Abstract
Particulate matter mass (PM), trace gaseous pollutants, and select volatile organic compounds (VOCs) with meteorological variables were measured in Logan, Utah (Cache Valley), for >4 weeks during winter 2017 as part of the Utah Winter Fine Particle Study (UWFPS). Higher PM levels for short time periods and lower ozone (O3) levels were present due to meteorological and mountain valley conditions. Nitrogenous pollutants were relatively strongly correlated with PM variables. Diurnal cycles of NOx, O3, and fine PM(PM 2.5) (aerodynamic diameter <2.5 μm [PM2.5]) suggested formation from NOx. O3 levels increased from early morning into midafternoon, and NOx and PM2.5 increased throughout the morning, followed by sharp decreases. Toluene/benzene and xylenes/benzene ratios and VOC correlations with nitrogenous and PM species were indicative of local traffic sources. Wind sector comparisons suggested that pollutant levels were lower when winds were from nearby mountains to the east versus winds from northerly or southerly origins.
Implications:
The Cache Valley in Idaho and Utah has been designated a PM2.5 nonattainment area that has been attributed to air pollution buildup during winter stagnation events. To inform state implementation plans for PM2.5 in Cache Valley and other PM2.5 nonattainment areas in Utah, a state and multiagency federal research effort known as the UWFPS was conducted in winter 2017. As part of the UWFPS, the U.S. Environmental Protection Agency (EPA) measured ground-based PM species and their precursors, VOCs, and meteorology in Logan, Utah. Results reported here from the EPA study in Logan provide additional understanding of wintertime air pollution conditions and possible sources of PM and gaseous pollutants as well as being useful for future PM control strategies in this area.
Introduction
The Cache Valley is narrow (<20 km wide), enclosed by steep mountains, and is oriented in primarily a north-south direction in southern Idaho and northern Utah; Logan is the largest city in the valley, with an approximate population of 50,000 (U.S. Census 2016) and is about 30 km south of the Idaho-Utah border. Together with other valleys in the Rocky Mountain West of the United States, since 2000 Cache Valley has experienced high concentrations of fine particulate matter (aerodynamic diameter <2.5 μm [PM2.5]) and related precursors such as ammonia and oxides of nitrogen (NOx, NOy) from agricultural, traffic, and home heating emissions in winter (Malek et al. 2006). (NOy is defined as NOx plus compounds formed from the oxidation of NOx, which can include nitric acid and organic nitrates (Seinfeld and Pandis 2016).) Wintertime cold surface temperatures, low wind speeds, low solar radiation, and snow cover can result in consecutive, multiday stagnation events that trap air pollutants in valleys (Baasandorj et al. 2018; Green et al. 2015; Malek et al. 2006; Silva et al. 2007; Wang et al. 2012). Exceedances of the National Ambient Air Quality Standard (NAAQS) for the 2006 24-hr PM2.5 of 35 μg/m3 have occurred in Cache Valley during winter stagnation events in the 2008–2013 time frame. This resulted in this area being designated a PM2.5 moderate nonattainment area (Baasandorj et al. 2018; Idaho Department of Environmental Quality 2014; U.S. Environmental Protection Agency [EPA] 2018a; Utah Air Quality Board 2014). In October, 2018 the EPA (2018b) modified the status of the area to be in attainment based on monitoring data from 2015–2017.
To inform state implementation plans for PM2.5 in Cache Valley and other PM2.5 nonattainment areas in Utah, a state-federal research effort led by Utah and the National Oceanic and Atmospheric Administration (NOAA) and known as the Utah Winter Fine Particulate Study, or UWFPS (Baasandorj et al. 2018), was undertaken. As part of this effort, the EPA measured ground-based PM species and their precursors and meteorology in Logan during January–February 2017. Federal reference and equivalent methods (FRMs and FEMs, respectively), other particle and gas-phase measurement methods, and methods for meteorological parameters were used in this study. Particulate matter concentration (i.e., PM2.5, PM10-2.5, PM10) and trace gases such as nitrogen dioxide (NO2), other oxides of nitrogen (nitric oxide [NO], NOy), and ozone (O3) were measured on a minute-to-minute basis to see how PM2.5 and other pollutants varied throughout the day. Volatile organic compounds (VOCs) were measured in 12-hr integrals and compared with the other pollutants. This paper reports on the PM, non-VOC gaseous, selected VOC, and meteorological measurements made by the EPA.
Methods
Details on the methods used and quality assurance are provided elsewhere (Baasandorj et al. 2018). In brief, Cache Valley air pollution and meteorological data were measured at coordinates 41°45′32.0″N, 111° 48′54.2″W on Utah State University property in northeastern Logan. With the exception of VOCs, pollutant and meteorological data were measured continuously from noon January 16 to 7:47 a.m. February 16, 2017. VOCs were measured on a 12-hr basis (6 a.m.–6 p.m.; 6 p.m.–6 a.m.) from 6 p.m. January 16 to 6 a.m. February 13, 2017.
PM2.5 and PM10 mass were measured with a model T640 mass monitor (Teledyne API, San Diego, CA; EPA Federal Equivalent Method (FEM) EQPM-0516-236). NO2 was measured using cavity-attenuated phase shift (CAPS) spectroscopy (model T500U, Teledyne; FEM EQNA-0514-212). Nitrogen oxide gaseous species were measured using a chemiluminescence analyzer (model T200U, Teledyne; FRM RFNA-1194-099) in accordance with Title 40, Part 50 of the Code of Federal Regulations (40 CFR Part 50 Appendix F). O3 was measured with a model 211 Scrubberless Ozone Monitor (2B Technologies, Boulder, CO; FEM EQOA-0514-215). Nitrogen oxides, O3, and VOCs were obtained from individual glass manifold ports ~5 m above ground level through 6.4-mm Teflon lines. Wind speed, wind direction, temperature, relative humidity, and precipitation were measured with a Vaisala weather transmitter (model WXT520; Vaisala, Helsinki, Finland); mixing layer height was measured with a Vaisala ceilometer (model CL51).
Measurement units were as follows: PM2.5 and PM10 (μg/m3); trace gaseous pollutants NO2, NO, NOx, NOy, and O3 (ppb); and meteorological variables: temperature (°C), atmospheric pressure (mbar), precipitation (mm of water), relative humidity (%), wind speed (m/sec), wind direction (degrees with due north at 0°), and mixing layer height (cloud height in m). These data were recorded on a minute-by-minute basis. NOx was calculated as the sum of NO (T200U) and NO2 (T500U CAPS). In addition, values were calculated for NOz as NOy minus NOx and for PM10-2.5 as the difference of PM10 and PM2.5. Values of these pollutants reported as <0 were changed to 0 for the analyses described.
