Abstract
Serotonin (5-HT) 5-HT2C receptor (5-HT2CR) is recognized as a critical mediator of disease-related pathways and behaviors based upon actions in the central nervous system (CNS). Since 5-HT2CR is a class A G protein-coupled receptor (GPCR), drug discovery efforts have traditionally pursued the activation of the receptor through synthetic ligands with agonists proposed for the treatment of obesity, substance use disorders and impulse control disorders while antagonists may add value for the treatment of anxiety, depression and schizophrenia. The most significant agonist discovery to date is the FDA-approved anti-obesity medication lorcaserin. In recent years, efforts towards developing other mechanisms to enhance receptor function have resulted in the discovery of Positive Allosteric Modulators (PAMs) for the 5-HT2CR, with several molecule series now reported. The biological significance and context for signaling and function of the 5-HT2CR, and the current status of 5-HT2CR agonists and PAMs are discussed in this review.
Keywords: 5-HT2C receptor, Agonists, Allosteric modulation, Positive allosteric modulators, Target selectivity, Signaling bias, Drug discovery, Ligand development, Pharmacological probes, Central nervous system disorders, Neurotherapeutics
1. INTRODUCTION TO THE 5-HT2CR PROTEIN STRUCTURE AND FUNCTION
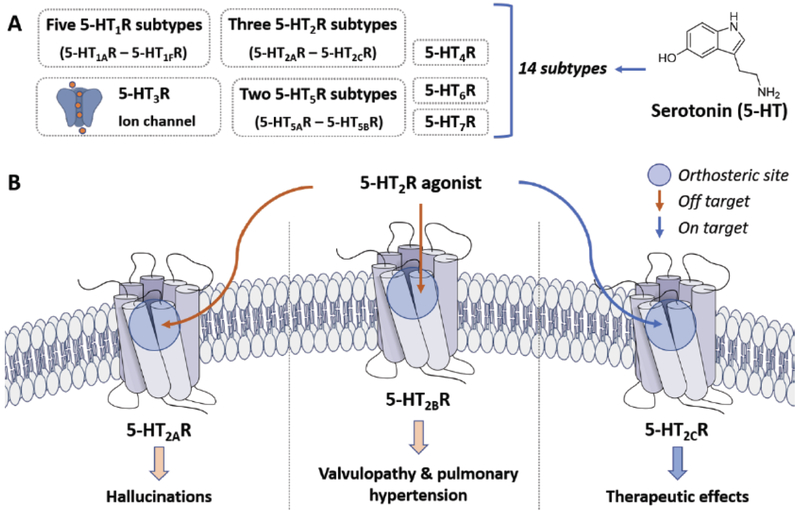
The G protein-coupled receptor (GPCR) super family, a vast group of proteins imbedded in cellular membranes, represents nearly one-third of all therapeutic targets [1]. Out of the approximately 800 different GPCRs identified in mammals, only 25% have been exploited for therapeutic development and the function of ~200 have yet to be elucidated [2]. The family of serotonin (5-HT) receptors (5-HTXRs) are believed to be, from an evolutionary standpoint, among the oldest GPCRs [3]. There are seven classes of 5-HTXRs: 5-HT1R, 5-HT2R, 5-HT3R (a ligand-gated ion channel), 5-HT4R, 5-HT5R, 5-HT6R, and 5-HT7R, which are classified based on sequence homology and functional aspects. The 5-HT2C receptor (5-HT2CR), a receptor subtype in the 5-HT2R family (5-HT2AR, 5-HT2BR, 5-HT2CR) has been increasingly investigated as a therapeutic target in recent years. Like all other GPCRs, 5-HT2CR is characterized by seven transmembrane spanning helices (TM I – VII), three extracellular (ECL 1–3) and three intracellular loops (ICL 1–3), an intracellular carboxy-terminus, and an extracellular amino-terminus (Fig. 1) [4, 5]. Importantly, the X-ray crystal structure of the 5-HT2CR has recently been solved with non-selective 5-HT agonist ergotamine (“active-like” state) or the 5-HT2R antagonist/inverse agonist ritanserin (inactive state), further allowing a critical review of the receptor’s structural features and conformations for structure-based drug design (PDB: 6BQG) [5]. Reviewing the 5-HT2CR sequence, there is approximately 80% sequence homology in the TM region between members of the 5-HT2R family, the region that forms the orthosteric binding site for 5-HT, while the ECL and ICL sequences are known to vary across receptor subtypes [6]. In addition to the sequence similarity of the orthosteric sites across the 5-HT2R family that makes the selective chemotype targeting of 5-HT2Rs difficult, there is the ubiquitous issue that 5-HT binding to all 5-HT2R, including the 5-HT2CR, results in activation of complex webs of intracellular signaling processes, several of which are inadequately appreciated at present.
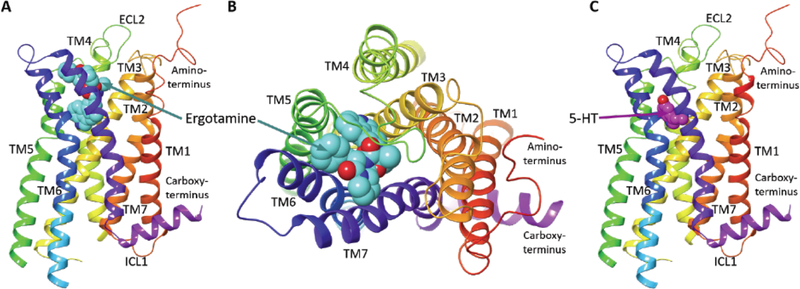
Fig. (1).
The recently solved X-ray crystal structure of the “active-like” state of the 5-HT2CR bound to ergotamine (PDB code: 6BQG) and 5-HT2CR model bound to 5-HT is depicted. (A) The side view of the ergotamine-bound 5-HT2CR crystal structure with the N- and C-termini, extracellular and intracellular loops, and the transmembrane helices labeled; ECL = extracellular loop, ICL = intracellular loop, TM = transmembrane domain helix, ergotamine (cyan space fill representation) at the orthosteric site [5]. (B) Top/extracellular-view of the ergotamine binding site. (C) The side view of a 5-HT2CR structure model based on the crystal structure with 5-HT docked to the orthosteric site; 5-HT (magenta space fill representation). The 5-HT-bound 5-HT2CR was generated via induced fit docking protocols on 6BQG using the Schrödinger Drug Discovery Suite. All ligands shown in space filling representation and CPK coloring scheme for N and O atoms.
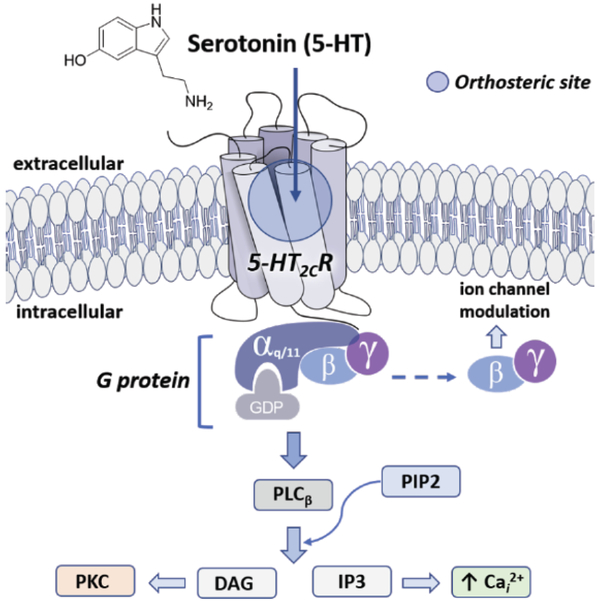
The canonical G protein-dependent signaling through the 5-HT2CR is engendered by 5-HT-stimulated coupling to Gαq/11 to activate the enzyme phospholipase Cβ (PLCβ) mediated hydrolysis of phosphatidylinositol 4,5-biphosphate (PIP2) to generate the intracellular second messenger inositol-1,4,5-trisphosphate (IP3), accumulation of the downstream IP3 metabolite inositol monophosphate (IP1), and diacylglycerol (DAG) (Fig. 2). Intracellular calcium (Cai2+) mobilization, frequently measured with calcium-binding fluorescent dyes, and IP1 levels, assessed with [3H]-inositol, are well-characterized to be elevated following the activation of the 5-HT2CR (for reviews) [7, 8]. In fact, the release of Cai2+ and/or IP1 is often utilized as a readout in functional cellular assays to measure the extent to which the 5-HT2CR is activated [9].
Fig. (2). A cross-section of the 5-HT2CR signaling webs initiated by 5-HT binding to the orthosteric site is presented.
Activation of Gαq/11 promotes phospholipase Cβ (PLCβ) mediated hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) into diacylglycerol (DAG) and inositol-1,4,5-triphosphate (IP3). IP3 promotes release of intracellular calcium (Cai2+) while DAG binds to downstream effector protein kinase C (PKC).
5-HT2CR has also been shown to regulate ion channels and transport processes as well activate other downstream effectors, including Phospholipase A2 (PLA2), Phospholipase D (PLD), cyclic nucleotides, and extracellular signal-regulated kinases (ERK1/2) [4, 10]. For instance, stimulation of the 5-HT2CR is thought to activate cytosolic PLA2 which hydrolyzes arachidonic acid-containing phospholipids to produce free arachidonic acid and a host of its metabolites that are functionally relevant in this signaling web (for review) [11]. Agonist signaling bias can result in the activation of PLCβ over PLA2, and vice versa, depending on the ligand that binds to the receptor [12]. Stimulation of the 5-HT2CR leads to the activation of protein kinase C (PKC) and downstream stimulation of the mitogen-activated protein kinase cascade resulting in the phosphorylation of ERK1/2 [13, 14]. In fact, 5-HT2CR was shown to couple ERK1/2 via a PLD-and PKC-dependent pathway possibly through Gα12/13 proteins [14]. The PLD and PKC involvement in ERK1/2 phosphorylation evoked by 5-HT2CR stimulation has recently been validated in a mouse hypothalamic cell line [15].
The signaling web for the 5-HT2CR is influenced by the fact that this receptor is the only known GPCR that under-goes RNA editing [16]. Five closely spaced adenosines within the second intracellular loop of the 5-HT2CR are subject to deamination by Adenosine Deaminases that Act on RNA (ADAR), resulting in adenosine to inosine substitution which alters the coupling efficiency between the receptor and its G protein and restricts its ability to activate intracellular cascades [17–21]. Editing allows the 5-HT2CR to exist in 32 mRNA variants that encode up to 24 predicted proteoforms [22, 23]. Thus, mRNA editing is fundamentally important to normal 5-HT2CR biological function [24]. In addition to the increased diversity of signaling afforded by RNA editing of the 5-HT2CR, evidence is accumulating that signaling via the 5-HT2CR is further diversified by the formation of oligomers, in fact the 5-HT2CR appears to function as a homodimer [25]. Heterodimers are reported to form between the different isoforms of the 5-HT2CR generated by RNA editing [26]. Likewise, 5-HT2CR has been reported to heterodimerize with the ghrelin growth hormone secretagogue receptor 1α, the melatonin MT2 receptor, and the N-methyl-D-aspartate-gated ion channel subunit GluN2A, and most recently with the 5-HT2AR and 5-HT2BR [27–32]. Intriguingly, the 5-HT2AR:5-HT2CR complex did not modify the Gαq/11 coupling of the receptor subunits, but rather the 5-HT2CR exerted dominance when in complex with the 5-HT2AR, such that only the 5-HT2CR coupled with the G protein to generate intracellular signaling; the 5-HT2AR signaling is ‘masked’ [31]. Thus, 5-HT2AR:5-HT2CR protein complex appears to be a distinct molecular species, which contributes to cellular signaling, generating unique properties when co-expressed in vitro [31].
5-HT2CR, as for other GPCRs, acts via the “receptor-some,” the composition of membrane, cytosolic and accessory proteins through which protein-protein interactions interface GPCR coupling to downstream intracellular signaling cascades to tailor cellular responsivity. For example, 5-HT2CR interacts with PSD-95/disk large/zonula occludens domain-containing proteins, calmodulin, β-arrestins, and phosphatase and tensin homolog, and all of them have been shown to modulate receptor kinetics by varying mechanisms [4, 10, 33]. Of relevance, β-arrestins are known to play a key role in desensitization and resensitization processes that regulate the functional activity of 5-HT2CR, and edited 5-HT2CR isoforms have been shown to modify the kinetics of these processes due to divergent magnitudes of association with β-arrestin [34]. Agonist-dependent desensitization is associated with 5-HT2CR phosphorylation involving G protein receptor kinase2 (GRK2), binding of β-arrestin and uncoupling of the receptor from the G protein to result in receptor internalization into endosomes; resensitization and recycling to the plasma membrane occur with dephosphorylation [17, 23]. Thus, there is a rich opportunity to regulate the biological function of the 5-HT2CR.
