Abstract
Musculoskeletal pain is a cause of disability in older individuals and is commonly associated with executive function deficits. In particular, verbal fluency deficits have been previously reported in older individuals with and without musculoskeletal pain, however, no studies have examined non-verbal fluency. The present study investigated non-verbal fluency performance in younger and older individuals and associations with clinical and experimental pain. The NEPAL study included older (n=63) and younger (n=28) individuals who completed demographic, and clinical pain assessments followed by a multi-modal QST battery. A subset of participants (older n=39/63, younger n=1½8) underwent a structural 3T MRI to extract cortical thickness and subcortical gray matter volumes. The Ruff Figural Fluency Test was administered to assess fluid/divergent thinking, ability to shift cognitive set, and planning strategies. Total Unique Designs drawn and Error Ratio assessed participants’ ability to minimize repetition while maximizing unique productions. Adjusting for race and education, older participants with chronic pain had significantly lower Total Unique Designs (67.1 ± 20.3) compared to older adults without chronic pain (78.8 ± 15.9) and younger controls (93.8 ± 20.3, p<0.001). Within the older sample, those with chronic pain had a significantly greater Error Ratio (0.22 ± 0.3) compared to those without chronic pain (0.09 ± 0.06) and younger controls (0.05 ± 0.05, p=0.002). In older participants, greater Total Unique Design scores were significantly associated only with lower pressure pain sensitivity (r = 0.300, p = 0.031) while greater Error Ratio scores were significantly associated with greater thermal pain sensitivity (r = 0.304, p = 0.027). However, after accounting for sleep quality, clinical and experimental pain associations were eliminated. Across all participants, non-verbal fluency performance was associated with cortical thickness in frontal, parietal and temporal regions as well as several subcortical gray matter structures even after adjusting for multiple comparisons (p’s<0.001). Our findings suggest a pain-related deficit in non-verbal fluency beyond the established age-related decrements that may be dependent on sleep quality and was associated with specific patterns of gray matter structure.
Keywords: Pain, age, fluency, cognition, executive function, brain structure, gray matter
Introduction
It is widely known that pain has the ability to interrupt and demand attention (Eccleston & Crombez, 1999), impacting physical and cognitive function. A number of characteristics of pain contribute to this phenomenon including pain intensity, predictability, novelty, and environmental factors among others (Eccleston & Crombez, 1999). Acute pain, or pain that persists less than three months, has been known to negatively impact cognition (Mather et. al, 2015, Seminowicz & Davis, 2007). Chronic pain persisting over three months, may have the ability to further hinder cognitive and physical performance, which may be supported by previous studies reporting lower cognitive function in persons with chronic pain (Dick & Rashiq, 2007, Oosterman et al., 2011). Specifically, it has been found that pain interferes with, and is a distraction from, cognitive tasks, both verbal and non-verbal (Eccleston, 1994).
It is known that aging confers psychological and biological changes that can vary greatly from person to person. Older individuals may not display the same age-related changes in cognition across different cognitive domains (Glisky, 2007). In particular, cognitive fluency, part of executive function, changes across the lifespan (Tomer et al., 1993; Crossley et al., 1997). Fluency has been defined as one’s ability to utilize one or more strategies to avoid or minimize response repetition, while maximizing response production, with verbal (i.e., semantic category naming and letter fluency) and non-verbal fluency (i.e., semantic category drawing and design fluency) domains. Verbal fluency has been assessed frequently over the years and has been shown to reveal cognitive impairments among many populations (Tombaugh et al., 1999). Verbal fluency has been demonstrated to be sensitive to lesions in the frontal lobe, temporal lobe, and caudate nucleus; Alzheimer’s disease; Huntington’s disease; amnesia, and traumatic brain injury (Tombaugh et al., 1999). Evidence suggests that verbal fluency measures are sensitive to the effect of years of education and age, but are relatively insensitive to gender (Tombaugh et al., 1999). However, very few studies have investigated non-verbal fluency specifically in older individuals. The Ruff Figural Fluency Test (RFFT) was developed to assess this domain of executive functioning (Ruff et al., 1987). It requires the subject to create as many unique designs as possible, while minimizing repetition of designs. This plays into different areas of executive function such as planning strategies, fluid and divergent thinking skills, and ability to shift cognitive set (Ruff et al., 1987).
Given the negative reciprocal interactions between pain and executive function along with the known age-related decrement in executive functions, the present study aimed to elucidate age and pain-related differences in non-verbal fluency and its underlying structural brain correlates. We tested the hypothesis that older adults would have worse performance on a measure of non-verbal fluency (i.e., RFFT) when compared to young, healthy controls. We also tested the hypothesis that the older individuals who experienced chronic pain (i.e., pain over 3 months in duration) would have significantly poorer non-verbal fluency performance when compared to older individuals with no chronic pain and that poorer non-verbal fluency performance would be significantly associated with greater clinical and experimental pain sensitivity using Quantitative Sensory Testing. Finally, we sought to explore non-verbal fluency task performance associations with gray matter cortical thickness and subcortical volumes.
Patients and Methods
This is a secondary data analyses including ninety-one individuals (ages 18–26 and 60–93 years of age) enrolled as part of the screening process for an ongoing project at the University of Florida (UF) studying pain, aging and mobility function (Neuromodulatory Examination of Pain and Mobility Across the Lifespan [NEPAL]). Participants were recruited through posted fliers, print ads, and word of mouth referrals between January 2016 and January 2018. The study was approved by the UF Institutional Review Board (IRB) and reviewed bi-annually by the UF Pepper Center Data Safety Monitoring Board. Participants were screened over the phone and excluded if they reported 1) uncontrolled hypertension (blood pressure >150/95 mm Hg), heart failure, or history of acute myocardial infarction; 2) systemic rheumatic disorders (i.e., rheumatoid arthritis, systemic lupus erythematosus, fibromyalgia); 3) excessive anxiety regarding protocol procedures; 4) hospitalization within the preceding year for psychiatric illness; 5) reports at any time in their life of a diagnosis of schizophrenia, bipolar disorder, a major depressive episode; or 6) inability to consent for study participation. Older adults were enrolled as long as they met study inclusion/exclusion criteria and they were later categorized into pain groups based on their responses to the pain questions at the Health Assessment Session (HAS). Participants attended separate HAS, Quantitative Sensory Testing (QST) and Neuroimaging sessions. At the HAS, individuals were further excluded if they exhibited significant cognitive impairment as assessed by 3MS score < or = 77 (Teng & Chui, 1987).
