Abstract
Background
Quality improvement initiatives have been developed to improve acute coronary syndrome (ACS) care largely in high-income country settings. We sought to synthesize the effect size and quality of evidence from randomized controlled trials (RCTs) and non-randomized studies for hospital-based ACS quality improvement interventions on clinical outcomes and process of care measures for their potential implementation in low- and middle-income country settings.
Methods and Results
We conducted bibliometric search of databases and trial registers and hand searching in 2016 and performed an updated search in May 2018 and May 2019. We performed data extraction, risk of bias assessment, and quality of evidence assessments in duplicate. We assessed differences in outcomes by study design comparing RCTs to non-randomized quasi-experimental studies and by country income status. A meta-analysis was not feasible due to substantial, unexplained heterogeneity among the included studies and thus, we present a qualitative synthesis. We screened 5,858 records and included 32 studies (14 RCTs [n=109,763] and 18 non-randomized quasi-experimental studies [n=54,423]. In-hospital mortality ranged from 2.1%-4.8% in the intervention groups versus 3.3-5.1% in the control groups in 5 RCTs (n=55,942). Five RCTs (n=64,313) reported a 3.0%-31.0% higher rates of reperfusion for STEMI patients in the intervention groups. The effect sizes for in-hospital and discharge medical therapies in a majority of RCTs were 3.0%-10.0% higher in the intervention groups. There was no significant difference in 30-day mortality evaluated by 4 RCTs (n=42,384), which reported 2.5%-15.0% vs. 5.9-22% 30-day mortality rates in the intervention vs. control groups. In contrast, non-randomized quasi-experimental studies reported larger effect sizes compared to RCTs. There were no significant consistent differences in outcomes between high-income and middle-income countries. Low-income countries were not represented in any of the included studies.
Conclusions
Hospital-based ACS quality improvement interventions have a modest effect on process of care measures but not on clinical outcomes with expected differences by study design. Although quality improvement programs have an ongoing and important role for ACS quality of care in high-income country settings, further research will help to identify key components for contextualizing and implementing such interventions to new settings to achieve their desired effects.
In 2015, the estimated global prevalence of ischemic heart disease was 111 million (95% uncertainty interval: 101 to 122 million) with 7.3 million global cases of fatal acute myocardial infarction (95% uncertainty interval: 6.8 to 7.8 million).1 In response to delays and deficiencies in acute myocardial infarction and acute coronary syndrome care associated with high morbidity and mortality rates, professional organizations have developed quality improvement initiatives. These quality improvement programs are complex interventions that frequently include clinical pathways, audits, performance feedback, education, checklists. Non-randomized studies have evaluated the efficacy of various hospital-based acute coronary syndrome quality improvement interventions on clinical outcomes and process of care measures. There is evidence for temporal improvement of evidence-based management and outcomes for acute coronary syndrome including a reduction in disparities of care. For example, the joint American Heart Association’s and American College of Cardiology Chest Pain-MI Registry (formerly known as ACTION-Registry) demonstrated temporal improvements in process of care measures from 2006 to 2014, such as the use of aspirin (94% to 99%), beta blockers (93% to 98%), and lipid lowering medications (85% to 99%) at discharge.2
To overcome the potential confounding and uncertainty inherent in non-randomized studies and to understand which components of these complex quality improvement interventions are effective, several teams have performed randomized or quasi-randomized trials of quality improvement interventions, largely in high-income countries. However, questions remain about their generalizability across and implementation in different settings, including low- and middle-income countries.
The objective of this systematic review was to estimate the effect size and quality of evidence for hospital-based acute coronary syndrome quality improvement interventions on clinical outcomes and process of care measures using data from RCTs and to summarize differences in the effect estimates between RCTs and non-randomized studies. We also contextualize the findings on how quality improvement interventions may be particularly useful in low- and middle-income country health systems where the presentation and management of acute coronary syndrome is more heterogeneous than in high-income countries, evidence-practice gaps are frequently greater, and clinical outcomes are generally, but not always, poorer.3
METHODS
We developed and published our systematic review protocol on the international prospective register of systematic reviews (PROSPERO)4 a priori and performed our review according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines illustrated in Figure 1. All supporting data and methods used for this systematic review are available within the article and supplemental files and can be used to replicate the study.
Figure 1.
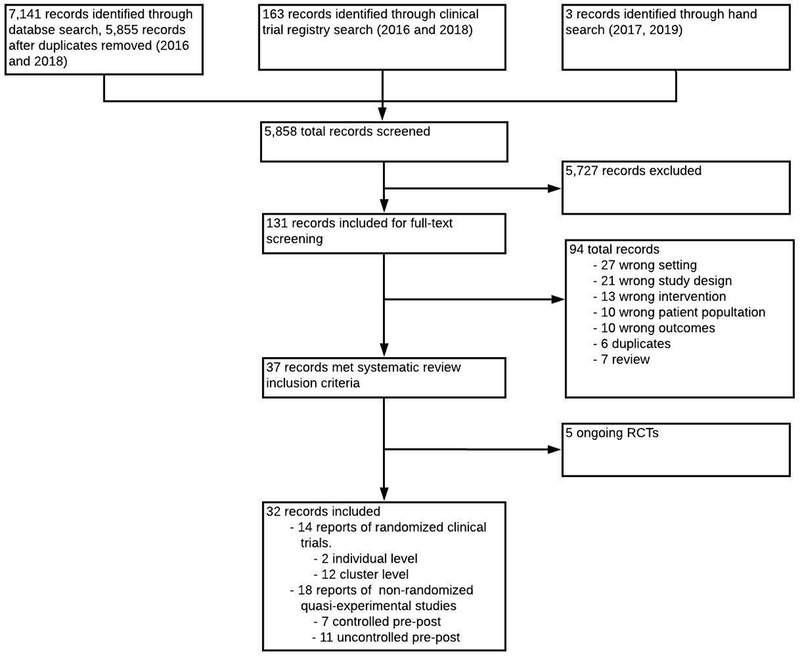
PRISMA flow chart of included studies.
