Abstract
Although it is generally accepted that the acid–base ratio of tissue, as represented by the pH, is strictly regulated to maintain normal function, recent studies in the mammalian nervous system have shown that neuronal activity can result in significant shifts in pH. In the mammalian retina, many cellular phenomena, including neuronal activity, are regulated by a circadian clock. We thus investigated whether a clock regulates retinal pH, using pH-sensitive microelectrodes to measure the extracellular pH (pHo) of the in vitro rabbit retina in the subjective day and night, that is, under conditions of constant darkness. These measurements demonstrated that a circadian clock regulates the pHo of the rabbit retina so that the pHo is lower at night than in the day. This day/night difference in retinal pHo was observed when the rabbits were maintained on a normal light/dark cycle and after they were maintained on a light/dark cycle that was phase-delayed by 9 hr. Continuous recordings of retinal pHo around subjective dusk indicated that the change from daytime to nighttime pHo is relatively fast and suggested that the clock that regulates pHo is located in the retina. The lowest pHorecorded in the retina in both the day and night was in the vicinity of the inner segments of photoreceptor cells, supporting the idea that photoreceptors serve as the primary source of protons. The circadian-induced shift in pHo was several times greater than light-induced pHo changes. These findings suggest that a circadian clock in the mammalian retina regulates retinal pH.
Keywords: protons, circadian rhythm, energetic metabolism, photoreceptors, acid–base, diurnal, mammal, ion-selective microelectrodes
Although all living tissue produces acid because of metabolic activity, it has been accepted that the acid–base ratio of tissue, as represented by the pH, is strictly regulated to maintain normal function. In fact, a loss of control of pH can have devastating consequences, because a wide variety of neuronal processes from regulatory enzymes to ion channels are highly sensitive to acid–base dynamics. In the mammalian nervous system, however, recent studies have shown that neuronal activity can result in shifts in pH that are large enough to influence enzyme and channel functions (Chesler and Kaila, 1992). For example, in the mammalian retina, light stimulation produces changes in the pH of the extracellular space (Yamamoto et al., 1992).
In addition to the effects of light and dark adaptation, many cellular phenomena in the mammalian retina are now known to be regulated by a circadian clock (Reme et al., 1991; Cahill and Besharse, 1995), a type of biological oscillator that is intrinsic to neural tissue and has persistent rhythmicity with a period of ∼24 hr in the absence of external timing cues (e.g., constant darkness) (Block et al., 1993). For example, in the mammalian retina, a circadian clock regulates dopamine content (Wirz-Justice et al., 1984), melatonin production and release (Tosini and Menaker, 1996), photoreceptor disk shedding (La Vail and Ward, 1978), and neuronal light responses (Mangel and Wang, 1997). Because neuronal activity in the mammalian retina is regulated by a circadian clock, we thus investigated whether a circadian clock regulates the pH of the rabbit retina. Our findings suggest that a circadian clock in the mammalian retina regulates retinal pH. Thus, an intrinsic oscillator in neural tissue modulates metabolic activity and pH as part of normal daily function.
MATERIALS AND METHODS
Experiments were performed on superfused retinas obtained from pigmented adult rabbits weighing 2.0–4.0 kg. The rabbits were maintained for at least 10 d on a 12 hr light/dark cycle or a similar cycle that had been phase-delayed by 9 hr (Fig. 2). That is, “lights on” occurred at 5 A.M. in most of the experiments but at 2 P.M. in some experiments (Fig. 2). The rabbits were then kept in constant darkness for at least 24 hr before an experiment; all experiments were conducted during the second circadian cycle of constant darkness. The care and use of the rabbits were in accordance with federal and institutional guidelines. The rabbits were deeply anesthetized with urethane (1.5 gm/kg, i.p.), and an eye was enucleated after additional local intraorbital injections of 2% xylocaine. Surgery was performed under dim red illumination. Isolated eyecups were placed in a superfusion chamber, vitreal side up, and superfused in the dark for up to 4 hr at 35.5–36.0°C. The superfusion solution was made according to the formula of Ames and Nesbett (1981), including organics and amino acids but excluding horse serum. The superfusate flowed by gravity at ∼3 ml/min and was constantly bubbled by a gas mixture of CO2 and O2. The proportion of the gases was adjusted so that the warmed solution in the experimental chamber had a pH of 7.8.
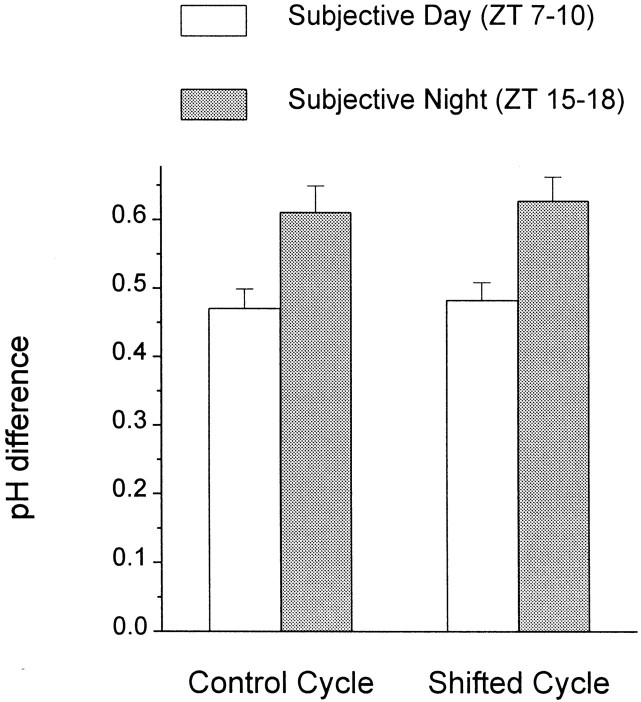
Fig. 2.