Health Assessment Session
Upon verbal and written informed consent, participants completed general health and demographic information questionnaires. The following instruments were also administered during this session to assess domains related to self-reported pain, cognitive and other potential covariates:
Self-reported Pain:
Consistent with the consensus of the Task Force for the Classification of Chronic Pain for the 11th version of the International Classification of Diseases (ICD-11) of the World Health Organization (WHO) (Treede et al., 2015), participants reporting pain during the past 3 months with any degree of difficulty in performing activities on a daily basis (i.e., pain during walking, using stairs, in bed, sitting or lying, and standing) were categorized as having chronic pain. Participants also completed the Graded Chronic Pain Scale (GCPS) (Von Korff et al., 1992) assessing pain-related interference and global pain severity over the past 6 months. The GCPS consists of seven items related to pain interference and pain intensity (i.e., interference in daily activities, current/average/worst pain in last 6 months). Each question is a 0–10 numeric rating scale where participants rated their current, average and worst pain during the past 6 months. An average of these three items were calculated and multiplied by 10 to create a characteristic pain intensity score. Participants also rated the extent to which their pain interfered with their daily activities in the past 6 months (3 items), which were calculated in the same way as reported above to produce a disability score.
Dependent Variables:
To measure non-verbal fluency, the Ruff Figural Fluency Test (RFFT) (Ruff, 2011) was administered. This test examines planning strategies, fluid and divergent thinking, and ability to flexibly shift cognitive set in relation to non-verbal fluency. The participants were asked to draw as many unique designs as possible within 60 seconds by connecting the dots in different patterns for each of the five parts. The total number of unique designs drawn constituted the main score (i.e., Total Unique Designs). The Error Ratio score accounts for the number of repetitions of the same pattern drawn by the participants providing a measure of the individual’s ability to minimize repetition while maximizing unique productions. The test booklet and accompanying professional manual acquired from Psychological Assessment Resources (PAR) provides normative information to obtain education-corrected values for Total Unique Designs and Error Ratio scores.
Potential Covariates:
The Center for Epidemiologic Studies Depression Scale (CES-D) was used to assess depressive symptoms experienced by participants during the last week. The questionnaire consists of twenty questions relating to positive and negative thoughts/feelings. Each question ranges from 0–3 points and total scores ranged from 0–60, with higher scores indicating more depressive symptoms (Radloff, 1977). The State and Trait Anxiety Inventory (STAI) was used to assess trait and state anxiety (Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983) and to distinguish it from depressive syndromes. Participants also filled out the Edinburgh Handedness Inventory (EHI), a robust and validated handedness questionnaire that generates a lateralization index, ranging from 1 to −1 indicating the direction and degree of hand-preference (Oldfield, 1971). In addition, At the end of the Health Assessment Session, participants were given the Pittsburg Sleep Quality Inventory (PSQI) to take and fill out at home and return to study staff upon their next lab visit. The PSQI assesses the quality and patterns of sleep by measuring seven domains: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction over the last month. For the present analysis, the global sum score was used were “5” or greater indicates a “poor” sleeper.
Quantitative Sensory Testing (QST) Session
QST was used to assess the functional properties of peripheral receptor and somatosensory pathways, similar to previous methodology reported by our group (Cruz-Almeida et al., 2013). All QST procedures were performed in a quiet room with an approximate temperature between 21°C and 23°C. All subjects were seated in a comfortable chair with armrests and a semi-reclining back. An overview of the testing procedures was then explained to the subject. For each different modality, specific instructions were recited immediately before beginning the test. Measurement of a particular type of threshold was first demonstrated, and at least one practice trial was conducted to ensure that subjects understood the testing procedures.
After the practice trials were completed, data collection began for each test modality. All QST stimuli were administered to standardized testing sites with pressure applied to the right trapezius and quadriceps, and all other stimuli applied to the left second metatarsal head and thenar eminence. Tactile and vibratory detection testing was always conducted first, followed by thermal (detection and pain), and pressure (pain), the order of which was randomized and counterbalanced between participants. Skin surface temperature was measured using a DT1001 DermaTemp infrared scanner (Exergen) at the thenar eminence and metatarsal head immediately before threshold measurements commenced, to ensure temperature was within the range of 27–37°C. QST thresholds have been reported to be independent of skin temperature within this range (Hagander et al., 2000). Thermal detection and pain threshold measurements were obtained using a 30 × 30 mm thermode connected to the TSA-II Neurosensory Analyzer (Medoc Ltd., Ramat Yishai, Israel).
Vibration:
The handheld VSA-3000 probe of the Medoc system was used to measure vibratory detection thresholds for a 100 Hz stimulus frequency. Subjects were instructed to click a button as soon as they felt the vibratory sensation. The probe’s circular contact tip (1.22 cm2) was held in place by the experimenter during testing so that there was a slight and maintained indentation of ∼1–2 mm. Stimulus presentations were programmed with the software accompanying the vibratory equipment to control the rise rate of stimulus amplitude, the number of trials, and the time between each trial. Three trials, separated by ∼10 sec each, were conducted using the ascending method of limits: vibratory amplitude began at 0 μm at a rate of 0.5 μm/sec and increased until the subject indicated that the stimulus was felt or until the maximum amplitude of 130 μm was reached. Subjects were asked to indicate the “first moment” that they felt the vibration at each test site. The mean value across the three trials was calculated as the vibratory threshold for each site.
Tactile Detection:
Thresholds were tested using von Frey monofilaments (Touch Test™ Sensory Evaluator, North Coast Medical, Inc., Morgan Hill, CA), ranging in force from 0.008g to 300g. Subjects were instructed to keep their eyes closed during this part of testing and to respond verbally with a “yes” if they could feel the stimulus or “no” if they did not feel the stimulus. The method of limits was used in four stimulus series. An average was calculated for the two descending and two ascending series. The descending average was calculated by using the force from the first monofilament that was not detected and the last monofilament that was detected. Calculations of ascending averages were similar in that they used the force of the first monofilament that was detected and the last monofilament that was not detected.
Thermal Detection:
The method of limits was used to obtain measures of cool and warm detection thresholds. These thresholds were recorded using a mouse button that the participant clicked when the requested sensation was perceived. Cool detection consisted of the thermode starting at a baseline temperature (32 degrees Celsius) and moving downward until the participant perceived the sensation becoming cooler, at which point they responded by pressing the mouse button. The warm detection was similar in that the stimulation also started at the baseline temperature and moved upward until the participant perceived it was getting warmer, followed by a press of the mouse button. Each cool/warm detection stimulus was preceded by a five second rest period.
