Abstract
The possibility that R-type calcium channels contribute to fast glutamatergic transmission in the hippocampus has been assessed using low concentrations of NiCl2 and the peptide toxin SNX 482, a selective antagonist of the pore-forming α1E subunit of R-type calcium channel. EPSPs or EPSCs were recorded in the whole-cell configuration of the patch-clamp technique mainly from CA3 hippocampal neurons. Effects of both NiCl2 and SNX 482 were tested on large (composite) EPSCs evoked by mossy and associative–commissural fiber stimulation. NiCl2 effects were also tested on minimal EPSPs–EPSCs. Both substances reduced the amplitude of EPSPs–EPSCs. This effect was associated with an increase in the number of response failures of minimal EPSPs–EPSCs, an enhancement of the paired-pulse facilitation ratios of both minimal and composite EPSCs, and a reduction of the inverse squared coefficient of variation (CV−2). The reduction of CV−2 was positively correlated with the decrease in EPSC amplitude. The inhibitory effect of NiCl2 was occluded by SNX 482 but not by ω-conotoxin-MVIIC, a broad-spectrum antagonist thought to interact with N- and P/Q-type calcium channels, supporting a specific action of low concentrations of NiCl2 on R-type calcium channels. Together, these observations indicate that both NiCl2 and SNX 482 act at presynaptic sites and block R-type calcium channels with pharmacological properties similar to those encoded by the α1E gene. These channels are involved in fast glutamatergic transmission at hippocampal synapses.
Keywords: hippocampus, mossy fibers, associative–commissural fibers, single-fiber EPSPs, single-fiber EPSCs, composite EPSC, ω-conotoxin-MVIIC, SNX 482
Synaptic transmission is triggered by calcium entry through voltage-dependent calcium channels (VDCCs) into presynaptic nerve terminals (Katz, 1969). Using type-specific calcium channel blockers, it has been found that most VDCCs involved in transmitter release in the mammalian CNS belong to N- and P/Q-type channels (Takahashi and Momiyama, 1993; Wheeler et al., 1994; Dunlap et al., 1995). These channels are localized at presynaptic release sites at which they synergistically control synaptic function. The relationship between presynaptic calcium concentration ([Ca2+]) and transmitter release is nonlinear and can be approximated by a power function with the exponent that typically varies between 3 and 4 in different synapses (Dodge and Rahamimoff, 1967; Augustine and Charlton, 1986; Takahashi and Momiyama, 1993; Wu and Saggau, 1997). The relative contribution of VDCCs to transmitter release can be estimated by monitoring changes in [Ca2+] in presynaptic nerve endings, loaded with calcium indicators in the presence of selective VDCC antagonists. Using this method, Wu and Saggau (1995) have found that, at hippocampal CA3–CA1 synapses, complete block of both N- and P/Q-type VDCCs with ω-conotoxin-MVIIC (ω-CTx-MVIIC) does not completely suppress presynaptic calcium transient. This indicates that, in addition to N- and P/Q-type, other VDCC types contribute to presynaptic calcium entry and fast glutamatergic synaptic transmission (Luebke et al., 1993; Takahashi and Momiyama, 1993; Wu and Saggau, 1995). Approximately one-quarter of presynaptic calcium influx is resistant to ω-CTx-MVIIC in both hippocampus (Wu and Saggau, 1995) and cerebellum (Mintz et al., 1995). The residual ω-CTx-MVIIC-resistant calcium transient might be mediated by T- or R-type VDCCs (Wu and Saggau, 1994, 1995; Mintz et al., 1995, 1997). However, the involvement of T-type VDCC seems unlikely because this channel type is localized mainly on the soma and dendrites (Huguenard, 1996; Craig et al., 1999). A possible candidate is the R-type VDCC first described by Zhang et al. (1993) and involved in excitation–secretion coupling in both central neurons and chromaffin cells (Wu et al., 1998; Wang et al., 1999; Albillos et al., 2000).
In the present experiments, we have investigated whether R-type calcium channels contribute to trigger transmitter release at hippocampal mossy and associative–commissural fibers synapses. To this aim, we have used low concentrations of NiCl2 and the polypeptide toxin SNX 482, a selective antagonist of recombinant α1E channels (Newcomb et al., 1998), which inhibits some native R-type channels (Wang et al., 1999; Tottene et al., 2000). We found that, at these synapses, R-type calcium channels constitute a significant fraction of presynaptic VDCCs controlling fast glutamatergic transmission.
MATERIALS AND METHODS
Slice preparation. Experiments were performed on hippocampal slices obtained from postnatal day 14 (P14) to P19 Wistar rats as described previously (Gasparini et al., 2000). Briefly, animals were decapitated after being anesthetized with an intraperitoneal injection of urethane (2 gm/kg). The brain was quickly removed from the skull and placed in an ice-cold artificial CSF (ACSF) containing (in mm): 130 NaCl, 3.5 KCl, 1.2 NaH2PO4, 25 NaHCO3, 1.3 MgCl2, 2 CaCl2, and 11 glucose (saturated with 95% O2 and 5% CO2), pH 7.3–7.4. Transverse hippocampal slices (300–400 μm thick) were cut with a vibratome and stored at room temperature in a holding bath containing the same saline solution as above. After a recovery period of at least 1 hr, an individual slice was transferred to the recording chamber in which it was continuously superfused with oxygenated ACSF at a rate of 2–3 ml/min.
Electrophysiological recordings. EPSPs or EPSCs were recorded at 32°C from CA1 or CA3 pyramidal neurons using the patch-clamp technique in the whole-cell configuration. Patch pipettes were filled with a solution containing (in mm): 125 Cs-methanesulphonate, 10 CsCl, 10 HEPES, 0.6 EGTA, 5N-(2,6-dimethylphenylcarbamoylmethyl)triethylammonium-bromide (Alomone Labs, Jerusalem, Israel), 2 MgATP, and 0.3 NaGTP (resistance of 3–5 MΩ). Bicuculline methiodide (5–10 μm) (Sigma-Aldrich, Milan, Italy), 3-[(R)-2-carboxypiperazin-4-yl]-propyl-1-phosphonic acid (20 μm; Tocris Cookson, Bristol, UK), and tetrodotoxin (10 nm; Affiniti Research Products, Exeter, UK) were routinely added to the bathing solution to block GABAA and NMDA receptors and to reduce polysynaptic activity, respectively. Bipolar twisted NiCr-insulated electrodes were placed in stratum radiatum to activate Schaffer collaterals in the CA1 region and associative–commissural fibers in the CA3 area or in stratum lucidum to stimulate mossy fibers (MFs). Paired stimuli (50 msec interval, 100 μsec duration) were usually applied at 0.05 Hz (or 0.1 Hz in the case of pressure application of drugs). Two sets of experiments were performed with recordings of large (composite) EPSCs and minimal EPSPs–EPSCs, respectively. In the first set of experiments, stimulation intensity was adjusted to evoke composite (multifibers) EPSCs with no response failures. In another set, minimal afferent stimulation was used to activate one or few fibers. The minimal EPSPs–EPSCs were associated with occasional response failures. Their number was usually estimated by visual discrimination. MF–EPSCs were characterized by their fast rise time and by their sensitivity to (2S,2′R,3′R)-2-(2′,3′-dicarboxycyclopropyl)glycine (DCG-IV) (1 μm; Tocris Cookson), a selective agonist of metabotropic glutamate receptors (mGluRs) 2/3, known to selectively block mossy fibers transmission (Kamiya et al., 1996), whereas associative–commissural fiber EPSPs were characterized by their slower rise time and their resistance to DCG-IV (Berretta et al., 2000). Because the effects of NiCl2 on MF–EPCSs and associative–commissural fiber–EPSCs were very similar, data obtained from activation of these two inputs were pooled together.