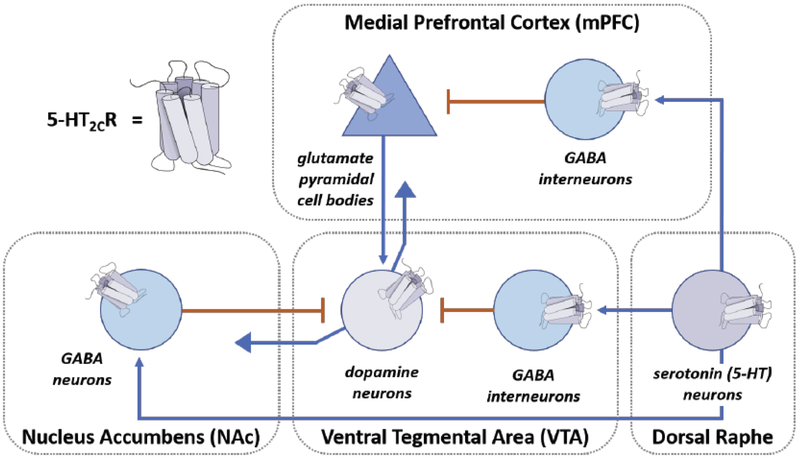
The localization of the family of 5-HT receptors is region- and cell-specific throughout the body, and this is true for 5-HT2CR, whose expression and function in the central nervous system (CNS) drives its primary, known biology. Serotonergic cell bodies project from the dorsal raphe nuclei in the midbrain to key brain regions associated with the reward pathway (e.g., ventral tegmental area, VTA; nucleus accumbens, NAc) and higher executive function (e.g., prefrontal cortex, PFC) [35, 36]. Postsynaptic expression of the 5-HT2CR is reported in various neuronal cell types including those that employ acetylcholine, dopamine, and γ-aminobutyric acid (GABA) as neurotransmitters, with a major outcome of 5-HT2CR stimulation defined as the modulation of dopamine neuronal function (for review) [35]. For example, stimulation of the 5-HT2CR in the VTA increases the firing rate of GABA interneurons, enhances basal GABA release in the VTA in a brain slice preparation, and decreases firing rates of dopaminergic neurons [37–39]. Interestingly, while 5-HT2CR antagonists have been reported to increase dopaminergic neurotransmission and dopamine levels in the NAc, local activation of the NAc 5-HT2CR in vivo suppressed potassium-stimulated GABA release [40–42]. Intriguingly, 5-HT2CR has also been shown to be expressed on VTA dopamine neurons, including those that project to the NAc [43, 44]. 5-HT2CR localized to VTA dopamine receptors has recently been demonstrated as a key regulator of their physiology as well as binge-like eating behavior in mice [45]. Thus, 5-HT2CR controls dopamine neurotransmission within the VTA as well as its target regions in the limbic-corticostriatal circuit, including the NAc and medial prefrontal cortex (mPFC) (Fig. 3) [46, 47]. These data are but a fraction of research findings that illustrate the nature and complexity of cell- and region-specific roles of this receptor in neurobiology.
Fig. (3). A schematic presentation of the proposed 5-HT2CR modulation of limbic-corticoaccumbens circuit is represented.
Serotonin (5-HT) neurons originating from the dorsal raphe nuclei innervate GABA interneurons in the medial prefrontal cortex (mPFC), nucleus accumbens (NAc), and the ventral tegmental area (VTA). Inhibitory GABAergic neurons facilitate decreased dopaminergic neuronal firing in the VTA both directly and indirectly. 5-HT2CR localizes to neurons within each of the nodes in this circuitry.
The interaction between the 5-HT2CR and 5-HT2AR was demonstrated in mPFC and the resulting signaling control in vivo is an underappreciated area of research that has recently provided clues to suggest therapeutic modalities for neuropsychiatric disorders [35, 36, 48]. A review of early work on this topic indicated a likely oppositional relationship between the 5-HT2AR and 5-HT2CR in the control of certain behaviors [49]. However, the relationship is now proving to be more interactive and synergistic in studies that show a combination of a selective 5-HT2AR antagonist and selective 5-HT2CR agonist can work in concert to suppress behaviors associated with substance use disorders (SUDs) in rodents, at doses far below the effective doses needed when administered independently [50]. This result also suggests a delicate balance of 5-HT2R signaling in healthy individuals that necessitates reliable, selective 5-HT2CR tool compounds for use in probing the mechanisms that underlie chronic psychiatric conditions. The clinical significance of selective 5-HT2CR modulation alone has recently been validated by the approval of the 5-HT2CR agonist lorcaserin (14, Fig. 5) for the treatment of obesity [51, 52]. Given the notion that 5-HT2CR can exert inhibitory control over dopaminergic tone in brain regions that drive reward-related behaviors and cortical areas of the brain responsible for higher order executive functions related to acting impulsively and reacting to external cues, the development of 5-HT2CR ligands is a promising approach to treating obesity, SUDs, and other neuropsychiatric conditions [53–55]. Additionally, improved and functionally diverse 5-HT2CR agonists may enable beneficial combination therapies with 5-HT2AR-acting medications. However, the development of selective small molecule 5-HT2CR agonists remains a challenge and the chemical diversity of known synthetic 5-HT2CR agonists is minimal.
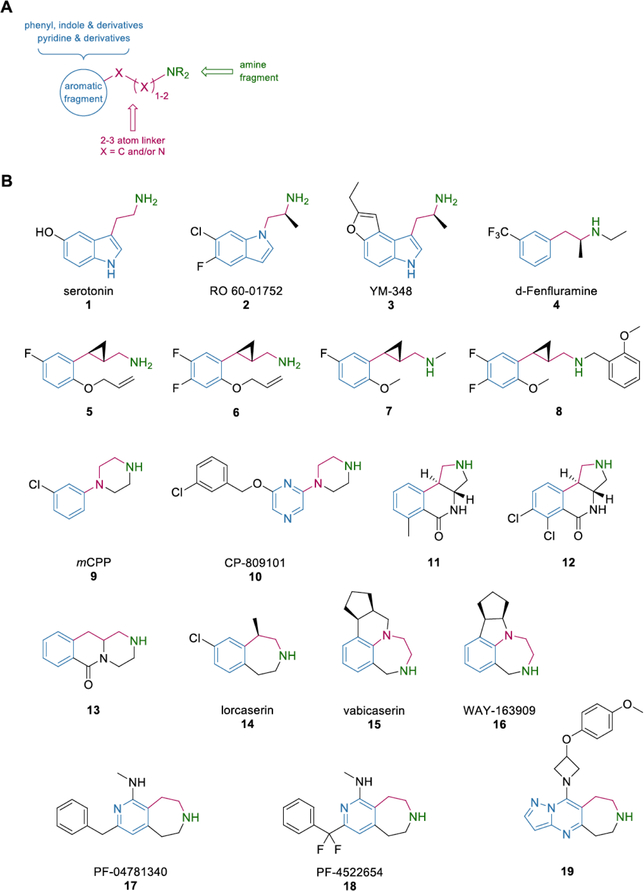
Fig. (5). Current synthetic 5-HT2CR agonists share similarities in their pharmacophore.
(A) The general chemical scheme describing structural requirements for 5-HT2CR agonists is presented. (B) Selected 5-HT2CR agonists are organized based on chemotype. Blue indicates the common aromatic fragment, green indicates the requisite ionizable amine, and magenta highlights the linker.
2. 5-HT2CR AS A THERAPEUTIC TARGET
5-HT2CR has been targeted for the development of therapeutics for several chronic pathological disorders. Agonists have been suggested for the treatment of obesity, SUDs and impulse control disorders while antagonists may add value in the treatment of anxiety, depression and schizophrenia. In particular, a strong case for the use of a selective 5-HT2CR agonist was made for obesity. The constitutive 5-HT2CR knockout mouse exhibited hypophagia and increased body mass in the context of both insulin resistance and late-onset obesity. Weight gain as well as a greater relative risk of metabolic dysfunction and diabetes occurs upon chronic treatment with atypical antipsychotics with 5-HT2CR antagonist properties (e.g., olanzapine) in humans and animals [56–59]. Selective 5-HT2CR agonists have been consistently demonstrated to suppress food intake [56, 60–62], in fact, the selective 5-HT2CR agonist WAY163909 dose-dependently decreased food intake in normal Sprague–Dawley rats, obese Zuker rats and mice with diet-induced obesity [63]. Investigations of 5-HT involvement in the mechanisms underlying satiety have focused predominantly on neural loci in the hypothalamus and midbrain/hindbrain circuits which synchronize energy balance and glucose homeostasis in concert with peripheral systems (for reviews) [64, 65]. Based upon these findings, lorcaserin (Belviq®) was approved by the U.S. Federal Drug Administration (FDA) as the first-in-class 5-HT2CR agonist marketed for weight reduction in patients with a body-to-mass (BMI) index of >30 or with a BMI >27 comorbid with type-2 diabetes, hypertension or dyslipidemia [51, 66].
Extensive preclinical studies have also demonstrated the role of the 5-HT2CR in the rewarding and incentive-salience value of cocaine, other psychostimulants, ethanol and, most recently, opioids as well as factors involved in relapse vulnerability during recovery from SUDs [35, 48, 67–69]. For example, employing the self-administration assay, the preclinical model with the best validity for human drug-taking, systemic administration of a selective 5-HT2CR agonist suppressed cocaine intake and the resurgence of drug-seeking evoked by pretreatment with cocaine or exposure to cocaine-associated cues [i.e., drug-taking environment, and the discrete cues associated with previous cocaine delivery (e.g., lights, tones)] [70–72]. These effects of the 5-HT2CR agonist are reversed by a selective 5-HT2CR antagonist. Conversely, pretreatment with a selective 5-HT2CR antagonist enhanced self-administration of low doses of cocaine and cocaine-evoked reinstatement of drug-seeking, while the selective 5-HT2CR antagonist SB242084 is self-administered in primates [73–76]. In humans, lorcaserin improved smoking cessation rates and significantly decreased corticolimbic activation elicited by palatable food cue exposure, further supporting the role of the 5-HT2CR in as a common mediator of reward and cue-associated events across abused drugs and palatable food [77, 78]. The added value of lorcaserin to suppress the rewarding effects of cocaine and other abused drugs imputes a further dimension of likely therapeutic utility [36, 48, 50], and lorcaserin is currently in clinical trials for Cocaine Use Disorder (CUD) and other SUDs (clinicaltrials.gov; accessed May 6, 2019).
Serotonin is one of the primary regulators of behavioral inhibition, a fundamental aspect of impulse control. Impulsivity, a predisposition toward rapid unplanned reactions to stimuli without regard to the negative consequences contributes to initial drug use and is perpetrated by continued use of the abused drug (for reviews) [79, 80]. Intriguingly, individuals with poor impulse control may be more vulnerable to drug-associated stimuli, and less capable of engaging processes that override heightened attentional bias for cues [81, 82]. The role of the 5-HT2CR in these interlocked behavioral phenotypes has been demonstrated in that selective 5-HT2CR agonists consistently reduce and the 5-HT2CR antagonist SB242084 enhance motor impulsivity, while mice with a constitutive loss of the 5-HT2CR exhibit elevated motor impulsivity [33, 71, 83–85]. The status of 5-HT2CR function in the mPFC appears to be a contributor to the vulnerability of impulsive rats to cocaine reward and sensitivity to cue-associated relapse vulnerability, which is interwoven with vulnerability to endogenous factors (e.g., craving, stress, withdrawal), all of which can serve as immediate antecedents to relapse [48, 86]. Relapse is a major hurdle for the successful treatment of chronic SUD and a pharmacotherapy that can improve inhibitory control would represent a first-in-class drug for those sufferers [87, 88]. Thus, the 5-HT2CR is a potential therapeutic target at the intersection of disinhibited behaviors that contribute to obesity and SUD. Given that obesity is at epidemic levels in the U.S. while drug overdoses and SUDs continue to drive unprecedented mortality and morbidity rates, targeting the 5-HT2CR is an attractive and urgent approach towards the development of a neuropharmacotherapy to treat these intractable public health concerns.
Selective targeting of the 5-HT2CR remains a challenge for medicinal chemists primarily due to the fourteen 5-HT receptor subtypes distributed throughout the body in various cell types and tissues and that each receptor accommodates 5-HT at a conserved orthosteric binding site. It is well established that, when only comparing the 5-HT2R subtypes (5-HT2AR, 5-HT2BR, and 5-HT2CR), receptor subtype selectivity is a major determining factor with regard to the scale of potential therapeutic utility (Fig. 4). With the understanding that 5-HT2AR or 5-HT2BR agonists are expected to evoke hallucinations or cardiac valvulopathy, respectively, the need for 5-HT2CR orthosteric agonists that lack demonstrable efficacy at 5-HT2AR and 5-HT2BR sharpened [89, 90]. For example, the potent 5-HT releaser fenfluramine was approved and marketed in the U.S. as an appetite suppressant in combination with the norepinephrine releaser phentermine (Fen-Phen) in 1983. A proportion of patients treated with fenfluramine developed pulmonary hypertension and valvular heart disease, resulting in its withdrawal from the market in 1997 and subsequent legal damages exceeding $10 billion U.S. [91]. Activation of the 5-HT2BR on heart values by the fenfluramine metabolite norfenfluramine is the primary pathogenic mechanism [90]. An additional example of this scale in clinical utility stems from concern over lorcaserin which has a low potential for abuse but could lead to positive subjective effects at supratherapeutic doses, based upon its action as a partial agonist at the 5-HT2AR, which is responsible for hallucinogenic actions [92]. The FDA deliberations let to a Schedule IV classification for lorcaserin under the Controlled Substances Act. Thus, the therapeutic utility of lorcaserin (14; Fig. 5) in the general population is limited by concern of side effects [92, 93].
Fig. (4). Proposed outcomes mediated by activation of specific members of the 5-HT2R family are depicted.
(A) The 5-HT receptor family is comprised of 13 GPCRs and one ligand-gated ion channel (divided into seven subtype categories). Each receptor is activated by endogenous 5-HT. Therefore, the orthosteric site is highly conserved across receptor subtypes. (B) Agonist-mediated activation of 5-HT2AR accounts for the hallucinogenic actions of such abused drugs as d-lysergic acid diethylamide. Agonist-mediated activation of 5-HT2BR can lead to pulmonary hypertension and valvular heart disease. Agonist-mediated activation of 5-HT2CR represents an attractive therapeutic approach to treat obesity, substance use disorders and impulse control disorders. However, the high sequence homology at the orthosteric sites of these receptors present challenges to selectively target the 5-HT2CR.
3. THE CURRENT STATE OF 5-HT2CR AGONISTS
Targeting the 5-HT2CR for therapeutic purposes has led to the discovery of many ligands that vary in activity and selectivity yet, in a general sense, come from very similar chemotypes. A brief structural survey of the most selective 5-HT2CR agonists highlights the lack of chemical diversity, which in turn leads to a limited selection of tool compounds (Fig. 5). Coupled with the reality that the 5-HT2R subtype orthosteric sites share high homology, agonists that lack diverse scaffolds are historically unlikely to overcome the subtype selectivity challenge. Virtually all compounds shown in (Fig. 5) rely on the phenethylamine moiety or a derivative thereof. More accurately, each of the indicated agonists require a general scaffold that incorporates an aromatic ring attached to an ionizable nitrogen via a two-three atom linker, usually carbon (Fig. 5). While many examples of 5-HT2CR agonists from the aforementioned chemotypes are known, most do not demonstrate an acceptable functional selectivity for the 5-HT2CR over the 5-HT2AR and 5-HT2BR (Table 1). Though several compounds show promise, even the most selective compounds are similar to 14, while the most selective compound listed herein, CP-809101 (10), has been shown to produce genotoxic effects [94]. As observed in early work on 5-HT2CR agonist discovery, novel scaffolds that deviate from the traditional chemotype and can selectively target the 5-HT2CR are difficult to discover and further exploration of chemical space may yield therapeutically important scaffolds.