Thermal Pain:
The method of limits was also used to determine cold pain and heat pain thresholds using the same equipment and in a similar manner as the thermal detection thresholds. Subjects were instructed to indicate as soon as the sensation changed from “just being cold to being painfully cold” or from “just being hot to being painfully hot.” Each cold/heat pain stimulus was preceded by a ten second rest period, in order for the thermode to return to baseline temperature, as well as the participant’s skin temperature.
Pressure Pain:
An AlgoMed computerized pressure algometer (Medoc Ltd., Ramat Yishai, Israel) was used to deliver calibrated pressure through a 10 mm rubber tip. Testing was done on the right trapezius and right quadriceps muscles with the order of testing sites randomized and counterbalanced. Pressure applied to each site was increased at a constant rate of 1 kg/sec until participants clicked a button to indicate when the stimulus “first became painful” and that threshold was recorded. This procedure was repeated 3–5 times to obtain an average pressure pain threshold for each test site.
Neuroimaging Session
Participants that were eligible for an MRI underwent an additional session at the University of Florida’s McKnight Brain Institute on the Advanced Magnetic Resonance Imaging and Spectroscopy (AMRIS) facility’s Philips (Best, the Netherlands) 3-Tesla scanner with a 32-channel radio-frequency coil. A high resolution, T1-weighted turbo field echo anatomical scan was collected using the following parameters: TR = 7.0 ms, TE = 3.2 ms, 170 slices acquired in a sagittal orientation, flip angle = 8 degrees, resolution = 1 mm3. Head movement was minimized via cushions positioned inside the head coil and specific instructions to participants.
Image analysis was carried out with the stable version (v.5.3.0) of the FreeSurfer software (http://surfer.nmr.mgh.harvard.edu). In short, the procedure included: motion correction, intensity normalization, Talairach registration, skull stripping, segmentation of subcortical white matter, tessellation of the GM/white matter (WM) boundary, automated topology correction, and surface deformation. We used a 10 mm (full-width at half-maximum) Gaussian kernel to smooth maps. Finally, FreeSurfer created a surface 3D model of the cortex using intensity and continuity information.
Cortical thickness analysis
We visually checked the cortical reconstruction of each subject for inaccuracies and manually corrected major topological inaccuracies with vertex edits or control points and subsequently repeated the processing. Cortical thickness was calculated as the shortest distance between the GM/WM boundary and pial surface at each vertex across the cortical mantle, measured in millimeters (mm). In addition to vertex-based reconstruction, FreeSurfer automatically parcellated the cortex into 34 gyral-based regions-of-interest (ROIs) per hemisphere, according to the Desikan-Killiany atlas. For each of the 68 cortical parcellations, FreeSurfer calculated average cortical thickness in mm.
Subcortical volume analysis
Subcortical volumes were calculated with FreeSurfer’s automated procedure for volumetric measures. Each voxel in the normalized brain volume was assigned to one of 40 labels, using a probabilistic atlas obtained from a manually labeled training set. The labels we used for further analysis were the putamen, caudate nucleus, globus pallidus, nucleus accumbens, brainstem, thalamus, amygdala, and hippocampus.
Statistical analyses
QST data were z-transformed for each modality at each test site and were combined for analysis due to the multicollinearity within thermal, mechanical and pain modalities. Thus, standardized scores were created for thermal detection (i.e., greater Z-Scores reflect lower detection thresholds or more thermal detection sensitivity), thermal pain (i.e., greater Z-Scores reflect lower pain thresholds or more thermal pain sensitivity), mechanical detection (i.e., greater Z-Scores reflect higher detection thresholds or less mechanical detection sensitivity), and pressure pain (i.e., greater Z-Scores reflect higher pressure pain thresholds or less pressure pain sensitivity), and these were used for further statistical analysis. The combination of warm and cold modalities is appropriate based on the physiological properties of sensory channels, from peripheral receptors to ascending pathways (Willis & Coggeshall, 2004).
One-way analysis of variance (ANOVAs) were used to compare groups with respect to continuous/discrete ordinal variables while χ2 analyses were used to assess associations with nominal variables. Assumptions underlying each statistical test were examined. One-way analysis of covariance (ANCOVA) procedures were conducted with Pain Group as between subject factors controlling for variables that were significantly different in the pairwise comparisons, between the older participants only. Bonferroni post-hoc corrections were implemented to control for multiple comparisons. If any ANOVA/ANCOVA assumptions were not met, we also performed the nonparametric Kruskal-Wallis test. We applied partial correlations adjusting for race, education and other potential covariates to examine associations between RFFT performance and clinical pain using the GCPS subscales as well as standardized experimental pain scores. Partial correlations were used to examine associations between education corrected-RFFT performance and gray matter brain measures adjusting for race, handedness and estimated intracranial volume. We applied a Bonferroni correction by the number of cortical areas per hemisphere (p < (0.05/34) = ~0.001) and by the number of sub-cortical structures per hemisphere (p < (0.05/8) = ~0.006) in order to correct for multiple comparisons.
Results
Detailed demographic and clinical characteristics of our older (n=63) and younger (n=28) participants are presented in Table 1. Younger controls included individuals 18 to 24 years of age, older controls were 61 to 85 years of age while older individuals with chronic pain were 56 to 82 years old. Study participants were significantly different with respect to race, and education, STAI and PSQI (which was completed only by a subset: younger n=13, older controls n=19 and older chronic pain n=30). Given that anxiety, depression and sleep disturbances can significantly contribute to cognitive performance above and beyond demographics and education, we employed a stepwise statistical approach. In all analyses, we first included race and education as covariates followed by the subsequent addition of the CES-D, STAI and PSQI.
Table 1.
Clinical and demographic characteristics of the NEPAL study participants
| Younger Controls (n=28) | Older No Chronic Pain (n=24) | Older Chronic Pain (n=39) | Sig. | |
|---|---|---|---|---|
| Age, mean ± SD | 20.4 ± 1.6 | 71.4 ± 6.8 | 70.4 ± 6.0 | 0.001 |
| Sex, no. (%) | 0.090 | |||
| Female | 15 (53.6) | 12 (50.0) | 29 (74.4) | |
| Male | 13 (46.4) | 12 (50.0) | 10 (25.6) | |
| Race, no. (%) | ||||
| Caucasian | 14 (50.0) | 24 (100.0) | 32 (82.1) | 0.001 |
| African American | 3 (10.7) | 0 (0.0) | 3 (7.7) | |
| Hispanic | 10 (35.7) | 0 (0.0) | 1 (2.5) | |
| Other | 1 (3.6) | 0 (0.0) | 3 (7.7) | |
| Education, no. (%) | ||||
| High School Degree | 19 (67.8) | 2 (8.3) | 13 (33.3) | 0.001 |
| Some College | 4 (14.3) | 2 (8.3) | 8 (20.5) | |
| Bachelor’s Degree | 5 (17.9) | 6 (25.0) | 7 (18.0) | |
| Graduate Degree | 0 (0.0) | 14 (58.3) | 11(28.2) | |
| CES-D, mean ± SD | 6.4 ± 4.7 | 6.2 ± 5.3 | 8.6 ± 7.9 | 0.230 |
| STAI, mean ± SD | 30.4 ± 11.1 | 24.4 ± 10.6 | 22.9 ± 11.7 | 0.029 |
| Total PSQI, mean ± SD | 7.2 ± 2.5 | 4.6 ± 2.5 | 7.0 ± 3.7 | 0.020* |
| 3MS, mean ± SD | 99.1 ± 2.5 | 99.4 ± 3.1 | 96.2 ± 4.5 | 0.001 |
(n=91).