NiCl2 was applied either in the bath via a three-way tap system or by pressure (5–10 psi) from a glass pipette (outer diameter, 5–10 μm) positioned close to the recording cell. In the bath, NiCl2 was applied at concentrations of 30–50 μm and usually started to produce an effect after 2–3 min. Therefore, responses obtained during bath application of NiCl2 were averaged only starting from the third minute of NiCl2 application. Lower concentrations of NiCl2 (10 μm) did not produce any effect. We did not use higher (100 μm) concentrations of this divalent cation to avoid interference with other types of channels. Pressure application facilitated accessibility of NiCl2 to the cell membrane and reduced the application time (Miledi and Thies, 1971). In these experiments, the concentration of NiCl2 in the pipette was raised 10 times to obtain the same blocking effect as with bath application. ω-Conotoxin-MVIIC (Bachem AG, Bubendorf, Switzerland) and SNX 482 (gift of G. Miljanich and L. Nadasdi, Elan Pharmaceuticals Inc., Menlo Park, CA) were applied by pressure. In some of these experiments, when two inputs (MFs and associative–commissural fibers) were alternatively activated, drugs delivered through the pressure pipette were found to affect, in the majority of the cases, only EPSCs evoked by stimulation of nerve fibers running closer to the tip of the pipette. A subsequent displacement of the pipette closer to the other afferent pathway was able to suppress EPSCs elicited by stimulation of the second input.
Rise and decay time constants were evaluated on average responses, using a single exponential fit. Paired-pulse facilitation (PPF) was measured as the ratio between the amplitude of the second and first response before and during drug application after averaging all respective traces. In some (n = 21) experiments, the coefficient of variation (CV) of response amplitude was determined as CV = SD/mean, and its inverse squared value (CV−2) was calculated. This measure is traditionally used to detect changes in presynaptic transmitter release (Katz, 1969; Voronin, 1993; Chavez-Noriega and Stevens, 1994) (for limitations, see Faber and Korn, 1991).
Data are presented as mean ± SEM. Statistical comparisons were made with the use of paired t test or Wilcoxon signed rank test (p < 0.05 was taken as significant).
RESULTS
NiCl2 reduces composite EPSCs evoked by stimulation of mossy or associative–commissural fibers
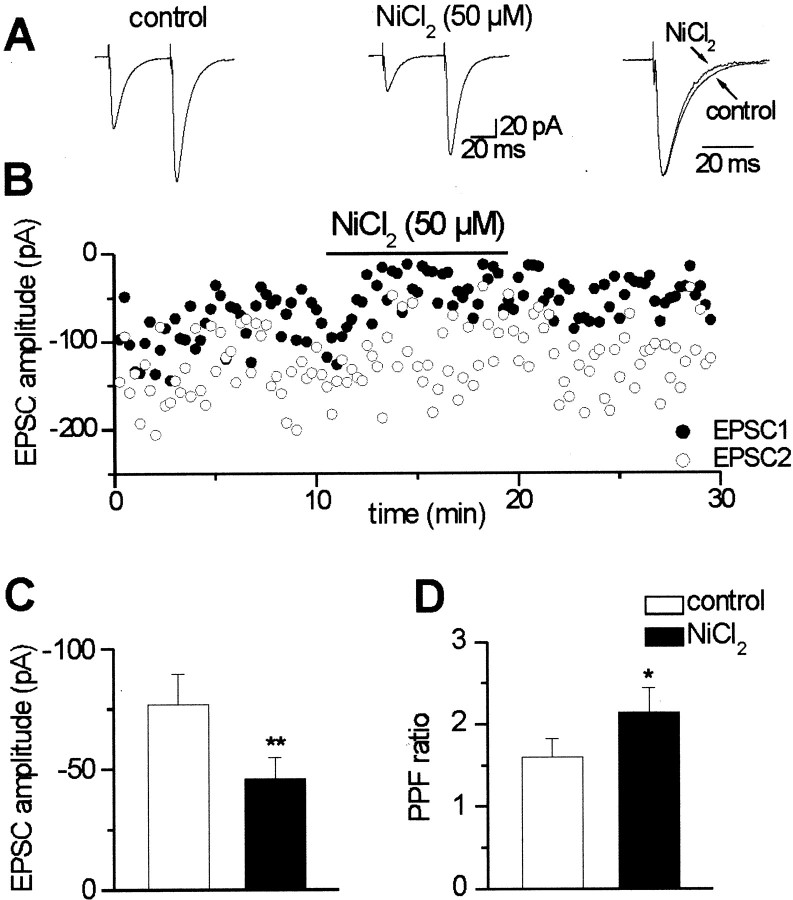
Bath application of NiCl2 at concentrations (30–50 μm) known to block some R-type calcium channels (Randall and Tsien, 1995; Tottene et al., 2000) significantly (p < 0.005) depressed the mean peak amplitude of composite EPSCs, elicited by stimulation of mossy or associative–commissural fibers in CA3 pyramidal neurons from 77.0 ± 12.6 to 45.9 ± 9.0 pA (n = 5) (Fig.1). NiCl2 induced also a significant (p < 0.05) increase in the paired-pulse facilitation ratio from 1.62 ± 0.22 to 2.14 ± 0.30 (n = 4) (Fig. 1D). The effect of NiCl2 was not associated with significant changes in holding current (values before and during NiCl2 were −123.4 ± 14.8 and −130.9 ± 12.6 pA, respectively; p > 0.05) or membrane input resistance (293.5 ± 9.1 and 287.4 ± 14.8 MΩ, before and during NiCl2 application, respectively;p > 0.5). The kinetic properties of the EPSCs before and during NiCl2 application were examined in five cells. As exemplified in Figure 1A, no significant (p > 0.5) modifications in the rise or decay time constants were detected (the mean rise and decay time constants were 3.5 ± 0.5 and 3.4 ± 0.5 msec or 12.6 ± 2.3 and 11.0 ± 1.2 msec before and during NiCl2, respectively). As illustrated in Figure1B, the effects of NiCl2 were only partially reversible.
Fig. 1.
NiCl2 reduces the amplitude of composite EPSCs. A, Average of EPSCs evoked in a CA3 neuron by stimulation of mossy fibers in control conditions (40 traces;left) and in the presence of NiCl2 (24 traces; middle). On the right, EPSCs (to the first stimulus) recorded in control conditions and in the presence of NiCl2 have been averaged, normalized, and superimposed.B, Plot of EPSC1 (filled symbols) and EPSC2 (open symbols) amplitude versus time before, during (horizontal bar), and after application of NiCl2. C, Mean peak amplitude of the first EPSC before (white column) and during (black column) superfusion of NiCl2 (n= 5). D, Mean paired-pulse facilitation ratio in control conditions and during NiCl2 application (n = 4). In this and the following figures, error bars refer to SEM. *p < 0.05; **p < 0.005.