VOCs were sampled with 6-L Summa and silicon-ceramic–coated canisters using a TM1200S sampling system (Entech Instruments, Simi Valley, CA). Collected samples were sent back to the laboratory for analysis using a gas chromatography–flame ionization (GC-FID) procedure. Compound identifications were determined by comparing observed compound peak retention time with those provided in a developed calibration table containing more than 400 compounds. Units for VOCs were reported as ppbC. Details of the sample collection and the GC-FID methodology procedures are provided elsewhere (Krug et al. 2018). The VOCs examined in this study were benzene, toluene, ethylbenzene, m,p-xylene, o-xylene, and methyl chloride. (The first five of these VOCs are collectively referred to as BTEX species.) In addition, based on molecular weight, the ratios of toluene to benzene, m, p-xylene to ethylbenzene, and xylenes to benzene were also calculated to assess traffic and other anthropogenic impacts. Methyl chloride is known to be emitted from wood and grassland burning (Agency for Toxic Substances and Disease Registry 1998). Wood combustion is considered an aerosol source in mountain valleys such as Cache Valley (Brown and Baasandorj 2017).
The data collected each minute were averaged to an hourly basis and on the 12-hr VOC sampling periods of 6 a.m.–6 p.m. (designated as day) and 6 p.m.–6 a.m. (designated as night); all times were local standard. For the wind directions, these were vector averages (Mardia and Jupp 2000; Saucier 1955). Summary statistics were calculated for the variables on hourly and 12-hr bases. A data completeness criterion of 75% was imposed for a summary statistic to be considered valid.
Wind direction data were used to indicate the compass sector from which the wind was blowing. The eight sectors were N, NE, E, SE, S, SW, W, and NW. Each sector subtended an arc of 45° with N extending from 337.5° to 22.5°.
Spearman correlation coefficients were used to indicate the strength of association between the variables. Comparisons of the pollutant levels between day and night values and between wind sectors were made using the Wilcoxon rank-sum test, with the magnitude of the differences calculated as Hodges-Lehmann estimates. The pairwise wind sector comparisons were preceded by an overall Kruskal-Wallis test. The choice of nonparametric statistical procedures was driven by the non-normality of many of the distributions (Hollander, Wolfe, and Chicken 2013). All statistical calculations were done using SAS version 9.4 software (SAS 2012).
Results
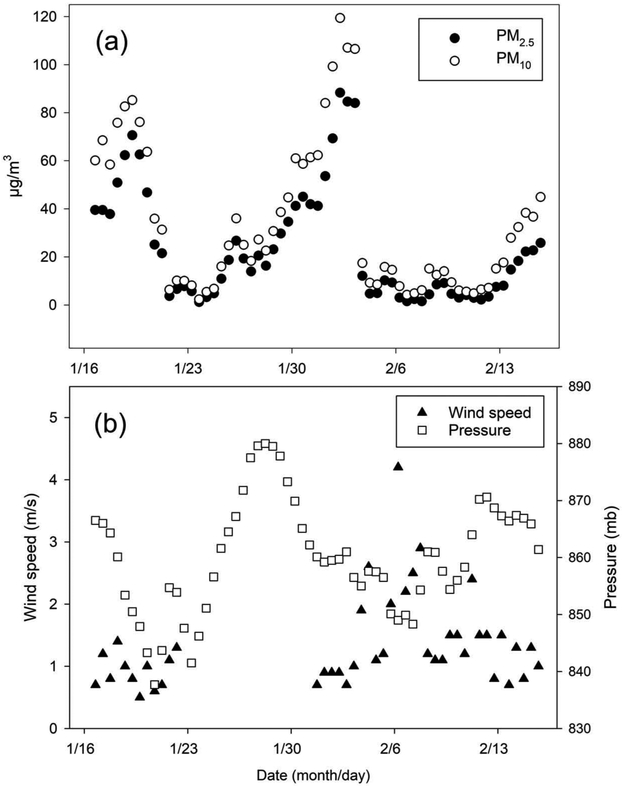
Summary statistics for all the variables are provided in Tables 1 and 2 for hourly and 12-hr time frames, respectively. Summary statistics for minute-by-minute data are in Table S1 in the supplemental material. As revealed in these tables, data completeness was very high for all the pollutants measured and for most of the meteorological variables. Summary statistics for NOx and NOz are based on the calculated NOx values. Instrumentation issues resulted in the wind data being missed during a stretch of time. In addition, instrument problems led to low data capture for the mixing height, and because of this, results relating to this variable should be cautiously interpreted. Figure 1a displays the 12-hr averages of PM2.5 and PM10; Figure 1b displays the 12-hr averages of wind speed and pressure.
Table 1.
Summary of trace gaseous and PM pollutant and meteorological data for Logan, Utah from January 16 to February 16, 2017, based on hourly averages.a
| Pollutant/meteorological variable | nb | Median | Mean | Coefficient of variation (%) | Minimum | 25th percentile | 75th percentile | Maximum |
|---|---|---|---|---|---|---|---|---|
| NO2 | 720 | 14.2 | 16.5 | 69 | 0.5 | 6.4 | 25.6 | 45.0 |
| NO | 720 | 1.8 | 7.4 | 172 | 0 | 0.1 | 9.1 | 101.2 |
| NOxc | 700 | 17.9 | 24.0 | 88 | 0.5 | 7.6 | 35.8 | 137.0 |
| NOy | 720 | 22.3 | 29.3 | 86 | 0 | 8.9 | 44.3 | 150.9 |
| NOzc | 700 | 3.6 | 5.6 | 104 | 0 | 0.6 | 8.9 | 25.0 |
| O3 | 724 | 22.8 | 21.3 | 61 | 0.0 | 9.4 | 32.4 | 46.5 |
| PM10 | 740 | 24.1 | 34.9 | 91 | 0.4 | 8.6 | 55.9 | 145.0 |
| PM2.5 | 740 | 16.3 | 24.1 | 99 | 0.2 | 4.5 | 38.3 | 103.7 |
| PM10-2.5 | 740 | 7.6 | 10.7 | 84 | 0.1 | 3.7 | 16.0 | 46.6 |
| Temperature | 740 | −2.4 | −3.0 | 217 | −18.7 | −7.3 | 1.6 | 11.4 |
| Humidity | 740 | 80.8 | 77.6 | 14 | 42.9 | 70.6 | 86.3 | 91.6 |
| Pressure | 740 | 859.8 | 860.1 | 1 | 836.9 | 854.0 | 866.7 | 881.2 |
| Wind speed | 542 | 1.0 | 1.4 | 77 | 0.3 | 0.8 | 1.5 | 7.5 |
| Total precipitation | 740 | 0.0 | 0.0 | 511 | 0 | 0 | 0 | 3.0 |
| Mixing height | 286 | 273.4 | 664.0 | 113 | 97.2 | 194.9 | 780.8 | 2976.3 |
Notes. aUnits are trace gases, ppb; PM, μg/m3; temperature, °C; humidity, %; pressure, mbar; wind speed, m/sec; precipitation, mm water; and mixing height, m.
