Abstract
The role played by genetic components in the etiology of the Class III phenotype, a class of dental malocclusion, is not yet understood. Regions that may be related to the development of Class III malocclusion have been suggested previously. The aim of this study was to search for genetic linkage with 6 microsatellite markers (D1S234, D4S3038, D6S1689, D7S503, D10S1483, and D19S566), near previously proposed candidate regions for Class III. We performed a two-point parametric linkage analysis for 42 affected individuals from 10 Brazilian families with a positive Class III malocclusion segregation. Analysis of our data indicated that there was no evidence for linkage of any of the 6 microsatellite markers to a Class III locus at θ = zero, with data supporting exclusion for 5 of the 6 markers evaluated. The present work reinforces that Class III is likely to demonstrate locus heterogeneity, and there is a dependency of the genetic background of the population in linkage studies.
Keywords: linkage analysis, Class III malocclusion, microsatellite markers, candidate loci, mandibular prognathism, genetic heterogeneity
Introduction
Class III phenotype, a class of skeletal malocclusion also known as mandibular prognathism, is a heterogeneous complex trait that is skeletally characterized by an overgrowth of the mandible, an undergrowth of the maxilla, or a combination of both. This phenotype may be noticeable at an early age, and generally becomes progressively more evident with growth. It is one of the main factors that leads a potential patient to seek orthodontic and surgical treatment (Singh, 1999). The frequency of Class III malocclusion varies among worldwide populations, presenting the lowest frequencies in Euro-American populations (0.48 to 4%), intermediary frequencies in Sub-Saharan African populations (3 to 8%), and higher frequencies in far-eastern Asian populations such as Korea, China, and Japan (15 to 23%) (El-Gheriani et al., 2003; Yamaguchi et al., 2005).
Class III malocclusion has been observed segregating within families, and several inheritance patterns have been suggested, including autosomal-recessive (Downs, 1927), autosomal-dominant (Bertram et al., 1959; Wolff et al., 1993), and a polygenic threshold model (Litton et al., 1970). Furthermore, the presence of phenotype subtypes, phenocopies, and incomplete penetrance was also observed (Bui et al., 2006; Cruz et al., 2008). A previous study in Brazilian families estimated the heritability of Class III malocclusion as 0.316, and also reported the influence of a major gene with a clear sign of Mendelian inheritance and a multifactorial component (Cruz et al., 2008). In summary, genetic and environmental factors play roles in the etiology of Class III malocclusion, but the relative contribution of each of these components in the etiology of non-syndromic Class III malocclusion is unclear (Singh, 1999).
Few studies have focused on the genomic location of the genes that influence the development of Class III malocclusion. A genome-wide scan linkage analysis detected nominal statistical significance of linkage to Class III malocclusion at loci D1S234, D6S305, and D19S884 in a set of Japanese and Korean affected sibling-pairs (Yamaguchi et al., 2005). Another study with a genome-wide scan and linkage analysis in 4 Colombian families reported suggestion of linkage in chromosomes 1p, 3q, 11q, and 12q (Frazier-Bowers et al., 2009). One way to minimize the overall cost of investigation is to look for linkage between the trait and candidate genes previously related in the literature. Since these studies indicated that multiple genomic areas may be involved in Class III malocclusion, several genes may be suggested as candidate genes. Three markers in this study (D1S234, D6S1689, and D19S566) were selected based on the investigation by Yamaguchi et al. (2005), being the same (D1S234), or relatively near with higher heterozygosity (> 0.8). The 3 additional markers (D4S3038, D7S503, and D10S1483) were selected based on their proximity to genes related to craniofacial growth, development, and malformation—FGFR3 (4p16.3), TWIST (7p21.2), and FGFR2 (10q26), respectively—and on their high values of heterozygosity (> 0.8). Mutations in FGFR3 are related to the development of craniosynostosis and multiple types of skeletal dysplasia (Jacob et al., 2006; Rump et al., 2006), while TWIST and FGFR2 are related to Saethre-Chotzen and Pfeiffer syndromes, which also present craniosynostosis and midfacial hypoplasia as clinical features (Freitas et al., 2006; Stevens and Roeder, 2006). In addition, there can be an interaction between the TWIST and FGFR protein families during craniofacial development and malformation (Kress et al., 2006).
