Abstract
The development of neuronal excitability involves the coordinated expression of different voltage-gated ion channels. We have characterized the expression of two sensory neuron-specific tetrodotoxin-resistant sodium channel α subunits, Nav1. (SNS/PN3) and Nav1.9 (SNS2/NaN), in developing rat lumbar dorsal root ganglia (DRGs). Expression of both Nav1.8 and Nav1.9 increases with age, beginning at embryonic day (E) 15 and E17, respectively, and reaching adult levels by postnatal day 7. Their distribution is restricted mainly to those subpopulations of primary sensory neurons in developing and adult DRGs that give rise to unmyelinated C-fibers (neurofilament 200 negative). Nav1.8 is expressed in a higher proportion of neuronal profiles than Nav1.9 at all stages during development, as in the adult. At E17, almost all Nav1.8-expressing neurons also express the high-affinity NGF receptor TrkA, and only a small proportion bind to IB4, a marker for c-ret-expressing (glial-derived neurotrophic factor-responsive) neurons. Because IB4 binding neurons differentiate from TrkA neurons in the postnatal period, the proportion of Nav1.8 cells that bind to IB4 increases, in parallel with a decrease in the proportion of Nav1.8–TrkA co-expressing cells. In contrast, an equal number of Nav1.9 cells bind IB4 and TrkA in embryonic life. The differential expression of Nav1.8 and Nav1.9 in late embryonic development, with their distinctive kinetic properties, may contribute to the development of spontaneous and stimulus-evoked excitability in small diameter primary sensory neurons in the perinatal period and the activity-dependent changes in differentiation they produce.
Keywords: sodium channels, TTXr, dorsal root ganglia (DRG), development, sensory neurons, growth factors
Primary somatosensory neurons show three specific temporal patterns of action potential firing in development (Fitzgerald, 1987; Meister et al., 1991; Fitzgerald and Fulton, 1992). During the period of neurite outgrowth in early embryonic development, dorsal root ganglion (DRG) neurons are electrically inexcitable (Fitzgerald, 1987). This is followed by a period of low-frequency spontaneous activity at embryonic day (E) 16–E20, largely coinciding with peripheral target innervation (E14–E19) (Fitzgerald, 1987; Meister et al., 1991; Fitzgerald and Fulton, 1992). Finally, after the formation of central synapses and peripheral terminals, the spontaneous activity is replaced by higher frequency sensory-evoked activity at which time the neurons adapt to a more differentiated pattern of excitability (Fitzgerald, 1987; Meister et al., 1991). This activity contributes to selective gene expression (Dietzel, 1995; Spitzer et al., 1995; Fields, 1996) promoting neuronal differentiation of both primary sensory (Gu and Spitzer, 1995;Brosenitsch et al., 1998) and spinal cord neurons (Holliday and Spitzer, 1990).
The sodium currents of DRG neurons are divided into two types on the basis of their sensitivity to tetrodotoxin (TTX) (Waxman et al., 1999). All primary sensory neurons express a low activation threshold, fast-inactivating, TTX-sensitive (TTXs) current (Kostyuk et al., 1981;Caffrey et al., 1992; Catterall, 1992; Roy and Narahashi, 1992). Small DRG neurons also display a number of high activation threshold, slowly activating and inactivating TTX-resistant (TTXr) currents (TTX-R1, R2, R3) (McLean et al., 1988; Roy and Narahashi, 1992; Elliott and Elliott, 1993; Arbuckle and Docherty, 1995; Rush et al., 1998). In DRG neurons, the TTXs current is mediated by one or more of the following sodium channel α-subunits known, according to the new standardized nomenclature (Goldin et al., 2000), as Nav1.1 (rat I), Nav1.2 (rat IIa), Nav1.3 (rat III), Nav1.7 (PN-1/hNE) (Toledo-Aral et al., 1997;Cummins et al., 1998), and Nav1.6 (NaCh6/SCN8A/PN4) (Black et al., 1996; Waxman et al., 1999; Tzoumaka et al., 2000), whereas the TTXr current is mediated by two sensory neuron-specific sodium channels, Nav1.8(SNS/PN3) (Akopian et al., 1996; Sangameswaran et al., 1996) and Nav1.9 (SNS2/NaN) (Dib-Hajj et al., 1998b, 1999; Tate et al., 1998; Fjell et al., 2000). The ratio of the TTXs/TTXr current will affect excitability by influencing action potential kinetics, including activation threshold, rate of rise, peak amplitude, duration, and capacity to fire repetitively (Rush el al., 1998). In the adult DRG, Nav1.8 is expressed in both A- and C-fiber neurons (Amaya et al., 2000; Renganathan et al., 2000), whereas Nav1.9 is preferentially expressed in C-fiber neurons (Tate et al., 1998; Amaya et al., 2000; Fjell et al., 2000). Both channels are strongly implicated in the molecular mechanisms of nociception (Okuse et al., 1997; McCleskey and Gold, 1999).
Nav1.3 has a developmentally regulated pattern of expression, peaking at E17 and downregulated by birth (Waxman et al., 1994; Felts et al., 1997). The expression of both Nav1.6 and Nav1.7 increase postnatally, whereas the Nav1.2 and Nax (NaG) α-subunits, which are expressed in large DRG neurons, are constitutively expressed throughout development (Felts et al., 1997). We have now investigated the onset and pattern of expression of the TTXr sodium channels Nav1.8 and Nav1.9 in late embryonic and early postnatal periods.
MATERIALS AND METHODS
Time-mated pregnant Sprague Dawley rats were used for all procedures in accordance with the United Kingdom Animals (Scientific Procedures) Act (1986) and following Massachusetts General Hospital animal care guidelines. Rats were designated postnatal day (P) 0 on the day of parturition.
Tissue preparation. For embryonic tissue, time-mated pregnant rats were terminally anesthetized with CO2 and exsanguinated, and the embryos were harvested and collected in cold PBS. For Northern blot analysis, all lumbar DRGs were removed from two rat litters per age category (E15, E17, E19, and P0) and processed for RNA extraction. For immunohistochemistry, the L4 and L5 DRGs were dissected from overnight post-fixed [4% paraformaldehyde in 0.1 mphosphate buffer (PB), pH 7.4] whole embryos (E15, E17, and E19). DRGs (L4, L5) were dissected from neonatal (P0 and P7) and adult animals after they were killed with intraperitoneal injection of sodium pentobarbital (200 mg/kg) (Duphar) and transcardiac perfusion with 4% paraformaldehyde in 0.1 m PB. DRGs were post-fixed overnight and cryoprotected with 20% sucrose for 3 hr.
Northern blot analysis. Total RNA was extracted from homogenized tissue samples by acid phenol extraction according to the technique of Chomczynski and Sacchi (1987). The RNA (15 μg per sample) was separated on 1.5% formaldehyde–agarose gels and blotted onto Hybond N+ nylon membranes. RNA markers ranging from 0.24 to 9.5 kb (Life Technologies, Gaithersburg, MD) were used to determine transcript size. Filters were hybridized in a solution containing 50% formaldehyde, 5× SSC, 5× Denhardt's solution, 1% SDS, and 100 μg/ml sheared herring sperm DNA at 42°C. The filters were washed in 0.1× SSC and 0.1% SDS at 42°C. Nav1.8 (650 bp), Nav1.9 (500 bp), and cyclophilin (240 bp) probes were prepared as described inCostigan et al. (1998) and Amaya et al. (2000). A 50 ng sample of each probe was radiolabeled by incorporation of 50 μCi of [32P]dCTP and separated from unincorporated nucleotides on Sephadex G-50 columns. At least two independent Northern blots obtained from RNA that was extracted from different pools of animals were used for each observation.
Immunocytochemistry. For colocalization of Nav1.8 or Nav1.9 with IB4 (isolectin B4 from Griffonia simplicifolia) or the A-fiber marker NF200, sections were blocked for 1 hr at room temperature with PBS containing 0.3% Triton X-100 (Tx-100; Sigma, St. Louis, MO) and 20% normal goat serum (Vector Laboratories, Burlingame, CA). Embryonic tissue was pretreated with 3% hydrogen peroxidase (Sigma) for 30 min to reduce endogenous peroxidase background. Primary antibody incubations with rabbit polyclonal antibodies against Nav1.8 or Nav1.9 (1:500 and 1:750 dilutions, respectively) (Amaya et al., 2000) and the mouse monoclonal antibody NF200 (1:200; Sigma) or biotin-conjugated IB4 (40 μg/ml; Sigma) were performed in PBS containing 0.1% Tx-100 overnight at 4°C. Secondary antibody incubation with CY3-conjugated anti-rabbit IgG (1:300; Jackson ImmunoResearch, West Grove, PA) and FITC-conjugated anti-mouse IgG (1:200; Vector Laboratories) or FITC-congugated avidin (1:200; Vector Laboratories) was performed in PBS containing 0.1% Tx-100 for 3 hr at room temperature after three 10 min washes with PBS.