Search methods
In November 2016, we conducted bibliometric search of nine databases. We hand searched references of included trials to identify additional studies. This search was updated in May 2018 and May 2019 to include trials that may have been published since the initial search. We placed no restrictions on language of publication. See Data Supplement 1 for the detailed list and search strategies for each database.
Included studies
Two authors (EB, AA) independently conducted title and abstract screening. Differences between the two initial reviewers regarding inclusion of studies were resolved by consensus or review with a third author (MDH). We included individual- and cluster-level RCTs and non-randomized quasi-experimental studies of acute coronary syndrome quality improvement interventions. We included a variety of interventions including audit and feedback reporting systems, admission and discharge checklists, chart case management, patient educational or behavioral change materials, health care quality training that are directed as the hospital system, doctors, nurses, or allied health professionals, or information management systems with the goal of being inclusive in the type and target of intervention. The classifications of the included study settings into high-, middle-, and low-income were made based on the World Bank’s Atlas calculation methods using gross national income per capita.5
Study outcomes
We included a combination of clinical outcomes and process of care measures for our outcomes. The co-primary outcomes included rates of: 1) in-hospital major adverse cardiovascular events (fatal and non-fatal myocardial infarction, fatal and non-fatal stroke, and major bleeding, combined and separate), 2) reperfusion for patients with ST-segment elevation myocardial infarction (STEMI), and 3) in-hospital and discharge medical therapy including anti-platelets, anticoagulants, beta-blockers, and statins (combined and separate). Secondary outcomes included: 1) time from hospital presentation to initial electrocardiogram (ECG), 2) time to reperfusion (STEMI only), 3) 30-day and 1-year major adverse cardiovascular events. We also attempted to evaluate rates of behavioral counseling for diet, activity, and tobacco cessation, uptake of quality improvement intervention components, patient-level health related quality of life, patient-related costs as additional secondary outcomes; however, we did not identify any studies that reported these outcomes.
Data extraction
Data extraction, risk of bias assessment, and quality of evidence assessments were performed in duplicate by two authors (EB, AA) using standardized forms. Differences were resolved by consensus or review with a third author (MDH). The risk of bias assessment was performed using the Cochrane Risk of Bias Tool across the domains of selection, performance, detection, attrition, reporting, and other biases. The quality of evidence assessment was performed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework checklist, which accounts for issues related to internal validity (risk of bias, inconsistency, imprecision, publication bias) and to external validity, such as directness of results.6
Statistical analyses
This systematic review presents a qualitative, narrative synthesis of data from both individual- and cluster-level RCTs and non-randomized quasi-experimental studies of acute coronary syndrome quality improvement interventions. We sought to perform a meta-analysis but did not do so because of substantial, unexplained heterogeneity across the different studies.
RESULTS
Summary of included studies
Figure 1 demonstrates the PRISMA flowchart. After de-duplication, we identified 5,858 records to screen using our search methods. We excluded 5,727 studies through title/abstract screening and reviewed the full texts of the 131 remaining studies. We excluded 94 records after full text review. 5 of the 37 studies that met the inclusion criteria were ongoing trials and thus we present 32 studies in this systematic review. Among the included studies, we identified 14 RCTs (2 individual level and 12 cluster level) consisting of 109,763 patients (Table 1).7–20 We also identified and included 18 studies (n=54,423) that used either a non-randomized controlled or uncontrolled quasi-experimental study design (Data Supplements 2 and 3).21–38 22 of the 32 studies were conducted in high-income countries including 10 in the U.S, 5 in Australia, 6 in Western Europe, and 1 in Canada; while the remaining 10 studies were conducted in middle-income countries including 3 in China, 3 in India, 2 in Taiwan, 1 in Brazil, and 1 in Iran.
Table 1.
Summary of included randomized controlled trials.
| Study | Setting | N | Population | Intervention and comparator | 1° and key 2° outcomes |
|---|---|---|---|---|---|
| Bailey 2007 2000-20017 |
Patient-level; Missouri, USA |
I: 488 patients C: 365 patients TP: 853 |
AMI |
Intervention: Pharmacist-led recommendations for secondary prevention at discharge. Comparator: Usual care |
Guideline directed discharge medications |
| Berner 2007 1999-20008 |
Cluster; Alabama, USA |
I1: 7 hospitals I2: 8 hospitals C: 6 hospitals TP: 2,210 |
Unstable angina |
Intervention1: QI intervention developed and implemented by opinion leaders. Intervention 2: General blinded hospital performance feedback Comparator: Usual care |
ECG within 20 minutes after arrival, guideline directed in-hospital and discharge medications |
| Berwanger 2012 2011-20129 |
Cluster; urban hospitals in Brazil |
I:19 hospitals C: 17 hospitals TP: 1,150 |
AMI |
Intervention: Reminders, checklists, case management, and educational materials Comparator: Usual care |