PSQI questionnaires were returned by a subset of the participants (younger n=13, older controls n=19 and older chronic pain n=30)
1. Age and Pain Differences in RFFT Performance
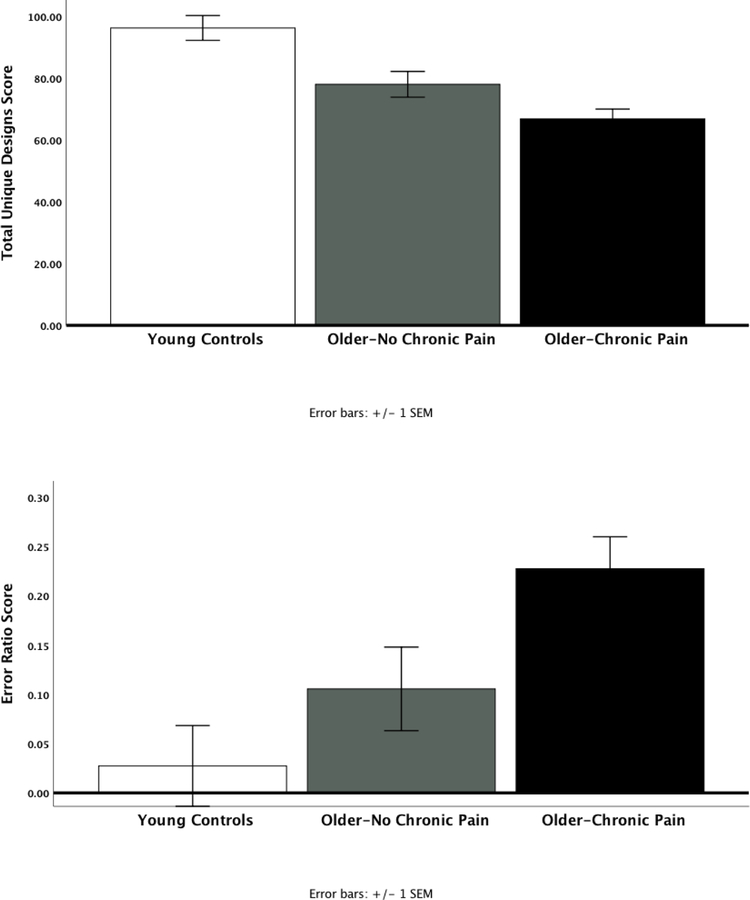
As expected, older participants had significantly lower Total Unique Designs (70.7 ± 20.3) as well as greater Error Ratio (0.18 ± 0.3) compared to younger controls (97.1 ± 19.6, p<0.001 and 0.02 ± 0.03, p = 0.002, respectively), even after adjusting for education and race. In addition, older participants with chronic pain had significantly lower Total Unique Designs (67.1 ± 20.3) compared to older adults without chronic pain (78.8 ± 15.9) and younger controls (97.1 ± 19.6, p<0.001, Figure 1a). Similarly, Error Ratio was significantly higher in our older participants with chronic pain (0.22 ± 0.3) versus those without chronic pain (0.09 ± 0.06) and younger controls (0.05 ± 0.05, p = 0.002, Figure 1b). Given that Error Ratio violated normality assumptions, we also performed a Kruskal-Wallis test where there were statistically significant differences between the groups (p=0.003). Total Unique Designs remained highly significantly different even after accounting for CES-D, STAI and PSQI (p<0.001). On the other hand, differences in Error Ratio were significantly attenuated only after accounting for PSQI (p=0.099), but not CES-D and STAI.
Figure 1.
Age and Pain differences in Total Unique Designs (a) and Error Ratio (b) scores controlling for race and education (mean ± SEM) (p<0.001).
2. Associations of RFFT Performance with Clinical and Experimental Pain in Older Adults
Within the older sample, greater GCPS Characteristic Pain Intensity Scale, but not the Pain Disability scale, was significantly correlated with lower Total Unique Designs adjusting for race and education (r = −0.293, p = 0.020). There was no statistically significant association between Error Ratio with GCPS Characteristic Pain Intensity (r = 0.230, p=0.07), or GCPS Pain Disability (r=0.067 p=0.601). After accounting for CES-D, STAI and PSQI scores, there were no significant associations between clinical pain and RFFT performance (p’s>0.05). Greater Total Unique Designs was significantly associated with lower pressure pain sensitivity (r = 0.300, p = 0.031), but not with any other experimental pain measure (Table 2). Higher Error Ratio was significantly associated with greater thermal pain sensitivity (r = 0.304, p = 0.027) but not any other measure, Table 2). Accounting for CES-D, STAI and PSQI scores, these associations were diminished and no longer statistically significant (Total Unique Design/pressure pain r=0.271, p=0.095 and Error Ratio/thermal pain r=0.270, p=0.092).
Table 2.
Associations between Total Unique Designs and Error Ratio with experimental pain measures controlling for race and education.
| Total Unique Designs | Error Ratio | |
|---|---|---|
| Std. Mechanical Detection | 0.065, p = 0.640 | −0.063, p = 0.642 |
| Std. Thermal Detection | −0.036, p = 0.796 | 0.134, p = 0.339 |
| Std. Pressure Pain | 0.300, p = 0.031 | −0.133, p = 0.349 |
| Std. Thermal Pain | −0.053, p = 0.706 | 0.304, p = 0.027 |
3. Associations of RFFT Performance with Cortical Thickness and Subcortical Volumes
A subset of participants (younger controls, n=1½8 and older adults, n=39/63) completed the RFFT and were eligible for an MRI. Table 3 summarizes associations between Total Unique Designs and Error Ratios with the brain measures of cortical thickness and subcortical volumes across all participants (n=50) adjusted for education, race, handedness and estimated intracranial volume. Total Unique design scores were significantly associated with most of the cortical thickness measures in regions of the prefrontal, frontal, parietal and temporal areas even after adjusting for multiple comparisons. Similarly, Total Unique Design scores were significantly associated with most subcortical regions including the thalamus, Accumbens, Putamen, Hippocampus and Amygdala. On the other hand, Error Ratios were not strongly associated with cortical or subcortical regions after multiple comparison adjustments.
