Abstract
We examined the effect of zinc on rat neuronal nicotinic acetylcholine receptors (nAChRs) expressed in Xenopusoocytes as simple heteromers of α2, α3, or α4 and β2 or β4. Coapplication of zinc with low concentrations of acetylcholine (≤EC10) resulted in differential effects depending on receptor subunit composition. The α2β2, α2β4, α3β4, α4β2, and α4β4 receptors exhibited biphasic modulation by zinc, with potentiation of the acetylcholine response occurring at 1–100 μm zinc and inhibition occurring at higher zinc concentrations. In contrast, α3β2 receptors were only inhibited by zinc (IC50 = 97 ± 16 μm). The greatest potentiating effect of zinc was seen with α4β4 receptors that were potentiated to 560 ± 17% of the response to ACh alone, with an EC50 of 22 ± 4 μm zinc. Cadmium, but not nickel, was also able to potentiate α4β4 receptors. Both zinc potentiation of α4β4 receptors and zinc inhibition of α3β2 receptors were voltage independent. The sensitivity of zinc potentiation of α4β4 to diethylpyrocarbonate treatment and alterations in pH suggested the involvement of histidine residues. Zinc continued to inhibit α4β4 and α3β2 after diethylpyrocarbonate treatment. Application of a potentiating zinc concentration increased the response of α4β2 and α4β4 receptors to saturating ACh concentrations. The rate of Ach-induced desensitization of these receptors was unaffected by zinc. Our results reveal zinc potentiation as a new mode of neuronal nAChR modulation.
Keywords: neuronal nicotinic receptors, zinc, potentiation, inhibition, modulation, acetylcholine
Nicotinic acetylcholine receptors (nAChRs) are ligand-gated ion channels found at the neuromuscular junction and throughout the CNS and PNS. Neuronal nAChRs are similar to muscle nAChRs: they are formed as pentameric assemblies of subunits (Anand et al., 1991; Cooper et al., 1991). To date, the neuronal nAChR subunit family consists of nine α subunits (α2–α10) and three β subunits (β2–β4) (Corringer et al., 2000). These subunits can assemble, in exogenous expression systems, in various combinations to form receptors with varying functional and pharmacological properties (Role, 1992). In the nervous system, neuronal nAChRs can form as pentameric homomers (such as α7 receptors) (Chen and Patrick, 1997; Drisdel and Green, 2000), as simple heteromers composed of a single type of α subunit and a single type of β subunit (such as α4β2 receptors) (Whiting et al., 1991;Flores et al., 1992), or as complex heteromers of three or more subunits (such as α3α5β4 receptors) (Conroy and Berg, 1995).
Ionic zinc has been found in neurons throughout the brain, with highest concentrations in the cerebral cortex and limbic areas (Frederickson et al., 2000). Zinc is localized to small, clear vesicles in synaptic terminals and is released in a calcium-dependent manner (Assaf and Chung, 1984; Howell et al., 1984). The extracellular concentration of zinc is estimated to reach concentrations as high as 300 μm (Assaf and Chung, 1984). Zinc modulates the functions of members of several ligand-gated ion channel families, including glutamate, GABA, glycine, and ATP receptors (Mayer et al., 1989;Draguhn et al., 1990; Rassendren et al., 1990; Cloues et al., 1993;Bloomenthal et al., 1994; Paoletti et al., 1997; Krishek et al., 1998;Harvey et al., 1999; Xiong et al., 1999; Laube et al., 2000). Both potentiation and inhibition of agonist-induced responses have been observed. The ability to modulate ligand-gated ion channel function suggests that zinc may be an important modulator of synaptic activity.
Relatively few studies have examined the effect of zinc on neuronal nAChRs. Zinc was shown to block ACh responses of rat intracardiac parasympathetic neurons (Nutter and Adams, 1995). Zinc also attenuates the Ach-induced response of homomeric α7 nAChRs exogenously expressed in Xenopus oocytes (Palma et al., 1998). We have now examined the effect of zinc on the several neuronal nAChRs that can be formed by pairwise expression of the α2, α3, or α4 subunits with the β2 or β4 subunits in Xenopus oocytes. We find that although all subunit combinations are inhibited by high concentrations of zinc, some subunit combinations are potentiated by low zinc concentrations (≤100 μm). Moreover, the extent of potentiation by zinc varies markedly depending on receptor subunit composition. Potentiation by zinc represents a new mode of neuronal nAChR modulation.
MATERIALS AND METHODS
Materials. Xenopus laevis frogs were purchased from Nasco (Fort Atkinson, WI). The care and use of X. laevis frogs in this study were approved by the University of Miami Animal Research Committee and meet the guidelines of the National Institutes of Health. RNA transcription kits were from Ambion (Austin, TX). Collagenase B was from Boehringer Mannheim (Indianapolis, IN). All other reagents were from Sigma (St. Louis, MO).
Neuronal nAChR expression in X. laevis oocytes. m7G(5′)ppp(5′)G-capped cRNA transcripts encoding nAChR subunits were prepared by in vitrotranscription from linearized template DNA encoding the α2, α3, α4, β2, β4, α3–216-α4, and α4–216-α3 subunits. Chimeric subunits were constructed previously (Harvey et al., 1996). MatureX. laevis frogs were anesthetized by submersion in 0.1% 3-aminobenzoic acid ethyl ester, and oocytes were surgically removed. Follicle cells were removed by treatment with collagenase B for 2 hr at room temperature. Stage V oocytes were individually injected with 0.5–10 ng of each cRNA in 15–50 nl of water and incubated at 19°C in modified Barth's saline (88 mm NaCl, 1 mm KCl, 2.4 mmNaHCO3, 0.3 mmCaNO3, 0.41 mmCaCl2, 0.82 mmMgSO4, 100 μg/ml gentamicin, 15 mm HEPES, pH 7.6) for 2–7 d.
Electrophysiological methods. Current responses were measured under two-electrode voltage clamp, using a TEV-200 voltage-clamp unit (Dagan, Minneapolis, MN). Micropipettes were filled with 3 m KCl and had resistances of 0.3–2.0 MΩ. Current responses were sampled at 100 Hz and filtered (four-pole, Bessel low-pass) at 20 Hz. Current responses were captured, stored, and analyzed on a Macintosh Power PC 7100 computer using AxoData 1.2.2 and AxoGraph 4.0 software (Axon Instruments, Foster City, CA). All experiments were performed at a holding potential of −70 mV, except for voltage-dependence studies (see Fig. 7), ACh concentration–response experiments (see Fig. 8), and desensitization rate measurements. The ACh concentration–response experiments were performed at a holding potential of −40 mV to decrease current amplitudes at higher ACh concentrations and to minimize the contribution of the calcium-activated chloride channel to the ACh response. As demonstrated in Figure 7, the Zn2+ effect is independent of membrane holding potential. Desensitization rate measurements for α4β4 and α4β2 receptors were performed at −40 mV with Ba2+ substituting for Ca2+ in all solutions to further minimize any contribution of the calcium-activated chloride channels. Each oocyte was exposed to an EC50 concentration of ACh with or without 50 μmZn2+ for 60 sec. The order of application was alternated from oocyte to oocyte. The desensitizing phase of each response was fit to a single exponential function.
Fig. 7.
Zn2+ potentiation of α4β4 receptors and inhibition of α3β2 receptors are voltage independent.A, Current responses of an α4β4-expressing oocyte to 1 μm ACh before, during, and after coapplication of 50 μm Zn2+ at membrane holding potentials of −40 mV (left trace, calibration: 50 nA, 20 sec) and −90 mV (right trace, calibration: 200 nA, 20 sec). The traces were normalized for comparison. B, Current responses of α4β4-expressing oocytes to 1 μm ACh in the presence of 50 μm Zn2+ were recorded at various holding potentials and plotted as a percentage of the response to ACh alone (mean ± SEM of 3 oocytes). Current responses of α3β2 expressing oocytes to 4 μm ACh in the presence of 100 μm Zn2+ were recorded at various holding potentials and plotted as a percentage of the response to ACh alone (mean ± SEM of 3 oocytes).