The average difference between retinal and superfusate pH exhibits a circadian rhythm. Before an experiment, the rabbits were entrained for at least 10 d to a 12 hr light/dark cycle or a 12 hr light/dark cycle that had been phase-delayed by 9 hr. At the start of an experiment, the rabbits were placed in constant darkness for at least 24 hr, after which the retinas were prepared in either the subjective day or night. The time of subjective day (ZT 7–10) or night (ZT 15–18) that is indicated corresponds to the time that pH measurements were made. Retinal pHo was defined as the lowest measured pHo in the retina. Each data point represents mean value ± SEM for 7–12 measurements.
Extracellular pH (pHo) in the retina was measured with double-barreled pH-selective microelectrodes based on the Hydrogen Ionophore I–Cocktail B (Fluka, Buchs, Switzerland). The microelectrodes were constructed using a modified version of a procedure described previously (Dmitriev et al., 1999b; Dmitriev and Mangel, 2000). Briefly, the microelectrodes were pulled from borosilicate “theta” glass capillaries (WPI, Sarasota, FL). After the glass was pulled, the reference electrode barrel was backfilled with distilled water. Several micropipettes were exposed overnight to a silane atmosphere obtained by dropping 0.4–0.5 ml of a solution of dimethyldichlorosilane in carbon tetrachloride (10% in volume, both from Sigma, St. Louis, MO) in a tightly closed jar at room temperature. After silanization, the ion-selective barrel was backfilled with 0.5 μl of the Fluka mixture. The tip of the microelectrode was broken gently on a piece of soft paper, thus allowing the ion exchanger to move spontaneously to the tip of the microelectrode. Finally, the distilled water in the reference barrel was replaced by backfilling it with a solution containing (in mm): 140.0 NaCl, 5.0 KCl, 2.0 CaCl2, and 5.0 Tris, pH 7.5. The same solution was also used to fill the remainder of the ion-selective barrel. Microelectrodes that were used for the experiments had tip diameters of ∼5–10 μm and were shaped like medicine needles. The resistance of the pH-selective barrel was 5–10 GΩ, whereas the reference barrel had a resistance between 20 and 50 MΩ, depending on the diameter of the tip. Microelectrodes were calibrated in the experimental chamber at 36°C before and after measurements on the retinal tissue. The calibration solutions contained the same concentrations of the principal cations (Na+, K+, Ca2+, Mg2+, all as Cl-salts) found in Ames medium, plus 5 mm Tris. HCl was added to obtain pH values of 7.0 and 8.0. All microelectrodes had slopes that were always 56 mV/pH unit or better. Absolute pH values in the superfusate and retina were determined by calibrating measurements of the pH microelectrode with those of a commercially available glass pH electrode (Corning, Corning, NY) placed in the superfusate.
The electroretinogram (ERG) was recorded with a pair of Ag–AgCl macroelectrodes, one positioned on the vitreal side of the eyecup and the second in contact with the sclera. When an ion-selective microelectrode was advanced through the retina, the reference barrel of the microelectrode was used for recording the proximal and distal parts of the intraretinal ERG via the macroelectrodes in the bath and on the scleral surface, respectively. The position of the ion-selective microelectrode in the tissue could be estimated using the proximal and distal intraretinal ERG. In addition, fast changes of the reference barrel potential, which occurred on penetration of the inner and outer limiting membranes, also aided in determining microelectrode position in the retina. Voltage signals were digitized and sent to computer memory via a DigiData 1200 Interface (Axon Instruments, Foster City, CA) under control of AxoScope 1.1 software (Axon Instruments).
After surgery, the superfused rabbit eyecup preparation was maintained in the dark for 1 hr before pH measurements were obtained. The physiological condition of the tissue was assessed by occasionally recording the ERG to short (5–10 sec) monochromatic (550 nm) light flashes of scotopic intensity. These flashes were also occasionally used to verify microelectrode position, but most of the time the retina was kept in complete darkness. In experiments devoted to light-induced retinal activity (see Fig. 5), longer duration light stimuli (1 and 5 min) were used. The maximum, unattenuated intensity (Io) of the full field monochromatic (550 nm) stimulus from a 100 W tungsten–halogen lamp was 60 μW/cm2. Intensity values indicated in the text are relative toIo. Calibrated neutral density filters were used to control light intensity, and narrow-band interference filters were used to control stimulus wavelength.
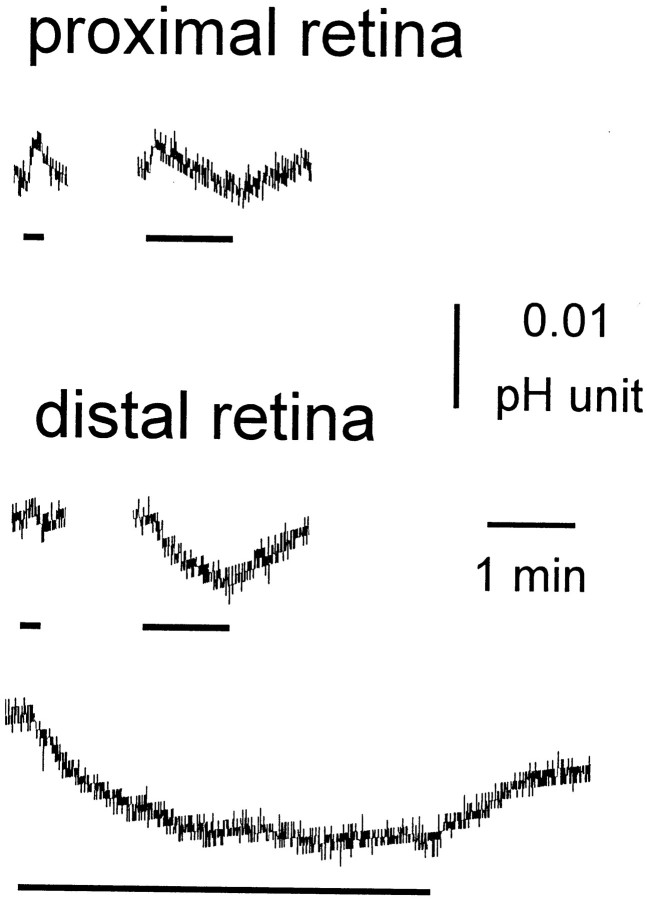
Fig. 5.