The aim of this study was to search for genetic linkage with highly polymorphic markers in 6 previously identified candidate genes/regions with Class III malocclusion in ten Brazilian families.
Materials & Methods
Family Enrollment
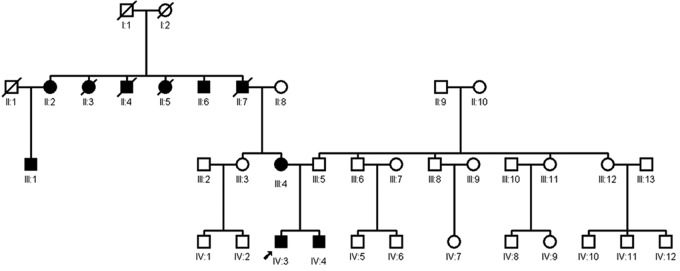
The project was approved by the National Ethical Committee in Research of Brazil (CONEP 9177). Consent to participate in the research was obtained from every adult or legal guardian, in the case of minors. Ten families were selected from a previous study (Cruz et al., 2008), based on total family size, number of affected members, and an autosomal-dominant pattern of inheritance (Fig. and Appendix).
Figure.
Pedigree of a Brazilian family with Class III malocclusion showing an autosomal dominant inheritance based upon multiple generations and approximately equal occurrence in males and females, although there is no male to male transmission.
Clinical Assessment
Class III malocclusion status was demonstrated by lateral cephalometric radiographs, dental casts, and/or facial and intra-oral photographs. The individuals were diagnosed with Class III malocclusion when they presented a negative ANB angle (relative sagittal position of the maxilla and mandible). The SNGoGn (mandibular plane) angle was also taken into account, since a high value may suggest a predominant pattern of vertical facial growth, and a low value is related to a predominant pattern of horizontal facial growth. In an attempt to reduce phenotype heterogeneity, we excluded individuals with severe undergrowth of the maxilla – evaluated by the ANS-Po (anterior nasal spine-porion) measurement – and normal mandible (Appendix Table 1). Also, individuals who presented malformations, such as cleft palate, or syndromes were excluded.
DNA Isolation and Genotyping Assay
Venous blood was collected from 42 affected individuals from those families. Genomic DNA was isolated by the ‘salting out’ method (Miller et al., 1988) and diluted to 20 ng/µL. Six microsatellite markers were selected, as previously described, for analysis: D1S234 (1p36.11), D4S3038 (4p16.3), D6S1689 (6p21), D7S503 (7p21.2), D10S1483 (10q26), and D19S566 (19p13.1). As discussed above, they were chosen for their high heterozygosity, and for their previous linkage to Class III malocclusion (Yamaguchi et al., 2005) or proximity to candidate regions to skeletal disorders (Stein et al., 2004; Yoshida et al., 2004; Yamaguchi et al., 2005; Freitas et al., 2006; Jacob et al., 2006; Kress et al., 2006; Rump et al., 2006; Stevens and Roeder, 2006). D1S234 is located relatively close to RUNX3, D6S1689 to RUNX2, D4S3038 to FGFR3, D7S503 to TWIST, and D10S1483 to FGFR2.