Fig. 8.
Zn2+ increases the response of neuronal nAChRs to saturating ACh concentrations. ACh concentration–response relationships of α4β4- and α4β2-expressing oocytes in the absence (filled symbols) and presence (open symbols) of 50 μm Zn2+ are plotted as a percentage of the fit maximum response to ACh alone (mean ± SEM of 3 oocytes).A, Zn2+ (50 μm) significantly decreased the EC50 of α4β4 receptors for ACh from 74 ± 22 to 23 ± 8 μm(p < 0.05) and significantly increased the maximal response to 160 ± 11% of the maximal response to ACh alone (p < 0.02). B, Zn2+ (50 μm) significantly increased the maximal response of α4β2 receptors to ACh to 140 ± 14% of the maximal response to ACh alone (p < 0.02).
Oocytes were perfused at room temperature (20−25°C) with perfusion solution (115 mm NaCl, 1.8 mmCaCl2, 2.5 mm KCl, 0.1 μm atropine, 10 mm HEPES, pH 7.2) in a chamber constructed from 1/8 inch inner diameter Tygon tubing. Perfusion was continuous at a rate of ∼20 ml/min. Agonists and metals were diluted in the perfusion solution and then applied to oocytes using solenoid valves. All experiments, except for desensitization rate measurements and experiments shown in Figs. 3 and 8, were performed as follows. ACh alone was applied for 30 sec, followed by a 30 sec application of solutions containing both ACh and the metal ion of interest, and then by 30 sec of ACh alone. Between applications, oocytes were perfused continuously. In cases in which no desensitization was evident (α4β4 responses in Figs. 1-7), defined as a current decrease of <5% over 30 sec, control current in response to agonist was determined from a 1 sec average beginning 29 sec after initiation of agonist application. Current levels during metal coapplication were determined from a 1 sec average beginning 29 sec after initiation of metal application and compared with the control current. In cases in which desensitization was evident (all experiments involving α2β2, α2β4, α3β2, α3β4, and α4β2), defined as a current decrease of >5% over 30 sec, the following analysis method was used. The initial 30 sec ACh response in the absence of metal was fit to a single or dual exponential and was projected over the next 30 sec in which both ACh and metal were coapplied. The degree of modulation was measured by taking a 1 sec average 29 sec after initiation of metal application and comparing it with a 1 sec average of the projected response to ACh alone at the same time period. Thus, both metal and control values were taken 59 sec after the initiation of the experiment.
Fig. 3.

Zn2+ preincubation eliminates potentiation transients seen with high Zn2+concentrations. A, Current responses of an α4β4-expressing oocyte preincubated with 0, 100 μm, and 1 mm Zn2+ for 20–30 sec before coapplication of 1 μm ACh. Calibration: 50 nA, 10 sec.B, Current responses during coapplication of various concentrations of Zn2+ and 1 μm ACh were plotted as a percentage of the response to 1 μm ACh alone recorded immediately before Zn2+ preincubation (mean ± SEM of 3 oocytes).
Fig. 1.
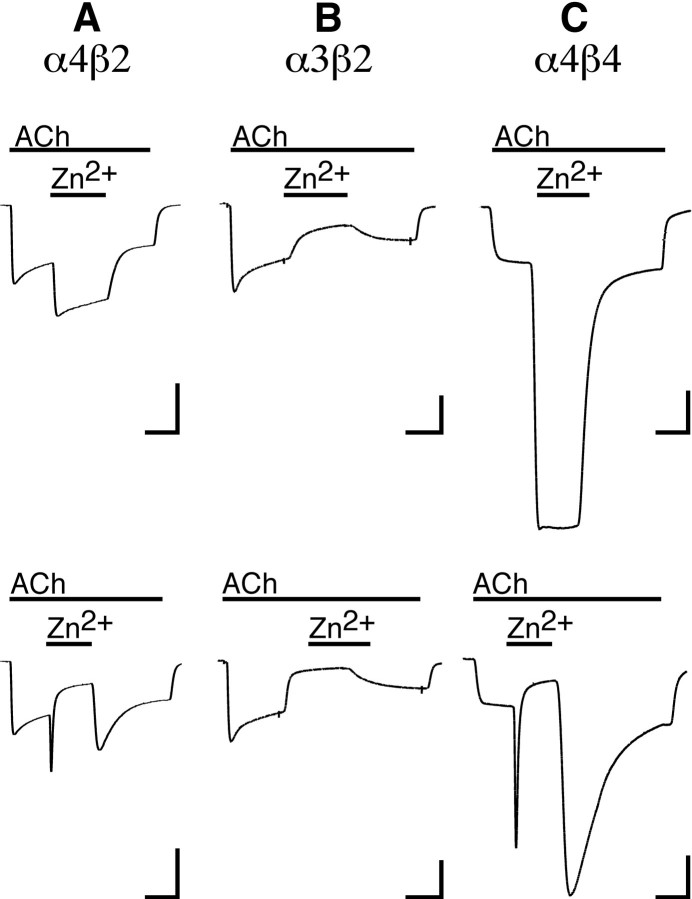
Zn2+ potentiates and inhibits neuronal nAChRs. A, Current responses of an α4β2-expressing oocyte to 10 μm ACh before, during, and after coapplication of 70 μm(top trace) or 1 mm Zn2+(bottom trace). Calibration: 60 nA, 20 sec.B, Current responses of an α3β2-expressing oocyte to 4 μm ACh before, during, and after coapplication of 70 μm Zn2+(top trace) or 1 mm Zn2+(bottom trace). Calibration: 300 nA, 20 sec. C, Current responses of an α4β4-expressing oocyte to 1 μm ACh before, during, and after coapplication of 100 μm Zn2+(top trace) or 3 mm Zn2+(bottom trace). Calibration: 200 nA, 20 sec.
Fig. 2.

The nature of neuronal nAChR modulation by Zn2+ is subunit dependent. The effects of Zn2+ coapplication on Ach-induced current responses are plotted as a percentage of the response to ACh alone (mean ± SEM of 3–6 oocytes). Some error bars are obscured by the symbols. The potentiating and inhibiting phases of the Zn2+effect were fit separately as described in Materials and Methods. Note the increase in axis range in C to accommodate the greater potentiation seen with α4β4 nAChRs.
Fig. 4.
Cd2+ and Ni2+ are less effective than Zn2+at potentiating neuronal nAChRs. The effects of Cd2+(▿) and Ni2+ (⋄) on ACh (1 μm)-induced current responses of α4β4-expressing oocytes are plotted as a percentage of the current obtained with 1 μm ACh alone (mean ± SEM of 3–5 oocytes). Potentiating and inhibiting phases were fit separately as described in Materials and Methods. Data from Figure 2C showing the effect of Zn2+ (○) are included for comparison. Some error bars are obscured by symbols.
Fig. 5.
