Staphylococcus aureus is an important human pathogen in both community and health care settings. One of the challenges with S. aureus as a pathogen is its acquisition of antibiotic resistance. Previously, we showed that deletion of the msaABCR operon reduces cell wall thickness, resulting in decreased resistance to vancomycin in vancomycin-intermediate S. aureus (VISA).
KEYWORDS: cell wall, antibiotic resistance, msaABCR, peptidoglycan, Staphylococcus aureus
ABSTRACT
Staphylococcus aureus is an important human pathogen in both community and health care settings. One of the challenges with S. aureus as a pathogen is its acquisition of antibiotic resistance. Previously, we showed that deletion of the msaABCR operon reduces cell wall thickness, resulting in decreased resistance to vancomycin in vancomycin-intermediate S. aureus (VISA). In this study, we investigated the nature of the cell wall defect in the msaABCR operon mutant in the Mu50 (VISA) and USA300 LAC methicillin-resistant Staphylococcus aureus (MRSA) strains. Results showed that msaABCR mutant cells had decreased cross-linking in both strains. This defect is typically due to increased murein hydrolase activity and/or nonspecific processing of murein hydrolases mediated by increased protease activity in mutant cells. The defect was enhanced by a decrease in teichoic acid content in the msaABCR mutant. Therefore, we propose that deletion of the msaABCR operon results in decreased peptidoglycan cross-linking, leading to increased susceptibility toward cell wall-targeting antibiotics, such as β-lactams and vancomycin. Moreover, we also observed significantly downregulated transcription of early cell wall-synthesizing genes, supporting the finding that msaABCR mutant cells have decreased peptidoglycan synthesis. More specifically, the msaABCR mutant in the USA300 LAC strain (MRSA) showed significantly reduced expression of the murA gene, whereas the msaABCR mutant in the Mu50 strain (VISA) showed significantly reduced expression of glmU, murA, and murD. Thus, we conclude that the msaABCR operon controls the balance between cell wall synthesis and cell wall hydrolysis, which is required for maintaining a robust cell wall and acquiring resistance to cell wall-targeting antibiotics, such as vancomycin and the β-lactams.
INTRODUCTION
Staphylococcus aureus is an important human pathogen in both community and health care settings. Staphylococcus infections can range from mild, superficial skin and soft tissue infections to life-threatening infections, such as toxic shock, endocarditis, and septicemia (1, 2). Most of the drugs used in Staphylococcus-related infections target cell wall biosynthesis, and this has resulted in increased prevalence of resistance to these drugs, which is a major health concern (3). Methicillin-resistant Staphylococcus aureus (MRSA) strains are resistant to most β-lactam antibiotics because of the acquisition and expression of a penicillin-binding protein (PBP2a), which has a lower affinity for β-lactams; however, there are a number of additional auxiliary factors that are critical for resistance to β-lactam antibiotics (3–5). The Centers for Disease Control and Prevention (CDC) estimates that in 2014, 72,444 invasive MRSA infections and 9,194 MRSA-related deaths occurred in the United States (6). While the glycopeptide antibiotic vancomycin has been used successfully to treat MRSA infections, vancomycin-intermediate Staphylococcus aureus (VISA) and vancomycin-resistant Staphylococcus aureus (VRSA) are also becoming increasingly prevalent.
As is characteristic of Gram-positive bacteria, the staphylococcal cell wall is composed of a highly complex meshwork of peptidoglycan chains, teichoic acids, and cell wall-associated surface proteins. Any compromise of the integrity of the cell wall also plays an important role in antibiotic resistance, because it is targeted by many antibiotics, such as β-lactams (e.g., penicillins and cephalosporins), glycopeptides (e.g., vancomycin and teicoplanin), and other cell wall-active antibiotics (e.g., fosfomycin, daptomycin, nisin, and bacitracin). Peptidoglycan is a macromolecule made of glycan chains cross-linked by pentapeptide chains, which are further cross-linked by pentaglycine bridges. Peptidoglycan is essential for maintaining an intact cell wall to withstand internal turgor pressure as well as to maintain enough plasticity to allow for growth, division, and survival of the bacterial population (7–9). Wall teichoic acids (WTAs) are the most abundant anionic glycopolymers linked to the peptidoglycans in Gram-positive cell walls. WTAs are involved in a variety of processes, such as stress resistance (antimicrobial peptides, antimicrobial fatty acids, cationic antibiotics, heat, and osmolarity) and pathogenesis (host attachment, colonization, infection, and biofilm formation) (10). WTAs also determine the localization of penicillin-binding proteins (PBPs) in the cell wall, coordinate the localization, stability, and/or activity of murein hydrolases, and confer resistance against host-produced lytic enzymes, such as lysozymes (7, 9, 11). Thus, peptidoglycan and WTAs are critical bacterial components for maintaining cell wall structure and promoting antibiotic resistance.
Studies have shown that β-lactam and glycopeptide antibiotics disrupt peptidoglycan cross-linking, which ultimately weakens the integrity of the bacterial cell wall, leading to cell lysis. Due to the efficacy and safety profiles of these antibiotics, resensitization of resistant pathogens to these drugs is a promising approach to treatment (12). Several recent studies have uncovered the role of other factors/genes involved in the biosynthesis of cell envelope components (such as peptidoglycan and teichoic acids), in modulating cell wall hydrolase activities of β-lactams, and in vancomycin resistance. These reports have rekindled interest in targeting genes involved in the maintenance of cell wall integrity and ultimately resensitizing resistant strains (4, 7, 13–20). Previously, we showed that the msaABCR operon plays a role in biofilm development, virulence, persister cell formation, and antibiotic resistance in S. aureus (21–26). The msaABCR operon is a four-gene operon composed of msaA, msaB, msaC, and an antisense RNA, msaR (23). The msaB gene is the only protein-coding gene whose product, MsaB, is both a transcription factor and an RNA chaperone (24, 27). Deletion of the msaABCR operon resulted in decreases in the vancomycin MIC in VISA strains (Mu50, HIP6297, and LIM2) and in a MRSA strain (USA300 LAC) of S. aureus (22, 26). In addition, we also observed significant reductions of cell wall thickness in the Mu50 and HIP6297 strains (22). We have also shown that msaABCR mutants are defective in persister cell formation under cell wall-targeting antibiotic (vancomycin and daptomycin) stress conditions in the USA300 LAC strain background (26). Increases in Triton X-100-induced autolysis and protease production are also major phenotypes of the msaABCR-deletion mutant (21, 22). We previously showed that the msaABCR operon represses protease activity regulating the expression of four extracellular proteases (Aur, Scp, Ssp, and Spl) in various growth phases, including the early exponential growth phase (21, 23). The serine protease Ssp, the cysteine proteases, and other proteases have been found to be involved in the processing of AtlA, thereby regulating murein hydrolase activities and autolysis (21, 28–30). These findings indicate roles for the msaABCR operon in cell wall synthesis, autolysis, and the development of vancomycin resistance. We therefore hypothesized that the msaABCR operon plays a role in maintaining cell wall integrity by regulating both cell wall synthesis and cell wall hydrolase activity. In this study, we investigated the mechanism behind the cell wall defect (reduction of cell wall thickness), the increased cell autolysis, and the vancomycin/β-lactam susceptibility in the msaABCR-deletion mutant in both the VISA Mu50 and MRSA USA300 LAC strains.
RESULTS
Deletion of the msaABCR operon leads to increased susceptibility to antimicrobials that target the cell wall.
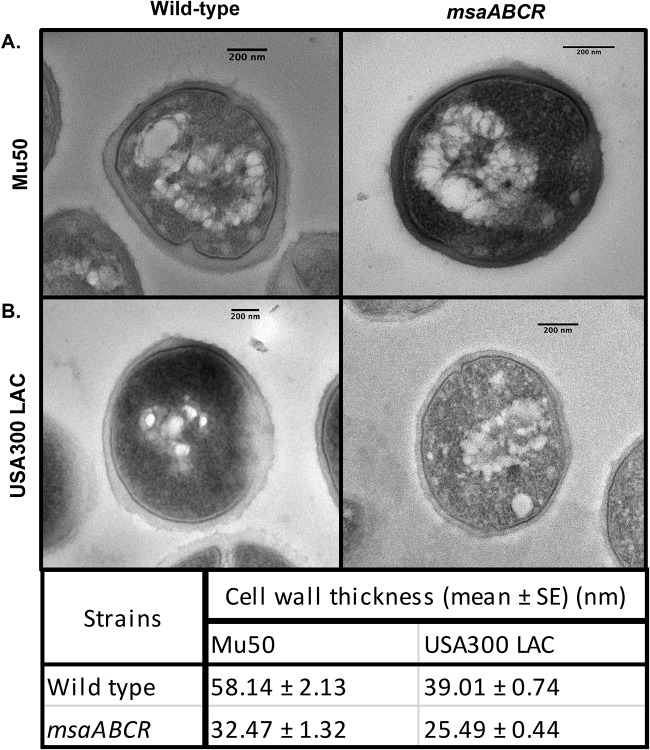
To study cell wall defects in the msaABCR-deletion mutant, we first examined the cell structure and cell wall thickness of msaABCR operon mutants using transmission electron microscopy in both the highly virulent community-acquired MRSA strain, USA300 LAC, and the clinical vancomycin intermediate-resistance (VISA) isolate strain, Mu50. Mutants of USA300 LAC and Mu50 showed significant decreases (approximately 45%) in cell wall thickness, with a smoother texture than their respective wild-type strains (Fig. 1A and B). These results suggest that the msaABCR operon plays important roles in cell wall synthesis and maintenance in both MRSA and VISA strains. We then investigated the impact of msaABCR operon deletion on resistance to cell wall-active antibiotics. We measured the susceptibility of the msaABCR deletion mutants to cell wall-targeting antibiotics (vancomycin, methicillin, oxacillin, cloxacillin, cefoxitin, bacitracin, and fosfomycin) relative to that of the wild type by using a microdilution assay to determine the MIC (Table 1). We found that deletion of the msaABCR operon led to an increase in susceptibility to antibiotics that target cell wall synthesis and cross-linking (vancomycin [binding to lipid II], methicillin, cefoxitin, cloxacillin, and oxacillin) in both the USA300 LAC and Mu50 strains. In the USA300 LAC strain, the msaABCR deletion mutant was more susceptible than the wild type to fosfomycin; however, this was not observed in the Mu50 strain. The increased susceptibility of msaABCR mutants to several cell wall-targeting antimicrobials suggests that the integrity of the mutant bacterium cell wall is compromised.
FIG 1.
The msaABCR operon regulates cell wall morphology. Transmission electron micrographs of cells of the wild type and msaABCR operon deletion mutant in the Mu50 (A) and USA300 LAC (B) strains. (Bottom) Cell wall thicknesses of the wild type and msaABCR operon deletion mutant in the USA300 LAC and Mu50 strains.
TABLE 1.
MICs for different cell wall-targeting antimicrobials in msaABCR mutant Mu50 (VISA) and USA300 LAC (MRSA) strains
| Antibiotic tested | MIC (μg/ml)a
|
Mode of action | |||||
|---|---|---|---|---|---|---|---|
| Mu50 | msaABCR | Complementation | USA300 LAC | msaABCR | Complementation | ||
| Vancomycin | 6.5 | 1.65 | 3.25 | 0.825 | 0.412 | 0.825 | Binds to d-Ala and prevents peptidoglycan cross-linking |
| Methicillin | 1,000 | 500 | 1,000 | 25 | 12.5 | 25 | Inhibits PBPs and peptidoglycan cross-linking |
| Cefoxitin | 250 | 125 | 250 | 25 | 12.5 | 25 | Inhibits PBPs and peptidoglycan cross-linking (PBP4) |
| Cloxacillin | 500 | 62.5 | 500 | 1.56 | 0.195 | 0.781 | Inhibits PBPs (Tpases) and peptidoglycan cross-linking |
| Oxacillin | 500 | 125 | 500 | 50 | 12.5 | 50 | Inhibits PBPs (Tpases) and peptidoglycan cross-linking |
| Fosfomycin | >1,000 | >1,000 | >1,000 | 50 | 25 | 50 | Inhibits bacterial cell wall biogenesis by inactivating the enzyme MurA |
| Bacitracin | 250 | 250 | 250 | 100 | 200 | 200 | Interferes with the dephosphorylation of bactoprenol, which transports the building blocks of the peptidoglycan bacterial cell wall outside the inner membrane |
| Lysostaphin | 1.25 | 0.312 | 0.625 | 0.156 | 0.078 | 0.156 | Cleaves the cross-linking pentaglycine bridges in the cell wall peptidoglycan |
These results represent three independent experiments performed in triplicates.
msaABCR deletion mutants are more susceptible to cell wall-active enzymes.