All primers were purified by a de-salting method and diluted to 10 µM. Forward primers were labeled with 6-FAM, HEX, or NED according to their polymerase chain reaction (PCR) product size, to be distinguished during genotyping procedures. Amplification was done separately and then run together in an ABI377 (Perkin Elmer, Waltham, MA, USA). PCR was performed in a total volume of 12.5 µL, containing 40 ng of genomic DNA, 0.3 µM of forward and reverse primers, 0.250 mmol dNTPs, 1 mmol MgCl2, 1 U of Taq polymerase, and 1X amplification buffer. The amplification process was carried out starting with a hot start at 95°C for 5′ out followed by 29 cycles of 94°C for 1 min, annealing melting temperature (TM) for 1 min, and 72°C for 1 min, and the process was finished, after the cycles, with one additional step at 72°C for 30 sec. For D1S234 and D10S1483, the annealing temperature was 51°C, and for D4S3038, D6S1689, D7S503, and D19S566, it was 50°C, 53°C, 53°C, and 52°C, respectively. PCR products were run in agarose gel 2%, and bands were evaluated and photographed through UV light to verify amplification. Amplification products were added with Rox markers and evaluated by an ABI PRISM® 377 genetic analyzer (Applied Biosystems by Life Technologies, Carlsbad, CA, USA). Data from genotyping were then analyzed with Genotyper (Applied Biosystems by Life Technologies, Carlsbad, CA, USA). Information was analyzed independent of pedigree structure, and all genotyped individuals were checked for Mendelian segregation in the pedigrees as a genotyping control.
Linkage Analysis
A two-point parametric linkage analysis was performed, with the penetrance set at 90% for an autosomal-dominant inheritance pattern, according to the results of segregation analysis (Cruz et al., 2008). Allele frequency was assumed to be 1%, which corresponds to about a 2% prevalence of Class III malocclusion in the Brazilian population.
Data were analyzed over an array of θ values, with MLINK (Lathrop and Lalouel, 1984) software, as implemented in the FASTLINK package, version 3.0 (Cottingham et al., 1993). Theoretically, the families and samples should be sufficient to show significant linkage (LOD = 3.4) to the major locus for Class III malocclusion in these families if the polymorphic marker is essentially in the same location as the locus (θ = zero), and they should be sufficient to show a suggestion of linkage (LOD = 2.3) if θ is 0.05. Evaluation of the power of the study, used to evaluate whether the families collected were likely to be informative for linkage to the set of pedigrees, was accessed by a simulation performed with the SIMLINK software package (Botstein and Risch, 2003). This evaluation is indicated for general likelihood calculations.
Results
The Table summarizes the LOD score obtained for all families. There was no evidence or suggestion of linkage observed. In contrast, analysis of our data supports evidence of exclusion in 5 of the 6 markers evaluated here. A LOD score of -15.8 (θ = 0) was estimated for D1S234, with evidence of exclusion up to a θ of 0.1 (LOD = -3.28). For D4S3038, a LOD of -1.44 was estimated at θ = 0 and of 0.44 at θ = 0.1; therefore, no linkage or exclusion could be determined, although there is an apparent trend to exclusion of this region as well. A LOD score of -7.06 (θ = 0) was estimated for D6S1689, with no evidence of linkage up to a θ of 0.01. Concerning D7S503, a LOD score of -8.94 (θ = 0) was estimated, and there was evidence of exclusion up to a θ of 0.01. In relation to D10S1483, a LOD score of -7.73 (θ = 0) was estimated. Finally, D19S566 presented a LOD score of -9.21 (θ = 0), with evidence of exclusion up to a θ of 0.01. The values of LOD score for each marker in each family can be observed in Appendix Tables 2 through 7.
Table.