Table 1.
Functional profiling of numerous 5-HT2CR agonists and respective profiles at the 5-HT2AR and 5-HT2BR to show subtype selectivity among 5-HT2Rs.
| Compound | 5-HT2CR | 5-HT2AR | 5-HT2BR | Refs. | |||||
|---|---|---|---|---|---|---|---|---|---|
| EC50 (nM) | Emax (%) | EC50 (nM) | Emax (%) | EC50 (nM) | Emax (%) | 2A/2C ratio | 2B/2C ratio | ||
| 5-HT (1) | 3.48 | 100 | 14.04 | 100 | 20 ± 2.8 | 100 | 4.03 | 5.75 | [95] |
| RO 60–0175 (2) | 52 ± 3 | 88 ± 20 | 400 ± 20 | 91 ± 5 | 2.4 ± 1 | 130 ± 30 | 7.69 | 0.05 | [96] |
| YM-348 (3) | 1.0 ± 0.2 | 76 ± 1 | 93 ± 10 | 97 ± 2 | 3.2 ± 3 | 110 ± 10 | 93.0 | 3.2 | [97] |
| d-fenfluramine (4) | 300 ± 29 | 90 ± 9 | 720 ± 77 | 80 ± 4 | 23 ± 4 | 100 ± 10 | 2.4 | 0.08 | [98] |
| 5 | 4.2 | 87 | 374 | 56 | NE | NE | 89.0 | - | [99] |
| 6 | 8.7 | 97 ± 1 | 491 | 21 ± 0 | 1745 | 35 ± 7 | 57.1 | 200.6 | [100] |
| 7 | 7.65 | 71 | 6.57 | 79 | 7.05 | 54 | 0.86 | 0.92 | [101] |
| 8 | 7.63 | 92 | NE | NE | 6.86 | 63 | - | 0.90 | [63] |
| mCPP (9) | 120 ± 10 | 63 ± 3 | 150 ± 20 | 18 ± 2 | 93 ± 50 | 21 ± 9 | 1.25 | 0.78 | [97] |
| CP-809101 (10) | 0.11 | 93 | 153 | 67 | 65.3 | 57 | 1391 | 593.6 | [102] |
| 11 | 1.1 ± 0.4 | 90 | 127 ± 82 | 100 | 94 ± 68 | 50 | 115.5 | 85.5 | [103] |
| 12 | 3.3 ± 1.8 | 90 | 128 ± 44 | 100 | 414 ± 468 | 130 | 38.8 | 125.5 | [103] |
| 13 | 36 | 100 | 707 | 100 | >10000 | - | 19.6 | >277.8 | [104] |
| lorcaserin (14) | 9 ± 0.5 | 100 | 168 ± 11 | 75 | 943 ± 90 | 100 | 18.7 | 104.8 | [51] |
| vabicaserin (15) | 8 | 100 | 1650 | -a | >10,000 | 80 | 206.3 | 0.19 | [105] |
| WAY-163909 (16) | 8 ± 3 | 90 ± 6 | NE | NE | 185 ± 105 | 40 ± 3 | - | 23.1 | [63] |
| PF-04781340 (17) | 9 | 99 | - | - | 1484 | 69 | - | 164 | [106] |
| PF-4522654 (18) | 16 | 58 | NE | NE | >10000 | <10 | - | >625 | [107] |
| 19 | 95 | 76 | 2634 | 21 | 503 | 31 | 27.7 | 5.3 | [108] |
| 20 | 5 ± 3 | - | 635 ± 862 | - | 48 ± 41 | - | 127 | 9.6 | [109] |
Functional output indicated antagonist activity; Values with error represent the mean ± SD; For 5-HT2AR and 5-HT2BR, large reported errors indicate inconsistent results from compounds showing no effect and intermittent positive effects; No effect (NE); Not applicable or not tested (−).
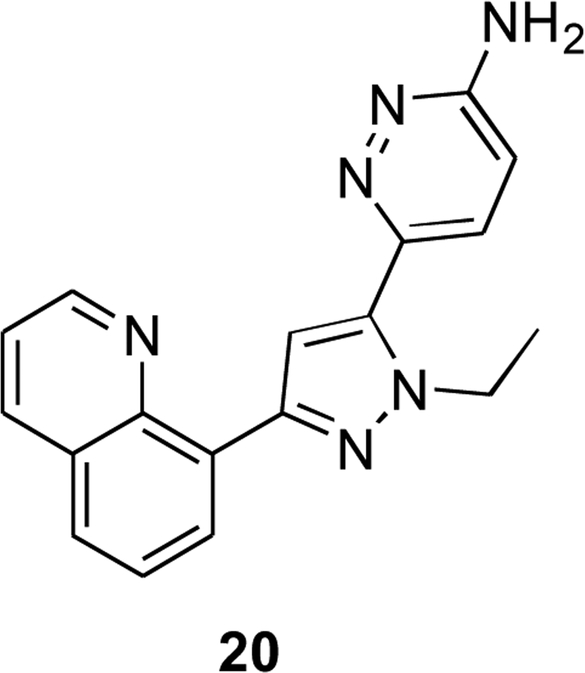
Within the past few years, there has been renewed attention on the 5-HT2CR and innovative drug discovery strategies have been employed to probe the chemical space for 5-HT2CR agents and also to uncover unique functional characteristics of the 5-HT2CR. Towards novel chemotype discovery, Wacker and co-workers at Bristol-Myers Squibb reported a series of analogues lacking a basic amine interaction at the active site Asp 134 residue, which is a requisite interaction for traditional 5-HT2CR synthetic agonists and 5-HT [109]. Noteworthy contributions from this work included the screening of ligands in cells expressing the edited (VNV) isoform of the 5-HT2CR containing a residue mutation, replacing the active site Asp 134 residue with Ala (D134A). This mutant allowed the subsequent screening of agonist hits, found via traditional compound library screening techniques, to selectively identify and optimize atypical agonists. Compound 20 was obtained after rounds of optimization to a non-basic heterocyclic amide agonist hit and was administered orally to rats, displaying a reduction in food intake that could be reversed via a 5-HT2CR antagonist (Fig. 6). Additionally, Wacker and co-workers reported that the initial hit compounds were discovered during a search for 5-HT2CR positive allosteric modulators (PAMs), further demonstrating the increased interest in diverse approaches for potentiating 5-HT2CR signaling.
Fig. (6). A novel pharmacophore for 5-HT2CR agonists is described.
Compound 20 is the result of an intentional screening and optimization study which sought to discover non-traditional 5-HT2CR ligands, as defined by an aromatic ring joined to an amine fragment by a short aliphatic linker [109].
A phenomenon in GPCR signaling known as signaling bias or functional selectivity is evident under conditions in which an agonist displays divergent levels of activation through the multiple signaling pathways linked to receptor activation. Functional selectivity is well-characterized for the 5-HT2CR [23, 101]. For example, compounds 7 and 8 reported by Kozikowski and colleagues display preferential activation through the Gαq-mediated signaling pathway (Fig. 5) [101]. Significantly, incorporation of the N-benzyl moiety that differentiates 8 from 7 leads to a complete loss of function at the 5-HT2BR, while retaining efficacy for the 5-HT2CR. This compound (8) suppressed amphetamine-induced hyperactivity in rodents, consistent with the efficacy of other 5-HT2CR agonists [101]. Additionally, a recent manuscript by Booth and coworkers provides a further investigation into 5-HT2CR desensitization after agonist activation, which is understood to be mediated to some extent by the β-arrestin signaling pathway [110]. Multiple agonists were tested in a PLCβ-activation desensitization assay, including 5-HT (1), 14 and m-chlorophenylpiperazine (mCPP) (9) (Fig. 5). The magnitude of 5-HT2CR-mediated PLCβ activation correlated with desensitization in their assay. Furthermore, the selection of agonists was assessed in a β-arrestin recruitment assay, where a correlation between desensitization and β-arrestin recruitment was observed. Expanded chemical space, functional selectivity, and 5-HT2CR desensitization are concepts that, when incorporated into ligand discovery, will greatly aid in the development of novel 5-HT2CR agonists with improved selectivity and functional profiles. However, there continues to be a lack of subtype selective, novel chemotypes that display significant clinical potential, which is evidenced by a recent surge of interest in targeting spatially distinct, allosteric binding sites.
4. BACKGROUND AND RATIONALE FOR 5-HT2CR ALLOSTERIC MODULATORS
Agonists and antagonists targeted to GPCRs that are traditionally designed to bind to a receptor orthosteric site, which has co-evolved with an endogenous ligand for a given receptor. However, in many cases, endogenous signaling ligands activate multiple receptors that are classified in a family. To accommodate the same signaling ligand, the orthosteric site is highly conserved among GPCR family members, which may modulate diverse physiological functions in distinct tissues. Exemplary GPCR families include the metabotropic glutamate receptors (mGluRs) and 5-HTXRs which are comprised of eight and 14 receptor subtypes respectively [67, 111]. Thus, targeting the orthosteric site of the 5-HT2CR among closely related subtypes, 5-HT2AR and 5-HT2BR, has remained a challenge for medicinal chemists.
One strategy that has emerged over the past decade for the selective modulation of GPCRs is the identification and targeting of allosteric sites, which are defined as ligand binding sites that are spatially distinct from an orthosteric site [112]. In many cases, allosteric sites have proven to be less conserved than the respective receptor orthosteric sites, leading to improved ligand selectivity across subtypes [113–116]. Thus, allosteric modulation provides an opportunity to specifically target receptors that belong to a subfamily of similar GPCRs, potentially minimizing off-target effects, a significant advantage over typical agonists that at the endogenous ligand binding pocket. Additionally, most GPCR-targeted neurotherapeutic regimens result in chronic exposure of the receptor to orthosteric ligands, differing from the temporal control of endogenous ligands, which has been shown to alter 5-HT2CR trafficking kinetics due to internalization and desensitization and resensitization processes [110, 117]. An allosteric modulator may minimize the aforementioned detrimental effects of synthetic agonists by maintaining the natural spatial and temporal signaling characteristics that are key features of neuronal circuitry [118]. Additionally, the fine-tuning of GPCR signaling may be afforded by allosteric ligand structural modifications that result in separate control of orthosteric ligand affinity or efficacy [119].
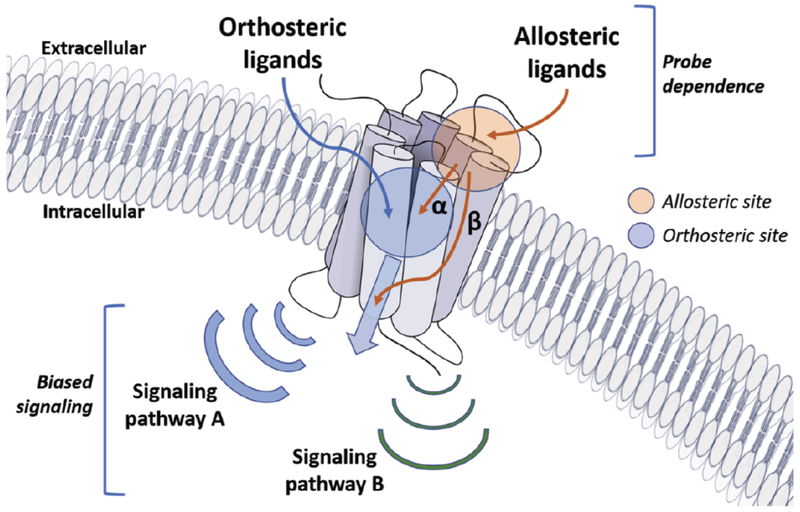
Theoretically, small molecule allosteric modulators of the 5-HT2CR can promote a conformational change in the receptor or stabilize certain conformational populations of the receptor that produce several possible outcomes when coupled with agonist (e.g., 5-HT) binding: (i) positive allosteric modulators (PAMs) increase the binding affinity and/or efficacy of orthosteric ligands, (ii) negative allosteric modulators (NAMs) decrease binding affinity and/or efficacy of the orthosteric ligand, and (iii) neutral allosteric ligands (NALs) bind to the allosteric site without actuating a change in orthosteric ligand binding or efficacy [120–122]. Another important, yet understudied aspect of 5-HT2CR allosteric modulation is the potential leveraging of biased signaling (i.e., promotion of one signaling pathway over another at the same receptor) or probe dependence (differing signaling outcomes based on the identity of the orthosteric ligand), which may be exploited as a novel modality toward the treatment of complex neuropsychiatric disorders (Fig. 7) [123]. Importantly, from a structural biology perspective, rapid progress is being made in the analysis of structural determinants for allosteric modulator function at well-studied GPCRs and an X-ray crystal structure of the 5-HT2CR is now available that is amenable to allosteric modulator docking and modelling [5, 124]. From the pharmacology and medicinal chemistry perspectives, key concepts with regard to GPCR allosteric modulator discovery and development have been extensively analyzed and reported, with a major focus on mGluR and muscarinic receptor allosteric modulators [125, 126]. These insights on how to approach the development of GPCR allosteric modulators lay the groundwork for the exploration of allosteric modulation at other GPCRs, including efforts to develop allosteric modulators for the 5-HT2CR [127].
Fig. (7). A schematic presentation of potential allosteric modulation of GPCR signal transduction is depicted.
The allosteric ligand binds to a site (orange circle) that is distinctly different than the orthosteric site (blue circle) which accommodates the orthosteric ligand. Actual binding site locations are generally dictated by the specific GPCR family member. Allosteric modulators can modulate binding affinity (α) and/or efficacy (β) of orthosteric ligands in a positive (PAM) or negative manner (NAM), or may simply occupy the site as a neutral allosteric ligand (NAL). The nature of the modulation is determined by several features of signaling including the orthosteric ligand employed (i.e., probe dependence) as well as the ligand-dependent selectivity for certain downstream transduction mechanisms within a given cell (i.e., biased signaling).