Table 3.
Associations between Total Unique Designs and Error Ratio with brain measures adjusted for education, handedness, race and estimated total intracranial volume.
| Total Unique Designs (r, p-value) | Error Ratio (r, p-value) | |
|---|---|---|
| Cortical Thickness | ||
| Caudal anterior cingulate | ||
| Right | 0.339, p=0.010 | −0.270, p=0.042 |
| Left | 0.316, p=0.017 | −0.119, p=0.379 |
| Caudal middle frontal | ||
| Right | 0.575, p=0.000 | −0.331, p=0.012 |
| Left | 0.312, p=0.018 | −0.213, p=0.112 |
| Lateral orbitofrontal | ||
| Right | 0.537, p=0.000 | −0.414, p=0.001 |
| Left | 0.551, p=0.000 | −0.428, p=0.001 |
| Medial orbitofrontal | ||
| Right | 0.608, p=0.000 | −0.229, p=0.086 |
| Left | 0.607, p=0.000 | −0.406, p=0.002 |
| Pars opercularis | ||
| Right | 0.638, p=0.000 | −0.410, p=0.002 |
| Left | 0.597, p=0.000 | −0.170, p=0.206 |
| Pars orbitalis | ||
| Right | 0.478, p=0.000 | −0.444, p=0.001 |
| Left | 0.594, p=0.000 | −0.556, p=0.000 |
| Pars triangularis | ||
| Right | 0.634, p=0.000 | −0.311, p=0.018 |
| Left | 0.614, p=0.000 | −0.266, p=0.046 |
| Postcentral | ||
| Right | 0.520, p=0.000 | −0.212, p=0.113 |
| Left | 0.533, p=0.000 | −0.338, p=0.010 |
| Precentral | ||
| Right | 0.525, p=0.000 | −0.165, p=0.220 |
| Left | 0.481, p=0.000 | −0.195, p=0.147 |
| Rostral anterior cingulate | ||
| Right | 0.437, p=0.001 | −0.421, p=0.001 |
| Left | 0.578, p=0.000 | −0.178, p=0.185 |
| Rostral middle frontal | ||
| Right | 0.539, p=0.000 | −0.346, p=0.008 |
| Left | 0.472, p=0.000 | −0.256, p=0.055 |
| Superior frontal | ||
| Right | 0.696, p=0.000 | −0.451, p=0.000 |
| Left | 0.629, p=0.000 | −0.464, p=0.000 |
| Frontal pole | ||
| Right | 0.308, p=0.020 | −0.283, p=0.033 |
| Left | 0.212, p=0.113 | −0.210, p=0.117 |
| Insula | ||
| Right | 0.544, p=0.000 | −0.172, p=0.200 |
| Left | 0.508, p=0.000 | −0.265, p=0.046 |
| Cuneus | ||
| Right | 0.300, p=0.023 | −0.161, p=0.232 |
| Left | 0.228, p=0.088 | −0.215, p=0.108 |
| Entorhinal | ||
| Right | 0.106, p=0.411 | 0.081, p=0.549 |
| Left | 0.176, p=0.192 | 0.095, p=0.483 |
| Fusiform | ||
| Right | 0.535, p=0.000 | −0.240, p=0.072 |
| Left | 0.531, p=0.000 | −0.255, p=0.056 |
| Inferior parietal | ||
| Right | 0.585, p=0.000 | −0.145, p=0.282 |
| Left | 0.619, p=0.000 | −0.318, p=0.016 |
| Inferior temporal | ||
| Right | 0.512, p=0.000 | −0.328, p=0.013 |
| Left | 0.442, p=0.001 | −0.336, p=0.011 |
| Isthmus cingulate | ||
| Right | 0.553, p=0.000 | −0.329, p=0.013 |
| Left | 0.440, p=0.001 | −0.330, p=0.012 |
| Lateral occipital | ||
| Right | 0.478, p=0.000 | −0.386, p=0.003 |
| Left | 0.352, p=0.007 | −0.177, p=0.187 |
| Lingual | ||
| Right | 0.418, p=0.000 | −0.232, p=0.083 |
| Left | 0.231, p=0.084 | −0.057, p=0.675 |
| Middle temporal | ||
| Right | 0.709, p=0.000 | −0.323, p=0.014 |
| Left | 0.617, p=0.000 | −0.259, p=0.052 |
| Parahippocampal | ||
| Right | 0.172, p=0.202 | −0.093, p=0.493 |
| Left | 0.228, p=0.088 | −0.025, p=0.856 |
| Paracentral | ||
| Right | 0.395, p=0.002 | −0.240, p=0.072 |
| Left | 0.285, p=0.032 | −0.169, p=0.209 |
| Pericalcarine | ||
| Right | 0.073, p=0.588 | 0.066, p=0.625 |
| Left | 0.135, p=0.315 | −0.057, p=0.673 |
| Posterior cingulate | ||
| Right | 0.516, p=0.000 | −0.332, p=0.012 |
| Left | 0.474, p=0.000 | −0.435, p=0.000 |
| Precuneus | ||
| Right | 0.525, p=0.000 | −0.239, p=0.073 |
| Left | 0.560, p=0.000 | −0.278, p=0.036 |
| Superior parietal | ||
| Right | 0.427, p=0.001 | −0.225, p=0.092 |
| Left | 0.417, p=0.001 | −0.266, p=0.046 |
| Superior temporal | ||
| Right | 0.724, p=0.000 | −0.252, p=0.059 |
| Left | 0.637, p=0.000 | −0.286, p=0.031 |
| Supramarginal | ||
| Right | 0.671, p=0.000 | −0.398, p=0.002 |
| Left | 0.589, p=0.000 | −0.297, p=0.025 |
| Temporal pole | ||
| Right | 0.351, p=0.008 | −0.051, p=0.707 |
| Left | 0.343, p=0.009 | −0.118, p=0.383 |
| Transverse temporal | ||
| Right | 0.492, p=0.000 | −0.184, p=0.170 |
| Left | 0.439, p=0.001 | −0.140, p=0.299 |
| Subcortical Volumes | ||
| Thalamus | ||
| Right | 0.432, p=0.001 | −0.180, p=0.179 |
| Left | 0.432, p=0.001 | −0.159, p=0.236 |
| Accumbens | ||
| Right | 0.378, p=0.004 | −0.173, p=0.198 |
| Left | 0.427, p=0.001 | −0.338, p=0.010 |
| Caudate | ||
| Right | 0.231, p=0.084 | −0.065, p=0.633 |
| Left | 0.207, p=0.122 | −0.025, p=0.851 |
| Putamen | ||
| Right | 0.443, p=0.001 | −0.112, p=0.406 |
| Left | 0.415, p=0.001 | −0.084, p=0.535 |
| Pallidum | ||
| Right | 0.011, p=0.937 | 0.144, p=0.287 |
| Left | −0.159,p=0.236 | 0.215, p=0.109 |
| Hippocampus | ||
| Right | 0.359, p=0.006 | −0.092, p=0.498 |
| Left | 0.386, p=0.003 | −0.050, p=0.710 |
| Amydgala | ||
| Right | 0.445, p=0.001 | −0.106, p=0.434 |
| Left | 0.505, p=0.000 | −0.149, p=0.270 |
| Brainstem | 0.077, p=0.571 | −0.019, p=0.890 |
Bolded values survived Bonferroni correction for multiple comparisons
Discussion
The present study sought to elucidate age and pain-related differences in non-verbal fluency, a type of executive cognitive function. As hypothesized, older adults with chronic pain had worse performance on a measure of non-verbal fluency (i.e., RFFT) when compared to pain-free older and younger, healthy controls. Indeed, worse non-verbal figural fluency performance was significantly associated with greater clinical pain intensity as well as greater pressure and thermal experimental pain sensitivity in older individuals with chronic pain, although these associations were eliminated after accounting for self-reported sleep quality. Finally, non-verbal figural fluency tasks may provide information about the extent and localization of brain dysfunction. Our study specifically suggests that non-verbal fluency performance is associated with cortical thickness in frontal, parietal and temporal regions as well as several subcortical gray matter structures, some of which have not been typically associated with this executive function domain, highlighting the need for further understanding of neurobiological mechanisms.