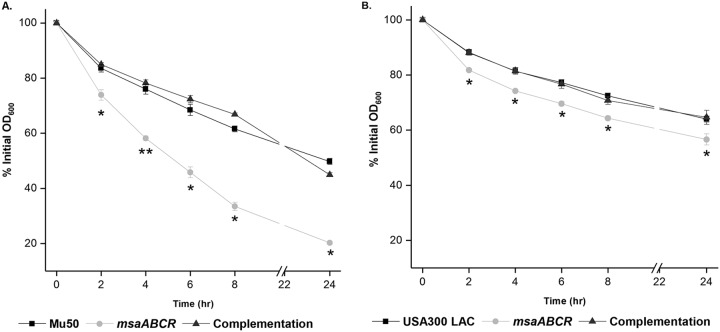
We investigated the nature of the cell wall perturbation in the msaABCR deletion mutant by measuring its growth kinetics in the presence of lysozyme, a hydrolase that catalyzes the hydrolysis of 1,4-beta-linkages in the glycan chain in peptidoglycan. By measuring the optical density at 600 nm (OD600), we monitored the effect of lysozyme (500 μg/ml) on msaABCR mutant cultures and compared them with their respective wild-type and complementation counterparts in both Mu50 (Fig. 2A) and USA300 LAC (Fig. 2B) strains. Lysozyme was added when the starter culture reached an OD600 of 1 in tryptic soy broth (TSB). We observed that the msaABCR deletion mutant cells were lysed significantly faster than their respective wild-type and complementation counterparts (Fig. 2). In addition, population analysis showed a >2- to 3-fold reduction in the msaABCR mutant population after 6 h (4 h postaddition of 500 μg/ml lysozyme treatment) compared to that of their respective wild-type counterparts (Fig. 3A and B). We also measured the rate of cell lysis in the presence of lysozyme. For this, we harvested exponentially growing cells and measured whole-cell lysis as the percentage of the initial OD600 at different time points over 24 h in the presence of 350 μg/ml lysozyme. We observed that the msaABCR deletion mutants lysed at a significantly higher rate than their respective wild-type and complementation counterparts in both Mu50 (Fig. 4A) and USA300 LAC (Fig. 4B) strains.
FIG 2.
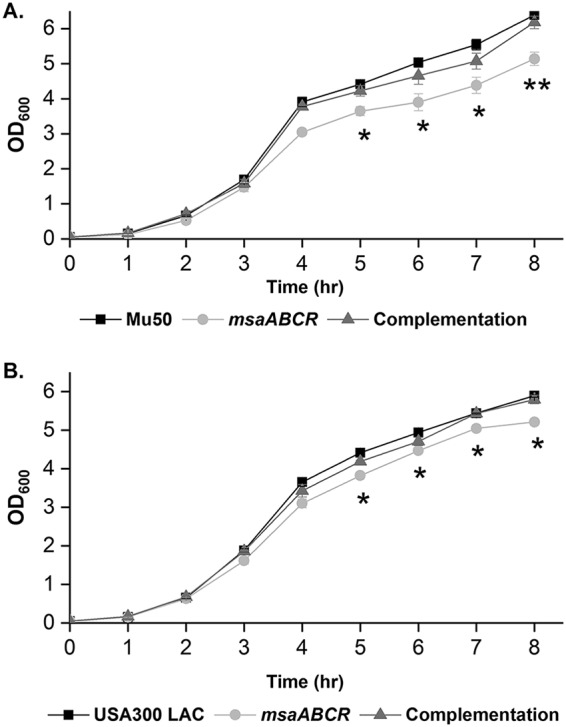
msaABCR mutant growth yield is lower than the wild type in the presence of lysozyme. Growth curves in the presence of lysozyme. The starter cultures for all test strains were normalized to an OD600 of 0.05 and incubated further until an OD600 reading of 1.0. The cultures were then treated with 500 μg/ml lysozyme, and the growth kinetics were measured by taking the OD600 reading every hour. Mu50 (A) and USA300 LAC (B) strain backgrounds and their respective msaABCR mutant and complementation strains. These results represent the means from three independent experiments. Error bars represent the standard errors (SEs). Student’s t test and one-way analysis of variance (ANOVA) were used to compare the results from the wild types with those from their corresponding mutants. *, P < 0.05; **, P < 0.005.
FIG 3.
msaABCR mutant S. aureus is lysed with lysozyme at a higher rate than for the wild type. Population profiles at different time points when 500 μg/ml lysozyme was added to cultures of the Mu50 (A) and USA300 LAC (B) strain backgrounds and their respective msaABCR mutant and complementation strains after 2 h of growth of the starter culture. These results represent the means from three independent experiments performed in triplicates. Error bars represent the SEs. Student’s t test and one-way ANOVA were used to compare the results from the wild types with those from their corresponding mutants. *, P < 0.05; **, P < 0.005.
FIG 4.
msaABCR mutant cells are lysed at a higher rate than for the wild type in the presence of lysozyme. Lysozyme-induced whole-cell lysis. S. aureus cells were suspended in PBS and digested with 350 μg/ml lysozyme. Cell lysis was measured as a decrease in OD600 in Mu50 (A) and USA300 LAC (B) strain backgrounds and in their respective msaABCR mutant and complementation strains. These results represent the means from three independent experiments. Error bars represent the SEs. Student’s t test and one-way ANOVA were used to compare the results from the wild types with those from their corresponding mutants. *, P < 0.05; **, P < 0.005.
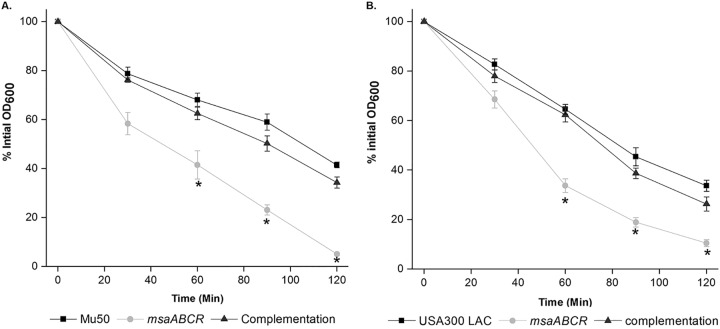
We then measured the susceptibility of msaABCR mutant cells to a second cell wall-lytic enzyme, lysostaphin, which hydrolyzes the pentaglycine chain in peptidoglycan. We found that deletion of the msaABCR operon led to an increase in susceptibility to lysostaphin in both the USA300 LAC and Mu50 strains (Table 1). We then measured cell lysis in the presence of lysostaphin. In this assay, we harvested exponentially growing cells and measured whole-cell lysis as a percentage of the initial OD600 over 1 h at regular 15-min intervals in the presence of 0.5 μg/ml lysostaphin. However, we observed a significant increase in whole-cell lysis in the Mu50 strain msaABCR mutant (Fig. 5A) but not in the USA300 LAC strain msaABCR mutant (Fig. 5B). Increased lysostaphin susceptibility in the msaABCR mutant in the Mu50 background indicates a defect in the mutant cell wall that may be due to a reduction in cell wall thickness and/or a reduction in cell wall cross-linking.
FIG 5.
The msaABCR mutant of the Mu50 strain is lysed with lysostaphin at a higher rate than for the wild type. Lysostaphin lysis assay. S. aureus wild-type, msaABCR mutant, and complementation strains in the Mu50 (A) and USA300 LAC (B) strain backgrounds were grown to an OD of 1 at 37°C in TSB medium. Bacteria were harvested by centrifugation, washed twice with Tris buffer, and suspended to an initial OD600 of ∼1. Lysostaphin lysis assays were performed with 0.5 μg/ml lysostaphin, and lysis was measured as a decline in OD600 over time. These results represent the means from three independent experiments. Error bars represent the SEs. Student’s t test and one-way ANOVA were used to compare the results from the wild types with those from their corresponding mutants. *, P < 0.05
Previous studies have shown that the increased lysozyme sensitivity of S. aureus peptidoglycan is dependent on its degree of O-acetylation, teichoic acid content, and/or cross-linking (31–33). To evaluate which of these three factors contributes to lysozyme sensitivity in the mutants, we measured the expression of the oatA gene, which is involved in O-acetylation of peptidoglycan, in the msaABCR mutants relative to their wild-type counterparts using quantitative real-time PCR (Table 2). Our results showed that both Mu50 and USA300 LAC wild types and the msaABCR deletion mutants have similar expression of the oatA gene. This result shows that lysozyme sensitivity in the mutants is not due to O-acetylation. In addition, we treated peptidoglycan preparations from all test strains with 10% trichloroacetic acid (TCA) and 80 mM NaOH to remove teichoic acid and O-acetyl groups, as described in Materials and Methods. These peptidoglycan preparations (0.5 mg/ml) were then resuspended in 80 mM sodium phosphate-buffered saline and digested with 350 μg/ml lysozyme. We observed that the peptidoglycan preparation from msaABCR mutants lysed at a higher rate than the respective wild-type cell walls in both Mu50 (Fig. 6A) and USA300 LAC (Fig. 6B) strains. These experiments show that the increased lysis rate of the mutant cell wall is mainly due to reduced cross-linking and is not related to O-acetylation. We further investigated the contribution of teichoic acid content to the mutant phenotype in the last section of Results.
TABLE 2.
Expression of cell wall biosynthesis and hydrolase genes in the msaABCR mutant relative to their expression in the wild type
| Gene | Mean fold change ± SEa
|
|
|---|---|---|
| Mu50 | USA300 LAC | |
| glmU | –2.77 ± 0.09 | –1.19 ± 0.06 |
| murA | –3.49 ± 0.18 | –2.5 ± 0.38 |
| murB | –1.29 ± 0.14 | –1.48 ± 0.01 |
| murD | –2.74 ± 0.03 | 1.47 ± 0.19 |
| fmhB (femX) | 1.20 ± 0.04 | 1.42 ± 0.20 |
| femA | –1.15 ± 0.03 | 1.04 ± 0.02 |
| femB | –1.19 ± 0.02 | –1.12 ± 0.04 |
| oatA | 1.28 ± 0.16 | –1.49 ± 0.13 |
| mecA | –1.63 ± 0.04 | 1.21 ± 0.04 |
| pbp1 | 1.38± 0.05 | 1.21 ± 0.07 |
| pbp2 | –1.1 ± 0.03 | 1.26 ± 0.12 |
| pbp3 | 1.11±0.06 | –1.12 ± 0.05 |
| pbp4 | –1.34 ± 0.23 | –1.21 ± 0.21 |
| atlA | 2.49 ± 0.15 | 1.68 ± 0.13 |
| lytM | 1.52 ± 0.08 | 1.59 ± 0.13 |
| sle-1 | 1.05 ± 0.12 | 1.34 ± 0.07 |
| lytN | 1.77 ± 0.23 | –1.09 ± 0.09 |
| lytH | –1.06 ± 0.2 | 1.3 ± 0.43 |
| lytX | –1.06 ± 0.23 | –1.06 ± 0.53 |
| lytY | –1.01 ± 0.21 | –1.07 ± 0.09 |
| lytZ | 1.42 ± 0.24 | 1.37 ± 0.31 |
| tarO | –1.56 ± 0.13 | –1.67 ± 0.10 |
| tarA | –1.82 ± 0.14 | –1.59 ± 0.23 |
These results represent the means from three independent experiments performed in triplicates. Genes up- or downregulated by ≥ or ≤2.0-fold are shown in boldface font.
FIG 6.
msaABCR mutant peptidoglycans are lysed in the presence of lysozyme at a higher rate than for the wild type. Digestion of peptidoglycan devoid of teichoic acid and acetyl groups with lysozyme. Peptidoglycan was suspended in 80 mM sodium phosphate-buffered saline (0.5 mg/ml) and digested with 350 μg/ml lysozyme. Lysis of peptidoglycan was measured as a decrease in OD600 in the Mu50 (A) and USA300 LAC (B) strain backgrounds and their respective msaABCR mutant and complementation strains. These results represent the means from three independent experiments. Error bars represent the SEs. Student’s t test and one-way ANOVA were used to compare the results from the wild types with those from their respective mutants. *, P < 0.05.
The msaABCR operon plays a role in cell wall cross-linking.