Certain distinctive challenges exist in the development of GPCR allosteric modulators, which are applicable to the 5-HT2CR (see reviews) [113, 116, 128]. For instance, less-conserved allosteric sites across a subfamily of receptors (e.g., 5-HT2Rs) that are a result of decreased evolutionary pressure at these sites might lead to residue or structural differences in the allosteric site of the 5-HT2CR between species. Additionally, most drug discovery initiatives in this arena ascribe pharmacological profiles to discovered compounds based upon screening in cells expressing the human unedited (INI) 5-HT2CR, however, this is not the most abundant isoform localized in brain and the importance of the diversity of signaling afforded by RNA editing of the 5-HT2CR is left out of the equation of developing new ligands in this chemical space. The potential of probe dependence of novel 5-HT2CR allosteric modulators necessitates careful selection of orthosteric ligands for assays in which the endogenous ligand 5-HT is a preferred choice. However, efforts to selectively potentiate the FDA-approved anti-obesity medication and 5-HT2CR agonist 14 at the 5-HT2CR over other 5-HT2Rs should preferentially employ 14 in cell-based screening assays. Further, allosteric modulator design may suffer from a featureless or “flat” structure-activity relationship (SAR) if only binding affinity or efficacy is considered without appreciation of other parameters such as the cooperativity between the affinity and efficacy of the allosteric and orthosteric ligands. Elegant work has been accomplished to aid in the quantification of allosteric effects through the development of the “operational model of allostery”, which may be employed to aid the analysis of SAR and appropriately define allosteric modulator mechanism [114]. Given that 5-HT2CR agonists interact at the conserved orthosteric site of the receptor and, in general, 5-HT2CR agonists are of similar chemotypes, PAMs targeting a topologically distinct site may be ideal for the discovery of selective small molecules with expanded clinical utility.
5. THE CURRENT STATE OF 5-HT2CR POSITIVE ALLOSTERIC MODULATORS
The design of allosteric modulators for GPCRs is a relatively recent endeavor and interest in designing allosteric modulators for numerous, diverse GPCRs is increasing (see reviews) [112, 113, 129]. Very few examples of allosteric modulators of 5-HTXRs are known. Provided with consistent evidence that activation of the 5-HT2CR will afford therapeutic benefits, it is presumed that 5-HT2CR PAMs will engender therapeutic benefits alongside the intrinsic advantages of allosteric modulation.
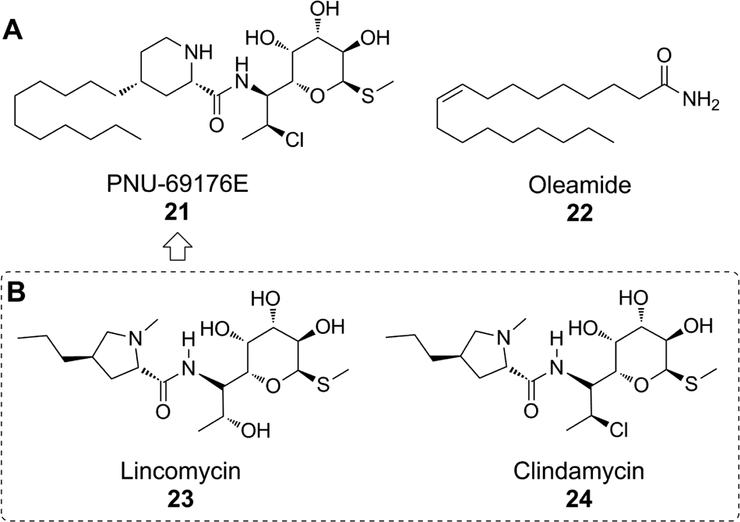
The discovery of PNU-69176E (21) as the first known subtype-selective 5-HT2CR PAM was the result of an early, undescribed screening of a chemical library by Pharmacia (now Pfizer) in 1999 (Fig. 8) [130]. Structurally, 21 is an analogue of the natural product antibiotic lincomycin (23) and a derivative of the clinically available clindamycin (24) (Fig. 8) [131]. As such, 21 was likely the product of an antibiotic medicinal chemistry campaign. The compound consists of three readily identifiable fragments. At the core is a piperidinyl carboxamide displaying a 2,4-cis geometry. A distinct Polar Head (PH) moiety comprised of an α-D-galactopyranoside and an undecyl lipophilic tail (LT) contribute to the stereochemistry- and sp3-rich (tetrahedral) framework, a significant departure from known 5-HT2CR activators and small molecule therapeutics in general [132]. The natural product-like framework can allow for a more robust exploration of the chemical space in three-dimensions versus the typical sp2-rich (planar) carbon frameworks commonly employed in medicinal chemistry as a result of the current state of metal-mediated coupling chemistry. However, the calculated physicochemical parameters of 21 are less than ideal according to Lipinski’s Rule of Five [e.g., total polar surface area (TPSA) = 111.04, ClogP = 4.90, molecular weight (MW) = 537.21, hydrogen bond donors (HBD) = 5].
Fig. (8).
(A) The earliest reported small molecules with allosteric modulatory activity at the 5-HT2CR are PNU-69176E (21) and oleamide (22). (B) PNU-69176E is an analogue of the natural product antibiotic lincomycin (23) and a derivative of the clinically available antibiotic clindamycin (24).
Our team validated 5-HT2CR PAM activity for compound 21 via in-house synthesis and pharmacological characterization in a cell-based assay used to measure Cai2+ release as a result of 5-HT2CR activation [132]. Compound 21 potentiated Cai2+ release evoked by 5-HT [0.3 nM, a concentration that induced ~20% of maximal Cai2+ release (EC20)] in cells stably expressing physiological levels of the unedited (INI), human isoform of the 5-HT2CR. Subsequently, 21 or its diastereomer were added to provide titration curves used to elucidate the extent to which 21 potentiated Cai2+ release in the presence of 5-HT [132]. We found that 21 enhanced 5-HT-induced Cai2+ release over a range of concentrations. On the other hand, the diastereomer did not potentiate 5-HT2CR-mediated signaling under the same conditions, indicating a stereochemical requirement for the PAM activity. Consistent with PAM activity, 21 did not display intrinsic activity as a 5-HT2CR agonist. Furthermore, the 21 did not alter Cai2+ release in cells stably expressing the highly homologous human 5-HT2AR, underscoring that this scaffold-type can allow for subtype selective targeting of 5-HT2Rs.
An additional compound that was characterized in earlier work as an allosteric modulator of the 5-HT2CR was oleamide (22), the primary amide of oleic acid (Fig. 8). Structurally, oleamide contains a primary amide and a long hydrophobic tail that contains 17 carbons and a cis-double bond at the 9-position. Compound 22 was discovered to exist in the cerebrospinal fluid of sleep-deprived cats and has been shown to modulate sleep in rats [127]. Evidence of its de novo synthesis from rat brain microsomes has been reported [133–135]. This naturally occurring compound has been reported to exhibit a complex pharmacological profile interacting with systems including cannabinoid receptors and various 5-HTXRs [136, 137]. Initial investigations illustrated that 22 positively modulated 5-HT-induced 5-HT2AR- and 5-HT2CR-mediated chloride currents in Xenopus oocytes [138]. A follow-up study demonstrated that 22 may act as an allosteric modulator at the 5-HT2AR by increasing 5-HT-induced potentiation of phosphoinositide hydrolysis in vitro while no effect was observed in the absence of 5-HT. Additionally, in the same study, 22 acted as a NAM at the 5-HT7R, leading to a decreased production of cyclic adenosine monophosphate (cAMP) in the presence of 5-HT. However, in the absence of 5-HT, 22 displayed partial agonist activity and promoted cAMP production via an allosteric site [139]. An analysis of 22 and related compounds was conducted to establish ligand subtype selectivity with respect to the 5-HT2AR versus the 5-HT1AR. Compound 22 displayed no selectivity and potentiated signaling through both receptor subtypes in the presence of 5-HT [137]. In addition to functional modulation of the 5-HT7R, 22 induced a robust increase in 5-HT binding affinity to the 5-HT7R orthosteric site [140]. Behavioral pharmacological studies in rodents agree with the in vitro observations [135, 136, 141]. Given the complex pharmacology of 22, further investigation into the optimization of this compound as a potential therapeutic is warranted.
Recently, three groups have embarked on drug discovery campaigns to identify 5-HT2CR PAMs and as a result, there are three new synthetic 5-HT2CR lead PAMs that display interesting scaffold diversity (Fig. 9). In a trend seen among other GPCR allosteric modulators, these compounds are characterized as generally lipophilic, with each displaying a CLogP greater than 4, as calculated by the BioByte algorithm via ChemDraw Professional. While lipophilic small molecules are understood to engender an enhanced ability to pass through the hydrophobic cell phospholipid bilayer and passively diffuse into the CNS, additional pharmacodynamic obstacles are faced when compounds are highly lipophilic [142–144]. Thus, these 5-HT2CR PAMs represent the beginning stages of drug discovery in this space.
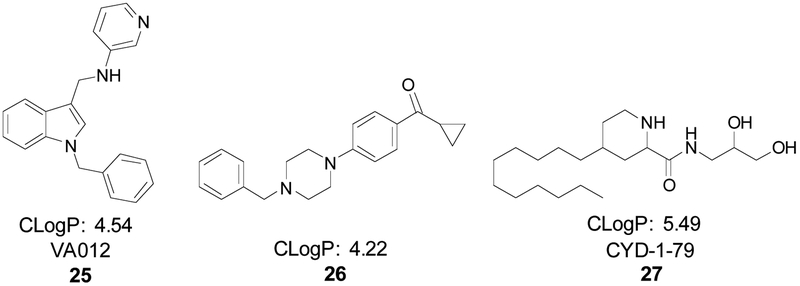
Fig. (9). A new generation of 5-HT2CR PAMs has emerged.
The lead compounds from recent 5-HT2CR PAM discovery projects include VA012 (25) [142], 26 [143], and CYD-1–79 (27) [121] respectively. The CLogPs are reported to demonstrate that elevated lipophilicity is common among these newly discovered 5-HT2CR PAMs.
López-Rodríguez and colleagues reported the first of the recent synthetic 5-HT2CR PAMs, VA012 (25), in a manuscript published in 2017 (Fig. 9) [142]. The team from Vivia Biotech implemented a proprietary high-throughput screening method based on whole-cell flow cytometry, termed Ex-viTech, in which ~1,600 small molecules from an in-house library were screened against the 5-HT2CR at 10 μM concentration in HeLa cells stably expressing physiological levels of the human 5-HT2CR. In the same manner, hits from the 5-HT2CR-based assay were counter-screened at the 5-HT2AR and 5-HT2BR. Hit compound activity was validated via a functional cell-based assay designed to measure IP1 levels, a well-known component of the Gαq signaling cascade. The reported compounds are characterized by a common 1-benzyl-1H-indole scaffold with various cyclic and heterocyclic moieties substituted at the 3 position. The necessity of an aromatic moiety at the indole 1 position was probed by substituting a cyclopropane ring in this position, which resulted in the loss of PAM activity. The project yielded 25, which was subsequently evaluated for off-target effects showing no effects in a GPCR panel and brain penetration showing a brain-plasma ratio of 3.8 after 120 min at 10 mg/kg in rodents. In an in vitro IP1-based functional assay, 25 potentiated a 5-HT-induced functional response in a concentration-dependent manner at the 5-HT2CR and did not display significant 5-HT binding inhibition at 10 μM, as tested in an in vitro competition binding assay. In vivo, 25 reduced food intake and body weight gain in a rodent feeding model without producing CNS-related malaise or resulting in taste aversion to 25. These results support the assertion that 5-HT2CR PAMs may hold therapeutic potential for obesity.
Our group reported a different strategy towards identifying novel 5-Ht2CR PAMs in 2019 [121]. Following on our work characterizing 21, we sought to deconstruct this hit into a simplified pharmacophore in which a piperidine core was flanked by an undecyl carbon chain at the 4 position and a simplified alcohol connected by an amide linker at the 2 position [132]. Our primary goal was to improve the drug-likeness of 21, which was already shown to be subtype selective, by replacing the complex α-D-galactopyranoside with a simplified fragment that retained activity to investigate the therapeutic potential of 5-HT2CR PAMs in vivo. The resulting lead compound, CYD-1–79 (27), was discovered by substituting a propanediol moiety in place of the α-D-galactopyranoside, which engendered a more drug-like profile and improved synthetic scale-up feasibility. Compound 27 was confirmed to be an in vivo rodent model candidate following in vitro pharmacological evaluation, off-target panel screening, and pharmacokinetic/pharmacodynamic profiling (Fig. 9).
Specifically, 27 enhanced in vitro 5-HT2CR functional response to 5-HT in a concentration-dependent manner and was inactive in a similar assay at the 5-HT2AR. A radioligand competition binding assay was used to profile off-target interactions and resulted in no significant binding inhibition at any 5-HTXR family member. Compound 27 was assessed in a battery of rat behavioral assays following an in vivo rat pharmacokinetic evaluation that showed reasonable oral bioavailability (F% = 39.1) and half-life (T1/2 = 5.82 ± 0.37 h). We found that 27 suppressed motor activity in the presence of exogenous 5-HT2CR stimulation with the selective 5-HT2CR agonist 16. In rats trained to discriminate the 5-HT2CR agonist 16 from saline, compound 27 partially substituted for 16 and synergized with a low dose of 16 to substitute fully for the stimulus effects of 16. Given that the drug discrimination assay has face validity for modeling the subjective effects of drugs that penetrate the blood-brain barrier and has been employed to establish allosteric modulator effects on the interoceptive effects of receptor-selective agonists, these data support the contention that 27 is a 5-HT2CR PAM [145–147]. Lastly, in a cocaine self-administration model in rats, 27 suppressed cocaine-seeking provoked by exposure to the cocaine-taking environment and cocaine-associated cues. The sensitivity to cocaine-associated cues increases the risk of craving and relapse during abstinence, thus, these findings suggest that 27 may provide value as a therapeutic strategy to extend recovery in CUD.