740 maximum possible hr.
NOx was calculated as the sum of NO2 and NO, and NOz as the difference NOy – NOx.
Table 2.
Summary of trace gaseous, PM, and VOC pollutant and meteorological data for Logan, Utah from January 16 to February 16, 2017, based on 12-hr averages.a
| Pollutant/meteorological variable | nb | Median | Mean | Coefficient of variation (%) | Minimum | 25th percentile | 75th percentile | Maximum |
|---|---|---|---|---|---|---|---|---|
| NO2 | 61 | 14.2 | 16.6 | 60 | 2.7 | 9.4 | 24.3 | 40.8 |
| NO | 61 | 2.9 | 7.4 | 127 | 0.0 | 0.6 | 9.9 | 36.4 |
| NOxc | 61 | 20.0 | 24.1 | 71 | 2.9 | 10.6 | 36.6 | 63.1 |
| NOy | 61 | 24.8 | 29.4 | 76 | 1.7 | 11.9 | 43.3 | 83.0 |
| NOzc | 61 | 3.7 | 5.7 | 101 | 0.1 | 0.8 | 8.8 | 21.3 |
| O3 | 61 | 22.7 | 21.3 | 51 | 0.8 | 13.9 | 30.3 | 41.2 |
| PM10 | 61 | 24.7 | 34.6 | 89 | 2.2 | 9.2 | 58.7 | 119.4 |
| PM2.5 | 61 | 16.3 | 24.0 | 98 | 1.2 | 4.8 | 39.5 | 88.3 |
| PM10-2.5 | 61 | 7.6 | 10.6 | 79 | 1.0 | 4.2 | 16.1 | 31.1 |
| Temperature | 61 | −2.2 | −2.9 | 214 | −15.3 | −7.1 | 1.6 | 7.4 |
| Humidity | 61 | 79.3 | 77.6 | 12 | 52.2 | 71.5 | 83.6 | 90.4 |
| Pressure | 61 | 860.1 | 860.1 | 1 | 837.7 | 854.3 | 866.5 | 880.0 |
| Wind speed | 43 | 1.1 | 1.3 | 55 | 0.5 | 0.8 | 1.5 | 4.2 |
| Total precipitation | 61 | 0 | 0.6 | 357 | 0 | 0 | 0 | 14.8 |
| Mixing height | 19 | 236.7 | 541.3 | 107 | 160.5 | 196.8 | 481.8 | 1693.8 |
| Benzene | 54 | 1.64 | 1.91 | 46 | 0.63 | 1.19 | 2.57 | 4.04 |
| Toluene | 54 | 4.46 | 5.75 | 64 | 0.77 | 2.70 | 8.18 | 15.11 |
| Ethylbenzene | 54 | 0.71 | 0.87 | 54 | 0.17 | 0.58 | 1.19 | 2.20 |
| m,p-Xylene | 54 | 2.54 | 3.03 | 61 | 0.61 | 1.70 | 3.54 | 7.54 |
| o-Xylene | 54 | 1.06 | 1.27 | 58 | 0.21 | 0.75 | 1.55 | 3.91 |
| Methyl chloride | 54 | 1.00 | 1.06 | 30 | 0.74 | 0.86 | 1.17 | 2.57 |
| Toluene/benzene | 54 | 2.67 | 2.96 | 50 | 0.41 | 2.30 | 3.38 | 10.42 |
| m,p-xylene/ethylbenzene | 54 | 3.61 | 3.46 | 23 | 0.80 | 3.22 | 3.85 | 6.24 |
| Sum of xylenes/benzene | 54 | 2.21 | 2.44 | 23 | 1.20 | 1.92 | 2.60 | 3.50 |
Notes. a Units are gases, ppb; PM, μg/m3 ; temperature, °C; humidity, %; pressure, mbar; wind speed, m/sec; precipitation, mm water; mixing height, m; and VOCs, ppbC; except ratios, unitless.
61 maximum possible 12-hr periods for gaseous, PM, and meteorological variables and 57 maximum for VOCs.
NOx was calculated as the sum of NO2 and NO, and NOz as the difference NOy – NOx.
Figure 1.
The 12-hr averages of (a) PM2.5 and PM10 and (b) wind speed and pressure.
The frequency of the wind direction sectors is reported in Table 3 for hourly and 12-hr time frames. Both the individual counts and percentages are provided.
Table 3.
Wind sector frequencies for Logan, Utah from January 16 to February 16, 2017.
| Hourly averages (740 maximum possible hr) |
12-hr averages (61 maximum possible 12-hr periods) |
|||
|---|---|---|---|---|
| Sector | Frequency | Percentagea | Frequency | Percentagea |
| N | 39 | 7 | 6 | 14 |
| NE | 81 | 15 | 5 | 12 |
| E | 62 | 11 | 4 | 9 |
| SE | 24 | 4 | 1 | 2 |
| S | 45 | 8 | 3 | 7 |
| SW | 106 | 20 | 12 | 28 |
| W | 72 | 13 | 1 | 2 |
| NW | 113 | 21 | 11 | 26 |
Notes. a Rounded to nearest whole percent.
As reflected in Tables 1 and 2, the summaries of the meteorological variables indicate generally cold temperatures, with 75% of the values being below or just above freezing; generally high humidity, with the 75th percentile being around 85%; atmospheric pressure, with a mean and a median of approximately 860 mbar and only minor fluctuation with a coefficient of variation just above 1%; mostly very light wind speeds; little precipitation (90% of the hours had no measured precipitation); and mixing height (for the data captured) being generally low, with medians approximately from 230 to 270 m, but exhibiting noticeable variability, with a coefficient of variation over 100% and means roughly between 650 and 540 m. Although not indicated in the tables, precipitation that did occur was in the form of snow. Wind directions were most often from the NW and SW (see Table 3).
Based on Tables 1 and 2, the following observations about the pollutant distributions are made. The coefficients of variation indicate fairly large variability (even for the 12-hr time frame) for all pollutants, although this variability was less for the VOCs. For both 1-hr and 12-hr time frames, the relative levels of the means and medians show evidence of a slight skewness to higher values for the gaseous (except O3) and PM pollutants. However, this skewness was not apparent for the VOCs.