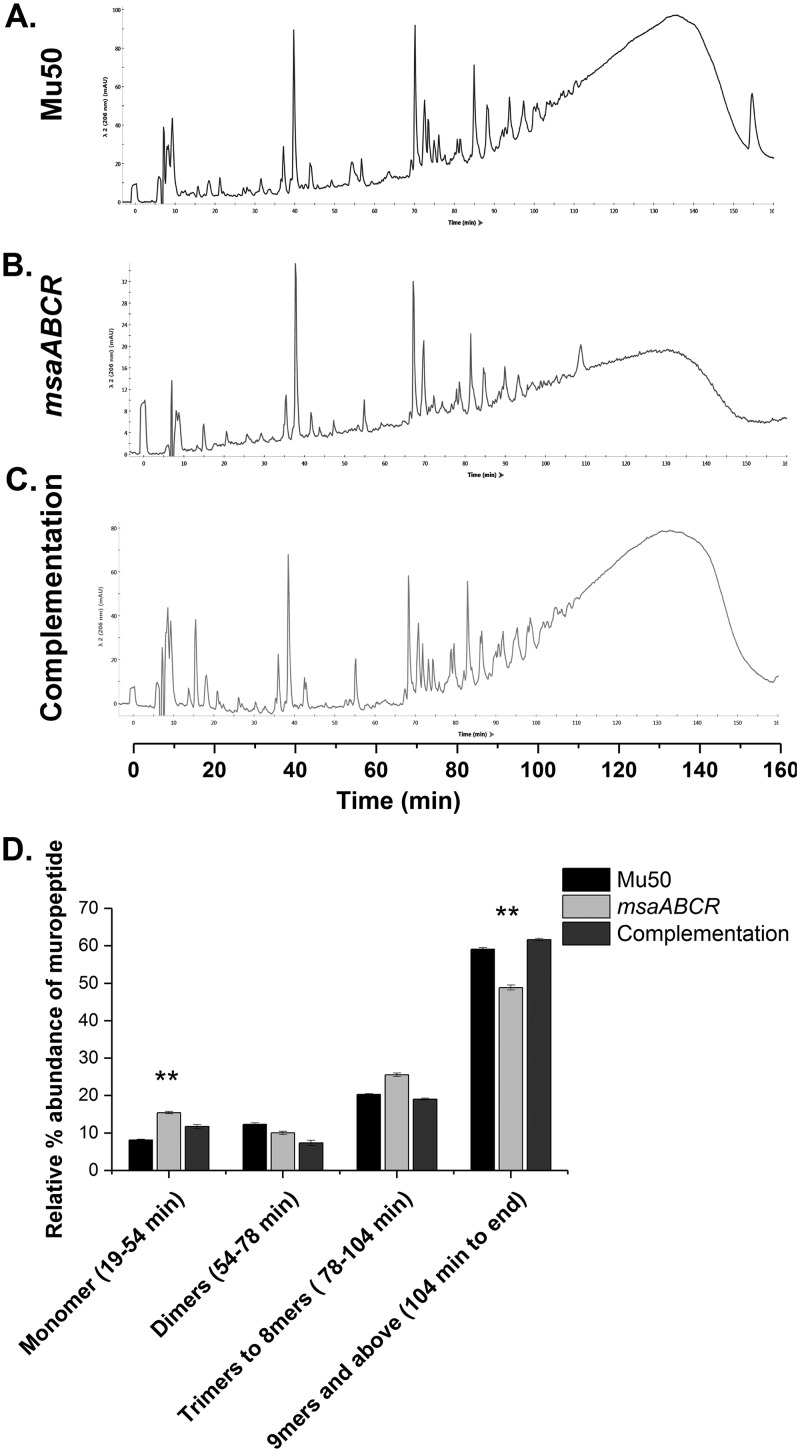
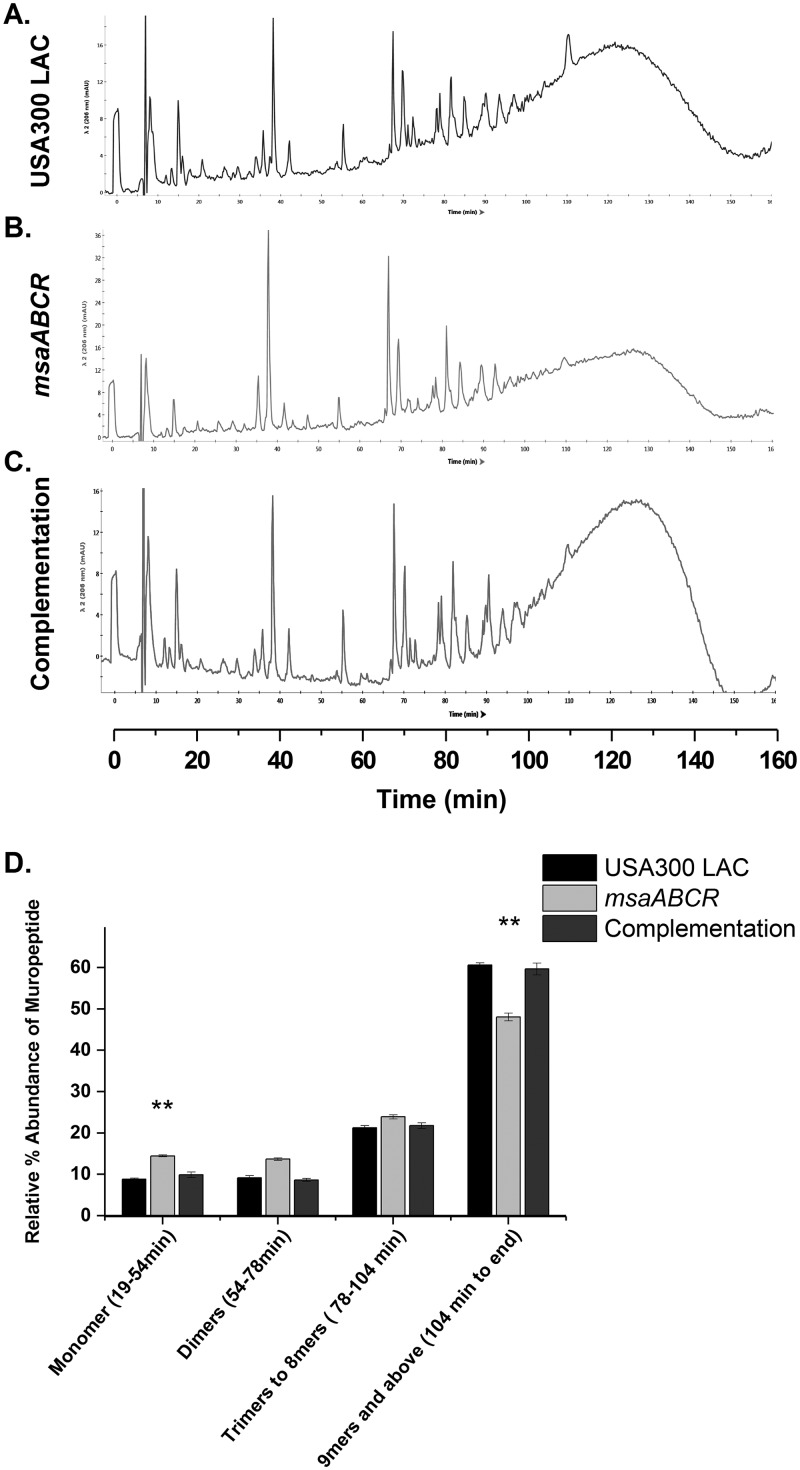
To gain a deeper insight into peptidoglycan composition, we purified peptidoglycan from the Mu50 and USA300 LAC strains and their respective msaABCR deletion mutants to determine their muropeptide composition. We digested the purified peptidoglycan with mutanolysin and analyzed the muropeptide composition using high-pressure liquid chromatography (HPLC). The HPLC profiles of the mutants from both strains showed a marked difference in peptidoglycan composition relative to that of the wild type (Fig. 7 and 8). We observed a significant reduction in the amount of oligomeric muropeptide with a retention time >104 min, which corresponds to those oligomers greater than or equal to 9-mers in both msaABCR mutants relative to that in their corresponding wild-type counterparts. Furthermore, we observed differences in the relative abundance of the monomeric peaks that elute between 19 min and 54 min between mutants and wild types. Specifically, the monomeric fraction was significantly higher in the msaABCR mutants than in the wild type (Fig. 7 and 8). As mutanolysin cleaves glycan chains in peptidoglycan, digestion of highly cross-linked peptidoglycan by mutanolysin produces more highly cross-linked muropeptides than less highly cross-linked peptidoglycan. Thus, a decreased relative abundance of oligomeric muropeptides and a concomitant increased abundance of monomeric muropeptides from digestion of msaABCR mutant peptidoglycan with mutanolysin demonstrate that the msaABCR mutants have cell walls with reduced cross-linking in both the VISA Mu50 and MRSA USA300 LAC strains relative to that in their respective wild-type counterparts.
FIG 7.
msaABCR mutant peptidoglycan is less cross-linked than the wild type. (A to C) HPLC chromatograms of mutanolysin-digested peptidoglycan purified from the indicated strains in the Mu50 strain background. The peaks were identified according to de Jonge et al. (67). Representative results of three individual experiments are shown. (D) Muropeptide composition expressed as the percentage of the total area under the curve for each strain. The figure shows percentages indicating the relative abundances of monomers, dimers, trimers to 8-mers, and higher oligomers in each strain. These results represent the means from three independent experiments. Error bars represent the SEs. Student’s t test and one-way ANOVA were used to compare the results from the wild type with those from mutants. **, P < 0.005.
FIG 8.
msaABCR mutant peptidoglycan is less cross-linked than the wild type. (A to C) HPLC chromatograms of mutanolysin-digested peptidoglycan purified from the indicated strains in the USA300 LAC strain background. The peaks were identified according to de Jonge et al. (67). Representative results of three individual experiments are shown. (D) Muropeptide composition expressed as the percentage of the total area under the curve for each strain. The figure shows percentages indicating the relative abundances of monomers, dimers, trimers to 8-mers, and higher oligomers in each strain. These results represent the means from three independent experiments. Error bars represent the SEs. Student’s t test and one-way ANOVA were used to compare the results from the wild type with those from the deletion mutant. **, P < 0.005.
Deletion of the msaABCR operon affects the expression of cell wall biosynthesis genes.
Several studies have shown that many factors affect cross-linking within the staphylococcal cell wall. Reduction in cell wall cross-linking may be due to a defect in the pentapeptide chains, decreased activities of penicillin-binding proteins (PBPs) (11–16), defects in pentaglycine bridges (10), and/or a decrease in peptidoglycan teichoic acid content (9). To identify the cause of the decreased cross-linking and decreased cell wall thickness in msaABCR mutants, we first measured the relative expression of genes involved in early cell wall synthesis, including pentapeptide and pentaglycine synthesis genes as well as peptidoglycan cross-linking genes (PBPs), using quantitative real-time PCR (Table 2).
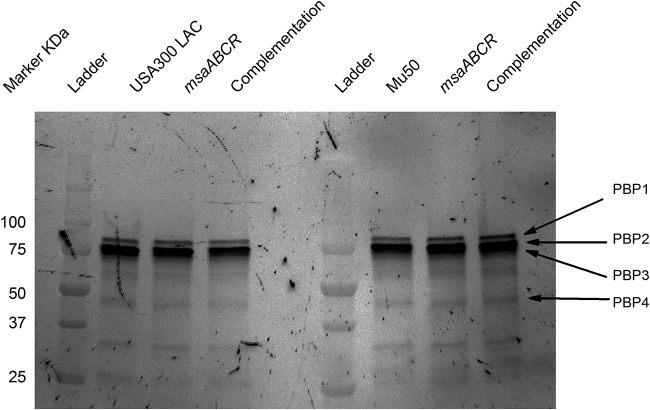
The results of quantitative reverse transcription-PCR (qRT-PCR) analysis indicate that reduction in the peptidoglycan cross-linking of msaABCR mutants is independent of the actions of penicillin-binding proteins, since the mecA, pbp1, pbp2, pbp3, and pbp4 genes were not significantly differentially expressed (<2.0-fold change) in msaABCR mutants compared with that in the wild type (Table 2). We also performed a fluorescence penicillin-binding assay with Bocillin FL to measure the levels of penicillin-binding protein. This assay showed that the msaABCR mutant and its complementation counterparts in the USA300 LAC and Mu50 strains did not have significantly different levels of penicillin-binding proteins compared with that in the wild type (Fig. 9), confirming that the cross-linking defect in the msaABCR mutant is independent of PBP activity.
FIG 9.
Decreased cross-linking in msaABCR mutant peptidoglycan is independent of PBPs. Fluorescence penicillin-binding assay with purified cell membranes from the wild type, its msaABCR mutant, and its complementation mutant in the USA300 LAC and Mu50 strain backgrounds. Gradient gel visualized with Alexa Fluor 488. Representative results of three individual experiments are shown.
The femXAB genes produce a family of nonribosomal peptidyltransferases involved in pentaglycine synthesis and cell wall cross-linking (34–36). Mutations in femAB reduce the amount of peptidoglycan cross-linking and the length of the pentaglycine chain, thus leading to a difference in tolerance to lysostaphin treatment (35–37). Our results showed that there are no significant changes in the expression levels of these genes between the msaABCR mutant and wild type in both strains (Table 2). Thus, the increased lysostaphin-induced lysis rate in the Mu50 msaABCR mutant is not due to a difference in femXAB gene expression.
In addition, we also measured the relative expression of several early peptidoglycan and teichoic acid biosynthesis genes (Table 2). We found that murA, glmU, and murD were significantly downregulated in the msaABCR deletion mutant in Mu50; however, only murA was downregulated in the msaABCR mutant in USA300 LAC. murA codes for UDP-N-acetylglucosamine-1-carboxyvinyl-transferase-2, which catalyzes the conversion of UDP-N-acetylglucosamine (UDP-GlcNAc) to UDP-N-acetyl-3-(1-carboxyvinyl)-d-glucosamine. Deletion of murA in S. aureus causes a significant reduction in peptidoglycan content (38). Since murA is involved in the rate-limiting steps of cell wall biosynthesis, the downregulation of this gene in the msaABCR mutant may contribute to decreased cell wall synthesis, leading to decreased cell wall thickness. glmU encodes a bifunctional protein that catalyzes the last two sequential reactions in the de novo biosynthetic pathway for UDP-N-acetylglucosamine (UDP-GlcNAc) (39). UDP-GlcNAc is also a precursor for wall teichoic acid (WTA), along with peptidoglycan synthesis. Thus, decreased expression of glmU in the Mu50 msaABCR mutant may also contribute to decreased cell wall thickness. MurD (UDP-N-acetylmuramyl-l-alanine:d-glutamate ligase) is the bacterial d-glutamate-adding enzyme that catalyzes the attachment of d-glutamate to a cytoplasmic peptidoglycan precursor, UDP-N-acetylmuramyl-l-alanine (39). This step is important for pentapeptide chain synthesis during cell wall biosynthesis. Thus, downregulation of murD in the Mu50 msaABCR mutant may also contribute to decreased cell wall synthesis rates and decreased cross-linking. Furthermore, we did not observe any significant changes in expression of teichoic acid synthesis genes (tarO and tarA) in the msaABCR mutants compared with that in the wild type in either the Mu50 or USA300 LAC strains. Therefore, results show that decreases in peptidoglycan cross-linking of msaABCR mutants is independent of the actions of penicillin-binding proteins or any peptide chain synthesis defect. However, deletion of the msaABCR operon caused decreased expression of early biosynthetic genes in both the USA300 LAC and Mu50 strains, suggesting a lower peptidoglycan synthesis rate and/or lower peptidoglycan content in the mutant cell wall.