For colocalization of Nav1.8 or Nav1.9 and TrkA, sections were immunostained using red-direct tyramide signal amplification (TSA-red; NEN, Boston, MA). The technique enables double labeling with two antibodies raised in the same host animal without cross-reactivity (Michael et al., 1997). Sections were incubated with primary antibodies Nav1.8 (1:1500) or Nav1.9 (1:3000) in TNB buffer (0.1 m Tris-buffered saline, pH 7.4, containing 1% blocking reagent) overnight at 4°C, followed by three 5 min washes in wash buffer (0.1 m Tris, pH 7.5, 0.15m NaCl, 0.05% Tween 20) and secondary antibody incubation with biotin-conjugated anti-rabbit antiserum (1:100; Vector Laboratories) in TNB buffer for 2 hr at room temperature. After washing (three 5 min washes in wash buffer), sections were incubated with streptavidin-HRP (1:100 in TNB buffer) followed by incubation with flurophore tyramide (1:50 in amplification diluent) for 7–10 min at room temperature. After three washes in TSA wash buffer, sections were incubated with TrkA (1:750, generously provided by Louis Reichardt, University of California at San Francisco) in PBS containing 0.1% Tx-100 at 4°C overnight and processed as for secondary antibody incubation as mentioned above, except using FITC-conjugated anti-rabbit secondary antibody (1:200, Vector Laboratories). Fluorescent images were captured by use of a digital (Nikon Spot) camera, except where confocal microscopy (Leica, Nussloch, Germany) was used for high magnification of embryonic tissue images.
For comparative purposes, duplicate slides were prepared by collecting consecutive 10 μm sections on sequential slides, allowing different antibody combinations to be tested on the same DRG. Sections collected on an individual slide were equally spaced throughout the ganglion (L4) as follows: every 40 μm for E15–E19, 100 μm for P0, and 1200 μm for P7–adult. The first section to be placed on the first slide was chosen randomly in each case.
Data analysis. Nav1.8 and Nav1.9 colocalization with each neuronal marker was performed by computer analysis using Freehand (Macromedia), which enabled positively immunostained cell profiles (cells with a clearly identifiable nucleus) for each antibody to be marked and counted, and overlaying the two images was used to count double-labeled cells. Total cell profiles were determined by enhancing the brightness of the green (FITC) image and counting all cell profiles not previously marked as being single or double immunostained. For analysis only of the E17 age category, the image of the entire DRG was divided into four equal quarters, and one quarter was counted at random. The first section to be photomicrographed for each age category was selected at random, and four to six sections evenly spaced throughout the DRG were counted (n = 5 per age category).
Change in expression of the neuronal markers (TrkA, IB4, and NF200) and Nav1.8 or Nav1.9 in the DRG during development is presented as the proportion of positive profiles per total cell profiles. This was determined as follows: the percentage of positive immunostained profiles per section was averaged for each animal (5–6 sections per animal). Five separate averages (n = 5 animals) were expressed as the mean and SEM to give the final values. No statistically significant variance was found by the ANOVA paired test of the values for TrkA, IB4, and NF200, when expressed as percentage of total profiles, between the two data sets obtained from independent experiments (Nav1.8 or Nav1.9 at different ages). Therefore, these essentially identical data sets were pooled (Table1, % neuronal marker in the total cell population).
Table 1.
The expression of Nav1.8 and Nav1.9 within sensory neuronal subtypes in the DRG with increasing development
| Neuronal marker | Age category | % neuronal marker in total cell population | % Nav1.8 in neuronal marker population | % Nav1.9 in neuronal marker population |
|---|---|---|---|---|
| TrkA | E17 | 79.5 (±1.0) | 78.8 (±1.3) | 55.0 (±3.0) |
| P0 | 78.6 (±0.6) | 63.0 (±3.5) | 48.9 (±2.5) | |
| P7 | 60.2 (±3.2) | 51.1 (±2.0) | 43.7 (±2.6) | |
| P21 | 45.3 (±1.8) | 41.8 (±2.6) | 43.4 (±3.5) | |
| Adult | 42.0 | 49.2 (±5.3) | 45.5 (±6.0) | |
| IB4 | E17 | 15.0 (±0.8) | 13.4 (±1.9) | 44.1 (±3.1) |
| P0 | 28.2 (±0.8) | 21.4 (±2.8) | 64.8 (±3.8) | |
| P7 | 42.3 (±1.2) | 31.5 (±1.6) | 60.5 (±1.3) | |
| P21 | 39.3 (±1.2) | 45.2 (±1.8) | 59.9 (±1.7) | |
| Adult | 40.0 | 55.0 (±6.7) | 59.8 (±6.0) | |
| NF200 | E17 | 21.2 (±1.7) | 11.0 (±1.4) | 0.83 (±2.2) |
| P0 | 35.7 (±1.0) | 14.5 (±1.6) | 8.62 (±2.0) | |
| P7 | 42.4 (±1.5) | 12.1 (±2.6) | 6.64 (±1.4) | |
| P21 | 47.0 (±1.9) | 13.0 (±1.8) | 2.64 (±0.5) | |
| Adult | 40.0 | 18.4 (±2.7) | 0 (±0) |
The change in expression of the neuronal markers (TrkA, IB4, and neurofilament NF200) in the DRG during development is represented as a proportion of positive profiles from the total cell profile population. The change in the distribution of Nav1.8 and Nav1.9 expression within each neuronal subtype is represented as a proportion of Nav1.8 and Nav1.9 positive profiles within the total neuronal marker profile population. Each value represents the mean percentage of positive profiles from 10 animals per age category for the % neuronal marker column, and 5 animals per age group for the Nav1.8 and Nav1.9 columns, with the SEM between animals in parentheses.
RESULTS
Developmental expression of Nav1.8 and Nav1.9 in the DRG
Changes in Nav1.8 and Nav1.9 mRNA and protein expression in rat lumbar DRGs were analyzed at late embryonic (E15, E17, E19) and early neonatal stages (P0) of development.
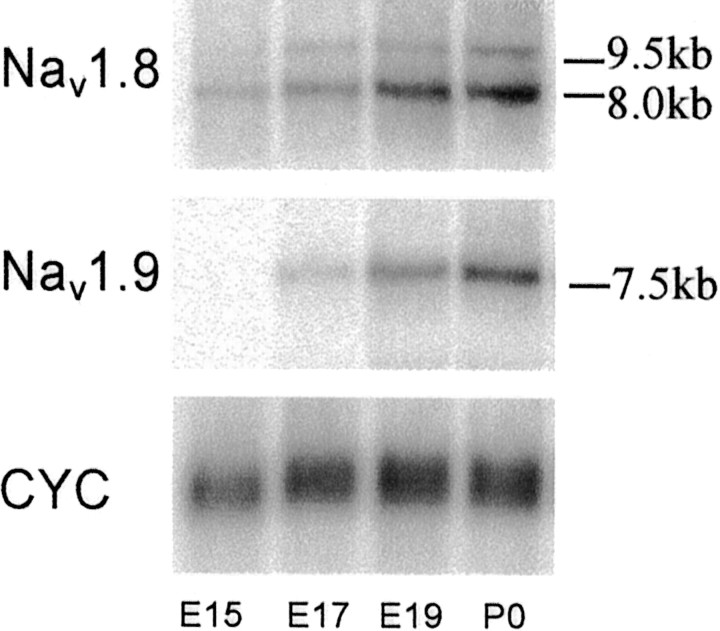
An 8.0 kb Nav1.8 mRNA transcript was detected at low levels in lumbar DRGs at E15. At this stage, no Nav1.9 transcript could be seen. At E17, however, both an Nav1.8 and an Nav1.9 mRNA transcript (7.5 kb) were detectable in the DRG (Fig. 1). A second alternatively spliced Nav1.8 transcript of 9.5 kb, similar to that previously described in the adult (Amaya et al., 2000) could be seen by E17 and at all later stages, which was not present at E15. The relative amount of both the Nav1.8 and Nav1.9 mRNA transcripts increased from E17 to P0 (Fig. 1).
Fig. 1.
The developmental regulation of Nav1.8 and Nav1.9 expression in embryonic DRG. Northern blot analysis of Nav1.8 (top) and Nav1.9 (middle) α-subunit mRNA transcripts in lumbar DRG between developmental ages E15 and P0. Cyclophilin mRNA (CYC, bottom) is a loading control.
The proportion of DRG neuronal profiles expressing Nav1.8 and Nav1.9 protein was assessed at developmental stages E17, P0, P7, and P21, as well as in the adult (Amaya et al., 2000). While a few Nav1.8-labeled profiles could be seen in E15 DRGs (data not shown), Nav1.9-labeled profiles could not be detected until E17, which indicates a coordinated temporal pattern of expression of messenger mRNA with protein expression for both Nav1.8 and Nav1.9. At E17, ∼25% of DRG neurons expressed Nav1.8, and this increased to ∼50% (adult levels) by P7 (Fig.2A). The proportion of Nav1.9-positive neuronal profiles increased from ∼20% at E17 to ∼40% (adult levels) at P7 (Fig.2A). At all developmental ages, significantly fewer neurons express Nav1.9 than Nav1.8 (E17 to P0, p < 0.01; P7 to adult, p < 0.005; ANOVA test) (Fig.2A).