Fluency is a well-recognized measure of executive function (Luo, Luk, & Bialystok, 2010; Pennington & Ozonoff, 1996) and describes the ability to generate non-verbal items according to certain rules. Non-verbal figural fluency tasks require individuals to draw straight lines between constellations of printed dots to form as many novel patterns as they can within a set time limit. Executive non-verbal fluency tasks provide an opportunity to explore and separate phonological processes from executive control processes (Ruff, Light, & Evans, 1987). Age-related deficits in non-verbal fluency are consistent with the existing literature in age-related decrements across most cognitive functional domains including non-verbal figural fluency (Izaks et al., 2011, Federmeier & Kutas, 2005, Wecker et al., 2005). In neurological populations, figural fluency was linearly related to the severity of trauma, from mild to severe, with severe subjects generating the fewest figures (Ruff, Evans, & Marshall, 1986). Ruff, Allen, Farrow, Niernann, and Wylie (1994) reported that subjects with right frontal damage scored more impaired than left frontal and posterior subjects. Similarly, nonverbal fluency has been selectively associated with bilateral frontal gray matter volumes in persons with Alzheimer’s Disease (Fama et al., 2000).
To our knowledge, there is limited research on the association of pain with non-verbal fluency performance in older individuals. Nonetheless, our findings are consistent with the growing literature where acute and chronic pain are reciprocally associated with executive function (Moriarty et al., 2011) and are also congruent with the executive function performance decline associated with aging. Specifically, the current study adds to the existing literature by suggesting that both acute and chronic pain processing is negatively associated with the non-verbal fluency domain of executive function, above and beyond the effects of aging in a sample of cognitively intact community-dwelling older individuals. Previously, several investigations report deficits in various measures of executive function in fibromyalgia (Chou et al., 2018; de Guevara et al., 2018) with frontal cortical activity reduced during a verbal fluency test (Chou et al., 2016). Moriarty and colleagues (Moriarty et al., 2011) suggest that pain leads to cognitive impairment via competition for limited neural resources, neuroplasticity or neurochemical mediation. The perception of pain is highly dependent on cognitive processes such as attention and long-term memory, such that impairment in these, or the executive functions that coordinate them (e.g. working memory), are likely to contribute to pain intensity and pain-related disability (Berryman et al., 2013, Simon et al., 2016). Given that there were some associations between non-verbal fluency with self-reported pain as well as experimental pressure and thermal pain, it is plausible that chronic pain impacts non-verbal fluency performance, which in turn impairs the processing of experimental pain stimulation. However, after accounting for sleep quality, but not depression or anxiety, the RFFT-pain associations were eliminated, suggesting more complex interactions. For example, individuals with chronic pain often suffer from sleep disturbances, which in turn may affect daytime cognitive performance. However, the directionality of pain-sleep associations are not currently well-understood (Finan et al., 2013). Future longitudinal studies are needed to establish to what extent sleep contributes to how chronic pain impacts cognitive performance, and/or vice-versa.
Finally, we wanted to explore associations between the brain’s gray matter and non-verbal fluency performance in our sample of younger and older adults given the limited understanding of its neurobiological substrates in healthy states. Specifically, frontal, parietal and temporal brain regions were significantly associated with RFFT performance, even after accounting for multiple comparisons. These findings are consistent with previous studies where nonverbal fluency has been associated with anterior cerebral function, (Jones-Gotman and Milner, 1977; Ruff et al, 1994), although more bilaterally in our sample (similar to Baldo et al, 2001). However, our findings do not support the idea that the RFFT (Ruff et al, 1987) is exclusively a measure of general frontal lobe function (Baldo et al, 2001) and it may not discriminate between frontal and temporal regional abnormalities (Suchy et al, 2003). Indeed, nonverbal fluency deficits have also been found in persons with predominantly mesial temporal pathology, such as Alzheimer’s disease (Bigler et al, 1988; Fama et al, 1998). Further, our findings also support that figural fluency performance likely requires additional brain resources such as visuomotor and spatial capabilities involving fronto-parietal regions as well as creativity-related mental representations (Bristol and Viskontas, 2006; Damasio, 2001) likely involving the hippocampal declarative memory system (Eichenbaum et al., 1996; Strange et al., 2014). However, from the present study it is difficult to elucidate why cortical (i.e., insula) and subcortical (i.e., thalamus, basal ganglia, amygdala) regions not typically associated with executive functional processes were strongly associated with RFFT performance in our sample. An alternative explanation based on the behavioral data where the pain-RFFT performance was eliminated by accounting for sleep quality, is that these regions are also involved in sleep processes in aging (Liu et al., 2018). Future mechanistic investigations are further needed to inform these patterns of results.