Guideline directed in-hospital and discharge medications, in-hospital and 30-day MACE outcomes |
| Du 2014 2007-201010 |
Cluster; urban hospitals in China |
I: 32 hospitals C: 38 hospitals TP: 3,500 |
AMI |
Intervention: Clinical care pathway Comparator: Usual care |
Guideline directed discharge medications, reperfusion therapy, and in-hospital MACE |
| Flather 2012 2007-200911 |
Cluster; France, Italy, Poland, Spain, UK |
I: 19 hospitals C: 19 hospitals TP: 2,622 |
AMI |
Intervention: QI tools specific to individual hospitals led by senior cardiologists Comparator: Usual care |
Guideline directed In-hospital and discharge medications, risk stratification, coronary-angiography |
| Guenancia 2016 2012-201312 |
Multicenter patient-level; Burgundy, France |
I: 286 patients C: 286 patients TP: 572 |
NSTEMI |
Intervention: GRACE score + clinical assessment to guide clinical decision Comparator: Clinical assessment only |
Guideline directed in-hospital medications and in-hospital MACE. |
| Heller 2001 1996-199813 |
Cluster; New South Wales, Australia |
I: 19 hospitals C: 19 hospitals TP: 3,242 |
AMI, angina, chest pain |
Intervention: Educational sessions performance feedback Comparator: Educational session only |
Guideline directed in-hospital and discharge medications, coronary angiography, and echocardiography |
| Huffman 2018 2014-201716 |
Cluster; stepped wedge; Kerala, India |
I: 63 hospitals C: 63 hospitals TP: 21,374 |
AMI |
Intervention: Multi-component QI toolkit Comparator: Usual care |
30-day MACE, health-related quality of life, in-hospital and discharge medical therapy |
| Kinsman 2012 2008-200914 |
Cluster; rural Victoria, Australia |
I: 3 hospitals C: 3 hospitals TP: 108 |
AMI eligible for thrombolysis |
Intervention: Clinical pathways, reminders, education sessions, audit and feedback Comparator: Usual care |
Eligible AMI patients receiving thrombolysis, time to thrombolysis and ECG |
| Lytle 2015 2008-201015 |
Cluster; multiple participants in GWTG registry, USA |
I: 19 hospitals C: 17 hospitals TP: 19,579 |
STEMI or NSTEMI |
Intervention: Targeted feedback reports based on 3 lowest performing metrics Comparator: Standard performance feedback |
Guideline directed in-hospital and discharge medications, reperfusion therapy |
| Sauaia 2000 1994-199617 |
Cluster; Colorado, USA |
I: 9 hospitals C: 9 hospitals TP: 1,367 |
AMI |
Intervention: Written feedback + 2-hour on-site performance feedback Comparator: Written feedback only |
Guideline directed in-hospital and discharge medications, and reperfusion within 12 hours of arrival |
| Soumerai 1998 1992-199618 |
Cluster; Minnesota, USA |
I: 20 hospitals C: 16 hospitals TP: 5,347 |
AMI |
Intervention: Small and large group discussion, informal consultations revision of protocols, clinical pathways Comparator: Usual care |
Guideline directed in-hospital and discharge medications |
| Tu 2009 2001-200519 |
Cluster; Ontario, Canada |
I: 42 hospitals C: 39 hospitals TP: 18,492 |
AMI |
Intervention: Early feedback Comparator: Delayed feedback |
12 process of care indicators for AMI |
| Wu 2019 2011-201420 |
Cluster; stepped wedge; China | I: 101 hospitals C: 101 hospitals TP: 29,346 |
Final diagnosis of ACS |
Intervention: QI team, clinical pathways, education session, audit and feedback Comparator: Usual care |
In-hospital mortality, in-hospital MACE, 16 key performance indicators |
Total number of participants: 109,763
Abbreviations: I: intervention, C: comparator, TP: Total number of participants, MACE: major adverse cardiovascular events, RCT: randomized controlled trial, AMI: acute myocardial infarction, STEMI: ST-elevation myocardial infarction, NSTEMI: non-ST elevation myocardial infarction. GRACE: Global Registry of Acute Coronary Events, GWTG: Get with the Guidelines Registry. QI: quality improvement, 1°: primary, 2°: secondary
Risk of bias assessment of randomized controlled trials.
Summaries of trial specific risk of bias assessment and documentation supporting risk of bias assessment for included RCTs are listed in Data Supplement 4. Six of 14 RCTs had low risk of selection bias based on reported methods of sequence generation and or allocation concealment,9, 10, 14, 16, 19, 20 whereas eight studies had unclear or high risk of selection bias.7, 8, 11–13, 15, 17, 18 None of the 14 trials blinded the study personnel and thus had a high risk of performance bias. Though only one RCT20 blinded the outcome assessors, we determined there to be a low risk of detection bias for the remaining 13 RCTs given the objective nature of these outcomes, which are less likely to be influenced by unblinding of outcome assessors.39 We categorized nine trials as having low risk of attrition bias due to differential missingness across groups,7, 8, 10–12, 14, 16, 17, 20 while four trials were unclear risk of bias for this domain.13, 15, 18, 19 Six studies had low risk of reporting bias based on previously published protocols and adherence to those protocols,9, 10, 14–16, 20 while seven had unclear risk of reporting bias,7, 8, 11–13, 17, 18 and one study had high risk of reporting bias.19 We also identified four studies as having high risk of recruitment bias due to randomization at the cluster level with recruitment at the individual level.7, 12, 13, 18
Summary of findings by outcome
We summarized selected outcomes from individual studies of included randomized controlled trials in Table 2 and a comprehensive summary is listed in Data Supplement 5. We present a summary of findings in Table 3. Outcomes from non-randomized studies are summarized in Data Supplements 6–8.
Table 2.
Summary of outcomes of randomized controlled trials.