Fig. 2.
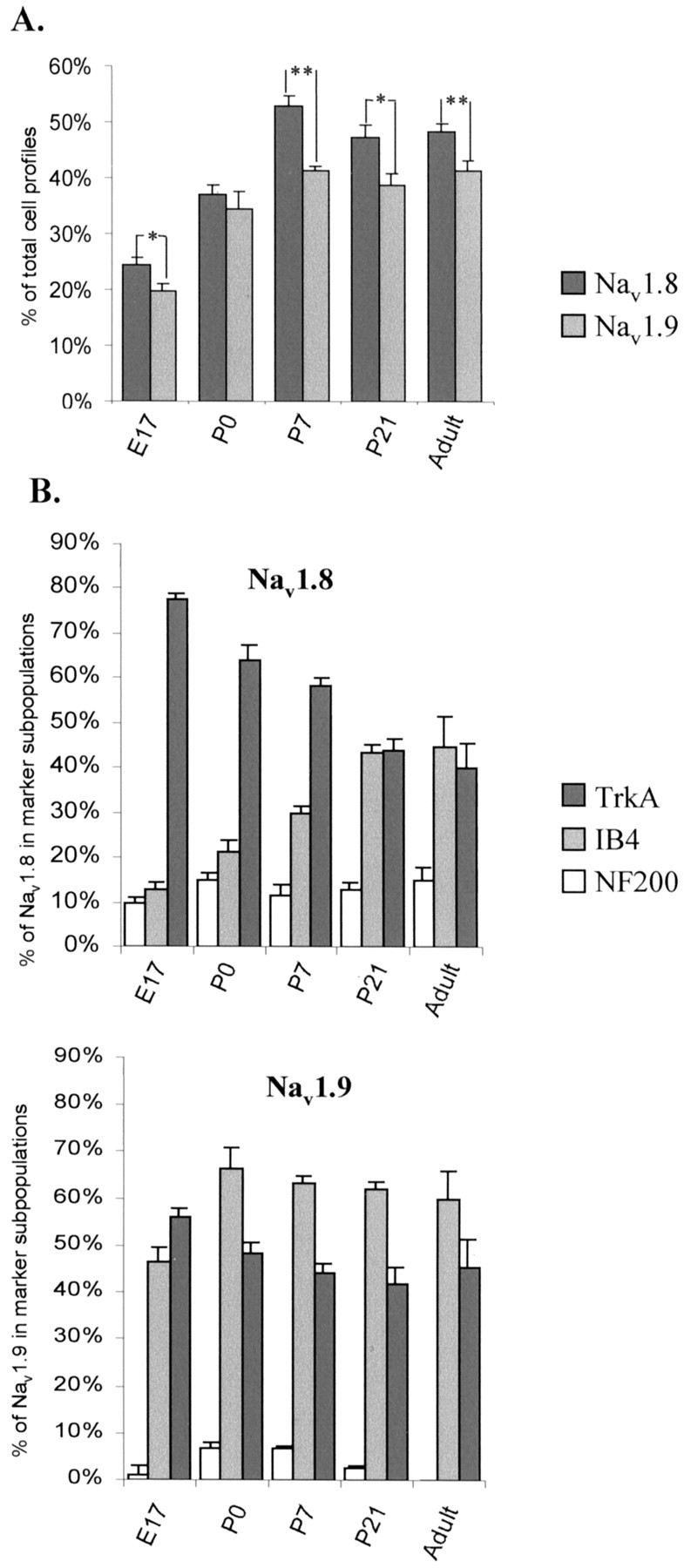
Quantitative analysis of Nav1.8 and Nav1.9 protein expression levels through embryonic and neonatal development. A, The percentage of Nav1.8- or Nav1.9-positive cell profiles as a proportion of the total number of DRG neuronal profiles in the developing DRG. Error bars represent SEM. (*p < 0.01, **p < 0.001, for values for Nav1.8 vs Nav1.9; ANOVA paired test). Levels of SNS and SNS2 protein expression are significantly different (p = 0.001; ANOVA) from E17 to P7, compared with adult Nav1.8 and Nav1.9 expression.B, The proportion of the Nav1.8- and Nav1.9-positive profiles expressed in neuronal subpopulations expressing NF 200, TrkA, and IB4 binding, with increasing development.
Developmental expression of Nav1.8 and Nav1.9 in neuronal subpopulations
Co-expression of Nav1.8 and Nav1.9 with markers of different neuronal subpopulations was studied in the developing DRG. These included TrkA (to identify NGF-responsive, small and medium diameter, peptide-containing neurons) (McMahon et al., 1994; Averill et al., 1995; Molliver et al., 1995), IB4 [to identify nonpeptide containing, glia-derived neurotrophic factor (GDNF) responsive small diameter neurons] (Plenderleith et al., 1988; Plenderleith and Snow, 1993), and neurofilament (NF200; to identify large diameter cells with myelinated axons) (Price, 1985; Lawson and Waddell, 1991;Lawson et al., 1993).
Although the proportion of NF200-positive profiles increased from 21% at E17 to adult levels by P7 (Table 1), the proportion of Nav1.8-expressing neurons that co-expressed this marker was low at all stages of development (11% at E17 and 13% at P7), which is comparable with adult levels of 18% (Fig.2B, Table 1). A very small proportion of Nav1.9-expressing cells (<9%) were found to co-express NF200 at all ages, indicating that this ion channel, as in the adult (Tate et al., 1998; Amaya et al., 2000; Fjell et al., 2000), is predominantly found in cells destined to have unmyelinated axons.
At E17, Nav1.8 was found mainly in TrkA-positive neurons (∼80%) with only 13% IB4-positive cells. The proportion of Nav1.8 cells that were IB4-positive increased postnatally, reaching adult levels of 45% at P21. This was accompanied by a parallel decrease in the proportion of Nav1.8-positive cells also expressing TrkA, so that eventually Nav1.8 is equally represented in both sets of neurons (Fig. 2B, Table 1). As reported previously, the proportion of TrkA-positive cells in the total population decreased from 80% at E17 to 45% at P21, whereas that of IB4 increased from 15 to 40% over this time (Table 1) (Bennett et al., 1996).
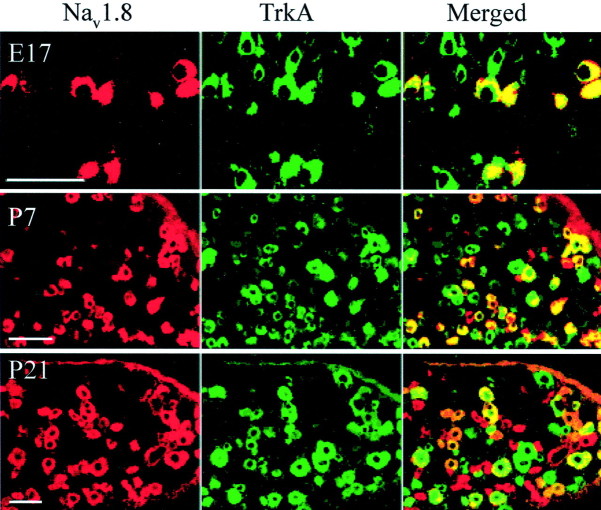
Typical examples of Nav1.8 co-expression with TrkA are shown in Figure 3, illustrating the transition from the majority of Nav1.8-expressing cells being TrkA-positive at E17 to ∼40% colocalization at P21. As in the adult (Amaya et al., 2000), all medium diameter Nav1.8-positive cells were TrkA-positive, although this does not hold true for the reverse situation; some medium TrkA-positive cells did not co-express Nav1.8. In contrast to the Nav1.8 population, many Nav1.9-positive neurons were also IB4-positive at E17 (Fig. 2B, Table 1), although the overall IB4-positive population is low at this stage (15%). The proportion of Nav1.9–IB4 co-expressing cells reached adult levels at P0, despite the fact that the upregulation of IB4 to adult levels is only reached at P7 (Table 1). Figure4 shows representative illustrations of the pattern of colocalization of Nav1.9 with the three different markers at developmental ages E17, P0, and P7. As in the adult, all Nav1.9 profiles possess a small diameter at all ages, unlike Nav1.8 in which ∼20% of the cells have medium-sized diameters.
Fig. 3.
Distribution of Nav1.8 changes with phenotypic changes in the developing DRG. Double immunocytochemistry of Nav1.8 labeled (red), TrkA labeled (green), and composite image (far right panel) showing double labeled (yellow) DRG cells at specific developmental ages. Scale bars, 50 μm.
Fig. 4.