The present study has some limitations. First, our study investigated a very specific cognitive domain, non-verbal fluency. While this domain has been scarcely researched in the past, future studies may benefit from testing multiple, different cognitive domains in relation to clinical and experimental pain to obtain a more comprehensive idea of the cognitive deficits these individuals are facing. Second, the present cross-sectional analyses do not provide any temporal information on which to infer directionality or causality. Third, both sleep and brain measures were only available on a subset of study participants, thus, we cannot ascertain that these data were missing completely-at-random. Lastly, the majority of the sample was Caucasian, community-dwelling adults who are highly educated. A more diverse cohort with regards to socioeconomic status including education would be beneficial in future studies to gain a greater insight into the relationship between pain, age, and cognitive function that can be generalizable to other populations.
In summary, our study further supports the view that chronic pain is associated with cognitive function beyond the effects of acute pain or aging. However, our analysis of these results show that this phenomenon varies from person to person, and it exists even in very high functioning individuals consistent with their underlying frontal, parietal and temporal gray matter structure. An individualized treatment approach to chronic pain prevention, treatment, and management and cognition-based therapy should prove advantageous if they are targeted towards a population comparable to the present study. Future studies would benefit from studying different populations and comprehensive evaluation of responses to various treatment modalities.
Highlights.
Older adults reporting chronic musculoskeletal pain performed worse on a non-verbal fluency test compared to older and younger controls.
Although some pain measures were associated with non-verbal fluency performance, these associations were eliminated after controlling for self-reported sleep quality.
Non-verbal fluency performance was associated with cortical thickness in frontal, parietal and temporal regions as well as several subcortical gray matter structures including the hippocampus, thalamus, amygdala and sections of the basal ganglia.
ACKNOWLEDGEMENTS:
We are grateful to our volunteers for their participation and the NEPAL study team (Darlin Ramirez, Brandon Apagueno and Rachna Sannegowda). This work was supported by the National Institutes on Aging (K01AG048259/R01AG059809 to YCA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTERESTS: All authors report no conflict of interest.
References
- Baldo JV, Shimamura AP, Delis DC, Kramer J, Kaplan E. Verbal and design fluency in patients with frontal lobe lesions. J Int Neuropsychol Soc 2001;7:586–96. [DOI] [PubMed] [Google Scholar]
- Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, & Stitt LW (1988). Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. The Journal of rheumatology, 15(12), 1833–1840. [PubMed] [Google Scholar]
- Berryman C, Stanton TR, Bowering KJ, Tabor A, McFarlane A, & Moseley GL (2013). Evidence for working memory deficits in chronic pain: a systematic review and meta-analysis. PAIN®, 154(8), 1181–1196. [DOI] [PubMed] [Google Scholar]
- Bigler ED, Schultz R, Grant M, Knight G, Lucas J, Roman M, Hall S, Sullivan M. Design fluency in dementia of the Alzheimer’s type: Preliminary findings. Neuropsychology, 2 (1988), pp. 127–133. [Google Scholar]
- Bristol AS & Viskontas I (2006). Dynamic processes within associative memory stores: Piecing together the neural basis of creative cognition. Creativity and Reason in Cognitive Development 60–80. 10.1017/CBO9780511606915.005. [DOI] [Google Scholar]
- Chou PH, Lin WH, Hung CA, Chang CC, Li WR, Lan TH, & Huang MW (2016). Perceived occupational stress is associated with decreased cortical activity of the prefrontal cortex: a multichannel near-infrared spectroscopy study. Scientific reports, 6, 39089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou PH, Tang KT, Chen YH, Sun CW, Huang CM, Chen DY. Reduced frontal activity during a verbal fluency test in fibromyalgia: A near-infrared spectroscopy study. J Clin Neurosci 2018. April;50:35–40. doi: 10.1016/j.jocn.2018.01.030. Epub 2018 Feb 14. [DOI] [PubMed] [Google Scholar]
- Crossley M, D’Arcy C, Rawson NS. Letter and category fluency in community-dwelling Canadian seniors: a comparison of normal participants to those with dementia of the Alzheimer or vascular type. J Clin Exp Neuropsychol 1997;19:52–62. [DOI] [PubMed] [Google Scholar]
- Cruz‐Almeida Y, King CD, Goodin BR, Sibille KT, Glover TL, Riley JL, … & Redden DT (2013). Psychological profiles and pain characteristics of older adults with knee osteoarthritis. Arthritis care & research, 65(11), 1786–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio H (2001). “Neural basis of language disorders,” in Language Intervention Strategies in Aphasia and Related Neurogenic Communication Disorders, (4th Edn.) ed. Chapey R, editor. (Philadelphia, PA: Lippincott William and Wilkins;), 18–36. [Google Scholar]
- de Guevara CML, Fernández-Serrano MJ, del Paso GAR, & Duschek S (2018). Executive function impairments in fibromyalgia syndrome: Relevance of clinical variables and body mass index. PloS one, 13(4), e0196329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick B, Eccleston C, & Crombez G (2002). Attentional functioning in fibromyalgia, rheumatoid arthritis, and musculoskeletal pain patients. Arthritis Care & Research, 47(6), 639–644. [DOI] [PubMed] [Google Scholar]
- Dick BD, & Rashiq S (2007). Disruption of attention and working memory traces in individuals with chronic pain. Anesthesia & Analgesia, 104(5), 1223–1229. [DOI] [PubMed] [Google Scholar]
- Eccleston C (1995). Chronic pain and distraction: an experimental investigation into the role of sustained and shifting attention in the processing of chronic persistent pain. Behaviour research and therapy, 33(4), 391–405. [DOI] [PubMed] [Google Scholar]
- Eccleston C, & Crombez G (1999). Pain demands attention: A cognitive–affective model of the interruptive function of pain. Psychological bulletin, 125(3), 356. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Schoenbaum G, Young B, Bunsey M. Functional organization of the hippocampal memory system. Proc Natl Acad Sci U S A 1996. November 26;93(24):13500–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fama R, Sullivan EV, Shear PK, Cahn-Weiner DA, Yesavage JA, Tinklenberg JR, & Pfefferbaum A (1998). Fluency performance patterns in Alzheimer’s disease and Parkin-son’s disease. The Clinical Neuropsychologist,12, 487–499. [Google Scholar]