The cross-linking defect in the msaABCR mutant is primarily due to increased protease activity.
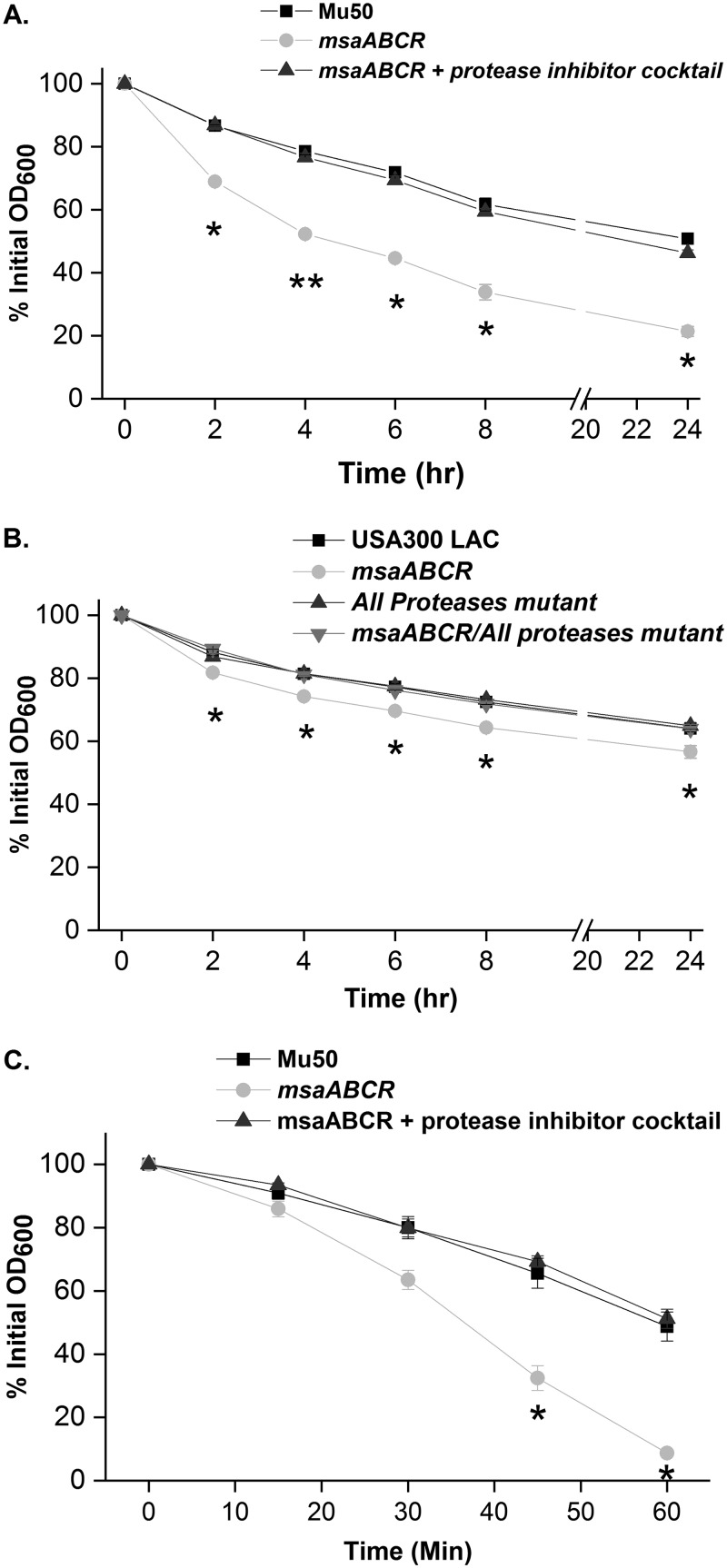
We previously reported that treatment of msaABCR deletion mutants with Triton X-100 results in increased autolysis (21, 22) and have shown that the msaABCR operon negatively regulates the production of proteases, resulting in increased processing of murein hydrolases and thus affecting the rate of cell wall autolysis in the USA300 LAC and Mu50 strains (21, 40). In this study, we sought to investigate the potential role of protease activity in the cross-linking defect in the msaABCR mutants. Specifically, we hypothesized that the msaABCR operon plays a role in maintaining cell wall integrity via regulating the activity of murein hydrolases. To test the potential role of protease activity in the cross-linking defect, we measured the lysozyme lysis rate in msaABCR deletion/all-protease knockout strains. In this study, we observed that the lysozyme-induced lysis phenotype of the msaABCR deletion mutant was restored to the wild-type level in the msaABCR deletion/all-protease knockout strains in the USA300 LAC background (Fig. 10B). Since we did not have these mutants in the Mu50 strain, we grew Mu50 cells in the presence of a protease inhibitor cocktail. When Mu50 msaABCR mutant cells were grown in the presence of protease inhibitors, both lysozyme lysis (Fig. 10A) and lysostaphin lysis (Fig. 10C) rates were reversed to wild-type levels. These results suggest that increased protease activity is responsible for the cross-linking defect in the msaABCR mutants.
FIG 10.
Increased lysis in the msaABCR mutant is reversed to wild-type levels when all proteases are deleted or inhibited in the mutant. Lysozyme-induced whole-cell lysis. S. aureus cells were suspended in PBS and digested with 350 μg/ml lysozyme. Lysis of Mu50 wild type (WT), its msaABCR mutant, and its msaABCR mutant grown in the presence of a protease inhibitor cocktail (A) and USA300 LAC WT, its msaABCR-deletion mutant, its all-protease deletion mutant, and its msaABCR deletion/all-protease deletion double mutant (B) was measured as a decrease in OD600. (C) Lysostaphin lysis assay. Mu50 WT, its msaABCR mutant, and its msaABCR mutant grown in the presence of a protease inhibitor cocktail were grown to an OD of 1 at 37°C in TSB medium. Bacteria were harvested by centrifugation, washed twice with Tris buffer, and suspended to obtain an initial OD600 of ∼1. Lysostaphin lysis assays were performed with 0.5 μg/ml (A), and lysis was measured as a decline in OD600 over time. These results represent the means from three independent experiments. Error bars represent the SEs. Student’s t test and one-way ANOVA were used to compare the results from the wild types with those from their mutants. *, P < 0.05; **, P < 0.005.
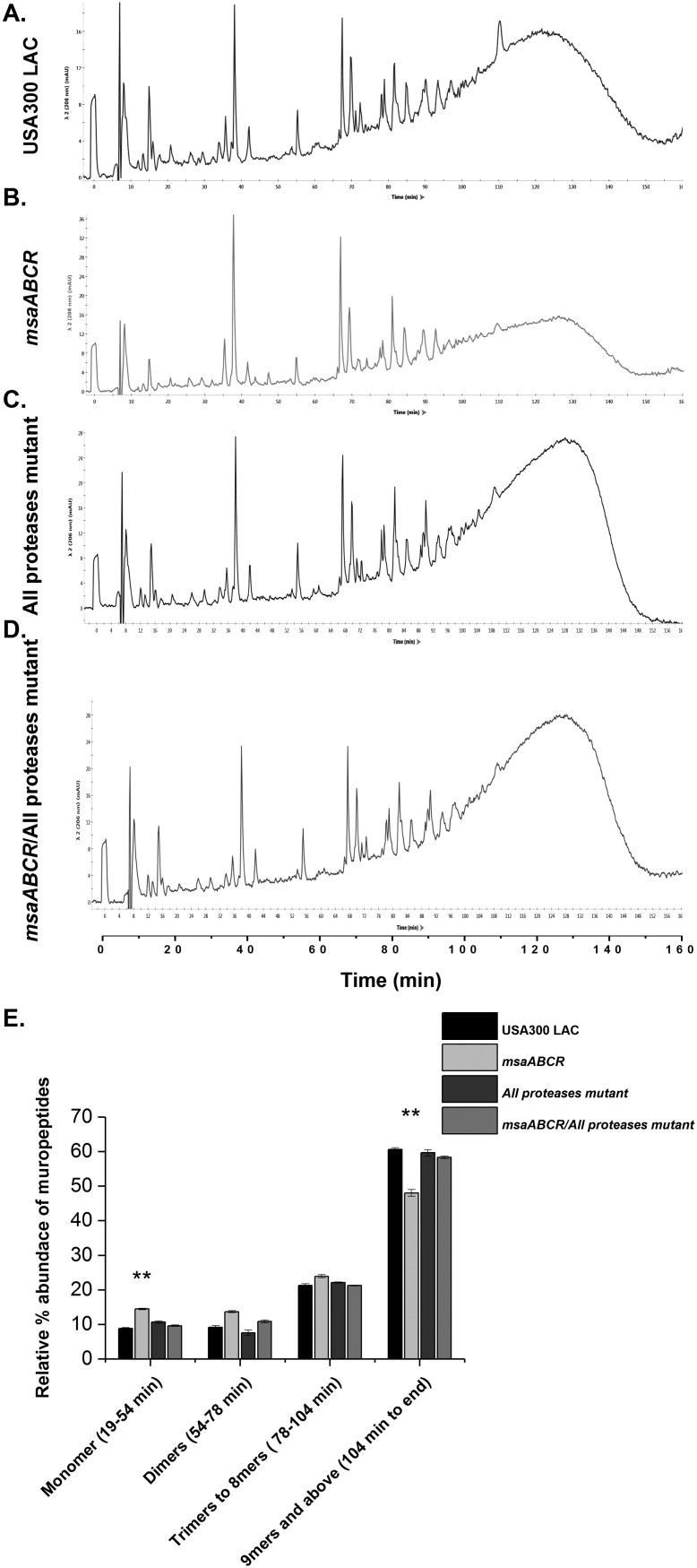
To further confirm the role of the increased presence of proteases in the msaABCR deletion mutant in contributing to a defect in cell wall cross-linking, we performed muropeptide composition analysis by HPLC on all strains, including all-protease knockout mutants and the msaABCR/protease double deletion mutant in the USA300 LAC strain background. We observed that the relative composition of oligomeric muropeptides in these double mutants reverts to wild-type levels in the USA300 LAC strain background (Fig. 11). There was no significant difference in the relative amount of oligomeric muropeptides in USA300 LAC, USA300 LAC all-protease knockout strains, and msaABCR deletion/all-protease knockout strains. However, there was a significant reduction in the amount of oligomeric muropeptides, which represents the degree of cross-linking, with a retention time of >104 min in the msaABCR deletion mutant, and this reverted back to the wild-type level in the msaABCR deletion/all-protease knockout strains. This result further confirms that increased protease activity contributes to defects in peptidoglycan cross-linking in msaABCR deletion mutants in the USA300 LAC strain.
FIG 11.
Cross-linking defect in the msaABCR deletion mutant is due to increased protease activity. (A to D) HPLC chromatograms of mutanolysin-digested peptidoglycan purified from the indicated strains in the LAC strain background. The peaks were identified according to de Jonge et al. (67). Representative results of three individual experiments are shown. (E) Muropeptide composition expressed as the percentage of the total area under the curve for each strain. The figure shows percentages indicating the relative abundances of monomers, dimers, trimers to 8-mers, and higher oligomers in each strain. These results represent the means from three independent experiments. Error bars represent the SEs. Student’s t test and one-way ANOVA were used to compare the results from the wild type with those from its mutant. **, P < 0.005.
The cell wall cross-linking defect in the msaABCR mutant is mediated by increased processing of the major autolysin, AtlA.
Our previous study showed that deletion of the msaABCR operon in the USA300 LAC strain does not have any significant effect on expression of any of the known S. aureus murein hydrolase genes (atlA, lytM, lytN, lytX, lytY, lytZ, and sle1) (21). In this study, we measured the relative expression of these hydrolase genes in both the USA300 LAC and Mu50 strains by qRT-PCR (Table 2). We confirmed similar results in the USA300 LAC strain background, as reported previously (21). However, atlA expression was upregulated by 2.49-fold in the Mu50 msaABCR mutant, while other genes were not differentially expressed.