In three cases in which NiCl2 was tested on composite EPSCs evoked in CA1 neurons by stimulation of Schaffer collaterals, it reduced the mean amplitude EPSCs by 26.8 ± 14.7% (n = 3).
Pressure application of NiCl2 induces a reversible reduction of composite EPSCs
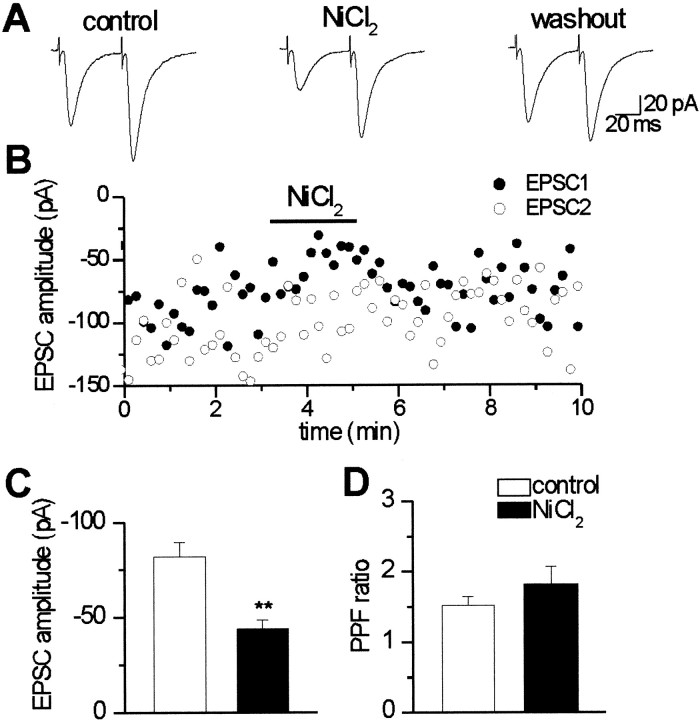
Poor accessibility of divalent cations to the cell membrane may account for the slow onset and the only partial washout of NiCl2 effects (Miledi and Thies, 1971). To facilitate accessibility and to reduce the time of application, in another set of experiments, NiCl2 was applied by pressure from a glass pipette positioned close to the recording cell. To obtain the same inhibition as with bath application, it was necessary to raise 10 times the concentration of NiCl2 in the pipette, which therefore was 300 μm. In the example shown in Figure2, pressure application of NiCl2 reduced the peak amplitude of the EPSCs evoked in a CA1 pyramidal cell by stimulation of the Schaffer collateral by 45.3%, and this effect was almost completely reversed during washout. NiCl2 produced also an increase in the paired-pulse facilitation ratio from 1.4 to 2.1. On average, in three CA1 cells, NiCl2 reduced the peak amplitude of EPSCs evoked by Schaffer collateral stimulation by 40.5 ± 5.2% and increased the paired-pulse facilitation ratio from 1.7 ± 0.1 to 2.2 ± 0.3 (data not shown). In 10 CA3 neurons, NiCl2 significantly (p < 0.001) reduced the mean amplitude of EPSCs evoked by mossy fiber stimulation by 46.2 ± 2.4% and increased the paired-pulse facilitation ratio from 1.5 ± 0.1 to 1.8 ± 0.3 (Fig.2C,D).
Fig. 2.
Pressure application of NiCl2reversibly reduces the peak amplitude of composite EPSCs evoked in a CA1 pyramidal neuron by stimulation of the Schaffer collateral.A, Average of 10 EPSCs recorded in control conditions, in the presence of NiCl2, and during washout. NiCl2 reduced the amplitude of the first EPSC from 77.7 to 42.5 pA. B, The amplitudes of EPSC1 (filled symbols) and EPSC2 (open symbols) are plotted against time before, during (horizontal bar), and after application of NiCl2. The concentration of NiCl2 in the pressure pipette was 300 μm. C, Mean peak amplitude of EPSC1 evoked in CA3 neurons by stimulation of mossy fibers in control conditions (white column) and during (black column) superfusion of NiCl2(n = 10). D, Mean paired-pulse facilitation ratio of mossy fiber EPSCs in control conditions and during NiCl2 application (n = 10). **p < 0.001.
The neurotoxin SNX 482 depresses EPSCs evoked by stimulation of the mossy and associative–commissural fibers
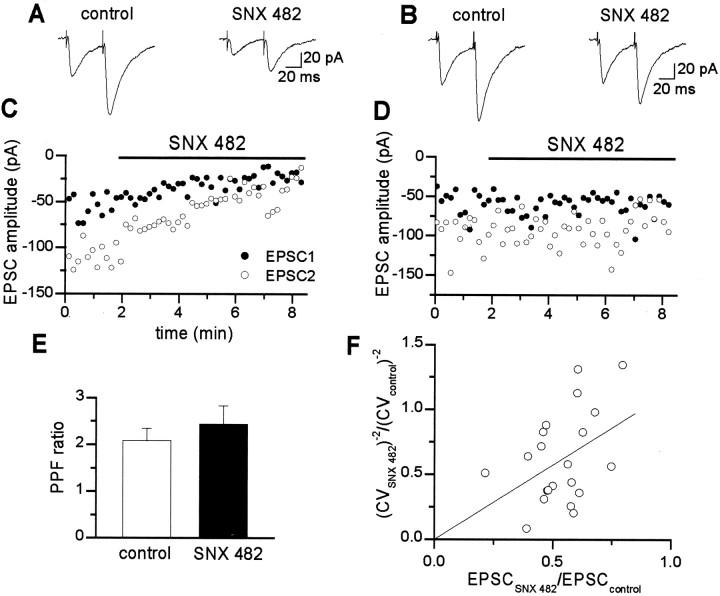
SNX 482, a toxin isolated from the tarantula Histerocrates gigas venom, at low concentrations, is a selective antagonist of recombinant VDCCs containing α1E subunits and inhibits some native R-type channels (Newcomb et al., 1998; Wang et al., 1999; Tottene et al., 2000). To see whether R-type calcium channels containing the α1E subunit contribute to glutamate release in the hippocampus, SNX 482 at 0.3 or 1 μm was applied by pressure from a pipette located close to the mossy fibers in the vicinity of the patched cell. These concentrations should be equivalent to 30 and 100 nm toxin applied by bath perfusion (see Materials and Methods). Figure 3, A andC, shows the effects of SNX 482 (1 μm) on the amplitude of EPSCs evoked in a CA3 neuron by mossy fiber stimulation. The toxin reduced the peak amplitude of the EPSC by 62.7%. The effect of the toxin was localized; EPSCs evoked in the same neuron by stimulation of the associative–commissural fibers were unaffected (Fig.3B,D). The mean peak amplitude of the associative–commissural EPSC was 59.0 and 58.8 pA before and after SNX 482, respectively. However, when the pressure pipette was moved closer to the associative–commissural fiber terminals, a 54.9% reduction of EPSCs amplitude was observed (data not shown). Overall, in seven CA3 cells, SNX 482 (1 μm) produced a reduction of the peak EPSC amplitude of 55.9 ± 4.5%. A slightly weaker effect on EPSC amplitude (42.3 ± 2.9% reduction) was induced by lower concentrations of SNX 482 (0.3 μm; n = 14). The blocking effect produced by the toxin was very similar to that obtained with NiCl2 and was irreversible. The decrease in the mean EPSC amplitude produced by SNX 482 (1 μm) was accompanied by an increase in the paired-pulse facilitation ratio in four of seven cells tested. However, the mean increase in the PPF ratio did not reach statistical significance (Fig. 3E). Therefore, to further test the involvement of presynaptic mechanisms in SNX 482 action, an additional analysis based on calculation of the inverse squared coefficient of variation of response amplitude (CV−2; see Materials and Methods) was performed. CV−2 decreased in all cells tested with 1 μm drug concentration (n = 7) and in the majority of the cells tested with 0.3 μm (n = 11/14) (Fig.3F). Importantly, the reduction of CV−2 produced by SNX 482 significantly correlated with the decrease in the mean amplitude of EPSCs (r = 0.43; n = 21; p < 0.05; one-tailed test). These observations support a presynaptic site of action of the toxin.