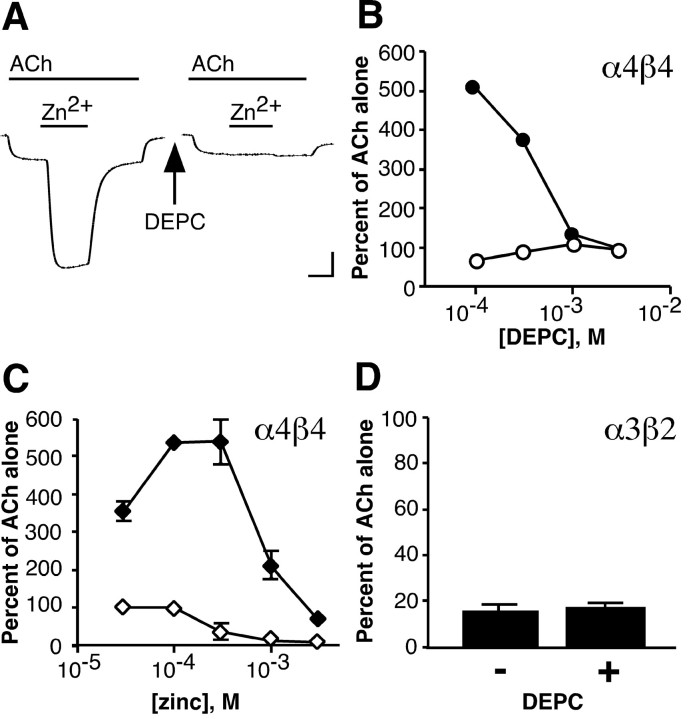
Diethylpyrocarbonate treatment abolishes Zn2+ potentiation but not Zn2+inhibition of neuronal nAChRs. A, The current response of an α4β4-expressing oocyte to 1 μm ACh before, during, and after coapplication of 100 μmZn2+ is shown on the left. After a 10 min treatment with 3 mm DEPC and a 10 min wash period, application of 100 μm Zn2+ to the same oocyte no longer potentiates the ACh response (right trace). Calibration: 250 nA, 20 sec. B, The effect of a 10 min incubation with various concentrations of DEPC on ACh (1 μm)-induced current responses in the presence of 100 μm Zn2+ (●) is plotted as a percentage of the response to ACh immediately before Zn2+ application. The effect of DEPC on current responses to 1 μm ACh alone (○) is plotted as a percentage of the response to ACh before DEPC treatment. Symbols and error bars represent the mean ± SEM of four sets of oocytes, each set consisting of three oocytes. Error bars are obscured by symbols.C, The effect of various concentrations of Zn2+ on the response of α4β4 receptors to 1 μm ACh before (♦) and after (⋄) treatment with 3 mm DEPC is plotted as a percentage of the response to ACh alone immediately before Zn2+ application (mean ± SEM of 3 oocytes). D, Block of α3β2-expressing oocytes by 1 mm Zn2+ was measured before (−) and after (+) treatment with 3 mm DEPC for 10 min and is plotted as a percentage of the response to 4 μm ACh immediately before Zn2+ application (mean ± SEM of 3 oocytes).
Fig. 6.
Zn2+ potentiation of α4β4 is sensitive to alterations in pH. Potentiation of the response to 1 μm ACh by 100 μm Zn2+ at pH 5.5, 7.2, and 8.0 is plotted as a percentage of the response to ACh alone (mean ± SEM of 3 oocytes; significant differences from pH 7.2: *p < 0.0001, **p < 0.005).
For the ACh concentration–response experiments shown in Figure 8, experiments were performed and measurements were taken as follows. Because receptor expression levels vary from oocyte to oocyte, it is necessary to normalize concentration–response data from different oocytes to allow comparison. However, desensitization makes the maximal response an unreliable standard with which to normalize data. For this reason we normalized each response to the response to a low concentration of ACh (3 μm) that produced little or no desensitization. To compare and display the results we then renormalized the data to the fit maximal response to ACh alone. To determine the effect of Zn2+ on the ACh concentration–response relationship we used our standard protocol (30 sec of ACh alone, followed by 30 sec of ACh + Zn2+, followed by 30 sec of ACh alone), but with a normalizing ACh application interleaved between experimental applications. Data were then normalized to the immediately preceding application of 3 μm ACh. We measured the effect of Zn2+ in two ways. First, because most responses of both receptors showed appreciable desensitization, we measured the effect of Zn2+ as described above for desensitizing receptors. Second, the peak response to Zn2+ was compared with a 1 sec average of the response to ACh alone taken immediately before Zn2+ application. Because both methods yielded similar results, only the results of the first method are plotted in Figure 8.
Because neuronal nAChRs differ in their sensitivity to ACh, it is important to ensure that the effects of metal application are measured with ACh concentrations that elicit a similar fraction of the maximal response. Therefore, unless noted otherwise, all ACh concentrations were within a range from the EC2 to the EC10 for each receptor. ACh EC2 and EC10 values were calculated from data presented previously (Harvey et al., 1996). Within this range, a single ACh concentration was used for each subunit combination (12 μm for α2β2, 15 μm for α2β4, 4 μm for α3β2, 17 μm for α3β4, 10 μm for α4β2, 1 μm for α4β4).
Zinc solutions used in all experiments shown in this study were prepared from zinc acetate stock solutions. To rule out an effect of the acetate, zinc concentration effect curves for the α3β2, α4β2, and α4β4 receptors were replicated using ZnCl2 with similar results (data not shown). In the absence of ACh, zinc concentrations <70 μm had no effect on α4β4-expressing, α3β2-expressing, or uninjected oocytes. Zinc concentrations ≥70 μm elicited small, slow, variable (inward and outward) currents. Because these currents were always <5% of ACh responses, they were disregarded in our analysis.
Diethylpyrocarbonate treatment of nAChRs. Diethylpyrocarbonate (DEPC) was diluted in perfusion solution immediately before application. Preliminary experiments determined that a 10 min incubation achieved a maximal DEPC effect (data not shown). During incubation, the DEPC solution was exchanged twice at regular intervals. After incubation, the DEPC was washed out of the chamber for an additional 10 min with perfusion solution. Electrophysiological measurements were taken before DEPC application and immediately after the 10 min washout period.
Data analysis. Data from metal concentration–response experiments for α2β2, α2β4, α3β4, α4β2, and α4β4 receptors were analyzed as follows. Concentration–potentiation curves were fit according to the following equation for concentrations up to and including those concentrations of metals that achieved maximal potentiation: I =Imax/(1 + (EC50/X)n), where I represents the current response at a given metal concentration, X; Imax is the maximal current; EC50 is the concentration of metal yielding half-maximal potentiation; n is the Hill coefficient. Concentration–inhibition curves were fit according to the following equation for concentrations of metals at or above those concentrations that achieved maximal potentiation:I = Imax/(1 + (X/IC50)n), where I represents the current response at a given metal concentration, X; Imax is the maximal current; IC50 is the concentrations of metal yielding half-maximal inhibition; n is the Hill coefficient. Because no potentiation was apparent for the α3β2 receptor, the entire data set was fit with the concentration–inhibition equation.
The data presented in Figure 2 suggest that at some concentrations zinc may be exerting both potentiating and inhibiting effects on neuronal nAChRs. If this is true, then the maximal potentiation that we observe may be an underestimate. To examine this issue, we fit the zinc concentration–effect data to a more complex equation that included both a potentiating and an inhibitory site: I =Imin + (Imax − Imin){[1/(1 + (EC50/X)n)] − [1/(1 + (IC50/X)m)]}, where I represents the current response at a given metal concentration, X; Imin is the minimal current; Imax is the maximal current; EC50 and IC50 are the concentrations of metal yielding half-maximal potentiation and inhibition, respectively; n and m are the Hill coefficients for potentiation and inhibition, respectively (Harvey et al., 1999). Results of this analysis suggest that the maximal potentiation that we observe is indeed an underestimate. Fitting to this equation suggests that the true maximal potentiation is severalfold greater than what we observe. However, because the measured data covers only the lower portion of the putative full curve, we have reported only the results of fitting the potentiating and inhibiting data sets separately.
ACh concentration–response curves in Figure 8 were fit to the following equation: I =Imax/(1 + (EC50/X)n), where I represents the current response at a given ACh concentration, X; Imax is the maximal current; EC50 is the concentration of ACh yielding half-maximal response; n is the Hill coefficient.