- Fama R, Sullivan EV, Shear PK, Cahn-Weiner DA, Marsh L, Lim KO, Yesavage JA, Tinklenberg JR, Pfefferbaum A. Structural brain correlates of verbal and nonverbal fluency measures in Alzheimer’s disease. Neuropsychology 2000. January;14(1):29–40. [PubMed] [Google Scholar]
- Federmeier KD, & Kutas M (2005). Aging in context: age‐related changes in context use during language comprehension. Psychophysiology, 42(2), 133–141. [DOI] [PubMed] [Google Scholar]
- Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain 2013. December;14(12):1539–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisky EL (2007). Changes in cognitive function in human aging. Brain aging: Models, methods, and mechanisms, 3–20. [Google Scholar]
- Hagander LG, Midani HA, Kuskowski MA, and Parry GJ (2000). Quantitative sensory testing: effect of site and skin temperature on thermal thresholds. Clin. Neurophysiol 111, 17–22. [DOI] [PubMed] [Google Scholar]
- Izaks GJ, Joosten H, Koerts J, Gansevoort RT, & Slaets JP (2011). Reference data for the Ruff Figural Fluency Test stratified by age and educational level. PLoS One, 6(2), e17045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Gotman M, Milner B. Design Fluency: The invention of nonsense drawings after focal cortical lesions. Neuropsychologia, 15 (1977), pp. 653–664. [DOI] [PubMed] [Google Scholar]
- Kewman DG, Vaishampayan N, Zald D, & Han B (1991). Cognitive impairment in musculoskeletal pain patients. The International Journal of Psychiatry in Medicine, 21(3), 253–262. [DOI] [PubMed] [Google Scholar]
- Liu YR, Fan DQ, Gui WJ, Long ZL, Lei X, Yu J. Sleep-related brain atrophy and disrupted functional connectivity in older adults. Behav Brain Res 2018. July 16;347:292–299. doi: 10.1016/j.bbr.2018.03.032. Epub 2018 Mar 22. [DOI] [PubMed] [Google Scholar]
- Luo L, Luk G, & Bialystok E (2010). Effect of language proficiency and executive control on verbal fluency performance in bilinguals. Cognition, 114(1), 29–41. [DOI] [PubMed] [Google Scholar]
- Mathur VA, Khan SA, Keaser ML, Hubbard CS, Goyal M, & Seminowicz DA (2015). Altered cognition-related brain activity and interactions with acute pain in migraine. NeuroImage: Clinical, 7, 347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickanin J, Grossman M, Onishi K, Auriacombe S, & Clark C (1994). Verbal and nonverbal fluency in patients with probable Alzheimer’s disease. Neuropsychology, 8(3), 385–394. [Google Scholar]
- Moriarty O, McGuire BE, & Finn DP (2011). The effect of pain on cognitive function: a review of clinical and preclinical research. Progress in neurobiology, 93(3), 385–404. [DOI] [PubMed] [Google Scholar]
- O’Neill S, Manniche C, Graven-Nielsen T, & Arendt-Nielsen L (2014). Association between a composite score of pain sensitivity and clinical parameters in low-back pain. The Clinical journal of pain, 30(10), 831–838. [DOI] [PubMed] [Google Scholar]
- Oosterman JM, Derksen LC, van Wijck AJ, Veldhuijzen DS, & Kessels RP (2011). Memory functions in chronic pain: examining contributions of attention and age to test performance. The Clinical journal of pain, 27(1), 70–75. [DOI] [PubMed] [Google Scholar]
- Pennington BF, & Ozonoff S (1996). Executive functions and developmental psychopathology. Journal of child psychology and psychiatry, 37(1), 51–87. [DOI] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied psychological measurement, 1(3), 385–401. [Google Scholar]
- Ruff RM, Allen CC, Farrow CE, Niemann H, & Wylie T (1994). Figural fluency: Differential impairment in patients with left versus right frontal lobe lesions, Archives of Clinical Neuropsychology, 9(1), 41–55. [PubMed] [Google Scholar]
- Ruff RM, Evans R, & Marshall LF (1986). Impaired verbal and figural fluency after head injury. Archives of Clinical Neuropsychology, 1(2), 87–101. [PubMed] [Google Scholar]
- Ruff RM, Light RH, & Evans RW (1987). The Ruff Figural Fluency Test: a normative study with adults. Developmental neuropsychology, 3(1), 37–51. [Google Scholar]
- Ruff R (2011) Figural Fluency Test. In: Kreutzer JS, DeLuca J, Caplan B (eds) Encyclopedia of Clinical Neuropsychology Springer, New York, NY [Google Scholar]
- Seminowicz DA, & Davis KD (2007). A re-examination of pain–cognition interactions: implications for neuroimaging. Pain, 130(1), 8–13. [DOI] [PubMed] [Google Scholar]
- Simon CB, Lentz TA, Bishop MD, Riley JL III, Fillingim RB, & George SZ (2016). Comparative associations of working memory and pain catastrophizing with chronic low back pain intensity. Physical therapy, 96(7), 1049–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Witter MP, Lein ES, Moser EI. Functional organization of the hippocampal longitudinal axis. Nat Rev Neurosci 2014. October;15(10):655–69. doi: 10.1038/nrn3785. [DOI] [PubMed] [Google Scholar]
- Suchy Y, Sands K, Chelune GJ. Verbal and nonverbal fluency performance before and after seizure surgery. J Clin Exp Neuropsychol 2003. April;25(2):190–200. [DOI] [PubMed] [Google Scholar]
- Teng EL, & Chui HC (1987). The Modified Mini-Mental State (3MS) examination. The Journal of clinical psychiatry, 48(8), 314. [PubMed] [Google Scholar]
- Thurstone LL Primary mental abilities, University of Chicago Press, Chicago: (1938). [Google Scholar]
- Thurstone LL, Thurstone TG Examiner manual for the SRA Primary Mental Abilities test, Science Research Associates, Chicago: (1949). [Google Scholar]
- Tombaugh TN, Kozak J, & Rees L (1999). Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Archives of clinical neuropsychology, 14(2), 167–177. [PubMed] [Google Scholar]
- Tomer R, Levin BE. Differential effects of aging on two verbal fluency tasks. Percept Mot Skills 1993;76:465–6 [DOI] [PubMed] [Google Scholar]
- Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, … & Giamberardino MA (2015). A classification of chronic pain for ICD-11. Pain, 156(6), 1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Korff M, Ormel J, Keefe FJ, Dworkin SF. Grading the severity of chronic pain. Pain 1992;50:133–49. [DOI] [PubMed] [Google Scholar]
- Wecker NS, Kramer JH, Hallam BJ, & Delis DC (2005). Mental flexibility: age effects on switching. Neuropsychology, 19(3), 345. [DOI] [PubMed] [Google Scholar]
- Willis WD, & Coggeshall RE (2004). Functional organization of dorsal horn interneurons. In Sensory mechanisms of the spinal cord (pp. 271–560). Springer, Boston, MA. [Google Scholar]