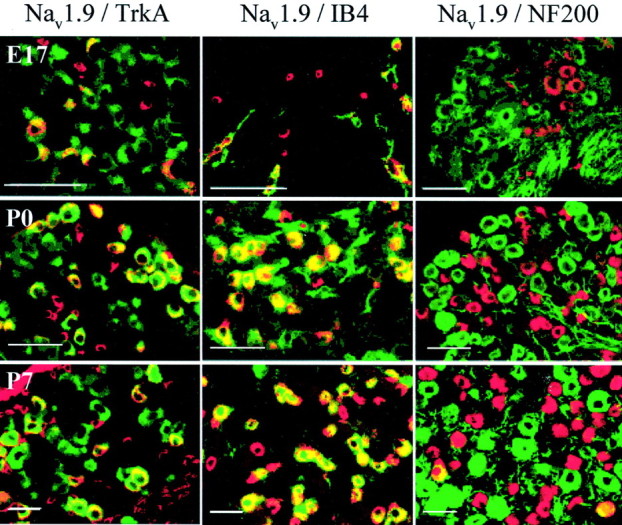
Distribution of Nav1.9 in subpopulations of DRG neurons through development. Immunoreactivity of cells positive for Nav1.9 (red), marker population (green), or both (yellow) (composite image) in Nav1.9 colocalization studies with TrkA (left), IB4 (middle), and NF200 (right) in DRG neurons at developmental ages E17, P0, and P7. Scale bars, 50 μm.
DISCUSSION
The expression of voltage-gated ion channels is fundamental to neuronal excitability and, therefore, to spontaneous and stimulus-evoked activity in primary sensory neurons. The regulation of ion channel expression may be influenced by both intrinsic developmental signals, such as time-dependent changes in transcription factors, and extrinsic developmental events, such as changes in neurotrophic factor expression, so that the distribution of ion channels may differ within the developing and mature nervous systems. In this study, we characterized the developmental expression of two TTXr sensory neuron-specific sodium channels, Nav1.8 (SNS) and Nav1.9 (SNS2), within defined neuronal populations of the DRG.
Nav1.8 is the first TTXr sodium channel to be expressed, at E15, before both its larger putative alternatively spliced transcript and Nav1.9, which becomes detectable only at E17. The distribution of Nav1.9 in small C-fiber neurons (Dib-Hajj et al., 1998a; Amaya et al., 2000) may explain its later onset of expression compared with Nav1.8, because C-fiber cells develop later than larger A-fiber neurons (Altman and Bayer, 1984). The level of expression of both Nav1.8 and Nav1.9 increased with increasing development, reaching adult levels by P7, with a higher proportion of total neuronal profiles expressing Nav1.8 than Nav1.9 at all stages in development. Nav1.9, as in the adult, is effectively restricted to small diameter sensory neurons at all stages, whereas Nav1.8 is expressed mainly in small diameter DRG neurons but in some medium-sized NF200-positive neurons as well. By P21, both Nav1.8 and Nav1.9 exhibit similar distribution patterns to that found in the adult (Amaya et al., 2000).
Nav1.8 and Nav1.9 appear to be regulated by distinct mechanisms
Despite similar and largely overlapping distribution patterns of both the TTXr channels in small diameter adult sensory neurons (Amaya et al., 2000), the expression of Nav1.8 and Nav1.9 within defined neuronal subpopulations differed substantially in early development. At E17, Nav1.8 is expressed mostly in TrkA-positive, NGF-responsive neurons, with little expression in the IB4-binding population. Since TrkA neurons differentiate into IB4-positive, c-ret-expressing GDNF-responsive neurons at the time of birth (Bennett et al., 1996; Molliver and Snider, 1997; Molliver et al., 1997), the proportion of Nav1.8 colocalization within this IB4-binding population increases proportionally, with a simultaneous relative decrease in TrkA–Nav1.8-expressing neurons. In contrast, at the onset of Nav1.9 expression at E17, approximately half of the Nav1.9-positive population co-express IB4, although only a very small minority of total DRG neurons are IB4-positive at this time. The remaining half colocalize with TrkA, which is the neuronal population that constitutes the vast majority of small diameter sensory neurons at this stage (Bennett et al., 1996; Molliver and Snider, 1997). This difference in the embryonic distribution pattern of Nav1.8 and Nav1.9 suggests that the cellular pattern of expression of each channel is likely to be controlled by distinct promoter regions, responding to different transcription factors in the late embryonic period.
In the adult, there is evidence to suggest an NGF- and GDNF-dependent regulation of Nav1.8 (Hinson et al., 1997;Dib-Hajj et al., 1998a; Fjell et al., 1999a,b) and Nav1.9 (Fjell et al., 1999c; Cummins et al., 2000), respectively, although both channels are expressed by TrkA and IB4-positive cells (Amaya et al., 2000). At the time of IB4-positive (GDNF-responsive) cell differentiation (at E17), these neurons express Nav1.9 before Nav1.8. The onset of Nav1.9 expression at E17 coincides, moreover, with the release of GDNF from Schwann cells between E14 and E16 (Wright and Snider, 1996) (Fig. 5). Nav1.8 expression at E15 coincides with the beginning of A- and C-fiber distal target innervation (Molliver and Snider, 1997). This suggests that Nav1.8 may be regulated by target-derived neurotrophic factors (Dib-Hajj et al., 1998a; Fjell et al., 1999a,b,c) (Fig. 5) because NGF expression begins in the skin at time of innervation (Thoenen et al., 1988;Elkabes et al., 1994). Both Nav1.8 and Nav1.9 are downregulated in the adult by peripheral axotomy (Dib-Hajj et al., 1998b; McCleskey and Gold, 1999) but not by rhizotomy (Sleeper et al., 2000), supporting the hypothesis of a regulatory mechanism arising from peripheral target-derived signals.
Fig. 5.
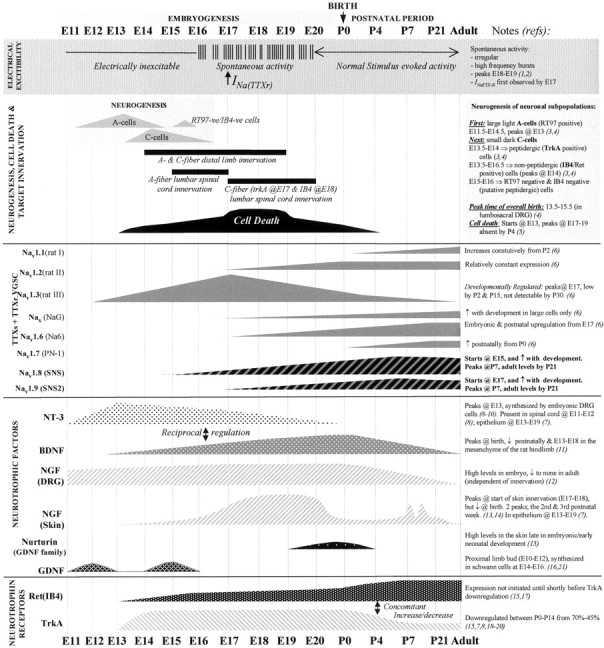
A summary of significant events defining rat DRG sensory neuron development. A schematic representation showing a time scale of developmentally regulated events of a typical DRG is illustrated from embryonic age 11 (E11) to postnatal age 21 (P21) and in adult animals (left toright of the diagram; not to scale). This is an attempt to correlate the major changes that occur in the developing DRG, with particular emphasis on acquisition of neuronal electrical excitability and changes in the expression of voltage-gated sodium channels (VGSCs). The expression of the sodium channels may be regulated by the developmental expression of specific neurotrophic factors. The top (shaded)panel illustrates the distinct patterns of electrical activity in the developing DRG, marking the period of developmentally regulated ectopic spontaneous discharge from E16 to E20, and the earliest detection of a TTX-resistant (TTXr) sodium current. The second panel outlines key developmental events, including the birth of specific neuronal subpopulations; the large light A-cell population (future A fibers) are born from E11.5 to E14.5, in advance of the birth of small, dark C-cell neurons (giving rise to C-fibers) which occurs later at E13.5–E16.5. A third unidentified subpopulation of putative nonpeptide-containing neurons (RT97-negative and IB4-negative) are generated between E14 and E15. Almost immediately after neuronal birth, the onset of axon outgrowth begins from E14 onward, followed by peripheral innervation of both A- and C-fibers simultaneously from E14 and central target innervation from E15 or E18 for A- and C-fibers, (Figure legend continues.) (Figure legend continued.) respectively. Themiddle panel shows changes in mRNA expression of known TTX-sensitive (TTXs) and the TTX-resistant (TTXr) voltage-gated sodium channels (VGSC) in the DRG, which may be correlated with developmentally regulated changes in neurotrophic factors (second panel from bottom) or changes in neuronal phenotype, which is indicated by a shift in the expression of specific neurotrophic receptors (bottom panel).Dotted lines indicate that expression at earlier ages has not yet been investigated. Arrows in the text denote an increase or decrease in expression, respectively. References are indicated by numbers on right side of figure: 1.Fitzgerald, 1987; 2.Fitzgerald and Fulton, 1992; 3. Jackman and Fitzgerald, 2000; 4.Altman and Bayer, 1984; 5.Coggeshall et al., 1994; 6.Felts et al., 1997;7. Ernfors et al., 1992; 8.Ernfors et al., 1989; 9.ElShamy and Ernfors, 1996;10.Schecterson and Bothwell, 1992; 11.Sebert and Shooter, 1993; 12.Constantinou et al., 1994.13. Davis et al., 1987; 14.Rohrer et al., 1998; 15.Molliver et al., 1997b;16.Alvares and Fitzgerald, 1999; 17.Bennett et al., 1996; 18.Phillips and Armanini, 1996;19.Farinas et al., 1998; 20.Ehrhard and Otten, 1994; 21. Wright and Snider, 1996.