| Outcome | Trial | Event Rates, No (%) | Significance | ||
|---|---|---|---|---|---|
| Intervention | Comparator | *Effect (95% CI) | P-value | ||
| In-hospital MACE | Berwanger 20129 | 33 (5.5) | 38 (7.0) | OR: 0.72 (0.36, 1.43) | 0.35 |
| Du 201410 | 92 (5.8) | 122 (6.4) | RR: 1.12 (0.58, 2.14) | 0.74 | |
| Guenancia 201612 | 26 (9.1) | 31 (10.8) | OR: 1.59 (0.61, 4.17) | 0.49 | |
| Wu 201920 | 559 (3.8) | 655 (4.4) | aOR: 0.93 (0.75, 1.15) | NR | |
| In-hospital mortality | Berwanger 20129 | 29 (4.8) | 28 (5.1) | OR: 0.82 (0.37, 1.82) | 0.62 |
| Du 201410 | 41 (2.6) | 78 (4.1) | RR: 1.60 (0.97, 2.64) | 0.07 | |
| Guenancia 201612 | 6 (2.1) | 11 (3.8) | OR: 1.16 (0.68, 2.01) | NR | |
| Huffman 201816 | 321 (2.8) | 331 (3.3) | aOR: 0.98 (0.82, 1.17) | NR | |
| Rates of reperfusion for STEMI | Du 201410 | 290 (42.7) | 229 (31.8) | RR: 1.24 (0.98, 1.55) | 0.70 |
| Huffman 201816 | 4805 (71.0) | 5067 (73.2) | OR: 1.24 (1.06, 1.46) | NR | |
| Kinsman 201214 | Thrombolysis Baseline: 80% Post-intervention: 78% |
Thrombolysis Baseline: 96% Post-intervention: 84% |
NR | I: 0.86 C: 0.19 NR |
|
| Lytle 201515 | 730 (97.2) | 228 (94.2) | 0.03 | ||
| Sauaia 200017 | Baseline: 12 (55.0) Post-intervention: 9 (75.0) |
Baseline: 31 (84) Post-intervention: 4 (44) |
Control 6.5 times worse compared to baseline | I: 0.01 C: 0.02 |
|
| Tu 200919 | % change (95% CI): 6.7 (−0.8, 14.2) |
% change (95% CI): 7.2 (−0.5, 15.1) |
†Absolute % difference: 3.3 (−5.7, 12.4) |
0.47 | |
| Wu 201920 | 1414 (48.9) | 1683 (52.2) | aOR: −2.2 (−4.7, 0.30) | NR | |
| 30-day total mortality | Berwanger 20129 | 42 (7.0) | 46 (8.4) | OR: 0.79 (0.46, 1.34) | 0.38 |
| Huffman 201816 | 445 (3.9) | 509 (5.1) | aOR: 0.87 (0.75, 1.00) | NR | |
| Sauaia 200017 | Baseline: 81 (19.0) Post-intervention: 33 (15.0) |
Baseline: 85 (17.0) Post-intervention: 46 (22.0) |
NR | NR | |
| Tu 200919 | Absolute % change: (95% CI): −1.9 (−3.8, −0.1) |
Absolute % change: (95% CI): 0 (−2.3, 2.3) |
†Absolute % difference: (95% CI): −2.5 (−4.9, 0.1) | 0.50 | |
| 30-day MACE | Berwanger 20129 | 49 (8.1) | 55 (10.1) | OR: 0.76 (0.45, 1.27) | 0.30 |
| Huffman 201816 | 445 (3.9) | 645 (6.4) | OR: 0.92 (0.81, 1.04) | NR | |
Abbreviations: CI: confidence interval, STEMI: ST-elevation myocardial infarction, MACE: major adverse cardiovascular events, TP: total participants. NS: not significant, aOR: adjusted odds ratio. NR: not reported.
Odds ratios or adjusted odd ratios represent the effect estimates of the intervention compared with the control.
Absolute % differences represent the effect estimates using a difference in difference analysis.
Table 3.
Summary of outcomes and quality of evidence of randomized controlled trials.
| Hospital-based acute coronary syndrome quality improvement interventions vs. usual care | ||||
|---|---|---|---|---|
| Outcomes | Effect on outcome | Studies/total participants | Quality of the evidence | Comments |
| In-hospital MACE | Absolute in-hospital mortality ranged from 2.1%-4.8% in the intervention vs. 3.3%-5.1% in the control and the unadjusted mortality rates were 0.3%-1.7% lower in the intervention groups.9, 10, 12, 16, 20 | 5 RCTs TP: 55,942 |
MODERATE* | Unblinded studies* |
| Rates of reperfusion for STEMI | In five studies, absolute rate of reperfusion was 3%-10% higher in the intervention except for one outlier study with 31% higher rate in the intervention. Two studies showed no difference.10, 14–17, 19,20 | 7 RCTs TP: 93,659 |
MODERATE*,† | Unblinded studies* with some inconsistency† |
| Rates of in-hospital and discharge medical therapy | In-hospital medical therapy: Effect estimates ranged from no difference to 15.2% except one outlier study with 31% increase in the intervention vs. 9.1% in the control.8, 9, 11, 13, 16, 17, 19, 20 | 8 RCTs TP: 79,803 |
MODERATE*,† | Unblinded studies* with some inconsistency† |
| Discharge medical therapy: Effect estimates ranged from no difference to 7.2% higher in the intervention groups.7, 8, 9, 10, 11, 15, 16, 17, 18, 19, 20 | 11 RCTs TP: 100,511 |
MODERATE*,† | Unblinded studies* with some inconsistency† | |
| Door to ECG time | ‖One RCT showed 10% higher rate of ECGs done in time in the intervention group. One small sized study showed no difference between the intervention and control.14, 20 | 2 RCT TP: 29,454 |
LOW*,† | Unblinded study*, Unable to assess for inconsistency† |
| Door to any reperfusion for STEMI time | Two studies reported a 2%-7% higher rate of reperfusion under 90 min in the intervention group. Three studies reported no difference in reperfusion time between groups. 10, 14, 15, 16, 20 | 5 RCTs TP: 73,908 |
LOW*,† | Unblinded studies* Significantly variable in outcome measures† |
| 30-day MACE | The effect estimates ranged from 3.9%-15% and 5.1%-22% in the intervention and control groups respectively.9, 16, 17, 19 | 4 RCTs TP: 42,384 |
LOW*,†,‡ | Unblinded studies*, Significant inconsistency in estimates† |
Downgraded due to study limitations.
Downgraded due to inconsistency.
Downgraded due to imprecision
GRADE Working Group grades of evidence.6 High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect.
ECG done in time means the patient received the first ECG within 10 minutes of hospital arrival.