The latest 5-HT2CR PAM in this trio is 26 which was reported by Yadav and colleagues in 2019 (Fig. 9) [143]. This work identifies a phenyl cyclopropyl-linked N-heterocycle pharmacophore as producing multiple derivatives with PAM activity at the 5-HT2CR and one compound (26) with additional NAM activity at the 5-HT2BR. Robust SAR studies were reported for their scaffold of interest, however the origin of this pharmacophore and the rationale for designing N-heterocycle compounds as potential 5-HT2CR PAMs was not fully disclosed. Interestingly, using an in vitro luciferase-based assay, 27 was shown to enhance a 5-HT-mediated functional response at the 5-HT2CR up to 139% Emax, while additionally reducing 5-HT potency at the 5-HT2BR with no effect on 5-HT Emax at the 5-HT2BR. In agreement with its in vitro characterization as a 5-HT2CR PAM, 26 decreased food intake with approximately equivalent efficacy as lorcaserin in Sprague Dawley rats. Thus, this report provides additional in vivo evidence that 5-HT2CR PAMs may prove to be therapeutically important molecules for obesity and additional optimization should proceed.
CONCLUSION AND FUTURE DIRECTIONS
5-HT2CR is an important clinical target for obesity and several chronic pathological disorders [122], and will certainly continue to be actively pursued for therapeutic development as well as further study of the complex neurobiology that is coordinated through the 5-HT2CR. One such aspect that requires further study is the signaling and trafficking mechanisms of class A GPCRs in their oligomeric forms. Specifically for the 5-HT2CR, it is important to understand the functional implications of homodimerization (two 5-HT2CR proteins) or heterodimerization (one 5-HT2CR protein interacting with, for example, one 5-HT2AR protein), which is only beginning to be unraveled by a few research groups [28, 29, 148]. As new biological information is available, medicinal chemists will be enabled to strategically design new compounds to modulate oligomerization or biased signaling, which may lead to future breakthrough therapies. The 5-HT2CR PAMs reported to date share an interesting characteristic in that significant increases in 5-HT efficacy (Emax), but not potency, at the 5-HT2CR are observed in vitro. Observations such as this will direct structural biology studies in the future to investigate conformational shifts responsible for efficacy increases and solving the structure of a PAM-agonist-5-HT2CR co-complex will provide valuable information towards this goal. It is conceivable that a structure such as this will be available in the coming years due to recent successes in solving 5-HT2CR active and inactive state crystal structures [5]. Additionally, the discovery of 5-HT2CR PAMs that enhance 5-HT potency will enable the investigation of similar or contrasting in vivo effects compared to 5-HT2CR PAMs that enhance 5-HT efficacy alone. In light of the complex modulatory role for the 5-HT2CR in neurobiological processes, a diverse array of ligand modulation modalities should improve our understanding of the specific ligand profiles needed to pursue clinical endpoints.
As an important therapeutic target for diverse neurological and psychiatric disorders, medicinal chemists should pursue diverse mechanisms of receptor engagement at the 5-HT2CR and groups have begun to rise to this challenging task. For agonist discovery, expansion of available pharmacophores is critically important and significant work towards this goal has recently been reported by Wacker and coworkers at Bristol-Myers Squibb utilizing an orthosteric-site mutant version of the 5-HT2CR. Additionally, further investigation into the complexity of 5-HT2CR signaling and oligomerization, as studied recently by Cunningham and colleagues [110] will help guide future compound design. In conclusion, there has been considerable recent progress in targeting the 5-HT2CR, as shown by the FDA-approved agonist 14, yet significant gaps in understanding 5-HT2CR biology remain as do pharmacophore challenges for medicinal chemists in moving towards safer, more efficacious clinical therapies targeting the 5-HT2CR.
ACKNOWLEDGEMENTS
FUNDING
This work was supported by grants R01 DA038446, T32 DA07287, and F31 DA045511 from the National Institute on Drug Abuse and the Ruth L. Kirschstein National Research Service Award of the National Institutes of Health.
LIST OF ABBREVIATIONS
- GPCR
G protein-Coupled Receptor
- 5-HT
Serotonin
- 5-HT2AR
Serotonin5-HT2A Receptor
- 5-HT2BR
Serotonin 5-HT2B Receptor
- 5-HT2CR
Serotonin 5-HT2C Receptor
- 5-HTXR
Serotonin Receptor Subtypes
- AA
Arachidonic Acid
- Cai2+
Intracellular Calcium
- CaM
Calmodulin
- cAMP
Cyclic Adenosine Monophosphate
- CNS
Central Nervous System
- CUD
Cocaine Use Disorder
- DAG
Diacylglycerol
- ECL
Extracellular Loop
- GABA
γ-Aminobutyric Acid
- ICL
Intracellular Loop
- IP1
Inositol Monophosphate
- IP3
Inositol-1,4,5-Triphosphate
- NAc
Nucleus Accumbens
- NAL
Neutral Allosteric Ligand
- NAM
Negative Allosteric Modulator
- PAM
Positive Allosteric Modulator
- PKC
Protein Kinase C
- PLA2
Phospholipase A2
- PLCβ
Phospholipase Cβ
- PFC
Prefrontal Cortex
- SUD
Substance Use Disorder
- TM
Transmembrane Domain
- VTA
Ventral Tegmental Area
Footnotes
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- [1].Hauser AS; Attwood MM; Rask-Andersen M; Schiöth HB; Gloriam DE Trends in GPCR drug discovery: New agents, targets and indications. Nat. Rev. Drug Discov, 2017, 76(12), 829–842. [ 10.1038/nrd.2017.178] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wild C; Cunningham KA; Zhou J Allosteric modulation of G protein-coupled receptors: An emerging approach of drug discovery. Austin J. Pharmacol. Ther, 2014, 2(1), 1–8. [PMC free article] [PubMed] [Google Scholar]
- [3].Hannon J; Hoyer D Molecular biology of 5-HT receptors. Behav. Brain Res, 2008, 195(1), 198–213. [ 10.1016/j.bbr.2008.03.020] [DOI] [PubMed] [Google Scholar]
- [4].Kroeze WK; Roth BL Molecular Biology and Genomic Organization of G Protein-Coupled Serotonin Receptors In: Roth BL (eds) The Serotonin Receptors. The Receptors. Humana Press, 2006, pp 1–38. [DOI: 10.1007/978-1-59745-080-5_1] [DOI] [Google Scholar]
- [5].Peng Y; McCorvy JD; Harpsoe K; Lansu K; Yuan S; Popov P; Qu L; Pu M; Che T; Nikolajsen LF; Huang XP; Wu Y; Shen L; Bjorn-Yoshimoto WE; Ding K; Wacker D; Han GW; Cheng J; Katritch V; Jensen AA; Hanson MA; Zhao S; Gloriam DE; Roth BL; Stevens RC; Liu ZJ 5-HT2C receptor structures reveal the structural basis of GPCR polypharmacology. Cell, 2018, 172(4), 719–730. [DOI: 10.1016/j.cell.2018.01.001] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Heifetz A; Storer RI; McMurray G; James T; Morao I; Aldeghi M; Bodkin MJ; Biggin PC Application of an integrated GPCR SAR-modeling platform to explain the activation selectivity of human 5-HT2C over 5-HT2B. ACS Chem. Biol, 2016, 11(5), 1372–1382. [ 10.1021/acschembio.5b01045] [DOI] [PubMed] [Google Scholar]
- [7].Raymond JR; Mukhin YV; Gelasco A; Turner J; Collinsworth G; Gettys TW; Grewal JS; Garnovskaya MN Multiplicity of mechanisms of serotonin receptor signal transduction. Pharmacol. Ther, 2001, 92(2–3), 179–212. [ 10.1016/S0163-7258(01)00169-3] [DOI] [PubMed] [Google Scholar]
- [8].Millan MJ; Marin P; Bockaert J; Mannoury la Cour C Signaling at G-protein-coupled serotonin receptors: recent advances and future research directions. Trends Pharmacol. Sci, 2008, 29(9), 454–464. [ 10.1016/j.tips.2008.06.007] [DOI] [PubMed] [Google Scholar]
- [9].Seitz PK; Bremer NM; McGinnis AG; Cunningham KA; Watson CS Quantitative changes in intracellular calcium and extracellular-regulated kinase activation measured in parallel in CHO cells stably expressing serotonin (5-HT) 5-HT2A or 5-HT2C receptors. BMC Neurosci, 2012, 15(25), 25 [ 10.1186/1471-2202-13-25] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Roth BL; Willins DL; Kristiansen K; Kroeze WK 5-Hydroxytryptamine2-family receptors (5-hydroxytryptamine2A, 5-hydroxytryptamine2B, 5-hydroxytryptamine2C): Where structure meets function. Pharmacol. Ther, 1998, 79(3), 231–257. [ 10.1016/S0163-7258(98)00019-9] [DOI] [PubMed] [Google Scholar]
- [11].Burke JE; Dennis EA Phospholipase A2 structure/function, mechanism, and signaling. J. Lipid Res, 2009, 50(Suppl), S237–242. [DOI: 10.1194/jlr.R800033-JLR200] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Berg KA; Maayani S; Goldfarb J; Scaramellini C; Leff P; Clarke WP Effector pathway-dependent relative efficacy at serotonin type 2A and 2C receptors: evidence for agonist-directed trafficking of receptor stimulus. Mol. Pharmacol, 1998, 54(1), 94–104. [ 10.1124/mol.54.1.94] [DOI] [PubMed] [Google Scholar]
- [13].Werry TD; Stewart GD; Crouch MF; Watts A; Sexton PM; Christopoulos A Pharmacology of 5HT(2C) receptor-mediated ERK1/2 phosphorylation: agonist-specific activation pathways and the impact of RNA editing. Biochem. Pharmacol, 2008, 76(10), 1276–1287. [ 10.1016/j.bcp.2008.08.024] [DOI] [PubMed] [Google Scholar]
- [14].Werry TD; Gregory KJ; Sexton PM; Christopoulos A Characterization of serotonin 5-HT2C receptor signaling to extracellular signal-regulated kinases 1 and 2. J. Neurochem, 2005, 93(6), 1603–1615. [ 10.1111/j.1471-4159.2005.03161.x] [DOI] [PubMed] [Google Scholar]
- [15].Lauffer L; Glas E; Gudermann T; Breit A Endogenous 5-HT2C receptors phosphorylate the cAMP response element binding protein via protein kinase C-promoted activation of extracellular-regulated kinases-1/2 in hypothalamic mHypoA-2/10 cells. J. Pharmacol. Exp. Ther, 2016, 358(1), 39–49. [ 10.1124/jpet.116.232397] [DOI] [PubMed] [Google Scholar]
- [16].O’Neil RT; Emeson RB Quantitative analysis of 5HT(2C) receptor RNA editing patterns in psychiatric disorders. Neurobiol. Dis, 2012, 45(1), 8–13. [ 10.1016/j.nbd.2011.08.026] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Marion S; Weiner DM; Caron MG RNA editing induces variation in desensitization and trafficking of 5-hydroxytryptamine 2c receptor isoforms. J. Biol. Chem, 2004, 279(4), 2945–2954. [ 10.1074/jbc.M308742200] [DOI] [PubMed] [Google Scholar]
- [18].Werry TD; Loiacono R; Sexton PM; Christopoulos A RNA editing of the serotonin 5HT2C receptor and its effects on cell signalling, pharmacology and brain function. Pharmacol. Ther, 2008, 119(1), 7–23. [ 10.1016/j.pharmthera.2008.03.012] [DOI] [PubMed] [Google Scholar]
- [19].Schaub M; Keller W RNA editing by adenosine deaminases generates RNA and protein diversity. Biochimie, 2002, 84(8), 791–803. [ 10.1016/S0300-9084(02)01446-3] [DOI] [PubMed] [Google Scholar]
- [20].Chen CX; Cho DS; Wang Q; Lai F; Carter KC; Nishikura K A third member of the RNA-specific adenosine deaminase gene family, ADAR3, contains both single- and double-stranded RNA binding domains. RNA, 2000, 6(5), 755–767. [ 10.1017/S1355838200000170] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Valente L; Nishikura K ADAR gene family and A-to-I RNA editing: Diverse roles in posttranscriptional gene regulation In: Progress in Nucleic Acid Research and Molecular Biology; Academic Press, 2005, Vol. 79, pp. 299–338. [DOI] [PubMed] [Google Scholar]
- [22].Herrick-Davis K; Grinde E; Niswender CM Serotonin 5-HT2C receptor RNA editing alters receptor basal activity: implications for serotonergic signal transduction. J. Neurochem, 1999, 73(4), 1711–1717. [ 10.1046/j.1471-4159.1999.731711.x] [DOI] [PubMed] [Google Scholar]
- [23].Berg KA; Cropper JD; Niswender CM; Sanders-Bush E; Emeson RB; Clarke WP RNA-editing of the 5-HT(2C) receptor alters agonist-receptor-effector coupling specificity. Br. J. Pharmacol, 2001, 134(2), 386–392. [ 10.1038/sj.bjp.0704255] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Maydanovych O; Beal PA Breaking the central dogma by RNA editing. Chem. Rev, 2006, 106(8), 3397–3411. [ 10.1021/cr050314a] [DOI] [PubMed] [Google Scholar]
- [25].Herrick-Davis K; Grinde E; Lindsley T; Teitler M; Mancia F; Cowan A; Mazurkiewicz JE Native serotonin 5-HT2C receptors are expressed as homodimers on the apical surface of choroid plexus epithelial cells. Mol. Pharmacol, 2015, 87(4), 660–673. [ 10.1124/mol.114.096636] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Herrick-Davis KF; Dinah T 5-HT2C receptors in the pathophysiology of CNS disease; British Journal of Clinical Pharmacology: Blackwell Science Inc, 2011, 72, pp. 129–155. [Google Scholar]
