes,” IEEE Trans. Biomed. Eng, vol. 63, no. 6, pp. 1192–1200, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Toffanin C, et al. , “Toward a run-to-run adaptive artificial pancreas: In silico results,” IEEE Trans. Biomed. Eng, vol. 65, no. 3, pp. 479–488, 2018. [DOI] [PubMed] [Google Scholar]
- [24].Laguna Sanz A. J., et al. , “An enhanced model predictive control for the artificial pancreas using a confidence index based on residual analysis of past predictions,” J. Diabetes Sci. Technol, vol. 11, no. 3, pp. 537–544, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chakrabarty A, et al. , “Event-triggered model predictive control for embedded artificial pancreas systems,” IEEE Trans. Biomed. Eng, vol. 65, no. 3, pp. 575–586, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Turksoy K and Cinar A, “Adaptive control of artificial pancreas systems-a review,” J. Healthc. Eng, vol. 5, no. 1, pp. 1–22, 2014. [DOI] [PubMed] [Google Scholar]
- [27].Grosman B, et al. , “Multi-zone-MPC: Clinical inspired control algorithm for the artificial pancreas,” in 18th IFAC World Congr, 2011, pp. 7120–7125. [Google Scholar]
- [28].Shi D, et al. , “Zone model predictive control with glucose- and velocity-dependent control penalty adaptation for an artificial pancreas,” in 2018 Amer. Control Conf, 2018, pp. 3577–3582. [Google Scholar]
- [29].Dalla Man C., et al. , “The UVA/PADOVA type 1 diabetes simulator: New features,” J. Diabetes Sci. Technol, vol. 8, no. 1, pp. 26–34, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wytock M, et al. , “Dynamic energy management with scenario-based robust mpc,” in 2017 Amer. Control Conf, 2017, pp. 2042–2047. [Google Scholar]
- [31].Maahs DM, et al. , “Outcome measures for artificial pancreas clinical trials: A consensus report,” Diabetes Care, vol. 39, no. 7, pp. 1175–1179, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].International Hypoglycemia Study Group, “Glucose concentrations of less than 3.0 mmol/L (54 mg/dL) should be reported in clinical trials: A joint position statement of the American Diabetes Association and the European Association for the Study of Diabetes,” Diabetes Care, vol. 40, no. 1, pp. 155–157, 2017. [DOI] [PubMed] [Google Scholar]
- [33].Gillis R, et al. , “Glucose estimation and prediction through meal responses using ambulatory subject data for advisory mode model predictive control,” J. Diabetes Sci. Technol, vol. 1, no. 6, pp. 825–833, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lee JB, et al. , “Enhanced model predictive control (eMPC) strategy for automated glucose control,” Ind. Eng. Chem. Res, vol. 55, no. 46, pp. 11857–11868, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.