Fig. 3.
Pressure application of the toxin SNX 482 close to mossy fiber terminals reduces the peak amplitude of mossy but not associative–commissural fiber EPSCs. A, Average of composite EPSCs evoked in a CA3 neuron by stimulation of the mossy fibers before (left) and during (right) SNX 482 application. C, Plot of the peak amplitude of EPSC1 (filled symbols) and EPSC2 (open symbols) before and during application of SNX 482 (horizontal bar; concentration of SNX 482 into the pressure pipette, 1 μm). B,D, Similar presentation for EPSCs evoked in the same CA3 neuron by stimulation of associative–commissural fibers. Note that SNX 482 reduced the amplitude of mossy fiber EPSCs but did not affect associative–commissural EPSCs (mean peak amplitude values before and during SNX 482 were 76.7 and 26.5 pA for mossy fiber EPSCs and 59.0 and 58.8 pA for associative–commissural fiber EPSCs). Mean PPF ratio of mossy fiber EPSCs recorded in seven CA3 pyramidal cells before and during toxin application. F, Plot of the ratio of CV−2 (toxin over control) versus relative EPSC amplitudes (expressed as the ratio of the amplitude during SNX 482 application to the baseline amplitude). The regression line has been fitted through the data (least-square approximation; regression coefficient is 0.43; p < 0.05). Eachsymbol represents one cell (n = 21).
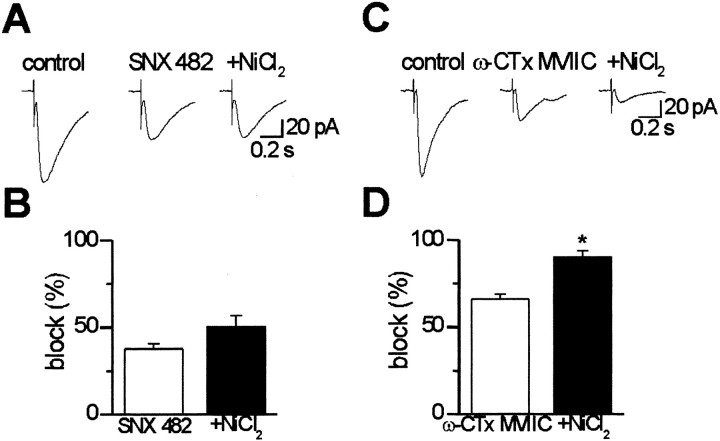
If NiCl2 and SNX 482 act on the same target, namely on the α1E subunit of the R-type of calcium channel, application of NiCl2 (30 μm) after the toxin should not produce any additional effect. Indeed in five cases, bath application of NiCl2 after the toxin did not significantly (p > 0.1) modify the EPSC amplitude, suggesting that both the toxin and the divalent cation act on the same target. The mean peak amplitude reduction of the EPSCs was 37.8 ± 3.1% after application of SNX 482 (0.3 μm) and 50.4 ± 6.4% after addition of NiCl2 (Fig.4A).
Fig. 4.
Occlusion between the effects of SNX 482 and NiCl2 indicates a common site of action. A, Examples of composite EPSC (average of 10 traces) evoked in a CA3 pyramidal neuron by stimulation of mossy fibers before (left) and during (middle) pressure application of SNX 482 (concentration in the pipette, 0.3 μm). Nine minutes after toxin application, superfusion of NiCl2 (30 μm) did not affect the amplitude or shape of the EPSC (right). EPSC peak amplitude values were 94.5, 58.0, and 55.0 pA in control, in SNX 482 and in NiCl2, respectively. B, Summary data from five cells. Differences in percentage block between SNX 482 and NiCl2 are not statistically significant (p > 0.1). C, Composite EPSCs (average of 10 responses) evoked in a CA3 pyramidal neuron by stimulation of mossy fibers before (left) and during (middle) pressure application of ω-CTx-MVIIC (concentration in the pipette, 10 μm). Ten minutes after the toxin, bath application of NiCl2 (30 μm) produced an additional reduction of EPSC amplitude (right). EPSCs peak amplitude values were 103.4, 33.8, and 12.0 pA in control, in ω-CTx-MVIIC, and in NiCl2, respectively. D, Summary data from five cells. Differences in percentage block between ω-CTx-MVIIC and NiCl2 were statistically significant (p < 0.001).
As a control for the above occlusive interaction, we performed additional experiments (n = 5) using ω-conotoxin-MVIIC known to block N- and P/Q-type calcium channels (Wu and Saggau, 1995). In line with previous reports (Wu and Saggau, 1995), pressure application of the toxin (concentration in the pressure pipette, 10 μm) produced a 66.1 ± 2.8% reduction of the peak amplitude EPSC, which was irreversible. When NiCl2 (30 μm) was applied in the bath after ω-conotoxin-MVIIC, an additional significant (p < 0.001) reduction of EPSC amplitude to 90.2 ± 3.6% was observed (Fig. 4B). This indicates that NiCl2 at this concentration suppresses a set of channels different from that blocked by ω-conotoxin-MVIIC. The small EPSC that remains after ω-conotoxin-MVIIC and NiCl2 might be mediated by R-type calcium channels less sensitive to NiCl2, as suggested by previous experiments at the calyx synapses in the rat medial nucleus of the trapezoid body (Wu et al., 1998).
The data presented so far clearly show that presynaptic α1E channels contribute to glutamate release at mossy and associative–commissural fiber synapses. The question then rises whether all presynaptic terminals could have R-type channels commingled with N- and P/Q-type or approximately half of all terminals could have only R-type channels, whereas the other half posses only N- and P/Q-type. To address this point, another set of experiments using minimal stimulation, which presumably activates only a few afferent fibers or even a single fiber, were performed. In these experiments, we used NiCl2 at concentrations (30–50 μm) known to block the same R-type calcium channels inhibited by SNX 482 (see occlusion by SNX 482 on NiCl2 block).