Prism software (GraphPad, San Diego, CA) was used to fit the data and to assess statistical significance using a two-tailed unpairedt test.
RESULTS
Zn2+ modulates neuronal nAChRs
Simple heteromeric neuronal nAChRs consisting of one type of α subunit (α2, α3, or α4) and one type of β subunit (β2 or β4) were expressed in Xenopus oocytes. Current responses were recorded under two-electrode voltage clamp on application of ACh (at or below the EC10 for each receptor) in the absence or presence of various concentrations of Zn2+. The effect of Zn2+ varied depending on the subunit composition of the receptor.
Zn2+ application had a biphasic effect on ACh responses of α4β2-expressing oocytes. Zn2+ concentrations in the range of 1–100 μm increased the inward current elicited by 10 μm ACh (Figs.1A, top trace, 2C). A maximal potentiation of 260 ± 17% was achieved with 70 μmZn2+, with an EC50for potentiation of 16 ± 4 μmZn2+. At higher Zn2+ concentrations, the degree of potentiation by Zn2+ was diminished until at 1 mm Zn2+, steady current in response to ACh + Zn2+ was less than current in response to ACh alone (Figs. 1A,bottom trace, 2C). This apparent inhibition of α4β2 receptors by Zn2+ had an IC50 of 440 ± 140 μm.
In contrast to the biphasic effect of Zn2+on α4β2 nAChRs, α3β2 receptors exhibited only inhibition by Zn2+ (Fig. 1B,2B). Reduction in the current elicited by 4 μm ACh occurred with Zn2+ concentrations ranging from 1 μm to 3 mm, with an IC50 of 97 ± 16 μm. Zn2+ concentrations ≥1 mm almost completely blocked the response to ACh. The loss of Zn2+ potentiation on changing the α subunit from α4 to α3 supports a role for the α subunit in mediating potentiation.
To determine whether β subunits also play a role in Zn2+ potentiation of nAChRs, we examined the effect of Zn2+ on the α3β4 receptor (Fig. 2B). Current in response to 17 μm ACh was potentiated to a maximum of 140 ± 2% at 200 μmZn2+, with an EC50of 47 ± 9 μmZn2+. Similar to our results with α4β2 receptors, high concentrations of Zn2+inhibited the α3β4 receptor (IC50 = 3200 ± 1400 μmZn2+).
The ability of α4β2 and α3β4, and the failure of α3β2 nAChRs, to be potentiated by Zn2+ suggests that the α4 and β4 subunits are each capable of supporting Zn2+ potentiation when they are present in a receptor. When both of these subunits were present in the same receptor (α4β4), the effect of Zn2+was dramatic (Figs. 1C, 2C). The α4β4 receptor was potentiated to a maximum of 560 ± 17% at 100 μm Zn2+, with an EC50 of 22 ± 4 μmZn2+. Again, higher concentrations of Zn2+ were found to be inhibitory, with an IC50 of 510 ± 37 μmZn2+.
The α2 subunit is highly homologous to the α4 subunit. As might be expected, α2β2 and α2β4 receptors were also potentiated by low Zn2+ concentrations and inhibited by high Zn2+ concentrations (Fig.2A; Table 1).
Table 1.
Potentiation and inhibition of neuronal nAChRs by Zn2+
| Receptor | Potentiation | Inhibition | |||
|---|---|---|---|---|---|
| Maximum (%) | EC50(μm) | nH | IC50(μm) | nH | |
| α2β2 | 140 ± 4 | 13 ± 7 | 1.5 ± 0.7 | 52 ± 30 | 1.3 ± 0.4 |
| α2β4 | 200 ± 5 | 45 ± 6 | 1.4 ± 0.2 | 590 ± 180 | 1.8 ± 0.3 |
| α3β2 | 97 ± 16 | 0.6 ± 0.1 | |||
| α3β4 | 140 ± 2 | 47 ± 9 | 1.2 ± 0.2 | 3200 ± 1400 | 1.3 ± 0.6 |
| α4β2 | 260 ± 17 | 16 ± 4 | 1.5 ± 0.5 | 440 ± 140 | 1.3 ± 0.6 |
| α4β4 | 560 ± 17 | 22 ± 4 | 1.4 ± 0.3 | 510 ± 37 | 2.5 ± 0.4 |
| α3-216-α4β4 | 170 ± 5 | 9 ± 3 | 1.6 ± 0.7 | 1100 ± 700 | 1.4 ± 0.9 |
| α4-216-α3β4 | 250 ± 10 | 26 ± 4 | 1.6 ± 0.4 | 660 ± 62 | 2.3 ± 0.4 |
Maximum ACh-induced current in the presence of Zn2+is presented as a percentage of the control response to ACh alone (see Materials and Methods). For potentiation, EC50 and Hill coefficient (nH) values were determined by fitting to a Hill equation, and for inhibition, IC50 and Hill coefficient (nH) values were determined by fitting to a separate Hill equation (see Materials and Methods). All values are the mean ± SEM of three to six oocytes.
When high concentrations of Zn2+ are coapplied with ACh to some subunit combinations, the current responses can be complex. A good example can be seen when 3 mmZn2+ is coapplied to the α4β4 receptor (Fig. 1C, bottom trace). On coapplication of Zn2+, a rapid transient potentiation is seen, followed by a rapid decrease in the response. On removal of Zn2+, there is again a rapid potentiation of the current followed by a slow decline toward the response amplitude seen with ACh alone. These potentiation transients can be explained by considering the relatively slow fluid exchange rate in the perfusion chamber. When a high concentration of Zn2+is applied, the concentration of Zn2+ in the chamber will pass through a concentration range that potentiates but does not inhibit the receptors, before reaching a final concentration that both potentiates and inhibits the receptors. Similarly, when Zn2+ is withdrawn, the declining concentration in the chamber will again pass through a potentiating, but not inhibiting, concentration range. To determine whether this explanation is valid, we used a revised protocol in which Zn2+ alone was applied to α4β4-expressing oocytes first, followed by coapplication of Zn2+ and ACh. With this protocol, the potentiation transients were eliminated (Fig.3A). The Zn2+ concentration–effect relationship obtained with this modified protocol (Fig. 3B) was similar to results presented in Figure 2C.
Modulation of nAChRs by cadmium and nickel
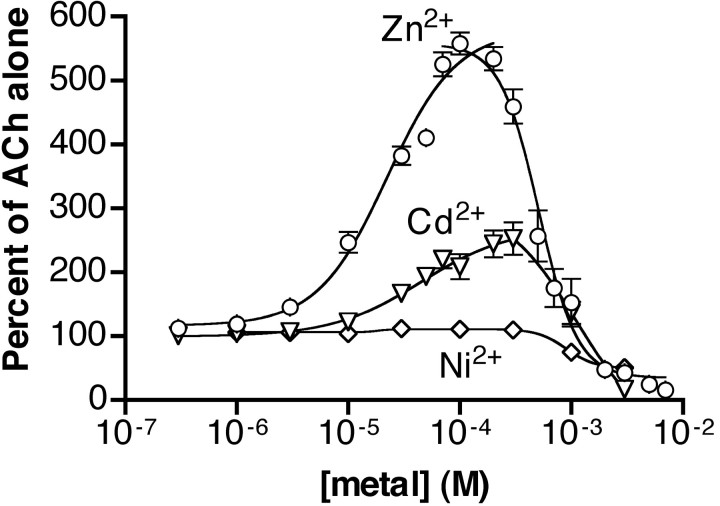
We explored the selectivity of the potentiation by examining the effect of additional transition metals on the α4β4 receptor (Fig.4). Cd2+ was able to potentiate α4β4 receptors with a maximal potentiation to 250 ± 26% and an EC50 of 45 ± 24 μm Cd2+. At 1 mmCd2+ the potentiation was diminished, and at 3 mm Cd2+ the Ach-induced response was almost completely inhibited. In contrast to Zn2+ and Cd2+, Ni2+had almost no ability to potentiate the ACh response. The maximal potentiation by Ni2+ was only to 112 ± 5% of the response to ACh alone. Concentrations of Ni2+ at or above 1 mminhibited the ACh response.