In-hospital major adverse cardiovascular events
Five RCTs (n=55,942) assessed the effect of hospital-based quality improvement interventions on in-hospital major adverse cardiovascular events (MACE) consisting of fatal and non-fatal myocardial infarction, fatal and non-fatal stroke, and major bleeding, combined and separate).9, 10, 12, 16, 20 The overall absolute rate of in-hospital mortality ranged from 2.1%-4.8% in the intervention groups compared to 3.3%-5.1% in the control groups. The unadjusted mortality rates were 0.3%-1.7% lower in the intervention groups compared to the control (Table 2). In comparison, seven non-randomized quasi-experimental studies (n=42,013) showed an absolute event rate reduction in in-hospital mortality ranging from 0.2%-13% post intervention (Data Supplements 6–8).21, 23, 31, 34, 36–38
Rates of reperfusion for STEMI
Seven RCTs (n=93,659) assessed the effect of hospital-based acute coronary syndrome quality improvement interventions on rates of reperfusion for patients with STEMI.10, 14–17, 19 Five RCTs (n=64,313) showed an overall 3.0%−31.0% higher absolute rate of reperfusion in the intervention groups compared to the control groups.10, 15–17, 19 Two RCTs (n=29,454) showed no difference between the intervention and control groups.14, 20 One17 of the five RCTs (n=1,367) was an outlier with 31% higher rate in reperfusion in the intervention group compared to the other four,10, 15, 16, 19, 20 (n=62,946) which showed a 3.0%−10.9% higher rate of reperfusion in the intervention groups (Table 2). Five non-randomized controlled and uncontrolled quasi-experimental studies (n=28,083) showed no increase in rates of reperfusion post-intervention (Data Supplements 6–8).21, 27, 36–38
Rates of in-hospital and discharge medical therapy
Table 3 and Data Supplement 5 describe the results for in-hospital and discharge medical therapy. Eight RCTs (n= 79,803) 8, 9, 11, 13, 16, 17, 19, 20 evaluated in-hospital aspirin, beta blocker, and anticoagulant use. The effect estimates reported in seven studies ranged from no difference to 15.2% higher rates in the intervention, and one outlier RCT8 (n=2,210) showed a substantially larger effect on in-hospital aspirin and anticoagulation therapy in the intervention group. Eleven RCTs (100,511)7–11, 15–20 evaluated discharge medical therapy including aspirin, beta-blocker, statin, and angiotensin converting enzyme-inhibitor/angiotensin receptor blockers (ACE-I/ARB). The effect estimates from nine trials ranged from no difference to 7.2% higher rates in the intervention groups, and one outlier RCT18 (n=5,347) showed a substantially larger effect on discharge aspirin and beta blocker use in the intervention group. One RCT10 (n=3,500) reported combined recommended discharged therapies showed a 11% absolute higher rate in the intervention compared to control (unadjusted RR (95% CI) 1.23 (1.06, 1.42); P=0.007).
In contrast, the results from non-randomized quasi-experimental studies showed a 2.6%-25% increase of in-hospital medical therapy and a 2.0%-80.0% increase in discharge medical therapy with most studies reporting a greater than 10% increase in in-hospital or discharge medical therapy post intervention (Data Supplements 6–8).22–24, 27, 30, 33, 37, 38
Hospital presentation to ECG time
One RCT (n=29,346)20 showed 10% higher rate of ECGs completed in time, i.e. within 10 minutes after arrival, in the intervention group compared to the control (adjusted OR: 1.12 (0.90, 1.39)) while another RCT (n=108) showed no difference in door to ECG time between the intervention and control groups (Data Supplement 5).14 Four non-randomized quasi-experimental studies21, 25, 31, 38 (n= 5,058) showed minimal differences.
Door to any reperfusion time for STEMI patients
Five RCTs (73,908) evaluated door to any reperfusion time for STEMI patients.10, 14–16, 20 Three RCTs 10, 14, 16 (n=24,983) reported no difference in mean or median door to balloon time, while two RCT15, 20 (n=48,925) showed an absolute 2.0%-7% higher rate of reperfusion in less than 90 minutes in the intervention groups compared to the control groups. In contrast, 7 non-randomized quasi-experimental studies21, 25, 26, 31, 33, 34, 37, 38 (n=7,039) showed a significant reduction in door to any reperfusion time or an increase in rates of reperfusion within 60 minutes of presentation (Data Supplements 6–8).
30-day and 1-year major adverse cardiovascular events
Four RCTs (n=42,384) reported 30-day mortality rates of 3.9%-15% in the intervention groups compared to the 5.1%-22.0% in the control groups 9, 16, 17, 19 The 30-day mortality rates from the more recent three RCTs9, 16, 19 are comparable and less than 10% in comparison to one RCT which reported a markedly higher 15% and 22% 30-day mortality rates in the intervention and control groups respectively. This relatively small RCT (n=1,397) was completed between 1994-1996 and the lower 30-day mortality rates in the more recent trials may be a reflection of time trends in improvements in clinical outcomes of acute coronary syndrome due to better clinical management. One non-randomized quasi-experimental study (n= 420) showed a 2.5% reduction of total 30-day mortality.34 No RCTs reported differences in 1-year major adverse cardiovascular event rates. In contrast, four non-randomized quasi-experimental studies (n= 14,824) showed a 1.2%-4.0% lower mortality rate at 1 year in the intervention groups (Data Supplements 6–8).22, 24, 30, 34
Ten of the fourteen RCTs were conducted in high-income countries and four were conducted in middle-income countries. Overall, there were no consistent significant differences in the effect estimates on clinical outcomes and process of care measures between the high-income and middle-income countries. Additionally, twelve out of the eighteen quasi-experimental studies were conducted in high-income countries while the remaining were in middle-income countries. There were significant variabilities in the representation of high-income vs. middle-income countries for each study outcome and together with the inconsistencies in the effect estimates, they limit the ability to confidently assess differences in outcomes by country income status from the quasi-experimental studies.
Study quality assessment
Table 3 describes the outcome-specific quality of evidence assessment for RCTs. We graded the quality of evidence moderate for four out of the seven outcomes and low or very low for the remainder, downgrading because of study limitations and between-study heterogeneity. We also present the quality of evidence for nonrandomized quasi-experimental studies in Data Supplement 8, which were considered very low given study designs, study limitations, and heterogeneity.
DISCUSSION
This systematic review is the first, to our knowledge, of RCTs and non-randomized quasi-experimental studies on hospital-based quality improvement interventions for patients with acute coronary syndrome on clinical outcomes and process of care measures. There was substantial heterogeneity across studies in the types of in-hospital quality improvement interventions studied, which limited our ability to identify what types of interventions were most efficacious. Despite a large number of RCTs that reported on the primary outcomes, the heterogeneity in how the results are reported limited the ability to perform a pooled analysis and thus, we present a qualitative analysis of the data.