NiCl2 depresses EPSPs or EPSCs evoked by minimal stimulation of the mossy or associative–commissural fibers
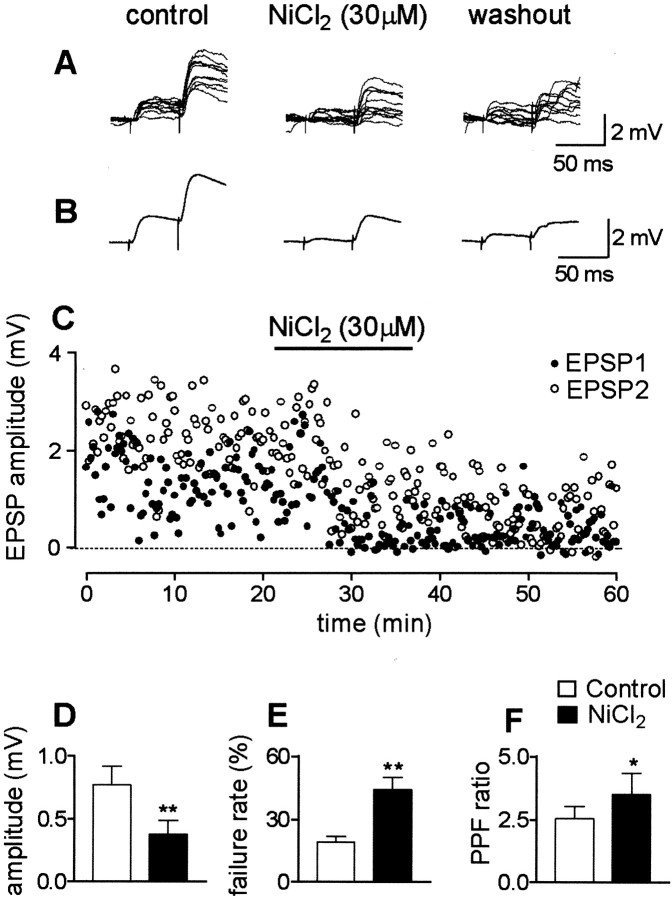
Figure 5(control) exemplifies EPSPs evoked by minimal stimulation of the associative–commissural fibers at resting membrane potentials (ranging from −58 to −63 mV). The EPSPs fluctuated in amplitude from trial to trial with occasional failures. When two stimuli were applied at 50 msec intervals, the second one triggered higher-amplitude responses, with a reduced number of failures. EPSPs were completely blocked by CNQX (10 μm), indicating that they were generated by glutamate acting on non-NMDA receptors (data not shown). Bath application of NiCl2 (30–50 μm) significantly reduced the peak amplitude of the EPSPs evoked by mossy or associative–commissural fibers by ∼50% (Fig. 5D) in 13 of 15 recorded cells (in two cells, NiCl2 was ineffective). Thus, during NiCl2 application, the mean EPSP amplitudes changed from 0.77 ± 0.15 to 0.38 ± 0.11 mV (n = 13; p < 0.001) (Fig.5D). This effect was associated with a significant increase in the number of failures from 19 ± 3 to 44 ± 6% (n = 13; p < 0.001) (Fig.5E) and in the paired-pulse facilitation ratio from 2.5 ± 0.4 to 3.5 ± 0.9 (n = 12; p < 0.05) (Fig. 5F). NiCl2 did not affect membrane input resistance; it was 220 ± 32 and 273 ± 28 MΩ before and during NiCl2 application, respectively (n = 13; p > 0.5).
Fig. 5.
NiCl2 reduces the peak amplitude of EPSPs evoked in a CA3 pyramidal neuron by minimal stimulation of the associative–commissural fibers. A, Ten individual traces obtained by paired stimuli (50 msec interval) delivered to associative–commissural fibers at 0.05 Hz from −60 mV in control conditions, during NiCl2 perfusion, and 13 min after wash. Note the increased number of response failures during NiCl2application. B, Averages of responses obtained before (n = 87), during (n = 60), and 13 min after wash (n = 40). C, Plot of EPSP amplitudes evoked by the first (EPSP1;filled symbols) and second (EPSP2;open symbols) pulse in the paired-pulse paradigm before, during (horizontal bar), and after application of NiCl2. D, Mean peak amplitude of the first EPSP before (white column) and during (black column) superfusion of NiCl2 (n= 13). E, Mean failure rates before (white column) and during (black column) application of NiCl2 (n = 13). F, Mean paired-pulse facilitation ratio in control conditions and during NiCl2 application (n = 12). *p < 0.05; **p < 0.001.
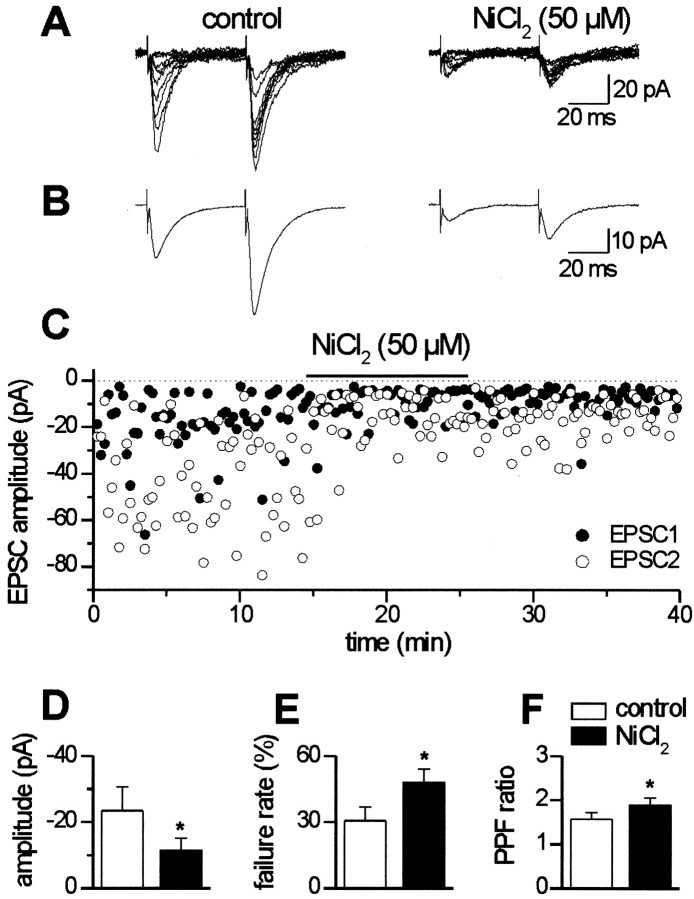
Similar blocking effect of NiCl2 (∼50%) was found in five of six cells clamped at a holding potential of −60 mV. In these cells, EPSCs were evoked by minimal stimulation of MF or associative–commissural fibers in stratum lucidum or radiatum, respectively (Fig. 6). In the presence of NiCl2, a significant reduction in the mean peak amplitude of the EPSCs was detected (from 23.4 ± 7.1 to 11.5 ± 3.7 pA; n = 5; p < 0.05) (Fig.6D). A significant (p < 0.05) increase in the number of failures and paired-pulse facilitation ratio was also found (from 30.6 ± 6.3 to 48.0 ± 6.1% and from 1.67 ± 0.15 to 1.89 ± 0.16%, respectively) (Fig.6E,F). All of these effects were rarely reversible.
Fig. 6.
NiCl2 reduces the peak amplitude of EPSCs evoked in CA3 pyramidal neurons by minimal stimulation of the mossy fibers. A, Ten individual traces of EPSCs recorded from a holding potential of −60 mV, in control conditions and in the presence of NiCl2. Note decreased EPSC amplitudes during NiCl2 application and increased number of response failures after the first pulse. In this experiment, failure rate increased from 18.7 to 58.7%. B, Average EPSCs from the same experiment (n = 56 in control andn = 32 during application of NiCl2). C, Plot of EPSC1 (filled symbols) and EPSC2 (open symbols) amplitude versus time before, during (horizontal bar), and after application of NiCl2. D–F, Mean values of the EPSC1 amplitude (D), failure rate (E), and paired-pulse facilitation ratio (F) before (white columns) and during (black columns) NiCl2 application (n = 5). *p < 0.05.