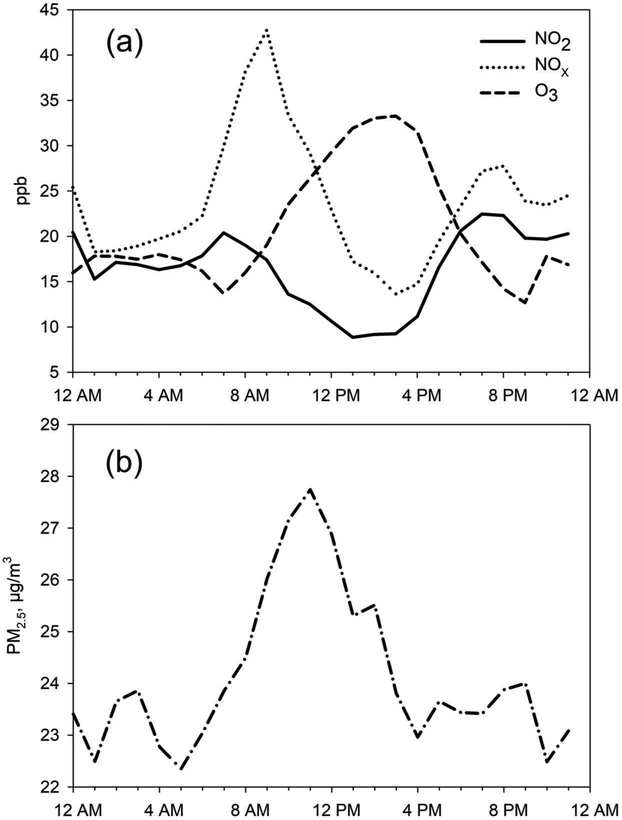
Concentrations of all pollutants were compared between day and night time frames using the Wilcoxon rank-sum test. No statistically significant differences (10% level) were found for any of the PM or VOC variables. Among the gaseous pollutants, NO2 was higher at night (10% level) and O3 (5% level) and NO (1% level) were higher during daytime hours; these observations were expected based on photochemistry. In addition, Spearman correlation coefficients between the pollutants were very similar for day and night periods. Given this general lack of differences on a day/night basis, the remaining statistical testing made no distinction between day and night data. However, intraday behavior of pollutants is presented graphically in Figure 2a and b.
Figure 2.
Hour-of-the day averages of (a) NO2, NOx, and O3 and (b) PM2.5.
Table 4 reports Spearman correlation coefficients between gaseous, PM, and meteorological variables on an hourly basis. As seen in the table, nitrogenous pollutants were generally moderately to strongly positively correlated among themselves and with the PM variables. Ozone was moderately or strongly negatively correlated with each of the other pollutants. The PM species concentrations were strongly correlated with each other (on a 12-hr basis as well; see Figure 1a).
Table 4.
Spearman correlation coefficients (%) between trace gaseous pollutants, particulate matter pollutants, and meteorological variables from Logan, Utah based on 1-hr summaries.a
| Pollutant/meteorological variable |
NO2 | NO | NOx | NOy | NOzb | O3 | PM10 | PM2.5 | PM10-2.5 | Wind speedc |
Temperature | Humidity | Pressure | Mixing heightd |
Total precipitatione |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NO2 | 100 | 69 | 95 | 93 | 73 | −92 | 75 | 75 | 66 | −60 | −53 | 34 | 26 | −64 | −14 |
| NO | 100 | 85 | 84 | 63 | −60 | 69 | 69 | 65 | −33 | −38 | 7 | 22 | −61 | −14 | |
| NOx | 100 | 98 | 74 | −87 | 80 | 80 | 72 | −54 | −51 | 28 | 25 | −70 | −17 | ||
| NOy | 100 | 83 | −84 | 88 | 88 | 79 | −54 | −55 | 24 | 25 | −76 | −22 | |||
| NOzb | 100 | −61 | 94 | 95 | 82 | −50 | −60 | 12 | 21 | −77 | −31 | ||||
| O3 | 100 | −64 | −65 | −56 | 59 | 47 | −44 | −21 | 58 | 4f | |||||
| PM10 | 100 | 98 | 94 | −44 | −53 | 2f | 22 | −78 | −28 | ||||||
| PM2.5 | 100 | 87 | −47 | −57 | 11 | 20 | −78 | −28 | |||||||
| PM10-2.5 | 100 | −36 | −42 | −16 | 24 | −67 | −27 | ||||||||
| Wind speedc | 100 | 49 | −50 | −6f | 42 | 21 | |||||||||
| Temperature | 100 | −20 | −57 | 59 | 36 | ||||||||||
| Humidity | 100 | −24 | −52 | 17 | |||||||||||
| Pressure | 100 | −34 | −17 | ||||||||||||
| Mixing heightd | 100 | 4f | |||||||||||||
| Total precipitatione | 100 |
Notes. All correlations statistically significant at the 1% level, except as indicated.
Sample size varied between 684 and 740, except for variable pairs involving wind speed or mixing height. Excluding mixing height, sample sizes involving wind speed ranged from 508 to 531. Excluding wind speed, sample sizes involving mixing height were between 273 and 286. The value of n for the wind speed/mixing height pair was 193.
NOz = NOy – NOx
Wind speed had lower data capture (542 of 740 possible hr or 73%).
Mixing height suffered from poor data capture (286 of 740 possible hr or 39%).
Most of the hours had no precipitation (673 of 740 possible hr or 90%).
Not statistically significant (10% level).
Table 4 also indicates that mixing height was generally moderately or relatively strongly negatively correlated with each of the pollutants, except for O3 where there was a moderate positive correlation; again, low data capture tempers the conclusions regarding mixing height. Although not as strong as for mixing height, pollutant correlations were generally negative with temperature and wind speed. Only weak correlations were seen between the pollutants and humidity, atmospheric pressure, and precipitation total. Finally, correlations among the meteorological variables were not strong, with all being <60%.
Table 5 presents the Spearman correlation coefficients among VOC species. As seen there, each of the VOCs, except methyl chloride, was relatively strongly positively correlated with the others. On the other hand, the correlation of methyl chloride with any of the other VOCS was not statistically significantly different from 0.
Table 5.
Spearman correlation coefficients (%) between VOCs from Logan, Utah based on 12-hr samples (n = 54).a
| VOC | Benzene | Toluene | Ethylbenzene | m,p-Xylene | o-Xylene | Methyl chloride |
|---|---|---|---|---|---|---|
| Benzene | 100 | 81 | 82 | 86 | 78 | 10b |
| Toluene | 100 | 79 | 82 | 69 | 12b | |
| Ethylbenzene | 100 | 87 | 68 | 18b | ||
| m,p-Xylene | 100 | 77 | 17b | |||
| o-Xylene | 100 | 13b | ||||
| Methyl chloride | 100 |
Notes. aAll correlations without a superscript letter are significantly different from 0 (P < 0.0001).