Role of Nav1.8 and Nav1.9 in sensory neurons during development
The earliest electrophysiological recordings of TTXr currents have been from E17 rat DRG neurons (Ogata and Tatebayashi, 1992), fetal mouse, and cultured fetal human DRG neurons (Orozco et al., 1988; Caviedes et al., 1990). When Nav1.8 sodium channels are first expressed in rat lumbar DRG at E15, sensory neurons are electrically inexcitable (Fig.5). However, the characteristic fast repriming kinetics of Nav1.8 (Rush et al., 1998; Waxman, 1999; Waxman et al., 1999; Renganathan et al., 2000), which allows sustained action potential firing during prolonged depolarization, may mean that it contributes to the observed spontaneous activity in DRG neurons between E16 and E20 (Fitzgerald, 1987; Meister et al., 1991;Fields, 1998). Although the role of this period of spontaneous activity remains unclear, it spans the period of peripheral target innervation (Jackman and Fitzgerald, 2000), C-fiber central target innervation and synaptogenesis (Jackman and Fitzgerald, 2000), and has been suggested to play an important role in promoting neuronal differentiation (Gu and Spitzer, 1997), activity-dependent regulation of specific genes (Holliday and Spitzer, 1990; Dutton et al., 1993; Fields, 1996,1998), and the stabilization of synapses (Greensmith and Vrbova, 1996). Furthermore, the onset of expression of Nav1.8 coincides with the period of time when sodium channel β1-subunit mRNA is upregulated (Zur et al., 1995), which may contribute to the regulation and stability of the functional sodium channel (Catterall, 2000). However, as Nav1.8 null mutant mice display no obvious evidence of developmental abnormalities (Akopian et al., 1999; Cummins et al., 1999), it is possible that Nav1.8 contributes only slightly (Matsuda et al., 1978), if at all, to the differentiation of primary sensory neurons and the spinal neurons they innervate. A transgenic knockdown of Nav1.9 and double knockouts will help establish whether TTXr sodium currents play a role in the maturation of the peripheral nervous system.
TTXr sodium currents have been shown to contribute to the initiation of action potential depolarization in immature neurons (Orozco et al., 1988), so expression of Nav1.8 and Nav1.9 in embryonic and neonatal sensory neurons may contribute to the capacity of these neurons to conduct electrical signals to the spinal cord. In the adult, the TTXr sodium currents in the peripheral terminals of nociceptors contribute to the regulation of the excitability of the terminals. Inflammatory mediators such as Prostaglandin E2(PGE2), for example, appear to contribute to peripheral sensitization by a PKA-dependent phosphorylation of Nav1.8, increasing the sodium current density (Jeftinija, 1994, England et al., 1996; Gold et al., 1996, 1998; Tanaka et al., 1998; Gold, 1999). The contribution of TTXr sodium channels to injury- and inflammatory-induced pain hypersensitivity at early developmental stages remains to be examined.
Reciprocal regulation of TTXr sodium channels and Nav1.3
The onset of Nav1.9 expression at E17 correlates with the start of embryonic Nav1.3 sodium channel downregulation (Waxman et al., 1994; Felts et al., 1997). The opposite pattern occurs after adult peripheral nerve injury in which downregulation of both Nav1.8 and Nav1.9 is accompanied by a simultaneous upregulation of Nav1.3 (Waxman et al., 1994;Rizzo et al., 1995; Black et al., 1997, 1999; Cummins and Waxman, 1997). Our data are in agreement with the findings by Black et al. (1997), suggesting reciprocal developmental and injury-induced regulation of Nav1.8 and Nav1.9 with Nav1.3. Future studies directed toward the isolation and functional analysis of transcriptional promoter regions from Nav1.3, Nav1.8, and Nav1.9 genes may shed light on potential mechanisms that underlie this reciprocal regulation.
Conclusions
Figure 5 illustrates the relative timing of key events in the developing rat lumbar DRGs, including electrical activity, target innervation, exposure to growth factors, and sodium channel expression. This figure is an attempt to correlate the major changes that occur in the developing DRG, with particular emphasis on the link between the acquisition of neuronal electrical excitability and the expression of specific voltage-gated sodium channels (VGSCs). Expression of the VGSCs may be controlled by the developmental expression of specific neurotrophic factors that may regulate the differentiation of the neurons. Responsiveness of the neurons to these neurotrophic factors is contingent on expression of their cognate receptors. Key events, including birth of different subsets of neurons, natural cell death, axonal outgrowth, and peripheral and central target innervation are illustrated. The transcriptional regulation of the sodium channels, their expression only in particular subsets of neurons, their specific contribution to the electrical activity of immature neurons, and the consequence of this for differentiation is still poorly understood. Nevertheless, elucidation of the differential developmental patterns of expression of these channels, including Nav1.8 and Nav1.9, will provide a basis for beginning to understand the onset and maturation of excitability in primary sensory neurons.
Footnotes
This work was supported by GlaxoWellcome and grants from the National Institutes of Health (NS38253-01/NS39518-01 to C.J.W.) and the Medical Research Council (United Kingdom). We thank Isabelle Decosterd, Andrew Allchorne, Simon Beggs, Jacqueta Meredith-Middleton, Fumi Amaya, and Chris Plumpton for their technical assistance and advice.
Correspondence should be addressed to Clifford J. Woolf, Neural Plasticity Research Group, Department of Anesthesia and Critical Care, Massachusetts General Hospital and Harvard Medical School, 13th Street, Building 149 (#4309), Charlestown, MA 02129. E-mail:Woolf.clifford@mgh.harvard.edu.