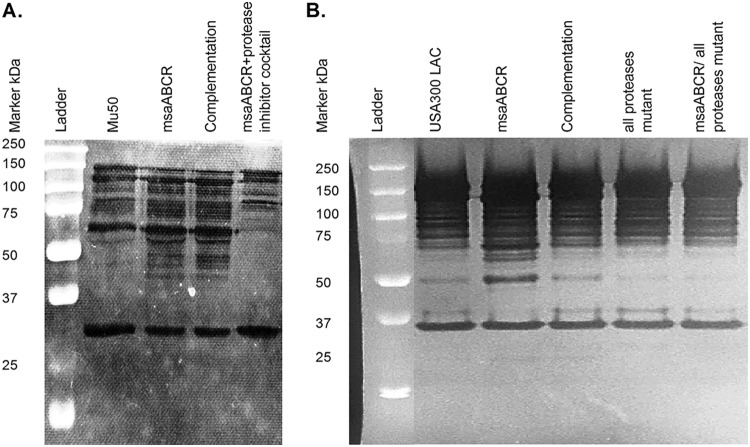
Peptidoglycan hydrolase cellular concentration, localization, and activity are believed to be intricately regulated and to play key roles in bacterial cell wall metabolism, daughter cell separation, antibiotic-mediated cell lysis, and pathogenicity (21, 24). The major autolytic enzymes, AtlA and LytM, have been shown to undergo proteolytic processing to produce active hydrolytic enzymes and determine their localization. AtlA is a bifunctional autolysin initially produced as a 138-kDa protein that subsequently undergoes proteolytic processing to generate the two major autolytic enzymes, 62-kDa N-acetylmuramyl-l-alanine amidase (AM) and 51-kDa N-acetylglucosaminidase (GL) (10). Similarly, LytM (34 kDa), like other lysostaphin-type peptidases, undergoes proteolytic processing, producing smaller-sized active enzymes (19 and 22 kDa) (41, 42). Previously, we showed that the msaABCR mutant produces more and differentially proteolytically processed cell wall-bound murein hydrolases during the late exponential growth phase and extracellular murein hydrolases during the stationary growth phase in the USA300 LAC strain background in a protease-dependent manner (21). Since we saw cross-linking defects and increased autolysis in early-exponential-growth cells, we further investigated the role of the msaABCR operon in protease-mediated processing of the cell wall-bound murein hydrolases during the early exponential growth phase. We performed a zymogram analysis of cell wall-bound murein hydrolases from early-exponential-growth cells and measured their activity using S. aureus (RN4220) whole cells as the substrates. The msaABCR deletion mutant showed a significantly altered pattern of AM and GL activities relative to its wild-type and complementation counterparts in both Mu50 (Fig. 12A) and USA300 LAC strains (Fig. 12B). In the zymogram assay, the msaABCR deletion mutants showed a significantly higher number of bands corresponding to amidases (AM) than their wild-type counterparts. Also, the msaABCR mutants showed higher-intensity bands of glucosaminidase (GL) activity compared with that in the wild type. Overall, these results confirm that the msaABCR mutants have increased processing and/or activity of murein hydrolases relative to their respective wild-types in both the Mu50 USA300 LAC strains (Fig. 12A and B).
FIG 12.
Cross-linking defect in the msaABCR mutant may be mediated by increased processing of the major autolysin, AtlA, and increased activity. SDS-extracted cell wall-bound murein hydrolase zymogram. (A) Cell wall-bound murein hydrolase zymogram of Mu50 WT, its msaABCR deletion mutant, and its msaABCR complement. (B) Cell wall-bound murein hydrolase zymogram of USA300 LAC WT, its msaABCR deletion mutant, its msaABCR complement, its all-protease mutant, and its msaABCR deletion/all-protease deletion double mutant. Representative results of three individual experiments are shown.
Furthermore, zymogram analysis also showed that the processing and/or activity of murein hydrolase bands in the msaABCR deletion/all-protease knockout strain reverted to the wild-type level (Fig. 12B). Increased processing and/or activity of murein hydrolase bands in msaABCR mutant Mu50 cells reverted back to wild-type levels when grown in the presence of a protease inhibitor cocktail (Fig. 12A). This observation further supports our result and confirms our previous findings that increased protease activity in the msaABCR mutant is responsible for increased processing and/or activity of murein hydrolases.
Overall, these findings suggest that the extra murein hydrolase bands in zymogram analysis produced by the msaABCR mutants in both VISA and MRSA strains were due to increased AtlA production as well as increased processing of AtlA and/or other murein hydrolases by the increased activity of proteases. Therefore, increased protease-mediated murein hydrolase activity may be responsible for the defective cell wall cross-linking in the msaABCR mutants.
Previous studies have shown that WTAs are cell-surface glycopolymers that are covalently attached to peptidoglycan and modulate AtlA murein hydrolase activity as well as its localization (19, 43). In addition, WTAs are also known to play a role in PBP4 localization, lysozyme resistance, and cell wall cross-linking (10, 11, 31). To examine the potential role of WTAs in the msaABCR mutant cell wall cross-linking defect, we estimated the teichoic acid content of the cell wall by measuring the inorganic phosphate concentration of WTA extracted from msaABCR mutants in comparison with that in their wild-type and complementation strains in the Mu50 (Fig. 13A) and USA300 LAC (Fig. 13B) backgrounds. We observed a significant reduction in the inorganic phosphate concentration of WTA in msaABCR mutant cells (76%) compared with that in its wild-type (100%) and complementation (98%) counterparts in Mu50. However, we observed a significant but smaller difference in inorganic phosphate concentration of WTA between the msaABCR mutant (87%) and the wild type (100%) in the USA300 LAC background (Fig. 13). This observation suggests that the msaABCR mutant might have reduced teichoic acid content affecting autolysin activity and cross-linking. However, further investigation is required to fully understand the relationship between the decreased WTA content and decreased cross-linking in the msaABCR mutants.
FIG 13.
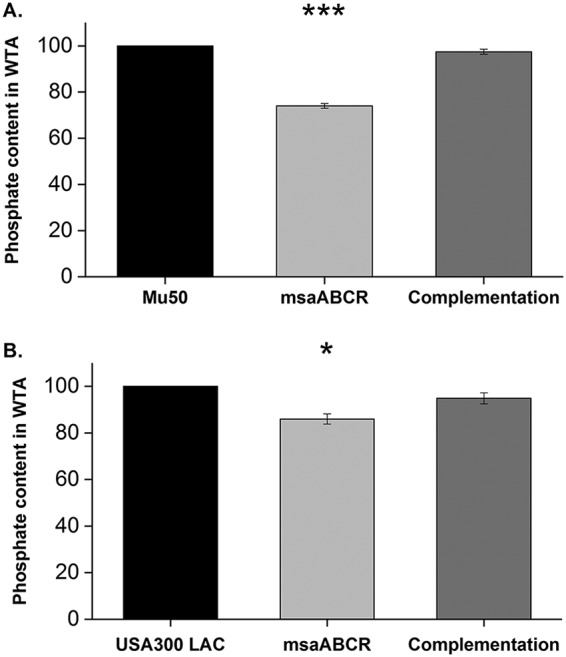
Decreased teichoic acid in msaABCR mutant cells increases their susceptibility to cleavage by murein hydrolases. (A) Comparison of the percentages of phosphate contents in wall teichoic acid (WTA) in the wild type, its msaABCR mutant, and its msaABCR complement in the Mu50 strain background. (B) Comparison of the percentages of phosphate contents in wall teichoic acid (WTA) in the wild type, its msaABCR mutant, and its msaABCR complement in the USA300 LAC strain background. These results represent the means from three independent experiments performed in triplicates. Error bars represent the standard errors. Student’s t test (OriginPro) and one-way ANOVA were used to compare the results from the wild type with those from its mutant. *, P < 0.05; ***, P < 0.0005.
DISCUSSION
In this study, we investigated the role of the msaABCR operon in S. aureus cell wall biosynthesis and cell wall lysis. We showed that the msaABCR operon mutants have reduced cell wall thickness and peptidoglycan cross-linking compared with that in wild-type S. aureus in both MRSA (USA300 LAC) and VISA (Mu50) strains. We have shown that the reduced peptidoglycan cross-linking in msaABCR mutant cells is mainly due to the nonspecific processing and/or increased activity of murein hydrolases due to increased protease activity. Decreased teichoic acid content in the msaABCR mutant cell wall may also be an additional factor for making it more susceptible to lysis by murein hydrolases. In addition, transcriptional expression of murA, which is responsible for the early rate-limiting step in cell wall synthesis, was significantly decreased in the msaABCR mutant of both strains. Other early peptidoglycan biosynthesis genes (glmU and murD) were also significantly downregulated in the Mu50 msaABCR deletion mutant but not in the USA300 LAC background. Although we observed decreased PG cross-linking, cell wall thickness, and teichoic acid content along with an increased rate of cell wall lysis in the msaABCR mutants, these cell wall-associated phenotypes were found to be more prominent in the VISA (Mu50) strain than in the MRSA (USA300 LAC) strain. These observations correlate with our findings that the msaABCR mutants are more susceptible to cell wall-targeting antibiotics, such as vancomycin, methicillin, several β-lactams, and the enzyme lysostaphin (Table 1). The marked deleterious effects following the deletion of msaABCR in both the USA300 LAC and the Mu50 strains under cell wall-related stresses suggest that a key role of the msaABCR operon is the maintenance of cell wall integrity.
VISA strains are phenotypically and genotypically different from vancomycin-susceptible strains (VSSA) strains. Increased cell wall thickness (44), reduced autolysis (45), altered cell WTA content (46, 47), and decreased virulence (48) are examples of altered phenotypes associated with VISA strains, which have been explained in detail in reviews (49, 50). Differentially expressed or mutated genes involved in Staphylococcus metabolism (47, 51), cell wall synthetic pathways, and murein hydrolases are examples of altered genotypes in VISA strains (47, 49, 50, 52). Mu50 was shown to have lower autolytic activity in whole-cell lysis assays and zymogram analysis relative to its vancomycin-susceptible counterpart (45). The decreased autolysis in VISA strains is thought to contribute to cell wall thickening, leading to increased resistance to vancomycin. Increased expression and nonspecific processing of atlA and decreased cell wall thickness in the Mu50 msaABCR deletion strain shows the importance of the msaABCR operon in acquiring vancomycin resistance and the complexities of autolysis regulation during VISA development. In our studies, we also observed several distinct and characteristic differences between the USA300 LAC (MRSA) and the Mu50 (VISA) strains. The USA300 LAC strain had a shorter lag growth phase, a thinner cell wall, increased expression of atlA, increased activity of murein hydrolases, and higher susceptibility to cell wall-targeting antibiotics than the Mu50 strain. We also observed that the cell wall of the msaABCR deletion mutant of USA300 LAC was thinner than the cell wall of the msaABCR mutant of the Mu50 strain. With the Mu50 strain, we observed increased lysostaphin susceptibility in the msaABCR mutant, which was not the case with USA300 LAC. These strain-dependent genotypic and phenotypic variations may contribute to some of the differences in phenotype observed between the msaABCR deletion mutant in the Mu50 (VISA) versus that in the USA300 LAC (MRSA) background, namely, pronounced reductions in cell wall thickness, cell wall lysis rate, teichoic acid phosphate content, and expression of cell wall synthetic and hydrolase genes as well as a different lysostaphin lysis pattern. These findings also corroborate the finding that deletion of the msaABCR operon has more pronounced effects in the Mu50 (VISA) than in the USA300 LAC (MRSA) strain with cell wall-related stresses.
Several studies have established a link between the degree of cross-linking of peptidoglycan and resistance to several cell wall-targeting antibiotics, such as β-lactams and glycopeptides, in a PBP-independent manner (4, 5, 53). One such study by Bæk et al. showed that, of the highly conserved, intracellular ATP-dependent proteases, ClpPX affects cell wall thickness, cell wall cross-linking, and the activity of murein hydrolases (54). Moreover, altered processing of the major autolysin, AtlA, was also observed in the clpP and ClpX mutants (54). In this study, we showed that deletion of all proteases in the msaABCR mutant reversed the nonspecific processing of murein hydrolases and decreased cross-linking, suggesting that the cross-linking defect is a result of increased murein hydrolase activity via increased protease activity. Thus, the msaABCR operon regulates cell wall cross-linking and cell wall thickness by modulating protease activity and murein hydrolase activity, which further confirms the role of proteases and the processing of murein hydrolases in cell wall metabolism and antibiotic resistance in S. aureus.
WTAs play a crucial role in septal localization of autolysins in S. aureus cells and interact with them, thereby regulating autolysis (4, 19, 20, 43, 55, 56). WTAs are also known to have an important role in peptidoglycan cross-linking, as well as in β-lactam and vancomycin resistance, determining septal localization of PBP4 and FmtA (9–11, 16, 17). The reduction in WTA inorganic phosphate content (surrogate measure of teichoic acid content) and the concomitant increase in autolysin activity within the cell wall might be responsible for increased peptide chain lysis in msaABCR mutant cells leading to decreased cross-linking. Quantitative real-time PCR analysis showed a consistent but small decrease (<2-fold) in gene expression levels of teichoic acid biosynthesis genes (tarO and tarA) in msaABCR mutants in both the USA300 LAC and Mu50 strains. Caballero et al. also showed that the MsaB protein binds to undecaprenyl-phosphate-N-acetylglucosaminyl-1-phosphatetransferase (tarO) mRNA (27), suggesting the possibility of posttranscriptional regulation of teichoic acid production by the msaABCR operon. A bifunctional protein, GlmU, catalyzes the de novo biosynthetic pathway for UDP-N-acetylglucosamine (UDP-GlcNAc) (39). UDP-GlcNAc is also a precursor for WTA, along with peptidoglycan synthesis. Thus, decreased expression of glmU in the Mu50 msaABCR mutant may also contribute to decreased teichoic content in the Mu50 strain. This also correlates with the larger decrease in teichoic acid content in the Mu50 msaABCR mutant than in the USA300 LAC msaABCR mutant. Given that msaABCR mutant cells exhibit decreased WTA content, this may be an additional contributing factor in the decreased cross-linking and increased antibiotic susceptibility observed in both the Mu50 and USA300 LAC strain backgrounds.
In this study, we observed 2.49- and 1.68-fold increases in atlA gene transcription levels in the msaABCR-deleted Mu50 and USA300 LAC strains, respectively, compared with that in their respective wild-type strains. A recent study by Caballero et al. showed that the msaB deletion mutant produces greater amounts of N-acetylmuramoyl-l-alanine amidase, Sle1, and the bifunctional autolysin AtlA, based on their proteomics analysis (27). They also showed that the MsaB (also known as CspA) protein binds to these mRNAs according to their RNA-binding protein immunoprecipitation-microarray profiling (RIP-ChIP) (27). Thus, increased activity of murein hydrolases in the msaABCR mutants of both strains may also be due to posttranscriptional regulation by the MsaB protein as an RNA chaperone.