Not significantly different from 0 (P > 0.20).
Table 6 reports Spearman correlation coefficients for VOCs with trace gaseous and PM pollutants. Table 6 reveals that for the VOC species, except methyl chloride, positive strong or moderate correlations were found for each of the PM and gaseous pollutants, except O3. The correlations with O3 were negative and at least moderate. None of the correlations of methyl chloride with the other pollutants were statistically significantly different from 0.
Table 6.
Spearman correlation coefficients (%) between VOCs and nitrogenous species, ozone, and PM from Logan, Utah based on 12-hr samples (6 a.m.–6 p.m. and 6 p.m.–6 a.m.; n = 54).a
| VOC | NO2 | NO | NOx | NOy | NOz | O3 | PM10 | PM2.5 | PM10-2.5 |
|---|---|---|---|---|---|---|---|---|---|
| Benzene | 87 | 81 | 99 | 94 | 79 | −79 | 84 | 84 | 78 |
| Toluene | 77 | 76 | 82 | 81 | 70 | −70 | 72 | 74 | 62 |
| Ethylbenzene | 78 | 70 | 84 | 85 | 72 | −71 | 78 | 80 | 68 |
| m,p-Xylene | 87 | 81 | 93 | 92 | 72 | −80 | 79 | 79 | 76 |
| o-Xylene | 69 | 58 | 72 | 69 | 52 | −60 | 56 | 56 | 57 |
| Methyl chloride | 9b | 12b | 8b | 11b | 20b | −3b | 10b | 12b | 9b |
Notes. aAll correlations without a superscript letter are significantly different from 0 (1% level).
Not statistically significantly different from 0 (10% level).
Figure 2a displays the diurnal cycles, indicated by hour-of-the-day averages of NO2, NOx, and O3 concentrations; Figure 2b is the same presentation for PM2.5. As seen in the Salt Lake Valley of Utah (Baasandorj et al. 2017; Brown and Baasandorj 2017), Logan averages for NOx showed a sharp increase during morning hours, followed by a sharp decline to early afternoon. Average O3 levels increased from approximately 7 a.m. to 4 p.m. This is consistent with the formation of daytime O3 from elevated morning levels of nitrogen oxides and other precursors. (NO2 showed a similar, but less pronounced, diurnal pattern as NOx.) PM2.5 concentrations in Logan increased during the morning hours, followed by a sharp decrease beginning in midday. Average hour-of-the-day PM concentrations were in a narrow range of 22–28 μg/m3, and the standard errors of these means were relatively constant throughout the day.
On both hourly and 12-hr time frames, the pollutant levels were compared between wind sectors. In each case, pairwise comparisons using the Wilcoxon rank-sum test were preceded by the Kruskal-Wallis test. The results are presented in Tables 7–9 for all 28 possible pairwise comparisons. These tables are all read as follows. For ease of readability, blank entries in the table indicate no statistically significant (10% level) difference was found. When a statistically significant difference was found, the cell entry indicates the significance level and the Hodges-Lehmann estimate of the magnitude of the difference. The cell entries indicate the difference between sectors in the leftmost column as first sector minus second sector. For example, Table 7 has an entry of −5.0 for NO2 for the SE-W cell; this indicates a significant difference at the 10% level and an estimated difference of 5.0 ppb lower when winds are from the SE sector versus the W sector.
Table 7.
Comparison of hourly average pollutant concentrations from Logan, Utah between wind sectors.a
| Sectors | NO2 | NO | NOx | NOy | NOz | O3 | PM10 | PM2.5 | PM10-2.5 |
|---|---|---|---|---|---|---|---|---|---|
| E-SE | |||||||||
| E-NE | |||||||||
| E-NW | 1.5b | ||||||||
| E-S | −2.1c | −5.6c | −12.4d | −8.5d | −4.0d | ||||
| E-SW | 3.8c | −6.5d | −8.0b | −4.4c | −2.9c | ||||
| E-W | −3.9b | ||||||||
| E-N | 0.7c | 3.6b | 2.2c | ||||||
| SE-NE | |||||||||
| SE-NW | |||||||||
| SE-S | −12.3c | −7.6b | −4.8c | ||||||
| SE-SW | −6.4c | −3.6b | |||||||
| SE-W | −5.0b | ||||||||
| SE-N | |||||||||
| N-NW | 4.0b | ||||||||
| N-S | −2.9d | −6.6c | −11.9c | −2.9d | −21.4d | −15.4d | −6.6d | ||
| N-SW | −1.5d | −6.1b | −1.8d | −14.9d | −9.4d | −6.0d | |||
| N-W | −6.3c | −1.3d | −10.8d | −14.2d | −1.5c | 7.2d | −11.3d | −7.4d | −3.3d |
| N-NE | |||||||||
| NW-S | −2.4d | −9.4d | −2.7d | −5.9d | −17.6d | −12.8d | −5.7d | ||
| NW-SW | 2.2b | −1.2d | −1.6d | −7.5d | −13.0d | −7.9d | −5.0d | ||
| NW-W | −4.8d | −0.7d | −8.2d | −11.3d | −1.3d | 3.1c | −9.1d | −6.1d | −2.8d |
| NW-NE | |||||||||
| S-SW | |||||||||
| S-W | −5.3c | 10.1d | 9.2b | 3.1b | |||||
| S-NE | 2.1d | 5.8b | 9.7c | 2.4d | 15.6d | 11.4d | 5.2d | ||
| W-SW | 7.3d | 7.0c | 7.3c | −11.0d | |||||
| W-NE | 5.5c | 0.5c | 9.2d | 11.4d | 1.0c | −6.2d | 7.4c | 5.2d | 1.9b |
| NE-SW | −1.0d | −1.4c | −3.4b | −11.4d | −6.7d | −4.6d |
Notes. The Hodges-Lehmann estimates of the magnitude of the differences are given as A – B, where A-B is the wind sector pair in the leftmost column. Units are ppb for the trace gaseous species and μg/m3 for PM.
Empty cells indicate no statistically significant difference (10% level).
Statistically significant difference (10% level).
Statistically significant difference (5% level).
Statistically significant difference (1% level).
Table 9.