Light-evoked pHo changes are smaller than clock-induced changes, and the polarity of light-evoked changes in pHo depends on retinal depth. A decrease in pHois indicated by a downward movement of the trace. Typical light-evoked changes in retinal pH are shown for theproximal retina (inner nuclear layer) and distal retina (photoreceptor layer). Light stimulation altered retinal pHo by 0.01–0.02 pH units. In the proximal retina, light flashes produced an initial acidification followed by an alkalinization. In the distal retina, light flashes produced an alkalinization. Bars under the records indicate the occurrence of monochromatic (550 nm) light flashes (−3.6 log Io).
The Kolmogorov–Smirnov two-sample test was used for all statistical comparisons between control and experimental conditions.
RESULTS
The pHo of the rabbit retina was measured with pH-sensitive microelectrodes in the subjective day and night, that is, under conditions of constant darkness (Fig.1). The electrodes were placed in the superfusate above the retina and moved toward the tissue in 100 μm steps. After recording pHo in the various layers of the retina, we withdrew the pH-sensitive microelectrodes from the retina in a series of 100 μm steps. When the pH electrodes were within 600–800 μm of the vitreal surface of the retina, the pH of the superfusion solution began to decrease because of the influence of the tissue. The closer the microelectrodes were to the retina, the lower the recorded pH. In all of the experiments, a clear pH gradient was recorded in the solution above the retina (Fig. 1). Thus, retinal pHo was always lower than the pH of the superfusate. This difference in pH between the retina and the superfusate was a fundamental characteristic of viable tissue; when the retina lost its ability to respond to light, the pH difference was much smaller.
Fig. 1.
Extracellular pH of the rabbit retina is lower in the subjective night, compared with the subjective day. pHowas measured as a function of distance (micrometers) from a superfused rabbit retina in the subjective day and night. pH-sensitive microelectrodes were advanced through the superfusate to the retina, then through the retina, and finally withdrawn. Retinal pHowas always lower than superfusate pH, and the difference between retinal and superfusate pH was larger in the subjective night than in the subjective day. The electrodes were moved in 100 μm steps every 30 sec. Fast “spikes” on the records are movement artifacts.
The magnitude of the pH difference between the retina and the superfusate depended on the time of day (Figs. 1, 2). During the subjective day [Zeitgeber Time (ZT) 7–10, where ZT 0 is dawn], the average difference between retinal pHo and superfusate pH was 0.47 ± 0.03 (SEM) (n = 11). In contrast, during the subjective night (ZT 15–18), the average difference between retinal pHo and superfusate pH was 0.61 ± 0.04 (n = 9; p < 0.01). A similar day/night difference was obtained after the light/dark cycle was phase-delayed by 9 hr for a period of 1 week. As shown in Figure2, the phase-shifted retinas exhibited a difference in retinal and superfusate pH of 0.48 ± 0.03 (n = 8) during the subjective day and 0.63 ± 0.03 (n = 8) during the subjective night (p < 0.01). These data thus clearly indicate that a circadian clock regulates pHo in the rabbit retina so that more acid is produced at night than in the day.
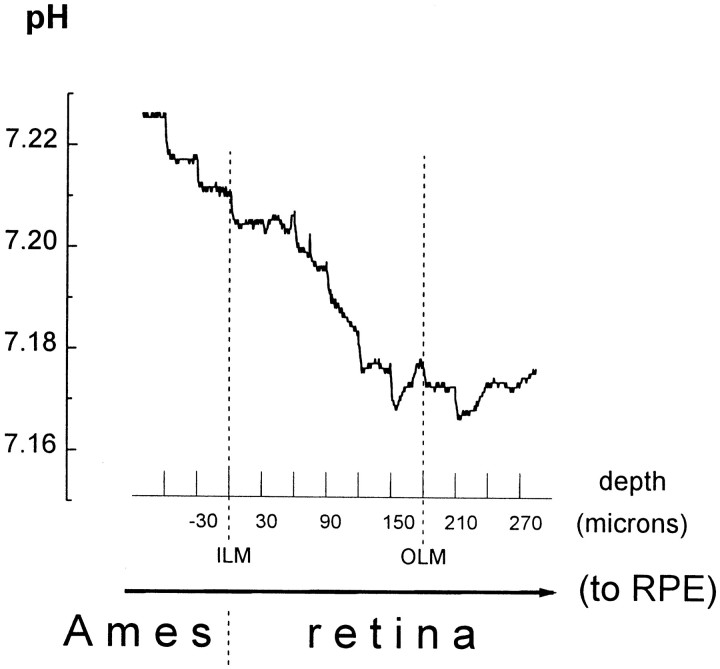
Retinal pHo varies with retinal layer in both the day and night and is lowest in the vicinity of the outer limiting membrane (OLM), a structure in the retina located in the vicinity of the inner segments of photoreceptor cells. During penetration of the retina from the vitreal side, pHo decreased until it reached a minimum value at the OLM, after which it increased slightly as the microelectrode approached the retinal pigment epithelium (Fig. 3). The pHo gradient across the retina was 13.7 ± 1.3% (n = 8) of the total difference between the minimal retinal pHo and the superfusate pH. There was no statistically significant day/night difference in the magnitude of the transretinal pHo gradient or inthe portion of transretinal pHo gradient in the total pH difference between retina and superfusate (14.5 ± 2.1%,n = 4 during subjective day; and 12.9 ± 1.5%,n = 4 during subjective night). In addition, the general character of the transretinal pHogradient was also similar in the night and day. After penetration of the inner limiting membrane (ILM), a structure located at the vitreal surface of the retina, recorded pHo decreased monotonically as the pH-selective microelectrode moved deeper into the retina in 30 μm steps until it reached a minimum at the OLM. Because pHo was lowest at the OLM, photoreceptor cells are likely the primary source of acid production in the retina.