NiCl2 was able to reduce to approximately the same extent EPSCs evoked in CA1 neurons by minimal stimulation of Schaffer collaterals (from 18.4 ± 8.6 to 7.7 ± 3.9 pA;n = 3/4; data not shown).
In conclusion, although a certain degree of variability of NiCl2 block on single fiber EPSPs–EPSCs was observed in different cells (on average, ∼50%), a complete suppression was never achieved, suggesting that presynaptic terminals bearing only R-type channels are highly unlikely.
DISCUSSION
The present experiments show that R-type VDCCs highly sensitive to both SNX 482 and NiCl2 contribute to fast excitatory transmission in the hippocampus, at mossy and associative–commissural fiber synapses. The first clue that presynaptic VDCCs of the R-type trigger transmitter release has been provided by Wu et al. (1998) in the medial nucleus of the trapezoid body in brainstem slices (calyx of Held synapses). At these synapses, approximately one-quarter of calcium current evoked by a presynaptic action potential was resistant to ω-conotoxin-MVIIC but sensitive to relatively low concentrations of NiCl2 (Randall and Tsien, 1995), supporting the idea that R-type VDCCs cooperate with MVIIC-sensitive N- and P/Q-type to regulate synaptic transmission. In this preparation, the lower effectiveness of VDCC of R- and N-type in triggering release compared with P/Q-type channels could be attributable to the distant location of a substantial fraction of R- and N-type channels from the release sites (Wu et al., 1999). More recently, R-type VDCCs have been found to control both oxytocin release in isolated neurohypophysial terminals (Wang et al., 1999) and the rapid secretory response coupled to exocytotic release of catecholamines in mouse adrenal slice chromaffin cells (Albillos et al., 2000).
Native R-type VDCCs with different biophysical and pharmacological properties have been described in different types of neurons (Tottene et al., 1996; Hilaire et al., 1997; Magnelli et al., 1998; Wu et al., 1998; Wang et al., 1999) and have been shown to be coexpressed in the same type of neuron (Forti et al., 1994; Tottene et al., 1996; Wu et al., 1998; Tottene et al., 2000). Only some of the native R-type VDCCs are inhibited by SNX 482, the first selective (at concentrations <300–500 nm) antagonist of recombinant α1E (CaV 2.3) channels (Newcomb et al., 1998; Wang et al., 1999; Tottene et al., 2000).Tottene et al. (2000) have shown that, in cerebellar granule cells, the component of R-type calcium current inhibited by a low concentration of SNX 482 (fully inhibited by 200 nm toxin) is also highly sensitive to NiCl2 block (fully inhibited by 30 μm NiCl2), whereas the R-type component inhibited by larger concentrations of toxin [IC50 of 500 nm, called SNX 482-resistant component, because at these concentrations SNX 482 is not a selective antagonist of α1E(CaV 2.3) channels] is also less sensitive to NiCl2 block. Both of these components are absent in α1E knock-out mice (Wilson et al., 2000), because the calcium current that remains in cerebellar granule cells of knock-out mice in the presence of ω-agatoxin-IVA (ω-AgaIVA), ω-conotoxin-GVIA, and nimodipine is not inhibited by 1 μm SNX 482 (a concentration that would completely inhibit the so called toxin-resistant component of Tottene et al., 2000). Because both components are suppressed after transfection of the neurons with specific α1E antisense oligonucleotides (Tottene et al., 2000) and are completely absent in α1E knock-out mice (Wilson et al., 2000), the different R-type VDCCs coexpressed in cerebellar granule cells appear to be all encoded by the α1E gene. Alternatively, spliced isoforms of the α1Esubunit most likely account for their different biophysical and pharmacological properties (Schramm et al., 1999; Tottene et al., 2000). The origin of the additional current component not inhibited by very high concentrations of SNX 482 described by Wilson et al. (2000)remains unclear. It is possible that this current may actually be attributable to Q-type channels not completely inhibited by 200 nmω-AgaIVA (Randall and Tsien, 1995).
In the present experiments, the observation that low concentrations of SNX 482 were able to reduce the amplitude of EPSCs to the same extent (∼50%) as low concentrations of NiCl2 strongly favors the conclusion that R-type calcium channels are involved in transmitter release. Additional evidence in favor of R-type VDCCs in fast synaptic transmission was provided by occlusion experiments in which NiCl2 was applied after SNX 482. No additional significant reduction in EPSC amplitude was detected after addition of NiCl2, suggesting that both NiCl2 and the toxin act on the same site. On the contrary, an additional reduction of EPSC amplitude was observed when NiCl2 was applied after ω-CTx-MVIIC, at a saturating concentration known to completely inhibit N- and P/Q-type VDCCs (Castillo et al., 1994; Wu and Saggau, 1995), further supporting a specific action of NiCl2 on R-type channels. These results contradict previous findings that NiCl2, at the same concentration, failed to modify field EPSPs evoked in the CA1 hippocampal region by Schaffer collateral stimulation (Oliet et al., 1997). This apparent discrepancy may be attributable to differences in the experimental conditions. To obtain a mGluR form of long-term depression, a Ca2+/Mg2+ratio of 4:4 mm was used in experiments by Oliet et al. (1997) (instead of 2:1.3 mm used in the present experiments). This condition that reduces basal synaptic transmission to 62% may have affected the driving force for calcium and cooperativity within presynaptic calcium channels.
Within the hippocampus, using in situ hybridization and immunocytochemical techniques, high levels of the α1E mRNA transcript and protein have been detected mainly postsynaptically at the somatic and dendritic level (Yokoyama et al., 1995; Day et al., 1996). The postsynaptic localization of the α1E protein is further supported by electrophysiological experiments showing the presence of NiCl2-sensitive R-type calcium channels on the dendrites of CA1 pyramidal neurons. These channels together with the T-type are supposed to contribute to action potential burst firing (Christie et al., 1995; Magee and Johnston, 1995; Kavalali et al., 1997; Magee and Carruth, 1999). However, immunoreactivity for class E calcium channels has been detected also presynaptically at the level of mossy fiber terminals in stratum lucidum, suggesting, in agreement with the present findings, a major role of these channels in cell communication (Day et al., 1996). Although we cannot entirely exclude that low concentrations of NiCl2 or SNX 482 may affect T- or R-type calcium channels located postsynaptically, the following observations support the conclusion that their blocking action on EPSP–EPSC amplitude can be mostly accounted for by a presynaptic depression of transmitter release.
(1) NiCl2 did not modify membrane potential, input resistance, or EPSCs kinetics. (2) NiCl2and SNX 482 reduced EPSPs–EPSCs evoked from −60 mV, a holding potential at which most low-voltage-activated calcium channels are inactivated. (3) In the case of alternative activation of two distinct inputs to the same cell, the toxin affected preferentially EPSCs evoked by stimulation of fibers localized closer to the pressure pipette, leaving the others almost unaffected. In the case of a postsynaptic action, SNX 482 should have affected EPSCs evoked by both inputs. (4) In the presence of NiCl2, the reduction of the amplitude of EPSPs–EPSCs evoked by minimal stimulation was associated with a significant increase in the number of failures. (5) In the presence of SNX 482, the decrease in the amplitude of EPSC was accompanied by a reduction of CV−2, which is a traditional measure of transmitter release. (6) The reduction of EPSP–EPSC amplitude by NiCl2 and SNX 482 was associated with an increase in the paired-pulse facilitation ratio, another traditional index of changes in presynaptic release probability.