REFERENCES
- 1.Akopian AN, Sivilotti L, Wood JN. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature. 1996;379:257–262. doi: 10.1038/379257a0. [DOI] [PubMed] [Google Scholar]
- 2.Akopian AN, Souslova V, England S, Okuse K, Ogata N, Ure J, Smith A, Kerr BJ, McMahon SB, Boyce S, Hill R, Stanfa LC, Dickenson AH, Wood JN. The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways. Nat Neurosci. 1999;2:541–548. doi: 10.1038/9195. [DOI] [PubMed] [Google Scholar]
- 3.Altman J, Bayer SA. The development of the rat spinal cord. Adv Anat Embryol Cell Biol. 1984;85:1–164. doi: 10.1007/978-3-642-69537-7. [DOI] [PubMed] [Google Scholar]
- 4.Alvares D, Fitzgerald M. Building blocks of pain: the regulation of key molecules in spinal sensory neurones during development and following peripheral axotomy. Pain. 1999;6:S71–85. doi: 10.1016/S0304-3959(99)00140-2. [DOI] [PubMed] [Google Scholar]
- 5.Amaya F, Decosterd I, Samad TA, Plumpton C, Tate S, Mannion RJ, Costigan M, Woolf CJ. Diversity of expression of the sensory neuron-specific TTX-resistant voltage-gated sodium ion channels SNS and SNS2. Mol Cell Neurosci. 2000;15:331–342. doi: 10.1006/mcne.1999.0828. [DOI] [PubMed] [Google Scholar]
- 6.Arbuckle JB, Docherty RJ. Expression of tetrodotoxin-resistant sodium channels in capsaicin-sensitive dorsal root ganglion neurons of adult rats. Neurosci Lett. 1995;185:70–73. doi: 10.1016/0304-3940(94)11227-a. [DOI] [PubMed] [Google Scholar]
- 7.Averill S, McMahon SB, Clary DO, Reichardt LF, Priestley JV. Immunocytochemical localization of trkA receptors in chemically identified subgroups of adult rat sensory neurons. Eur J Neurosci. 1995;7:1484–1494. doi: 10.1111/j.1460-9568.1995.tb01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bennett DL, Averill S, Clary DO, Priestley JV, McMahon SB. Postnatal changes in the expression of the trkA high-affinity NGF receptor in primary sensory neurons. Eur J Neurosci. 1996;10:2204–2208. doi: 10.1111/j.1460-9568.1996.tb00742.x. [DOI] [PubMed] [Google Scholar]
- 9.Black JA, Dib-Hajj S, McNabola K, Jeste S, Rizzo MA, Kocsis JD, Waxman SG. Spinal sensory neurons express multiple sodium channel α-subunit mRNAs. Brain Res Mol Brain Res. 1996;43:117–131. doi: 10.1016/s0169-328x(96)00163-5. [DOI] [PubMed] [Google Scholar]
- 10.Black JA, Langworthy K, Hinson AW, Dib-Hajj SD, Waxman SG. NGF has opposing effects on Na+ channel III and SNS gene expression in spinal sensory neurons. NeuroReport. 1997;8:2331–2335. doi: 10.1097/00001756-199707070-00046. [DOI] [PubMed] [Google Scholar]
- 11.Black JA, Cummins TR, Plumpton C, Chen YH, Hormuzdiar W, Clare JJ, Waxman SG. Upregulation of a silent sodium channel after peripheral, but not central, nerve injury in DRG neurons. J Neurophysiol. 1999;82:2776–2785. doi: 10.1152/jn.1999.82.5.2776. [DOI] [PubMed] [Google Scholar]
- 12.Brosenitsch T, Salgado-Commissariat D, Kunze DL, Katz DM. A role for L-type calcium channels in developmental regulation of transmitter phenotype in primary sensory neurons. J Neurosci. 1998;18:1047–1055. doi: 10.1523/JNEUROSCI.18-03-01047.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caffrey JM, Eng DL, Black JA, Waxman SG, Kocsis JD. Three types of sodium channels in adult rat dorsal root ganglion neurons. Brain Res. 1992;592:283–297. doi: 10.1016/0006-8993(92)91687-a. [DOI] [PubMed] [Google Scholar]
- 14.Catterall WA. Cellular and molecular biology of voltage-gated sodium channels. Physiol Rev. 1992;72:S15–48. doi: 10.1152/physrev.1992.72.suppl_4.S15. [DOI] [PubMed] [Google Scholar]
- 15.Catterall WA. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron. 2000;26:13–25. doi: 10.1016/s0896-6273(00)81133-2. [DOI] [PubMed] [Google Scholar]
- 16.Caviedes P, Ault B, Rapoport SI. The role of altered sodium currents in action potential abnormalities of cultured dorsal root ganglion neurons from trisomy 21 (Down syndrome) human fetuses. Brain Res. 1990;510:229–236. doi: 10.1016/0006-8993(90)91372-n. [DOI] [PubMed] [Google Scholar]
- 17.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 18.Coggeshall RE, Pover CM, Fitzgerald M. Dorsal root ganglion cell death and surviving cell numbers in relation to the development of sensory innervation in the rat hindlimb. Brain Res Dev Brain Res. 1994;82:193–212. doi: 10.1016/0165-3806(94)90163-5. [DOI] [PubMed] [Google Scholar]
- 19.Constantinou J, Reynolds ML, Woolf CJ, Safieh-Garabedian B, Fitzgerald M. Nerve growth factor levels in developing rat skin: upregulation following skin wounding. NeuroReport. 1994;5:2281–2284. doi: 10.1097/00001756-199411000-00019. [DOI] [PubMed] [Google Scholar]
- 20.Costigan M, Mannion RJ, Kendall G, Lewis SE, Campagna JA, Coggeshall RE, Meridith-Middleton J, Tate S, Woolf CJ. Heat shock protein 27: developmental regulation and expression after peripheral nerve injury. J Neurosci. 1998;18:5891–5900. doi: 10.1523/JNEUROSCI.18-15-05891.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cummins TR, Waxman SG. Downregulation of tetrodotoxin-resistant sodium currents and upregulation of a rapidly repriming tetrodotoxin-sensitive sodium current in small spinal sensory neurons after nerve injury. J Neurosci. 1997;17:3503–3514. doi: 10.1523/JNEUROSCI.17-10-03503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cummins TR, Howe JR, Waxman SG. Slow closed-state inactivation: a novel mechanism underlying ramp currents in cells expressing the hNE/PN1 sodium channel. J Neurosci. 1998;18:9607–9619. doi: 10.1523/JNEUROSCI.18-23-09607.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cummins TR, Dib-Hajj SD, Black JA, Akopian AN, Wood JN, Waxman SG. A novel persistent tetrodotoxin-resistant sodium current in SNS-null and wild-type small primary sensory neurons. J Neurosci 19 1999. RC43(1–6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cummins TR, Black JA, Dib-Hajj SD, Waxman SG. Glial-derived neurotrophic factor upregulates expression of functional SNS and NaN sodium channels and their currents in axotomized dorsal root ganglion neurons. J Neurosci. 2000;20:8754–8761. doi: 10.1523/JNEUROSCI.20-23-08754.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis AM, Bandtlow C, Heumann R, Korsching S, Rohrer, Theonen H. Timing and site of nerve growth factor synthesis in developing skin in relation to innervation and expression of the receptor. Nature. 1987;326:353–358. doi: 10.1038/326353a0. [DOI] [PubMed] [Google Scholar]
- 26.Dib-Hajj SD, Black JA, Cummins TR, Kenney AM, Kocsis JD, Waxman SG. Rescue of α-SNS sodium channel expression in small dorsal root ganglion neurons after axotomy by nerve growth factor in vivo. J Neurophysiol. 1998a;79:2668–2676. doi: 10.1152/jn.1998.79.5.2668. [DOI] [PubMed] [Google Scholar]
- 27.Dib-Hajj SD, Tyrrell L, Black JA, Waxman SG. NaN, a novel voltage-gated Na channel, is expressed preferentially in peripheral sensory neurons and down-regulated after axotomy. Proc Natl Acad Sci USA. 1998b;95:8963–8968. doi: 10.1073/pnas.95.15.8963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dib-Hajj SD, Tyrrell L, Cummins TR, Black JA, Wood PM, Waxman SG. Two tetrodotoxin-resistant sodium channels in human dorsal root ganglion neurons. FEBS Lett. 1999;462:117–120. doi: 10.1016/s0014-5793(99)01519-7. [DOI] [PubMed] [Google Scholar]
- 29.Dietzel ID. Voltage-gated ion currents in embryogenesis. Perspect Dev Neurobiol. 1995;2:293–308. [PubMed] [Google Scholar]
- 30.Dutton EK, Simon AM, Burden SJ. Electrical activity-dependent regulation of the acetylcholine receptor delta-subunit gene, MyoD, and myogenin in primary myotubes. Proc Natl Acad Sci USA. 1993;90:2040–2044. doi: 10.1073/pnas.90.5.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ehrhard PB, Otten U. Postnatal ontogeny of the neurotrophin receptors trk and trkB mRNA in rat sensory and sympathetic ganglia. Neurosci Lett. 1994;166:207–210. doi: 10.1016/0304-3940(94)90487-1. [DOI] [PubMed] [Google Scholar]
- 32.Elkabes S, Dreyfus CF, Schaar DG, Black IB. Embryonic sensory development: local expression of neurotrophin-3 and target expression of nerve growth factor. J Comp Neurol. 1994;341:204–213. doi: 10.1002/cne.903410206. [DOI] [PubMed] [Google Scholar]