Fig. 3.
Retinal pHo varies with retinal depth. Depth profiles of retinal pHo were obtained with pH-selective microelectrodes that were advanced through the superfusate (Ames medium) and retina to the retinal pigment epithelium (toRPE) in 30 μm steps every 30 sec. Retinal pHo was lowest in the vicinity of the outer limiting membrane (OLM). The vitreal surface of the retina was defined as a position of 0. The twovertical dashed lines indicate the depth at which the microelectrode penetrated the inner limiting membrane (ILM) and OLM. pH values are shown on the y-axis. Because the retina is a metabolically active tissue that produces acid, there is a pH gradient in its vicinity. When the pH-selective microelectrode was at a distance of 600 μm or more from the retina, the recorded pHo was 7.80 (Fig. 1). When the pH electrode was closer to the retinal surface, as shown in Figure 3, the pHo recorded in the Ames medium was < 7.80.
The circadian clock that modulates retinal pHo is probably located in the retina itself. Because pHo can be measured continuously with pH-selective microelectrodes, the time course of clock-induced changes in retinal pHo could be determined accurately. These experiments indicated that pHo in thein vitro rabbit eyecup decreased at dusk but remained relatively constant during the subjective day and night. Specifically, in these experiments, pH-selective microelectrodes were moved into the retina to the depth of the OLM during the second circadian cycle of constant darkness. The microelectrodes were then left there for ∼2 hr to monitor changes in pHo before and after ZT 12 (Fig. 4a), that is, at the time of subjective dusk, or during the subjective day (ZT 7–10) or night (ZT 14–18) (Fig. 4b). As shown in Figure4a, retinal pHo remained at approximately the same level between ZT 11 and 12 but decreased relatively quickly between ZT 12 and 13, that is, at the start of subjective night. Moreover, the magnitude of the average change in pHo between ZT 12 and 13 was 0.13 ± 0.02 (n = 4), which is similar to the total day/night difference in pHo (0.14–0.15 pH units) described above. Although retinal pHo decreased during the transition from subjective day to subjective night at dusk, it was relatively stable when it was measured at other, nontransition times during the subjective day (ZT 7–10) and night (ZT 14–18). For example, Figure 4b illustrates that when pHo was monitored continuously during the subjective night, beginning at ZT 14, the pHoincreased by 0.02 pH units over the course of ∼2 hr. In addition, repeated measurements of retinal pHo, which were separated in time by 20–60 min, demonstrated that retinal pHo increased slightly on average (0.01 pH units/hr) when it was measured during these nontransition times (ZT 7–10, n = 12; ZT 14–18, n = 13). This small increase in retinal pHo, which was observed during nontransition times in the subjective day and night, is probably caused by a slow decrease in the viability of the in vitroeyecup preparation with time. These results thus suggest that a circadian clock in the mammalian retina modulates retinal pHo.
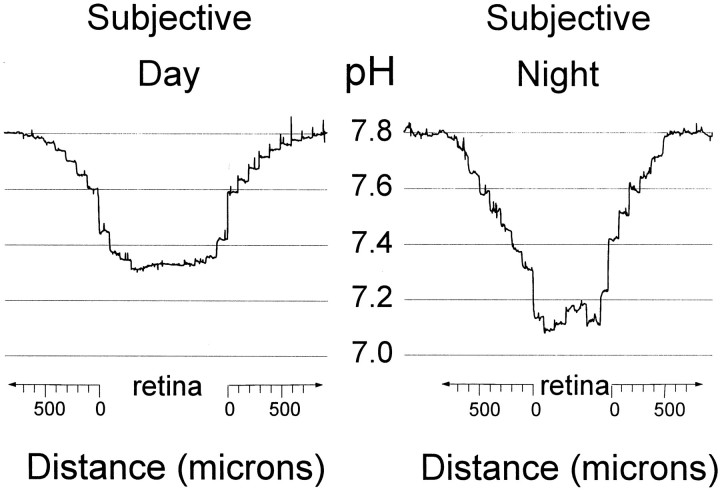
Fig. 4.