Comparison of 12-hr average VOC concentrations from Logan, Utah between wind sectors.
| Sectors | Benzene | Toluene | Ethyl benzene |
m, p-Xylene |
o-Xylene | Methyl chloride |
|---|---|---|---|---|---|---|
| E-SE | ||||||
| E-NE | ||||||
| E-NW | −6.2c | −0.4c | −1.8b | |||
| E-S | −1.9b | −9.2b | −1.1b | −4.3b | ||
| E-SW | −0.4c | |||||
| E-W | ||||||
| E-N | ||||||
| SE-NE | ||||||
| SE-NW | ||||||
| SE-S | ||||||
| SE-SW | ||||||
| SE-Wa | No test | No test | No test | No test | No test | No test |
| SE-N | ||||||
| N-NW | ||||||
| N-S | ||||||
| N-SW | ||||||
| N-W | ||||||
| N-NE | ||||||
| NW-S | −2.1b | −0.7b | ||||
| NW-SW | ||||||
| NW-W | ||||||
| NW-NE | 0.5d | 6.5c | 0.2b | 1.2c | 0.5c | |
| S-SW | 5.0b | |||||
| S-W | ||||||
| S-NE | 2.0b | 9.9b | 0.9b | 4.4b | 1.8b | |
| W-SW | ||||||
| W-NE | ||||||
| NE-SW |
Notes. The Hodges-Lehmann estimates of the magnitude of the differences are given as A – B, where A-B is the wind sector pair in the leftmost column. Units are ppbC for the VOCs; ratios are unitless.
No comparison possible because there was only one observation for each wind direction.
Statistically significant difference (10% level).
Statistically significant difference (5% level).
Statistically significant difference (1% level).
For each of the gaseous and PM variables, the Kruskal-Wallis test found a significant (at least at the 5% level) difference among wind sectors for the hourly time frame. Table 7 reports the results of the pairwise wind sector comparisons on an hourly basis. Some general observations from this table are that most pollutant levels were lower when winds were from a northerly sector as opposed to a southerly or westerly one, excepting O3 from a westerly sector. In addition, PM and O3 levels were lower with winds from an easterly sector versus a southerly one.
Table 8 presents the results of the wind sector comparisons on a 12-hr basis for the gaseous and PM pollutants. The Kruskal-Wallis test indicated a significant (at least at the 5% level) difference among the wind sectors for each pollutant. This table suggests lower gaseous and PM levels with winds from easterly sectors as opposed to northerly or southerly ones.
Table 8.
Comparison of 12-hr average chemical concentrations from Logan, Utah between wind sectors.a
| Sectors | NO2 | NO | NOx | NOy | NOz | O3 | PM10 | PM2.5 | PM10-2.5 |
|---|---|---|---|---|---|---|---|---|---|
| E-SE | |||||||||
| E-NE | −0.5d | −4.0c | −2.9d | ||||||
| E-NW | −9.7d | −2.6c | −12.0d | −15.0d | −4.1d | 14.3c | −22.5e | −12.7e | −6.9d |
| E-S | −18.1c | −22.6c | −39.4c | −54.2c | −13.7c | −16.2c | −78.5c | −51.6c | −22.1c |
| E-SW | −5.6c | −6.6c | −12.6c | −17.1d | −3.5e | −29.0d | −18.2d | −8.9d | |
| E-W | |||||||||
| E-N | −18.5d | −22.5d | −31.3d | −7.6d | 21.1d | −45.2d | −29.5c | −11.3d | |
| SE-NE | |||||||||
| SE-NW | |||||||||
| SE-S | |||||||||
| SE-SW | |||||||||
| SE-Wb | No test | No test | No test | No test | No test | No test | No test | No test | No test |
| SE-N | |||||||||
| N-NW | |||||||||
| N-S | −18.9c | ||||||||
| N-SW | −14.7d | ||||||||
| N-W | |||||||||
| N-NE | 15.7c | 4.4c | 20.5d | 29.5d | −22.9d | 10.8c | |||
| NW-S | −18.8d | −27.3c | −38.8d | −9.6d | −56.1c | −44.3c | −11.1c | ||
| NW-SW | −9.0c | ||||||||
| NW-W | |||||||||
| NW-NE | 8.4c | 4.3e | 10.9e | 13.4d | −16.9d | ||||
| S-SW | 11.3c | −9.7c | |||||||
| S-W | |||||||||
| S-NE | 17.2d | 23.2d | 38.1d | 52.2c | 13.2d | −18.8d | 70.3c | 48.9c | 20.1c |
| W-SW | |||||||||
| W-NE | |||||||||
| NE-SW | −4.5c | −7.7e | −13.5c | −15.6d | −3.0c | −21.7c | −8.7d |
Notes. The Hodges-Lehmann estimates of the magnitude of the differences are given as A – B, where A-B is the wind sector pair in the leftmost column. Units are ppb for trace gaseous species and μg/m3 for PM.
Empty cells indicate no statistically significant difference (10% level).
No comparison possible because there was only one observation for each wind direction.
Statistically significant difference (10% level).
Statistically significant difference (5% level).
Statistically significant difference (1% level).
Table 9 contains the results of the 12-hr wind sector comparisons for the VOCs. The Kruskal-Wallis test found significant (5% level) differences for each VOC variable except o-xylene and methyl chloride. However, for completeness, the results of the Wilcoxon rank-sum pairwise comparisons are reported in Table 9 for o-xylene and methyl chloride. For the few pairwise comparisons that yielded a significant difference, the results for the VOCs were much the same as for the 12-hr average gaseous and PM pollutants. That is, there were generally higher levels from the northerly or southerly sectors compared with the easterly ones.
Discussion and conclusion
As reference points, the 24-hr NAAQS for PM2.5 is 35 μg/m3 for the 98th percentile, and the annual standard is a mean of 12 μg/m3. However, these values are based on averages over multiple years and are not directly comparable to the values reported here. Each of the minute, hourly, and 12-hr time frames considered here yielded some values that would be considered high relative to the PM2.5 NAAQS. Given this study’s weather conditions (cold temperatures, light winds, generally lower mixing heights, little precipitation), some higher PM levels on these short time periods are not surprising. (Similar meteorological conditions have been attributed to inversions in Logan resulting in high PM levels (Malek et al. 2006).) Similarly, the cold wintertime conditions would be expected to generate relatively low ozone levels, and indeed that was the case in this study. Light winds and lack of atmospheric pressure changes may explain the lack of day/night differences during the study, although the observed lower nighttime O3 levels would still be expected based on photochemistry. The somewhat unexpected moderately positive correlations of O3 with wind speed and mixing height must be interpreted in light of the fact the monitoring period was characterized by light winds and low mixing heights (also bearing in mind the low data capture for the mixing height).