- [27].Schellekens H; van Oeffelen WE; Dinan TG; Cryan JF Promiscuous dimerization of the growth hormone secretagogue receptor (GHS-R1a) attenuates ghrelin-mediated signaling. J. Biol. Chem, 2013, 288(1), 181–191. [ 10.1074/jbc.M112.382473] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Felsing DE; Anastasio NC; Miszkiel JM; Gilbertson SR; Allen JA; Cunningham KA Biophysical validation of serotonin 5-HT2A and 5-HT2C receptor interaction. PLoS One, 2018, 13(8)e0203137 [ 10.1371/journal.pone.0203137] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Price AE; Sholler DJ; Stutz SJ; Anastasio NC; Cunningham KA Endogenous serotonin 5-HT2A and 5-HT2C receptors associate in the medial prefrontal cortex. ACS Chem. Neurosci, 2019, 21(5), 1532–1537. [ 10.1021/acschemneuro.8b00669] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kamal M; Gbahou F; Guillaume JL; Daulat AM; Benleulmi-Chaachoua A; Luka M; Chen P; Kalbasi Anaraki D; Baroncini M; Mannoury la Cour C; Millan MJ; Prevot V; Delagrange P; Jockers R Convergence of melatonin and serotonin (5-HT) signaling at MT2/5-HT2C receptor heteromers. J. Biol. Chem, 2015, 290(18), 11537–11546. [ 10.1074/jbc.M114.559542] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Moutkine I; Quentin E; Guiard BP; Maroteaux L; Doly S Heterodimers of serotonin receptor subtypes 2 are driven by 5-HT2C protomers. J. Biol. Chem, 2017, 292(15), 6352–6368. [ 10.1074/jbc.M117.779041] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bigford GE; Chaudhry NS; Keane RW; Holohean AM 5-Hydroxytryptamine 5HT2C receptors form a protein complex with N-methyl-D-aspartate GluN2A subunits and activate phosphorylation of Src protein to modulate motoneuronal depolarization. J. Biol. Chem, 2012, 287(14), 11049–11059. [ 10.1074/jbc.M111.277806] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Anastasio NC; Gilbertson SR; Bubar MJ; Agarkov A; Stutz SJ; Jeng Y; Bremer NM; Smith TD; Fox RG; Swinford SE; Seitz PK; Charendoff MN; Craft JW Jr; Laezza FM; Watson CS; Briggs JM; Cunningham KA Peptide inhibitors disrupt the serotonin 5-HT2C receptor interaction with phosphatase and tensin homolog to allosterically modulate cellular signaling and behavior. J. Neurosci, 2013, 33(4), 1615–1630. [ 10.1523/JNEUROSCI.2656-12.2013] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bohn LM; Schmid CL Serotonin receptor signaling and regulation via β-arrestins. Crit. Rev. Biochem. Mol. Biol, 2010, 45(6), 555–566. [ 10.3109/10409238.2010.516741] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Howell LL; Cunningham KA Serotonin 5-HT2 receptor interactions with dopamine function: implications for therapeutics in cocaine use disorder. Pharmacol. Rev, 2015, 67(1), 176–197. [ 10.1124/pr.114.009514] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Anastasio NC; Stutz SJ; Fink LH; Swinford-Jackson SE; Sears RM; DiLeone RJ; Rice KC; Moeller FG; Cunningham KA Serotonin (5-HT) 5-HT2A receptor (5-HT2AR):5-HT2CR imbalance in medial prefrontal cortex associates with motor impulsivity. ACS Chem. Neurosci, 2015, 6(7), 1248–1258. [ 10.1021/acschemneuro.5b00094] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Prisco S; Pagannone S; Esposito E Serotonin-dopamine interaction in the rat ventral tegmental area: an electrophysiological study in vivo. J. Pharmacol. Exp. Ther, 1994, 271(1), 83–90. [PubMed] [Google Scholar]
- [38].Di Giovanni G; Di Matteo V; La Grutta V; Esposito E m-Chlorophenylpiperazine excites non-dopaminergic neurons in the rat substantia nigra and ventral tegmental area by activating serotonin-2C receptors. Neuroscience, 2001, 103(1), 111–116. [ 10.1016/S0306-4522(00)00561-3] [DOI] [PubMed] [Google Scholar]
- [39].Theile JW; Morikawa H; Gonzales RA; Morrisett RA Role of 5-hydroxytryptamine2C receptors in Ca2+-dependent ethanol potentiation of GABA release onto ventral tegmental area dopamine neurons. J. Pharmacol. Exp. Ther, 2009, 329(2), 625–633. [ 10.1124/jpet.108.147793] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kasper JM; Booth RG; Peris J Serotonin-2C receptor agonists decrease potassium-stimulated GABA release in the nucleus accumbens. Synapse, 2015, 69(2), 78–85. [ 10.1002/syn.21790] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Di Giovanni G; De Deurwaerdére P; Di Mascio M; Di Matteo V; Esposito E; Spampinato U Selective blockade of serotonin-2C/2B receptors enhances mesolimbic and mesostriatal dopaminergic function: a combined in vivo electrophysiological and microdialysis study. Neuroscience, 1999, 91(2), 587–597. [ 10.1016/S0306-4522(98)00655-1] [DOI] [PubMed] [Google Scholar]
- [42].Gobert A; Rivet J-M; Lejeune F; Newman-Tancredi A; Adhumeau-Auclair A; Nicolas J-P; Cistarelli L; Melon C; Millan MJ Serotonin(2C) receptors tonically suppress the activity of mesocortical dopaminergic and adrenergic, but not serotonergic, pathways: a combined dialysis and electrophysiological analysis in the rat. Synapse, 2000, 36(3), 205–221. [] [DOI] [PubMed] [Google Scholar]
- [43].Bubar MJ; Stutz SJ; Cunningham KA 5-HT(2C) receptors localize to dopamine and GABA neurons in the rat mesoaccumbens pathway. PLoS One, 2011, 6(6)e20508 [ 10.1371/journal.pone.0020508] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Bubar MJ; Cunningham KA Distribution of serotonin 5-HT2C receptors in the ventral tegmental area. Neuroscience, 2007, 146(1), 286–297. [ 10.1016/j.neuroscience.2006.12.071] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Xu P; He Y; Cao X; Valencia-Torres L; Yan X; Saito K; Wang C; Yang Y; Hinton A Jr; Zhu L; Shu G; Myers MG Jr; Wu Q; Tong Q; Heisler LK; Xu Y Activation of serotonin 2C receptors in dopamine neurons inhibits binge-like eating in mice. Biol. Psychiatry, 2017, 81(9), 737–747. [ 10.1016/j.biopsych.2016.06.005] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Santana N; Artigas F Expression of serotonin2C receptors in pyramidal and GABAergic neurons of rat prefrontal cortex: a comparison with striatum. Cereb. Cortex, 2017, 27(6), 3125–3139. [DOI] [PubMed] [Google Scholar]
- [47].Liu S; Bubar MJ; Lanfranco MF; Hillman GR; Cunningham KA Serotonin2C receptor localization in GABA neurons of the rat medial prefrontal cortex: implications for understanding the neurobiology of addiction. Neuroscience, 2007, 146(4), 1677–1688. [ 10.1016/j.neuroscience.2007.02.064] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Anastasio NC; Stutz SJ; Fox RG; Sears RM; Emeson RB; DiLeone RJ; O’Neil RT; Fink LH; Li D; Green TA; Moeller FG; Cunningham KA Functional status of the serotonin 5-HT2C receptor (5-HT2CR) drives interlocked phenotypes that precipitate relapse-like behaviors in cocaine dependence. Neuropsychopharmacology, 2014, 39(2), 370–382. [ 10.1038/npp.2013.199] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Bubar MJ; Cunningham KA Serotonin 5-HT2A and 5-HT2C receptors as potential targets for modulation of psychostimulant use and dependence. Curr. Top. Med. Chem, 2006, 6(18), 1971–1985. [ 10.2174/156802606778522131] [DOI] [PubMed] [Google Scholar]
- [50].Cunningham KA; Anastasio NC; Fox RG; Stutz SJ; Bubar MJ; Swinford SE; Watson CS; Gilbertson SR; Rice KC; Rosenzweig-Lipson S; Moeller FG Synergism between a serotonin 5-HT2A receptor (5-HT2AR) antagonist and 5-HT2CR agonist suggests new pharmacotherapeutics for cocaine addiction. ACS Chem. Neurosci, 2013, 4(1), 110–121. [ 10.1021/cn300072u] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Thomsen WJ; Grottick AJ; Menzaghi F; Reyes-Saldana H; Espitia S; Yuskin D; Whelan K; Martin M; Morgan M; Chen W; Al-Shamma H; Smith B; Chalmers D; Behan D Lorcaserin, a novel selective human 5-hydroxytryptamine2C agonist: in vitro and in vivo pharmacological characterization. J. Pharmacol. Exp. Ther, 2008, 325(2), 577–587. [ 10.1124/jpet.107.133348] [DOI] [PubMed] [Google Scholar]
- [52].Hoy SM Lorcaserin: A review of its use in chronic weight management. Drugs, 2013, 73(5), 463–473. [ 10.1007/s40265-013-0035-1] [DOI] [PubMed] [Google Scholar]
- [53].Harvey-Lewis C; Li Z; Higgins GA; Fletcher PJ The 5-HT(2C) receptor agonist lorcaserin reduces cocaine self-administration, reinstatement of cocaine-seeking and cocaine induced locomotor activity. Neuropharmacology, 2016, 101, 237–245. [ 10.1016/j.neuropharm.2015.09.028] [DOI] [PubMed] [Google Scholar]
- [54].Di Giovanni G; De Deurwaerdère P New therapeutic opportunities for 5-HT2C receptor ligands in neuropsychiatric disorders. Pharmacol. Ther, 2016, 157, 125–162. [ 10.1016/j.pharmthera.2015.11.009] [DOI] [PubMed] [Google Scholar]
- [55].Higgins GA; Fletcher PJ Therapeutic potential of 5-HT2C receptor agonists for addictive disorders. ACS Chem. Neurosci, 2015, 6(7), 1071–1088. [ 10.1021/acschemneuro.5b00025] [DOI] [PubMed] [Google Scholar]
- [56].Tecott LH; Sun LM; Akana SF; Strack AM; Lowenstein DH; Dallman MF; Julius D Eating disorder and epilepsy in mice lacking 5-HT2c serotonin receptors. Nature, 1995, 374(6522), 542–546. [ 10.1038/374542a0] [DOI] [PubMed] [Google Scholar]
- [57].Nonogaki K; Ohba Y; Sumii M; Oka Y Serotonin systems upregulate the expression of hypothalamic NUCB2 via 5-HT2C receptors and induce anorexia via a leptin-independent pathway in mice. Biochem. Biophys. Res. Commun, 2008, 372(1), 186–190. [ 10.1016/j.bbrc.2008.05.010] [DOI] [PubMed] [Google Scholar]
- [58].Wirshing DA; Wirshing WC; Kysar L; Berisford MA; Goldstein D; Pashdag J; Mintz J; Marder SR Novel antipsychotics: comparison of weight gain liabilities. J. Clin. Psychiatry, 1999, 60(6), 358–363. [ 10.4088/JCP.v60n0602] [DOI] [PubMed] [Google Scholar]
- [59].Kirk SL; Glazebrook J; Grayson B; Neill JC; Reynolds GP Olanzapine-induced weight gain in the rat: role of 5-HT2C and histamine H1 receptors. Psychopharmacology (Berl.), 2009, 207(1), 119–125. [ 10.1007/s00213-009-1639-8] [DOI] [PubMed] [Google Scholar]
- [60].Ge T; Zhang Z; Lv J; Song Y; Fan J; Liu W; Wang X; Hall FS; Li B; Cui R The role of 5-HT2c receptor on corticosterone-mediated food intake. J. Biochem. Mol. Toxicol, 2017, 31(6) [ 10.1002/jbt.21890] [DOI] [PubMed] [Google Scholar]
- [61].Halford JC; Lawton CL; Blundell JE The 5-HT2 receptor agonist MK-212 reduces food intake and increases resting but prevents the behavioural satiety sequence. Pharmacol. Biochem. Behav, 1997, 56(1), 41–46. [ 10.1016/S0091-3057(96)00152-9] [DOI] [PubMed] [Google Scholar]
- [62].Wacker DA; Miller KJ Agonists of the serotonin 5-HT2C receptor: preclinical and clinical progression in multiple diseases. Curr. Opin. Drug Discov. Devel, 2008, 11(4), 438–445. [PubMed] [Google Scholar]
- [63].Dunlop J; Sabb AL; Mazandarani H; Zhang J; Kalgaonker S; Shukhina E; Sukoff S; Vogel RL; Stack G; Schechter L; Harrison BL; Rosenzweig-Lipson S WAY-163909 [(7bR, 10aR)-1,2,3,4,8,9,10,10a-octahydro-7bH-cyclopenta-[b][1,4]diazepino[6,7,1hi]indole], a novel 5-hydroxytryptamine 2C receptor-selective agonist with anorectic activity. J. Pharmacol. Exp. Ther, 2005, 313(2), 862–869. [ 10.1124/jpet.104.075382] [DOI] [PubMed] [Google Scholar]
- [64].Voigt JP; Fink H Serotonin controlling feeding and satiety. Behav. Brain Res, 2015, 277, 14–31. [ 10.1016/j.bbr.2014.08.065] [DOI] [PubMed] [Google Scholar]
- [65].Gautron L; Elmquist JK; Williams KW Neural control of energy balance: Translating circuits to therapies. Cell, 2015, 161(1), 133–145. [ 10.1016/j.cell.2015.02.023] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Smith SR; Prosser WA; Donahue DJ; Morgan ME; Anderson CM; Shanahan WR; Group, A.P.D.S. Lorcaserin (APD356), a selective 5-HT(2C) agonist, reduces body weight in obese men and women. Obesity (Silver Spring), 2009, 17(3), 494–503. [ 10.1038/oby.2008.537] [DOI] [PubMed] [Google Scholar]
- [67].Bubar MJ; Cunningham KA Prospects for serotonin 5-HT2R pharmacotherapy in psychostimulant abuse. Prog. Brain Res, 2008, 172, 319–346. [ 10.1016/S0079-6123(08)00916-3] [DOI] [PubMed] [Google Scholar]