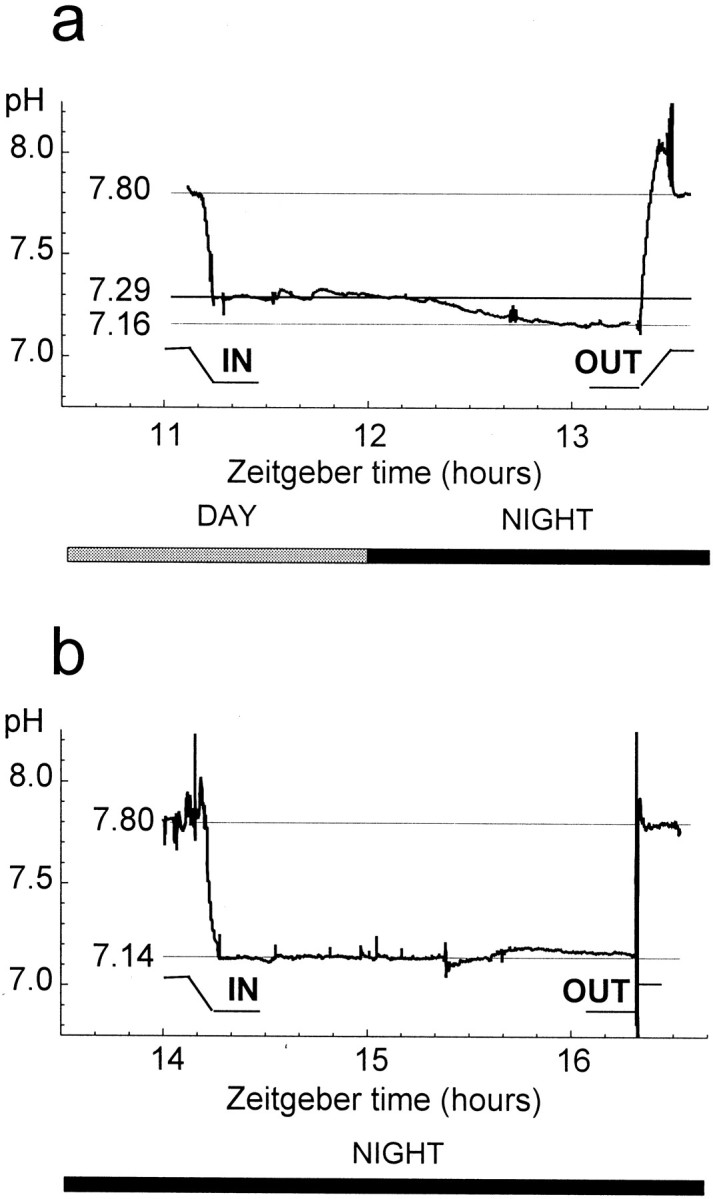
A circadian clock in the rabbit retina regulates retinal pH. a, Extracellular pH was monitored continuously from 1 hr before subjective dusk (ZT 12) until 1 hr after by placing microelectrodes into the in vitro retina at the level of the OLM. Retinal pHodecreased from a maximum (7.29) at ZT 12 to a minimum (7.16) at ZT 13. b, In contrast to the transition between subjective day and night at dusk, retinal pHo increased by 0.02 pH units over the course of 2 hr when it was measured during the subjective night (ZT 14–18), that is, at a nontransition time. Before and after penetration of thein vitro retina, the microelectrodes were moved to the superfusate, pH 7.8.
Although light stimulation modulates retinal pHo(Borgula et al., 1989; Oakley and Wen, 1989; Yamamoto et al., 1992), circadian-induced changes in retinal pHo are several times greater than light-induced changes, and unlike light-evoked pH changes, the polarity of the circadian-induced pH change does not depend on retinal depth. As shown in Figure5, light stimulation produced changes in pHo of ∼0.01–0.02 pH units. In the proximal (inner nuclear layer) retina, a 10 sec light flash produced a slight alkalinization. When stimulus duration was increased (60 sec), the initial alkalinization was followed by a slow acidification. In the distal retina (photoreceptor layer), the initial alkalinization was smaller and sometimes hardly visible. The delayed acidification was more prominent in the distal, compared with the proximal, retina. In the distal retina, the acidification had a very slow time course, requiring ∼3 min of light stimulation to produce a maximal response (Fig. 5). Finally, no obvious difference in the effect of light stimulation on pHo was observed in the day, compared with the night.
DISCUSSION
The results of this study indicate that a circadian clock regulates rabbit retinal pHo so that it is lower in the night, compared with the day. The lowest pHo recorded in the retina in both the day and night was in the vicinity of the inner segments of photoreceptor cells, supporting the idea that photoreceptor cells are the principal consumers of energy in the retina and, as a consequence, serve as the primary source of protons. In addition, the clock-induced shift in pHo is several times greater than light-induced pHo changes. Moreover, the findings suggest that the clock that modulates retinal pHo is located in the rabbit retina itself. These findings are discussed in more detail below with respect to the circadian phenomenon itself and how it might affect retinal function.
Relationship of the clock, pH, and energetic metabolism
Although shifts in pHo attributable to neuronal activity have been reported in studies of in vitroCNS tissue (Kraig et al., 1983; Chesler and Kaila, 1992), the current findings are the first demonstration that pHo in the mammalian CNS can be modulated by a circadian clock, an endogenous neural process. Moreover, the fact that an endogenous process produces acid in the retina suggests that the circadian-induced changes in pHo observed in thein vitro rabbit retina also occur in vivo. In fact, in support of this idea, Kraig et al. (1983) have shown that stimulus-induced shifts in the pHo of rat cortex occur to a similar extent both in vivo and in vitro, suggesting that changes in blood flow in vivo do not alter or eliminate activity-dependent and circadian clock-induced pHo shifts.
The finding that the clock decreases retinal pHoat night indicates that the clock increases the production of acid in the retina at night in a sustained manner over the course of many hours. Such a sustained increase in acid production at night is most likely caused by an increase in energetic metabolism at night, and in fact, such an increase has been demonstrated recently (Dmitriev et al., 1999a). It is well known that energetic metabolism is a proton-producing process (Krebs et al., 1975; Alberti and Cuthbert, 1982), and there is no other known sustained source of acid in the brain. This metabolic source is powerful enough to generate pH gradients that can be measured. During oxidative degradation of glucose, in which one CO2 molecule is released for six ATP molecules, brain tissues consume 1.5–5.0 μm O2 each minute (Mellergard and Siesjo, 1998) and produce an equivalent amount of CO2. The acidic cost of energetic metabolism during glycolysis, when one lactic acid molecule is released for each ATP molecule made, is even higher. Thus, both the sustained difference in pH between retina and superfusate that is present in the day and the even greater difference in pH between retina and superfusate that is present at night are likely to be caused by energetic metabolic processes.