Figure 1a indicates notable drops in PM2.5 and PM10 levels around January 20 and February 3. One potential reason for these drops in PM concentration might be the co-occurrence of higher wind speeds and a concomitant pressure drop (e.g., the breakup of a persistent cold air pool [PCAP]). However, this explanation is not well supported by Figure 1b. The pressure dropped around January 20; although there was also a pressure drop a few days before February 3, the pressure had leveled out and was fairly constant by that date. For the days around both time points, the winds were very light and steady. Precipitation scavenging also does not appear to have played a role in either decline. Six of the 7 hr between hour 19 on February 3 and hour 1 on February 4 did have some light snowfall, totaling about 3.1 cm of snow. Aside from a few stray hours, the only other snowfall events were February 6 and early February 7 (27 hr with 12 hr having snowfall yielding a total of approximately 7.1 cm of snow) and late afternoon February 8 into early morning February 11 (57 hr with 44 hr having snow, totaling about 25.5 cm). Neither of these latter events was associated with a drop in particulate matter.
The typical urban-suburban NOx levels of 10 ppb and greater reported by Seinfeld and Pandis (2016) are similar to the median and mean hourly NOx levels measured here (see Tables 1 and 2). Using hour-of-the-day averages, diurnal cycles of NOx, O3, and PM2.5 suggested O3 formation from nitrogen oxides (and other precursors); this was consistent with the basic photochemical cycle of nitrogen oxides and O3 (Seinfeld and Pandis 2016). Figure 2b indicates that PM2.5 shows an increase in levels during the morning hours when one would expect mixing height to be increasing. Yet Table 4 reports a negative correlation between mixing height and the PM variables. This apparent contradiction of increasing concentration with presumably increasing mixing height may simply be an artifact of the poor data capture for the mixing height (Table 4) combined with the narrow range of PM2.5 (Figure 2b). We note in Table 4 that the correlation between PM2.5 and NOz is strong (95%); Baasandorj et al. (2018) provide information on the creation of NOz (oxidation products of NOx) and suggest that Cache Valley was more nitrate limited (versus ammonia limited) in formation of ammonium nitrate.
Like O3, VOC levels were low. The strong correlations of BTEX species with themselves and with the nitrogenous and PM species suggest that these pollutants are associated with traffic. This idea is reinforced by the concomitant results from the wind direction analyses and the location of the site in northeastern Logan. The more developed portion of the city and the major roadways lie to the south and west of the site. Gasoline and diesel fuel are known sources of all these pollutants. Mean and median toluene/benzene and m,p-xylene/ethylbenzene ratios were approximately a value of 3.0, suggesting traffic and other anthropogenic emissions (Gelencsér, Siszler, and Hlavay 1997; Nelson, Quigley, and Smith 1983). Furthermore, the mean and median sum of xylenes/benzene ratios were >2, suggesting a local traffic impact (Zielinska and Fujita 2003). In addition, there are no major industrial sources in the immediate area of Logan. BTEX levels in Logan were lower than levels measured in the Salt Lake Valley during the UWFPS (Baasandorj et al. 2018); the Salt Lake Valley has an industrial (refinery) influence and much larger contribution from mobile sources. (The strong BTEX correlations with nitrogenous species observed in Logan may also result from the role of VOCs in the formation of certain nitrogen compounds as suggested by Silva et al. (2007) for nitric acid and Baasandorj et al. (2018) for ammonium nitrate.)
It was considered a possibility that wood burning for heating purposes might have been a cause of elevated PM levels. Of the VOCs monitored in this study, methyl chloride was thought to be the best indicator of wood burning (Agency for Toxic Substances and Disease Registry 1998). But the lack of day/night differences for methyl chloride and the lack of correlation of this VOC with the PM species do not support this suggestion.
The strong VOC-PM correlations observed here support the suggestion of Baasandorj et al. (2018) and Silva et al. (2007) that when high levels of PM are of concern, co-monitoring of VOCs may be informative. In this study, the only measured VOC indicative of wood burning was methyl chloride. In future studies, it would be worthwhile to monitor other VOC tracers of wood burning, such as formaldehyde or levoglucosan. If PM speciation is done, soluble potassium might also be considered as a wood burning indicator. In addition, an aethalometer to measure wood smoke might be used.
Although large differences between wind directions (e.g., the PM variables for the E-S and NW-S pairs in Table 8) are estimated, these specific numbers should be cautiously regarded because of the small sample sizes (see Table 3) for some wind sectors (e.g., E, S). Thus, it is advisable to focus on qualitative differences, such as higher versus lower. Wind sector comparisons indicate that winds from an easterly direction tend to have lower pollutant levels than winds from northerly or southerly origins. This was found for the VOC, gaseous, and PM variables on a 12-hr basis, and for the PM species on an hourly time frame. This might be due, in part, to the fact that Logan is located on the eastern edge of the Cache Valley near steep mountains and thus somewhat sheltered from pollution originating to the east. Thus, lower concentrations may result from winds from the east carrying clean mountain air through canyons to Logan. In addition, hourly wind sector comparisons indicated generally lower pollutant levels with winds from a northerly sector versus a southerly or westerly area, although O3 was an exception, being somewhat higher from western areas than northerly ones. Given the location of the site in northeastern Logan, these results may reflect greater development and the major roadways, and hence traffic volume, in southern and western areas of Logan.
Supplementary Material
Acknowledgment
For their roles in various aspects of the study, the authors thank Jim Szykman and Andrew Whitehill of EPA and Andrew Habel of Jacobs Technology Inc. The authors also thank David Olson and Jason Weinstein of EPA for reviewing the manuscript.
Funding
The U.S. Environmental Protection Agency through its Office of Research and Development funded and managed the research described here under contracts EP-C-15-008 to Jacobs Technology Inc. and EP-W-13-024 to Alion Science and Technology. The paper has been subjected to Agency review and approved for publication. The views expressed in this paper are those of the authors and do not necessarily represent the views or policies of the U.S. Environmental Protection Agency. Mention of trade names or commercial products does not constitute an endorsement or recommendation for use.
Biography
Shaibal Mukerjee is a Research Physical Scientist in the National Exposure Research Laboratory.
Luther Smith is a Senior Statistician at Alion Science and Technology, Inc. with a focus on statistical analysis of environmental data.
Russell Long is a Research Chemist in the National Exposure Research Laboratory.
William Lonneman is a Research Chemist in the Senior Environmental Employment Program at EPA and is responsible for GC measurements of ambient air VOCs.
Surender Kaushik is a Supervisory Physical Scientist in the National Exposure Research Laboratory.
Maribel Colon is a Research Chemist involved in Wildland Fire and Emergency Response Team member, methods development for VOCs, PM, sensors, etc.
Karen Oliver is a Research Chemist, Lab Manager responsible for the daily lab operations for the VOC lab and analytical method development.