Overall LOD Score for θ Values for All 10 Brazilian Families with Class III Malocclusion
| θ Values | ||||||||
|---|---|---|---|---|---|---|---|---|
| Marker | 0.00 | 0.01 | 0.05 | 0.10 | 0.20 | 0.30 | 0.40 | |
| D1S234 | −15.84 | −9.04 | −5.12 | −3.28 | −1.57 | −0.73 | −0.25 | |
| D4S3038 | −1.44 | −0.17 | 0.35 | 0.44 | 0.31 | 0.11 | −0.01 | |
| D6S1689 | −7.06 | −3.54 | −1.15 | −0.24 | 0.34 | 0.37 | 0.20 | |
| D7S503 | −8.94 | −3.77 | −1.39 | −0.51 | 0.03 | 0.10 | 0.05 | |
| D10S1483 | −7.73 | −1.90 | −0.63 | −0.20 | 0.07 | 0.11 | 0.06 | |
| D19S566 | −9.21 | −4.71 | −1.94 | −0.89 | −0.19 | −0.02 | 0.00 | |
Discussion
Evidence suggestive of linkage in chromosomes 1p, 6p, and 19p with Class III malocclusion was observed in a set of Japanese and Korean sib-pairs (Yamaguchi et al., 2005). Contradictory evidence of exclusion of these regions was observed in our set of Brazilian families. This finding may be explained because of different genetic backgrounds in these populations and the fact that Class III malocclusion is likely to be a heterogeneous and, to some degree, polygenic trait. The present Brazilian population, as well as most populations present in America, was formed with the admixture and genetic contribution of major parental groups—European, African, and Amerindian (Godinho et al., 2008; Wang et al., 2008)—while the Asian contribution to the genetic pool of the Brazilian population is very recent and could be disregarded. Therefore, it can be thought that populations with different genetic backgrounds – such as Brazil and Japan/Korea – may present different gene(s) implicated in Class III malocclusion etiology.
As previously mentioned, Class III malocclusion can be the result of an overgrowth of the mandible and/or maxillary retrognathism. The genes FGFR3 (4p16.3) and FGFR2 (10q26), as well as the TWIST gene (7p21.2)—located near D4S3038, D10S1483, and D7S503 markers, respectively—may be related more to maxillary retrognathism/hypoplasia, as evidenced by their involvement in cranial suture biology and craniosynostosis. The observed absence of evidence for linkage to these genes in the Brazilian families may be explained by most of the families evaluated presenting a phenotype of mainly mandibular overgrowth.
In conclusion, no evidence of linkage in 6 previously suggested region/genes with Class III malocclusion was observed in a set of 10 Brazilian families. An exclusion region was delimited in either direction for all markers, except D4S3038. Our results strongly reinforce locus heterogeneity in the development of Class III malocclusion, and the genes implicated in the etiology of this trait may be correlated with the ethnicity of the population.
Supplementary Material
Acknowledgments
We sincerely thank the patients and their families for making this study possible, as well as all the colleagues who helped in gathering the sample
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
We also thank the University of Brasilia (UnB), Catholic University of Brasília (UCB), and Indiana University for financial support; and Luciana Rollemberg Nogueira for laboratory support. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sector.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Bertram SK, William JW, Richard HF. (1959). Heredity and the craniofacial complex. Am J Orthodont 45:172-217. [Google Scholar]
- Botstein D, Risch N. (2003). Discovering genotypes underlying human phenotypes: past successes for Mendelian disease, future approaches for complex disease. Nat Genet 33(Suppl):228S-237S. [DOI] [PubMed] [Google Scholar]
- Bui C, King T, Proffit W, Frazier-Bowers S. (2006). Phenotypic characterization of Class III patients. Angle Orthod 76:564-569. [DOI] [PubMed] [Google Scholar]
- Cottingham RW, Jr, Idury RM, Schaffer AA. (1993). Faster sequential genetic linkage computations. Am J Hum Genet 53:252-263. [PMC free article] [PubMed] [Google Scholar]