Bacteria can take up and utilize sugars and amino sugars in the cytoplasm for ATP production through glycolysis and the synthesis of bacterial components (e.g., peptidoglycan, lipoteichoic acid, WTA, capsule, and nucleic acids) (57–59). UDP-N-acetylglucosamine serves as a biosynthetic precursor for peptidoglycan, biofilm, the bacterial capsule, and teichoic acids (57). Thus, allocation of sugar into different pathways is critical for virulence regulation and survival of bacteria (57–59). Several adjustments influencing the balance between cell wall synthesis and hydrolysis have been reported, which eventually lead to cell wall thickening in VISA strains compared with that in their counterpart VSSA strains. Also, cell wall synthesis consumes large amounts of substrate and energy (49). To build a thickened cell wall, the demand for cell wall biosynthetic precursors is increased in VISA strains, which requires adjustments in cellular metabolism. These adjustments are directly or indirectly linked to the biosynthesis of cell wall precursors (49, 51, 60). Decreased cell wall thickness, decreased expression of early cell wall synthetic genes and teichoic acid content, and the absence of a capsule in msaABCR mutant (24) cells suggests the allocation of sugar away from the cell envelope synthetic pathway. Therefore, we hypothesize that the msaABCR operon also regulates the allocation and utilization of sugars in different pathways. We plan to further investigate the role of msaABCR in the allocation and utilization of sugar in several pathways (glycolysis, tricarboxylic acid cycle, gluconeogenesis, and the amino sugar pathway) to determine its role in peptidoglycan, teichoic acid, and other cell wall component synthesis as well as in determining cell wall thickness.
Peptidoglycan is a dynamic structure that undergoes simultaneous synthesis and degradation during cell growth. The balance between these two opposing processes is essential for maintaining an intact and robust bacterial cell wall. Overall, the defect in cell wall integrity in the msaABCR mutants is due to a combination of two contributing factors. First, msaABCR mutant cells have increased extracellular protease activity, which leads to increased and unregulated processing of murein hydrolases and subsequent autolysis. This results in defective cross-linking of mutant strain peptidoglycan and promotes cell wall lysis, which is enhanced by the decreased teichoic acid content within the cell wall. Second, msaABCR mutant cells have significantly reduced peptidoglycan synthesis, due to a decrease in transcription of early cell wall-synthesizing genes (glmU, murA, and murD). Based on these results, we propose that the decreased cell wall thickness and antibiotic resistance observed in the msaABCR mutant strain is due to its inability to maintain a balance between simultaneous cell wall synthesis and hydrolysis. Our new findings shed light on the role of the msaABCR operon in cell wall biosynthesis, autolysis, cell wall integrity, and antibiotic resistance in both VISA and MRSA strains. Therefore, we conclude that the msaABCR operon modulates β-lactam and vancomycin resistance by keeping a balance between cell wall synthesis and cell wall hydrolysis, which is required for maintaining a robust and thick cell wall in both MRSA and VISA strains.
MATERIALS AND METHODS
Bacteria and growth conditions.
The bacterial strains used in this study are described in Table 3. We used a representative strain for each of the two clinically significant antibiotic-resistant S. aureus strains. These were Mu50, a known vancomycin-intermediate S. aureus (VISA) isolate, and community-acquired MRSA strain USA300 LAC, a vancomycin-sensitive S. aureus isolate (VSSA). The allelic replacement method was used to generate msaABCR deletion mutants in strains Mu50 and USA300 LAC (22, 61). For trans-complementation, the msaABCR region was cloned into the pCN34 low-copy vector with the modification of changing the kanamycin selectable marker to a chloramphenicol-resistant marker, as described previously (22). S. aureus strains were grown in tryptic soy agar (TSA) or tryptic soy broth (TSB). Overnight bacterial cultures were prepared by inoculating cells from frozen culture stocks into culture tubes containing 5 ml of freshly prepared TSB or Mueller-Hinton broth (MHB) and incubating at 37°C with continuous shaking at 225 rpm. The overnight cultures were diluted 1:10 in fresh medium, incubated for 2 h, and then normalized to an optical density at 600 nm (OD600) of 0.05 to use as the starting culture for experiments. For growth in the presence of protease inhibitors, one Mini EDTA-free protease inhibitor cocktail tablet (Roche) was added to 10 ml of TSB.
TABLE 3.
Strains used in the study
| Strain or plasmid | Relevant characteristics | Reference or source |
|---|---|---|
| RN4220 | Restriction-deficient mutant of 8325-4 | NARSA |
| USA300 LAC | CA-MRSA USA300 strain | L. Shaw |
| USA300 LAC msaABCR | USA300 LAC msaABCR deletion mutant | G. Sahukhal and M. Elasri |
| USA300 LAC msaABCR complementation | pCN34-msaABCR operon complementation into USA300 LAC msaABCR deletion mutant | G. Sahukhal and M. Elasri |
| MOE 458 | LAC/proteases | M. Smeltzer |
| MOE 466 | msaABCR/protease mutant | G. Sahukhal and M. Elasri |
| Mu50 | VISA, MRSA, mecA+ Ermr, ST5 | ATCC |
| Mu50 msaABCR | msaABCR operon deletion | This study |
| Mu50 msaABCR complementation | pCN34-msaABCR operon complementation in Mu50 msaABCR deletion mutant | This study |
Transmission electron microscopy.
Preparation and examination of S. aureus cells by transmission electron microscopy were performed as described previously with some modifications (22). Briefly, exponentially growing cells were fixed in 2% paraformaldehyde and 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.4) for 2 h at room temperature and treated with 1% osmium tetroxide in 0.1 M sodium cacodylate buffer for 1 h at 4°C. Cells were dehydrated in a graded ethanol series, infiltrated with (1:1) acetone-(Spurs) epoxy resin overnight, immersed in freshly made 100% epoxy resin, placed in molds with epoxy resin, and polymerized at 60°C for 2 to 3 days. The 50-nm ultrathin sections were cut using an Ultracut E microtome by Reichert-Jung. The USA300 LAC strains were imaged on a JEOL 2100 200 KeV transmission electron microscope (TEM), while Mu50 strains were imaged on a JEOL 1230 120 KeV TEM. Cell wall thicknesses were measured using the biological image analysis platform Fiji ImageJ (62) at a final magnification of ×60,000. At least fifteen cells of each strain, with approximately medial cuts, were measured, and the results were expressed as the mean ± standard error (SE).
Antibiotic susceptibility assay.
The antibiotic susceptibility of all strains was measured in triplicates by the broth microdilution method, per CLSI guidelines (63). MHB supplemented with 2% NaCl was used for all broth microdilution experiments. Antibiotic-containing wells were inoculated with 5 × 105 CFU/ml (64). After overnight incubation at 35°C, the wells were analyzed for visible bacterial growth, as indicated by turbidity. The lowest concentration of antibiotics that prevented bacterial growth was considered the MIC.
Growth curves and CFU count.
An aliquot of fresh bacterial culture was diluted to an OD of approximately 0.05 in 25 ml of fresh TSB in a 250-ml conical flask and grown at 37°C with shaking at 225 rpm. The OD600 was read at various time points. To analyze the growth of the msaABCR operon mutants in the presence of lysozyme, 500 μg/ml lysozyme was added when the culture OD600 reached 1, and the OD600 was measured hourly for up to 8 h. The population profile at 2-, 4-, and 6-h time points was determined by serially diluting culture aliquots to the appropriate dilution and plating them on TSA plates. Lysozyme (500 μg/ml) was added to the culture after 2 h of growth for population profile determination (31).
Lysozyme-induced lysis assay.
Lysozyme-induced lysis of S. aureus wild-type and msaABCR mutant strains was measured after suspending the cells in phosphate-buffered saline (PBS; pH 7.4) containing lysozyme (350 μg/ml). Briefly, overnight cultures of S. aureus strains were pelleted, washed twice with ice-cold PBS, and suspended in buffer containing 350 μg/ml of lysozyme. The initial OD600 was adjusted to 0.8, and the decrease in OD600 was measured at hourly intervals for 6 h and finally at 24 h, all at 37°C (32).
Lysostaphin susceptibility assay.
A lysostaphin susceptibility assay was performed as described previously (65). Exponentially growing cultures of S. aureus were pelleted, washed once in lysostaphin buffer (50 mM Tris-HCl [pH 7.5]), and resuspended in fresh buffer to the original culture volume. Lysostaphin (Sigma-Aldrich) was added to each culture at a final concentration of 0.5 μg/ml. The OD600 of each culture was read at various time points and plotted as a percentage of the initial reading, which was set at 100% at 0 min.
Peptidoglycan preparation and lysis assay.
Peptidoglycan without teichoic acid and acetyl groups was prepared as described previously with some modifications (66). Briefly, bacteria were grown overnight in 50 ml of TSB, harvested by centrifugation, and washed twice with sodium acetate buffer (20 mM, pH 4.6). Cells were disrupted in the same buffer using glass beads. The resulting preparations were boiled in the presence of 2% (wt/vol) sodium dodecyl sulfate (SDS) for 45 min, followed by washing with water and buffer to remove the SDS. These cell wall preparations were then treated with RNase, DNase, and trypsin and then finally heated at 60°C with trichloroacetic acid (10% wt/vol) for 5 h to remove teichoic acids. The cell wall preparations were washed 3 times with water to wash off TCA. Finally, O-acetyl groups were removed by alkaline treatment of the peptidoglycan (3 h in 80 mM NaOH at 37°C) (31). Peptidoglycan preparations were stored frozen at –85°C. For the lysis assay, peptidoglycan preparations were thawed and suspended in PBS containing 350 μg/ml of lysozyme. The initial OD600 was adjusted to around 0.8, and the decrease in OD was measured at 30-min intervals over 2 h at 37°C.
Muropeptide analysis by HPLC.
For muropeptide analysis, 1 liter of TSB medium was inoculated with freshly grown culture and incubated to an OD600 of 0.05. The cultures were grown at 37°C to early exponential phase (OD600 of 0.7). The cells were cooled on ice and subsequently collected by centrifugation (18,000 × g for 10 min). Peptidoglycan was purified as previously described (37, 67). Briefly, the bacterial cell suspension was boiled in 4% (wt/vol) SDS and washed several times with water. The cells were then transferred to a tube containing 0.1-mm glass beads (Biospec Products, Inc., Bartlesville, OK) and lysed using a FastPrep instrument. Lysed cells were treated with DNase (10 μg/ml), RNase (50 μg/ml), trypsin (50 μg/ml), and finally with 48% hydrofluoric acid to remove teichoic acid, thereby generating the purified peptidoglycan fraction.
Muropeptides were prepared by digesting 5 mg of purified peptidoglycan with 25 μg of mutanolysin (Sigma-Aldrich), were reduced with sodium borohydride (Sigma-Aldrich), and then were analyzed by reverse-phase HPLC using a Hypersil ODS column (Thermo Electron Corporation), as described previously (68). We used reverse-phase HPLC with a 3-μm particle size, 120-Å-pore-size octyldecyl silane Hypersil (250 mm by 4.6 mm) C18 column equipped with a 10-mm by 4-mm guard column made of the same material (Thermo Electron Corporation). The column temperature was kept at 52°C. We used a sodium phosphate-methanol buffer system without sodium azide. HPLC traces were recorded at 206 nm, and the muropeptide profiles of three independently grown cultures were determined. For quantification purposes, the area of the muropeptide peaks was integrated and quantified using Bio-Rad ChromLab software and displayed as the percentage of the total (20 min to 155 min) peak area. The average values with the standard errors from the three experiments are shown as the results.
RNA extraction, reverse transcription, and quantitative reverse transcription-PCR.
An aliquot of overnight culture was normalized to an OD600 of 0.05, grown to exponential phase, and harvested by centrifugation. The bacterial pellet was treated with RNAprotect Bacteria reagent (Qiagen, Valencia, CA), and the total RNA was extracted as described previously (21, 22). Real-time PCR was performed on the cDNA prepared from the total RNA samples to quantify relative gene expression. We also used gyrB as the internal housekeeping gene control in all our experiments (21, 22, 69). The relative fold change in gene expression was calculated using the delta threshold cycle (CT) method. All the primers used for qRT-PCR are listed in Table 4.
TABLE 4.