In contrast to the sustained production of acid by energetic metabolic processes, changes in acid–base transport, for example, probably cannot account for the sustained decrease in pHothat occurs at night. Although changes in acid–base transport can alter pHo in a transient fashion, a sustained change in acid–base transport that lasted many hours would result in substantial depletion of intracellular acid, leading to neuronal dysfunction. In contrast to clock-induced changes in pHo, light-evoked changes in pHo, which are much smaller in size and more transient, species dependent, and often of mixed polarity (Fig. 5) (Borgula et al., 1989; Oakley and Wen, 1989; Yamamoto et al., 1992;Dmitriev and Mangel, 2000), might be caused by changes in acid–base transport or carbonic anhydrase activity.
The sustained production of acid during the day and the even greater production of acid at night require intracellular sources. The present findings suggest that photoreceptor cells are the primary source of protons in the retina, because the lowest recorded pHo in the retina was in the vicinity of the inner segments of photoreceptor cells (Fig. 3), the location with the highest concentration of mitochondria in the retina. The present findings are thus consistent with the previous suggestion that photoreceptor cells are the principal consumers of energy in the retina (Haugh-Scheidt et al., 1995; Demontis et al., 1997). However, until a more thorough analysis of the sources and sinks of acid flux in the retina is performed, the present findings cannot be considered conclusive.
Although the clock-induced decrease in retinal pH at night likely reflects a clock-induced increase in retinal metabolic activity (Dmitriev et al., 1999a), the sequence of events whereby the clock affects pH and metabolic activity is not known. It is well known that ATP is used primarily to support electrochemical ionic gradients across the cell membrane, both in the brain (Astrup, 1982; Erecinska and Silver, 1989) and in the retina (Ames et al., 1992). The decrease in pHo and the increase in metabolic activity at night may be caused by clock-induced ionic conductance changes that result from the action of neurotransmitters such as melatonin (Cassone et al., 1988; Cosci et al., 1997) and dopamine (Shulman and Fox, 1996). In fact, recent evidence has shown that the potassium conductance of neurons in the suprachiasmatic nucleus (Pennartz et al., 1999) and pineal (Hasegawa and Dryer, 1999) is under circadian control. Alternatively, the clock may be directly increasing the activity of specific enzymes in retinal metabolic pathways at night.
Location of the clock that regulates retinal pH
Our findings suggest that the clock that modulates retinal pHo is located in the rabbit retina itself. Specifically, we have demonstrated that the pHoof the in vitro rabbit retina changes from a daytime to a nighttime value when the retina is left in constant darkness and that this change occurs at dusk but not at other, nontransition times during the day and night (Fig. 4). Thus, our data suggest that an endogenous circadian clock in the in vitro rabbit retina produces a day/night difference in retinal pHo. The clock that regulates retinal pH may be identical to the clock in the photoreceptor cells that regulates retinal melatonin synthesis (Cahill and Besharse, 1993). As noted above, clock-induced ionic conductance changes, which might result from the action of melatonin, may increase metabolic activity and decrease pH at night. However, it is also possible that the clock that regulates retinal pH is not the clock that regulates melatonin. It is interesting to note that cryptochrome genes, which may form part of the circadian clock, are expressed in the ganglion cell layer and inner nuclear layer (Miyamoto and Sancar, 1998) of the mouse, suggesting the possibility of a second clock in the retina.
Although it is very likely that the day/night difference in retinal pHo is caused by the action of a circadian clock, it is possible that a clock is not involved. We have shown that under conditions of constant darkness, the pHo of the rabbit retina is lower during the subjective night than in the subjective day (Figs. 1, 2). We have also shown that phase-shifting the light/dark cycle by 9 hr phase-shifts the day/night difference in retinal pHo (Fig. 2). In addition, all of our data (Figs. 1-5) were obtained during the second cycle of constant darkness, demonstrating that the day/night difference in retinal pH persists for at least two 24 hr cycles. Thus, our data indicate that a rhythmic process, which is likely a circadian clock, produces a day/night difference in retinal pHo, persists for 2 d, and can be entrained by light. However, because we do not know the period of this process and because we have not monitored the pH of an individual retina for at least 24 hr, it is theoretically possible that the daily rhythmic change in retinal pHo that is observed in constant darkness is not attributable to a circadian clock. That is, it is possible that the day/night difference in retinal pHo is an aftereffect of a rhythmic series of events brought about by daily exposure of the eye to a light/dark cycle. Such a pH rhythm might damp out in a few hours of darkness and not persist in a self-sustaining manner. On the other hand, because it has also been shown that a circadian clock with a period of ∼24 hr regulates the pH of the fish retina (Dmitriev and Mangel, 2000), it is very likely that the rhythmic process that regulates pH in the rabbit retina is a circadian clock as well.