- 33.Elliott AA, Elliott JR. Characterization of TTX-sensitive and TTX-resistant sodium currents in small cells from adult rat dorsal root ganglia. J Physiol (Lond) 1993;463:39–56. doi: 10.1113/jphysiol.1993.sp019583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.ElShamy WM, Ernfors P. A local action of neurotrophin-3 prevents the death of proliferating sensory neuron precursor cells. Neuron. 1996;16:963–972. doi: 10.1016/s0896-6273(00)80119-1. [DOI] [PubMed] [Google Scholar]
- 35.England S, Bevan S, Docherty RJ. PGE2 modulates the tetrodotoxin-resistant sodium current in neonatal rat dorsal root ganglion neurones via the cyclic AMP-protein kinase A cascade. J Physiol (Lond) 1996;495:429–440. doi: 10.1113/jphysiol.1996.sp021604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ernfors P, Henschen A, Olsen L, Persson H. Expression of nerve growth factor mRNA is developmentally regulated and increased after axotomy in rat spinal cord neurons. Neuron. 1989;2:1605–1612. doi: 10.1016/0896-6273(89)90049-4. [DOI] [PubMed] [Google Scholar]
- 37.Ernfors P, Merlio J-P, Persson H. Cells expressing mRNA for neurotrophins and their receptors during embryonic rat development. Eur J Neurosci. 1992;4:1140–1158. doi: 10.1111/j.1460-9568.1992.tb00141.x. [DOI] [PubMed] [Google Scholar]
- 38.Farinas I, Wilkinson GA, Backus C, Reichardt LF, Patapoutian A. Characterization of neurotrophin and Trk receptor functions in developing sensory ganglia: direct NT-3 activation of TrkB neurons in vivo. Neuron. 1998;21:325–334. doi: 10.1016/s0896-6273(00)80542-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Felts PA, Yokoyama S, Dib-Hajj S, Black JA, Waxman SG. Sodium channel α-subunit mRNAs I, II, III, NaG, Na6 and hNE (PN1): different expression patterns in developing rat nervous system. Brain Res Mol Brain Res. 1997;45:71–82. doi: 10.1016/s0169-328x(96)00241-0. [DOI] [PubMed] [Google Scholar]
- 40.Fields RD. Signaling from neural impulses to genes. The Neuroscientist. 1996;2:315–325. [PMC free article] [PubMed] [Google Scholar]
- 41.Fields RD. Effects of ion channel activity on development of dorsal root ganglion neurons. J Neurobiol. 1998;37:158–170. doi: 10.1002/(sici)1097-4695(199810)37:1<158::aid-neu12>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 42.Fitzgerald M. Spontaneous and evoked activity of fetal primary afferents in vivo. Nature. 1987;326:603–605. doi: 10.1038/326603a0. [DOI] [PubMed] [Google Scholar]
- 43.Fitzgerald M, Fulton PB. The physiological properties of developing sensory neurons. In: Scott SA, editor. Sensory neurons. Oxford UP; New York: 1992. pp. 287–306. [Google Scholar]
- 44.Fjell J, Cummins TR, Davis BM, Albers KM, Fried K, Waxman SG, Black JA. Sodium channel expression in NGF-overexpressing transgenic mice. J Neurosci Res. 1999a;57:39–47. doi: 10.1002/(SICI)1097-4547(19990701)57:1<39::AID-JNR5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 45.Fjell J, Cummins TR, Dib-Hajj SD, Fried K, Black JA, Waxman SG. Differential role of GDNF and NGF in the maintenance of two TTX-resistant sodium channels in adult DRG neurons. Brain Res Mol Brain Res. 1999b;67:267–282. doi: 10.1016/s0169-328x(99)00070-4. [DOI] [PubMed] [Google Scholar]
- 46.Fjell J, Cummins TR, Fried K, Black JA, Waxman SG. In vivo NGF deprivation reduces SNS expression and TTX-R sodium currents in IB4-negative DRG neurons. J Neurophysiol. 1999c;81:803–810. doi: 10.1152/jn.1999.81.2.803. [DOI] [PubMed] [Google Scholar]
- 47.Fjell J, Hjelmstrom P, Hormuzdiar W, Milenkovic M, Aglieco F, Tyrrell L, Dib-Hajj S, Waxman SG, Black JA. Localization of the tetrodotoxin-resistant sodium channel NaN in nociceptors. NeuroReport. 2000;11:199–202. doi: 10.1097/00001756-200001170-00039. [DOI] [PubMed] [Google Scholar]
- 48.Greensmith L, Vrbova G. Motorneurone survival: a functional approach. Trends Neurosci. 1996;11:450–455. doi: 10.1016/s0166-2236(96)20034-7. [DOI] [PubMed] [Google Scholar]
- 49.Gold MS. Tetrodotoxin-resistant Na+ currents and inflammatory hyperalgesia. Proc Natl Acad Sci USA. 1999;96:7645–7649. doi: 10.1073/pnas.96.14.7645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gold MS, Reichling DB, Shuster MJ, Levine JD. Hyperalgesic agents increase a tetrodotoxin-resistant Na+ current in nociceptors. Proc Natl Acad Sci USA. 1996;93:1108–1112. doi: 10.1073/pnas.93.3.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gold MS, Levine JD, Correa AM. Modulation of TTX-R INa by PKC and PKA and their role in PGE2-induced sensitization of rat sensory neurons in vitro. J Neurosci. 1998;18:10345–10355. doi: 10.1523/JNEUROSCI.18-24-10345.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goldin AL, Barchi RL, Caldwell JH, Hofmann F, Howe JR, Hunter JC, Kallen RG, Mandel G, Meisler MH, Netter YB, Noda M, Tamkun MM, Waxman SG, Wood JN, Catterall WA. Nomenclature of voltage-gated sodium channels. Neuron. 2000;28:365–368. doi: 10.1016/s0896-6273(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 53.Gu X, Spitzer NC. Distinct aspects of neuronal differentiation encoded by frequency of spontaneous Ca2+ transients. Nature. 1995;375:784–787. doi: 10.1038/375784a0. [DOI] [PubMed] [Google Scholar]
- 54.Gu X, Spitzer NC. Breaking the code: regulation of neuronal differentiation by spontaneous calcium transients. Dev Neurosci. 1997;19:33–41. doi: 10.1159/000111183. [DOI] [PubMed] [Google Scholar]
- 55.Hinson AW, Gu XQ, Dib-Hajj S, Black JA, Waxman SG. Schwann cells modulate sodium channel expression in spinal sensory neurons in vitro. Glia. 1997;21:339–349. doi: 10.1002/(sici)1098-1136(199712)21:4<339::aid-glia1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 56.Holliday J, Spitzer NC. Spontaneous calcium influx and its roles in differentiation of spinal neurons in culture. Dev Biol. 1990;141:13–23. doi: 10.1016/0012-1606(90)90098-4. [DOI] [PubMed] [Google Scholar]
- 57.Jackman A, Fitzgerald M. Development of peripheral hindlimb and central spinal cord innervation by subpopulations of dorsal root ganglion cells in the embryonic rat. J Comp Neurol. 2000;418:281–298. [PubMed] [Google Scholar]
- 58.Jeftinija S. Bradykinin excites tetrodotoxin-resistant primary afferent fibers. Brain Res. 1994;665:69–76. doi: 10.1016/0006-8993(94)91153-3. [DOI] [PubMed] [Google Scholar]
- 59.Kostyuk PG, Veselovsky NS, Tsyndrenko AY. Ionic currents in the somatic membrane of rat dorsal root ganglion neurons. I. Sodium currents. Neuroscience. 1981;6:2423–2430. doi: 10.1016/0306-4522(81)90088-9. [DOI] [PubMed] [Google Scholar]
- 60.Lawson SN, Waddell PJ. Soma neurofilament immunoreactivity is related to cell size and fiber conduction velocity in rat primary sensory neurons. J Physiol (Lond) 1991;435:41–63. doi: 10.1113/jphysiol.1991.sp018497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lawson SN, Perry MJ, Prabhakar E, McCarthy PW. Primary sensory neurones: neurofilament, neuropeptides, and conduction velocity. Brain Res Bull. 1993;30:239–243. doi: 10.1016/0361-9230(93)90250-f. [DOI] [PubMed] [Google Scholar]
- 62.Matsuda Y, Yoshida S, Yonezawa T. Tetrodotoxin sensitivity and Ca component of action potentials of mouse dorsal root ganglion cells cultured in vitro. Brain Res. 1978;154:69–82. doi: 10.1016/0006-8993(78)91052-1. [DOI] [PubMed] [Google Scholar]
- 63.McCleskey EW, Gold MS. Ion channels of nociception. Annu Rev Physiol. 1999;61:835–856. doi: 10.1146/annurev.physiol.61.1.835. [DOI] [PubMed] [Google Scholar]
- 64.McLean MJ, Bennet PB, Thomas RM. Subtypes of dorsal root ganglion neurons based upon different inward currents as measured by whole cell voltage clamp. Mol Cell Biochem. 1988;80:90–107. doi: 10.1007/BF00231008. [DOI] [PubMed] [Google Scholar]
- 65.McMahon SB, Armanini MP, Ling LH, Phillips HS. Expression and coexpression of Trk receptors in subpopulations of adult primary sensory neurons projecting to identified peripheral targets. Neuron. 1994;12:1161–1171. doi: 10.1016/0896-6273(94)90323-9. [DOI] [PubMed] [Google Scholar]
- 66.Meister M, Wong RO, Baylor DA, Shatz CJ. Synchronous bursts of action potentials in ganglion cells of the developing mammalian retina. Science. 1991;252:939–943. doi: 10.1126/science.2035024. [DOI] [PubMed] [Google Scholar]