List of primers used in this study
| Primer for real-time PCR | Sequence (5′→3′) | Reference |
|---|---|---|
| RT gyrA F | GCCGTCAGTCTTACCTGCTC | 21 |
| RT gyrA R | AATAACGACACGCACACCAG | 21 |
| RT glmU F | TACAGTTAACTATGATGGTGAAAATAA | This study |
| RT glmU R | AATTGTTACAGGTGCTACTAAATTAAC | This study |
| RT murA F | TGTGCACCTTGCAATTGACT | This study |
| RT murA R | CCGTTTTATGCATGTTGCAG | This study |
| RT murB F | CATAAGCGCCAGCATTCATA | This study |
| RT murB R | CTCACGTGTTGCTCGTGATT | This study |
| RT murD F | AGCCTACAGATTATTTAGTTACAGAGT | This study |
| RT murD R | TAAATGTTAGTAATTATAGCAATGTGTG | This study |
| RT fmhB (femX) F | GTTCAAAACTATTTAATAACTCATCATC | This study |
| RT fmhB (femX) R | ATTTAAAGAAGGTTTATCAAAAGACTAC | This study |
| RT femA F | CGCAAACTGTTGGCCACTAT | This study |
| RT femA R | AAGCAAGCTGCAATGACCTC | This study |
| RT femB F | GCGAGAAAAATGATGCCCTA | This study |
| RT femB R | TACGCCCATCCATCGTACTT | This study |
| RT mecA F | CGACTTCACATCTATTAGGTTAT | This study |
| RT mecA R | ACACGATAGCCATCTTCA | This study |
| RT ppb1 F | GGATTAAATGTTGGTCGCTGA | This study |
| RT pbp1 R | TTATTTGCGGTTGTCATGGA | This study |
| RT pbp2 F | ATGTGAAGAGAACGATTATTAAGATTAT | This study |
| RT pbp2 R | AATAAGCAAACAATAAGATACCTAGTAA | This study |
| RT pbp3 F | GTGGACCAACCTCATCTTTA | This study |
| RT pbp3 R | CGGGAGACCCTTATTATTCT | This study |
| RT pbp4 F | CCGTTGGATTGACGAAATGT | 22 |
| RT pbp4 R | ACCAGCGATTTCGTTGATTT | 22 |
| RT tarO F | AATTGCCGCTGCCTTAGTAGTT | This study |
| RT tarO R | TGTACCCATTGGCAACGAAA | This study |
| RT tarA F | CTTACAGTAGATGGAGAGATACAGTTAT | This study |
| RT tarA R | AGATGTGTTACTCAAAAAGTTATAGAAG | This study |
| RT atlA F | ATAACCGCACTGGTTGGGTA | This study |
| RT atlA R | TTGGCAGCTGATGTAGTTGG | This study |
| RT lytM F | TCAAAATGGTGGATCAGCAA | 21 |
| RT lytM R | CACCACCGTGATATTGTCCA | 21 |
| RT sle 1 F | ACATGGGGTCAATGTACATATCATG | 21 |
| RT sle 1 R | CGTTATCCCAGTTATTAGCATTCCA | 21 |
| RT lytN F | TTTGGTGGAGGATATGGTCA | 21 |
| RT lytN R | GCCATCCACCATTATTCCAG | 21 |
| RT lytH F | ACCTCCATGACCAGGATCAA | 21 |
| RT lytH R | GATAGCTGGATGGCACACAA | 21 |
| RT lytX F | TCCCAACCATGCTTTTTAGC | 21 |
| RT lytX R | GAGTCGAAATTGATGGCAAA | 21 |
| RT lytY F | GATGTTGCGCAGGATTTTCT | 21 |
| RT lytY R | CGATTATTGGTGGTGCAAAG | 21 |
| RT lytZ F | CCGAAAATATCCAAGCACGA | 21 |
| RT lytZ R | TCCGATTTCCATGTTGGTTT | 21 |
| RT oatA F | TATTAAATAACCTGAAATAACGAAGAAT | This study |
| RT oatA R | AGTTATAGGAATCATTATTTATCACTTG | This study |
Membrane purification and PBP detection.
PBPs were detected using fluorography from membranes prepared from wild-type and msaABCR mutant strains according to the method described previously (22, 70), with some modifications. Briefly, cells were grown to exponential phase (OD600 of ∼0.7), harvested, washed once with wash buffer (50 mM Tris, 150 mM NaCl, 5 mM MgCl2 [pH 7.5]), and resuspended in the same buffer with 0.5 mM phenylmethylsulfonyl fluoride and 10 mM β-mercaptoethanol. Cells were labeled with 5 μg/ml Bocillin FL (Thermo Fisher Scientific) for 10 min, treated with lysostaphin (100 μg/ml), DNase (20 μg/ml), and RNase (10 μg/ml) for 30 min at 37°C, and then sonicated. Cell and membrane fragments were harvested by centrifugation at 110,000 × g for 40 min at 4°C. Membranes were solubilized with 2% Triton X-100. Protein concentrations were measured using the bicinchoninic acid (BCA) protein assay kit (Pierce), and 20 μg of membrane proteins was mixed with 4× SDS-PAGE sample buffer and boiled for 5 min. Samples were separated on a 4% to 20% gradient gel, and Bocillin FL-labeled proteins were detected with Alexa Fluor 488 using a Bio-Rad Versa Doc system. The gel images were analyzed and quantified using Image Lab software.
Zymographic analysis.
Zymographic analyses were performed as described previously (56, 71). Cells were grown until exponential growth phase and harvested as described above. The cell pellet was suspended in SDS sample buffer and boiled for 20 min. The boiled samples were centrifuged at 17,000 × g, and volumes of supernatant normalized to the OD of the initial culture were loaded onto 12% SDS-PAGE gels containing 3% (wt/vol) heat-killed S. aureus RN4220 cells. The gels were washed twice in Nanopure water and incubated in renaturation buffer (0.1% Triton X-100, 10 mM CaCl2, 10 mM MgCl2, 50 mM Tris-HCl, pH 7.5) at 37°C with gentle agitation. The gels were subsequently stained with 0.1% methylene blue.
Wall teichoic acid isolation, purification, and quantification.
WTA was specifically isolated as previously described (72, 73). Bacteria were grown overnight in 50 ml of TSB, harvested by centrifugation, and washed twice with sodium acetate buffer (20 mM, pH 4.6). Cells were disrupted in the same buffer using glass beads (with a Fast Prep instrument).
To isolate WTA, 500-μl aliquots of crude cell extracts from wild-type, mutant, and complementation strains having the same ODs were diluted 4-fold in sodium acetate buffer containing 2% SDS, sonicated for 15 min, and then vigorously shaken for 1 h at 60°C. The cell walls were sedimented by centrifugation, subjected to repeated washings with sodium acetate buffer to remove the SDS, and finally resuspended in 1 ml of sodium acetate buffer. WTA was extracted by diluting 250 μl of purified cell walls 4-fold in 5% trichloroacetic acid and incubating at 60°C for 4 h. The peptidoglycan was removed by centrifugation, and the WTA was quantified by determining its inorganic phosphate (Pi) content as described by Covas et al. (73).
Statistical analysis.
All statistical analyses to test significance in this study were performed using OriginPro software (Origin Lab, Northampton, MA). A statistical significance level of 0.05 was set as the cutoff value in statistical analyses. Student’s t tests and one-way analyses of variance (ANOVAs) were used to compare the results from the wild type with those from mutants.
ACKNOWLEDGMENTS
This work was supported by the Mississippi INBRE, funded by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM103476.
We thank Lindsey Shaw, Mark Smeltzer, and the ATCC for sharing the S. aureus strains and mutants. We also thank Shanti Pandey and Sarah E. Polley (undergraduate student) for their contributions to this study.
REFERENCES
- 1.Chatterjee SS, Otto M. 2013. Improved understanding of factors driving methicillin-resistant Staphylococcus aureus epidemic waves. Clin Epidemiol 5:205–217. doi: 10.2147/CLEP.S37071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dayan GH, Mohamed N, Scully IL, Cooper D, Begier E, Eiden J, Jansen KU, Gurtman A, Anderson AS. 2016. Staphylococcus aureus: the current state of disease, pathophysiology and strategies for prevention. Expert Rev Vaccines 15:1373–1392. doi: 10.1080/14760584.2016.1179583. [DOI] [PubMed] [Google Scholar]
- 3.Assis LM, Nedeljkovic M, Dessen A. 2017. New strategies for targeting and treatment of multi-drug resistant Staphylococcus aureus. Drug Resist Updat 31:1–14. doi: 10.1016/j.drup.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Roemer T, Schneider T, Pinho MG. 2013. Auxiliary factors: a chink in the armor of MRSA resistance to beta-lactam antibiotics. Curr Opin Microbiol 16:538–548. doi: 10.1016/j.mib.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 5.Berger-Bächi B, Rohrer S. 2002. Factors influencing methicillin resistance in staphylococci. Arch Microbiol 178:165–171. doi: 10.1007/s00203-002-0436-0. [DOI] [PubMed] [Google Scholar]
- 6.CDC. 2014. Active bacterial core surveillance report, Emerging Infections Program Network, methicillin-resistant Staphylococcus aureus, 2014. CDC, Atlanta, GA. [Google Scholar]
- 7.Sewell EW, Brown ED. 2014. Taking aim at wall teichoic acid synthesis: new biology and new leads for antibiotics. J Antibiot (Tokyo) 67:43–51. doi: 10.1038/ja.2013.100. [DOI] [PubMed] [Google Scholar]
- 8.Dmitriev BA, Toukach FV, Holst O, Rietschel ET, Ehlers S. 2004. Tertiary structure of Staphylococcus aureus cell wall murein. J Bacteriol 186:7141–7148. doi: 10.1128/JB.186.21.7141-7148.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rajagopal M, Walker S. 2017. Envelope structures of Gram-positive bacteria. Curr Top Microbiol Immunol 404:1–44. doi: 10.1007/82_2015_5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown S, Santa Maria JP Jr, Walker S. 2013. Wall teichoic acids of gram-positive bacteria. Annu Rev Microbiol 67:313–336. doi: 10.1146/annurev-micro-092412-155620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atilano ML, Pereira PM, Yates J, Reed P, Veiga H, Pinho MG, Filipe SR. 2010. Teichoic acids are temporal and spatial regulators of peptidoglycan cross-linking in Staphylococcus aureus. Proc Natl Acad Sci U S A 107:18991–18996. doi: 10.1073/pnas.1004304107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peacock SJ, Paterson GK. 2015. Mechanisms of methicillin resistance in Staphylococcus aureus. Annu Rev Biochem 84:577–601. doi: 10.1146/annurev-biochem-060614-034516. [DOI] [PubMed] [Google Scholar]
- 13.Komatsuzawa H, Sugai M, Ohta K, Fujiwara T, Nakashima S, Suzuki J, Lee CY, Suginaka H. 1997. Cloning and characterization of the fmt gene which affects the methicillin resistance level and autolysis in the presence of Triton X-100 in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 41:2355–2361. doi: 10.1128/AAC.41.11.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sobral RG, Ludovice AM, de Lencastre H, Tomasz A. 2006. Role of murF in cell wall biosynthesis: isolation and characterization of a murF conditional mutant of Staphylococcus aureus. J Bacteriol 188:2543–2553. doi: 10.1128/JB.188.7.2543-2553.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gardete S, Ludovice AM, Sobral RG, Filipe SR, de Lencastre H, Tomasz A. 2004. Role of murE in the expression of beta-lactam antibiotic resistance in Staphylococcus aureus. J Bacteriol 186:1705–1713. doi: 10.1128/jb.186.6.1705-1713.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell J, Singh AK, Maria JPS, Kim Y, Brown S, Swoboda JG, Mylonakis E, Wilkinson BJ, Walker S. 2011. Synthetic lethal compound combinations reveal a fundamental connection between wall teichoic acid and peptidoglycan biosyntheses in Staphylococcus aureus. ACS Chem Biol 6:106–116. doi: 10.1021/cb100269f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farha MA, Leung A, Sewell EW, D'Elia MA, Allison SE, Ejim L, Pereira PM, Pinho MG, Wright GD, Brown ED. 2013. Inhibition of WTA synthesis blocks the cooperative action of PBPs and sensitizes MRSA to beta-lactams. ACS Chem Biol 8:226–233. doi: 10.1021/cb300413m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szweda P, Schielmann M, Kotlowski R, Gorczyca G, Zalewska M, Milewski S. 2012. Peptidoglycan hydrolases-potential weapons against Staphylococcus aureus. Appl Microbiol Biotechnol 96:1157–1174. doi: 10.1007/s00253-012-4484-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu X, Zhang S, Sun B. 2016. SpoVG regulates cell wall metabolism and oxacillin resistance in methicillin-resistant Staphylococcus aureus strain N315. Antimicrob Agents Chemother 60:3455–3461. doi: 10.1128/AAC.00026-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boyle-Vavra S, Yin S, Daum RS. 2006. The VraS/VraR two-component regulatory system required for oxacillin resistance in community-acquired methicillin-resistant Staphylococcus aureus. FEMS Microbiol Lett 262:163–171. doi: 10.1111/j.1574-6968.2006.00384.x. [DOI] [PubMed] [Google Scholar]