Role of circadian clock regulation of retinal pH
Several lines of evidence suggest that a circadian clock-induced increase in the concentration of protons during the night may serve as a clock signal for the night. First, recent work has shown that the uncoupling action of dopamine on rabbit horizontal cells is pH dependent (Hampson et al., 1994). Although dopamine does not affect dye coupling between rabbit horizontal cells at a superfusate pH of 7.4, it does uncouple the cells when the superfusate pH is reduced to 7.2. It is thus possible that a clock-induced decrease in retinal pHo at night allows dopamine to uncouple rabbit horizontal cells or that the low pH at night itself uncouples horizontal cells. Second, recent work has shown that a circadian clock regulates the light responses of fish and rabbit horizontal cells (Wang and Mangel, 1996; Mangel and Wang, 1997). Because of the action of the clock, the responses are cone mediated during the day and rod mediated, slower, and smaller in size during the night. A decrease in Ringer's solution pH from 7.6 to 7.4, which reduces retinal pHo by ∼0.1 pH units, an amount comparable to the extent of circadian regulation, reduces the size of fish horizontal cell light responses by ∼50% (Harsanyi and Mangel, 1993; Dmitriev and Mangel, 2000). A decrease in retinal pHo may reduce cone horizontal cell light responses by decreasing transmitter release from cones (Barnes et al., 1993). Thus, the clock-induced decrease in retinal pHo during the night may contribute to the suppression of horizontal cell light responses observed during the night. Circadian clock regulation of the pHo and metabolic activity of the retina may therefore modulate synaptic transmission in the retina. The clock-induced decrease in retinal pHo during the night may also increase rod photoreceptor sensitivity by stabilizing rhodopsin (Barlow et al., 1993). Studies that have used 2-deoxyglucose to measure metabolic activity have indicated that a circadian clock also regulates metabolic activity in the suprachiasmatic nucleus (Schwartz and Gainer, 1977). Thus, it is possible that circadian clock regulation of pH and metabolic activity is a general property of neural circadian clock tissue.
In summary, a circadian clock, which is probably located in the rabbit retina itself, regulates retinal pH so that the pHo is lower at night than in the day. Photoreceptor cells may serve as the primary source of the protons. The clock-induced shift in pHo, which is likely caused by an increase in energetic metabolism at night, is several times greater than light-induced pHo changes. Finally, the circadian clock-induced increase in the concentration of protons during the night may serve as a clock signal for the night whereby neuronal signaling and coupling are modulated. Thus, an intrinsic oscillator in neural tissue modulates metabolic activity and pH as part of normal daily function.
Footnotes
This work was supported in part by National Institutes of Health Grant EY05102 and National Science Foundation Grant IBN-9819981 to S.C.M., and by National Eye Institute CORE Grant P30 EY03039 to the University of Alabama at Birmingham. We thank Dr. Christophe Ribelayga for helpful discussions.
Correspondence should be addressed to Dr. Stuart Mangel, Department of Neurobiology, University of Alabama School of Medicine, 1719 Sixth Avenue South, Birmingham, AL 35294. E-mail:mangel@nrc.uab.edu.
REFERENCES
- 1.Alberti K, Cuthbert C. The hydrogen ion in normal metabolism: a review. In: Porter R, Lawrenson G, editors. Metabolic acidosis. Pitman; Bath, UK: 1982. pp. 1–19. [DOI] [PubMed] [Google Scholar]
- 2.Ames A, III, Ying-Ying L, Heher EC, Kimble CR. Energy metabolism of rabbit retina as related to function: high cost of Na+ transport. J Neurosci. 1992;12:840–853. doi: 10.1523/JNEUROSCI.12-03-00840.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Astrup J. Energy-requiring cell functions in the ischemic brain. J Neurosurg. 1982;56:482–497. doi: 10.3171/jns.1982.56.4.0482. [DOI] [PubMed] [Google Scholar]
- 4.Barlow RB, Birge RR, Kaplan E, Tallent JR. On the molecular origin of photoreceptor noise. Nature. 1993;366:64–66. doi: 10.1038/366064a0. [DOI] [PubMed] [Google Scholar]
- 5.Barnes S, Merchant V, Mahmud F. Modulation of transmission gain by protons at the photoreceptor output synapse. Proc Natl Acad Sci USA. 1993;90:10081–10085. doi: 10.1073/pnas.90.21.10081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Block GD, Khalsa SB, McMahon DG, Michel S, Guesz M. Biological clocks in the retina: cellular mechanisms of biological timekeeping. Int Rev Cytol. 1993;146:83–144. doi: 10.1016/s0074-7696(08)60381-2. [DOI] [PubMed] [Google Scholar]
- 7.Borgula GA, Karwoski CJ, Steinberg RH. Light-evoked changes in extracellular pH in frog retina. Vision Res. 1989;29:1069–1077. doi: 10.1016/0042-6989(89)90054-0. [DOI] [PubMed] [Google Scholar]
- 8.Cahill GM, Besharse JC. Circadian clock functions localized in Xenopus retinal photoreceptors. Neuron. 1993;10:573–577. doi: 10.1016/0896-6273(93)90160-s. [DOI] [PubMed] [Google Scholar]
- 9.Cahill GM, Besharse JC. Circadian rhythmicity in vertebrate retinas: regulation by a photoreceptor oscillator. Prog Ret Eye Res. 1995;14:267. [Google Scholar]