- Cruz RM, Krieger H, Ferreira R, Mah J, Hartsfield J, Jr, Oliveira S. (2008). Major gene and multifactorial inheritance of mandibular prognathism. Am J Med Genet A 146(A):71-77. [DOI] [PubMed] [Google Scholar]
- Downs WG. (1927). Studies in the causes of dental anomalies. Genetics 12:570-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Gheriani AA, Maher BS, El-Gheriani AS, Sciote JJ, Abu-shahba FA, Al-Azemi R, et al. (2003). Segregation analysis of mandibular prognathism in Libya. J Dent Res 82:523-527. [DOI] [PubMed] [Google Scholar]
- Frazier-Bowers S, Rincon-Rodriguez R, Zhou J, Alexander K, Lange E. (2009). Evidence of linkage in a Hispanic cohort with a Class III dentofacial phenotype. J Dent Res 88:56-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas EC, Nascimento SR, de Mello MP, Gil-da-Silva-Lopes VL. (2006). Q289P mutation in FGFR2 gene causes Saethre-Chotzen syndrome: some considerations about familial heterogeneity. Cleft Palate Craniofac J 43:142-147. [DOI] [PubMed] [Google Scholar]
- Godinho N, Gontijo C, Diniz M, Falcao-Alencar G, Dalton G, Amorim C, et al. (2008). Regional patterns of genetic admixture in South America. Forensic Science International: Genetics Supplement Series 1:329-330. [Google Scholar]
- Jacob AL, Smith C, Partanen J, Ornitz DM. (2006). Fibroblast growth factor receptor 1 signaling in the osteo-chondrogenic cell lineage regulates sequential steps of osteoblast maturation. Dev Biol 296:315-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress W, Schropp C, Lieb G, Petersen B, Busse-Ratzka M, Kunz J, et al. (2006). Saethre-Chotzen syndrome caused by TWIST 1 gene mutations: functional differentiation from Muenke coronal synostosis syndrome. Eur J Hum Genet 14:39-48. [DOI] [PubMed] [Google Scholar]
- Lathrop GM, Lalouel JM. (1984). Easy calculations of lod scores and genetic risks on small computers. Am J Hum Genet 36:460-465. [PMC free article] [PubMed] [Google Scholar]
- Litton SF, Ackerman LV, Isaacson RJ, Shapiro BL. (1970). A genetic study of class III malocclusion. Am J Orthodont 58:565-577. [DOI] [PubMed] [Google Scholar]
- Miller SA, Dykes DD, Polesky HF. (1988). A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 16:1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rump P, Letteboer TG, Gille JJ, Torringa MJ, Baerts W, van Gestel JP, et al. (2006). Severe complications in a child with achondroplasia and two FGFR3 mutations on the same allele. Am J Med Genet A 140:284-290. [DOI] [PubMed] [Google Scholar]
- Singh GD. (1999). Morphologic determinants in the etiology of class III malocclusions: a review. Clin Anat 12:382-405. [DOI] [PubMed] [Google Scholar]
- Stein GS, Lian JB, van Wijnen AJ, Stein JL, Montecino M, Javed A, et al. (2004). Runx2 control of organization, assembly and activity of the regulatory machinery for skeletal gene expression. Oncogene 23:4315-4329. [DOI] [PubMed] [Google Scholar]
- Stevens CA, Roeder ER. (2006). Ser351Cys mutation in the fibroblast growth factor receptor 2 gene results in severe Pfeiffer syndrome. Clin Dysmorphol 15:187-188. [DOI] [PubMed] [Google Scholar]
- Wang S, Ray N, Rojas W, Parra MV, Bedoya G, Gallo C, et al. (2008). Geographic patterns of genome admixture in Latin American Mestizos. PLoS genetics 4:e1000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff G, Wienker TF, Sander H. (1993). On the genetics of mandibular prognathism: analysis of large European noble families. J Med Genet 30:112-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Park SB, Narita A, Maki K, Inoue I. (2005). Genome-wide linkage analysis of mandibular prognathism in Korean and Japanese patients. J Dent Res 84:255-259. [DOI] [PubMed] [Google Scholar]
- Yoshida CA, Yamamoto H, Fujita T, Furuichi T, Ito K, Inoue K, et al. (2004). Runx2 and Runx3 are essential for chondrocyte maturation, and Runx2 regulates limb growth through induction of Indian hedgehog. Genes Dev 18:952-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.