Potential involvement of histidine residues in mediating Zn2+ potentiation
Histidine residues are often involved in coordinating Zn2+ at Zn2+binding sites. DEPC, under some conditions (pH ≥ 6, low millimolar concentrations), can selectively modify the imidazole ring of histidine, eliminating its ability to coordinate Zn2+ ions. We examined the effects of DEPC treatment on the ability of Zn2+ to modulate α4β4 and α3β2 receptors (Fig.5). A 10 min incubation with DEPC concentrations ranging from 100 μm to 3 mmhad little effect on the response of α4β4 receptors to ACh. Although treatment with 100 μm DEPC had a minimal effect on potentiation by 100 μmZn2+ (Fig. 5B), treatment with 1 or 3 mm DEPC abolished the ability of Zn2+ to potentiate the α4β4 receptor (Fig. 5A,B). This result suggests the involvement of at least one histidine residue in mediating the potentiating effects of Zn2+ on neuronal nAChRs. We examined the effect of 3 mm DEPC on modulation of α4β4 receptors by a range of Zn2+ concentrations (Fig. 5C). Although potentiation was eliminated, Zn2+continued to inhibit α4β4 receptors after DEPC treatment. DEPC (3 mm) also failed to affect Zn2+ inhibition of α3β2 receptors (Fig. 5D). The ability of Zn2+to inhibit α4β4 or α3β2 after DEPC treatment suggests that the inhibitory site on these receptors may not involve histidine residues.
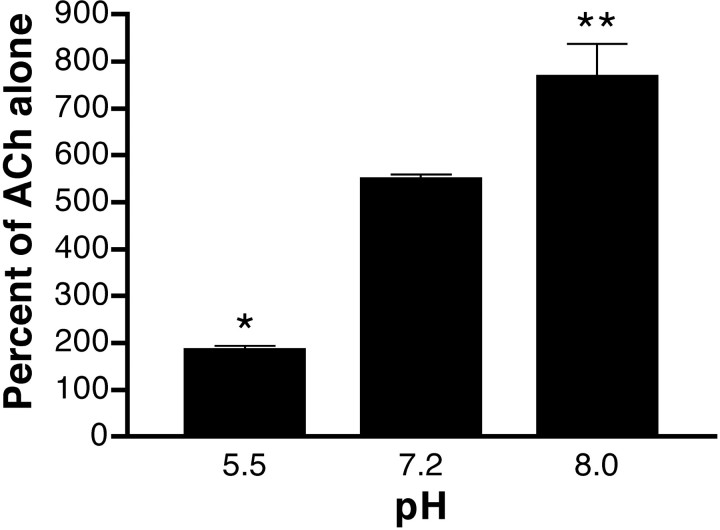
To provide further evidence that histidine residues are involved in mediating Zn2+ potentiation of α4β4, we examined the effect of altering the pH (Fig.6). At pH 5.5, the extent of potentiation by 100 μm Zn2+ was significantly reduced (180 ± 9%) as compared with potentiation at pH 7.2 (our standard conditions). Increasing the pH to 8.0 resulted in a significant increase in the magnitude of the Zn2+ effect (770 ± 70%). These results are consistent with a role for one or more histidine residues in mediating the potentiating effect of Zn2+.
Zn2+-mediated potentiation and inhibition of neuronal nAChRs are voltage independent
To assess the proximity of the Zn2+binding sites to the electrical field of the membrane, we examined potentiation of α4β4 and inhibition of α3β2 receptors over a range of holding potentials. The potentiation of the ACh response of α4β4 receptors by 50 μmZn2+ was examined at several holding potentials ranging from −90 mV to −40 mV (Fig.7B). This Zn2+ concentration was chosen to minimize any influence from the inhibition seen at higher Zn2+ concentrations. The extent of potentiation was similar at all holding potentials tested (Fig.7A,B). The degree of inhibition of α3β2 nAChR current responses by 100 μmZn2+ was also independent of the holding potential from −90 mV to −40 mV (Fig. 7B).
Zn2+ potentiates the response of neuronal nAChRs to saturating acetylcholine concentrations
We examined the effect of Zn2+ on the ACh concentration–response relationships of α4β4 and α4β2 receptors (see Materials and Methods). Again, 50 μmZn2+ was chosen to provide potentiation with minimal inhibition. Zn2+coapplications significantly increased the response of α4β4 to saturating ACh concentrations (160 ± 11% of the response to ACh alone; p < 0.02) and significantly decreased the EC50 for ACh activation from 74 ± 22 μm in the absence of Zn2+ to 23 ± 8 μm in the presence of Zn2+ (p < 0.05) (Fig. 8A). Zn2+ coapplication also significantly increased the response of α4β2 to saturating ACh concentrations (140 ± 14% of the response to ACh alone; p < 0.02) but had no significant effect on the apparent ACh affinity of α4β2 (Fig. 8B).
Zn2+ does not alter receptor desensitization rate
One possible mechanism for Zn2+potentiation is through an effect on receptor desensitization. If Zn2+ were to slow the desensitization rate of a receptor, an apparent potentiation of the agonist response would result. This possible mechanism seems unlikely to account for Zn2+ potentiation of α4β4, which can be dramatically potentiated even when no appreciable desensitization is evident (Fig. 1C). However, to examine this issue in more detail, we measured the desensitization rate of α4β4 and α4β2 receptors when exposed to an EC50 concentration of ACh in the absence and presence of 50 μmZn2+. Oocytes were held at −40 mV and Ba2+ was substituted for Ca2+ in all solutions to minimize the contribution of the Ca2+-activated Cl− channel (see Materials and Methods). For α4β4, there was no difference between desensitization rate for ACh alone (τ = 88 ± 13 sec) and the desensitization rate in the presence of Zn2+ (τ = 77 ± 17 sec) (n = 6). For α4β2, there was no difference between the desensitization rate for ACh alone (τ = 42 ± 3 sec) and the desensitization rate in the presence of Zn2+ (τ = 43 ± 7 sec) (n = 5). We conclude that Zn2+ does not affect the rate of Ach-induced desensitization for these receptors.
Determinants of Zn2+ potentiation are only partially localized to the N-terminal extracellular domain
We used chimeras of the α3 and α4 subunits to provide preliminary information regarding the location of amino acid residues involved in mediating Zn2+ potentiation (Fig. 9). The α4–216-α3 and α3–216-α4 subunits each consist of the N-terminal extracellular domain of one subunit joined to the remainder of the other subunit. Each of the chimeras was coexpressed with the β4 subunit, and the resulting receptors were then examined for sensitivity to a range of Zn2+ concentrations using our standard protocol (see Materials and Methods). Receptors in which the N-terminal extracellular domain of α4 has been replaced with the α3 sequence (α3–216-α4 β4) showed a dramatic loss in sensitivity to Zn2+ potentiation. However, 170 ± 5% potentiation at 100 μmZn2+ was still significantly greater than the potentiation seen with α3β4. Receptors in which the region of α4 containing the transmembrane and cytoplasmic domains has been replaced with the α3 sequence (α4–216-α3 β4) also showed a loss in sensitivity to Zn2+ potentiation when compared with α4β4. However, with a maximal potentiation of 250 ± 10% at 100 μmZn2+, the α4–216-α3 β4 receptors were potentiated to a greater degree than α3–216-α4 β4 or α3β4 receptors. These results suggest that although the most critical determinants of Zn2+ potentiation are located in the N-terminal extracellular domain of α4, important residues also reside in the remainder of the subunit.