- 10.Cassone VM, Roberts MH, Moore RY. Effects of melatonin on 2-deoxy-14C-glucose uptake within rat suprachiasmatic nucleus. Am J Physiol. 1988;255:R332–R337. doi: 10.1152/ajpregu.1988.255.2.R332. [DOI] [PubMed] [Google Scholar]
- 11.Chesler M, Kaila K. Modulation of pH by neuronal activity. Trends Neurosci. 1992;15:396–402. doi: 10.1016/0166-2236(92)90191-a. [DOI] [PubMed] [Google Scholar]
- 12.Cosci B, Longoni B, Marchiafava PL. Melatonin induces membrane conductance changes in isolated retinal rod receptor cells. Life Sci. 1997;60:1885–1889. doi: 10.1016/s0024-3205(97)00150-1. [DOI] [PubMed] [Google Scholar]
- 13.Demontis GC, Longoni B, Gargini C, Cervetto L. The energetic cost of photoreception in retinal rods of mammals. Arch Ital Biol. 1997;135:95–109. [PubMed] [Google Scholar]
- 14.Dmitriev AV, Mangel SC. A circadian clock regulates the pH of the fish retina. J Physiol (Lond) 2000;522:77–82. doi: 10.1111/j.1469-7793.2000.0077m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dmitriev AV, Nagy TR, Mangel SC. A circadian clock regulates the metabolic activity of the fish retina. Invest Ophthalmol Vis Sci [Suppl] 1999a;40:S611. [Google Scholar]
- 16.Dmitriev AV, Pignatelli A, Piccolino M. Resistance of retinal extracellular space to Ca2+ level decrease: implications for the synaptic effects of divalent cations. J Neurophysiol. 1999b;82:283–289. doi: 10.1152/jn.1999.82.1.283. [DOI] [PubMed] [Google Scholar]
- 17.Erecinska M, Silver I. ATP and brain function. J Cereb Blood Flow Metab. 1989;9:2–19. doi: 10.1038/jcbfm.1989.2. [DOI] [PubMed] [Google Scholar]
- 18.Hampson ECGM, Weiler R, Vaney DI. pH-gated dopaminergic modulation of horizontal cell gap junctions in mammalian retina. Proc R Soc Lond B Biol Sci. 1994;255:67–72. doi: 10.1098/rspb.1994.0010. [DOI] [PubMed] [Google Scholar]
- 19.Harsanyi K, Mangel SC. Modulation of cone to horizontal cell transmission by calcium and pH in the fish retina. Vis Neurosci. 1993;10:81–91. doi: 10.1017/s0952523800003242. [DOI] [PubMed] [Google Scholar]
- 20.Hasegawa Y, Dryer SE. Diurnal modulation of K+ channels in chick pineal cells. Soc Neurosci Abstr. 1999;25:351. [Google Scholar]
- 21.Haugh-Scheidt LM, Griff ER, Linsenmeier RA. Light-evoked oxygen responses in the isolated toad retina. Exp Eye Res. 1995;61:73–81. doi: 10.1016/s0014-4835(95)80060-3. [DOI] [PubMed] [Google Scholar]
- 22.Kraig RP, Ferreira-Filho CR, Nicholson C. Alkaline and acid transients in cerebellar microenvironment. J Neurophysiol. 1983;49:831–850. doi: 10.1152/jn.1983.49.3.831. [DOI] [PubMed] [Google Scholar]
- 23.Krebs H, Woods H, Alberti K. Hyperlactataemia and lactic acidosis. Essays Med Biochem. 1975;1:81–103. [Google Scholar]
- 24.Lavail MM, Ward PA. Studies on the hormonal control of circadian outer segment disc shedding in the rat retina. Invest Ophthalmol Vis Sci. 1978;17:1189–1193. [PubMed] [Google Scholar]
- 25.Mangel SC, Wang Y. Light responses of rabbit cone-connected horizontal cells exhibit a diurnal rhythm. Invest Ophthalmol Vis Sci [Suppl] 1997;38:S616. [Google Scholar]
- 26.Mellergard P, Siesjo BK. Cerebral energy metabolism and pH: pH and brain function (Kaila K, Ransom BR, eds), pp 67–91. Wiley-Liss; New York: 1998. [Google Scholar]
- 27.Miyamoto Y, Sancar A. Vitamin B2-based blue-light photoreceptors in the retinohypothalamic tract as the photoactive pigments for setting the circadian clock in mammals. Proc Natl Acad Sci USA. 1998;95:6097–6102. doi: 10.1073/pnas.95.11.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oakley B, Wen R. Extracellular pH in the isolated retina of the toad in darkness and during illumination. J Physiol (Lond) 1989;419:353–378. doi: 10.1113/jphysiol.1989.sp017876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pennartz CMA, De Jeu MTG, Geurtsen AMS, Schaap J, Bos NPA. Ionic mechanisms underlying the circadian rhythm in firing rate of rat suprachiasmatic nucleus. Soc Neurosci Abstr. 1999;25:2065. [Google Scholar]
- 30.Reme C, Wirz-Justice A, Terman M. The visual input stage of the mammalian circadian pacemaking system: I. Is there a clock in the mammalian eye? J Biol Rhythms. 1991;6:5–29. doi: 10.1177/074873049100600104. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz W, Gainer JH. Suprachiasmatic nucleus: use of 14C-labeled deoxyglucose uptake as a functional marker. Science. 1977;197:1089–1091. doi: 10.1126/science.887940. [DOI] [PubMed] [Google Scholar]
- 32.Shulman LM, Fox DA. Dopamine inhibits mammalian photoreceptor Na+, K+-ATPase activity via a selective effect on the alpha3 isozyme. Proc Natl Acad Sci USA. 1996;93:8034–8039. doi: 10.1073/pnas.93.15.8034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tosini G, Menaker M. Circadian rhythms in cultured mammalian retina. Science. 1996;272:419–421. doi: 10.1126/science.272.5260.419. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Mangel SC. A circadian clock regulates rod and cone input to fish retinal cone horizontal cells. Proc Natl Acad Sci USA. 1996;93:4655–4660. doi: 10.1073/pnas.93.10.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wirz-Justice A, Da Prada M, Reme CE. Circadian rhythm in rat retinal dopamine. Neurosci Lett. 1984;45:21–25. doi: 10.1016/0304-3940(84)90323-9. [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto F, Borgula GA, Steinberg RH. Effects of light and darkness on pH outside rod photoreceptors in the cat retina. Exp Eye Res. 1992;54:685–697. doi: 10.1016/0014-4835(92)90023-l. [DOI] [PubMed] [Google Scholar]