- 21.Sahukhal GS, Batte JL, Elasri MO. 2015. msaABCR operon positively regulates biofilm development by repressing proteases and autolysis in Staphylococcus aureus. FEMS Microbiol Lett 362:1–10. doi: 10.1093/femsle/fnv006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Samanta D, Elasri MO. 2014. The msaABCR operon regulates resistance in vancomycin-intermediate Staphylococcus aureus strains. Antimicrob Agents Chemother 58:6685–6695. doi: 10.1128/AAC.03280-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sahukhal GS, Elasri MO. 2014. Identification and characterization of an operon, msaABCR, that controls virulence and biofilm development in Staphylococcus aureus. BMC Microbiol 14:154. doi: 10.1186/1471-2180-14-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Batte JL, Samanta D, Elasri MO. 2016. MsaB activates capsule production at the transcription level in Staphylococcus aureus. Microbiology 162:575–589. doi: 10.1099/mic.0.000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Batte JL, Sahukhal GS, Elasri MO. 2018. MsaB and CodY interact to regulate Staphylococcus aureus capsule in a nutrient-dependent manner. J Bacteriol 200:e00294-18. doi: 10.1128/JB.00294-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sahukhal GS, Pandey S, Elasri MO. 2017. msaABCR operon is involved in persister cell formation in Staphylococcus aureus. BMC Microbiol 17:218. doi: 10.1186/s12866-017-1129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caballero CJ, Menendez-Gil P, Catalan-Moreno A, Vergara-Irigaray M, Garcia B, Segura V, Irurzun N, Villanueva M, Ruiz de Los Mozos I, Solano C, Lasa I, Toledo-Arana A. 2018. The regulon of the RNA chaperone CspA and its auto-regulation in Staphylococcus aureus. Nucleic Acids Res 46:1345–1361. doi: 10.1093/nar/gkx1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rice K, Peralta R, Bast D, de Azavedo J, McGavin MJ. 2001. Description of staphylococcus serine protease (ssp) operon in Staphylococcus aureus and nonpolar inactivation of sspA-encoded serine protease. Infect Immun 69:159–169. doi: 10.1128/IAI.69.1.159-169.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen C, Krishnan V, Macon K, Manne K, Narayana SVL, Schneewind O. 2013. Secreted proteases control autolysin-mediated biofilm growth of Staphylococcus aureus. J Biol Chem 288:29440–29452. doi: 10.1074/jbc.M113.502039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas VC, Thurlow LR, Boyle D, Hancock LE. 2008. Regulation of autolysis-dependent extracellular DNA release by Enterococcus faecalis extracellular proteases influences biofilm development. J Bacteriol 190:5690–5698. doi: 10.1128/JB.00314-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bera A, Biswas R, Herbert S, Kulauzovic E, Weidenmaier C, Peschel A, Gotz F. 2007. Influence of wall teichoic acid on lysozyme resistance in Staphylococcus aureus. J Bacteriol 189:280–283. doi: 10.1128/JB.01221-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pushkaran AC, Nataraj N, Nair N, Gotz F, Biswas R, Mohan CG. 2015. Understanding the structure-function relationship of lysozyme resistance in Staphylococcus aureus by peptidoglycan O-acetylation using molecular docking, dynamics, and lysis assay. J Chem Inf Model 55:760–770. doi: 10.1021/ci500734k. [DOI] [PubMed] [Google Scholar]
- 33.Bera A, Herbert S, Jakob A, Vollmer W, Gotz F. 2005. Why are pathogenic staphylococci so lysozyme resistant? The peptidoglycan O-acetyltransferase OatA is the major determinant for lysozyme resistance of Staphylococcus aureus. Mol Microbiol 55:778–787. doi: 10.1111/j.1365-2958.2004.04446.x. [DOI] [PubMed] [Google Scholar]
- 34.Climo MW, Ehlert K, Archer GL. 2001. Mechanism and suppression of lysostaphin resistance in oxacillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 45:1431–1437. doi: 10.1128/AAC.45.5.1431-1437.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stranden AM, Ehlert K, Labischinski H, Berger-Bachi B. 1997. Cell wall monoglycine cross-bridges and methicillin hypersusceptibility in a femAB null mutant of methicillin-resistant Staphylococcus aureus. J Bacteriol 179:9–16. doi: 10.1128/jb.179.1.9-16.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rohrer S, Ehlert K, Tschierske M, Labischinski H, Berger-Bächi B. 1999. The essential Staphylococcus aureus gene fmhB is involved in the first step of peptidoglycan pentaglycine interpeptide formation. Proc Natl Acad Sci U S A 96:9351–9356. doi: 10.1073/pnas.96.16.9351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grundling A, Schneewind O. 2006. Cross-linked peptidoglycan mediates lysostaphin binding to the cell wall envelope of Staphylococcus aureus. J Bacteriol 188:2463–2472. doi: 10.1128/JB.188.7.2463-2472.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blake KL, O'Neill AJ, Mengin-Lecreulx D, Henderson PJ, Bostock JM, Dunsmore CJ, Simmons KJ, Fishwick CW, Leeds JA, Chopra I. 2009. The nature of Staphylococcus aureus MurA and MurZ and approaches for detection of peptidoglycan biosynthesis inhibitors. Mol Microbiol 72:335–343. doi: 10.1111/j.1365-2958.2009.06648.x. [DOI] [PubMed] [Google Scholar]
- 39.van Heijenoort J. 1998. Assembly of the monomer unit of bacterial peptidoglycan. Cell Mol Life Sci 54:300–304. doi: 10.1007/s000180050155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Biswas R, Voggu L, Simon UK, Hentschel P, Thumm G, Gotz F. 2006. Activity of the major staphylococcal autolysin Atl. FEMS Microbiol Lett 259:260–268. doi: 10.1111/j.1574-6968.2006.00281.x. [DOI] [PubMed] [Google Scholar]
- 41.Ramadurai L, Jayaswal RK. 1997. Molecular cloning, sequencing, and expression of lytM, a unique autolytic gene of Staphylococcus aureus. J Bacteriol 179:3625–3631. doi: 10.1128/jb.179.11.3625-3631.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Firczuk M, Mucha A, Bochtler M. 2005. Crystal structures of active LytM. J Mol Biol 354:578–590. doi: 10.1016/j.jmb.2005.09.082. [DOI] [PubMed] [Google Scholar]
- 43.Schlag M, Biswas R, Krismer B, Kohler T, Zoll S, Yu W, Schwarz H, Peschel A, Gotz F. 2010. Role of staphylococcal wall teichoic acid in targeting the major autolysin Atl. Mol Microbiol 75:864–873. doi: 10.1111/j.1365-2958.2009.07007.x. [DOI] [PubMed] [Google Scholar]
- 44.Hanaki H, Kuwahara-Arai K, Boyle-Vavra S, Daum RS, Labischinski H, Hiramatsu K. 1998. Activated cell-wall synthesis is associated with vancomycin resistance in methicillin-resistant Staphylococcus aureus clinical strains Mu3 and Mu50. J Antimicrob Chemother 42:199–209. doi: 10.1093/jac/42.2.199. [DOI] [PubMed] [Google Scholar]
- 45.Utaida S, Pfeltz RF, Jayaswal RK, Wilkinson BJ. 2006. Autolytic properties of glycopeptide-intermediate Staphylococcus aureus Mu50. Antimicrob Agents Chemother 50:1541–1545. doi: 10.1128/AAC.50.4.1541-1545.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Katayama Y, Sekine M, Hishinuma T, Aiba Y, Hiramatsu K. 2016. Complete reconstitution of the vancomycin-intermediate Staphylococcus aureus phenotype of strain Mu50 in vancomycin-susceptible S. aureus. Antimicrob Agents Chemother 60:3730–3742. doi: 10.1128/AAC.00420-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsuo M, Cui LZ, Kim J, Hiramatsu K. 2013. Comprehensive identification of mutations responsible for heterogeneous vancomycin-intermediate Staphylococcus aureus (hVISA)-to-VISA conversion in laboratory-generated VISA strains derived from hVISA clinical strain Mu3. Antimicrob Agents Chemother 57:5843–5853. doi: 10.1128/AAC.00425-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gardete S, Kim C, Hartmann BM, Mwangi M, Roux CM, Dunman PM, Chambers HF, Tomasz A. 2012. Genetic pathway in acquisition and loss of vancomycin resistance in a methicillin resistant Staphylococcus aureus (MRSA) strain of clonal type USA300. PLoS Pathog 8:e1002505. doi: 10.1371/journal.ppat.1002505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu Q, Peng H, Rao X. 2016. Molecular events for promotion of vancomycin resistance in vancomycin intermediate Staphylococcus aureus. Front Microbiol 7:1601. doi: 10.3389/fmicb.2016.01601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McGuinness WA, Malachowa N, DeLeo FR. 2017. Vancomycin resistance in Staphylococcus aureus. Yale J Biol Med 90:269–281. [PMC free article] [PubMed] [Google Scholar]
- 51.Nelson JL, Rice KC, Slater SR, Fox PM, Archer GL, Bayles KW, Fey PD, Kreiswirth BN, Somerville GA. 2007. Vancomycin-intermediate Staphylococcus aureus strains have impaired acetate catabolism: implications for polysaccharide intercellular adhesin synthesis and autolysis. Antimicrob Agents Chemother 51:616–622. doi: 10.1128/AAC.01057-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cui L, Lian JQ, Neoh HM, Reyes E, Hiramatsu K. 2005. DNA microarray-based identification of genes associated with glycopeptide resistance in Staphylococcus aureus. Antimicrob Agents Chemother 49:3404–3413. doi: 10.1128/AAC.49.8.3404-3413.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maidhof H, Reinicke B, Blümel P, Berger-Bächi B, Labischinski H. 1991. femA, which encodes a factor essential for expression of methicillin resistance, affects glycine content of peptidoglycan in methicillin-resistant and methicillin-susceptible Staphylococcus aureus strains. J Bacteriol 173:3507–3513. doi: 10.1128/jb.173.11.3507-3513.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bæk KT, Gründling A, Mogensen RG, Thøgersen L, Petersen A, Paulander W, Frees D. 2014. Beta-lactam resistance in methicillin-resistant Staphylococcus aureus USA300 is increased by inactivation of the ClpXP protease. Antimicrob Agents Chemother 58:4593–4603. doi: 10.1128/AAC.02802-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Neuhaus FC, Baddiley J. 2003. A continuum of anionic charge: structures and functions of d-alanyl-teichoic acids in Gram-positive bacteria. Microbiol Mol Biol Rev 67:686–723. doi: 10.1128/mmbr.67.4.686-723.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Atilano ML, Pereira PM, Vaz F, Catalao MJ, Reed P, Grilo IR, Sobral RG, Ligoxygakis P, Pinho MG, Filipe SR. 2014. Bacterial autolysins trim cell surface peptidoglycan to prevent detection by the Drosophila innate immune system. Elife 3:e02277. doi: 10.7554/eLife.02277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Komatsuzawa H, Fujiwara T, Nishi H, Yamada S, Ohara M, McCallum N, Berger-Bächi B, Sugai M. 2004. The gate controlling cell wall synthesis in Staphylococcus aureus. Mol Microbiol 53:1221–1231. doi: 10.1111/j.1365-2958.2004.04200.x. [DOI] [PubMed] [Google Scholar]
- 58.Kawada-Matsuo M, Oogai Y, Komatsuzawa H. 2017. Sugar allocation to metabolic pathways is tightly regulated and affects the virulence of Streptococcus mutans. Genes 8:11. doi: 10.3390/genes8010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kawada-Matsuo M, Mazda Y, Oogai Y, Kajiya M, Kawai T, Yamada S, Miyawaki S, Oho T, Komatsuzawa H. 2012. GlmS and NagB regulate amino sugar metabolism in opposing directions and affect Streptococcus mutans virulence. PLoS One 7:e33382. doi: 10.1371/journal.pone.0033382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alexander EL, Gardete S, Bar HY, Wells MT, Tomasz A, Rhee KY. 2014. Intermediate-type vancomycin resistance (VISA) in genetically-distinct Staphylococcus aureus isolates is linked to specific, reversible metabolic alterations. PLoS One 9:e97137. doi: 10.1371/journal.pone.0097137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bae T, Schneewind O. 2006. Allelic replacement in Staphylococcus aureus with inducible counter-selection. Plasmid 55:58–63. doi: 10.1016/j.plasmid.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 62.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. 2012. Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cockerill F, Clinical and Laboratory Standards Institute. 2015. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: approved standard, 10th ed Clinical and Laboratory Standards Institute, Wayne, Pa. [Google Scholar]
- 64.Memmi G, Filipe SR, Pinho MG, Fu ZB, Cheung A. 2008. Staphylococcus aureus PBP4 is essential for beta-lactam resistance in community-acquired methicillin-resistant strains. Antimicrob Agents Chemother 52:3955–3966. doi: 10.1128/AAC.00049-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grundling A, Missiakas DM, Schneewind O. 2006. Staphylococcus aureus mutants with increased lysostaphin resistance. J Bacteriol 188:6286–6297. doi: 10.1128/JB.00457-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reifsteck F, Wee S, Wilkinson BJ. 1987. Hydrophobicity-hydrophilicity of staphylococci. J Med Microbiol 24:65–73. doi: 10.1099/00222615-24-1-65. [DOI] [PubMed] [Google Scholar]
- 67.de Jonge BL, Chang YS, Gage D, Tomasz A. 1992. Peptidoglycan composition of a highly methicillin-resistant Staphylococcus aureus strain. The role of penicillin binding protein 2A. J Biol Chem 267:11248–11254. [PubMed] [Google Scholar]
- 68.Corrigan RM, Abbott JC, Burhenne H, Kaever V, Grundling A. 2011. c-di-AMP is a new second messenger in Staphylococcus aureus with a role in controlling cell size and envelope stress. PLoS Pathog 7:e1002217. doi: 10.1371/journal.ppat.1002217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goerke C, Campana S, Bayer MG, Doring G, Botzenhart K, Wolz C. 2000. Direct quantitative transcript analysis of the agr regulon of Staphylococcus aureus during human infection in comparison to the expression profile in vitro. Infect Immun 68:1304–1311. doi: 10.1128/iai.68.3.1304-1311.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kocaoglu O, Carlson EE. 2013. Penicillin-binding protein imaging probes. Curr Protoc Chem Biol 5:239–250. doi: 10.1002/9780470559277.ch130102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vaz F, Filipe SR, Xavier BA. 2015. Preparation and analysis of crude autolytic enzyme extracts from Staphylococcus aureus. Bio Protoc 5:e1687. doi: 10.21769/BioProtoc.1687. [DOI] [Google Scholar]
- 72.Peschel A, Otto M, Jack RW, Kalbacher H, Jung G, Gotz F. 1999. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J Biol Chem 274:8405–8410. doi: 10.1074/jbc.274.13.8405. [DOI] [PubMed] [Google Scholar]
- 73.Covas G, Vaz F, Henriques G, Pinho MG, Filipe SR. 2016. Analysis of cell wall teichoic acids in Staphylococcus aureus. Methods Mol Biol 1440:201–213. doi: 10.1007/978-1-4939-3676-2_15. [DOI] [PubMed] [Google Scholar]