Fig. 9.
Zn2+ modulation of receptors formed by chimeric α subunits. The effects of Zn2+coapplication on the Ach-induced current responses of α4–216-α3 β4 (●) and α3–216-α4 β4 (▴) receptors are plotted as a percentage of the response to ACh alone (mean ± SEM of 3 oocytes). Some error bars are obscured by symbols. The potentiation and inhibition curves for α4β4 and α3β4 taken from Figure 2 are shown for comparison (dashed lines).
DISCUSSION
We have demonstrated that neuronal nAChRs are modulated by Zn2+ in a subunit-dependent manner. The α2β2, α2β4, α3β4, α4β2, and α4β4 receptors are potentiated by low Zn2+ concentrations (1–100 μm) and inhibited by high concentrations of Zn2+ (>100 μm). In contrast, the α3β2 receptors exhibit only inhibition of Ach-induced currents on Zn2+ coapplication. Cd2+ coapplication also modulated the ACh response of α4β4 receptors in a biphasic manner, but potentiation by Cd2+ was much less than that seen with Zn2+. A role for histidine residues in mediating Zn2+ potentiation was suggested by the ability of DEPC to abolish potentiation of α4β4 receptors and by the sensitivity of potentiation to alterations in pH. Zn2+ coapplication potentiated the α4β4 and α4β2 receptors even at saturating ACh concentrations. However, Zn2+ had no effect on the rate of Ach-induced desensitization of either receptor.
Zn2+ has been shown to act as a subtype-dependent modulator of other classes of ligand-gated ion channels such as ATP, GABA, glutamate, and glycine receptors (Mayer et al., 1989; Rassendren et al., 1990; Cloues et al., 1993; Bloomenthal et al., 1994; Krishek et al., 1998; Harvey et al., 1999; Xiong et al., 1999; Laube et al., 2000). Within the glutamate and ATP receptor families, for example, some subtypes are modulated in a biphasic manner (showing potentiation, then inhibition as the Zn2+ concentration is increased), whereas others are inhibited only by Zn2+. This is similar to what we have observed with neuronal nAChRs. The effects of Zn2+ on NMDA-type glutamate receptors are particularly complex. Both voltage-dependent and voltage-independent inhibition as well as potentiation of NMDA receptors have been observed, depending on the particular receptor subunit combination, subunit splice variant, and Zn2+concentration (Mayer et al., 1989; Hollmann et al., 1993; Paoletti et al., 1997; Traynelis et al., 1998).
Neuronal nAChRs have previously been shown to be potentiated by extracellular calcium (Mulle et al., 1992; Vernino et al., 1992). Calcium exerts its potentiating effect on neuronal nAChRs by increasing the probability of channel opening (Amador and Dani, 1995). Calcium appears to be bound by a series of EF-hand binding domains, and two critical glutamate residues on the α7 subunit have been identified (Galzi et al., 1996). These glutamate residues, as well as other components of the putative EF-hand structures, are conserved in the α and β subunits used in our study. We think it is unlikely that Zn2+ potentiation operates through these Ca2+ binding sites for the following reasons. First, both α7 and α3β2 receptors are potentiated by Ca2+ and contain the putative EF-hand structures (Vernino et al., 1992; Galzi et al., 1996) but are not potentiated by Zn2+ (Palma et al., 1998) (Fig. 2B). Second, the ability of DEPC and pH changes to reduce Zn2+ potentiation (Figs. 5, 6) suggests the involvement of histidine residues in coordinating Zn2+. Histidines are not generally found in EF-hand Ca2+ binding structures and are not present in the putative EF-hand structures in any of the subunits used in our study.
Neuronal nAChRs have also been shown to be modulated by various other agents. A group of compounds typified by physostigmine and galanthamine can activate exogenously expressed α4β2 receptors and α7-containing receptors expressed in hippocampal neurons by interacting with an allosteric site distinct from the ACh binding site (Pereira et al., 1993, 1994; Schrattenholz et al., 1996). Lead inhibits α3β4 and α4β2 receptors at submicromolar concentrations and potentiates α3β2 receptors at concentrations >100 μm(Zwart et al., 1995). Low concentrations (1–10 μm) of (+)-tubocurarine potentiate β4-containing receptors (α2β4 and α3β4) while inhibiting β2-containing receptors (α2β2 and α3β2) (Cachelin and Rust, 1994). Atropine potentiates α4β2 and α4β4 receptors (but not α2β2, α2β4, α3β2, or α3β4 receptors) responding to low (1 μm) ACh concentrations while inhibiting responses to high (1 mm) ACh concentrations (Zwart and Vijverberg, 1997). The pattern of potentiation and inhibition of the various receptor subtypes seen with these agents varies markedly from what we have found with Zn2+, suggesting that Zn2+ modulation of neuronal nAChRs is unrelated to modulation caused by these other agents.
We used DEPC to test for the involvement of histidine residues in Zn2+ potentiation of nAChRs. Reaction of DEPC with histidine results in modification of the imidazole ring, rendering it incapable of coordinating Zn2+ (Miles, 1977; Lundblad and Noyes, 1984). A role for histidine residues in Zn2+ modulation of glycine receptors has been revealed using this technique (Harvey et al., 1999). DEPC can also affect arginine, lysine, cysteine, serine, and tyrosine residues when used under conditions of high concentration (≥10 mm) and low pH (pH 4). However, under our conditions (≤3 mm, pH 7.2), DEPC should be selective for histidine residues (Miles, 1977). We found that Zn2+ potentiation of neuronal nAChRs could be abolished by DEPC treatment. In contrast, Zn2+ continued to inhibit α4β4 and α3β2 after DEPC treatment.
Histidine residues are also prevented from coordinating Zn2+ by protonation of both imidazole nitrogens. The first pKa ranges from 6.0 to 6.5, depending on the local environment. If we assume apKa of 6.25, then at pH 7.2 (our standard conditions), 89% of the imidazole rings would have an unprotonated nitrogen capable of coordinating Zn2+. Shifting the pH below thepKa would reduce the fraction of unprotonated histidines and should reduce histidine coordination of Zn2+. At pH 5.5, at which only 18% of histidines would be unprotonated, potentiation of α4β4 was reduced to only 180 ± 9% of ACh alone. At pH 8.0, at which 98% of histidine residues would be unprotonated, potentiation of α4β4 increased to 770 ± 70% of ACh alone. The sensitivity of Zn2+ potentiation to both DEPC treatment and pH changes strongly suggests a role for histidine residues. These histidine residues may be involved in directly coordinating the Zn2+ ion. However, we cannot rule out the possibility that DEPC and changes in pH are affecting a histidine within an allosteric pathway through which Zn2+ might exert its potentiating effects.
To approximate the location of the Zn2+binding sites in relation to the electric field of the membrane, we examined Zn2+ potentiation and inhibition at several holding potentials (Fig. 7). The degree of potentiation of α4β4 receptors and inhibition of α3β2 receptors remained constant across a range of holding potentials. This result suggests that potentiation and inhibition are not under the influence of the electric field of the membrane, and thus the relevant binding sites are not likely to be closely associated with the transmembrane domains of the receptor. We also examined the Zn2+sensitivity of receptors formed by chimeras of the α3 and α4 subunits (Fig. 9). Results of these experiments suggest that determinants of Zn2+ potentiation are located within the N-terminal extracellular domain, as well as in the remainder of the protein.