Overall, we found the quality of the evidence from randomized controlled trials moderate to low, which showed modest to no effect of the interventions studied on clinical outcomes, including in-hospital and 30-day mortality and combined major adverse cardiovascular events. In contrast, non-randomized studies demonstrated larger, but overall modest effect sizes on clinical outcomes. Similarly, RCTs showed modest to no effect of the interventions on rates of reperfusion for STEMI patients and rates of guideline directed in-hospital and discharge medical therapy, although overall the effects sizes were higher compared to the effects on clinical outcomes. Non-randomized quasi-experimental studies showed a greater effect size on process of care outcome measures in comparison to randomized controlled trials, although with greater inconsistency in the size of the effect estimates. Overall, both randomized and non-randomized studies demonstrated larger effect estimates for process of care measures compared with clinical outcomes. Only 13 out of 32 studies (7 RCTs and 6 non-randomized studies) reported clinical outcome measures and only 4 RCTs reported the primary clinical outcomes of this review (i.e. in-hospital mortality and in-hospital MACE). The evidence base for acute coronary syndrome quality improvement interventions on clinical outcomes could be improved if future studies include clinical outcomes measures more consistently to help identify and test which interventions may have greater impact on clinical outcomes. There was a significant heterogeneity in the interventions included in this review, ranging from education programs, targeted performance feedback, clinical pathways, and audits among others, which did not allow to assess specific interventions that are potentially more efficacious than others. One important area of future research will be process evaluation of existing interventions to better understand which interventions might be more effective in different clinical settings.
A range of high-income, high- and low- middle-income countries were represented in this review, both in the randomized and non-randomized studies. There was no significant difference in the effect estimates with the various interventions studied between high-income and middle-income countries including Brazil, China, and India. However, low-income settings, including countries in sub-Saharan Africa, are not well represented in the studies included in this systematic review. Despite the growing burden of ischemic heart disease, there is minimal understanding on implementation and utilization of evidence-based acute coronary syndrome management in low-income countries.3 Low-income countries may potentially have higher gain, both in clinical and process of care outcome measures, from acute coronary syndrome quality improvement interventions compared to middle- or high-income countries that typically have higher baseline use of guideline-directed management and lower event rates. Therefore, having more low-income countries represented in future clinical trials could help understand which clinical settings may benefit the most from quality improvement interventions.
Implementation of acute coronary syndrome quality improvement interventions in the context of low-income countries, additional to process of care and outcome evaluations, need to also consider structural interventions, including at the health worker (e.g. adequate staffing and training), hospital (e.g. functioning diagnostic and treatment equipment), and pre-hospital (e.g. available emergency response system) levels to enhance performance. Evidence from a systematic review that assessed strategies to improve health-care provider performance in low-and middle-income countries shows that the efficacy of strategies to improve health-care provider performance in low resource settings was highly variable. The effect estimates were the largest for multifaceted strategies that incorporated several elements including improving infrastructure, training and group problem solving, and emphasizes the need for future research to generate better evidence using standardized methodologies of outcome analysis and robust study designs including randomized controlled trials.39
Strengths and limitations
There are several strengths to this systematic review, including providing a summary of multiple experimental study designs. The concurrent summary of evidence from RCTs and non-randomized quasi-experimental studies also allows for comparison of the evidence between the different study designs. Title screening, data extraction, and quality assessments were performed in duplicate to minimize error and a pre-specified protocol prior to the initiation of review was published to guide the search strategy and minimize the risk of bias.
This review also has limitations. First, the study duration of the RCTs may not have been implemented long enough to observe changes in health systems, culture and attitude from providers and administrators that could influence implementation of interventions, quality of care and outcomes. Although this review included a wide range of countries of varying economic status, there remains underrepresentation of low-income countries, which limits the generalizability of the findings to those settings potentially most in need of health system strengthening. Understanding effective elements of quality improvement interventions is important to improve quality and safety of acute coronary syndrome in diverse clinical and resource settings. Second, the process of performing RCTs of quality improvement interventions could have led to improvements in baseline care through a Hawthorne effect. Third, the review tried to synthesize complex interventions, which may not be possible.
CONCLUSIONS
This systematic review demonstrates that RCTs of hospital-based acute coronary syndrome quality improvement interventions have a modest effect on process of care measures but not on clinical outcomes. Overall, non-randomized quasi-experimental studies showed larger effect sizes compared to randomized clinical trials particularly for process of care measures. Understanding which components of quality improvement interventions are more effective and their role in low-resource settings, which were largely not included in these trials, would be important future directions. Further research will also help to identify key components for contextualizing successful acute coronary syndrome quality improvement interventions to new settings.
Supplementary Material
What is known:
-
§
Hospital-based quality improvement programs have been implemented to improve quality of care for acute coronary syndrome, particularly in high-income country settings.
-
§
The evidence base evaluating the efficacy of these programs on process of care measures and clinical outcomes has largely been derived from non-randomized studies, though randomized controlled trials of quality improvement interventions have been undertaken more recently.
What this study adds:
-
§
This systematic review synthesizes the evidence base for hospital-based acute coronary syndrome quality improvement interventions on process of care measures and clinical outcomes.
-
§
Randomized trial data show more modest effects on process measures like reperfusion rates and medication use compared with non-randomized studies without clear effects on improving clinical outcomes.
-
§
This study demonstrates substantial heterogeneity across study reports, expected differences in effect size by study type, and estimated direction and magnitude of effects, which are relevant to settings where quality improvement programs may be newly implemented, including low- and middle-income countries.
Acknowledgments
Sources of funding: National Institutes of Health/Fogarty International Center: R25TW009345
Disclosures: MDH receives funding from the World Heart Federation to serve as its senior program advisor for the Emerging Leaders program, which is supported by Boehringer Ingelheim and Novartis with previous support from BUPA and AstraZeneca. MDH also receives support from the American Heart Association, Verily, AstraZeneca and American Medical Associations for work unrelated to this research. The remaining authors have no disclosures.
Footnotes
Registration: https://www.crd.york.ac.uk/PROSPERO/ ; CRD42016047604
References:
- 1.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JHY, Alger HM, Wong SS, Muntner P. Heart Disease and Stroke Statistics‚ 2018 Update: A Report From the American Heart Association. Circulation. 2018;137:1–426. [DOI] [PubMed] [Google Scholar]
- 2.Masoudi FA, Ponirakis AP, De Lemos JA, Jollis JG, Kremers M, Messenger JC, Moore JW, Moussa I, Oetgen WJ, Varosy PD, Vincent RN, Wei J, Curtis JP, Roe MT, Spertus JA. Trends in U.S. cardiovascular care: 2016 Report from 4 ACC National Cardiovascular Data Registries. J Am Coll Cardiol 2017;69:1427–1450. [DOI] [PubMed] [Google Scholar]
- 3.Vedanthan R, Seligman B, Fuster V. Global perspective on acute coronary syndrome: a burden on the young and poor. Circ Res 2014;114:1959–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.PROSPERO International Prospective Register of Systematic Reviews. Hospital-based quality improvement interventions for patients with acute coronary syndrome: a systematic review. CRD42016047604. Available at: www.crd.york.ac.uk/PROSPERO/DisplayPDF.php?ID=CRD42016047604 Accessed on November 23 2018.