- [68].Neelakantan H; Holliday ED; Fox RG; Stutz SJ; Comer SD; Haney M; Anastasio NC; Moeller FG; Cunningham KA Lorcaserin suppresses oxycodone self-administration and relapse vulnerability in rats. ACS Chem. Neurosci, 2017, 8(5), 1065–1073. [ 10.1021/acschemneuro.6b00413] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Moeller FG; Cunningham KA Innovative therapeutic intervention for opioid use disorder. Neuropsychopharmacology, 2018, 43(1), 220–221. [ 10.1038/npp.2017.192] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Swinford-Jackson SE; Anastasio NC; Fox RG; Stutz SJ; Cunningham KA Incubation of cocaine cue reactivity associates with neuroadaptations in the cortical serotonin (5-HT) 5-HT2C receptor (5-HT2CR) system. Neuroscience, 2016, 324, 50–61. [ 10.1016/j.neuroscience.2016.02.052] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Cunningham KA; Fox RG; Anastasio NC; Bubar MJ; Stutz SJ; Moeller FG; Gilbertson SR; Rosenzweig-Lipson S Selective serotonin 5-HT(2C) receptor activation suppresses the reinforcing efficacy of cocaine and sucrose but differentially affects the incentive-salience value of cocaine- vs. sucrose-associated cues. Neuropharmacology, 2011, 61(3), 513–523. [ 10.1016/j.neuropharm.2011.04.034] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Grottick AJ; Fletcher PJ; Higgins GA Studies to investigate the role of 5-HT(2C) receptors on cocaine- and food-maintained behavior. J. Pharmacol. Exp. Ther, 2000, 295(3), 1183–1191. [PubMed] [Google Scholar]
- [73].Manvich DF; Kimmel HL; Cooper DA; Howell LL The serotonin 2C receptor antagonist SB 242084 exhibits abuse-related effects typical of stimulants in squirrel monkeys. J. Pharmacol. Exp. Ther, 2012, 342(3), 761–769. [ 10.1124/jpet.112.195156] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Manvich DF; Kimmel HL; Howell LL Effects of serotonin 2C receptor agonists on the behavioral and neurochemical effects of cocaine in squirrel monkeys. J. Pharmacol. Exp. Ther, 2012, 341(2), 424–434. [ 10.1124/jpet.111.186981] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Pelloux Y; Dilleen R; Economidou D; Theobald D; Everitt BJ Reduced forebrain serotonin transmission is causally involved in the development of compulsive cocaine seeking in rats. Neuropsychopharmacology, 2012, 37(11), 2505–2514. [ 10.1038/npp.2012.111] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Fletcher PJ; Grottick AJ; Higgins GA Differential effects of the 5-HT(2A) receptor antagonist M100907 and the 5-HT(2C) receptor antagonist SB242084 on cocaine-induced locomotor activity, cocaine self-administration and cocaine-induced reinstatement of responding. Neuropsychopharmacology, 2002, 27(4), 576–586. [DOI] [PubMed] [Google Scholar]
- [77].Shanahan WR; Rose JE; Glicklich A; Stubbe S; Sanchez-Kam M Lorcaserin for smoking cessation and associated weight gain: a randomized 12-week clinical trial. Nicotine Tob. Res, 2017, 19(8), 944–951. [DOI] [PubMed] [Google Scholar]
- [78].Farr OM; Upadhyay J; Gavrieli A; Camp M; Spyrou N; Kaye H; Mathew H; Vamvini M; Koniaris A; Kilim H; Srnka A; Migdal A; Mantzoros CS Lorcaserin administration decreases activation of brain centers in response to food cues and these emotion- and salience-related changes correlate with weight loss effects: a 4-week-long randomized, placebo-controlled, double-blind clinical trial. Diabetes, 2016, 65(10), 2943–2953. [ 10.2337/db16-0635] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Cunningham KA; Anastasio NC Serotonin at the nexus of impulsivity and cue reactivity in cocaine addiction. Neuropharmacology, 2014, 76(Pt B), 460–478. [ 10.1016/j.neuropharm.2013.06.030] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Moeller FG; Barratt ES; Dougherty DM; Schmitz JM; Swann AC Psychiatric aspects of impulsivity. Am. J. Psychiatry, 2001, 158(11), 1783–1793. [ 10.1176/appi.ajp.158.11.1783] [DOI] [PubMed] [Google Scholar]
- [81].Liu S; Lane SD; Schmitz JM; Waters AJ; Cunningham KA; Moeller FG Relationship between attentional bias to cocaine-related stimuli and impulsivity in cocaine-dependent subjects. Am. J. Drug Alcohol Abuse, 2011, 37(2), 117–122. [ 10.3109/00952990.2010.543204] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Leung D; Staiger PK; Hayden M; Lum JA; Hall K; Manning V; Verdejo-Garcia A Meta-analysis of the relationship between impulsivity and substance-related cognitive biases. Drug Alcohol Depend, 2017, 172, 21–33. [ 10.1016/j.drugalcdep.2016.11.034] [DOI] [PubMed] [Google Scholar]
- [83].Fletcher PJ; Tampakeras M; Sinyard J; Higgins GA Opposing effects of 5-HT(2A) and 5-HT(2C) receptor antagonists in the rat and mouse on premature responding in the five-choice serial reaction time test. Psychopharmacology (Berl.), 2007, 195(2), 223–234. [ 10.1007/s00213-007-0891-z] [DOI] [PubMed] [Google Scholar]
- [84].Winstanley CA; Theobald DE; Dalley JW; Glennon JC; Robbins TW 5-HT2A and 5-HT2C receptor antagonists have opposing effects on a measure of impulsivity: interactions with global 5-HT depletion. Psychopharmacology (Berl.), 2004, 176(3–4), 376–385. [ 10.1007/s00213-004-1884-9] [DOI] [PubMed] [Google Scholar]
- [85].Pennanen L; van der Hart M; Yu L; Tecott LH Impact of serotonin (5-HT)2C receptors on executive control processes. Neuropsychopharmacology, 2013, 38(6), 957–967. [ 10.1038/npp.2012.258] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Besson M; Pelloux Y; Dilleen R; Theobald DE; Lyon A; Belin-Rauscent A; Robbins TW; Dalley JW; Everitt BJ; Belin D Cocaine modulation of frontostriatal expression of Zif268, D2, and 5-HT2c receptors in high and low impulsive rats. Neuropsychopharmacology, 2013, 38(10), 1963–1973. [ 10.1038/npp.2013.95] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Anastasio NC; Liu S; Maili L; Swinford SE; Lane SD; Fox RG; Hamon SC; Nielsen DA; Cunningham KA; Moeller FG Variation within the serotonin (5-HT) 5-HT2C receptor system aligns with vulnerability to cocaine cue reactivity. Transl. Psychiatry, 2014, 4, e369 [ 10.1038/tp.2013.131] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Everitt BJ; Robbins TW Drug addiction: updating actions to habits to compulsions ten years on. Annu. Rev. Psychol, 2016, 67, 23–50. [ 10.1146/annurev-psych-122414-033457] [DOI] [PubMed] [Google Scholar]
- [89].Nichols DE Hallucinogens. Pharmacol. Ther, 2004, 101(2), 131–181. [ 10.1016/j.pharmthera.2003.11.002] [DOI] [PubMed] [Google Scholar]
- [90].Fitzgerald LW; Burn TC; Brown BS; Patterson JP; Corjay MH; Valentine PA; Sun JH; Link JR; Abbaszade I; Hollis JM; Largent BL; Hartig PR; Hollis GF; Meunier PC; Robichaud AJ; Robertson DW Possible role of valvular serotonin 5-HT(2B) receptors in the cardiopathy associated with fenfluramine. Mol. Pharmacol, 2000, 57(1), 75–81. [PubMed] [Google Scholar]
- [91].Connolly HM; Crary JL; McGoon MD; Hensrud DD; Edwards BS; Edwards WD; Schaff HV Valvular heart disease associated with fenfluramine-phentermine. N. Engl. J. Med, 1997, 337(9), 581–588. [ 10.1056/NEJM199708283370901] [DOI] [PubMed] [Google Scholar]
- [92].Shram MJ; Schoedel KA; Bartlett C; Shazer RL; Anderson CM; Sellers EM Evaluation of the abuse potential of lorcaserin, a serotonin 2C (5-HT2C) receptor agonist, in recreational polydrug users. Clin. Pharmacol. Ther, 2011, 89(5), 683–692. [ 10.1038/clpt.2011.20] [DOI] [PubMed] [Google Scholar]
- [93].Greenway FL; Shanahan W; Fain R; Ma T; Rubino D Safety and tolerability review of lorcaserin in clinical trials. Clin. Obes, 2016, 6(5), 285–295. [ 10.1111/cob.12159] [DOI] [PubMed] [Google Scholar]
- [94].Kalgutkar AS; Dalvie DK; Aubrecht J; Smith EB; Coffing SL; Cheung JR; Vage C; Lame ME; Chiang P; McClure KF; Maurer TS; Coelho RV Jr; Soliman VF; Schildknegt K Genotoxicity of 2-(3-chlorobenzyloxy)-6-(piperazinyl)pyrazine, a novel 5-hydroxytryptamine2c receptor agonist for the treatment of obesity: role of metabolic activation. Drug Metab. Dispos, 2007, 35(6), 848–858. [ 10.1124/dmd.106.013649] [DOI] [PubMed] [Google Scholar]
- [95].Kelly CR; Sharif NA Pharmacological evidence for a functional serotonin-2B receptor in a human uterine smooth muscle cell line. J. Pharmacol Exp. Ther, 2006, 317(3), 1254–1261. [ 10.1124/jpet.105.100172] [DOI] [PubMed] [Google Scholar]
- [96].Bös M; Jenck F; Martin JR; Moreau JL; Sleight AJ; Wichmann J; Widmer U Novel agonists of 5HT2C receptors. Synthesis and biological evaluation of substituted 2-(indol-1-yl)-1-methylethylamines and 2-(indeno[1,2-b]pyrrol-1-yl)-1-methylethylamines. Improved therapeutics for obsessive compulsive disorder. J. Med. Chem, 1997, 40(17), 2762–2769. [ 10.1021/jm970030l] [DOI] [PubMed] [Google Scholar]
- [97].Kimura Y; Hatanaka K; Naitou Y; Maeno K; Shimada I; Koakutsu A; Wanibuchi F; Yamaguchi T Pharmacological profile of YM348, a novel, potent and orally active 5-HT2C receptor agonist. Eur. J. Pharmacol, 2004, 483(1), 37–43. [ 10.1016/j.ejphar.2003.10.004] [DOI] [PubMed] [Google Scholar]
- [98].Fitzgerald LW; Burn TC; Brown BS; Patterson JP; Corjay MH; Valentine PA; Sun J-H; Link JR; Abbaszade I; Hollis JM; Largent BL; Hartig PR; Hollis GF; Meunier PC; Robichaud AJ; Robertson DW Possible role of valvular serotonin 5-HT(2B) receptors in the cardiopathy associated with fenfluramine. Mol. Pharmacol, 2000, 57(1), 75–81. [PubMed] [Google Scholar]
- [99].Cheng J; Giguère PM; Onajole OK; Lv W; Gaisin A; Gunosewoyo H; Schmerberg CM; Pogorelov VM; Rodriguiz RM; Vistoli G; Wetsel WC; Roth BL; Kozikowski AP Optimization of 2-phenylcyclopropylmethylamines as selective serotonin 2C receptor agonists and their evaluation as potential antipsychotic agents. J. Med. Chem, 2015, 58(4), 1992–2002. [ 10.1021/jm5019274] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Cheng J; Giguere PM; Schmerberg CM; Pogorelov VM; Rodriguiz RM; Huang XP; Zhu H; McCorvy JD; Wetsel WC; Roth BL; Kozikowski AP Further advances in optimizing (2-phenylcyclopropyl)methylamines as novel serotonin 2C agonists: effects on hyperlocomotion, prepulse inhibition, and cognition models. J. Med. Chem, 2016, 59(2), 578–591. [ 10.1021/acs.jmedchem.5b01153] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Zhang G; Cheng J; McCorvy JD; Lorello PJ; Caldarone BJ; Roth BL; Kozikowski AP Discovery of N-substituted (2-phenylcyclopropyl)methylamines as functionally selective serotonin 2C receptor agonists for potential use as antipsychotic medications. J. Med. Chem, 2017, 60(14), 6273–6288. [ 10.1021/acs.jmedchem.7b00584] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Siuciak JA; Chapin DS; McCarthy SA; Guanowsky V; Brown J; Chiang P; Marala R; Patterson T; Seymour PA; Swick A; Iredale PA CP-809,101, a selective 5-HT2C agonist, shows activity in animal models of antipsychotic activity. Neuropharmacology, 2007, 52(2), 279–290. [ 10.1016/j.neuropharm.2006.07.024] [DOI] [PubMed] [Google Scholar]
- [103].Fevig JM; Feng J; Rossi KA; Miller KJ; Wu G; Hung CP; Ung T; Malmstrom SE; Zhang G; Keim WJ; Cullen MJ; Rohrbach KW; Qu Q; Gan J; Pelleymounter MA; Robl JA Synthesis and SAR of 2,3,3a,4-tetrahydro-1H-pyrrolo[3,4-c]isoquinolin-5(9bH)-ones as 5-HT2C receptor agonists. Bioorg. Med. Chem. Lett, 2013, 23(1), 330–335. [ 10.1016/j.bmcl.2012.10.091] [DOI] [PubMed] [Google Scholar]
- [104].Zhao G; Kwon C; Bisaha SN; Stein PD; Rossi KA; Cao X; Ung T; Wu G; Hung CP; Malmstrom SE; Zhang G; Qu Q; Gan J; Keim WJ; Cullen MJ; Rohrbach KW; Devenny J; Pelleymounter MA; Miller KJ; Robl JA Synthesis and SAR of potent and selective tetrahydropyrazinoisoquinolinone 5-HT(2C) receptor agonists. Bioorg. Med. Chem. Lett, 2013, 23(13), 3914–3919. [ 10.1016/j.bmcl.2013.04.061] [DOI] [PubMed] [Google Scholar]
- [105].Dunlop J; Watts SW; Barrett JE; Coupet J; Harrison B; Mazandarani H; Nawoschik S; Pangalos MN; Ramamoorthy S; Schechter L; Smith D; Stack G; Zhang J; Zhang G; Rosenzweig-Lipson S Characterization of vabicaserin (SCA-136), a selective 5-hydroxytryptamine 2C receptor agonist. J. Pharmacol. Exp. Ther, 2011, 337(3), 673–680. [ 10.1124/jpet.111.179572] [DOI] [PubMed] [Google Scholar]