- 67.Michael GJ, Averill S, Nitkunan A, Rattray M, Bennett DL, Yan Q, Priestley JV. Nerve growth factor treatment increases brain-derived neurotrophic factor selectively in TrkA-expressing dorsal root ganglion cells and in their central terminations within the spinal cord. J Neurosci. 1997;17:8476–8490. doi: 10.1523/JNEUROSCI.17-21-08476.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Molliver DC, Snider WD. Nerve growth factor receptor TrkA is down-regulated during postnatal development by a subset of dorsal root ganglion neurons. J Comp Neurol. 1997;381:428–438. doi: 10.1002/(sici)1096-9861(19970519)381:4<428::aid-cne3>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 69.Molliver DC, Radeke MJ, Feinstein SC, Snider WD. Presence or absence of TrkA protein distinguishes subsets of small sensory neurons with unique cytochemical characteristics and dorsal horn projections. J Comp Neurol. 1995;361:404–416. doi: 10.1002/cne.903610305. [DOI] [PubMed] [Google Scholar]
- 70.Molliver DC, Wright DE, Leitner ML, Parsadanian AS, Doster K, Wen D, Yan Q, Snider WD. IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron. 1997;19:849–861. doi: 10.1016/s0896-6273(00)80966-6. [DOI] [PubMed] [Google Scholar]
- 71.Ogata N, Tatebayashi H. Ontogenic development of the TTX-sensitive and TTX-insensitive Na+ channels in neurons of the rat dorsal root ganglia. Brain Res Dev Brain Res. 1992;65:93–100. doi: 10.1016/0165-3806(92)90012-l. [DOI] [PubMed] [Google Scholar]
- 72.Okuse K, Chaplan SR, McMahon SB, Luo ZD, Calcutt NA, Scott BP, Akopian AN, Wood JN. Regulation of expression of the sensory neuron-specific sodium channel SNS in inflammatory and neuropathic pain. Mol Cell Neurosci. 1997;10:196–207. doi: 10.1006/mcne.1997.0657. [DOI] [PubMed] [Google Scholar]
- 73.Orozco CB, Epstein CJ, Rapoport SI. Voltage-activated sodium conductances in cultured normal and trisomy 16 dorsal root ganglion neurons from the fetal mouse. Brain Res. 1988;466:265–274. doi: 10.1016/0165-3806(88)90052-1. [DOI] [PubMed] [Google Scholar]
- 74.Phillips HS, Armanini MP. Expression of the trk family of neurotrophin receptors in developing and adult dorsal root ganglion neurons. Philos Trans R Soc Lond B Biol Sci. 1996;351:413–416. doi: 10.1098/rstb.1996.0036. [DOI] [PubMed] [Google Scholar]
- 75.Plenderleith MB, Snow PJ. The plant lectin Bandeiraea simplicifolia I-B4 identifies a subpopulation of small diameter primary sensory neurones which innervate the skin in the rat. Neurosci Lett. 1993;159:17–20. doi: 10.1016/0304-3940(93)90787-l. [DOI] [PubMed] [Google Scholar]
- 76.Plenderleith MB, Cameron AA, Key B, Snow PJ. Soybean agglutinin binds to a subpopulation of primary sensory neurones in the cat. Neurosci Lett. 1988;86:257–262. doi: 10.1016/0304-3940(88)90492-2. [DOI] [PubMed] [Google Scholar]
- 77.Price J. An immunohistochemical and quantitative examination of dorsal root ganglion neuronal subpopulations. J Neurosci. 1985;5:2051–2059. doi: 10.1523/JNEUROSCI.05-08-02051.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Renganathan M, Cummins TR, Hormuzdiar WN, Waxman SG. α-SNS produces the slow TTX-resistant sodium current in large cutaneous afferent DRG neurons. J Neurophysiol. 2000;84:710–718. doi: 10.1152/jn.2000.84.2.710. [DOI] [PubMed] [Google Scholar]
- 79.Rizzo MA, Kocsis JD, Waxman SG. Selective loss of slow and enhancement of fast Na+ currents in cutaneous afferent dorsal root ganglion neurones following axotomy. Neurobiol Dis. 1995;2:87–96. doi: 10.1006/nbdi.1995.0009. [DOI] [PubMed] [Google Scholar]
- 80.Rohrer H, Heumann H, Theonen H. The synthesis of nerve growth factor (NGF) in developing skin is independent of innervation. Dev Biol. 1998;128:240–244. doi: 10.1016/0012-1606(88)90286-2. [DOI] [PubMed] [Google Scholar]
- 81.Roy ML, Narahashi T. Differential properties of tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels in rat dorsal root ganglion neurons. J Neurosci. 1992;12:2104–2111. doi: 10.1523/JNEUROSCI.12-06-02104.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rush AM, Brau ME, Elliott AA, Elliott JR. Electrophysiological properties of sodium current subtypes in small cells from adult rat dorsal root ganglia. J Physiol (Lond) 1998;511:771–789. doi: 10.1111/j.1469-7793.1998.771bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sangameswaran L, Delgado SG, Fish LM, Koch BD, Jakeman LB, Stewart GR, Sze P, Hunter JC, Eglen RM, Herman RC. Structure and function of a novel voltage-gated, tetrodotoxin-resistant sodium channel specific to sensory neurons. J Biol Chem. 1996;271:5953–5956. doi: 10.1074/jbc.271.11.5953. [DOI] [PubMed] [Google Scholar]
- 84.Schecterson LC, Bothwell M. Novel roles for neurotrophins are suggested by BDNF and NT-3 mRNA expression in developing neurons. Neuron. 1992;9:449–463. doi: 10.1016/0896-6273(92)90183-e. [DOI] [PubMed] [Google Scholar]
- 85.Sebert ME, Shooter EM. Expression of mRNA for neurotrophic factors and their receptors in the rat dorsal root ganglion and sciatic nerve following nerve injury. J Neurosci Res. 1993;36:357–367. doi: 10.1002/jnr.490360402. [DOI] [PubMed] [Google Scholar]
- 86.Sleeper AA, Cummins TR, Dib-Hajj SD, Hormuzdiar W, Tyrrell L, Waxman SG, Black JA. Changes in expression of two tetrodotoxin-resistant sodium channels and their currents in dorsal root ganglion neurons after sciatic nerve injury but not rhizotomy. J Neurosci. 2000;20:7279–7289. doi: 10.1523/JNEUROSCI.20-19-07279.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Spitzer NC, Olson E, Gu X. Spontaneous calcium transients regulate neuronal plasticity in developing neurons. J Neurobiol. 1995;26:316–324. doi: 10.1002/neu.480260304. [DOI] [PubMed] [Google Scholar]
- 88.Tanaka M, Cummins TR, Ishikawa K, Dib-Hajj SD, Black JA, Waxman SG. SNS Na+ channel expression increases in dorsal root ganglion neurons in the carrageenan inflammatory pain model. NeuroReport. 1998;9:967–972. doi: 10.1097/00001756-199804200-00003. [DOI] [PubMed] [Google Scholar]
- 89.Tate S, Benn S, Hick C, Trezise D, John V, Mannion RJ, Costigan M, Plumpton C, Grose D, Gladwell Z, Kendall G, Dale K, Bountra C, Woolf CJ. Two sodium channels contribute to the TTX-R sodium current in primary sensory neurons. Nat Neurosci. 1998;1:653–659. doi: 10.1038/3652. [DOI] [PubMed] [Google Scholar]
- 90.Thoenen H, Bandtlow C, Heumann R, Lindholm D, Meyer M, Rohrer H. Nerve growth factor: cellular localization and regulation of synthesis. Cell Mol Neurobiol. 1988;8:35–40. doi: 10.1007/BF00712909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Toledo-Aral JJ, Moss BL, He ZJ, Koszowski AG, Whisenand T, Levinson SR, Wolf JJ, Silos-Santiago I, Halegoua S, Mandel G. Identification of PN1, a predominant voltage-dependent sodium channel expressed principally in peripheral neurons. Proc Natl Acad Sci USA. 1997;94:1527–1532. doi: 10.1073/pnas.94.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tzoumaka E, Tischler AC, Sangameswaran L, Eglen RM, Hunter JC, Novakovic SD. Differential distribution of the tetrodotoxin-sensitive rPN4/NaCh6/Scn8a sodium channel in the nervous system. J Neurosci Res. 2000;60:37–44. doi: 10.1002/(SICI)1097-4547(20000401)60:1<37::AID-JNR4>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 93.Waxman SG. The molecular pathophysiology of pain: abnormal expression of sodium channel genes and its contributions to hyperexcitability of primary sensory neurons. Pain. 1999;6:S133–140. doi: 10.1016/S0304-3959(99)00147-5. [DOI] [PubMed] [Google Scholar]
- 94.Waxman SG, Kocsis JD, Black JA. Type III sodium channel mRNA is expressed in embryonic but not adult spinal sensory neurons, and is reexpressed following axotomy. J Neurophysiol. 1994;72:466–470. doi: 10.1152/jn.1994.72.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Waxman SG, Dib-Hajj S, Cummins TR, Black JA. Sodium channels and pain. Proc Natl Acad Sci USA. 1999;96:7635–7639. doi: 10.1073/pnas.96.14.7635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wright DE, Snider WD. Focal expression of glial cell line-derived neurotrophic factor in developing mouse limb bud. Cell Tissue Res. 1996;286:209–217. doi: 10.1007/s004410050689. [DOI] [PubMed] [Google Scholar]
- 97.Zur KB, Oh Y, Waxman SG, Black JA. Differential up-regulation of sodium channel α- and β 1-subunit mRNAs in cultured embryonic DRG neurons following exposure to NGF. Brain Res Mol Brain Res. 1995;30:97–105. doi: 10.1016/0169-328x(94)00283-k. [DOI] [PubMed] [Google Scholar]