Regarding the problem of whether R-type calcium channels are commingled or not with N and P/Q on the same presynaptic terminal, our data on single fiber EPSPs–EPSCs favor the hypothesis of a colocalization of multiple type of calcium channels on the same nerve ending. In fact, single fiber EPSP–EPSC reduction by NiCl2 varied from 51 to 59% but never achieved 100%, as expected for R-type calcium channels alone. Moreover, although the present experiments do not allow to reliably estimate the fraction of presynaptic calcium entry because of α1E channels, assuming that transmitter release is proportional to [Ca2+]m and m = 3.5 (according to Wu and Saggau, 1995, at least for CA3-CA1 synapses), we can estimate that ∼15% of R-type calcium channels contribute to glutamate release. This value is slightly lower than that of 25% given by Wu and Saggau (1995) forω-CTx-MVIIC-resistant calcium channels. This difference could be explained by several factors, including differences in m value that can vary from synapse to synapse and in the same synapse in relation to the localization of calcium channels, more or less close to the active zones (Wu et al., 1999).
In conclusion, our data demonstrate that R-type calcium channels with pharmacological properties similar to those encoded by the α1E gene highly sensitive to NiCl2 and to SNX 482 substantially contribute to fast glutamatergic synaptic transmission at mossy and associative–commissural fiber synapses.
Footnotes
This work was supported by grants from Ministero Università e Ricerca Scientifica e Tecnologica to E.C. and D.P., International Association for the Promotion of Cooperation with Scientists from the New Independent States of the Former Soviet Union to E.C. and L.L.V., and Wellcome Trust to L.L.V. We thank H. Arechiga for participating in some experiments and J. C. Magee for carefully reading this manuscript.
Correspondence should be addressed to Enrico Cherubini, Neuroscience Program and Istituto Nazionale Fisica della Materia Unit, International School for Advanced Studies, Via Beirut 2-4, 34014 Trieste, Italy. E-mail: cher@sissa.it.
S. Gasparini's present address: Louisiana State University Neuroscience Center, 2020 Gravier Street, New Orleans, LA 70112.
REFERENCES
- 1.Albillos A, Neher E, Moser T. R-type Ca2+ channels are coupled to the rapid component of secretion in mouse adrenal slice chromaffin cells. J Neurosci. 2000;20:8323–8330. doi: 10.1523/JNEUROSCI.20-22-08323.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Augustine GJ, Charlton MP. Calcium dependence of presynaptic calcium current and postsynaptic response at the squid giant synapse. J Physiol (Lond) 1986;381:619–640. doi: 10.1113/jphysiol.1986.sp016347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berretta N, Rossokhin AV, Kasyanov AM, Sokolov MV, Cherubini E, Voronin LL. Postsynaptic hyperpolarization increases the strength of AMPA-mediated synaptic transmission at large synapses between mossy fibres and CA3 pyramidal cells. Neuropharmacology. 2000;39:2288–2301. doi: 10.1016/s0028-3908(00)00076-9. [DOI] [PubMed] [Google Scholar]
- 4.Castillo PE, Weisskopf MG, Nicoll RA. The role of Ca2+ channels in hippocampal mossy fiber synaptic transmission and long-term potentiation. Neuron. 1994;12:261–269. doi: 10.1016/0896-6273(94)90269-0. [DOI] [PubMed] [Google Scholar]
- 5.Chavez-Noriega LE, Stevens CF. Increased transmitter release at excitatory synapses produced by direct activation of adenylate cyclase in rat hippocampal slices. J Neurosci. 1994;14:310–317. doi: 10.1523/JNEUROSCI.14-01-00310.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christie BR, Eliot LS, Ito K, Miyakawa H, Johnston D. Different Ca2+ channels in soma and dendrites of hippocampal pyramidal neurons mediate spike-induced Ca2+ influx. J Neurophysiol. 1995;73:2553–2557. doi: 10.1152/jn.1995.73.6.2553. [DOI] [PubMed] [Google Scholar]
- 7.Craig PJ, Beattie RE, Folly EA, Banerjee MD, Reeves MB, Priestley JV, Carney SL, Sher E, Perez-Reyes E, Volsen SG. Distribution of the voltage-dependent calcium channel α1G subunit mRNA and protein throughout the mature rat brain. Eur J Neurosci. 1999;11:2949–2964. doi: 10.1046/j.1460-9568.1999.00711.x. [DOI] [PubMed] [Google Scholar]
- 8.Day NC, Shaw AL, McCormack AL, Craig PJ, Smith W, Beattie R, Williams TL, Ellis SB, Ince PG, Harpold MM, Lodge D, Volsen SG. Distribution of α1A, α1B and α1E voltage-dependent calcium channel subunits in the human hippocampus and parahippocampal gyrus. Neuroscience. 1996;71:1013–1024. doi: 10.1016/0306-4522(95)00514-5. [DOI] [PubMed] [Google Scholar]
- 9.Dodge FA, Rahamimoff R. Co-operative action of calcium ions in transmitter release at the neuromuscular junction. J Physiol (Lond) 1967;193:419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunlap K, Luebke JI, Turner TJ. Exocytotic Ca2+ channels in mammalian central neurons. Trends Neurosci. 1995;18:89–98. [PubMed] [Google Scholar]
- 11.Faber DS, Korn H. Applicability of the coefficient of variation method for analyzing synaptic plasticity. Biophys J. 1991;60:1288–1294. doi: 10.1016/S0006-3495(91)82162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forti L, Tottene A, Moretti A, Pietrobon D. Three novel types of voltage-dependent calcium channels in rat cerebellar neurons. J Neurosci. 1994;14:5243–5256. doi: 10.1523/JNEUROSCI.14-09-05243.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gasparini S, Saviane C, Voronin LL, Cherubini E. Silent synapses in the developing hippocampus: lack of functional AMPA receptors or low probability of glutamate release? Proc Natl Acad Sci USA. 2000;97:9741–9746. doi: 10.1073/pnas.170032297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hilaire C, Diochot S, Desmadryl G, Richard S, Valmier J. Toxin-resistant calcium currents in embryonic mouse sensory neurons. Neuroscience. 1997;80:267–276. doi: 10.1016/s0306-4522(97)00101-2. [DOI] [PubMed] [Google Scholar]
- 15.Huguenard JR. Low-threshold calcium currents in central nervous system neurons. Annu Rev Physiol. 1996;58:329–348. doi: 10.1146/annurev.ph.58.030196.001553. [DOI] [PubMed] [Google Scholar]
- 16.Kamiya H, Shinozaki H, Yamamoto C. Activation of metabotropic glutamate receptors type 2/3 suppresses transmission at rat hippocampal mossy fiber synapses. J Physiol (Lond) 1996;493:447–455. doi: 10.1113/jphysiol.1996.sp021395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katz B. The release of neural transmitter substance. Thomas; Springfield, IL: 1969. [Google Scholar]