- 5.World Bank Country and Lending Groups. Available at: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups Accessed on June 24 2018.
- 6.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schunemann HJ, GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bailey TC, Noirot LA, Blickensderfer A, Rachmiel E, Schaiff R, Kessels A, Braverman A, Goldberg A, Waterman B, Dunagan WC. An intervention to improve secondary prevention of coronary heart disease. Arch Intern Med 2007;167:586–90. [DOI] [PubMed] [Google Scholar]
- 8.Berner ES, Baker CS, Funkhouser E, Heudebert GR, Allison JJ, Fargason CA Jr., Li Q, Person SD, Kiefe CI. Do local opinion leaders augment hospital quality improvement efforts? A randomized trial to promote adherence to unstable angina guidelines. Med Care. 2003;41:420–31. [DOI] [PubMed] [Google Scholar]
- 9.Berwanger O, Guimaraes HP, Laranjeira LN, Cavalcanti AB, Kodama AA, Zazula AD, Santucci EV, Victor E, Tenuta M, Carvalho V, Mira VL, Pieper KS, Weber B, Mota LH, Peterson ED, Lopes RD. Effect of a multifaceted intervention on use of evidence-based therapies in patients with acute coronary syndromes in Brazil: the BRIDGE-ACS randomized trial. JAMA. 2012;307:2041–9. [DOI] [PubMed] [Google Scholar]
- 10.Du X, Gao R, Turnbull F, Wu Y, Rong Y, Lo S, Billot L, Hao Z, Ranasinghe I, Iedema R, Kong L, Hu D, Lin S, Shen W, Huang D, Yang Y, Ge J, Han Y, Lv S, Ma A, Gao W, Patel A, CPACS-2 Investigators. Hospital quality improvement initiative for patients with acute coronary syndromes in China: a cluster randomized, controlled trial. Circ Cardiovasc Qual Outcomes. 2014;7:217–26. [DOI] [PubMed] [Google Scholar]
- 11.Flather MD, Babalis D, Booth J, Bardaji A, Machecourt J, Opolski G, Ottani F, Bueno H, Banya W, Brady AR, Bojestig M, Lindahl B. Cluster-randomized trial to evaluate the effects of a quality improvement program on management of non-ST-elevation acute coronary syndromes: The European Quality Improvement Programme for Acute Coronary Syndromes (EQUIP-ACS). Am Heart J. 2011;162:700–707 [DOI] [PubMed] [Google Scholar]
- 12.Guenancia C, Stamboul K, Hachet O, Yameogo V, Garnier F, Gudjoncik A, Cottin Y, Lorgis L. Clinical effectiveness of the systematic use of the GRACE scoring system (in addition to clinical assessment) for ischaemic outcomes and bleeding complications in the management of NSTEMI compared with clinical assessment alone: a prospective study. Heart Vessels. 2016;31:897–906. [DOI] [PubMed] [Google Scholar]
- 13.Heller RF, D’Este C, Lim LL, O’Connell RL, Powell H. Randomised controlled trial to change the hospital management of unstable angina. Med J Aust. 2001;174:217–21. [DOI] [PubMed] [Google Scholar]
- 14.Kinsman LD, Rotter T, Willis J, Snow PC, Buykx P, Humphreys JS. Do clinical pathways enhance access to evidence-based acute myocardial infarction treatment in rural emergency departments? Aust J Rural Health. 2012;20:59–66. [DOI] [PubMed] [Google Scholar]
- 15.Lytle BL, Li S, Lofthus DM, Thomas L, Poteat JL, Bhatt DL, Cannon CP, Fonarow GC, Peterson ED, Wang TY, Alexander KP. Targeted versus standard feedback: Results from a randomized quality improvement trial. Am Heart J. 2015;169:132–141. [DOI] [PubMed] [Google Scholar]
- 16.Huffman MD, Mohanan PP, Devarajan R, Baldridge AS, Kondal D, Zhao L, Ali M, Krishnan MN, Natesan S, Gopinath R, Viswanathan S, Stigi J, Joseph J, Chozhakkat S, Lloyd-Jones DM, Prabhakaran D. Effect of a Quality Improvement Intervention on Clinical Outcomes in Patients in India With Acute Myocardial Infarction: The ACS QUIK Randomized Clinical Trial. JAMA. 2018;319:567–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sauaia A, Ralston D, Schluter WW, Marciniak TA, Havranek EP, Dunn TR. Influencing care in acute myocardial infarction: a randomized trial comparing 2 types of intervention. Am J Med Qual 2000;15:197–206. [DOI] [PubMed] [Google Scholar]
- 18.Soumerai SB, McLaughlin TJ, Gurwitz JH, Guadagnoli E, Hauptman PJ, Borbas C, Morris N, McLaughlin B, Gao X, Willison DJ, Asinger R, Gobel F. Effect of local medical opinion leaders on quality of care for acute myocardial infarction: a randomized controlled trial. JAMA. 1998;279:1358–63. [DOI] [PubMed] [Google Scholar]
- 19.Tu JV, Donovan LR, Lee DS, Wang JT, Austin PC, Alter DA, Ko DT. Effectiveness of public report cards for improving the quality of cardiac care: the EFFECT study: a randomized trial. JAMA. 2009;302:2330–7. [DOI] [PubMed] [Google Scholar]
- 20.Wu Y, Li S, Patel A, Li X, Du X, Wu T, Zhao Y, Feng L, Billot L, Peterson ED, Woodward M, Kong L, Huo Y, Hu D, Chalkidou K, Gao R. Effect of a Quality of Care Improvement Initiative in Patients With Acute Coronary Syndrome in Resource-Constrained Hospitals in China: A Randomized Clinical Trial. JAMA Cardiol 2019;4:418–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alexander T, Mullasari AS, Joseph G, Kannan K, Veerasekar G, Victor SM, Ayers C, Thomson VS, Subban V, Gnanaraj JP, Narula J, Kumbhani DJ, Nallamothu BK. A system of care for patients with ST-segment elevation myocardial infarction in India. JAMA Cardiol 2017;2:498–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aziz EF, Javed F, Pulimi S, Pratap B, De Benedetti Zunino ME, Tormey D, Hong MK, Herzog E. Implementing a pathway for the management of acute coronary syndrome leads to improved compliance with guidelines and a decrease in angina symptoms. J Healthc Qual 2012;34:5–14. [DOI] [PubMed] [Google Scholar]