- [106].Rouquet G; Moore DE; Spain M; Allwood DM; Battilocchio C; Blakemore DC; Fish PV; Jenkinson S; Jessiman AS; Ley SV; McMurray G; Storer RI Design, synthesis, and evaluation of tetrasubstituted pyridines as potent 5-HT2C receptor agonists. ACS Med. Chem. Lett, 2015, 6(3), 329–333. [ 10.1021/ml500507v] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Storer RI; Brennan PE; Brown AD; Bungay PJ; Conlon KM; Corbett MS; DePianta RP; Fish PV; Heifetz A; Ho DK; Jessiman AS; McMurray G; de Oliveira CA; Roberts LR; Root JA; Shanmugasundaram V; Shapiro MJ; Skerten M; Westbrook D; Wheeler S; Whitlock GA; Wright J Multiparameter optimization in CNS drug discovery: design of pyrimido[4,5-d]azepines as potent 5-hydroxytryptamine 2C (5-HT2C) receptor agonists with exquisite functional selectivity over 5-HT2A and 5-HT2B receptors. J. Med. Chem, 2014, 57(12), 5258–5269. [ 10.1021/jm5003292] [DOI] [PubMed] [Google Scholar]
- [108].Tye H; Mueller SG; Prestle J; Scheuerer S; Schindler M; Nosse B; Prevost N; Brown CJ; Heifetz A; Moeller C; Pedret-Dunn A; Whittaker M Novel 6,7,8,9-tetrahydro-5H-1,4,7,10a-tetraaza-cyclohepta[f]indene analogues as potent and selective 5-HT(2C) agonists for the treatment of metabolic disorders. Bioorg. Med. Chem. Lett, 2011, 21(1), 34–37. [ 10.1016/j.bmcl.2010.11.089] [DOI] [PubMed] [Google Scholar]
- [109].Carpenter J; Wang Y; Wu G; Feng J; Ye XY; Morales CL; Broekema M; Rossi KA; Miller KJ; Murphy BJ; Wu G; Malmstrom SE; Azzara AV; Sher PM; Fevig JM; Alt A; Bertekap RL Jr; Cullen MJ; Harper TM; Foster K; Luk E; Xiang Q; Grubb MF; Robl JA; Wacker DA Utilization of an active site mutant receptor for the identification of potent and selective atypical 5-HT2C receptor agonists. J. Med. Chem, 2017, 60(14), 6166–6190. [ 10.1021/acs.jmedchem.7b00385] [DOI] [PubMed] [Google Scholar]
- [110].Felsing DE; Canal CE; Booth RG Ligand-directed serotonin 5-HT2C receptor desensitization and sensitization. Eur. J. Pharmacol, 2019, 848, 131–139. [ 10.1016/j.ejphar.2019.01.037] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Menniti FS; Lindsley CW; Conn PJ; Pandit J; Zagouras P; Volkmann RA Allosteric modulators for the treatment of schizophrenia: targeting glutamatergic networks. Curr. Top. Med. Chem, 2013, 13(1), 26–54. [ 10.2174/1568026611313010005] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Christopoulos A Allosteric binding sites on cell-surface receptors: novel targets for drug discovery. Nat. Rev. Drug Discov, 2002, 1(3), 198–210. [ 10.1038/nrd746] [DOI] [PubMed] [Google Scholar]
- [113].Conn PJ; Christopoulos A; Lindsley CW Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat. Rev. Drug Discov, 2009, 8(1), 41–54. [ 10.1038/nrd2760] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Gregory KJ; Sexton PM; Christopoulos A Overview of receptor allosterism. Curr Protoc Pharmacol, 2010, 1, 1.21.1–1.21.34. [ 10.1002/0471141755.ph0121s51] [DOI] [PubMed] [Google Scholar]
- [115].Melancon BJ; Hopkins CR; Wood MR; Emmitte KA; Niswender CM; Christopoulos A; Conn PJ; Lindsley CW Allosteric modulation of seven transmembrane spanning receptors: theory, practice, and opportunities for central nervous system drug discovery. J. Med. Chem, 2012, 55(4), 1445–1464. [ 10.1021/jm201139r] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Wootten D; Christopoulos A; Sexton PM Emerging paradigms in GPCR allostery: implications for drug discovery. Nat. Rev. Drug Discov, 2013, 12(8), 630–644. [ 10.1038/nrd4052] [DOI] [PubMed] [Google Scholar]
- [117].Schlag BD; Lou Z; Fennell M; Dunlop J Ligand dependency of 5-hydroxytryptamine 2C receptor internalization. J. Pharmacol. Exp. Ther, 2004, 310(3), 865–870. [ 10.1124/jpet.104.067306] [DOI] [PubMed] [Google Scholar]
- [118].Foster DJ; Conn PJ Allosteric modulation of GPCRs: new insights and potential utility for treatment of schizophrenia and other CNS disorders. Neuron, 2017, 94(3), 431–446. [ 10.1016/j.neuron.2017.03.016] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Kenakin TP ‘7TM receptor allostery: putting numbers to shape-shifting proteins. Trends Pharmacol. Sci, 2009, 30(9), 460–469. [ 10.1016/j.tips.2009.06.007] [DOI] [PubMed] [Google Scholar]
- [120].Christopoulos A Advances in G protein-coupled receptor allostery: from function to structure. Mol. Pharmacol, 2014, 86(5), 463–478. [ 10.1124/mol.114.094342] [DOI] [PubMed] [Google Scholar]
- [121].Wild CT; Miszkiel JM; Wold EA; Soto CA; Ding C; Hartley RM; White MA; Anastasio NC; Cunningham KA; Zhou J Design, synthesis, and characterization of 4-undecylpipe-ridine-2-carboxamides as positive allosteric modulators of the serotonin (5-HT) 5-HT2C receptor. J. Med. Chem, 2019, 62(1), 288–305. [ 10.1021/acs.jmedchem.8b00401] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Zhou J; Cunningham KA Positive-allosteric modulation of the 5-HT2C receptor: implications for neuropsychopharmacology and neurotherapeutics. Neuropsychopharmacology, 2019, 44(1), 230–231. [ 10.1038/s41386-018-0190-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Wold EA; Zhou J GPCR allosteric modulators: mechanistic advantages and therapeutic applications. Curr. Top. Med. Chem, 2018, 18(23), 2002–2006. [ 10.2174/1568026619999190101151837] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Dror RO; Green HF; Valant C; Borhani DW; Valcourt JR; Pan AC; Arlow DH; Canals M; Lane JR; Rahmani R; Baell JB; Sexton PM; Christopoulos A; Shaw DE Structural basis for modulation of a G-protein-coupled receptor by allosteric drugs. Nature, 2013, 503(7475), 295–299. [ 10.1038/nature12595] [DOI] [PubMed] [Google Scholar]
- [125].Bridges TM; Lindsley CW G-protein-coupled receptors: from classical modes of modulation to allosteric mechanisms. ACS Chem. Biol, 2008, 3(9), 530–541. [ 10.1021/cb800116f] [DOI] [PubMed] [Google Scholar]
- [126].Lindsley CW; Emmitte KA; Hopkins CR; Bridges TM; Gregory KJ; Niswender CM; Conn PJ Practical strategies and concepts in GPCR allosteric modulator discovery: recent advances with metabotropic glutamate receptors. Chem. Rev, 2016, 116(11), 6707–6741. [ 10.1021/acs.chemrev.5b00656] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Merkler DJ; Merkler KA; Stern W; Fleming FF Fatty acid amide biosynthesis: a possible new role for peptidylglycine alpha-amidating enzyme and acyl-coenzyme A: glycine N-acyltransferase. Arch. Biochem. Biophys, 1996, 330(2), 430–434. [ 10.1006/abbi.1996.0272] [DOI] [PubMed] [Google Scholar]
- [128].Conn PJ; Lindsley CW; Meiler J; Niswender CM Opportunities and challenges in the discovery of allosteric modulators of GPCRs for treating CNS disorders. Nat. Rev. Drug Discov, 2014, 13(9), 692–708. [ 10.1038/nrd4308] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Wold EA; Chen J; Cunningham KA; Zhou J Allosteric modulation of class A GPCRs: targets, agents, and emerging concepts. J. Med. Chem, 2019, 62(1), 88–127. [ 10.1021/acs.jmedchem.8b00875] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Im WB; Chio CL; Alberts GL; Dinh DM Positive allosteric modulator of the human 5-HT2C receptor. Mol. Pharmacol, 2003, 64(1), 78–84. [ 10.1124/mol.64.1.78] [DOI] [PubMed] [Google Scholar]
- [131].Spízek J; Novotná J; Rezanka T Lincosamides: chemical structure, biosynthesis, mechanism of action, resistance, and applications. Adv. Appl. Microbiol, 2004, 56, 121–154. [ 10.1016/S0065-2164(04)56004-5] [DOI] [PubMed] [Google Scholar]
- [132].Ding C; Bremer NM; Smith TD; Seitz PK; Anastasio NC; Cunningham KA; Zhou J Exploration of synthetic approaches and pharmacological evaluation of PNU-69176E and its stereoisomer as 5-HT2C receptor allosteric modulators. ACS Chem. Neurosci, 2012, 3(7), 538–545. [ 10.1021/cn300020x] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Sugiura T; Kondo S; Kodaka T; Tonegawa T; Nakane S; Yamashita A; Ishima Y; Waku K Enzymatic synthesis of oleamide (cis-9, 10-octadecenoamide), an endogenous sleep-inducing lipid, by rat brain microsomes. Biochem. Mol. Biol. Int, 1996, 40(5), 931–938. [DOI] [PubMed] [Google Scholar]
- [134].Boger DL; Sato H; Lerner AE; Austin BJ; Patterson JE; Patricelli MP; Cravatt BF Trifluoromethyl ketone inhibitors of fatty acid amide hydrolase: a probe of structural and conformational features contributing to inhibition. Bioorg. Med. Chem. Lett, 1999, 9(2), 265–270. [ 10.1016/S0960-894X(98)00734-3] [DOI] [PubMed] [Google Scholar]
- [135].Fedorova I; Hashimoto A; Fecik RA; Hedrick MP; Hanus LO; Boger DL; Rice KC; Basile AS Behavioral evidence for the interaction of oleamide with multiple neurotransmitter systems. J. Pharmacol. Exp. Ther, 2001, 299(1), 332–342. [PubMed] [Google Scholar]
- [136].Soria-Gómez E; Márquez-Diosdado MI; Montes-Rodríguez CJ; Estrada-González V; Prospéro-García O Oleamide administered into the nucleus accumbens shell regulates feeding behaviour via CB1 and 5-HT2C receptors. Int. J. Neuropsychopharmacol, 2010, 13(9), 1247–1254. [ 10.1017/S1461145710000702] [DOI] [PubMed] [Google Scholar]
- [137].Boger DL; Patterson JE; Jin Q Structural requirements for 5-HT2A and 5-HT1A serotonin receptor potentiation by the biologically active lipid oleamide. Proc. Natl. Acad. Sci. USA, 1998, 95(8), 4102–4107. [ 10.1073/pnas.95.8.4102] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Huidobro-Toro JP; Harris RA Brain lipids that induce sleep are novel modulators of 5-hydroxytrypamine receptors. Proc. Natl. Acad. Sci. USA, 1996, 93(15), 8078–8082. [ 10.1073/pnas.93.15.8078] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Thomas EA; Carson MJ; Neal MJ; Sutcliffe JG Unique allosteric regulation of 5-hydroxytryptamine receptor-mediated signal transduction by oleamide. Proc. Natl. Acad. Sci. USA, 1997, 94(25), 14115–14119. [ 10.1073/pnas.94.25.14115] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Hedlund PB; Carson MJ; Sutcliffe JG; Thomas EA Allosteric regulation by oleamide of the binding properties of 5-hydroxytryptamine7 receptors. Biochem. Pharmacol, 1999, 58(11), 1807–1813. [ 10.1016/S0006-2952(99)00274-9] [DOI] [PubMed] [Google Scholar]
- [141].Cheer JF; Cadogan AK; Marsden CA; Fone KC; Kendall DA Modification of 5-HT2 receptor mediated behaviour in the rat by oleamide and the role of cannabinoid receptors. Neuropharmacology, 1999, 38(4), 533–541. [ 10.1016/S0028-3908(98)00208-1] [DOI] [PubMed] [Google Scholar]
- [142].García-Cárceles J; Decara JM; Vázquez-Villa H; Rodriguez R; Codesido E; Cruces J; Brea J; Loza MI; Alén F; Botta J; McCormick PJ; Ballesteros JA; Benhamú B; Rodriguez de Fonseca F; López-Rodríguez ML A positive allosteric modulator of the serotonin 5-HT2C receptor for obesity. J. Med. Chem, 2017, 60(23), 9575–9584. [ 10.1021/acs.jmedchem.7b00994] [DOI] [PubMed] [Google Scholar]
- [143].Singh K; Sona C; Ojha V; Singh M; Mishra A; Kumar A; Siddiqi MI; Tripathi RP; Yadav PN Identification of dual role of piperazine-linked phenyl cyclopropyl methanone as positive allosteric modulator of 5-HT2C and negative allosteric modulator of 5-HT2B receptors. Eur. J. Med. Chem, 2019, 164, 499–516. [ 10.1016/j.ejmech.2018.12.070] [DOI] [PubMed] [Google Scholar]
- [144].Mikitsh JL; Chacko AM Pathways for small molecule delivery to the central nervous system across the blood-brain barrier. Perspect. Medicin. Chem, 2014, 6, 11–24. [ 10.4137/PMC.S13384] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Marona-Lewicka D; Nichols DE Complex stimulus properties of LSD: a drug discrimination study with alpha 2-adrenoceptor agonists and antagonists. Psychopharmacology (Berl.), 1995, 120(4), 384–391. [ 10.1007/BF02245809] [DOI] [PubMed] [Google Scholar]
- [146].Kleven MS; Assié MB; Koek W Pharmacological characterization of in vivo properties of putative mixed 5-HT1A agonist/5-HT(2A/2C) antagonist anxiolytics. II. Drug discrimination and behavioral observation studies in rats. J. Pharmacol. Exp. Ther, 1997, 282(2), 747–759. [PubMed] [Google Scholar]
- [147].Mohler EG; Franklin SR; Rueter LE Discriminative-stimulus effects of NS9283, a nicotinic α4β2* positive allosteric modulator, in nicotine-discriminating rats. Psychopharmacology (Berl.), 2014, 231(1), 67–74. [ 10.1007/s00213-013-3207-5] [DOI] [PubMed] [Google Scholar]
- [148].Ward RJ; Pediani JD; Godin AG; Milligan G Regulation of oligomeric organization of the serotonin 5-hydroxytryptamine 2C (5-HT2C) receptor observed by spatial intensity distribution analysis. J. Biol. Chem, 2015, 290(20), 12844–12857. [ 10.1074/jbc.M115.644724] [DOI] [PMC free article] [PubMed] [Google Scholar]