- 18.Kavalali ET, Zhuo M, Bito H, Tsien RW. Dendritic Ca2+ channels characterized by recordings from isolated hippocampal dendritic segments. Neuron. 1997;18:651–663. doi: 10.1016/s0896-6273(00)80305-0. [DOI] [PubMed] [Google Scholar]
- 19.Luebke J, Dunlap K, Turner TJ. Multiple calcium channel types control glutamatergic synaptic transmission in the hippocampus. Neuron. 1993;11:895–902. doi: 10.1016/0896-6273(93)90119-c. [DOI] [PubMed] [Google Scholar]
- 20.Magee JC, Carruth M. Dendritic voltage-gated ion channels regulate the action potential firing mode of hippocampal CA1 pyramidal neurons. J Neurophysiol. 1999;82:1895–1901. doi: 10.1152/jn.1999.82.4.1895. [DOI] [PubMed] [Google Scholar]
- 21.Magee JC, Johnston D. Characterization of single voltage-gated Na+ and Ca2+ channels in apical dendrites of rat CA1 pyramidal neurons. J Physiol (Lond) 1995;487:67–90. doi: 10.1113/jphysiol.1995.sp020862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magnelli V, Baldelli P, Carbone E. Antagonists-resistant calcium currents in rat embryo motoneurons. Eur J Neurosci. 1998;10:1810–1825. doi: 10.1046/j.1460-9568.1998.00178.x. [DOI] [PubMed] [Google Scholar]
- 23.Miledi R, Thies RE. Tetanic and post-tetanic rise in frequency of miniature end-plate potentials in low calcium solution. J Physiol (Lond) 1971;212:245–257. doi: 10.1113/jphysiol.1971.sp009320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mintz IM, Sabattini BL, Regehr WG. Calcium control of transmitter release at a cerebellar synapse. Neuron. 1995;15:675–688. doi: 10.1016/0896-6273(95)90155-8. [DOI] [PubMed] [Google Scholar]
- 25.Newcomb R, Szoke B, Palma A, Wang G, Chen X, Hopkins W, Cong R, Miller J, Tarczy-Hornoch K, Loo JA, Dooley DJ, Nadasdi L, Tsien RW, Lemos JR, Miljanich G. A selective peptide antagonist of the class E calcium channel from the venom of tarantula, Hysterocrates gigas. Biochemistry. 1998;37:15353–15362. doi: 10.1021/bi981255g. [DOI] [PubMed] [Google Scholar]
- 26.Oliet SHR, Malenka RC, Nicoll RA. Two distinct forms of long term depression coexist in CA1 hippocampal pyramidal cells. Neuron. 1997;18:969–982. doi: 10.1016/s0896-6273(00)80336-0. [DOI] [PubMed] [Google Scholar]
- 27.Randall AD, Tsien RW. Pharmacological dissection of multiple types of Ca2+ channel currents in rat cerebellar granule neurons. J Neurosci. 1995;15:2995–3012. doi: 10.1523/JNEUROSCI.15-04-02995.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schramm M, Vajna R, Pereverzev A, Tottene A, Klockner U, Pietrobon D, Hescheler J, Schneider T. Isoforms of α1E voltage-gated calcium channels in rat cerebellar granule cells: detection of major calcium channel α1-transcripts by reverse transcription-polymerase chain reaction. Neuroscience. 1999;92:565–575. doi: 10.1016/s0306-4522(99)00013-5. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi T, Momiyama A. Different types of calcium channels mediate central synaptic transmission. Nature. 1993;366:156–158. doi: 10.1038/366156a0. [DOI] [PubMed] [Google Scholar]
- 30.Tottene A, Moretti A, Pietrobon D. Functional diversity of P-type and R-type calcium channels in rat cerebellar neurons. J Neurosci. 1996;16:6353–6363. doi: 10.1523/JNEUROSCI.16-20-06353.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tottene A, Volsen S, Pietrobon D. α1E subunits form the pore of three cerebellar R-type calcium channels with different pharmacological and permeation properties. J Neurosci. 2000;20:171–178. doi: 10.1523/JNEUROSCI.20-01-00171.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Voronin LL. On the quantal analysis of hippocampal long-term potentiation and related phenomena of synaptic plasticity. Neuroscience. 1993;56:275–304. doi: 10.1016/0306-4522(93)90332-a. [DOI] [PubMed] [Google Scholar]
- 33.Wang G, Dayanithi G, Newcomb R, Lemos JR. An R-type Ca2+ current in neurohypophysial terminals preferentially regulates oxytocin secretion. J Neurosci. 1999;19:9235–9241. doi: 10.1523/JNEUROSCI.19-21-09235.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wheeler DB, Randall A, Tsien RW. Roles of N-type and Q-type Ca2+ channels in supporting hippocampal synaptic transmission. Science. 1994;264:107–111. doi: 10.1126/science.7832825. [DOI] [PubMed] [Google Scholar]
- 35.Wilson SM, Toth PT, Oh SB, Gillard SE, Volsen S, Ren D, Phipson LH, Lee EC, Fletcher CF, Tessarollo L, Copeland NG, Jenkins NA, Miller RJ. The status of voltage-dependent calcium channels in α1E knock-out mice. J Neurosci. 2000;20:8566–8571. doi: 10.1523/JNEUROSCI.20-23-08566.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu LG, Saggau P. Presynaptic calcium is increased during normal synaptic transmission and paired-pulse facilitation, but not in long-term potentiation in area CA1 of hippocampus. J Neurosci. 1994;14:645–654. doi: 10.1523/JNEUROSCI.14-02-00645.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu LG, Saggau P. Block of multiple presynaptic calcium channel types by ω-conotoxin-MVIIC at hippocampal CA3 to CA1 synapses. J Neurophysiol. 1995;73:1965–1972. doi: 10.1152/jn.1995.73.5.1965. [DOI] [PubMed] [Google Scholar]
- 38.Wu LG, Saggau P. Presynaptic inhibition of elicited neurotransmitter release. Trends Neurosci. 1997;20:204–212. doi: 10.1016/s0166-2236(96)01015-6. [DOI] [PubMed] [Google Scholar]
- 39.Wu LG, Borst JG, Sakmann B. R-type Ca2+ currents evoke transmitter release at a rat central synapse. Proc Natl Acad Sci USA. 1998;95:4720–4725. doi: 10.1073/pnas.95.8.4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu LG, Westenbroek RE, Borst JG, Catterall WA, Sakmann B. Calcium channel types with distinct presynaptic localization couple differentially to transmitter release in single calix-type synapses. J Neurosci. 1999;19:726–736. doi: 10.1523/JNEUROSCI.19-02-00726.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yokoyama CT, Westenbroek RE, Hell JW, Soong TW, Snutch TP, Catterall WA. Biochemical properties and subcellular distribution of the neuronal class E calcium channel α1 subunit. J Neurosci. 1995;15:6419–6432. doi: 10.1523/JNEUROSCI.15-10-06419.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang JF, Randall AD, Ellinor PT, Horne WA, Sather WA, Tanabe T, Schwarz TL, Tsien RW. Distinctive pharmacology and kinetics of cloned neuronal Ca2+ channels and their possible counterparts in mammalian CNS neurons. Neuropharmacology. 1993;32:1075–1088. doi: 10.1016/0028-3908(93)90003-l. [DOI] [PubMed] [Google Scholar]