It is clear that both α and β subunits make contributions to Zn2+ potentiation of neuronal nAChRs. Possible explanations for this observation include a Zn2+ binding site on each individual subunit yielding five binding sites, or Zn2+ binding sites formed at the interface between α and β subunits yielding at least two binding sites. The Hill coefficient values between 1.0 and 2.0 that we have observed for Zn2+ potentiation (Table 1) suggest that a neuronal nAChR may have one or two Zn2+potentiation sites. If nAChRs have a single site, it might be similar to the Ni2+ binding site of retinal cyclic nucleotide-gated (CNG) channels (Shammat and Gordon, 1999). At least two subunits contribute a histidine residue to form a single Ni2+ site during the open state of the CNG channel (Gordon and Zagotta, 1995a,b). If neuronal nAChRs have two Zn2+ potentiation sites, the sites might be formed similarly to agonist binding sites (at the interface between two subunits). In either case, stabilization of the open state would explain our observation that Zn2+increases the response to saturating ACh concentrations.
Knowledge regarding the effect of Zn2+ on neuronal nAChRs expressed in a neuronal context is limited. Nutter and Adams (1995) reported inhibition of ACh-evoked currents in cultured rat parasympathetic neurons. Although the neuronal nAChR subunit expression in these neurons is heterogeneous, the predominant subunits are α3, α7, and a varying ratio of β2 and β4 (Poth et al., 1996, 1997). Thus, based on our results and the results of Palma et al., (1998), many of the nAChRs expressed by these neurons would be expected to be inhibited by Zn2+.
CNS synaptic terminals have been shown to be capable of taking up, storing, and releasing Zn2+ (Huang, 1997;Frederickson et al., 2000). Extracellular Zn2+ is estimated to reach concentrations as high as several hundred micromolar during neuronal activity (Frederickson et al., 1983; Assaf and Chung, 1984). Neuronal nAChRs are located both presynaptically and postsynaptically in many parts of the CNS and PNS. Our finding that Zn2+modulates neuronal nAChRs at concentrations that may be achieved in the nervous system suggests that Zn2+ may affect synaptic activity through modulation of neuronal nAChRs.
Footnotes
This work was supported by a grant to C.W.L. from the National Institute on Drug Abuse (DA08102). B.H. and D.D. were supported in part by National Heart, Lung, and Blood Institute T32HL07188. B.H. was supported in part by a PhRMA Foundation Medical Student Research Fellowship. B.H. is a Lois Pope LIFE Fellow. We thank Drs. Richard Kramer and Jeff Krajewski for helpful discussions and Ana Mederos for technical assistance.
Correspondence should be addressed to Dr. Charles W. Luetje, Department of Molecular and Cellular Pharmacology (R-189), University of Miami School of Medicine, P.O. Box 016189, Miami, FL 33101. E-mail:cluetje@chroma.med.miami.edu.
REFERENCES
- 1.Amador M, Dani JA. Mechanism for modulation of nicotinic acetylcholine receptors that can influence synaptic transmission. J Neurosci. 1995;15:4525–4532. doi: 10.1523/JNEUROSCI.15-06-04525.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anand R, Conroy WG, Schoepfer R, Whiting P, Lindstrom J. Neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes have a pentameric quaternary structure. J Biol Chem. 1991;266:11192–11198. [PubMed] [Google Scholar]
- 3.Assaf SY, Chung S-H. Release of endogenous Zn2+ from brain tissue during activity. Nature. 1984;308:734–736. doi: 10.1038/308734a0. [DOI] [PubMed] [Google Scholar]
- 4.Bloomenthal AB, Goldwater E, Pritchett DB, Harrison NL. Biphasic modulation of the strychnine-sensitive glycine receptor by zinc. Mol Pharmacol. 1994;46:1156–1159. [PubMed] [Google Scholar]
- 5.Cachelin AB, Rust G. Unusual pharmacology of (+)-tubocurarine with rat neuronal nicotinic acetylcholine receptors containing β4 subunits. Mol Pharmacol. 1994;46:1168–1174. [PubMed] [Google Scholar]
- 6.Chen D, Patrick JW. The alpha-bungarotoxin-binding nicotinic acetylcholine receptor from rat brain contains only the alpha7 subunit. J Biol Chem. 1997;272:24024–24029. doi: 10.1074/jbc.272.38.24024. [DOI] [PubMed] [Google Scholar]
- 7.Cloues R, Jones S, Brown DA. Zinc potentiates ATP-activated currents in rat sympathetic neurons. Pflügers Arch. 1993;424:152–158. doi: 10.1007/BF00374606. [DOI] [PubMed] [Google Scholar]
- 8.Conroy WG, Berg DK. Neurons can maintain multiple classes of nicotinic acetylcholine receptors distinguished by different subunit compositions. J Biol Chem. 1995;270:4424–4431. doi: 10.1074/jbc.270.9.4424. [DOI] [PubMed] [Google Scholar]
- 9.Cooper E, Couturier S, Ballivet M. Pentameric structure and subunit stoichiometry of a neuronal nicotinic acetylcholine receptor. Nature. 1991;350:235–238. doi: 10.1038/350235a0. [DOI] [PubMed] [Google Scholar]
- 10.Corringer PJ, Le Novere N, Changeux JP. Nicotinic receptors at the amino acid level. Annu Rev Pharmacol Toxicol. 2000;40:431–458. doi: 10.1146/annurev.pharmtox.40.1.431. [DOI] [PubMed] [Google Scholar]
- 11.Draguhn A, Verdorn TA, Ewert M, Seeburg PH, Sakmann B. Functional and molecular distinction between recombinant rat GABAA receptor subtypes by Zn2+. Neuron. 1990;5:781–788. doi: 10.1016/0896-6273(90)90337-f. [DOI] [PubMed] [Google Scholar]
- 12.Drisdel RC, Green WN. Neuronal α-bungarotoxin receptors are α7 subunit homomers. J Neurosci. 2000;20:133–139. doi: 10.1523/JNEUROSCI.20-01-00133.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flores CM, Rogers SW, Pabreza LA, Wolfe BB, Kellar KJ. A subtype of nicotinic cholinergic receptor in rat brain is composed of α4 and β2 subunits and is up-regulated by chronic nicotine treatment. Mol Pharmacol. 1992;41:31–37. [PubMed] [Google Scholar]
- 14.Frederickson CJ, Klitenick MA, Manton WI, Kirkpatrick JB. Cytoarchitectonic distribution of zinc in the hippocampus of man and the rat. Brain Res. 1983;273:335–339. doi: 10.1016/0006-8993(83)90858-2. [DOI] [PubMed] [Google Scholar]
- 15.Frederickson CJ, Suh SW, Silva D, Frederickson CJ, Thompson RB. Importance of zinc in the central nervous system: the zinc-containing neuron. J Nutr. 2000;130:1471S–1483S. doi: 10.1093/jn/130.5.1471S. [DOI] [PubMed] [Google Scholar]
- 16.Galzi JL, Bertrand S, Corringer PJ, Changeux JP, Bertrand D. Identification of calcium binding sites that regulate potentiation of a neuronal nicotinic acetylcholine receptor. EMBO J. 1996;15:5824–5832. [PMC free article] [PubMed] [Google Scholar]
- 17.Gordon SE, Zagotta WN. A histidine residue associated with the gate of the cyclic nucleotide-activated channels in rod photoreceptors. Neuron. 1995a;14:177–183. doi: 10.1016/0896-6273(95)90252-x. [DOI] [PubMed] [Google Scholar]