Donald Whitaker is a Research Physical Scientist responsible for overseeing the VOC analysis laboratory and development of sample collection methods.
References
- Agency for Toxic Substances and Disease Registry. 1998. Toxicological profile for chloromethane. Public Health Service, US Department of Health and Human Services, Atlanta, GA: Accessed July, 2018 https://www.atsdr.cdc.gov/toxprofiles/tp106.pdf. [Google Scholar]
- Baasandorj M, Brown S, Hoch S, Crosman E, Long R, Silva P, Mitchell L, Hammond I, Martin R, Bares R, et al. 2018. Utah winter fine particulate study. Final Report. Accessed May, 2018 https://documents.deq.utah.gov/air-quality/planning/technical-analysis/research/northern-utah-airpollution/utah-winter-fine-particulate-study/DAQ-2018-004037.pdf.
- Baasandorj M, Hoch SW, Bares R, Lin JC, Brown SS, Millet DB, Martin R, Kelly K, Zarzana KJ, Whiteman D, et al. 2017. Coupling between chemical and meteorological processes under persistent cold-air pool conditions: Evolution of wintertime PM2.5 pollution events and N2O5 observations in Utah’s Salt Lake valley. Environ. Sci. Technol. 51:5941–50. doi: 10.1021/acs.est.6b06603. [DOI] [PubMed] [Google Scholar]
- Brown SS, and Baasandorj M. 2017. Utah winter fine particulate study (UWFPS) research flights of the NOAA twin otter to address particulate matter pollution in urban areas of the Great Salt Lake Basin. Accessed May, 2018 https://www.esrl.noaa.gov/csd/groups/csd7/measurements/2017uwfps/whitepaper.pdf.
- Gelencsér A, Siszler K, and Hlavay J. 1997. Toluene–benzene concentration ratio as a tool for characterizing the distance from vehicular emission sources. Environ. Sci. Technol. 31:2869–72. doi: 10.1021/es970004c. [DOI] [Google Scholar]
- Green MC, Chow JC, Watson JG, Dick K, and Inouye D. 2015. Effects of snow cover and atmospheric stability on winter PM2.5 concentrations in western U.S. valleys. J. Appl. Meteorol. Climatol 54:1191–201. doi: 10.1175/JAMC-D-14-0191.1. [DOI] [Google Scholar]
- Hollander M, Wolfe DA, and Chicken E. 2013. Nonparametric statistical methods, 848 New York: John Wiley & Sons. [Google Scholar]
- Idaho Department of Environmental Quality. 2014. Cache valley Idaho PM2.5 nonattainment area state implementation plan amendment. Accessed February, 2018 https://www.deq.idaho.gov/media/1118467/cache-valley-pm25-nonattainment-area-sip-amendment.pdf.
- Krug JD, Lewandowski M, Offenberg JH, Turlington J, Lonneman WA, Modak N, Krantz QT, King C, Gavett SH, Gilmour MI, et al. 2018. The photochemical conversion of surrogate emissions for use in toxicological studies: Role of particulate- and gas-phase products. Environ. Sci. Technol. 52:3037–44. doi: 10.1021/acs.est.7b04879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek E, Davis T, Martin RS, and Silva PJ. 2006. Meteorological and environmental aspects of one of the worst national air pollution episodes (January 2004) in Logan, Cache Valley, Utah, USA. Atmos. Res. 79:108–22. doi: 10.1016/j.atmosres.2005.05.003. [DOI] [Google Scholar]
- Mardia KV, and Jupp PE. 2000. Directional statistics, 429 Chichester, England: Wiley. [Google Scholar]
- Nelson PF, Quigley SM, and Smith MY. 1983. Sources of atmospheric hydrocarbons in Sydney: A quantitative determination using a source reconciliation technique. Atmos. Environ. 17:439–49. doi: 10.1016/0004-6981(83)90117-8. [DOI] [Google Scholar]
- SAS. 2012. Version 9.4. SAS Institute, Cary, NC. doi: 10.1094/PDIS-11-11-0999-PDN. [DOI] [Google Scholar]
- Saucier WJ 1955. Principles of meteorological analysis, 438 Chicago: Univ. Chicago Press. [Google Scholar]
- Seinfeld JH, and Pandis SN. 2016. Atmospheric chemistry and physics: From air pollution to climate change, 176, 179 3rd ed. Hoboken, NJ: Wiley. [Google Scholar]
- Silva PJ, Vawdrey EL, Corbett M, and Erupe E. 2007. Fine particle concentrations and composition during wintertime inversions in Logan, Utah, USA. Atmos. Environ. 41:5410–22. doi: 10.1016/j.atmosenv.2007.02.016. [DOI] [Google Scholar]
- U.S. Census. 2016. QuickFacts Logan. Utah: Accessed July, 2018 https://www.census.gov/quickfacts/fact/table/logancityutah/INC110216? [Google Scholar]
- U.S. Environmental Protection Agency. 2018a. Nonattainment Areas for Criteria Pollutants (Green Book). Accessed September, 2018 https://www3.epa.gov/airquality/greenbook/anayo_ut.html (Utah) and https://www3.epa.gov/airquality/greenbook/anayo_id.html (Idaho).
- U.S. Environmental Protection Agency. 2018b. 40 CFR Part 52 [EPA–R08–OAR–2018–0309 and EPA–R10–OAR–2018–0316:FRL–9985–28–Region 8 and Region 10]. Determination of attainment by the attainment date and clean data determination for the Logan, UT-ID 2006 24-Hour PM2.5 nonattainment area. Fed. Reg. 83 (203):52983 accessed December 2018). https://www.federalregister.gov/documents/2018/10/19/2018-22284/determination-of-attainment-by-the-attainment-date-and-clean-data-determination-for-the-logan-ut-id. [Google Scholar]
- Utah Air Quality Board. 2014. Utah State Implementation Plan: Control Measures for Area and Point Sources, Fine Particulate Matter, PM2.5 SIP for the Logan, UT-ID Nonattainment Area, Section IX, Part A.23, 69 Salt Lake City, UT: Publisher name: Utah Department of Environmental Quality. [Google Scholar]
- Wang SY, Gillies RR, Martin R, Davies RE, and Booth MR. 2012. Connecting subseasonal movements of the winter mean ridge in Western North America to inversion climatology in Cache Valley, Utah. J. Appl. Meteor. Climatol. 51:617–27. doi: 10.1175/JAMC-D-11-0101.1. [DOI] [Google Scholar]
- Zielinska B, and Fujita EM. 2003. Characterization of ambient volatile organic compounds at the western boundary of the SCOS97-NARSTO modeling domain. Atmos. Environ. 37:S171–S180. doi: 10.1016/S1352-2310(03)00389-3. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.