- 23.Carlhed R, Bojestig M, Wallentin L, Lindstrom G, Peterson A, Aberg C, Lindahl B, Quality Improvement in Coronary Care Study Group. Improved adherence to Swedish national guidelines for acute myocardial infarction: the Quality Improvement in Coronary Care (QUICC) study. Am Heart J. 2006;152:1175–81. [DOI] [PubMed] [Google Scholar]
- 24.Carlhed R, Bojestig M, Peterson A, Aberg C, Garmo H, Lindahl B, Quality Improvement in Coronary Care Study Group. Improved clinical outcome after acute myocardial infarction in hospitals participating in a Swedish quality improvement initiative. Circ Cardiovasc Qual Outcomes. 2009;2:458–64. [DOI] [PubMed] [Google Scholar]
- 25.Chen KC, Yen DHT, Chen CD, Young MS, Yin WH. Effect of emergency department in-hospital tele-electrocardiographic triage and interventional cardiologist activation of the infarct team on door-to-balloon times in st-segment-elevation acute myocardial infarction. Am J of Cardiol 2011;107:1430–1435. [DOI] [PubMed] [Google Scholar]
- 26.Dai X, Meredith D, Sawey E, Kaul P, Smith SC Jr, Stouffer GA. a quality improvement program for recognition and treatment of inpatient ST-segment elevation myocardial infarctions. JAMA Cardiol 2016;1:1077–2. [DOI] [PubMed] [Google Scholar]
- 27.Ellerbeck EF, Kresowik TF, Hemann RA, Mason P, Wiblin RT, Marciniak TA. Impact of quality improvement activities on care for acute myocardial infarction. Int J Qual Health Care. 2000;12:305–10. [DOI] [PubMed] [Google Scholar]
- 28.Fakhr-Movahedi A, Soleimani M, Ghazvininejad R, Maher MK, Ghorbani R. Effect of patient-focused clinical pathway on anxiety, depression and satisfaction of patients with coronary artery disease: a quasi-experimental study. Iran Red Crescent Med J. 2015;17:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuo F-Y, Huang W-C, Chiou K-R, Mar G-Y, Cheng C-C, Chung C-C, Tsai H-L, Jiang C-H, Wann S-R, Lin S-L, Liu C-P. The effect of failure mode and effect analysis on reducing percutaneous coronary intervention hospital door-to-balloon time and mortality in ST segment elevation myocardial infarction. BMJ Qual Saf 2013;22:626–638. [DOI] [PubMed] [Google Scholar]
- 30.Fonarow GC, Gawlinski A, Watson K. In-hospital initiation of cardiovascular protective therapies to improve treatment rates and clinical outcomes. Crit Path Cardiol 2003;2:61–70. [DOI] [PubMed] [Google Scholar]
- 31.Khot UN, Johnson ML, Ramsey C, Khot MB, Todd R, Shaikh SR, Berg WJ. Emergency department physician activation of the catheterization laboratory and immediate transfer to an immediately available catheterization laboratory reduce door-to-balloon time in ST-elevation myocardial infarction. Circulation. 2007;116:67–76. [DOI] [PubMed] [Google Scholar]
- 32.Lai C-L, Fan C-M, Liao P-C, Tsai K-C, Yang C-Y, Chu S-H, Chien K-L. Impact of an audit program and other factors on door-to-balloon times in acute ST-elevation myocardial infarction patients destined for primary coronary intervention. Acad Emerg Med 2009;16:333–342. [DOI] [PubMed] [Google Scholar]
- 33.Prabhakaran D, Jeemon P, Mohanan PP, Govindan U, Geevar Z, Chaturvedi V, Reddy KS. Management of acute coronary syndromes in secondary care settings in Kerala: Impact of a quality improvement programme. Natl Med J India 2008;21:107–111. [PubMed] [Google Scholar]
- 34.Scholz KH, Maier SKG, Jung J, Fleischmann C, Werner GS, Olbrich HG, Ahlersmann D, Keating FK, Jacobshagen C, Moehlis H, Hilgers R, Maier LS. Reduction in treatment times through formalized data feedback. JACC Cardiovasc Interv 2012;5:848–857. [DOI] [PubMed] [Google Scholar]
- 35.Robinson MB, Thompson E. Evaluation of the effectiveness of guidelines, audit and feedback: improving the use of intravenous thrombolysis in patients with suspected acute myocardial infarction. Int J Qual Health Care. 2005;8:211–222. [DOI] [PubMed] [Google Scholar]
- 36.Scott IA, Eyeson-Annan ML, Huxley SL,West MJ. Optimising care of acute myocardial infarction: results of a regional quality improvement project. J Qual Clin Pract 2000;20:12–9. [DOI] [PubMed] [Google Scholar]
- 37.Scott IA, Coory MD, Harper CM. The effects of quality improvement interventions on inhospital mortality after acute myocardial infarction. Med J Aust 2001;175:465–70. [DOI] [PubMed] [Google Scholar]
- 38.Scott IA. Optimising care of acute coronary syndromes in three Australian hospitals. Int J Qual Health Care. 2004;16:275–284. [DOI] [PubMed] [Google Scholar]
- 39.Rowe AK, Rowe SY, Peters DH, Holloway KA, Chalker J, Ross-Degnan D. Effectiveness of strategies to improve health-care provider practices in low-income and middle-income countries: a systematic review. Lancet Glob Health. 2018;6:e1163–e1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