- 18.Gordon SE, Zagotta WN. Subunit interactions in coordination of nickel in cyclic nucleotide-gated channels. Proc Natl Acad Sci USA. 1995b;92:10222–10226. doi: 10.1073/pnas.92.22.10222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harvey SC, Maddox FN, Luetje CW. Multiple determinants of dihydro-β-erythroidine sensitivity on rat neuronal nicotinic receptor α subunits. J Neurochem. 1996;67:1953–1959. doi: 10.1046/j.1471-4159.1996.67051953.x. [DOI] [PubMed] [Google Scholar]
- 20.Harvey RJ, Thomas P, James CH, Wilderspin A, Smart TG. Identification of an inhibitory Zn2+ binding site on the human glycine receptor α1 subunit. J Physiol (Lond) 1999;520.1:53–64. doi: 10.1111/j.1469-7793.1999.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hollmann M, Boulter J, Maron C, Beasley L, Sullivan J, Pecht G, Heinemann S. Zinc potentiates agonist-induced currents at certain splice variants of the NMDA receptor. Neuron. 1993;10:943–954. doi: 10.1016/0896-6273(93)90209-a. [DOI] [PubMed] [Google Scholar]
- 22.Howell GA, Welch MG, Frederickson CJ. Stimulation-induced uptake and release of zinc in hippocampal slices. Nature. 1984;308:736–738. doi: 10.1038/308736a0. [DOI] [PubMed] [Google Scholar]
- 23.Huang EP. Metal ions and synaptic transmission: think zinc. Proc Natl Acad Sci USA. 1997;94:13386–13387. doi: 10.1073/pnas.94.25.13386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krishek BJ, Moss SJ, Smart TG. Interaction of H+ and Zn2+ on recombinant and native rat neuronal GABAA receptors. J Physiol (Lond) 1998;507.3:639–652. doi: 10.1111/j.1469-7793.1998.639bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laube B, Kuhse J, Betz H. Kinetic and mutational analysis of Zn2+ modulation of recombinant human inhibitory glycine receptors. J Physiol (Lond) 2000;522.2:215–230. doi: 10.1111/j.1469-7793.2000.t01-1-00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lundblad RL, Noyes CM. Chemical reagents for protein modification. CRC; Boca Raton, FL: 1984. The modification of histidine residues. pp. 105–125. [Google Scholar]
- 27.Mayer ML, Vyklicky L, Jr, Westbrook GL. Modulation of excitatory amino acid receptors by group IIB metal cations in cultured mouse hippocampal neurones. J Physiol (Lond) 1989;415:329–350. doi: 10.1113/jphysiol.1989.sp017724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miles EW. Modification of histidyl residues in proteins with diethylpyrocarbonate. Methods Enzymol. 1977;47:431–442. doi: 10.1016/0076-6879(77)47043-5. [DOI] [PubMed] [Google Scholar]
- 29.Mulle C, Choquet D, Korn H, Changeux JP. Calcium influx through nicotinic receptors in rat central neurons: its relevance to cellular regulation. Neuron. 1992;8:135–143. doi: 10.1016/0896-6273(92)90115-t. [DOI] [PubMed] [Google Scholar]
- 30.Nutter TJ, Adams DJ. Monovalent and divalent cation permeability and block of neuronal nicotinic receptor channels in rat parasympathetic ganglia. J Gen Physiol. 1995;105:701–723. doi: 10.1085/jgp.105.6.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palma E, Maggi L, Miledi R, Eusebi F. Effects of Zn2+ on wild and mutant neuronal α7 nicotinic receptors. Proc Natl Acad Sci USA. 1998;95:10246–10250. doi: 10.1073/pnas.95.17.10246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paoletti P, Ascher P, Neyton J. High-affinity zinc inhibition of NMDA NR1–NR2A receptors. J Neurosci. 1997;17:5711–5725. doi: 10.1523/JNEUROSCI.17-15-05711.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pereira EFR, Reinhardt-Maelicke S, Schrattenholz A, Maelicke A, Albuquerque EX. Identification and functional characterization of a new agonist site on nicotinic acetylcholine receptors of cultured hippocampal neurons. J Pharmacol Exp Ther. 1993;265:1474–1491. [PubMed] [Google Scholar]
- 34.Pereira EFR, Alkondon M, Reinhardt S, Maelicke A, Peng X, Lindstrom J, Whiting P, Albuquerque EX. Physostigmine and galanthamine: probes for a novel binding site on the α4β2 subtype of neuronal nicotinic acetylcholine receptors stably expressed in fibroblast cells. J Pharmacol Exp Ther. 1994;270:768–778. [PubMed] [Google Scholar]
- 35.Poth K, Bookman RJ, Luetje CW. Individual rat intrinsic cardiac neurons display variable nicotinic pharmacologies and neuronal nAChR β subunit mRNA expression patterns. Soc Neurosci Abstr. 1996;22:1172. [Google Scholar]
- 36.Poth K, Nutter TJ, Cuevas J, Parker MJ, Adams DJ, Luetje CW. Heterogeneity of nicotinic receptor class and subunit mRNA expression among individual parasympathetic neurons from rat intracardiac ganglia. J Neurosci. 1997;17:586–596. doi: 10.1523/JNEUROSCI.17-02-00586.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rassendren F-A, Lory P, Pin J-P, Nargeot J. Zinc has opposite effects on NMDA and non-NMDA receptors expressed in Xenopus oocytes. Neuron. 1990;4:733–740. doi: 10.1016/0896-6273(90)90199-p. [DOI] [PubMed] [Google Scholar]
- 38.Role LW. Diversity in primary structure and function of neuronal nicotinic acetylcholine receptor channels. Curr Opin Neurobiol. 1992;2:254–262. doi: 10.1016/0959-4388(92)90112-x. [DOI] [PubMed] [Google Scholar]
- 39.Schrattenholz A, Pereira EFR, Roth U, Weber KH, Albuquerque EX, Maelicke A. Agonist responses of neuronal nicotinic acetylcholine receptors are potentiated by a novel class of allosterically acting ligands. Mol Pharmacol. 1996;49:1–6. [PubMed] [Google Scholar]
- 40.Shammat IM, Gordon SE. Stoichiometry and arrangement of subunits in rod cyclic nucleotide-gated channels. Neuron. 1999;23:809–819. doi: 10.1016/s0896-6273(01)80038-6. [DOI] [PubMed] [Google Scholar]
- 41.Traynelis SF, Burgess MF, Zheng F, Lyuboslavsky P, Powers JL. Control of voltage-independent zinc inhibition of NMDA receptors by the NR1 subunit. J Neurosci. 1998;18:6163–6175. doi: 10.1523/JNEUROSCI.18-16-06163.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vernino S, Amador M, Luetje CW, Patrick J, Dani JA. Calcium modulation and high calcium permeability of neuronal nicotinic acetylcholine receptors. Neuron. 1992;8:127–134. doi: 10.1016/0896-6273(92)90114-s. [DOI] [PubMed] [Google Scholar]
- 43.Whiting PJ, Schoepfer R, Conroy WG, Gore MJ, Keyser KT, Shimasaki S, Esch F, Lindstrom JM. Expression of nicotinic acetylcholine receptor subtypes in brain and retina. Brain Res Mol Brain Res. 1991;10:61–70. doi: 10.1016/0169-328x(91)90057-5. [DOI] [PubMed] [Google Scholar]
- 44.Xiong K, Peoples RW, Montgomery JP, Chiang Y, Stewart RR, Weight FF, Li C. Differential modulation by copper and zinc of P2X2 and P2X4 receptor function. J Neurophysiol. 1999;81:2088–2094. doi: 10.1152/jn.1999.81.5.2088. [DOI] [PubMed] [Google Scholar]
- 45.Zwart R, Vijverberg HPM. Potentiation and inhibition of neuronal nicotinic receptors by atropine: competitive and noncompetitive effects. Mol Pharmacol. 1997;52:886–895. doi: 10.1124/mol.52.5.886. [DOI] [PubMed] [Google Scholar]
- 46.Zwart R, Van Kleef RGDM, Milikan JM, Oortgiesen M, Vijverberg HPM. Potentiation and inhibition of subtypes of neuronal nicotinic acetylcholine receptors by Pb2+. Eur J Pharmacol. 1995;291:399–406. doi: 10.1016/0922-4106(95)90082-9. [DOI] [PubMed] [Google Scholar]